Abstract
Purpose
While there is a rising focus on sleep issues among athletes, a notable gap exists in the comparative analysis of sleep patterns between male and female athletes. This study aims to evaluate the sleep patterns of collegiate swimmers during a specific period (pre-competition training phase) based on the National Sleep Foundation’s recommendations and compares sleep differences between males and females.
Patients and Methods
15 swimmers (6 males and 9 females) completed the Athlete Sleep Screening Questionnaire (ASSQ) and wore actigraphy devices for 8 consecutive nights to record objective sleep patterns including bedtime, wake time, sleep onset latency, total sleep time, wake after sleep onset, and sleep efficiency.
Results
The total sleep time of collegiate male (5.0±0.4 h, 4.6 to 5.4h) and female (6.0±0.7 h, 5.5 to 6.5h) swimmers was less than 7 hours per night, and male swimmers’ sleep efficiency (76.7±8.9%, 67.4 to 86.0%) was lower than the 85% standard. Male swimmers had less objectively measured sleep duration (p=0.006, d=1.66, large effect), lower sleep efficiency (p=0.013, d=1.51, large effect), and longer wake after sleep onset (p=0.096, d=0.94, moderate effect). Female swimmers had higher sleep difficulty scores (p=0.06, d=1.08, moderate effect), and there was a significant difference in the distribution of sleep difficulty scores between male and female swimmers (p=0.033, V=0.045, small effect).
Conclusion
Collegiate swimmers exhibited poor sleep patterns during pre-competition preparation, and the sleep fragmentation of male swimmers was more pronounced. There were sex differences in both subjective and objective measured sleep patterns, with male swimmers having less sleep and low efficiency, while female swimmers experienced more significant sleep disturbances.
Keywords: sex, sleep, monitor, swimmer, pre-competition
Introduction
Sleep is essential for athletes to balance the demands of training and competition and achieve optimal performance. It plays a pivotal role in athletes’ physiological and psychological recovery processes1 The National Sleep Foundation recommends healthy population obtain 7–9 hours of sleep per night with a sleep efficiency of 85% to maintain good condition,2 while athletes may need more sleep than non-active individuals for effective recovery and adaptation between exercises.3 However, a meta-analysis found that athletes often failed to achieve ≥7 hours of sleep and ≥85% sleep efficiency during training or on game night.4 Remarkably, athletes maintaining an average nightly sleep of less than 8 hours were 70% more susceptible to injuries5 and nearly three times more prone to get infected6 compared to those securing more than 8 hours of sleep. Acute sleep loss not only adversely affects subsequent athletic performance but also hampers cognitive abilities, thereby influencing the academic performance of collegiate athletes. The diverse negative repercussions of sleep loss highlight the imperative for athletic teams to implement sleep monitoring for their collegiate athletes.
Compared to athletes from other sports disciplines, swimmers are notably susceptible to sleep-related problems, characterized by insufficient sleep, poor sleep quality, and longer sleep onset latency.7 Additionally, sex is considered a contributing factor to sleep issues.8–10 Among the general population, objective measurements indicate that females exhibit higher sleep quality, shorter sleep onset latency, and greater sleep efficiency compared to males,9 yet females also report more sleep-related complaints such as insomnia and more nocturnal awakenings.10 A survey of 632 German athletes revealed that female athletes tended to get less sleep before competition than male athletes do.11 In contrast, Silva et al12 employed PSG to find that male elite athletes had longer sleep latencies, more wake after sleep onset, and lower sleep efficiency. However, it is still uncertain whether the sex differences observed in previous studies persist among collegiate swimmers.
To date, most studies examining sex differences in sleep among athletes have relied on self-report data, potentially overlooking objective sleep variations. Given the significance of sleep for athletes and the paucity of research on sex differences in sleep patterns among swimmers, this study aims to: (1) assess the sleep patterns of collegiate swimmers during the pre-competition phase, and (2) compare sex differences in the sleep patterns of collegiate swimmers. We hypothesized that both subjective and objective sleep of female swimmers was worse than that of male swimmers.
Materials and Methods
Participants
Our research complies with the Helsinki Declaration. Following a thorough understanding of the study procedure, 17 Chinese collegiate swimmers voluntarily participated in this study. All participants were National Level 1 athletes or above and qualified for the 21st Chinese University Swimming Championships. All participants gave informed consent, and the Wuhan Institute of Physical Education Ethics Committee granted institutional approval for ethics (approval number: 2023070). Before the study began, athletes went through a 3-day sleep familiarization phase. During this phase, two athletes failed to adapt to wearing the actigraphy device to sleep and thus were excluded from the study. Consequently, the study ultimately included 15 collegiate swimmers (female: n=9; age: 19.9±1.1 years).
Study Design
This cross-sectional study was carried out during the preparation for the 21st Chinese University Swimming Championships. The study process is shown in Figure 1. Participants wore actigraphy devices to record sleep for eight continuous nights, with the final day as rest and the preceding seven as training. On each training day, participants engaged in 2–3 hours of swimming training (14:00 h-16:00 h), with daily training volumes ranging from 4000 m to 5000 m. All participants shared the same sleep environment in standardized dormitories, with four athletes assigned to each room. All the athletes lived in their dormitories for at least one semester, so there would be no effect of the sleep environment on the athletes’ sleep.
Figure 1.
Study flowchart.
Participants were required to maintain their usual sleep routines during the sleep monitoring period and were allowed to use medications, caffeine, and training supplements according to their individual preferences. Due to the lack of accuracy of short-term sleep monitoring of athletes by actigraphy, this study exclusively recorded nighttime sleep, and the analysis did not include daytime naps.
