Abstract
Few studies have described chimeric antigen receptor (CAR) T-cell therapy for patients with B-cell acute lymphoblastic leukemia (B-ALL) with central nervous system leukemia (CNSL) because of concerns regarding poor response and treatment-related neurotoxicity. Our study included 48 patients with relapsed/refractory B-ALL with CNSL to evaluate the efficacy and safety of CD19-specific CAR T cell–based therapy. The infusion resulted in an overall response rate of 87.5% (95% confidence interval [CI], 75.3-94.1) in bone marrow (BM) disease and remission rate of 85.4% (95% CI, 72.8-92.8) in CNSL. With a median follow-up of 11.5 months (range, 1.3-33.3), the median event-free survival was 8.7 months (95% CI, 3.7-18.8), and the median overall survival was 16.0 months (95% CI, 13.5-20.1). The cumulative incidences of relapse in BM and CNS diseases were 31.1% and 11.3%, respectively, at 12 months (P = .040). The treatment was generally well tolerated, with 9 patients (18.8%) experiencing grade ≥3 cytokine release syndrome. Grade 3 to 4 neurotoxic events, which developed in 11 patients (22.9%), were associated with a higher preinfusion disease burden in CNS and were effectively controlled under intensive management. Our results suggest that CD19-specific CAR T cell–based therapy can induce similar high response rates in both BM and CNS diseases. The duration of remission in CNSL was longer than that in BM disease. CD19 CAR T-cell therapy may provide a potential treatment option for previously excluded patients with CNSL, with manageable neurotoxicity. The clinical trials were registered at www.clinicaltrials.gov as #NCT02782351 and www.chictr.org.cn as #ChiCTR-OPN-16008526.
Key Points
-
•
Neurotoxicity of CD19 CAR T-cell therapy was acceptable in patients with relapsed/refractory B-ALL with CNSL.
-
•
CD19-specific CAR T cells could induce similar high response rates in both BM and CNS diseases.
Introduction
Relapsed/refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL) in adult patients is associated with poor prognosis after salvage chemotherapy. Approximately 1% of these patients develop recurrent central nervous system (CNS) relapse, and there are few treatment options for patients with CNS leukemia (CNSL) refractory to conventional CNS-directed therapies.1, 2, 3 Chimeric antigen receptor (CAR) T-cell therapy represents one of the most promising immunotherapeutic approaches for hematologic malignancies currently. Multiple clinical trials with CD19-specific CAR T-cell therapy have demonstrated complete remission (CR) rates of 70% to 90% in children and adults with R/R B-ALL.4, 5, 6, 7
Despite the great success, the poor clearance rate of leukemia cells in immune-privileged sites and the development of significant adverse events (AEs) frequently complicate administration of CAR T-cell therapy.8, 9, 10, 11 CNSL has rarely been the focus of any clinical trial of CAR T-cell therapy because of concerns regarding poor response and treatment-related neurotoxicity. Furthermore, advanced CNS disease accompanied by active neurologic symptoms is generally an exclusion criterion for most CAR T-cell studies.4, 5, 12 In 2017, a report described for the first time a patient with refractory secondary CNS diffuse large B-cell lymphoma who received CD19 CAR T-cell treatment and achieved CR with controllable and reversible neurotoxicity,13 confirming the effective trafficking of CAR T cells into the CNS. Sparse clinical reports have revealed cases of IV-delivered CD19 CAR T cells mediating leukemia cell clearance from the cerebrospinal fluid (CSF) with fully reversible toxicity, which further indicates that patients with R/R B-ALL with CNSL may benefit from CAR T-cell therapy, with an acceptable safety profile.10, 11, 14, 15 However, to our knowledge, there are currently no large-scale specialized case series reports of response assessment or long-term survival regarding CAR T-cell therapy for CNSL. In addition, the occurrence and severity of CAR T-cell therapy–related AEs, especially neurotoxicity, must be evaluated in a larger number of patients diagnosed with CNS involvement. We report the analysis of response, toxicity, and feasibility of CD19-specific CAR T cell–based therapy in patients with R/R B-ALL with CNSL. In this study, we attempted to pursue an optimal CAR T cell–based therapeutic strategy for CNSL.
Methods
Study design and patients
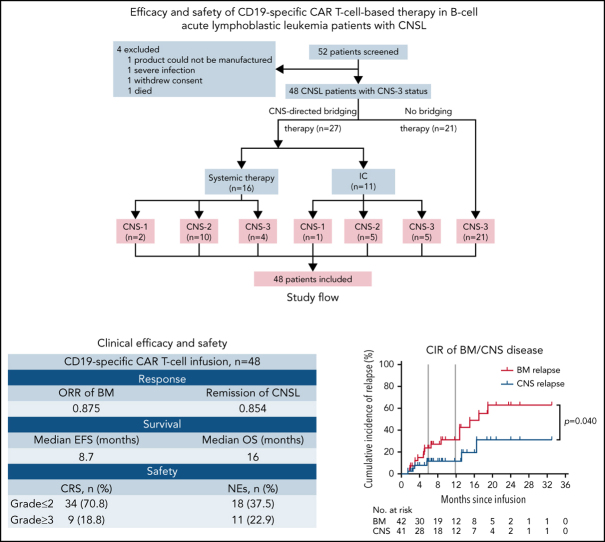
From November 2016 to April 2021, 52 patients with R/R B-ALL confirmed with CNSL involved in 2 clinical trials at 5 centers in China were successively screened for this study. All patients matched the diagnostic criteria of B-ALL according to World Health Organization classification16 and had R/R disease defined according to National Comprehensive Cancer Network Guidelines, version 1.2016.17 Definition of CNS status for B-ALL was as follows: CNS-1 (no detectable blasts on cytology in a sample of CSF), CNS-2 (white blood cells [WBCs] <5/μL; cytology positive for blasts), or CNS-3 (WBCs ≥5/μL; cytology positive for blasts or solid mass). CNSL was confirmed as CNS-3 status17 at the time of the most recent relapse or within 30 days before screening. As a result of CAR T-cell production failure (n = 1), severe infection (n = 1), withdrawal of consent (n = 1), and death (n = 1), 4 patients who did not receive CAR T-cell infusion were excluded. A total of 48 patients received CAR T-cell infusion and were included in the analysis. After leukapheresis, 27 patients received CNS-directed bridging treatment, and 21 patients did not receive any CNS-directed bridging treatment until CAR T-cell infusion. Patients were categorized as CNS-1 (n = 3), CNS-2 (n = 15), or CNS-3 status (n = 30) by the last assessment before CAR T-cell infusion (Figure 1). This study was approved by the institutional ethics review board of each participating center, and informed consent was obtained from each participant, in compliance with the Declaration of Helsinki. Additional details regarding the study design are provided in the data supplement.
