Summary
The skin is a common site of infection by Staphylococcus aureus, but the host-pathogen interactions that control pathogen growth and invasion remain poorly understood. We found a differential requirement for quorum-sensing Agr virulence and specifically PSMα peptides for pathogen growth and induction of inflammation in the skin. In neutrophil-deficient mice, S. aureus growth on the epidermis was unaffected, but the pathogen penetrated into the dermis and subcutaneous tissue which required PSMα. In the dermis, pathogen expansion required Agr virulence in wild-type, but not neutrophil-deficient mice. Mechanistically, Agr virulence limited oxidative and non-oxidative killing in neutrophils by inhibiting pathogen late endosome localization and promoting phagosome escape into the cytosol. Unlike Agr, SaeR/S virulence was not required for pathogen growth in the epidermis, and promoted dermal pathogen growth independently of neutrophils. Thus, S. aureus growth and invasion are regulated distinctly by different virulence systems in the skin. Agr limits intracellular pathogen killing in neutrophils to promote pathogen expansion in the dermis and subcutaneous tissue.
Keywords: Agr virulence, neutrophils, phenol-soluble modulins, SaeR/S virulence, Staphylococcus aureus, skin infection, skin inflammation
Introduction
Staphylococcus aureus, a Gram-positive bacterium, is a major human pathogen causing significant morbidity and mortality in both community and hospital-acquired infections (Lowy, 1998). The skin is a major infection site for S. aureus that can colonize the epidermis of about 10% of healthy individuals (Lowy, 1998). S. aureus can produce several virulence factors which can help the pathogen to breach the epidermal barrier and invade the dermis and deeper tissues thereby causing a wide range of systemic infections (Balasubramanian et al., 2017). One major S. aureus virulence program is the quorum-sensing (QS) accessory gene regulatory (Agr) system that is activated in response to bacterial density (Novick, 2003). Upon stimulation by the auto-inducing peptide (AIP), the receptor kinase AgrC activates the response regulator AgrA to induce the expression of the agrBDCA operon that induces virulence factors via the regulatory RNAIII (Boisset et al., 2007). In addition, AgrA activates the promoters of genes encoding phenol-soluble modulins (PSMs), a group of amphipathic α-helical peptides that include PSMα, PSMβ and δ-toxin (Queck et al., 2008). The four PSMα1-4 peptides are highly cytotoxic to a wide variety of cells including keratinocytes and phagocytes (Nakagawa et al., 2017; Nakamura et al., 2013; Wang et al., 2007). In the epidermis, Agr-regulated PSMα induces keratinocyte damage leading to the release of the alarmins IL-36 and IL-1α that triggers skin inflammation (Liu et al., 2017; Nakagawa et al., 2017). Agr is involved in the development of S. aureus infection in several tissues (Abdelnour et al., 1993; Gillaspy et al., 1995; Heyer et al., 2002) and Agr and PSMs are particularly crucial for the development of subcutaneous infection, epidermal S. aureus colonization and induction of skin inflammation (Cheung et al., 2011; Kobayashi et al., 2011; Li et al., 2016; Nakagawa et al., 2017; Nakamura et al., 2020; Wang et al., 2007). However, the host immune mechanisms that limit pathogen invasion into the dermis remain unclear. Furthermore, the pathogen-host interactions that regulate S. aureus expansion in the dermis remain poorly understood.
Neutrophils play a critical role in the initial phase of host defense against bacterial pathogens. The importance of neutrophils in limiting S. aureus infection is highlighted by the observation that patients with neutropenia or genetic defects that impair pathogen killing are highly susceptible to infections (Curnutte et al., 1974; Hill et al., 1974; Howard et al., 1977; Introne et al., 1999). Upon infection with S. aureus, neutrophils are rapidly recruited to the site of infection where they engulf and kill the internalized pathogen via several mechanisms. These include the production of reactive oxygen species (ROS) through the assembly of the phagocyte nicotinamide adenine dinucleotide phosphate (NADP+) oxidase complex, and the delivery of a plethora of bactericidal molecules inside the phagosome (Ley et al., 2018). To counter these immune protective mechanisms, S. aureus has evolved multiple and often redundant strategies to evade pathogen killing inside the phagosome including production of molecules that counter ROS and modifications of the cell wall that increase the resistance of the pathogen to antimicrobial molecules (Beavers and Skaar, 2016; Foster et al., 2014). Phagosomal escape is another strategy by which S. aureus can limit intracellular killing (Horn et al., 2018). Several pathogen factors have been suggested to play a critical role in inducing the translocation of S. aureus from the phagosome to the cytosol (Horn et al., 2018). Of these factors, Agr-induced PSMα peptides have been identified as critical mediators of S. aureus phagosomal escape in both epithelial cells and phagocytes in vitro (Blattner et al., 2016; Grosz et al., 2014; Munzenmayer et al., 2016; Surewaard et al., 2013). However, the host cells targeted by Agr virulence to promote pathogen growth in vivo remain unclear. In the current work, we show specific interaction of Agr virulence with neutrophils regulates pathogen expansion and inflammation in the skin. Furthermore, we show a different function of Agr virulence in the epidermis and the dermis during S. aureus infection.
Results
Differential role of neutrophils in epidermal and intradermal S. aureus infection.
To assess the role of neutrophils in epidermal infection, we compared the epicutaneous and intradermal models of S. aureus colonization using S. aureus (strain LAC, pulsed-field type USA300). In the intradermal model, 106 colony-forming units (cfu) of S. aureus were inoculated into the dermis and lesion size, neutrophil infiltration and pathogen loads were assessed overtime after infection. We observed a peak in lesion size, skin inflammation and neutrophil infiltration on day 2 which correlated with pathogen loads (Figures 1A-1D). In the epicutaneous model, 106 cfu of S. aureus are applied to the surface of the epidermis with a gauze to enhance colonization, but without physical disruption of the epidermis using a protocol that induces Agr virulence (Nakamura et al., 2013). In this gauze-supported skin colonization model, we found that disease score, pathogen loads and neutrophil infiltration increased overtime when assessed on day 0, 2, 4 and 7 after epidermal colonization (Figures 1E-1H). To examine the role of neutrophils, WT mice were treated with anti-Ly6G monoclonal antibody (Mab) to deplete neutrophils or isotype-matched control Mab. Administration of anti-Ly6G Mab depleted ~80% of CD11b+Gr1+ neutrophils in the skin of infected mice when compared with mice treated with control antibody (Figure S1A). As expected, depletion of neutrophils was associated with an increase in the size of the skin lesions after intradermal inoculation which correlated with increased pathogen loads (Figures 1I-1K). In contrast, we found that in the epicutaneous colonization model, depletion of neutrophils was associated with reduced skin inflammation and disease scores, but similar pathogen loads when compared with that observed in mice treated with control antibody (Figures 1L-1N).
Figure 1. Differential role of neutrophils in epidermal and intradermal S. aureus infection.
A-H, C57BL/6 mice were intradermally (A-D) and epicutaneously (E-H) inoculated with S. aureus. Representative macroscopic images of mouse skin and hematoxylin and eosin (HE)-stained skin sections on day 7 after colonization (n=3 to 5 mice per group) (A and E). Skin lesion size (B), numbers of S. aureus colony forming unit (cfu) (C and G), numbers of neutrophils (D and H) and skin disease scores (F) in the skin of WT mice at indicated time point after infection. I-N, Mice treated with control Mab and anti-Ly6G Mab were intradermally (I-K) and epicutaneously (L-N) inoculated with S. aureus. Representative macroscopic images of mouse skin and HE-stained skin sections on day 7 after colonization (n=5 to 7 mice per group) (I and L). Skin lesion size at indicated time point (J). Number of S. aureus cfu (K and N) and skin disease score (M) in the skin of mice 7 days after infection. Data are presented as mean ± SEM (B, C, D, F, G, H, J and K). Each dot represents a mouse (B, C, D, F, G, H, K, M and N). Skin lesions are highlighted by dotted red circles (A and I). Data are representative of at least two independent experiments. Scale bars, 100 μm. n.s.; not significant, *P<0.05; **P<0.01 versus control Mab or day 0, by unpaired two-tailed Mann-Whitney U test (K, M and N), two-tailed Student’s t test (J), one-way ANOVA test with Bonferroni’s correction (F and G) or Kruskal-Wallis test (B, C, D and H). See also Figure S1.
