Abstract
Elastography is an imaging technology capable of measuring tissue stiffness and consistency. The technology has achieved widespread use in the workup and management of diseases of the liver, breast, thyroid, and prostate. Although elastography is increasingly being applied in neurosurgery, it has not yet achieved widespread adoption and many clinicians remain unfamiliar with the technology. Therefore, we sought to summarize the range of applications and elastography modalities available for neurosurgery, report its effectiveness in comparison with conventional imaging methods, and offer recommendations. All full-text English-language manuscripts on the use of elastography for neurosurgical procedures were screened using the PubMed/MEDLINE, Embase, Cochrane Library, Scopus, and Web of Science databases. Thirty-two studies were included with 990 patients, including 21 studies on intracranial tumors, 5 on hydrocephalus, 4 on epilepsy, 1 on spinal cord compression, and 1 on adolescent scoliosis. Twenty studies used ultrasound elastography (USE) whereas 12 used magnetic resonance elastography (MRE). MRE studies were mostly used in the preoperative setting for assessment of lesion stiffness, tumor–brain adherence, diagnostic workup, and operative planning. USE studies were performed intraoperatively to guide resection of lesions, determine residual microscopic abnormalities, assess the tumor–brain interface, and study mechanical properties of tumors. Elastography can assist with resection of brain tissue, detection of microscopic lesions, and workup of hydrocephalus, among other applications under investigation. Its sensitivity often exceeds that of conventional MRI and ultrasound for identifying abnormal tissue and lesion margins.
Keywords: Elastography, neurosurgery, stiffness, ultrasound, tumor, hydrocephalus, epilepsy
Introduction
Advances in computing power and processing techniques in recent decades have enabled the incorporation of sophisticated imaging tools into neurosurgical operative planning and guidance. These developments have led to improvements in the efficacy and safety of operative neurosurgery [1]. Although attempts were made as early as the 1950s to use intraoperative ultrasound for brain tumor localization, it was not until the 1980s that B-mode ultrasound achieved mainstream recognition for intraoperative use [2]. Around the same time, magnetic resonance imaging (MRI) was emerging as a scanning tool for preoperative planning and postoperative monitoring. Intraoperative MRI was adopted the following decade, with early efforts highlighting its effectiveness in improving the extent of tumor resection [3,4].
In the mid-1990s, elastography emerged as a new imaging technology that could assess the stiffness of tissue, providing improved spatial localization and resolution compared with manual palpation. Elastography can be generated by magnetic resonance scanners, known as magnetic resonance elastography (MRE), or by an ultrasound transducer, known as ultrasound elastography (USE) [5]. Some original indications for elastography included the assessment of liver fibrosis and detection of prostate and breast cancers, but it has increasingly been studied in the context of neurosurgery, for purposes including resection of brain lesions and identification of hydrocephalus [5,6].
Given the recent emergence of elastography as a new technique in neurosurgery, surgeons have limited experience with this modality. Moreover, several modalities are available to neurosurgeons, such as MRE and USE, with USE further subdivided into shear-wave elastography (SWE), involving propagation of shear waves generated by a transducer across tissue, and strain elastography, involving physical compression of tissue by a transducer. Therefore, the goal of this systematic review is to summarize the current literature on elastography in neurosurgery and offer recommendations for future study design. Our primary objectives are to determine the range of applications and types of elastography for neurosurgery, and to compare its effectiveness with conventional imaging methods.
Methods
Literature Search
A systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The English-language peer-reviewed literature published as of October 22, 2021 was queried using the following databases: PubMed/MEDLINE, Embase, Cochrane Library, Scopus, and Web of Science. Full search queries are listed in Supplementary Table 1. The bibliographies of included studies were also assessed to identify additional relevant studies.
Studies were included if they were a full-text English manuscript examining USE or MRE in the human brain or spinal cord preoperatively, intraoperatively, or postoperatively for patient management. Both pediatric and adult populations were included. Studies were excluded if they: 1) only examined patients without a neurological disease, 2) studied animals or were performed ex vivo or in vitro, or 3) did not feature a neurosurgical operation.
Data Extraction
Each eligible article was independently screened against these criteria by 3 reviewers (CWL, KJ, LY) using the Covidence systematic review application (Covidence), with disagreements adjudicated by a fourth independent reviewer (AMH). Articles satisfying criteria for full-text review then underwent data extraction by 4 authors (AMH, CWL, KJ, LY) using Microsoft Excel (Microsoft Corporation). Details extracted included sample size, sample demographics, pathology examined, type of elastography, and imaging parameters (ie, machine, transducer, and frequency). Outcomes of interest included quantitative elasticity measurements, sensitivity and accuracy of elastography, qualitative comparisons with intraoperative findings, and efficacy of elastography for surgical management.
Study quality was assessed using the National Institutes of Health Quality Assessment Tool for Case Series studies (Supplementary Table 2) [7]. Additionally, a level of evidence rating was determined for each study in accordance with guidelines from the North American Spine Society [8].
Results
We identified 2008 unique articles, of which 123 underwent full-text review, and 32 were deemed eligible for data extraction (Figure 1). A total of 990 patients were included across the 32 studies, with participants’ mean ages ranging from 6 weeks to 72 years old (Table 1). Four studies investigated exclusively pediatric populations [9–12], whereas the remaining 28 studies examined adult cohorts. All studies were published within the past 2 decades, with nearly 72% published from 2016 onward. Ultrasound elastography was used in 20 (62.5%) studies, and MRE was used in 12 (37.5%) studies. By design, all MRE studies generated shear waves for determination of tissue stiffness, although 2 studies by Yin et al incorporated slip-interface imaging, a modality derived from the differential shear-wave motion of tissue across an interface [13,14]. Ultrasound studies were evenly split between those using SWE (n=10) and those using strain elastography (n=10). All studies were classified as Level III or IV studies (Supplementary Table 2).
Figure 1.
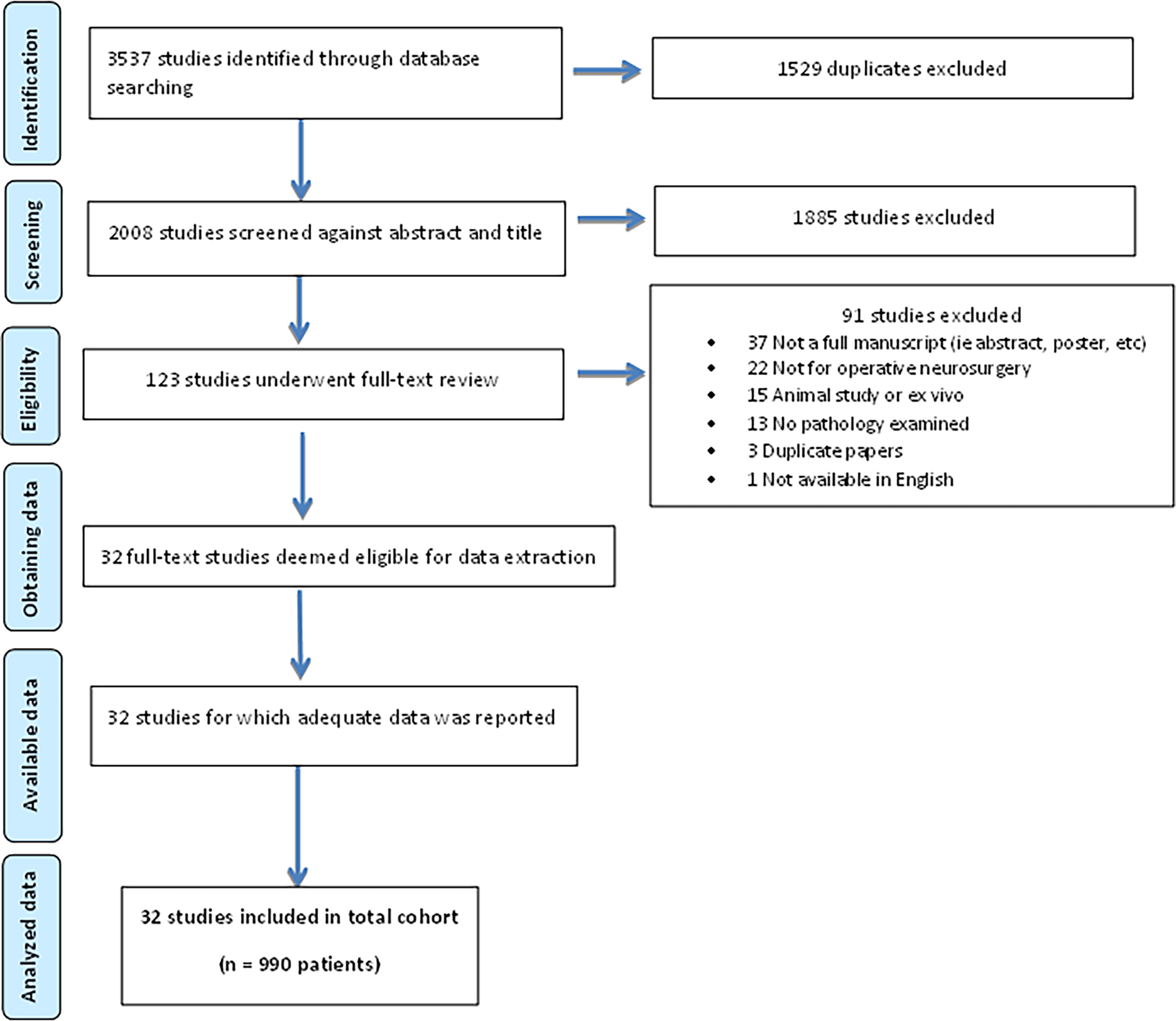
PRISMA diagram for systematic review.
