Abstract
Processes by which human herpesviruses penetrate and are released from polarized epithelial cells, which have distinct apical and basolateral membrane domains differing in protein and lipid content, are poorly understood. We recently reported that human cytomegalovirus (CMV) mutants with deletions of the gene US9 formed wild-type plaques in cultures of human fibroblasts but were impaired in the capacity for cell-to-cell spread in polarized human retinal pigment epithelial cells. Unlike the glycoproteins that are required for infection, the protein encoded by CMV US9 plays an accessory role by promoting dissemination of virus across cell-cell junctions of polarized epithelial cells. To identify the product and investigate its specialized functions, we selected Madine-Darby canine kidney II (MDCK) epithelial cells that constitutively express CMV US9 or, as a control, US8. The gene products, designated gpUS9 and gpUS8, were glycosylated proteins of comparable molecular masses but differed considerably in intracellular distribution and solubility. Immunofluorescence laser scanning confocal microscopy indicated that, like gpUS8, gpUS9 was present in the endoplasmic reticulum and Golgi compartments of nonpolarized cells. In polarized epithelial cells, gpUS9 also accumulated along lateral membranes, colocalizing with cadherin and actin, and was insoluble in Triton X-100, a property shared with proteins that associate with the cytoskeleton. We hypothesize that gpUS9 may enhance the dissemination of CMV in infected epithelial tissues by associating with the cytoskeletal matrix.
Human cytomegalovirus (CMV) is a ubiquitous pathogen that can cause severe disease in immunocompromised patients (particularly those with AIDS), transplant recipients, and congenitally infected infants (7, 15, 24). Following primary infection, CMV persists in a latent state in the monocyte/macrophage lineage (35, 51) and can be reactivated by allogeneic stimulation from macrophages (48). CMV has been detected in secretions (saliva, urine, semen, and breast milk) from organs composed of epithelial cells (reviewed in reference 7). CMV retinitis is a potentially vision-threatening complication of the posterior segment of the eye in patients undergoing bone marrow transplantation (11). In AIDS patients, CMV retinopathy is the major cause of vision loss late in the course of disease (24, 26). In some cases, endothelial cells of the retinal vasculature are infected, supporting the idea that retinal infection may result from CMV reactivation in macrophages. Although most patients with AIDS are CMV seropositive, not all develop clinical complications, which suggests that the CMV strain, virus load, and immune status may modulate disease progression (58). Despite lifelong maintenance therapy, CMV retinitis frequently progresses in patients with AIDS (6), a phenomenon which is attributed to the development of resistance to the nucleoside analogs ganciclovir and foscarnet (27).
CMV is the largest human herpesvirus, having a DNA genome of 230 to 235 kbp that consists of two components, a unique short (US) and a unique long sequence, flanked by inverted repeats. The CMV genome has the capacity to encode more than 220 proteins, of which 70 have the sequence characteristics of glycoproteins (9, 36). As in other herpesviruses, CMV genes encoding glycoproteins are divided into two groups: genes that are essential and genes that are dispensable for infection of human fibroblasts (HF) in culture (reviewed in reference 41). Using deletion mutagenesis, it was demonstrated that the US component of the CMV genome contains genes encoding proteins that play an accessory role in infection by increasing pathogenesis in vivo (30–32). At least four glycoproteins encoded by the US sequence allow CMV-infected cells to evade T-cell immunity by downregulating the major histocompatibility complex class I molecules from the cell surface (1, 29, 33, 34, 38, 59, 60). Cell-to-cell dissemination of virus in polarized cells of epithelial or neuronal tissues may also require specialized functions that are adapted to a particular niche. We reported that CMV strain AD169 enters polarized human retinal pigment epithelial (ARPE-19) cells by fusion of the virion envelope with apical membranes, which is facilitated by glycoprotein B (gB), but that gB does not play a role in virus spread from cell to cell (55). In contrast, we found that CMV mutants with deletions of US9, a gene in the US component, form small plaques in cultures of polarized ARPE- 19 cells, suggesting that the mutants are impaired in their capacity for cell-to-cell spread (39, 45). Mutants of herpes simplex virus type 1 (HSV-1) with deletions of gE are also impaired in cell-to-cell spread in epithelial cells (3, 14). These results indicate that dissemination of infection across lateral membranes of polarized epithelial cells differs from penetration of apical membranes and requires specialized gene products.
To perform their vectorial function, polarized epithelial cells have evolved a plasma membrane divided by tight junctions into apical and basolateral domains that differ in protein and lipid composition (20, 42, 46). Cell-cell junctions, complex structures that establish and maintain the polarized morphology of epithelial cells, are classified into three groups: tight, adherens, and gap junctions. Tight junctions, the most apical component, regulate the flux of ions and hydrophilic molecules through the paracellular pathway and are mediated by the transmembrane protein occludin and membrane-associated proteins ZO-1 and ZO-2 (2, 17, 18, 20–22). Adherens junctions are highly specialized regions where cadherins—transmembrane proteins that function in cell-cell adhesion and establishment of polarized cell morphology—and their associated catenins and actin filaments are densely aligned with the plasma membrane through a well-developed protein undercoat (19, 50, 53). Prominent bundles of actin filaments encircle the apexes of lateral membranes in association with adherens junctions (40). Binding properties of the cadherin extracellular domain that contacts cadherins on adjacent cells depend on the formation of protein complexes with the intracellular carboxyl terminus, which links the actin cytoskeleton to the membrane.
In the present study, we addressed the role of US9 in cell-to-cell spread of CMV in polarized epithelial cells by expressing the gene product and, as a control, that of US8. We identified novel glycoproteins of the predicted molecular mass, which we designated gpUS9 and gpUS8. Immunofluorescence microscopy of Madine-Darby canine kidney II (MDCK) cells showed that gpUS9 and gpUS8 were present in the endoplasmic reticulum (ER) and Golgi compartments in nonpolarized and polarized epithelial cells. Moreover, in polarized cells gpUS9 exhibited a unique staining pattern, accumulating along lateral membranes, colocalizing with E-cadherin and the cortical actin cytoskeleton, and forming Triton X-insoluble protein complexes that were enriched along lateral membranes. Changes induced in cells expressing gpUS9 may shed light on the role of this accessory glycoprotein in facilitating the spread of CMV in polarized epithelial cells and consequently on pathogenesis in vivo.
(Portions of this report were presented at the 22nd International Herpesvirus Workshop, De Kalb, Ill., 1996, and the 23rd International Herpesvirus Workshop, San Diego, Calif., 1997.)
MATERIALS AND METHODS
Cells, culture medium, and propagation of polarized MDCK cells on microporous filters.
MDCK type II cells were grown in T-75 cm2 flasks (Costar) at 37°C in minimal essential medium containing 5% fetal calf serum, 200 mM l-glutamine, 0.1 mg of streptomycin per ml, and 100 U of penicillin per ml. To form polarized monolayers, the MDCK cells were grown on filters (Transwell; Costar) with pore sizes of 0.4 μm. Transepithelial resistance of MDCK cell monolayers was measured with a Millicell electrical resistance system (Millipore) and reached approximately 350 Ω/cm2 by 4 days of growth on permeable filter supports. The paracellular permeability of polarized MDCK cells on the filters was monitored by measuring the passage of [3H]inulin from the apical to the basolateral chambers of the filters as previously described (16). Five hundred microliters of medium containing a total of 45,000 cpm of [3H]inulin was added to the apical chambers of the filters. After 8 h, duplicate 10-μl aliquots were removed from the basolateral chambers and radioactivity was counted in a Beckman LS1701 scintillation counter.
Cloning and epitope tagging of US9 and US8.
