Abstract
Background:
Amphicrine prostate carcinoma (AMPC) is a poorly defined subset of prostate cancer in which cells co-express luminal prostate epithelial and neuroendocrine markers. The optimal treatment strategy is unknown. We sought to further characterize the clinical, histomorphologic, and molecular characteristics of AMPC and to identify areas of potential future treatment investigations.
Methods:
We retrospectively identified 17 cases of AMPC at a single institution, defined as synaptophysin expression in >70% of cells and co-expression of AR signaling markers (either AR, PSA, or NKX3.1) in >50% of cells. Clinical and histologic features of AMPC cases as well as response to treatment and clinical outcomes were described.
Results:
Five AMPC cases arose de novo in the absence of prior systemic treatment and behaved distinctly from cases that were treatment-emergent. In these de novo cases, despite expression of neuroendocrine markers, prognosis appeared more favorable than high-grade neuroendocrine carcinoma, with two (40%) patients with de novo metastatic disease, universal response to androgen deprivation therapy, and no deaths at a median follow-up of 12.3 months. Treatment-emergent AMPC arose a median of 41.1 months after ADT initiation and was associated with poor response to therapy.
Conclusions:
We show that amphicrine prostate cancer is a unique entity and differs in clinical and molecular features from high-grade neuroendocrine carcinomas of the prostate. Our study highlights the need to recognize AMPC as a unique molecularly defined subgroup of prostate cancer.
Keywords: Amphicrine prostate cancer, castration resistant prostate cancer, neuroendocrine, synaptophysin
Introduction
Prostate cancer is an androgen dependent malignancy, with the androgen receptor (AR) driving a variety of cell growth and proliferation processes. The cornerstone of treatment for advanced prostate cancer is androgen deprivation therapy (ADT), which lowers testosterone production and therefore AR activation. Resistance to ADT inevitably develops, a disease state known as castration resistant prostate cancer (CRPC). It is increasingly recognized that CRPC is a heterogenous entity, with a variety of evolved mechanisms of resistance to both ADT and second-generation hormonal manipulations such as abiraterone, apalutamide, darolutamide or enzalutamide.1
One well-described subset of metastatic castration-resistant prostate cancer (mCRPC) is high-grade neuroendocrine carcinoma which most commonly occurs as a result of selective pressure after treatment with ADT for typical prostate adenocarcinoma.2 Neuroendocrine prostate cancer (NEPC) is characterized by the expression of neuroendocrine markers (e.g. synaptophysin, INSM1, chromogranin), the absence of prostatic lineage markers (e.g. AR, NKX3.1, and HOXB13) and histomorphologic features that are similar to high-grade neuroendocrine and small cell carcinomas in other sites.3 Increasingly, other distinct phenotypes of mCRPC are being recognized.
Labrecque and colleagues characterized 5 individual CRPC subtypes based on both immunohistochemical (IHC) staining and gene expression profiling.4 In addition to AR driven CRPC and high-grade neuroendocrine prostate cancer, there is an AR-low phenotype, a double negative phenotype which lacks both AR and neuroendocrine signaling, and an amphicrine phenotype in which cancer cells co-express AR signaling and neuroendocrine markers.4 Importantly, some of these subtypes have clinically important considerations, e.g. NEPC is treated with platinum chemotherapy and the double negative phenotype is driven by fibroblast growth factor receptor signaling, making pharmacologic inhibition of this pathway an attractive area for clinical trial exploration.5
Although amphicrine prostate carcinoma (AMPC) has previously been described in the literature, it remains poorly understood. AMPC is defined by the co-expression of AR signaling and neuroendocrine markers and maintenance of luminal prostate epithelial differentiation. Importantly, AMPC needs to be differentiated from biphasic carcinomas, in which intermixed but phenotypically distinct neoplastic cell populations with either adenocarcinoma or high-grade neuroendocrine features co-exist within the same tumor mass.2,6 Molecularly, AMPC appears to be driven either by attenuation of the RE1- silencing transcription factor (REST), a transcriptional repressor of neuronal genes, or by increased ASCL1 activity.7 In one of the first clinicopathologic series of AMPC, Prendeville and colleagues reported a series of five cases of high-grade prostate cancer with amphicrine features, all of which had immunohistochemical positivity for PSA, AR, and prostate-specific acid phosphatase as well as non-focal positivity for chromogranin-A and synaptophysin.8 All five cases lacked morphologic features of either small-cell or large-cell neuroendocrine carcinoma and had a clinically aggressive phenotype. A more recent cohort of 79 cases of prostate cancer with NE differentiation identified 15 cases which had features of high-grade adenocarcinoma and diffuse positivity for at least one prostatic and one NE marker.9 Although greater recognition is being paid to AMPC, the clinical outcomes and responses to treatment of this unique subset of prostate cancer have not been previously well described.
In this study, we report on the histologic and clinical characteristics of a series of men with amphicrine prostate carcinoma, focusing on response to treatment and potential areas of future treatment opportunities. We differentiate between AMPC that is present at diagnosis (i.e. de novo) and AMPC that has arisen as a result of selective treatment pressure.
