Abstract
The complete nucleotide sequence of a novel enteric virus, Aichi virus, associated with nonbacterial acute gastroenteritis in humans was determined. The Aichi virus genome proved to be a single-stranded positive-sense RNA molecule with 8,251 bases excluding a poly(A) tail; it contains a large open reading frame with 7,302 nucleotides that encodes a potential polyprotein precursor of 2,433 amino acids. The genome contains a 5′ nontranslated region (NTR) with 712 bases and a 3′ NTR with 240 bases followed by a poly(A) tail. The structure of the genome, VPg–5′ NTR–leader protein–structural proteins–nonstructural proteins–3′ NTR–poly(A), was found to be typical of a picornavirus. The VP0-VP3 and VP3-VP1 cleavage sites were determined to be Q-H and Q-T, respectively, by N-terminal amino acid sequence analyses using purified virion proteins. Possible cleavage sites, Q-G, Q-A, and Q-S, which cleave P2 and P3 polyproteins were found to be similar to those of picornaviruses. A dendrogram based on 3Dpol proteins indicated that Aichi virus is genetically distinct from the known six genera of picornaviruses including entero-, rhino-, cardio-, aphtho-, and hepatovirus and echovirus 22. Considering this together with other properties of the virus (T. Yamashita, S. Kobayashi, K. Sakae, S. Nakata, S. Chiba, Y. Ishihara, and S. Isomura, J. Infect. Dis. 164:954–957, 1991), we propose that Aichi virus be regarded as a new genus of the family Picornaviridae.
In fecal specimens of patients with nonbacterial acute gastroenteritis, morphologically distinct round-structured viruses (approximately 20 to 40 nm in diameter) have been detected by electron microscopy (9, 10). Of these viruses, Norwalk or Norwalk-like viruses (18–22, 38) and astroviruses (17, 24, 39) have been recognized as major etiologic agents of human gastroenteritis (4), and recent genetic analyses have revealed that they can be classified into the families Caliciviridae and Astroviridae, respectively (8, 23).
In 1989, we isolated a novel cytopathic small round virus, designated Aichi virus, from a stool specimen of a patient with oyster-associated nonbacterial gastroenteritis. The virus caused apparent cytopathic effects of BS-C-1 cells (40). By using an enzyme-linked immunosorbent assay, 13 of 47 (28%) stool specimens collected from five different oyster-associated gastroenteritis outbreaks were shown to be positive for the Aichi virus antigen. Seroconversion, detected by an increase of the neutralizing antibody titer up to four times or more, was observed in 20 of 43 (47%) paired sera from these outbreaks (41). Furthermore, Aichi virus was isolated from Pakistani children with gastroenteritis as well as from Japanese travelers who developed gastroenteritis after having traveled in Southeast Asian countries (42). Although direct evidence for the pathogenesis of the virus has not been obtained yet, these findings strongly suggested that Aichi virus is one of the causative agents of human gastroenteritis.
A morphological study on purified Aichi virus virions indicated that the surface structure is characteristic of a small round-structured virus (40). However, the ability to grow in cultured cells, along with other biological properties, i.e., resistance to treatment with chloroform and stability under a low pH (pH 3.5), suggested that Aichi virus was a member of the enteroviruses. However, none of the enterovirus antisera neutralized Aichi virus, and, conversely, the antiserum to Aichi virus did not react with any other enterovirus or other enteric viruses such as Norwalk-like viruses and astroviruses (40, 41). Neither a nucleotide nor amino acid sequence has been reported yet. Therefore, definitive classification of this virus remains unclear.
In this study, we performed molecular cloning and complete nucleotide sequence analysis as well as genetic analysis of Aichi virus genome RNA to define the phylogenetic relationship between Aichi virus and other RNA viruses. Our results indicated that Aichi virus is a distinct member of the known picornaviruses.
