Highlights
-
•
FNDC1 plays a crucial role in tumors progression and can be used as a therapeutic target.
-
•
FNDC1 is implicated in poor prognosis of a diverse range of malignant tumors.
-
•
FNDC1 could promote lung adenocarcinoma cell invasion and migration through the JAK2-STAT3 signaling pathway.
-
•
Interference with FNDC1 blocks the activation of the JAK2-STAT3 pathway, thereby inhibiting the invasion and migration of lung adenocarcinoma cells.
Keywords: FNDC1; Lung cancer, GSEA; JAK2/STAT3; pathway
Abstract
Background
Fibronectin type III domain containing 1 (FNDC1) has been associated with the metastasis of many tumors, but its function in lung cancer remains uncertain.
Methods
FNDC1 expression was analyzed in The Cancer Genome Atlas (TCGA) and Genotype-Tissue Expression (GTEx), evaluate its prognostic value. Gene Set Enrichment Analysis (GSEA) enrichment analysis of differential expression of FNDC1 in lung cancer. The expression of FNDC1 was detected in five types of lung cancer cells, and screened to establish FNDC1 stable knockdown cell strains. To observe the migration and invasion ability of lung cancer cells after FNDC1 knockdown. Finally, we used rhIL-6 to interfere with the stable knockdown of FNDC1 in A549 cells and observed the recovery of migration and invasion.
Result
Our results showed that FNDC1 expression was increased in 21 tumor tissues, including lung cancer, and was associated with poor prognosis in five cancers, including lung adenocarcinoma (LUAD) (P < 0.05). GSEA enrichment analysis showed that FNDC1 was related to the pathways involved the JAK-STAT signaling pathway. Stable knockdown of FNDC1 in A549 and H292 cells resulted in decreased migration and invasion ability of both cells, accompanied by decreased expression of MMP-2 and Snail, and a significant decline in the expression of p-JAK2 and p-STAT3. The suppressive effect of FNDC1 knockdown on lung cancer cell metastasis counteracted by the JAK-STAT agonist rhIL-6 were presented in the nude mouse metastatic tumor model.
Conclusion
FNDC1 is implicated in poor prognosis of a diverse range of malignant tumors, which can promote metastasis and invasion of lung cancer through the JAK2-STAT3 signaling pathway.
Introduction
As shown in the Global Cancer Epidemiology Database, it is estimated that there are 2.1 million new cases of lung cancer and 1.8 million deaths annually [1]. In the United States, non-small cell lung cancer (NSCLC) accounts for more than 85 % [2]. The most common histological subtypes of NSCLC are LUAD and lung squamous cell carcinoma (LUSC). Although new diagnostic techniques and biotherapy have made great progress in lung cancer, about 47.3 % of lung cancer patients have been diagnosed with different organ metastasis [3], resulting in an increasing number of lung cancer deaths. American Cancer Society statistics indicate that lung cancer is the leading cause of cancer deaths in the United States, with a 5 year survival rate of approximately 5 % when distant metastases occur [4]. So far, the mechanism of lung cancer metastasis has not been fully revealed in the medical community, and blocking metastasis is still one of the main challenges in the field of cancer research.
Extracellular matrix macromolecules (ECM) are the major component of the extracellular microenvironment and play an important role in tumor invasion and metastasis [5]. ECM mainly includes three categories: structural protein, connexin and proteoglycan. FNDC1 is one of the main members of ECM connexins and has been reported to be associated with invasion and migration of various tumors [6], [7], [8], [9]. The FNDC1 gene, located on human chromosome 6q25.3, is a class of non-receptor-dependent activators of G-protein signaling, whose encoded product is involved in the construction of protein multimers, such as complexes with G-protein and channel protein connexin 43, which can induce apoptosis [10]. Under physiological conditions, FNDC1 is expressed in different tissues such as thyroid, heart, kidney, lung, and digestive tract. The earliest differential expression was found in human skin fibroblasts [11]. Recent studies have shown that FNDC1 expression is increased in gastric cancer tissues and promotes the proliferation, migration and invasion of gastric cancer cells. The higher the expression level of FNDC1, the poorer the disease-free survival of gastric cancer patients [12], [13], [14]. FNDC1 can also promote the migration and invasion of cancer cells in breast cancer [15], prostate cancer [12] and colorectal cancer [16], but its role and mechanism in lung cancer remain to be revealed. Based on the increased expression of FNDC1 in wide range of malignant tumors, we hypothesized it as a pan-cancer marker and further investigated its role in lung cancer.
The Janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) signaling pathway is continuously activated in a variety of malignant tumors, which is closely related to tumor occurrence, metastasis, and poor prognosis [17], [18], [19]. Typically, STAT3 activation is transient and momentary. In tumor cells, STAT3 exhibits persistent aberrant activation and induces the expression of VEGF and MMPs, promoting angiogenesis, invasion, and metastasis [20]. D. Harada et al. [21]. showed that phosphorylation of STAT3 in lung cancer is associated with angiogenesis, cancer cell proliferation and metastasis. FNDC1 activates G protein-coupled receptors with regulatory effects on the JAK-STAT3 signaling pathway through the interaction of G protein Gβγ subunits [22], [23], [24], [25]. In recent years, anti-tumor drugs targeting STAT3 inhibition have also successfully entered the clinical trial stage, and have good prospects in tumor treatment [26,27]. Therefore, this study first analyzed the pan-cancer attributes of FNDC1 and its prognostic correlation with various malignant tumors. Then, in vivo experiments revealed the correlation between the expression of FNDC1 in lung cancer cells and the migration and invasion of lung cancer cells. A stable knockdown of FNDC1 lung cancer cell line was constructed, and the regulatory effect of FNDC1 on the JAK-STAT3 signaling pathway in lung cancer was revealed from in vivo and in vitro experiments. This study revealed the pan-cancer properties of FNDC1 and its mechanism of action in lung cancer. Fig. 1 shows a schematic diagram of our flow chart.
