Highlights
-
•
Combination treatment with anti-PD-L1 blockade and IL-15 superagonism enhanced NK-cell killing of SNUC cells.
-
•
Untreated SNUC patient tumor samples were found to have an NK cell infiltrate and PD-L1+ tumor cells.
-
•
A striking 55.7-fold increase in CKlow tumor cell/NK cell interactions was observed in patients without disease recurrence after treatment.
-
•
Patients with higher CD3+CD8+ in the stroma had a significantly improved 5-year overall survival.
-
•
A significant increase in CKlow tumor cell/CD8+ cytotoxic T cell interactions was noted in long-term survivors.
Keywords: Sinonasal undifferentiated carcinoma, Immunotherapy, Natural killer cells, Immune microenvironment, Antibody-dependent cellular cytotoxicity, IL-15
Abstract
Purpose
Sinonasal undifferentiated carcinoma (SNUC) is a rare, aggressive malignancy of the sinonasal cavity with poor prognosis and limited treatment options. To investigate the potential for SNUC sensitivity to combinatory immunotherapy, we performed in vitro studies with SNUC cell lines and used multi-spectral immunofluorescence to characterize the in vivo patient SNUC tumor immune microenvironment (TIME).
Experimental design
Human-derived SNUC cell lines were used for in vitro studies of tumor cell susceptibility to natural killer (NK) cell-based immunotherapeutic strategies. Tumor samples from 14 treatment naïve SNUC patients were examined via multi-spectral immunofluorescence and clinical correlations assessed.
Results
Anti-PD-L1 blockade enhanced NK cell lysis of SNUC cell lines ∼5.4 fold (P ≤ 0.0001). This effect was blocked by a CD16 neutralizing antibody demonstrating activity through an antibody-dependent cellular cytotoxicity (ADCC) mediated pathway. ADCC-dependent lysis of SNUC cells was further enhanced by upregulation of PD-L1 on tumor cells by exogenous interferon-gamma (IFN-γ) administration or interleukin-15 (IL-15) stimulated IFN-γ release from NK cells. Combination treatment with anti-PD-L1 blockade and IL-15 superagonism enhanced NK-cell killing of SNUC cells 9.6-fold (P ≤ 0.0001). Untreated SNUC patient tumor samples were found to have an NK cell infiltrate and PD-L1+ tumor cells at a median of 5.4 cells per mm2. A striking 55.7-fold increase in CKlow tumor cell/NK cell interactions was observed in patients without disease recurrence after treatment (P = 0.022). Patients with higher CD3+CD8+ in the stroma had a significantly improved 5-year overall survival (P = 0.0029) and a significant increase in CKlow tumor cell/CD8+ cytotoxic T cell interactions was noted in long-term survivors (P = 0.0225).
Conclusion
These data provide the pre-clinical rationale for ongoing investigation into combinatory immunotherapy approaches for SNUC.
Introduction
Sinonasal undifferentiated carcinoma (SNUC) is a rare, high-grade epithelial malignancy of the sinonasal cavity with an annual incidence ranging from 0.5 – 2.6 cases per 100,000 per year [1], [2], [3]. The World Health Organization (WHO) classifies SNUC as a clinicopathologically distinct carcinoma of unknown histogenesis that lacks squamous or glandular differentiation [4]. The male to female incidence ratio is approximately 2:1 [3,5,6]. SNUC is highly aggressive, and patients tend to present with locally advanced disease that frequently invades critical structures of the skull base, brain, and orbit [1,3,7]. High rates of locoregional recurrence and distant metastasis further contribute to the poor prognosis of this tumor type, with reported median overall survival rates of 22.1 months [6].
Aggressive multimodal combinations of chemotherapy, radiation, and surgical resection comprises the current standard of care for SNUC, but the proximity of these tumors to critical structures at the cranial base can hinder the efficacy of current treatment paradigms. While recent studies by our groups show induction chemotherapy may offer additional survival benefit in select patients [1,3], efforts to discover novel treatment approaches are necessary to reduce morbidity and improve survival of patients with SNUC [8,9]. Recent focuses in molecular characterization of SNUC have identified genomic alterations that may inform the discovery of diagnostic markers, prognostic factors, and targeted therapies [8], [9], [10], [11], [12], [13]. However, the clinical need for effective therapeutic options to combat this aggressive malignancy remains urgent and unmet.
The potential applicability of immunotherapeutic approaches in the treatment of SNUC is largely unstudied and the SNUC tumor immune microenvironment (TIME) remains poorly described. Immune checkpoint inhibitors targeting the Programmed Cell Death Protein 1 (PD-1) and Programmed Cell Death Ligand 1 (PD-L1) pathway have earned FDA approval in recurrent/metastatic head and neck squamous cell carcinoma [14,15]; however, only a single case report has investigated targeting this pathway in SNUC [16]. Resistance to checkpoint inhibition therapies is an ongoing focus of investigation, and aberrancies in tumor cell antigen processing and presentation play a role in this immune evasion [17], [18], [19]. Immunotherapy approaches that are functionally independent of both antigen and HLA specificity are one potential method of overcoming such challenges.
Natural killer cells are effector cells of the innate immune system that uniquely kill multiple adjacent target cells independent of antigen presentation or specificity and catalyze immune responses with antibodies and T cells, making them a promising anti-cancer immune compartment to circumvent mechanisms of T cell escape [18,20,21]. Avelumab is a commercially available antibody that uniquely disrupts PD-1/PD-L1 immune checkpoint signaling and promotes antibody-dependent cellular cytotoxicity (ADCC) with NK cells [22]. N-601 is a newly engineered structural analog of Avelumab that has been previously demonstrated by our group to coordinate ADCC with NK cells to lyse chordoma cells in vitro [23]. Furthermore, IFNγ release from activated NK and T cells upregulates PD-L1 on tumor cells, thus further sensitizing tumor cells to PD-L1 targeted immunotherapies and contributing to positive feedback loop of immunogenicity [18,[23], [24], [25]].
To further enhance the potential for ADCC, this study also evaluated the IL-15 superagonist N-803, IL-15N72D:IL-15RαSu/Fc complex consisting of IL-15 N72D and a dimeric IL-15Rα sushi domain-IgG1 Fc fusion protein [24,[26], [27], [28]]. IL-15 itself has been investigated as a potential immunotherapeutic option for treatment of malignancies due to its ability to enhance proliferation and activation of NK cells and CD8+ cytotoxic T cells [29,30], and N-803 has been demonstrated to show greater effects than IL-15 in its ability to increase NK and T-cell proliferation and activity, and exert anti-tumor effects in vivo.
We hypothesized that SNUC may be amenable to targeting the PD-1/PD-L1 pathway and using the only available models of SNUC sought to conduct the first pre-clinical investigation evaluating immunotherapeutic targeting of this aggressive malignancy. Given that SNUC cells in vitro do not fully re-capitulate the tumor immune microenvironment (TIME) and thus all the components that may influence response to immunotherapy in vivo, we conducted a multi-spectral immunofluorescence evaluation of tumor tissue samples from untreated SNUC patients to identify prognostic biomarkers and help guide future development of rational combinatory immunotherapy approaches.
Materials and methods
Cell lines and culture
Human-derived SNUC cell lines, labeled MDA8788–6 and MDA8788–7, were obtained from the Department of Head and Neck Surgery at MD Anderson Cancer Center (Houston, TX), where they were generated [31]. All cell lines were passaged twice per week, used at a low (<30) passage number, and verified to be free of mycoplasma infection.
Immune reagents and NK cells
The anti-PD-L1 antibody (N-601), the IL-15/IL-15r superagonist (N-803), and PD-L1 t-haNKs were provided by ImmunityBio under a Cooperative Research and Development Agreement with the National Cancer Institute of the National Institutes of Health. PD-L1 t-haNK cells were irradiated (15 Gy) prior to cryopreservation and shipment from ImmunityBio to the NIH for experimental use and were thawed on the same day as the experiment. Human IgG1 isotype control antibody and human anti-CD16 antibody were obtained from BioLegend. Healthy donor PBMCs were obtained from the NIH Clinical Center Blood Bank (NCT00001846). NK cells were isolated from PBMCs using the Miltenyi Biotec Human NK Cell Isolation (negative selection) Kit according to the manufacturer's protocol with >90 % purity.
