Background
Background
Greece is among the countries characterized by high rates of antimicrobial resistance and high consumption of antibiotics, including carbapenems.
Objectives
To measure the impact of a carbapenem-focused antimicrobial stewardship programme (ASP) on the antibiotic consumption and patient outcomes in a Greek tertiary hospital during the COVID-19 pandemic.
Methods
A quasi-experimental, before–after study, comparing a 12 month pre-intervention period with a 12 month intervention period in which a carbapenem-focused ASP was implemented.
Results
A total of 1268 patients were enrolled. The proportion of admitted patients who received carbapenems decreased from 4.1% (842 of 20 629) to 2.3% (426 of 18 245) (−1.8%; P < 0.001). A decrease of −4.9 DDD/100 patient-days (PD) (95% CI −7.3 to −2.6; P = 0.007) in carbapenem use and an increase in the use of piperacillin/tazobactam [+2.1 DDD/100 PD (95% CI 1.0–3.3; P = 0.010)] were observed. Thirty-day mortality following initiation of carbapenem treatment and all-cause in-hospital mortality remained unaltered after ASP implementation. In contrast, length of hospital stay increased (median 17.0 versus 19.0 days; P < 0.001), while the risk of infection-related readmission within 30 days of hospital discharge decreased (24.6% versus 16.8%; P = 0.007). In the post-implementation period, acceptance of the ASP intervention was associated with lower daily hazard of in-hospital death [cause-specific HR (csHR) 0.49; 95% CI 0.30–0.80], lower odds of 30 day mortality (OR 0.36; 95% CI 0.18–0.70) and higher rate of treatment success (csHR 2.45; 95% CI 1.59–3.77).
Conclusions
Implementing and maintaining a carbapenem-focused ASP is feasible, effective and safe in settings with high rates of antimicrobial resistance, even during the COVID-19 pandemic.
Introduction
Carbapenems are important elements of the antibiotic armamentarium, with established efficacy against most infections caused by MDR Gram-negative bacteria (GNB). Their efficacy is mainly due to (i) their stability against most β-lactamases, including the AmpCs and the ESBLs, and (ii) the broad spectrum of their activity.1 In addition, carbapenems have a better safety profile compared with other last-line antibiotics, such as polymyxins.1 However, inappropriate use of these broad-spectrum β-lactam antibiotics aggravates the problem of antimicrobial resistance (AMR) by promoting the emergence of XDR and pandrug-resistant Gram-negative nosocomial pathogens through the induction of selective pressure.2,3
Over the last decade, Greece has ranked among the countries with the highest consumption of antibiotics in Europe, including carbapenems and other broad-spectrum antibiotics, both in community and hospital settings.4 In parallel, the country’s AMR rates, including carbapenem-resistant GNB, have been extremely high, and this is also the case in some other European countries.5 The observed infections due to MDR-GNB in these countries pose a significant challenge for clinicians and a major threat for healthcare systems due to their high attributable mortality and hospital costs.6
The threat of AMR may become more evident in the years to come, in part due to the high and often inappropriate use of antimicrobials during the coronavirus disease 2019 (COVID-19) pandemic.7 Especially in the early phases of the pandemic, antimicrobials were widely used as repurposed drugs and as empirical coverage of coinfections and superinfections in COVID-19 patients.8 However, no reliable scientific evidence supports the use of antibiotics, antiretrovirals and antiparasitics as repurposed drugs against severe acute respiratory syndrome coronavirus 2.9 In addition, almost three-quarters of COVID-19 patients received antibiotics during the first months of the pandemic, but only a minority of them had documented bacterial coinfection or superinfection.10 This observed overprescription included carbapenems in several studies of hospitalized patients, especially in ICU.11–14
Accordingly, a pivotal target of antimicrobial stewardship programmes (ASPs) is to decrease unnecessary administration of carbapenems. To optimize carbapenem prescription in our hospital, a setting with high rates of MDR-GNB, a carbapenem-focused ASP was implemented during the first year of the COVID-19 pandemic. The aim of our study was to examine the impact of this ASP on the consumption of antibiotics used to treat MDR-GNB and on patient safety and outcomes.
Materials and methods
Study design, setting and population
This retrospective-prospective, before–after, quasi-experimental study was conducted at a 770 bed tertiary university hospital that covers all surgical and medical specialties, including cardiac surgery, neurosurgery, surgical oncology, rheumatology, oncology, haematology and ICU. Before January 2020 there was no formal ASP implemented in the study site. The pre-implementation period from January 2019 to December 2019 was retrospectively evaluated and was compared with the intervention period of January 2020 to December 2020.
The study enrolled all patients ≥16 years of age who received carbapenems (i.e. meropenem, imipenem or ertapenem) for at least 24 h during the 24 month study period. Those who received more than one course of carbapenems during each of the study subperiods were only included once in the corresponding subperiod analysis, the first time they received the carbapenem antibiotic. Patients who died within 24 h of carbapenem administration or had been transferred from another hospital and had received carbapenem therapy during their hospitalization at that hospital were excluded.
The study was approved by the hospital’s Review Board. The need for the patient’s informed consent was waived because the study represented customary medical practice and the ASP complied with national medical guidelines and legislation for the control of AMR in Greece.
Intervention
Starting 1 January 2020, a multifaceted ASP was implemented to optimize the prescription of carbapenems with regard to indication, dosage and duration of administration. Whenever appropriate, the intervention promoted recommendation for judicious use of carbapenem-sparing antibiotics. The ASP team comprised of an infectious disease (ID) specialist, an ID fellow, a microbiologist and a pharmacist. The programme was based on the strategy of prospective audit and feedback to prescribers and was supplemented by parallel case-based educational sessions, meetings and presentations on proper use of antibiotics.