The CMV US9 gene was excised from plasmid pHXSH and tagged with a synthetic oligonucleotide encoding epitope H943 of HSV-1 α4 (25) in an EarI site at the carboxyl terminus-encoding portion of US9 to construct plasmid pHXSH(Ear)-US9ep. This α4 epitope is recognized by murine monoclonal antibody (MAb) H943. The double-stranded oligonucleotide 5′GAC GAG TAC GAC GAC GCA GCC GAC GCC GCC GGC GAC CGG GCC CCG 3′, coding for the α4 epitope, was inserted near the portion of the gene encoding the carboxyl terminus of the US9 product following amino acid (aa) 241. The insertion site was verified by DNA sequence analysis. The resulting gene, US9ep, which encoded aa 1 to 241 followed by the H943 epitope, Asp Glu Tyr Asp Asp Ala Ala Asp Ala Ala Gly Asp Arg Ala Pro, and aa 242 to 247 of the US9 product, was cloned into the EcoRV site of pcDNA3 (Invitrogen). The US8 gene was subcloned by double digestion of pHXSH with EarI and BamHI to generate a 930-bp fragment which was purified, blunt ended with Klenow polymerase (New England Biolabs), and ligated into the EcoRV site of pcDNA3. An oligonucleotide tag encoding epitope H170 of HSV-1 gD (10) was inserted into a unique Eco47III site modified with ClaI linkers. The resulting gene, US8ep, encoded aa 1 to 208 tagged by the H170 epitope, Ser Leu Lys Met Ala Asp Pro Asn Arg Phe Arg Gly Lys Asp Leu Pro, at the 3′ terminus; these amino acids were preceded by Arg Tyr from the ClaI linker.
Selection of MDCK cells expressing US9 and US8.
MDCK cells transfected with the US9ep and US8ep genes in pcDNA3 were selected as described previously (56). Approximately 106 MDCK cells at 50 to 60% confluence were transfected with 10 μg of plasmid DNA by the calcium phosphate precipitation method. After 4 to 6 h, fresh medium containing 10% fetal calf serum was added. The next day, cells were trypsinized and 5 × 104 cells/ml were plated into 24-well culture dishes in medium containing G418 (1.2 mg/ml; Gibco). Resistant clones were evaluated for expression of US9ep and US8ep by immunofluorescence with MAbs to the respective epitopes. The properties of three MUS9ep cell clones (designated 102, 105, and 109) and three MUS8ep cell clones (designated 1D5, 15D5, and 1G7) were studied in detail.
Immunological reagents.
Immunofluorescence assays were done as previously described (56). US9ep and US8ep were detected with MAbs H943 and H170 to their respective epitope tags. Cellular proteins in tight junctions were stained with rabbit antisera to ZO-1 (Zymed Laboratories); for adherens junctions, rat MAb to E-cadherin (Sigma) and rabbit antisera to β-catenin (gift from Inka Nathke, Harvard University) were used. ER was stained with antiserum to GRP94 (Stressgene), and the Golgi network was reacted with LcH agglutinin from Lens culinaris conjugated with fluorescein isothiocyanate (FITC) (E-Y Lab Inc.). FITC- and Texas red (Sigma)-labeled anti-mouse, -rat, and -rabbit immunoglobulin antisera were purchased from Jackson ImmunoResearch Laboratories. Actin was stained with phalloidin conjugated with FITC or Texas red. Cells were incubated with primary antibodies (1 h, 37°C) and then with secondary antibodies conjugated with FITC or Texas red (30 min). For double staining, cells were simultaneously incubated with primary antibodies from different species and secondary antibodies labeled with FITC or Texas red.
ECL Western blotting and endoglycosidase treatment.
Proteins encoded by US8ep and US9ep were analyzed with the enhanced chemiluminescence (ECL) immunoblot detection system (Amersham). Cells (in T-25 cm2 flasks) were extracted with 1.0% Nonidet P-40–1.0% deoxycholic acid–0.1% sodium dodecyl sulfate (SDS) in phosphate-buffered saline (PBS) containing the protease inhibitors Nα-p-tosyl-l-lysine chloromethyl ketone (TLCK) (10 μM), tolylsulfonyl phenylalanyl chloromethyl ketone (TPCK) (10 μM), and 1 mM phenylmethylsulfonyl fluoride (PMSF) for 30 min. Sample buffer containing 2% SDS was then added. Cell extracts were subjected to SDS–12% polyacrylamide gel electrophoresis (PAGE) in gels cross-linked with N,N-diallyltartardiamide. Proteins were electrophoretically transferred to nitrocellulose for immunoblot reactions with murine MAbs (1:1,000). Filters were washed and incubated with peroxidase-conjugated anti-mouse immunoglobulin antiserum (1:10,000). Bands were visualized by ECL Western blotting followed by exposure to Hyperfilm. For endoglycosidase H (endo-H) and peptide-N-glycosidase F (PNGase F) analysis, COS- 1 cells were transfected with pHXSH(Ear)-US8ep and pHXSH-US9ep. Cells were lysed with 1.0% SDS–1.0% Nonidet P-40–1.0% deoxycholic acid in PBS containing protease inhibitors (TLCK, TPCK, and PMSF) and boiled in the presence of 5% β- mercaptoethanol. Samples were incubated with enzymes overnight at 37°C according to the manufacturer’s directions. Markers for molecular mass were carbonic anhydrase (30 kDa) and ovalbumin (46 kDa) (Amersham).
Immunofluorescence laser scanning confocal microscopy.
For immunofluorescence assays, polarized MUS8 and MUS9 cells grown on permeable filter supports were fixed with fresh 3% paraformaldehyde–0.1% Triton X-100 (5 min) and then reacted with 3% paraformaldehyde–2% sucrose (15 min). Paraformaldehyde was quenched by incubating cells with 50 mM NH4Cl. Fixed cells were incubated with antibodies on ice as described above, cut, and mounted on glass slides in Mowiol solution (Calbiochem-Behring). Cells were analyzed by using a krypton-argon laser coupled with a Bio-Rad MRC 600 confocal head attached to a Nikon Optiphot II microscope with a Plane Apo 60 1.4× objective lens. Cells were scanned simultaneously for FITC and Texas red emission by using K1 and K2 filter blocks. For serial optical section analysis, cells were scanned from apical to basolateral membranes with increments of 0.5 to 1.0 μm between sections. The data were analyzed with Comos software.
Extraction of proteins in MDCK cells.
Immunofluorescence analysis was carried out as follows. Cells were extracted with modified CSK buffer [50 mM NaCl, 300 mM sucrose, 10 mM 1,4-piperazinebis(ethanesulfonic acid) (pH 6.8), 3 mM MgCl2, 0.5% Triton X-100, 1 mM PMSF] for 15 min at 4°C and then fixed with 3.75% formaldehyde in PBS for 30 min as previously described (43). Control cells were fixed with 3.75% formaldehyde and then permeabilized with CSK buffer for 15 min at 4°C. Cells were blocked in PBS–0.2% bovine serum albumin–1% normal goat serum for 1 h and then incubated with specific MAbs for immunofluorescence analysis. Western blot analysis was carried out as follows. MUS9ep and MUS8ep cells were grown on glass or permeable filter supports, washed with PBS, and solubilized in 1.0% Triton X-100 or 65 mM octylglucoside in 2-(N-morpholino)ethanesulfonic acid (MES)-buffered saline (MBS, 25 mM MES [pH 6.5], 15 M NaCl, 1 mM PMSF) for 20 min at 4°C on a rocking platform as previously described (47). Cells were removed with a rubber policeman, transferred to Eppendorf tubes, and centrifuged at 14,000 rpm for 15 min in a Sorvall RMG 14 refrigerated microcentrifuge (Dupont). The supernatant and the insoluble pellet were resuspended in immunoprecipitation buffer [15 mM Tris (pH 7.5), 5 mM EDTA, 2.5 mM EGTA, 1% SDS], and then subjected to PAGE and analyzed by ECL Western blotting.