Figure 1.
Screening and enrollment of patients. Fifty-two patients with R/R B-ALL with CNSL were screened for eligibility between November 2016 and April 2021 in the study. CNS-directed bridging therapy included systemic chemotherapy (high-dose methotrexate; range, 3-5 g/m2) and triple intrathecal chemotherapy (IC; 10 mg of methotrexate, 50 mg of cytarabine, and 5 mg of dexamethasone each time). Patients were categorized as CNS-1 (n = 3), CNS-2 (n = 15), and CNS-3 status (n = 30) by the last assessment before CAR T-cell infusion. A total of 48 patients received CAR T-cell infusion and were included in the analysis for response evaluation, survival, and toxicity assessment.
CAR T-cell manufacturing
The CD19 CAR used in isolated CD19 CAR T-cell infusion in the NCT02782351 trial contained a CD28 or 4-1BB costimulatory domain and a CD3-ζ signaling domain constructed as previously described.10, 18 The third generation of the CD19 CAR and CD22 CAR used in combined CD19 and CD22 CAR T-cell infusion in the ChiCTR-OPN-16008526 trial comprised tandem CD28 and 4-1BB costimulatory domains and a CD3-ζ signaling domain.19 Peripheral blood (PB) mononuclear cells were isolated from the PB of patients, and CD3+ T cells were separated by magnetic beads. After activation, T cells were infected with lentiviral vectors carrying the CD19 CAR or CD22 CAR, and CAR T cells were expanded and evaluated for transduction efficiency. The detailed procedures for cell manufacture and quality control assays are described in the data supplement.
Clinical procedures
After leukapheresis, CNS-directed bridging therapy was administered to patients with high disease burden at screening (WBCs >20/μL in CSF; cytology positive for blasts or solid mass), after which a CNS reassessment was required before CAR T-cell infusion. All included patients were given fludarabine (30 mg/m2 per day on days −5 to −3) and cyclophosphamide (750 mg/m2 on day −5) for lymphodepletion chemotherapy. Patients were required to undergo a final assessment of bone marrow (BM) and CNS diseases before infusion; 27 patients had received bridging chemotherapy after leukapheresis and underwent the last assessment after bridging therapy and before CAR T-cell infusion. Patterns of infusion included isolated CD19 CAR T-cell therapy and combined CD19 and CD22 CAR T-cell therapy. The patients were followed for response and toxicity assessment via clinical manifestation, PB, BM, and CSF analysis, and diagnostic imaging until death or loss to follow-up. Data cutoff for evaluation of outcomes was 20 April 2021. Additional details regarding the clinical procedures are provided in the supplemental Data.
Response and toxicity assessment
Response assessment of BM disease was defined according to National Comprehensive Cancer Network guidelines on B-ALL.17 CNS remission refers to achievement of CNS-1 status in a patient with CNS-2/3 status, and CNS relapse requires new development of CNS-3 status or clinical signs of CNSL, brain/eye involvement, or hypothalamic syndrome.17 Cytokine release syndrome (CRS) was graded according to the ASTCT CRS Consensus Grading.20 CAR T cell–related neurotoxic events (NEs) and other AEs were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events, v.4.03.21 Additional details regarding the evaluation and management of CRS and NEs are provided in the supplemental Data.
Laboratory assessments
The proportions of circulating CAR T cells in CD3+ T lymphocytes in the PB and CSF were measured by flow cytometry. CAR DNA copies were measured as copies per microgram of genomic DNA sample by quantitative real-time polymerase chain reaction. Multiparameter flow cytometry was used to screen and quantitate minimal residual disease in BM and CSF. Cytokines were assessed according to the manufacturer's instructions. Cytogenetic and genomic aberrations were identified by karyotyping, real-time quantitative polymerase chain reaction, fluorescence in situ hybridization, and next-generation exome sequencing.
Statistical analysis
The analysis of categorical variables was performed with the Clopper-Pearson 95% confidence interval (CI) and Fisher's exact test. The Wilcoxon rank-sum test was applied to continuous variables. Cumulative incidence of relapse (CIR) was calculated according to the Fine and Gray method, with nonrelapse mortality and subsequent allogeneic hematopoietic stem cell transplantation (allo-HSCT) considered competing risks.22 Duration of remission (DOR) was defined as time from CR to relapse or death without documented relapse. Event-free survival (EFS) was defined as time from the date of CAR T-cell infusion to the earliest occurrence of any of the following: failure to achieve response, death resulting from any cause, relapse at any site, development of second malignant disease, or last follow-up. Overall survival (OS) was defined as time from the date of CAR T-cell infusion to death resulting from any cause or last follow-up. EFS and OS probabilities were estimated by the Kaplan-Meier method and were compared by the log-rank test. Ordinal logistic regression was used to estimate risk factors of the occurrence of CRS and NEs. Spearman's rank correlation analysis was used to evaluate the correlation of neurotoxicity with the presence and severity of CRS. CIR was calculated with Statistical Analysis Software 9.4, and SPSS Statistics 17.0 was applied for other statistical analyses. alues of P < .05 were considered statistically significant.
Results
Baseline characteristics
A total of 48 patients with R/R B-ALL diagnosed with CNSL finished CAR T-cell infusion and were included in the study. Clinical characteristics of patients are summarized in Table 1. Of the 48 patients, 46 were confirmed as having CD19+ B-ALL, and the other 2 had chronic myeloid leukemia with B-ALL blast crisis. The median age was 31 years (range, 6-68), with >60% of the patients age <40 years. Thirty-six patients (75.0%) had combined BM and CNS involvement, whereas 12 patients (25.0%) had isolated CNSL. Poor-risk cytogenetic aberrations were detected in 31 patients (64.6%). All patients had received prior intensive cytotoxic chemotherapy, with a median of 4 lines of therapy (range, 3-16), and 10 patients (20.8%) had experienced relapse after allo-HSCT. Six patients (12.5%) relapsed ≥3 times, and 4 patients (8.3%) had primary refractory disease. Twenty-seven patients (56.3%) received CNS-directed bridging treatment, 9 of whom retained CNS-3 disease before CAR T-cell infusion. According to the last assessment before CAR T-cell infusion, 3 patients were identified with CNS-1 (6.3%), 15 with CNS-2 (31.2%), and 30 with CNS-3 status (62.5%). Extramedullary diseases other than CNS disease were observed in 9 patients (18.8%), including kidney, pleura, or skeleton involvement or testicular leukemia. Thirty-seven patients (77.1%) were administered isolated anti-CD19 CAR T cells, and 11 patients (22.9%) received combined anti-CD19 and anti-CD22 CAR T cells.
Table 1.