Table 1 –
Summary of 32 studies identified in systematic review
| Author, year | N | F% | Mean age | US or MRE | Elastography method | Machine | Transducer / Frequency |
|---|---|---|---|---|---|---|---|
| Freimann et al., 2012 [35] | 20 | 60 | 71 yo | MRE | Shear wave | 1.5-T Sonata MRI, Siemens | 60 Hz |
| Hughes et al., 2015 [25] | 14 | 71 | 59 yo | MRE | Shear wave | Signa Excite (3T MRI) | 60 Hz |
| Hughes et al., 2016 [29] | 10 | 50 | 60 yo | MRE | Shear wave | Signa Excite (3T MRI) | 60 Hz |
| Lagerstrand et al., 2021 [30] | 10 | 20 | 60 yo | MRE | Shear wave | Achieva (3T MRI), Philips | NA |
| Murphy et al., 2013 [38] | 12 | NR | NR | MRE | Shear wave | Signa Excite (3T) | 60 Hz |
| Olivero et al., 2016 [12] | 1 | 100 | 19 yo | MRE | Shear wave | Siemens Trio (3T) | 50 Hz |
| Perry et al., 2017 [36] | 10 | 60 | 72 yo | MRE | Shear wave | GE Healthcare (3T MRI) | 60 Hz |
| Sakai et al., 2016 [27] | 34 | 68 | 54 yo | MRE | Shear wave | Discovery MR750W | MR Touch, 60 Hz |
| Solamen et al., 2021 [37] | 22 | 18 | 67 yo | MRE | Shear wave | Achieva (3T MRI), Philips | NA |
| Takamura et al., 2021 [28] | 32 | 78 | 62 yo | MRE | Shear wave | Discovery 750 | 60 Hz |
| Yin et al., 2015 [13] | 9 | 78 | 54 yo | MRE | SII | Signa Excite | 60 Hz |
| Yin et al., 2017 [14] | 25 | 68 | 61 yo | MRE | SII | Signa Excite | 60 Hz |
| Alawaji et al., 2021 [39] | 4 | 0 | 32 yo | USE | Shear wave | Aixplorer, Supersonic Imagine | Multifrequency (SE12–3, 3–12 MHz) or linear (SL10–2, 4–15 MHz) |
| Al-Habib et al., 2021 [31] | 25 | 32 | 48 yo | USE | Shear wave | Aixplorer, Supersonic Imagine | Linear transducer array (SuperLinear 15–4), 2 MHz |
| Cepeda et al., 2020 [15] | 36 | 28 | 65 yo | USE | Strain | Hitachi-Noblus | C42 convex probe, 8–10 MHz |
| Cepeda, Arrese et al., 2021 [24] | 18 | 50 | 62 yo | USE | Strain | Hitachi-Noblus | Neonatal convex, 8 MHz |
| Cepeda, Garcia-Garcia et al., 2021 [17] | 16 | 19 | 66 yo | USE | Strain | Hitachi-Noblus | C42 probe, 4–8 MHz |
| Cepeda, Garcia-Garcia et al., 2021 [16] | 40 | 53 | 59 yo | USE | Strain | Hitachi-Noblus | C42 probe, 4–8 MHz |
| Chakraborty et al., 2012 [18] | 24 | NS | NR | USE | Strain | Acuson 128 XP10 | NR |
| Chan et al., 2014 [9] | 1 | 0 | 7 yo | USE | Shear wave | Aixplorer, SuperSonic Imagine | Multifrequency (SE12–3, 3–12 MHz), center frequency at 7.5 MHz |
| Chan et al., 2021 [19] | 34 | 56 | 21 yo | USE | Shear wave | Aixplorer, SuperSonic Imagine | Multifrequency (SE12–3, 3–12 MHz) or linear (SL10–2, 4–15 MHz) |
| Chauvet et al., 2015 [20] | 63 | 61 | 53 yo | USE | Shear wave | Aixplorer, Supersonic Imagine | Linear (SL10–2), 6 MHz |
| Dirrichs et al., 2019 [10] | 166 | 46 | 6 wks | USE | Shear wave | Aixplorer, Supersonic Imagine | High-resolution linear, 10.2-MHz |
| Mathon et al., 2019 [32] | 28 | 54 | 28 yo | USE | Shear wave | Aixplorer, Supersonic Imagine | SuperMicroConvex (SMC 12–3) |
| Mathon et al., 2021 [33] | 18 | 61 | 26 yo | USE | Shear wave | Aixplorer, Supersonic Imagine | SuperMicroConvex (SMC 12–3) |
| Pepa et al., 2020 [40] | 36 | 58 | 61 yo | USE | Strain | MyLabTwice | Linear, 3–11 MHz |
| Prada et al., 2019 [26] | 64 | 42 | 55 yo | USE | Strain | MyLabTwice | Linear, 3–11 MHz |
| Prada et al., 2020 [34] | 4 | 25 | 26 yo | USE | Strain | MyLab Twice | Linear, 3–11 MHz |
| Selbekk et al., 2005 [23] | 2 | NR | NR | USE | Strain | System FiVe, GE Vingmed | Flat linear-array, 10-MHz |
| Selbekk et al., 2012 [21] | 15 | NR | NR | USE | Strain | System FiVe, GE Vingmed | Flat linear-array, 10-MHz |
| Vergari et al., 2020 [11] | 25 | 67 | 15 yo | USE | Shear wave | Aixplorer, Supersonic Imagine | SL 10–2 MHz probe |
| Yin et al., 2021 [22] | 172 | 37 | 44 yo | USE | Shear wave | Aixplorer, Supersonic Imagine | SMC12–3 convex array, 3–12 MHz |
F – female; MEG – motion-encoding gradient; MRE – Magnetic Resonance Elastography; MRI – Magnetic Resonance Imaging; NA – not applicable; NR – not reported; SII – slip interface imaging; T – Tesla; US – ultrasound; USE – ultrasound elastography
The distribution of pathologies is depicted in Figure 2. Twenty-one studies (65.6%) investigated elastography for resection of intracranial tumors (Table 2). The most common intracranial tumors encountered included high-grade gliomas (HGG) (n=199 patients) [15–22], low-grade gliomas (n=127) [17,19–23], meningiomas (n=191) [14,16,18–20,24–28], metastases (n=34) [15,18–20,23,26], pituitary adenomas (n=32) [26,27,29,30], and vestibular schwannomas (n=16) [13,19,27]. Of the HGGs, 54 tumors were identified as glioblastomas [15,17,18]. Overall, 49 gliomas were unspecified [26,27]. The remaining 11 studies (Table 3) included those investigations in patients with spinal cord compression (n=25) [31], epilepsy (n=51) [9,32–34], hydrocephalus (n=109) [10,12,35–37], and adolescent idiopathic scoliosis (n=25) [11].
Figure 2.
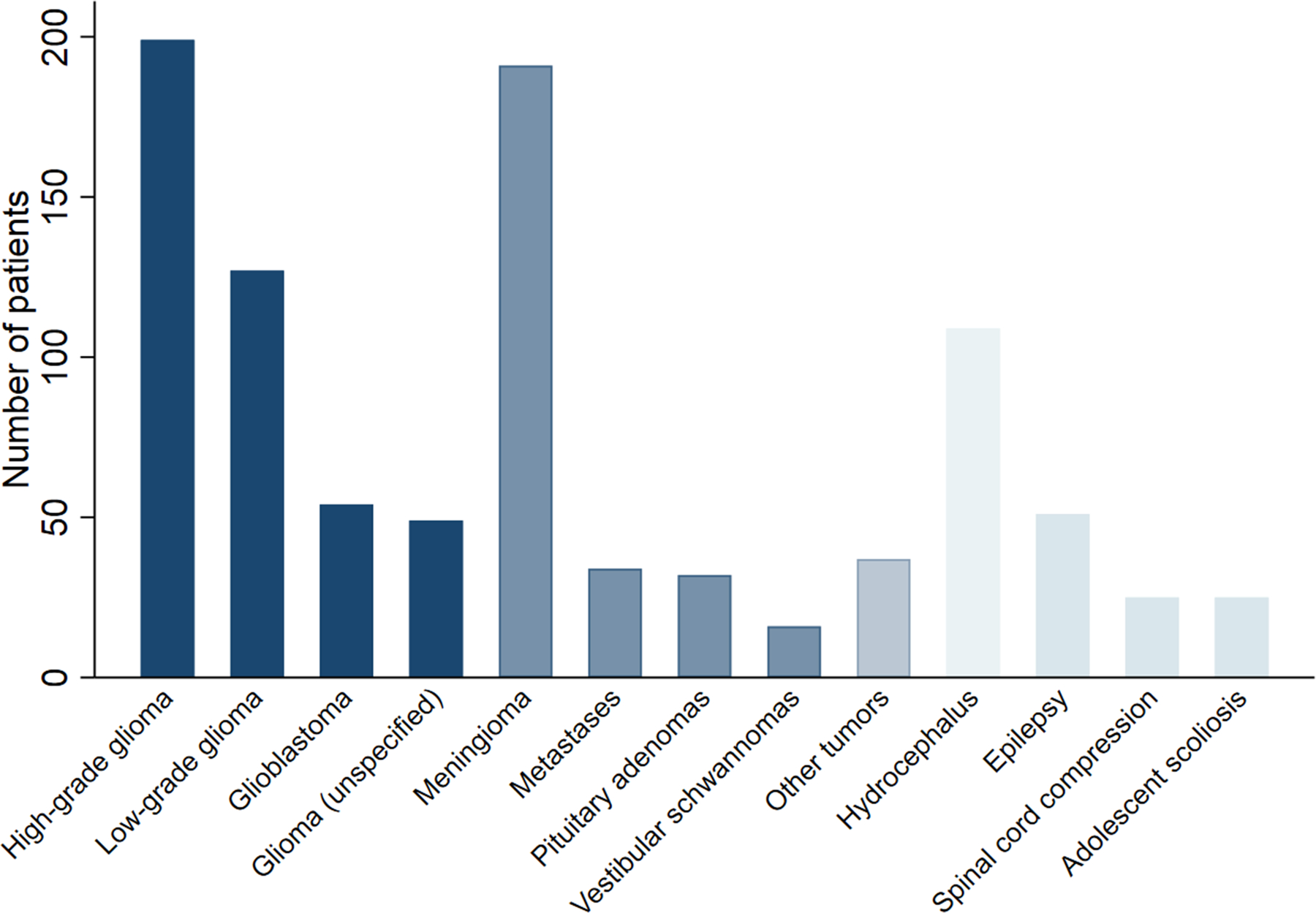
Distribution of pathologies across patients identified in the systematic review.
