Abstract
Background.
The response to antimalarial treatment is assessed using serial microscopy. New techniques for accurate measurement of the Plasmodium falciparum histidine-rich protein 2 (HRP2) antigen have allowed for monitoring of the antigen concentration over time, offering a potential alternative for assessing treatment response.
Methods.
Posttreatment HRP2 concentrations were measured in samples obtained longitudinally from 537 individuals with P. falciparum malaria who were participating in efficacy trials in Angola, Tanzania, and Senegal. The HRP2 half-life was estimated using a first-order kinetics clearance model. The association between the HRP2 concentration 3 days after treatment and recrudescence of infection was assessed.
Results.
Despite substantial variation in HRP2 concentrations among participants at baseline, concentrations consistently showed a first-order exponential decline. The median half-life of HRP2 was estimated to be 4.5 days (interquartile range [IQR], 3.3–6.6 days) in Angola, 4.7 days (IQR, 4.0–5.9 days) in Tanzania, and 3.0 days (IQR, 2.1–4.5 days) in Senegal. The day 3 HRP2 concentration was predictive of eventual recrudescence, with an area under the receiver operating characteristic curve of 0.86 (95% confidence interval, .73–.99).
Conclusions.
Consistent HRP2 clearance dynamics following successful antimalarial treatment imply a common underlying mechanism of biological clearance. Patients who ultimately did not respond to treatment did not exhibit this same pattern of clearance, even in the absence of other indications of inadequate response to treatment.
Keywords: Antimalarial resistance, efficacy monitoring, antigen concentration
Use of light microscopy to count asexual parasites in a stained peripheral blood film is the gold standard for determining the parasite burden in malaria parasite infections. The parasite density can be used to grade the severity of disease, and its rise and fall is crucial for monitoring the response to antimalarial treatment. The gold standard for determining the efficacy of antimalarials is serial microscopy to determine whether the parasite density declines as rapidly and completely as expected after treatment [1].
Estimation of the parasite density by microscopy relies on the availability of reagents of ample quality, functioning equipment, and competent microscopists. However, the laboratory conditions necessary to offer quality malaria microscopy are often unavailable in rural sub-Saharan Africa, where the malaria burden is highest. Instead, most confirmed malaria cases are now diagnosed on the basis of rapid diagnostic test (RDT) results; RDTs accounted for 74% of all malaria testing in 2015 [2]. The low cost and ease of use of RDTs, as well as their sensitivity for detecting clinical malaria, are factors that contributed to the World Health Organization’s universal recommendation for diagnostic confirmation of suspected malaria cases [3]. RDTs rely on the detection of malaria parasite antigens, in most cases the Plasmodium falciparum histidine-rich protein 2 (HRP2) antigen. However, because currently available RDTs are purely qualitative, indicating the presence or absence of the malaria parasite antigen, they do not provide quantitative information about parasite burden. Although there have been efforts to characterize and use the duration of RDT positivity to monitor the response to treatment [4], this approach has not been useful because the duration of antigenemia is a relatively crude measure of antimalarial efficacy and because the persistence of detectable antigen lags behind clearance of asexual intraerythrocytic parasites.
The dynamics of HRP2 concentration during and immediately after malaria parasite infection have to date not been well characterized. Several studies have shown that the HRP2 concentration can be used as a marker of severity [5, 6], and recent work has shown the HRP2 concentration to also be predictive of posttreatment hemolysis in severe cases [7]. Previous work using a semiquantitative enzyme-linked immunosorbent assay (ELISA) for determining the HRP2 concentration has provided some initial data on HRP2 dynamics, including an estimated half-life of 3.67 days [8]. However, because of the cost and infrequent use of ELISA-based HRP2 detection, fundamental gaps around HRP2 dynamics remain, and unlike for parasite density, there is no clear understanding of how HRP2 levels change and ultimately persist after the start of antimalarial treatment. The recent development of a high-throughput, bead-based assay for HRP2 detection and quantification allows accurate detection of HRP2 at concentrations nearing 1 pg/mL [9] and provides the opportunity to characterize HRP2 dynamics more completely. Here, we describe posttreatment HRP2 dynamics in symptomatic patients with uncomplicated falciparum malaria from southern, eastern, and western Africa.
METHODS
Sample Collection
We analyzed samples from therapeutic efficacy studies in Angola, collected during 2013 [10] and 2015 [11]; in Tanzania, collected during 2010 and 2011; and Senegal, collected during 2011–2015. Persons aged 6 months to 9 years (in Angola), 6 months to 5 years (in Tanzania), and 4 years to 20 years (in Senegal) with uncomplicated P. falciparum monoinfection (range of initial parasite densities, 1000–200 000 parasites/μL) were treated with artemisinin-based combination therapy and followed for 28–42 days to monitor the response to treatment. Blood specimens were collected at enrollment (ie, day 0), on day 3 (except for the 2013 Angola study), on day 7, and weekly thereafter for analysis by the dried blood spot technique. Additionally, in Senegal, blood specimens were collected on days 1 and 2. Whatman 903 filter paper was used in Angola, Whatman 3 paper was used in Tanzania, and Whatman FTA cards were used in Senegal. Participants were classified by microscopy as having one of the following: adequate clinical and parasitological response (ACPR), indicating treatment success; reinfection, indicating acquisition of a new infection during follow-up that was unrelated to the original infection; or recrudescence, indicating true treatment failure following inadequate clearance of the original infection. Cases of reinfection and recrudescence were differentiated by comparing parasite genotypes at enrollment and on the day of treatment failure. All participants or their parents or guardians provided written informed consent for blood specimen collection during the original studies, and analysis of anonymized stored blood samples was approved by Centers for Disease Control and Prevention Human Subjects Review Board. The original studies were reviewed and approved by human subjects review boards at the Angolan Ministry of Health, the Tanzanian Ministry of Health, and the University Cheikh Anta Diop in Senegal.