Fig. 1.
Flow Chart.
Materials and methods
FNDC1 and malignant tumor bioinformatics data collection and analysis
The Cancer Genome Atlas (TCGA) and The Genotype-Tissue Expression (GTEx) data were downloaded from the University of California, Santa Cruz (UCSC) Xena (https://xena.ucsc.edu/) database, including gene expression and clinical data for LUAD and LUSC. Among them, tumor tissue data were obtained from TCGA, and normal tissue data were obtained from TCGA and GTEx. 526 LUAD tumor samples and 59 normal samples; 501 LUSC tumor samples and 49 normal samples were obtained from the TCGA database. 288 normal samples were obtained from the GTEx database. The data was corrected by the normalize Between Arrays function of limma R package.
Clinical prognostic analysis of FNDC1
Integrating the clinical information of LUAD and LUSC patients, Kaplan-Meier analysis was performed using the log-rank test through the Survival package. The “surv_cutpoint” function in the Survminer package was used to determine the optimal cut-off value of the risk score, and the data visualization was conducted by the ggplot2 package.
GSEA gene enrichment analysis of FNDC1 in lung cancer
GSEA (Gene Set Enrichment Analysis) is used to explore the signaling pathways associated with FNDC1 in LUAD and LUSC. The median FNDC1 expression was used as the cutoff point between the high and low expression groups. Gene expression enrichment analysis was performed between datasets with low or high FNDC1 mRNA expression. Use the Perl software to obtain the corresponding expression datasets and phenotypic data from FNDC1 in the expression matrices of LUAD and LUSC, respectively. Differentially expressed genes in the high and low expression groups were used as phenotypic data for GSEA. The functional gene set was set to c2.cp.kegg.v7.5.1.symbols.gmt, the analysis parameters were "No collapse", the number of permutations was set to "1000", the permutation type was set to "Phenotype", and the above files were analyzed by GSEA_4.2.3 software. Normalized enrichment score (NES), normal P-value and false discovery rate (FDR) q-values indicate association between genomes and pathways.
Cell culture and intervention
Five lung cancer cell lines (H1975, H292, H358, A549, HCC827) and human normal bronchial epithelial cell lines (16HBE) and 293 T cells were purchased from Kilton Biotechnology Co., Ltd. (JRDUN, Shanghai, CHINA). All lung cancer cells were cultured with RPMI-1640 (Hyclone, Logan, UT, USA) medium containing 10 % fetal bovine serum (Gibco, Grand Island, NY) and 1 % bispecific antibody (penicloptomycin mixture) in an incubator at 37 °C and 5 % CO2. 16 HBE and 293 T were cultured in DMEM culture medium (Hyclone, Logan, UT, USA), and experiments were performed when the cells grew to about 70 %.
In the cell transfection assay, FNDC1 interference vector was constructed, then 293T cells were used to package lentivirus, and H292 and A549 were infected to construct FNDC1 stable knockdown cell lines. The construction process of lentiviral interference vector includes: designing FNDC1 stem targets (shown in Table 1), sequencing (Shanghai Meiji Biotechnology Co., Ltd.). The sequencing results showed no base deletions, mutations or shifts, indicating that the vector was successfully constructed. The FNDC1 siRNA was then synthesized, annealed, vector linearized, vector recovered and ligated, vector transformed and positive clone identified, and the plasmid was extracted and lentiviral packaged using 293 T cells. The ratio of core and packaging plasmids was: pLKO.1-shFNDC1 1000 ng, psPAX2 900 ng, pMD2G 100 ng. Viral fluids were collected after 72 h of transfection, and 2ul of viral fluids were used to re-infect 293T cells, which were tested for transfection efficiency against PBS and null-loading groups, and then extracted for the construction of stable transfer strains of A549 and H292
Table 1.
Design results of FNCD1 interfering targets.
