Abstract
It is well-known that the pregnane X receptor (PXR)/Nr1i2 is a critical xenobiotic-sensing nuclear receptor enriched in liver and intestine and is responsible for drug-drug interactions, due to its versatile ligand binding domain (LBD) and target genes involved in xenobiotic biotransformation. PXR can be modulated by various xenobiotics including pharmaceuticals, nutraceuticals, dietary factors, and environmental chemicals. Microbial metabolites such as certain secondary bile acids (BAs) and the tryptophan metabolite indole-3-propionic acid (IPA) are endogenous PXR activators. Gut microbiome is increasingly recognized as an important regulator for host xenobiotic biotransformation and intermediary metabolism. PXR regulates and is regulated by the gut-liver axis. This review summarizes recent research advancements leveraging pharmaco- and toxico-metagenomic approaches that have redefined the previous understanding of PXR. Key topics covered in this review include: (1) genome-wide investigations on novel PXR-target genes, novel PXR-DNA interaction patterns, and novel PXR-targeted intestinal bacteria; (2) key PXR-modulating activators and suppressors of exogenous and endogenous sources; (3) novel bidirectional interactions between PXR and gut microbiome under physiologic, pathophysiological, pharmacological, and toxicological conditions; and (4) modifying factors of PXR-signaling including species and sex differences and time (age, critical windows of exposure, and circadian rhythm). The review also discusses critical knowledge gaps and important future research topics centering around PXR.
SIGNIFICANCE STATEMENT
This review summarizes recent research advancements leveraging O’mics approaches that have redefined the previous understanding of the xenobiotic-sensing nuclear receptor pregnane X receptor (PXR). Key topics include: (1) genome-wide investigations on novel PXR-targeted host genes and intestinal bacteria as well as novel PXR-DNA interaction patterns; (2) key PXR modulators including microbial metabolites under physiological, pathophysiological, pharmacological, and toxicological conditions; and (3) modifying factors including species, sex, and time.
Introduction
Brief Historical Perspective
Prior to the discovery of the pregnane X receptor (PXR), it was initially discovered that newborn rats exhibited higher sensitivity to nonmetabolized cardiac glycoside ouabainin in comparison with adults (Klaassen, 1972; Klaassen, 1973). This increased sensitivity was due to the lower hepatic uptake in newborns, whereas pretreatment of both newborn and adult rats with pregnenolone-16α-carbonitrile (PCN) markedly increased the hepatic uptake and biliary excretion of ouabain (Klaassen, 1974a; Klaassen, 1974b). PCN treatment in isolated rat hepatocytes markedly enhanced the uptake of another glycoside [3H] digoxin, which was a substrate of the organic anion transporter 2 (Oatp2, now known as solute carrier organic anion transporter family 1a4/Oatp1a4) (Eaton and Klaassen, 1979). It was later noted that PCN stimulated the enzyme activity of a single form of cytochrome P450 (P-450PCN) that was immunochemically distinguishable from another cytochrome P450 (CYP) inducible by phenobarbital in rat liver (P450-PB) (Newman and Guzelian, 1983). In addition, glucocorticoids such as dexamethasone could also induce P-450PCN in rat liver, and the notion of the existence of a class of microsomal enzyme inducers was proposed (Schuetz et al., 1984). These pioneer studies together supported the existence of a receptor that can be activated by chemicals such as PCN, which in turn coregulates the xenobiotic-inducible drug metabolism and transport systems (Staudinger et al., 2001).
At the turn of the century, the nuclear receptor PXR was discovered and was initially recognized as a major receptor for xenobiotics that transactivate many genes involved in drug metabolism and transport including Cyp3a (previously known as P-450PCN) and Oatp1a4 (previously known as Oatp2) (Moore and Kliewer, 2000; Kliewer et al., 2002; Tirona et al., 2003; Cheng et al., 2005; Maher et al., 2005; Kurihara et al., 2007; Petrick and Klaassen, 2007; Alnouti and Klaassen, 2008; Buckley and Klaassen, 2009; Hernandez et al., 2009; Aleksunes and Klaassen, 2012). PXR is highly expressed in the liver and intestine and can be activated by various drugs, nutraceuticals, dietary factors, and environmental chemicals (Moore et al., 2003), leading to drug-drug, food-drug, and drug-toxicant interactions. Although the DNA-binding domain of PXR is highly conserved across species (Kliewer et al., 2002), its ligand-binding domain displays more variability and versatility, which contribute to the species difference in PXR activation and target gene expression. Although being well-recognized as a classic drug-receptor over the years, studies using PXR-null and humanized PXR transgenic mice have demonstrated that PXR also contributes to intermediary metabolism through worsening diet-induced obesity, hepatic steatosis, and hepatic inflammation (Spruiell et al., 2014a; Spruiell et al., 2014b; Kim et al., 2021). However, pharmacological activation of PXR has antiinflammatory effects (Cheng et al., 2012; Huang et al., 2018). The physiologic functions of PXR have also included transactivating genes involved in lipid metabolism (Zhou et al., 2006; Zhou et al., 2008) and cell cycle (Guzelian et al., 2006).
Recent research advancements leveraging pharmaco- and toxico-metagenomic approaches have redefined our understanding of PXR. First, genome-wide investigations using chromatin immunoprecipitation coupled with second-generation sequencing have expanded the list of direct-PXR target genes and identified novel PXR-DNA interaction patterns (Cui et al., 2010; Smith et al., 2014). Although it was widely recognized based on in vitro investigations that PXR binds to AGGTCA-like direct repeats separated by 3 or 4 base pairs (DR-3 or DR-4) as well as everted repeats 6 (ER-6) and ER-8 (Kliewer et al., 2002), an in vivo roadmap of PXR bindings in mouse liver with extended search for longer response elements have identified that DR-4 is the most prevalent PXR-DNA binding motif in vivo, and more importantly, there is a novel DR-(5n+4) periodic pattern of PXR-DNA interactions (defined as an “accordion model”) (Cui et al., 2010). In addition, PXR-DNA interactions within the target gene loci are not limited to promoter regions but are also localized to regions such as distal enhancers, downstream, and introns (Cui et al., 2010). Second, although it was previously thought that PXR interacts with its binding partner retinoic X receptor (RXR) as a dimer, computational approaches have demonstrated that PXR and RXR function as a hetero-tetramer, with the unique β-stranded interface of the PXR homodimer in the middle and two RXR proteins on the outside (Noble et al., 2006; Teotico et al., 2008). In addition, the key amino acids forming the PXR homodimer interface are highly conserved during evolution, and disruption of these amino acids results in decreased transcription activity of PXR (Noble et al., 2006; Teotico et al., 2008). Third, novel endogenous PXR ligands and/or activators have been identified, which are microbial metabolites of bile acids (BAs) and tryptophan (Staudinger et al., 2001; Xie et al., 2001; Venkatesh et al., 2014). Along the same lines, novel bidirectional interactions of PXR and the gut microbiome have been discovered under physiologic, pathophysiological, pharmacological, and toxicological conditions (Cheng et al., 2018; Li et al., 2018; Dempsey et al., 2019; Scoville et al., 2019; Lim et al., 2020; Kim et al., 2021; Little et al., 2021). Last but not least, modifying factors including sex (Fu et al., 2012; Barretto et al., 2021); time, including liver development (Hart et al., 2009); early-life exposures to PXR-activating xenobiotics (Li et al., 2016a; Li et al., 2016b); aging (Fu et al., 2012); and circadian rhythm have been further elucidated. Ongoing research studies continue to expand our knowledge base regarding endogenous functions of PXR and novel target genes, novel PXR ligands/modulators, and PXR versus gut microbiome crosstalk.
This review will therefore provide an updated summary of the recent discoveries of PXR with a primary focus on PXR and the gut-liver axis within the context of exposures and discuss important further research topics in this area from our perspective.
PXR-Modulating Xenobiotics
PXR Activators
PXR is responsible for the regulation of various drug-metabolizing enzymes after activation by various ligands and/or activators (Kliewer et al., 1998). Due to its promiscuous ligand binding domain (LBD), a wide variety of possible ligands can interact with PXR and modulate complex drug-metabolizing enzymes expression through drug-drug interaction (Lehmann et al., 1998). Many synthetic drugs, natural products, environmental chemicals, as well as microbial metabolites and diets have been recognized as regulators of PXR signaling (Table 1) (Chang and Waxman, 2006). It should be noted that chemicals can only be classified as PXR ligands if the direct binding to the LBD of PXR has been established. It should also be noted that PXR-activating xenobiotics may have off-target effects that are not related to PXR signaling.
TABLE 1.
PXR-modulating chemicals
| Chemical IDs | Activator/Repressor | Species | Reference | ||
|---|---|---|---|---|---|
| Exogenous molecules | Drugs | Rifampicin | Activator and ligand | Human | (Blumberg et al., 1998; Li and Chiang, 2005; Li and Chiang, 2006) |
| RU486 | Activator | Human | (Banerjee et al., 2015; Kliewer, 2015; | ||
| Etomidoline | Activator | Human | Lynch et al., 2021) | ||
| Clotrimazole | Activator | Human, weak activatorin rodent | (Jones et al., 2000; Lehmann et al., 1998; Banerjee et al., 2015; Kliewer, 2015) | ||
| Colfibrate | Activator and ligand | Rat | (Daujat-Chavanieu and Gerbal-Chaloin, 2020) | ||
| Phenobarbital | Activator | Human | (Li et al., 2019; Tamasi et al., 2009) | ||
| Dexamethasone | Activator | Human androdent | (Pascussi et al., 2001; Yueh et al., 2005; Pascussi et al., 2000; Shi et al., 2010) | ||
| Cyclophosphamide (CPA) | Activator | Human | (Lindley et al., 2002; Zhuo et al., 2014) | ||
| Ritanovir | Activator | Human | (Chang and Waxman, 2006; Shehu et al., 2019; Brewer and Chen, 2016) | ||
| Paclitaxel | Activator | Human | (Kostrubsky et al., 1998) | ||
| Felodipine | Activator | Human | (Dybdahl et al., 2012; Pinne et al., 2016) | ||
| Nifedipine | Activator | Human | (Bertilsson et al., 1998; Chang and Waxman, 2006; Lynch et al., 2021) | ||
| Bendipine | Activator | Human | (Bertilsson et al., 1998; Chang and Waxman, 2006; Lynch et al., 2021) | ||
| Carbamazepine | Activator | Human | (El-Sankary et al., 2001; Luo et al., 2002) | ||
| Bosentan | Activator | Human | (van Giersbergen et al., 2002) | ||
| Mevastatin | Activator | Human | (Raucy et al., 2002) | ||
| Tamoxifen | Activator | Human | (Desai et al., 2002; Zhuo et al., 2014) | ||
| Topotecan | Activator | Human | (Schuetz et al., 2002) | ||
| Lacidipine | Activator | Human | (Bertilsson et al., 1998; Chang and Waxman, 2006; Lynch et al., 2021) | ||
| Resveratrol | Inhibitor | Human | (Smutny and Pavek, 2014), (Deng et al., 2014) | ||
| Ketoconazole | Inhibitor | Human | (Mani et al., 2013; Huang et al., 2007) | ||
| Exogenous molecules | Drugs | ET-743 | Inhibitor | Human | (Synold et al., 2001) |
| SPA70 | Inhibitor | Human | (Lin et al., 2017) | ||
| Phytochemicals | St. John’s wort (hyperforin) | Activator | Human | (Watkins et al., 2003; Kliewer, 2015) | |
| Forskolin | Activator | Human, mouse | (Seamon et al., 1981; Ding and Staudinger, 2005a) | ||
| Guggulsterones | Activator | Human, mouse | (Chang and Waxman, 2006; Brobst et al., 2004; Ding and Staudinger, 2005b) | ||
| Artemisinin | Activator | Human | (Burk et al., 2005) | ||
| Kava extract | Activator | Human | (Raucy, 2003), (Ma et al., 2004) | ||
| Coumestrol | Repressor | Human and humanized mouse | (Wang et al., 2008) | ||
| Sulforaphane | Repressor | Human | (Zhou et al., 2007) | ||
| Schiandrol B (SolB) | Activator | Human | (Zeng et al., 2017) | ||
| Environmental chemicals | Bisphenol A (BPA) | Activator | Human | (Sui et al., 2012; Barrett, 2012) | |
| PBDEs | Activator | Human, rodents | (Pacyniak et al., 2007; Sueyoshi et al., 2014) | ||
| PCBs | Activator | Human, rodents | (Egusquiza et al., 2020; Wahlang et al., 2014; Jacobs et al., 2005) | ||
| PFOA | Activator | Rodents | (Bjork et al., 2011; Ren et al., 2009) | ||
| PFOS | Activator | Human, rodents | (Bjork et al., 2011; Ren et al., 2009) | ||
| Phthalate monoesters | Activator | Human, rodents | (Hurst and Waxman, 2004; Cooper et al., 2008) | ||
| Metal ions (Pb+, Ag+), As | Activator | Zebrafish, humanized mouse | (Liu et al., 2019; Medina-Diaz et al., 2009) | ||
| Uranium | Activator | Rat | (Souidi et al., 2005) | ||
| Exogenous molecules | Pesticides | Chlordane (trans-nonachlor) | Activator | Human | (Jacobs et al., 2005) |
| Alachlor | Activator | Human, rat | (Mikamo et al., 2003; Lemaire et al., 2006) | ||
| Nonylphenol | Activator | Fish | (Meucci and Arukwe, 2006) | ||
| Cis-bifenthrin | Activator | Human | (Xiang et al., 2018) | ||
| DDT | Activator | Human, mouse | (Lemaire et al., 2004) | ||
| Dieldrin | Activator | Human | (Milnes et al., 2008) | ||
| Cholpyrifos | Activator | Mouse | (Lemaire et al., 2004) | ||
| Cypermethrine | Activator | Human | (Lemaire et al., 2004; Abass et al., 2012) | ||
| Endosulfan | Activator | Human | (Lemaire et al., 2004; Coumoul et al., 2002) | ||
| Dane | Activator | Human, rat | (Lemaire et al., 2004; Mikamo et al., 2003) | ||
| Methoxychlor | Activator | Human, rat | (Lemaire et al., 2004; Mikamo et al., 2003; Lemaire et al., 2006) | ||
| Miscellaneous | CITCO | Activator | Humans | (Lin et al., 2020) | |
| SR12813 | Activator | Human, rabbit, canine | (Jones et al., 2000; Pinne et al., 2016) | ||
| Pregnenolone 16Chlorpyrifosile (PCN) | Activator and ligand | Rodents | (Ma et al., 2008; Marek et al., 2005) | ||
| FKK6 | Activator and ligand | Human, mouse | (Dvořák et al., 2020) | ||
| Food components | Aflatoxin B1 | Activator | Human | (Ayed-Boussema et al., 2012a; Daujat-Chavanieu and Gerbal-Chaloin, 2020) | |
| Patulin | Activator | Human | (Ayed-Boussema et al., 2012b) | ||
| Isorhamnetin | Activator and ligand | Mouse | (Dou et al., 2014) | ||
| High fat diet | Activator | Mouse | (Saraswathi et al., 2017) | ||
| Alpha-ketoglutarate | Activator | Pig | (He et al., 2017) | ||
| Ochratoxin A | Inhibitor | Human | (Doricakova and Vrzal, 2015; Daujat-Chavanieu and Gerbal-Chaloin, 2020) | ||
| Vitamins | Vit K | Activator and ligand | Mouse | (Sultana et al., 2018; Tabb et al., 2003; Avior et al., 2015) | |
| Vit E | Activator | Human | (Landes et al., 2003) | ||
| Endogenous molecules | Microbial metabolites | Lithocholic acid (LCA) | Activator | Human, rodents | (Wistuba et al., 2007; Staudinger et al., 2001) |
| 3-keto LCA | Activator | Human, rodents | (Xie et al., 2001; Staudinger et al., 2001) | ||
| Deoxycholic acid (DCA) | Activator | Mouse | (Xie et al., 2001) | ||
| Indole-3-propionic acid | Ligand and activator | Human, mouse | (Venkatesh et al., 2014) | ||
| Skatole (3-methyl-indole) | Partial agonist and low affinity ligand | Human | (Vyhlidalova et al., 2020) | ||
| Lipopolysaccharides (LPS) | Inhibitor | Mouse | (Xu et al., 2004; Xu et al., 2005) |
Synthetic Drugs
The antituberculosis drug rifampicin is a well-known ligand and activator of human pregnane X receptor (hPXR), which binds and activates the receptor at a relatively low concentration (Blumberg et al., 1998; Moore et al., 2000a); however, rifampicin does not activate the rodent PXR (Gibson et al., 2002). In rodents, the prototypical known PXR ligand is PCN (Marek et al., 2005; Ma et al., 2008). Cell-based reporter assays have shown that activation of hPXR by rifampicin upregulates the expression of CYP3A4 in human, rabbit, pig, dog, and rhesus monkeys (Jones et al., 2000; Moore et al., 2000; Gibson et al., 2002). In vitro ligand assays showed that hPXR binds rifampicin (Jones et al., 2000), which then triggers the release of corepressors and initiates recruitment of coactivators to promote transcription of PXR-target genes (Takeshita et al., 2002; Ourlin et al., 2003).
