Abstract
Objectives
To analyze and compare the available data about the outcomes of endoscopic and microscopic type I tympanoplasty.
Data sources
PubMed, Cochrane library Ovid, Scopus, Google scholar, and ClinicalTrials.
Methods
We conducted a meta-analysis in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines. We included comparative studies describing type I tympanoplasty, and comparing surgical outcomes of the endoscope with the microscope in terms of efficacy and safety.
Results
Our systematic search yielded 22 studies meeting the inclusion criteria and eligible for analysis. The pooled graft uptake rates and audiological results of endoscopic and microscopic tympanoplasty demonstrated non-significant differences. In contrast, endoscopic type I tympanoplasty outperforms microscopic tympanoplasty regarding a highly significant decrease not only in pooled mean operative time but also in the pooled complications rate.
Conclusions
Based on our meta-analysis, the surgical outcomes of endoscope-assisted and microscope-assisted type I tympanoplasty in terms of postoperative hearing outcomes and the graft uptake rate were comparable. On the contrary, operative time and complications rate proved to be significantly reduced with endoscopy compared to microscopy. Hence, the endoscope is as efficient as the microscope in type I tympanoplasty but less invasive, fewer in complications and shorter in operative time.
Keywords: Meta-analysis, Tympanoplasty, Myringoplasty, Endoscope, Microscope, Tympanic membrane perforation
Key Points
-
•
Can the endoscope be a good alternative to the microscope in type I tympanoplasty?
-
•
Using the microscope in tympanoplasty has been the conventional procedure for repairing perforated tympanic membranes since the 1950s. However, ear surgeons have increasingly practised endoscope-assisted tympanoplasty since the late 1990s.
-
•
In this study, surgical outcomes of both techniques in terms of postoperative audiological results and the graft uptake rate were comparable.
-
•
In contrast, the endoscopic technique was superior to the microscopic one in terms of operative time and complications rate.
-
•
According to our study, the endoscope-Assisted type I tympanoplasty proved to be as effective as the microscopic technique but safer and less invasive.
Introduction
Tympanoplasty is a surgical procedure aiming at the eradication of infection, repair of the perforated tympanic membrane (TM), and hearing rehabilitation in patients with chronic otitis media (COM) [1]. Middle ear infections, trauma or iatrogenic injury are the principal causes of TM perforation. Up to 80% of TM perforations heal spontaneously [2]; as for the remaining, surgical repair is usually required [3].
With endoscope assistance, minimally invasive techniques of ear surgery have arisen and evolved since the 1990s [4]. Analogous to functional endoscopic sinus surgery, so too did the concept of functional endoscopic ear surgery (FEES). The philosophy of FEES fundamentally supports three essential principles: 1. using the external auditory canal (EAC) as the natural conduit to the tympanic cavity; 2. restoring normal ventilation routes of the middle ear and the mastoid; and 3. conserving as much normal anatomy as possible [5]. Consequently, endoscopic ear surgery has become widely accepted with anatomical and physiological concepts [6].
Despite the well-known merits of endoscopic techniques, some concerns about their efficiency and safety are still exist among some ear surgeons and hinder the transition from conventional microscopic tympanoplasty to endoscopic tympanoplasty for those surgeons [7].
Therefore, there is a need for a meta-analysis comparing the outcomes of both endoscopy and microscopy techniques of type I tympanoplasty in terms of efficacy and safety.
Objectives
In this study, we aimed to make a comparison between endoscopic and microscopic type I tympanoplasty in relation to the duration of surgery, outcomes and complications through a meta-analysis.
Materials and methods
We conducted a meta-analysis using the standard methodology outlined in the Cochrane Handbook [8] and reported the findings in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement guidelines. [9] A PRISMA flow diagram was used to describe the flow of information through the various phases of the systematic review.
Eligibility criteria
Inclusion criteria for our meta-analysis were as follows: (1) journal articles published in English; (2) articles concerned with TM perforation especially due to COM; (3) studies describing myringoplasty or type I tympanoplasty, and comparing surgical outcomes of the endoscope with the microscope; (4) either temporalis fascia or perichondrium as a source of the graft tissue; (5) graft placement method via underlay technique.
Exclusion criteria for our meta-analysis were as follows: (1) articles describing other types of otitis media (e.g. acute OM or OM with effusion) or other pathologies (e.g. cholesteatoma or middle ear tumours); (2) the aid of an endoscope holder; (3) cadaver studies; (4) animal studies; (5) irrelevant publications to our study.
Outcome measures
The outcome measures, which we considered in terms of efficacy and safety, were average operative time (intraoperative outcome); average postoperative air-bone gaps (ABGs) improvement and graft uptake rate (primary efficacy outcomes); complications rate (secondary safety outcomes).