Table 2 –
Summary of pathologies and findings for 21 studies investigating elastography in patients with intracranial tumors
| Author, year | Pathology (n) | Elasticity results | Conclusions |
|---|---|---|---|
| Alawaji et al., 2021 [39] | Epidermoid cyst (4) | YM: Epidermoid cyst - 167 kPA, adjacent brain tissue - 20 kPa | Can differentiate epidermoid cysts + adjacent tissue; detect residual microscopic tumors |
| Cepeda et al., 2020 [15] | GBM (26), brain metastases (10) | Elastography AUCs: 0.85–0.99, B-mode AUCs: 0.79–0.94 | Machine learning algorithms can differentiate GBM and metastases |
| Cepeda, Arrese et al., 2021 [24] | Meningioma (18) | MTE: Soft - 95 ± 13, hard - 131 ± 7; algorithm AUC=0.96 | Machine learning algorithm can predict meningioma consistency; MTE provides a semi-quantitative analysis of elasticity |
| Cepeda, Garcia-Garcia et al., 2021 [17] | GBM (16) | NR | A radiomic feature of USE is significantly associated with overall survival; quantitative texture analysis of USE is feasible. |
| Cepeda, Garcia-Garcia et al., 2021 [16] | HGG (21), LGG (9), meningioma (10) | MTE: HGG - 85, LGG - 84, meningioma - 120; Ki-67 < 10% - 110.34, Ki-67 > 10% - 80 | Tumor MTE positively correlated with fractional anisotropy and negatively with Ki-67 index. Developed regression model to calculate MTE from fractional anisotropy |
| Chakraborty et al., 2012 [18] | GBM (12), intracranial metastases (2), other (10) | NA | USE and surgical findings of tumor stiffness are comparable, but USE did not demarcate the brain-tumor interface as accurately as B-mode US. |
| Chan et al., 2021 [19] | HGG (8), LGG (9), malignant embryonal tumor (6), meningioma (3), other (7) | YM: Tumors - 33.5 kPa, normal brain - 14.9 kPa. Residual tumor detection sensitivity: SWE - 94%, US - 73%, surgeon - 36% | YM measurements correlated with surgeons’ stiffness grading. SWE significantly outperformed surgeons in detecting residual tumor. |
| Chauvet et al., 2015 [20] | Meningioma (16), metastasis (15), HGG (18), LGG (14) | YM: Meningioma - 33.1 kPa, LGG - 23.7 kPa, HGG - 11.4 kPa, metastasis - 16.7 kPa. | Low and high-grade glioma stiffness differ significantly |
| Hughes et al., 2015 [25] | Meningioma (14) | MRE sensitivity: heterogenous tumors – 75%, hard tumors – 60%, soft tumors – 100% | MRE effective in ruling-in, but not out, heterogenous tumors. Less consistency between MRE and operative findings for small/vascular tumors. |
| Hughes et al., 2016 [29] | Pituitary macroadenoma (10) | MRE: soft tumors - 1.38 kPa; intermediate tumors - 1.94 kPa | Pituitary macroadenomas designated by surgeons as soft or intermediate differ significantly in elasticity. |
| Lagerstrand et al., 2021 [30] | Pituitary adenoma (10) | Virtual MRE: 8.32 | Virtual MRE constructed to display high resolution images and identify regions of stiffness that affect surgical outcomes. |
| Murphy et al., 2013 [38] | Meningioma (12) | Scatter chart only, averages not provided | MRE tumor stiffness correlated with surgeon’s assessment, superior to conventional MRI. |
| Pepa et al., 2020 [40] | Meningioma (36) | NA; elastograms qualitatively categorized | USE has greater accuracy and sensitivity than MRI for meningioma consistency and brain-tumor interface. |
| Prada et al., 2019 [26] | Glioma (45), meningioma (8), metastases (4), other (7) | NA; elastograms qualitatively categorized | LGG is stiffer while HGG is softer than normal brain. Elastography superior to B-mode US for identifying lesion margins |
| Sakai et al., 2016 [27] | Meningioma (13), pituitary adenoma (11), vestibular schwannoma (6), glioma (4) | MRE: Meningiomas - 1.9 kPa, pituitary adenomas - 1.2 kPa, vestibular schwannomas - 2.0 kPa, gliomas - 1.5 kPa. | MRE can discriminate tumors; stiffness significantly correlated with surgeon’s qualitative assessment of tumor consistency |
| Selbekk et al., 2005 [23] | Metastasis (1), low-grade astrocytoma (1) | NR | Tumors had lower strain than surrounding tissue. Elastography qualitatively similar to B-mode US but detected tumors absent on B-mode US. |
| Selbekk et al., 2012 [21] | LGG (8), high-grade astrocytoma (7) | Average contrast: Strain – 0.60, B-mode: 0.39 | Elastography offers better discrimination between tumors and healthy brain than B-mode US, but appears noisier. |
| Takamura et al., 2021 [28] | Meningiomas: Meningothelial (15), fibrous (12), transitional (8), angiomatous (1) | MRE: Meningioma – 3.12 kPa; stiffness inversely correlated with tumor thickness | Stiffness and intraoperative consistency significantly correlated, but did not significantly differentiate histologic subtypes. |
| Yin et al., 2015 [13] | Vestibular schwannomas (9) | NA; tumor-brain adhesion qualitatively categorized as no, partial, or complete slip interface | Slip interface imaging can reliably predict tumor adhesion and may help in preoperative planning. |
| Yin et al., 2017 [14] | Meningiomas (25) | NA; tumor-brain adhesion qualitatively categorized as no, partial, or complete slip interface | Slip interface imaging agreed with surgical findings in 72% of cases and can preoperatively evaluate tumor adhesion to brain, helping predict the surgical resection plane. |
| Yin et al., 2021 [22] | LGG (86), HGG (86) | YM: LGG – 19.7 kPa, HGG – 9.6 kPa, peritumor tissue – 8.2 kPa; HGG AUC: 0.86 | SWE can reliably differentiate low and high-grade gliomas. Optimal cutoff value for HGG is 12.1 kPa. |
AUC – area under the curve; GBM – glioblastoma; HGG – high-grade glioma; LGG – low-grade glioma; MRE – Magnetic Resonance Elastography; MTE – Mean Tissue Elasticity; NA – not applicable; NR – not reported; SWE – shear wave elastography; US – ultrasound; USE – ultrasound elastography; YM – Young’s Modulus
Table 3 –
Summary of pathologies, interventions, and findings for 11 studies investigating elastography in patients with conditions other than intracranial tumors
| Author, year | Pathology (n) | Surgery | Elasticity results | Conclusions |
|---|---|---|---|---|
| Al-Habib et al., 2021 [31] | Degenerative disease (10), tumors (6), trauma (3), infection (1), syndromic (4), tethered cord (1) | Laminectomy or corpectomy | Spinal cord: Compressed group - 93.8 kPa, decompressed group - 9.35 kPa. Dura: compressed group - 121.8 kPa, decompressed group - 29.8 kPa | Compression significantly increases stiffness of spinal cord and dura mater. Elasticity is significantly reduced following decompression. |
| Chan et al., 2014 [9] | MRI-negative epilepsy (1) | Resection of FCD | YM: Lesion - 74.7 kPA; gray matter - 36.2 kPa; white matter - 20.8 kPa | USE can identify MRI-negative and ultrasound-negative epileptogenic lesions. Histology confirmed type 11b focal cortical dysplasia. |
| Dirrichs et al., 2019 [10] | Hydrocephalus/increased ICP (56), controls (110) | Invasive ventricular drainage | YM: Hydrocephalus – 21.8 kPa, controls – 14.1 kPa | Increased stiffness in hydrocephalus, correlation between ICP measurements and SWE |
| Freimann et al., 2012 [35] | NPH (20) | CSF drainage, VP shunting | Shear elasticity: before shunt – 2.24 kPa, after shunt – 2.26 kPa; connectivity parameter: before shunt – 0.26, after shunt – 0.28 | Viscoelastic parameters lower in NPH than healthy controls; elasticity did not change after shunt treatment but connectivity parameter returned to normal. |
| Mathon et al., 2019 [32] | Epilepsy (28) | Epileptogenic zone resection | Lesion/normal brain stiffness ratio: MRI- FCD – 2.8 kPa, MRI+ FCD – 3.9 kPa; SWE sensitivity: 93% | SWE improves intraoperative detection of epileptogenic lesions and can detect FCD not visible on B-mode US. |
| Mathon et al., 2021 [33] | FCD (18): MRI- FCD (8), MRI+ FCD (10) | FCD resection | FCD/normal brain stiffness ratio: MRI- FCD – 2.2 kPa, MRI+ FCD – 3.6 kPa; SWE sensitivity: 78% | SWE outperforms B-mode US in intraoperative FCD detection, detected 60% of MRI- FCD |
| Olivero et al., 2016 [12] | Hydrocephalus (1) | Shunt drainage | MRE: 1.62 kPa (normal ~ 3.0 kPa) | Observed extremely low brain tissue stiffness in low pressure hydrocephalus, even after shunt treatment |
| Perry et al., 2017 [36] | Idiopathic NPH (10) | VP shunt placement | MRE (vs healthy control): Cerebrum – 2.64 (higher), occipital – 2.97 (higher), parietal – 2.63 (higher), periventricular – 1.74 (lower) | Abnormal brain stiffness correlates with symptoms. Postoperative improvement associated with increased deep gray matter stiffness, and failure associated with increased temporal stiffness. |
| Prada et al., 2020 [34] | Type IIb FCD of right frontal lobe (4) | FCD resection | Qualitative results only; FCD mean stiffness similar to adjacent healthy brain | Dysplastic foci contain heterogenous stiffness; neither elastography nor contrast-enhanced US superior to B-mode US in identifying boundaries. |
| Solamen et al., 2021 [37] | NPH (22) | Lumbar drain placement | Increased stiffness in cerebral and periventricular regions (p=0.01) | NPH drain responders showed initial increase followed by a later decrease, suggesting mechanical changes from drainage. |
| Vergari et al., 2020 [11] | AIS (25) | Fusion | Shear wave speed: AIS – 4.0 m/s, controls – 3.1 m/s, AIS patients 1 year after treatment – 3.3 m/s | Significant differences between AIS and healthy discs, which normalizes 1 year after treatment. |