Quantification of the HRP2 Concentration
Dried blood spots were shipped to the Centers for Disease Control and Prevention Malaria Branch laboratory in Atlanta, Georgia, and blood was eluted in a solution of phosphate-buffered saline, 0.05% Tween20, and 0.02% NaN3 by gentle shaking overnight to a final concentration of 1 part blood to 20 parts solution. The presence and quantification of HRP2 was performed as described previously [9], using the bead-based Luminex platform (Austin, TX). Briefly, samples were incubated with polystyrene microspheres (BioRad, Hercules, CA) coated with monoclonal antibodies against HRP2 (mouse immunoglobulin G antiHRP2 [MPFM-55A]; Abcam, Cambridge, United Kingdom). Beads were incubated with a detection antibody (mouse immunoglobulin anti-HRP2 [MPFG-55P]; Abcam, Cambridge, United Kingdom) that was previously biotinylated by the Thermo Scientific EZ-Link Micro Sulfo-NHS-Biotinylation Kit (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s protocol and were subsequently incubated with streptavidin-phycoerythrin (Invitrogen, Carlsbad, CA). After a final wash step with reagent diluent, beads were resuspended in 100 µL of phosphate-buffered saline; fluorescence was read on a Bio-Plex 200 instrument (BioRad, Hercules, CA) by generating the median fluorescence signal for 50 beads and then the mean fluorescence intensity (MFI) of the medians among replicates. The final measure, denoted as MFI-bg, was reported by subtracting MFIs from beads on each plate only exposed to sample diluent during the sample incubation step. A MFI-bg value was extrapolated to a concentration of HRP2 by use of a standard curve created by recombinant type B HRP2 (GST-W2-HRP2; MicroMol, Karlsruhe, Germany).
Estimation of the Clearance Rate
The HRP2 clearance rate was estimated from patients cured following treatment. A first-order kinetics clearance model [12] was fit to the HRP2 concentration versus time (in days) data for each participant, resulting in a unique estimate for the clearance rate for each individual. Patients who had <4 collection time points, experienced reinfection or recrudescence, or showed an increase in the HRP2 level of >25% or 1000 pg/mL between subsequent samples were excluded in the estimation of clearance rates. For comparability across countries, data on day 1 and day 2 HRP2 concentrations in Senegal were not included in the estimation of clearance rates. For each individual, the clearance rate (in days−1), the corresponding half-life, and the goodness of fit (ie, the R2 statistic) were estimated. The distribution, median, and interquartile range (IQR) of the estimated parameters were calculated. The relationship between age and HRP2 clearance rate was investigated in the Senegal data set because it had the widest age range among the countries. The Pearson correlation coefficient for the relationship between age and clearance rate was calculated, and a Kolmogorov-Smirnov test was used to test whether the distribution of clearance rate constants was different between participants aged 5–9 years and those aged 10–20 years.
Estimation of the Duration of HRP2 Positivity
For each individual for whom a clearance rate constant was estimated, the duration of HRP2 positivity was directly calculated from the fitted clearance model as the expected time to reach a certain concentration of HRP2 that would be reliably detected by a hypothetical HRP2 detection test with a specified limit of detection, ranging from 1 to 10 000 pg/mL. To estimate each individual’s duration of HRP2 positivity, the individual’s pretreatment HRP2 concentration and estimated clearance rate constant were used. The median and IQR for the duration of HRP2 positivity for each value of the detection threshold were calculated separately for each site.
Modeling the Association Between HRP2 Clearance and Treatment Outcome
The dynamics of HRP2 concentration were characterized by stratifying by treatment outcome in the data sets from Angola, where there was a significant number of treatment failures. For each patient from the Angola studies, the log-transformed HRP2 concentration for each day of follow up for which a blood specimen was available was standardized by dividing that concentration by the HRP2 concentration at day 0. The median normalized HRP2 concentration was calculated for each time point, stratifying by the following treatment outcomes: ACPR, reinfection, or recrudescence. For reinfections and recrudescences, data were censored to exclude the day of treatment failure and any subsequent data, to avoid confounding the estimation of the clearance rate. Receiver operating characteristic (ROC) curves estimating the relationship between the sensitivity and specificity of the normalized day 3 and day 7 HRP2 concentrations as a predictor for patients ultimately classified as experiencing recrudescence versus those experiencing ACPR or reinfection were created [13]. The area under the ROC curve was estimated together with its 95% confidence interval (CI) [13].
