Abstract
Background:
The prevalence of revision surgery due to aseptic loosening and periprosthetic joint infection (PJI) following total hip and knee arthroplasty is growing. Strategies to prevent the need for revision surgery and its associated health-care costs and patient morbidity are needed. Therapies that modulate the gut microbiota to influence bone health and systemic inflammation are a novel area of research.
Methods:
A literature review of preclinical and clinical peer-reviewed articles relating to the role of the gut microbiota in bone health and PJI was performed.
Results:
There is evidence that the gut microbiota plays a role in maintaining bone mineral density, which can contribute to osseointegration, osteolysis, aseptic loosening, and periprosthetic fractures. Similarly, the gut microbiota influences gut permeability and the potential for bacterial translocation to the bloodstream, increasing susceptibility to PJI.
Conclusions:
Emerging evidence supports the role of the gut microbiota in the development of complications such as aseptic loosening and PJI after total hip or knee arthroplasty. There is a potential for microbial therapies such as probiotics or fecal microbial transplantation to moderate the risk of developing these complications. However, further investigation is required.
Clinical Relevance:
Modulation of the gut microbiota may influence patient outcomes following total joint arthroplasty.
The number of total knee arthroplasty (TKA) and total hip arthroplasty (THA) procedures in the United States is projected to surpass 1 million annually by 20301,2. The economic cost of osteoarthritis is estimated to be up to 2.5% of the gross domestic product (GDP) of high-income countries2-4. Revision surgery, with aseptic loosening of implants and periprosthetic joint infections (PJIs) as the leading causes, accounts for 10% of these costs2,5. Implant failures requiring revision cause pain and require hospital stays for patients1,6,7. Consequently, strategies to reduce aseptic loosening and PJIs are needed.
One of the most fascinating developments in science and medicine over the past 2 decades has been the study of the gut microbiota. With the numbers of microbes dwarfing the totality of cells in the human body they inhabit, this ecosystem of microbes populating our gastrointestinal tract has been implicated in an array of conditions, including metabolic disorders such as diabetes and obesity, cancers, and depression8,9. Treating diseases via the manipulation of the gut microbiota has thus gained tremendous interest.
In the past decade, the so-called “gut-bone axis” has been hailed as a key mediator in bone health10. Healthy host bones are needed for implant osseointegration and to avoid aseptic loosening and periprosthetic fractures following THA and TKA11. Emerging research has also implicated the gut microbiota in PJIs12. Therefore, this review will focus on the potential for interventional microbial therapies that may one day reduce the need for revision surgery following THA and TKA.
The Gut-Bone Axis: A Brief Overview
Bone metabolism is primarily mediated by osteoclasts and osteoblasts, which resorb and install new bone matrix, respectively. The contribution of the gut microbiota to this process is complex (Fig. 1). Germ-free mice were found to have increased bone mass, due to a reduced number of osteoclasts, compared with conventionally raised mice13. Disruption of the microbiota via antibiotics reduces bone quality and strength14. Additionally, the microbiota is a key mediator of bone metabolism in fracture healing, osteoporosis, inflammatory bowel disease, and rheumatoid arthritis. The gut microbiota communicates with distant sites of bone metabolism through immune mediators, regulation of hormones, extracellular vesicles, short-chain fatty acids (SCFAs), vitamins, and aromatic amino acids, among other mechanisms15-19.
Fig. 1.
Microbial involvement in bone homeostasis. Well-known mediators of the gut-bone axis involve the immune system, microbial metabolites such as SCFAs and vitamins, and hormones.
An example of the role of the microbiota is the important contribution made by the segmented filamentous bacteria (SFB) in the gut to fracture healing20. Fracture healing requires an inflammatory phase, and the SFB induce the production of the proinflammatory cytokines tumor necrosis factor (TNF)-α and interleukin (IL)-17 from Th (T helper) 17 cells, aiding in this healing process. Accordingly, the administration of broad-spectrum antibiotics disrupts this pathway and severely blunts the bone healing response20,21. SFB and their downstream proinflammatory cytokines also stimulate osteoclast activity, contributing to osteoporosis. Inflammatory bowel disease (IBD), characterized by an increased profile of proinflammatory bacteria and gut dysbiosis, elevates osteoporosis fracture risk by 40%, with the prevalence of osteopenia and osteoporosis being up to 77% in patients with IBD22-24.
SCFAs, which are the byproducts of indigestible carbohydrates metabolized by microbes, improve bone mineral density (BMD) by promoting osteoblast numbers25. Production by microbes or delivery of SCFAs to parts of the digestive tract promote beneficial bacteria such as Akkermansia muciniphila26. Such organisms have been shown to be critical in metabolism, weight control, and response to immunotherapies in oncology27-32. Extracellular vesicles from A. muciniphila improve bone strength and mass in osteoporotic mice19. The production of vitamins and other important nutrients by the gut microbiota also likely plays an important role in bone health. The absorption of vitamin D, important for bone health due to its role in calcium acquisition and storage, can be increased with the administration of the probiotic bacteria Limosilactobacillus reuteri33. Vitamin K2 is produced by the gut microbiota and inhibits osteoclast differentiation while stimulating osteoblast activity and numbers34-37. Parathyroid hormone (PTH)-induced bone loss is also microbiota-dependent, with germ-free mice and antibiotic-treated mice protected from the effects of the hormone38. Bone health depends on gut barrier integrity as well, and bacteria within the Clostridium, Enterococcus, and Streptococcus genera have been implicated in the metabolism of vitamin A, which is known to improve barrier function18. Finally, proinflammatory bacteria such as Streptococcus species are postulated to contribute to joint pain by the secretion of immunologic factors that pass from the gut into the circulation39.
Given the intimate involvement of the gut microbiota with bone health, manipulating the gut microbiota to achieve better outcomes prior to and following joint arthroplasty is an exciting possibility.
Aseptic Loosening
Aseptic loosening of implants is one of the most common reasons for revision total joint arthroplasty40. The production of wear debris at the joint interface causes inflammation41-43. Wear debris stimulates macrophages to produce proinflammatory cytokines, which increase osteoclast numbers and activity, causing osteolysis (Fig. 2)44. Manipulation of macrophages to an anti-inflammatory state may reduce osteolysis and improve implant longevity45. Suboptimal osseointegration predisposes patients to aseptic loosening46. Low BMD delays osseointegration and reduces the initial stability of the implants46. Poor bone quality also increases implant migration and heightens the risk of revision surgery47,48. This decreases patient satisfaction and slows recovery after the surgery11,49. While use of bisphosphonates such as zoledronic acid reduces implant migration, it is associated with many side effects50-53. Poor bone quality and osteolysis also increase the risk of periprosthetic fractures and are a common cause of revision surgery54,55. Mortality rates following periprosthetic femoral fractures have been reported to be 15.8% (at 18 months) and 16.5% (at 12 months), respectively, with enduring pain and decreased ambulatory function several years after revision surgery54,56-58. While poor surgical technique and the use of cementless implants increase the risk of periprosthetic fractures, implant loosening is a very common cause of these fractures, with up to 66% of patients presenting with implant loosening at the time of their fracture59,60.