In addition to the prototypical ligands, many other therapeutic compounds can activate PXR either as direct ligands or indirect activators (Chang and Waxman, 2006). Dexamethasone is another PXR activator in both mice and humans (Pascussi et al., 2001; Yueh et al., 2005). The barbiturates anticonvulsant drugs, such as phenobarbital, are PXR activators in a species-specific manner. For example, phenobarbital preferably activates PXR in humans, pigs, and rabbits but has little effect in rodents including mice and rats (Jones et al., 2000; Moore and Kliewer, 2000; Smirlis et al., 2001; Wei et al., 2002; Chang and Waxman, 2006; Pinne et al., 2016). Specifically, phenobarbital enhances the recruitment of the steroid receptor coactivator-1 to hPXR but not to mouse pregnane X receptor (mPXR) (Li et al., 2019). The antifungal drug clotrimazole has been reported to bind to hPXR at a concentration of 10 µM, stimulating coactivator recruitment and enhancing the transcription of PXR target genes (Lehmann et al., 1998; Jones et al., 2000), whereas clotrimazole is a weaker activator of rat and mouse PXR (Bertilsson et al., 1998; Jones et al., 2000). The protease inhibitor and anti-HIV drug ritonavir acts as a ligand of hPXR and upregulates PXR-target gene expression in primary human hepatocyte cultures (Chang and Waxman, 2006). Cancer chemotherapeutic drugs, such as paclitaxel, activate hPXR in cell-based reporter assays (Synold et al., 2001) where it acts as an hPXR ligand and upregulates the expression of PXR-target genes including CYP3A4 and the multidrug resistance 1 (MDR1) transporter (Kostrubsky et al., 1998). The muscle relaxant etomidoline has been identified as a novel selective PXR agonist in a stable hPXR-Luc HepG2 cell line and upregulated CYP3A4 mRNA expression in HepaRG cells in a PXR-dependent manner (Lynch et al., 2021). The calcium channel blockers, such as nifedipine, bendipine, and lacidipine, are used in the treatment of high blood pressure. Studies have reported that these drugs are associated with hPXR activation (Bertilsson et al., 1998; Chang and Waxman, 2006; Lynch et al., 2021). Several other drugs including carbamazepine (El-Sankary et al., 2001; Luo et al., 2002), bosentan (van Giersbergen et al., 2002), mevastatin (Raucy et al., 2002), tamoxifen (Desai et al., 2002; Zhuo et al., 2014), and topotecan (Schuetz et al., 2002) have all been reported to be activators of hPXR in cell-based reporter gene assays. In addition, 11 additional drugs have recently been identified as novel hPXR activators (Lynch et al., 2021).
The activation of PXR by chemotherapeutic drugs can result in drug resistance and lower efficacy, partly through upregulating CYP3A4 (Chen, 2010; Mani et al., 2013). Lin et al. investigated the therapeutic value of hPXR antagonists and the expression of CYP3A4. They found that SPA70 is a potent antagonist of hPXR, which ablated the expression of CYP3A4, suggesting that SPA70 can be a potential therapeutic as an hPXR antagonist (Lin et al., 2017).
Natural Products
Several plant-derived compounds have been identified as agonists and/or activators of hPXR or mPXR in cell-based reporter assays. For example, the active ingredient of St. John’s wort, namely hyperforin, has been reported as a ligand of hPXR and the direct binding of hyperforin to the PXR ligand-binding domain has been visualized by X-ray crystallography (Watkins et al., 2003). Hyperforin upregulates the PXR-target gene CYP3A/Cyp3a expression of both human and mouse origins in cell-based reporter assays (Cantoni et al., 2003; Komoroski et al., 2004). Guggulsterones are plant-derived sterol compounds that activate hPXR and mPXR, as evidenced by increased expression of PXR-target genes (Brobst et al., 2004; Ding and Staudinger, 2005; Chang and Waxman, 2006). The plant-derived diterpene forskolin is used in traditional medicine to treat cardiovascular disorders (Seamon et al., 1981) and acts as a ligand for both hPXR and mPXR, as evidenced by increased PXR-target gene expression in cell-based reporter gene assays (Ding and Staudinger, 2005). Artemisinin, a naturally occurring sesquiterpene, is an hPXR ligand that promotes interactions between PXR and its coactivator DRIP205 (Burk et al., 2005). However, artemisinin is known to decrease the basal expression of mPXR (Burk et al., 2005). Other plant-derived compounds such as the kava extract desmethoxyyangonin and dihydromethysticin also activate hPXR in cell-based reporter gene assays (Raucy, 2003; Ma et al., 2004).
Environmental Chemicals
Several environmental contaminants have been reported to activate PXR signaling or increase the expression of PXR in various animal species. These environmental contaminants include pesticides, steroids, plasticizers, heavy metals, etc. Several insecticides including organochlorines and pyrethroids have been reported to activate hPXR and upregulate CYP3A4 mRNA expression (Milnes et al., 2008). The herbicide alachlor has been reported to activate PXR in vitro and upregulates CYP3A mRNA expression in rat liver (Mikamo et al., 2003; Lemaire et al., 2006). Using in vitro gene expression assays, 15 different classes of pesticides that act as hPXR activators have been identified as summarized in Table 1 (Lemaire et al., 2006). Activation of PXR and the corresponding increase in Cyp3a gene expression occur after exposure to nonylphenol in juvenile fish liver (Meucci and Arukwe, 2006). The pyrethroid insecticide cis-bifenthrin upregulates the mRNA and protein expression of PXR in the HepG2 cell line (Xiang et al., 2018). The heavy metal ions Ag+ and Pb2+ upregulate the PXR mRNA in embryonic zebrafish fibroblasts (Liu et al., 2019). In CYP3A4 transgenic (TG) mice, arsenic has been reported to increase the mRNA expression of both PXR and CYP3A4, as well as CYP3A4 protein expression (Medina-Diaz et al., 2009). Chronic uranium exposure has been reported to increase PXR mRNA expression in the brain, liver, and kidney of rats (Souidi et al., 2005). The noncoplanar polychlorinated biphenyls (PCBs) have been reported to activate hPXR (Jacobs et al., 2005; Al-Salman and Plant, 2012). Bisphenol A is an agonist of hPXR but not mPXR (Sui et al., 2012). Several formerly used flame retardants, such as the polybrominated diphenyl ethers (PBDEs) are activators of PXR of both human and mouse origins (Pacyniak et al., 2007; Sueyoshi et al., 2014). Phthalate esters are used in the manufacture of plastic products and are activators of both human and rodent PXR (Chang and Waxman, 2006).
Food Components
Several dietary components have been reported as activators of PXR of either humans or of other organisms. Mycotoxins are found in food of humans and animals and have been reported as activators of PXR (Ding et al., 2006; Ayed-Boussema et al., 2012a; Ayed-Boussema et al., 2012b). Aflatoxin B1, which is a potent liver carcinogen is a PXR activator and upregulates the expression of PXR-target genes (Ayed-Boussema et al., 2012a; Daujat-Chavanieu and Gerbal-Chaloin, 2020). Patulin, another mycotoxin associated with fruits, has been reported to activate PXR and its target genes in primary human hepatocytes (Ayed-Boussema et al., 2012b). Molecular docking showed isorhamnetin, a flavonol compound present in various fruits and vegetables, is a hPXR ligand and subsequently represses the NF-ĸB pathway (Dou et al., 2014). Menaquinone-4, a vitamin K2 analog and medication for osteoporosis, is a hPXR ligand and upregulates PXR-target gene expression in both in vivo and in vitro experiments (Tabb et al., 2003; Avior et al., 2015; Sultana et al., 2018). Vitamin E is a potent activator of hPXR with similar efficacy as rifampicin (Landes et al., 2003). A high fat diet (HFD) can lead to enhanced PXR signaling in mice (Saraswathi et al., 2017). The nutritional factor alpha-ketoglutarate can enhance PXR activity and downregulate the NF-ĸB signaling pathway, although the molecular mechanism needs to be further explored (He et al., 2017).
PXR Repressors
Although most studies in the literature have focused on PXR activators, PXR repressors have also been identified as summarized in Table 1. Phytoestrogens and phytochemicals including coumestrol and sulforaphane bind to the LBD of hPXR and inhibit the expression of PXR-target genes in both hepatocytes and in livers of humanized PXR-transgenic (hPXR-TG) mice (Wang et al., 2008). Sulforaphane, a phytochemical present in broccoli, binds to the LBD of hPXR and antagonizes the PXR signaling (Zhou et al., 2007). The antifungal drug ketoconazole is a well-known repressor of PXR activity in HepG2 liver cancer-derived cells, human hepatocytes, LS174T colon cancer-derived cells, as well as CV-1 cells cotransfected with plasmids expressing either pCMX-hPXR or pCMX-mPXR along with a CYP3A4 luciferase reporter plasmid (Huang et al., 2007). The antineoplastic chemical ET-743 suppresses hPXR signaling and downregulates MDR1 gene expression in vitro (Synold et al., 2001). The specific PXR antagonist # 70 is another compound that serves as a potent and selective repressor of hPXR (Lin et al., 2017). Lipopolysaccharides, which is produced by resident gram-negative bacteria, inhibits PXR expression and downregulates Cyp3a11 mRNA expression in mouse liver (Xu et al., 2004; Xu et al., 2005). Ochratoxin A, a potent mycotoxin produced by Aspergillus sp, is a repressor of PXR in the HepG2 cell line (Doricakova and Vrzal, 2015; Daujat-Chavanieu and Gerbal-Chaloin, 2020; Shen et al., 2020).
Regulation of Complex Gut-Liver Crosstalk
Previously, it was well-known that the major bile acid sensor farsenoid X receptor (FXR) is the primary receptor regulating the expression of host bile acid processing genes within the gut-liver axis. The intestinal activation of FXR by bile acids activate fibroblast growth factor 15 in mice and fibroblast growth factor 19 in humans, which provides a negative feedback regulation of the host hepatic bile acid biosynthesis through downregulating Cyp7a1, the rate-limiting bile acid synthetic enzyme in liver (Wang et al., 2012; Li and Chiang 2015; Xiang et al., 2021). In addition to FXR, emerging research showed that PXR also contributes to the regulation of gut-liver crosstalk. The nuclear receptor PXR is expressed in several tissues in the body including kidney, lung, placenta, and ovary, but PXR is predominantly expressed in the liver and small intestine (Mohandas, 2017; Kliewer et al, 2002; Burk et al, 2004). The intestinal-derived secondary bile acid, lithocholic acid (LCA) activates PXR in mouse liver and thus increases the expression of the PXR-target gene Cyp3a11 (Staudinger et al., 2001). In addition, PXR transcriptionally regulates several bile acid transporters within the gut-liver axis (Ntcp, Ostα, Ostβ, and Oatp1a4) (Beaudoin et al., 2020; Stauginder et al., 2001). However, organ-specific PXR-signaling has been observed. For example, the microbial tryptophan metabolite indole 3-propionic acid (IPA) is a PXR activator in intestine as evidenced upregulation of the PXR-target gene Mdr1 in mouse jejunum (Venkatesh et al., 2014); however, it does not readily activate PXR in mouse liver (Morgan et al., 2018). In contrast, other ligands such as St. John’s wort extract (with hyperforin being one of the active ingredients) increase the expression and activity of PXR-target genes in both the intestine and liver (Durr et al., 2000; Moore et al., 2000b; Cantoni et al., 2003). In summary, in addition to FXR, PXR also contributes to the regulation of genes involved in bile acid homeostasis and xenobiotic biotransformation within the gut-liver axis.
PXR, Novel Endogenous PXR Activators, and the Gut Microbiome
The interactions between host PXR and gut microbiome are bidirectional. First, distinct microbial metabolites act as PXR activators (Table 1) under pathophysiological conditions to modulate the expression of PXR-target genes in a tissue-specific manner. Second, PXR of the host impacts the gut microbiome composition and functions under physiologic, pharmacological, and toxicological conditions. This section will summarize the key findings regarding the bidirectional interactions between the host PXR signaling and the gut microbiome.
Regulation of the Host PXR Signaling by Microbial Metabolite
The most hydrophobic secondary BA generated from dehydroxylation reactions by the gut microbiota is LCA, which is the most toxic BA to hepatocytes at high concentrations, such as during cholestasis (Fickert et al., 2006; Woolbright et al., 2014; Xu et al., 2020). Toxic levels of LCA and other secondary BAs produce liver damage through inflammation, oxidative stress, DNA damage, and cell death, leading to both liver and colon cancers (Allen et al., 2011; Ajouz et al., 2014; Woolbright et al., 2014; Jia and Jeon, 2019). Dietary supplement with 0.5% LCA increased the mRNA of the prototypical PXR-target gene cytochrome P450 3a11 (Cyp3a11) in a PXR-dependent manner in livers of adult male mice; in addition, PXR serves as a sensor of LCA to protect against hepatotoxicity (Staudinger et al., 2001). LCA transactivates PXR of both human and mouse origins in luciferase reporter assays, and it increased Cyp3a11 mRNA in a PXR-dependent manner (8 mg per day for 4 days via oral gavage) (Xie et al., 2001). Gastrectomy increased the endogenous LCA, LCA-producing intestinal bacteria Bacteroides fragilis, PXR nuclear translocation, and Cyp3a11 mRNA expression in mouse liver (Ishii et al., 2014). Because the major BA sensor FXR is not activated by LCA, it is thought that LCA-mediated activation of PXR provides an important compensatory protective mechanism to reduce LCA overload and ameliorate liver injury (Jung et al., 2006; Ishii et al., 2014). In addition to LCA, deoxycholic acid (DCA), another microbially derived secondary BA, can also transactivate PXR (Xie et al., 2001).