We next assessed the localization of S. aureus after epicutaneous and intradermal inoculation using immunohistochemical analysis. As expected, the bacterium was detected in the dermis in close association with neutrophils after intradermal infection (Figure S1B). In contrast, S. aureus localized to the epidermal surface after epicutaneous colonization (Figure S1B). We reasoned that antibiotics will be unable to kill S. aureus in the epicutaneous model given its superficial localization. Intraperitoneal administration of vancomycin abrogated S aureus colonization and pathogen-induced inflammation in the intradermal model (Figures S1C-S1E), but had no effect in either disease score or S. aureus loads in the epicutaneous model (Figures S1F-S1H). The nicotinamide adenine dinucleotide phosphate (NADPH) oxidase is critical for phagosomal bacteria killing via oxidative burst in neutrophils (Soehnlein and Lindbom, 2010). Deficiency in Cybb/Nox2, an essential component of the multi-unit NADPH oxidase complex, impaired S. aureus clearance and enhanced lesion size after intradermal inoculation (Figures S1I-S1L). In contrast, Cybb−/− mice and WT mice exhibited comparable pathogen loads and disease scores after epicutaneous inoculation (Figures S1M-S1P). These results indicate that neutrophils play a differential role in the epicutaneous and intradermal S. aureus colonization models.
Neutrophils are required to prevent S. aureus invasion into the dermis.
Administration of anti-Ly6G Mab induces ~ 80% depletion of neutrophils (Figure S1A). We hypothesized that residual neutrophils in mice treated with anti-Ly6G Mab may be sufficient to control pathogen infection in the presence of the epidermal barrier. To test this hypothesis, we generated mice lacking neutrophils by deleting Mcl1, an antiapoptotic Bcl-2 member that is required for neutrophil survival (Dzhagalov et al., 2007). To generate neutrophil-deficient mice, we crossed Mcl1 floxed mice (Mcl1f/f) with Mrp8Cre/+ mice that drive Cre expression specifically in neutrophils (Csepregi et al., 2018). Flow cytometric analysis revealed that CD11b+Ly6G+ neutrophils were absent in the bone marrow, blood and spleen of Mrp8Cre/+Mcl1f/f mice (Figures S2A and S2B). Furthermore, epicutaneous inoculation with S. aureus induced robust recruitment of neutrophils in the skin of Mcl1f/f mice, but minimal or no neutrophil infiltration was observed in Mrp8Cre/+Mcl1f/f mice while the numbers of monocytes and macrophages in the skin were comparable in both groups of mice (Figure S2C). Neutrophil-deficient Mrp8Cre/+Mcl1f/f mice inoculated intradermally with S. aureus developed large ulcerated skin lesions associated with abundant numbers of bacteria below the epidermis and a marked increase in pathogen loads when compared with neutrophil-sufficient Mcl1f/f mice (Figures 2A-2C). While S. aureus was only detected on the surface of the epidermis in Mcl1f/f mice after epicutaneous inoculation, Mrp8Cre/+Mcl1f/f mice showed marked invasion of S. aureus into the dermis and underlying soft tissue (Figure 2D and S2D). Although the skin lesions were smaller in Mrp8Cre/+Mcl1f/f mice than in Mcl1f/f mice, they contained numerous erosions in the epidermis (Figure 2E). The total pathogen loads in the skin tissue were comparable in Mrp8Cre/+Mcl1f/f and Mcl1f/f mice (Figure 2F). Consistent with local penetration of S. aureus into the dermis and underlying soft tissue, we detected higher pathogen numbers in the spleen, kidney and liver of Mrp8Cre/+Mcl1f/f mice than in Mcl1f/f mice after epicutaneous colonization (Figure S2D). We also compared in the same experiments the phenotype of Mrp8Cre/+Mcl1f/f neutrophil-deficient and Cybb−/− mice after S. aureus dermal inoculation. Both mutant mice showed larger skin lesion and increased pathogen loads compared to WT mice, but neutrophil-deficient mice exhibited larger lesions with more pathogen loads than Cybb−/− mice (Figures S2E-S2G). These results indicate that neutrophils are required to prevent invasion of S. aureus into the dermis and systemic tissues after epidermal colonization. Furthermore, the studies suggest that neutrophils control pathogen growth through oxidative and non-oxidative killing in the dermis.
Figure 2. Neutrophils are required to prevent S. aureus invasion into the dermis.
A-F, Mcl1f/f and Mrp8Cre/+Mcl1f/f mice were intradermally (A-C) and epicutaneously (D-F) inoculated with S. aureus. Representative macroscopic images of mouse skin, immunohistochemical sections and HE-stained skin sections on day 7 after infection (n=7 to 8 mice per group (A) and n=13 to 14 mice per group (D)). Skin lesion size at indicated time point (B). The number of S. aureus cfu (C and F) and skin disease scores (E) in the skin of Mcl1f/f and Mrp8Cre/+Mcl1f/f mice 7 days after infection. Data are presented as mean ± SEM (B and C). Each dot represents a mouse (C, E and F). Skin lesions are highlighted by dotted red circles. Black arrowheads show bacteria in HE sections (purple areas), white arrowheads show immune-labeled bacteria. Data represent combined results from at least two independent experiments. Scale bars, 100 μm. n.s.; not significant, *P<0.05; **P<0.01 versus Mcl1f/f mice, by unpaired two-tailed Mann-Whitney U test (B, E and F) or two-tailed Student’s t test (C). See also Figure S2.
Agr-regulated PSMα peptides are required for pathogen dermal invasion in the absence of neutrophils.
The QS Agr system regulates the production of a wide array of virulence factors including PSMs that are important for pathogen growth in animal tissues (Novick, 2003; Le et al. 2015) We next asked whether Agr virulence is required for S. aureus invasion into the dermis and soft tissue in the absence of neutrophils. In these experiments, we inoculated Mcl1f/f and Mrp8Cre/+Mcl1f/f mice epicutaneously with WT or isogenic S. aureus strains deficient in Agr virulence or the production of Agr-regulated PSMα or PSMβ peptides. In neutrophil-sufficient mice, the agr and psmα deficient strains, but not the psmβ mutant strain were impaired in inducing inflammatory skin disease which correlated with reduced S. aureus colonization after epicutaneous inoculation (Figures 3A-3C). While the WT bacterium and the psmβ mutant invaded the dermis and underlying soft tissue in Mrp8Cre/+Mcl1f/f neutrophil-deficient mice, the agr and psmα deficient strains did not (Figures 3D-3F). These results indicate that Agr virulence and specifically PSMα peptides are required for S. aureus invasion into the dermis and soft tissue in neutrophil-deficient mice colonized epicutaneously with the pathogen.
Figure 3. Agr-regulated PSMα peptides are required for pathogen dermal invasion in the absence of neutrophils.
A-F, Mcl1f/f and Mrp8Cre/+Mcl1f/f mice were epicutaneously inoculated with WT or the indicated isogenic mutant S. aureus strains. Representative macroscopic images of mouse skin and HE-stained skin sections on day 7 after infection (n=5 to 6 mice per group) (A and D). Skin disease scores (B and E) and the number of S. aureus cfu (C and F) in the skin of Mcl1f/f and Mrp8Cre/+Mcl1f/f mice 7 days after infection. Each dot represents a mouse (B, C, E and F). Arrowheads show bacteria. Thickened epidermis is outlined by dotted line. Data are representative of at least two independent experiments. n.s.; not significant, *P<0.05; **P<0.01, by Kruskal-Wallis test (B, C, E and F).
Agr virulence is not required for intradermal S. aureus growth in the absence of neutrophils.
We next assessed the role of Agr virulence in intradermal S. aureus infection in the presence or absence of neutrophils. We inoculated Mcl1f/f and Mrp8Cre/+Mcl1f/f mice intradermally with WT and isogenic S. aureus agr mutant strains and assessed the ability of bacteria to colonize the skin and underlying soft tissue and induce skin disease. In neutrophil-sufficient Mcl1f/f mice, the agr and psmα deficient strains, but not the psmβ mutant were impaired in inducing skin disease which correlated with a marked reduction in pathogen colonization (Figures 4A-4C). In contrast, the agr and psmα deficient strains colonized the dermis and subcutaneous soft tissue and elicited skin lesions to levels that were comparable to those observed with the WT bacterium in neutrophil-deficient Mrp8Cre+Mcl1f/f mice (Figures 4D-4F and S3). These results indicate that Agr virulence is required for S. aureus growth and skin pathology after intradermal inoculation in the presence, but not in the absence of neutrophils.