The ID specialist and the ID fellow were alerted by the pharmacy upon prescription order for a carbapenem and provided unsolicited in-person (‘handshake’) consultation within 72 h for all adult patients receiving a carbapenem antibiotic. Further ID consultation service was available 24/7 through telephone or in person upon request by the treating doctors. Unsolicited follow-up bedside ID consultation was provided daily or every other day for patients whose treating physicians had accepted the intervention. After examining each eligible patient and reviewing their medical record, the ID specialist or the ID fellow discussed with the prescribers whether continuing carbapenems or using non-carbapenem antibiotics for empirical treatment would be appropriate. Whenever relevant microbiological data were available, the options of targeted de-escalation to narrow-spectrum antibiotics or targeted escalation to ceftazidime/avibactam, tigecycline or colistin were considered. Of note, and only when susceptibility data were available, ceftolozane/tazobactam was used as a carbapenem-sparing treatment option while ceftazidime/avibactam was used only for the targeted treatment of carbapenem-resistant GNB. Treating physicians were not obligated to comply with ASP team’s recommendations.
Variables
Antibiotic consumption data per calendar quarter for carbapenems, piperacillin/tazobactam, ceftolozane/tazobactam, ceftazidime/avibactam, tigecycline and colistin were retrieved from the hospital pharmacy records and were expressed as DDD per 100 patient-days (PD).
Patient demographics, clinical characteristics, length of hospital stay, and outcomes were retrospectively reviewed during the pre-intervention period and prospectively collected during the intervention period. Outcome endpoints included inpatient death, death within 30 days of carbapenem initiation (including post-discharge cases) and infection-related readmission within 30 days of hospital discharge. Outcome during or at the end of the antibiotic treatment could be assessed only for the post-implementation cohort and was classified as death, new/recurrent infection or favourable outcome. For every patient in the post-implementation cohort, it was recorded whether the treating physician accepted the ASP recommendation or not.
Statistical analysis
The effect of the ASP implementation on hospital antibiotic use was assessed using interrupted time series analyses. A segmented Poisson regression model was employed to examine the extent to which the ASP was associated with an immediate level change and/or a gradual trend change of the monthly numbers of carbapenem-treated patients. In this model, the series of monthly counts of carbapenem-treated patients formed the dependent variable. Independent variables were the time elapsed since the start of the study, the ASP implementation indicator (post- versus pre-ASP) and the time after the intervention. The monthly series of hospital admissions (log transformed) was used as an offset variable to convert the outcome into a rate that accounts for variation in the hospital population size over time. Two pairs of sine–cosine Fourier functions of time were included to capture seasonality. The model coefficients were estimated using the maximum-likelihood method. Residual autocorrelation was ruled out by examining autocorrelation graphs. Sensitivity analyses were conducted by using the monthly numbers of hospitalized patients and PD as alternative denominators for the treatment rate, and by inflating the standard errors by the scaled Pearson chi-squared statistics to adjust for the possibility of overdispersion.
In addition, we examined the temporal trends in the consumption of carbapenems and other selected antibiotics with activity against MDR-GNB by using quarterly hospital data. A level-change linear regression model for interrupted time series was used for this purpose. Stratification per quarter was employed to adjust for seasonality. The model was estimated using the ordinary least squares method, and Newey–West standard errors were used to account for autocorrelation.
The impact on patient outcomes was assessed on an ITT principle by comparing all carbapenem-treated patients between the pre-implementation period and the ASP intervention period. Pearson's chi-squared test was used to assess between-group differences in overall proportions of in-hospital mortality, total mortality within 30 days of initiation of carbapenem treatment, and infection-related readmission within 30 days of hospital discharge. The Wilcoxon rank-sum test was used to assess between-group differences in length of hospital stay. Multivariable Cox regression was employed to obtain cause-specific HRs (csHRs) for in-hospital death and discharge alive, adjusting for differences in baseline covariates. The time origin was set to hospital admission. Discharge alive from the hospital was treated as a competing event to in-hospital death. In this analysis, a low csHR for discharge alive reflects a low daily rate of discharge resulting in prolonged hospital stay. Multivariable logistic regression was employed to estimate OR for total mortality within 30 days of initiation of carbapenem treatment and OR for infection-related readmission within 30 days of hospital discharge, correcting for differences in baseline covariates. All models adjusted for patient sex, age, ward of hospitalization, and history of previous hospitalization.
A series of sensitivity analyses were performed to assess the likely clinical impact of the ASP intervention under different conditions. On a modified ITT analysis, we compared the pre-implementation cohort to the post-implementation cohort, excluding patients for whom the intervention was not accepted. On per-protocol analysis, we compared patients who did not receive the intervention in either the pre- or the post-implementation period with those who received the intervention. Finally, restricting the analysis within the post-implementation period, we compared patients for whom the intervention was accepted with patients for whom the intervention was not. In the latter analysis, we additionally compared the clinical outcome at the end of therapy.
None of the study variables had missing data. Statistical significance was considered at the usual P < 0.05 threshold. Data processing and statistical modelling were performed using Stata version 17 (Stata Corp., College Station, TX, USA).