RESULTS
Cloning and expression of the epitope-tagged products of the US9 and US8 genes.
CMV mutants with deletions of the US9 gene, but not those with deletions of the US8 gene, were impaired in their capacity for cell-to-cell spread in epithelial cells, suggesting that the US9-encoded protein plays a role in dissemination of infection in polarized cells (39, 45). Analyses of the amino acid sequences encoded by CMV US9 and the adjacent gene, US8, indicate that their products have the features of type I membrane-anchored glycoproteins (9). CMV US9 is predicted to encode a protein 247 aa in length that contains a 24-aa signal sequence, a 168-aa ectodomain with two N-glycosylation sites, a 30-aa transmembrane anchor, and a 25-aa cytoplasmic domain. CMV US8 is predicted to encode a protein 227 aa in length that contains a 19-aa signal sequence, a 160-aa ectodomain, one N-glycosylation site, a 22-aa transmembrane anchor, and a 26-aa cytoplasmic domain. Thus, the products of the US9 and US8 genes have similar properties inasmuch as they are predicted to be transmembrane glycoproteins, comparable in molecular mass and number of N-glycosylation sites, whose transcripts were detected with early kinetics in CMV-infected cells (30). Since we wished to select a CMV glycoprotein as a control for US9 expression in polarized epithelial cells and US8 did not alter cell-to-cell spread of virus, US8 was selected. To identify the proteins, the genes were cloned and tagged at the carboxyl terminus-encoding portions with epitopes from HSV proteins (Fig. 1). An epitope from HSV-1 α4, a nuclear protein recognized by MAb H943, was used to tag US9. After tagging US9 with the α4 epitope, we found that the antibody reacted with tagged protein in Western blotting but not in immunoprecipitation experiments. To facilitate both types of analyses, US8 was tagged with an epitope from HSV-1 gD, an envelope glycoprotein recognized by MAb H170. These epitopes do not contain known motifs for binding calcium or actin or nuclear transport signals, which might alter the subcellular localization of these proteins.
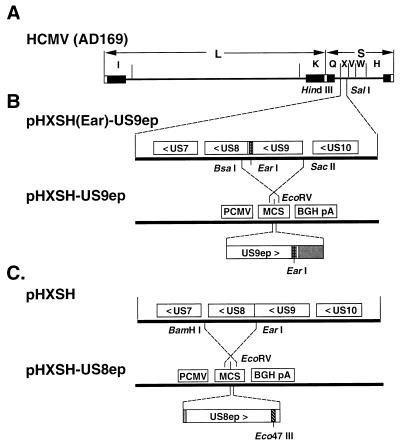
FIG. 1.
Construction of plasmids containing CMV US9ep and CMV US8ep. (A) Organization of the CMV (AD169) genome and HindIII fragments H, I, K, Q, V, W, and X. (B) Expanded region of the HindIII X fragment contained in pHXSH(Ear)-US9ep. Plasmid p111-3, which expresses the US9 gene tagged with a synthetic oligomer corresponding to an epitope in HSV-1 ICP4 (hatched box), was constructed by double digestion of pHXSH(Ear)-US9ep with BsaI and SacII. The fragment containing the epitope-tagged gene was inserted in the rightward direction into the EcoRV site in the pcDNA3 vector. (C) Expanded region of the HindIII X fragment containing CMV US8. pHXSH was double digested with EarI and BamHI, and the fragment containing US8 was inserted in the rightward direction into the EcoRV site of pcDNA3. US8 was tagged at the Eco47III site with a synthetic oligomer corresponding to epitope H170 from HSV-1 gD (hatched box) flanked by ClaI sites, after modification of the Eco47III site with the ClaI linker. Residual noncoding sequences of US9 (B) and US8 (C) are represented by gray boxes, and the epitope tags, which were added to the carboxyl terminus, are represented by hatched boxes. PCMV, human CMV promoter-enhancer; MCS, multiple cloning site; BGH pA, bovine growth hormone polyadenylation signal.
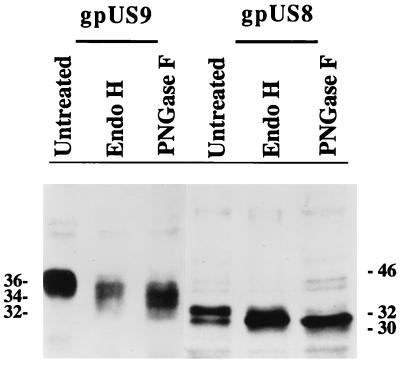
To determine whether these CMV genes encode glycoproteins, tagged US9 and US8 were expressed transiently in COS- 1 cells and the proteins were detected by electrophoresis and Western blotting (Fig. 2). Two protein bands of the expected size, 36 and 34 kDa, were detected for US9, and two bands of 32 and 30 kDa were detected for US8. To determine whether these proteins were glycosylated, they were digested with endo- H, which cleaves mannose-rich complex carbohydrates, and PNGase F, which removes all N-linked carbohydrates. Endo-H-treated US9 protein had an approximate molecular mass of 34 kDa, and endo-H-treated US8 protein had an approximate molecular mass of 31 kDa. Following PNGase F treatment, the mobility of the US9 product increased to 32 kDa and that of the US8 product increased to 30 kDa. The results of these transient-expression experiments showed that the US9 and US8 genes encoded glycoproteins that contained both simple and complex carbohydrates. Based on the recommended nomenclature for CMV gene products (49), these glycoproteins were named gpUS9 and gpUS8.
FIG. 2.
Western blot analysis of CMV gpUS9 and gpUS8 expressed in transfected COS- 1 cells. Electrophoretically separated proteins were reacted with MAbs to the epitope tags and detected in ECL Western blots (untreated). gpUS9 and gpUS8 were digested with endo-H and PNGase F. Molecular mass markers, in kilodaltons, are shown in the margins.
Analysis of MDCK cells constitutively expressing gpUS9 and gpUS8.
To evaluate the properties of gpUS9 and gpUS8 in epithelial cells, we selected stably transfected MDCK cells that constitutively expressed these glycoproteins. MDCK cells were transfected with pHXSH-US9ep and pHXSH-US8ep DNA containing the genes for gpUS9 and gpUS8, respectively (Fig. 1B and C), and the selectable marker neomycin transferase. G418-resistant MUS9ep and MUS8ep cells that formed isolated colonies in 24-well plates were expanded and tested for expression by immunofluorescence as described in Materials and Methods. Colonies of clones in which 40 to 80% of the cells expressed the glycoproteins were expanded for detailed analysis of cell polarity and colocalization of the CMV glycoproteins with cellular proteins.
