Abstract
Apoptosis is a central host defense mechanism to eliminate virus-infected cells. Activation of NF-κB suppresses apoptosis following some types of stimulation in vitro. To test the physiological importance of this pathway in vivo, we studied murine encephalomyocarditis virus (EMCV) infection in mice and cell lines defective in NF-κB1 (p50) signaling. As previously reported, we find that all p50 knockout (p50 −/−) mice survive an EMCV infection that readily kills normal mice. By introducing the p50 mutation into interferon (IFN) type I receptor knockout (IFNRI −/−) mice, we find that this resistance is not mediated by IFN-β as previously thought. While no IFNRI −/− mice survive, the double-knockout mice survive 60% of the time. The survival is tightly linked to the animals’ ability to clear the virus from the heart in vivo. Using murine embryonic fibroblasts (MEF) derived from wild-type, p50 −/−, and p65 −/− embryos, we found that NF-κB is not required for the replication cycle of EMCV. However, during these experiments we observed that p50 −/− and p65 −/− MEF infected with EMCV undergo enhanced, premature cytotoxicity. Upon examination of this cell death, we found that EMCV infection induced both plasma membrane and nuclear changes typical of apoptosis in all cell lines. These apoptotic processes occurred in an accelerated and pronounced way in the NF-κB-defective cells, as soon as 6 h after infection, when virus is beginning to be released. Previously, only the RelA (p65) subunit of NF-κB has been shown to play a role in suppressing apoptosis. In our studies, we find that p50 is equally important in suppressing apoptosis during EMCV infection. Additionally, we show that suppression of apoptosis by NF-κB1 is required for EMCV virulence in vivo. The attenuation in p50 −/− mice can be explained by rapid apoptosis of infected cells which allows host phagocytes to clear infected cells before the viral burst leading to a reduction of the viral burden and survival of the mice.
Programmed cell death, or apoptosis, is a fundamental biochemical process that plays an essential role in normal development and tissue homeostasis (15, 34). Apoptosis is also utilized by the host to defend against invading microbes. In particular, the relationship between viruses and apoptosis has been well established, and the subject has been reviewed (37, 44, 46). Viruses can trigger apoptosis through recognition by cytotoxic T cells, by viral disruption of cellular metabolism and cell cycle regulation, and by induction of proinflammatory cytokines such as tumor necrosis factor (TNF) (44). Perhaps the best evidence demonstrating the importance of apoptosis in controlling viral replication are the many different strategies that viruses have evolved to avoid host programmed cell death. Virus-encoded antiapoptotic proteins include Bcl-2 homologs, like adenovirus E1B, that indirectly inhibit activation of caspases (2, 9, 10, 14, 35), cowpox virus CrmA and baculovirus p35, which are direct inhibitors of the caspases (13, 36, 55), and a new class of proteins called viral FLICE-inhibitory proteins (v-FLIPs) encoded by gammaherpesviruses and poxvirus (8, 47). Infection of cells with mutant viruses lacking these genes often results in premature apoptotic death and altered yields of progeny virus, indicating that antiapoptotic proteins are necessary for efficient virus replication (11, 23, 33). In addition, suppression of apoptotic cell death is important for viral persistence (26) and may be required for the establishment of certain latent infections (22). Therefore, a better understanding of the signaling pathways important for apoptosis of virally infected cells will provide further insight into virus replication and persistence and facilitate development of novel drug targets.
NF-κB is a transcription factor central to immune and inflammatory responses as well as viral replication (3, 4, 49, 52). Viral infection or cell stimulation by proinflammatory cytokines like TNF-α allow rapid nuclear translocation of NF-κB through degradation of IκB inhibitory cytoplasmic retention proteins. A dominant negative IκBα that lacks both constitutive (5, 42) and inducible (12, 49) phosphorylation sites is capable of eliminating NF-κB activity in transfected cells and rendering the cells highly susceptible to TNF-α-induced apoptosis (50). Similar results were obtained by stimulating p65 −/− mouse embryo fibroblasts (MEF) with TNF-α (6). These experiments demonstrate that activation of NF-κB inhibits some forms of apoptosis. In its absence, the cell dies. Less is known regarding the role of p50 in NF-κB-mediated inhibition of apoptosis. At present, the importance of the NF-κB signaling pathway in vivo is still in question.
In their initial reporting of mice with targeted disruption of the NF-κB1 subunit, p50 (p50 −/− mice), Sha et al. discovered that while these mice are more susceptible to certain bacterial pathogens, they are resistant to murine encephalomyocarditis virus (EMCV) infections; which cause myocarditis and dilated cardiomyopathy and which kill normal healthy mice (43, 53). They suggested that the resistance may be caused by elevated beta interferon (IFN-β) levels in the mice. While IFN-β signaling is clearly important for control of picornavirus replication, another possibility to explain the inhibition of virulence following EMCV infection of p50 −/− mice is that p50 −/− cells undergo premature apoptosis during viral infection. Recent data suggests that under certain conditions and in certain cell types infection with picornaviruses can activate markers of apoptosis (25, 48). Since NF-κB is activated with picornavirus infection (56), we hypothesized that the attenuation of EMCV virulence in p50 −/− mice is related to early induction of markers of apoptosis and cell death from the lack of NF-κB signaling and can therefore occur independent of type I IFN (IFN-α/β) signaling. Premature activation of apoptosis in infected cells in vivo may limit viral replication through phagocytosis of infected apoptotic cells (19, 21, 31).