All statistical analyses were done in R, version 3.3.0 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
The HRP2 concentration in 3513 samples from 537 participants was measured (Table 1). The median pretreatment HRP2 concentration was 498 000 pg/mL (IQR, 196 000–1 171 000 pg/mL) in Angola, 249 000 pg/mL (IQR, 154 000–394 000 pg/mL) in Tanzania, and 212 000 pg/mL (IQR, 63 000–1 293 000 pg/mL) in Senegal. In each population, the median log HRP2 concentration declined linearly over time (Figure 1), indicative of a first-order kinetics clearance process. The first-order kinetics clearance model, when fit to individual participant-time courses, was well specified, with a median R2 of 0.98 (IQR, 0.96–0.99) in Angola, 0.98 (IQR, 0.96–0.99) in Tanzania, and 0.94 (IQR, 0.91–0.97) in Senegal (Table 1 and Supplemental Figure 1). Similarly, the distribution of estimated clearance rate constants and, consequently, half-lives was narrow and overlapped among the 3 countries (Table 1 and Figure 2). The median clearance rate constants were 0.15 day−1 (IQR, 0.10–0.21 day−1) in Angola, 0.15 day−1 (IQR, 0.12–0.17 day−1) in Tanzania, and 0.23 day−1 (IQR, 0.15–0.33 day−1) in Senegal, resulting in median blood circulation half-lives of 4.5 days (IQR, 3.3–6.6 days) in Angola, 4.7 days (IQR, 4.0–5.9 days) in Tanzania, and 3.0 days (IQR, 2.1–4.5 days) in Senegal (Table 1). There were 2 outliers with estimated half-lives of >20 days, 1 in Angola and 1 in Tanzania. There was no statistically significant association between age and clearance rate in Senegal, with a Pearson correlation P value of .58, and a Kolmogorov–Smirnov test P value of .60.
Table 1.
Clearance of Histidine-Rich Protein 2 (HRP2) in Patients Followed Longitudinally After Antimalarial Treatment in 3 Different Sites in Sub-Saharan Africa
| Variable | Angola (n = 311) | Tanzania (n = 95) | Senegal (n = 131) |
|---|---|---|---|
| Monotonically decreasing HRP2 concentrationa | 230 | 68 | 85 |
| Increase in HRP2 concentration after day 7b | 52 | 20 | 32 |
| Data from <4 time pointsc | 29 | 7 | 14 |
| Estimated clearance rate, d−1 | 0.15 (0.10–0.21) | 0.15 (0.12–0.17) | 0.23 (0.15–0.33) |
| Estimated half-life, d | 4.5 (3.3–6.6) | 4.7 (4.0–5.9) | 3.0 (2.1–4.5) |
| Estimated time to reach 1000 pg/mL, d | 40 (29–59) | 37 (31–50) | 24 (17–33) |
| Goodness of fit of first-order kinetics clearance model, R2 | 0.98 (0.96–0.99) | 0.98 (0.96–0.99) | 0.94 (0.91–0.97) |
Data are no. of participants or median value (interquartile range).
Within a 25% or 1000 pg/mL margin of error.
Indication of possible treatment failure. Data denote no. of participants who were excluded from calculation of clearance rate.
Data denote no. of participants who were excluded from calculation of clearance rate.
Figure 1.
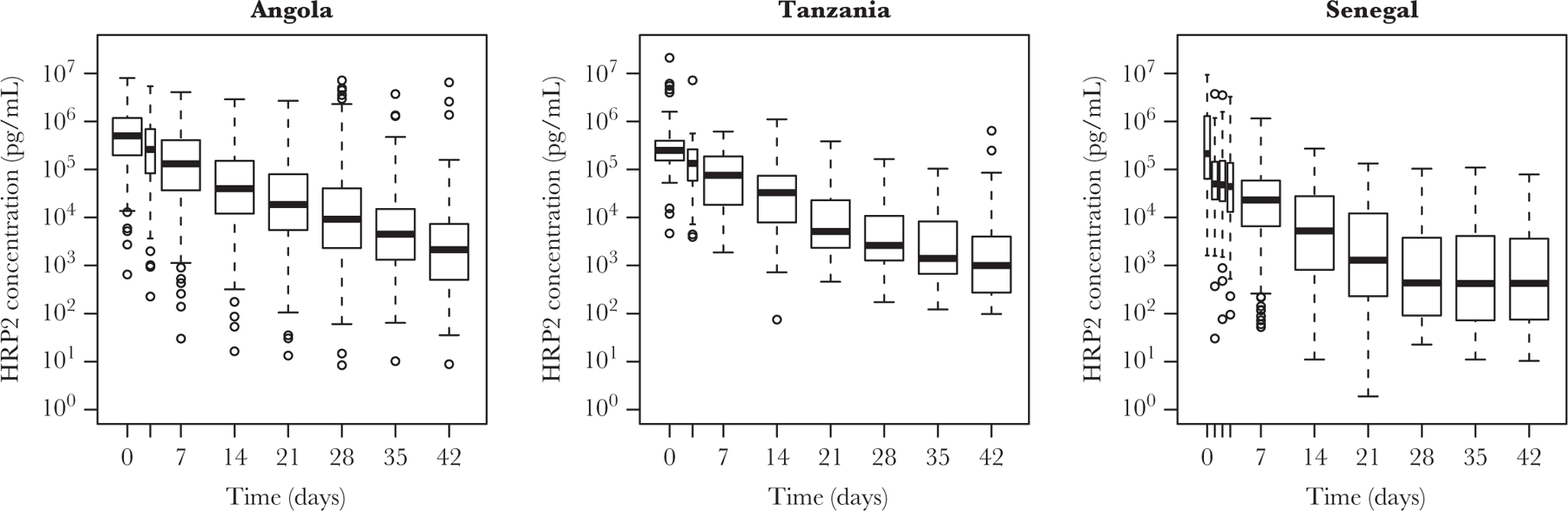
Histidine-rich protein 2 (HRP2) concentrations over time following initiation of antimalarial treatment at day 0 in patients with malaria who were followed longitudinally in Angola, Tanzania, and Senegal. Boxes show median values and interquartile ranges. Whiskers show extreme values and points represent outliers.