Characteristics of patients with R/R B-ALL with CNSL at baseline (N = 48)
| Characteristic | Value |
|---|---|
| Median age (range), y | 31 (6-68) |
| Age group, y | |
| <40 | 32 (66.7) |
| ≥40 | 16 (33.3) |
| Sex | |
| Male | 30 (62.5) |
| Female | 18 (37.5) |
| Disease status | |
| BM + CNS | 36 (75.0) |
| Isolated CNS | 12 (25.0) |
| Other EMDs* | |
| Yes | 9 (18.8) |
| No | 39 (81.2) |
| Poor-risk cytogenetics† | |
| Ph+‡ | 20 (41.7) |
| T315I mutation | 3 (6.3) |
| FLT3/ITD mutation | 1 (2.1) |
| IKZF1 mutation | 3 (6.3) |
| Ph-like§ǁ | 3 (9.1) |
| TP53 aberrationǁ | 2 (6.1) |
| MLL rearrangementǁ | 4 (12.2) |
| Complex karyotype‡ | 2 (4.2) |
| BM blasts before infusion, % | |
| <5 | 22 (45.8) |
| ≥5 | 26 (54.2) |
| CNS status before infusion | |
| 1 | 3 (6.3) |
| 2 | 15 (31.2) |
| 3 | 30 (62.5) |
| Median prior treatment lines (range), n | 4 (3-16) |
| No. of relapses | |
| 1 | 25 (52.1) |
| 2 | 13 (27.1) |
| ≥3 | 6 (12.5) |
| Primary refractory¶ | 4 (8.3)* |
| Prior allo-HSCT | |
| Yes | 10 (20.8) |
| No | 38 (79.2) |
| Prior cranial irradiation | |
| Yes | 5 (10.4) |
| No | 43 (89.6) |
| CNS-directed bridging treatment | |
| Yes | 27 (56.3) |
| No | 21 (43.7) |
| Costimulator | |
| 4-1BB | 32 (66.7) |
| CD28 | 5 (10.4) |
| CD28 + 4-1BB | 11 (22.9) |
| Infused cells | |
| Isolated CD19 | 37 (77.1) |
| Combined CD19 + CD22 | 11 (22.9) |
| Average dose, ×106/kg | |
| <3 | 26 (54.2) |
| 3-5 | 13 (27.0) |
| >5 | 9 (18.8) |
Data are presented as n (%) unless otherwise specified.
Extramedullary diseases other than CNS involvement.
From cytogenetic analysis performed at any time after diagnosis; some patients had >1 abnormality identified. All patients were screened for cytogenetic abnormalities using karyotyping, and 33 patients underwent next-generation sequencing covering gene fusions and pathogenic mutations recommended by National Comprehensive Cancer Network guidelines.
Screening assessment in 48 patients.
Including 3 Ph− patients with EBF1-PDGFRB+ fusion (n = 2) and JAK2/PAX5 rearrangement (n = 1).
Screening assessment in 33 patients.
Patients experiencing less than CR after initial induction therapy.
Response rates
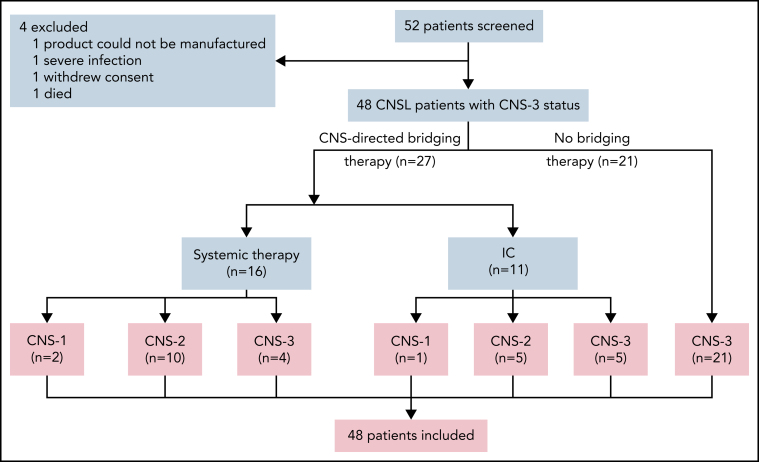
At the day-30 assessment, 42 (87.5%) of 48 patients (95% CI, 75.3-94.1) had achieved CR/CR with incomplete blood count recovery for BM disease, and minimal residual disease–negative CR was observed in 35 patients (72.9%; 95% CI, 66.1-90.7). Forty-one patients (85.4%; 95% CI, 72.8-92.8) achieved remission in CNSL. One patient had CR solely in BM disease, and 4 patients had no response in both BM and CNS diseases. Two patients, who died on days 13 and 14 after CAR T-cell infusion, respectively, were unavailable for response assessment and considered not to have had a response but were included in the toxicity and survival assessments. The response rates across all subgroups are provided in Figure 2. The remission rate from CNSL in patients receiving CNS-directed bridging treatment was higher than the rate in those receiving no bridging treatment (96.3% vs 71.4%; P = .034). The response rates from BM and CNS diseases were similar across the remaining subgroups.
Figure 2.
Subgroup analysis of response rates in patients with R/R B-ALL with CNSL. The forest plots showed the subgroup analysis of the rate of ongoing responses according to demographic and clinical characteristics at baseline. The analysis of response rate across subgroups was performed using the Clopper-Pearson 95% CI and Fisher's exact test. CRi, CR with incomplete blood count recovery; EMD, extramedullary disease; MLL, mixed-lineage leukemia; ORR, overall response rate.