Fig. 2.

Mechanism of wear particle-induced osteolysis. Pathophysiology includes debris (pictured as green circular particles) causing macrophage recruitment and inflammation, and concurrent osteolysis (the red lesion in the bone).
Altering the gut microbiota could affect osseointegration and the risk of aseptic loosening and periprosthetic fracture. The gut microbiota influences the inflammatory capacity of the immune system, which is a key mediator of wear particle-induced osteolysis61-65. The gut microbiota of rats with wear particle-induced osteolysis had an increased Firmicutes-to-Bacteroidetes ratio and a reduced abundance of SCFA-producing bacteria, both of which are associated with an increased inflammatory profile61. The administration of the probiotic Lacticaseibacillus casei, known for its immunomodulatory and anti-inflammatory properties, protected mice from wear particle-induced osteolysis while also reducing inflammatory markers and osteoclast number62,63. SCFAs such as propionate and butyrate also inhibited wear particle-induced osteolysis in a mouse calvarium via multiple mechanisms, one being the negative regulation of osteoclast differentiation64. The probiotic Lactobacillus reuteri prevented bone loss in estrogen-depleted mice66, likely by increasing 25-hydroxyvitamin D levels to aid in the absorption of calcium necessary for bone growth33. The treatment of 75 to 80-year-old women presenting with low BMD with the same strain of L. reuteri decreased tibial bone loss over a span of 12 months67. In contrast, supplementation with a multispecies probiotic formulation that included various Lactobacillus and Bifidobacterium species had no effect on the hip and spine BMD of patients 50 to 72 years of age with osteopenia; however, outcomes were measured only at 6 months68. The levels of the inflammatory cytokine TNF-α and of osteoclast-inducing PTH were significantly reduced in the serum, indicating that the multispecies probiotic might play a positive role in bone quality in the long term68. While 4 species of Lactobacillus were used, L. reuteri was not part of the formulation, suggesting that treatment effectiveness could depend on the species and strains of probiotics.
Use of anti-inflammatory probiotics should be evaluated for its potential to protect against osteolysis and aid in increasing BMD, allowing for proper osseointegration and the avoidance of aseptic loosening. Longitudinal clinical trials studying the prevalence of aseptic loosening in patients given probiotics containing the Lactobacillus and Bifidobacterium species before, during, and after arthroplasty are indicated. Fecal microbial transplantation (FMT) may also be considered as an intensive option to protect high-risk patients, given its ability to produce persistent changes in the gut microbiota69. Preclinical animal data show that FMT can help treat osteoporosis, but data on its efficacy and concurrent risks are still lacking70,71. Even with the advent of oral capsules, which are more easily administered than an enema or nasal gastric delivery, FMT remains unpopular for patients without life-threatening conditions, given its expense and required screening of both the donor and the recipient69,72,73.
Periprosthetic Joint Infections
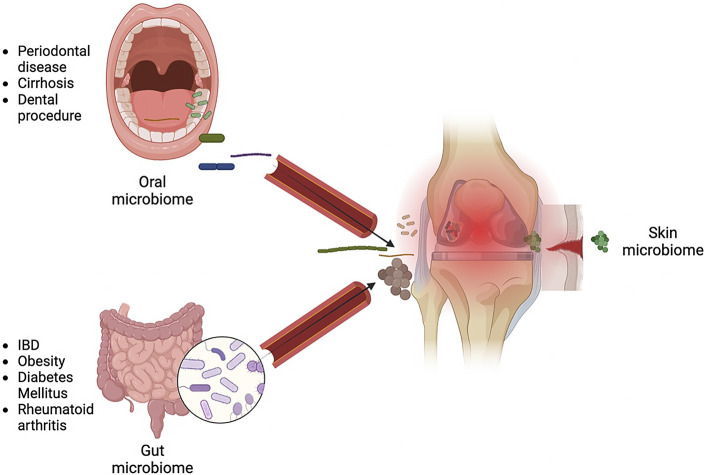
Although its prevalence is <2%, the impact of PJI on the individual patient is severe, with a 5-fold increase in mortality, a higher risk of morbidity, and a reduced quality of life due to repeat surgical procedures and loss of ambulatory capacity74. Treatment of PJI entails a combination of antibiotic therapy and surgery, but up to 35% of these interventions fail75,76. Bacteria can adhere to and colonize the implant, forming a biofilm that is challenging to disrupt via conventional antibiotic courses77,78. While bacterial colonization and subsequent PJI were previously thought to occur solely due to contamination from skin during the initial surgical procedure, recent work has revealed the presence of microbes in the joints of patients even prior to surgery12. This section of the review will focus on the lesser-known sources of microbes within joint spaces: the oral and gut microbiota (Fig. 3)12,79-82.
Fig. 3.
Three sources of bacteria in periprosthetic joint infections and risk factors.
It was postulated that 6% to 13% of PJIs are caused by bacteria resident in the oral cavity and saliva83. The oral bacterial species Fusobacterium nucleatum and Peptostreptococcus have been reported to cause PJI in patients following dental surgery84,85. Oral bacteria such as Prevotella intermedia and Porphyromonas gingivalis have been found in both the subgingival dental plaques and the synovial fluid of patients with both rheumatoid arthritis and periodontal disease81. Additionally, identical clones of the oral bacterial species F. nucleatum and Serratia proteamaculans were found in both dental plaques and synovial fluid of patients with both rheumatoid arthritis and periodontal disease82. The 2 diseases are thought to have similar pathophysiological mechanisms, and since rheumatoid arthritis has been associated with an increased risk of PJI, the identification of oral microbiota constituents in these joints causes considerable concern86,87. Despite this, no association between antibiotic prophylaxis and the incidence of PJI following dental procedures has been found88,89. The American Academy of Orthopaedic Surgeons recommends antibiotic prophylaxis only in immunocompromised patients, patients with poor glycemic control, those who have had an arthroplasty within the past year, and those with a history of PJI90.