Figure 4. PSMα peptides are not required for intradermal S. aureus growth in the absence of neutrophils.
A-F, WT, Mcl1f/f and Mrp8Cre/+Mcl1f/f mice were intradermally inoculated with WT or the indicated isogenic mutant S. aureus strains. Representative macroscopic images and HE-stained skin sections on day 7 after infection (n=4 to 6 mice per group) (A and D). Skin lesion size at indicated time point (B and E). The number of S. aureus cfu in the skin of mice 7 days after infection (C and F). Data are presented as mean ± SEM (B, C, E and F). Skin lesions are highlighted by dotted red circles. Arrowheads show bacteria. Data represent combined results from at least two independent experiments. Scale bars, 100 μm. n.s.; not significant, *P<0.05; **P<0.01 versus, by Kruskal-Wallis test (B, C, E and F). See also Figure S3.
Contrasting roles of Agr and SaeR/S virulence in pathogen colonization and skin inflammation.
S. aureus also uses the SaeR/S regulatory system to produce a variety of virulence factors that are important for immune evasion and pathogen growth in host tissues (Guerra et al., 2017). SaeR/S does not impact on psm expression, but is required for expression of many toxins and immune modulatory proteins import for pathogen growth (Munzenmayer et al., 2016). We next assessed the ability of SaeR/S virulence to promote S. aureus skin colonization in the presence and absence of neutrophils. In WT mice, the saeR/S mutant strain elicited reduced skin disease after epidermal colonization, but pathogen colonization was comparable to that observed with the WT bacterium (Figures 5A-5C). The reduced inflammation observed with the saeR/S mutant correlated with impaired recruitment of neutrophils to the skin (Figure S4A). In contrast to the agr mutant, the S. aureus strain lacking saeR/S virulence retained its ability to invade into the dermis and soft tissue after epidermal colonization in neutrophil-deficient Mrp8Cre/+Mcl1f/f mice (Figures 5A-5C). In the intradermal model, the saeR/S-deficient strain was impaired in eliciting skin disease and neutrophil infiltration which was associated with markedly reduced pathogen colonization in WT mice (Figures 5D-5F and Figure S4B). In contrast to the agr mutant, the saeR/S-deficient strain was greatly impaired in inducing skin disease after intradermal inoculation in Mrp8Cre/+Mcl1f/f neutrophil-deficient mice (Figures 5D-5F). Furthermore, unlike the agr mutant, the ability of the S. aureus saeR/S mutant to colonize the dermis and subcutaneous tissue was also reduced impaired in Mrp8Cre/+Mcl1f/f mice (Figures 5D-5F). These results indicate that SaeR/S virulence is not required for dermal invasion after epicutaneous inoculation in the absence of neutrophils. Furthermore, SaeR/S-regulated virulence is critical for intradermal pathogen growth and skin disease both in the presence and absence of neutrophils.
Figure 5. SaeR/S virulence is critical for intradermal S. aureus growth in the absence of neutrophils.
A-F, Mcl1f/f and Mrp8Cre/+Mcl1f/f mice were epicutaneously (A-C) and intradermally (D-F) inoculated with WT or the isogenic saeR/S mutant S. aureus strain. Representative macroscopic images and HE-stained skin sections on day 7 after infection (n=4 to 6 mice per group) (A and D). Skin disease scores (B) and the number of S. aureus cfu (C and F) in the skin of Mcl1f/f and Mrp8Cre/+Mcl1f/f mice 7 days after infection. Skin lesion size at indicated time point (E). Each dot represents a mouse (B, C and F). Data are presented as mean ± SEM (E and F). Skin lesions are highlighted by dotted red circles (D). Black arrowheads show bacteria. Data represent combined results from at least two independent experiments. Scale bars, 100 μm. n.s.; not significant, *P<0.05; **P<0.01, by Kruskal-Wallis test (B, C, E and F). See also Figure S4.
Agr virulence limits neutrophil phagosomal killing in neutrophils.
The finding that Agr virulence is not required for pathogen growth in the absence of neutrophils suggested that a main function of Agr is to evade pathogen killing by neutrophils. Quantitative RT-PCR analysis revealed that expression of psmα was increased in a time-dependent manner in neutrophils after S. aureus infection (Figure 6A). This is in contrast to saeR and saeS expressions that declined in neutrophils after infection (Figure 6A). The expression of Agr virulence in vitro was consistent with the observation that psmα was detected in vivo after intradermal inoculation of a psmα-lux S. aureus reporter strain in WT mice, but much less in neutrophil-deficient mice (Figure 6B). The reduced expression of psmα in neutrophil-deficient mice was verified in dermal tissue using quantitative RT-PCR analysis (Figure 6C). These results suggest that expression of S. aureus PSMα is positively regulated in neutrophils in vivo. To assess the function of Agr virulence in neutrophils, we incubated primary mouse neutrophils with WT and agr mutant S. aureus strains at a moi of 5 for 15 min, removed extracellular bacteria by extensive washing and assessed the number of intracellular and extracellular bacteria after pathogen uptake. We found that the uptake of the WT and mutant S. aureus strains by neutrophils was comparable when assessed 20 min after incubation (Figure S5A). In contrast, the numbers of intracellular and extracellular agr and psmα mutant, but not psmβ-deficient, bacteria were reduced compared to the WT bacterium when assessed 80 min after infection (Figure 6D). In contrast, the numbers of intracellular and extracellular seaR/S mutant bacteria were comparable to those observed with the WT bacterium when assessed 80 min after incubation with neutrophils (Figure S5B). To determine whether Agr virulence regulates the localization of S. aureus inside neutrophils, we incubated primary neutrophil preparations with yellow fluorescence protein (YFP)-labeled WT and agr mutant strains and assessed their intracellular localization by fluorescence microscopy. Because the neutrophil preparations contain ~ 80% neutrophils, we incubated the cells with APC-labeled anti-Ly6G antibody to identify neutrophils in these preparations. At 20 min post incubation, the number of bacteria inside neutrophils and the percentage of neutrophils in which the intracellular pathogen co-localized with LAMP-1, a marker of late-endosomes and lysosomes, were comparable in neutrophils incubated with the WT and the agr mutant strains (Figure S5C). Likewise, the numbers of WT and agr mutant strain co-localizing with Rab5, a marker of early endosomes was comparable 20 min after incubation (Figure S5D). After 80 min incubation, the percentage of neutrophils containing high number (greater than 10) bacteria of the WT strain was higher than of the agr mutant strain (Figure 6E). Conversely, the percentage of neutrophils harboring low number (5 or less) of the agr mutant strain was higher than that of the WT strain (Figure 6E). Notably, the percentage of neutrophils in which the pathogen co-localized with LAMP-1 was higher in the agr mutant than in the WT without an increase in neutrophil cytotoxicity (Figure 6E, right panel, and S5E). These results suggest that Agr virulence limits the localization of S. aureus in late endosome promoting phagosome escape into the cytosol and extracellular space.
Figure 6. Agr virulence limits the localization of S. aureus in late endosomes and promotes phagosome escape into the cytosol.