Figure 2.
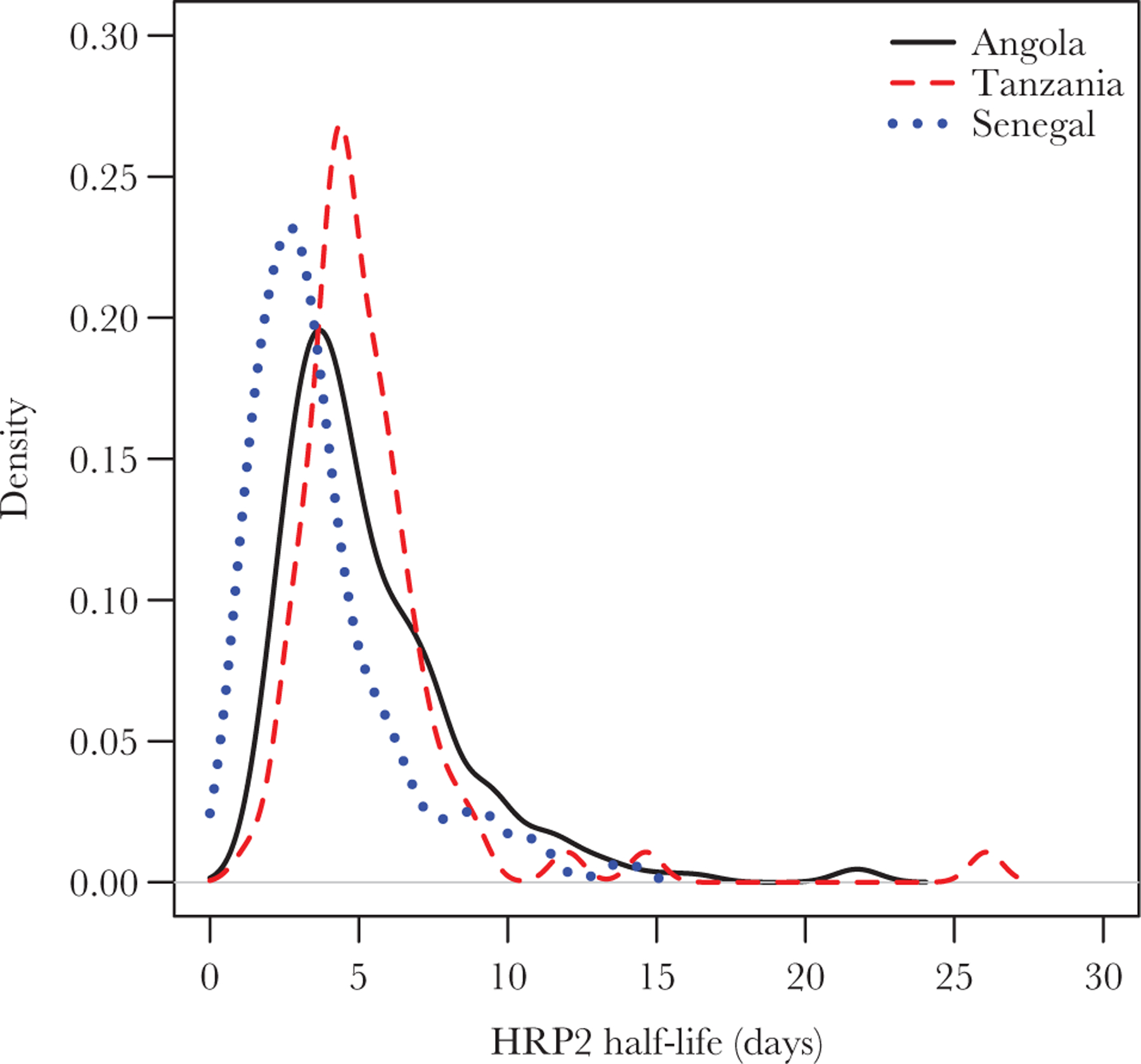
Probability density function of estimated histidine-rich protein 2 (HRP2) half-lives in Plasmodium falciparum–infected patients who were followed longitudinally after antimalarial therapy in Angola, Tanzania, and Senegal.