A standard Aichi virus strain, A846/88, was isolated in BS-C-1 cells, as described previously (40), plaque purified, and then grown in Vero cells. The virion was purified by CsCl and sucrose density gradient centrifugation, as described elsewhere (40), and RNA was extracted by proteinase K treatment followed by phenol-chloroform extraction and ethanol precipitation (36). One microgram of the RNA was converted into cDNA with a mixture of random and oligo(dT)15 primers (Promega Corp., Madison, Wis.) by using a reverse transcriptase from Moloney murine leukemia virus (Gibco BRL, Gaithersburg, Md.), which was subsequently cloned into pBR322 (Gibco BRL) by a dC–dG-tailing method, as previously described (35). Screening was carried out by dot blot hybridization with the virion RNA as a template and biotinylated inserts as probes. Clones containing the 5′ end of the genome were obtained with the 5′ RACE System for Rapid Amplification of cDNA Ends (Gibco BRL). The following nucleotide sequences were obtained from GenBank: bovine enterovirus (BEV), D00214; coxsackievirus A16 (CA16), U05876; coxsackievirus B3 (CB3), M16572; enterovirus 70 (EV70), D00820; poliovirus type 1 (PV1), J02281; human rhinovirus 2 (HRV2), X02316; human rhinovirus 14 (HRV14), X01087; human rhinovirus 89 (HRV89), M16248; encephalomyocarditis virus (EMCV), M81861; Theiler murine encephalomyelitis virus (TMEV), M20301; foot-and-mouth disease virus type A12 (FMDV-A), M10975; foot-and-mouth disease virus type OK1 (FMDV-O), X00871; foot-and-mouth disease virus type SAT3 (FMDV-S), M28719; hepatitis A virus (HAV), M14707; echovirus 22 (E22), L02971; swine vesicular disease virus (SVDV), X54521; and simian hepatitis A virus (SHAV), D00924.
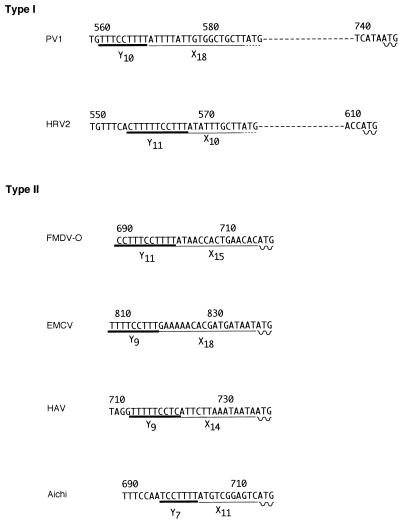
Previous studies have shown that the Aichi virus virion contains a single-stranded RNA molecule as the genome (40). The length was roughly estimated to be 8.2 kb by agarose gel electrophoresis under a denatured condition (data not shown). Twelve overlapping cDNA clones spanning the entire genome were obtained, and their nucleotide sequences were determined. The RNA genome of Aichi virus consists of 8,251 nucleotides (nt), excluding a poly(A) tract. A large open reading frame with 7,302 nt that encodes a potential polyprotein precursor of 2,433 amino acids (aa) was found; it is preceded by 712 nt and followed by 240 nt and a poly(A) tail. The genome organization was analogous to that of other picornaviruses, and the deduced amino acid sequence of the C-terminal one-third of the polyprotein had a high degree of sequence conservation with picornaviruses. These observations made it possible to suggest that the first 712 nt are a 5′ nontranslated region (NTR). The length is similar to that of other picornaviruses and must encode cis-acting elements and an internal ribosome entry site (IRES) (25). The base composition was found to be 19.5% adenine, 21.1% guanine, 37.8% cytosine, and 21.6% uracil. This high G+C content (≈59%) was relatively similar to that of aphthoviruses (in FMDV-O, G+C is 53%) rather than of enteroviruses (in PV1, G+C is 45%), rhinoviruses (in HRV14, G+C is 41%), hepatoviruses (in HAV, G+C is 38%), cardioviruses (in EMCV, G+C is 49%), or E22 (G+C is 39%). In the Aichi virus 5′ NTR, neither a poly(C) tract, as found in aphtho- and cardioviruses, nor a relatively long pyrimidine-rich sequence (approximately 40 bases), structurally analogous to the poly(C) tract found in hepatoviruses, was observed. Although the precise secondary structure of the 5′ NTR could not be defined, the location of the pyrimidine tract (nt 695 to 701) and the initiator methionine (nt 713) suggested that the IRES of Aichi virus belongs to type II, similar to aphtho-, cardio- and hepatoviruses (Fig. 1). RNA folding analysis of the extreme 5′ end of the RNA with the MFOLD program in CGC (version 9.0, December 1996; Genetics Computer Group, Madison, Wis.) suggested the presence of a hairpin structure followed by pseudonots, such as found in aphtho-, cardio-, and hepatoviruses (data not shown). The picornavirus 3′ NTRs differ in length, ranging from 40 nt in HRV to 126 nt in EMCV (32), and the 3′ NTR of the Aichi virus genome was longer than that of EMCV, the longest in the picornavirus family, by more than 100 nt. Whether the 3′ NTR of Aichi virus consists of three double-stranded hairpin stems, as seen in EMCV, two stems, as seen in PV1 and FMDV, or a single stem, similar to HRV and HAV, could not be determined (2, 27).