AIS – adolescent idiopathic scoliosis; FCD – Focal Cortical Dysplasia; MRI – Magnetic Resonance Imaging; NA – not applicable NPH – normal pressure hydrocephalus; SWE – shear-wave elastography; US – ultrasound; VP – ventriculoperitoneal shunting; YM – Young’s Modulus
The timing and purpose of elastography were largely contingent on the decision to use MRE or USE. MRE studies were performed preoperatively to assist with operative planning and/or to determine accuracy of MRE relative to intraoperative results. An exception includes a study by Olivero et al, in which MRE was performed 3 weeks after shunt revision surgery for low-pressure hydrocephalus and revealed that the abnormally low stiffness in low-pressure hydrocephalus persists even after patients exhibit a symptomatic response to treatment and ventricular size returns to baseline [12]. In contrast, nearly all USE studies were performed intraoperatively to guide resection of tumors or epileptic lesions or to assess changes after spinal cord decompression. Two exceptions included a study by Vergari et al, who measured elasticity of lumbar annulus fibrosus in patients with adolescent idiopathic scoliosis preoperatively and at 3 months and 1 year after surgery to determine disc properties in scoliosis [11], and Dirrichs et al, who performed USE on neonates with hydrocephalus prior to invasive ventricular drainage [10].
Outcome data varied depending on the method of elastography used. Eight studies using MRE reported shear elasticity measurements by processing acquired images and using computer-generated maps of stiffness known as elastograms [12,14,25,27–29,35,36,38], whereas 2 MRE studies categorized stiffness qualitatively [13,14]. Lagerstrand et al used diffusion-weighted imaging to generate a virtual MRE and quantify stiffness [30]. All 10 studies that used ultrasound SWE reported quantitative results but differed in their selected outcome, with 6 reporting the Young’s modulus measure of stiffness [9,10,19,20,22,39], 2 reporting stiffness ratios between epileptic lesions and normal brain tissue [32,33], 1 reporting the shear modulus measure of stiffness [31], and 1 reporting shear-wave speed (from which stiffness is traditionally calculated) [11]. In contrast, only 2 ultrasound strain elastography studies quantitated tissue elasticity [16,24]. The remaining 8 strain elastography studies qualitatively assessed tissue stiffness, with metrics including sensitivity, specificity, accuracy, and feasibility [15,17,18,21,23,26,34,40]. For example, Pepa et al categorized meningioma consistency and meningioma-brain interface on a scale from 0 to 2 using strain elastography and compared results with MRI analysis and intraoperative findings [40].
Discussion
Palpation of tissue is a critical technique dating back to ancient times, as it provides valuable information on soft tissue physiology and the presence of disease. The development of elastography in the 1990s improved upon manual palpation with vastly improved resolution, quantitative measurements, and the capacity to assess tissue otherwise inaccessible to traditional palpation [41]. Elastography captures information about tissue displacement and relates it to measures of stiffness. Elastography is now used routinely in several domains of medicine. For example, it is recommended by the World Federation for Ultrasound in Medicine and Biology as the preferred first-line assessment for the severity of liver fibrosis in patients with chronic viral hepatitis C [42]. It can noninvasively stage chronic liver disease, quantify portal hypertension, and predict the progression and prognosis of patients with liver disease [43]. Measurements of elasticity have also proven valuable in assessing breast cancer [44], thyroid nodules [45], chronic kidney disease [46], and prostate cancer [47], among other organs. Increasingly, elastography is being applied to neurosurgery. Here we systematically review the literature on elastography in neurosurgery, finding that elastography is not only feasible and correlates with surgical findings, but is often superior to conventional MRI and ultrasound for identifying lesion margins and abnormal tissue [9,21,23,26,32,33,38,40]. For example, Murphy et al found that MRE findings of meningioma stiffness in 12 patients were superior to MRI T1- and T2-weighted imaging [38], while Prada et al found that glioma margins were sharper in 76% of cases with ultrasound elastography compared to B-mode ultrasound.
Elastography can be generated using either a MR scanner or ultrasound machine, with important implications for potential applications. Table 4 provides a comparison of MRE and USE technologies, noting potential applications for each modality and associated advantages and limitations. We found a preference for USE over MRE in neurosurgery, likely reflecting the ease of availability of intraoperative ultrasound, fast image acquisition time, and lower cost compared with MRE [48]. However, USE generally requires bone removal, such as craniectomy or laminectomy, as ultrasound waves are reflected off bone, and can only sample tissue within the plane of the probe [49]. In contrast, MRE was preferred for preoperative assessment of intracranial pathology and postoperative monitoring. Cohen-Cohen et al write that knowledge of tumor consistency gleaned from MRE can be used for operative planning, counseling patients about surgical risk, and estimating operative time [50]. Important exceptions permitting noninvasive USE include neonatal cranial sonography, which is feasible in the first year of life due to the acoustic windows provided by the fontanelles [51], and assessments of non-bony structures, such as intervertebral discs [52]. MRE may be less desirable than USE in neonates, due to the presence of motion artifacts and potential need for sedation [53,54].
Table 4 –
Comparison of magnetic resonance and ultrasound elastography, with applications in neurosurgery, advantages, and limitations for each modality
| Modality | Applications | Advantages | Limitations |
|---|---|---|---|
| Magnetic resonance elastography | Preoperative
assessment: •Diagnose abnormal lesions •Tumor consistency and heterogeneity •Classify tumors as hard or soft •Tumor-brain adhesion •Differentiate types of tumors •Postoperative monitoring after shunt treatment for hydrocephalus •Investigate pathophysiology of disease |
•Penetrates bone
non-invasively •Can perform routinely as part of follow-up care at scheduled intervals •Samples large area of tissue |
•Expensive •May require sedation in pediatric patients •Motion artifacts •Long acquisition times •Availability can be challenging for intraoperative cases |
| Ultrasound
elastography (Shear-wave or strain) |
Intraoperative
assessment: •Tumor boundaries •Tumor consistency and heterogeneity •Tumor-brain adhesion •Differentiate high- and low-grade gliomas •Presence of residual tumor •MRI-negative epileptogenic lesions •Spinal cord compression Preoperative and postoperative assessment: •Stiffness of intervertebral disks in scoliosis patients •Neonatal hydrocephalus (<1 year old) |
•Inexpensive •Ultrasound readily available intraoperatively •Fast acquisition time •Real-time imaging |
•Invasive, requires bone
removal (exceptions: infants, intervertebral disk
pathology) •Sample limited to acoustic window in plane of the probe •Limited depth of measurements due to tissue attenuation, tradeoff between spatial resolution and depth of penetration •Interobserver variability •Applies pressure to tissue, requires care for fragile tissue |
Although MRE relies on the propagation of shear waves, USE can be subdivided between strain elastography and SWE (Figure 4) [55]. Strain elastography involves mechanical external compression of tissue by an ultrasound transducer, and the resulting tissue deformation, or strain, is captured relative to its surroundings [56,57]. Since the external force in strain elastography is unknown and may not be uniform, the elasticity results are qualitative, or at best semiquantitative when ratios of average tissue strain are computed [58,59]. Furthermore, the external force can vary across ultrasound operators, potentially affecting study reproducibility [39]. In SWE, an acoustic radiation force impulse generates shear waves that propagate across tissue, and the resulting tissue displacement is used to calculate shear-wave velocity, which in turn can be used to calculate the Young’s modulus, providing a quantitative measure of tissue stiffness [57,60]. Shear waves travel faster in stiffer, contracted tissue. The Young’s modulus is related to the shear modulus obtained in MRE [61]. Studies in the neurosurgery literature are evenly split between strain elastography and SWE, although SWE has been favored in recent years. Nonetheless, qualitative strain elastography studies still demonstrated the capacity of elastography to detect brain-lesion interfaces and residual tumors [23,26], highlighting that both strain and SWE can be useful for operative neurosurgery.
