Abstract
Purpose
Surgery for recurrent glioma provides cytoreduction and tissue for molecularly informed treatment. With mostly heavily pretreated patients involved, it is unclear whether the benefits of repeat surgery outweigh its potential risks.
Methods
Patients receiving surgery for recurrent glioma WHO grade 2–4 with the goal of tissue sampling for targeted therapies were analyzed retrospectively. Complication rates (surgical, neurological) were compared to our institutional glioma surgery cohort. Tissue molecular diagnostic yield, targeted therapies and post-surgical survival rates were analyzed.
Results
Between 2017 and 2022, tumor board recommendation for targeted therapy through molecular diagnostics was made for 180 patients. Of these, 70 patients (38%) underwent repeat surgery. IDH-wildtype glioblastoma was diagnosed in 48 patients (69%), followed by IDH-mutant astrocytoma (n = 13; 19%) and oligodendroglioma (n = 9; 13%). Gross total resection (GTR) was achieved in 50 patients (71%). Tissue was processed for next-generation sequencing in 64 cases (91%), and for DNA methylation analysis in 58 cases (83%), while immunohistochemistry for mTOR phosphorylation was performed in 24 cases (34%). Targeted therapy was recommended in 35 (50%) and commenced in 21 (30%) cases. Postoperatively, 7 patients (11%) required revision surgery, compared to 7% (p = 0.519) and 6% (p = 0.359) of our reference cohorts of patients undergoing first and second craniotomy, respectively. Non-resolving neurological deterioration was documented in 6 cases (10% vs. 8%, p = 0.612, after first and 4%, p = 0.519, after second craniotomy). Median survival after repeat surgery was 399 days in all patients and 348 days in GBM patients after repeat GTR.
Conclusion
Surgery for recurrent glioma provides relevant molecular diagnostic information with a direct consequence for targeted therapy under a reasonable risk of postoperative complications. With satisfactory postoperative survival it can therefore complement a multi-modal glioma therapy approach.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11060-024-04595-5.
Keywords: Recurrent glioma, Molecular diagnostics, Targeted therapy, Precision oncology, Surgical complications
Introduction
Glial tumors make up about 70% of primary intracranial tumors [1]. The most aggressive, glioblastoma (GBM), isocitrate dehydrogenase (IDH) wildtype, is a therapy-resistant systemic brain disease with dismal prognosis [2, 3]. Despite multimodal treatment including maximal safe resection, radio- and chemotherapy, recurrence of diffuse glioma is almost inevitable. Many trials on systemic therapy for recurrent glioma have failed in the past [4, 5]. Latest data indicate a median overall survival of 15 months for patients with GBM, isocitrate dehydrogenase (IDH) wildtype [6]. Even IDH-mutant diffuse gliomas of lower WHO grades, albeit showing longer survival rates, almost always involve a long path of multimodal salvage therapies [7, 8].
Over the past years, genomic studies have revolutionized our understanding of the biology, diagnosis and classification of intracranial tumors. The 5th edition of the WHO classification of tumors of the central nervous system (CNS) has combined molecular and histopathological features to provide integrated diagnoses of CNS tumors [9]. Indeed, current high-throughput sequencing pipelines do not only increase the reliability of current tumor diagnoses but also provide a workup for a more personalized approach in neurooncological therapy [10–12].
However, most salvage therapies do not account for possible post-therapeutic changes in tumor biology especially in IDH-mutant glioma and show limited treatment efficacy and inevitable further tumor progression [13–17]. Therefore, sampling of current tumor tissue at progression to inform on relevant molecular alterations for targeted therapy has hence become an increasingly relevant modality of last-line therapy [18].
At the same time, repeat surgery has been established as an option for amenable patients with recurrent glioma, with multiple studies on a viable post-surgery survival benefit of patients with GBM and low-grade glioma evolving in the past [19–23]. This is especially true for patients with a high extent of resection, under a reasonable rate of permanent neurological deficits, ranging from 4 to 8%, and surgical complication rates of 9 to 22% [24–29].
In this regard, surgery for recurrent glioma could provide, in addition to cytoreduction, tissue that recapitulates the real-time post-therapeutic tumor biology to help provide further targeted therapy options at tumor recurrence. However, with mostly heavily pretreated patients involved, little is known as to whether benefits of repeat surgery aiming at molecularly informed treatment outweigh its potential surgical and neurological complications.
Methods
In this retrospective study, we analyzed clinical, histopathological, molecular, surgical and follow-up data of 180 patients evaluated by the interdisciplinary tumor board of our center with the recommendation of tissue sampling of recurrent intracranial tumors for molecularly informed personalized treatment. Only patients undergoing repeat surgery for diffuse glioma (gross total resection (GTR), subtotal resection (STR), open biopsy or stereotactic biopsy) at our center between 2017 and 2022 were included (n = 70). In resection cases, the extent of resection (GTR or STR) was evaluated on early postoperative MRIs (within 48 h), with no residual contrast-enhancing (in case of GBM) or FLAIR (in case of IDH-mutant gliomas FLAIR-hyperintense FLAIR-hyperintense) tumor volume defined as GTR.. Cases with repeat surgery at other centers and cases in which repeat surgery did not take place were excluded (n = 102). In addition, a reference cohort of patients undergoing surgery for newly diagnosed diffuse glioma = ‘primary reference cohort’ and recurrent gliomas = ‘recurrent reference cohort’ (each as a separate group) treated at our center in 2022 was used to compare complication rates of repeat surgery with primary surgery for intracranial gliomas. This reference data is available under [30].
Statistical analysis
Patient characteristics were analyzed using descriptive statistics. Continuous variables are reported as mean ± standard deviation or median (and interquartile range (IQR)). Ordinal and nominal variables are presented as numbers and percentages. Missing data are designated as such. Comparison of nominal variables between groups was performed using Fisher’s exact test. Survival analysis was performed using the Gehan-Breslow-Wilcoxon test and the Log-rank (Mantel-Cox) test. All statistical analyses were performed using Graphpad PRISM (Version 9).