Results
Antibiotic consumption
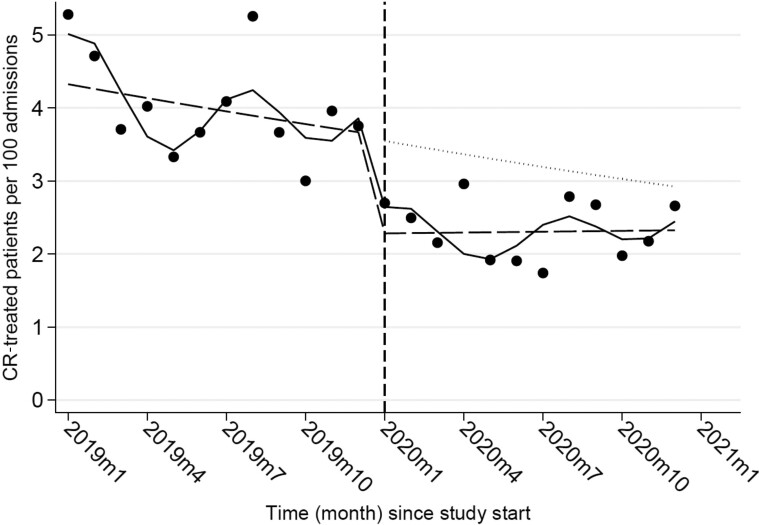
In all, 1329 carbapenem courses were administered to 1268 patients during the 2 year study period, 55 of whom received more than one course of carbapenems in any of the two study subperiods. After the ASP implementation, the proportion of admitted patients who received carbapenem treatment decreased significantly, from 4.1% (842 of 20 629) to 2.3% (426 of 18 245) (−1.8%; P < 0.001). The interrupted time series analysis confirmed that the implementation of the carbapenem-focused ASP was associated with an overall level reduction in the rate of carbapenem treatments per 100 hospital admissions [incidence rate ratio (IRR) 0.63; 95% CI 0.50–0.80; P < 0.001], while no substantial trend change occurred after the ASP implementation (IRR 1.02; 95% CI 1.00–1.04; P = 0.117; Figure 1). Sensitivity analyses confirmed that the estimated level change in the rate of carbapenem-treated patients was robust against different statistical modelling specifications and rate denominators (Table 1).
Figure 1.
Monthly rates of carbapenem (CR)-treated patients per 100 hospital admissions, pre- and post-implementation of the ASP. Dots show observed rates, the solid line shows predicted rates from Poisson regression model adjusted for seasonality and overdispersion, the dashed line shows the deseasonalized trend, the dotted line shows the counterfactual scenario assuming the intervention was not implemented, and the vertical dashed line shows the time of the beginning of the intervention.
Table 1.
Sensitivity analyses of estimating the effect of the ASP implementation on the rate of carbapenem prescription for various types of model specifications
| Outcome being modelled | Poisson model specifications | IRR | 95% CI | P value |
|---|---|---|---|---|
| Monthly rate of carbapenem-treated patients, per 100 hospital admissions | Unadjusted | 0.70 | 0.56–0.87 | 0.002 |
| Adjusted for seasonality | 0.63 | 0.50–0.80 | <0.001 | |
| Adjusted for seasonality, overdispersion | 0.63 | 0.39–1.01 | 0.056 | |
| Monthly rate of carbapenem-treated patients, per 100 inpatients | Unadjusted | 0.71 | 0.57–0.88 | 0.002 |
| Adjusted for seasonality | 0.69 | 0.55–0.86 | 0.001 | |
| Adjusted for seasonality, overdispersion | 0.69 | 0.44–1.08 | 0.103 | |
| Monthly rate of carbapenem-treated patients, per 1000 PD | Unadjusted | 0.68 | 0.55–0.85 | 0.001 |
| Adjusted for seasonality | 0.71 | 0.57–0.88 | 0.002 | |
| Adjusted for seasonality, overdispersion | 0.71 | 0.46–1.08 | 0.107 |
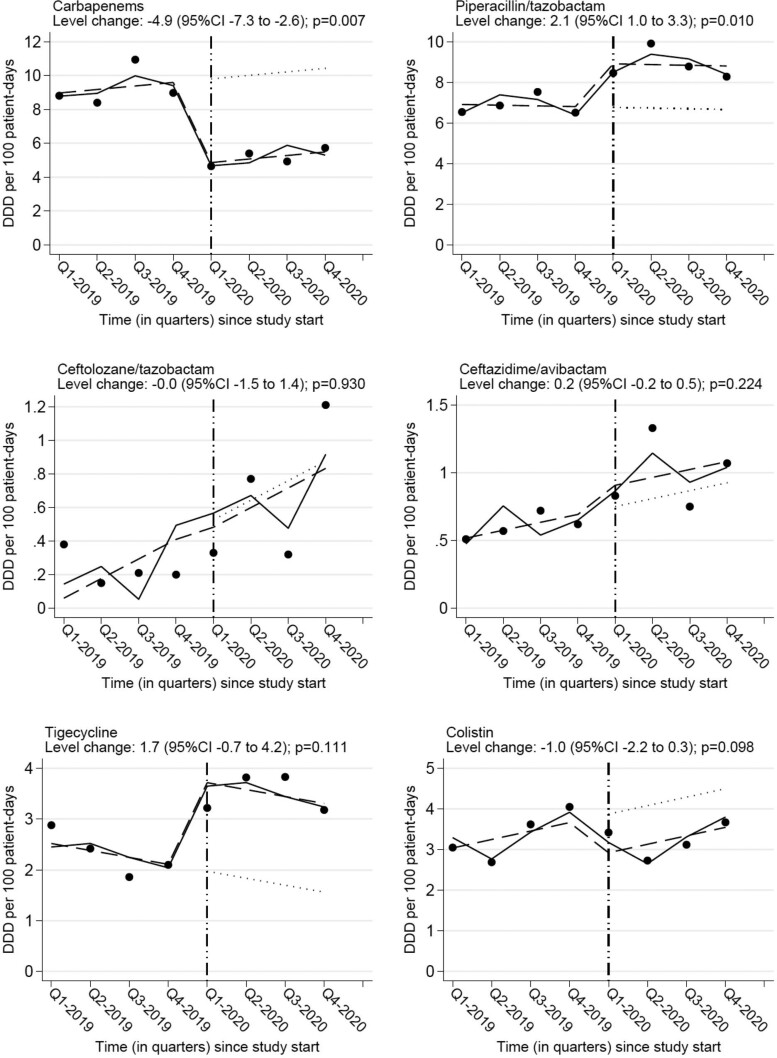
Analysis of quarterly data on hospital consumption of carbapenems showed that the ASP was associated with a decrease of −4.9 DDD/100 PD (95%CI −7.3 to −2.6; P = 0.007). A concurrent increase in the consumption of piperacillin/tazobactam was noted [+2.1 DDD/100 PD (95% CI 1.0–3.3; P = 0.010)]. There was also a non-statistically significant increase of tigecycline consumption and decrease of colistin consumption. The consumption of ceftolozane/tazobactam and ceftazidime/avibactam remained largely unaffected (Table 2 and Figure 2).