| Name | Sequences |
|---|---|
| FNDC1 Site 1(410–428) | GCATTACAACATTGCCTAT |
| FNDC1-F | CCGGTGCATTACAACATTGCCTATCTCGAGATAGGCAATGTTGTAATGCTTTTTG |
| FNDC1-R | AATTCAAAAAGCATTACAACATTGCCTATCTCGAGATAGGCAATGTTGTAATGCA |
| FNDC1 Site 2 (1586–1604) | GCAGAACACGGAGGACAAT |
| FNDC1-F | CCGGTGCAGAACACGGAGGACAATCTCGAGATTGTCCTCCGTGTTCTGCTTTTTG |
| FNDC1-R | AATTCAAAAAGCAGAACACGGAGGACAATCTCGAGATTGTCCTCCGTGTTCTGCA |
| FNDC1 Site3 (4149–4167) | CCCTCTTACAGACAAGGTT |
| FNDC1-F | CCGGTCCCTCTTACAGACAAGGTTCTCGAGAACCTTGTCTGTAAGAGGGTTTTTG |
| FNDC1-R | AATTCAAAAACCCTCTTACAGACAAGGTTCTCGAGAACCTTGTCTGTAAGAGGGA |
Validation of FNDC1 inhibition efficiency, cancer cell metastasis markers, replication assays and animal experiments by using A549 and H292 stable transplants. In the validation of FNDC1 inhibition efficiency, A549 and H292 cells were categorized into five groups, including control, siNC, siFNDC1–1, siFNDC1–2, and siFNDC1–3. qRT-PCR and Westen Blot assays were performed to detect FNDC1 expression. A549 and H292 cells were categorized into 3 groups of siNC, siFNDC1–1, and siFNDC1–2 in the cancer cell metastasis-related marker assay; Westen Blot assay for MMP2, Snail, JAK2, p-JAK2, STAT3 and p-STAT3 expression; Transwell assay for cell migration and invasive ability. Verification of FNDC1 inhibition of the JAK-STAT pathway using rhIL-6, an agonist of JAK-STAT, in a reply experiment. A549 cells were divided into four groups, including rhIL-6 siNC, rhIL-6 siFNDC1, Vehicle siFNDC1 and Vehicle siNC, with the final concentration of rhIL-6 (MedChemExpress, Shanghai, China) was 10 ng/ml, and Transwell assay was used to detect cell migration and invasion ability.
Animal model construction and transfer assays
In vivo experiments were used to explore the role of FNDC1 in hematogenous metastasis of lung cancer. Thymus-free nude mice (BALB/cNude) males, 5 weeks old, weight 20±2 g, purchased from Chengdu Dashuo Laboratory Animal Co, animal license number: SCXK (Chuan) 2020–030. Nude mice are housed in a barrier environment with a relative humidity of 50 %∼70 % and a temperature of 22∼25 °C without specific pathogens, maintaining a light and dark alternating rhythm for 12 h, with eating and drinking freely. After 1 week of adaptive feeding, BALB/cNude was randomly divided into siNC group and siFNDC1 group, with 6 animals in each group. The siFNDC1 group was injected with 2 × 106 A549 cells by tail vein injection. The siNC group was injected 2 × 106 unloaded A549 cells in the tail vein. Nude mice were executed by cervical dislocation 4 weeks after tail vein injection, and intact lungs were removed for weighing and photographing. After fixation of the lungs with Bouin's solution for 24 h, the number of nodules on the lung surface was counted. After paraffin-embedded sections, the microstructure of metastases was observed by HE staining, the expression of FNDC1 in pulmonary nodules was detected by Westen blot, and the expressions of FNDC1, MMP2, Snail, STAT3 and p-STAT3 were detected by immunofluorescence. The above animal experiment protocol was reviewed and approved by the Ethics Committee of the Affiliated Hospital of Chengdu University of Traditional Chinese Medicine, No. 2022KL-055–01, and the experimental process strictly abides by international and national animal welfare guidelines.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Reverse transcription was performed after extraction of total cellular RNA using Trizol (Invitrogen, Carlsbad, Ca, USA). The PCR primer sequences are as follows: FNDC1-Forward: 5′-CAGTAAGGCGGATGTTGAGC-3′, FNDC1-Reverse: 5′-TTGGGGAGAAGCAGGCAC-3′, GAPDH-Forward: 5′-AATCCCATCACCATCTTC-3′, GAPDH-Reverse: 5′-AGGCTGTTGTCATACTTC-3′. The amplification parameters of qRT-PCR were: 95 °C for 10 min, 95 °C for 15s+ 60 °C for 45 s, and 40 amplification cycles were performed. The parameters of the solubility curve were as follows: 95 °C 15 s, 60 °C 1 min, 95 °C 15 s, 60 °C 15 s. The relative expression of FNDC1 mRNA was calculated using the 2−ΔΔCT method.
Western blotting
The total protein of lung cancer cells and lung tissue was extracted, and the protein concentration was measured by the BCA protein assay kit (Thermor, Waltham, MA, USA). Next, cell or tissue lysates are loaded onto SDA-PAGE for isolation and transfer to NC (Millipore, Billerica, CA, USA) membranes. Closed with 5 % skim milk for 1 h at room temperature, then the relevant primary antibody was diluted according to the instructions in the following proportions: FNDC1 (Thermor, Waltham, MA, USA) 1:1000, MMP2 (Abcam, Cambridge, MA, USA) 1:1000, Snail (Proteintech, Wuhan, Hubei, CHINA) 1:1000, JAK2 (Cell Signaling Technology, Danvers, MA, USA) 1:1000, p-JAK2 (Cell Signaling Technology) 1:1000, STAT3 (Abcam, Cambridge, MA, USA) 1:2000, p-STAT3 (Abcam, Cambridge, MA, USA) 1:5000, β-actin (Abcam, Cambridge, MA, USA) 1:5000 and incubate with membrane overnight at 4 °C. Then wash 3 times with TBST, dilute the HRP-labeled secondary antibody (Beyotime, Shanghai, China) at 1:1000 and incubate with the membrane at 37 °C for 1 h, and after color development, it was placed in the imaging system for band scanning, and ImageJ software read the band gray value.