Similarly, enteric pathogens from the gut can spread to joint spaces via the hematogenous route to seed and infect implant sites. This is thought to be the mechanism of the increased PJI risk in patients with IBD91,92. The dysregulated and inflamed intestinal microbiota paves the way to a disrupted gut barrier, leading to bacterial translocation into the bloodstream. Since only a small amount of bacteria is required to establish a PJI, this phenomenon puts patients at high risk for implant failure93. Patients with IBD are often on immunosuppressive therapies, which may also increase their risk of PJI. While the most recent guidelines for prophylactic antibiotic therapy provide recommendations for immunocompromised patients and those who have obesity, liver cirrhosis, type-2 diabetes, and other conditions associated with gut dysbiosis must also be considered in the decision whether to administer such therapy8,9,94-96. Prophylactic antibiotic therapy in patients with those conditions may contribute to dysbiosis and worsen the risk of PJI in immunocompetent patients with gut dysbiosis. The duration of treatment also matters, as the long-term use of antibiotics in women >60 years of age was shown to significantly increase the risk of all-cause mortality, while treatment for <2 months did not97. Persistent changes in the microbiota have been documented following even short-term antibiotic use98. For example, a 7-day course of clindamycin caused reductions in the Bacteroides species that persisted for 2 years, while a 10-day course reduced Bifidobacterium to levels that could not be restored until 1 year post-treatment99-101. Although the link has yet to be explored, such profound disturbances in the enteric microbiota might predispose patients with comorbidities to altered gut microbial homeostasis, further compromised barrier function, and a subsequently increased risk of PJI. Comorbidities such as congestive heart failure, diabetes, obesity, renal disease, rheumatoid arthritis, and liver cirrhosis are just some of the many risk factors for the development of PJI102-104. Many of these comorbidities are also associated with gut microbiota perturbation that may increase these patients’ susceptibility for PJI39,94.
Use of probiotics to alter gut microbiota composition, improving the gut barrier and decreasing bacterial translocation to the bloodstream, is an area requiring further research. For example, obesity is strongly associated with an increased abundance of the Firmicutes phylum at the expense of Bacteroidetes within the gut of patients, with this shift reversed by weight loss95. Mice with this shift in their microbiota composition after antibiotic therapy are more vulnerable to PJI than mice with normal microbiota78. Likewise, patients with IBD are at a higher risk for PJI. However, their microbiota has the opposite shift: an increased abundance of Bacteroidetes compared with Firmicutes91,105. As such, use of specific probiotics to restore a healthy Firmicutes-to-Bacteroidetes balance for patients at higher risk for PJI could be explored.
Limitations in the Field
Although an ample number of studies indicate a strong possibility of microbial therapies in the future of orthopaedics, presently this field does have some limitations. Most microbial therapeutic modalities such as FMT, prebiotics, and fermented food remain greatly understudied regarding their direct impact on human bone health70,106,107. Moreover, the long-term efficacy of probiotics in functionally altering the gut microbiota remains controversial, with most studies demonstrating no substantial changes in overall microbial diversity following probiotic supplementation in humans108. Since PJIs can occur anytime during the life of an arthroplasty recipient, the therapy’s inability to be efficacious over a patient’s lifespan could be a limitation108-110. If probiotics fail, FMT or other microbial therapeutics (prebiotic, phage, defined microbial consortia, fermented food, and fermentation products) may be explored as an alternative; FMT has been shown to produce persistent changes in the recipient microbial signature69. However, since it remains unknown whether FMT will help or worsen the incidence of aseptic loosening and PJI, exploration of probiotics continues to be the preferred route.
It is also possible for probiotic therapies to have functional effects without creating major alterations in the microbial ecosystem, making their mechanism of action challenging to study105,106,111. Consequently, the reliability of probiotic treatment in humans with differing basal microbial signatures can become hard to predict105,106,111. The present literature also has scant information on the appropriate dosages of microbial therapies for the bone health of humans. This is primarily because studies in this developing field have largely employed preclinical animal models, indicating the need for future studies in humans106.
Conclusions
The involvement of the gut microbiota in the body’s physiology and pathophysiology has made it a target in the treatment of various diseases. Its effect on bone and joint conditions, especially with respect to its control of systemic inflammation, should not be ignored. Probiotics are presently being investigated to treat a wide range of conditions, ranging from bloating and traveler’s diarrhea to atopic dermatitis and clinical depression112,113. Presently popular and available probiotic formulations include the Lactobacillus and Bifidobacterium genera of bacteria, and emerging research shows that various other bacteria such as A. muciniphila and other species could be promising, with multifarious health effects19,112-114. Given the contributions of the gut microbiota to bone health, probiotics are a potential future therapeutic option in populations of patients requiring or living with hip and knee implants. However, dosages and therapeutic timelines are far from being elucidated, and the ability of probiotic interventions to cause long-term changes in the gut microbiota remains controversial108-110,113. Finally, nonspecific probiotic treatment may worsen gut health in certain circumstances, highlighting the need for personalized therapies in the future115. Still, with preclinical and clinical studies strongly suggesting that the manipulation of the gut microbiota may reduce the incidence of aseptic loosening and potentially even PJIs, further investigation of probiotic supplementation in this patient population is supported.
Footnotes
Investigation performed at the London Health Sciences Centre, Schulich School of Medicine & Dentistry, Western University, London, Ontario, Canada
Disclosure: Funding for this study was provided by an Arthritis Society of Canada Stars Career Development Award and the Schulich School of Medicine & Dentistry Summer Research Training Program. The Article Processing Charge for open access publication was funded by the Arthritis Society. The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article (http://links.lww.com/JBJSOA/A623).
Contributor Information
Arjuna Srikrishnaraj, Email: asrikrishnaraj2026@meds.uwo.ca.
Brent A. Lanting, Email: brent.lanting@lhsc.on.ca.
Jeremy P. Burton, Email: jeremy.burton@lawsonresearch.com.