A, psmα, saeR and saeS expression levels in neutrophils after incubation with WT S. aureus at a moi of 5 for 15 min, removal of extracellular bacteria (time 0 min) and further incubation for indicated times. B, Mcl1f/f and Mrp8Cre/+Mcl1f/f mice were intradermally inoculated with psmα-lux S. aureus for 2 days. Luminescence of psmα-lux (relative light units) is indicated on the right panel. (n=4 mice per group). C, psmα expression in the skin of Mcl1f/f and Mrp8Cre/+Mcl1f/f mice intradermally inoculated with WT S. aureus. D, The numbers of S. aureus in intracellular and extracellular compartments of WT neutrophils cultured with WT and the indicated mutant S. aureus strains (moi =5) for 15 min, washed and then incubated in the presence of recombinant TNF-α for 1 hour to induce oxidative burst (total 80 min). E, Representative microscopic images of WT neutrophils cultured with YFP-WT and YFP-agr mutant S. aureus (moi=5) for 15 min and then incubated in the presence of recombinant TNF-α for 1 h (total 80 min). Neutrophils with YFP-S. aureus (green) were stained with DAPI (blue) and fluorescence-labeled antibodies against Ly6G (white) and LAMP-1 (red). The percentage of neutrophils containing different numbers of WT (white bars) and agr mutant (black bars) YFP-S. aureus and the percentage of neutrophils in which bacteria co-localized with LAMP-1 inside neutrophils are indicated on the right panel. White arrowheads show neutrophils in which intracellular bacteria co-localize with LAMP-1. Yellow arrowheads show neutrophils with high (greater than 10) intracellular bacteria. Neutrophils cultured with YFP-WT (n=233) and YFP-agr S. aureus (n=193) were enumerated from 8 fields per group. Data are presented as mean ± SEM. Data are representative of at least two independent experiments. n.s.; not significant, *P<0.05; **P<0.01, by one-way ANOVA test with Bonferroni’s correction (A), unpaired two-tailed Mann-Whitney U test (B, C and E) or Kruskal-Wallis test (D). See also Figure S5.
Agr virulence limits oxidative and non-oxidative killing in vivo.
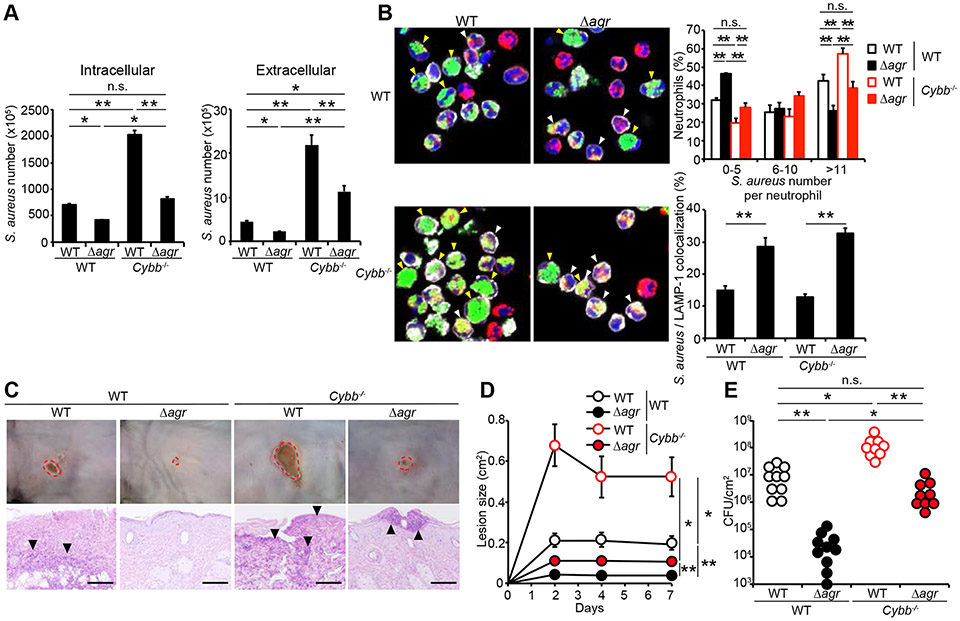
A main mechanism of pathogen killing inside the neutrophil phagosome is mediated via NADPH oxidase-dependent oxidative burst (Ley et al. 2018). To assess the role of Agr virulence in the regulation of oxidative killing, we infected WT and neutrophils deficient in Cybb/Nox2, an essential molecule of the NAPDH oxidase complex with WT and agr mutant S. aureus strains. We found that the uptake of the WT and agr mutant S. aureus strains by WT and Cybb−/− neutrophils was comparable when assessed 20 min after incubation (Figure S6A). After 80 min incubation, however, the numbers of intracellular and extracellular WT and agr mutant S. aureus were higher in Cybb−/− neutrophils than in WT neutrophils (Figure 7A). Notably, the numbers of intracellular and extracellular agr mutant bacteria in Cybb−/− neutrophils were comparable or higher than of the WT bacteria in WT neutrophils (Figure 7A). Cytotoxicity of Cybb−/− neutrophils treated with WT and agr mutant S. aureus was higher than that observed in WT neutrophils (Figure S6B). We next assessed the number and localization of WT and agr mutant S. aureus in WT and Cybb−/− neutrophils by fluorescence microscopy. After 80 min incubation, the percentage of neutrophils containing greater than 10 bacteria of the WT strain was higher than of the agr mutant strain in both WT and Cybb−/− neutrophils (Figure 7B). Conversely, the percentage of neutrophils harboring low number of bacteria (5 or less) of the WT strain was lower than of the agr mutant strain in both WT and Cybb−/− neutrophils (Figure 7B). Notably, the percentage of neutrophils harboring high number (more than 10) of agr mutant S. aureus in Cybb−/− neutrophils was comparable to that of the WT bacterium in WT neutrophils (Figure 7B). Furthermore, the percentage of neutrophils in which bacteria co-localized with LAMP-1 was higher in the agr mutant than in the WT bacterium in both WT and Cybb−/− neutrophils (Figure 7B, right lower panel).
Figure 7. Agr virulence limits oxidative and non-oxidative pathogen killing in vivo.
A, Numbers of S. aureus in intracellular and extracellular compartments of WT and Cybb−/− neutrophils cultured with WT and agr mutant S. aureus strains (moi =5) for 15 min and then incubated in the presence of recombinant TNF-α for 1 hour (total 80 min). B, Representative microscopic images of WT and Cybb−/− neutrophils cultured with YFP-WT and YFP-agr mutant S. aureus (moi =5) for 15 min, washed and further incubated in the presence of recombinant TNF-α for 1 h (total 80 min). Neutrophils with YFP-S. aureus (green) were stained with DAPI (blue) and fluorescence-labeled antibodies against Ly6G (white) and LAMP-1 (red). The percentage of neutrophils containing different numbers of WT (open bars) and agr mutant (solid bars) YFP-S. aureus and the percentage of neutrophils in which bacteria co-localized with LAMP-1 inside neutrophils are indicated on right panels. White arrowheads show neutrophils in which intracellular bacteria co-localized with LAMP-1. Yellow arrowheads show neutrophils with high (greater than 10) intracellular bacteria. The percentage of bacteria in WT neutrophils cultured with YFP-WT (n=156) and YFP-agr mutant S. aureus (n=172) and Cybb−/− neutrophils cultured with YFP-WT (n=127) and YFP-agr mutant S. aureus (n=166) were enumerated from 8 different fields per group. C-E, WT and Cybb−/− mice were intradermally inoculated with WT and agr mutant S. aureus strains. Representative macroscopic images of mouse skin and HE-stained skin sections on day 7 after colonization (n=9 to 10 mice per group) (C). Skin lesion size at indicated time point (D). The number of S. aureus cfu in the skin of WT and Cybb−/− mice 7 days after infection (E). Data are presented as mean ± SEM (A, B, D and E). Skin lesions are highlighted by dotted red circles and arrowheads show bacteria (C). Data are representative of at least two independent experiments. n.s.; not significant, *P<0.05; **P<0.01, by Kruskal-Wallis test (A, B, D and E). See also Figure S6.
We showed in Figure 4 that Agr virulence is not required for growth of S. aureus in the dermis in the absence of neutrophils suggesting that this virulence programs targets killing by neutrophils. Therefore, we assessed whether Agr virulence limits pathogen killing via the phagosomal NAPDH oxidase complex in vivo. In these experiments, we inoculated WT and Cybb−/− mice with WT and agr mutant S. aureus intradermically and assessed skin disease and pathogen loads. In accord with results shown in Figures S1I-S1K, the disease scores and pathogen loads observed after inoculation with the WT bacterium were higher in Cybb−/− mice than in WT animals (Figures 7C-7E). In accord with results shown in Figures 4A-4C, the agr mutant bacterium was impaired in triggering skin disease which was associated with markedly reduced pathogen loads in WT mice (Figures 7C-7E). Importantly, the loads of the agr mutant strain were ~ 100-fold higher in Cybb−/− mice than in WT mice which was associated with higher lesion sizes (Figure 7E). However, the loads and lesion sizes with the agr mutant strain in Cybb−/− mice were almost comparable to those observed with the WT bacterium in WT mice (Figures 7D and 7E). Together the results suggest that Agr virulence limits both oxidative and non-oxidative killing in neutrophils to expand in the dermis.