Results
Repeat surgery of recurrent glioma for molecularly informed treatment
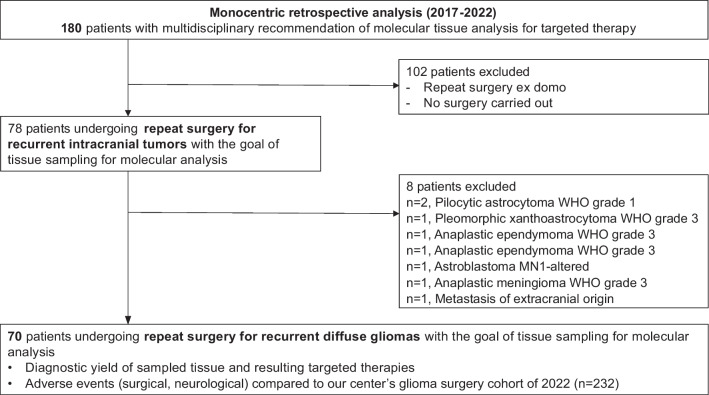
Between 2017 and 2022, the multidisciplinary tumor board at our center recommended analysis of current tumor tissue for targeted therapy in 180 patients with recurrent intracranial tumors. Of those, 102 patients (57%) were excluded from the analysis, either because repeat surgery took place at a different institution or because patients did not undergo repeat surgery at all, e.g. because the patient restrained from it or because molecular diagnostics was performed on tumor tissue derived from previous surgery. Overall, there was no significant difference in the clinical condition of patients not undergoing surgery at our institution as informed by their Karnofsky Performance Status (KPS) compared to the analyzed cohort (82.3% vs 86.1%, p = 0.06, two-tailed t-test). However, males were overrepresented in the cohort of excluded patients (68% vs. 44% in the study cohort, p = 0.002, Chi-square test). In addition, a higher proportion of deeply located lesions (basal ganglia and brain stem) with a significantly higher rate of white matter involvement was noted in the group not undergoing surgery at our institution (21% vs. 6% in the study cohort, p = 0.03, Chi-square test, Supplementary Table 1). Because we focused our further analysis on diffuse gliomas, 8 additional cases (two cases of pilocytic astrocytoma WHO grade 1, one case of each: pleomorphic xanthoastrocytoma, WHO grade 3; anaplastic meningioma WHO grade 3; anaplastic ependymoma WHO grade 3; Astroblastoma, MN1 altered; metastasis of extracranial origin and pituitary adenocarcinoma) were excluded (Fig. 1).
Fig. 1.
Study flow-chart. WHO = World Health Organization
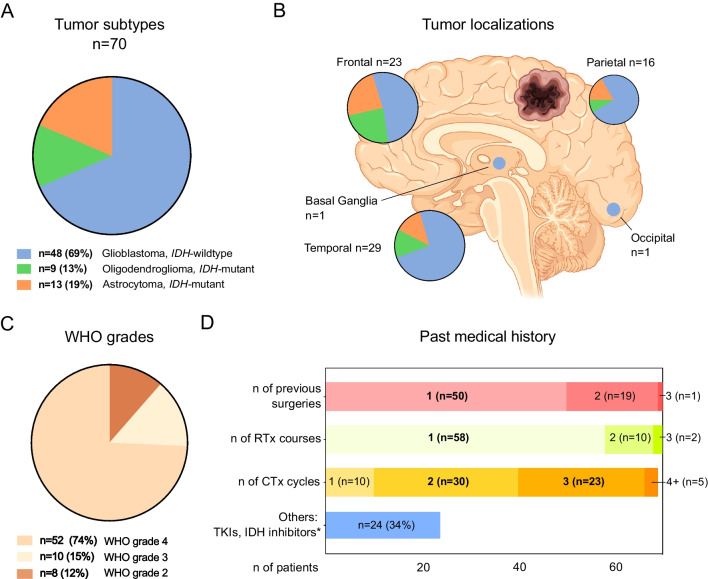
All 70 remaining cases were diagnosed as diffuse gliomas: 48 cases of WHO grade 4 IDH-wildtype glioblastoma, including three cases of gliosarcoma), 13 cases of IDH-mutant astrocytoma WHO grade 2, 3, and 4, and 9 cases of IDH-mutant oligodendroglioma, WHO grade 2 and 3. About one third of the diagnosed cases (n = 22) included tumors harboring IDH mutations. Demographically, this patient cohort included 39 females and 31 males with a median age of 54 years (interquartile range, IQR of 49 to 63). Tumors were mostly localized within the frontal (33%) and temporal (41%) lobes, (Fig. 2). Most patients showed a similar past medical history. The “average” patient included in the analysis had undergone one surgical procedure for tumor resection (n = 50, 71%), with further 27% of patients showing a history of two previous craniotomies for tumor resection. In most of the cases, one course of radiotherapy (n = 58, 83%) and two (n = 30, 46%) to three (n = 23, 39%) courses of chemotherapy had taken place prior to repeat surgery. Most patients had received primarily temozolomide as adjuvant chemotherapy (64 patients, 91%). 24 patients (34%) had also received further therapies prior to repeated surgery, including anti-angiogenic drugs, tumor neo-antigen vaccines and small molecular inhibitors, like IDH-inhibitors (Table 1, Fig. 2).
Fig. 2.
Patient and tumor characteristics. A: Tumor subtypes, IDH = isocitrate dehydrogenase. B: Tumor (predominant) localizations, color coding according to legend (A) Figure created in part using biorender.com. C: Tumor WHO grades. D: Past medical history. RTx = Radiotherapy, CTx = Chemotherapy, TKI = Tyrosine kinase inhibitor, *Includes Bevacizumab (n = 6), tumor peptide vaccinations (n = 6), IDH-inhibitors (n = 2)
Table 1.