References
- 1.Sloan M, Premkumar A, Sheth NP. Projected Volume of Primary Total Joint Arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg Am. 2018. Sep 5;100(17):1455-60. [DOI] [PubMed] [Google Scholar]
- 2.Canadian Institute for Health Information. CJRR annual report: Hip and knee replacements in Canada. Accessed 2023 Jun 19. https://www.cihi.ca/en/cjrr-annual-report-hip-and-knee-replacements-in-canada. [Google Scholar]
- 3.Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019. Apr 27;393(10182):1745-59. [DOI] [PubMed] [Google Scholar]
- 4.Hunter DJ, Schofield D, Callander E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol. 2014. Jul;10(7):437-41. [DOI] [PubMed] [Google Scholar]
- 5.Delanois RE, Mistry JB, Gwam CU, Mohamed NS, Choksi US, Mont MA. Current Epidemiology of Revision Total Knee Arthroplasty in the United States. J Arthroplasty. 2017. Sep;32(9):2663-8. [DOI] [PubMed] [Google Scholar]
- 6.Klug A, Gramlich Y, Rudert M, Drees P, Hoffmann R, Weißenberger M, Kutzner KP. The projected volume of primary and revision total knee arthroplasty will place an immense burden on future health care systems over the next 30 years. Knee Surg Sports Traumatol Arthrosc. 2021. Oct;29(10):3287-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keswani A, Lovy AJ, Robinson J, Levy R, Chen D, Moucha CS. Risk Factors Predict Increased Length of Stay and Readmission Rates in Revision Joint Arthroplasty. J Arthroplasty. 2016. Mar;31(3):603-8. [DOI] [PubMed] [Google Scholar]
- 8.Adak A, Khan MR. An insight into gut microbiota and its functionalities. Cell Mol Life Sci. 2019. Feb;76(3):473-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomaa EZ. Human gut microbiota/microbiome in health and diseases: a review. Antonie Van Leeuwenhoek. 2020. Dec;113(12):2019-40. [DOI] [PubMed] [Google Scholar]
- 10.Lu L, Chen X, Liu Y, Yu X. Gut microbiota and bone metabolism. FASEB J. 2021. Jul;35(7):e21740. [DOI] [PubMed] [Google Scholar]
- 11.Aro HT, Engelke K, Mattila K, Löyttyniemi E. Volumetric Bone Mineral Density in Cementless Total Hip Arthroplasty in Postmenopausal Women: Effects on Primary Femoral Stem Stability and Clinical Recovery. J Bone Joint Surg Am. 2021. Jun 16;103(12):1072-82. [DOI] [PubMed] [Google Scholar]
- 12.Fernández-Rodríguez D, Baker CM, Tarabichi S, Johnson EE, Ciccotti MG, Parvizi J. Mark Coventry Award: Human Knee Has a Distinct Microbiome: Implications for Periprosthetic Joint Infection. J Arthroplasty. 2023. Jun;38(6S):S2-6. [DOI] [PubMed] [Google Scholar]
- 13.Sjögren K, Engdahl C, Henning P, Lerner UH, Tremaroli V, Lagerquist MK, Bäckhed F, Ohlsson C. The gut microbiota regulates bone mass in mice. J Bone Miner Res. 2012. Jun;27(6):1357-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castaneda M, Smith KM, Nixon JC, Hernandez CJ, Rowan S. Alterations to the gut microbiome impair bone tissue strength in aged mice. Bone Rep. 2021. Apr 8;14:101065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sassi F, Tamone C, D'Amelio P. Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients. 2018. Nov 3;10(11):1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan J, Herzog JW, Tsang K, Brennan CA, Bower MA, Garrett WS, Sartor BR, Aliprantis AO, Charles JF. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci U S A. 2016. Nov 22;113(47):E7554-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, Ho WTP, Liu C, Chow SK, Ip M, Yu J, Wong HS, Cheung WH, Sung JJY, Wong RMY. The role of gut microbiota in bone homeostasis. Bone Joint Res. 2021. Jan;10(1):51-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chmiel JA, Stuivenberg GA, Al KF, Akouris PP, Razvi H, Burton JP, Bjazevic J. Vitamins as regulators of calcium-containing kidney stones - new perspectives on the role of the gut microbiome. Nat Rev Urol. 2023. Oct;20(10):615-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu JH, Chen CY, Liu ZZ, Luo ZW, Rao SS, Jin L, Wan TF, Yue T, Tan YJ, Yin H, Yang F, Huang FY, Guo J, Wang YY, Xia K, Cao J, Wang ZX, Hong CG, Luo MJ, Hu XK, Liu YW, Du W, Luo J, Hu Y, Zhang Y, Huang J, Li HM, Wu B, Liu HM, Chen TH, Qian YX, Li YY, Feng SK, Chen Y, Qi LY, Xu R, Tang SY, Xie H. Extracellular Vesicles from Child Gut Microbiota Enter into Bone to Preserve Bone Mass and Strength. Adv Sci (Weinh). 2021. Feb 17;8(9):2004831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dar HY, Perrien DS, Pal S, Stoica A, Uppuganti S, Nyman JS, Jones RM, Weitzmann MN, Pacifici R. Callus γδ T cells and microbe-induced intestinal Th17 cells improve fracture healing in mice. J Clin Invest. 2023. Apr 17;133(8):e166577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ono T, Okamoto K, Nakashima T, Nitta T, Hori S, Iwakura Y, Takayanagi H. IL-17-producing γδ T cells enhance bone regeneration. Nat Commun. 2016. Mar 11;7:10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ali T, Lam D, Bronze MS, Humphrey MB. Osteoporosis in inflammatory bowel disease. Am J Med. 2009. Jul;122(7):599-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pollak RD, Karmeli F, Eliakim R, Ackerman Z, Tabb K, Rachmilewitz D. Femoral neck osteopenia in patients with inflammatory bowel disease. Am J Gastroenterol. 1998. Sep;93(9):1483-90. [DOI] [PubMed] [Google Scholar]