The bacteria-derived tryptophan metabolite IPA is a PXR ligand in the mouse intestine (Venkatesh et al., 2014). Produced by the intestinal bacteria Clostridium sporogenes, IPA downregulates the proinflammatory cytokine tumor necrosis factor-α) in enterocytes and upregulates the mRNAs encoding tight junction proteins and protects the gut barrier integrity in a PXR-dependent manner (Venkatesh et al., 2014). Although IPA activates PXR in the mouse intestine, it does not activate PXR in the mouse liver (Morgan et al., 2018). However, circulating IPA activates PXR in the vascular endothelium of mice to reduce the vasodilatory responses to nitric oxide in isolated and cultured vessels in a PXR-dependent manner. This suggests that IPA-mediated PXR activity plays a key role in endothelial function (Pulakazhi Venu et al., 2019). This also indicates that PXR activation by IPA is tissue specific.
Based on the conclusion above that IPA activates PXR, it was proposed that microbial indole metabolite mimicry might be a novel strategy for drug discovery. In fact, the functionalized indole derivatives have generated first-in-class noncytotoxic PXR agonists. The lead compounds are Felix Kopp Kortagere (FKK) 5 and FKK6 that activate PXR to reduce inflammation. FKK6, later called CVK003, is a direct PXR-binding ligand and induces PXR-target gene expression in cells, human organoids, and mice. Through PXR activation, FKK6 represses proinflammatory cytokines in humanized PXR transgenic mice (Dvořák et al., 2020a). The removal of the phenyl-sulfonyl group of FKK6 shifts the agonist activity away from PXR toward the aryl hydrocarbon receptor, whereas the addition of the imidazole pyridyl group preserves PXR activity in vitro (Li et al., 2021). Targeting the microbial indole metabolite mimicry serves as an important mechanism to understand the crosstalk between PXR and other important xenobiotic-sensing transcription factors such as the aryl hydrocarbon receptor (Dvořák et al., 2020b; Li et al., 2021).
Necessity of the Gut Microbiome in the Host PXR Signaling under Basal and Toxicological Conditions
The role of the gut microbiome in modulating the basal PXR signaling was first noted by quantifying the expression and enzyme activities of the prototypical PXR-target gene Cyp3a in livers of germ free (GF) mice (Toda et al., 2009b) and in livers of antibiotic-treated conventional (CV) mice (Toda et al., 2009a). Ciprofloxacin, which is a quinolone antibiotic, decreases Cyp3a11 expression and triazolam metabolism in mouse liver by reducing LCA-producing intestinal bacteria, whereas LCA treatment increased Cyp3a11 expression in GF mice (Toda et al., 2009a). Another study showed that most of the major Cyps and the PXR gene expression were lower in the livers of GF mice (Toda et al., 2009b), suggesting that intestinal bacteria maintain constitutive PXR-signaling in mouse liver. Transcriptomic-wide investigations including microarray (Bjorkholm et al., 2009) and RNA-Seq sequencing (Selwyn et al., 2015b) in livers of CV and GF mice further demonstrated that gut microbiome is necessary for maintaining the basal expression of various xenobiotic-processing genes and PXR-signaling. In fact, xenobiotic metabolism ranked among the topmost differentially regulated networks in the entire liver transcriptome of adult male GF mice (Selwyn et al., 2015a). The GF-mediated downregulation of Cyp3a11 gene expression is modified by age and blunted the ontogenic increase of Cyp3a11 in mouse liver (Selwyn et al., 2015a). Interestingly, conventionalization through exposing the GF mice with feces from CV mice restored the constitutive expression of Cyp3a11 mRNA and protein in the liver (Selwyn et al., 2016), and this observation further confirmed the necessity of gut microbiome in the basal expression of this PXR-target gene in the liver. Extended investigations in various sections of the intestine of CV and GF mice showed that the gut microbiome is also important in maintaining the constitutive expression of Cyp3a in an intestinal section-specific manner (Fu et al., 2017). Therefore, the gut microbiome is a functional modifier of PXR and Cyp3a-mediated drug metabolism in the liver and intestine under basal conditions (Dempsey and Cui, 2019).
After exposure to known PXR-activating chemicals, the gut microbiome may serve as a novel modifier of PXR-target gene expression in the liver. The previously used flame retardants PBDEs are bioaccumulative and thus persistent in the environment long after their use was banned. Among the 209 possible PBDE congeners, the noncoplanar BDE-47 and BDE-99 are known to activate PXR in mouse liver (Pacyniak et al., 2007). Interestingly, oral exposure to BDE-47 and BDE-99 upregulated the mRNA of many drug-processing genes, including the PXR-target gene Cyp3a11 in mouse liver. This upregulation was further augmented by the lack of gut microbiome (Li et al., 2017), suggesting that the gut microbiome inactivates the PBDE parent compounds or metabolites, thus less PXR activators are available under CV conditions. In serum of CV mice, oral exposure to BDE-47 and BDE-99 decreased the tryptophan microbial metabolite IPA (Scoville et al., 2019), which is a known endogenous PXR ligand in mouse intestine (Venkatesh et al., 2014). The imbalance between endogenous versus exogenous PXR activators after xenobiotic exposure may serve as a contributing mechanism for drug-drug and food-drug interactions.
Regulation of Gut Microbiome by PXR-Activating Xenobiotics
Although the intestinal microbiome modulates the basal and inducible PXR signaling as discussed above, the host PXR gene and exposure to PXR activators can reciprocally modulate the gut microbiome composition and metabolism. After oral exposure to PCN, which is the prototypical PXR ligand in mice, there was a distinct change in the gut microbiome as evidenced by the principal coordinate analysis of beta diversity (Dempsey et al., 2019). At the class level, PCN decreased Actinobacteria and a taxon in the Firmicutes phylum but increased Gammaproteobacteria. At the genus level, PCN decreased Dorea, Anaeroplasma, and a taxon in the Peptococcaceae family, but increased Ruminococcus. In addition, PCN decreased two taxa in Bifidobacterium, which is known to have BA deconjugation activities (Foley et al., 2019), which corresponds to decreased gene abundance of the bile salt hydrolase in the microbial DNA from PCN-exposed mice and reduced expression (Dempsey et al., 2019). PCN also tended to decrease the microbial DNA encoding the bile acid-inducible operon CD and baiJ, although a statistical significance was not achieved. In livers of PCN-exposed CV mice, there was a decrease in the total secondary BAs, and this was due to a significant reduction in DCA, 3-dehydrocholic acid, 12-dehydrocholic acid, and a trend of a decrease (not significant) in tauro-lithocholic acid and tauro-hyodeoxycholic acid (Dempsey et al., 2019). In summary, pharmacological activation of PXR by its prototypical ligand PCN downregulates distinct BA-metabolizing intestinal bacteria and alters BA homeostasis.
After exposure to PXR-activating therapeutic drugs, the gut microbiome can also be modulated. The cholesterol-lowering statins are known PXR activators (Howe et al., 2011; Hoffart et al., 2012). Two PXR-activating statins, namely atorvastatin and pravastatin, produce gut dysbiosis and reduced butyrate production in a PXR-dependent manner in mice (Caparros-Martin et al., 2017).
After exposure to PXR-activating environmental chemicals, gut dysbiosis has been observed (Cheng et al., 2018; Li et al., 2018; Scoville et al., 2019; Cruz et al., 2020; Lim et al., 2020; Gomez et al., 2021), although caution is needed in interpreting the results, as these environmental toxicants may also have off-target effects on the gut microbiome independent from PXR activation. Oral exposure to BDE-47 and BDE-99, which are known to activate PXR and CAR in mouse liver (Pacyniak et al., 2007), produced profound gut dysbiosis as evidenced by decreased alpha diversity and 45 differentially regulated bacteria in the large intestinal content of adult male mice (Li et al., 2018). Most notably, there was an increase in Akkermansia muciniphila and Erysipelotrichaceae Allobaculum spp., which have been reported to have anti-inflammatory and antiobesity functions (Png et al., 2010; Ravussin et al., 2012; Derrien et al., 2017). BDE-99 increased many unconjugated BAs in multiple biocompartments in a gut microbiome-dependent manner, which correlates with an increase in microbial 7α-dehydroxylation enzymes for secondary BA synthesis and increased expression of host intestinal transporters for BA absorption. PBDEs also downregulated host BA-synthetic enzymes and transporters in mouse livers in a gut microbiome-dependent manner (Li et al., 2018). In addition to BAs, BDE-47 and BDE-99 also modulated other intermediary metabolites such as those from amino acid and carbohydrate metabolism (Scoville et al., 2019). Among serum, liver, as well as small and large intestinal content, the large intestinal content is the biocompartment where PBDEs altered the largest number of aqueous metabolites, and most of the differential regulation was observed in the GF mice. For example, the gut microbiome was necessary for PBDE-mediated downregulation in branched-chain and aromatic amino acid metabolites, whereas gene-metabolite networks revealed a positive association between the mRNA expression of the hepatic glycan synthesis gene α-1,6-mannosyltransferase and mannose, which are important for protein glycosylation (Scoville et al., 2019). Thus, the lack of gut microbiota augmented the PBDE-mediated effects on intermediary metabolism in adult male mice. Maternal exposure to BDE-47 produced persistent gut dysbiosis in adult male mouse pups and persistently increased fecal and hepatic BAs within the 12α hydroxylation pathway, which corresponds to the upregulation of the hepatic rate-limiting BA-synthetic enzyme Cyp7a1 (Gomez et al., 2021). For all the studies described above, follow-up investigations using PXR-null mice are necessary to determine whether the observed effects by PBDEs are PXR-dependent. The PBDE-mediated dysbiosis in human microbiome was further investigated in a fermentation system using fresh human stool, and it was shown that PBDEs produced an imbalance in sulfur, short-chain fatty acids, and aromatic organic compounds and altered the microbial volatolome in a dose- and time-dependent manner (Cruz et al., 2020).
Several noncoplaner congeners of the Fox River PCBs mixture are known PXR activators, and oral exposure to the Fox River mixture (6 mg/kg) produced gut dysbiosis including an upregulation in A. muciniphila, Clostridium scindens, and Enterococcus in large intestinal pellet of adult female CV mice. There was also an increase in multiple BAs in serum and small intestinal pellets in a gut microbiota-dependent manner, and Pearson’s correlation analysis identified a positive correlation between 5 taxa and most secondary BAs (Cheng et al., 2018). At the dose of 30 mg/kg of PCBs, NADP and arginine are predicted to interact with the drug-metabolizing enzymes within the Cyp1-3 family, and this was also highly correlated with the presence of Ruminiclostridium and Roseburia. This suggests a novel role of the gut-liver axis in PCB-mediated effect on intermediary metabolism (Lim et al., 2020). In addition to the Fox River PCB mixture, the Aroclor1260 PCB mixture also altered the composition of the gut microbiome in a diet-induced obese mouse model and altered beta diversity in a PXR-dependent manner (Wahlang et al., 2021).
Regulation of Gut Microbiome by the Host PXR Gene under Basal and Pathophysiological Conditions
Although PXR has been extensively studied and well-recognized as a xenobiotic-sensing receptor for drugs, environmental chemicals, and nutraceuticals, very recent studies showed that this classic drug receptor also plays important biologic functions under basal conditions (Little et al., 2021) and pathophysiological conditions, such as diet-induced nonalcoholic steatohepatitis and toxicant-induced nonalcoholic steatohepatitis (Spruiell et al., 2014a; Spruiell et al., 2014b; Wahlang et al., 2021). Pharmacological activation of PXR also has anti-inflammatory functions (Cheng et al., 2012). Interestingly, such novel PXR-mediated functions are associated with PXR-dependent modulation of the gut microbiome and microbial metabolites.
Under physiologic conditions, studies using PXR-null mice have shown that the absence of PXR increased the microbial richness and the proinflammatory bacteria (Helicobacteraceae and Helicobacter) and decreased the fecal levels of many abundant taurine-conjugated BAs. Thus, PXR may function to maintain gut flora and immune surveillance under basal conditions (Little et al., 2021). The suppression of microbial abundance by PXR under basal conditions was also independently observed in another study (Wahlang et al., 2021). Interestingly, the basal effect of PXR on the gut microbiome was distinct from pharmacological and toxicological activation of PXR (Cheng et al., 2018; Li et al., 2018; Dempsey et al., 2019; Lim et al., 2020; Little et al., 2021). Bona fide PXR-targeted intestinal bacteria Dorea, Mogibacteriaceae, Ruminococcaceae, Streptococcus, and Anaeroplasma were consistently suppressed by PXR under both basal and PXR-activated conditions (Cheng et al., 2018; Li et al., 2018; Dempsey and Cui, 2019; Lim et al., 2020; Little et al., 2021). Interestingly, hPXR-TG mice had a distinct microbial profile and a general trend of reduced fecal DCA compared with wild type mice, suggesting that the species difference in the PXR protein may lead to different gut microbiome configurations and functions (Little et al., 2021).
The PXR gene can produce adverse effects in pathophysiological conditions. In a HFD-induced obese mouse model, it was demonstrated that the presence of PXR worsens obesity as evidenced by protection from weight gain and liver steatosis in PXR-null mice (Spruiell et al., 2014a; Spruiell et al., 2014b). PXR activation is also detrimental in the regulation of glucose metabolism (Hukkanen et al., 2014). Specifically, in mice, PXR-deficiency improves HFD-induced obesity and genetically induced obesity (i.e., the ob/ob mice), which are models for type 2 diabetes (He et al., 2013; Zhao et al., 2017). Interestingly, hPXR-TG mice also carry a genetic predisposition for type 2 diabetes (Spruiell et al., 2014a). Pregnane X receptor-transgenic mice have impaired glucose utilization, elevated fasting glucose levels, and severely impaired glucose tolerance during high fat diet treatment, suggesting there is a species difference of PXR in regulating obesity and glucose signaling (Spruiell et al., 2014a; Spruiell et al., 2014b). Interestingly, the HFD-mediated increase in intestinal Firmicutes/Bacteroidetes ratio, which is a hallmark for obesity and increased energy harvest from diet (Turnbaugh et al., 2006), was completely abolished in male PXR-null mice. There was also a PXR-dependent, HFD-mediated decrease in the antiobese Allobaculum and the anti-inflammatory Bifidobacterium (Kim et al., 2021). The PXR-dependent gut dysbiosis after an HFD-induced obesity was associated with PXR-enhanced weight gain, hepatic steatosis, inflammation, as well as PXR-dependent upregulation in hepatic genes involved in microbial response, inflammation, oxidative stress, and cancer (Kim et al., 2021). In contrast to the higher susceptibility to HFD-induced nonalcoholic fatty liver disease phenotype in male mice, the resistance to non-alcoholic fatty liver disease in females may be explained by a PXR-dependent decrease in proinflammatory bacteria (Ruminococcus gnavus and Peptococcaceae) (Kim et al., 2021). In a toxicant-induced nonalcoholic steatohepatitis mouse model exposed to both HFD and the Aroclor1260 PCB mixture, PXR-dependent gut dysbiosis was also noted including increased Actinobacteria and Verrucomicrobia abundance in PXR-null mice (Wahlang et al., 2021). In conclusion, PXR exacerbates hepatic steatosis and inflammation accompanied by obesity- and inflammation-prone gut microbiome signature, suggesting that gut microbiome may contribute to PXR-mediated exacerbation of obesity.