FIG. 1.
Comparison of conserved sequence elements in the IRES of picornaviruses. A short stretch including the Yn-Xm-ATG motif and the initiator methionine, according to Jang et al. (16), is shown. Yn, pyrimidine-rich tract; Xm, nonconserved sequence; ATG, initiator methionine.
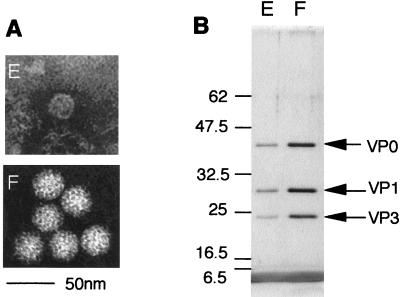
Aichi virus virions contain three, not four, capsid proteins, of 42, 30, and 22 kDa. No protein band corresponding to VP4 (usually 7 to 8 kDa) was observed in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), even when only 1 μg of purified virions was loaded onto the gel and visualized by silver staining. Because it has been reported that preparations of highly purified picornavirus particles invariably contain small amounts of VP0, intact Aichi virus particles were separated from the empty particles by sedimentation in sucrose, and the capsid proteins were separated by SDS-PAGE. The proteins composing these two particles were found to be similar (Fig. 2). This clearly illustrated that the Aichi virus virion has no VP4, as previously shown for E22 (15).
FIG. 2.
Electron micrograph (A) and SDS-PAGE analysis (B) of Aichi virus full particle (F) and empty particle (E). The intact virions and the empty particles were purified by banding at 100,000 × g for 22 h in CsCl with an initial density of 1.36g/ml followed by 5 to 30% (wt/vol) sucrose density gradient centrifugation at 100,000 × g for 100 min (40). The proteins were analyzed by SDS–12% PAGE, and the bands were visualized by silver staining. For N-terminal sequence analysis, the protein band was transferred to a polyvinylidene difluoride membrane (Millipore Corporation, Bedford, Mass.) and analyzed by an Applied Biosystems model 476A automated protein sequencer.
To further characterize each capsid protein and identify some of the cleavage sites, 30- and 22-kDa proteins from the intact particles were separated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane, and then the N-terminal sequence was determined. The analysis provided the results TLTEDLDAPQDTGNI and HWKTRAVPGAG for the 30- and 22-kDa proteins, respectively. These sequences were found at aa 765 to 779 and 542 to 552 in the predicted polyprotein sequence. These residues unambiguously localized the N-terminal end of VP1 and VP3 in Aichi virus P1 protein (Table 1). The largest, 42 kDa, provided no signal in the analysis, indicating that the N-terminal amino acid was blocked. This was not surprising, because the N-terminal end of picornavirus VP4 is myristylated, and it has been shown that a characteristic consensus myristylation sequence (GXXX[T/S], where X is a nonconserved amino acid) is conserved in all picornaviruses. This motif was easily found at aa 171 to 175 of the Aichi virus polyprotein. Therefore, glycine at aa 171 was probably myristylated like other VP4 proteins of picornaviruses (32). Because the molecular mass calculated for aa 171 to 541 (≈39 kDa) was close to the molecular mass obtained in SDS-PAGE and no VP4 was found on the gel, we concluded that the 42-kDa protein is VP0 and that no VP4-VP2 cleavage occurred. The Aichi virus 42-kDa protein strongly reacted with convalescent-phase serum from patients (40); therefore, it probably constitutes the surface of the virions. We concluded that the VP0-VP3 and VP3-VP1 cleavage sites are Q-H and Q-T, respectively. These observations further indicated that a leader (L) protein consisting of 170 aa is present upstream of VP0. The length of the L protein is a little shorter than that of FMDV (217 aa) and more than two times longer than that of EMCV (67 aa). However, neither the catalytic dyad (Cys and His) conserved in a papain-like thiol protease and found in the FMDV L protein (12, 26) nor a putative zinc-binding motif, Cys-His-Cys-Cys, found in EMCV or TMEV (6) could be identified. The function of the Aichi virus L protein is unknown at the moment. Although there was no consensus amino acid sequence around the VP1-2A junctions among the picornaviruses, the P1-P2 cleavage site of Aichi virus was tentatively determined to be Y-V, located at aa 1042 and 1043, based on the molecular mass of VP1 and the known P1-P2 cleavage site of HRV2 (31).