- 24.Bjarnason I, Macpherson A, Mackintosh C, Buxton-Thomas M, Forgacs I, Moniz C. Reduced bone density in patients with inflammatory bowel disease. Gut. 1997. Feb;40(2):228-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallimann A, Magrath W, Thompson K, Moriarty T, Richards RG, Akdis CA, O’Mahony L, Hernandez CJ. Gut microbial-derived short-chain fatty acids and bone: a potential role in fracture healing. Eur Cell Mater. 2021. Apr 21;41:454-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daisley BA, Chanyi RM, Abdur-Rashid K, Al KF, Gibbons S, Chmiel JA, Wilcox H, Reid G, Anderson A, Dewar M, Nair SM, Chin J, Burton JP. Abiraterone acetate preferentially enriches for the gut commensal Akkermansia muciniphila in castrate-resistant prostate cancer patients. Nat Commun. 2020. Dec 1;11(1):4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Tang L, Feng Y, Zhao S, Han M, Zhang C, Yuan G, Zhu J, Cao S, Wu Q, Li L, Zhang Z. A purified membrane protein from Akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of CD8+ T cells in mice. Gut. 2020. Nov;69(11):1988-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Derosa L, Routy B, Thomas AM, Iebba V, Zalcman G, Friard S, Mazieres J, Audigier-Valette C, Moro-Sibilot D, Goldwasser F, Silva CAC, Terrisse S, Bonvalet M, Scherpereel A, Pegliasco H, Richard C, Ghiringhelli F, Elkrief A, Desilets A, Blanc-Durand F, Cumbo F, Blanco A, Boidot R, Chevrier S, Daillère R, Kroemer G, Alla L, Pons N, Le Chatelier E, Galleron N, Roume H, Dubuisson A, Bouchard N, Messaoudene M, Drubay D, Deutsch E, Barlesi F, Planchard D, Segata N, Martinez S, Zitvogel L, Soria JC, Besse B. Intestinal Akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat Med. 2022. Feb;28(2):315-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoon HS, Cho CH, Yun MS, Jang SJ, You HJ, Kim JH, Han D, Cha KH, Moon SH, Lee K, Kim YJ, Lee SJ, Nam TW, Ko G. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat Microbiol. 2021. May;6(5):563-73. [DOI] [PubMed] [Google Scholar]
- 30.Rao Y, Kuang Z, Li C, Guo S, Xu Y, Zhao D, Hu Y, Song B, Jiang Z, Ge Z, Liu X, Li C, Chen S, Ye J, Huang Z, Lu Y. Gut Akkermansia muciniphila ameliorates metabolic dysfunction-associated fatty liver disease by regulating the metabolism of L-aspartate via gut-liver axis. Gut Microbes. 2021. Jan-Dec;13(1):1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013. May 28;110(22):9066-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hasani A, Ebrahimzadeh S, Hemmati F, Khabbaz A, Hasani A, Gholizadeh P. The role of Akkermansia muciniphila in obesity, diabetes and atherosclerosis. J Med Microbiol. 2021. Oct;70(10). [DOI] [PubMed] [Google Scholar]
- 33.Jones ML, Martoni CJ, Prakash S. Oral supplementation with probiotic L. reuteri NCIMB 30242 increases mean circulating 25-hydroxyvitamin D: a post hoc analysis of a randomized controlled trial. J Clin Endocrinol Metab. 2013. Jul;98(7):2944-51. [DOI] [PubMed] [Google Scholar]
- 34.Iwamoto J, Yeh JK, Takeda T. Effect of vitamin K2 on cortical and cancellous bones in orchidectomized and/or sciatic neurectomized rats. J Bone Miner Res. 2003. Apr;18(4):776-83. [DOI] [PubMed] [Google Scholar]
- 35.Iwamoto J, Matsumoto H, Tadeda T, Sato Y, Yeh JK. Comparison of the effect of vitamin K(2) and risedronate on trabecular bone in glucocorticoid-treated rats: a bone histomorphometry study. Yonsei Med J. 2009. Apr 30;50(2):189-94. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Myneni VD, Mezey E. Regulation of bone remodeling by vitamin K2. Oral Dis. 2017. Nov;23(8):1021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chatterjee K, Mazumder PM, Sarkar SR, Saha R, Chatterjee A, Sarkar B, Banerjee S. Neuroprotective effect of Vitamin K2 against gut dysbiosis associated cognitive decline. Physiol Behav. 2023. Oct 1;269:114252. [DOI] [PubMed] [Google Scholar]
- 38.Yu M, Malik Tyagi A, Li JY, Adams J, Denning TL, Weitzmann MN, Jones RM, Pacifici R. PTH induces bone loss via microbial-dependent expansion of intestinal TNF+ T cells and Th17 cells. Nat Commun. 2020. Jan 24;11(1):468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boer CG, Radjabzadeh D, Medina-Gomez C, Garmaeva S, Schiphof D, Arp P, Koet T, Kurilshikov A, Fu J, Ikram MA, Bierma-Zeinstra S, Uitterlinden AG, Kraaij R, Zhernakova A, van Meurs JBJ. Intestinal microbiome composition and its relation to joint pain and inflammation. Nat Commun. 2019. Oct 25;10(1):4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tarazi JM, Chen Z, Scuderi GR, Mont MA. The Epidemiology of Revision Total Knee Arthroplasty. J Knee Surg. 2021. Nov;34(13):1396-401. [DOI] [PubMed] [Google Scholar]
- 41.Cong Y, Wang Y, Yuan T, Zhang Z, Ge J, Meng Q, Li Z, Sun S. Macrophages in aseptic loosening: Characteristics, functions, and mechanisms. Front Immunol. 2023. Mar 8;14:1122057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abu-Amer Y, Darwech I, Clohisy JC. Aseptic loosening of total joint replacements: mechanisms underlying osteolysis and potential therapies. Arthritis Res Ther. 2007;9(Suppl 1):S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang Y, Jia T, Wooley PH, Yang SY. Current research in the pathogenesis of aseptic implant loosening associated with particulate wear debris. Acta Orthop Belg. 2013. Feb;79(1):1-9. [PubMed] [Google Scholar]
- 44.Gallo J, Goodman SB, Konttinen YT, Raska M. Particle disease: biologic mechanisms of periprosthetic osteolysis in total hip arthroplasty. Innate Immun. 2013;19(2):213-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goodman SB, Gibon E, Pajarinen J, Lin TH, Keeney M, Ren PG, Nich C, Yao Z, Egashira K, Yang F, Konttinen YT. Novel biological strategies for treatment of wear particle-induced periprosthetic osteolysis of orthopaedic implants for joint replacement. J R Soc Interface. 2014. Jan 29;11(93):20130962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aro HT Alm JJ Moritz N Mäkinen TJ,Lankinen P. Low BMD affects initial stability and delays stem osseointegration in cementless total hip arthroplasty in women: a 2-year RSA study of 39 patients. Acta Orthop. 2012. Apr;83(2):107-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Voort P, Pijls BG, Nieuwenhuijse MJ, Jasper J, Fiocco M, Plevier JW, Middeldorp S, Valstar ER, Nelissen RG. Early subsidence of shape-closed hip arthroplasty stems is associated with late revision. A systematic review and meta-analysis of 24 RSA studies and 56 survival studies. Acta Orthop. 2015;86(5):575-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pijls BG, Valstar ER, Nouta KA, Plevier JWM, Fiocco M, Middeldorp S, Nelissen RGHH. Early migration of tibial components is associated with late revision: a systematic review and meta-analysis of 21,000 knee arthroplasties. Acta Orthop. 2012. Dec;83(6):614-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andersen MR, Winther NS, Lind T, Schrøder HM, Flivik G, Petersen MM. Low Preoperative BMD Is Related to High Migration of Tibia Components in Uncemented TKA-92 Patients in a Combined DEXA and RSA Study With 2-Year Follow-Up. J Arthroplasty. 2017. Jul;32(7):2141-6. [DOI] [PubMed] [Google Scholar]