Transwell assays
H292 and A549 cells were cultured with RPMI-1640 culture medium containing 10 % fetal bovine serum, 1 % dual antibody (penicillin-streptomycin mixture) in an incubator at 37 °C, 5 % CO2. The cells were observed as adherent cells under the microscope, and the rate of viable cells stained with Trypan blue was more than 95 %, which was changed to serum-free medium for 24 h. The serum-free medium and Matrigel were diluted in a 2:1 ratio, 80 ul was plated into the Transwell chamber, and 0.3 ml of 2 × 105/mL cell suspension was inoculated into the chamber after coagulation. After fixation and staining after 24 h of incubation, the number of cells in each field of view was observed and counted using a microscope.
Immunofluorescence
Paraffin sections of nude mouse lung tissue were deparaffinized and rehydrated and placed in 0.01 M sodium citrate buffer solution for high-pressure retrieval of antigens for 15 min, then washed with PBS and cooled to room temperature. Primary antibodies were titrated according to the instructions to select the optimal dilution ratio, FNDC1 (Thermor, Waltham, MA, USA) 1:1000, MMP2 (Abcam, Cambridge, MA, USA) 1:1000, Snail (Proteintech, Wuhan, Hubei, CHINA) 1:1000, STAT3 (Abcam, Cambridge, MA, USA) 1:2000, p-STAT3 (Abcam, Cambridge, MA, USA) 1:5000, with 4 °C humidity box incubation overnight. After rinsing with PBS, fluorescent secondary antibody diluted in the appropriate ratio was added dropwise and incubated in a wet box at room temperature for 1 h. The tablets were sealed with a 1:500 dilution of anti-quenching sealer and DAPI (Beyotime), stored in the refrigerator at −20 °C, and finally photographed with a 400 × fluorescence microscope.
Statistical methods
Bioinformatics analysis was performed using the R software. Survival curves were plotted by the Kaplan-Meier method, and were compared using log-rank tests. GraphPad Prism 8.0.2 software (GraphPad, Inc., La Jolla, CA, USA) was used for statistical analysis of cell and animal experiments. The t-test was used for comparison between two groups, and one-way ANOVA was used for comparison between multiple groups. The data were expressed as mean ± standard deviation, with the P-value < 0.05 was considered significant.
Result
The pan-cancer property of FNDC1
We first evaluated the expression of FNDC1 in the TCGA and GTEx pan-cancer databases, and performed a log2 (x + 0.001) transformation on the expression value. Finally, we eliminated the cancer species with less than 3 samples in a single cancer species. It was found that the expression of FNDC1 in 21 tumor tissues was different from that in normal tissues, including BRCA, CHOL, ESCA, GBM, HNSC, KICH, KIRC, LAML, LGG, LIHC, LUAD, LUSC, OV, PAAD, SKCM, STAD, STES, TGCT, THCA, UCS and WT (Fig. 2. A). Then we analyzed the expression differences of FNDC1 in LUAD and LUSC, and found that FNDC1 was highly expressed in both LUAD and LUSC (Fig. 2b). Subsequently, we performed paired sample test and visual analysis on the expression differences of FNDC1 in tumor tissues and adjacent tissues of LUAD and LUSC patients. It was found that FNDC1 was highly expressed in tumor tissues compared with adjacent normal tissues (Fig. 2c, d), indicating that FNDC1 may play a role in the pathogenesis of LUAD and LUSC.
Fig. 2.
(a) FNDC1 pan-cancer attribute difference analysis: FNDC1 expression in tumor tissues and normal tissues in TCGA and GTEx pan-cancer data. (b) Expression of FNDC1 in lung cancer: The expression levels of FNDC1 in LUAD and LUSC and normal tissues in TCGA and GTEx databases. (c, d) The expression of FNDC1 in LUAD and LUSC paired tumor and normal tissues in TCGA database.
Survival analysis
In order to evaluate the utility of FNDC1 expression in predicting cancer prognosis, we analyzed the association between FNDC1 expression and overall survival in the TCGA cohort. The results showed that higher FNDC1 expression was significantly associated with the prognosis of cancer patients with LUAD (P = 0.017), BRCA (P = 0.05), STAD (P = 0.005), STES (P = 0.044), and KIRP (P < 0.001), but not in LUSC (P = 0.189) (shown in Fig. 3). It indicates that FNDC1 is a potential oncogene in these types of cancers.
Fig. 3.
Kaplan-Meier survival curves: TCGA database was used to analyze the correlation between FNDC1 and the prognosis of various cancers.
GSEA gene enrichment analysis
The enrichment pathways of LUAD and LUSC were intersected, and significant differences were considered when the normal P value < 0.05 and the FDR q value<0.05. We found that these pathways involved focal adhesion pathway, actin cytoskeleton regulation pathway, TGF-β signaling pathway, ECM-receptor interaction pathway, melanoma pathway, gap junction pathway, dilated cardiomyopathy pathway, cancer pathway, vascular smooth muscle contraction pathway, hypertrophic cardiomyopathy pathway, prostate cancer pathway, arrhythmogenic right ventricular cardiomyopathy pathway, glycosaminoglycan biosynthesis-chondroitin sulfate pathway, small cell lung cancer pathway and the JAK-STAT signaling pathway. The purpose of the NES (Normalized Enrichment Score) average is to assess the overall enrichment of a gene set in a sample, and its computation relates to the ordering and integration of genes in the gene set. The top 15 pathways of the average NES were selected as significantly enriched signaling pathways, as shown in Table 2.
Table 2.