Table 2.
Quarterly hospital consumption of carbapenems and other selected antibiotics with activity against MDR-GNB and level changes due to the ASP implementation (measured in DDD per 100 PD)
| Antibiotic group or agent | 2019 (pre-intervention year) | 2020 (intervention year) | After–before level change | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | Estimate | 95% CI | P value | |
| Carbapenems | 8.81 | 8.40 | 10.94 | 8.98 | 4.65 | 5.40 | 4.93 | 5.73 | −4.9 | −7.3 to −2.6 | 0.007 |
| ȃMeropenem | 8.67 | 8.33 | 10.80 | 8.91 | 4.56 | 5.20 | 4.65 | 5.44 | −5.0 | −7.3 to −2.6 | 0.007 |
| ȃImipenem | 0.02 | 0.00 | 0.02 | 0.01 | 0.02 | 0.00 | 0.04 | 0.00 | 0.0 | −0.0 to 0.1 | 0.145 |
| ȃErtapenem | 0.12 | 0.07 | 0.12 | 0.06 | 0.07 | 0.20 | 0.24 | 0.29 | 0.0 | −0.4 to 0.4 | 0.995 |
| Piperacillin/tazobactam | 6.54 | 6.86 | 7.53 | 6.51 | 8.46 | 9.91 | 8.78 | 8.28 | 2.1 | 1.0–3.3 | 0.010 |
| Ceftolozane/tazobactam | 0.38 | 0.15 | 0.21 | 0.20 | 0.33 | 0.77 | 0.32 | 1.21 | 0.0 | −1.5 to 1.4 | 0.930 |
| Ceftazidime/avibactam | 0.51 | 0.57 | 0.72 | 0.62 | 0.83 | 1.33 | 0.75 | 1.07 | 0.2 | −0.2 to 0.5 | 0.224 |
| Tigecycline | 2.88 | 2.42 | 1.86 | 2.10 | 3.22 | 3.82 | 3.83 | 3.18 | 1.7 | −0.7 to 4.2 | 0.111 |
| Colistin | 3.05 | 2.69 | 3.62 | 4.05 | 3.42 | 2.73 | 3.12 | 3.67 | −1.0 | −2.2 to 0.3 | 0.098 |
Level changes were estimated by segmented linear regression adjusting for seasonality and autocorrelation. Q, quarter.
Figure 2.
Interrupted time series graphs showing level changes in the consumption of carbapenems and other selected antibiotics with activity against MDR-GNB following the ASP implementation. The dots correspond to quarterly antibiotic consumption rates measured in DDD per 100 PD. The solid line shows the predicted rates from a segmented linear regression model adjusted for seasonality and autocorrelation. The dashed line shows the deseasonalized trend. The dotted line corresponds to the counterfactual scenario assuming the intervention was not implemented.
Clinical outcomes
Demographic and clinical characteristics of the patients before and after ASP implementation did not differ significantly, as summarized in Table 3. Mortality within 30 days of initiation of carbapenem treatment (22.4% versus 23.1%; P = 0.798) and all-cause in-hospital mortality (23.6% versus 28.4%; P = 0.065) remained unaltered after ASP implementation. In contrast, length of hospital stay increased (median 17.0 versus 19.0 days; P < 0.001), while the risk of infection-related readmission within 30 days of hospital discharge decreased (24.6% versus 16.8%; P = 0.007). Multivariable regression analyses showed similar effect sizes after adjustment for baseline differences in patient sex, age, ward of hospitalization, and history of previous hospitalization (Tables S1–S19, available as Supplementary data at JAC Online). The results of sensitivity analyses in Table 4 confirmed that the effects of the ASP on patient outcomes were consistent under different assumed conditions.
Table 3.
Comparison of patient characteristics and outcomes on an ITT principle (pre- versus post-implementation of the ASP) and per acceptance of the antimicrobial stewardship intervention
| ITT analysis | Acceptance analysis (post-implementation cohort) |
|||||
|---|---|---|---|---|---|---|
| Variables | Pre-implementation cohort (n = 842) | Post-implementation cohort (n = 426) | P value | Intervention not accepted (n = 46) | Intervention accepted (n = 380) | P value |
| Male sex, n (%) | 540 (64.1) | 266 (62.4) | 0.554 | 33 (71.7) | 233 (61.3) | 0.168 |
| Age, years, median (IQR) | 68.0 (56.0–78.0) | 69.0 (58.0–79.0) | 0.422 | 73.0 (64.0–84.0) | 69.0 (56.0–78.0) | 0.048 |
| Ward of hospitalization, n (%) | 0.591 | 0.200 | ||||
| ȃIntensive care | 92 (10.9) | 54 (12.7) | 2 (4.4) | 52 (13.7) | ||
| ȃMedicine | 529 (62.8) | 263 (61.7) | 29 (63.0) | 234 (61.5) | ||
| ȃSurgery | 217 (25.8) | 105 (24.6) | 15 (32.6) | 90 (23.7) | ||
| ȃOther | 4 (0.5) | 4 (0.9) | 0 (0.0) | 4 (1.1) | ||
| Previous hospitalization, n (%) | 493 (58.6) | 236 (55.4) | 0.284 | 20 (43.5) | 216 (56.8) | 0.085 |
| CCI, median (IQR) | NA | 2.0 (1.0–4.0) | NA | 3.0 (2.0–6.0) | 2.0 (1.0–4.0) | 0.104 |
| Length of hospital stay (days) before carbapenem therapy, median (IQR) | 5.0 (1.0–11.0) | 5.0 (1.0–12.0) | 0.272 | 6.0 (3.0–14.0) | 4.5 (1.0–12.0) | 0.279 |
| Length of hospital stay (days), median (IQR) | 17.0 (9.0–31.0) | 19.0 (12.0–37.0) | <0.001 | 17.0 (10.0–33.0) | 20.0 (12.0–38.0) | 0.497 |
| In-hospital any-cause death, n (%) | 199 (23.6) | 121 (28.4) | 0.065 | 21 (45.7) | 100 (26.3) | 0.006 |
| Death within 30 days of carbapenem initiation, n (%) | 187 (22.4) | 98 (23.1) | 0.798 | 18 (39.1) | 80 (21.1) | 0.006 |
| Infection-related readmission within 30 days of discharge alive, n (%) | 153 (24.6) | 51 (16.8) | 0.007 | 6 (24.0) | 45 (16.1) | 0.313 |
| Treatment outcome, n (%) | <0.001 | |||||
| ȃDeath | 15 (32.6) | 62 (16.3) | ||||
| ȃNew or recurrent infection | 8 (17.4) | 9 (2.4) | ||||
| ȃSuccess | 23 (50.0) | 309 (81.3) | ||||
NA, not available; CCI, Charlson comorbidity index.