- 50.Liu Y, Xu JW, Li MY, Wu LM, Zeng Y, Shen B. Zoledronic Acid for Periprosthetic Bone Mineral Density Changes in Patients With Osteoporosis After Hip Arthroplasty-An Updated Meta-Analysis of Six Randomized Controlled Trials. Front Med (Lausanne). 2021. Dec 23;8:801282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fink HA, MacDonald R, Forte ML, Rosebush CE, Ensrud KE, Schousboe JT, Nelson VA, Ullman K, Butler M, Olson CM, Taylor BC, Brasure M, Wilt TJ. Long-Term Drug Therapy and Drug Discontinuations and Holidays for Osteoporosis Fracture Prevention: A Systematic Review. Ann Intern Med. 2019. Jul 2;171(1):37-50. [DOI] [PubMed] [Google Scholar]
- 52.Xu Q, Li D, Chen J, Yang J, Yan J, Xia Y, Zhang F, Wang X, Cao H. Crosstalk between the gut microbiota and postmenopausal osteoporosis: Mechanisms and applications. Int Immunopharmacol. 2022. Sep;110:108998. [DOI] [PubMed] [Google Scholar]
- 53.Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR; HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007. May 3;356(18):1809-22. [DOI] [PubMed] [Google Scholar]
- 54.Capone A, Congia S, Civinini R, Marongiu G. Periprosthetic fractures: epidemiology and current treatment. Clin Cases Miner Bone Metab. 2017. May-Aug;14(2):189-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Della Rocca GJ, Leung KS, Pape HC. Periprosthetic fractures: epidemiology and future projections. J Orthop Trauma. 2011. Jun;25 2:S66-70. [DOI] [PubMed] [Google Scholar]
- 56.Drew JM, Griffin WL, Odum SM, Van Doren B, Weston BT, Stryker LS. Survivorship After Periprosthetic Femur Fracture: Factors Affecting Outcome. J Arthroplasty. 2016. Jun;31(6):1283-8. [DOI] [PubMed] [Google Scholar]
- 57.Bhattacharyya T, Chang D, Meigs JB, Estok DM, 2nd, Malchau H. Mortality after periprosthetic fracture of the femur. J Bone Joint Surg Am. 2007. Dec;89(12):2658-62. [DOI] [PubMed] [Google Scholar]
- 58.Zheng L, Lee WY, Hwang DS, Kang C, Noh CK. Could Patient Undergwent Surgical Treatment for Periprosthetic Femoral Fracture after Hip Arthroplasty Return to Their Status before Trauma? Hip Pelvis. 2016. Jun;28(2):90-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lindahl H, Garellick G, Regnér H, Herberts P, Malchau H. Three hundred and twenty-one periprosthetic femoral fractures. J Bone Joint Surg Am. 2006;88(6):1215-22. [DOI] [PubMed] [Google Scholar]
- 60.Abdel MP, Watts CD, Houdek MT, Lewallen DG, Berry DJ. Epidemiology of periprosthetic fracture of the femur in 32 644 primary total hip arthroplasties: a 40-year experience. Bone Joint J. 2016. Apr;98-B(4):461-7. [DOI] [PubMed] [Google Scholar]
- 61.Moran MM, Wilson BM, Li J, Engen PA, Naqib A, Green SJ, Virdi AS, Plaas A, Forsyth CB, Keshavarzian A, Sumner DR. The gut microbiota may be a novel pathogenic mechanism in loosening of orthopedic implants in rats. FASEB J. 2020. Nov;34(11):14302-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Z, Xue K, Bai M, Deng Z, Gan J, Zhou G, Qian H, Bao N, Zhao J. Probiotics protect mice from CoCrMo particles-induced osteolysis. Int J Nanomedicine. 2017. Jul 27;12:5387-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aghamohammad S, Sepehr A, Miri ST, Najafi S, Pourshafie MR, Rohani M. Anti-inflammatory and immunomodulatory effects of Lactobacillus spp. as a preservative and therapeutic agent for IBD control. Immun Inflamm Dis. 2022. Jun;10(6):e635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu YL, Zhang CH, Teng Y, Pan Y, Liu NC, Liu PX, Zhu X, Su XL, Lin J. Propionate and butyrate attenuate macrophage pyroptosis and osteoclastogenesis induced by CoCrMo alloy particles. Mil Med Res. 2022. Aug 23;9(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang F, Pan H, Tan Z, Chen L, Li T, Liu Y. Prevotella histicola Prevented Particle-Induced Osteolysis via Gut Microbiota-Dependent Modulation of Inflammation in Ti-Treated Mice. Probiotics Antimicrob Proteins. 2023. Mar 10. [DOI] [PubMed] [Google Scholar]
- 66.Britton RA, Irwin R, Quach D, Schaefer L, Zhang J, Lee T, Parameswaran N, McCabe LR. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J Cell Physiol. 2014. Nov;229(11):1822-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nilsson AG, Sundh D, Bäckhed F, Lorentzon M. Lactobacillus reuteri reduces bone loss in older women with low bone mineral density: a randomized, placebo-controlled, double-blind, clinical trial. J Intern Med. 2018. Sep;284(3):307-17. [DOI] [PubMed] [Google Scholar]
- 68.Jafarnejad S, Djafarian K, Fazeli MR, Yekaninejad MS, Rostamian A, Keshavarz SA. Effects of a Multispecies Probiotic Supplement on Bone Health in Osteopenic Postmenopausal Women: A Randomized, Double-blind, Controlled Trial. J Am Coll Nutr. 2017. Sep-Oct;36(7):497-506. [DOI] [PubMed] [Google Scholar]