Sleep Assessment
Objective Sleep Measurement
In this study, sleep was assessed using a commercially available actigraphy device (ActiGraph wGT3X-BT, USA) worn on athlete’s non-dominant wrist. Sleep data analysis was conducted using the manufacturer’s software (Actilife v6.12). Actigraphy is a non-invasive wrist-worn sleep monitoring device, and the GT3x model has previously been employed to track sleep patterns among athletes. Compared to Polysomnography (PSG), GT3x exhibits 90% sensitivity and 84% accuracy.13
Participants were instructed to wear the actigraphy devices throughout the day, except when swimming or bathing. The actigraphy devices were set to a sampling rate of 30 Hz and a 1-minute epoch for activity counts. Sleep variables were calculated using the Sadeh algorithm, which is more accurate for wrist-worn sleep monitoring in younger populations (under 30 years old).14 The following sleep variables were collected from the actigraphy: Bedtime (hh: mm), Wake Time (hh: mm), Sleep Onset Latency (min), Total Sleep Time (h), Wake After Sleep Onset (min), and Sleep Efficiency (%). Definitions for all sleep variables are provided in Table 1.
Table 1.
Sleep Variables and Definitions
| Sleep Variables | Definitions |
|---|---|
| Bedtime (hh:mm)* | Clock time at which a participant went to bed to attempt to sleep. |
| Wake Time (hh:mm)* | Clock time at which a participant got out of bed and stopped attempting to sleep. |
| Sleep Onset Latency (SOL, min)* | Total duration between bedtime and sleep onset. |
| Total Sleep Time (TST, h) | Total duration of sleep obtained during a sleep period. |
| Wake After Sleep Onset (WASO, min) | Total duration of time spent awake during a sleep period. |
| Sleep Efficiency (SE, %) | Percentage of time in bed spent sleeping after sleep onset. |
Notes: *Adjusted according to sleep diary.
Sleep diaries were used in conjunction with the actigraphy devices to calibrate participants’ actual bedtime and wake time. The sleep diary entries included the participants’ previous night’s lights-off time, lights-on time, and execution of the wristwatch wearing, along with additional information (eg, usage of electronic devices before sleep, alcohol and caffeine intake, etc.). Participants were required to complete the sleep diary within 30 minutes of waking up each day to ensure the accuracy of the information. Researchers used the actigraphy data and sleep diaries to determine participants’ sleep/wake states. When the actigraphy data fell below the sleep threshold and the sleep diary indicated that participants attempted to sleep, that time point was defined as “sleep”.
Subjective Sleep Measurement
The Athlete Sleep Screening Questionnaire (ASSQ), a sleep screening tool developed specifically for athletes, can provide the most accurate assessment of athletes’ sleep health and intervention needs.15 ASSQ calculates a Sleep Difficulty Score (SDS) based on participants’ answers to designated questions. The higher the SDS, the more severe the sleep-related problem is. ASSQ classified athletes’ sleep problems into four different categories based on SDS: none (SDS 0–4), mild (SDS 5–7), moderate (SDS 8–10), and severe (SDS 11–17). Our study used the Chinese version of ASSQ to evaluate athletes’ sleep problems. The SDS of this version has validated a significant positive correlation with PSQI (r=0.776, p<0.001) and has been shown to be highly reliable through internal consistency (Cronbach’s α=0.82).16 Participants completed the ASSQ questionnaire at the same time under the same environment on the first day of the study.
Statistical Analyses
All data were subjected to statistical analysis using SPSS software (SPSS 24.0, IBM SPSS, Armonk, NY). The Shapiro–Wilk test was applied to check the normality of data distribution. Sleep variables and SDS were presented as mean ± SD and an independent samples t-test was used to examine differences between sexes. Non-normally distributed data were reported as Median (IQR) and analyzed with the Mann–Whitney U-test. Cohen’s d was computed to quantify the magnitude of differences, with interpretation as negligible: >0.2, small effect: 0.2–0.59, moderate effect: 0.6–1.19, large: 1.2–1.99, and very large effect: >2.17 Categorical data was described as numbers (n) and proportions (%) and analyzed with Fisher’s exact test. Fisher’s exact test was employed to evaluate disparities in SDS categories based on sex. Cramer’s V was utilized for estimating the effect size, categorized as small, medium, or large.18 A significance level was set as p≤0.05.