Flow cytometric analysis
For assessment of survival, SNUC cells were labeled with LIVE/DEAD 488 Fixable Green Dead Cell Stain Kit (Life Technologies #L34970) according to the manufacturer's protocol. Human Fc block (BD #564,219) was added to 1 × 106 cells and incubated for 10 min on ice. To characterize PD-L1 expression in the SNUC cell lines, cells were stained with PD-L1-BV421 (BD #563,738) and fixed with Cytofix (BD #554,655). Marker expression was quantified by percent positive cells and mean fluorescence intensity (MFI). Flow cytometry was performed on BD FACSCanto (BD Biosciences) and analyzed using FlowJo v10.7.1 (TreeStar).
Indium-111 NK cell killing assays
NK cells (effector cells) were isolated from healthy donor PBMCs as previously described [23]. NK cells isolated from PBMCs were cultured for 24 h in the NK cell media described above with or without 50 ng/mL of N-803. Cryopreserved PD-L1 t-haNK cells were thawed on the day of the experiment and washed two times in the previously described NK cell media before being adjusted to the desired assay concentration and plated.
SNUC cells (target cells) were harvested from cell culture at approximately 80 % confluence and labeled with 111In (10 L/100,000 cells)(Supplementary Fig. 1). SNUC cells were plated at 2000 cells/well in 96-well round-bottom culture plates and incubated with 2 μg/mL of isotype control antibody or N-601 at room temperature for 30 min. NK cells (healthy donor or PD-L1 t-haNKs) were added to wells at an effector-to-target (E:T) ratio of 10:1. In experiments assessing the role of CD16 and ADCC in the mechanism of NK cell killing, NK cells were incubated with 2 μg/mL of anti-CD16 mAb for 2 h before being added to target cells. For experiments with IFNγ pretreatment, tumor cell lines were incubated with 20 ng/mL of IFNγ for 24 h prior to labeling with 111In. For experiments evaluating the effect of N-803 on IFNγ production from NK cells and subsequent upregulation of PD-L1 on tumor cells, healthy donor NK cells were cultured with 100 ng (50 ng/mL in 2 mL) immobilized N-803 for 24 h or left untreated. Supernatants from NK cell cultures were quantified for IFNγ using a human ELISA kit (Life Technologies). SNUC cells were then incubated for 24 h in supernatant harvested from N-803-treated or untreated NK cell cultures. As a positive control, SNUC cells were incubated with 20 ng/mL of IFNγ for 24 h. The SNUC cells were then analyzed by flow cytometry for PD-L1 expression and used as targets for NK cell-mediated lysis via 111In-release assay. To focus on the indirect effects of N-803 on tumor cell sensitivity to N-601-mediated ADCC, NK cells in this lysis experiment were not treated with N-803.
After 20 h, assay plates were centrifuged at 1500 RPM and supernatants were quantified for the presence of 111In using a PerkinElmer (Waltham, MA) WIZARD2 Automatic Gamma Counter. Spontaneous 111In release was determined by incubating target cells without effector cells, and complete lysis was determined by incubating target cells with 0.05 % Triton X-100. Experimental lysis was standardized using the following equation: Percent lysis = [(experimental cpm - spontaneous cpm) / (complete cpm - spontaneous cpm)] x 100. Negative control values in each bar graph represent spontaneous lysis of target cells without effector cells. All experiments were carried out in technical triplicate, with each individual experiment being repeated at least three separate times unless otherwise specified.
Patient tissue
Tumor samples obtained from 14 previously untreated patients with SNUC were used for this study. In accordance with the Declaration of Helsinki, all patients provided written informed consent and this study was approved by the MD Anderson Institutional Review Board. All samples were formalin-fixed paraffin-embedded (FFPE) and were re-reviewed by a single head and neck pathologist (D.B.). Patient data were collected from an MD Anderson Cancer Center institutional database. No patients had received treatment by the time of tissue collection, and 12 of them subsequently received platinum-based induction chemotherapy. Eight of the patients had responses to induction chemotherapy, and four did not. The patient demographics and clinical characteristics are summarized in Supplementary Table 1. The median follow-up time from the initial diagnosis to death or last contact was 32.2 months (range: 8.1–176.2 months).
Multi-spectral immunofluorescence staining
Four-µm-thick sections obtained from the 14 FFPE SNUC samples were labeled using the Opal Polaris 7 Color IHC Manual Detection Kit (Akoya Biosciences, Marlborough, MA). Antigen retrieval was performed in Citra Plus Antigen Solution (pH6.2, Biogenex Laboratories, Fremont, CA, HK0809K) for 15 min at 95 °C before each staining cycle. A multi-spectral immunofluorescence panel of antibodies was then used (Supplementary Table 2). After the 6 cycles of labeling, nuclei were counter-stained with DAPI. The slides were scanned at 40x magnification using the Vectra Polaris imaging system (Akoya Biosciences) to create high-resolution digital images.
Cellular identification and phenotyping
Images were analyzed using the Visiopharm software (Visiopharm, Horsholm, Denmark) platform version 2022.09. Tissue segmentation (tumor and stroma) was initially performed based on cell morphology, tissue structures, and CK signals, then confirmed using H&E staining as a reference after the cell detection process by using Visiopharm Deep Learning U-Net detecting DAPI to identify the cell nuclei. To generate the cell mask, the nuclei mask was dilated by 10 pixels to approximate the cell boundary for accurate phenotyping. As not all CD8+ cells were CD3+ positive, the CD8+ single positive branch was separately added to the cell identification map. Phenotyping was done using Visiopharm change by intensity option, which uses two measurements that include marker intensity (threshold) and percent coverage (object%) for each of the markers to identify the populations of interest. Tumor cells with low expression of pan CK were defined as ‘CKlow’ cells. We found that some cancer cells were NCAM/CD56 positive. To distinguish NK cells from NCAM/CD56 positive cancer cells, a combination of morphometric measurements such as cell size and forming factor was used to minimize the false positive signals.
Neighborhood analysis
After phenotyping the cell centroids (e.g. center-x and center-y) were extracted for each cell on the whole-slide image per slide as a tab-serpated file (.tsv) and transferred to RStudio for downstream analysis. The x and y coordinates were then converted to spatial point patterns where each centroid is considered using the R package spatstat [32]. A radius of 50 µm was used to assess cell-to-cell interaction and count the type and the number of each cell type within the measured radius determined from the x and y coordinates [33,34]. The final required cell-to-cell interactions were computed by counting the total number of different cell types found within the radius by group and dividing by the total tissue size (mm2). The effect of cell-to-cell interactions on clinical outcomes (e.g. response to induction chemotherapy, disease recurrence, and short-term survival and long-term survival using 12 months as a threshold) were assess by Wilcoxon test using the R package rstatix [35].
Statistical analyses
Comparisons in NK cell killing models or flow cytometric data between two independent groups were performed using a Student's t-test with a 2-tailed distribution at a confidence level of 95 %. Comparison of more than two independent groups was performed using one-way analysis of variance (ANOVA) with Tukey's multiple comparisons test. All error bars indicate standard error of the mean (SEM). Test results are reported as P-values with a significance cutoff set at P < 0.05. Expression of each marker versus overall survival status, response to induction chemotherapy, and recurrence status were assessed by Wilcoxon test, and the survival rate differences were calculated using Log-Rank testing. All analyses were performed using GraphPad 9.0 software (San Diego, CA).
Results
SNUC cell lines express PD-L1
Our first step in evaluating the feasibility of therapies targeting the PD-1/PD-L1 axis was to quantify PD-L1 expression in the only two available SNUC cell lines. Both cell lines expressed PD-L1, with the percentage of PD-L1 positive cells being 42.1 % and 73.2 %, and MFI 531 and 897, for MDA8788–6 and MDA8788–7 cell lines respectively (Table 1).
Table 1.