Patient characteristics
| Patient Characteristic | n = 70 |
|---|---|
| Age at repeat surgery (years) | |
| Median, (IQR) | 54.0 (13.9) |
| Mean, (SD) | 53.8 (11.3) |
| Gender | |
| Male, (%) | 31 (44%) |
| Female, (%) | 39 (56%) |
| Molecular Diagnoses | |
| Glioblastoma, IDH-wildtype, WHO grade 4, (%) | 48 (73%) |
| Diffuse astrocytoma, IDH-mutant, (%) | 13 (19%) |
| WHO grade 2 | 6 |
| WHO grade 3 | 3 |
| WHO grade 4 | 4 |
| Oligodendroglioma, IDH-mutant, (%) | 9 (13%) |
| WHO grade 2 | 2 |
| WHO grade 3 | 7 |
| Tumor localizations, (%) | |
| Frontal | 23 (33%) |
| Parietal | 16 (23%) |
| Temporal | 29 (41%) |
| Occipital | 1 (1%) |
| Basal ganglia | 1 (1%) |
| Tumor side, (%) | |
| Right | 35 (50%) |
| Left | 35 (50%) |
| N of previous surgeries | |
| 1 | 50 (71%) |
| 2 | 19 (27%) |
| 3 | 1 (2%) |
| N of previous chemotherapy cycles | |
| None | 2 (3%) |
| 1 | 10 (14%) |
| 2 | 30 (43%) |
| 3 | 23 (33%) |
| 4 or more | 5 (6%) |
| Applied agents | |
| TMZ (temozolomide) | 64 (91%) |
| CCNU (lomustine)/ VP-16 (etoposid) | 48 (69%) |
| BCNU (carmustine) | 1 (1%) |
| PCV (procarbazine, lomustine and vincristine) | 1 (1%) |
| Other modalities | |
| Bevacizumab | 6 (9%) |
| IDH-inhibitors | 2 (3%) |
| Tumor-treating fields | 6 (9%) |
| Tumor vaccines | 2 (3%) |
| Other* | 6 (9%) |
| N of previous radiotherapy courses | |
| 1 | 58 (83%) |
| 2 | 10 (14%) |
| 3 | 2 (3%) |
IQR Inter-quartile range, SD Standard deviation, IDH isocitrate dehydrogenase, WHO World Health Organization. *Therapy agents include Chloroquine, Irinotecan, Palbociclib, Atezolizumab
Diagnostic yield of repeatsurgery and its implication for targeted therapies
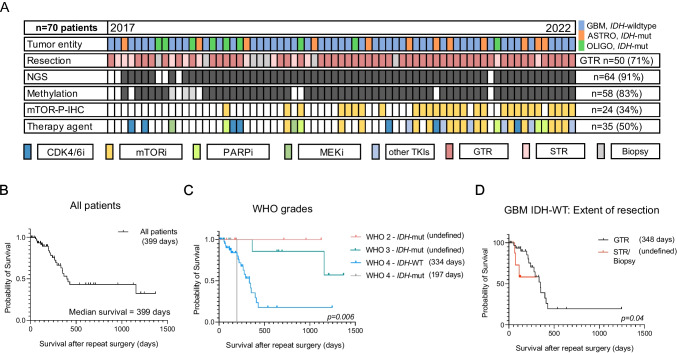
In 66 cases (94%), tissue samples from repeat surgery were further processed for next-generation sequencing (NGS, performed in n = 64, 91% of the cases) and DNA methylation profiling (performed in n = 58, 83% of the cases), with methylation data from previous tissue used in 3 further cases). In four cases (6%), further molecular workup was omitted, either because radiation necrosis instead of vital tumor tissue was diagnosed (n = 1) or because the postoperative condition of the patients deteriorated rapidly, rendering them non-amenable for further therapies (n = 3). Immunohistochemistry for mTOR phosphorylation (mTOR-P-IHC) was carried out in 34% of the cases (n = 24). A statistically non-significant difference was noted in the proportion of patients with actionable targets in patients undergoing biopsies (2/9, 22%) compared to patients undergoing gross total and subtotal resection (33/61 cases, 54%, p = 0.151, Fisher’s exact test). In 35 cases (50%), a recommendation for targeted therapy was made based on tissue analyses. Recommended agents included mTOR inhibitors (n = 15), CDK 4/6 and PARP inhibitors (n = 6 each), MEK inhibitors (n = 3), and further tyrosine kinase inhibitors (TKIs, n = 5). Targeted therapy was commenced in 21 cases (30% of the complete cohort). In the remaining 14 cases (20%) patients either deceased or their general condition deteriorated before targeted therapy could be initiated. This included 8 cases where patients were lost to follow up and further 6 cases of patients that succumbed to tumor progress while applications for health insurance approval were either pending or refused (Fig. 3A).
Fig. 3.
Oncoplot of available tissue data with overall survival (OS) after surgery in different patient groups in the patient cohort. A: Consecutive patients are listed horizontally in chronological order (time of surgery from 2017 to 2022). NGS = Next-generation sequencing. mTOR-P-IHC = Phospho-mTOR Immunohistochemistry. IDH = isocitrate dehydrogenase. CDK4/6i = Cycline-dependent Kinase 4/6 inhibitors. mTORi = mammalian target of rapamycin inhibitor. PARPi = Poly (ADP-ribose) polymerase inhibitor. MEKi = Mitogen-activated protein kinase kinase inhibitor. GTR = Gross Total Resection, STR = Subtotal resection B: All patients in the cohort (median survival 399 days). C: Survival after surgery based on WHO classification grades (WHO 2: undefined, WHO 3: undefined, WHO 4, isocitrate dehydrogenase wild type, IDH-WT: 334 days, WHO 4, IDH-mut: 197 days. D: Survival in GBM, IDH-WT patients after gross total resection (GTR, 348 days) vs all other surgical modalities (subtotal resection – STR and biopsies, survival undefined) in this cohort, p = 0.04, Gehan-Breslow-Wilcoxon test
Next, we examined the post-surgery survival of the patients of this cohort. At the time of this analysis, 44 of 70 patients were censored. Across all tumor types and WHO grades, the median survival after repeat surgery was 399 days (Fig. 3B), indicating a median survival long enough for patients to benefit from the personalized targeted therapies that were determined based on the sampled tumor tissue. As expected, patients with WHO grade 4 tumors showed the worst survival after surgery, compared to WHO grade 2 and grade 3 tumors (348 days in WHO grade 4 vs. undefined in other groups, p = 0.03, Gehan-Breslow-Wilcoxon test, Fig. 3C). Indeed, patients with IDH-wildtype GBM conferred a post-surgery survival rate of 334 days. To confirm the prognostic benefit of GTR in the context of this study, we compared survival after repeat surgery in GBM patients undergoing GTR (348 days, n = 34) to that of all other GBM patients (undergoing STR, open or stereotactic biopsies), with undefined survival due to small sample size in the latter (n = 10, p = 0.04, Gehan-Breslow-Wilcoxon test, Fig. 3D).
Surgical and neurological risk after repeat surgery: A comparative analysis
In this cohort, GTR was possible in 50 cases (71%). Other surgical modalities included subtotal resection (n = 11, 16%), and open or stereotactic biopsies (n = 9, 13%). This is in line with the number of cases where tissue sampling was the primary goal of surgery (n = 9, see Table 2). To establish a workable frame for a risk–benefit analysis in this cohort, we compared the rate of postoperative surgical complications and neurological deterioration with that of all other patients undergoing surgery for newly diagnosed or first glioma recurrence at our center in 2022 (n = 232). This ‘reference cohort’ was further divided into patients with repeat surgery on first tumor recurrence, i. e. patients receiving their second craniotomy. (n = 50, recurrent reference cohort) and patients receiving surgery (first craniotomy) for newly diagnosed diffuse glioma (n = 182, primary reference cohort).