TABLE 1.
Comparisons of amino acid and nucleic acid homologies of Aichi virus with representatives of other picornaviruses
| Region | No. of amino acid or RNA residues (% homology)
|
||||||
|---|---|---|---|---|---|---|---|
| Aichi | PV1 | HRV2 | EMCV | FMDV-O | HAV | E22 | |
| 5′ NTR | 712 | 742 (47) | 610 (48) | 833 (45) | 713 (48) | 735 (48) | 709 (46) |
| L | 170 | —a | — | 67 (14) | 217 (18) | 6 (2) | 12 (4) |
| VP0 | 371 | 341 (22) | 330 (21) | 326 (23) | 287 (25) | 239 (19) | 289 (20) |
| VP3 | 223 | 238 (27) | 237 (27) | 231 (32) | 220 (23) | 246 (21) | 253 (24) |
| VP1 | 278 | 302 (23) | 289 (23) | 277 (23) | 213 (19) | 300 (19) | 234 (20) |
| 2A | 111 | 149 (18) | 136 (19) | 143 (15) | 16 (5) | 189 (20) | 147 (20) |
| 2B | 165 | 97 (18) | 95 (15) | 150 (21) | 154 (24) | 107 (20) | 122 (18) |
| 2C | 335 | 329 (28) | 322 (27) | 325 (30) | 318 (32) | 335 (29) | 329 (28) |
| 3A | 95 | 87 (22) | 77 (20) | 88 (17) | 153 (17) | 74 (17) | 117 (20) |
| 3B (VPg) | 27 | 22 (32) | 21 (33) | 20 (19) | 23/24 (19/28)b | 23 (22) | 20 (30) |
| 3C | 190 | 183 (24) | 183 (25) | 205 (23) | 213 (22) | 219 (24) | 200 (17) |
| 3D | 468 | 461 (36) | 460 (32) | 460 (35) | 470 (36) | 489 (26) | 469 (27) |
| 3′ NTR | 240 | 71 (21) | 42 (12) | 126 (30) | 95 (25) | 63 (18) | 90 (23) |
—, no leader protein is encoded.
Three VPg’s in tandem are encoded, each with 23 or 24 aa.
Possible cleavage sites of nonstructural proteins were determined from amino acid alignment with known picornaviruses. Cleavage sites 2A-2B, 2B-2C, 2C-3A, 3A-3B (VPg), 3B-3Cpro, and 3Cpro-3Dpol are similar to those well conserved among picornaviruses, and they were tentatively assigned as Q-G, Q-G, Q-G, Q-A, Q-G, and Q-S, respectively. The 2A protein of picornavirus is known to have a cis-acting proteolytic activity and has been classified into two types. In entero- and rhinoviruses, 2A functions autocatalytically to cleave the P1 polyprotein at its own N terminus and mediates the cleavage of the p220 component of the cap-binding complex eIF-4γ, leading to the shutoff of host cellular protein synthesis. Like 3C protease, a catalytic triad conserved in trypsin-like protease has been identified (3, 43). In aphtho- and cardioviruses, on the other hand, 2A mediates the cleavage at its own C terminus and the autocleavage motif, NPEG, is conserved in a C-terminal 2A protein (11, 30). In Aichi virus 2A protein, neither the critical GXCG motif of trypsin-like protease nor the NPEG motif could be found. Further study is necessary to elucidate the function and capability of Aichi virus 2A in virus replication. Amino acid sequences of the 2C, 3Cpro, and 3Dpol regions were well aligned with other picornaviruses (data not shown). Although the function of 2C protein was not completely elucidated, a highly conserved motif (GxxGXGKT [X, uncharged, x, nonconserved]) in the nucleotide binding domain of the putative picornavirus helicase was found in Aichi virus 2C protein. 3B (VPg) of Aichi virus (27 aa) was longer than that of other picornaviruses by 3 to 7 aa. A tyrosine residue was conserved at the third amino acid, as observed in other VPg proteins of picornaviruses. We found only one copy of the VPg sequence; therefore, Aichi virus was determined to be different from aphthoviruses, which have three copies of the VPg sequence in tandem. The 3Cpro that participates in most of the cleavages of picornavirus polyprotein contains a catalytic triad formed by histidine, aspartate/glutamate, and cysteine. These amino acids are conserved in all picornaviruses, and they were seen in Aichi virus 3Cpro at positions 42, 84, and 143, respectively. A motif, GXCGG, conserved at the C terminus of enterovirus and rhinovirus 3Cpro was considered to form a part of the active site, and this motif was present but altered to GXCGS in Aichi virus (X, nonconserved amino acid). An identical change was observed in cardioviruses. A histidine residue that probably participates in the substrate binding pocket in the trypsin-like protease was found at aa 161 in Aichi virus 3Cpro (3, 5). Highly conserved motifs (KDELR, YGDD, and FLKR) in picornavirus 3Dpol were found at aa 160, 328, and 377, respectively, in Aichi virus 3Dpol.