Long-term survival
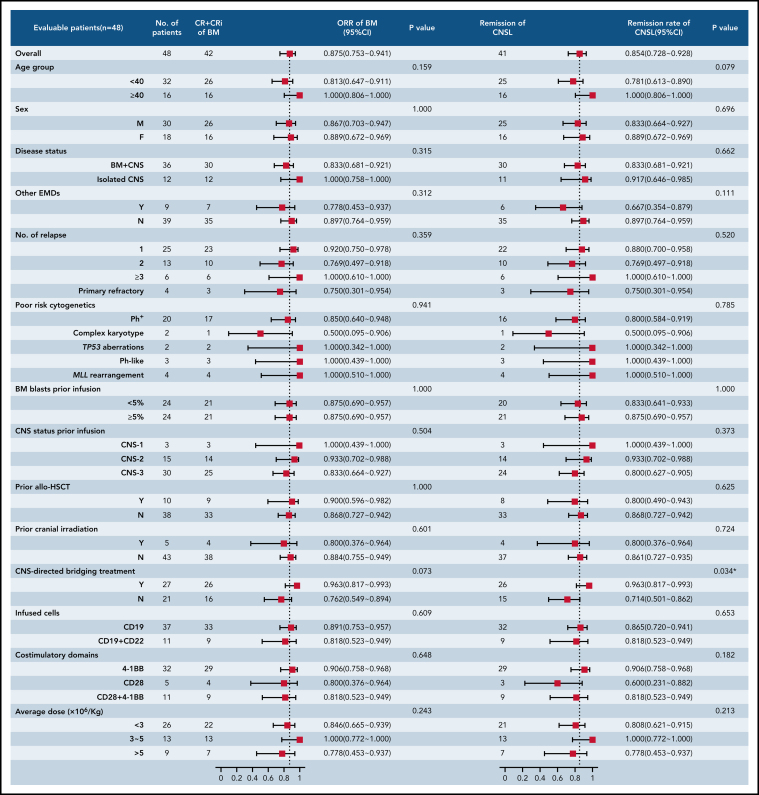
The median follow-up duration was 11.5 months (range, 1.3-33.3). Among the 42 patients achieving CR/CR with incomplete blood count recovery, the CIR of BM disease was 23.7% (95% CI, 20.7-27.2) at 6 months and 31.1% (95% CI, 26.6-36.4) at 12 months after CAR T-cell therapy. Compared with CIR of BM disease, relapse was less frequent in CNSL, with a CIR of CNSL of 11.3% (95% CI, 10.2-12.6) at 12 months (P = .040). The median DOR for BM disease was 17.0 months (95% CI, 9.8-24.2), and the median DOR for CNSL was not reached (NR; Figure 3A).
Figure 3.
Long-term survival in patients with R/R B-ALL with CNSL after CAR T-cell therapy. (A) The cumulative incidence of BM relapse and CNS relapse in patients achieving remission in terms of BM (n = 42) or CNS (n = 41) disease. Event of interest was defined as any pattern of relapse occurring by the cutoff day, and nonrelapse mortality and subsequent allo-HSCT were considered competing risks. Patients who did not experience relapse at last follow-up were censored. (B-F) OS analysis (B) and EFS analysis (C); EFS analysis according to CNS status before CAR T-cell infusion (D); and OS analysis (E) and EFS analysis (F) according to Ph status. The probabilities of OS and EFS were estimated by means of the Kaplan-Meier method and were compared using the log-rank test.
The median OS of all 48 patients was 16.0 months (95% CI, 13.5-20.1), and the median EFS was 8.7 months (95% CI, 3.7-18.8; Figure 3B-C). The 6-month OS and EFS rates were 72.0% (95% CI, 55.6-86.1) and 53.3% (95% CI, 36.5-68.1), respectively. Patients with CNS-3 status before CAR T-cell infusion had inferior median EFS than those with CNS-1/2 status (5.1 vs NR vs 15.0 months, respectively; P = .049; Figure 3D), but the difference in OS was not statistically significant among the 3 cohorts (CNS-1 vs CNS-2 vs CNS-3: NR vs 18.8 vs 14.4 months, respectively; P = .545). Patients with Ph+ B-ALL had a significantly reduced median OS compared with those without BCR/ABL rearrangement (8.0 vs 18.8 months; P = .014; Figure 3E), and the same was true in EFS analysis (3.5 vs 13.6 months; P = .008; Figure 3F). Other subgroup-specific prognostic impacts were investigated, and no significant difference in either EFS or OS was found across all subgroups (supplemental Figure 1A-H).
Safety
Common AEs within the first 30 days are shown in Table 2. CRS occurred in 43 (89.6%) of 48 patients, and 9 (18.8%) had grade 3 to 5 CRS. The occurrence and severity of CRS among different subgroups are displayed in Table 3. Univariate analysis showed that peak CAR DNA copies in PB (P < .001) and level of serum interleukin-6 (sIL-6; P = .012) were factors influencing the occurrence and severity of CRS. These factors were further included into the multivariate logistic regression model, and data showed that higher peak CAR DNA copies (odds ratio, 4.230; 95% CI, 1.043-10.425; P = .002) and increased level of sIL-6 (odds ratio, 2.355; 95% CI, 1.430-5.026; P = .035) were independent risk factors for severe CRS (grade 3-5).
Table 2.
AEs within the first 30 d after CAR T-cell infusion
| No. of patients | ||
|---|---|---|
| All grades | Grade ≥3 | |
| AEs | ||
| Fatigure | 1 (2.1) | 0 |
| Dyspnea | 4 (8.3) | 2 (4.2) |
| Nausea + anorexia | 22 (45.8) | 4 (8.3) |
| Diarrhea + constipation | 1 (2.1) | 0 |
| Myalgia | 2 (4.2) | 0 |
| Oral mucositis | 1 (2.1) | 0 |
| Infection | 6 (12.5) | 2 (4.2) |
| Fever | 44 (91.7) | 33 (68.8) |
| Hypotension | 8 (16.7) | 4 (8.3) |
| Hypoxia | 6 (12.5) | 4 (8.3) |
| Tachycardia | 3 (6.3) | 2 (4.2) |
| Capillary leak syndrome | 1 (2.1) | 1 (2.1) |
| CRS | 43 (89.6) | 9 (18.8) |
| Hematologic AEs | ||
| Neutropenia | 42 (87.5) | 34 (70.8) |
| Lymphocytopenia | 40 (83.3) | 35 (72.9) |
| Thrombocytopenia | 29 (60.4) | 16 (33.3) |
| Hypoglobinemia | 32 (66.7) | 22 (45.8) |
| Multisystem AEs | ||
| Gastrointestinal bleeding | 1 (2.1) | 1 (2.1) |
| Hemoptysis | 1 (2.1) | 1 (2.1) |
| Intracranial hemorrhage | 1 (2.1) | 1 (2.1) |
| Acute pancreatitis | 1 (2.1) | 1 (2.1) |
| Heart failure | 3 (6.3) | 3 (6.3) |
| Renal failure | 2 (4.2) | 2 (4.2) |
| Multiple organ and system failure | 1 (2.1) | 1 (2.1) |
| Laboratory abnormalities | ||
| ALT/AST elevation | 28 (58.3) | 11 (22.9) |
| Blood bilirubin elevation | 7 (14.6) | 5 (10.4) |
| Creatintine elevation | 8 (16.7) | 2 (4.2) |
| Electrolyte disturbance | 1 (2.1) | 1 (2.1) |
| Myocardial enzyme/BNP elevation | 14 (29.2) | 4 (8.3) |
| Fibrinogen decreased | 3 (6.3) | 3 (6.3) |
| NEs | ||
| Encephalopathy | 11 (22.9) | 4 (8.3) |
| Disturbance in attention | 6 (12.5) | 1 (2.1) |
| Cognitive disorder | 6 (12.5) | 2 (4.2) |
| Mental status changes | 1 (2.1) | 0 |
| Memory impairment | 2 (4.2) | 1 (2.1) |
| Lethargy | 4 (8.3) | 1 (2.1) |
| Depressed level of consciousness | 10 (20.8) | 3 (6.3) |
| Headache/dizziness | 7 (14.6) | 2 (4.2) |
| Tremor | 2 (4.2) | 1 (2.1) |
| Aphasia | 1 (2.1) | 1 (2.1) |
| Delirium | 8 (16.7) | 2 (4.2) |
| Motor dysfunction | 1 (2.1) | 1 (2.1) |
| Ataxia | 2 (4.2) | 0 |
| Seizure | 4 (8.3) | 4 (8.3) |
| Vision disorder | 3 (6.3) | 3 (6.3) |
| Tinnitus | 1 (2.1) | 1 (2.1) |
Data are presented as n (%). AEs were captured for all treated patients within the first 30 d after CAR T-cell infusion.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BNP, brain natriuretic peptide.