PD-L1 characterization of SNUC cell lines. *Bold denotes increase of ≥20 %.
| Cell line | Derivation | PD-L1 Expression |
|||
|---|---|---|---|---|---|
| IFN |
|||||
| - |
+ |
||||
| % positive | MFI | % positive | MFI | ||
| MDA8788–6 | 74-year-old female T4N0M0 SNUC Right maxillary sinus | 42.1 | 531 | 91.2 | 1543 |
| MDA8788–7 | 73.6 | 897 | 95.5 | 1938 | |
We hypothesized that IFNγ co-culture would enhance PD-L1 expression in these SNUC cell lines based on reports of this effect on tumor cells of other tissue types [18,[23], [24], [25]]. Indeed, co-culture of both SNUC cell lines with IFNγ resulted in upregulation of PD-L1 to 91.2% (1543 MFI) and 95.5 % (1938 MFI) for lines MDA8788–6 and MDA8788–7, respectively (Table 1). This upregulation in expression of PD-L1 was statistically significant when analyzed across three individual flow cytometric experiments (P ).
Anti-PD-L1 antibody enhances NK cell killing of SNUC cells through an ADCC-dependent mechanism
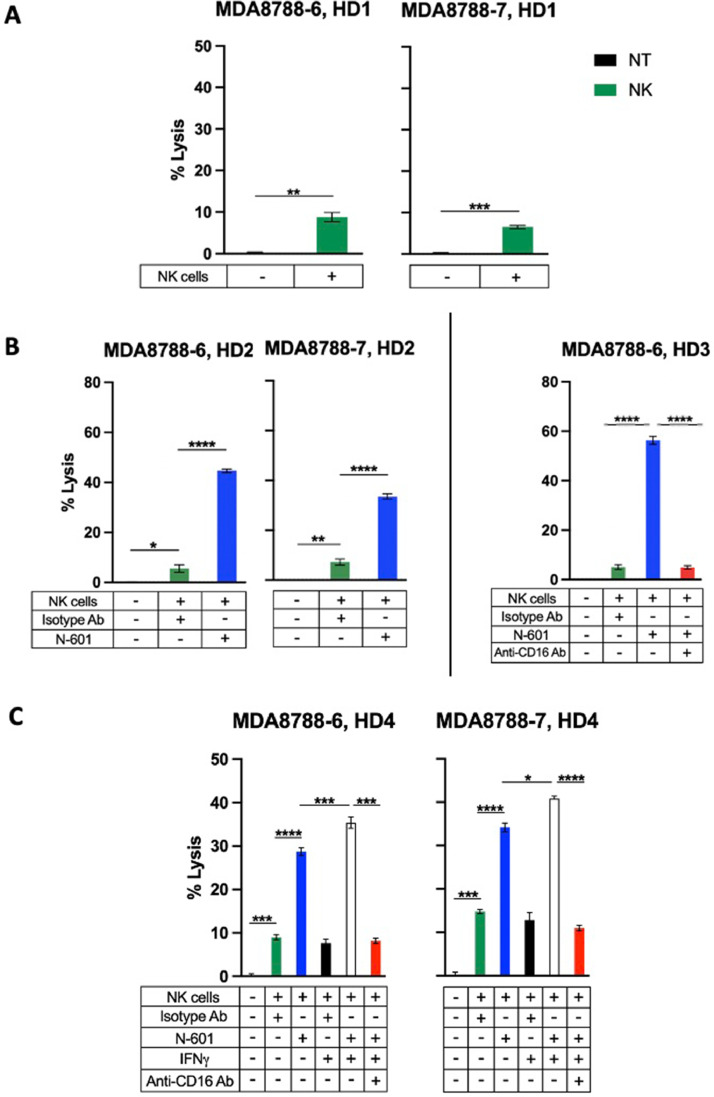
The susceptibility of SNUC cell lines to lysis by immune cells has not yet been demonstrated. Healthy donor NK cell–mediated lysis of both SNUC cell lines was significantly higher than spontaneous lysis nontreated controls but remained low overall (average: 7.8 %; SEM: 1.0 %) across >25 independent experiments (Fig. 1A). This modest cytotoxic effect of NK cells highlights the potential for augmentation of NK cell activity through immunomodulation.
Fig. 1.
SNUC cell lines are modestly susceptible to lysis by healthy donor natural killer (NK) cells at baseline, and cytotoxicity is enhanced by IFNγ-mediated PD-L1 upregulation and ligand targeting with N-601 through an ADCC-dependent mechanism. A. SNUC cell lines were used as targets for healthy donor NK cells versus non-treated (NT) controls. B. N-601 enhances NK cell killing of SNUC cells through antibody-dependent cellular cytotoxicity ADCC. N-601-mediated ADCC assays were performed by co-incubating SNUC cells with N-601 or isotype control antibody (Isotype Ab) prior to exposure to healthy donor NK cells (left). NK cells were treated with anti-CD-16 antibody for ADCC blocking experiments where indicated (right). C. IFNγ-treated SNUC cells show increased sensitivity to NK-cell lysis via N-601-mediated ADCC. SNUC cells were treated with 20 ng/mL of IFNγ or untreated as controls for 24 h prior to co-culture with N-601 or isotype control antibody (Isotype Ab). SNUC cells were used as targets for healthy donor NK cells in 111In-release killing assays. NK cells were treated with anti-CD-16 antibody for ADCC blocking experiments where indicated. E:T ratios are 10:1. **P ≤ 0.01, ***P ≤ 0.001.
Given the surface expression of PD-L1 in the SNUC cell lines (Table 1), we hypothesized that anti-PD-L1 blockade would similarly sensitize SNUC cells to NK-cell mediated lysis through mediation of ADCC. Co-culture of SNUC cells with anti-PD-L1 prior to exposure to healthy donor NK cells significantly increased tumor cell lysis of both SNUC cell lines compared to untreated controls (Fig. 1B). Across >20 independent experiments, N-601 treatment enhanced NK cell-mediated lysis of SNUC cells by an average of 5.4-fold (P ≤ 0.0001) compared to isotype control antibody (Fig. 1B). To illustrate the mechanism of N-601 enhancing NK cell killing of SNUC cells, we treated heathy donor NK cells with a CD16 neutralizing antibody before co-culture with tumor cells to inhibit ADCC. Blocking of CD16 abrogated the increase in tumor cell lysis seen with anti-PD-L1 treatment (Fig. 1B), thus demonstrating that N-601 enhances NK cell lysis of SNUC cells through an ADCC-dependent mechanism.
IFNγ-induced upregulation of PD-L1 on SNUC cells further enhances ADCC with anti-PD-L1 antibody (N-601)
After demonstrating that SNUC cell lysis by NK cells is amenable to improvement by an ADCC-coordinating antibody, our next aim was to utilize a biologically relevant mechanism to further enhance the sensitivity of tumor cells to such targeting. Tumor cells may be exposed to IFNγ from activated immune cells in a proinflammatory tumor immune microenvironment. Given the evidence of IFNγ’s ability to upregulate SNUC cell surface PD-L1 expression (Table 1), we hypothesized that this change would increase the efficacy of anti-PD-L1-coordinated ADCC with NK cells. Across 9 independent experiments, co-culture of SNUC cells with IFNγ enhanced NK cell-mediated lysis of both SNUC cell lines in the presence of N-601 by an average of 32 % (P ≤ 0.001) (Fig. 1C). Importantly, IFNγ exposure provided no cytotoxic advantage in the absence of N-601, suggesting that IFNγ functions through a PD-L1-dependent mechanism with N-601 and NK cells. Further, the enhanced cytotoxic effect of IFNγ treatment is entirely abrogated in the presence of a CD16 neutralizing antibody. Together, these findings suggest that exposure of tumor cells to IFNγ increases tumor cell lysis through PD-L1 upregulation and subsequent ligand targeting with N-601 through an ADCC-dependent mechanism.