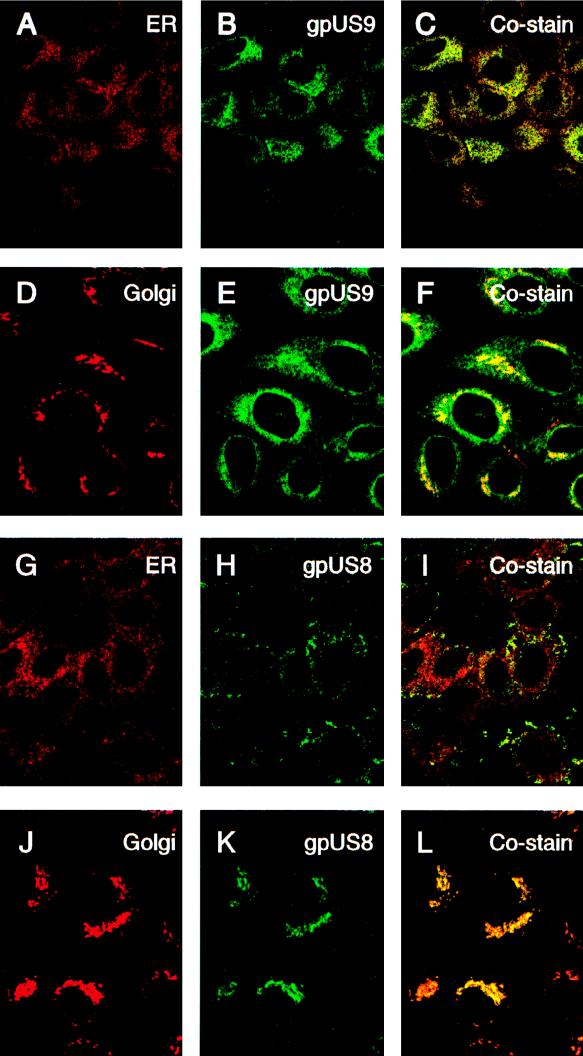
In the first series of experiments, we assessed the presence of gpUS9 and gpUS8 in the ER and Golgi compartments of nonpolarized cells grown on glass. MUS9ep and MUS8ep cells were stained with markers for the ER (GRP94) and Golgi complex (LcH agglutinin of L. culinaris) and with MAbs to the epitope tags on gpUS9 (Fig. 3A to F) and gpUS8 (Fig. 3G to L). Immunofluorescence assessments with confocal microscopy showed that both glycoproteins colocalized with proteins in the ER and Golgi compartments of nonpolarized cells. gpUS9 showed a reticular pattern that overlay the ER (Fig. 3A to C). A comparable pattern was obtained for gpUS8 (Fig. 3G to I). The glycoproteins move through the Golgi compartment, as indicated by coincident staining patterns obtained for gpUS9 (Fig. 3D to F) and gpUS8 (Fig. 3J to L) with the marker for this secretory compartment. These results confirmed that both gpUS9 and gpUS8 were transported from the ER to the Golgi compartment in the secretory pathway in nonpolarized cells.
FIG. 3.
Immunofluorescence analysis of CMV gpUS9 and gpUS8 distribution in the ER and Golgi compartments of subconfluent nonpolarized cells. MUS9ep clone 102 is shown in panels A to F, and MUS8ep clone 1D5 is shown in panels G to L. gpUS9 stained with MAb H943 is shown in panels B, C, E, and F. gpUS8 stained with MAb H170 is shown in panels H, I, K, and L. Antiserum to GRP94 in the ER was used for panels A and G, and LcH agglutinin reactive with Golgi proteins was used for panels D and J. Costaining of gpUS9 in the ER (C) and Golgi compartments (F) and of gpUS8 in the ER (I) and Golgi compartments (L) is also shown.
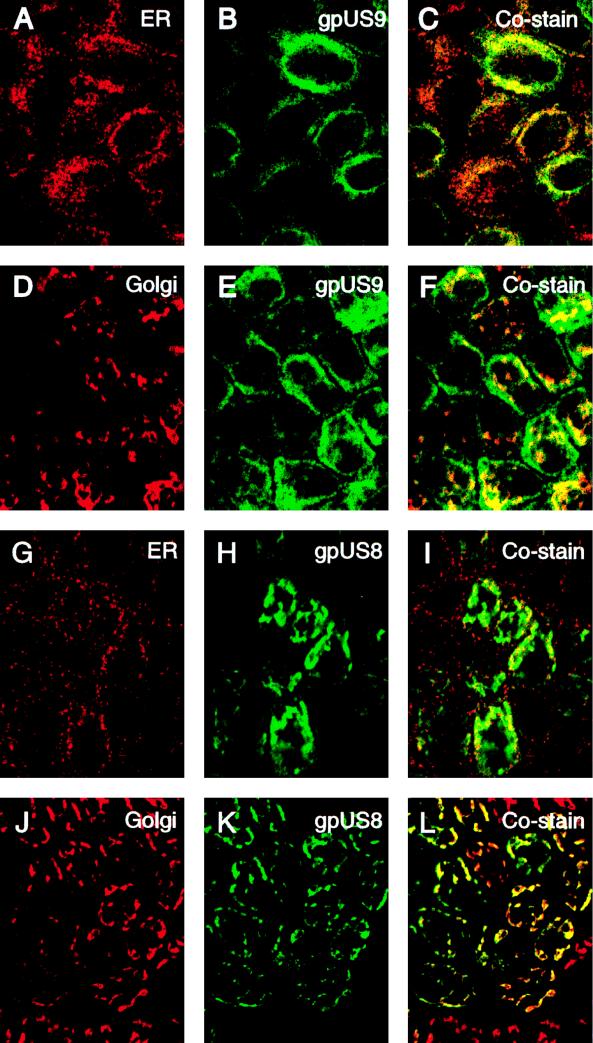
Next, we examined MUS9ep and MUS8ep cells after 4 days’ growth on permeable filter supports. Again, we found that the CMV glycoproteins colocalized with the ER and the Golgi compartments, but the staining patterns were noticeably different from those in nonpolarized cells (Fig. 4). The gpUS9 staining pattern overlay the ER (Fig. 4A to C) and the Golgi compartments (Fig. 4D to F), but a substantial fraction of gpUS9 accumulated at the peripheries of the cells (Fig. 4E to F). In contrast, gpUS8 colocalized with the ER (Fig. 4G to I) and Golgi compartments (Fig. 4J to L) in polarized and in nonpolarized MDCK cells. Results of these experiments suggested that both gpUS9 and gpUS8 overlay the ER and Golgi compartments, but a substantial portion of gpUS9 was found along lateral membranes of polarized MDCK cells.
FIG. 4.
Immunofluorescence analysis of CMV gpUS9 and gpUS8 distribution in the ER and Golgi compartments of polarized epithelial cells on permeable filter supports at 4 days. MUS9ep clone 102 (A to F), MUS8ep clone 1D5 (G to L), gpUS9 (B, C, E, and F), and gpUS8 (H, I, K, and L) are shown. Antiserum to GRP94 in the ER was used for panels A and G, and LcH agglutinin reactive with Golgi proteins was used for panels D and J. Costaining of gpUS9 in the ER (C) and Golgi compartments (F) and of gpUS8 in the ER (I) and Golgi compartments (L) is also shown.
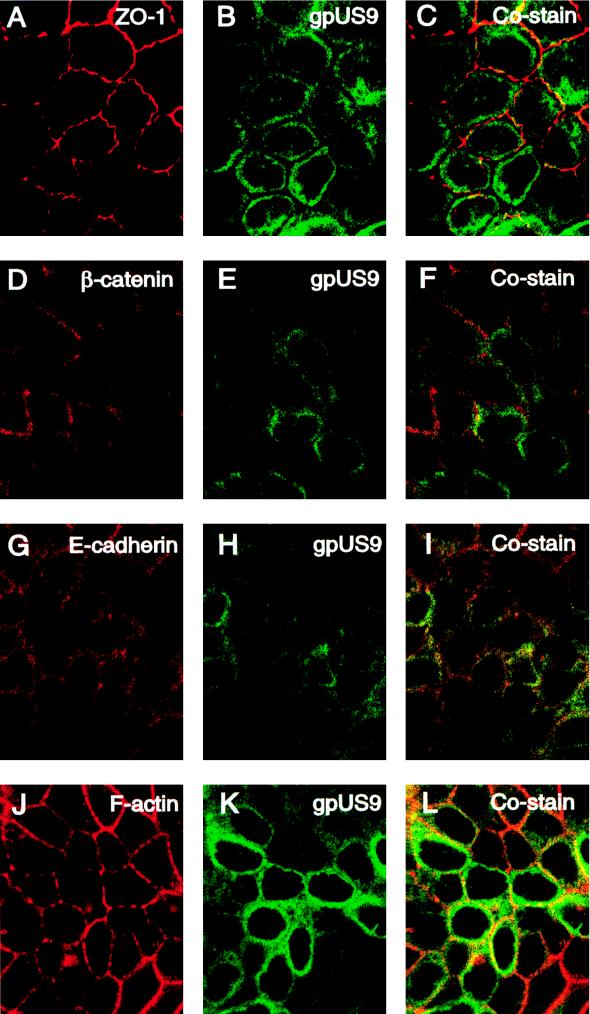
gpUS9 colocalizes with proteins in lateral membranes and the actin cytoskeleton.