Figure 4.
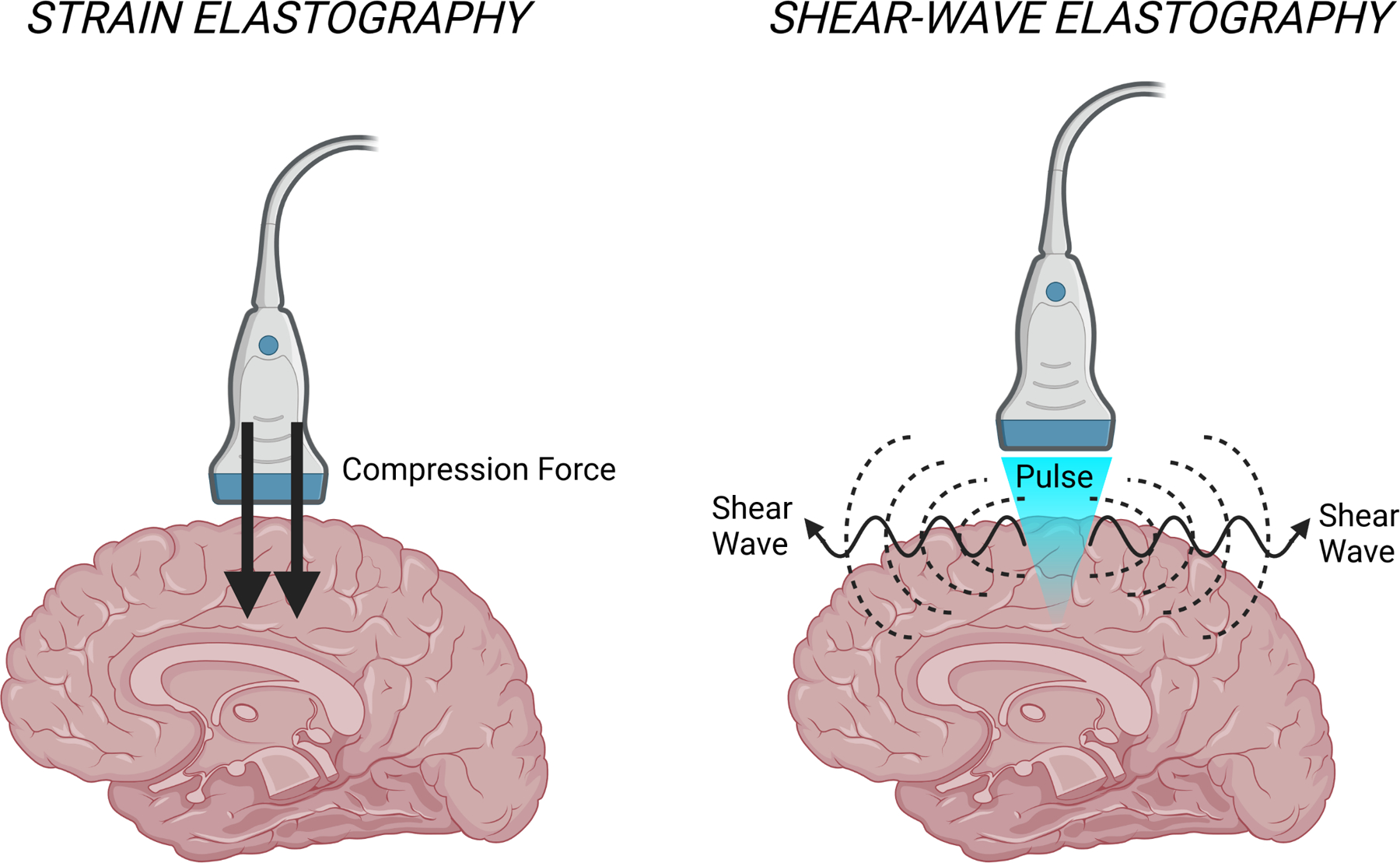
Comparison of strain and shear-wave elastography modalities. Strain elastography involves compression of tissue, while shear-wave elastography records the propagation of waves across tissue.
Elastography applications
Elastography has been used to detect residual tumors or lesions otherwise invisible to surgeons and conventional imaging techniques [9,19,23,32,33,39]. In a study of 34 patients with brain tumors, Chan et al determined that SWE outperformed surgeons and B-mode ultrasound in detecting residual tumors, with sensitivities of 93%, 73%, and 36% for SWE, B-mode ultrasound, and surgeons, respectively [19]. Studies also found that elastography can be particularly useful in treatment of MR-negative epilepsy, a condition affecting 20% to 40% of patients undergoing surgery for drug-resistant epilepsy [62]. Although single-photon emission tomography and fluorodeoxyglucose positron emission tomography are commonly used to localize epileptogenic zones, these techniques are expensive and require radiotracers [62]. Chan et al illustrated that SWE can identify epileptogenic lesions absent on MRI and conventional ultrasound by observing greater stiffness in these lesions compared with healthy brain tissue [9]. Moreover, studies by Mathon et al reported differences in lesion stiffness even between MRI-negative and MRI-positive focal cortical dysplasia [32,33].
Furthermore, elastography can differentiate between tumors and World Health Organization grade categories [15,20,22,27], Sakai et al used MRE to illustrate that pituitary adenomas were significantly less stiff than meningiomas [27], while Prada et al noted that low-grade gliomas were often stiffer than normal brain tissue and HGGs were softer [26]. In contrast, Takamura et al did not identify a role for MRE in differentiating meningioma subtypes, suggesting that elastography may be most useful in differentiating between, rather than within, tumor categories [28]. Cepeda et al furthered these studies by training a machine learning algorithm using elastography images, showing that the algorithm could reliably differentiate glioblastomas from metastases [15].
Elastography can also guide surgical resection by providing information on tumor heterogeneity and the degree of tumor–brain adherence [13,14,26,40]. For example, Pepa et al presented several meningioma cases illustrating how elastography can inform surgical strategy (Figure 3), including cases in which the intraoperative elastography conflicted with assessments of tumor–brain interface on preoperative MRI. The new information provided by elastography allowed the surgeon to replan the resection strategy, such as in a case where the resection was changed from piecemeal to en bloc, and a case where the surgeons could identify a well-preserved interface from the elastography images to begin debulking [40]. Overall, these studies suggest that elastography can be used in the preoperative setting to narrow the differential diagnosis prior to biopsy, can assist with preoperative planning by providing measures of tumor consistency and adherence to normal brain tissue, and can provide real-time intraoperative findings and guide the resection strategy and extent of resection [13].
Figure 3.
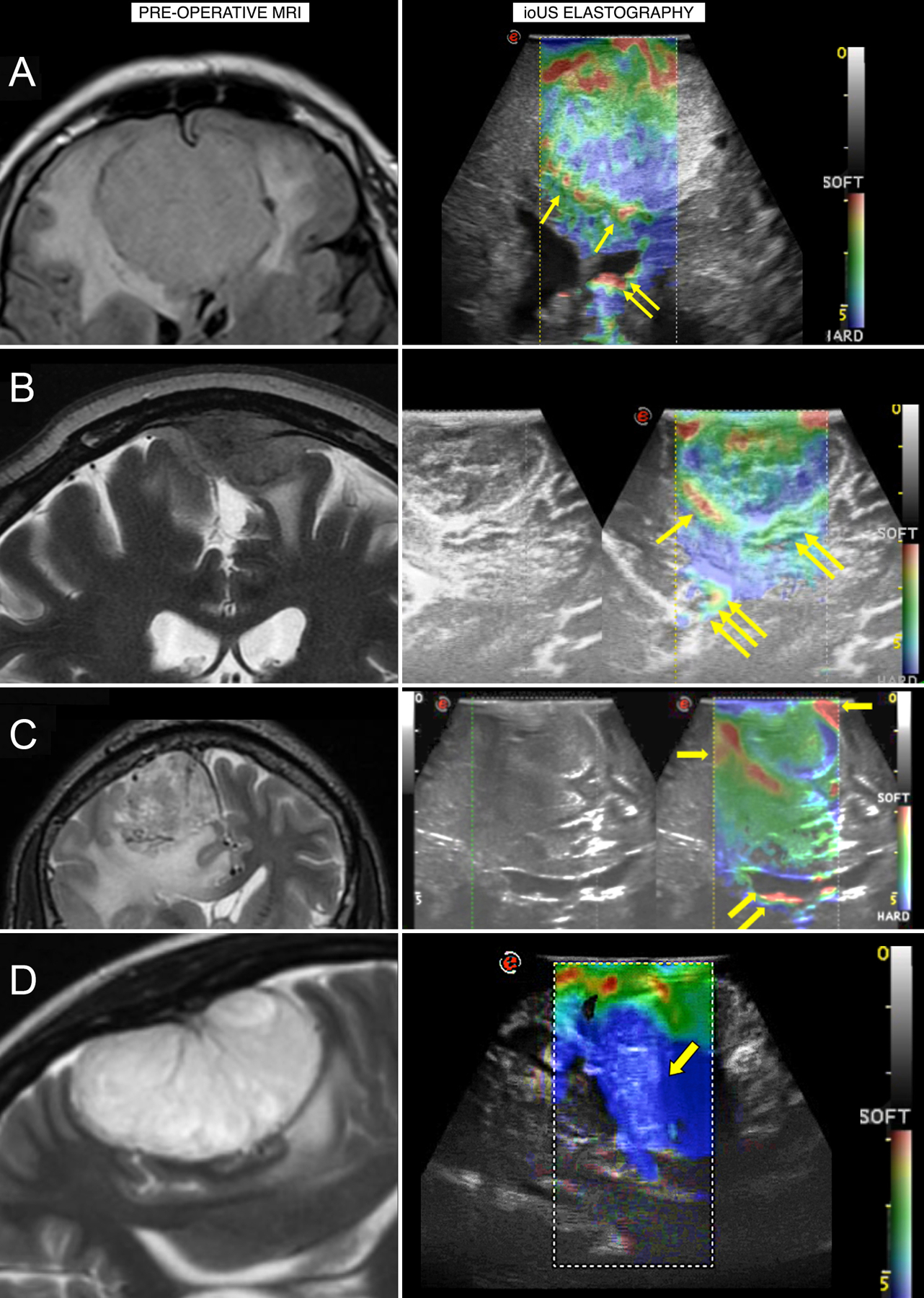
A) An ethmoidal meningioma displaying a heterogeneous consistency on elastography, suggesting that debulking should begin from softer areas. B) A parietal meningioma with a mixed brain–tumor interface, suggesting that dissection should progress from the better-preserved interface to the most adherent interface. C) A parasagittal meningioma where the elastography assessment of interface conflicts with the preoperative MRI and illustrates a well-preserved interface to begin debulking. D) A convexity meningioma shown on elastography to be harder than visualized on MRI, allowing the surgeon to change from a piecemeal to en bloc resection. ioUS, intraoperative ultrasound. Reprinted from Pepa GM Della, Menna G, Stifano V, et al. Predicting meningioma consistency and brain-meningioma interface with intraoperative strain ultrasound elastography: a novel application to guide surgical strategy. Neurosurg Focus. 2021;50(1):1–11.[40] Permission will be obtained at next stage of manuscript.