Accordingly, we present data showing that loss of p50 signaling during picornavirus infection can attenuate the virulence of EMCV infection in vivo during the acute phase of viral replication through an IFN-α/β-independent mechanism. This was done by showing that mice deficient in type I IFN receptor (IFNRI) signaling (IFNRI −/− mice) are extremely sensitive to EMCV infection, while p50 −/− IFNRI −/− double-knockout mice exhibit a significant resistance to EMCV infection. Additionally, we show that p50 is not required for viral replication and that EMCV infection of cells that lack p50 results in accelerated cell death with activation of markers of apoptosis. This finding suggests that NF-κB signaling is required for inhibition of apoptosis and of premature cell death and that these mechanisms are required for unrestricted viral replication in vivo and for EMCV virulence.
MATERIALS AND METHODS
Cell and cell culture.
The cells used in these studies were L929 cells (24) (a kind gift from S. A. Huber, University of Vermont, Burlington) and primary S129 wild-type, p50 −/−, and p65 −/− MEF (7, 43) (kind gifts from D. Baltimore). The cells were cultured in Dulbecco’s modified Eagle medium with high glucose (4,500 mg/liter) supplemented with 10% heat-inactivated fetal bovine serum, 1 mM sodium pyruvate, 2 mM l-glutamine, streptomycin (100 mg/ml), and penicillin G (100 U/ml) (Irvine Scientific, Santa Ana, Calif.) at 37°C and 5% CO2 under humidified conditions. Cells were routinely subcultured every 2 to 3 days.
Virus and viral assays.
EMCV was isolated from murine heart and was a kind gift from S. A. Huber (24). Infected murine hearts were aseptically harvested and homogenized in high-glucose Dulbecco’s modified Eagle medium supplemented with 2% heat-inactivated fetal bovine serum; after three cycles of freeze-thawing for release of intracellular virus, the supernatant was used for plaque-forming assay, with titers determined on L929 cells (24). For in vitro studies, EMCV was passaged through L929 cells and MEF were infected at a multiplicity of infection of 5. After 30 min of incubation and three washing steps to remove residual virus, new medium was added to the cells; at the time points indicated, supernatants were assayed for viral progeny.
Mice.
Wild-type S129, IFNRI −/− (30), and p50 −/− (43) mice were maintained in a germ-free environment and housed in microisolators during the challenges. Double-knockout mice lacking both the IFNRI and the p50 subunit of NF-κB (p50 −/− IFNRI −/− mice) were generated, and the genotype of the offspring was verified by PCR. At 4 to 6 weeks of age, mice were challenged with an intraperitoneal (i.p.) injection of EMCV (103 PFU) as previously described (41, 43). These mice were monitored daily for the survival study or sacrificed 2 days later for the measurements of viral titers.
Annexin V assay.
At various time points after infection, cells were harvested by trypsinization, pelleted, resuspended in Annexin V buffer (29), washed, and then resuspended in Annexin V buffer containing 1 μg of Annexin V-fluorescein isothiocyanate (Bender Med Systems, Vienna, Austria) per ml. After 30 min of incubation at room temperature, the cells were immediately analyzed with FACScan (Becton Dickinson, San Jose, Calif.) and LYSIS II software.
Assays for DNA fragmentation.
To detect host cell DNA degradation, total cellular DNA was collected by using a Puregene DNA isolation kit (Gentra Systems, Minneapolis, Minn.), and 3 μg of DNA per lane was run on a 1.5% agarose gel. The gel was stained with ethidium bromide and photographed. To detect DNA strand breaks in situ, cells were grown on Lab-Tek two-chamber slides (Nalgene, Naperville, Ill.), and the TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling) assay was performed by using an Apotag Plus kit (Oncor, Gaithersburg, Md.), with the following modifications (32): prior to the terminal deoxytidyltransferase reaction, endogenous alkaline phosphatase was quenched with levamisole (Vector Laboratories, Burlingame, Calif.), and the detection system was changed to alkaline phosphatase, using anti-digoxigenin-alkaline phosphatase Fab fragments (Boehringer Mannheim, Indianapolis, Ind.) diluted 1:500 in phosphate-buffered saline containing 3% bovine serum albumin (Sigma) and Vector Red (Vector) as a substrate.
Microscopy and immunocytochemistry.
EMCV-infected cells were identified with murine monoclonal antibody 10D3 (18) at a 1:400 dilution in phosphate-buffered saline–3% bovine serum albumin (a kind gift from Ann C. Palmenberg, University of Wisconsin, Madison). Antibody 10D3 was originally raised against mengovirus 3D polymerase but exhibits strong cross-reactivity against the 3D polymerase of EMCV (18). Bound antibody was detected with polyclonal fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin G at a 1:100 dilution (Jackson Immunoresearch Laboratories, West Grove, Pa.). Nuclear morphology was visualized with Hoechst 33342 dye (Sigma) at 1 μg/ml for 2 min. Finally, slides were mounted with Vectashield mounting medium (Vector) and imaged in an MRC 1024 confocal microscope (Bio-Rad, Hercules, Calif.) equipped with Lasersharp software.
RESULTS
Deficiency of the p50 subunit of NF-κB in vivo can limit the early phase of EMCV replication and confer resistance by an IFNRI-independent signaling mechanism.