Table 4.
Results of multivariable Cox proportional hazards regression and logistic regression quantifying the effects of the ASP on patient outcomes under different conditions (sensitivity analyses)
| ITT analysisa | Modified ITT analysisb | Per-protocol analysisc | Per-acceptance analysisd | |||||
|---|---|---|---|---|---|---|---|---|
| Clinical outcomes and effect measure | ES (95% CI) | P value | ES (95% CI) | P value | ES (95% CI) | P value | ES (95% CI) | P value |
| In-hospital death, csHR | 0.99 (0.79–1.24) | 0.922 | 0.91 (0.71–1.16) | 0.450 | 0.87 (0.69–1.11) | 0.256 | 0.49 (0.30–0.80) | 0.004 |
| Discharge alive, csHR | 0.80 (0.70–0.92) | 0.002 | 0.83 (0.72–0.95) | 0.009 | 0.85 (0.74–0.98) | 0.022 | 1.32 (0.87–2.00) | 0.187 |
| 30 day death, ORe | 1.26 (0.95–1.67) | 0.107 | 1.10 (0.82–1.49) | 0.513 | 1.03 (0.77–1.38) | 0.852 | 0.36 (0.18–0.70) | 0.003 |
| 30 day infection-related readmission, ORf | 0.60 (0.42–0.86) | 0.006 | 0.57 (0.39–0.83) | 0.003 | 0.57 (0.39–0.83) | 0.003 | 0.57 (0.20–1.61) | 0.290 |
| Treatment outcomeg | ||||||||
| ȃDeath, csHR | 0.74 (0.40–1.37) | 0.337 | ||||||
| ȃRe-infection, csHR | 0.26 (0.09–0.74) | 0.011 | ||||||
| ȃSuccess, csHR | 2.45 (1.59–3.77) | <0.001 | ||||||
All effects are corrected for baseline differences in patient sex, age, ward of hospitalization, and history of previous hospitalization. ES, effect size attributed to the intervention.
Compares the pre-implementation cohort (n = 842) with the post-implementation cohort (n = 426).
Compares the pre-implementation cohort (n = 842) with the post-implementation cohort excluding patients for whom the intervention was not accepted (n = 380).
Compares patients who did not receive the intervention in either the pre- or the post-implementation period (n = 888) with those who received the intervention (n = 380).
Compares patients for whom the intervention was accepted (n = 380) with patients for whom the intervention was not accepted (n = 46) in the post-implementation period.
Includes death from any cause within 30 days of initiation of carbapenem therapy.
Assessed within 30 days of hospital discharge alive.
Assessed during or at the end of treatment with carbapenems.
Apart from age, demographic and clinical characteristics did not differ substantially between patients with and without acceptance of ASP recommendations during the post-implementation period, but patient outcomes were worse for the latter (Table 3). Multivariable analysis confirmed that patients for whom ASP recommendations were accepted had lower daily hazard of in-hospital death (csHR 0.49; 95% CI 0.30–0.80), lower odds of 30 day mortality (OR 0.36; 95% CI 0.18–0.70) and, albeit not statistically significant, lower odds of infection-related readmission (OR 0.57; 95% CI 0.20–1.61) compared with patients for whom the intervention was not accepted. Moreover, acceptance of the ASP intervention was associated with a higher rate of treatment success (csHR 2.45; 95% CI 1.59–3.77) (Table 4).
Discussion
This study describes the implementation of a carbapenem-focused ASP during the first year of the COVID-19 pandemic and its impact on the consumption of several broad-spectrum antibiotics with activity against MDR-GNB and on patient outcomes. It is one of the few studies to assess a hospital ASP for carbapenems in Greece, a country with high rates of antibiotic consumption and AMR. The results demonstrate that judicious use of carbapenems in a setting with high rates of MDR-GNB was feasible and led to a significant decrease of their consumption and, importantly, improvement of patient outcomes.
The implementation and maintenance of the ASP in our hospital during the first phases of the COVID-19 pandemic was a challenging and laborious process. At its beginning, the pandemic caused a tremendous depletion of human and structural resources in many hospitals worldwide, compromising their antimicrobial stewardship activities.15 This resulted in increased consumption of antimicrobials in hospitals, including carbapenems,16 even though medical guidelines regarding the administration of this class of antibiotics had not changed during the COVID-19 pandemic. In some cases, the increased antimicrobial consumption was mitigated by reinstating stewardship activities17 or by intensifying ongoing ASPs.14 These observations are in accordance with the findings of our study regarding the feasibility of an effective ASP during the COVID-19 period.