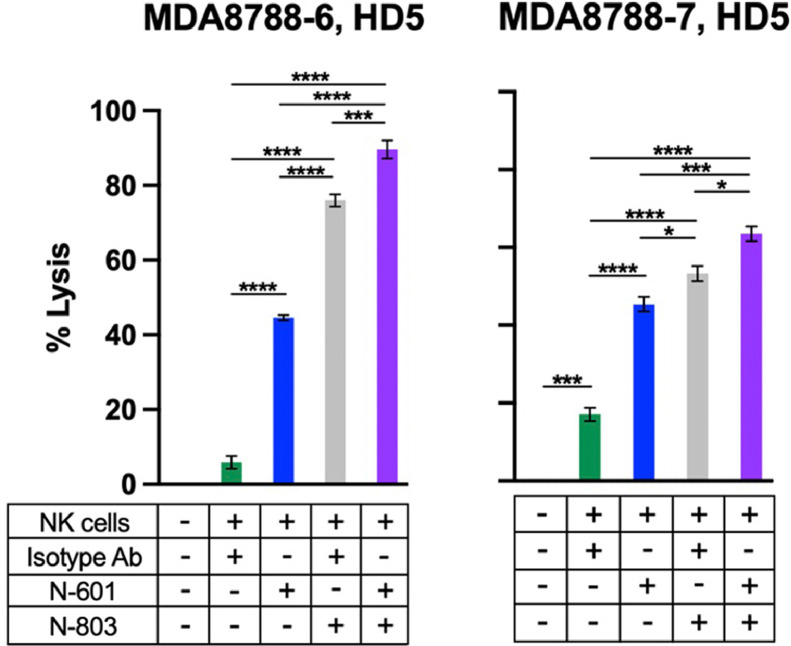
IL-15 superagonism (N-803) enhances NK cell killing of SNUC cells
We hypothesized that stimulation of NK cells with IL-15 superagonism would enhance their cytotoxic efficacy against SNUC cells. NK cells co-cultured with IL-15 superagonism were significantly more lethal against both SNUC cell lines compared to untreated NK cell controls (Fig. 2). The average increase in tumor cell lysis by N-803 treatment of NK cells was 8.2-fold (P ≤ 0.0001) compared to untreated controls (Fig. 2).
Fig. 2.
N-803-activated NK cells show enhanced killing of SNUC cells, and combinatory treatment with N-803 and N-601 further enhances cytotoxicity. N-601-mediated ADCC assays were performed by co-incubating SNUC cells with N-601 or isotype control antibody. Combination treatment assays were performed by co-incubating SNUC cells with N-601 or isotype control antibody and treating NK cells with N-803. E:T ratios are 10:1. *P ≤ 0.05, ***P ≤ 0.001, ****P ≤ 0.0001.
ADCC and cytokine-mediated activation are independent immunomodulatory mechanisms of anti-tumor immunity. Our group recently demonstrated the efficacy of a combinatorial NK cell-oriented approach using both N-601 and N-803 in preclinical models of chordoma [23]. Based on these findings and the expression of PD-L1 in this preclinical model of SNUC, we hypothesized that the combinatorial treatment of SNUC cells with N-601 and NK cells with N-803 would similarly enhance tumor cell lysis by NK cells compared to monotherapy treatments with either of these agents. The average increase in SNUC cell lysis by combinatory treatment with N-601 and N-803 was 9.6-fold (P ≤ 0.0001) compared to untreated controls (Fig. 2). Combination treatment with N-601 and N-803 significantly enhanced NK-cell killing of both SNUC cell lines compared to monotherapy with N-601 (P ≤ 0.001) or N-803 (P ≤ 0.05) (Fig. 2).
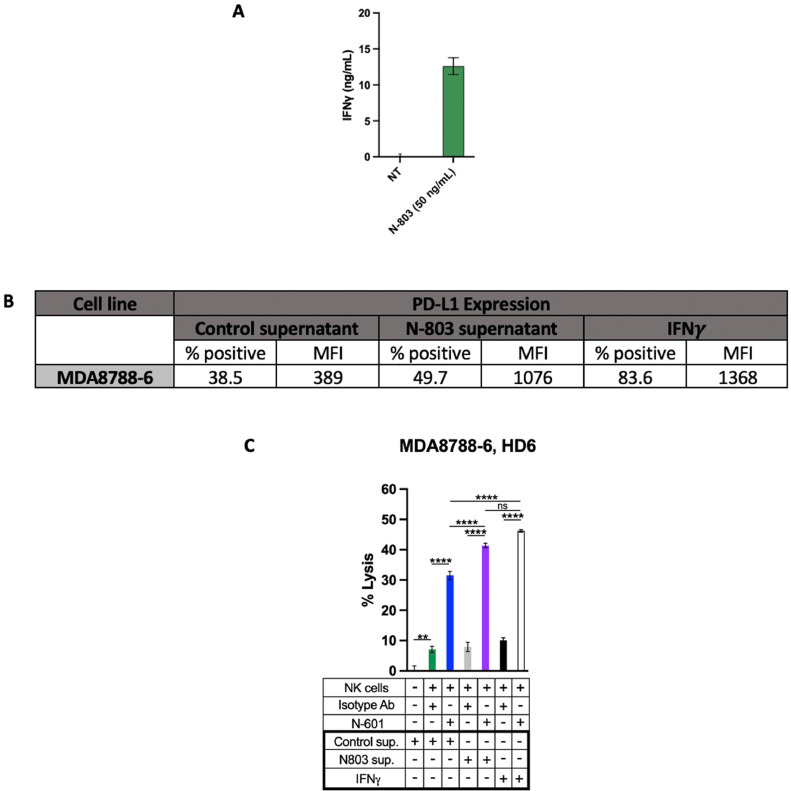
SNUC cell sensitivity to ADCC with anti–PD-L1 antibody is further enhanced by IL-15/IL-15r superagonist-induced IFNγ secretion
After demonstrating the anti-tumor activity of combinatory approaches with anti-PD-L1 and IL-15 superagonism, we sought to further model the dynamic role of IL-15 superagonism in augmenting SNUC cell susceptibility to NK cell-mediated ADCC. As evidenced in Fig. 2, IL-15 superagonism directly primes NK cells for tumor cell lysis. This increased cytotoxic NK cell function is related to increased expression of cytolytic molecules such as granzyme B and perforin [36,37]. In addition, NK cells stimulated by N-803 have been shown to increase secretion of IFNγ, a critical cytokine in a proinflammatory cascade intended to bolster host defense [36,38,39]. We thus hypothesized that co-culture of SNUC cells with supernatants of NK cells stimulated with an IL-15 superagonist would indirectly enhance tumor cell lysis through IFNγ-mediated PD-L1 upregulation.
Supernatants harvested from NK cells treated with immobilized N-803 contained significantly higher levels of IFNγ than supernatants from untreated NK cell controls (Fig. 3A). SNUC cells co-cultured in supernatant from N-803-treated NK cells exhibited greater surface expression of PD-L1 (both percent% and MFI) compared to untreated controls (Fig. 3B). SNUC cell PD-L1 upregulation corresponded with tumor cell lysis when SNUC cells were exposed to ADCC-coordinating antibody N-601 and untreated NK cells (Fig. 3C). Together, these data demonstrate the ability of IL-15 superagonoism to indirectly enhance SNUC cell lysis through inducing IFNγ release from NK cells and subsequent upregulation of PD-L1, the target for an ADCC-mediating antibody.
Fig. 3.
N-803 stimulates IFNy production from NK cells, which upregulates PD-L1 expression on SNUC cells and increases their sensitivity to N-601-mediated NK cell lysis. A. Healthy donor NK cells were cultured in 100 ng immobilized N-803 for 24 h or left untreated as controls. ELISA was used to quantify IFNy secretion within the supernatants of each NK cell culture. B. SNUC cells from the MDA8788–6 line were then cultured for 24 h in supernatant harvested from the N-803-treated NK cells, non-treated control supernatant, or in 20 ng/mL of IFNy as a positive control. SNUC cells were analyzed by flow cytometry for percent positive cells and MFI. C. SNUC cells from part B were used as targets for NK cell-mediated lysis via 111In-release assay. SNUC cells were treated with N-601 or isotype control antibody, and combination treatment assays were performed by treating SNUC cells with N-601 or isotype control antibody and treating NK cells with N-803. E:T ratios are 10:1. **P ≤ 0.01, ****P ≤ 0.0001.