Search strategy
We performed a systematic search for all available studies comparing surgical outcomes of the endoscope with the microscope in the databases of PubMed, Cochrane library Ovid, Scopus, Google scholar, and ClinicalTrials; dating from inception until 22 November 2019. We used the following keywords (in different combinations): microscopic, endoscopic, type I tympanoplasty, myringoplasty, chronic otitis media. Review articles and bibliographies of each identified study were searched for additional references that may contain further related studies.
Study selection
Abstracts of articles identified using the above search strategy were reviewed; articles that appeared to fulfill the inclusion criteria were retrieved in full. We excluded duplicate records and irrelevant reports at this stage. When there was a doubt, a second reviewer assessed the article, and a consensus was reached.
Data extraction
Data were independently extracted onto a previously edited Excel table by two reviewers and cross-checked; any discrepancies were resolved by consensus. For the meta-analysis, we retrieved the following information: author, year of publication, study design, number of patients, and outcomes regarding efficacy and safety.
Statistical analysis
Data entry, processing and statistical analysis were carried out using Review Manager 5.3 (RevMan 2014) [10]. A meta-analysis was performed to calculate direct estimates of each treatment technique. Interventions for patients, who achieved favourable outcomes, were pooled to evaluate efficacy that was measured by standardized mean difference (SMD) with a 95% confidence interval (CI) for operative time and postoperative ABG improvement; and odds ratio (OR) with 95% CI for graft uptake rate. In addition, interventions for patients, who reached serious adverse events, were pooled to evaluate safety that was measured by OR with 95% CI for complications rate.
According to the heterogeneity of treatment effect across trials using the Chi2 test results and I2-statistics; a fixed-effect model (P ≥ 0.1) or random-effects model (P < 0.1) was used. In addition, we used a random-effects model for subgroup analysis.
Assessment of risk of publication bias across studies
We assessed the publication bias across studies using the funnel plot method for each pooled analysis that included more than or equal to 10 studies [11].
Results
Study selection
Figure 1 represents the PRISMA flow diagram for the review process and study selection. We found 150 records by searching the database; of these, sixty records were left after removing the duplicates and after the exclusion of ninety records based on the title and the abstract review. We searched 60 articles for eligibility by full-text review; 15 articles cannot be accessed or obtain full-text; 10 studies were reviews and case reports; eight studies did not describe the functional outcome; the desired procedure was not used in five studies leaving 22 studies [12–33] that met all inclusion criteria.
Fig. 1.
PRISMA flow diagram for study selection
Study characteristics
Table 1 shows the demographic characteristics and clinical data of all 22 studies [12–33]. Regarding their design, these 22 studies included ten randomized controlled trials, six prospective comparative studies, five retrospective comparative studies and one retrospective cohort study. The enrolled studies were published between 2008 and 2019. The total number of interventions was 1578 interventions; with 766 interventions in the endoscopic group, and 812 in the microscopic group.