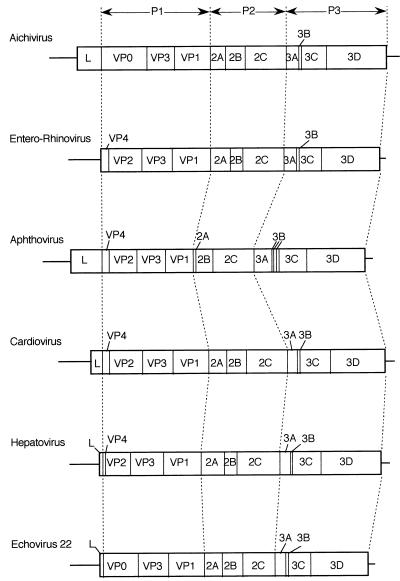
The genome structure of Aichi virus, VPg–5′ NTR–leader protein–structural proteins–nonstructural proteins–3′ NTR–poly(A) tail, is typical of a picornavirus (Fig. 3). The overall structure of the Aichi virus genome most closely resembled an aphthovirus, except for VPg. The genome organization was apparently different from that of caliciviruses, including small round-structured viruses, or that of astroviruses. These two viruses contain three open reading frames, and 3Dpol is located more than 2,000 nt upstream of the poly(A) tail due to the presence of a capsid protein gene (17, 19, 39).
FIG. 3.
Genome organization of Aichi virus and comparison of the structure among picornaviruses. The genome organizations have been shown according to the L434 system (29). P1 represents viral structural proteins. P2 and P3 represent nonstructural proteins. P1 proteins of hepatovirus and E22 produce 6- and 12-aa leaders, respectively (5 and 11 aa without the initiator methionine).
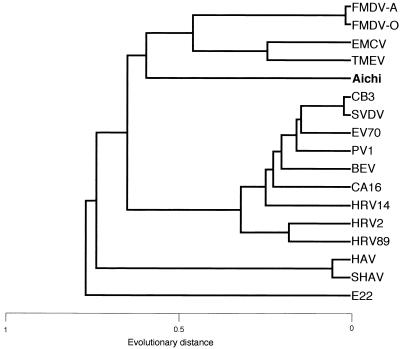
The nucleotide sequence homologies of the 5′ and 3′ NTRs and the amino acid sequence homologies between Aichi virus and representative viruses from other picornaviruses are shown in Table 1. The 5′ NTR of Aichi virus exhibits a similar and relatively high degree of sequence homology with other viruses. The homology of Aichi virus proteins with corresponding polypeptides of other picornaviruses varied between 15 and 36%, except for a short leader of HAV and E22. This value is of the same order as that seen when HAV or E22 is compared with other picornaviruses (7, 15). The dendrogram based on 3Dpol proteins is depicted in Fig. 4, indicating that Aichi virus should be separated from the known six genera of picornaviruses including entero-, rhino-, cardio-, aphtho-, and hepatoviruses and E22 (15, 28, 32, 33).
FIG. 4.
Relationships between Aichi virus and other picornaviruses based on amino acid differences of 3Dpol proteins. The dendrogram was generated by evolutionary distances computed by UPGMA.