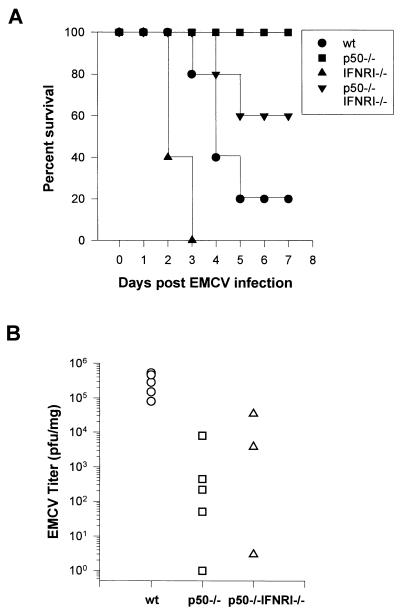
A previous report demonstrates that p50 −/− mice are highly resistant to EMCV infection (43). The precise mechanism for this resistance to infection has not been thoroughly established. To determine in vivo whether the attenuated virulence in EMCV-infected mice might be secondary to more rapid induction of apoptosis during the early phase of viral replication, through an IFNRI-independent mechanism, we bred p50 −/− mice with IFNRI −/− mice. The resulting double-knockout animals were infected with EMCV, and the mortality data from these experiments are shown in Fig. 1A. As expected, the p50 −/− mice had no significant mortality upon an i.p. injection with 103 PFU of EMCV, while the IFNRI −/− mice had 100% mortality by day 3 postinfection. Surprisingly, the p50 −/− IFNRI −/− double-knockout mice survived 60% of the time, while the wild-type mice had only a 20% survival rate by day 5. Mice heterozygous for the p50 knockout and homozygous for the IFNRI knockout (p50 +/− IFNRI −/− mice) had an intermediate mortality (data not shown), demonstrating a gene dose effect.
FIG. 1.
Survival curves and viral titers in the hearts of mice infected with EMCV. (A) Wild-type (wt), p50 −/−, IFNRI −/−, and double-knockout (p50 −/− IFNRI −/−) mice, 4 to 6 weeks old, were challenged with an i.p. injection of 103 PFU of EMCV. Survival was monitored at daily intervals. Ten mice were infected in each group. (B) Viral titers in the hearts of mice at day 2 after infection with EMCV. Viral titers were determined as detailed in Materials and Methods. Titers are expressed as PFU per milligram of cardiac tissue. Data points from each heart are shown.
We measured viral titers in the hearts of mice 2 days postinfection to see if the resistance to EMCV infection in p50 −/− mice is related to the ability of the virus to replicate in vivo (Fig. 1B). This time point is prior to activation of a significant cellular immune response and is a measure of the initial phase of viral replication (57). Wild-type mice consistently had titers of about 105 to 106 PFU per mg of cardiac tissue. The p50 −/− mice could generate only significantly lower titers, with 2 to 3 orders of magnitude fewer PFU/milligram of cardiac tissue, and in some cases we were unable to recover any virus from the hearts of these animals. The p50 −/− IFNRI −/− mice had slightly higher titers than the p50 −/− animals, which were still significantly lower than those found in the wild-type mice.
From these data, we conclude that IFN-α and -β are important for protection against EMCV, but the attenuated virulence conferred by targeted disruption of p50 is independent of IFN-α/β signaling. These data also demonstrate that EMCV can replicate in p50 −/− mice; however, in these animals the virus is unable to reach titers that are associated with mortality.
NF-κB is not required for the replication cycle of EMCV.
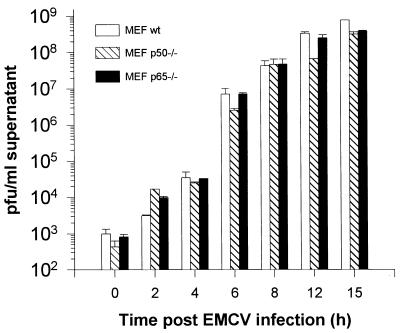
One potential explanation for the in vivo results for p50 −/− mice is that p50 is required for efficient EMCV replication. To test this possibility, we determined in vitro the kinetics of EMCV replication in infected MEF derived from wild-type and p50 −/− mice. Wild-type, p50 −/−, and p65 −/− cells were able to support productive viral replication to comparable levels (Fig. 2), as were cells expressing a dominant negative IκBα (data not shown). The viral burst occurred between 6 and 12 h postinfection. These results demonstrate that EMCV can establish a productive infection independent of NF-κB signaling.
FIG. 2.
Viral titers in vitro after EMCV infection. MEF derived from wild-type (wt), p50 −/−, and p65 −/− mice were infected with EMCV at a multiplicity of infection of 5. At the time points indicated, an aliquot of the supernatant was assayed for infectious viral progeny. Titers are expressed as PFU per milliliter of supernatant. The data represent the averages and standard errors of three independent experiments.
NF-κB-deficient cells die prematurely with EMCV infection.
Recently it has been shown that mammalian cells require p65 to induce an antiapoptotic response following TNF-α stimulation (6, 28, 50, 52). Therefore, we hypothesized that the altered virulence observed in p50 −/− mice might be related to the ability of the virus to induce cell death, thus limiting viral replication in vivo.