If a detection limit of 1000 pg/mL for a diagnostic test was assumed, the duration of HRP2 persistence was modeled to be 40 days (IQR, 29–59 days) in Angola, 37 days (IQR, 31–50 days) in Tanzania, and 24 days (IQR, 17–33 days) in Senegal. The relationship between the duration of HRP2 positivity and the log of the limit of detection was linear in all 3 countries (Figure 3). If the lower limit of detection of 1 pg/mL provided by the bead assay [9] was assumed, HRP2 antigen in samples would be predicted to persist up to 86 days (IQR, 64–124 days) in Angola, 82 days (IQR, 72–106 days) in Tanzania, and 60 days (IQR, 40–82 days) in Senegal.
Figure 3.

Modeled posttreatment duration of test positivity as a function of the limit of detection of a histidine-rich protein 2 (HRP2) detection test, estimated from HRP2 concentrations measured in patients who were followed longitudinally in Angola, Tanzania, and Senegal. IQR, interquartile range.
Treatment failure in Angola was associated with a sharp increase in the HRP2 concentration on the day the recurrent infection was detected through microscopy (Figure 4A). Before the day of treatment failure, the shape of the decay curve was similar for cases of ACPR and reinfections (Figure 4B). In contrast, for patients who eventually experienced recrudescence, the median HRP2 concentration on day 3 was higher than on day 0, and the median HRP2 concentration in cases of recrudescence was higher than in cases of treatment success and cases of reinfection for all time points, even before treatment failure was observed at a follow-up visit. The normalized HRP2 concentration on day 3 was predictive of treatment outcome, with an area under the ROC curve of 0.859 (95% CI, .731–.988). A day 3 HRP2 concentration that was >96% of the day 0 HRP2 concentration was 90% sensitive and 74% specific in predicting future recrudescence (Figure 5). On day 7, an HRP2 concentration that was >80% of the day 0 HRP2 concentration was 74% sensitive and 72% specific in predicting recrudescence, with an area under the ROC curve of 0.767 (95% CI, .649–.884).
Figure 4.
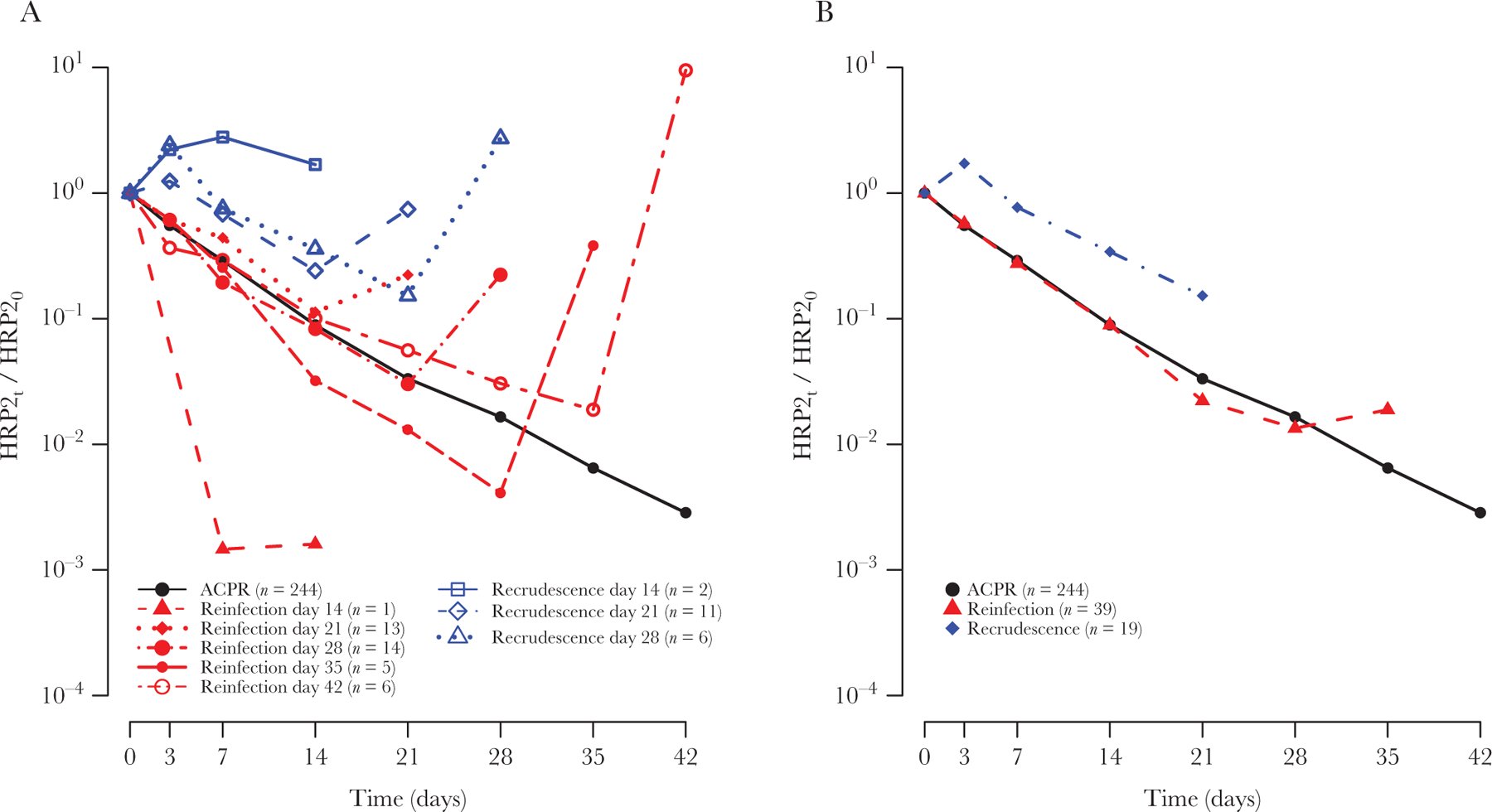
A, Median normalized histidine-rich protein 2 (HRP2) concentrations over time since initiation of antimalarial treatment at day 0, stratified by treatment outcome in patients from Angola, showing a spike in the HRP2 concentration on the day of treatment failure. B, After exclusion of day of treatment failure data, cases of adequate clinical and parasitological response (ACPR) and reinfection followed a similar linear decline, but cases of recrudescence showed a different clearance behavior.