- 69.Goloshchapov OV, Olekhnovich EI, Sidorenko SV, Moiseev IS, Kucher MA, Fedorov DE, Pavlenko AV, Manolov AI, Gostev VV, Veselovsky VA, Klimina KM, Kostryukova ES, Bakin EA, Shvetcov AN, Gumbatova ED, Klementeva RV, Shcherbakov AA, Gorchakova MV, Egozcue JJ, Pawlowsky-Glahn V, Suvorova MA, Chukhlovin AB, Govorun VM, Ilina EN, Afanasyev BV. Long-term impact of fecal transplantation in healthy volunteers. BMC Microbiol. 2019. Dec 30;19(1):312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang YW, Cao MM, Li YJ, Zhang RL, Wu MT, Yu Q, Rui YF. Fecal microbiota transplantation as a promising treatment option for osteoporosis. J Bone Miner Metab. 2022. Nov;40(6):874-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang YW, Cao MM, Li YJ, Lu PP, Dai GC, Zhang M, Wang H, Rui YF. Fecal microbiota transplantation ameliorates bone loss in mice with ovariectomy-induced osteoporosis via modulating gut microbiota and metabolic function. J Orthop Translat. 2022. Sep 26;37:46-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wynn AB, Beyer G, Richards M, Ennis LA. Procedure, Screening, and Cost of Fecal Microbiota Transplantation. Cureus. 2023. Feb 17;15(2):e35116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chauhan U, Popov J, Farbod Y, Kalantar M, Wolfe M, Moayyedi P, Marshall JK, Halder S, Kaasalainen S. Fecal Microbiota Transplantation for the Treatment of Ulcerative Colitis: A Qualitative Assessment of Patient Perceptions and Experiences. J Can Assoc Gastroenterol. 2021. Mar 26;4(6):e120-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zmistowski B, Karam JA, Durinka JB, Casper DS, Parvizi J. Periprosthetic joint infection increases the risk of one-year mortality. J Bone Joint Surg Am. 2013. Dec 18;95(24):2177-84. [DOI] [PubMed] [Google Scholar]
- 75.Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE; Foreign-Body Infection (FBI) Study Group. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. JAMA. 1998. May 20;279(19):1537-41. [DOI] [PubMed] [Google Scholar]
- 76.Chaussade H, Uçkay I, Vuagnat A, Druon J, Gras G, Rosset P, Lipsky BA, Bernard L. Antibiotic therapy duration for prosthetic joint infections treated by Debridement and Implant Retention (DAIR): Similar long-term remission for 6 weeks as compared to 12 weeks. Int J Infect Dis. 2017. Oct;63:37-42. [DOI] [PubMed] [Google Scholar]
- 77.Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004. Oct 14;351(16):1645-54. [DOI] [PubMed] [Google Scholar]
- 78.Hernandez CJ, Yang X, Ji G, Niu Y, Sethuraman AS, Koressel J, Shirley M, Fields MW, Chyou S, Li TM, Luna M, Callahan RL, Ross FP, Lu TT, Brito IL, Carli AV, Bostrom MPG. Disruption of the Gut Microbiome Increases the Risk of Periprosthetic Joint Infection in Mice. Clin Orthop Relat Res. 2019. Nov;477(11):2588-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Luo JM, Guo L, Chen H, Yang PF, Xiong R, Peng Y, Yang L. A study of pre-operative presence of micro-organisms in affected knee joints of rheumatoid arthritis patients who need total knee arthroplasty. Knee. 2017. Mar;24(2):409-18. [DOI] [PubMed] [Google Scholar]
- 80.Torchia MT, Amakiri I, Werth P, Moschetti W. Characterization of native knee microorganisms using next-generation sequencing in patients undergoing primary total knee arthroplasty. Knee. 2020. Jun;27(3):1113-9. [DOI] [PubMed] [Google Scholar]
- 81.Martinez-Martinez RE, Abud-Mendoza C, Patiño-Marin N, Rizo-Rodríguez JC, Little JW, Loyola-Rodríguez JP. Detection of periodontal bacterial DNA in serum and synovial fluid in refractory rheumatoid arthritis patients. J Clin Periodontol. 2009. Dec;36(12):1004-10. [DOI] [PubMed] [Google Scholar]
- 82.Témoin S, Chakaki A, Askari A, El-Halaby A, Fitzgerald S, Marcus RE, Han YW, Bissada NF. Identification of oral bacterial DNA in synovial fluid of patients with arthritis with native and failed prosthetic joints. J Clin Rheumatol. 2012. Apr;18(3):117-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moreira AI, Mendes L, Pereira JA. Is there scientific evidence to support antibiotic prophylaxis in patients with periodontal disease as a means to decrease the risk of prosthetic joint infections? A systematic review. Int Orthop. 2020. Feb;44(2):231-6. [DOI] [PubMed] [Google Scholar]
- 84.Shi TB, Fang XY, Wang CX, Cai YQ, Li WB, Zhang WM. Rare Occurrence of Acute Hematogenous Periprosthetic Joint Infection Due to Fusobacterium Nucleatum in the Background of a Dental Procedure: A Case Report. Orthop Surg. 2020. Dec;12(6):2026-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bartz H, Nonnenmacher Cb, Bollmann C, Kuhl M, Zimmermann S, Heeg K, Mutters R. Micromonas (Peptostreptococcus) micros: unusual case of prosthetic joint infection associated with dental procedures. Int J Med Microbiol. 2005. Jan;294(7):465-70. [DOI] [PubMed] [Google Scholar]
- 86.Premkumar A, Morse K, Levack AE, Bostrom MP, Carli AV. Periprosthetic Joint Infection in Patients with Inflammatory Joint Disease: Prevention and Diagnosis. Curr Rheumatol Rep. 2018. Sep 10;20(11):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morrison TA, Figgie M, Miller AO, Goodman SM. Periprosthetic joint infection in patients with inflammatory joint disease: a review of risk factors and current approaches to diagnosis and management. HSS J. 2013. Jul;9(2):183-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kao FC, Hsu YC, Chen WH, Lin JN, Lo YY, Tu YK. Prosthetic joint infection following invasive dental procedures and antibiotic prophylaxis in patients with hip or knee arthroplasty. Infect Control Hosp Epidemiol. 2017. Feb;38(2):154-61. [DOI] [PubMed] [Google Scholar]
- 89.Berbari EF, Osmon DR, Carr A, Hanssen AD, Baddour LM, Greene D, Kupp LI, Baughan LW, Harmsen WS, Mandrekar JN, Therneau TM, Steckelberg JM, Virk A, Wilson WR. Dental procedures as risk factors for prosthetic hip or knee infection: a hospital-based prospective case-control study. Clin Infect Dis. 2010. Jan 1;50(1):8-16. [DOI] [PubMed] [Google Scholar]