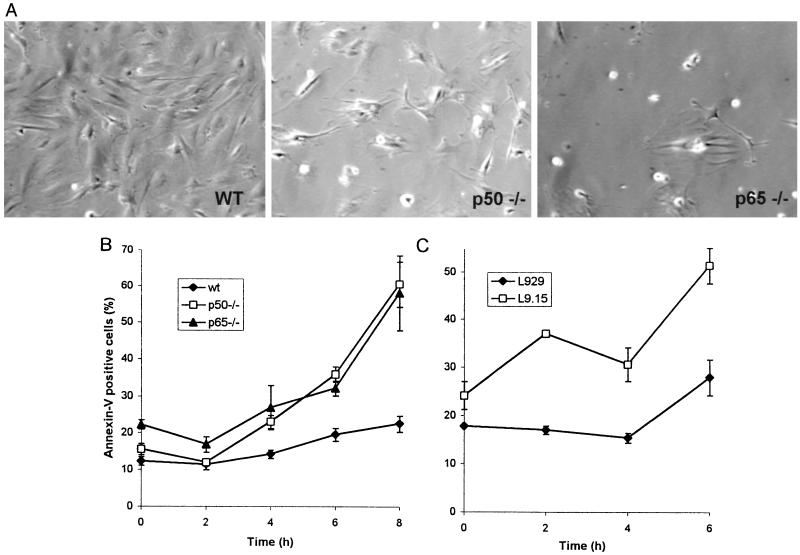
A close examination of the cells in culture at early time points during EMCV infection revealed that the cells that lacked p50 or p65 displayed a cytopathic effect with rounding and detachment from the plate well before the wild-type cells (Fig. 3A). To quantitate the rate of total cell death, flow cytometric evaluation of Annexin+ cells was performed. By 6 h postinfection, a significantly higher percentage of p50 −/− and p65 −/− MEF showed evidence of cell death (Fig. 3B), similar to results for cells expressing a dominant negative IκBα (Fig. 3C).
FIG. 3.
EMCV infection of cells deficient in NF-κB results in an enhanced cytopathic effect and in premature cell death. (A) MEF from wild-type, p50 −/−, and p65 −/− mice were plated at the same density, infected with EMCV, and observed under the microscope 8 h postinfection. Representative fields from wild-type (wt), p50 −/−, and p65 −/− cells are shown. The cells defective in NF-κB signaling round and detach from the plate earlier and at a higher rate than the wild-type cells. (B) Quantitation of this enhanced cytopathic effect, using Annexin V staining and flow cytometry. The lines show the average percentage and standard error of Annexin V-positive wild-type (wt), p50 −/−, and p65 −/− cells. (C) L929 cells with stable transfection of a dominant negative IκBα (L9.15) had a higher rate of cell death than untransfected L929 cells. Three independent experiments were performed, and duplicates of 10,000 cells each were analyzed for each experiment. The mutant cells show premature cell death with externalization of phosphatidylserine significantly earlier than the wild-type cells. (Data were analyzed with a two-way analysis of variance with repeated measures; P < 0.01 for the statistical effect of cell type on the percentage of Annexin V-positive cells versus time in panels B and C.)
EMCV infection induces markers of apoptosis in MEF.
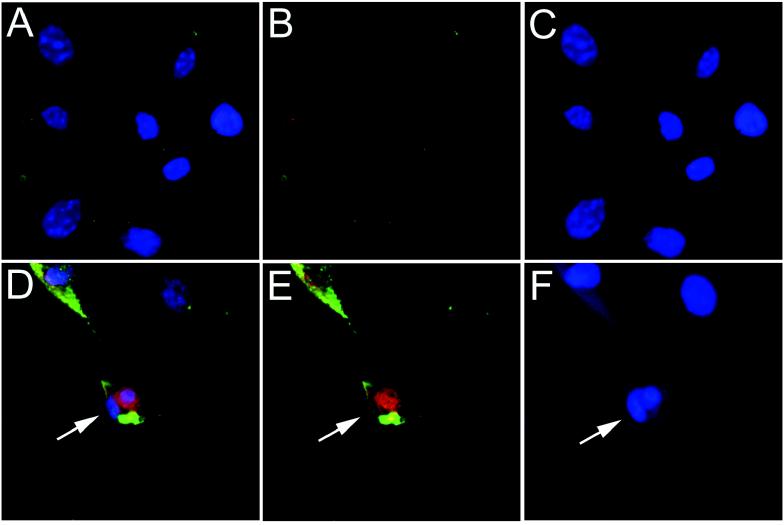
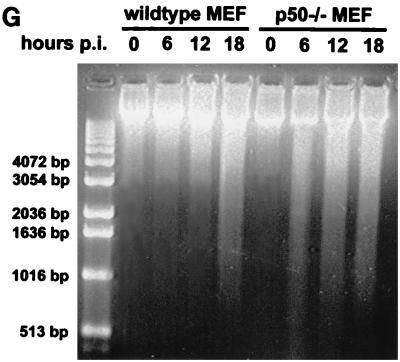
Since picornaviruses can activate both lytic and apoptotic features of cell death (25, 40, 48), we sought to demonstrate that EMCV infection could activate markers of apoptosis. To this end, we investigated nuclear hallmarks of apoptosis in EMCV-infected MEF. Morphologically, nuclei of infected cells from wild-type and p50 −/− MEF displayed nuclear shrinkage with chromatin condensation and margination as well as formation of apoptotic bodies with DNA strand breaks, as evidenced by in situ TUNEL staining 12 h postinfection (Fig. 4A to F). Quantitative flow cytometry of TUNEL-stained cells demonstrated that 70% of infected cells became TUNEL positive at 12 h postinfection, compared to less than 10% of uninfected cells (data not shown). Furthermore, EMCV infection led to degradation of host cell genomic DNA, as shown by gel electrophoresis (Fig. 4G). There was degradation of genomic DNA in EMCV-infected p50 −/− MEF before it was observed in infected wild-type MEF. Significant degradation of genomic DNA does not occur in wild-type MEF until 18 h postinfection. From these results, we conclude that EMCV infection of MEF induces an apoptotic phenotype with nuclear changes typical of apoptosis and DNA degradation that occurs earlier in cells that lack p50.