Table 3.
Factors related to occurrence and severity of CRS/NEs
| CRS | NEs | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade | Analysis | Grade | Analysis | ||||||||||
| Univariate | Multivariate* | Univariate | Multivariate* | ||||||||||
| 0-2 | 3-4 | 5 | P | OR | 95% CI | P | 0-2 | 3-4 | P | OR | 95% CI | P | |
| Median age (range), y | 32 (8-68) | 26 (6-66) | 22 | .543 | 27 (8-68) | 23 (6-61) | .635 | ||||||
| Disease status | .843 | .705 | |||||||||||
| BM + CNS | 29 (80.6) | 6 (16.7) | 1 (2.8) | 27 (75.0) | 9 (25.0) | ||||||||
| Isolated CNS | 10 (83.3) | 2 (16.7) | 0 | 10 (83.3) | 2 (16.7) | ||||||||
| Infused cells | .145 | 1.000 | |||||||||||
| Isolated CD19 | 30 (81.1) | 7 (18.9) | 0 | 28 (75.7) | 9 (24.3) | ||||||||
| Combined CD19 + CD22 | 9 (81.8) | 1 (9.1) | 1 (9.1) | 9 (81.8) | 2 (18.2) | ||||||||
| Costimulator | .425 | .611 | |||||||||||
| 4-1BB | 26 (81.3) | 6 (18.8) | 0 | 25 (78.1) | 7 (21.9) | ||||||||
| CD28 | 4 (80) | 1 (20) | 0 | 3 (60) | 2 (40) | ||||||||
| CD28 + 4-1BB | 9 (81.8) | 1 (9.1) | 1 (9.1) | 9 (81.8) | 2 (18.2) | ||||||||
| Average dose, ×106/kg | .373 | .615 | |||||||||||
| <3 | 19 (73.1) | 7 (26.9) | 0 | 20 (76.9) | 6 (23.1) | ||||||||
| 3-5 | 12 (92.3) | 1 (7.7) | 0 | 11 (84.6) | 2 (15.4) | ||||||||
| >5 | 8 (88.9) | 0 | 1 (11.1) | 6 (66.7) | 3 (33.3) | ||||||||
| CNS-directed bridging treatment | .465 | 1.000 | |||||||||||
| Yes | 23 (85.2) | 4 (14.8) | 0 | 21 (77.8) | 6 (22.2) | ||||||||
| No | 16 (76.2) | 4 (19.0) | 1 (4.8) | 16 (76.2) | 5 (23.8) | ||||||||
| CNS status before infusion | .798 | .083 | 1.530 | 1.260-5.171 | .034† | ||||||||
| 1 | 3 (100) | 0 | 0 | 3 (100) | 0 | ||||||||
| 2 | 13 (86.7) | 2 (13.3) | 0 | 14 (93.3) | 1 (6.7) | ||||||||
| 3 | 23 (76.7) | 6 (20.0) | 1 (3.3) | 20 (66.7) | 10 (33.3) | ||||||||
| BM blasts before infusion, % | .636 | .514 | |||||||||||
| <5 | 18 (81.8) | 4 (18.2) | 0 | 18 (81.8) | 4 (18.2) | ||||||||
| ≥5 | 21 (80.8) | 4 (15.4) | 1 (3.8) | 19 (73.1) | 7 (26.9) | ||||||||
| Median peak CAR DNA copies in PB (range), per μg | 50 118 (2 733-108 747) |
296 728 (10 699-5 888 436) |
981 213 | <.001† | 4.230 | 1.043-10.425 | .002† | 66 069 (2 733-630 957) |
398 107 (63 095-5 888 436) |
<.001† | 2.017 | 0.972-4.216 | .014† |
| Median peak sIL-6 (range), pg/mL | 107 (29-375) |
349 (135 to >2 000) |
>2 000 | .012† | 2.355 | 1.430-5.026 | .035† | 209 (29-491) |
1 589 (135 to >2 000) |
.037† | 3.103 | 2.987-6.260 | .001† |
| Median blasts in CSF (range), % | 10.9 (6.4-35.6) | 14.6 (3.8-50.2) | 15.6 | .270 | 10.6 (3.8-26.8) | 26.3 (10.6-50.2) | .038† | 1.642 | 1.051-3.730 | .037† | |||
Data are presented as n (%) unless otherwise specified.
OR, odds ratio.
Ordinal logistic regression was used to estimate the risk factors of the occurrence and severity of CRS/NEs. The variables that entered the multivariate regression model include variables identified by univariate analysis with P < .1.
P < .05.
NEs of any grade were observed in 18 patients (37.5%), with severe NEs (grade 3-4) in 11 patients (22.9%). Common NEs were encephalopathy (22.9%), depressed level of consciousness (20.8%), delirium (16.7%), headache (14.6%), and seizure (8.3%; Table 2). Univariate analysis showed that higher peak CAR DNA copies in PB (P < .001), increased level of sIL-6 (P = .037), and higher percentage of CNS blasts (P = .038) were associated with severe neurotoxicity, separately. Multivariate analysis identified the aforementioned factors, as well as CNS-3 status, as independent risk factors for occurrence of severe NEs (Table 3).
Severe CRS was effectively managed according to protocol-specific guidelines, with 6 (66.7%) of the 9 patients with severe CRS receiving the IL-6 receptor antagonist tocilizumab with (n = 3) or without corticosteroids (n = 3). Among the 18 patients developing neurotoxicity within the first 30 days after CAR T-cell infusion, the median time from CAR T-cell infusion to the onset of NEs in cohorts of grade 1 to 2 and grade 3 to 4 was 5 days (range, 5-9) and 4 days (range, 2-10), respectively (P = .574; supplemental Figure 2A). Of the 11 patients with grade 3 to 4 NEs, 10 (90.9%) received systemic corticosteroids, and other management included tocilizumab (n = 3), mannitol (n = 7), antiepileptics (n = 6), levetiracetam prophylaxis (n = 1), and intensive care admission (n = 2). No patients developed lethal diffuse cerebral edema confirmed by diagnostic imaging. Spearman's analysis showed that there was a significant correlation of neurotoxicity with the incidence and severity of CRS (r = 0.553; P = .024; supplemental Figure 2B).