Results
Objective Sleep Variables
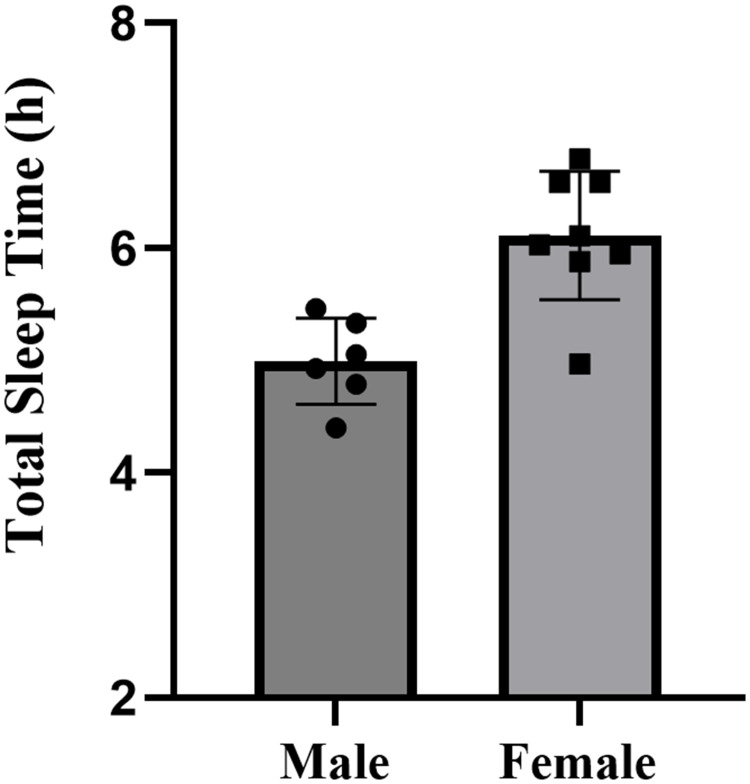
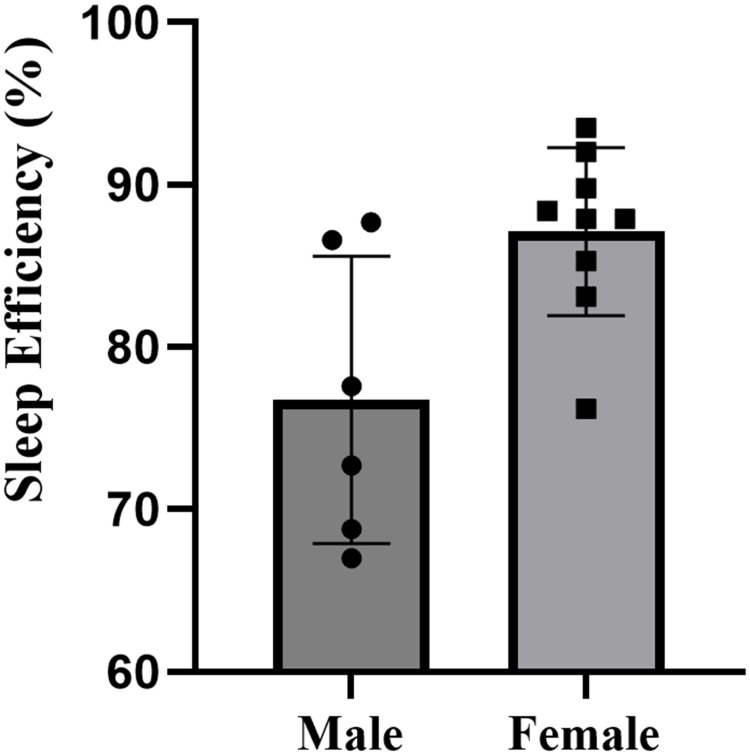
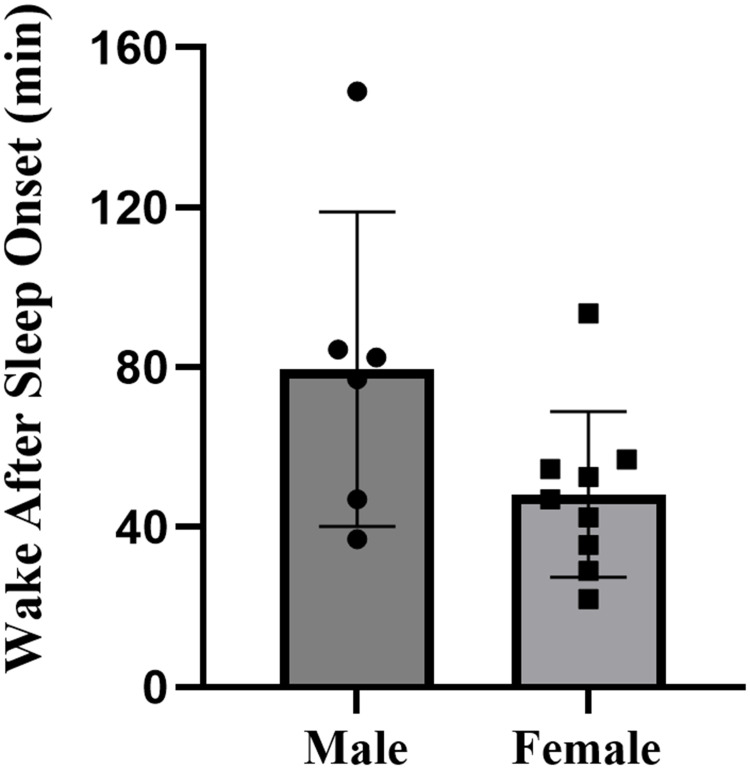
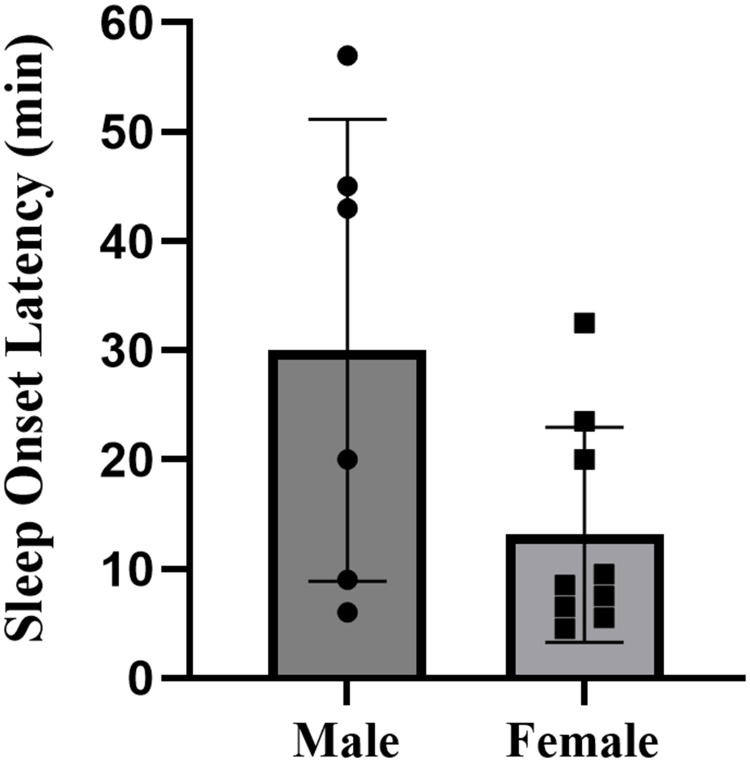
The descriptive statistics for all sleep variables are presented in Table 2. There were no missing cases in the formal study period. The study documented the sleep patterns of 15 collegiate swimmers, consisting of 6 males and 9 females, totaling 120 nights of sleep data. Overall, swimmers went to bed at 00:53, and woke up at 7:42, with an average sleep duration of 5.6 hours, sleep efficiency of 83.0%±84%, and wake after sleep onset of 55.9±22.1 minutes. Specifically, male swimmers had a total sleep time of 5.0±0.4 hours, with a sleep efficiency of 76.7%±8.9%. Female swimmers had a total sleep time of 6.0±0.7 hours, with a sleep efficiency of 87.1%±5.2%. Compared to females, male swimmers exhibited significantly shorter total sleep time (p=0.006, d=1.66, large effect) (Figure 2), lower sleep efficiency (p=0.013, d=1.51, large effect) (Figure 3), and more wake after sleep onset (p=0.096, d=0.94, moderate effect) (Figure 4). The sleep latency of male swimmers was slightly longer than that of females, but the difference was not significant (p=0.38, d=0.47, small effect) (Figure 5). There were no significant differences in bedtime (p=0.68, d=0.22, small effect) and wake-up time (p=0.76, d=0.46, small effect) between male and female collegiate swimmers.