The two core strategies of an ASP for inpatient populations include formulary restriction and pre-authorization, and prospective audit and feedback to prescribers. These strategies can be applied separately or in combination. In the pre-pandemic era, both strategies have been shown to effectively and safely reduce unnecessary in-hospital antibiotic use.18 However, few relevant data exist during the COVID-19 pandemic. The initial lack of evidence on optimal management of COVID-19 and the accompanying fear of it, the overwhelmed hospitals amid COVID-19 surges, the shortage of available skilled doctors for the implementation or maintenance of ASPs, and medication supply problems, had created an entirely new situation in the hospital sector that may have affected the performance of ASPs. In a recent study from an academic medical centre in the USA, introduction of restriction criteria regarding meropenem use for 2 months during the third year of the pandemic successfully reduced inappropriate meropenem utilization and hospital length of stay, contributing to significant cost savings for the institution.19 On the other hand, the results of the present study confirm the preservation of the efficiency of an ASP based on a prospective audit and feedback strategy. Recently, another study showed that the strengthening of an ASP that was already in place, by using a combination of restrictive policies and persuasive techniques, was successful in safely controlling the observed increase of carbapenem consumption during the first wave of COVID-19 pandemic.14 Thus, there is evidence to suggest that both core antimicrobial stewardship strategies continue to be safe and effective during the COVID-19 era.
Several reports before the COVID-19 pandemic described reduced carbapenem use without negatively affecting patient outcomes through the implementation of carbapenem-focused ASPs.20–25 However, few studies on antimicrobial stewardship were performed in Greece,26–28 and only one study examined a carbapenem-focused intervention.27 None of these studies addressed the impact of the intervention on the consumption of newer non-carbapenem antibiotics with activity against MDR-GNB. Our study adds new evidence as the sharp and sustained decrease in carbapenem use achieved by the ASP was associated with an increase only in the consumption of piperacillin/tazobactam, which has a lower ecological impact than carbapenems. Moreover, the carbapenem-focused ASP in this study did not significantly affect the consumption of tigecycline and colistin, which are associated with several toxicities and adverse effects, or the consumption of ceftolozane/tazobactam and ceftazidime/avibactam. The latter is important, considering the need to preserve the efficacy of new antibiotics through their judicious use.
Following the ASP implementation in this study, there were no significant changes in all-cause in-hospital mortality and 30 day mortality after carbapenem initiation. On the contrary, the infection-related readmission rate was lower over the post-implementation period. In addition, when the analysis was restricted to the post-implementation cohort, acceptance of the intervention was associated with reduced in-hospital and 30 day mortality after carbapenem initiation, as well as better treatment outcome. These findings are in accordance with the results of the great majority of hospital ASP studies that measured patient outcomes and reported statistically significant reductions or at least non-significant changes in patient mortality and infection-related readmissions.29–31
The difference in treatment outcome between cases with accepted and non-accepted intervention in the post-implementation subgroup was probably due to the optimization of diagnostic work-up and antimicrobial treatment through the acceptance of the intervention, reflecting the benefits of ID consultation on this parameter.32–34 However, other factors might have acted as potential sources of bias on the estimation of treatment outcome. First, contrary to cases where ASP team recommendation was declined and further consultation was provided only upon request, unsolicited follow-up consultation was given regularly until completion of antimicrobial treatment when the intervention was accepted, thus enhancing the prompt, continuous and efficient handling of possible new-onset complications in these patients. Furthermore, the intervention was not accepted for a number of patients with terminal illness and without proof of concurrent infection for whom the treating physician hesitated to withdraw antibiotics, thus defying the ASP team recommendation.
Previous studies have reported reductions in length of hospital stay after implementing ASPs.18,30 However, we found that patients in the post-implementation group experienced longer length of hospital stay, on average, compared with the pre-implementation group. This finding cannot be fully explained. A possible reason could be found in the decreased proportion of admitted patients that received carbapenems during the intervention period, indicating that treating physicians used carbapenems more judiciously then, reserving them only for severe cases, which, however, required longer hospitalization compared with the cases in the pre-intervention period. Unfortunately, we were unable to retrieve more data on the severity of patients’ illness during the pre-implementation period to test this hypothesis.
A key strength of our study is the use of a multidimensional methodology to assess the impact of a carbapenem-focused ASP on antibiotic use and clinical outcomes. Another important feature is the high rate (89%) of acceptance of the intervention by treating physicians. Furthermore, a cross-sectional survey in our hospital near the end of the post-implementation period showed that the ASP described here had positive impact on doctors’ perceptions, attitudes and practices regarding the management of infections due to MDR microorganisms, and 98.5% of respondents wanted the ASP to continue during the COVID-19 pandemic.35 Finally, our study can be easily replicated in settings where targeting a specific antibiotic class is needed and ID physicians are available for this purpose.
The present study is not exempt from limitations. Although our segmental regression analysis of interrupted time series is recommended as a powerful tool to assess temporal trends following an intervention,36,37 it shares the same limitations as any analysis of observational data. Our analysis examined level and slope changes in the rates of use of carbapenems and other antibiotics following ASP implementation, by accounting for potential confounding effects by seasonality and varying inpatient population size over time. The absence of differences in demographic and clinical characteristics of the patients before and after ASP implementation provides some assurance that our results are unlikely to have been confounded by differences in local epidemiology between the pre-intervention and intervention periods. However, we cannot completely exclude the possibility of residual confounding by unmeasured factors, such as varying frequency and severity of infections with highly resistant bacteria that would require carbapenems. Our sensitivity analyses produced consistent estimates of the relative reduction in the number of patients treated with carbapenems following the ASP under different statistical modelling specifications, but we must note that adjustment for overdispersion resulted in less precise estimates and higher P values. We do not view the latter as a major concern as the P values remained relatively low (ranging from 0.056 to 0.107) after we inflated the standard errors for overdispersion, and because a separate analysis of hospital volume data of carbapenem consumption confirmed a significant reduction following the ASP implementation. Furthermore, we did not evaluate the impact of the ASP on AMR or the incidence of Clostridioides difficile infection, because we considered that the strengthening of infection prevention and control measures due to COVID-19 in the post-implementation period would be an important confounder. In addition, the retrospective nature of the study in the pre-intervention period did not allow the retrieval of reliable data on patient comorbidities during that period. Moreover, paediatric patients were not included in our study. Lastly, this was a single-centre study in a large academic hospital whose capacity was not exceeded during the study period because of the COVID-19 pandemic, thus limiting the generalizability of our results to hospitals of different size and characteristics.