Furthermore, fatal AEs within the first 30 days after CAR T-cell infusion occurred in 2 patients; 1 died as a result of grade 5 CRS manifested as refractory hypotension/hypoxia together with grade 4 organ toxicity (patient 37), despite receiving tocilizumab, high-dose corticosteroid, and plasma exchange, and 1 died as a result of refractory Escherichia coli sepsis confirmed by positive blood culture (patient 42). Detailed information about the management and prognosis of these 2 patients is provided in supplemental Figure 2A.
CAR T-cell kinetics
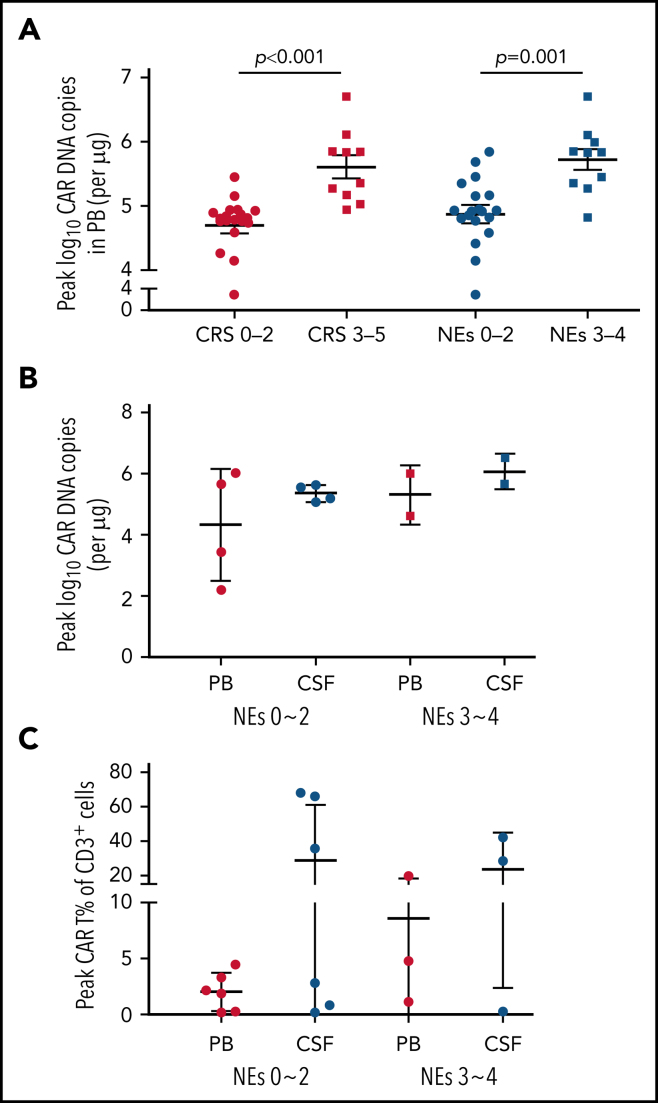
The expansion and persistence of CAR T cells were evaluated in patients with PB and CSF specimens adequate for analysis. The median peak number of CAR DNA copies in PB among patients with severe (grade ≥3) CRS/NEs was remarkably higher than that in those with mild (grade 0-2) CRS/NEs (Figure 4A). In patients developing different grades of NEs, the peak expansion of CAR T cells seemed to be higher in CSF than in PB in both cohorts of severe and mild NEs, although the difference was not statistically significant (Figure 4B-C).
Figure 4.
Expansion of CAR T cells in both PB and CSF. (A) The peak CAR T-cell expansion in PB after CAR T-cell infusion assessed by real-time quantitative polymerase chain reaction (qPCR) categorized by severity of CRS and NEs. (B) The comparison of peak CAR T-cell expansion in PB and CSF assessed by qPCR categorized by NE grade. (C) The comparison of peak CAR T-cell expansion in PB and CSF assessed by flow cytometry categorized by NE grade. Patients were not completely overlapped as presented in panels B and C. Data represent median ± standard deviation. The Mann-Whitney U test was used for statistical analysis. Grade given on x-axis labels.
The dynamic expansion and persistence of CAR T cells in both PB and CSF were monitored in 9 evaluable patients after CAR T-cell infusion (supplemental Figure 3). All 9 patients achieved CR and exhibited peak expansion of CAR T cells in CSF within the first month after CAR T-cell infusion, along with clearance of blasts in CSF. At the data cutoff date, 7 of the 9 patients maintained their CR status for a median of 93 days (range, 41-242), and CAR T cells remained detectable in CSF for a median of 85 days (range, 41-183). The other 2 patients (patients 13 and 20) relapsed during follow-up. Patient 13 with isolated CNSL at baseline before infusion showed significantly higher CAR copies in CSF than in PB. However, CAR T cells were undetectable in PB and CSF when CD19+ blasts recurred in BM 5 months after CAR T-cell treatment. Patient 20 with combined BM/CNS involvement at baseline before infusion exhibited remarkable expansion of CAR T cells in both PB and CSF, and then CAR T cells dropped significantly in both PB and CSF, with CAR copies lower than the detection limit at 27 days in CSF and 62 days in PB after CAR T-cell treatment. CNS relapse was first observed on day 62, and CD19+ blasts reoccurred subsequently in BM 6 months later.
Discussion
CNS relapse is still a common cause of treatment failure in R/R B-ALL, although chemotherapy, cranial irradiation, and allo-HSCT are all modalities that can be incorporated into the management of CNSL.3, 23, 24, 25 In the present study, we report the efficacy, toxicity, and clinical feasibility of CD19-specific CAR T cell–based therapy in patients with R/R B-ALL with CNSL. This study is the first to include children and adults with CNS-3 status on a relatively large scale in a clinical trial of CAR T-cell treatment.
Robust responses were achieved in this study. An overall response rate of 87.5% for BM disease and remission rate of 85.4% for CNSL were observed, which were similar to those previously reported in pivotal CD19-specific CAR T-cell therapy trials, with overall response rates published as 81% to 93%.11, 26, 27 However, it is worth noting that all patients included in our study had CNS disease of CNS-3 status before screening, and 62.5% of patients remained in CNS-3 status after bridging therapy and before CAR T-cell infusion. Most patients had CNS involvement in the multiple-relapse setting, particularly after radiotherapy (10.4%) and allo-HSCT (20.8%), which is challenging to treat and generally leads to poor prognosis. The above analysis suggests that CD19-specific CAR T cell–based therapy can induce high response in patients with R/R B-ALL with CNS involvement.