Figure 5.
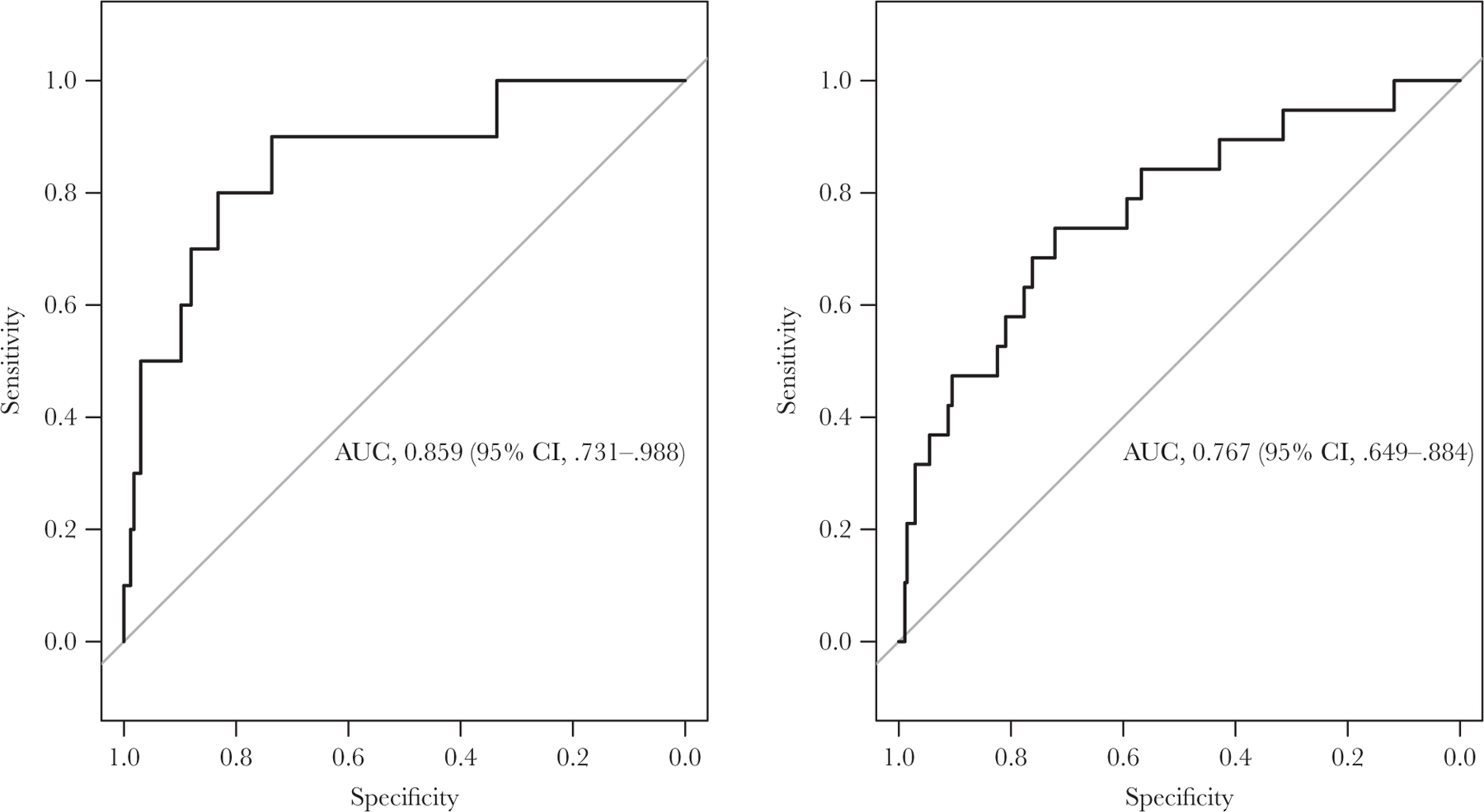
Areas under the receiver operating characteristic curve (AUCs) for predicting recrudescent infections on the basis of normalized histidine-rich protein 2 (HRP2) concentrations on day 3 (left; n = 177) and day 7 (right; n = 293) after initiation of antimalarial treatment among patients in Angola. CI, confidence interval.