Table 2.
Surgical and neurological outcomes
| Outcome | Repeat surgery cohort n = 70 (%) | Recurrent reference cohort n = 50 (%) | p-Value* | Primary reference cohort n = 182 (%) | p-Value* |
|---|---|---|---|---|---|
| Extent of resection | 0.02 | 0.002 | |||
| Gross total resection (GTR)** | 50 (71) | 27 (54) | 77 (42) | ||
| Subtotal resection (STR)** | 11 (16) | 19 (38) | 57 (31) | ||
| Biopsy | 9 (13) | 4 (8) | 48 (26) | ||
| Previous intent of mere tissue sampling | 9 (13) | ||||
| Complications requiring surgical intervention | 7 (11) | 3 (6) | 0.519 | 12 (7) | 0.359 |
| Wound healing disorder without CSF fistula | 3 (4) | 2 (4) | 4 (2) | ||
| CSF fistula | 2 (3) | 0 (0) | 2 (1) | ||
| CSF circulation disorder | 2 (3) | 0 (0) | 1 (< 1) | ||
| Postoperative intracranial hemorrhage | 0 (0) | 1 (2) | 5 (3) | ||
| Postoperative neurological deterioration | 13 (19) | 6 (12) | 0.448 | 23 (13) | 0.233 |
| Hemiparesis | 6 (9) | 2 (4) | 5 (3) | ||
| Aphasia | 4 (7) | 2 (4) | 11 (6) | ||
| Hemianopsia | 1 (1) | 1 (2) | 3 (2) | ||
| Aggravation of focal seizures | 2 (3) | 1 (2) | 2 (1) | ||
| Other | 0 (0) | 0 (0) | 2 (1) | ||
| Non-resolving neurological deterioration | 7 (10) | 3 (4) | 0.519 | 14 (8) | 0.612 |
Please note that all cases of aggravated focal seizures were transient. CSF = cerebrospinal fluid. SDH = subdural hematoma. *Fisher’s exact test. **verified through early postoperative MRI as a nodular contrast enhancing/ FLAIR-hyperintense residual tumor (within 48 h). Data from recurrent reference and primary reference cohort available under [30]
The surgical revision rate in the current study cohort (repeat surgery cohort) was 11% (n = 7), compared to 7% and 6% in the recurrent and primary reference cohorts, respectively (p = 0.519 and p = 0.359, Fisher's exact test). Complications requiring revision surgery in the repeat surgery study cohort included superficial wound healing disorders (n = 3), CSF (cerebrospinal fluid) fistula (n = 2) or CSF circulation disorders requiring placement of a drain into the resection cavity (n = 2). No postoperative hematomas were observed in the repeat surgery study cohort. We next examined the role of repeat surgery on newly developed or aggravated neurological deficits. Postoperative neurological deterioration mainly included hemiparesis and aphasia (n = 6 and n = 4, respectively). In two patients, an aggravation of focal seizure frequency and in one case a postoperative hemianopsia were noted, setting the rate of immediate postoperative neurological deterioration to 19% (13/70 cases), with no statistically significant differences to the recurrent and primary ‘therapy-naïve’ reference cohorts, which showed postoperative neurological deterioration rates of 12% and 13% (p = 0.448 and 0.233, respectively, Fisher’s exact test). Importantly, in all cohorts, deficits resolved in almost half of the patients, with non-significant differences in permanent deficits between patients of the repeat surgery (10%) vs. 4% and 8% in patients of the reference cohorts, p = 0.612, Fisher’s exact test, Table 2).
Discussion
In the current analysis, 50% of all patients undergoing repeat surgery after a tumor board recommendation for molecularly informed treatment emerged with a recommendation for targeted therapy. In 40% of these patients, therapy could not be initiated for reasons not related to the surgical procedure itself but because of rapid tumor progression while health insurance approval for therapy was pending, impeding commencement of treatment. Ultimately, one third of all patients undergoing repeat surgery received targeted therapies. The slightly higher risk for perioperative complications and transient neurological deterioration compared to our institutional reference cohort (including both recurrent and therapy-naïve, newly diagnosed glioma patients) was non-significant. Similarly, differences in rates of post-surgical permanent neurological deficits were also non-significant.
Although tumor tissue sampling was the primary intention of surgical intervention in this cohort, it was usually not the mere achievement: GTR was possible in 70% of the cases. In our study, patients with IDH-wildtype GBM conferred a median survival after repeat surgery of 348 days (11.6 months) after GTR. This is comparable to survival rates reported in previous trials focusing on GBM patients receiving their first re-resection, which range between 11.9 and 12.9 months [20, 29]. It is important to note that, due to the small sample size and heterogenous treatment regimens, a direct prognostic benefit from the choice of targeted therapy after surgery cannot be deduced in this cohort.
In the light of recent advances in molecular diagnostics and their potential to deliver personalized therapeutic strategies this study aimed to highlight a further dimension of the benefits of repeat surgery: The potential of tissue sampling to inform on therapeutic targets under a reasonable perioperative risk of surgical complications and neurological deterioration. Procedures like STR or even biopsy were still able to inform on therapeutic targets according to our findings, rendering them amenable intervention options in individualized settings when GTR is not viable. Nevertheless, given the high proportion of patients undergoing GTR in this cohort, our data indicate that even in the context of tissue sampling for molecularly informed treatment as a primary intent, it is worth evaluating the possibility of a maximized re-resection when weighed out against a ‘mere’ biopsy.
In this cohort, the diagnostic yield for actionable targets was 55% in resection cases and 22% in biopsy cases (difference non-significant, primarily limited by the small sample size in the biopsy group). The overall rate of actionable targets in this current cohort was 50%, which is comparable to what is found in reports identifying targetable mutations in recurrent glioma patients. To our knowledge, only one comparable analysis has been described in the literature. In a study by Blobner et al. [31] the rate of actionable targets identified in glioma patients was 69%, but also included IDH mutations (30%), which was regarded as pre-known in our analysis, making the rate of ‘novel’ mutations similar in both studies (at about 40% to 50%). Of note, in the study by Blobner et al., only 17% of all patients (n = 72) could commence targeted therapy, which is slightly lower than our study (30%). Beyond the molecular work-up including methylome profiling and panel sequencing of tumor material, Phospho-mTOR-IHC was applied on tumor samples to detect mTOR activation. Because phosphorylations are dynamic post-translational modifications that could point to adaptive resistance mechanisms of current therapy [32, 33], sampling of tumors at their recurrence could therefore also provide an opportunity to inform on such mechanisms, as shown in this analysis. Most likely, given the advancements in molecular diagnostics and our increasing understanding of glioma biology, the therapeutic yield will naturally increase beyond 50% in the coming years.