Table 1.
Summary of interventions and study characteristics
| Author, year | Study design | Number of interventions | Comparative parameters | ||||
|---|---|---|---|---|---|---|---|
| ET | MT | Operative time | ABG improvement | Graft uptake | Complications rate | ||
| Harugop et al., 2008 [12] | RCT | 50 | 50 | √ | √ | √ | |
| Lade et al., 2014 [13] | RCT | 30 | 30 | √ | √ | ||
| Kumar et al., 2015 [14] | Prospective comparative | 30 | 30 | √ | √ | √ | √ |
| Nassif et al., 2015 [15] | Retrospective cohort | 22 | 23 | √ | √ | √ | |
| Patel et al., 2015 [16] | RCT | 22 | 22 | √ | √ | √ | |
| Ahmed et al., 2016 [17] | Prospective comparative | 50 | 50 | √ | √ | √ | |
| Gadag et al., 2016 [18] | RCT | 30 | 30 | √ | √ | ||
| Gaur et al., 2016 [19] | Prospective comparative | 30 | 30 | √ | √ | √ | |
| Huang et al., 2016 [20] | Retrospective comparative | 50 | 50 | √ | √ | √ | √ |
| Kumar et al., 2016 [21] | RCT | 30 | 30 | √ | √ | √ | √ |
| Lakpathi et al., 2016 [22] | Prospective comparative | 30 | 30 | √ | √ | √ | |
| Sanji et al., 2016 [23] | Retrospective comparative | 16 | 28 | √ | √ | ||
| Shoeb et al., 2016 [24] | Prospective comparative | 30 | 30 | √ | √ | ||
| Choi et al., 2017 [25] | Retrospective comparative | 25 | 48 | √ | √ | √ | |
| Jyothi et al., 2017 [26] | RCT | 60 | 60 | √ | √ | √ | √ |
| Kuo and Wu, 2017 [27] | Retrospective comparative | 62 | 44 | √ | √ | √ | √ |
| Sinha et al., 2017 [28] | RCT | 22 | 22 | √ | √ | ||
| Khaliq et al., 2018 [29] | RCT | 30 | 30 | √ | √ | √ | |
| Maran et al., 2018 [30] | RCT | 30 | 30 | √ | √ | √ | |
| Saggu et al., 2018 [31] | Prospective comparative | 30 | 30 | √ | √ | ||
| Ohki et al., 2019 [32] | Retrospective comparative | 47 | 75 | √ | √ | √ | |
| Sundararajan et al., 2019 [33] | RCT | 40 | 40 | √ | √ | √ | √ |
ET endoscopic tympanoplasty; MT microscopic tympanoplasty; ABG air–bone gap; RCT randomized controlled trial
Effects of interventions
Operative time
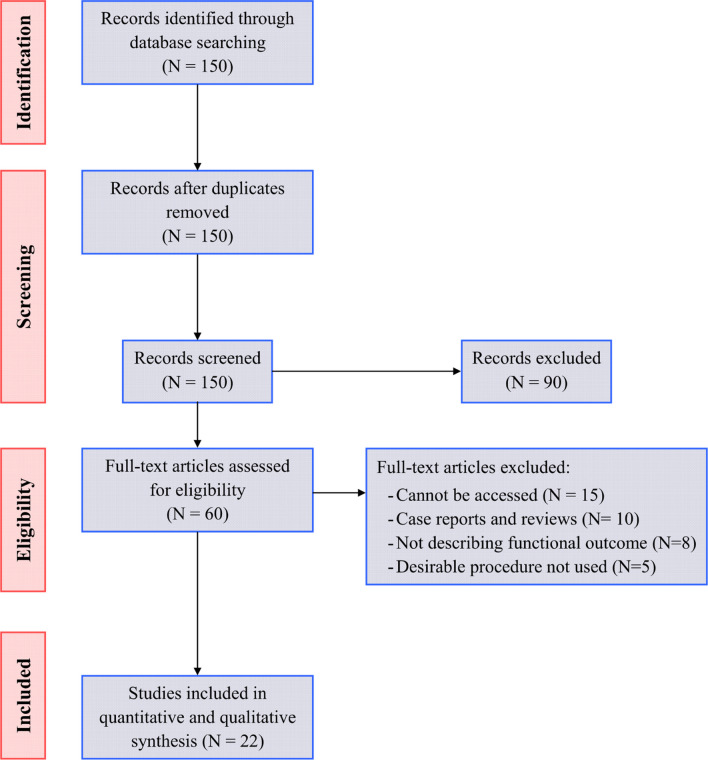
We found nineteen studies that reported operative time with a total number of interventions (n = 1414). The average operative time was (77.7 ± 24.5) min for the ET group and (91.7 ± 18.8) min for the MT group. In the pooled analysis (Fig. 2A), endoscopic tympanoplasty (ET) showed a highly significant decrease in mean operative time compared to microscopic tympanoplasty (MT) (SMD: −1.33; 95% CI −1.95 to −0.72; p < 0.0001). However, highly significant heterogeneity (I2 = 96%, p < 0.00001) and publication bias were found (Fig. 2B).
Fig. 2.
A Forest plot comparing the operative time of endoscopic tympanoplasty and microscopic tympanoplasty. B Funnel plot of the operative time. CI confidence interval; IV inverse variance; SE standard error; SMD standardized mean difference
ABG improvement
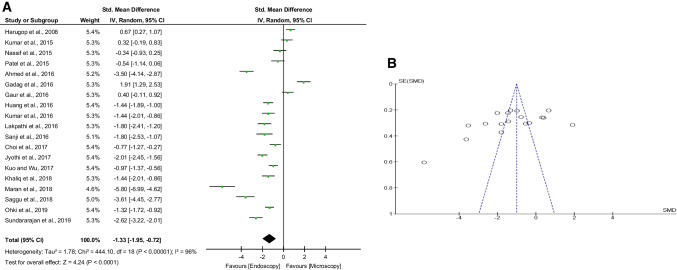
Fifteen studies exhibited ABG improvement with a total number of interventions (n = 1135). Based on the pooled analysis (Fig. 3A), ET showed a non-significant difference in mean ABGs improvement compared to MT (SMD: 0.03; 95% CI −0.33 to 0.39; p = 0.87). However, highly significant heterogeneity (I2 = 89%, p < 0.00001) and publication bias were found (Fig. 3B).
Fig. 3.