Material and Methods
We retrospectively identified men with prostate cancer who had at least one tissue biopsy which showed positivity for synaptophysin in >70% of cells and co-expression of an AR signaling marker (either AR, PSA, or NKX3.1) in >50% of cells. These cutoffs were chosen as a way to identify tumors that were dominantly positive for each of those markers and to provide a numerical definition for future studies of AMPC. Furthermore, only tumors without morphological features of small cell or large cell carcinoma (including nuclear molding or prominent geographic necrosis) as described previously were included.6 Men were initially identified using the UW genitourinary-Caisis database and querying for the word “synaptophysin” and then excluded if they did not meet the criteria above. After the initial database query, four additional patients were identified who had metastatic biopsies that met criteria. Clinical and histopathologic data was abstracted retrospectively. Next-generation sequencing of tumor tissue was performed on either primary prostate or metastatic tissue using the clinically validated UW-OncoPlex platform.10 Differential expression analyses of publicly available RNA-Seq data re- aligned to the hg38 human genome using STAR v2.7.3a were carried out in R using limma v3.40.6 with the default settings for the voom, lmFit, eBayes, and topTable functions.4,7,11,12 Androgen receptor and neuroendocrine signature scores were calculated using GSVA v1.32.0 using log2 fragments per kilobase of transcript per million mapped reads (FPKM) values as input.13
Analyses were descriptive in nature. The Kaplan-Meier method was used to estimate overall survival and results are reported with 95% CI. Analyses were conducted using the statistical software STATA. Pathologic slides were reviewed and independently scored by an expert genitourinary pathologist (M.C.H.). For all immunohistochemical studies, immunoreactivities were scored in a blinded manner using a previously established H- score system, whereby the optical density level (“0” for no brown color, “1” for faint and fine brown chromogen deposition, “2” for intermediate chromogen deposition, and “3” for prominent chromogen deposition) was multiplied by the percentage of cells at each staining level, resulting in a total H-score (range 0–300).14,15
Results
Baseline Characteristics
We identified 17 patients that met our inclusion criteria for AMPC. Four patients had only histopathologic data available without clinical follow-up and were included only in those analyses. Eight patients had amphicrine characteristics that emerged after prior treatment (i.e. “treatment-emergent”) and five patients had amphicrine features present either at time of diagnosis or after prior localized therapy without systemic therapy (i.e. “de novo”). One patient with treatment-emergent disease was primarily treated outside the country with limited data available, and was included only in survival and histologic analyses. Table 1 shows the baseline characteristics for all patients included in the series. Table 2 shows characteristics at time of amphicrine diagnosis for patients with de novo AMPC (Table 2A) and treatment-emergent AMPC (Table 2B). Patients with de novo disease presented at a median age of 56 (range 54–75 years). Three of these patients had locally advanced disease only whereas two had metastatic disease at diagnosis. Of the three patients with locally advanced disease, two had very locally aggressive T4 tumors and one had a pathologic T2c tumor at the time of prostatectomy. All patients with treatment-emergent disease had metastatic disease at the time of AMPC recognition, and the median time from initiation of ADT to diagnosis of AMPC was 41.1 months (Interquartile range 21.1–74.4 months). In contrast, although reports in the literature vary, median time from diagnosis to NEPC is estimated between 18–25 months.16 Most patients in this series had received prior second-generation hormonal therapy and 5 (71%) had received prior docetaxel chemotherapy.
Table 1.
Baseline Characteristics.
| Total, N | 13 |
| Age at Diagnosis- yr: Median(Range) | 62 (44–75) |
| Gleason score- no. (%) | |
| 7 | 4 (31) |
| 8 | 1 (8) |
| 9 | 4 (31) |
| 10 | 1 (8) |
| Unknown | 3 (23) |
| De Novo Amphicrine Disease, no. (%) | 5 (38) |
| Treatment-Emergent Amphicrine Disease, no. (%) | 8 (62) |
| Presented with metastatic disease at diagnosis (%) | 5 (38) |
| Use of standard ADT no. (%) | 13 (100) |
| Use of abiraterone, no. (%) | 7 (54) |
| Use of enzalutamide, no. (%) | 4 (31) |
| Use of docetaxel, no. (%) | 7 (54) |
| Use of cabazitaxel, no. (%) | 1 (8) |
| Use of taxane + platinum chemotherapy, no. (%) | 4 (31) |
| Use of sipeleucel-T, no. (%) | 3 (23) |
| Use of radium-223, no. (%) | 1 (8) |
| Clinical Trial Participation, no. (%)* | 4 (31) |
Clinical Trials: Phase I Taiho TO-TASS3681–101 (NCT02566772) (Pt 2), Phase I DuoBody PSMA bispecific antibody (NCT03926013) (Pt 2), Phase II BAT + Olaparib (NCT03516812) (Pt 4), Phase II Bipolar Androgen Therapy (NCT02286921) (Pt 7), Inovio DNA Vaccine (NCT02514213) (Pt 14)
Table 2.
Characteristics at time of AMPC diagnosis.