Discussion
Although most S. aureus infections originate in the skin, the pathogen-host interactions that regulate pathogen invasion from the skin surface to the dermis and underlying tissues remain poorly understood. In the current work, we show that neutrophils play a critical role in preventing the invasion of the pathogen from the epidermis to the dermis. Although QS Agr virulence was required for pathogen invasion into the dermis and for pathogen expansion in both the epidermis and the dermis, we found a differential requirement for Agr virulence in the absence of neutrophils. In the epidermis, Agr-regulated cytopathic PSMα peptides were required for pathogen growth independently of the presence of neutrophils while in the dermis Agr virulence was dispensable for pathogen expansion in the absence of neutrophils. Cytopathic PSMα peptides are important for keratinocyte damage and the subsequent release of keratinocyte alarmins to induce inflammation after epidermal colonization (Nakagawa et al., 2017). Because the surface of the epidermis is scarce in nutrients, the requirement of Agr-regulated PSMα peptides suggests that these cytopathic peptides also induce the release of nutrients or other factors from keratinocytes to sustain robust growth of S. aureus on the epidermal surface. The host molecules that enable pathogen growth in the epidermis remain to be determined.
Earlier studies showed that PSMα peptides induce robust killing of a variety of immune and non-immune cells in vitro (Nakagawa et al., 2017; Nakamura et al., 2013; Wang et al., 2007), suggesting that Agr virulence is used by the pathogen to target host cells. However, the relevance of these findings for the regulation of pathogen growth in vivo remained unclear. The lack of requirement of Agr virulence for pathogen growth and induction of skin disease after dermal inoculation in the absence of neutrophils suggests that a main function of Agr virulence in vivo is to evade killing of the pathogen by neutrophils. Our studies are consistent with previous work that identified Agr-induced PSMα peptides as critical mediators of S. aureus phagosomal escape in phagocytic cells in vitro (Blattner et al., 2016; Grosz et al., 2014; Surewaard et al., 2013), although these previous studies did not assess the relevance of these findings in vivo. We further show that the presence of Agr virulence reduced the co-localization of S. aureus with LAMP1, a marker of late endosomes, in neutrophils. Collectively, these observations suggest that membrane-rupturing PSMα peptides induced via Agr virulence limit the degradation of the pathogen in late endosomes and lysosomes by promoting its escape from the phagosome to the cytosol. We found an increased in extracellular bacteria after infection of neutrophils with WT S. aureus compared with agr mutant bacteria, suggesting that Agr virulence also promotes the release of S. aureus from the cytosol to the extracellular compartment. The release of WT S. aureus into the extracellular space mediated by Agr is likely to promote pathogen spread in vivo. Previous work with human neutrophils showed marked lysis of the cells after S. aureus infection with CA-MRSA strain MW2 which was largely dependent on Agr virulence (Surewaard et al., 2013). We did not observe increased cytotoxicity of mouse neutrophils incubated with WT S. aureus compared with the agr mutant bacterium under our in vitro conditions. The reason for the difference in results is not clear, but it could be explained by host species, pathogen strain or subtle differences in the experimental conditions. Analyses of neutrophils and mice deficient in the NAPDH oxidase complex that kills bacteria via oxidative burst in the phagosome revealed that Agr virulence limits the killing of S. aureus via both oxidative and non-oxidative mechanisms in neutrophils. Although the non-oxidative killing mechanisms are regulated by Agr virulence, neutrophils can kill bacteria through several processes including activation of myeloperoxidase, delivery of anti-microbial molecules into the phagosome and the release of neutrophil extracellular traps (Ley et al., 2018). Further studies are needed to understand the individual contribution of the non-oxidative processes and whether Agr virulence can subvert such mechanisms to promote pathogen growth in vivo.
Our studies revealed a contrasting role of Agr and SaeR/S virulence in the regulation of pathogen growth and inflammatory disease in the skin (Figure S6C). While Agr virulence was required for pathogen growth in WT mice and dermal invasion in neutrophil-deficient mice, SaeR/S was dispensable for both activities after epidermal colonization. Furthermore, although both virulence programs were required for dermal pathogen expansion in WT mice, the SaeR/S system was necessary for dermal expansion and skin disease in the absence of neutrophils while Agr virulence was not. These results suggest that a main function of Agr virulence in the dermis and subcutaneous tissue is to promote pathogen growth by subverting pathogen killing mechanisms in neutrophils while SaeR/S virulence may act by targeting different cells or processes in the host. In the absence of neutrophils and Agr virulence, these observations suggest that SaeR/S drives pathogen expansion in the dermis as this virulence program was required for S. aureus growth after dermal inoculation. The contrasting role of Agr and SaeR/S virulence in skin infection may be explained by differences in the expression and function of these virulence programs in host cells during infection. Consistent with previous studies (Surewaard et al., 2012), we found that psmα is induced after phagocytosis in neutrophils in vitro. In addition, we observed reduced expression of psmα in the skin of neutrophil-deficient mice supporting the notion that Agr virulence is induced in neutrophils in vivo. Inside host cells and presumably neutrophils the concentration of the quorum sensing pheromone AIP can reach the critical concentration necessary for agr activation (Carnes et al., 2010; Pang et al., 2010). Furthermore, S. aureus produces (p)ppGpp as a messenger of environmental stress conditions which appears important for the induction of PSMs in the neutrophil phagosome (Geiger et al., 2012). While Agr-induced PSMα peptides form α-helical structures that disrupt hydrophobic membranes without host cell specificity (Peschel and Otto, 2013), the cytolytic activity of SaeR/S-regulated pore-forming toxins including Panton-Valentine leucocidin (PVL) and the leukocidins LukSF and LukAB recognize specific receptors on host cells and display a more restricted species and host cell specificity (Cho et al., 2015; Geiger et al., 2012; Nygaard et al., 2010). For example, a major role of SaeR/S-regulated LukAB and PVL in the escape of S. aureus from human macrophages (Munzenmayer et al., 2016). Thus, SaeR/S-regulated virulence may act to subvert pathogen killing in macrophages or other host cells. However, further studies are needed to determine the mechanism by which SaeR/S virulence limits pathogen killing in vivo.
In conclusion, we show that a distinct role of Agr virulence in the colonization of the epidermis and dermis and a key role of neutrophils in preventing invasion of S. aureus into the dermis. Notably, Agr virulence was not required for pathogen expansion in the dermis in the absence of neutrophils suggesting that this virulence program targets neutrophils to limit pathogen killing via oxidative and non-oxidative killing. The function of Agr virulence is in contrast to that of the saeR/S system that is not required for epidermal colonization or pathogen invasion into the dermis and promotes pathogen expansion independently of neutrophils. Further studies are needed to understand the mechanism by which Agr promotes pathogen growth in the epidermis and limits non-oxidative killing by neutrophils.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCING SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Gabriel Nunez (gabriel.nunez@umich.edu).
EXPERIMENTAL MODELS AND SUBJECTS DETAILS
Animals
C57BL/6 mice were purchased from the Jackson Laboratory and expanded in our mouse facility at the University of Michigan. Cybb−/− mice were purchased from the Jackson Laboratory. Mrp8Cre/+Mcl1f/f mice on C57BL/6 background were kindly provided by Dr. Andres Hidalgo, Centro Nacional de Investigaciones Cardiovasculares (CNIC), Madrid. All mice were maintained under specific pathogen-free conditions and were used at 8 to 12 weeks of age. All animal studies were performed according to approved protocols by the University of Michigan Review Board for Animal Care.
METHOD DETAILS
Antibody treatment
For antibody-mediated depletion, antibodies were purchased from BioXCell. To deplete neutrophils, mice were treated with anti-Ly6G antibody (250 μg) (clone: 1A8) intraperitoneally on day −1, 1, 3 and 5 before and after S. aureus infection.