A Forest plot comparing the air-bone gaps improvement of both techniques. B Funnel plot of the air-bone gaps improvement. CI confidence interval; IV inverse variance; SE standard error; SMD standardized mean difference
Graft uptake rate
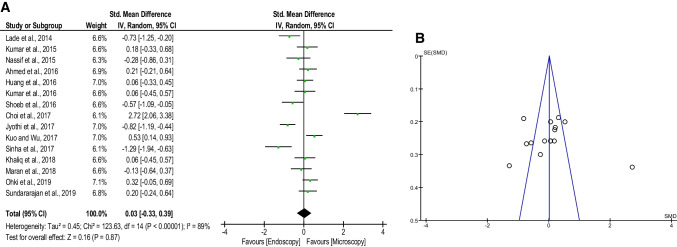
All 22 studies included data about graft uptake rate with a total number of interventions (n = 1559). Accrued results (Fig. 4A) showed that ET was as effective as MT (89.8% vs. 90.2%; OR: 0.95; 95% CI 0.68–1.34; p = 0.79). In addition, no heterogeneity (I2 = 0%, p = 0.99) or publication bias was found (Fig. 4B).
Fig. 4.
A Forest plot comparing the graft uptake rate of endoscopic tympanoplasty and microscopic tympanoplasty. B Funnel plot of the graft uptake rate. CI confidence interval; M–H Mantel–Haenszel; OR odds ratio; SE standard error
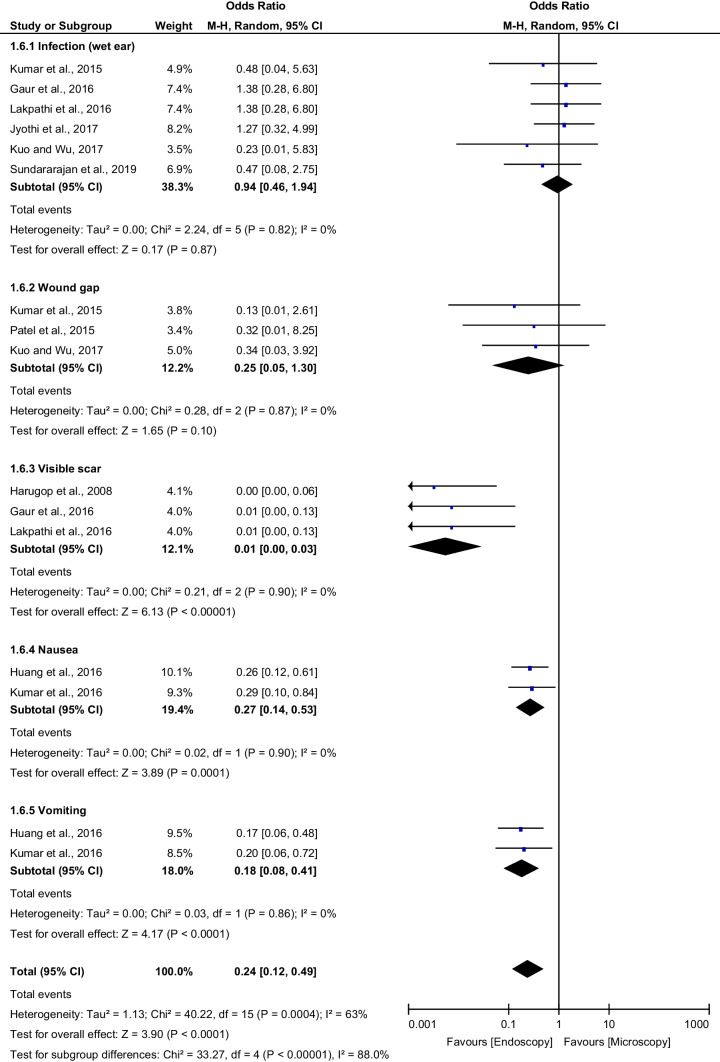
Postoperative complications
Various postoperative complications were retrieved from enrolled studies. We analyzed the incidence of postoperative complications, with a particular focus on the infection (wet ear), wound gap, visible scar, nausea and vomiting. Incidence of postoperative complications was reported in 10 studies.
Data regarding the infection (wet ear) were available from six studies with a total of 486 interventions. Three studies reported data on the wound gap with a total of 210 interventions. Based on the pooled analysis, ET showed a non-significant difference compared to MT in terms of the rates of infection (6.3% vs 7.3%; OR: 0.94; 95% CI 0.46–1.94; p = 0.87) and wound gap (0.9% vs 6.3%; OR: 0.25; 95% CI 0.05–1.30; p = 0.10). Also, no heterogeneity was found.