Table 2.
Comparison of Objectively Measured Sleep Variables and SDS in Male and Female Swimmers
| Male (CI95%) | Female (CI95%) | p | d | |
|---|---|---|---|---|
| Bedtime (hh: mm) | 00:59±0:53 (00:03 to 1:56) | 00:49±0:41 (00:17 to 1:21) | 0.68 | 0.22 |
| Wake Time (hh: mm) | 7:54±0:18 (7:26 to 8:04) | 7:40±0:36 (7:12 to 8:08) | 0.76 | 0.46 |
| Total Sleep Time (h) | 5.0±0.4 (4.6 to 5.4) | 6.0±0.7 (5.5 to 6.5) | 0.006 | 1.66 |
| Sleep Efficiency (%) | 76.7±8.9 (67.4 to 86.0) | 87.1±5.2 (83.2 to 91.1) | 0.013 | 1.51 |
| Wake After Sleep Onset (min) | 67.6±20.3 (46.3 to 88.9) | 48.2±20.7 (32.2 to 64.1) | 0.096 | 0.94 |
| Sleep Onset Latency (min) | 14.0 (21.0) (0.3 to 39.7) | 8.5 (15.8) (5.6 to 20.7) | 0.38 | 0.47 |
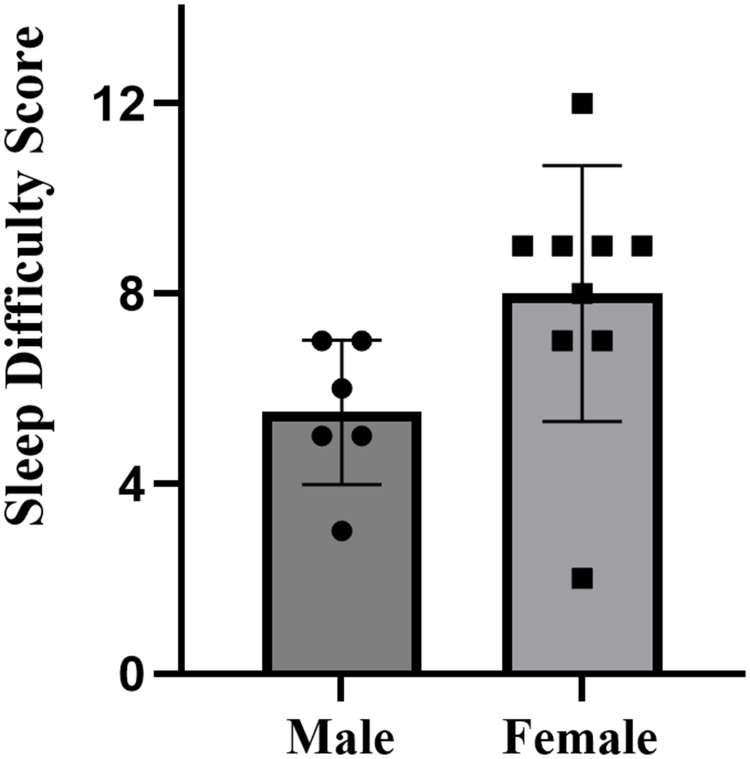
| Sleep Difficulty Score | 5.5±1.5 (4.7 to 7.3) | 8.0±2.7 (5.1 to 9.7) | 0.06 | 1.08 |
Figure 2.
Total sleep time of male and female collegiate swimmers.
Figure 3.
Sleep efficiency of male and female collegiate swimmers.
Figure 4.
Wake after sleep onset of collegiate swimmers.
Figure 5.
Sleep onset latency of male and female collegiate swimmers.