| A. De Novo AMPC | |
| Age at Diagnosis- yr: Median(Range) | 56 (54–75) |
| Gleason score- no. (%) | |
| 7 | 1 (20) |
| 9 | 1 (20) |
| 10 | 1 (20) |
| Unknown | 2 (40) |
| Sites of metastasis | |
| None | 3 (60) |
| Lymph Node Only | 1 (20) |
| Visceral Metastases | 1 (20) |
| B. Treatment-Emergent AMPC | |
| PSA, median (range) | 33.33 (0.61–3660) |
| Sites of Metastasis | |
| Bone +/− Lymph Node | 2 |
| Visceral +/− Bone +/− Lymph Node | 6 |
| Time since initiation of ADT, months | 41.1 (95% CI: 11.1–71.4) |
| Prior Treatments | |
| ADT | 7 (100) |
| Abiraterone | 4 (57) |
| Enzalutamide | 4 (57) |
| Docetaxel | 5 (71) |
| Cabazitaxel | 1 (14) |
| Docetaxel/Carboplatin | 1 (14) |
| Radium-223 | 1 (14) |
| Sipuleucel-T | 3 (43) |
Histopathologic and Molecular Characteristics
Fourteen cases had pathologic specimens that were available for re-review. Representative cases are shown in Figure 1. Architecturally, tumors showed mostly features of high-grade adenocarcinoma with sheets, small nests or cords of cells. Focal glandular and rosette-like cribiform structures were noted. Cytologically, we observed large nuclei with mostly open chromatin and often prominent nucleoli. Most tumors showed abundant cytoplasm which varied in staining characteristics from pale eosinophilic to dense amphophilic. Mitotic activity was noted in most cases. As per our inclusion criteria (see methods section), none of the tumors showed features of small cell carcinoma (Figure 1). Notably, these histomorphologic features are similar to other prior reports that described tumors with amphicrine features.8,9 No distinct morphology unique to AMPC was identified and by definition (see Methods) none of the cases showed features of high-grade neuroendocrine carcinoma (including nuclear molding or extensive geographic necrosis). H-scores for relevant immunostains are shown in Table 3. For eight cases immunostains for the AR signaling marker NKX3.1 and the neuroendocrine marker synaptophysin were available for review. Both NKX3.1 and synaptophysin showed high expression levels, mean 279.4 (range 215–300) and mean 212.5 (range 140–280), respectively. For five cases, PSA and chromogranin expression was assessed and mean H-scores were 128 (range 10–190) for PSA and 102.5 (range 50–150) for chromogranin. Androgen receptor staining was available in one case, with an H-score of 240.
Figure 1.
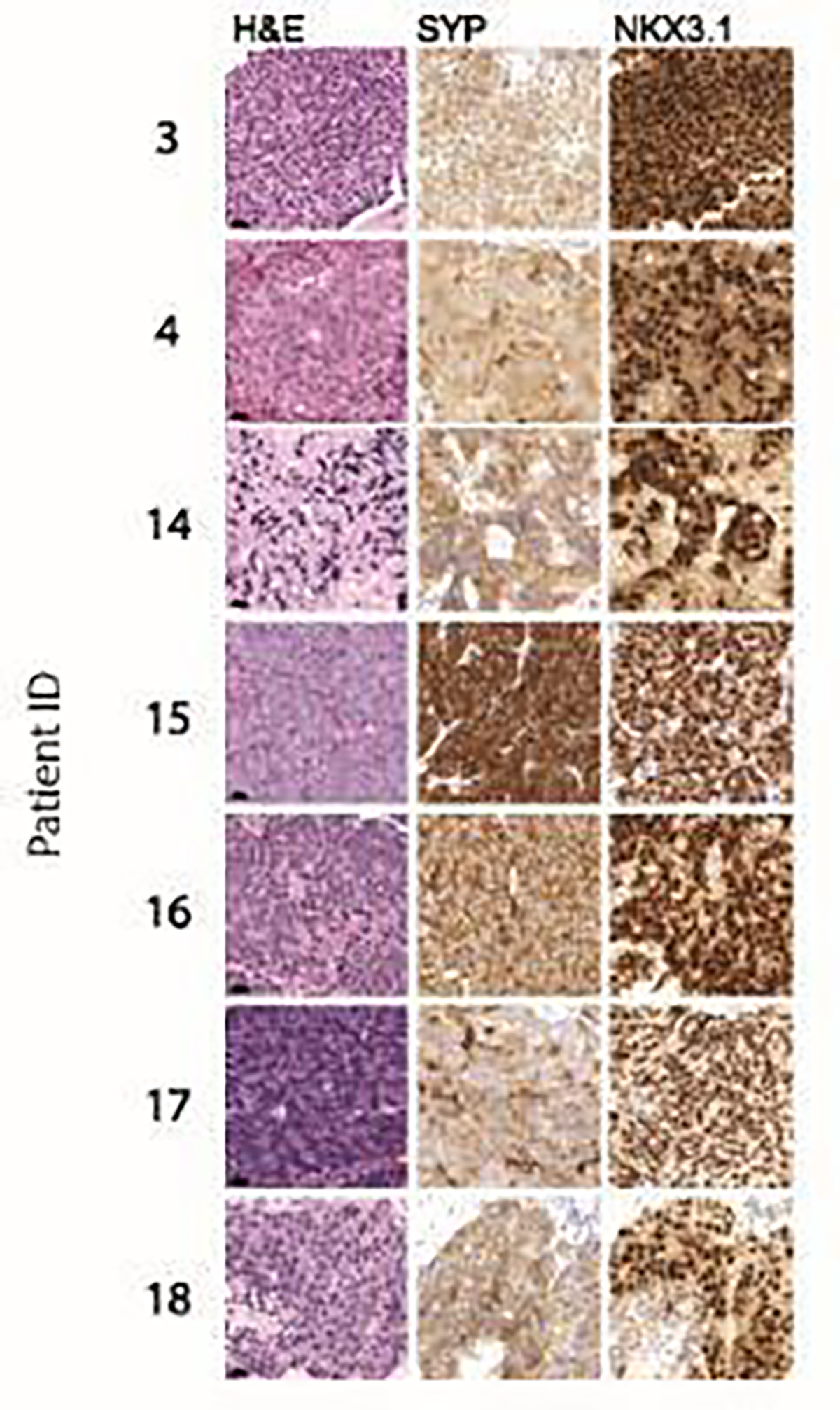
Histomorphological and immunophenotypic characterization of amphicrine prostate cancers. Representative micrographs show H&E, synaptophysin (SYP) and NKX3.1 immunohistochemistry. Note the diffuse positivity for SYP and NKX3.1 in all cases. Scale bars denote 20 um.