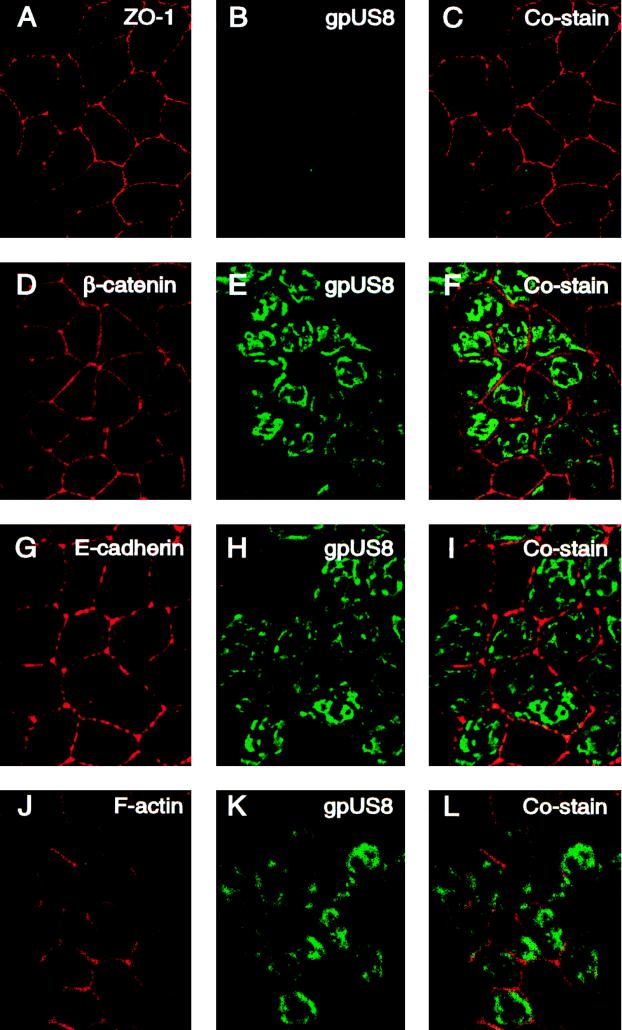
We reported that mutants with deletions of the US9 gene are impaired in cell-to-cell spread in polarized epithelial cells grown on permeable filters but form wild-type plaques in cultures of nonpolarized HF (39, 45, 55). Since a substantial fraction of gpUS9 appeared adjacent to lateral membranes, we next monitored the distribution patterns of the viral glycoproteins and cellular proteins in the cytoskeleton (Fig. 5). Cells were propagated on permeable filters for 4 days and reacted with antibodies to the tagged glycoproteins and key proteins in the epithelial cell architecture, including ZO-1, E-cadherin, β- catenin, and the cortical actin cytoskeleton. In MUS9ep cells, ZO-1, a tight junction protein, stained in a tight ringlike pattern at the interface between apical and basolateral membranes (Fig. 5A). We observed that gpUS9 stained with a broad ringlike pattern indicative of basolateral distribution (Fig. 5B) and found that gpUS9 was present at the level of ZO-1 but did not overlie this component of tight junction complexes (Fig. 5C). β- Catenin (Fig. 5D) and E-cadherin (Fig. 5G) in adherens junctions and F-actin in the cortical actin cytoskeleton (Fig. 5J) also stained in a basolateral pattern. gpUS9 was detected at the level of adherens junctions but did not costain significantly with β- catenin (Fig. 5F). In contrast, gpUS9 codistributed with E-cadherin, a transmembrane protein in adherens junctions (Fig. 5I). A particularly strong costaining pattern was obtained between gpUS9 and F-actin in the cortical cytoskeleton, which underlies lateral membranes (Fig. 5L). Examination of gpUS8 showed that it was not present at the level of ZO-1 in tight junctions (Fig. 6B) and did not overlie any proteins in junctional complexes or the actin cytoskeleton (Fig. 6C, F, I, and L). It should be mentioned that, like gpUS8, CMV gB is transported to apical membranes of polarized MDCK cells (55) and fails to costain with components of tight junctions, adherens junctions, or the actin cytoskeleton (54). These experimental results indicate that gpUS9 is present in large amounts at the level of tight junctions and adherens junctions, accumulates along lateral membranes, and colocalizes with E-cadherin and F-actin, which are cytoskeletal components of polarized epithelial cells.
FIG. 5.
Immunofluorescence analysis showing colocalization of CMV gpUS9 with junctional proteins and cortical actin of polarized epithelial cells. Cells were grown on microporous filters for 4 days and then fixed and stained for gpUS9, ZO-1, E-cadherin, β- catenin, and F-actin. ZO-1 accumulated at tight junctions of the apical-basolateral cell border. Optical sections of E-cadherin and β- catenin in adherens junctions were taken 1.5 μm below the apical membrane; the optical section of F-actin was taken 2.0 μm below the apical membrane.
FIG. 6.
Immunofluorescence analysis showing CMV gpUS8 distribution in polarized epithelial cells. Cells were grown on microporous filters for 4 days and then fixed and stained for gpUS8, ZO-1, E-cadherin, β- catenin, and F-actin as described in the legend to Fig. 5.
Insolubility of gpUS9 in polarized epithelial cells.
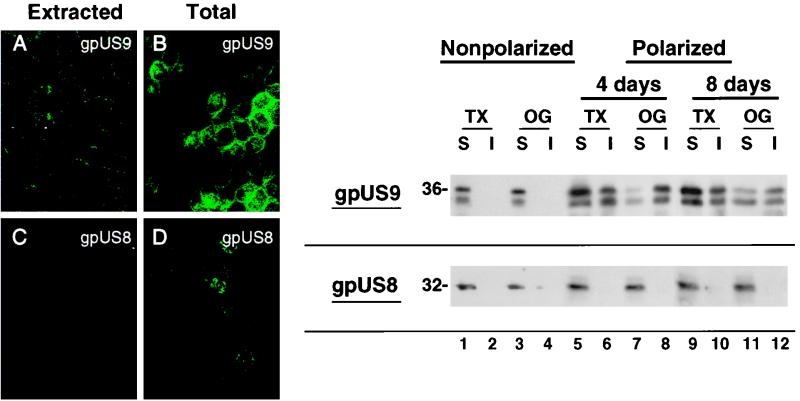
The finding that gpUS9 colocalizes with the cortical actin cytoskeleton of epithelial cells suggested that gpUS9 might partition with cellular proteins in a detergent-insoluble fraction and that it might differ in solubility from gpUS8. To investigate this possibility, we extracted polarized MUS9ep and MUS8ep cells on filters with Triton X-100 prior to fixation as described in Materials and Methods. Control cells were fixed and then extracted and stained for gpUS9 and gpUS8 (Fig. 7A to D). Comparison of the protein remaining following extraction showed that gpUS9 was in the Triton X-insoluble fraction, whereas gpUS8 was not (Fig. 7A and C). gpUS9 appeared as a discontinuous ring along lateral membranes. Both gpUS9 and gpUS8 were detected when the total cellular proteins were fixed before extraction (Fig. 7B and D).