FIG. 4.
EMCV infection of MEF induces markers typical of apoptosis. (A to F) MEF were infected with EMCV and stained 12 h postinfection. The data shown are from wild-type, uninfected (A to C) and EMCV-infected (D to F) MEF. Cells were simultaneously stained for EMCV infection (green fluorescence), TUNEL stained for DNA strand breaks (red fluorescence), and stained with Hoechst 33342 dye (blue fluorescence) for nuclear morphology. Three-color composites (A and D), combined fluorescence for EMCV and TUNEL (B and E), and Hoechst 33342 only (C and F) are displayed. The marked cell (→) is EMCV infected, stains positive for DNA strand breaks, and shows formation of an apoptotic body. Similar results were obtained with p50 −/− MEF (data not shown). (G) Agarose gel electrophoresis of EMCV-infected MEF. At the time points indicated, DNA was isolated from the cells and run on a 1.5% agarose gel. Both wild-type and p50 −/− MEF show extensive breakdown of genomic DNA following EMCV infection. In p50 −/− cells, the DNA is fragmented before that observed in the wild-type cells (6 versus 18 h postinfection [p.i.]).
Collectively, our results argue that p50 is required to suppress or delay cell death and markers of apoptosis during EMCV infection. At 6 h postinfection, when infectious progeny virus are starting to be released, p50-deficient cells have already initiated a program of cell death whereas wild-type cells do not die until a later time point, allowing additional replication of the virus in vivo.
DISCUSSION
During infection, many viruses, including picornaviruses, induce a cytopathic effect ultimately leading to host cell death and release of viral progeny (40). In principal, an infected cell can die like any other cell, of apoptosis or lysis or a combination of these two pathways (16). Picornavirus infection often results in lysis of the host cell (40, 48). However, under nonpermissive conditions (i.e., infection with a guanidine-sensitive virus variant in the presence of guanidine), poliovirus has been reported to induce apoptosis of the host cell (48), and recently coxsackievirus B3 infection of cultured cardiac myocytes has been shown to induce markers of apoptosis (25). However, little is known regarding the role of apoptotic mechanisms during infection with picornaviruses. Our data indicate that NF-κB signaling is required to prevent premature death of infected cells and is required for in vivo virulence by an IFN-α/β-independent mechanism.
Given the important antiviral properties of IFN-α/β we anticipated that mice that lacked the IFNRI would have early and consistent mortality by days 2 to 3 after EMCV infection, a time point that precedes activation of the cellular immune response (39, 57). Based on the fact that loss of NF-κB leads to accelerated apoptosis (6, 7, 50), we hypothesized that if disruption of p50 increased the rate of apoptotic cell death in infected mice that lacked the IFNRI, there would be an improvement in survival of the infected mice in spite of loss of IFNRI signaling. If such were the case, EMCV titers would be lower in infected mice during the early phase of viral replication. Our results are consistent with this hypothesis, demonstrating a substantial improvement in survival in IFNRI −/− mice that also lacked p50 and showing that survival from the EMCV challenge was linked to the animals’ ability to limit viral replication.
To identify the mechanism(s) responsible for these in vivo findings, we studied p50-deficient MEF in culture. EMCV is able to establish a productive viral infection in MEF lacking the p50 subunit of NF-κB that is similar to that which occurs in wild-type MEF, indicating that the p50 subunit of NF-κB is not required for EMCV infectivity and replication.
In support of the hypothesis that EMCV infection of p50-deficient cells leads to earlier cell death, we noted that p50 −/− MEF had an accelerated cytopathic effect with premature cell death compared to the wild-type cells. This was apparent at the microscopic level, with detachment of cells from the plate, and was quantitated by flow cytometric analysis of Annexin+ cells. Since death of virally infected cells can be mediated by lysis and/or apoptosis, and since cells which are initially apoptotic later progress to have disrupted cell membranes, we quantitated the rate of total cell death by using Annexin V. Annexin+ cells include cells in the early stages of apoptosis with externalized phosphatidylserine (29) and cells that are at a late stage in the apoptotic process with disrupted cell membranes or have lysed secondary to viral replication. Most importantly, this assay has been demonstrated to reliably predict a cell’s susceptibility to phagocytosis in vivo (21).