Table 3.
Relevant immunostains for AMPC cases with available pathology slides.
| Cases Evaluated | H-score, mean | H-score, median (range) | |
|---|---|---|---|
| Androgen Receptor (AR) | 1 | 240 | 240 |
| PSA | 5 | 128 | 140 (10–200) |
| NKX3.1 | 8 | 279 | 285 (215–300) |
| Synaptophysin (SYP) | 8 | 213 | 205 (140–280) |
| INSM1 | 1 | 200 | 200 |
| Chromogranin (CG) | 4 | 103 | 105 (50–150) |
Six patients had next-generation sequencing of either a primary or metastatic lesion (Table 4). Three had treatment-emergent AMPC and three had de novo AMPC. Four (66%) of six patients had TP53 mutations. Three (50%) patients had TMPRSS2-ERG rearrangements. Two patients with de novo AMPC had TMPRSS2-ERG rearrangements only, without other alterations detected. One patient with de novo disease had a germline MSH2 mutation with associated loss of heterozygosity in the tumor and associated elevated tumor mutational burden and microsatellite instability. One patient with treatment-emergent disease had somatic BRCA2 deletion with loss of heterozygosity.
Table 4.
Next-generation sequencing of either primary or metastatic tissue from patients with AMPC.
| Patient ID | Tissue Source (1=primary, 2=metastatic) | Germline Mutation | Somatic Mutations |
|---|---|---|---|
| 2 | 1 | Not tested | TP53 (p.R273C) |
| 2 | 2 | Not tested | TP53 (p.R273C), TMPRSS2-ERG fusion, AR amplification |
| 3 | 1 | MSH2 | Biallelic mutation in MSH2 (exon 3–16 dletion, with associated LOH); positive for microsatellite instability; TP53 (p.R273C), hypermutation with TMB of 20 mutations/Mb |
| 5 | 1 | None | TMPRSS2-ERG rearrangement |
| 9 | 2 | Unknown | BRCA2 deletions in exons 3–11 with LOH; PTEN deletion (exons 6- 8); FOXA1 in- frame insertion (c.797ins24); TP53 (p.C2777Gfs*63); APC (p.G520X) with LOH; AR amplification |
| 11 | 2 | Not Tested | None |
| 13 | 2 | Not Tested | TMPRSS2-ERG rearrangement |
This patient also had a PTEN deletion and TP53 mutation. Two patients had AR amplification. None of the cases sequenced had RB1 loss.
Clinical Outcomes
The clinical course of each patient with available treatment data is shown in Figure 2A. The clinical course of patients with treatment-emergent AMPC was variable. Prior to diagnosis of AMPC, time to CRPC after surgical or medical castration was 7.9 months (Interquartile Range: 4.9–11.9 months). Six patients received either abiraterone or enzalutamide as first-line therapy for castration resistant disease. Of these, three (50%) experienced a PSA50 response (i.e. PSA decline of at least 50% from pre-treatment baseline). Median time until clinical/radiographic progression on first-line abiraterone or enzalutamide was 8.6 months (Interquartile range: 8–11 months).
Figure 2.
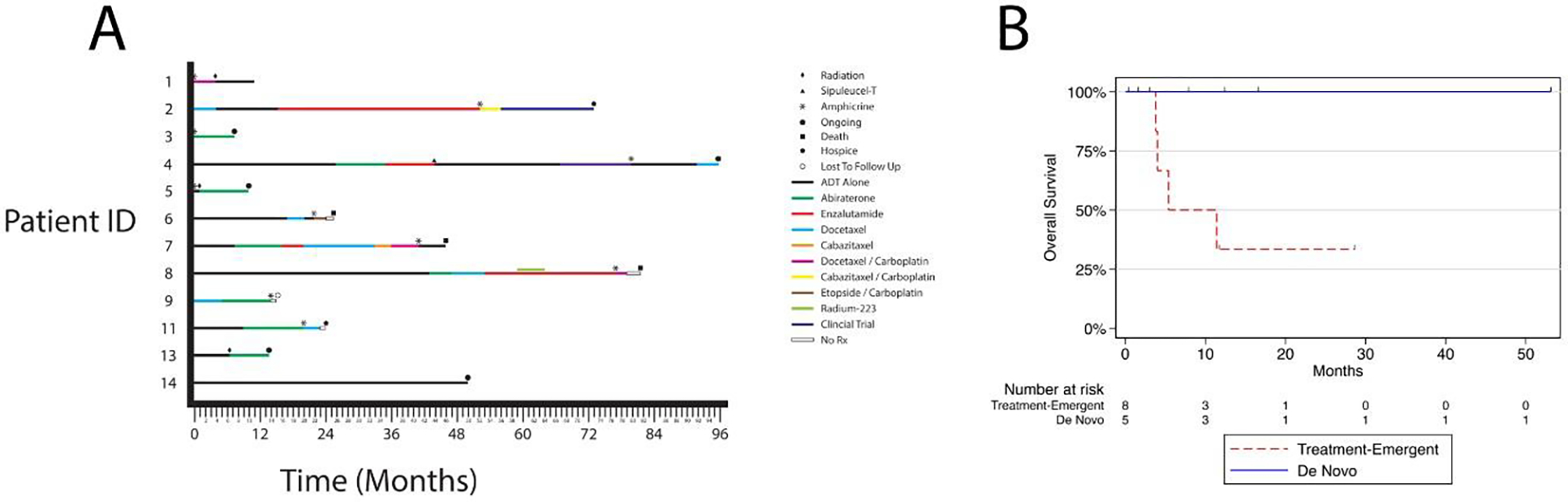
Treatment and clinical outcomes of men with AMPC. A. Swimmers plot showing treatment before and after amphicrine diagnosis (noted as *). B. Overall survival from time of amphicrine diagnosis. Red= Treatment-Emergent AMPC, blue = de novo AMPC.