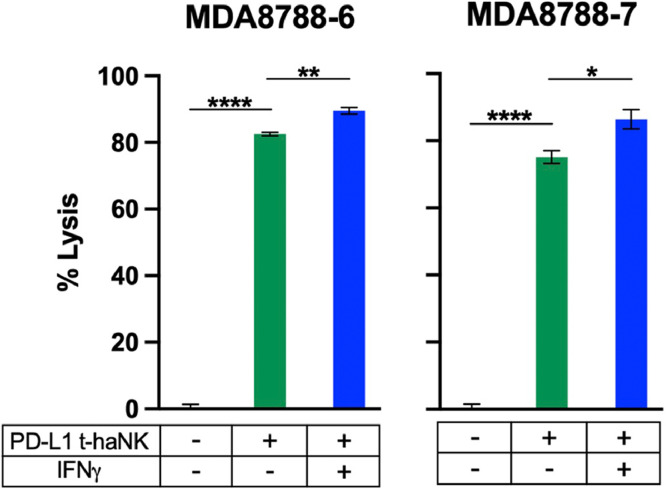
PD-L1 t-haNK cells induce lysis of SNUC cells
While NK cell-mediated immunotherapy offers a potential solution to the problem of T cell escape, endogenous NK cells are limited in number and function. NK cells comprise between just 5–20 % of circulating lymphocytes in humans [40]. Moreover, only about 14 % of humans express the high affinity allele of the NK cell CD16 receptor that engages IgG1 antibodies to initiate ADCC [41]. As a potential adoptive cellular therapy option, we interrogated the efficacy of an “off the shelf” human NK cell line (PD-L1 t-haNK) that has been genetically engineered to express the high-affinity CD16 receptor and a chimeric antigen receptor (CAR) specific for PD-L1. Based on our previous work showing the anti-tumor activity of PD-L1 t-haNKs against PD-L1-expressing tumor cells [23,42], we hypothesized that SNUC cells would be particularly sensitive to lysis by this engineered NK cell line. Both SNUC cell lines exhibited sensitivity to killing PD-L1 t-haNK cells, and such sensitivity was enhanced by upregulation of PD-L1 on SNUC cells through co-culture with IFNγ by an average of 8.2 % (P ≤ 0.05) (Fig. 4, Table 1).
Fig. 4.
PD-L1 t-haNKs induce lysis of SNUC cell lines in a PD-L1 dependent manner. SNUC cell lines were used as targets for PD-L1 t-haNK cells in 111In-release killing assays. SNUC cells were co-cultured in 20 ng/mL of IFNγ or left untreated as controls. E:T ratios are 10:1. *P ≤ 0.05, **P ≤ 0.01, ****P ≤ 0.0001.
The SNUC tumor immune microenvironment (TIME)
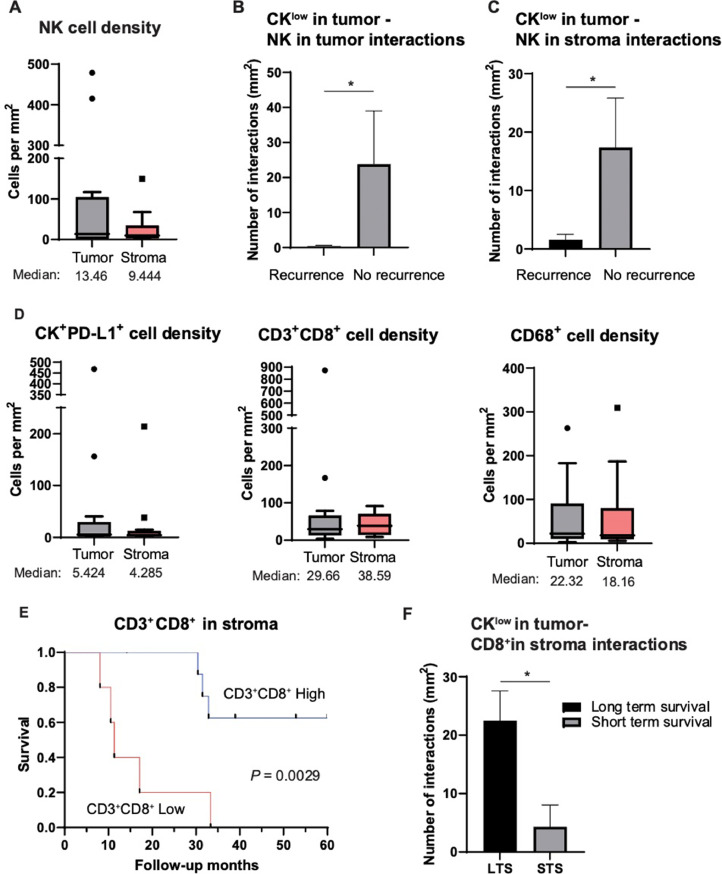
We next examined the SNUC TIME using multi-spectral immunofluorescence to determine the translational applicability of immunotherapeutic approaches to the SNUC TIME. To date, characterization of the SNUC TIME with multi-spectral immunofluorescence has not been performed. First, tissue sections from 14 clinically annotated cases of SNUC were stained with antibodies against CD3, CD8, PD-L1, CD68, NCAM/CD56, and cytokeratin (CK) (Supplementary Fig. 2). First, we focused on NK cells and found the presence of an NK cell infiltrate in SNUC which was similar between the tumor parenchyma and stroma, median of 13.46 NK cells per mm2 in tumor parenchyma versus 9.44 NK cells per mm2 in stroma (Fig. 5A). No correlation was observed between NK cell density and response to induction chemotherapy, disease recurrence, and overall survival status. Multi-spectral immunofluorescence also allows for spatial interaction analysis and to examine the effects of cell-cell interactions on clinical outcomes. Interestingly, when comparing the number of interactions between CKlow tumor cells and NK cells in the tumor parenchyma, a striking 55.7-fold increase in CKlow tumor cell/NK cell interactions was observed in patients without disease recurrence (P = 0.022) (Fig. 5B; Supplementary Fig. 3). A significant 10.9-fold increase in CKlow tumor cell interactions with NK cells in the stroma was also observed in patients without disease recurrence (P = 0.023) (Fig. 5C). When comparing interactions between CKhigh tumor cells and NK cells no significant difference was observed in disease recurrence (Supplementary Table 3). Collectively, these results suggest that SNUC tumor cell interactions with NK cells are important for limiting disease recurrence and that methods to augment NK cell activity may be an effective treatment strategy for SNUC.
Fig. 5.
The tumor immune microenvironment analysis in SNUC tumor samples. A. Quantification of NK cells per mm2 in 14 SNUC tumor samples. B. Increased interactions between CKlow tumor cells and NK cells in the tumor parenchyma are associated with no disease recurrence C. Increased interactions between CKlow tumor cells and NK cells in stroma are associated with no disease recurrence. D. Quantification of CK+PD-L1+, CD3+CD8+ and CD68+ cells per mm2 in 14 SNUC tumor samples. E. Patients with high CD3+CD8+ in stroma show significantly better 5-year overall survival rate (P = 0.0029). F. High CKlow cells in tumor and CD8+ in stroma interactions are associated with longer term survival. *P ≤ 0.05.
In further evaluation of the SNUC TIME, we assessed PD-L1+ tumor cells and found a median of 5.42 CK+/PD-L1+ cells per mm2 in the tumor parenchyma (Fig. 5D). As SNUC is a highly invasive malignancy, we regularly observed CK+ tumor cells in the stroma and identified 4.23 CK+/PD-L1+ cells per mm2 in the tumor stroma (Fig. 5D). A significant infiltration of CD3+CD8+cytotoxic T cells was noted in the tumor parenchyma and stroma at medians of 29.66 and 38.59 cells per mm2, respectively (Fig. 5D). A significant infiltration of CD68+ tumor-associated macrophages was noted in the tumor parenchyma and stroma at medians of 22.32 and 18.16 cells per mm2, respectively (Fig. 5D). We next assessed the clinical correlation of these immune cell densities and did not identify an association with response to induction chemotherapy or disease recurrence. However, we did observe that patients with high CD3+CD8+ in the stroma had a significantly improved 5-year overall survival (P = 0.0029) (Fig. 5E) while patients with high CD3+CD8+ in total tissue area had a non-statistically significant improvement in 5-year overall survival (P = 0.065) (Supplementary Fig. 4). An association with increased stromal CD8+ T cell infiltration and improved survival has also been reported in other types of head and neck cancer [43,44]. Neighborhood analysis was also performed to examine cell-cell interactions and interestingly a significant increase in CKlow interactions with CD8+ cytotoxic T cells was noted in long-term survivors compared to short-term survivors (P = 0.0225) (Fig. 5F). These data suggest that while NK cell activity is important for limiting disease recurrence, CD8+ cytotoxic T cell activity may aid in improving survival in patients with SNUC.