Data regarding visible scar, we found three studies with a total of 220 interventions. Two studies reported data on postoperative nausea and vomiting, with a total of 160 interventions for each. In the pooled analysis, ET showed a highly significant decrease compared to MT in terms of the rates of visible scar (0% vs 72.7%; OR: 0.01; 95% CI 0.00–0.03; p < 0.00001), nausea (36.3% vs 67.5%; OR: 0.27; 95% CI 0.14–0.53; p = 0.0001), and vomiting (12.5% vs 43.8%; OR: 0.18; 95% CI 0.08–0.41; p < 0.0001). In addition, no heterogeneity was found.
The overall effect of pooling the previous five subgroups showed that the rate of complications is significantly lower in ET than in MT (8.8% vs 32%; OR: 0.24; 95% CI 0.12–0.49; p < 0.0001) (Fig. 5).
Fig. 5.
Forest plot comparing the postoperative complications rate of endoscopic tympanoplasty and microscopic tympanoplasty with regard to infection (wet ear), wound gap, visible scar, nausea and vomiting. CI confidence interval; M–H Mantel–Haenszel
Discussion
In this study, we compared the outcomes of endoscopic with microscopic type I tympanoplasty through a systematic review meta-analysis. Hearing improvement and graft uptake rate of ET were comparable to those of MT. On the other hand, ET was superior to MT in terms of operative time and complications.
For decades, Microscope-assisted surgery was the main modality for ear surgery, allowing two-handed manipulation, binocular vision and an excellent stereoscopic surgical view. However, the vision of a microscope is along a straight line and may be limited in hidden areas and the deep recesses of the middle ear, so the surgeon has to use the post-auricular approach instead of the transcanal approach to obtain a wider surgical view.
One of the primary advantages of the endoscope is the panoramic and wide-angle surgical view with magnification. The endoscope can be approximated to the surgical field, bypassing the narrowing parts of the EAC, and the angled endoscope can be rotated to obtain all-round vision without the requirement of these invasive maneuvers, thereby reducing morbidity and operative time. In contrast, the microscope has a straight-line view, which can be limited when encountering variations of the EAC, such as a tortuous, stenotic ear canal and bony overhangs. Therefore, surgeons may need to drill out or curette bony overhangs during canaloplasty and canal wall curettage for complete visualization and assessment of the TM and the status of ossicles.
Endoscope-assisted surgery provides a wide field of view with magnified images, uses a smaller surgical incision, and preserves more tissue. In addition, endoscopes with different angles enable ‘‘around the corner’’ visualization of hidden areas and middle ear recesses. However, the endoscope lacks binocular vision (i.e. lost depth perception) and requires training besides being a one-handed technique, and therefore it is difficult with limited value in case of excessive bleeding in which the blood soils the tip of the endoscope obscuring the surgical field. Moreover, neck strain and backache related to direct vision through the endoscope and arm fatigue due to the weight of the scope and its camera may be demerits that can be overcome by developing a stand for the endoscope.
Comparable effects concerning graft uptake rate and hearing improvement results
With regard to the graft uptake, no significant difference was found between ET and MT. Similar results were also reported in previous meta-analytic studies of Tseng et al. [34], Lee et al. [35] and Pap et al. [36]. In this meta-analysis, we selected included studies that used similar operative techniques such as grafting material (temporalis fascia or perichondrium) and the graft placement method by underlay technique to obtain more accurate results about graft uptake rate.
Audiological results resembled graft uptake outcomes. Not unexpectedly, ABGs improvement demonstrated no significant difference between ET and MT, despite discrepancies in hearing evaluations. Remarkable TM closure rates between ET and MT may explain comparable audiological outcomes. However, potential publication bias with highly significant heterogeneity may have negatively impacted the integrity of this analysis. Tseng et al. [34], Lee et al. [35] and Pap et al. [36] reported similar analytic results.
Advantages of ET over MT
In agreement with Lee et al. [35] and Pap et al. [36], another significant advantage regarding ET is that the operative time for ET was significantly shorter than for MT. The surgeon's experience and the learning curve generally have an impact on the operative time. However, MT consumes more time due to frequent manipulation of the patient’s head or repeated microscope adjustment for a better view, using the post-auricular approach, or performing canaloplasty and curettage. According to Hsu et al. the relatively short time required for surgery and under anaesthesia results in significantly fewer medical resources expended on ET and decreased complications from prolonged exposure to anaesthesia [37]. In our meta-analysis, the analysis for operative time data suffers from significant heterogeneity and publication bias.