Median overall survival (OS) for patients with treatment-emergent AMPC after amphicrine diagnosis was 5.3 months (Interquartile Range: 4-Not Reached months) (Figure 2B). After diagnosis of AMPC in those with treatment-emergent disease, two patients were treated with docetaxel alone, one with a PSA50 response and one with progressive disease. Two patients received a taxane chemotherapy in combination with carboplatin after AMPC diagnosis. Neither had a PSA50 response. Two patients received carboplatin and etoposide, both with a PSA50 response. No patients that received platinum chemotherapy had a known alteration in a DNA damage repair gene.
There were no deaths in the five cases of de novo AMPC, with a median follow up of 12.3 months (Figure 2B). Three patients did not have distant metastatic disease at diagnosis. One patient had T4N1 disease and underwent neoadjuvant chemotherapy with docetaxel and carboplatin in addition to androgen deprivation therapy (ADT) followed by radiation therapy. He remains on ADT with an undetectable PSA and no evidence of disease one year after diagnosis. A second patient with T4bN0 disease underwent therapy with ADT and abiraterone with an undetectable PSA seven months following diagnosis, with therapy still ongoing. The third patient had a local recurrence nine years following prostatectomy and salvage radiation and is being treated with ADT alone. In the two cases of de novo AMPC that presented with distant metastatic disease, both received abiraterone in addition to ADT, with therapy ongoing nine months and seven months after initiation.
In order to contextualize the survival findings in our small cohort of AMPC patients, we examined publicly available data from The Cancer Genome Atlas (TCGA) as well as data collected as part of the SU2C-PCF (Stand Up to Cancer/Prostate Cancer Foundation) dream team consortium.12 Patients in the TCGA dataset had primary, nonmetastatic, prostate cancer, undergoing radical prostatectomy.17 The 16 men with amphicrine tumors had a statistically significant trend towards earlier biochemical recurrence, but no difference in disease specific survival (Figure 3).
Figure 3.
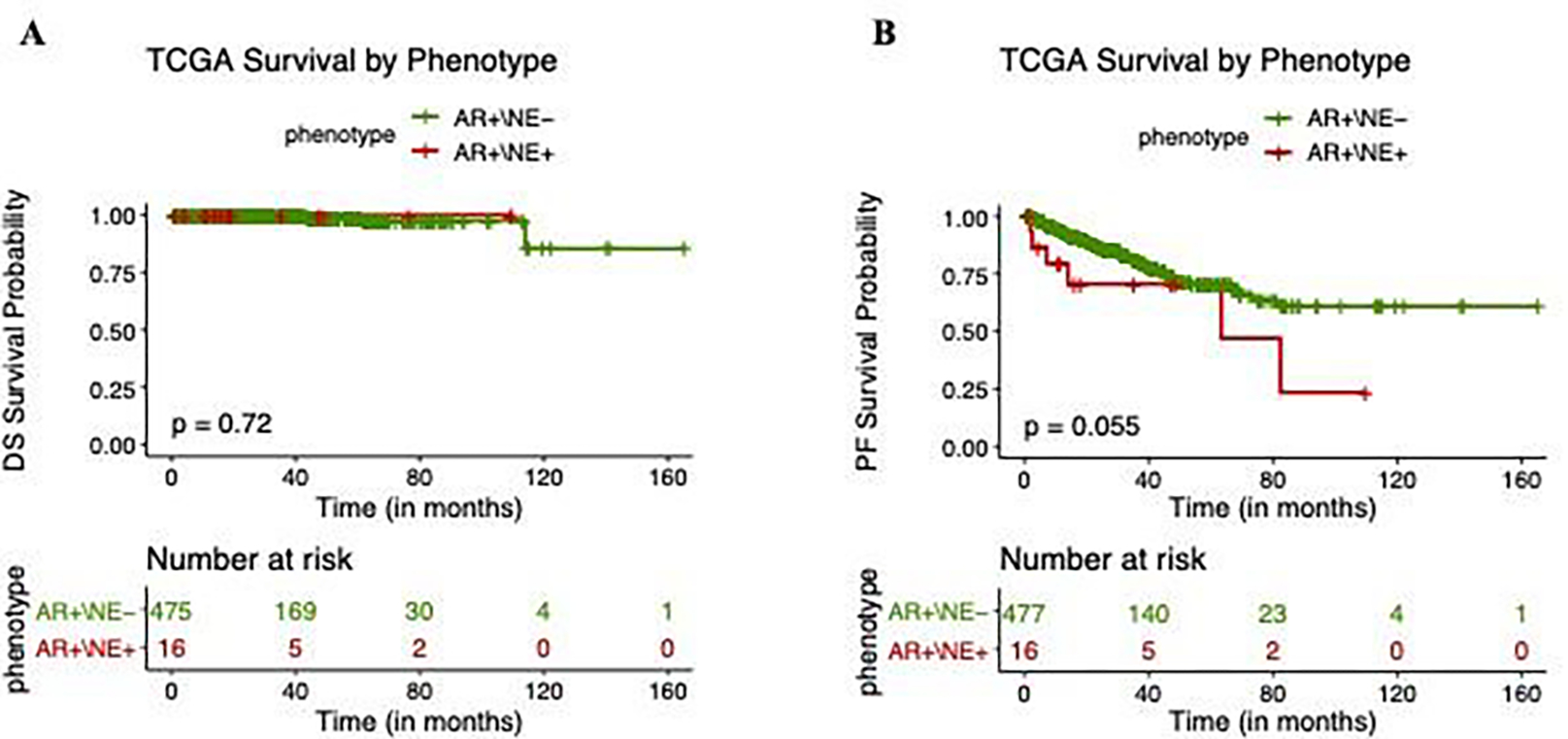
A. Disease specific (DS) survival of AR+/NE- and AR+/NE+ (amphicrine) tumors in The Cancer Genome Atlas (TCGA) dataset. B. Progression free (PF) survival of AR+/NE- and AR+/NE+ (amphicrine) tumors in the TCGA dataset.