Subgroup analysis of response showed that a better remission rate in CNSL was achieved among patients receiving CNS-directed bridging therapy. This result implies that optimal bridging therapy during the CAR T-cell manufacturing interval may serve to improve the remission rate in CNSL. CNS-directed bridging chemotherapy in the study included systemic chemotherapy and triple intrathecal chemotherapy; the dose and timing of different bridging therapies administered before CAR T-cell therapy were not explored in the present study. Future investigation is required for optimization of bridging treatment before CAR T-cell infusion.
The median OS and EFS achieved in our study were 16.0 and 8.7 months, respectively. Compared with previously reported EFS (ranging from 6.1 months to >1 year) achieved in patients with R/R B-ALL,26, 27 CD19-specific CAR T cell–based therapy also provided relatively durable remission in those with R/R B-ALL with CNSL. The median DOR for BM and CNS diseases was 17 months and not reached, respectively. There seems to be a longer DOR in CNSL than in BM disease, which should be confirmed in additional trials. Subgroup analysis showed that patients with CNS-3 status before CAR T-cell infusion had shorter EFS compared with those with CNS-1/2 status, emphasizing preinfusion disease burden in CNS might serve as a potential prognostic factor for long-term survival. Patients with Ph+-B-ALL had remarkably reduced EFS and OS compared with their counterparts, which implies that post–CAR T-cell consolidation therapy should be considered to attain long-term survival in this high-risk cohort.
Because of the risk of treatment-related neurotoxicity, there has been significant concern about the safety profile of CAR T-cell therapy in patients with CNSL.28 In this study, the incidence of grade ≥3 CRS and NEs was 18.8% and 22.9%, respectively. The safety analysis revealed that high-grade CRS and NEs occurred at incidences comparable to those previously reported, suggesting that CAR T-cell therapy for CNSL may act as an effective therapeutic strategy without significantly increased risk of neurotoxicity.29, 30 Prior studies have shown that neurotoxicity is associated with preinfusion disease burden and in vivo CAR T-cell expansion20, 28, 31, 32; our study also showed that patients with CNS-3 status at infusion had a higher probability of developing severe NEs. These observations indicate that high-burden CNSL before CAR T-cell infusion may predispose patients to severe neurotoxicity, and therefore, patients with high-burden CNSL should be intensively monitored and managed. In addition, CAR DNA copies in PB and sIL-6 concentrations were remarkably elevated in patients with severe NEs; these may serve as dynamic and readily accessible indicators for the development and progression of severe NEs. We also found neurotoxicity to be correlated with occurrence and severity of CRS, which is consistent with the findings from multiple prior studies using different CAR constructs.28, 32, 33 This association suggests that prevention or mitigation of earlier severe CRS may lead to a lower incidence of severe neurotoxicity.
The expansion and persistence of CAR T cells in CNS comprise another concern regarding treatment-related toxicity and clinical response in CAR T-cell therapy for CNSL. CAR T-cell expansion in CSF was described based on NE grade, and we did not observe any statistically significant association between the severity of NEs and peak CAR T-cell expansion in CSF. This may be partially attributable to sampling difficulty. CSF specimens were sometimes unavailable at the acute phase of severe NEs because of patients' intolerance of lumbar puncture. Dynamic CAR T-cell expansion was monitored in 9 evaluable patients based on response assessment during follow-up. The data showed a predominant presence of CAR T cells in CSF and seemingly longer persistence after CAR T-cell infusion, compared with CAR T-cell expansion in PB. In 2 cases of postinfusion relapse (patients 13 and 20) among the 9 patients, we did not find antigen loss of CD19, and CAR copies in CNS were detected below the inferior limit of quantitation at relapse. As a result, we tend to attribute relapse in these 2 patients to the extinction of CAR T cells in the CNS.
In summary, our study suggests that CD19-specific CAR T cell–based therapy can induce similar high response rates in both BM and CNS diseases, with an acceptable safety profile under intensive management. CD19-specific CAR T-cell therapy provides a potential treatment option for a cohort of previously excluded patients with CNSL with otherwise poor prognosis. However, our study is limited by incomplete information on CAR T-cell kinetics in the CNS and the retrospective nature of the analysis. Considering these promising results, prospective studies featuring a larger cohort of patients with high-burden CNSL are warranted to optimize this strategy for the treatment of R/R B-ALL with CNSL.
Acknowledgments
The authors thank the patients and families who participated in this trial and the staff of the clinical and laboratory units (Affiliated Hospital of Xuzhou Medical University, First Affiliated Hospital of Zhejiang University, Tianjin First Central Hospital, Tongji Hospital of Tongji University, and Tongji Hospital of Huazhong University of Science and Technology) for clinical and technical support.
This work is supported by funding from the Key Program of the National Natural Science Foundation of China (grant 81930005), the National Natural Science Foundation of China (grants 81800124, 81800159, 81800179, 81900177, and 81900179), the Natural Science Foundation of Jiangsu Province (grant BK20190985), and the Natural Science Foundation of Jiangsu Higher Education Institutions (grant KJA320003).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Chimeric antigen receptor (CAR) T-cell therapy in patients with B-cell acute lymphoblastic leukemia (B-ALL) with central nervous system (CNS) involvement has been hampered by the concern for poor response and treatment-related neurotoxicity. Qi and colleagues report on 48 relapsed/refractory B-ALL patients with CNS leukemia treated with CD19-specific CAR T cells, demonstrating 85-90% response in both marrow and CNS, with manageable neurotoxicity and median overall survival of 16 months. CNS responses are more durable than marrow responses.