FIG. 7.
Solubility of CMV gpUS9 and gpUS8 in polarized epithelial cells extracted with nonionic detergents. (Left panel) immunofluorescence staining of gpUS9 (A) and gpUS8 (C) after extraction, compared with total gpUS9 (B) and gpUS8 (D). (Right panel) MUS9ep and MUS8ep cells were cultured on glass (nonpolarized) and for 4 and 8 days, respectively, on microporous filters (polarized). Western blot analysis of CMV gpUS9 and gpUS8 after extraction of cells in Triton X-100 (TX) and octylglucoside (OG) is shown. Molecular mass markers, in kilodaltons, are shown at the left.
To determine whether the insolubility of gpUS9 depended on cell polarity, we extracted cells grown under nonpolarization and polarization conditions. For these experiments, cells were propagated on glass (nonpolarized) or on permeable filter supports (polarized) and then extracted with Triton X-100 and octylglucoside as described in Materials and Methods. Following extraction, the soluble and insoluble fractions were denatured, subjected to PAGE, and analyzed by ECL Western blotting (Fig. 7, right panel). Analysis of extracts from nonpolarized cells showed that all of gpUS9 and gpUS8 was present in the soluble fraction (Fig. 7, lanes 1 to 4). In polarized cells, however, nearly equal amounts of gpUS9 partitioned with both the detergent-soluble and -insoluble fractions at 4 days (Fig. 7, lanes 5 to 8) and at 8 days (Fig. 7, lanes 9 to 12). In contrast, gpUS8 was entirely soluble in both nonpolarized and polarized detergent-treated cells (Fig. 7). Results of these experiments indicate that a substantial fraction of gpUS9 is insoluble in Triton X-100 and octylglucoside, in contrast to gpUS8, even though the glycoproteins are similar in molecular mass and carbohydrate composition. The insolubility of CMV gpUS9 in polarized epithelial cells supports the colocalization patterns, which suggest that it may associate with insoluble components of the cortical actin cytoskeleton that is assembled in fully polarized cells.
DISCUSSION
We recently reported that human CMV deletion mutants RV35, RV61, and RV80, which lack the US9 gene of the parent strain AD169, were impaired in cell-to-cell transmission of virus and in altering junctional complexes in polarized human ARPE-19 cells grown on permeable filter supports (39, 45) but did not exhibit a defect in cell-to-cell transmission in nonpolarized HF (31). The small-plaque phenotype exhibited by CMV mutants with deletions of US9 indicated that this gene product plays an accessory role in infection, facilitating virus spread across lateral membranes in infected epithelial cells, and suggested that the highly structured junctions between polarized cells were altered. In the present study, we tested this hypothesis and found that gpUS9 became insoluble in polarized MDCK cells and associated with cytoskeletal proteins.
We expressed the products of US9 and, as a control, US8 in COS- 1 cells and identified novel glycoproteins of approximately 36 and 32 kDa, as predicted from the nucleotide sequence of the CMV genome (9). These we designated gpUS9 and gpUS8, respectively. MUS9ep and MUS8ep cells, which were selected from transfected MDCK cells that constitutively express these glycoproteins independently of other CMV proteins, were polarized after 4 days’ growth on permeable filters. gpUS9 was enriched at the level of apical junctions and along lateral membranes and codistributed with E-cadherin and F-actin in the cortical cytoskeleton in MDCK cells. The Jones laboratory has shown that US9 transcripts with β- class kinetics of synthesis are detected in CMV-infected HF (30). It remains to be determined whether CMV gpUS9 is produced in CMV-infected polarized ARPE-19 cells. Based on the impaired cell-to-cell spread of deletion mutants lacking gpUS9 (39), we speculate that accumulation of CMV gpUS9 along lateral membranes of polarized epithelial cells may alter the associations between cadherin and the actin cytoskeleton and promote the spread of infection.
The tight junction is an element of epithelial and endothelial cell-cell junctions that functions as a barrier to the diffusion of solutes through the paracellular pathway as well as a “fence” between the apical and basolateral membranes to create and maintain cell polarity (reviewed in reference 20). Tight junction assembly is regulated in part by E-cadherin-mediated assembly of the cell-cell adhesion complex at the adherens junction (reviewed in reference 23). In MDCK cells grown on permeable filters, well-developed polarity forms between 4 and 8 days and proteins in the actin cytoskeleton associate with proteins at cellular junctions, forming Triton X-100-insoluble protein complexes (43). ZO-1, E-cadherin, and α-, β- , and γ- catenins, which are assembled into cellular junctions, are insoluble, but the nonassembled protein fraction remains soluble. On the basis of our observation that CMV gpUS9 colocalizes with the actin cytoskeleton and forms a Triton X-100-insoluble protein complex, we speculate that it may bind to proteins in adherens junctions, perhaps E-cadherin, disrupting its association with cellular partners and thereby facilitating virus transmission. Constitutive expression of gpUS9 and gpUS8 did not block the development of epithelial cell polarity; in fact, the presence of gpUS9 in large amounts at adherens junction complexes was dependent on polarization. When MUS9ep cells were fully polarized, the pattern of gpUS9 overlay that of the cortical actin cytoskeleton. Thus, gpUS9 accumulated along the undercoat of lateral membranes, where proteins in adherens junction complexes bind to the actin cytoskeleton.
It is not known whether CMV gpUS9 interacts directly with F-actin or with proteins that bind the actin cytoskeleton or whether it alters proteins in signalling pathways that maintain the apical junctional complex. It has been recently appreciated that intracellular bacterial pathogens and viruses manipulate the actin cytoskeleton in epithelial cells to promote efficient entry and cell-to-cell spread (reviewed in references 12 and 52). It was reported that CMV causes rapid cytoskeletal disruption early in infection, that actin depolymerization facilitates viral infectivity, and that an isoform of cellular actin was associated with purified CMV virions (4, 28). Assembly of proteins in junctional complexes of polarized cells and their link to the actin cytoskeleton are highly regulated and involve signal transduction pathways (5, 13, 37, 57). In view of changes in the organization of the cytoskeleton that occur in CMV-infected polarized ARPE-19 cells (39), it is possible that signalling pathways regulating F-actin assembly in polarized epithelial cells may also be affected (44).
Several important questions relevant to CMV pathogenesis and the transmission of infection in epithelial cells and in human tissues in vivo are unresolved. Whether CMV gpUS9 is a structural component of the virion envelope that directs transport of progeny virions across lateral membranes, forms a complex with other viral glycoproteins that modulate its function, or is functionally redundant in strains that contain the set of putative glycoprotein genes lost during passage of the laboratory strain AD169 (8) must be clarified. We are in the process of producing MAbs to purified gpUS9 in order to examine the properties of this glycoprotein in CMV-infected human ARPE-19 cells. Regarding the relationship between spread of CMV in tissues and gpUS9 expression, it is possible that transport of this glycoprotein to lateral membranes destabilizes proteins in adherens junction complexes and the actin cytoskeleton in CMV-infected cells, enabling virus to spread in tissues composed of polarized cells. This is an attractive hypothesis that remains to be proved.
ACKNOWLEDGMENTS
These studies were supported by Public Health Service grants EY10138 and EY11223 from the National Institutes of Health (L.P.) and a grant from the University of California San Francisco AIDS Clinical Research Center.