In addition to the premature cell death that was observed in p50 −/− MEF, it was clear that infected cells also had an increase of both membrane and nuclear alterations consistent with apoptosis. The cell death and induction of apoptosis in EMCV-infected p50 −/− mice are very similar to that observed with mutant adenoviruses lacking the E1B 19-kDa protein. This protein is a homolog of the cellular proto-oncoprotein Bcl-2 and suppresses apoptosis caused by the adenovirus E1A protein (35). Infections with viruses carrying the E1B 19-kDa protein deletion lead to a phenotype characterized by premature and enhanced cytopathic effect as well as degradation of host cell genomic DNA (33). The DNA degradation upon adenovirus infection can be observed as either a smear or a ladder of chromosomal DNA, depending on multiple parameters such as the multiplicity of infection, the host cell type used, and the time course of the infection (54). Subsequent experiments with adenovirus and adenovirus gene products have shown that both forms of DNA degradation are due to apoptosis (35). It is also known that in many model systems of apoptosis, oligonucleosomal DNA fragments may be apparent only after more than 24 h following initiation of apoptosis (1). Taken together, our results show that EMCV-infected MEF exhibit nuclear changes typical of apoptosis, such as chromatin alterations, DNA strand breaks, and degradation of the genomic DNA. However, the cells do not reach the later stages of apoptosis because of the simultaneous viral replication that lyses the cells. While previous experiments have clearly demonstrated that p65 is required to suppress apoptosis following stimulation with TNF-α, this is the first example of a stimulus that can induce accelerated cell death in p50-deficient cells.
The data for cultured MEF make a compelling argument that the protective effect in the p50 −/− mice is secondary to accelerated cell death with activation of markers of apoptosis. Inhibition of apoptosis is a well-recognized strategy by which viruses can evade one component of the host defense system. The difference in the kinetics of cell death and activation of apoptotic markers following infection may be very relevant to viral production in vivo. It is known that apoptotic cells in vivo are cleared by phagocytic cells in a way that toxic cellular components (i.e., virus) are not released (19, 31). Reports in the literature have described at least five separate membrane changes that may lead to recognition of apoptotic cells by phagocytes (29). Of particular relevance to this study are the convincing data for the involvement of phosphatidylserine in the recognition of apoptotic cells by macrophages (20, 21). Thus, premature apoptosis with externalization of phosphatidylserine in p50-deficient cells may allow clearance of infected cells by host phagocytes before the virus is able to complete its full replication cycle. In this way, apoptosis acts as a defense mechanism used by the host to limit viral spread and to allow survival of the mice upon picornavirus infection.
In summary, EMCV infection of MEF induces a cytopathic effect that displays features of both apoptotic and lytic cell death. Thus, a picornavirus infection can induce markers of apoptosis in the host cell. The observed apoptotic events included plasma membrane and nuclear changes characteristic of apoptosis. Cell death following picornavirus infection is accelerated in cells lacking the p50 subunit of NF-κB. A likely explanation for our findings in vivo and in vitro is that NF-κB is required for the expression of yet unknown antiapoptotic survival genes during EMCV infection. This may explain the resistance to EMCV in the p50 −/− mice that occurs during early phases of viral replication independent of IFNRI signaling. It will be of great interest if future studies can provide us with a method to capitalize on this mechanism as novel drug targets for the treatment of viral heart disease.
ACKNOWLEDGMENTS
Edward M. Schwarz and Cornel Badorff contributed equally to this project.
We thank D. Baltimore, S. A. Huber, and A. C. Palmenberg for the kind provision of reagents used in this study. We are indebted to D. Young and D. Peterson for assistance with flow cytometry and confocal microscopy, respectively.
This work was supported by grants from the Arthritis Foundation to E.M.S., grant Ba 1668/1-1 from the Deutsche Forschungsgemeinschaft to C.B., and grants from the American Heart Association (96-303A) and UCSD Biotechnology Star Project (S96-38) to K.U.K. I.M.V. is an American Cancer Society Professor of Molecular Biology and receives funding from an OIG award from the National Institutes of Health.
REFERENCES