Sleep Difficulty Score (SDS) and Sleep Problem Categories Distribution
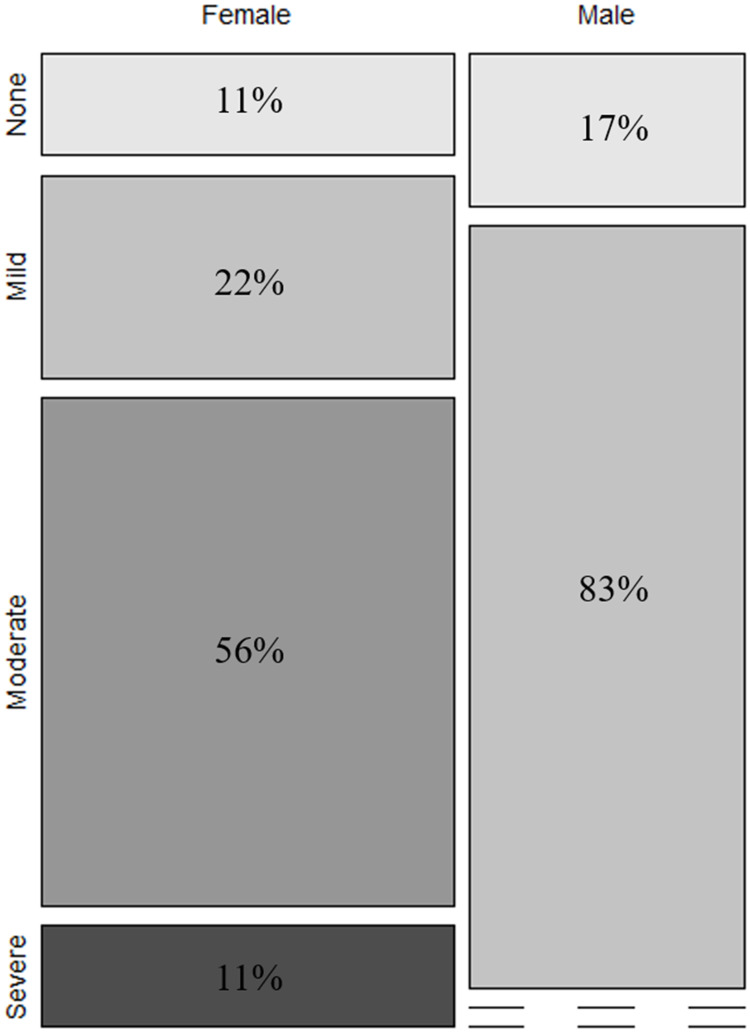
Tables 3 display the distribution of sleep problems for male and female collegiate swimmers. On the whole, the sleep difficulty score for swimmers is 6.7±2.6 (Table 2). The mean SDS of male swimmers was 5.5±1.5 (mild), while that of female swimmers was 8.0±2.7 (moderate), indicating that female swimmers suffered more sleep-related difficulties than males (p=0.06, d=1.08, large effect) (Figure 6). There was a small significant difference in the distribution of sleep problem categories between male and female swimmers according to Fisher’s exact test (p=0.03, V=0.05, small effect). Among male swimmers, 11% were in the category of no clinical sleep problems, and 83% were in the mild clinical sleep problems category. None of male swimmers were diagnosed with moderate or severe clinical sleep problems. In contrast, 22% of female swimmers were in mild category, over half of the female swimmers experienced moderate clinical sleep problems (56%), and 11% of females were categorized as having severe clinical sleep problems (Figure 7).
Table 3.
Clinical Sleep Problem Categories Distribution of Male and Female Swimmers
| Sleep Difficulty Score* | Male (n=6) | Female (n=9) |
|---|---|---|
| None (0–4) | 1 (17%) | 1 (11%) |
| Mild (5–7) | 5 (83%) | 2 (22%) |
| Moderate (8–10) | 0 (0%) | 6 (56%) |
| Severe (11–17) | 0 (0%) | 1 (11%) |
Notes: *Fisher’s exact test p=0.033, V=0.045.
Figure 6.
SDS (sleep difficulty score) of male and female collegiate swimmers.
Figure 7.
The distribution of male and female collegiate swimmers’ SDS.
Discussion
To our knowledge, this study is the first to utilize both subjective and objective methods to investigate the differences in the sleep of male and female collegiate swimmers during the pre-competition training phase. The findings reveal that collegiate swimmers, of both sexes, had sleep durations far below the recommended 7–9 hours, and male swimmers had sleep efficiencies below the healthy threshold (85%). Male swimmers, as objectively measured by actigraphy, exhibited shorter total sleep time, lower sleep efficiencies, and longer wake after sleep onset compared to female ones. Moreover, ASSQ suggested female swimmers suffered more severe sleep difficulties than males.
In contrast to athletes from other sports, swimmers tend to experience more prominent sleep issues, characterized by insufficient sleep quantity, poor sleep quality, excessive daytime sleepiness,7 and a higher prevalence of sleep disturbances.19 In this study, male and female collegiate swimmers had average sleep length of 5 hours and 6 hours, respectively, falling short of the 7–9-hour sleep target recommended by the National Sleep Foundation.2 Such inadequate amounts of sleep may not meet the recovery demands during high-intensity training periods and can add to psychological and physiological burdens. Less than 6 hours of sleep defined as sleep restriction, has significant negative effects on various aspects of athletic performance, including aerobic and anaerobic capacities, explosiveness, and specialized skills.20 Additionally, it compromises athletes’ immune function, elevating the risk of illness and injury,21 and is highly detrimental to overall health and training performance. Judging from the actigraphy data, swimmers’ bedtime was close to 01:00 AM, indicating a common trend of staying up late, which can be a major contributing factor to restricted sleep duration. Late bedtime is a typical feature of contemporary college student sleep patterns, and this is especially true for collegiate athletes who are susceptible to sacrificing sleep due to academic, training, and competition pressures,6 as well as travel-related commitments.22 Late bedtime, as a behavioral pattern, is primarily influenced by athletes’ sleep preferences and individual lifestyle habits and can be a primary target for sleep education. It has been demonstrated that basketball players who experience several weeks of sleep extension show significant improvements in athletic performance, reaction time, sleepiness, and mood.23 Given that sleep deficiency is treatable, coaches can promote the necessary sleep hygiene education and encourage collegiate athletes to use time management techniques to create a regular sleep-wake cycle. This would improve sleep health and boost academic and athletic performance.