DISCUSSION
The consistency in exponential clearance of HRP2 in 537 individuals across 3 regions of sub-Saharan Africa suggests a common underlying biological mechanism governing clearance of HRP2 following resolution of P. falciparum infection. While there was substantial variation in HRP2 concentrations at enrollment (ie, on day 0), with the IQR spanning 2 logs in each study population (reflecting a wide range of parasite densities at enrollment), the HRP2 clearance rate was remarkably stable, with the IQRs for HRP2 half-lives spanning only 2–3 days in the 3 countries. Moreover, there was significant similarity across patients of different ages in the 3 countries, with the distribution of half-lives largely overlapping. However, despite a narrow distribution of half-lives and a low level of interpatient variation overall, there were several notable outliers. For example, one individual in Tanzania exhibited a consistently slow HRP2 clearance rate, corresponding to a half-life of 26 days. With an initial HRP2 concentration of 204 000 pg/mL, RDT results for this individual would be expected to still be positive 200 days after treatment, assuming a conservative limit of detection of 1000 pg/mL.
Overall, the duration of HRP2 positivity was found to be more variable than the clearance rate, with the IQR for the time to reach an HRP2 concentration of 1000 pg/mL spanning 30 days in Angola, 19 days in Tanzania, and 16 days in Senegal. The duration of HRP2 positivity is a function of both the decay rate and the initial HRP2 concentration, which could vary because of a variety of factors, including parasite density, duration of infection, and parasite-specific expression of HRP2. Nevertheless, a corollary of first-order clearance kinetics is that the duration of HRP2 positivity will vary linearly with the log of the limit of detection for an HRP2 test. For example, the results show that a test that is 10 times more sensitive (eg, 100 pg/mL vs 1000 pg/mL) would be expected to increase the median duration of posttreatment RDT positivity by only 14 days in Angola, 16 days in Tanzania, and 13 days in Senegal. This observation is particularly important for the interpretation of results from highly sensitive HRP2 detection tests, whose introduction is the topic of recent debate [14].
While small, some differences were observed across the 3 countries, particularly between Senegal and the other 2 countries. The preponderance of older children and adults in the Senegal data set and the consequent partial immunity of the participants could explain the differences in clearance rates between the Senegal sites and the 2 other countries. Further factors could also account for the variation, including differences in sample collection materials and procedures, such as filter paper brand, storage time, and storage conditions. Alternately, this could reflect true differences in HRP2 clearance dynamics in the countries, resulting from host genetics, host immunity, the expression or size of the HRP2 gene [15], or the relative efficacy of the antimalarials. In addition, anti-HRP2 antibodies can cross-react with HRP3 antigens because of antigenic similarity between the proteins, a further potential confounding factor.
When juxtaposed against the relative consistency of HRP2 dynamics across the countries, the contrasting HRP2 levels in patients with different treatment outcomes is striking. Cases of recrudescence had remarkably different patterns in HRP2 levels across time, especially in the first 3 days, a likely consequence of incomplete parasite killing by the antimalarial regimen and continued production of HRP2 by surviving parasites. In fact, rather than decreasing exponentially, on average HRP2 levels increased from day 0 to day 3 in cases of recrudescence. Overall, treated infections that later recrudesced (ie, cases in which parasites never cleared) were characterized by HRP2 levels that were 1.9-fold higher on day 3 than levels in successfully treated infections. The difference between the dynamics of HRP2 in cases of ACPR or reinfection and cases of recrudescence is such that early changes in the HRP2 concentration could potentially be used as a tool for monitoring the response to treatment.
Currently available methods for monitoring the response to treatment, microscopy and RDT, are hampered by poor resolution. The high parasite killing rate of artemisinins means that, in most settings, the vast majority of patients will be microscopy negative by day 3, which was the case for 18 of 19 recrudescent infections from Angola analyzed here. Conversely, the sensitivity of antigen-detection tests and the binary nature of their results mean that RDT results for most patients remain positive for at least 3 weeks after treatment, independent of treatment outcome [4]. Similarly, the presence of gametocytes and lingering parasite DNA means that many patients will continue to have positive PCR results for days to weeks after successful clearance of asexual blood-stage parasites [16, 17]. In contrast, the ability of the bead-based HRP2 assay to accurately quantify HRP2 concentrations ranging from picograms/milliliter to micrograms/milliliter provides sufficiently high resolution to separate patients with adequate clearance of parasites from those with incomplete clearance.