Patients in the SU2C-PCF dataset had a metastatic biopsy while undergoing treatment for mCRPC. Samples were characterized as AMPC, NEPC, DNPC, or ARPC based on RNA-seq analysis as described previously.4,7 Overall survival curves are shown in Figure 4A. Notably, AMPC showed a distinctly different prognosis compared to NEPC. Median OS from time of biopsy for AMPC was 20.3 months (IQR 6.9–30 months) compared to a median OS of 5.4 months for NEPC (IQR 2.8–7.7 months). Whereas NEPC had a worse prognosis in comparison to the other described subtypes, AMPC showed survival similar to ARPC (Median OS 17.4 months; IQR 10–29.6 months).
Figure 4.
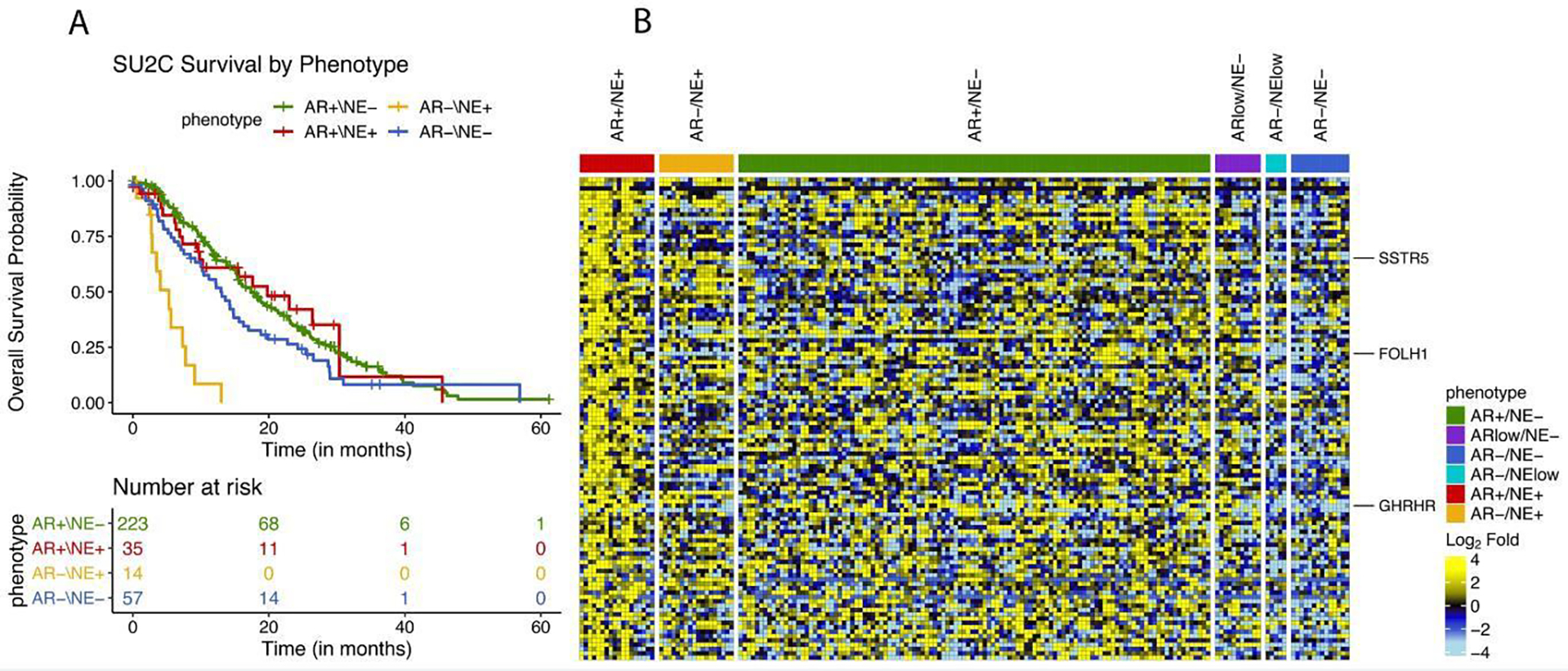
A. Overall survival in SU2C/PCF cohort with four molecularly defined subsets of prostate cancer. B. Gene expression heatmap of tumor specimens from the University of Washington rapid autopsy program.