Modifying Factors of PXR-Signaling
Species Difference
Overview
It’s well-known that there are species differences in PXR activity (Jones et al., 2000; LeCluyse, 2001; Xie and Evans, 2001). Activation and repression of PXR among various species contribute to species-specific efficacy and toxicity of pharmaceuticals and other xenobiotics in humans, animals, and the ecosystem (Pinne et al., 2016; Creusot et al., 2021). As discussed earlier, rifampicin, an antituberculosis drug, and SR12813 selectively activates PXR in humans and rabbits, whereas PCN is a classic synthetic compound that activates PXR in rodents (Li and Chiang, 2005; Marek et al., 2005; Li and Chiang, 2006; Ma et al., 2008). Using PXR and its prototypical target gene CYP3A/Cyp3a expression as a read-out, previous investigations revealed similarities and differences in activation of PXR and toxicities from using endogenous and exogenous compounds in hPXR, mPXR, rat PXR (rPXR), and zebrafish PXR (zfPXR) (Reschly and Krasowski, 2006; Reschly et al., 2007; Krasowski et al., 2011). For example, tropanyl 3,5-dimethylbenzoate, a 5-HT3 receptor agonist, activates hPXR but not rPXR (Shukla et al., 2011). The gut microbiota is capable of producing vitamin K2 (Conly and Stein, 1992). Interestingly, vitamin K2 acts as a PXR activator in humans but not in zebrafish (Creusot et al., 2021).
Although many chemicals activate PXR in a species-specific manner, common PXR-activating compounds across multiple species have also been identified. Using human cell lines including LS174T, LoVo, HCT116, and a mouse xenograft model, activation of PXR has been observed in both humans and mice using rifampicin and PCN, respectively, along with enhanced colon cancer cell growth, proliferation, invasion, and metastasis through activation of fibroblast growth factor 19 signaling (Wang et al., 2011). The microbial-derived secondary BAs LCA and DCA, as well as the primary BA chenodeoxycholic acid (CDCA) activates PXR in humans and rodents (Xie et al., 2001). Benzo[a]pyrene, a polyaromatic hydrocarbon, activates hPXR and mPXR, but not zfPXR (Cui et al., 2017; Creusot et al., 2021). Pharmaceutics, such as hyperforin, mevastatin, and n-butyl-4-aminobenzoate, activates hPXR but not zfPXR (Creusot et al., 2021). Other chemicals within classes of pharmaceutics, phytochemicals, environmental chemicals, endogenous compounds, and pesticides, and their examples of PXR activation are listed in Table 1.
Evolutionary Differences of PXR Across Species
The vitamin D receptor and PXR are suggested to have been separated from a duplication process of a common ancestral gene (Reschly and Krasowski, 2006). Due to speciation and evolutionary pressure, a distinction of functional differences in PXR and its LBD have likely arisen (Reschly and Krasowski, 2006; Reschly et al., 2007; Krasowski et al., 2011). It was shown that hPXR, mRXR, rPXR, and rbPXR share approximately 95% identity in their DNA binding domain ut share only 75%–80% in their amino acid sequences in the LBD (LeCluyse, 2001). This indicates that species-specific PXR activation is likely a result of adaptation to different environmental exposures (LeCluyse, 2001; Ekins et al., 2008), and it is supported by structural analysis of the PXR LBD (Watkins et al., 2001; Ngan et al., 2009). The amino acid substitution may result in changes in the LBD. In the PXR LBD, five out of nine amino acids in mPXR are conserved in hPXR, and the remaining four residues, Phe184, Arg203, Lys334, and Ser414, are substituted by Ser187, Leu203, Glu334, and Ile414, respectively (LeCluyse, 2001). Conservation of the five amino acids between mPXR and hPXR supports activation of PXR by certain chemicals, such as LCA in humans and mice. Therefore, substitutions in amino acid residues are likely responsible for the differential response of PXR in various species.
Sex Difference in PXR Activity
The expression and activity of PXR and its targets differ between males and females. In mouse models, the expression of Cyp3a11 and Cyp3a25, the prototypical targets of PXR in mice, is higher in females than males (Cui et al., 2012; Lu et al., 2013). Likewise, expression levels of PXR targets are slightly different between male and females in humans (Wolbold et al., 2003; Scandlyn et al., 2008). Because PXR is a critical component in xenobiotic biotransformation, characterizing and investigating the sex difference of PXR from xenobiotic exposures and diseases remain an important task. Correspondingly, drugs that are substrates of CYP3A4, such as cyclosporine, erythromycin, nifedipine, and ifosfamide have higher clearance rates in women (Austin et al., 1980; Kahan et al., 1986; Krecic-Shepard et al., 2000; Schmidt et al., 2001), which may lead to gender-dependent dosage regimens and toxicological effects because of species difference in the PXR-CYP3A4 pathway (Tanaka, 1999; Parkinson et al., 2004; Scandlyn et al., 2008).
Regarding the sex difference in toxicological responses related to PXR signaling, the hepatic Cyp3a11 gene expression from exposure to PCBs is higher in female mice than in male mice (Wahlang et al., 2019). Exposure to LCA to PXR-null mice produced cholestasis more prominently in males than in females (Uppal et al., 2005). When wild type (WT) and Cyp3a null mice were fed 60% high fat diet, female Cyp3a knockout (KO) mice gained 50% less weight than WT mice, but male Cyp3a KO mice gained more weight than the WT control. Interestingly, HFD increased the expression of PXR in Cyp3a KO female mice; whereas PXR was downregulated in male Cyp3a KO mice (Kumar et al., 2018). In humans, the risk of drug-induced hepatotoxicity during antituberculosis treatment from rifampicin is higher in women than in men (Shehu et al., 2016). Wang et al. hypothesized that sex-specific single nucleotide polymorphisms genotype and the resulting haplotype of PXR differentially regulate the expression of CYP3A4, leading to the sex-specific influence of hepatotoxicity (Wang et al., 2015). Significant associations were found between two PXR haplotypes, h001101 and h000110, and increased hepatotoxicity during antituberculosis treatment with rifampicin, isoniazid, or pyrazinamide (Wang et al., 2015). Single nucleotide polymorphism and haplotype differences of PXR may be a significant reason for differences in PXR activity.
The gut microbiome is suggested to be a critical driver of gender differences of PXR. GF mice had attenuated sex differences in PXR and its target genes in the liver, compared with CV mice (Weger et al., 2019). In addition, PXR also reciprocally drives sexually dimorphic hepatic changes in lipid and xenobiotic metabolism in response to gut microbiota in mice, in that the expression of most microbiota-sensitive hepatic genes in response to antibiotic-mediated depletion of microbiota in mice were PXR-dependent in males, but not in females (Barretto et al., 2021). Pathway enrichment analysis suggests that that microbiota-PXR interaction regulates fatty acid and xenobiotic metabolism, and data on liver triglyceride content indicate that antibiotic treatment reduced liver triglyceride content and hampered xenobiotic metabolism in male mice in a PXR-dependent manner (Barretto et al., 2021).
PXR and Critical Time Windows
Using the prototypical PXR-target gene Cyp3a11 as a read-out for PXR activation, it was shown that Cyp3a11 mRNA undergoes extensive regulation at critical time windows. For example, during mouse liver development, Cyp3a11 mRNA is low before birth, peaks around postnatal day 5, and maintains at high levels until young adulthood (Hart et al., 2009). It should be noted that the ontogenic regulation of Cyp3a11 may not be solely PXR dependent, as it has been shown that district chromatin epigenetic signatures, such as increased permissive mark histone H3 lysine 4 di-methylation positively associates with the ontogenic increase of Cyp3a11 mRNA in developing mouse liver (Li et al., 2009). During human liver development, the Cyp3a11 ortholog CYP3A4 gene locus is also extensively modified by the histone code, and the postnatal liver has reduced occupancy of repressive histone marks and higher occupancy of active histone marks compared with the fetal liver (Giebel et al., 2016). During aging, Cyp3a11 mRNA displays a consistent male-predominant pattern and decreases earlier in the livers of female mice than age-matched male mice (Fu et al., 2012). Cyp3a11 mRNA also exhibits diurnal variations between day and night, with higher expression around 4 AM followed by a decrease to relatively steady levels during the daytime (Zhang et al., 2009). Activation of PXR using its prototypical ligand PCN at various developmental ages showed that the highest fold-induction occurred in the 60-day-old young adult, followed by postnatal day 5 (Li et al., 2016b). Neonatal activation of PXR also persistently downregulated the mRNA and protein expression of multiple CYP4A isoforms, which are prototypical target genes of the lipid-sensing nuclear receptor peroxisome proliferator-activated receptor-α, in livers of adult male mouse pups in a PXR-dependent manner (Li et al., 2016a).
Pharmacological and Mathematical Models of PXR
There have been a few mathematical models developed to describe PXR activation and CYP3A upregulation in humans and rodents. These mathematical models implemented a system of ordinary differential equations to organize and simulate the molecular activation of PXR, signaling pathway, and induction of its targets. One of the first to depict a compartmental model of CYP3A4 gene regulation by PXR considered the standard cyclical process of PXR activation: rifampicin enters the cell and binds to PXR, which forms a heterodimer with RXRα; the PXR/RXRα binds to DNA and induces CYP3A4 mRNA, which is translated into protein; CYP3A4 mRNA is degraded and translated protein metabolizes the rifampicin in the cell (Luke et al., 2010). This model included a feedback loop and used zero- and first-order kinetics to model the steady-state mRNA levels of CYP3A4 and incorporated plasma and liver in their compartmental model (Luke et al., 2010).
Similar to the model by Luke et al., the rPXR pharmacokinetic/pharmacodynamic (PK/PD) model developed by Li et al. describes the change in levels of CYP3A1 and CYP3A2 with respect to time after the activation of rPXR from dexamethasone exposure at various drug concentrations (Li et al., 2012). The model formulated by Li et al. comprised of in vivo exposure to dexamethasone by intraperitoneal injection (100 mg/kg), measurement of total 6β-testosterone hydroxylation formation enzyme activity, and CYP3A1 and CYP3A2 mRNAs and protein levels, and contained two compartments, i.e., blood and liver (Li et al., 2012). The resulting model, upon estimation and calculation of parameters, such as clearance, apparent volume of distribution, and duration of zero-order absorption, and using transit compartments, was able to show the concentration-induction response relationship between dexamethasone and the prototypical rPXR targets (Li et al., 2012).
Data for the conceptual models for PXR can be obtained relatively easily by RT-qPCR (reverse trancription-quantitative polymmerase chain reaction) and western blots (Luke et al., 2010; Bailey et al., 2011; Kolodkin et al., 2013). Additional parameters, such as cytoplasm-to-nucleus mRNA translocation and RNA processing, can be performed to further improve physiologic and molecular relevance. These improved models can serve as important first-pass detection of CYP3A induction and PXR activation in novel compounds and can be used as a start of investigating a systems approach of PXR and other receptors and their targets, i.e., PXR and the glucocorticoid receptor (Kolodkin et al., 2013). However, no mathematical or statistical model describing the PXR-CYP3A relationship across species has been developed. Therefore, the development and expansion of such models will allow future research to investigate CYP3A and PXR mRNA or protein induction from cellular experiments in newly studied organisms have a good potential in assessing toxicity and drug-drug interactions across species.
Current Challenges and Knowledge Gaps
With the expansion of research on PXR recently, several challenges and knowledge gaps are noted in this area. First, prototypical PXR-target genes (e.g., Cyp3a) may be regulated by other mechanisms such as epigenetic modifiers and other transcription factors, thus they may not be a bona fide biomarker for PXR activation. Second, species differences in both PXR and the PXR-modulating endogenous molecules between humans and animal models continue to be a challenge in translational research. In addition, although several novel endogenous PXR ligands are being resolved, there is a knowledge gap regarding how other endogenous small molecules among the vast majority of poorly characterized “dark matter” within the metabolome modulate PXR. Certain endogenous molecules may serve as potent PXR ligands in animal models but only weak PXR ligands or display no activity toward PXR in humans; in addition, these molecules may serve as potent PXR ligands in certain tissues (e.g., intestine) but only have weak PXR-modulating activities in other tissues (liver). Last but not least, the consequence of PXR activation may be context specific and can be either beneficial (e.g., chemical detoxification and anti-inflammation) or harmful (e.g., drug-drug interactions and promoting nonalcoholic steatohepatitis). Therefore, it is very important to understand the complexity of the species difference, tissue specificity, and context-specificity duality of PXR modulation, and additional research efforts are needed to further characterize PXR target genes in different organs and species as well as novel PXR-modulating molecules.
Within the challenges discussed above, we may find new opportunities to address them: strategies in derivatizing endogenous PXR ligands to improve their activities toward human PXR and tissues other than their original functional sites may provide novel therapeutic options to treat complex human disease while bypassing the adverse drug reactions. In addition, with the availability of single cell transcriptomics and single cell metabolomics, it is possible to improve the precision and resolution of understanding the PXR functions and modulating factors.
Overall Conclusion and Closing Remarks
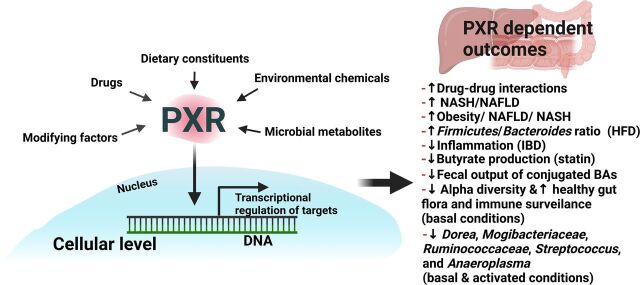
Taken together, as summarized in Fig. 1, PXR is a versatile xenobiotic-sensing nuclear receptor that has been rediscovered as a critical modulator for intermediary metabolism and metabolic diseases. Notably, PXR plays critical roles within the gut-liver axis within the context of exposures and disease outcomes. PXR activity can be modulated under basal, physiologic, pathophysiological, pharmacological, and toxicological conditions by a variety of environmental factors. These factors include drugs, environmental chemicals, dietary constituents, microbial metabolites, etc. Modifying factors such as sex, age, exposure time windows, and species specificity may interact the PXR signaling within the gut liver axis. Although the well-known outcomes of PXR activation with are drug-drug interactions through transcriptional regulation of various host genes involved in xenobiotic biotransformation, studies in the literature have also discovered bona fide PXR-targeted intestinal bacteria that are suppressed by PXR, as well as the importance of PXR in modulating inflammation and intermediary metabolism. Additional research using targeted and untargeted metabolomics is needed to further expand our knowledge regarding novel PXR-modulating small molecules from the diet, microbiome, and other exposures. It is also important to discover individual microbes and complex microbial symbiotic/competitive interactions that are involved in the production or consumption of PXR-modulating molecules. The discovery of novel endogenous PXR modulators is expected to aid in developing novel therapeutic modalities for drug-drug interactions, food-drug interactions, drug-toxicant interactions, as well as PXR-mediated metabolic diseases. We think it is also important to study the modulating of PXR-signaling by context-specific chemical mixtures, involving coexposures of both exogenous and endogenous PXR-modulating molecules. Due to the known species difference of PXR, it is important to continue utilizing humanized PXR transgenic models and in vitro systems to crossreference findings in various animal species. Development and expansion of mathematical models will allow future research to investigate PXR-CYP3A induction to investigate species and gender differences. Characterizing the sex difference in PXR-signaling continues to be a research priority, and incorporating additional metagenomics and metabolomics approaches will facilitate the identification of novel regulators that drive the gender-divergent PXR modulation and response. It is also important to further investigate the interactions between PXR and other transcription factors as well as cofactors. Additionally, relatively less is known about PXR and the gut-liver axis among the pediatric population. Further understanding the role of PXR in pediatric pharmaco-metagenomics is important to improve the safety and efficacy of using drugs in this vulnerable population.