Results of GSEA pathway analysis.
| Gene set name | AVG NES | LUAD |
LUSC |
|||||
|---|---|---|---|---|---|---|---|---|
| NES | NOM p-val | FDR q-val | NES | NOM p-val | FDR q-val | |||
| 1 | KEGG_FOCAL_ADHESION | 2.585 | 2.53 | 0 | 0 | 2.64 | 0 | 0.001 |
| 2 | KEGG_REGULATION_OF_ACTIN_CYTOSKELETON | 2.465 | 2.27 | 0.002 | 0.002 | 2.66 | 0 | 0.002 |
| 3 | KEGG_TGF_BETA_SIGNALING_PATHWAY | 2.42 | 2.46 | 0 | 0.001 | 2.38 | 0 | 0.001 |
| 4 | KEGG_ECM_RECEPTOR_INTERACTION | 2.41 | 2.37 | 0 | 0 | 2.45 | 0 | 0.001 |
| 5 | KEGG_MELANOMA | 2.365 | 2.39 | 0 | 0 | 2.34 | 0 | 0.001 |
| 6 | KEGG_GAP_JUNCTION | 2.345 | 2.17 | 0.002 | 0.004 | 2.52 | 0 | 0.001 |
| 7 | KEGG_DILATED_CARDIOMYOPATHY | 2.305 | 2.11 | 0.002 | 0.006 | 2.5 | 0 | 0.001 |
| 8 | KEGG_PATHWAYS_IN_CANCER | 2.3 | 2.18 | 0.002 | 0.004 | 2.42 | 0 | 0.001 |
| 9 | KEGG_VASCULAR_SMOOTH_MUSCLE_CONTRACTION | 2.29 | 2.1 | 0 | 0.006 | 2.48 | 0 | 0.001 |
| 10 | KEGG_HYPERTROPHIC_CARDIOMYOPATHY_HCM | 2.265 | 2.15 | 0 | 0.004 | 2.38 | 0 | 0.001 |
| 11 | KEGG_PROSTATE_CANCER | 2.245 | 2.17 | 0 | 0.004 | 2.32 | 0 | 0.001 |
| 12 | KEGG_ARRHYTHMOGENIC_RIGHT_VENTRICULAR_CARDIOMYOPATHY_ARVC | 2.245 | 2.13 | 0 | 0.005 | 2.36 | 0 | 0.001 |
| 13 | KEGG_GLYCOSAMINOGLYCAN_BIOSYNTHESIS_CHONDROITIN_SULFATE | 2.21 | 2.25 | 0 | 0.002 | 2.17 | 0 | 0.003 |
| 14 | KEGG_SMALL_CELL_LUNG_CANCER | 2.165 | 2.2 | 0 | 0.004 | 2.13 | 0.004 | 0.003 |
| 15 | KEGG_JAK_STAT_SIGNALING_PATHWAY | 2.15 | 1.85 | 0 | 0.002 | 2.38 | 0 | 0.001 |
The GSEA pathway enrichment results of FNDC1 in LUAD and LUSC mainly involve three aspects: 1. Tumor migration: including focal adhesion pathway, actin cytoskeleton regulation pathway, TGF-β signaling pathway, ECM-receptor interaction pathway, gap junction pathway, the JAK-STAT signaling pathway (Fig. 4); 2. Cancer occurrence and development: cancer pathway, melanoma pathway, prostate cancer pathway, small cell lung cancer pathway; 3. Heart disease: dilated cardiomyopathy pathway, hypertrophic cardiomyopathy pathway, vascular smooth muscle contraction pathway, arrhythmogenic right ventricular cardiomyopathy pathway. Giving the correlation between FNDC1 and STAT3, this study selected the JAK-STAT signaling pathway as the downstream pathway of FNDC1 to study the mechanism of this gene and lung cancer metastasis.
Fig. 4.
LUAD migration related pathways. (a) JAK-STAT signaling pathway. (b)TGF-β signaling pathway. (c) Regulation of action cytoskeleton. (d)Focal adhesion. (e)Gap junction. (f) ECM-receptor interaction.
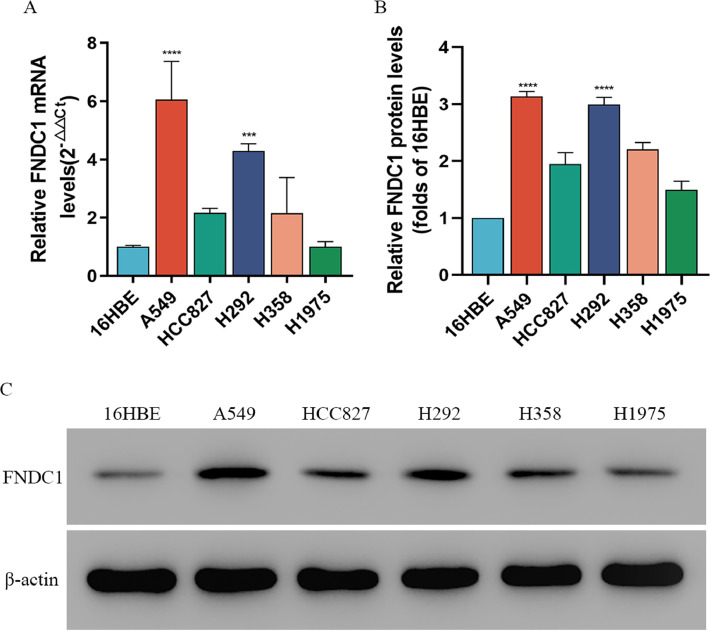
Expression of FNDC1 in lung cancer cell lines
qRT-PCR was used to detect differences in FNDC1 mRNA transcription between five lung cancer cell lines (H1975, H292, H358, A549, HCC827) and human normal bronchial epithelial cell lines (16HBE). The results showed a significant increase in FNDC1 mRNA transcription in the LUAD cell line compared to 16HBE, with A549 and H292 (P < 0.001) being the most significant (Fig. 5a). Subsequently, we measured the expression of FNDC1 protein in lung cancer cells by WB. The results showed that the protein expression of FNDC1 in the LUAD cell line (A549, H292) was significantly increased compared to bronchial epithelial cells (16HBE) (P < 0.001) (Fig. 5b, c). Thus, we demonstrated a significant increase in mRNA transcription levels and protein expression levels of FNDC1 in LUAD cells compared to 16HBE.