Acute epidemic gastroenteritis outbreaks after consumption of raw oysters are reported every winter in Japan (1, 13, 14, 34, 37), and most of them are suspected to be caused by Norwalk-like viruses. However, it has become obvious that some of them have been caused by Aichi virus (41). The primary structure of Aichi virus elucidated in this study will be unambiguously useful for developing sensitive and specific detection of Aichi virus sequences by PCR. By such a detection system, the significance of Aichi virus as a causative agent in acute nonbacterial gastroenteritis will be clarified.
Nucleotide sequence accession number.
The complete nucleotide sequence of Aichi virus has been submitted to the DDBJ, EMBL, and GenBank databases under accession no. AB010145.
REFERENCES
- 1.Ando T, Mulders M N, Lewis D C, Estes M K, Monroe S S, Glass R I. Comparison of the polymerase region of small round structured virus strains previously classified in three antigenic types by solid-phase immune electron microscopy. Arch Virol. 1994;135:217–226. doi: 10.1007/BF01309781. [DOI] [PubMed] [Google Scholar]
- 2.Auvinen P, Hyypiä T. Echoviruses include genetically distinct serotypes. J Gen Virol. 1990;71:2133–2139. doi: 10.1099/0022-1317-71-9-2133. [DOI] [PubMed] [Google Scholar]
- 3.Bazan J F, Fletterick R J. Viral cysteine proteases are homologous to the trypsin-like family of serine proteases: structural and functional implications. Proc Natl Acad Sci USA. 1988;85:7872–7876. doi: 10.1073/pnas.85.21.7872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blacklow N R. Medical virology of small round gastroenteritis viruses. In: de la Maza L M, Peterson E M, editors. Medical virology, no. 9. New York, N.Y: Plenum Press; 1990. pp. 111–128. [Google Scholar]
- 5.Boniotti B, Wirblich C, Sibilia M, Meyers G, Thiel H-J, Rossi C. Identification and characterization of a 3C-like protease from rabbit hemorrhagic disease virus, a calicivirus. J Virol. 1994;68:6487–6495. doi: 10.1128/jvi.68.10.6487-6495.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H H, Kong W P, Roos R P. The leader peptide of Theiler’s murine encephalomyelitis virus is a zinc-binding protein. J Virol. 1995;69:8076–8078. doi: 10.1128/jvi.69.12.8076-8078.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen J I, Ticehurst J R, Feinstone S M, Rosenblum B, Purcell R H. Hepatitis A virus cDNA and its RNA transcripts are infectious in cell culture. J Virol. 1987;61:3035–3039. doi: 10.1128/jvi.61.10.3035-3039.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cubitt D, Bradley D W, Carter M J, Chiba S, Estes M K, Saif L J, Schaffer F L, Smith A W, Studdert M J, Thiel H J. Caliciviridae. In: Murphy F A, Fauquet C M, Bishop D H L, Ghabrial S A, Jarvis A W, Martelli G P, Mayo M A, Summers M D, editors. Virus taxonomy: sixth report of the International Committee on Taxonomy of viruses. Vienna, Austria: Springer-Verlag; 1995. pp. 359–363. [Google Scholar]
- 9.Cukor G, Blacklow N R. Human viral gastroenteritis. Microbiol Rev. 1984;48:157–179. doi: 10.1128/mr.48.2.157-179.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dolin R, Treanor J J, Madore H P. Novel agents of viral enteritis in humans. J Infect Dis. 1987;155:365–376. doi: 10.1093/infdis/155.3.365. [DOI] [PubMed] [Google Scholar]
- 11.Donnelly M L, Gani D, Flint M, Monaghan S, Ryan M D. The cleavage activities of aphthovirus and cardiovirus 2A proteins. J Gen Virol. 1997;78:13–21. doi: 10.1099/0022-1317-78-1-13. [DOI] [PubMed] [Google Scholar]