Cell isolation and flow cytometric analysis
For isolation of skin mononuclear cells, skin tissues were digested with 1.0 Wunsch units/ml Liberase DH (Roche) and 100 μg/ml DNase I (Sigma) at 37°C for 1h. The cells were resuspended on a Percoll gradient (75%/40%) (GE Healthcare) and centrifuged at 2000 rpm for 20 min at 25°C. Single-cell suspensions from skin, blood, bone marrow and spleen were stained with the following fluorochrome-conjugated antibodies purchased from BD Biosciences, Biolegend, or eBioscience: Allophycocyanin (APC)-conjugated anti-Ly6G (1A8) and anti-Ly6C (AL-21); Fixable Viability Dye eFluor 780; fluorescein isothiocyanate (FITC)-conjugated anti-CD11b (M1/70); Peridinin chlorophyll protein complex-cyanin 5.5 (PerCP5.5)-conjugated anti-CD4 (RM4-5); phycoerythrin (PE)-conjugated anti-B220 (RA3-6B2), anti-F4/80 (BM8) and anti-Gr1 (RB6-8C5); PE-Cy7-conjugated anti-CD8 (53-6.7); violet 450-conjugated anti-Ly6G (1A8). The cells were then analyzed on a LSRII flow cytometer (BD Biosciences).
Immunohistochemical analysis
Frozen skin sections were fixed with acetone, labeled with polyclonal biotin-conjugated anti-S. aureus antibody (Abcam) at 4°C overnight and then stained with FITC-conjugated streptavidin (Invitrogen). In some experiments, skin sections were stained with PE-conjugated anti-Ly6G antibody (1A8). For nuclear staining, DAPI (Invitrogen) was used. Images of stained sections were viewed with a fluorescence microscope BZ-X700 (Keyence).
S. aureus colonization
The methicillin-resistant Staphylococcus aureus strain USA300 (LAC), the isogenic agr, psma, psmβ and saeR/S mutant strains and YFP-WT and YFP-agr mutant strains have been described (Nygaard et al., 2010; Wang et al., 2007). For epicutaneous colonization, bacteria were grown for 4 hrs in tryptic soy broth with shaking at 37°C. Mice were colonized on the shaved dorsal skin of mice by applying a 1 cm2 sterile gauze containing 1 x 106 cfu of S. aureus which was covered with occlusive plastic dressing (Tegaderm; 3M). In some experiments, mice were intradermally injected with 1 x 106 efu of S. aureus. For treatment with vancomycin, vancomycin (100 mg/kg) was injected intraperitoneally 30 min before S. aureus inoculation. To determine bacterial numbers in the colonized skin, spleen, kidney and liver, the tissues were collected from individual mice, homogenized in cold PBS and plated at serial dilution onto Mannitol-salt agar containing 10% egg yolk. The number of cfu was determined after 48 h of incubation at 37°C. Mice were sacrificed on day 7 day after colonization, and skins were assessed in a blinded fashion using a scoring system described previously (Nakamura et al., 2013). Briefly, 4 points were used to denote the severity of erythema (0, none; 1, mild; 2, moderate; 3, severe), scaling (0, none; 1, mild; 2, moderate; 3, severe), erosion (0, none; 1, mild; 2, moderate; 3, severe), edema (0, none; 1, mild; 2, moderate; 3, severe), and thickness (0, none; 1, mild; 2, moderate; 3, severe). Skin samples were fixed in 10% formalin and processed for HE and Gram staining or frozen sections were obtained for immunohistochemistry.
Quantitative real-time PCR with reverse transcription
S. aureus strains were grown overnight in tryptic soy broth with shaking at 37°C. In some experiments, neutrophils cultured with the serum-treated S. aureus were treated with recombinant TNF-α (Biolegend; 1 μg/ml) for the indicated time, washed with RPMI medium 2 times and then lysed with 0.1% TritonX-100 in PBS prior to isolation of S. aureus RNA. RNA isolation of S. aureus treated with lysozyme (15 mg/ml) and lysostaphin (1 mg/ml) for 30 min at 37°C was performed with E.Z.N.A. Bacterial RNA kit according to the manufacturer’s instructions (OMEGA). The complementary DNA was synthesized using a High Capacity RNA-to-cDNA Kit (Applied Biosystems) according to the manufacturer’s instructions. Quantitative real time (RT)-PCR was performed using a TaqMan PCR master mix (Applied Biosystems) and StepOne Real-time PCR system (Applied Biosystems). Primers to amplify bacterial genes (psmα, saeR, saeS and gyrB) have been described (Dastgheyb et al., 2015; Nygaard et al., 2010). psmα, saeR and saeS expression was normalized to that of S. aureus gyrB and relative expression calculated by the 2-ΔΔCt method.
S. aureus killing assay
WT LAC and its isogenic mutant strains (109 cfu/ml) were incubated with equal amount of serum from WT mice for 15 min. Neutrophils (5 x 106) enriched from the bone marrow of WT mice by Neutrophil Isolation Kit (Miltenyi Biotec) were cultured with serum-treated S. aureus at a moi of 5 for 15 min, washed with RPMI medium and then treated with recombinant TNF-α (1 μg/ml) for 1 h. After centrifugation, the supernatant and neutrophils were diluted with 0.1% TritonX-100 in PBS and the numbers of extracellular bacteria in supernatant and intracellular bacteria in neutrophils were assessed by serial plating on MSA plates. In some experiments, neutrophils cultured with YFP-A aureus were stained with APC-conjugated anti-Ly6G antibody, fixed with 4% paraformaldehyde, treated with 0.1% saponin, incubated with rabbit anti-Rab5 antibody, stained with DAPI and Alexa Fluor 555-conjugated anti-rabbit IgG or PE-labeled anti-LAMP-1 antibody, and viewed with confocal microscope A1 (Nikon).
Cytotoxicity Assay
Cytotoxicity was measured by a lactate dehydrogenase (LDH) cytotoxicity detection kit (Takara Biochemicals). Neutrophils were cultured with S. aureus and LDH release assay was performed according to the manufacturer’s instructions.
Measurement of psmα-lux expression
For in vivo bioluminescence imaging, mice were intradermally inoculated with 1 x 106 LAC psmα-lux strain (Dastgheyb et al., 2015), killed on day 2 after infection, and immediately placed into the light-tight chamber of the CCD (charge-coupled device) camera system (IVIS200, PerkinElmer). Luminescence emitted from lux-expressing bacteria in the tissue was quantified using the software program Living Image (PerkinElmer).