Fig. 5.
(a) mRNA expression of FNDC1 in LUAD cell line was detected by qRT-PCR. (b, c) FNDC1 protein expression level in LUAD cell line was detected by Western-blot.
FNDC1 expression was reduced in A549 and H292 cells after FNDC1 inhibition
To explore the role of FNDC1 expression in LUAD, we selected A549 and H292 cells as lentivirus-transfected cell models and used qRT-PCR and WB to detect FNDC1 interference efficiency. The results showed that the mRNA transcription (Fig. 6a, b) and protein (Fig. 6c, d, e, f) expression of FNDC1 were significantly reduced after lentivirus transfection of interfering cells, indicating that the FNDC1 stable knockdown cell lines of A549 and H292 were successfully established.
Fig. 6.
(a, b) mRNA expression levels of FNDC1 in A549 and H292 cells were detected by qRT-PCR. (c, d, e, f) The expression level of FNDC1 protein in A549 and H292 cells was detected by Western-blot.
Diminished migration and invasion of A549 and H292 cells after FNDC1 inhibition
In the Transwell assay, we found that transfected siFNDC1 resulted in a significant reduction in the migration (Fig. 7a, b) and invasion (Fig. 7c, d) abilities of A549 and H292 cells compared with siNC. The expression of cancer metastasis-related factors MMP2 and Snail was significantly reduced by Western blot (Fig. 7e, f). The prerequisite for tumor metastasis is to break through the natural barrier of ECM, and matrix metalloproteinase 2 (MMP2) has unique enzymatic activity, which uses ECM to degrade and promote the occurrence of tumor metastasis. Snail can inhibit the expression of E-cadherin and promote epithelial-mesenchymal transition, thereby promoting tumor invasion and metastasis. After inhibition of FNDC1, the migration and invasion ability of A549 and H292 cells decreased, and the expression of MMP2 and Snail decreased, indicating that FNDC1 may promote the migration and invasion of cancer cells through MMP2 and Snail.
Fig. 7.
Inhibition of FNDC1 inhibits the migration and invasion of A549 and H29 cells. (a, b) Migration results for A549 and H29. (c, d) Results of A549 and H29 infestation. (e, f) Expression of MMP2 and Snail in A549 and H29.
Inhibition of FNDC1 weakens the metastatic ability of lung cancer cells in mouse lung tissue outstanding
Based on the promotional effect of FNDC1 on lung cancer cell migration and invasion, we further explored the role of FNDC1 on LUAD metastasis in vivo using a nude mouse metastatic tumor model. Compared with A549 cells in the no-load group (siNC group), the expression of FNDC1 in the pulmonary metastatic nodules in the siFNDC1 group was significantly reduced in the tail vein injection of A549 cells with stable knockdown of FNDC1 (siFNDC1 group), indicating that the model of A549 cell metastases with stable knockdown of FNDC1 was successfully constructed (Fig. 8a, b). The number of metastatic nodules in the lungs was significantly reduced in the siFNDC1 group, 18.83±5.64 in the siFNDC1 group and 68.50±14.57 in the siNC group (Fig. 8c, d), suggesting that the metastatic ability of A549 cells was weakened by inhibiting FNDC1. Immunofluorescence results showed that the expression of cancer metastasis-related factors MMP2 and Snail in pulmonary nodules was significantly reduced when FNDC1 was inhibited (Fig. 8e, f, g).
Fig. 8.
Inhibition of FNDC1 expression significantly attenuates the metastatic ability of LUAD. (a, b) The expression level of FNDC1 in the siFNDC1 group was lower than that in the siNC group. (c, d) The number of metastatic nodules in the lung tissue of nude mice in the siFNDC1 group was significantly lower than that in the siNC group. (e, f, g) The expressions of FNDC1, MMP2 and Snail in the siFNDC1 group were decreased.
FNDC1 inhibits LUAD metastasis through the JAK2 / STAT3 signaling pathway
GSEA results showed that the expression of FNDC1 in lung cancer was related to the JAK-STAT pathway. In order to reveal the correlation between FNDC1 promoting LUAD metastasis and the JAK-STAT pathway, we further verified it in vitro and in vivo. The results of in vitro experiments showed that the activation of the JAK2-STAT3 pathway was inhibited in A549 and H292 cells with stable knockdown of FNDC1, and the expression of pJAK2 and pSTAT3 was significantly lower than that of siNC (Fig. 9a, b). The results of in vivo experiments showed that the expression of p-STAT3 in lung metastatic nodules of nude mice caused by FNDC1 knockdown A549 cells was lower than that of siNC group, and the activation of STAT3 was reduced (Fig. 9c, d).