Given the intractable situation of a recurrent glioma without further standard treatment options, patients in this cohort show a considerable post-surgical median survival of about 13 months and about 12 months for GBM patients. This postoperative survival was, according to the data we present, long enough for patients to receive targeted therapy. It has to be noted, however, that 40% of patients with a treatment target identified had deceased before treatment could be commenced, amongst others because of rapid postoperative tumor progression. This observation emphasizes the importance of patient selection.
The notion that patients with recurrent glioma are at a higher risk of surgical and neurological complications [34] because of intensive pre-treatment, especially in high-grade gliomas, necessitates weighing out potential benefits of such individualized treatment approaches against the presumed risks of surgical intervention. In this cohort, the surgical complication rate (11%) did not significantly differ from rates of therapy-naïve patients with newly diagnosed gliomas in our primary reference cohort (6%) or glioma patients undergoing repeat surgery in our recurrent reference cohort (7%). Moreover, the surgical complication rate we describe is comparable to what has been reported in the literature for similar patient cohorts (surgical complication rates of up to between 8 to 30%) [29, 35, 36]. It is of note that the surgical complications observed in this analysis belonged to the less severe spectrum (Clavien-Dindo classification ≤ 3), and did not involve prolonged intensive care unit stays or death [37].
Permanent neurological deficits were observed in 10% of the study cohort, which is slightly higher than in the reference cohort of newly diagnosed glioma patients (4%, differences statistically non-significant). This effect could be attributed to the higher GTR (71%) rate in this cohort, compared to newly diagnosed glioma patients (42%). The observation is comparable to previous studies on recurrent glioma, citing neurological deterioration rates of 8% to 20% [29, 38–40].
Care should be taken when interpreting the data presented due to several limitations, especially with regards to postoperative survival rates. Owing to this individualized approach and the retrospective study design, the patient cohort was heterogeneous in terms of tumor subtypes, WHO grades and targeted therapies, each of them with a potential prognostic impact. For instance, only 9 patients with IDH-mutant oligodendroglioma and 13 patients with IDH-mutant astrocytoma were included, and even in the largest subgroup of IDH-wildtype GBM patients, the variety of surgical approaches, tumor localizations and targeted therapies applied does not provide data with enough ground to reliably assess the efficacy of targeted therapy on patient survival, which is also beyond the intended scope of this analysis. When examining survival rates, it should be noted that a high number of patients was censored, mostly due to loss of follow up. However, it is safe to assume that a certain proportion of these patients, especially with IDH-mutant WHO 2 and 3 gliomas (these WHO grades have the highest proportion of censored patients in this analysis), had not yet been deceased by the time of this analysis. Still, general observations on survival rates in this cohort could still be made and do confirm previous reports [29].
From a surgical point of view, this analysis did not investigate specific tumor locations pertaining to, for example, eloquence, which would deem the perioperative risk for neurological deterioration higher than in non-eloquent locations [41]. Also, as known from previous studies, volumetric analysis of residual tumor instead of qualitative assessment of GTR vs STR could have helped stratify patients with regards to the effect of the absolute residual tumor volume on survival [42]. This study also does not examine the effect of these interventions on the quality of life or the cognitive performance of affected patients, especially in patients with lower-grade tumors expected to survive longer with a potentially higher burden of disease [43, 44]. From the patients’ point of view, the question as to whether the risks taken and efforts spent under this individualized approach are justified may not be potentially answered by the duration but rather by the quality of prolonged survival. In addition, the aspect of progressive disease itself causing neurological deterioration is not addressed by our data. Here, a control group of patients receiving targeted therapies without previous surgery for tissue sampling may help address this confounding factor. We also acknowledge that this is a highly advanced and individualized approach that is not readily available in other regions or healthcare systems, and therefore has wide implications for the generalizability of this approach in an international setting and the standard of care that could be provided to these patients [45, 46].
Nevertheless, the data we present lays out that in the case of treatment-refractory recurrent glioma, surgical resection for tissue sampling bears a realistic potential of providing relevant therapeutic targets in addition to a survival-relevant cytoreduction, with a reasonable postoperative surgical and neurological complication rate. With a sizable proportion of patients also commencing personalized targeted therapy, this work helps involved neuro-oncologists and neurooncological surgeons weigh out the risks and benefits of surgery and provide patients, families and health-care providers with realistic expectations when offering such surgical interventions in the future.
Conclusion
Surgery for recurrent glioma aiming at molecularly informed treatment is associated with a reasonable surgical morbidity and an acceptable risk of neurological deficits that does not seem to be significantly higher than in primary surgery. With GTR achieved in most cases and druggable targets identified in about half of the patients, a targeted therapy could be part of a multimodal approach in patients with recurrent glioma. Further subgroup analyses with a larger patient cohort could help provide optimized patient stratification for predicting risks of peri-operative complications to aid tumor-board based decision making in this patient cohort.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
Study concept and design: CJ, OTH. Data collection and analysis: OTH, PDT, MK. Data interpretation: OTH, CJ, AW, TK, LK, FS. Writing the manuscript and Figure preparation: OTH, CJ. Reviewing and editing: CJ, OTH, AU, AWW, LK, FS, AVD, JD, WW, SMK, AWU. Supervision: CJ. All authors reviewed the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflicts of interest
FS is a co-founder and shareholder of Heidelberg Epignostix GmbH. The authors have no further relevant financial or non-financial interests to disclose.
Ethics approval
The committee of ethical practice at the University of Heidelberg approved the protocol of this retrospective analysis under S-455/2023 which has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments and for which patient consent was not required.