We thank Zoya Kharitonov for excellent technical assistance, Keith Mostov (University of California, San Francisco) for kindly providing MDCK cells, and Inka Nathke (Harvard University) and James Nelson (Stanford University) for their generous gifts of antisera to β- catenin. We thank Caroline Damsky and Ed Mocarski for advice and critical discussions of this work.
REFERENCES
- 1.Ahn K, Gruhler A, Galocha B, Jones T R, Wiertz E J, Ploegh H L, Peterson P A, Yang Y, Fruh K. The ER-luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP. Immunity. 1997;6:613–621. doi: 10.1016/s1074-7613(00)80349-0. [DOI] [PubMed] [Google Scholar]
- 2.Anderson J M, Stevenson B R, Jesaitis A, Goodenough D A, Mooseker M S. Characterisation of ZO-1, a protein component of the tight junction from mouse liver and Madin-Darby canine kidney cells. J Cell Biol. 1988;106:1141–1149. doi: 10.1083/jcb.106.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balan P, Davis P N, Bell S, Atkinson H, Browne H, Minson T. An analysis of the in vitro and in vivo phenotypes of mutants of herpes simplex virus type 1 lacking glycoproteins gG, gE, gI or the putative gJ. J Gen Virol. 1994;75:1245–1258. doi: 10.1099/0022-1317-75-6-1245. [DOI] [PubMed] [Google Scholar]
- 4.Baldick C J, Jr, Shenk T. Proteins associated with purified human cytomegalovirus particles. J Virol. 1996;70:6097–6105. doi: 10.1128/jvi.70.9.6097-6105.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bockholt S M, Otey C A, Glenney J R, Burridge K. Localization of a 215-kDa tyrosine-phosphorylated protein that cross-reacts with tensin antibodies. Exp Cell Res. 1992;203:39–46. doi: 10.1016/0014-4827(92)90037-9. [DOI] [PubMed] [Google Scholar]
- 6.Bowen E F, Wilson P, Cope A, Sabin C, Griffiths P, Davey C, Johnson M, Emery V. Cytomegalovirus retinitis in AIDS patients: influence of cytomegaloviral load on response to ganciclovir, time to recurrence and survival. AIDS. 1996;10:1515–1520. doi: 10.1097/00002030-199611000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Britt W J, Alford C A. Cytomegalovirus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2493–2523. [Google Scholar]
- 8.Cha T-A, Tom E, Kemble G W, Duke G M, Mocarski E S, Spaete R R. Human cytomegalovirus clinical isolates carry at least 19 genes not found in laboratory strains. J Virol. 1996;70:78–83. doi: 10.1128/jvi.70.1.78-83.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chee M S, Bankier A T, Beck S, Bohni R, Brown C M, Cerny R, Horsnell T, Hutchison C A, Kouzarides T, Martignetti J A, Preddie E, Satchwell S C, Tomlinson P, Weston K M, Barrell B G. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr Top Microbiol Immunol. 1990;154:125–170. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- 10.Cohen G H, Dietzschold B, Ponce de Leon M, Long D, Golub E, Varrichio A, Pereira L, Eisenberg R J. Localization and synthesis of an antigenic determinant of herpes simplex virus glycoprotein D that stimulates the production of neutralizing antibody. J Virol. 1984;49:102–108. doi: 10.1128/jvi.49.1.102-108.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coskuncan N M, Jabs D A, Dunn J P, Haller J A, Green W R, Vogelsang G B, Santos G W. The eye in bone marrow transplantation. VI. Retinal complications. Arch Ophthalmol. 1994;112:372–379. doi: 10.1001/archopht.1994.01090150102031. [DOI] [PubMed] [Google Scholar]
- 12.Cudmore S, Reckmann I, Way M. Viral manipulations of the actin cytoskeleton. Trends Microbiol. 1997;5:142–148. doi: 10.1016/S0966-842X(97)01011-1. [DOI] [PubMed] [Google Scholar]
- 13.Davis S, Lu M L, Lo S H, Lin S, Butler J A, Druker B J, Roberts T M, An Q, Chen L B. Presence of an SH2 domain in the actin-binding protein tensin. Science. 1991;252:712–715. doi: 10.1126/science.1708917. [DOI] [PubMed] [Google Scholar]
- 14.Dingwell K S, Brunetti C R, Hendricks R L, Tang Q, Tang M, Rainbow A J, Johnson D C. Herpes simplex virus glycoproteins E and I facilitate cell-to-cell spread in vivo and across junctions of cultured cells. J Virol. 1994;68:834–845. doi: 10.1128/jvi.68.2.834-845.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drew L. Cytomegalovirus infection in patients with AIDS. J Infect Dis. 1988;158:449–456. doi: 10.1093/infdis/158.2.449. [DOI] [PubMed] [Google Scholar]
- 16.Dunn K C, Aotaki K A, Putkey F R, Hjelmeland L M. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996;62:155–169. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- 17.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol. 1994;127:1617–1626. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geiger B, Ayalon O. Cadherins. Annu Rev Cell Biol. 1992;8:307–332. doi: 10.1146/annurev.cb.08.110192.001515. [DOI] [PubMed] [Google Scholar]
- 20.Gumbiner B. Structure, biochemistry, and assembly of epithelial tight junctions. Am J Physiol. 1987;253:C749–C758. doi: 10.1152/ajpcell.1987.253.6.C749. [DOI] [PubMed] [Google Scholar]
- 21.Gumbiner B, Lowenkopf T, Apatira D. Identification of a 160-kDa polypeptide that binds to the tight junction protein ZO-1. Proc Natl Acad Sci USA. 1991;88:3460–3464. doi: 10.1073/pnas.88.8.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gumbiner B M. Breaking through the tight junction barrier. J Cell Biol. 1993;123:1631–1633. doi: 10.1083/jcb.123.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gumbiner B M. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 24.Holland G N. AIDS: retinal and choroidal infections. In: Lewis H, Ryan S J, editors. Medical and surgical retina: advances, controversies, and management. St. Louis, Mo: Mosby; 1994. pp. 415–433. [Google Scholar]
- 25.Hubenthal-Voss J, Houghten R A, Pereira L, Roizman B. Mapping of functional and antigenic domains of the α4 protein of herpes simplex virus 1. J Virol. 1988;62:454–462. doi: 10.1128/jvi.62.2.454-462.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jabs D A. Ocular manifestations of HIV infection. Trans Am Ophthalmol Soc. 1995;93:623–683. [PMC free article] [PubMed] [Google Scholar]
- 27.Jabs D A, Dunn J P, Enger C, Forman M, Bressler N, Charache P. Cytomegalovirus retinitis and viral resistance. Prevalence of resistance at diagnosis, 1994. Cytomegalovirus Retinitis and Viral Resistance Study Group. Arch Ophthalmol. 1996;114:809–814. doi: 10.1001/archopht.1996.01100140023002. [DOI] [PubMed] [Google Scholar]
- 28.Jones N L, Lewis J C, Kilpatrick B A. Cytoskeletal disruption during human cytomegalovirus infection of human lung fibroblasts. Eur J Cell Biol. 1986;41:304–312. [PubMed] [Google Scholar]