- 1.Ankarcrona M, Dypbukt J M, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton S A, Nicotera P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–973. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong R C, Aja T, Xiang J, Gaur S, Krebs J F, Hoang K, Bai X, Korsmeyer S J, Karanewsky D S, Fritz L C, Tomaselli K J. Fas-induced activation of the cell death-related protease CPP32 Is Inhibited by Bcl-2 and by ICE family protease inhibitors. J Biol Chem. 1996;271:16850–16855. doi: 10.1074/jbc.271.28.16850. [DOI] [PubMed] [Google Scholar]
- 3.Baeuerle P A, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin A S., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 5.Barroga C F, Stevenson J K, Schwarz E M, Verma I M. Constitutive phosphorylation of I kappa B alpha by casein kinase II. Proc Natl Acad Sci USA. 1995;92:7637–7641. doi: 10.1073/pnas.92.17.7637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beg A A, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 7.Beg A A, Sha W C, Bronson R T, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 8.Bertin J, Armstrong R C, Ottilie S, Martin D A, Wang Y, Banks S, Wang G H, Senkevich T G, Alnemri E S, Moss B, Lenardo M J, Tomaselli K J, Cohen J I. Death effector domain-containing herpesvirus and poxvirus proteins inhibit both Fas- and TNFR1-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:1172–1176. doi: 10.1073/pnas.94.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boulakia C A, Chen G, Ng F W, Teodoro J G, Branton P E, Nicholson D W, Poirier G G, Shore G C. Bcl-2 and adenovirus E1B 19 kDa protein prevent E1A-induced processing of CPP32 and cleavage of poly(ADP-ribose)-polymerase. Oncogene. 1996;12:529–535. [PubMed] [Google Scholar]
- 10.Boyd J M, Malstrom S, Subramanian T, Venkatesh L K, Schaeper U, Elangovan B, D’Sa-Eipper C, Chinnadurai G. Adenovirus E1B 19 kDa and Bcl-2 proteins interact with a common set of cellular proteins. Cell. 1994;79:341–351. doi: 10.1016/0092-8674(94)90202-x. [DOI] [PubMed] [Google Scholar]
- 11.Brooks M A, Ali A N, Turner P C, Moyer R W. A rabbitpox virus serpin gene controls host range by inhibiting apoptosis in restrictive cells. J Virol. 1995;69:7688–7698. doi: 10.1128/jvi.69.12.7688-7698.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267:1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 13.Bump N J, Hackett M, Hugunin M, Seshagiri S, Brady K, Chen P, Ferenz C, Franklin S, Ghayur T, Li P, et al. Inhibition of ICE family proteases by baculovirus antiapoptotic protein p35. Science. 1995;269:1885–1888. doi: 10.1126/science.7569933. [DOI] [PubMed] [Google Scholar]
- 14.Chinnaiyan A M, Orth K, Duan O R K H, Poirier G G, Dixit V M. Molecular ordering of the cell death pathway. Bcl-2 and Bcl-xL function upstream of the CED-3-like apoptotic proteases. J Biol Chem. 1996;271:4573–4576. doi: 10.1074/jbc.271.9.4573. [DOI] [PubMed] [Google Scholar]
- 15.Cohen J J, Duke R C, Fadok V A, Sellins K S. Apoptosis and programmed cell death in immunity. Annu Rev Immunol. 1992;10:267–293. doi: 10.1146/annurev.iy.10.040192.001411. [DOI] [PubMed] [Google Scholar]
- 16.Darzynkiewicz Z, Juan G, Li X, Gorczyca W, Murakami T, Traganos F. Cytometry in cell necrobiology: analysis of apoptosis and accidental cell death (necrosis) Cytometry. 1997;27:1–20. [PubMed] [Google Scholar]
- 17.DiDonato J A, Hayakawa M, Rothwarf D M, Zandi E, Karin M. A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 18.Duque H, Palmenberg A C. Epitope mapping of monoclonal antibodies raised to recombinant mengo 3D polymerase. Virus Genes. 1996;13:159–168. doi: 10.1007/BF00568908. [DOI] [PubMed] [Google Scholar]
- 19.Duvall E, Wyllie A H, Morris R G. Macrophage recognition of cells undergoing programmed cell death (apoptosis) Immunology. 1985;56:351–358. [PMC free article] [PubMed] [Google Scholar]
- 20.Fadok V A, Savill J S, Haslett C, Bratton D L, Doherty D E, Campbell P A, Henson P M. Different populations of macrophages use either the vitrionectin reseptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. J Immunol. 1992;149:4029–4035. [PubMed] [Google Scholar]
- 21.Fadok V A, Voelker D R, Campbell P A, Cohen J J, Bratton D L, Henson P M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 22.Gregory C D, Dive C, Henderson S, Smith C A, Williams G T, Gordon J, Rickinson A B. Activation of Epstein-Barr virus latent genes protects human B cells from death by apoptosis. Nature. 1991;349:612–614. doi: 10.1038/349612a0. [DOI] [PubMed] [Google Scholar]
- 23.Hershberger P A, Dickson J A, Friesen P D. Site-specific mutagenesis of the 35-kilodalton protein gene encoded by Autographa californica nuclear polyhedrosis virus: cell line-specific effects on virus replication. J Virol. 1992;66:5525–5533. doi: 10.1128/jvi.66.9.5525-5533.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huber S A. VCAM-1 is a receptor for encephalomyocarditis virus on murine vascular endothelial cells. J Virol. 1994;68:3453–3458. doi: 10.1128/jvi.68.6.3453-3458.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeon, E.-S., Z. Sheng, and K. U. Knowlton. 1996. Coxsackievirus induces myocyte apoptosis. Circulation 94(Suppl. I):I–356.