Fig. 1.
Overall summary of PXR and the gut-liver axis within the context of exposures and outcomes. Briefly, PXR activity can be modulated under basal, physiologic, pathophysiological, pharmacological, and toxicological conditions by a variety of environmental factors. These factors include drugs, environmental chemicals, dietary constituents, microbial metabolites, etc. Modifying factors such as sex, age, exposure time windows, and species specificity may interact the PXR signaling within the gut liver axis. Although the well-known outcomes of PXR activation with are drug-drug interactions through transcriptional regulation of various host genes involved in xenobiotic biotransformation, studies in the literature have also discovered bona fide PXR-targeted intestinal bacteria that are suppressed by PXR as well as the importance of PXR in modulating inflammation and intermediary metabolism.
Acknowledgments
The authors would like to thank Dr. Curtis D. Klaassen for editing the manuscript.
ABBREVIATIONS
- BA
bile acid
- CV
conventional
- CYP
cytochrome P450
- DCA
deoxycholic acid
- FKK
Felix Kopp Kortagere
- FXR
farnesoid X receptor
- GF
germ free
- HFD
high fat diet
- hPXR
human pregnane X receptor
- hPXR-TG
human pregnane X receptor-transgenic
- IPA
indole-3-propionic acid
- KO
knock out
- LBD
ligand binding domain
- LCA
lithocholic acid
- MDR1
multidrug resistance 1
- mPXR
mouse pregnane X receptor
- Oatp
organic anion transporting polypeptide
- PBDE
polybrominated diphenyl ethers
- PCB
polychlorinated biphenyl
- PCN
pregnenolone-16α-carbonitrile
- PXR
pregnane X receptor
- rPXR
rat PXR
- RXR
retinoic X receptor
- TG
transgenic
- WT
wild type
- zfPXR
zebrafish PXR
Authorship Contributions
Participated in research design: Dutta, Lim, Cui. Performed data analysis: Dutta, Lim, Cui. Wrote or contributed to the writing of the manuscript: Dutta, Lim, Cui.
Footnotes
This work was supported by National Institutes of Health National Institute of Environmental Health Sciences [Grants R01-ES025708, R01-ES030197, and R01-ES031098]; National Institutes of Health National Institute of General Medical Sciences [Grant R01-GM111381]; the University of Washington Center for Exposures, Diseases, Genomics, and Environment [Grant P30ES007033]; Environmental Pathology/Toxicology Training Program [Grant T32ES007032]; the University of Washington Sheldon Murphy Endowment; and the University of Washington Environmental Health and Microbiome Research Center.
No author has an actual or perceived conflict of interest with the contents of this article.
M.D. and J.J.L. contributed equally to this work.
References
- Abass K, Lämsä V, Reponen P, Küblbeck J, Honkakoski P, Mattila S, Pelkonen O, Hakkola J. Characterization of human cytochrome P450 induction by pesticides. Toxicology. 2012. Mar 29;294(1):17–26. [DOI] [PubMed] [Google Scholar]
- Ajouz H, Mukherji D, Shamseddine A (2014) Secondary bile acids: an underrecognized cause of colon cancer. World J Surg Oncol 12:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksunes LM, Klaassen CD (2012) Coordinated regulation of hepatic phase I and II drug-metabolizing genes and transporters using AhR-, CAR-, PXR-, PPARα-, and Nrf2-null mice. Drug Metab Dispos 40:1366–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen K, Jaeschke H, Copple BL (2011) Bile acids induce inflammatory genes in hepatocytes: a novel mechanism of inflammation during obstructive cholestasis. Am J Pathol 178:175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnouti Y, Klaassen CD (2008) Regulation of sulfotransferase enzymes by prototypical microsomal enzyme inducers in mice. J Pharmacol Exp Ther 324:612–621. [DOI] [PubMed] [Google Scholar]
- Al-Salman F, Plant N (2012) Non-coplanar polychlorinated biphenyls (PCBs) are direct agonists for the human pregnane-X receptor and constitutive androstane receptor, and activate target gene expression in a tissue-specific manner. Toxicol Appl Pharmacol 263:7–13. [DOI] [PubMed] [Google Scholar]
- Austin KL, Mather LE, Philpot CR, McDonald PJ (1980) Intersubject and dose-related variability after intravenous administration of erythromycin. Br J Clin Pharmacol 10:273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avior Y, Levy G, Zimerman M, Kitsberg D, Schwartz R, Sadeh R, Moussaieff A, Cohen M, Itskovitz-Eldor J, Nahmias Y (2015) Microbial-derived lithocholic acid and vitamin K2 drive the metabolic maturation of pluripotent stem cells-derived and fetal hepatocytes. Hepatology 62:265–278. [DOI] [PubMed] [Google Scholar]
- Ayed-Boussema I, Pascussi JM, Maurel P, Bacha H, Hassen W (2012a) Effect of aflatoxin B1 on nuclear receptors PXR, CAR, and AhR and their target cytochromes P450 mRNA expression in primary cultures of human hepatocytes. Int J Toxicol 31:86–93. [DOI] [PubMed] [Google Scholar]
- Ayed-Boussema I, Pascussi JM, Rjiba K, Maurel P, Bacha H, Hassen W (2012b) The mycotoxin, patulin, increases the expression of PXR and AhR and their target cytochrome P450s in primary cultured human hepatocytes. Drug Chem Toxicol 35:241–250. [DOI] [PubMed] [Google Scholar]
- Bailey I, Gibson GG, Plant K, Graham M, Plant N (2011) A PXR-mediated negative feedback loop attenuates the expression of CYP3A in response to the PXR agonist pregnenalone-16α-carbonitrile. PLoS One 6:e16703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee M, Robbins D, Chen T. Targeting xenobiotic receptors P XR and CAR in human diseases. Drug Discov Today. 2015, May;20(5):618–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JR. BPA and P XR activation: human receptor is affected, mouse receptor is not. Environ Health Perspect. 2012, Mar;120(3):A122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barretto SALasserre FHuillet MRégnier MPolizzi ALippi YFougerat APerson EBruel SBétoulières C, et al. (2021) The pregnane X receptor drives sexually dimorphic hepatic changes in lipid and xenobiotic metabolism in response to gut microbiota in mice. Microbiome 9:93 10.1186/s40168-021-01050-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudoin JJ, Brouwer KLR, Malinen MM (2020) Novel insights into the organic solute transporter alpha/beta, OSTα/β: from the bench to the bedside. Pharmacol Ther 211:107542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertilsson G, Heidrich J, Svensson K, Asman M, Jendeberg L, Sydow-Bäckman M, Ohlsson R, Postlind H, Blomquist P, Berkenstam A (1998) Identification of a human nuclear receptor defines a new signaling pathway for CYP3A induction. Proc Natl Acad Sci USA 95:12208–12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JA, Butenhoff JL, Wallace KB. Multiplicity of nuclear receptor activation by P FOA and P FOS in primary human and rodent hepatocytes. Toxicology. 2011. Oct 9;288(1-3):8–17. [DOI] [PubMed] [Google Scholar]
- Björkholm B, Bok CM, Lundin A, Rafter J, Hibberd ML, Pettersson S (2009) Intestinal microbiota regulate xenobiotic metabolism in the liver. PLoS One 4:e6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg B, Sabbagh W Jr, Juguilon H, Bolado J Jr, van Meter CM, Ong ES, Evans RM (1998) SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev 12:3195–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer CT, Chen T. P XR variants: the impact on drug metabolism and therapeutic responses. Acta Pharm Sin B. 2016. Sep;6(5):441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brobst DE, Ding X, Creech KL, Goodwin B, Kelley B, Staudinger JL (2004) Guggulsterone activates multiple nuclear receptors and induces CYP3A gene expression through the pregnane X receptor. J Pharmacol Exp Ther 310:528–535. [DOI] [PubMed] [Google Scholar]
- Buckley DB, Klaassen CD (2009) Induction of mouse UDP-glucuronosyltransferase mRNA expression in liver and intestine by activators of aryl-hydrocarbon receptor, constitutive androstane receptor, pregnane X receptor, peroxisome proliferator-activated receptor alpha, and nuclear factor erythroid 2-related factor 2. Drug Metab Dispos 37:847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk O, Koch I, Raucy J, Hustert E, Eichelbaum M, Brockmöller J, Zanger UM, Wojnowski L. The induction of cytochrome P450 3A5 (CYP3A5) in the human liver and intestine is mediated by the xenobiotic sensors pregnane X receptor (P XR) and constitutively activated receptor (CAR). J Biol Chem. 2004. Sep 10;279(37):38379–85. [DOI] [PubMed] [Google Scholar]
- Burk O, Arnold KA, Nussler AK, Schaeffeler E, Efimova E, Avery BA, Avery MA, Fromm MF, Eichelbaum M (2005) Antimalarial artemisinin drugs induce cytochrome P450 and MDR1 expression by activation of xenosensors pregnane X receptor and constitutive androstane receptor. Mol Pharmacol 67:1954–1965. [DOI] [PubMed] [Google Scholar]
- Cantoni L, Rozio M, Mangolini A, Hauri L, Caccia S (2003) Hyperforin contributes to the hepatic CYP3A-inducing effect of Hypericum perforatum extract in the mouse. Toxicol Sci 75:25–30. [DOI] [PubMed] [Google Scholar]
- Caparrós-Martín JALareu RRRamsay JPPeplies JReen FJHeadlam HAWard NCCroft KDNewsholme PHughes JD, et al. (2017) Statin therapy causes gut dysbiosis in mice through a PXR-dependent mechanism. Microbiome 5:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TK, Waxman DJ (2006) Synthetic drugs and natural products as modulators of constitutive androstane receptor (CAR) and pregnane X receptor (PXR). Drug Metab Rev 38:51–73. [DOI] [PubMed] [Google Scholar]
- Chen T. Overcoming drug resistance by regulating nuclear receptors. Adv Drug Deliv Rev. 2010. Oct 30;62(13):1257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Shah YM, Gonzalez FJ (2012) Pregnane X receptor as a target for treatment of inflammatory bowel disorders. Trends Pharmacol Sci 33:323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SL, Li X, Lehmler HJ, Phillips B, Shen D, Cui JY (2018) Gut microbiota modulates interactions between polychlorinated biphenyls and bile acid homeostasis. Toxicol Sci 166:269–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Maher J, Dieter MZ, Klaassen CD (2005) Regulation of mouse organic anion-transporting polypeptides (Oatps) in liver by prototypical microsomal enzyme inducers that activate distinct transcription factor pathways. Drug Metab Dispos 33:1276–1282. [DOI] [PubMed] [Google Scholar]
- Conly JM, Stein K (1992) The production of menaquinones (vitamin K2) by intestinal bacteria and their role in maintaining coagulation homeostasis. Prog Food Nutr Sci 16:307–343. [PubMed] [Google Scholar]
- Cooper BW, Cho T M, Thompson P M, Wallace AD. Phthalate induction of CYP3A4 is dependent on glucocorticoid regulation of P XR expression. Toxicol Sci. 2008. Jun;103(2):268–77. [DOI] [PubMed] [Google Scholar]
- Coumoul X, Diry M, Barouki R. P XRdependent induction of human CYP3A4 gene expression by organochlorine pesticides. Biochem Pharmacol. 2002. Nov 15;64(10):1513-9. [DOI] [PubMed] [Google Scholar]
- Creusot N, Garoche C, Grimaldi M, Boulahtouf A, Chiavarina B, Bourguet W, Balaguer P (2021) A comparative study of human and zebrafish pregnane X receptor activities of pesticides and steroids using in vitro reporter gene assays. Front Endocrinol (Lausanne) 12:665521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz R, Palmeira JD, Martins ZE, Faria MA, Ferreira H, Marques A, Casal S, Cunha SC (2020) Multidisciplinary approach to determine the effect of polybrominated diphenyl ethers on gut microbiota. Environ Pollut 260:113920. [DOI] [PubMed] [Google Scholar]
- Cui HGu XChen JXie YKe SWu JGolovko AMorpurgo BYan CPhillips TD, et al. (2017) Pregnane X receptor regulates the AhR/Cyp1A1 pathway and protects liver cells from benzo-[α]-pyrene-induced DNA damage. Toxicol Lett 275:67–76. [DOI] [PubMed] [Google Scholar]
- Cui JY, Gunewardena SS, Rockwell CE, Klaassen CD (2010) ChIPing the cistrome of PXR in mouse liver. Nucleic Acids Res 38:7943–7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui JY, Renaud HJ, Klaassen CD (2012) Ontogeny of novel cytochrome P450 gene isoforms during postnatal liver maturation in mice. Drug Metab Dispos 40:1226–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daujat-Chavanieu M, Gerbal-Chaloin S (2020) Regulation of CAR and PXR expression in health and disease. Cells 9:2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey JL, Cui JY (2019) Microbiome is a functional modifier of P450 drug metabolism. Curr Pharmacol Rep 5:481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey JL, Wang D, Siginir G, Fei Q, Raftery D, Gu H, Yue Cui J (2019) Pharmacological activation of PXR and CAR downregulates distinct bile acid-metabolizing intestinal bacteria and alters bile acid homeostasis. Toxicol Sci 168:40–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng R, Xu C, Chen X, Chen P, Wang Y, Zhou X, Jin J, Niu L, Ying M, Huang M, Bi H. Resveratrol suppresses the inducible expression of CYP3A4 through the pregnane X receptor. J Pharmacol Sci. 2014;126(2):146–54. [DOI] [PubMed] [Google Scholar]
- Derrien M, Belzer C, de Vos WM (2017) Akkermansia muciniphila and its role in regulating host functions. Microb Pathog 106:171–181. [DOI] [PubMed] [Google Scholar]
- Desai PB, Nallani SC, Sane RS, Moore LB, Goodwin BJ, Buckley DJ, Buckley AR (2002) Induction of cytochrome P450 3A4 in primary human hepatocytes and activation of the human pregnane X receptor by tamoxifen and 4-hydroxytamoxifen. Drug Metab Dispos 30:608–612. [DOI] [PubMed] [Google Scholar]
- Ding X, Lichti K, Staudinger JL. The mycoestrogen zearalenone induces CYP3A through activation of the pregnane X receptor. Toxicol Sci. 2006. Jun;91(2):448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Staudinger JL (2005a) Induction of drug metabolism by forskolin: the role of the pregnane X receptor and the protein kinase a signal transduction pathway. J Pharmacol Exp Ther 312:849–856. [DOI] [PubMed] [Google Scholar]
- Ding X, Staudinger JL (2005b) The ratio of constitutive androstane receptor to pregnane X receptor determines the activity of guggulsterone against the Cyp2b10 promoter. J Pharmacol Exp Ther 314(1):120–7. [DOI] [PubMed] [Google Scholar]
- Doricakova A, Vrzal R (2015) A food contaminant ochratoxin A suppresses pregnane X receptor (PXR)-mediated CYP3A4 induction in primary cultures of human hepatocytes. Toxicology 337:72–78. [DOI] [PubMed] [Google Scholar]