- 29.Jones T R, Hanson L K, Sun L, Slater J S, Stenberg R M, Campbell A E. Multiple independent loci within the human cytomegalovirus unique short region down-regulate expression of major histocompatibility complex class I heavy chains. J Virol. 1995;69:4830–4841. doi: 10.1128/jvi.69.8.4830-4841.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones T R, Muzithras V P. Fine mapping of transcripts expressed from the US6 gene family of human cytomegalovirus strain AD169. J Virol. 1991;65:2024–2036. doi: 10.1128/jvi.65.4.2024-2036.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones T R, Muzithras V P. A cluster of dispensable genes within the human cytomegalovirus genome short component: IRS1, US1 through US5, and the US6 family. J Virol. 1992;66:2541–2546. doi: 10.1128/jvi.66.4.2541-2546.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones T R, Muzithras V P, Gluzman Y. Replacement mutagenesis of the human cytomegalovirus genome: US10 and US11 gene products are nonessential. J Virol. 1991;65:5860–5872. doi: 10.1128/jvi.65.11.5860-5872.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones T R, Sun L. Human cytomegalovirus US2 destabilizes major histocompatibility complex class I heavy chains. J Virol. 1997;71:2970–2979. doi: 10.1128/jvi.71.4.2970-2979.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones T R, Wiertz E J, Sun L, Fish K N, Nelson J A, Ploegh H L. Human cytomegalovirus US3 impairs transport and maturation of major histocompatibility complex class I heavy chains. Proc Natl Acad Sci USA. 1996;93:11327–11333. doi: 10.1073/pnas.93.21.11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kondo K, Kaneshima H, Mocarski E S. Human cytomegalovirus latent infection of granulocyte-macrophage progenitors. Proc Natl Acad Sci USA. 1994;91:11879–11883. doi: 10.1073/pnas.91.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LaFemina R L, Hayward G S. Structural organization of the DNA molecules from human cytomegalovirus. In: Fields B N, Jaenisch R, Fox C F, editors. Animal virus genetics. New York, N.Y: Academic Press; 1980. pp. 39–55. [Google Scholar]
- 37.Lo S H, Weisberg E, Chen L B. Tensin: a potential link between the cytoskeleton and signal transduction. Bioessays. 1994;16:817–823. doi: 10.1002/bies.950161108. [DOI] [PubMed] [Google Scholar]
- 38.Machold R P, Wiertz E J, Jones T R, Ploegh H L. The HCMV gene products US11 and US2 differ in their ability to attack allelic forms of murine major histocompatibility complex (MHC) class I heavy chains. J Exp Med. 1997;185:363–366. doi: 10.1084/jem.185.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maidji E, Tugizov S, Jones T, Zheng Z, Pereira L. Accessory human cytomegalovirus glycoprotein US9 in the unique short component of the viral genome promotes cell-to-cell transmission of virus in polarized epithelial cells. J Virol. 1996;70:8402–8410. doi: 10.1128/jvi.70.12.8402-8410.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mays R W, Beck K A, Nelson W J. Organization and function of the cytoskeleton in polarized epithelial cells: a component of the protein sorting machinery. Curr Opin Cell Biol. 1994;6:16–24. doi: 10.1016/0955-0674(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 41.Mocarski E S. Cytomegaloviruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2447–2492. [Google Scholar]
- 42.Mostov K E. Regulation of protein traffic in polarized epithelial cells. Histol Histopathol. 1995;10:423–431. [PubMed] [Google Scholar]
- 43.Nathke I S, Hinck L, Swedlow J R, Papkoff J, Nelson W J. Defining interactions and distributions of cadherin and catenin complexes in polarized epithelial cells. J Cell Biol. 1994;125:1341–1352. doi: 10.1083/jcb.125.6.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nusrat A, Giry M, Turner J R, Colgan S P, Parkos C A, Carnes D, Lemichez E, Boquet P, Madara J L. Rho protein regulates tight junctions and perijunctional actin organization in polarized epithelia. Proc Natl Acad Sci USA. 1995;92:10629–10633. doi: 10.1073/pnas.92.23.10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pereira L, Maidji E, Tugizov S, Jones T. Deletion mutants in human cytomegalovirus glycoprotein US9 are impaired in cell-cell transmission and in altering tight junctions of polarized human retinal pigment epithelial cells. Scand J Infect Dis Suppl. 1995;99:82–87. [PubMed] [Google Scholar]
- 46.Rodriguez-Boulan E, Powell S K. Polarity of epithelial and neuronal cells. Annu Rev Cell Biol. 1992;8:395–427. doi: 10.1146/annurev.cb.08.110192.002143. [DOI] [PubMed] [Google Scholar]
- 47.Sargiacomo M, Sudol M, Tang Z, Lisanti M P. Signal transducing molecules and glycosyl-phosphatidylinositol-linked proteins form a caveolin-rich insoluble complex in MDCK cells. J Cell Biol. 1993;122:789–807. doi: 10.1083/jcb.122.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soderberg-Naucler C, Fish K N, Nelson J A. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell. 1997;91:119–126. doi: 10.1016/s0092-8674(01)80014-3. [DOI] [PubMed] [Google Scholar]
- 49.Spaete R R, Gehrz R C, Landini M P. Human cytomegalovirus structural proteins. J Gen Virol. 1994;75:3287–3308. doi: 10.1099/0022-1317-75-12-3287. [DOI] [PubMed] [Google Scholar]
- 50.Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- 51.Taylor-Weideman J A, Sissons J G P, Borysiewicz L K, Sinclair J H. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J Gen Virol. 1991;72:2059–2064. doi: 10.1099/0022-1317-72-9-2059. [DOI] [PubMed] [Google Scholar]
- 52.Theriot J A. The cell biology of infection by intracellular bacterial pathogens. Annu Rev Cell Dev Biol. 1995;11:213–239. doi: 10.1146/annurev.cb.11.110195.001241. [DOI] [PubMed] [Google Scholar]
- 53.Tsukita S, Tsukita S, Nagafuchi A, Yonemura S. Molecular linkage between cadherins and actin filaments in cell-cell adherens junctions. Curr Opin Cell Biol. 1992;4:834–839. doi: 10.1016/0955-0674(92)90108-o. [DOI] [PubMed] [Google Scholar]
- 54.Tugizov, S., E. Maidji, and L. Pereira. Unpublished observation.
- 55.Tugizov S, Maidji E, Pereira L. Role of apical and basolateral membranes in replication of human cytomegalovirus in polarized retinal pigment epithelial cells. J Gen Virol. 1996;77:61–74. doi: 10.1099/0022-1317-77-1-61. [DOI] [PubMed] [Google Scholar]
- 56.Tugizov S, Navarro D, Paz P, Wang Y, Qadri I, Pereira L. Function of human cytomegalovirus glycoprotein B: syncytium formation in cells constitutively expressing gB is blocked by virus-neutralizing antibodies. Virology. 1994;201:263–276. doi: 10.1006/viro.1994.1291. [DOI] [PubMed] [Google Scholar]
- 57.Turner C E, Burridge K. Transmembrane molecular assemblies in cell-extracellular matrix interactions. Curr Opin Cell Biol. 1991;3:849–853. doi: 10.1016/0955-0674(91)90059-8. [DOI] [PubMed] [Google Scholar]
- 58.van der Meer J, Drew W L, Bowden R A, Galasso G J, Griffiths P D, Jabs D A, Katlama C, Spector S A, Whitley R J. Summary of the International Consensus Symposium on Advances in the Diagnosis, Treatment and Prophylaxis of Cytomegalovirus Infection. Antivir Res. 1996;32:119–140. doi: 10.1016/s0166-3542(96)01006-6. [DOI] [PubMed] [Google Scholar]
- 59.Wiertz E J, Jones T R, Sun L, Bogyo M, Geuze H J, Ploegh H L. The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell. 1996;84:769–779. doi: 10.1016/s0092-8674(00)81054-5. [DOI] [PubMed] [Google Scholar]
- 60.Wiertz E J, Tortorella D, Bogyo M, Yu J, Mothes W, Jones T R, Rapoport T A, Ploegh H L. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature. 1996;384:432–438. doi: 10.1038/384432a0. [DOI] [PubMed] [Google Scholar]