The actigraphy data indicate that male swimmers, in comparison to females, sleep approximately 1 hour less per night, with a 9% reduction in sleep efficiency and an increase in wake after sleep onset of nearly 20 minutes. There were no significant differences in bedtime and wake time between male and female swimmers, suggesting that their time in bed did not differ. Therefore, it can be inferred that shorter total sleep time in male athletes can be ascribed to longer sleep onset latency and more wake after sleep onset, both of which reduce sleep efficiency and ultimately result in a shortened total sleep time. The clinical threshold for low sleep efficiency is set at 85%,24 and male swimmers’ sleep efficiency falls well below this standard. These findings suggest that male swimmers may experience more nocturnal sleep disturbances, potentially compromising their ability to obtain restorative sleep. Our finding emphasizes that male swimmers may need more help in terms of sleep efficiency. The difference in objective sleep quality between male and female swimmers can be explained from two perspectives: sleep hygiene and physiological characteristics. Some male athletes may exhibit poorer personal sleep hygiene behaviors, such as consuming caffeine25 or alcohol26 close to bedtime and prolonged electronic screen time before bed27). These behaviors can increase arousal levels before bed, leading to fragmented nighttime sleep. Physiologically, delayed onset muscle soreness induced by high-intensity training is one potential factor contributing to poor sleep quality.28 In our study, male athletes tend to have greater muscle mass compared to female athletes, which may contribute to more susceptible to sleep disturbances. Additionally, studies have shown that swimmers are at a 2.5 times higher risk of developing obstructive sleep apnea (OSA) compared to the general population,7 while males have a higher prevalence of OSA than females.29 These risk factors may reduce male swimmers’ ability to sleep effectively. Future research should pay more attention to the objective sleep of male athletes, elucidate the risk factors contributing to low sleep efficiency, and develop personalized sleep prescriptions for athletes to optimize sleep health and performance.
In comparison to female swimmers, male swimmers have shorter objective sleep quantity and lower sleep efficiency, while reporting fewer sleep-related problems. This finding aligns with research on the general population.10 Despite similar sleep requirements to maintain optimal performance, the types and risks of sleep disturbances may differ between sexes. These distinctions are largely influenced by the effects of sex hormones on circadian rhythms.30 In the general population, males have a higher probability of developing obstructive sleep apnea (OSA), while females tend to experience central sleep disturbances, characterized by subjective difficulty initiating and maintaining sleep.31 However, in studies involving athlete populations, there have been conflicting reports regarding subjective sleep problems reported by both sexes. For instance, during pre-competition sleep surveys, Silva et al12 reported higher rates of insomnia complaints among male athletes, while Biggins et al22 observed greater sleep anxiety among female athletes. Juliff et al32 conducted research on 283 athletes and found no differences between male and female athletes in sleep disturbances. These discrepancies may be attributed to variations in assessment methods and the absence of sleep questionnaires specifically designed for athletes. It is worth noting that PSQI may not align with sleep recommendations in the field of sports science.15 In contrast, our study utilized the Athlete Sleep Screening Questionnaire (ASSQ), which provides the most accurate assessment of athlete sleep health and intervention needs. Based on the average Sleep Difficulty Score (SDS), male swimmers had mild sleep problems (5.5), while female swimmers generally experienced moderate clinical sleep problems (8.0). In addition, female athletes exhibited greater individual variability in sleep problems (±2.7 vs ±1.5). Regarding the distribution of sleep problem categories, none of male swimmers were categorized as having moderate or severe clinical sleep problems, whereas over half of female swimmers were classified as having moderate, and 11% of females had severe clinical sleep problems. These findings underscore the greater subjective sleep disturbances among female swimmers. Several factors may contribute to the subjective sleep issues in female athletes, including circadian rhythm disruption (eg, low core body temperature and melatonin secretion),33 pre-competition stress and anxiety,19 and the influence of ovarian steroid hormones.34 Female athletes tend to be more susceptible to experiencing anxiety and heightened arousal levels compared to male athletes.35 These emotional responses can become more pronounced during specific periods, such as competitions and travel,19 making it more challenging for female athletes to initiate and maintain sleep. Furthermore, a study by Hrozanova et al36 suggested that the menstrual cycle of female athletes may affect the quantity of sleep they need, which may account for sex disparities in subjective and objective sleep measurements. Further research is warranted to better understand the potential mechanisms that contribute to the variations in subjective and objective sleep among male and female athletes. Additionally, future studies should aim to address the sleep disparities between these two groups and provide more specific insights.
To the best of our knowledge, this is the first study that concurrently compares the objective and subjective sleep of male and female collegiate swimmers, and observed differences in both aspects of sleep between sexes. Compared to previous studies, we employed actigraphy to monitor the objective sleep of athletes, overcoming the potential information bias associated with subjective sleep assessment. Our finding implies that it may not be appropriate to use a single test to evaluate athletes’ sleep since male athletes may not be aware of their fragmented sleep, while relying solely on objective measures may fail to consider subjective disturbances in female athletes. This study also emphasizes the suboptimal sleep health of collegiate swimmers, which is characterized by insufficient sleep and poor sleep efficiency, during the pre-competition training phase. However, further research is still needed to fully understand the risk factors for collegiate swimmers’ poor sleep. The current research highlights two mechanisms of sports-related sleep disturbances, namely cognitive-physiological arousal prior to sleep, and sleep restriction.12 These two mechanisms can also account for the collegiate swimmers in the current study’s features of poor sleep quality and short sleep duration and therefore can serve as targets for sleep management within sports teams. Coaches and relevant personnel can employ a combination of subjective and objective methods to identify athletes’ sleep problems. This will enable them to establish a scientific basis for developing personalized sleep prescriptions, which can contribute to efficient recovery and enhance athletic performance for athletes.