- 12.Gorbalenya A E, Koonin E V, Lai M M. Putative papain-related thiol proteases of positive-strand RNA viruses. Identification of rubi- and aphthovirus proteases and delineation of a novel conserved domain associated with proteases of rubi-, alpha- and coronaviruses. FEBS Lett. 1991;288:201–205. doi: 10.1016/0014-5793(91)81034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haruki K, Seto Y, Murakami T, Kimura T. Pattern of shedding of small, round-structured virus particles in stools of patients of outbreaks of food-poisoning from raw oysters. Microbiol Immunol. 1991;35:83–86. doi: 10.1111/j.1348-0421.1991.tb01536.x. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi Y, Ando T, Utagawa E, Sekine S, Okada S, Yabuuchi K, Miki T, Ohashi M. Western blot (immunoblot) assay of small, round-structured virus associated with an acute gastroenteritis outbreak in Tokyo. J Clin Microbiol. 1989;27:1728–1733. doi: 10.1128/jcm.27.8.1728-1733.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hyypiä T, Horsnell C, Maaronen M, Khan M, Kalkkinen N, Auvinen P, Kinnunen L, Stanway G. A distinct picornavirus group identified by sequence analysis. Proc Natl Acad Sci USA. 1992;89:8847–8851. doi: 10.1073/pnas.89.18.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jang S K, Pestova T V, Hellen C U, Witherell G W, Wimmer E. Cap-independent translation of picornavirus RNAs: structure and function of the internal ribosomal entry site. Enzyme. 1990;44:292–309. doi: 10.1159/000468766. [DOI] [PubMed] [Google Scholar]
- 17.Jiang B, Monroe S S, Koonin E V, Stine S E, Glass R I. RNA sequence of astrovirus: distinctive genomic organization and a putative retrovirus-like ribosomal frameshifting signal that directs the viral replicase synthesis. Proc Natl Acad Sci USA. 1993;90:10539–10543. doi: 10.1073/pnas.90.22.10539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang X, Graham D Y, Wang K, Estes M K. Norwalk virus genome cloning and characterization. Science. 1990;250:1580–1583. doi: 10.1126/science.2177224. [DOI] [PubMed] [Google Scholar]
- 19.Jiang X, Wang M, Wang K, Estes M K. Sequence and genomic organization of Norwalk virus. Virology. 1993;195:51–61. doi: 10.1006/viro.1993.1345. [DOI] [PubMed] [Google Scholar]
- 20.Khan A S, Moe C L, Glass R I, Monroe S S, Estes M K, Chapman L E, Jiang X, Humphrey C, Pon E, Iskander J K, Schonberger L B. Norwalk virus-associated gastroenteritis traced to ice consumption aboard a cruise ship in Hawaii: comparison and application of molecular method-based assays. J Clin Microbiol. 1994;32:318–322. doi: 10.1128/jcm.32.2.318-322.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lambden P R, Caul E O, Ashley C R, Clarke I N. Sequence and genome organization of a human small round-structured (Norwalk-like) virus. Science. 1993;259:516–519. doi: 10.1126/science.8380940. [DOI] [PubMed] [Google Scholar]
- 22.Lew J F, Petric M, Kapikian A Z, Jiang X, Estes M K, Green K Y. Identification of minireovirus as a Norwalk-like virus in pediatric patients with gastroenteritis. J Virol. 1994;68:3391–3396. doi: 10.1128/jvi.68.5.3391-3396.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monroe S S, Carter M J, Herrmann J E, Kurtz J B, Matsui S M. Astroviridae. In: Murphy F A, Fauquet C M, Bishop D H L, Ghabrial S A, Jarvis A W, Martelli G P, Mayo M A, Summers M D, editors. Virus taxonomy: sixth report of the International Committee on Taxonomy of Viruses. Vienna, Austria: Springer-Verlag; 1995. pp. 364–367. [Google Scholar]
- 24.Monroe S S, Jiang B, Stine S E, Koopmans M, Glass R I. Subgenomic RNA sequence of human astrovirus supports classification of Astroviridae as a new family of RNA viruses. J Virol. 1993;67:3611–3614. doi: 10.1128/jvi.67.6.3611-3614.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pelletier J, Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988;334:320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- 26.Piccone M E, Zellner M, Kumosinski T F, Mason P W, Grubman M J. Identification of the active-site residues of the L proteinase of foot-and-mouth disease virus. J Virol. 1995;69:4950–4956. doi: 10.1128/jvi.69.8.4950-4956.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pöyry T, Kinnunen L, Hyypiä T, Brown B, Horsnell C, Hovi T, Stanway G. Genetic and phylogenetic clustering of enteroviruses. J Gen Virol. 1996;77:1699–1717. doi: 10.1099/0022-1317-77-8-1699. [DOI] [PubMed] [Google Scholar]