In conclusion, this study demonstrates that implementing and maintaining a carbapenem-focused ASP is feasible, effective and safe in settings with high rates of MDR-GNB, even during the COVID-19 pandemic. The ASP not only effectively reduced the use of carbapenems, but also led to improved patient outcomes, without increasing the consumption of newer antibiotics.
Supplementary Material
Contributor Information
Nikolaos Spernovasilis, School of Medicine, University of Crete, Heraklion, Greece; Department of Internal Medicine and Infectious Diseases, University Hospital of Heraklion, Heraklion, Greece.
Evangelos I Kritsotakis, School of Medicine, University of Crete, Heraklion, Greece; Laboratory of Biostatistics, School of Medicine, University of Crete, Heraklion, Greece.
Anna Mathioudaki, Department of Internal Medicine and Infectious Diseases, University Hospital of Heraklion, Heraklion, Greece.
Alexandra Vouidaski, Department of Internal Medicine and Infectious Diseases, University Hospital of Heraklion, Heraklion, Greece.
Christos Spanias, Department of Pharmacy, University Hospital of Heraklion, Heraklion, Greece.
Maria Petrodaskalaki, Department of Quality & Research, University Hospital of Heraklion, Heraklion, Greece.
Petros Ioannou, School of Medicine, University of Crete, Heraklion, Greece; Department of Internal Medicine and Infectious Diseases, University Hospital of Heraklion, Heraklion, Greece.
Georgios Chamilos, School of Medicine, University of Crete, Heraklion, Greece; Department of Clinical Microbiology, University Hospital of Heraklion, Heraklion, Greece.
Diamantis P Kofteridis, School of Medicine, University of Crete, Heraklion, Greece; Department of Internal Medicine and Infectious Diseases, University Hospital of Heraklion, Heraklion, Greece.
Funding
This study was carried out as part of our routine work.
Transparency declarations
None to declare.
Supplementary data
Tables S1 to S19 are available as Supplementary data at JAC Online.
References
- 1. Meletis G. Carbapenem resistance: overview of the problem and future perspectives. Ther Adv Infect Dis 2016; 3: 15–21. 10.1177/2049936115621709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pavez M, Vieira C, de Araujo MR et al. Molecular mechanisms of membrane impermeability in clinical isolates of Enterobacteriaceae exposed to imipenem selective pressure. Int J Antimicrob Agents 2016; 48: 78–85. 10.1016/j.ijantimicag.2016.04.016 [DOI] [PubMed] [Google Scholar]
- 3. Simner PJ, Antar AAR, Hao S et al. Antibiotic pressure on the acquisition and loss of antibiotic resistance genes in Klebsiella pneumoniae. J Antimicrob Chemother 2018; 73: 1796–803. 10.1093/jac/dky121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. ECDC . Antimicrobial Consumption in the EU/EEA (ESAC-Net)—Annual Epidemiological Report for 2020. 2021. https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-consumption-europe-2020.
- 5. ECDC . Surveillance of Antimicrobial Resistance in Europe, 2020 Data. 2022. https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2020.
- 6. Cassini A, Högberg LD, Plachouras D et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis 2019; 19: 56–66. 10.1016/S1473-3099(18)30605-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lai CC, Chen SY, Ko WC et al. Increased antimicrobial resistance during the COVID-19 pandemic. Int J Antimicrob Agents 2021; 57: 106324. 10.1016/j.ijantimicag.2021.106324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spernovasilis NA, Kofteridis DP. COVID-19 and antimicrobial stewardship: what is the interplay? Infect Control Hosp Epidemiol 2021; 42: 378–9. 10.1017/ice.2020.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. NIH . Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. 2022. https://www.covid19treatmentguidelines.nih.gov/.
- 10. Langford BJ, So M, Raybardhan S et al. Antibiotic prescribing in patients with COVID-19: rapid review and meta-analysis. Clin Microbiol Infect 2021; 27: 520–31. 10.1016/j.cmi.2020.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liew Y, Lee WHL, Tan L et al. Antimicrobial stewardship programme: a vital resource for hospitals during the global outbreak of coronavirus disease 2019 (COVID-19). Int J Antimicrob Agents 2020; 56: 106145. 10.1016/j.ijantimicag.2020.106145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Castro-Lopes A, Correia S, Leal C et al. Increase of antimicrobial consumption in a tertiary care hospital during the first phase of the COVID-19 pandemic. Antibiotics (Basel) 2021; 10: 778. 10.3390/antibiotics10070778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grau S, Echeverria-Esnal D, Gómez-Zorrilla S et al. Evolution of antimicrobial consumption during the first wave of COVID-19 pandemic. Antibiotics (Basel) 2021; 10: 132. 10.3390/antibiotics10020132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. AlBahrani S, Almogbel F, Alanazi W et al. Carbapenem use correlates with percentage of patients with COVID-19 in intensive care units. Infection 2022; 10.1007/s15010-022-01867-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tomczyk S, Taylor A, Brown A et al. Impact of the COVID-19 pandemic on the surveillance, prevention and control of antimicrobial resistance: a global survey. J Antimicrob Chemother 2021; 76: 3045–58. 10.1093/jac/dkab300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Macera M, Onorato L, Calò F et al. The impact of the SARS-Cov2 pandemic on a persuasive educational antimicrobial stewardship program in a University Hospital in Southern Italy: a pre-post study. Antibiotics (Basel) 2021; 10: 1405. 10.3390/antibiotics10111405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Meschiari M, Onorato L, Bacca E et al. Long-term impact of the COVID-19 pandemic on in-hospital antibiotic consumption and antibiotic resistance: a time series analysis (2015–2021). Antibiotics (Basel) 2022; 11: 826. 10.3390/antibiotics11060826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Davey P, Marwick CA, Scott CL et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev 2017; 2: CD003543. 10.1002/14651858.CD003543.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wells DA, Johnson AJ, Lukas JG et al. Criteria Restricting Inappropriate Meropenem Empiricism (CRIME): a quasi-experimental carbapenem restriction pilot at a large academic medical centre. Int J Antimicrob Agents 2022; 60: 106661. 10.1016/j.ijantimicag.2022.106661 [DOI] [PubMed] [Google Scholar]