- 90.Quinn RH, Murray JN, Pezold R, Sevarino KS; Members of the Writing and Voting Panels of the AUC for the Management of Patients with Orthopaedic Implants Undergoing Dental Procedures. The American Academy of Orthopaedic Surgeons appropriate use criteria for the management of patients with orthopaedic implants undergoing dental procedures. J Bone Joint Surg Am. 2017. Jan 18;99(2):161-3. [DOI] [PubMed] [Google Scholar]
- 91.Chisari E, D’mello D, Sherman MB, Parvizi J. Inflammatory Bowel Diseases Increase the Risk of Periprosthetic Joint Infection. J Bone Joint Surg Am. 2022. Jan 19;104(2):160-5. [DOI] [PubMed] [Google Scholar]
- 92.Remily EA, Sax OC, Douglas SJ, Salib CG, Salem HS, Monárrez RG, Delanois RE. Inflammatory bowel disease is associated with increased complications after total knee arthroplasty. Knee. 2023. Jan;40:313-8. [DOI] [PubMed] [Google Scholar]
- 93.Tande AJ, Patel R. Prosthetic joint infection. Clin Microbiol Rev. 2014. Apr;27(2):302-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Al Bander Z, Nitert MD, Mousa A, Naderpoor N. The Gut Microbiota and Inflammation: An Overview. Int J Environ Res Public Health. 2020. Oct 19;17(20):7618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006. Dec 21;444(7122):1022-3. [DOI] [PubMed] [Google Scholar]
- 96.Acharya C, Sahingur SE, Bajaj JS. Microbiota, cirrhosis, and the emerging oral-gut-liver axis. JCI Insight. 2017. Oct 5;2(19):e94416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Heianza Y, Ma W, Li X, Cao Y, Chan AT, Rimm EB, Hu FB, Rexrode KM, Manson JE, Qi L. Duration and Life-Stage of Antibiotic Use and Risks of All-Cause and Cause-Specific Mortality: Prospective Cohort Study. Circ Res. 2020. Jan 31;126(3):364-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Patangia DV, Anthony Ryan C, Dempsey E, Paul Ross R, Stanton C. Impact of antibiotics on the human microbiome and consequences for host health. Microbiologyopen. 2022. Feb;11(1):e1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rashid MU, Zaura E, Buijs MJ, Keijser BJF, Crielaard W, Nord CE, Weintraub A. Determining the long-term effect of antibiotic administration on the human normal intestinal microbiota using culture and pyrosequencing methods. Clin Infect Dis. 2015. May 15;60(Suppl 2):S77-84. [DOI] [PubMed] [Google Scholar]
- 100.Löfmark S, Jernberg C, Jansson JK, Edlund C. Clindamycin-induced enrichment and long-term persistence of resistant Bacteroides spp. and resistance genes. J Antimicrob Chemother. 2006. Dec;58(6):1160-7. [DOI] [PubMed] [Google Scholar]
- 101.Jernberg C, Löfmark S, Edlund C, Jansson JK. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. 2007. May;1(1):56-66. [DOI] [PubMed] [Google Scholar]
- 102.Jiang SL, Schairer WW, Bozic KJ. Increased rates of periprosthetic joint infection in patients with cirrhosis undergoing total joint arthroplasty. Clin Orthop Relat Res. 2014. Aug;472(8):2483-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bozic KJ, Lau E, Kurtz S, Ong K, Berry DJ. Patient-related risk factors for postoperative mortality and periprosthetic joint infection in Medicare patients undergoing TKA. Clin Orthop Relat Res. 2012. Jan;470(1):130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jämsen E, Nevalainen P, Kalliovalkama J, Moilanen T. Preoperative hyperglycemia predicts infected total knee replacement. Eur J Intern Med. 2010. Jun;21(3):196-201. [DOI] [PubMed] [Google Scholar]
- 105.Stojanov S, Berlec A, Štrukelj B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms. 2020. Nov 1;8(11):1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schmidt TSB, Raes J, Bork P. The Human Gut Microbiome: From Association to Modulation. Cell. 2018. Mar 8;172(6):1198-215. [DOI] [PubMed] [Google Scholar]
- 107.Whisner CM, Castillo LF. Prebiotics, Bone and Mineral Metabolism. Calcif Tissue Int. 2018. Apr;102(4):443-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kristensen NB, Bryrup T, Allin KH, Nielsen T, Hansen TH, Pedersen O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: a systematic review of randomized controlled trials. Genome Med. 2016. May 10;8(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gao R, Zhang X, Huang L, Shen R, Qin H. Gut Microbiota Alteration After Long-Term Consumption of Probiotics in the Elderly. Probiotics Antimicrob Proteins. 2019. Jun;11(2):655-66. [DOI] [PubMed] [Google Scholar]
- 110.McFarland LV. Use of probiotics to correct dysbiosis of normal microbiota following disease or disruptive events: a systematic review. BMJ Open. 2014. Aug 25;4(8):e005047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kadooka Y, Sato M, Imaizumi K, Ogawa A, Ikuyama K, Akai Y, Okano M, Kagoshima M, Tsuchida T. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr. 2010. Jun;64(6):636-43. [DOI] [PubMed] [Google Scholar]
- 112.Williams NT. Probiotics. Am J Health Syst Pharm. 2010. Mar 15;67(6):449-58. [DOI] [PubMed] [Google Scholar]
- 113.Suez J, Zmora N, Segal E, Elinav E. The pros, cons, and many unknowns of probiotics. Nat Med. 2019. May;25(5):716-29. [DOI] [PubMed] [Google Scholar]
- 114.Huck O, Mulhall H, Rubin G, Kizelnik Z, Iyer R, Perpich JD, Haque N, Cani PD, de Vos WM, Amar S. Akkermansia muciniphila reduces Porphyromonas gingivalis-induced inflammation and periodontal bone destruction. J Clin Periodontol. 2020. Feb;47(2):202-12. [DOI] [PubMed] [Google Scholar]
- 115.Suez J, Zmora N, Zilberman-Schapira G, Mor U, Dori-Bachash M, Bashiardes S, Zur M, Regev-Lehavi D, Ben-Zeev Brik R, Federici S, Horn M, Cohen Y, Moor AE, Zeevi D, Korem T, Kotler E, Harmelin A, Itzkovitz S, Maharshak N, Shibolet O, Pevsner-Fischer M, Shapiro H, Sharon I, Halpern Z, Segal E, Elinav E. Post-Antibiotic Gut Mucosal Microbiome Reconstitution Is Impaired by Probiotics and Improved by Autologous FMT. Cell. 2018. Sep 6;174(6):1406-1423.e16. [DOI] [PubMed] [Google Scholar]