Fig. 9.
FNDC1 promotes the JAK2/STAT3 pathway activation. (a, b) In A549 and H292 cells, knockdown of FNDC1 significantly reduced the expression of p-JAK2 and p-STAT3. (c, d) The activation of STAT3 in A549 cell line with stable knockdown of FNDC1 was weakened in nude mice, and the expression of p-STAT3 was decreased.
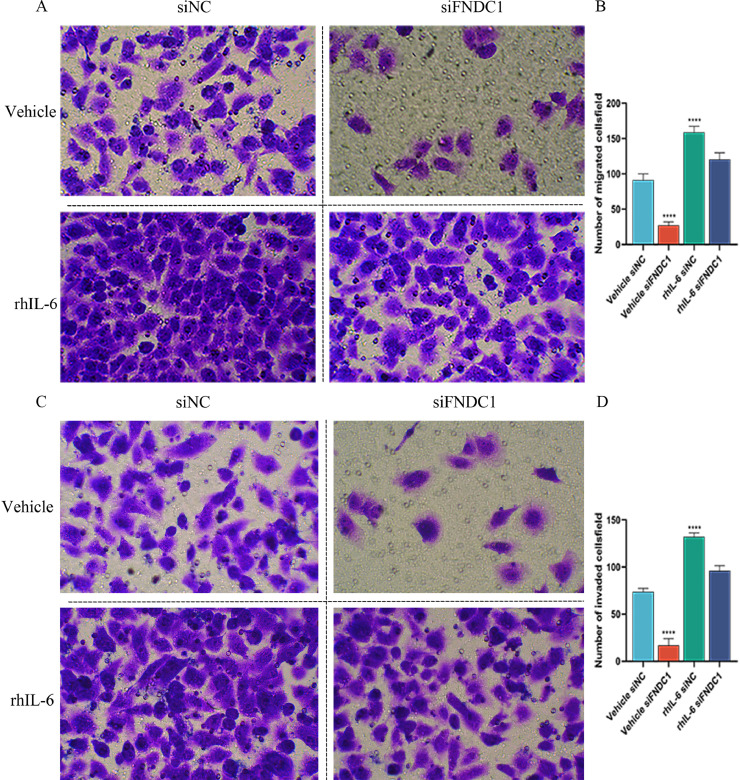
Subsequently, in order to prove that FNDC1 regulates the JAK2-STAT3 pathway to interfere with lung cancer cell metastasis, we conducted a recovery experiment. The JAK2-STAT3 pathway agonist rhIL-6 was used to treat A549 cells with FNCD1 stable knockdown, and Transwell method was used to detect the migration and invasion ability of cancer cells. The results showed that rhIL-6 offset the effect of FNDC1 knockdown. The migration and invasion ability of A549 was strengthened by the addition of rhIL-6 (Fig. 10), suggesting that FNDC1 facilitated the activation of the JAK2-STAT3 pathway and the metastasis of LUAD tumor cells.
Fig. 10.
The JAK2-STAT3 pathway agonist rhIL-6 could eliminate the inhibitory effect of siFNDC on the migration of lung cancer cells. Inhibition of FNDC1 decreased migration and invasion of A549 cells, while the migration and invasion ability of A549 was restored with rhIL-6.
Discussion
Lung cancer is known to have a high incidence and lethality. Despite recent advances in diagnosis and treatment methods, the overall cure rate and survival rate of metastatic LUAD and LUSC remain low [28,29]. Therefore, to find effective targets for tumor metastasis possesses vital therapeutic implications. In recent years, the role of ECM in tumor invasion and metastasis has attracted more and more attention [30,31], but the role of its member FNDC1 in tumor metastasis, especially the role mechanisms of FNDC1 in lung cancer metastasis, is still rarely reported.
Studies have shown that FNDC1 is involved in the occurrence and development of a variety of tumors and is an important regulator of tumor metastasis. For example, high expression of FNDC1 accelerates skin tumor progression and increases tumor thickness [32]. Knockdown of FNDC1 can inhibit the proliferation and migration of prostate cancer cells and induce apoptosis [12]. In patients with gastric cancer, high expression of FNDC1 is associated with poor clinical features and poor prognosis [33]. In breast cancer, FNDC1 expression is up-regulated, and inhibition of FNDC1 can inhibit the proliferation, migration, invasion and EMT of breast cancer cells [15]. Therefore, we analyzed the pan-cancer properties of FNDC1 and found that its expression was significantly increased in more than 20 malignant tumors, which was related to the poor prognosis of 5 cancers including LUAD, BRCA, STAD, STES and KIRP. It indicates that FNDC1 is an anti-tumor target worthy of study.
In order to further reveal the correlation between FNDC1 and lung cancer metastasis, GSEA enrichment analysis was carried out. It was found that the FNDC1 high expression group of LUAD and LUSC was mainly enriched in focal adhesion pathway, actin cytoskeleton regulation pathway, TGF-β signaling pathway, ECM-receptor interaction pathway, gap junction pathway, the JAK-STAT signaling pathway and other pathways related to cell migration and invasion (showed in Table 2). Among them, the actin cytoskeleton is connected to the ECM at the focal adhesion, providing a physical pathway for the migration and morphological changes of tumor cells [34]. STAT3 can activate the adhesion ability of focal adhesion [13], which is closely related to the invasion ability of tumor cells. The JAK/STAT3 pathway is also necessary for TGF-β-induced EMT and cancer cell migration and invasion [35]. Therefore, we chose the JAK-STAT signaling pathway to reveal the mechanism of FNDC1 promoting LUAD metastasis.