- Dou W, Zhang J, Li H, Kortagere S, Sun K, Ding L, Ren G, Wang Z, Mani S (2014) Plant flavonol isorhamnetin attenuates chemically induced inflammatory bowel disease via a PXR-dependent pathway. J Nutr Biochem 25:923–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürr D, Stieger B, Kullak-Ublick GA, Rentsch KM, Steinert HC, Meier PJ, Fattinger K (2000) St John’s Wort induces intestinal P-glycoprotein/MDR1 and intestinal and hepatic CYP3A4. Clin Pharmacol Ther 68:598–604. [DOI] [PubMed] [Google Scholar]
- Dvořák ZKopp FCostello CMKemp JSLi HVrzalová AŠtěpánková MBartoňková IJiskrová EPoulíková K, et al. (2020a) Targeting the pregnane X receptor using microbial metabolite mimicry. EMBO Mol Med 12:e11621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvořák Z, Sokol H, Mani S (2020b) Drug mimicry: promiscuous receptors PXR and AhR, and microbial metabolite interactions in the intestine. Trends Pharmacol Sci 41:900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybdahl M, Nikolov NG, Wedebye EB, Jónsdóttir SÓ, Niemelä JR. QSAR model for human pregnane X receptor (P XR) binding: screening of environmental chemicals and correlations with genotoxicity, endocrine disruption and teratogenicity. Toxicol Appl Pharmacol. 2012. Aug 1;262(3):301–9. [DOI] [PubMed] [Google Scholar]
- Eaton DL, Klaassen CD (1979) Effects of microsomal enzyme inducers on carrier-mediated transport systems in isolated rat hepatocytes. J Pharmacol Exp Ther 208:381–385. [PubMed] [Google Scholar]
- Egusquiza RJ, Ambrosio ME, Wang SG, Kay KM, Zhang C, Lehmler HJ, Blumberg B. Evaluating the Role of the Steroid and Xenobiotic Receptor (SXR/P XR) in P CB-153 Metabolism and Protection against Associated Adverse Effects during Perinatal and Chronic Exposure in Mice. Environ Health Perspect. 2020. Apr;128(4):47011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekins S, Reschly EJ, Hagey LR, Krasowski MD (2008) Evolution of pharmacologic specificity in the pregnane X receptor. BMC Evol Biol 8:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sankary W, Gibson GG, Ayrton A, Plant N (2001) Use of a reporter gene assay to predict and rank the potency and efficacy of CYP3A4 inducers. Drug Metab Dispos 29:1499–1504. [PubMed] [Google Scholar]
- Fickert P, Fuchsbichler A, Marschall HU, Wagner M, Zollner G, Krause R, Zatloukal K, Jaeschke H, Denk H, Trauner M (2006) Lithocholic acid feeding induces segmental bile duct obstruction and destructive cholangitis in mice. Am J Pathol 168:410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley MH, O’Flaherty S, Barrangou R, Theriot CM (2019) Bile salt hydrolases: Gatekeepers of bile acid metabolism and host-microbiome crosstalk in the gastrointestinal tract. PLoS Pathog 15:e1007581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu ZD, Csanaky IL, Klaassen CD (2012) Effects of aging on mRNA profiles for drug-metabolizing enzymes and transporters in livers of male and female mice. Drug Metab Dispos 40:1216–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu ZD, Selwyn FP, Cui JY, Klaassen CD (2017) RNA-Seq profiling of intestinal expression of xenobiotic processing genes in germ-free mice. Drug Metab Dispos 45:1225–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson GG, Plant NJ, Swales KE, Ayrton A, El-Sankary W (2002) Receptor-dependent transcriptional activation of cytochrome P4503A genes: induction mechanisms, species differences and interindividual variation in man. Xenobiotica 32:165–206. [DOI] [PubMed] [Google Scholar]
- Giebel NL, Shadley JD, McCarver DG, Dorko K, Gramignoli R, Strom SC, Yan K, Simpson PM, Hines RN (2016) Role of chromatin structural changes in regulating human CYP3A ontogeny. Drug Metab Dispos 44:1027–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez MV, Dutta M, Suvorov A, Shi X, Gu H, Mani S, Yue Cui J (2021) Early life exposure to environmental contaminants (BDE-47, TBBPA, and BPS) produced persistent alterations in fecal microbiome in adult male mice. Toxicol Sci 179:14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzelian J, Barwick JL, Hunter L, Phang TL, Quattrochi LC, Guzelian PS (2006) Identification of genes controlled by the pregnane X receptor by microarray analysis of mRNAs from pregnenolone 16alpha-carbonitrile-treated rats. Toxicol Sci 94:379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart SN, Cui Y, Klaassen CD, Zhong XB (2009) Three patterns of cytochrome P450 gene expression during liver maturation in mice. Drug Metab Dispos 37:116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Gao J, Xu M, Ren S, Stefanovic-Racic M, O’Doherty RM, Xie W (2013) PXR ablation alleviates diet-induced and genetic obesity and insulin resistance in mice. Diabetes 62:1876–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Li H, Huang N, Zhou X, Tian J, Li T, Wu J, Tian Y, Yin Y, Yao K (2017) Alpha-ketoglutarate suppresses the NF-κB-mediated inflammatory pathway and enhances the PXR-regulated detoxification pathway. Oncotarget 8:102974–102988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez JP, Mota LC, Baldwin WS (2009) Activation of CAR and PXR by dietary, environmental and occupational chemicals alters drug metabolism, intermediary metabolism, and cell proliferation. Curr Pharmacogenomics Person Med 7:81–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffart E, Ghebreghiorghis L, Nussler AK, Thasler WE, Weiss TS, Schwab M, Burk O (2012) Effects of atorvastatin metabolites on induction of drug-metabolizing enzymes and membrane transporters through human pregnane X receptor. Br J Pharmacol 165:1595–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe K, Sanat F, Thumser AE, Coleman T, Plant N (2011) The statin class of HMG-CoA reductase inhibitors demonstrate differential activation of the nuclear receptors PXR, CAR and FXR, as well as their downstream target genes. Xenobiotica 41:519–529. [DOI] [PubMed] [Google Scholar]
- Huang H, Wang H, Sinz M, Zoeckler M, Staudinger J, Redinbo MR, Teotico DG, Locker J, Kalpana GV, Mani S (2007) Inhibition of drug metabolism by blocking the activation of nuclear receptors by ketoconazole. Oncogene 26:258–268. [DOI] [PubMed] [Google Scholar]
- Huang K, Mukherjee S, DesMarais V, Albanese JM, Rafti E, Draghi Ii A, Maher LA, Khanna KM, Mani S, Matson AP (2018) Targeting the PXR-TLR4 signaling pathway to reduce intestinal inflammation in an experimental model of necrotizing enterocolitis. Pediatr Res 83:1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hukkanen J, Hakkola J, Rysä J (2014) Pregnane X receptor (PXR)—a contributor to the diabetes epidemic? Drug Metabol Drug Interact 29:3–15. [DOI] [PubMed] [Google Scholar]
- Hurst CH, Waxman DJ. Environmental phthalate monoesters activate pregnane X receptor-mediated transcription. Toxicol Appl Pharmacol. 2004. Sep 15;199(3):266–74. [DOI] [PubMed] [Google Scholar]
- Ishii M, Toda T, Ikarashi N, Kusunoki Y, Kon R, Ochiai W, Machida Y, Sugiyama K (2014) Gastrectomy increases the expression of hepatic cytochrome P450 3A by increasing lithocholic acid-producing enteric bacteria in mice. Biol Pharm Bull 37:298–305. [DOI] [PubMed] [Google Scholar]
- Jacobs MN, Nolan GT, Hood SR (2005) Lignans, bacteriocides and organochlorine compounds activate the human pregnane X receptor (PXR). Toxicol Appl Pharmacol 209:123–133. [DOI] [PubMed] [Google Scholar]
- Jia B, Jeon CO (2019) Promotion and induction of liver cancer by gut microbiome-mediated modulation of bile acids. PLoS Pathog 15:e1007954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SAMoore LBShenk JLWisely GBHamilton GAMcKee DDTomkinson NCLeCluyse ELLambert MHWillson TM, et al. (2000) The pregnane X receptor: a promiscuous xenobiotic receptor that has diverged during evolution. Mol Endocrinol 14:27–39. [DOI] [PubMed] [Google Scholar]
- Jung D, Mangelsdorf DJ, Meyer UA (2006) Pregnane X receptor is a target of farnesoid X receptor. J Biol Chem 281:19081–19091. [DOI] [PubMed] [Google Scholar]
- Kahan BD, Kramer WG, Wideman C, Flechner SM, Lorber MI, Van Buren CT (1986) Demographic factors affecting the pharmacokinetics of cyclosporine estimated by radioimmunoassay. Transplantation 41:459–464. [DOI] [PubMed] [Google Scholar]
- Kim S, Choi S, Dutta M, Asubonteng JO, Polunas M, Goedken M, Gonzalez FJ, Cui JY, Gyamfi MA (2021) Pregnane X receptor exacerbates nonalcoholic fatty liver disease accompanied by obesity- and inflammation-prone gut microbiome signature. Biochem Pharmacol 193:114698 10.1016/j.bcp.2021.114698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen CD (1972) Immaturity of the newborn rat’s hepatic excretory function for ouabain. J Pharmacol Exp Ther 183:520–526. [PubMed] [Google Scholar]
- Klaassen CD (1973) Comparison of the toxicity of chemicals in newborn rats to bile duct-ligated and sham-operated rats and mice. Toxicol Appl Pharmacol 24:37–44. [DOI] [PubMed] [Google Scholar]
- Klaassen CD (1974a) Effect of microsomal enzyme inducers on the biliary excretion of cardiac glycosides. J Pharmacol Exp Ther 191:201–211. [PubMed] [Google Scholar]
- Klaassen CD (1974b) Stimulation of the development of the hepatic excretory mechanism for ouabain in newborn rats with microsomal enzyme inducers. J Pharmacol Exp Ther 191:212–218. [PubMed] [Google Scholar]
- Kliewer SA, Goodwin B, Willson TM (2002) The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev 23:687–702. [DOI] [PubMed] [Google Scholar]
- Kliewer SAMoore JTWade LStaudinger JLWatson MAJones SAMcKee DDOliver BBWillson TMZetterström RH, et al. (1998) An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell 92:73–82. [DOI] [PubMed] [Google Scholar]
- Kliewer SA. Nuclear receptor P XR: discovery of a pharmaceutical anti-target. J Clin Invest. 2015. Apr;125(4):1388–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodkin A, Sahin N, Phillips A, Hood SR, Bruggeman FJ, Westerhoff HV, Plant N (2013) Optimization of stress response through the nuclear receptor-mediated cortisol signalling network. Nat Commun 4:1792 10.1038/ncomms2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komoroski BJZhang SCai HHutzler JMFrye RTracy TSStrom SCLehmann TAng CYCui YY, et al. (2004) Induction and inhibition of cytochromes P450 by the St. John’s wort constituent hyperforin in human hepatocyte cultures. Drug Metab Dispos 32:512–518. [DOI] [PubMed] [Google Scholar]
- Kostrubsky VE, Lewis LD, Strom SC, Wood SG, Schuetz EG, Schuetz JD, Sinclair PR, Wrighton SA, Sinclair JF (1998) Induction of cytochrome P4503A by taxol in primary cultures of human hepatocytes. Arch Biochem Biophys 355:131–136. [DOI] [PubMed] [Google Scholar]
- Krasowski MD, Ni A, Hagey LR, Ekins S (2011) Evolution of promiscuous nuclear hormone receptors: LXR, FXR, VDR, PXR, and CAR. Mol Cell Endocrinol 334:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krecic-Shepard ME, Park K, Barnas C, Slimko J, Kerwin DR, Schwartz JB (2000) Race and sex influence clearance of nifedipine: results of a population study. Clin Pharmacol Ther 68:130–142. [DOI] [PubMed] [Google Scholar]
- Kumar R, Litoff EJ, Boswell WT, Baldwin WS (2018) High fat diet induced obesity is mitigated in Cyp3a-null female mice. Chem Biol Interact 289:129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara I, Lee DK, Petit FG, Jeong J, Lee K, Lydon JP, DeMayo FJ, Tsai MJ, Tsai SY (2007) COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet 3:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landes N, Pfluger P, Kluth D, Birringer M, Rühl R, Böl GF, Glatt H, Brigelius-Flohé R (2003) Vitamin E activates gene expression via the pregnane X receptor. Biochem Pharmacol 65:269–273. [DOI] [PubMed] [Google Scholar]
- LeCluyse EL (2001) Pregnane X receptor: molecular basis for species differences in CYP3A induction by xenobiotics. Chem Biol Interact 134:283–289. [DOI] [PubMed] [Google Scholar]
- Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA (1998) The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Invest 102:1016–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire G, de Sousa G, Rahmani R. A P XR reporter gene assay in a stable cell culture system: CYP3A4 and CYP2B6 induction by pesticides. Biochem Pharmacol. 2004. Dec 15;68(12):2347–58. [DOI] [PubMed] [Google Scholar]
- Lemaire GMnif WPascussi JMPillon ARabenoelina FFenet HGomez ECasellas CNicolas JCCavaillès V, et al. (2006) Identification of new human pregnane X receptor ligands among pesticides using a stable reporter cell system. Toxicol Sci 91:501–509. [DOI] [PubMed] [Google Scholar]
- Li CY, Cheng SL, Bammler TK, Cui JY (2016a) Editor’s highlight: Neonatal activation of the xenobiotic-sensors PXR and CAR results in acute and persistent down-regulation of PPARα-signaling in mouse liver. Toxicol Sci 153:282–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CYDempsey JLWang DLee SWeigel KMFei QBhatt DKPrasad BRaftery DGu H, et al. (2018) PBDEs altered gut microbiome and bile acid homeostasis in male C57BL/6 mice. Drug Metab Dispos 46:1226–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CY, Lee S, Cade S, Kuo LJ, Schultz IR, Bhatt DK, Prasad B, Bammler TK, Cui JY (2017) Novel interactions between gut microbiome and host drug-processing genes modify the hepatic metabolism of the environmental chemicals polybrominated diphenyl ethers. Drug Metab Dispos 45:1197–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CY, Renaud HJ, Klaassen CD, Cui JY (2016b) Age-specific regulation of drug-processing genes in mouse liver by ligands of xenobiotic-sensing transcription factors. Drug Metab Dispos 44:1038–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HIllés PKarunaratne CVNordstrøm LULuo XYang AQiu YKurland IJLukin DJChen W, et al. (2021) Deciphering structural bases of intestinal and hepatic selectivity in targeting pregnane X receptor with indole-based microbial mimics. Bioorg Chem 109:104661 10.1016/j.bioorg.2021.104661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Welch MA, Li Z, Mackowiak B, Heyward S, Swaan PW, Wang H. Mechanistic Insights of Phenobarbital-Mediated Activation of Human but Not Mouse Pregnane X Receptor. Mol Pharmacol. 2019. Sep;96(3):345–354. doi: 10.1124/mol.119.116616. Epub 2019 Jul 10. P MID: 31436536; P MCID: P MC6701513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Li ZQ, Deng CH, Ning MR, Li HQ, Bi SS, Zhou TY, Lu W (2012) A mechanism-based pharmacokinetic/pharmacodynamic model for CYP3A1/2 induction by dexamethasone in rats. Acta Pharmacol Sin 33:127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Chiang JY (2005) Mechanism of rifampicin and pregnane X receptor inhibition of human cholesterol 7 alpha-hydroxylase gene transcription. Am J Physiol Gastrointest Liver Physiol 288:G74–G84. [DOI] [PubMed] [Google Scholar]