Discussion
SNUC is a rare, aggressive malignancy of the sinonasal cavity with poor prognosis and limited treatment options. While immunotherapy is a promising frontier of focus across multiple tumor types, including those of the sinonasal cavity and skull base [23,[45], [46], [47], [48]], no pre-clinical studies have investigated the potential role of immunotherapy for SNUC. Here, we perform the first pre-clinical evaluation of combinatory immunotherapy in SNUC cells lines and immune cell interactions in SNUC patient tissue TIME, correlating expression to response to standard of care therapy and patient survival.
PD-L1 expression on tumor tissue is used as a biomarker with prognostic and therapeutic implications in multiple solid tumor types, including HNSCC [14]. Recent efforts have characterized PD-L1 positivity in various neoplasms of the sinonasal cavity and anterior skull base such as sinonasal squamous cell carcinoma (SNSCC), sinonasal adenocarcinoma, and chordoma [47,48]; however, PD-L1 expression in SNUC remained uncharacterized. We found that not only did both SNUC cell lines express PD-L1, but that expression could be significantly increased to over 90 % of cells by IFN-γ co-culture, suggesting that SNUC cells may be amenable to immunotherapeutic targeting of the PD-1/PD-L1 axis.
Targeting of PD-L1 on SNUC cells using an anti-PD-L1 antibody significantly enhanced NK cell-mediated lysis of tumor cells via an ADCC-dependent mechanism (Fig. 1B). IFNγ effectively increased PD-L1 expression (% positivity and MFI) by at least 30 % in both SNUC cell lines, and further, induced de novo PD-L1 expression in cells that were previously PD-L1 negative (Table 1). When IFNγ-treated SNUC cells were co-cultured with anti-PD-L1 antibody in ADCC assays, they were significantly more sensitive to lysis by NK cells than controls without IFNγ (Fig. 1C). These findings demonstrate that IFNγ exposure increases PD-L1 on SNUC cells, which enhances the efficacy of an anti-PD-L1 antibody in mediating ADCC with NK cells.
Proinflammatory cytokines such as IFNγ are released from activated immune cells, particularly NK cells and T cells. IL-15 superagonism is a potent activator of NK cells and T cells [24,27], which led us to hypothesize that it would not only enhance the cytotoxic function of our NK cell effectors, but also improve the efficacy of an anti-PD-L1 antibody through inducing secretion of IFNγ. The direct stimulation of NK cells with IL-15 superagonism significantly enhanced their cytotoxicity against SNUC cells, and the combination of N-601 and N-803 provided superior SNUC cell lysis than monotherapy with either agent (Fig. 2). To demonstrate the indirect effect of IL-15 superagonism on target cell lysis by anti-PD-L1 antibody-coordinated ADCC, we treated NK cells with N-803 and quantified IFNγ secretion using ELISA (Fig. 3A). We then co-cultured SNUC cells with this supernatant and demonstrated that PD-L1 was upregulated (both% cells and MFI) compared to untreated controls (Fig. 3B). Co-culture of SNUC cells in this supernatant resulted in enhanced tumor cell lysis when subjected to ADCC with N-601 (Fig. 3C). Together, these data demonstrate that combinatory treatment with anti-PD-L1 antibody and IL-15 superagonism result in a complementary cytotoxic effect through multiple mechanisms. First, IL-15 superagonism directly enhances the cytotoxic capability of NK cells, which improves their ability to lyse SNUC cells altogether. Second, IL-15 superagonism induces IFNγ secretion from NK cells, which upregulates expression of N-601′s target, PD-L1, thus sensitizing SNUC cells to ADCC with N-601. These findings are essential to the translation of these immunotherapeutic agents to the controlled clinical setting for further investigation. While this study focuses on NK cell activity through ADCC and cytokine-mediated activation, it should be noted that the combination of N-601 and N-803 was selected based on its dynamic activity in both the NK and T cell immune compartments.
In this study we also performed the first analysis of the SNUC TIME using multi-spectral immunofluorescence. Importantly we found the presence of an NK, cytotoxic T cell, and macrophage infiltrate as well as PD-L1+ tumor cells (Fig. 5). Interaction analysis between CKlow tumor cells and NK and cytotoxic T cells revealed an increase in these interactions was associated with an improvement in disease recurrence and long-term survival, respectively. These same associations were not observed with CKhigh tumor cell interactions, collectively suggesting that strategies that improve these interactions with tumor cells may help to improve disease recurrence and survival. Sinonasal undifferentiated carcinoma represents a spectrum of differentiation, and it is feasible that CKlow tumor cells represent those tumor cells that are less differentiated than CKhigh tumor cells. These data may also suggest that more undifferentiated SNUC may be particularly amenable to immunotherapeutic approaches.
Ultimately, the data presented in this study provide the pre-clinical rationale for combinatory immunotherapy approaches for SNUC. Moving forward, the clinical efficacy of this approach could be investigated in the setting of recurrent/metastatic disease. Alternatively, our groups have advocated for an induction chemotherapy approach for the treatment of primary SNUC [1,3]. In this algorithm, patients who respond well to induction chemotherapy proceed to definitive chemoradiation whereas those patients whose tumor does not respond well proceed to surgery when feasible [1,3]. This treatment paradigm provides the opportunity of combination of immunotherapy and induction chemotherapy in the neoadjuvant setting in an effort to enhance disease response and long-term control. There is precedent for the combination of immunotherapy and chemotherapy in the neoadjuvant setting as recently demonstrated in lung cancer where the addition of neoadjuvant immunotherapy to chemotherapy increased pathologic complete response from 2.2 % to 24 % and resulted in significantly longer event-free survival [49]. We are actively working on clinical trial development to evaluate this approach in a clinical trial scenario for SNUC.
A limitation is the rare nature of SNUC and the preclinical in vitro model. While two-dimensional cellular models of cancer are suboptimal for experimentation with complex immune-based interactions, there are currently no established animal models of SNUC allowing for evaluation of immunotherapy. However, the multi-spectral immunofluorescence data demonstrating an association with increased CKlow tumor cell interactions with NK and CD8+cytotoxic T cells and improvement in disease recurrence and survival suggest this is a rational approach. Another limitation pertains to the 111In killing assay, which requires E:T ratios that exceed what one would expect to occur in a physiologic system with endogenous NK cells and tumor cells. However, the 10:1 E:T ratio used in this study is significantly lower than what our group has used in the past with other tumor types (20:1, 50:1) [22,23,41]. Moreover, we propose an adoptive NK cell therapy and an immunomodulating agent that promotes NK cell proliferation, which are potential solutions to increasing the physiologic ratio of NK cells to tumor cells [29,30]. Additionally, the dynamic activity of this proposed combinatory model to stimulate T cell responses through both immune checkpoint inhibition and IL-15 superagonism are not explored here. The role of checkpoint inhibition in T cell immunity is well characterized and has demonstrated promising clinical results in the setting of head and neck cancer, with a growing body of literature exploring their role in the neoadjuvant setting [50]. The literature demonstrating T cell expansion and stimulation in response to IL-15 superagonism is expanding [24,[27], [28], [29], [30]]. In conclusion, these data provide the scientific basis for ongoing investigation into combinatory immunotherapy concurrently targeting the NK and T cell compartments in SNUC.
Declaration
Portions of this study were presented at the North American Skull Base Society Annual meeting on February 18th, 2023, in Tampa, Florida.
Availability of data and material
Available upon request.
Ethics approval
IRB ethics approval was obtained for this study as described in the methods section.
CRediT authorship contribution statement
Austin T.K. Hoke: Writing – review & editing, Writing – original draft, Visualization, Validation, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Yoko Takahashi: Writing – review & editing, Writing – original draft, Visualization, Validation, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Michelle R. Padget: Writing – review & editing, Visualization, Validation, Methodology, Investigation, Formal analysis, Data curation. Javier Gomez: Writing – review & editing, Visualization, Validation, Methodology, Investigation, Formal analysis, Data curation. Moran Amit: Writing – review & editing, Methodology, Investigation. Jared Burks: Writing – review & editing, Methodology, Investigation. Diana Bell: Writing – review & editing, Resources, Investigation. Tongxin Xie: Writing – review & editing, Visualization, Methodology. Patrick Soon-Shiong: Writing – review & editing, Resources, Investigation. James W. Hodge: Writing – review & editing, Supervision, Resources, Methodology, Investigation, Funding acquisition, Conceptualization. Ehab Y. Hanna: Writing – review & editing, Visualization, Supervision, Resources, Investigation, Funding acquisition, Conceptualization. Nyall R. London: .