- 26.Levine B, Huang Q, Isaacs J T, Reed J C, Griffin D E, Hardwick J M. Conversion of lytic to persistent alphavirus infection by the bcl-2 cellular oncogene. Nature. 1993;361:739–742. doi: 10.1038/361739a0. [DOI] [PubMed] [Google Scholar]
- 27.Liou H C, Baltimore D. Regulation of the NF-kappa B/rel transcription factor and I kappa B inhibitor system. Curr Opin Cell Biol. 1993;5:477–487. doi: 10.1016/0955-0674(93)90014-h. [DOI] [PubMed] [Google Scholar]
- 28.Liu Z G, Hsu H, Goeddel D V, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 29.Martin S J, Reutelingsperger C P, McGahon A J, Rader J A, van Schie R C, LaFace D M, Green D R. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muller U, Steinhoff U, Reis L F, Hemmi S, Pavlovic J, Zinkernagel R M, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 31.Newman S L, Henson J E, Henson P M. Phagocytosis of senescent neutrophils by human monocyte derived macrophages and rabbit inflammatory macrophages. J Exp Med. 1982;156:430–442. doi: 10.1084/jem.156.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perry S W, Epstein L G, Gelbard H A. Simultaneous in situ detection of apoptosis and necrosis in monolayer cultures by TUNEL and trypan blue staining. BioTechniques. 1997;22:1102–1106. doi: 10.2144/97226st01. [DOI] [PubMed] [Google Scholar]
- 33.Pilder S, Logan J, Shenk T. Deletion of the gene encoding the adenovirus 5 early region 1b 21,000-molecular-weight polypeptide leads to degradation of viral and host cell DNA. J Virol. 1984;52:664–671. doi: 10.1128/jvi.52.2.664-671.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raff M C. Social controls on cell survival and cell death. Nature. 1992;356:397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- 35.Rao L, Debbas M, Sabbatini P, Hockenbery D, Korsmeyer S, White E. The adenovirus E1A proteins induce apoptosis, which is inhibited by the E1B 19-kDa and Bcl-2 proteins. Proc Natl Acad Sci USA. 1992;89:7742–7746. doi: 10.1073/pnas.89.16.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ray C A, Black R A, Kronheim S R, Greenstreet T A, Sleath P R, Salvesen G S, Pickup D J. Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1 beta converting enzyme. Cell. 1992;69:597–604. doi: 10.1016/0092-8674(92)90223-y. [DOI] [PubMed] [Google Scholar]
- 37.Razvi E S, Welsh R M. Apoptosis in viral infections. Adv Virus Res. 1995;45:1–60. doi: 10.1016/s0065-3527(08)60057-3. [DOI] [PubMed] [Google Scholar]
- 38.Regnier C H, Song H Y, Gao X, Goeddel D V, Cao Z, Rothe M. Identification and characterization of an IkappaB kinase. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 39.Rousseau V, Cremer I, Lauret E, Riviere I, Aguet M, De Maeyer E. Antiviral activity of autocrine interferon-beta requires the presence of a functional interferon type I receptor. J Interferon Cytokine Res. 1995;15:785–789. doi: 10.1089/jir.1995.15.785. [DOI] [PubMed] [Google Scholar]
- 40.Rueckert R R. Picornaviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 609–654. [Google Scholar]
- 41.Schwarz E M, Krimpenfort P, Berns A, Verma I M. Immunological defects in mice with a targeted disruption in Bcl-3. Genes Dev. 1997;11:187–197. doi: 10.1101/gad.11.2.187. [DOI] [PubMed] [Google Scholar]
- 42.Schwarz E M, Van Antwerp D, Verma I M. Constitutive phosphorylation of IκBα by casein kinase II occurs preferentially at serine 293: requirement for degradation of free IκBα. Mol Cell Biol. 1996;16:3554–3559. doi: 10.1128/mcb.16.7.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sha W C, Liou H C, Tuomanen E I, Baltimore D. Targeted disruption of the p50 subunit of NF-kappa B leads to multifocal defects in immune responses. Cell. 1995;80:321–330. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- 44.Shen Y, Shenk T E. Viruses and apoptosis. Curr Opin Genet Dev. 1995;5:105–11. doi: 10.1016/s0959-437x(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 45.Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 46.Teodoro J G, Branton P E. Regulation of apoptosis by viral gene products. J Virol. 1997;71:1739–1746. doi: 10.1128/jvi.71.3.1739-1746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, Mattmann C, Burns K, Bodmer J L, Schroter M, Scaffidi C, Krammer P H, Peter M E, Tschopp J. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 48.Tolskaya E A, Romanova L I, Kolesnikova M S, Ivannikova T A, Smirnova E A, Raikhlin N T, Agol V I. Apoptosis-inducing and apoptosis-preventing functions of poliovirus. J Virol. 1995;69:1181–1189. doi: 10.1128/jvi.69.2.1181-1189.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Traenckner E B, Pahl H L, Henkel T, Schmidt K N, Wilk S, Baeuerle P A. Phosphorylation of human I kappa B-alpha on serines 32 and 36 controls I kappa B-alpha proteolysis and NF-kappa B activation in response to diverse stimuli. EMBO J. 1995;14:2876–2883. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Antwerp D J, Martin S J, Kafri T, Green D R, Verma I M. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 51.Verma I M, Stevenson J K, Schwarz E M, Van Antwerp D, Miyamoto S. Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 52.Wang C Y, Mayo M W, Baldwin A S., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 53.Wang W Z, Matsumori A, Yamada T, Shioi T, Okada I, Matsui S, Sato Y, Suzuki H, Shiota K, Sasayama S. Beneficial effects of amlodipine in a murine model of congestive heart failure induced by viral myocarditis. A possible mechanism through inhibition of nitric oxide production. Circulation. 1997;95:245–251. doi: 10.1161/01.cir.95.1.245. [DOI] [PubMed] [Google Scholar]
- 54.White E, Stillman B. Expression of adenovirus E1B mutant phenotypes is dependent on the host cell and on synthesis of E1A proteins. J Virol. 1987;61:426–435. doi: 10.1128/jvi.61.2.426-435.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xue D, Horvitz H R. Inhibition of the Caenorhabditis elegans cell-death protease CED-3 by a CED-3 cleavage site in baculovirus p35 protein. Nature. 1995;377:248–251. doi: 10.1038/377248a0. [DOI] [PubMed] [Google Scholar]
- 56.Zhu Z, Tang W, Ray A, Wu Y, Einarsson O, Landry M L, Gwaltney J, Jr, Elias J A. Rhinovirus stimulation of interleukin-6 in vivo and in vitro. Evidence for nuclear factor kappa B-dependent transcriptional activation. J Clin Invest. 1996;97:421–430. doi: 10.1172/JCI118431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zinkernagel R M. Immunology taught by viruses. Science. 1996;271:173–178. doi: 10.1126/science.271.5246.173. [DOI] [PubMed] [Google Scholar]