- Li T, Chiang JY (2006) Rifampicin induction of CYP3A4 requires pregnane X receptor cross talk with hepatocyte nuclear factor 4alpha and coactivators, and suppression of small heterodimer partner gene expression. Drug Metab Dispos 34:756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Chiang JY (2015) Bile acids as metabolic regulators. Curr Opin Gastroenterol 31:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Cui Y, Hart SN, Klaassen CD, Zhong XB (2009) Dynamic patterns of histone methylation are associated with ontogenic expression of the Cyp3a genes during mouse liver maturation. Mol Pharmacol 75:1171–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JJ, Li X, Lehmler HJ, Wang D, Gu H, Cui JY (2020) Gut microbiome critically impacts PCB-induced changes in metabolic fingerprints and the hepatic transcriptome in mice. Toxicol Sci 177:168–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Bwayi M, Wu J, Li Y, Chai SC, Huber AD, Chen T. CIT CO Directly Binds to and Activates Human Pregnane X Receptor. Mol Pharmacol. 2020. Mar;97(3):180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Wang YM, Chai SC, Lv L, Zheng J, Wu J, Zhang Q, Wang YD, Griffin PR, Chen T (2017) SPA70 is a potent antagonist of human pregnane X receptor. Nat Commun 8:741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindley C, Hamilton G, McCune JS, Faucette S,, Shord SS, Hawke RL, Wang H, Gilbert D, Jolley S, Yan B, LeCluyse EL. The effect of cyclophosphamide with and without dexamethasone on cytochrome P450 3A4 and 2B6 in human hepatocytes. Drug Metab Dispos. 2002. Jul;30(7):814–22. [DOI] [PubMed] [Google Scholar]
- Little MDutta MLi HMatson AShi XMascarinas GMolla BWeigel KGu HMani S, et al. (2021) Understanding the physiological functions of the host xenobiotic-sensing nuclear receptors PXR and CAR on the gut microbiome using genetically modified mice. Acta Pharm Sin B, in press 10.1016/j.aspb.2021.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Tian J, Yin H, Yin J, Tang Y (2019) Self-protective transcriptional alterations in ZF4 cells exposed to Pb(NO3 )2 and AgNO3. J Biochem Mol Toxicol 33:e22408. [DOI] [PubMed] [Google Scholar]
- Lu YF, Jin T, Xu Y, Zhang D, Wu Q, Zhang YK, Liu J (2013) Sex differences in the circadian variation of cytochrome p450 genes and corresponding nuclear receptors in mouse liver. Chronobiol Int 30:1135–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke NS, DeVito MJ, Shah I, El-Masri HA (2010) Development of a quantitative model of pregnane X receptor (PXR) mediated xenobiotic metabolizing enzyme induction. Bull Math Biol 72:1799–1819. [DOI] [PubMed] [Google Scholar]
- Luo GCunningham MKim SBurn TLin JSinz MHamilton GRizzo CJolley SGilbert D, et al. (2002) CYP3A4 induction by drugs: correlation between a pregnane X receptor reporter gene assay and CYP3A4 expression in human hepatocytes. Drug Metab Dispos 30:795–804. [DOI] [PubMed] [Google Scholar]
- Lynch C, Sakamuru S, Huang R, Niebler J, Ferguson SS, Xia M (2021) Characterization of human pregnane X receptor activators identified from a screening of the Tox21 compound library. Biochem Pharmacol 184:114368 10.1016/j.bcp.2020.114368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Idle JR, Gonzalez FJ (2008) The pregnane X receptor: from bench to bedside. Expert Opin Drug Metab Toxicol 4:895–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Sachdeva K, Liu J, Ford M, Yang D, Khan IA, Chichester CO, Yan B (2004) Desmethoxyyangonin and dihydromethysticin are two major pharmacological kavalactones with marked activity on the induction of CYP3A23. Drug Metab Dispos 32:1317–1324. [DOI] [PubMed] [Google Scholar]
- Maher JM, Cheng X, Slitt AL, Dieter MZ, Klaassen CD (2005) Induction of the multidrug resistance-associated protein family of transporters by chemical activators of receptor-mediated pathways in mouse liver. Drug Metab Dispos 33:956–962. [DOI] [PubMed] [Google Scholar]
- Mani S, Dou W, Redinbo MR. P XR antagonists and implication in drug metabolism. Drug Metab Rev. 2013. Feb;45(1):60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek CJ, Tucker SJ, Konstantinou DK, Elrick LJ, Haefner D, Sigalas C, Murray GI, Goodwin B, Wright MC (2005) Pregnenolone-16alpha-carbonitrile inhibits rodent liver fibrogenesis via PXR (pregnane X receptor)-dependent and PXR-independent mechanisms. Biochem J 387:601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Díaz IM, Estrada-Muñiz E, Reyes-Hernández OD, Ramírez P, Vega L, Elizondo G (2009) Arsenite and its metabolites, MMA(III) and DMA(III), modify CYP3A4, PXR and RXR alpha expression in the small intestine of CYP3A4 transgenic mice. Toxicol Appl Pharmacol 239:162–168. [DOI] [PubMed] [Google Scholar]
- Meucci V, Arukwe A (2006) The xenoestrogen 4-nonylphenol modulates hepatic gene expression of pregnane X receptor, aryl hydrocarbon receptor, CYP3A and CYP1A1 in juvenile Atlantic salmon (Salmo salar). Comp Biochem Physiol C Toxicol Pharmacol 142:142–150. [DOI] [PubMed] [Google Scholar]
- Mikamo E, Harada S, Nishikawa J, Nishihara T (2003) Endocrine disruptors induce cytochrome P450 by affecting transcriptional regulation via pregnane X receptor. Toxicol Appl Pharmacol 193:66–72. [DOI] [PubMed] [Google Scholar]
- Milnes MRGarcia AGrossman EGrün FShiotsugu JTabb MMKawashima YKatsu YWatanabe HIguchi T, et al. (2008) Activation of steroid and xenobiotic receptor (SXR, NR1I2) and its orthologs in laboratory, toxicologic, and genome model species. Environ Health Perspect 116:880–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas S, Vairappan B. Role of pregnane X-receptor in regulating bacterial translocation in chronic liver diseases. World J Hepatol. 2017. Nov 18;9(32):1210–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JT, Kliewer SA (2000) Use of the nuclear receptor PXR to predict drug interactions. Toxicology 153:1–10. [DOI] [PubMed] [Google Scholar]
- Moore JT, Moore LB, Maglich JM, Kliewer SA (2003) Functional and structural comparison of PXR and CAR. Biochim Biophys Acta 1619:235–238. [DOI] [PubMed] [Google Scholar]
- Moore LBParks DJJones SABledsoe RKConsler TGStimmel JBGoodwin BLiddle CBlanchard SGWillson TM, et al. (2000a) Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J Biol Chem 275:15122–15127. [DOI] [PubMed] [Google Scholar]
- Moore LB, Goodwin B, Jones SA, Wisely GB, Serabjit-Singh CJ, Willson TM, Collins JL, Kliewer SA(2000b) St. John's wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc Natl Acad Sci USA 97:7500–7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan ET, Dempsey JL, Mimche SM, Lamb TJ, Kulkarni S, Cui JY, Jeong H, Slitt AL (2018) Physiological regulation of drug metabolism and transport: pregnancy, microbiome, inflammation, infection, and fasting. Drug Metab Dispos 46:503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SL, Guzelian PS (1983) Identification of the cyanopregnenolone-inducible form of hepatic cytochrome P-450 as a catalyst of aldrin epoxidation. Biochem Pharmacol 32:1529–1531. [DOI] [PubMed] [Google Scholar]
- Ngan CH, Beglov D, Rudnitskaya AN, Kozakov D, Waxman DJ, Vajda S (2009) The structural basis of pregnane X receptor binding promiscuity. Biochemistry 48:11572–11581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble SMCarnahan VEMoore LBLuntz TWang HIttoop ORStimmel JBDavis-Searles PRWatkins REWisely GB, et al. (2006) Human PXR forms a tryptophan zipper-mediated homodimer. Biochemistry 45:8579–8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ourlin JC, Lasserre F, Pineau T, Fabre JM, Sa-Cunha A, Maurel P, Vilarem MJ, Pascussi JM (2003) The small heterodimer partner interacts with the pregnane X receptor and represses its transcriptional activity. Mol Endocrinol 17:1693–1703. [DOI] [PubMed] [Google Scholar]
- Pacyniak EK, Cheng X, Cunningham ML, Crofton K, Klaassen CD, Guo GL (2007) The flame retardants, polybrominated diphenyl ethers, are pregnane X receptor activators. Toxicol Sci 97:94–102. [DOI] [PubMed] [Google Scholar]
- Parkinson A, Mudra DR, Johnson C, Dwyer A, Carroll KM (2004) The effects of gender, age, ethnicity, and liver cirrhosis on cytochrome P450 enzyme activity in human liver microsomes and inducibility in cultured human hepatocytes. Toxicol Appl Pharmacol 199:193–209. [DOI] [PubMed] [Google Scholar]
- Pascussi JM, Drocourt L, Fabre JM, Maurel P, Vilarem MJ. Dexamethasone induces pregnane X receptor and retinoid X receptoralpha expression in human hepatocytes: synergistic increase of CYP3A4 induction by pregnane X receptor activators. Mol Pharmacol. 2000. Aug;58(2):361–72. [DOI] [PubMed] [Google Scholar]
- Pascussi JM, Drocourt L, Gerbal-Chaloin S, Fabre JM, Maurel P, Vilarem MJ (2001) Dual effect of dexamethasone on CYP3A4 gene expression in human hepatocytes. Sequential role of glucocorticoid receptor and pregnane X receptor. Eur J Biochem 268:6346–6358. [DOI] [PubMed] [Google Scholar]
- Petrick JS, Klaassen CD (2007) Importance of hepatic induction of constitutive androstane receptor and other transcription factors that regulate xenobiotic metabolism and transport. Drug Metab Dispos 35:1806–1815. [DOI] [PubMed] [Google Scholar]
- Pinne M, Ponce E, Raucy JL (2016) Transactivation assays to assess canine and rodent pregnane X receptor (PXR) and constitutive androstane receptor (CAR) activation. PLoS One 11:e0164642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Png CW, Lindén SK, Gilshenan KS, Zoetendal EG, McSweeney CS, Sly LI, McGuckin MA, Florin TH (2010) Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am J Gastroenterol 105:2420–2428. [DOI] [PubMed] [Google Scholar]
- Pulakazhi Venu VK, Saifeddine M, Mihara K, Tsai YC, Nieves K, Alston L, Mani S, McCoy KD, Hollenberg MD, Hirota SA (2019) The pregnane X receptor and its microbiota-derived ligand indole 3-propionic acid regulate endothelium-dependent vasodilation. Am J Physiol Endocrinol Metab 317:E350–E361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raucy J, Warfe L, Yueh MF, Allen SW (2002) A cell-based reporter gene assay for determining induction of CYP3A4 in a high-volume system. J Pharmacol Exp Ther 303:412–423. [DOI] [PubMed] [Google Scholar]
- Raucy JL (2003) Regulation of CYP3A4 expression in human hepatocytes by pharmaceuticals and natural products. Drug Metab Dispos 31:533–539. [DOI] [PubMed] [Google Scholar]
- Ravussin Y, Koren O, Spor A, LeDuc C, Gutman R, Stombaugh J, Knight R, Ley RE, Leibel RL (2012) Responses of gut microbiota to diet composition and weight loss in lean and obese mice. Obesity (Silver Spring) 20:738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reschly EJ, Bainy AC, Mattos JJ, Hagey LR, Bahary N, Mada SR, Ou J, Venkataramanan R, Krasowski MD (2007) Functional evolution of the vitamin D and pregnane X receptors. BMC Evol Biol 7:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Vallanat B, Nelson DM, Yeung LWY, Guruge KS, Lam P KS, Lehman-McKeeman LD, Corton JC. Evidence for the involvement of xenobiotic-responsive nuclear receptors in transcriptional effects upon perfluoroalkyl acid exposure in diverse species. Reprod Toxicol. 2009. Jun;27(3-4):266–277. [DOI] [PubMed] [Google Scholar]
- Reschly EJ, Krasowski MD (2006) Evolution and function of the NR1I nuclear hormone receptor subfamily (VDR, PXR, and CAR) with respect to metabolism of xenobiotics and endogenous compounds. Curr Drug Metab 7:349–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraswathi V, Perriotte-Olson C, Ganesan M, Desouza CV, Alnouti Y, Duryee MJ, Thiele GM, Nordgren TM, Clemens DL (2017) A combination of dietary N-3 fatty acids and a cyclooxygenase-1 inhibitor attenuates nonalcoholic fatty liver disease in mice. J Nutr Biochem 42:149–159. [DOI] [PubMed] [Google Scholar]
- Scandlyn MJ, Stuart EC, Rosengren RJ (2008) Sex-specific differences in CYP450 isoforms in humans. Expert Opin Drug Metab Toxicol 4:413–424. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Baumann F, Hanschmann H, Geissler F, Preiss R (2001) Gender difference in ifosfamide metabolism by human liver microsomes. Eur J Drug Metab Pharmacokinet 26:193–200. [DOI] [PubMed] [Google Scholar]
- Schuetz E, Lan L, Yasuda K, Kim R, Kocarek TA, Schuetz J, Strom S (2002) Development of a real-time in vivo transcription assay: application reveals pregnane X receptor-mediated induction of CYP3A4 by cancer chemotherapeutic agents. Mol Pharmacol 62:439–445. [DOI] [PubMed] [Google Scholar]
- Schuetz EG, Wrighton SA, Barwick JL, Guzelian PS (1984) Induction of cytochrome P-450 by glucocorticoids in rat liver. I. Evidence that glucocorticoids and pregnenolone 16 alpha-carbonitrile regulate de novo synthesis of a common form of cytochrome P-450 in cultures of adult rat hepatocytes and in the liver in vivo. J Biol Chem 259:1999–2006. [PubMed] [Google Scholar]