Quantification and statistical analyses
Statistical analyses were performed using GraphPad Prism software version 5.0 (GraphPad Software Inc.). Differences between two groups were evaluated using Student’s t test (parametric) or Mann-Whitney U test (non-parametric). For multiple comparisons, statistical analysis was performed using one-way ANOVA (parametric) or Kruscal-Wallis test (non-parametric), and then Bonferroni test for parametric samples, or Dunn’s test for non-parametric samples as a post-hoc test. Differences at P<0.05 were considered significant.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Alexa Fluor 555 goat polyclonal anti-rabbit IgG | Abcam | Cat# ab150078 |
| APC anti-Ly6G | BioLegend | Cat# 127614; RRID: AB_2227348 |
| APC anti-Ly6C | BD Biosciences | Cat# 560595; RRID: AB_1727554 |
| Biotin rabbit polyclonal anti-S. aureus | Abcam | Cat# ab68954; |
| FITC anti-CD11b | BioLegend | Cat# 101205; RRID: AB_312788 |
| PE anti-CD107a (LAMP-1) | BioLegend | Cat# 121611; RRID: AB_1732051 |
| PE anti-B220 | BioLegend | Cat# 103208; RRID: AB_312993 |
| PE anti-F4/80 | eBioscience | Cat# 12-4801-80; RRID: AB_465923 |
| PE anti-Gr1 | BD Biosciences | Cat# 553128; RRID: AB_394644 |
| PE anti-Ly6G | BioLegend | Cat# 127608; RRID: AB_1186099 |
| PECy7 anti-CD8 | eBioscience | Cat# 25-0081-81; RRID: AB_469583 |
| PerCP5.5 anti-CD4 | BioLegend | Cat# 100540; RRID: AB_893326 |
| Rabbit monoclonal anti-Rab5 (clone EPR21801) | Abcam | Cat# ab218624 |
| Rat monoclonal anti-mouse CD16/32 (clone 93) | BioLegend | Cat# 101302; RRID: AB_312801 |
| Rat monoclonal anti-mouse Ly6G (clone 1A8) | BioXCell | Cat# BE0075-1 |
| Rat monoclonal IgG2a isotype control (clone 2A3) | BioXCell | Cat# BE0089 |
| Violet 450 anti-Ly6G | BD Biosciences | Cat# 560603; RRID: AB_1727564 |
| Bacterial and Virus Strains | ||
| Staphylococcus aureus LAC (USA300) wild-type | Dr. Michael Otto (Wang et al., 2007) | N/A |
| Staphylococcus aureus LAC (USA300) Δagr | Dr. Michael Otto (Wang et al., 2007) | N/A |
| Staphylococcus aureus LAC (USA300) Δpsmα | Dr. Michael Otto (Wang et al., 2007) | N/A |
| Staphylococcus aureus LAC (USA300) Δpsmβ | Dr. Michael Otto (Wang et al., 2007) | N/A |
| Staphylococcus aureus LAC (USA300) psmα-lux | Dr. Michael Otto (Dastgheyb et al., 2015) | N/A |
| Staphylococcus aureus JE2 (USA300) YFP-wild-type | Dr. Christian Wolz (Münzenmayer et al., 2016) | N/A |
| Staphylococcus aureus JE2 (USA300) YFP-Δagr | Dr. Christian Wolz (Münzenmayer et al., 2016) | N/A |
| Staphylococcus aureus LAC (USA300) wild-type | Dr. Jovanka M. Voyich (Nygaard et al., 2010) | N/A |
| Staphylococcus aureus LAC (USA300) ΔsaeR/S | Dr. Jovanka M. Voyich (Nygaard et al., 2010) | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| DAPI | BioLegend | Cat# 422801 |
| DNase I | Sigma-Aldrich | Cat# DN25 |
| FITC-streptavidin | BioLegend | Cat# 405202 |
| Fixable Viability Dye eFluor 780 | eBioscience | Cat# 65-0865-14 |
| Fluorescent mounting medium | Dako | Cat# S3023 |
| Liberase DH | Roche | Cat# 447529 |
| Lysostaphin | Sigma-Aldrich | Cat# L7386 |
| Mannitol salt agar | Nissui | Cat# 05236 |
| Percoll | GE Healthcare | Cat# 17-0891-01 |
| Recombinant mouse TNF-α | BioLegend | Cat# 575204 |
| Vancomycin Hydrochloride | Fresenius Kabi | NDC 63323-314-61 |
| Critical Commercial Assays | ||
| E.Z.N.A. Bacterial RNA kit | Omega Bio-tek | Cat# R6950-01 |
| High Capacity RNA-to-cDNA Kit | Applied Biosystems | Cat# 4387406 |
| LDH Cytotoxicity Detection Kit | Takara Biochemicals | Cat# MK401 |
| Neutrophil Isolation Kit | Miltenyi Biotec | Cat# 130-097-658 |
| RPMI Medium 1640 | Gibco | Cat# 21870-076 |
| TaqMan™ Fast Advanced Master Mix | Applied Biosystems | Cat# 4444556 |
| Tegaderm™ Film | 3M | Cat# 1624W |
| TritonX-100 | Fisher Scientific | Cat# BP151-500 |
| Tryptic Soy Broth | BD Difco | Cat# 211825 |
| Experimental Models: Organisms/Strains | ||
| Mouse: C57BL/6J: WT | The Jackson Laboratory | JAX: 000664 |
| Mouse: Cybb−/−: B6.129S-Cybbtm1Din/J | The Jackson Laboratory | JAX: 002365 |
| Mouse: Mrp8Cre/+ | Dr. Andres Hidalgo | MGI:4415239 |
| Mouse: Mcl1f/f | Dr. Andres Hidalgo | MGI:2684151 |
| Oligonucleotides | ||
| gyrB: forward 5’-CAAATGATCACAGCATTTGGTACAG-3’ | (Nygaard et al., 2010) | N/A |
| gyrB: reverse 5’-CGGCATCAGTCATAATGACGAT-3’ | (Nygaard et al., 2010) | N/A |
| gyrB: probe 5’-[FAM]-AATCGGTGGCGACTTTGATCTAGCGAAAG-[BHQ]-3’ | (Nygaard et al., 2010) | N/A |
| psmα: forward 5’-TATCAAAAGCTTAATCGAACAATTC-3’ | (Dastgheyb et al., 2015) | N/A |
| psmα: reverse 5’-CCCCTTCAAATAAGATGTTCATATC-3’ | (Dastgheyb et al., 2015) | N/A |
| psmα: probe 5’-[FAM]-AAAGACCTCCTTTGTTTGTTATGAAATCTTATTTACCAG-[BHQ]-3’ | (Dastgheyb et al., 2015) | N/A |
| saeS: forward 5’-CGTACATTCAGAGTAGAAAACTCTCGTAATAC-3’ | (Nygaard et al., 2010) | N/A |
| saeS: reverse 5’-GTTGCGCGAGTTCATTAGCTATATAT-3’ | (Nygaard et al., 2010) | N/A |
| saeS: probe 5’-[FAM]-AGCCTAATCCAGAACCACCCGTT-[BHQ]-3’ | (Nygaard et al., 2010) | N/A |
| saeR: forward 5’-CTGCCAAAACACAAGAACATGATAC-3’ | (Nygaard et al., 2010) | N/A |
| saeR: reverse 5’-CTTGGACTAAATGGTTTTTTGACATAGT-3’ | (Nygaard et al., 2010) | N/A |
| saeR: probe 5’-[FAM]-ATTTACGCCTTAACTTTAGGTGCAGAT-[BHQ]-3’ | (Nygaard et al., 2010) | N/A |
| Software and Algorithms | ||
| NIS-Elements-confocal software | NIKON | https://www.microscope.healthcare.nikon.com/products/software/nis-elements/nis-elements-confocal |
| Living image Software | PerkinElmer | https://www.perkinelmer.com/lab-products-and-services/resources/in-vivo-imaging-software-downloads.html#LivingImage |
| FlowJo | FlowJo LLC | https://www.flowjo.com |
| Gen5™ | BioTek | https://www.biotek.com/products/software-robotics-software/gen5-microplate-reader-and-imager-software/ |
| Prism (GraphPad Software) | GraphPad | https://www.graphpad.com |
Acknowledgments
The authors thank the University of Michigan Flow Cytometry Core for Flow cytometry analysis. This work was supported by JSPS KAKENHI; Grant Number 26713038 (Y.N.), 16H06252 (Y.N.), AMED-PRIME JP18gm6010016h0002 (Y.N.), the Naito Foundation (Y.N.), the Uehara Memorial Foundation (M.M.), Mochida Memorial Foundation for Medical and Pharmaceutical Research (M.M.) and NIH grant AR069303 (G.N.). Y.N. and S.N. were supported by the Institute for Global Prominent Research, Chiba University. S.N. was supported by the Leading Graduate School Program of Chiba University (Nurture of Creative Research Leaders in Immune System Regulation and Innovative Therapeutics). M.O. was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, US National Institutes of Health (grant number ZIA AI000904-16).
Footnotes
Competing Financial Interests
The authors declare no competing financial interests.