- 28.Rodrigo M J, Dopazo J. Evolutionary analysis of the picornavirus family. J Mol Evol. 1995;40:362–371. doi: 10.1007/BF00164022. [DOI] [PubMed] [Google Scholar]
- 29.Rueckert R R, Wimmer E. Systematic nomenclature of picornavirus proteins. J Virol. 1984;50:957–959. doi: 10.1128/jvi.50.3.957-959.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryan M D, Drew J. Foot-and-mouth disease virus 2A oligopeptide mediated cleavage of an artificial polyprotein. EMBO J. 1994;13:928–933. doi: 10.1002/j.1460-2075.1994.tb06337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skern T, Sommergruber W, Blaas D, Gruendler P, Fraundorfer F, Pieler C, Fogy I, Kuechler E. Human rhinovirus 2: complete nucleotide sequence and proteolytic processing signals in the capsid protein region. Nucleic Acids Res. 1985;13:2111–2126. doi: 10.1093/nar/13.6.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stanway G. Structure, function and evolution of picornaviruses. J Gen Virol. 1990;71:2483–2501. doi: 10.1099/0022-1317-71-11-2483. [DOI] [PubMed] [Google Scholar]
- 33.Stanway G, Kalkkinen N, Roivainen M, Ghazi F, Khan M, Smyth M, Meurman O, Hyypiä T. Molecular and biological characteristics of echovirus 22, a representative of a new picornavirus group. J Virol. 1994;68:8232–8238. doi: 10.1128/jvi.68.12.8232-8238.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugieda M, Nakajima K, Nakajima S. Outbreaks of Norwalk-like virus-associated gastroenteritis traced to shellfish: coexistence of two genotypes in one specimen. Epidemiol Infect. 1996;116:339–346. doi: 10.1017/s0950268800052663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Supanaranond K, Takeda N, Yamazaki S. The complete nucleotide sequence of a variant of Coxsackievirus A24, an agent causing acute hemorrhagic conjunctivitis. Virus Genes. 1992;6:149–158. doi: 10.1007/BF01703064. [DOI] [PubMed] [Google Scholar]
- 36.Takeda N. Complete nucleotide and amino acid sequences of enterovirus 70. In: Ishii K, Uchida Y, Miyamura K, Yamazaki S, editors. Acute hemorrhagic conjunctivitis. Basel, Switzerland: University of Tokyo Press, Tokyo/Karger; 1989. pp. 419–424. [Google Scholar]
- 37.Utagawa E T, Takeda N, Inouye S, Kasuga K, Yamazaki S. 3′-terminal sequence of a small round structured virus (SRSV) in Japan. Arch Virol. 1994;135:185–192. doi: 10.1007/BF01309777. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Jiang X, Madore H P, Gray J, Desselberger U, Ando T, Seto Y, Oishi I, Lew J F, Green K Y, Estes M K. Sequence diversity of small, round-structured viruses in the Norwalk virus group. J Virol. 1994;68:5982–5990. doi: 10.1128/jvi.68.9.5982-5990.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Willcocks M M, Brown T D, Madeley C R, Carter M J. The complete sequence of a human astrovirus. J Gen Virol. 1994;75:1785–1788. doi: 10.1099/0022-1317-75-7-1785. [DOI] [PubMed] [Google Scholar]
- 40.Yamashita T, Kobayashi S, Sakae K, Nakata S, Chiba S, Ishihara Y, Isomura S. Isolation of cytopathic small round viruses with BS-C-1 cells from patients with gastroenteritis. J Infect Dis. 1991;164:954–957. doi: 10.1093/infdis/164.5.954. [DOI] [PubMed] [Google Scholar]
- 41.Yamashita T, Sakae K, Ishihara Y, Isomura S, Utagawa E. Prevalence of newly isolated, cytopathic small round virus (Aichi strain) in Japan. J Clin Microbiol. 1993;31:2938–2943. doi: 10.1128/jcm.31.11.2938-2943.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamashita T, Sakae K, Kobayashi S, Ishihara Y, Miyake T, Mubina A, Isomura S. Isolation of cytopathic small round virus (Aichi virus) from Pakistani children and Japanese travelers from Southeast Asia. Microbiol Immunol. 1995;39:433–435. doi: 10.1111/j.1348-0421.1995.tb02225.x. [DOI] [PubMed] [Google Scholar]
- 43.Yu S F, Lloyd R E. Characterization of the roles of conserved cysteine and histidine residues in poliovirus 2A protease. Virology. 1992;186:725–735. doi: 10.1016/0042-6822(92)90039-r. [DOI] [PubMed] [Google Scholar]