The ability to predict long-term treatment outcome weeks before the recurrence of clinical symptoms and microscopy-detectable parasitemia would have myriad uses. With refinement of the technology, comparison of day 3 or day 7 antigen levels to the pretreatment level could provide sufficient information to determine the potential for drug failure and could be a viable and cost-saving alternative to current methods of therapeutic efficacy monitoring that necessitate following patients for up to 28 or 42 days to detect late treatment failures. In the clinical setting, management of malaria cases would benefit from a reliable test for cure only a few days after initiation of treatment. Inclusion of antigens in addition to HRP2 should improve sensitivity and specificity and make the technique more robust in the face of increasing reports of HRP2/HRP3 deletions [18–20]. The results reported here are from areas with full artemisinin susceptibility and rapid and complete clearance of parasites shortly after treatment. HRP2 dynamics in areas with higher rates of antimalarial resistance would be confounded by the continued presence of a substantial population of residual parasites still producing HRP2. Consequently, the test should also be validated in other settings, particularly where parasites resistant to artemisinins and partner drugs have been confirmed.
Supplementary Material
Financial support.
This work was supported by the US President’s Malaria Initiative.
Footnotes
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Publisher's Disclaimer: Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization (WHO). Methods for surveillance of antimalarial drug efficacy. Geneva: WHO, 2009. [Google Scholar]
- 2.World Health Organization (WHO). World malaria report 2015. Geneva: WHO, 2015. [Google Scholar]
- 3.World Health Organization (WHO). Guidelines for the treatment of malaria. 2nd ed. Geneva: WHO, 2010. [Google Scholar]
- 4.Aydin-Schmidt B, Mubi M, Morris U, et al. Usefulness of Plasmodium falciparum-specific rapid diagnostic tests for assessment of parasite clearance and detection of recurrent infections after artemisinin-based combination therapy. Malar J 2013; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinha I, Ekapirat N, Dondorp AM, Woodrow CJ. Use of a rapid test to assess plasma Plasmodium falciparum HRP2 and guide management of severe febrile illness. Malar J 2015; 14:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imwong M, Woodrow CJ, Hendriksen IC, et al. Plasma concentration of parasite DNA as a measure of disease severity in falciparum malaria. J Infect Dis 2015; 211:1128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ndour PA, Larréché S, Mouri O, et al. Measuring the Plasmodium falciparum HRP2 protein in blood from artesunate-treated malaria patients predicts post-artesunate delayed hemolysis. Sci Transl Med 2017; 9:eaaf9377. [DOI] [PubMed] [Google Scholar]
- 8.Dondorp AM, Desakorn V, Pongtavornpinyo W, et al. Estimation of the total parasite biomass in acute falciparum malaria from plasma PfHRP2. PLoS Med 2005; 2:e204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogier E, Plucinski M, Lucchi N, et al. Bead-based immuno-assay allows sub-picogram detection of histidine-rich protein 2 from Plasmodium falciparum and estimates reliability of malaria rapid diagnostic tests. PLoS One 2017; 12:e0172139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plucinski MM, Talundzic E, Morton L, et al. Efficacy of artemether-lumefantrine and dihydroartemisinin-piperaquine for treatment of uncomplicated malaria in children in Zaire and Uíge provinces, Angola. Antimicrob Agents Chemother 2015; 59:437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plucinski MM, Dimbu PR, Macaia AP, et al. Efficacy of artemether-lumefantrine, artesunate-amodiaquine, and dihydroartemisinin-piperaquine for treatment of uncomplicated Plasmodium falciparum malaria in Angola, 2015. Malar J 2017; 16:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowland M, Tozer TN. Clinical pharmacokinetics/pharmacodynamics. Lippincott Williams and Wilkins Philadelphia, 2005. [Google Scholar]
- 13.Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 2011; 12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plucinski MM, Rogier E, Dimbu PR, Fortes F, Halsey ES, Aidoo M. Estimating the added utility of highly sensitive histidine-rich protein 2 detection in outpatient clinics in Sub-Saharan Africa. Am J Trop Med Hyg 2017; 97:1159–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker J, Ho MF, Pelecanos A, et al. Global sequence variation in the histidine-rich proteins 2 and 3 of Plasmodium falciparum: implications for the performance of malaria rapid diagnostic tests. Malar J 2010; 9:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarra W, Snounou G. Only viable parasites are detected by PCR following clearance of rodent malarial infections by drug treatment or immune responses. Infect Immun 1998; 66:3783–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang H-H, Meibalan E, Zelin J, et al. Persistence of Plasmodium falciparum parasitemia after artemisinin combination therapy: evidence from a randomized trial in Uganda. Sci Rep 2016; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng Q, Gatton ML, Barnwell J, et al. Plasmodium falciparum parasites lacking histidine-rich protein 2 and 3: a review and recommendations for accurate reporting. Malar J 2014; 13:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization (WHO). False-negative RDT results and implications of new reports of P. falciparum histidine-rich protein. Geneva: WHO, 2016. [Google Scholar]
- 20.Kozycki CT, Umulisa N, Rulisa S, et al. False-negative malaria rapid diagnostic tests in Rwanda: impact of Plasmodium falciparum isolates lacking hrp2 and declining malaria transmission. Malar J 2017; 16:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