Competing interests
FS is a co-founder and shareholder of Heidelberg Epignostix GmbH.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Weller M, Wick W, Aldape K, Brada M, Berger M, Pfister SM, Nishikawa R, Rosenthal M, Wen PY, Stupp R, Reifenberger G. Glioma. Nat Rev Dis Primers. 2015;1:15017. doi: 10.1038/nrdp.2015.17. [DOI] [PubMed] [Google Scholar]
- 2.Fernandes C, Costa A, Osório L, Lago RC, Linhares P, Carvalho B, Caeiro C (2017) Current standards of care in glioblastoma therapy. In: De Vleeschouwer S (ed) Glioblastoma. Codon Publications. Copyright: The Authors, Brisbane (AU) [PubMed]
- 3.Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol. 2019;21:v1–v100. doi: 10.1093/neuonc/noz150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stupp R, Hegi ME, Gorlia T, Erridge SC, Perry J, Hong YK, Aldape KD, Lhermitte B, Pietsch T, Grujicic D, Steinbach JP, Wick W, Tarnawski R, Nam DH, Hau P, Weyerbrock A, Taphoorn MJ, Shen CC, Rao N, Thurzo L, Herrlinger U, Gupta T, Kortmann RD, Adamska K, McBain C, Brandes AA, Tonn JC, Schnell O, Wiegel T, Kim CY, Nabors LB, Reardon DA, van den Bent MJ, Hicking C, Markivskyy A, Picard M, Weller M, European Organisation for R, Treatment of C, Canadian Brain Tumor C, team Cs (2014) Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 15:1100-1108. 10.1016/S1470-2045(14)70379-1 [DOI] [PubMed]
- 5.Hilf N, Kuttruff-Coqui S, Frenzel K, Bukur V, Stevanović S, Gouttefangeas C, Platten M, Tabatabai G, Dutoit V, van der Burg SH, Thor Straten P, Martínez-Ricarte F, Ponsati B, Okada H, Lassen U, Admon A, Ottensmeier CH, Ulges A, Kreiter S, von Deimling A, Skardelly M, Migliorini D, Kroep JR, Idorn M, Rodon J, Piró J, Poulsen HS, Shraibman B, McCann K, Mendrzyk R, Löwer M, Stieglbauer M, Britten CM, Capper D, Welters MJP, Sahuquillo J, Kiesel K, Derhovanessian E, Rusch E, Bunse L, Song C, Heesch S, Wagner C, Kemmer-Brück A, Ludwig J, Castle JC, Schoor O, Tadmor AD, Green E, Fritsche J, Meyer M, Pawlowski N, Dorner S, Hoffgaard F, Rössler B, Maurer D, Weinschenk T, Reinhardt C, Huber C, Rammensee HG, Singh-Jasuja H, Sahin U, Dietrich PY, Wick W. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019;565:240–245. doi: 10.1038/s41586-018-0810-y. [DOI] [PubMed] [Google Scholar]
- 6.McKinnon C, Nandhabalan M, Murray SA, Plaha P. Glioblastoma: clinical presentation, diagnosis, and management. BMJ. 2021;374:n1560. doi: 10.1136/bmj.n1560. [DOI] [PubMed] [Google Scholar]
- 7.De Witt Hamer PC, De Witt Hamer PC, Klein M, Hervey-Jumper SL, Wefel JS, Berger MS. Functional outcomes and health-related quality of life following glioma surgery. Neurosurgery. 2021;88:720–732. doi: 10.1093/neuros/nyaa365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alemany M, Velasco R, Simó M, Bruna J. Late effects of cancer treatment: consequences for long-term brain cancer survivors. Neurooncol Pract. 2021;8:18–30. doi: 10.1093/nop/npaa039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller JJ, Gonzalez Castro LN, McBrayer S, Weller M, Cloughesy T, Portnow J, Andronesi O, Barnholtz-Sloan JS, Baumert BG, Berger MS, Bi WL, Bindra R, Cahill DP, Chang SM, Costello JF, Horbinski C, Huang RY, Jenkins RB, Ligon KL, Mellinghoff IK, Nabors LB, Platten M, Reardon DA, Shi DD, Schiff D, Wick W, Yan H, von Deimling A, van den Bent M, Kaelin WG, Wen PY. Isocitrate dehydrogenase (IDH) mutant gliomas: a Society for Neuro-Oncology (SNO) consensus review on diagnosis, management, and future directions. Neuro Oncol. 2022 doi: 10.1093/neuonc/noac207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim-Fat MJ, Song KW, Iorgulescu JB, Andersen BM, Forst DA, Jordan JT, Gerstner ER, Reardon DA, Wen PY, Arrillaga-Romany I. Clinical, radiological and genomic features and targeted therapy in BRAF V600E mutant adult glioblastoma. J Neurooncol. 2021;152:515–522. doi: 10.1007/s11060-021-03719-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weller M, van den Bent M, Hopkins K, Tonn JC, Stupp R, Falini A, Cohen-Jonathan-Moyal E, Frappaz D, Henriksson R, Balana C, Chinot O, Ram Z, Reifenberger G, Soffietti R, Wick W. EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol. 2014;15:e395–403. doi: 10.1016/s1470-2045(14)70011-7. [DOI] [PubMed] [Google Scholar]
- 13.Barthel FP, Johnson KC, Varn FS, Moskalik AD, Tanner G, Kocakavuk E, Anderson KJ, Abiola O, Aldape K, Alfaro KD, Alpar D, Amin SB, Ashley DM, Bandopadhayay P, Barnholtz-Sloan JS, Beroukhim R, Bock C, Brastianos PK, Brat DJ, Brodbelt AR, Bruns AF, Bulsara KR, Chakrabarty A, Chakravarti A, Chuang JH, Claus EB, Cochran EJ, Connelly J, Costello JF, Finocchiaro G, Fletcher MN, French PJ, Gan HK, Gilbert MR, Gould PV, Grimmer MR, Iavarone A, Ismail A, Jenkinson MD, Khasraw M, Kim H, Kouwenhoven MCM, LaViolette PS, Li M, Lichter P, Ligon KL, Lowman AK, Malta TM, Mazor T, McDonald KL, Molinaro AM, Nam DH, Nayyar N, Ng HK, Ngan CY, Niclou SP, Niers JM, Noushmehr H, Noorbakhsh J, Ormond DR, Park CK, Poisson LM, Rabadan R, Radlwimmer B, Rao G, Reifenberger G, Sa JK, Schuster M, Shaw BL, Short SC, Smitt PAS, Sloan AE, Smits M, Suzuki H, Tabatabai G, Van Meir EG, Watts C, Weller M, Wesseling P, Westerman BA, Widhalm G, Woehrer A, Yung WKA, Zadeh G, Huse JT, De Groot JF, Stead LF, Verhaak RGW. Longitudinal molecular trajectories of diffuse glioma in adults. Nature. 2019;576:112–120. doi: 10.1038/s41586-019-1775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Draaisma K, Chatzipli A, Taphoorn M, Kerkhof M, Weyerbrock A, Sanson M, Hoeben A, Lukacova S, Lombardi G, Leenstra S, Hanse M, Fleischeuer R, Watts C, McAbee J, Angelopoulos N, Gorlia T, Golfinopoulos V, Kros JM, Verhaak RGW, Bours V, van den Bent MJ, McDermott U, Robe PA, French PJ. Molecular evolution of IDH wild-type glioblastomas treated with standard of care affects survival and Design of Precision Medicine Trials: a report from the EORTC 1542 study. J Clin Oncol. 2020;38:81–99. doi: 10.1200/jco.19.00367. [DOI] [PubMed] [Google Scholar]