This study has several limitations. First of all, the sample size is somewhat small (6 males and 9 females), which can compromise the reliability of the findings. However, the study provides effect sizes (Cohen’s d) to further assess the magnitude of differences between sexes. Secondly, due to the limitations of actigraphy devices in monitoring short-duration sleep in athletes,37 this study solely examined swimmers’ overnight sleep while daytime naps were excluded from the analysis. This may underestimate the overall sleep swimmers obtain. Thirdly, the objective sleep variables were measured using actigraphy devices instead of polysomnography (PSG), which is considered the gold standard for sleep monitoring. Actigraphy has limitations in providing detailed information about the athletes’ sleep, and it is important to note that it may have certain flaws, such as underestimating sleep latency and overestimating sleep efficiency, which could lead to measurement inaccuracies.13
Conclusion
This is the first study to compare the sleep patterns of male and female swimmers simultaneously using both subjective and objective measures. During the pre-competition training phase, the sleep quantity of collegiate swimmers did not reach the recommended amount (7–9 hours), and the sleep efficiency of male swimmers was below the threshold for healthy sleep (85%). There were significant differences in both objective and subjective measured sleep between male and female collegiate swimmers. Male swimmers had shorter objectively measured sleep quantity, poorer sleep quality, and longer wake after sleep onset, while female swimmers reported more sleep-related problems via questionnaire. The present study highlights that when evaluating sleep in athletes, it is essential not to rely solely on a single monitoring method. Coaches should conduct precise assessments of athletes’ sleep, provide education on sleep hygiene, and develop personalized sleep prescriptions to enhance athletes’ sleep health, aiming to minimize the disparities in sleep patterns between male and female athletes. Further research is required to investigate the potential mechanisms underlying sex differences in athletes’ subjective and objective sleep patterns. This will contribute to a better comprehension of athletes’ recovery requirements, ultimately enhancing overall performance.
Acknowledgments
The research group is grateful to the team of athletes and coaches who actively cooperated with the experiment.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Halson SL. Nutrition, sleep and recovery. Eur J Sport Sci. 2008;8(2):119–126. doi: 10.1080/17461390801954794 [DOI] [Google Scholar]
- 2.Hirshkowitz M, Whiton K, Albert SM, et al. National sleep foundation’s updated sleep duration recommendations: final repor. Sleep Health. 2015;1(4):233–243. doi: 10.1016/j.sleh.2015.10.004 [DOI] [PubMed] [Google Scholar]
- 3.Bird SP. Sleep, recovery, and athletic performance: a brief review and recommendations. Strength Condition J. 2013;35(5):43–47. doi: 10.1519/SSC.0b013e3182a62e2f [DOI] [Google Scholar]
- 4.Roberts SSH, Teo WP, Warmington SA. Effects of training and competition on the sleep of elite athletes: a systematic review and meta-analysis. Br J Sports Med. 2019;53(8):513–522. doi: 10.1136/bjsports-2018-099322 [DOI] [PubMed] [Google Scholar]
- 5.Milewski MD, Skaggs DL, Bishop GA, et al. Chronic lack of sleep is associated with increased sports injuries in adolescent athletes. J Pediatr Orthop. 2014;34(2):129–133. doi: 10.1097/BPO.0000000000000151 [DOI] [PubMed] [Google Scholar]
- 6.Cohen S, Doyle WJ, Alper CM, Janicki-Deverts D, Turner RB. Sleep habits and susceptibility to the common cold. Arch Intern Med. 2009;169(1):62–67. doi: 10.1001/archinternmed.2008.505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Surda P, Putala M, Siarnik P, et al. Sleep in elite swimmers: prevalence of sleepiness, obstructive sleep apnoea and poor sleep quality. BMJ Open Sport Exerc Med. 2019;5(1):e000673. doi: 10.1136/bmjsem-2019-000673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lund HG, Reider BD, Whiting AB, Prichard JR. Sleep patterns and predictors of disturbed sleep in a large population of college students. J Adolesc Health. 2010;46(2):124–132. doi: 10.1016/j.jadohealth.2009.06.016 [DOI] [PubMed] [Google Scholar]
- 9.Goel N, Kim H, Lao RP. Gender differences in polysomnographic sleep in young healthy sleepers. Chronobiol Int. 2005;22(5):905–915. doi: 10.1080/07420520500263235 [DOI] [PubMed] [Google Scholar]
- 10.Tsai LL, Li SP. Sleep patterns in college students: gender and grade differences. J Psychosom Res. 2004;56(2):231–237. doi: 10.1016/S0022-3999(03)00507-5 [DOI] [PubMed] [Google Scholar]
- 11.Erlacher D, Ehrlenspiel F, Adegbesan OA, et al. Sleep habits in German athletes before important competitions or games. J Sports Sci. 2011;29(8):859–866. doi: 10.1080/02640414.2011.565782 [DOI] [PubMed] [Google Scholar]
- 12.Silva A, Narciso FV, Rosa JP, et al. Gender differences in sleep patterns and sleep complaints of elite athletes. Sleep Sci. 2019;12(4):242–248. doi: 10.5935/1984-0063.20190084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chase JD, Busa MA, Staudenmayer JW, Sirard JR. Sleep measurement using wrist-worn accelerometer data compared with polysomnography. Sensors. 2022;22(13):5041. doi: 10.3390/s22135041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Migueles JH, Cadenas-Sanchez C, Ekelund U, et al. Accelerometer data collection and processing criteria to assess physical activity and other outcomes: a systematic review and practical considerations. Sports Med. 2017;47(9):1821–1845. doi: 10.1007/s40279-017-0716-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bender AM, Lawson D, Werthner P, Samuels CH. The clinical validation of the athlete sleep screening questionnaire: an instrument to identify athletes that need further sleep assessment. Sports Med Open. 2018;4(1):23. doi: 10.1186/s40798-018-0140-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan CH, Lu JJ, Qiu J. Revision and validation of the Chinese version of the athlete sleeping screening questionnaire. Sport Sci Res. 2021;3(42):1006–1207. [Google Scholar]