Authorship
Contribution: K.X., J. Zhou, A.L., M. Zhang, H.H., and J. Zheng designed the study; Y.Q., Y.H., M. Zhao, X.X., P.L., and J.T. collected research data; Y.Q. and Y.W. performed the statistical analysis; Y.Q. drafted the manuscript; K.X. and J.Q. performed critical revision of the manuscript for important content; and all authors contributed to the manuscript and interpretation of the data and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Supplementary Material
REFERENCES
- 1.Richard-Carpentier G, Kantarjian H, Jabbour E. Recent advances in adult acute lymphoblastic leukemia. Curr Hematol Malig Rep. 2019;14(2):106–118. doi: 10.1007/s11899-019-00503-1. [DOI] [PubMed] [Google Scholar]
- 2.Bassan R, Pavoni C, Intermesoli T, et al. Updated risk-oriented strategy for acute lymphoblastic leukemia in adult patients 18-65 years: NILG ALL 10/07. Blood Cancer J. 2020;10(11):119. doi: 10.1038/s41408-020-00383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inaba H, Pui CH. Advances in the diagnosis and treatment of pediatric acute lymphoblastic leukemia. J Clin Med. 2021;10(9):1926. doi: 10.3390/jcm10091926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park JH, Rivière I, Gonen M, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378(5):449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hay KA, Gauthier J, Hirayama AV, et al. Factors associated with durable EFS in adult B-cell ALL patients achieving MRD-negative CR after CD19 CAR T-cell therapy. Blood. 2019;133(15):1652–1663. doi: 10.1182/blood-2018-11-883710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah BD, Bishop MR, Oluwole OO, et al. KTE-X19 anti-CD19 CAR T-cell therapy in adult relapsed/refractory acute lymphoblastic leukemia: ZUMA-3 phase 1 results. Blood. 2021;138(1):11–22. doi: 10.1182/blood.2020009098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu Y, Sun J, Wu Z, et al. Predominant cerebral cytokine release syndrome in CD19-directed chimeric antigen receptor-modified T cell therapy. J Hematol Oncol. 2016;9(1):70. doi: 10.1186/s13045-016-0299-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holstein SA, Lunning MA. CAR T-cell therapy in hematologic malignancies: a voyage in progress. Clin Pharmacol Ther. 2020;107(1):112–122. doi: 10.1002/cpt.1674. [DOI] [PubMed] [Google Scholar]
- 10.He X, Xiao X, Li Q, et al. Anti-CD19 CAR-T as a feasible and safe treatment against central nervous system leukemia after intrathecal chemotherapy in adults with relapsed or refractory B-ALL. Leukemia. 2019;33(8):2102–2104. doi: 10.1038/s41375-019-0437-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pehlivan KC, Duncan BB, Lee DW. CAR-T cell therapy for acute lymphoblastic leukemia: transforming the treatment of relapsed and refractory disease. Curr Hematol Malig Rep. 2018;13(5):396–406. doi: 10.1007/s11899-018-0470-x. [DOI] [PubMed] [Google Scholar]
- 12.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abramson JS, McGree B, Noyes S, et al. Anti-CD19 CAR T cells in CNS diffuse large-B-cell lymphoma. N Engl J Med. 2017;377(8):783–784. doi: 10.1056/NEJMc1704610. [DOI] [PubMed] [Google Scholar]
- 14.Jacoby E, Bielorai B, Avigdor A, et al. Locally produced CD19 CAR T cells leading to clinical remissions in medullary and extramedullary relapsed acute lymphoblastic leukemia. Am J Hematol. 2018;93(12):1485–1492. doi: 10.1002/ajh.25274. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Lu XA, Yang J, et al. Efficacy and safety of anti-CD19 CAR T-cell therapy in 110 patients with B-cell acute lymphoblastic leukemia with high-risk features. Blood Adv. 2020;4(10):2325–2338. doi: 10.1182/bloodadvances.2020001466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 17.Alvarnas JC, Brown PA, Aoun P, et al. Acute lymphoblastic leukemia, version 2.2015. J Natl Compr Canc Netw. 2015;13(10):1240–1279. doi: 10.6004/jnccn.2015.0153. [DOI] [PubMed] [Google Scholar]
- 18.Cao J, Wang G, Cheng H, et al. Potent anti-leukemia activities of humanized CD19-targeted chimeric antigen receptor T (CAR-T) cells in patients with relapsed/refractory acute lymphoblastic leukemia. Am J Hematol. 2018;93(7):851–858. doi: 10.1002/ajh.25108. [DOI] [PubMed] [Google Scholar]
- 19.Wang N, Hu X, Cao W, et al. Efficacy and safety of CAR19/22 T-cell cocktail therapy in patients with refractory/relapsed B-cell malignancies. Blood. 2020;135(1):17–27. doi: 10.1182/blood.2019000017. [DOI] [PubMed] [Google Scholar]
- 20.Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625–638. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127(26):3321–3330. doi: 10.1182/blood-2016-04-703751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. 2007;40(4):381–387. doi: 10.1038/sj.bmt.1705727. [DOI] [PubMed] [Google Scholar]
- 23.Jeha S, Pei D, Choi J, et al. Improved CNS control of childhood acute lymphoblastic leukemia without cranial irradiation: St Jude Total Therapy Study 16. J Clin Oncol. 2019;37(35):3377–3391. doi: 10.1200/JCO.19.01692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubinstein JD, Krupski C, Nelson AS, O'Brien MM, Davies SM, Phillips CL. Chimeric antigen receptor T cell therapy in patients with multiply relapsed or refractory extramedullary leukemia. Biol Blood Marrow Transplant. 2020;26(11):e280–e285. doi: 10.1016/j.bbmt.2020.07.036. [DOI] [PubMed] [Google Scholar]
- 25.Tang J, Yu J, Cai J, et al. Prognostic factors for CNS control in children with acute lymphoblastic leukemia treated without cranial irradiation. Blood. 2021;138(4):331–343. doi: 10.1182/blood.2020010438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martino M, Alati C, Canale FA, Musuraca G, Martinelli G, Cerchione C. A review of clinical outcomes of CAR T-cell therapies for B-acute lymphoblastic leukemia. Int J Mol Sci. 2021;22(4):2150. doi: 10.3390/ijms22042150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anagnostou T, Riaz IB, Hashmi SK, Murad MH, Kenderian SS. Anti-CD19 chimeric antigen receptor T-cell therapy in acute lymphocytic leukaemia: a systematic review and meta-analysis. Lancet Haematol. 2020;7(11):e816–e826. doi: 10.1016/S2352-3026(20)30277-5. [DOI] [PubMed] [Google Scholar]
- 28.Santomasso BD, Park JH, Salloum D, et al. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. 2018;8(8):958–971. doi: 10.1158/2159-8290.CD-17-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: Mechanisms, manifestations and management. Blood Rev. 2019;34:45–55. doi: 10.1016/j.blre.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy – assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santomasso B, Park JH, Riviere I, et al. Biomarkers associated with neurotoxicity in adult patients with relapsed or refractory B-ALL (R/R B-ALL) treated with CD19 CAR T cells [abstract]. J Clin Oncol., 2017;35(15_suppl):3019.
- 32.Gust J, Hay KA, Hanafi LA, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7(12):1404–1419. doi: 10.1158/2159-8290.CD-17-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gust J, Taraseviciute A, Turtle CJ. Neurotoxicity associated with CD19-targeted CAR-T cell therapies. CNS Drugs. 2018;32(12):1091–1101. doi: 10.1007/s40263-018-0582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.