Gene Expression in Amphicrine Carcinoma
To understand RNA expression patterns associated with AMPC, we leveraged previously published RNA-seq data from 180 cases of the University of Washington rapid autopsy cohort.4 Comparing AMPC with other molecular subtypes, we identified 110 genes which showed higher expression in AMPC (adj. P value <0.005) compared to AR-low carcinoma, neuroendocrine carcinoma, or double negative carcinoma. (Figure 4B).
Interestingly SSTR5 was upregulated, which encodes for a somatostatin receptor that can be bound by octreotide or Lu-177 dotatate, both of which are standardly used to treat pancreatic and gastrointestinal neuroendocrine carcinomas. Given the treatment availability of Lu-177 dotatate, this may be worthy of future study. Other potential targets of interest include FOLH1 which encodes for prostate specific membrane antigen (PSMA), and GHRHR which encodes for growth hormone releasing hormone.
Discussion
Limited published data exists characterizing amphicrine carcinoma of the prostate and the nomenclature around AMPC is still evolving. This series is, to our knowledge, the largest reported series of men with AMPC. Further, previous case reports have included patients with discrete populations of AR expressing cells and neuroendocrine cells spatially contained within the same tumor mass, which likely represents a different biologic entity. We utilized the more stringent criteria for AMPC of cells co-expressing both neuroendocrine markers (i.e. synaptophysin) and markers of AR-signaling (i.e. AR, PSA, or NKX3.1) without histologic features of neuroendocrine carcinoma. Our series dovetails with work by others that have identified amphicrine carcinoma as a small but unique histopathologic subset of both primary and metastatic prostate carcinoma.8,9,18 The prevalence of AMPC is unknown and its identification may be under-recognized in clinical practice, in part due to the lack of clearly defined diagnostic criteria and also mislabeling as neuroendocrine carcinoma due to the presence of synaptophysin on IHC. In addition, IHC evaluation for neuroendocrine markers is not routinely performed in the absence of traditional small-cell morphology.
Importantly, our work confirms that AMPC is distinct from its high-grade neuroendocrine counterpart. The prognosis of high-grade neuroendocrine carcinoma rem ains poor with few patients reaching second-line therapies.19 In our series, patients with de novo AMPC had better survival than would be expected if they behaved similarly to high-grade neuroendocrine tumors and appeared responsive to standard anti-androgen manipulations. The survival estimate in our series for treatment-emergent disease is likely to be unfavorably skewed as the diagnosis of amphicrine was made in most cases when there was a clinical indication for biopsy in late-stage CRPC. However, in the SU2C-PCF database, overall survival was significantly longer for AMPC compared to NEPC. In addition, although only a small subset of patients had genomic profiling, no patients had RB1 loss, which is characteristic of NEPC. Further, two patients had AR amplification, highlighting the ongoing AR signaling activity in these tumors.
Recent data has suggested that selective pressure from AR targeted therapeutics has shifted a significant proportion of CRPC tumors away from sole AR reliance.5 One proposed mechanism is the loss of the neural transcriptional repressor REST, which is associated with development of both NEPC and AMPC.4 It is unknown whether AMPC represents a transitional state wherein a second event occurs that leads to loss of AR signaling and the development of NEPC.
Our work was limited by its retrospective nature and small sample size. Recognition of the AMPC phenotype at the time of treatment selection was not even realized for some of the patients, which may have affected therapeutic selection. Our institution recently adopted a standardized set of IHC to be done on all pathology from men with metastatic prostate cancer, including INSM-1, AR, SYP, Ki-67, and NKX3.1. This series, however, included men who were identified before this practice was standardized, and the catalyst for performing IHC for neuroendocrine markers was not always explicit. In addition, survival analyses are reported from time of AMPC diagnosis which was only recognized at the time of biopsy, which may make survival estimates shorter since there was likely a clinical need for biopsy in CRPC. Challenges moving forward in understanding AMPC include the inter-tumoral heterogeneity within a single patient, and it may be that a multi- pronged therapeutic approach targeting different CRPC phenotypes will be most effective.
Conclusions
Our series indicates that AMPC is a unique subtype of prostate cancer that will likely benefit from specific treatment approaches. Further studies should be directed toward recognizing AMPC followed by further molecular characterization, and assessments of outcomes to current therapeutics. New approaches can be considered based on the identification of recurrent molecular features. As standard histologic studies may not identify AMPC, we suggest routine immunohistochemical evaluation for AR and neuroendocrine markers to be performed for all metastatic prostate cancers.
Funding:
This work was supported by the U.S. Department of Defense Prostate Cancer Research Program (W81XWH-20-1-0111, W81XWH-21-1-0229), the Pacific Northwest Prostate Cancer SPORE P50 CA097186, P01 CA 163227, R01 CA234715, Grant 2021184 from the Doris Duke Charitable Foundation, the V Foundation, the Prostate Cancer Foundation, and the Safeway Foundation.