Declaration of competing interest
N. London receives research funding from Merck Sharp & Dohme, LLC, regarding HPV-related sinonasal carcinomas not relevant to the present manuscript. P. Soon-Shiong is a founder of ImmunityBio. All other authors declare no competing interests.
Funding
This research was supported (in part) by the Intramural Research Program of the NIH, Center for Cancer Research, National Cancer Institute (NRL). This research was made possible through the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and contributions to the NIH from the Doris Duke Charitable Foundation (DDCF Grant #2014194), the American Association for Dental Research, the Colgate-Palmolive Company, Genentech, Elsevier, and other private donors. This research was supported by the Sinus Cancer Research Fund, Charles & Daneen Stiefel Chair in Cancer Research, Israel Omar Longoria Research Fund, NIH MD Anderson Cancer Center Support Grant (P30 CA016672), and National Cancer Institute Research Specialist Award 1 (R50 CA243707) (EYH).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2024.101943.
Appendix. Supplementary materials
References
- 1.Amit M., Abdelmeguid A.S., Watcherporn T., Takahashi H., Tam S., Bell D., et al. Induction chemotherapy response as a guide for treatment optimization in sinonasal undifferentiated carcinoma. J. Clin. Oncol. 2019;37(6):504–512. doi: 10.1200/JCO.18.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tyler M.A., Holmes B., Patel Z.M. Oncologic management of sinonasal undifferentiated carcinoma. Curr. Opin. Otolaryngol. Head Neck Surg. 2019;27(1):59–66. doi: 10.1097/MOO.0000000000000513. [DOI] [PubMed] [Google Scholar]
- 3.London N.R., Jr., Mohyeldin A., Daoud G., Gamez M.E., Blakaj D., Bonomi M., et al. Sinonasal undifferentiated carcinoma: institutional trend toward induction chemotherapy followed by definitive chemoradiation. Head Neck. 2020;42(11):3197–3205. doi: 10.1002/hed.26357. [DOI] [PubMed] [Google Scholar]
- 4.Ejaz A., Wenig B.M. Sinonasal undifferentiated carcinoma: clinical and pathologic features and a discussion on classification, cellular differentiation, and differential diagnosis. Adv. Anat. Pathol. 2005;12(3):134–143. doi: 10.1097/01.pap.0000163958.29032.56. [DOI] [PubMed] [Google Scholar]
- 5.Abdelmeguid A.S., Bell D., Hanna E.Y. Sinonasal undifferentiated carcinoma. Curr. Oncol. Rep. 2019;21(3):26. doi: 10.1007/s11912-019-0776-4. [DOI] [PubMed] [Google Scholar]
- 6.Chambers K.J., Lehmann A.E., Remenschneider A., Dedmon M., Meier J., Gray S.T., Lin D.T. Incidence and survival patterns of sinonasal undifferentiated carcinoma in the United States. J. Neurol. Surg. B Skull Base. 2015;76(2):94–100. doi: 10.1055/s-0034-1390016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Musy P.Y., Reibel J.F., Levine P.A. Sinonasal undifferentiated carcinoma: the search for a better outcome. Laryngoscope. 2002;112(8 Pt 1):1450–1455. doi: 10.1097/00005537-200208000-00023. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi Y., Gleber-Netto F.O., Bell D., Roberts D., Xie T.X., Abdelmeguid A.S., et al. Identification of markers predictive for response to induction chemotherapy in patients with sinonasal undifferentiated carcinoma. Oral Oncol. 2019;97:56–61. doi: 10.1016/j.oraloncology.2019.07.028. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi Y., Gleber-Netto F.O., Bell D., Roberts D., Xie T.X., Abdelmeguid A.S., et al. Identification of novel diagnostic markers for sinonasal undifferentiated carcinoma. Head Neck. 2019;41(8):2688–2695. doi: 10.1002/hed.25748. [DOI] [PubMed] [Google Scholar]
- 10.Jurmeister P., Gloss S., Roller R., Leitheiser M., Schmid S., Mochmann L.H., et al. DNA methylation-based classification of sinonasal tumors. Nat. Commun. 2022;13(1):7148. doi: 10.1038/s41467-022-34815-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dogan S., Vasudevaraja V., Xu B., Serrano J., Ptashkin R.N., Jung H.J., et al. DNA methylation-based classification of sinonasal undifferentiated carcinoma. Mod. Pathol. 2019;32(10):1447–1459. doi: 10.1038/s41379-019-0285-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chitguppi C., Rabinowitz M.R., Johnson J., Bar-Ad V., Fastenberg J.H., Molligan J., et al. Loss of SMARCB1 expression confers poor prognosis to sinonasal undifferentiated carcinoma. J. Neurol. Surg. B Skull Base. 2020;81(6):610–619. doi: 10.1055/s-0039-1693659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jo V.Y., Chau N.G., Hornick J.L., Krane J.F., Sholl L.M. Recurrent IDH2 R172X mutations in sinonasal undifferentiated carcinoma. Mod. Pathol. 2017;30(5):650–659. doi: 10.1038/modpathol.2016.239. [DOI] [PubMed] [Google Scholar]
- 14.Burtness B., Harrington K.J., Greil R., Soulieres D., Tahara M., de Castro G., Jr., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915–1928. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 15.Ferris R.L., Blumenschein G., Jr., Fayette J., Guigay J., Colevas A.D., Licitra L., et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2016;375(19):1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denaro N., Merlano M., Numico G., Garrone O., Bossi P. Complete response to immunotherapy in sinonasal undifferentiated carcinoma. Tumori. 2021;107(6):NP101–NPNP4. doi: 10.1177/03008916211026971. [DOI] [PubMed] [Google Scholar]
- 17.Zaretsky J.M., Garcia-Diaz A., Shin D.S., Escuin-Ordinas H., Hugo W., Hu-Lieskovan S., et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N. Engl. J. Med. 2016;375(9):819–829. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee M.Y., Robbins Y., Sievers C., Friedman J., Abdul Sater H., Clavijo P.E., et al. Chimeric antigen receptor engineered NK cellular immunotherapy overcomes the selection of T-cell escape variant cancer cells. J. Immunother. Cancer. 2021;9(3) doi: 10.1136/jitc-2020-002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garrido F., Perea F., Bernal M., Sanchez-Palencia A., Aptsiauri N., Ruiz-Cabello F. The escape of cancer from T cell-mediated immune surveillance: HLA class I loss and tumor tissue architecture. Vaccines. 2017;5(1) doi: 10.3390/vaccines5010007. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimasaki N., Jain A., Campana D. NK cells for cancer immunotherapy. Nat. Rev. Drug Discov. 2020;19(3):200–218. doi: 10.1038/s41573-019-0052-1. [DOI] [PubMed] [Google Scholar]
- 21.Kang S., Gao X., Zhang L., Yang E., Li Y., Yu L. The advances and challenges of NK cell-based cancer immunotherapy. Curr. Oncol. 2021;28(2):1077–1093. doi: 10.3390/curroncol28020105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujii R., Friedman E.R., Richards J., Tsang K.Y., Heery C.R., Schlom J., Hodge J.W. Enhanced killing of chordoma cells by antibody-dependent cell-mediated cytotoxicity employing the novel anti-PD-L1 antibody avelumab. Oncotarget. 2016;7(23):33498–33511. doi: 10.18632/oncotarget.9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoke A.T.K., Padget M.R., Fabian K.P., Nandal A., Gallia G.L., Bilusic M., et al. Combinatorial natural killer cell-based immunotherapy approaches selectively target chordoma cancer stem cells. Cancer Res. Commun. 2021;1(3):127–139. doi: 10.1158/2767-9764.CRC-21-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knudson K.M., Hicks K.C., Alter S., Schlom J., Gameiro S.R. Mechanisms involved in IL-15 superagonist enhancement of anti-PD-L1 therapy. J. Immunother. Cancer. 2019;7(1):82. doi: 10.1186/s40425-019-0551-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedman J., Padget M., Lee J., Schlom J., Hodge J., Allen C. Direct and antibody-dependent cell-mediated cytotoxicity of head and neck squamous cell carcinoma cells by high-affinity natural killer cells. Oral Oncol. 