References
- Abdelnour A, Arvidson S, Bremell T, Ryden C, and Tarkowski A (1993). The accessory gene regulator (agr) controls Staphylococcus aureus virulence in a murine arthritis model. Infect Immun 61, 3879–3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian D, Harper L, Shopsin B, and Torres VJ (2017). Staphylococcus aureus pathogenesis in diverse host environments. Pathog Dis 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavers WN, and Skaar EP (2016). Neutrophil-generated oxidative stress and protein damage in Staphylococcus aureus. Pathog Dis 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattner S, Das S, Paprotka K, Eilers U, Krischke M, Kretschmer D, Remmele CW, Dittrich M, Muller T, Schuelein-Voelk C, et al. (2016). Staphylococcus aureus Exploits a Non-ribosomal Cyclic Dipeptide to Modulate Survival within Epithelial Cells and Phagocytes. PLoS Pathog 12, e1005857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisset S, Geissmann T, Huntzinger E, Fechter P, Bendridi N, Possedko M, Chevalier C, Helfer AC, Benito Y, Jacquier A, et al. (2007). Staphylococcus aureus RNAIII coordinately represses the synthesis of virulence factors and the transcription regulator Rot by an antisense mechanism. Genes Dev 21, 1353–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnes EC, Lopez DM, Donegan NP, Cheung A, Gresham H, Timmins GS, and Brinker CJ (2010). Confinement-induced quorum sensing of individual Staphylococcus aureus bacteria. Nat Chem Biol 6, 41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung GY, Wang R, Khan BA, Sturdevant DE, and Otto M (2011). Role of the accessory gene regulator agr in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. Infect Immun 79, 1927–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Jeong DW, Liu Q, Yeo WS, Vogl T, Skaar EP, Chazin WJ, and Bae T (2015). Calprotectin Increases the Activity of the SaeRS Two Component System and Murine Mortality during Staphylococcus aureus Infections. PLoS Pathog 11, e1005026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csepregi JZ, Orosz A, Zajta E, Kasa O, Nemeth T, Simon E, Fodor S, Csonka K, Baratki BL, Kovesdi D, et al. (2018). Myeloid-Specific Deletion of Mcl-1 Yields Severely Neutropenic Mice That Survive and Breed in Homozygous Form. J Immunol 201, 3793–3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte JT, Whitten DM, and Babior BM (1974). Defective superoxide production by granulocytes from patients with chronic granulomatous disease. N Engl J Med 290, 593–597. [DOI] [PubMed] [Google Scholar]
- Dastgheyb SS, Villaruz AE, Le KY, Tan VY, Duong AC, Chatterjee SS, Cheung GY, Joo HS, Hickok NJ, and Otto M (2015). Role of Phenol-Soluble Modulins in Formation of Staphylococcus aureus Biofilms in Synovial Fluid. Infect Immun 83, 2966–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzhagalov I, St John A, and He YW (2007). The antiapoptotic protein Mcl-1 is essential for the survival of neutrophils but not macrophages. Blood 109, 1620–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TJ, Geoghegan JA, Ganesh VK, and Hook M (2014). Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol 12, 49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger T, Francois P, Liebeke M, Fraunholz M, Goerke C, Krismer B, Schrenzel J, Lalk M, and Wolz C (2012). The stringent response of Staphylococcus aureus and its impact on survival after phagocytosis through the induction of intracellular PSMs expression. PLoS Pathog 8, e1003016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillaspy AF, Hickmon SG, Skinner RA, Thomas JR, Nelson CL, and Smeltzer MS (1995). Role of the accessory gene regulator (agr) in pathogenesis of staphylococcal osteomyelitis. Infect Immun 63, 3373–3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosz M, Kolter J, Paprotka K, Winkler AC, Schafer D, Chatterjee SS, Geiger T, Wolz C, Ohlsen K, Otto M, et al. (2014). Cytoplasmic replication of Staphylococcus aureus upon phagosomal escape triggered by phenol-soluble modulin alpha. Cell Microbiol 16, 451–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra FE, Borgogna TR, Patel DM, Sward EW, and Voyich JM (2017). Epic Immune Battles of History: Neutrophils vs. Staphylococcus aureus. Front Cell Infect Microbiol 7, 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer G, Saba S, Adamo R, Rush W, Soong G, Cheung A, and Prince A (2002). Staphylococcus aureus agr and sarA functions are required for invasive infection but not inflammatory responses in the lung. Infect Immun 70, 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill HR, Ochs HD, Quie PG, Clark RA, Pabst HF, Klebanoff SJ, and Wedgwood RJ (1974). Defect in neutrophil granulocyte chemotaxis in Job's syndrome of recurrent "cold" staphylococcal abscesses. Lancet 2, 617–619. [DOI] [PubMed] [Google Scholar]
- Horn J, Stelzner K, Rudel T, and Fraunholz M (2018). Inside job: Staphylococcus aureus host-pathogen interactions. Int J Med Microbiol 308, 607–624. [DOI] [PubMed] [Google Scholar]
- Howard MW, Strauss RG, and Johnston RB Jr. (1977). Infections in patients with neutropenia. Am J Dis Child 131, 788–790. [DOI] [PubMed] [Google Scholar]
- Introne W, Boissy RE, and Gahl WA (1999). Clinical, molecular, and cell biological aspects of Chediak-Higashi syndrome. Mol Genet Metab 68, 283–303. [DOI] [PubMed] [Google Scholar]
- Kobayashi SD, Malachowa N, Whitney AR, Braughton KR, Gardner DJ, Long D, Bubeck Wardenburg J, Schneewind O, Otto M, and Deleo FR (2011). Comparative analysis of USA300 virulence determinants in a rabbit model of skin and soft tissue infection. J Infect Dis 204, 937–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K, Hoffman HM, Kubes P, Cassatella MA, Zychlinsky A, Hedrick CC, and Catz SD (2018). Neutrophils: New insights and open questions. Sci Immunol 3. [DOI] [PubMed] [Google Scholar]
- Li M, Dai Y, Zhu Y, Fu CL, Tan VY, Wang Y, Wang X, Hong X, Liu Q, Li T, et al. (2016). Virulence determinants associated with the Asian community-associated methicillin-resistant Staphylococcus aureus lineage ST59. Sci Rep 6, 27899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Archer NK, Dillen CA, Wang Y, Ashbaugh AG, Ortines RV, Kao T, Lee SK, Cai SS, Miller RJ, et al. (2017). Staphylococcus aureus Epicutaneous Exposure Drives Skin Inflammation via IL-36-Mediated T Cell Responses. Cell Host Microbe 22, 653–666 e655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy FD (1998). Staphylococcus aureus infections. N Engl J Med 339, 520–532. [DOI] [PubMed] [Google Scholar]
- Munzenmayer L, Geiger T, Daiber E, Schulte B, Autenrieth SE, Fraunholz M, and Wolz C (2016). Influence of Sae-regulated and Agr-regulated factors on the escape of Staphylococcus aureus from human macrophages. Cell Microbiol 18, 1172–1183. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Matsumoto M, Katayama Y, Oguma R, Wakabayashi S, Nygaard T, Saijo S, Inohara N, Otto M, Matsue H, et al. (2017). Staphylococcus aureus Virulent PSMalpha Peptides Induce Keratinocyte Alarmin Release to Orchestrate IL-17-Dependent Skin Inflammation. Cell Host Microbe 22, 667–677 e665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Oscherwitz J, Cease KB, Chan SM, Munoz-Planillo R, Hasegawa M, Villaruz AE, Cheung GY, McGavin MJ, Travers JB, et al. (2013). Staphylococcus delta-toxin induces allergic skin disease by activating mast cells. Nature 503, 397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Takahashi H, Takaya A, Inoue Y, Katayama Y, Kusuya Y, Shoji T, Takada S, Nakagawa S, Oguma R, et al. (2020). Staphylococcus Agr virulence is critical for epidermal colonization and associates with atopic dermatitis development. Sci Transl Med 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick RP (2003). Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol 48, 1429–1449. [DOI] [PubMed] [Google Scholar]
- Nygaard TK, Pallister KB, Ruzevich P, Griffith S, Vuong C, and Voyich JM (2010). SaeR binds a consensus sequence within virulence gene promoters to advance USA300 pathogenesis. J Infect Dis 201, 241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang YY, Schwartz J, Thoendel M, Ackermann LW, Horswill AR, and Nauseef WM (2010). agr-Dependent interactions of Staphylococcus aureus USA300 with human polymorphonuclear neutrophils. J Innate Immun 2, 546–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschel A, and Otto M (2013). Phenol-soluble modulins and staphylococcal infection. Nat Rev Microbiol 11, 667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queck SY, Jameson-Lee M, Villaruz AE, Bach TH, Khan BA, Sturdevant DE, Ricklefs SM, Li M, and Otto M (2008). RNAIII-independent target gene control by the agr quorum-sensing system: insight into the evolution of virulence regulation in Staphylococcus aureus. Mol Cell 32, 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soehnlein O, and Lindbom L (2010). Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol 10, 427–439. [DOI] [PubMed] [Google Scholar]
- Surewaard BG, de Haas CJ, Vervoort F, Rigby KM, DeLeo FR, Otto M, van Strijp JA, and Nijland R (2013). Staphylococcal alpha-phenol soluble modulins contribute to neutrophil lysis after phagocytosis. Cell Microbiol 15, 1427–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surewaard BG, Nijland R, Spaan AN, Kruijtzer JA, de Haas CJ, and van Strijp JA (2012). Inactivation of staphylococcal phenol soluble modulins by serum lipoprotein particles. PLoS Pathog 8, e1002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, Kennedy AD, Dorward DW, Klebanoff SJ, Peschel A, et al. (2007). Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med 13, 1510–1514. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.