In addition to its role in resection of intracranial tumors and epileptogenic lesions, elastography can provide insights into hydrocephalus [10,12,35–37]. Dirrichs et al noted a correlation between increased intracranial pressure and increased elasticity measurements in 56 patients with hydrocephalus, with an average elasticity of 21.8 kPa in hydrocephalus patients compared with 14.1 kPa in healthy controls. In contrast, a case report of low-pressure hydrocephalus illustrated substantively lower brain tissue stiffness that persisted even after shunt treatment. Olivero et al argued that this may suggest low stiffness as a precipitating factor for low pressure hydrocephalus [12]. Interestingly, Perry et al showed that patients with idiopathic normal pressure hydrocephalus have variations in tissue stiffness across brain regions, with the cerebrum, occipital cortex, and parietal cortex having higher stiffness than healthy controls, while the periventricular region had lower stiffness. Moreover, preoperative urinary incontinence was associated with increased stiffness in the cerebrum, frontal cortex, and cerebellum. Perry et al went on to demonstrate that elastography may potentially predict treatment response, with preoperative stiffness in the temporal and deep grey matter being associated with a greater likelihood of surgical failure [36]. In this manner, elastography could be used to provide a preoperative risk-assessment and prognosis, and allow physicians to tailor therapy accordingly.
Elastography has been far less studied for spine surgery than for intracranial surgery, with the systematic review only identifying 2 spine surgery studies. Al-Habib et al performed laminectomy or corpectomy in 25 patients diagnosed with degenerative disease, tumors, or trauma, and divided their cohort between a decompressed group (with sufficient bone removal) and a compressed group (despite bone removal) using B-mode ultrasound. They then recorded SWE measurements, finding significantly increased stiffness of the spinal cord and dura in the compressed group [31]. In a study of 25 patients with adolescent idiopathic scoliosis, Vergari et al illustrated significant differences in elasticity of the lumbar annulus fibrosus between patients with scoliosis and healthy controls, and that these differences trended toward normal 1 year postoperatively [11]. These studies highlight a potential role for elastography in diagnosing spinal pathology and monitoring treatment over time. Al-Habib et al also suggested that SWE can be used to assist in resection of spinal tumors, although to date this has not been reported [31]. Studies of spinal cord elastography have been limited due to the fragility of the cord compared with brain tissue, precluding the use of strain elastography, which relies on external compression. However, SWE, as used in the aforementioned studies, can overcome this limitation, and its use for spinal cord lesions may constitute a new area of study [63].
Recommendations
Neurosurgeons can consider elastography pre-, intra-, and post-operatively for a variety of purposes, including assessment of tumor adherence and possible histology, identification of brain-lesion interfaces, resection of tissue, improving extent of resection, workup of hydrocephalus or spinal cord pathology, and monitoring treatment response over time. In particular, USE is readily available and can provide real-time data during surgery, improving safety and efficacy. High-resolution transducers are available to improve imaging capabilities. Repeated measurements should be taken and averaged to minimize bias, and operators should be thoroughly trained prior to imaging with any modality. Evidence from the literature on liver elastography suggests that measurements can be affected by movement during breathing; therefore, capturing elastography during breath-holds may help minimize pulsatility [64]. Ideally, future studies should report quantitative values of stiffness (shear modulus for MRE or Young’s modulus for SWE) to facilitate comparison across the literature and to help establish evidence-based guidelines. Additional research should establish cutoff values for pathological processes, such as different tumor histologies, extending the work of Yin et al who determined a cutoff elasticity for HGG of 12.1 kPa. Finally, elastography should continue to be explored to treat all aspects of neurosurgery, particularly extracranial pathology, where it has been less studied.
Given the various modalities available, caution should be applied in comparing results. The shear modulus measured in MRE and the Young’s modulus in SWE are mathematically related but produce distinct elasticities, explaining discrepancies between Tables 2 and 3 [65]. Moreover, differences in elasticities may arise between studies depending on the frequencies and types of transducer, which should be reported [47]. As the technology is increasingly adopted, consensus evidence-based guideline on best practices can be formulated, as documented for elastography imaging of the liver [66], breast [67,68], prostate [69], and thyroid [70].
Limitations
Limitations of the study include the lack of consistent outcome measurements due to variation in elastography modalities. The studies in this systematic review are almost entirely Level III or IV evidence, indicating a risk for selection bias and restricting our ability to generalize to the broader neurosurgical patient population. The variation in study methodologies and outcomes prevented us from performing a quantitative analysis of lesion elasticity. Additionally, the methodologies of included studies ranged from case reports to prospective cohorts, but a lack of randomized controlled trials and long-term postoperative outcome data precludes a definitive assessment of the efficacy of elastography. Furthermore, few studies focused on extracranial pathology, preventing us from drawing meaningful conclusions for spine and peripheral nerve pathology. More high-quality studies are needed to compare outcomes across modalities and determine cutoffs for differentiating abnormal lesions and tumors.
Conclusion
Elastography is a new imaging technique that provides measurements of stiffness and tissue consistency. Here we report results from the first systematic review of elastography for operative neurosurgery. We find that elastography has a wide range of applications, including detecting residual tumors and lesions invisible on MRI, identifying lesion–brain interface and degree of adherence, predicting postoperative treatment response, diagnosing pathology, differentiating tumors, and assisting with operative planning. Elastography has been studied for neurosurgery using MR transducers and ultrasound modalities, including shear-wave and strain elastography. Studies have illustrated that elastography is often superior to conventional greyscale ultrasound or MR imaging. Recommendations for clinical practice are provided.
Supplementary Material
Funding:
None
Footnotes
Devices: None
Previous Presentations: None
Disclosures: Nicholas Theodore receives royalties from and owns stock in Globus Medical. He is a consultant for Globus Medical and has served on scientific advisory board/other office for Globus Medical. The remaining authors have no conflicts of interest to disclose.
IRB Approval: IRB approval was not required for this work.