Characteristically, ET is advantageous concerning safety, minimal invasiveness and the rate of complications. Because of a wide field of view with magnification, ear surgeons have obtained minimally invasive endoscope-assisted tympanoplasty accompanied by minimal complications. In our meta-analysis, we focused particularly on the following complications: the infection (wet ear), wound gap, visible scar, nausea and vomiting. No significant difference was found between both techniques with regard to infection and wound gap, but there was a highly significant decrease in the rates of visible scar, nausea and vomiting as well as the overall complications rate in favour of ET. Postoperatively, the wet ear results from a severe middle ear infection [37] and the wound gap following suture removal from early loose stitches [27] rather than the procedure itself. In the present study, a meta-analysis of cosmetic results through the presence or absence of a visible scar revealed that the endoscope was definitely preferred over the microscope. For ET, the transcanal approach to the middle ear and smaller incision with minimum tissue dissection for harvesting a graft lead to early wound healing and less scarring on the graft donor site [16, 18, 26]. Besides, avoiding the post-auricular route reduces the incidence of auricular displacement and asymmetry of the pinna yielding better cosmetic outcomes [12, 14, 19, 22]. Similar to meta-analytic results of visible scar, the rates of nausea and vomiting were significantly lower after ET than after MT. Nausea and vomiting are unpleasant events and are associated with patient discomfort and dissatisfaction during postoperative recovery [38]. These two adverse events require the administration of various treatment modalities and consequently can expand recovery room time, increase nursing care requests and the duration of hospital stay, and can further increase total healthcare costs [39].
In concordance with our results regarding complications, Lee et al. [35] reported that wound problems of ET were significantly lower than those of MT, but there was no significant difference between ET and MT regarding wet ear.
Strengths and limitations
Our meta-analysis possesses several strengths. Our findings were comparable to those presented in the previously published meta-analyses [34–36]. Unlike the preceding meta-analytic publications about the same topic, the present study included more randomized controlled trials and other comparative studies that the search had yielded. Aiming at a better assessment of efficacy and safety, we also added more parameters for comparison. As much as we could, we held some variable risk factors constant, such as the source of the graft tissue and the graft placement method, to reduce clinical heterogeneity.
Admittedly, our meta-analysis has a few limitations. There was a noticeable variance in the other risk factors influencing surgical outcomes (e.g. the age of patients, and the size or location of TM perforation). This variance resulted in raising concerns about clinical heterogeneity. Furthermore, publication bias with highly significant heterogeneity could limit the integrity of our analytic results regarding operative time and ABGs improvement. Nevertheless, this study provided results that may be beneficial to decision-making and outcome prediction in patients receiving ET.
Conclusion
Based on our meta-analysis, the surgical outcomes of endoscope-assisted and microscope-assisted type I tympanoplasty in terms of postoperative hearing results and the graft uptake rate were comparable. Operative time and complications rate, on the other hand, proved to be significantly reduced with endoscopy compared to microscopy. Hence, the endoscope is as efficient as the microscope in type I tympanoplasty but less invasive, fewer in complications and shorter in operative time.
Our results may be beneficial to decision-making and outcome prediction in patients receiving ET.
The current meta-analysis justifies the introduction of the endoscope to type I tympanoplasty and implies that the endoscope can be a better alternative to the conventional microscope technique. However, the potential effect of the location of TM perforation and the learning curve in surgical practice, besides other influencing factors, such as healthcare costs, intraoperative bleeding, postoperative hospital stay and the inner ear thermal damage, should be further investigated.
Author contributions
KBE: idea, data collection, statistics and presentation. MAH: idea, gathering data and revising manuscripts to include and exclude suitable ones, and revision. AMM: steps in the search for related articles, helping in the collection of data from relevant articles for statistical analysis and writing, and revising the manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
Available.