2019;90:38–44. doi: 10.1016/j.oraloncology.2019.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han K.P., Zhu X., Liu B., Jeng E., Kong L., Yovandich J.L., et al. IL-15:IL-15 receptor alpha superagonist complex: high-level co-expression in recombinant mammalian cells, purification and characterization. Cytokine. 2011;56(3):804–810. doi: 10.1016/j.cyto.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romee R., Cooley S., Berrien-Elliott M.M., Westervelt P., Verneris M.R., Wagner J.E., et al. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood. 2018;131(23):2515–2527. doi: 10.1182/blood-2017-12-823757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Margolin K., Morishima C., Velcheti V., Miller J.S., Lee S.M., Silk A.W., et al. Phase I trial of ALT-803, a novel recombinant IL15 complex, in patients with advanced solid tumors. Clin. Cancer Res. 2018;24(22):5552–5561. doi: 10.1158/1078-0432.CCR-18-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knudson K.M., Hodge J.W., Schlom J., Gameiro S.R. Rationale for IL-15 superagonists in cancer immunotherapy. Expert Opin. Biol. Ther. 2020;20(7):705–709. doi: 10.1080/14712598.2020.1738379. [DOI] [PubMed] [Google Scholar]
- 30.Isvoranu G., Surcel M., Munteanu A.N., Bratu O.G., Ionita-Radu F., Neagu M.T., Chiritoiu-Butnaru M. Therapeutic potential of interleukin-15 in cancer (Review) Exp. Ther. Med. 2021;22(1):675. doi: 10.3892/etm.2021.10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi Y., Kupferman M.E., Bell D., Jiffar T., Lee J.G., Xie T.X., et al. Establishment and characterization of novel cell lines from sinonasal undifferentiated carcinoma. Clin. Cancer Res. 2012;18(22):6178–6187. doi: 10.1158/1078-0432.CCR-12-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baddeley A.R.E, Turner R. Chapman and Hall/CRC Press; 2015. Spatial Point Patterns: Methodology and Applications with R. [Google Scholar]
- 33.Ferri-Borgogno S., Zhu Y., Sheng J., Burks J.K., Gomez J.A., Wong K.K., et al. Spatial transcriptomics depict ligand-receptor cross-talk heterogeneity at the tumor-stroma interface in long-term ovarian cancer survivors. Cancer Res. 2023;83(9):1503–1516. doi: 10.1158/0008-5472.CAN-22-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaufmann J., Biscio C.A.N., Bankhead P., Zimmer S., Schmidberger H., Rubak E., Mayer A. Using the R Package spatstat to assess inhibitory effects of microregional hypoxia on the infiltration of cancers of the head and neck region by cytotoxic T lymphocytes. Cancers. 2021;13(8) doi: 10.3390/cancers13081924. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kassambara A. Pipe-friendly framework for basic statistical tests. R package version 0.7.2. 2023 [Available from: https://CRAN.R-project.org/package=rstatix.
- 36.Rosario M., Liu B., Kong L., Collins L.I., Schneider S.E., Chen X., et al. The IL-15-based ALT-803 complex enhances FcgammaRIIIa-triggered NK cell responses and in vivo clearance of B cell lymphomas. Clin. Cancer Res. 2016;22(3):596–608. doi: 10.1158/1078-0432.CCR-15-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fantini M., David J.M., Wong H.C., Annunziata C.M., Arlen P.M., Tsang K.Y. An IL-15 superagonist, ALT-803, enhances antibody-dependent cell-mediated cytotoxicity elicited by the monoclonal antibody NEO-201 against human carcinoma cells. Cancer Biother. Radiopharm. 2019;34(3):147–159. doi: 10.1089/cbr.2018.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mathios D., Park C.K., Marcus W.D., Alter S., Rhode P.R., Jeng E.K., et al. Therapeutic administration of IL-15 superagonist complex ALT-803 leads to long-term survival and durable antitumor immune response in a murine glioblastoma model. Int. J. Cancer. 2016;138(1):187–194. doi: 10.1002/ijc.29686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinette A., McMichael E., Courtney N.B., Duggan M., Benner B.N., Choueiry F., et al. An IL-15-based superagonist ALT-803 enhances the NK cell response to cetuximab-treated squamous cell carcinoma of the head and neck. Cancer Immunol. Immunther. 2019;68(8):1379–1389. doi: 10.1007/s00262-019-02372-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langers I., Renoux V.M., Thiry M., Delvenne P., Jacobs N. Natural killer cells: role in local tumor growth and metastasis. Biologics. 2012;6:73–82. doi: 10.2147/BTT.S23976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujii R., Schlom J., Hodge J.W. A potential therapy for chordoma via antibody-dependent cell-mediated cytotoxicity employing NK or high-affinity NK cells in combination with cetuximab. J. Neurosurg. 2018;128(5):1419–1427. doi: 10.3171/2017.1.JNS162610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fabian K.P., Padget M.R., Donahue R.N., Solocinski K., Robbins Y., Allen C.T., et al. PD-L1 targeting high-affinity NK (t-haNK) cells induce direct antitumor effects and target suppressive MDSC populations. J. Immunother. Cancer. 2020;8(1) doi: 10.1136/jitc-2019-000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oguejiofor K., Hall J., Slater C., Betts G., Hall G., Slevin N., et al. Stromal infiltration of CD8 T cells is associated with improved clinical outcome in HPV-positive oropharyngeal squamous carcinoma. Br. J. Cancer. 2015;113(6):886–893. doi: 10.1038/bjc.2015.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gurin D., Slavik M., Hermanova M., Selingerova I., Kazda T., Hendrych M., et al. The tumor immune microenvironment and its implications for clinical outcome in patients with oropharyngeal squamous cell carcinoma. J. Oral Pathol. Med. 2020;49(9):886–896. doi: 10.1111/jop.13055. [DOI] [PubMed] [Google Scholar]
- 45.Park J.C., Faquin W.C., Durbeck J., Faden D.L. Immune checkpoint inhibitors in sinonasal squamous cell carcinoma. Oral Oncol. 2020;109 doi: 10.1016/j.oraloncology.2020.104776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yazici G., Gullu I., Cengiz M., Elmali A., Yilmaz M.T., Aksoy S., et al. The synergistic effect of immune checkpoint blockade and radiotherapy in recurrent/metastatic sinonasal cancer. Cureus. 2018;10(10):e3519. doi: 10.7759/cureus.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riobello C., Vivanco B., Reda S., Lopez-Hernandez A., Garcia-Inclan C., Potes-Ares S., et al. Programmed death ligand-1 expression as immunotherapeutic target in sinonasal cancer. Head Neck. 2018;40(4):818–827. doi: 10.1002/hed.25067. [DOI] [PubMed] [Google Scholar]
- 48.Garcia-Marin R., Reda S., Riobello C., Cabal V.N., Suarez-Fernandez L., Vivanco B., et al. Prognostic and therapeutic implications of immune classification by CD8(+) tumor-infiltrating lymphocytes and PD-L1 expression in sinonasal squamous cell carcinoma. Int. J. Mol. Sci. 2021;22(13) doi: 10.3390/ijms22136926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Forde P.M., Spicer J., Lu S., Provencio M., Mitsudomi T., Awad M.M., et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N. Engl. J. Med. 2022;386(21):1973–1985. doi: 10.1056/NEJMoa2202170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uppaluri R., Campbell K.M., Egloff A.M., Zolkind P., Skidmore Z.L., Nussenbaum B., et al. Neoadjuvant and adjuvant pembrolizumab in resectable locally advanced, human papillomavirus-unrelated head and neck cancer: a multicenter, phase II trial. Clin. Cancer Res. 2020;26(19):5140–5152. doi: 10.1158/1078-0432.CCR-20-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Available upon request.