List of Abbreviations
- ADT
Androgen Deprivation Therapy
- AMPC
Amphicrine Prostate Carcinoma
- APC
APC regulator of WNT signaling pathway
- AR
Androgen Receptor
- ARPC
Androgen receptor active prostate cancer
- ASCL1
Achaete-scute homolog 1
- BRCA2
Breast cancer gene 2
- CHEK2
Checkpoint kinase 2
- CRPC
Castration Resistant Prostate Cancer
- DNPC
Double Negative Prostate Cancer
- DS
Disease Specific
- FOLH1
Folate hydrolase 1
- FOXA1
Forkhead box A1
- GHRHR
Growth hormone releasing hormone
- IHC
Immunohistochemistry
- MSH2
MutS homolog 2
- NE
Neuroendocrine
- NEPC
Neuroendocrine Prostate Carcinoma
- NKX3.1
Homeobox protein Nkx-3.1
- OS
Overall survival
- PCF
Prostate Cancer Foundation
- PF
Progression Free
- PSA
Prostate Specific Antigen
- PTEN
Phosphatase and tensin homolog
- RB1
Retinoblastoma transcriptional corepressor 1
- REST
RE1-silencing transcription factor
- SSTR5
Somatostatin receptor 5
- SU2C
Stand Up to Cancer
- TMPRSS2-ERG
Transmembrane serine protease 2- ETS-related gene
- TP53
Tumor protein P53
Footnotes
Disclosure/Conflict of Interest Statement: MTS: Paid consultant and/or received Honoria from Sanofi, AstraZeneca, PharmaIn and Resverlogix. He has received research funding to his institution from Zenith Epigenetics, Bristol Myers Squibb, Merck, Immunomedics, Janssen, AstraZeneca, Pfizer, Madison Vaccines, Hoffman-La Roche, Tmunity, SignalOne Bio and Ambrx, Inc. EY: Consulting (personal) – Advanced Accelerator Applications, Bayer, Clovis, Exelixis, Janssen, Oncternal, Merck. Grant funding (to institution) – Bayer, Blue Earth, Daiichi-Sankyo, Dendreon, Lantheus, Merck, Seagen, Taiho
References
- 1.Graham L, Schweizer MT: Targeting persistent androgen receptor signaling in castration-resistant prostate cancer. Med Oncol 33:44, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal R, Huang J, Alumkal JJ, et al. : Clinical and Genomic Characterization of Treatment-Emergent Small-Cell Neuroendocrine Prostate Cancer: A Multi-institutional Prospective Study. J Clin Oncol 36:2492–2503, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamada Y, Beltran H: Clinical and Biological Features of Neuroendocrine Prostate Cancer. Curr Oncol Rep 23:15, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Labrecque MP, Coleman IM, Brown LG, et al. : Molecular profiling stratifies diverse phenotypes of treatment-refractory metastatic castration-resistant prostate cancer. J Clin Invest 130:4492–4505, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bluemn EG, Coleman IM, Lucas JM, et al. : Androgen Receptor Pathway- Independent Prostate Cancer Is Sustained through FGF Signaling. Cancer Cell 32:474–489 e6, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Epstein JI, Amin MB, Beltran H, et al. : Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. Am J Surg Pathol 38:756–67, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Labrecque MP, Brown LG, Coleman IM, et al. : RNA Splicing Factors SRRM3 and SRRM4 Distinguish Molecular Phenotypes of Castration-Resistant Neuroendocrine Prostate Cancer. Cancer Res 81:4736–4750, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prendeville S, Al-Bozom I, Comperat E, et al. : Prostate carcinoma with amphicrine features: further refining the spectrum of neuroendocrine differentiation in tumours of primary prostatic origin? Histopathology 71:926–933, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Gopalan A, Al-Ahmadie H, Chen YB, et al. : Neuroendocrine differentiation in the setting of prostatic carcinoma: contemporary assessment of a consecutive series. Histopathology 81:246–254, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuo AJ, Paulson VA, Hempelmann JA, et al. : Validation and implementation of a modular targeted capture assay for the detection of clinically significant molecular oncology alterations. Pract Lab Med 19:e00153, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dobin A, Davis CA, Schlesinger F, et al. : STAR: ultrafast universal RNA- seq aligner. Bioinformatics 29:15–21, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abida W, Cyrta J, Heller G, et al. : Genomic correlates of clinical outcome in advanced prostate cancer. Proc Natl Acad Sci U S A 116:11428–11436, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanzelmann S, Castelo R, Guinney J: GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics 14:7, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fedchenko N, Reifenrath J: Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue - a review. Diagn Pathol 9:221, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roudier MP, Winters BR, Coleman I, et al. : Characterizing the molecular features of ERG-positive tumors in primary and castration resistant prostate cancer. Prostate 76:810–22, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nadal R, Schweizer M, Kryvenko ON, et al. : Small cell carcinoma of the prostate. Nat Rev Urol 11:213–9, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cancer Genome Atlas Research N: The Molecular Taxonomy of Primary Prostate Cancer. Cell 163:1011–25, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdulfatah E, Reichert ZR, Davenport MS, et al. : De novo neuroendocrine transdifferentiation in primary prostate cancer-a phenotype associated with advanced clinico-pathologic features and aggressive outcome. Med Oncol 38:26, 2021 [DOI] [PubMed] [Google Scholar]
- 19.Wang HT, Yao YH, Li BG, et al. : Neuroendocrine Prostate Cancer (NEPC) progressing from conventional prostatic adenocarcinoma: factors associated with time to development of NEPC and survival from NEPC diagnosis-a systematic review and pooled analysis. J Clin Oncol 32:3383–90, 2014 [DOI] [PubMed] [Google Scholar]