Declarations
Conflict of interest
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dündar R, Kulduk E, Soy FK, Aslan M, Hanci D, Muluk NB, et al. Endoscopic versus microscopic approach to type 1 tympanoplasty in children. Int J Pediatr Otorhinolaryngol. 2014;78:1084–1089. doi: 10.1016/j.ijporl.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Gladstone HB, Jackler RK, Varav K. Tympanic membrane wound healing: an overview. Otolaryngol Clin North Am. 1995;28:913–932. doi: 10.1016/S0030-6665(20)30467-9. [DOI] [PubMed] [Google Scholar]
- 3.Ringenberg JC. Closure of tympanic membrane perforations by the use of fat. Laryngoscope. 1978;88:982–993. doi: 10.1288/00005537-197806000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Thomassin JM, Duchon-Doris JM, Emram B, Rud C, Conciatori J, Vilcoq P. Endoscopic ear surgery Initial evaluation. Ann Oto-Laryngol Chir Cerv Fac Bull Soc Oto-Laryngol Hopitaux Paris. 1990;107:564–570. [PubMed] [Google Scholar]
- 5.Pollak N. Principles of endoscopic ear surgery. In: Pollak N, editor. Endoscopic ear surgery. San Diego, CA: Plural Publishing; 2014. pp. 1–17. [Google Scholar]
- 6.Marchioni D, Alicandri-Ciufelli M, Piccinini A, Genovese E, Presutti L. Inferior retrotympanum revisited: an endoscopic anatomic study. Laryngoscope. 2010;120:1880–1886. doi: 10.1002/lary.20995. [DOI] [PubMed] [Google Scholar]
- 7.Doğan S, Bayraktar C. Endoscopic tympanoplasty: learning curve for a surgeon already trained in microscopic tympanoplasty. Eur Arch Otorhinolaryngol. 2017;274:1853–1858. doi: 10.1007/s00405-016-4428-0. [DOI] [PubMed] [Google Scholar]
- 8.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., editors. Cochrane handbook for systematic reviews of interventions. 2. Hoboken, NJ: Wiley-Blackwell; 2020. [Google Scholar]
- 9.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Review Manager (RevMan) [Computer program]. Version 5.3 (2014) The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen
- 11.Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002–d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 12.Harugop AS, Mudhol RS, Godhi RA. A comparative study of endoscope assisted myringoplasty and micrsoscope assisted myringoplasty. Indian J Otolaryngol Head Neck Surg. 2008;60:298–302. doi: 10.1007/s12070-008-0099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lade H, Choudhary SR, Vashishth A. Endoscopic vs microscopic myringoplasty: a different perspective. Eur Arch Otorhinolaryngol. 2014;271:1897–1902. doi: 10.1007/s00405-013-2673-z. [DOI] [PubMed] [Google Scholar]
- 14.Kumar M, Kanaujia S, Singh A. A comparative study of endoscopic myringoplasty vs conventional myringoplasty. Int J Otorhinolaryngol Clin. 2015;7:132–137. doi: 10.5005/jp-journals-10003-1209. [DOI] [Google Scholar]
- 15.Nassif N, Berlucchi M, de Zinis LOR. Tympanic membrane perforation in children: endoscopic type I tympanoplasty, a newly technique, is it worthwhile? Int J Pediatr Otorhinolaryngol. 2015;79:1860–1864. doi: 10.1016/j.ijporl.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 16.Patel J, Aiyer RG, Gajjar Y, Gupta R, Raval J, Suthar PP. Endoscopic tympanoplasty vs microscopic tympanoplasty in tubotympanic csom: a comparative study of 44 cases. Int J Res Med Sci. 2015;3:1953–1957. doi: 10.18203/2320-6012.ijrms20150307. [DOI] [Google Scholar]
- 17.Ahmed A, Alam S, Hashmi SF, Hasan SA. A prospective study comparing the results of endoscope assisted versus microscope assisted myringoplasty. Glob J Otolaryngol. 2016;1:66–71. [Google Scholar]
- 18.Gadag RP, Godse A, Narasaiah MD, shetty N, Salian PL, Comparative study of outcomes of microscopic versus endoscopic myringoplasty. Med Innov. 2016;5:3–6. [Google Scholar]
- 19.Gaur RS, Tejavath P, Chandel S. Comparative study of microscopic-assisted and endoscopic-assisted myringoplasty. Indian J Otol. 2016;22:177–182. doi: 10.4103/0971-7749.187976. [DOI] [Google Scholar]
- 20.Huang T-Y, Ho K-Y, Wang L-F, Chien C-Y, Wang H-M. A comparative study of endoscopic and microscopic approach type 1 tympanoplasty for simple chronic otitis media. J Int Adv Otol. 2016;12:28–31. doi: 10.5152/iao.2015.1011. [DOI] [PubMed] [Google Scholar]
- 21.Kumar D, Varunkumarthakur SSP. A comparative study of endoscopic and microscopic approach tympanoplasty for simple chronic otitis media. IOSR J Dent Med Sci. 2016;15:101–104. [Google Scholar]