- Scoville DK, Li CY, Wang D, Dempsey JL, Raftery D, Mani S, Gu H, Cui JY (2019) Polybrominated diphenyl ethers and gut microbiome modulate metabolic syndrome-related aqueous metabolites in mice. Drug Metab Dispos 47:928–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamon KB, Padgett W, Daly JW (1981) Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci USA 78:3363–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selwyn FP, Cheng SL, Bammler TK, Prasad B, Vrana M, Klaassen C, Cui JY (2015a) Developmental regulation of drug-processing genes in livers of germ-free mice. Toxicol Sci 147:84–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selwyn FP, Cheng SL, Klaassen CD, Cui JY (2016) Regulation of hepatic drug-metabolizing enzymes in germ-free mice by conventionalization and probiotics. Drug Metab Dispos 44:262–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selwyn FP, Cui JY, Klaassen CD (2015b) RNA-Seq quantification of hepatic drug processing genes in germ-free mice. Drug Metab Dispos 43:1572–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehu AI, Li G, Xie W, Ma X (2016) The pregnane X receptor in tuberculosis therapeutics. Expert Opin Drug Metab Toxicol 12:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehu AI, Lu J, Wang P, Zhu J, Wang Y, Yang D, McMahon D, Xie W, Gonzalez FJ, Ma X. Pregnane X receptor activation potentiates ritonavir hepatotoxicity. J Clin Invest. 2019. Apr 30;129(7):2898–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Shi Z, Fan JT, Yan B. Dechlorination and demethylation of ochratoxin A enhance blocking activity of P XR activation, suppress P XR expression and reduce cytotoxicity. Toxicol Lett. 2020. Oct 10;332:171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D, Yang D, Yan B. Dexamethasone transcriptionally increases the expression of the pregnane X receptor and synergistically enhances pyrethroid esfenvalerate in the induction of cytochrome P450 3A23. Biochem Pharmacol. 2010. Oct 15;80(8):1274–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla SJ, Sakamuru S, Huang R, Moeller TA, Shinn P, Vanleer D, Auld DS, Austin CP, Xia M (2011) Identification of clinically used drugs that activate pregnane X receptors. Drug Metab Dispos 39:151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirlis D, Muangmoonchai R, Edwards M, Phillips IR, Shephard EA (2001) Orphan receptor promiscuity in the induction of cytochromes p450 by xenobiotics. J Biol Chem 276:12822–12826. [DOI] [PubMed] [Google Scholar]
- Smith RPEckalbar WLMorrissey KMLuizon MRHoffmann TJSun XJones SLForce Aldred SRamamoorthy ADesta Z, et al. (2014) Genome-wide discovery of drug-dependent human liver regulatory elements. PLoS Genet 10:e1004648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smutny T, Pavek P. Resveratrol as an inhibitor of pregnane X receptor (P XR): another lesson in P XR antagonism. J Pharmacol Sci. 2014;126(2):177–8. [DOI] [PubMed] [Google Scholar]
- Souidi M, Gueguen Y, Linard C, Dudoignon N, Grison S, Baudelin C, Marquette C, Gourmelon P, Aigueperse J, Dublineau I (2005) In vivo effects of chronic contamination with depleted uranium on CYP3A and associated nuclear receptors PXR and CAR in the rat. Toxicology 214:113–122. [DOI] [PubMed] [Google Scholar]
- Spruiell K, Jones DZ, Cullen JM, Awumey EM, Gonzalez FJ, Gyamfi MA (2014a) Role of human pregnane X receptor in high fat diet-induced obesity in pre-menopausal female mice. Biochem Pharmacol 89:399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruiell K, Richardson RM, Cullen JM, Awumey EM, Gonzalez FJ, Gyamfi MA (2014b) Role of pregnane X receptor in obesity and glucose homeostasis in male mice. J Biol Chem 289:3244–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger JLGoodwin BJones SAHawkins-Brown DMacKenzie KILaTour ALiu YKlaassen CDBrown KKReinhard J, et al. (2001) The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci USA 98:3369–3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, Klaassen CD, Brown KK, Reinhard J, Willson T M, Koller BH, Kliewer SA. The nuclear receptor P XR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci U S A. 2001. Mar 13;98(6):3369–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueyoshi T, Li L, Wang H, Moore R, Kodavanti PR, Lehmler HJ, Negishi M, Birnbaum LS (2014) Flame retardant BDE-47 effectively activates nuclear receptor CAR in human primary hepatocytes. Toxicol Sci 137:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui Y, Ai N, Park SH, Rios-Pilier J, Perkins JT, Welsh WJ, Zhou C (2012) Bisphenol A and its analogues activate human pregnane X receptor. Environ Health Perspect 120:399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana H, Watanabe K, Rana MM, Takashima R, Ohashi A, Komai M, Shirakawa H (2018) Effects of vitamin K2 on the expression of genes involved in bile acid synthesis and glucose homeostasis in mice with humanized PXR. Nutrients 10:982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synold TW, Dussault I, Forman BM (2001) The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat Med 7:584–590. [DOI] [PubMed] [Google Scholar]
- Tabb MMSun AZhou CGrün FErrandi JRomero KPham HInoue SMallick SLin M, et al. (2003) Vitamin K2 regulation of bone homeostasis is mediated by the steroid and xenobiotic receptor SXR. J Biol Chem 278:43919–43927. [DOI] [PubMed] [Google Scholar]
- Takeshita A, Taguchi M, Koibuchi N, Ozawa Y (2002) Putative role of the orphan nuclear receptor SXR (steroid and xenobiotic receptor) in the mechanism of CYP3A4 inhibition by xenobiotics. J Biol Chem 277:32453–32458. [DOI] [PubMed] [Google Scholar]
- Tamasi V, Juvan P, Beer M, Rozman D, Meyer UA. Transcriptional activation of P PARalpha by phenobarbital in the absence of CAR and P XR. Mol Pharm. 2009. Sep-Oct;6(5):1573–81. [DOI] [PubMed] [Google Scholar]
- Tanaka E (1999) Gender-related differences in pharmacokinetics and their clinical significance. J Clin Pharm Ther 24:339–346. [DOI] [PubMed] [Google Scholar]
- Teotico DG, Frazier ML, Ding F, Dokholyan NV, Temple BR, Redinbo MR (2008) Active nuclear receptors exhibit highly correlated AF-2 domain motions. PLOS Comput Biol 4:e1000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirona RGLee WLeake BFLan LBCline CBLamba VParviz FDuncan SAInoue YGonzalez FJ, et al. (2003) The orphan nuclear receptor HNF4alpha determines PXR- and CAR-mediated xenobiotic induction of CYP3A4. Nat Med 9:220–224. [DOI] [PubMed] [Google Scholar]
- Toda T, Ohi K, Kudo T, Yoshida T, Ikarashi N, Ito K, Sugiyama K (2009a) Ciprofloxacin suppresses Cyp3a in mouse liver by reducing lithocholic acid-producing intestinal flora. Drug Metab Pharmacokinet 24:201–208. [DOI] [PubMed] [Google Scholar]
- Toda T, Saito N, Ikarashi N, Ito K, Yamamoto M, Ishige A, Watanabe K, Sugiyama K (2009b) Intestinal flora induces the expression of Cyp3a in the mouse liver. Xenobiotica 39:323–334. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI (2006) An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444:1027–1031. [DOI] [PubMed] [Google Scholar]
- Uppal H, Toma D, Saini SP, Ren S, Jones TJ, Xie W (2005) Combined loss of orphan receptors PXR and CAR heightens sensitivity to toxic bile acids in mice. Hepatology 41:168–176. [DOI] [PubMed] [Google Scholar]
- van Giersbergen PL, Gnerre C, Treiber A, Dingemanse J, Meyer UA (2002) Bosentan, a dual endothelin receptor antagonist, activates the pregnane X nuclear receptor. Eur J Pharmacol 450:115–121. [DOI] [PubMed] [Google Scholar]
- Venkatesh MMukherjee SWang HLi HSun KBenechet APQiu ZMaher LRedinbo MRPhillips RS, et al. (2014) Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and toll-like receptor 4. Immunity 41:296–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyhlídalová B, Bartonková I, Jiskrová E, Li H, Mani S, Dvorák Z. Differential activation of human pregnane X receptor P XR by isomeric mono-methylated indoles in intestinal and hepatic in vitro models. Toxicol Lett. 2020. May 15;324:104–110. [DOI] [PubMed] [Google Scholar]
- Wahlang B, Falkner KC, Clair HB, Al-Eryani L, Prough RA, States JC, Coslo DM, Omiecinski CJ, Cave MC. Human receptor activation by aroclor 1260, a polychlorinated biphenyl mixture. Toxicol Sci. 2014. Aug 1;140(2):283–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlang B, Alexander NC II, Li X, Rouchka EC, Kirpich IA, Cave MC (2021) Polychlorinated biphenyls altered gut microbiome in CAR and PXR knockout mice exhibiting toxicant-associated steatohepatitis. Toxicol Rep 8:536–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wistuba W, Gnewuch C, Liebisch G, Schmitz G, Langmann T. Lithocholic acid induction of the FGF19 promoter in intestinal cells is mediated by P XR. World J Gastroenterol. 2007. Aug 21;13(31):4230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlang B, Jin J, Hardesty JE, Head KZ, Shi H, Falkner KC, Prough RA, Klinge CM, Cave MC (2019) Identifying sex differences arising from polychlorinated biphenyl exposures in toxicant-associated liver disease. Food Chem Toxicol 129:64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DQ, Neuschwander-Tetri BA, Portincasa P, Pandak WM (2012) Interactions between bile acids and nuclear receptors and their effects on lipid metabolism and liver diseases. J Lipids 2012:560715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HLi HMoore LBJohnson MDMaglich JMGoodwin BIttoop ORWisely BCreech KParks DJ, et al. (2008) The phytoestrogen coumestrol is a naturally occurring antagonist of the human pregnane X receptor. Mol Endocrinol 22:838–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HVenkatesh MLi HGoetz RMukherjee SBiswas AZhu LKaubisch AWang LPullman J, et al. (2011) Pregnane X receptor activation induces FGF19-dependent tumor aggressiveness in humans and mice. J Clin Invest 121:3220–3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JY, Tsai CH, Lee YL, Lee LN, Hsu CL, Chang HC, Chen JM, Hsu CA, Yu CJ, Yang PC (2015) Gender-dimorphic impact of PXR genotype and haplotype on hepatotoxicity during antituberculosis treatment. Medicine (Baltimore) 94:e982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins RE, Maglich JM, Moore LB, Wisely GB, Noble SM, Davis-Searles PR, Lambert MH, Kliewer SA, Redinbo MR (2003) 2.1 a crystal structure of human PXR in complex with the St. John’s wort compound hyperforin. Biochemistry 42:1430–1438. [DOI] [PubMed] [Google Scholar]
- Watkins RE, Wisely GB, Moore LB, Collins JL, Lambert MH, Williams SP, Willson TM, Kliewer SA, Redinbo MR (2001) The human nuclear xenobiotic receptor PXR: structural determinants of directed promiscuity. Science 292:2329–2333. [DOI] [PubMed] [Google Scholar]
- Weger BDGobet CYeung JMartin EJimenez SBetrisey BFoata FBerger BBalvay AFoussier A, et al. (2019) The mouse microbiome is required for sex-specific diurnal rhythms of gene expression and metabolism. Cell Metab 29:362–382.e8 10.1016/j.cmet.2018.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei P, Zhang J, Dowhan DH, Han Y, Moore DD (2002) Specific and overlapping functions of the nuclear hormone receptors CAR and PXR in xenobiotic response. Pharmacogenomics J 2:117–126. [DOI] [PubMed] [Google Scholar]
- Zeng H, Jiang Y, Chen P, Fan X, Li D, Liu A, Ma X, Xie W, Liu P, Gonzalez FJ, Huang M, Bi H. Schisandrol B protects against cholestatic liver injury through pregnane X receptors. Br J Pharmacol. 2017. Apr;174(8):672–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolbold R, Klein K, Burk O, Nüssler AK, Neuhaus P, Eichelbaum M, Schwab M, Zanger UM (2003) Sex is a major determinant of CYP3A4 expression in human liver. Hepatology 38:978–988. [DOI] [PubMed] [Google Scholar]
- Woolbright BL, Li F, Xie Y, Farhood A, Fickert P, Trauner M, Jaeschke H (2014) Lithocholic acid feeding results in direct hepato-toxicity independent of neutrophil function in mice. Toxicol Lett 228:56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang D, Chu T, Li M, Wang Q, Zhu G (2018) Effects of pyrethroid pesticide cis-bifenthrin on lipogenesis in hepatic cell line. Chemosphere 201:840–849. [DOI] [PubMed] [Google Scholar]
- Xiang J, Zhang Z, Xie H, Zhang C, Bai Y, Cao H, Che Q, Guo J, Su Z (2021) Effect of different bile acids on the intestine through enterohepatic circulation based on FXR. Gut Microbes 13:1949095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Evans RM (2001) Orphan nuclear receptors: the exotics of xenobiotics. J Biol Chem 276:37739–37742. [DOI] [PubMed] [Google Scholar]
- Xie W, Radominska-Pandya A, Shi Y, Simon CM, Nelson MC, Ong ES, Waxman DJ, Evans RM (2001) An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc Natl Acad Sci USA 98:3375–3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu DX, Chen YH, Wang JP, Sun MF, Wang H, Wei LZ, Wei W (2005) Perinatal lipopolysaccharide exposure downregulates pregnane X receptor and Cyp3a11 expression in fetal mouse liver. Toxicol Sci 87:38–45. [DOI] [PubMed] [Google Scholar]
- Xu DX, Wei W, Sun MF, Wu CY, Wang JP, Wei LZ, Zhou CF (2004) Kupffer cells and reactive oxygen species partially mediate lipopolysaccharide-induced downregulation of nuclear receptor pregnane x receptor and its target gene CYP3a in mouse liver. Free Radic Biol Med 37:10–22. [DOI] [PubMed] [Google Scholar]
- Xu G, Dai M, Zheng X, Lin H, Liu A, Yang J (2020) Cholestatic models induced by lithocholic acid and α-naphthylisothiocyanate: different etiological mechanisms for liver injury but shared JNK/STAT3 signaling. Mol Med Rep 22:1583–1593. [DOI] [PubMed] [Google Scholar]
- Yueh MF, Kawahara M, Raucy J (2005) High volume bioassays to assess CYP3A4-mediated drug interactions: induction and inhibition in a single cell line. Drug Metab Dispos 33:38–48. [DOI] [PubMed] [Google Scholar]
- Zhang YK, Yeager RL, Klaassen CD (2009) Circadian expression profiles of drug-processing genes and transcription factors in mouse liver. Drug Metab Dispos 37:106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao LY, Xu JY, Shi Z, Englert NA, Zhang SY (2017) Pregnane X receptor (PXR) deficiency improves high fat diet-induced obesity via induction of fibroblast growth factor 15 (FGF15) expression. Biochem Pharmacol 142:194–203. [DOI] [PubMed] [Google Scholar]
- Zhou C, Poulton EJ, Grün F, Bammler TK, Blumberg B, Thummel KE, Eaton DL (2007) The dietary isothiocyanate sulforaphane is an antagonist of the human steroid and xenobiotic nuclear receptor. Mol Pharmacol 71:220–229. [DOI] [PubMed] [Google Scholar]
- Zhou JFebbraio MWada TZhai YKuruba RHe JLee JHKhadem SRen SLi S, et al. (2008) Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPARgamma in promoting steatosis. Gastroenterology 134:556–567. [DOI] [PubMed] [Google Scholar]
- Zhou J, Zhai Y, Mu Y, Gong H, Uppal H, Toma D, Ren S, Evans RM, Xie W (2006) A novel pregnane X receptor-mediated and sterol regulatory element-binding protein-independent lipogenic pathway. J Biol Chem 281:15013–15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo W, Hu L, Lv J, Wang H, Zhou H, Fan L (2014) Role of pregnane X receptor in chemotherapeutic treatment. Cancer Chemother Pharmacol 74:217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]