- 15.de Souza CF, Sabedot TS, Malta TM, Stetson L, Morozova O, Sokolov A, Laird PW, Wiznerowicz M, Iavarone A, Snyder J, deCarvalho A, Sanborn Z, McDonald KL, Friedman WA, Tirapelli D, Poisson L, Mikkelsen T, Carlotti CG, Jr, Kalkanis S, Zenklusen J, Salama SR, Barnholtz-Sloan JS, Noushmehr H. A distinct DNA methylation shift in a subset of glioma CpG Island methylator phenotypes during tumor recurrence. Cell Rep. 2018;23:637–651. doi: 10.1016/j.celrep.2018.03.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perry JR, Bélanger K, Mason WP, Fulton D, Kavan P, Easaw J, Shields C, Kirby S, Macdonald DR, Eisenstat DD, Thiessen B, Forsyth P, Pouliot JF. Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol. 2010;28:2051–2057. doi: 10.1200/jco.2009.26.5520. [DOI] [PubMed] [Google Scholar]
- 17.Wick W, Gorlia T, Bendszus M, Taphoorn M, Sahm F, Harting I, Brandes AA, Taal W, Domont J, Idbaih A, Campone M, Clement PM, Stupp R, Fabbro M, Le Rhun E, Dubois F, Weller M, von Deimling A, Golfinopoulos V, Bromberg JC, Platten M, Klein M, van den Bent MJ. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377:1954–1963. doi: 10.1056/NEJMoa1707358. [DOI] [PubMed] [Google Scholar]
- 18.van Opijnen MP, Broekman MLD, de Vos FYF, Cuppen E, van der Hoeven JJM, van Linde ME, Compter A, Beerepoot LV, van den Bent MJ, Vos MJ, Fiebrich HB, Koekkoek JAF, Hoeben A, Kho KH, Driessen CML, Jeltema HR, Robe P, Maas SLN. Study protocol of the GLOW study: maximising treatment options for recurrent glioblastoma patients by whole genome sequencing-based diagnostics-a prospective multicenter cohort study. BMC Med Genomics. 2022;15:233. doi: 10.1186/s12920-022-01343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmadi R, Dictus C, Hartmann C, Zürn O, Edler L, Hartmann M, Combs S, Herold-Mende C, Wirtz CR, Unterberg A. Long-term outcome and survival of surgically treated supratentorial low-grade glioma in adult patients. Acta Neurochir (Wien) 2009;151:1359–1365. doi: 10.1007/s00701-009-0435-x. [DOI] [PubMed] [Google Scholar]
- 20.Suchorska B, Weller M, Tabatabai G, Senft C, Hau P, Sabel MC, Herrlinger U, Ketter R, Schlegel U, Marosi C, Reifenberger G, Wick W, Tonn JC, Wirsching HG. Complete resection of contrast-enhancing tumor volume is associated with improved survival in recurrent glioblastoma-results from the DIRECTOR trial. Neuro Oncol. 2016;18:549–556. doi: 10.1093/neuonc/nov326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uppstrom TJ, Singh R, Hadjigeorgiou GF, Magge R, Ramakrishna R. Repeat surgery for recurrent low-grade gliomas should be standard of care. Clin Neurol Neurosurg. 2016;151:18–23. doi: 10.1016/j.clineuro.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 22.Leone A, Colamaria A, Fochi NP, Sacco M, Landriscina M, Parbonetti G, de Notaris M, Coppola G, De Santis E, Giordano G, Carbone F (2022) Recurrent glioblastoma treatment: State of the art and future perspectives in the precision medicine era. Biomedicines 10. 10.3390/biomedicines10081927 [DOI] [PMC free article] [PubMed]
- 23.Karschnia P, Young JS, Dono A, Häni L, Sciortino T, Bruno F, Juenger ST, Teske N, Morshed RA, Haddad AF, Zhang Y, Stoecklein S, Weller M, Vogelbaum MA, Beck J, Tandon N, Hervey-Jumper S, Molinaro AM, Rudà R, Bello L, Schnell O, Esquenazi Y, Ruge MI, Grau SJ, Berger MS, Chang SM, van den Bent M, Tonn JC. Prognostic validation of a new classification system for extent of resection in glioblastoma: a report of the RANO resect group. Neuro Oncol. 2023;25:940–954. doi: 10.1093/neuonc/noac193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramakrishna R, Hebb A, Barber J, Rostomily R, Silbergeld D (2015) Outcomes in reoperated low-grade gliomas. Neurosurgery 77:175–184; discussion 184. 10.1227/neu.0000000000000753 [DOI] [PubMed]
- 25.Ius T, Pauletto G, Cesselli D, Isola M, Turella L, Budai R, DeMaglio G, Eleopra R, Fadiga L, Lettieri C, Pizzolitto S, Beltrami CA, Skrap M. Second surgery in insular low-grade gliomas. Biomed Res Int. 2015;2015:497610. doi: 10.1155/2015/497610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bloch O, Han SJ, Cha S, Sun MZ, Aghi MK, McDermott MW, Berger MS, Parsa AT. Impact of extent of resection for recurrent glioblastoma on overall survival: clinical article. J Neurosurg. 2012;117:1032–1038. doi: 10.3171/2012.9.Jns12504. [DOI] [PubMed] [Google Scholar]
- 27.Shofty B, Haim O, Costa M, Kashanian A, Shtrozberg S, Ram Z, Grossman R. Impact of repeated operations for progressive low-grade gliomas. Eur J Surg Oncol. 2020;46:2331–2337. doi: 10.1016/j.ejso.2020.07.013. [DOI] [PubMed] [Google Scholar]