- 22.Lakpathi G, Reddy LS, Anand Comparative study of endoscope assisted myringoplasty and microscopic myringoplasty. Indian J Otolaryngol Head Neck Surg. 2016;68:185–190. doi: 10.1007/s12070-016-0970-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanji RR, Channegowda C, Patil SB. Retrospective evaluation of the surgical result of tympanoplasty for inactive chronic otitis media and comparison of endoscopic versus microscopic tympanoplasty. Indian J Otol. 2016;22:171–176. doi: 10.4103/0971-7749.187973. [DOI] [Google Scholar]
- 24.Shoeb M, Gite V, Bhargava S, Mhashal S. Comparison of surgical outcomes of tympanoplasty assisted by conventional microscopic method and endoscopic method. Int J Otorhinolaryngol Head Neck Surg. 2016;2:184–188. doi: 10.18203/issn.2454-5929.ijohns20163166. [DOI] [Google Scholar]
- 25.Choi N, Noh Y, Park W, Lee JJ, Yook S, Choi JE, et al. Comparison of endoscopic tympanoplasty to microscopic tympanoplasty. Clin Exp Otorhinolaryngol. 2017;10:44–49. doi: 10.21053/ceo.2016.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jyothi AC, Shrikrishna BH, Kulkarni NH, Kumar A. Endoscopic myringoplasty versus microscopic myringoplasty in tubotympanic CSOM: a comparative study of 120 cases. Indian J Otolaryngol Head Neck Surg. 2017;69:357–362. doi: 10.1007/s12070-017-1147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuo C, Wu H. Comparison of endoscopic and microscopic tympanoplasty. Eur Arch Otorhinolaryngol. 2017;274:2727–2732. doi: 10.1007/s00405-017-4570-3. [DOI] [PubMed] [Google Scholar]
- 28.Sinha M, Hirani N, Khilnani AK. Comparison of endoscopic underlay and microscopic underlay tympanoplasty: a prospective research at a tertiary care centre in Gujarat. Int J Otorhinolaryngol Head Neck Surg. 2017;3:874–877. doi: 10.18203/issn.2454-5929.ijohns20173137. [DOI] [Google Scholar]
- 29.Khaliq BA, Altaf S, Dar NH. A comparative study of endoscopic myringoplasty v/s conventional microscopic myringoplasty—our experience. Int J Sci Res. 2018;7:938–942. [Google Scholar]
- 30.Maran RK, Jain AK, Haripriya GR, Jain S. Microscopic versus endoscopic myringoplasty: a comparative study. Indian J Otolaryngol Head Neck Surg. 2018;71:1287–1291. doi: 10.1007/s12070-018-1341-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saggu S, Kuchhal V, Rawat A. A comparative study to compare the outcomes of myringoplasty (endoscopic versus microscopic) Ann Int Med Dent Res. 2018;4:16–20. doi: 10.21276/aimdr.2018.4.4.EN4. [DOI] [Google Scholar]
- 32.Ohki M, Kikuchi S, Tanaka S. Endoscopic type 1 tympanoplasty in chronic otitis media: comparative study with a postauricular microscopic approach. Otolaryngol Neck Surg. 2019;161:315–323. doi: 10.1177/0194599819838778. [DOI] [PubMed] [Google Scholar]
- 33.Sundararajan VS, Prabhakar Rao YS, Stephenson BR. A comparative study of microscopic myringoplasty and endoscopic myringoplasty in patients with mucosal type of chronic otitis media. Indian J Otol. 2019;25:81–84. doi: 10.4103/indianjotol.INDIANJOTOL_67_18. [DOI] [Google Scholar]
- 34.Tseng C-C, Lai M-T, Wu C-C, Yuan S-P, Ding Y-F. Comparison of the efficacy of endoscopic tympanoplasty and microscopic tympanoplasty: a systematic review and meta-analysis. Laryngoscope. 2017;127:1890–1896. doi: 10.1002/lary.26379. [DOI] [PubMed] [Google Scholar]
- 35.Lee S-Y, Lee DY, Seo Y, Kim YH. Can endoscopic tympanoplasty be a good alternative to microscopic tympanoplasty? A systematic review and meta-analysis. Clin Exp Otorhinolaryngol. 2019;12:145–155. doi: 10.21053/ceo.2018.01277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pap I, Tóth I, Gede N, Hegyi P, Szakács Z, Koukkoullis A, et al. Endoscopic type I tympanoplasty is as effective as microscopic type I tympanoplasty but less invasive—a meta-analysis. Clin Otolaryngol. 2019;44:942–953. doi: 10.1111/coa.13407. [DOI] [PubMed] [Google Scholar]
- 37.Hsu Y-C, Kuo C-L, Huang T-C. A retrospective comparative study of endoscopic and microscopic tympanoplasty. J Otolaryngol Head Neck Surg. 2018;47:44. doi: 10.1186/s40463-018-0289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Apfel CC, Roewer N. Postoperative nausea and vomiting. Anaesthesist. 2004;53:377–392. doi: 10.1007/s00101-004-0662-8. [DOI] [PubMed] [Google Scholar]
- 39.Eidi M, Kolahdouzan K, Hosseinzadeh H, Tabaqi R. A comparison of preoperative ondansetron and dexamethasone in the prevention of post-tympanoplasty nausea and vomiting. Iran J Med Sci. 2012;37:166–172. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available.