- 17.Varley MC, Elias GP, Aughey RJ. Current match-analysis techniques’ underestimation of intense periods of high-velocity running. Int J Sports Physiol Perform. 2012;7(2):183–185. doi: 10.1123/ijspp.7.2.183 [DOI] [PubMed] [Google Scholar]
- 18.Kim HY. Statistical notes for clinical researchers: chi-squared test and fisher’s exact test. Restor Dent Endod. 2017;42(2):152–155. doi: 10.5395/rde.2017.42.2.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biggins M, Purtill H, Fowler P, et al. Sleep, health, and well-being in elite athletes from different sports, before, during, and after international competition. Phys Sportsmed. 2021;49(4):429–437. doi: 10.1080/00913847.2020.1850149 [DOI] [PubMed] [Google Scholar]
- 20.Craven J, Mccartney D, Desbrow B, et al. Effects of acute sleep loss on physical performance: a systematic and meta-analytical review. Sports Med. 2022;52(11):2669–2690. doi: 10.1007/s40279-022-01706-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charest J, Grandner MA. Sleep and athletic performance: impacts on physical performance, mental performance, injury risk and recovery, and mental health. Sleep Med Clin. 2020;15(1):41–57. doi: 10.1016/j.jsmc.2019.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biggins M, Purtill H, Fowler P, et al. Impact of long-haul travel to international competition on sleep and recovery in elite male and female soccer athletes. Int J Sports Physiol Perform. 2022;17(9):1361–1370. doi: 10.1123/ijspp.2021-0165 [DOI] [PubMed] [Google Scholar]
- 23.Mah CD, Mah KE, Kezirian EJ, Dement WC. The effects of sleep extension on the athletic performance of collegiate basketball players. Sleep. 2011;34(7):943–950. doi: 10.5665/SLEEP.1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohayon M, Wickwire EM, Hirshkowitz M, et al. National sleep foundation’s sleep quality recommendations: first report. Sleep Health. 2019;3(1):6–19. doi: 10.1016/j.sleh.2016.11.006 [DOI] [PubMed] [Google Scholar]
- 25.Gardiner C, Weakley J, Burke LM, et al. The effect of caffeine on subsequent sleep: a systematic review and meta-analysis. Sleep Med Rev. 2023;69:101764. doi: 10.1016/j.smrv.2023.101764 [DOI] [PubMed] [Google Scholar]
- 26.Prentice C, Stannard SR, Barnes MJ. The effects of binge drinking behaviour on recovery and performance after a rugby match. J Sci Med Sport. 2014;17(2):244–248. doi: 10.1016/j.jsams.2013.04.011 [DOI] [PubMed] [Google Scholar]
- 27.Arora T, Broglia E, Thomas GN, Taheri S. Associations between specific technologies and adolescent sleep quantity, sleep quality, and parasomnias. Sleep Med. 2014;15(2):240–247. doi: 10.1016/j.sleep.2013.08.799 [DOI] [PubMed] [Google Scholar]
- 28.Hausswirth C, Louis J, Aubry A, et al. Evidence of disturbed sleep and increased illness in overreached endurance athletes. Med Sci Sports Exerc. 2014;46(5):1036–1045. doi: 10.1249/MSS.0000000000000177 [DOI] [PubMed] [Google Scholar]
- 29.Lin CM, Davidson TM, Ancoli-Israel S. Gender differences in obstructive sleep apnea and treatment implications. Sleep Med Rev. 2008;12(6):481–496. doi: 10.1016/j.smrv.2007.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mong JA, Baker FC, Mahoney MM, et al. Sleep, rhythms, and the endocrine brain: influence of sex and gonadal hormones. J Neurosci. 2011;31(45):16107–16116. doi: 10.1523/JNEUROSCI.4175-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orff HJ, Meliska CJ, Martinez LF, Parry BL. The influence of sex and gonadal hormones on sleep disorders. ChronoPhysiol Ther. 2014;4:15–25. [Google Scholar]
- 32.Juliff L, Halson SL, Peiffer JJ. Understanding sleep disturbance in athletes prior to important competitions. J Sci Med Sport. 2015;18(1):13–18. doi: 10.1016/j.jsams.2014.02.007 [DOI] [PubMed] [Google Scholar]
- 33.Cain SW, Dennison CF, Zeitzer JM, et al. Sex differences in phase angle of entrainment and melatonin amplitude in humans. J Biol Rhythms. 2010;25(4):288–296. doi: 10.1177/0748730410374943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Driver HS, Dijk DJ, Werth ES, Biedermann K, Borbély AA. Sleep and the sleep electroencephalogram across the menstrual cycle in young healthy women. J Clin Endocrinol Metab. 1996;81(2):728–735. doi: 10.1210/jcem.81.2.8636295 [DOI] [PubMed] [Google Scholar]
- 35.Schaal K, Tafflet M, Nassif H, et al. Psychological balance in high level athletes: gender-based differences and sport-specific patterns. PLoS One. 2016;6(5):e19007. doi: 10.1371/journal.pone.0019007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hrozanova M, Klöckner C, Sandbakk Ø, et al. Sex differences in sleep and influence of the menstrual cycle on women’s sleep in junior endurance athletes. PLoS One. 2021;16(6):e0253376. doi: 10.1371/journal.pone.0253376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galland B, Meredith-Jones K, Terrill P, et al. Challenges and emerging technologies within the field of pediatric actigraphy. Front Psychiatry. 2014;5:99. doi: 10.3389/fpsyt.2014.00099 [DOI] [PMC free article] [PubMed] [Google Scholar]