- 28.Chaichana KL, Zadnik P, Weingart JD, Olivi A, Gallia GL, Blakeley J, Lim M, Brem H, Quiñones-Hinojosa A. Multiple resections for patients with glioblastoma: prolonging survival. J Neurosurg. 2013;118:812–820. doi: 10.3171/2012.9.Jns1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ringel F, Pape H, Sabel M, Krex D, Bock HC, Misch M, Weyerbrock A, Westermaier T, Senft C, Schucht P, Meyer B, Simon M. Clinical benefit from resection of recurrent glioblastomas: results of a multicenter study including 503 patients with recurrent glioblastomas undergoing surgical resection. Neuro Oncol. 2016;18:96–104. doi: 10.1093/neuonc/nov145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dao Trong P, Olivares A, El Damaty A, Unterberg A. Adverse events in neurosurgery: a comprehensive single-center analysis of a prospectively compiled database. Acta Neurochir (Wien) 2023;165:585–593. doi: 10.1007/s00701-022-05462-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blobner J, Dengler L, Blobner S, Eberle C, Weller J, Teske N, Karschnia P, Rühlmann K, Heinrich K, Ziemann F, Greif PA, Jeremias I, Wuerstlein R, Hasselmann K, Dorostkar M, Harter PN, Quach S, Stoecklein V, Albert NL, Niyazi M, Tonn JC, Thon N, Christoph Westphalen B, von Baumgarten L (2023) Significance of molecular diagnostics for therapeutic decision-making in recurrent glioma. Neurooncol Adv 5:vdad060. 10.1093/noajnl/vdad060 [DOI] [PMC free article] [PubMed]
- 32.Wei W, Shin YS, Xue M, Matsutani T, Masui K, Yang H, Ikegami S, Gu Y, Herrmann K, Johnson D, Ding X, Hwang K, Kim J, Zhou J, Su Y, Li X, Bonetti B, Chopra R, James CD, Cavenee WK, Cloughesy TF, Mischel PS, Heath JR, Gini B. Single-cell phosphoproteomics resolves adaptive signaling dynamics and informs targeted combination therapy in glioblastoma. Cancer Cell. 2016;29:563–573. doi: 10.1016/j.ccell.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akhavan D, Cloughesy TF, Mischel PS. mTOR signaling in glioblastoma: lessons learned from bench to bedside. Neuro Oncol. 2010;12:882–889. doi: 10.1093/neuonc/noq052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stienen MN, Zhang DY, Broggi M, Seggewiss D, Villa S, Schiavolin S, Bozinov O, Krayenbühl N, Sarnthein J, Ferroli P, Regli L. The influence of preoperative dependency on mortality, functional recovery and complications after microsurgical resection of intracranial tumors. J Neurooncol. 2018;139:441–448. doi: 10.1007/s11060-018-2882-9. [DOI] [PubMed] [Google Scholar]
- 35.Berger A, Tzarfati G, Costa M, Serafimova M, Korn A, Vendrov I, Alfasi T, Krill D, Aviram D, Ben Moshe S, Kashanian A, Ram Z, Grossman R (2019) Incidence and impact of stroke following surgery for low-grade gliomas. J Neurosurg 1–9. 10.3171/2019.10.Jns192301 [DOI] [PubMed]
- 36.Djamel-Eddine Y-C, De Witte O, Mélot C, Lefranc F. Recurrent glioblastomas: should we operate a second and even a third time? Interdiscip Neurosurg. 2019;18:100551. doi: 10.1016/j.inat.2019.100551. [DOI] [Google Scholar]
- 37.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang SM, Parney IF, McDermott M, Barker FG, 2nd, Schmidt MH, Huang W, Laws ER, Jr, Lillehei KO, Bernstein M, Brem H, Sloan AE, Berger M. Perioperative complications and neurological outcomes of first and second craniotomies among patients enrolled in the glioma outcome project. J Neurosurg. 2003;98:1175–1181. doi: 10.3171/jns.2003.98.6.1175. [DOI] [PubMed] [Google Scholar]
- 39.Hoover JM, Nwojo M, Puffer R, Mandrekar J, Meyer FB, Parney IF. Surgical outcomes in recurrent glioma: clinical article. J Neurosurg. 2013;118:1224–1231. doi: 10.3171/2013.2.Jns121731. [DOI] [PubMed] [Google Scholar]
- 40.Yang K, Ellenbogen Y, Martyniuk A, Sourour M, Takroni R, Somji M, Gardiner E, Hui K, Odedra D, Larrazabal R, Algird A, Kachur E, Reddy K, Murty N, Farrokhyar F, Singh SK (2022) Reoperation in adult patients with recurrent glioblastoma: A matched cohort analysis. Neurooncol Adv 4: vdac115. 10.1093/noajnl/vdac115 [DOI] [PMC free article] [PubMed]
- 41.Park JK, Hodges T, Arko L, Shen M, Dello Iacono D, McNabb A, Olsen Bailey N, Kreisl TN, Iwamoto FM, Sul J, Auh S, Park GE, Fine HA, Black PM. Scale to predict survival after surgery for recurrent glioblastoma multiforme. J Clin Oncol. 2010;28:3838–3843. doi: 10.1200/jco.2010.30.0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woodroffe RW, Zanaty M, Soni N, Mott SL, Helland LC, Pasha A, Maley J, Dhungana N, Jones KA, Monga V, Greenlee JDW. Survival after reoperation for recurrent glioblastoma. J Clin Neurosci. 2020;73:118–124. doi: 10.1016/j.jocn.2020.01.009. [DOI] [PubMed] [Google Scholar]
- 43.Kirkman MA, Hunn BHM, Thomas MSC, Tolmie AK. Influences on cognitive outcomes in adult patients with gliomas: a systematic review. Front Oncol. 2022;12:943600. doi: 10.3389/fonc.2022.943600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lawrie TA, Gillespie D, Dowswell T, Evans J, Erridge S, Vale L, Kernohan A, Grant R (2019) Long-term neurocognitive and other side effects of radiotherapy, with or without chemotherapy, for glioma. Cochrane Database Syst Rev 8:Cd013047. 10.1002/14651858.CD013047.pub2 [DOI] [PMC free article] [PubMed]
- 45.Andreiuolo F, Mazeraud A, Chrétien F, Pietsch T. A global view on the availability of methods and information in the neuropathological diagnostics of CNS tumors: results of an international survey among neuropathological units. Brain Pathol. 2016;26:551–554. doi: 10.1111/bpa.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Del Bigio MR, Hainfellner JA, McLean CA, Powell SZ, Sikorska B, Takahashi H, Weis J, Xuereb JH. Neuropathology training worldwide-evolution and comparisons. Brain Pathol. 2014;24:285–298. doi: 10.1111/bpa.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.