References
- [1].Fahlbusch R, Golby A, Prada F, Zada G. Introduction: Utility of intraoperative imaging. Neurosurg Focus 2016;40:E1. 10.3171/2016.1.FOCUS1610. [DOI] [PubMed] [Google Scholar]
- [2].Chandler WF, Rubin JM. The application of ultrasound during brain surgery. World J Surg 1987;11:558–69. 10.1007/BF01655829. [DOI] [PubMed] [Google Scholar]
- [3].History Castillo M. and evolution of brain tumor imaging: Insights through radiology. Radiology 2014;273:S111–25. 10.1148/RADIOL.14140130/ASSET/IMAGES/LARGE/RADIOL.14140130.FIG12.JPEG. [DOI] [PubMed] [Google Scholar]
- [4].Swinney C, Li A, Bhatti I, Veeravagu A. Optimization of tumor resection with intra-operative magnetic resonance imaging. J Clin Neurosci 2016;34:11–4. 10.1016/J.JOCN.2016.05.030. [DOI] [PubMed] [Google Scholar]
- [5].Chan HW, Bamber J, Dorward N, Chakraborty A, Uff C. Ultrasound Elastography. In: Prada F, Solbiati L, Martegani A, DiMeco F, editors. Intraoperative Ultrasound Neurosurg. From Stand. B-mode to Elastosonography, Springer, Cham; 2016, p. 173–87. 10.1007/978-3-319-25268-1_14. [DOI] [Google Scholar]
- [6].Garra BS. Elastography: history, principles, and technique comparison. Abdom Imaging 2015;40:680–97. 10.1007/S00261-014-0305-8. [DOI] [PubMed] [Google Scholar]
- [7].National Institute of Health. Quality Assessment Tool for Case Series Studies 2020. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed November 28, 2021). [Google Scholar]
- [8].North American Spine Society. Levels of Evidence For Primary Research Question 2005. https://www.spine.org/Portals/0/Assets/Downloads/ResearchClinicalCare/LevelsofEvidence.pdf (accessed November 28, 2021). [Google Scholar]
- [9].Chan HW, Pressler R, Uff C, Gunny R, St Piers K, Cross H, et al. A novel technique of detecting MRI-negative lesion in focal symptomatic epilepsy: intraoperative ShearWave elastography. Epilepsia 2014;55. 10.1111/EPI.12562. [DOI] [PubMed] [Google Scholar]
- [10].Dirrichs T, Meiser N, Panek A, Trepels-Kottek S, Orlikowsky T, Kuhl CK, et al. Transcranial Shear Wave Elastography of Neonatal and Infant Brains for Quantitative Evaluation of Increased Intracranial Pressure. Invest Radiol 2019;54:719–27. 10.1097/RLI.0000000000000602. [DOI] [PubMed] [Google Scholar]
- [11].Vergari C, Chanteux L, Pietton R, Langlais T, Vialle R, Skalli W. Shear wave elastography of lumbar annulus fibrosus in adolescent idiopathic scoliosis before and after surgical intervention. Eur Radiol 2020;30:1980–5. 10.1007/S00330-019-06563-4. [DOI] [PubMed] [Google Scholar]
- [12].Olivero WC, Wszalek T, Wang H, Farahvar A, Rieth SM, Johnson CL. Magnetic Resonance Elastography Demonstrating Low Brain Stiffness in a Patient with Low-Pressure Hydrocephalus: Case Report. Pediatr Neurosurg 2016;51:257–62. 10.1159/000445900. [DOI] [PubMed] [Google Scholar]
- [13].Yin Z, Glaser KJ, Manduca A, Van Gompel JJ, Link MJ, Hughes JD, et al. Slip Interface Imaging Predicts Tumor-Brain Adhesion in Vestibular Schwannomas. Radiology 2015;277:507–17. 10.1148/RADIOL.2015151075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yin Z, Hughes JD, Glaser KJ, Manduca A, Van Gompel J, Link MJ, et al. Slip interface imaging based on MR-elastography preoperatively predicts meningioma-brain adhesion. J Magn Reson Imaging 2017;46:1007–16. 10.1002/JMRI.25623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cepeda S, García-García S, Arrese I, Fernández-Pérez G, Velasco-Casares M, Fajardo-Puentes M, et al. Comparison of Intraoperative Ultrasound B-Mode and Strain Elastography for the Differentiation of Glioblastomas From Solitary Brain Metastases. An Automated Deep Learning Approach for Image Analysis. Front Oncol 2021;10:1. 10.3389/FONC.2020.590756/FULL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cepeda S, García-García S, Velasco-Casares M, Fernández-Pérez G, Zamora T, Arrese I, et al. Is There a Relationship between the Elasticity of Brain Tumors, Changes in Diffusion Tensor Imaging, and Histological Findings? A Pilot Study Using Intraoperative Ultrasound Elastography. Brain Sci 2021;11:1–13. 10.3390/BRAINSCI11020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cepeda S, García-García S, Arrese I, Velasco-Casares M, Sarabia R. Relationship between the overall survival in glioblastomas and the radiomic features of intraoperative ultrasound: a feasibility study. J Ultrasound 2021. 10.1007/S40477-021-00569-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chakraborty A, Bamber JC, Dorward NL. Preliminary investigation into the use of ultrasound elastography during brain tumour resection: Http://DxDoiOrg/101258/Ult2011011057 2012;20:33–40. 10.1258/ULT.2011.011057. [DOI] [Google Scholar]
- [19].Chan HW, Uff C, Chakraborty A, Dorward N, Bamber JC. Clinical Application of Shear Wave Elastography for Assisting Brain Tumor Resection. Front Oncol 2021;11:112. 10.3389/FONC.2021.619286/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chauvet D, Imbault M, Capelle L, Demene C, Mossad M, Karachi C, et al. In Vivo Measurement of Brain Tumor Elasticity Using Intraoperative Shear Wave Elastography. Ultraschall Med 2016;37:584–90. 10.1055/S-0034-1399152. [DOI] [PubMed] [Google Scholar]
- [21].Selbekk T, Brekken R, Indergaard M, Solheim O, Unsgård G. Comparison of contrast in brightness mode and strain ultrasonography of glial brain tumours. BMC Med Imaging 2012;12:1–7. 10.1186/1471-2342-12-11/FIGURES/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yin L, Cheng L, Wang F, Zhu X, Hua Y, He W. Application of intraoperative B-mode ultrasound and shear wave elastography for glioma grading. Quant Imaging Med Surg 2021;11. 10.21037/QIMS-20-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Selbekk T, Bang J, Unsgaard G. Strain processing of intraoperative ultrasound images of brain tumours: Initial results. Ultrasound Med Biol 2005;31:45–51. 10.1016/J.ULTRASMEDBIO.2004.09.011. [DOI] [PubMed] [Google Scholar]
- [24].Cepeda S, Arrese I, García-García S, Velasco-Casares M, Escudero-Caro T, Zamora T, et al. Meningioma Consistency Can Be Defined by Combining the Radiomic Features of Magnetic Resonance Imaging and Ultrasound Elastography. A Pilot Study Using Machine Learning Classifiers. World Neurosurg 2021;146:e1147–59. 10.1016/J.WNEU.2020.11.113. [DOI] [PubMed] [Google Scholar]
- [25].Hughes JD, Fattahi N, Van Gompel J, Arani A, Meyer F, Lanzino G, et al. Higher-Resolution Magnetic Resonance Elastography in Meningiomas to Determine Intratumoral Consistency. Neurosurgery 2015;77:653. 10.1227/NEU.0000000000000892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Prada F, Del Bene M, Rampini A, Mattei L, Casali C, Vetrano IG, et al. Intraoperative Strain Elastosonography in Brain Tumor Surgery. Oper Neurosurg (Hagerstown, Md) 2019;17:227–36. 10.1093/ONS/OPY323. [DOI] [PubMed] [Google Scholar]
- [27].Sakai N, Takehara Y, Yamashita S, Ohishi N, Kawaji H, Sameshima T, et al. Shear Stiffness of 4 Common Intracranial Tumors Measured Using MR Elastography: Comparison with Intraoperative Consistency Grading. AJNR Am J Neuroradiol 2016;37:1851. 10.3174/AJNR.A4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Takamura T, Motosugi U, Ogiwara M, Sasaki Y, Glaser KJ, Ehman RL, et al. Relationship between Shear Stiffness Measured by MR Elastography and Perfusion Metrics Measured by Perfusion CT of Meningiomas. AJNR Am J Neuroradiol 2021;42:1216–22. 10.3174/AJNR.A7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hughes JD, Fattahi N, Van Gompel J, Arani A, Ehman R, Huston J. Magnetic resonance elastography detects tumoral consistency in pituitary macroadenomas. Pituitary 2016;19:286–92. 10.1007/S11102-016-0706-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lagerstrand K, Gaedes N, Eriksson S, Farahmand D, De Coursey E, Johansson G, et al. Virtual magnetic resonance elastography has the feasibility to evaluate preoperative pituitary adenoma consistency. Pituitary 2021;24:530. 10.1007/S11102-021-01129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Al-Habib A, Alhothali W, Albakr A, Elwatidy S, Alawaji G, Alabdulsalam H, et al. Effects of compressive lesions on intraoperative human spinal cord elasticity. J Neurosurg Spine 2021:1–10. 10.3171/2021.1.SPINE201482. [DOI] [PubMed] [Google Scholar]
- [32].Mathon B, Amelot A, Carpentier A, Clemenceau S. Intraoperative real-time guidance using ShearWave Elastography for epilepsy surgery. Seizure - Eur J Epilepsy 2019;71:24–7. 10.1016/J.SEIZURE.2019.06.001. [DOI] [PubMed] [Google Scholar]
- [33].Mathon B, Clemenceau S, Carpentier A. Intraoperative Ultrasound Shear-Wave Elastography in Focal Cortical Dysplasia Surgery. J Clin Med 2021;10:1–11. 10.3390/JCM10051049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Prada F, Gennari AG, Quaia E, D’Incerti L, de Curtis M, DiMeco F, et al. Advanced intraoperative ultrasound (ioUS) techniques in focal cortical dysplasia (FCD) surgery: A preliminary experience on a case series. Clin Neurol Neurosurg 2020;198. 10.1016/J.CLINEURO.2020.106188. [DOI] [PubMed] [Google Scholar]
- [35].Freimann FB, Streitberger KJ, Klatt D, Lin K, McLaughlin J, Braun J, et al. Alteration of brain viscoelasticity after shunt treatment in normal pressure hydrocephalus. Neuroradiology 2012;54:189–96. 10.1007/S00234-011-0871-1/FIGURES/3. [DOI] [PubMed] [Google Scholar]