- 20. García-Rodríguez JF, Bardán-García B, Peña-Rodríguez MF et al. Meropenem antimicrobial stewardship program: clinical, economic, and antibiotic resistance impact. Eur J Clin Microbiol Infect Dis 2019; 38: 161–70. 10.1007/s10096-018-3408-2 [DOI] [PubMed] [Google Scholar]
- 21. Faraone A, Poggi A, Cappugi C et al. Inappropriate use of carbapenems in an internal medicine ward: impact of a carbapenem-focused antimicrobial stewardship program. Eur J Intern Med 2020; 78: 50–7. 10.1016/j.ejim.2020.03.017 [DOI] [PubMed] [Google Scholar]
- 22. García-Rodríguez JF, Bardán-García B, Juiz-González PM et al. Long-term carbapenems antimicrobial stewardship program. Antibiotics (Basel) 2020; 10: 15. 10.3390/antibiotics10010015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. López-Viñau T, Peñalva G, García-Martínez L et al. Impact of an antimicrobial stewardship program on the incidence of carbapenem resistant Gram-negative bacilli: an interrupted time-series analysis. Antibiotics (Basel) 2021; 10:586. 10.3390/antibiotics10050586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rungsitsathian K, Wacharachaisurapol N, Nakaranurack C et al. Acceptance and outcome of interventions in a meropenem de-escalation antimicrobial stewardship program in pediatrics. Pediatr Int 2021; 63: 1458–65. 10.1111/ped.14703 [DOI] [PubMed] [Google Scholar]
- 25. Kit-Anan W, Boonsathorn S, Anantasit N et al. Handshake stewardship reduces carbapenem prescription in a pediatric critical care setting. Pediatr Int 2022; 64: e15227. 10.1111/ped.15227 [DOI] [PubMed] [Google Scholar]
- 26. Pitiriga V, Kanellopoulos P, Kampos E et al. Antimicrobial stewardship program in a Greek hospital: implementing a mandatory prescription form and prospective audits. Future Microbiol 2018; 13: 889–96. 10.2217/fmb-2018-0020 [DOI] [PubMed] [Google Scholar]
- 27. Makina A-A, Poulakou G, Sympardi S et al. Safety of a carbapenem-sparing approach as part of an antibiotic stewardship program, in a setting with increased carbapenem resistance. Infect Dis Clin Microbiol 2019; 1: 14–25. 10.5152/idcm.2019.19003 [DOI] [Google Scholar]
- 28. Chrysou K, Zarkotou O, Kalofolia S et al. Impact of a 4-year antimicrobial stewardship program implemented in a Greek tertiary hospital. Eur J Clin Microbiol Infect Dis 2022; 41: 127–32. 10.1007/s10096-021-04290-7 [DOI] [PubMed] [Google Scholar]
- 29. Schuts EC, Hulscher M, Mouton JW et al. Current evidence on hospital antimicrobial stewardship objectives: a systematic review and meta-analysis. Lancet Infect Dis 2016; 16: 847–56. 10.1016/S1473-3099(16)00065-7 [DOI] [PubMed] [Google Scholar]
- 30. Nathwani D, Varghese D, Stephens J et al. Value of hospital antimicrobial stewardship programs [ASPs]: a systematic review. Antimicrob Resist Infect Control 2019; 8: 35. 10.1186/s13756-019-0471-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carrara E, Sibani M, Barbato L et al. How to ‘SAVE’ antibiotics: effectiveness and sustainability of a new model of antibiotic stewardship intervention in the internal medicine area. Int J Antimicrob Agents 2022; 60: 106672. 10.1016/j.ijantimicag.2022.106672 [DOI] [PubMed] [Google Scholar]
- 32. Kim SH, Huh K, Cho SY et al. Factors associated with the recurrence of acute pyelonephritis caused by extended-spectrum β-lactamase-producing Escherichia coli: the importance of infectious disease consultation. Diagn Microbiol Infect Dis 2019; 94: 55–9. 10.1016/j.diagmicrobio.2018.11.019 [DOI] [PubMed] [Google Scholar]
- 33. Whittington KJ, Ma Y, Butler AM et al. The impact of infectious diseases consultation for children with Staphylococcus aureus bacteremia. Pediatr Res 2022; 92: 1598–605. 10.1038/s41390-022-02251-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mejia-Chew C, O’Halloran JA, Olsen MA et al. Effect of infectious disease consultation on mortality and treatment of patients with Candida bloodstream infections: a retrospective, cohort study. Lancet Infect Dis 2019; 19: 1336–44. 10.1016/S1473-3099(19)30405-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Spernovasilis N, Ierodiakonou D, Spanias C et al. Doctors’ perceptions, attitudes and practices towards the management of multidrug-resistant organism infections after the implementation of an antimicrobial stewardship programme during the COVID-19 pandemic. Trop Med Infect Dis 2021; 6: 20. 10.3390/tropicalmed6010020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stone SP, Cooper BS, Kibbler CC et al. The ORION statement: guidelines for transparent reporting of outbreak reports and intervention studies of nosocomial infection. J Antimicrob Chemother 2007; 59: 833–40. 10.1093/jac/dkm055 [DOI] [PubMed] [Google Scholar]
- 37. Schweizer ML, Braun BI, Milstone AM. Research methods in healthcare epidemiology and antimicrobial stewardship-quasi-experimental designs. Infect Control Hosp Epidemiol 2016; 37: 1135–40. 10.1017/ice.2016.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.