- [36].Perry A, Graffeo CS, Fattahi N, ElSheikh MM, Cray N, Arani A, et al. Clinical Correlation of Abnormal Findings on Magnetic Resonance Elastography in Idiopathic Normal Pressure Hydrocephalus. World Neurosurg 2017;99:695. 10.1016/J.WNEU.2016.12.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Solamen LM, McGarry MDJ, Fried J, Weaver JB, Lollis SS, Paulsen KD. Poroelastic Mechanical Properties of the Brain Tissue of Normal Pressure Hydrocephalus Patients During Lumbar Drain Treatment Using Intrinsic Actuation MR Elastography. Acad Radiol 2021;28:457–66. 10.1016/J.ACRA.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Murphy MC, Huston J, Glaser KJ, Manduca A, Meyer FB, Lanzino G, et al. Preoperative assessment of meningioma stiffness by magnetic resonance elastography. J Neurosurg 2013;118:643. 10.3171/2012.9.JNS12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Alawaji G, Alhothali W, Albakr A, Amer A, Al-Habib A, Ajlan A. Shear wave elastography for intracranial epidermoid tumors. Clin Neurol Neurosurg 2021;207:106531. 10.1016/J.CLINEURO.2021.106531. [DOI] [PubMed] [Google Scholar]
- [40].Pepa GM Della, Menna G, Stifano V, Pezzullo AM, Auricchio AM, Rapisarda A, et al. Predicting meningioma consistency and brain-meningioma interface with intraoperative strain ultrasound elastography: a novel application to guide surgical strategy. Neurosurg Focus 2021;50:1–11. 10.3171/2020.10.FOCUS20797. [DOI] [PubMed] [Google Scholar]
- [41].Sarvazyan A, Hall TJ, Urban MW, Fatemi M, Aglyamov SR, Garra BS. An overview of elastography - an emerging branch of medical imaging. Curr Med Imaging Rev 2011;7:255. 10.2174/157340511798038684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ferraioli G, Wong VWS, Castera L, Berzigotti A, Sporea I, Dietrich CF, et al. Liver Ultrasound Elastography: An Update to the World Federation for Ultrasound in Medicine and Biology Guidelines and Recommendations. Ultrasound Med Biol 2018;44:2419–40. 10.1016/J.ULTRASMEDBIO.2018.07.008. [DOI] [PubMed] [Google Scholar]
- [43].Gherlan GS. Liver ultrasound elastography: More than staging the disease. World J Hepatol 2015;7:1595. 10.4254/WJH.V7.I12.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhan J, Jin JM, Diao XH, Chen Y. Acoustic radiation force impulse imaging (ARFI) for differentiation of benign and malignant thyroid nodules--A meta-analysis. Eur J Radiol 2015;84:2181–6. 10.1016/J.EJRAD.2015.07.015. [DOI] [PubMed] [Google Scholar]
- [45].Chen L, He J, Liu G, Shao K, Zhou M, Li B, et al. Diagnostic performances of shear-wave elastography for identification of malignant breast lesions: a meta-analysis. Jpn J Radiol 2014;32:592–9. 10.1007/S11604-014-0349-2. [DOI] [PubMed] [Google Scholar]
- [46].Menzilcioglu MS, Duymus M, Citil S, Avcu S, Gungor G, Sahin T, et al. Strain wave elastography for evaluation of renal parenchyma in chronic kidney disease. Br J Radiol 2015;88. 10.1259/BJR.20140714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Sigrist RMS, Liau J, Kaffas A El, Chammas MC, Willmann JK. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics 2017;7:1303. 10.7150/THNO.18650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tang A, Cloutier G, Szeverenyi NM, Sirlin CB. Ultrasound Elastography and MR Elastography for Assessing Liver Fibrosis: Part 2, Diagnostic Performance, Confounders, and Future Directions. AJR Am J Roentgenol 2015;205:33. 10.2214/AJR.15.14553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Nicholson JA, Tsang STJ, MacGillivray TJ, Perks F, Simpson AHRW. What is the role of ultrasound in fracture management?: Diagnosis and therapeutic potential for fractures, delayed unions, and fracture-related infection. Bone Joint Res 2019;8:304. 10.1302/2046-3758.87.BJR-2018-0215.R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cohen-Cohen S, Helal A, Yin Z, Ball MK, Ehman RL, Gompel JJ Van, et al. Predicting pituitary adenoma consistency with preoperative magnetic resonance elastography. J Neurosurg 2021;1:1–8. 10.3171/2021.6.JNS204425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Gupta P, Sodhi KS, Saxena AK, Khandelwal N, Singhi P. Neonatal cranial sonography: A concise review for clinicians. J Pediatr Neurosci 2016;11:7. 10.4103/1817-1745.181261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Vergari C, Rouch P, Dubois G, Bonneau D, Dubousset J, Tanter M, et al. Non-invasive biomechanical characterization of intervertebral discs by shear wave ultrasound elastography: a feasibility study. Eur Radiol 2014;24:3210–6. 10.1007/S00330-014-3382-8. [DOI] [PubMed] [Google Scholar]
- [53].Afacan O, Erem B, Roby DP, Roth N, Roth A, Prabhu SP, et al. Evaluation of motion and its effect on brain magnetic resonance image quality in children. Pediatr Radiol 2016;46:1728. 10.1007/S00247-016-3677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Serai SD, Panganiban J, Dhyani M, Degnan AJ, Anupindi SA. Imaging Modalities in Pediatric NAFLD. Clin Liver Dis 2021;17:200. 10.1002/CLD.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Litwiller DV, Mariappan YK, Ehman RL. Magnetic Resonance Elastography. Curr Med Imaging Rev 2012;8:46. 10.2174/157340512799220562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Youk JH, Son EJ, Gweon HM, Kim H, Park YJ, Kim JA. Comparison of Strain and Shear Wave Elastography for the Differentiation of Benign From Malignant Breast Lesions, Combined With B-mode Ultrasonography: Qualitative and Quantitative Assessments. Ultrasound Med Biol 2014;40:2336–44. 10.1016/J.ULTRASMEDBIO.2014.05.020. [DOI] [PubMed] [Google Scholar]
- [57].Sowa Y, Numajiri T, Itsukage S, Nishino K. Comparison of Shear-Wave and Strain Ultrasound Elastography for Evaluating Fat Induration after Breast Reconstruction. Plast Reconstr Surg Glob Open 2016;4:e677. 10.1097/GOX.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Mutala TM, Ndaiga P, Aywak A. Comparison of qualitative and semiquantitative strain elastography in breast lesions for diagnostic accuracy. Cancer Imaging 2016;16. 10.1186/S40644-016-0070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Gennisson JL, Deffieux T, Fink M, Tanter M. Ultrasound elastography: Principles and techniques. Diagn Interv Imaging 2013;94:487–95. 10.1016/J.DIII.2013.01.022. [DOI] [PubMed] [Google Scholar]
- [60].Taljanovic MS, Gimber LH, Becker GW, Latt LD, Klauser AS, Melville DM, et al. Shear-wave elastography: Basic physics and musculoskeletal applications. Radiographics 2017;37:855–70. 10.1148/RG.2017160116/ASSET/IMAGES/LARGE/RG.2017160116.TBL1.JPEG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Nowicki A, Dobruch-Sobczak K. Introduction to ultrasound elastography. J Ultrason 2016;16:113. 10.15557/JOU.2016.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Gajdoš M, Říha P, Kojan M, Doležalová I, Mutsaerts HJMM, Petr J, et al. Epileptogenic zone detection in MRI negative epilepsy using adaptive thresholding of arterial spin labeling data. Sci Reports 2021 111 2021;11:1–6. 10.1038/s41598-021-89774-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Ganau M, Syrmos N, Martin AR, Jiang F, Fehlings MG. Intraoperative ultrasound in spine surgery: history, current applications, future developments. Quant Imaging Med Surg 2018;8:261. 10.21037/QIMS.2018.04.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Park SH, Kim SY, Suh CH, Lee SS, Kim KW, Lee SJ, et al. What we need to know when performing and interpreting US elastography. Clin Mol Hepatol 2016;22:406. 10.3350/CMH.2016.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Zhang YN, Fowler KJ, Ozturk A, Potu CK, Louie AL, Montes V, et al. Liver Fibrosis Imaging: A clinical review of Ultrasound and Magnetic Resonance Elastography. J Magn Reson Imaging 2020;51:25. 10.1002/JMRI.26716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ferraioli G, Filice C, Castera L, Choi BI, Sporea I, Wilson SR, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 3: liver. Ultrasound Med Biol 2015;41:1161–79. 10.1016/J.ULTRASMEDBIO.2015.03.007. [DOI] [PubMed] [Google Scholar]
- [67].Lee SH, Chang JM, Cho N, Koo HR, Yi A, Kim SJ, et al. Practice guideline for the performance of breast ultrasound elastography. Ultrasonography 2014;33:3. 10.14366/USG.13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Barr RG, Nakashima K, Amy D, Cosgrove D, Farrokh A, Schafer F, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 2: breast. Ultrasound Med Biol 2015;41:1148–60. 10.1016/J.ULTRASMEDBIO.2015.03.008. [DOI] [PubMed] [Google Scholar]
- [69].Barr RG, Cosgrove D, Brock M, Cantisani V, Correas JM, Postema AW, et al. WFUMB Guidelines and Recommendations on the Clinical Use of Ultrasound Elastography: Part 5. Prostate. Ultrasound Med Biol 2017;43:27–48. 10.1016/J.ULTRASMEDBIO.2016.06.020. [DOI] [PubMed] [Google Scholar]
- [70].Cosgrove D, Barr R, Bojunga J, Cantisani V, Chammas MC, Dighe M, et al. WFUMB Guidelines and Recommendations on the Clinical Use of Ultrasound Elastography: Part 4. Thyroid. Ultrasound Med Biol 2017;43:4–26. 10.1016/J.ULTRASMEDBIO.2016.06.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