FNDC1 is also known as Activators of G-Protein Signaling 8 (AGS8), a Gβγ subunit receptor non-dependent auxiliary protein [36]. Typically, heterotrimeric G proteins are activated by G protein-coupled receptors on the cell surface in response to extracellular stimuli. Such accessory proteins activate or inactivate the Gα subunit and act as alternative binding proteins to the Gα or Gβγ subunits [37], [38], [39], potentially providing alternative signal inputs for heterotrimeric G protein signaling [40]. It was shown that FNDC1 can bind directly to the Gβγ subunit in G proteins [24,25], leading to dissociation of the Gα and Gβγ subunits [41]. The Gi family of the α subunit includes three closely related members, among which Gαi2 can activate JAK to regulate the JAK-STAT signaling pathway [22,23]. Once JAK is activated, STAT protein will be phosphorylated and dimerized and then translocated to the nucleus [42], where gene transcription is regulated. D. Harada et al. showed that phosphorylation of STAT3 in lung cancer is associated with increased cell proliferation, angiogenesis, and metastasis [21], while the high expression of p-STAT3 plays an important role in mediating lymph node metastasis [43]. p-STAT3 can induce the expression of anti-apoptotic genes such as Bcl-2 and Bcl-XL and proto-oncogenes such as c-Myc in the nucleus, leading to sustained cell proliferation [44]. Persistent aberrant activation of STAT3 induces vascular endothelial cells to express VEGF and TWIST, which in turn promotes angiogenesis and epithelial-mesenchymal transition in tumor tissues [45,46]. In addition, p-STAT3 can also activate the transcriptional expression of MMP2, MMP9 and Snail in cancer cells [47,48]. Matrix metalloproteinases (MMPs) are a group of zinc-dependent metalloenzymes that regulate a variety of cellular processes, including tumor cell proliferation and metastasis [20,49]. Snail is a zinc-finger transcriptional repressor that triggers EMT, increases invasion of cancer cells, and also induces MMP-2 expression [50]. In this study, FNDC1 expression was increased in a variety of lung cancer cell lines, with A549 and H292 being the most significant. Therefore, we constructed A549 and H292 cell lines with stable knockdown of FNDC1.Then we further observed the interventional effects of FNDC1 on lung cancer cell migration and invasion, JAK2/STAT3 pathway activation, and metastasis model. Interference with FNDC1 was found to reduce the migratory and invasive abilities of both A549 and H292, as well as the expression of tumor metastasis markers MMP-2 and Snail. The same results were shown in the nude mouse metastasis model. The number of metastatic lung nodules was significantly reduced in the siFNDC1 model group compared to the siNC group, and the expression of cancer metastasis-associated factors MMP2 and Snail in lung nodules was significantly reduced. These results showed that inhibition of FNDC1 expression could reduce lung adenocarcinoma metastasis, and this effect could be counteracted by the JAK-STAT agonist rhIL-6, suggesting that FNDC1 promotes lung cancer cell metastasis through the JAK-STAT signaling pathway.
In conclusion, our results demonstrated that FNDC1 is highly expressed in several tumors, including LUAD and LUSC, and is significantly correlated with poor prognosis in various malignancies, including LUAD. Both GSEA analysis and experimental validation showed that FNDC1 could promote lung adenocarcinoma cell invasion and migration through the JAK2-STAT3 signaling pathway. Interfering with FNDC1 blocked the JAK2-STAT3 pathway activation, which inhibited the invasion and migration ability of lung adenocarcinoma cells, suggesting that FNDC1 is an essential target for the treatment of tumors such as LUAD.
Funding statement
This research was funded by the Science and Technology Planning Project of Sichuan Provincial Department of Science and Technology (NO. 2022YFS0405); National College Students' innovation and entrepreneurship training program (202210633028).
Availability of data and material
The original contributions presented in this study are included in the article, and further inquiries can be directed to the corresponding author.
Ethics approval statement
The animal study protocol was approved by the Animal Experiment Ethics Committee of Chengdu University of Traditional Chinese Medicine, number: 2022KL-055–01. The experimental procedures strictly adhered to international and national animal welfare guidelines.
CRediT authorship contribution statement
Yang Song: Conceptualization, Visualization, Writing – original draft. Jun-Feng Guo: Investigation, Validation, Writing – review & editing. Pei-Shu Lan: Methodology, Validation, Visualization, Writing – review & editing. Miao Wang: Methodology, Writing – review & editing. Quan-Yu Du: Project administration, Conceptualization, Data curation, Funding acquisition, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank the researchers and staff of the above software and databases including Xiantao Academic (www.xiantao.love).
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Molina J.R., Yang P., Cassivi S.D., Schild S.E., Adjei A.A. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008;83(5):584–594. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamura T., Kurishima K., Nakazawa K., Kagohashi K., Ishikawa H., Satoh H., Hizawa N. Specific organ metastases and survival in metastatic non-small-cell lung cancer. Mol. Clin. Oncol. 2015;3(1):217–221. doi: 10.3892/mco.2014.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J. Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 5.Luo Z., Wang Q., Lau W.B., Lau B., Xu L., Zhao L., Yang H., Feng M., Xuan Y., Yang Y., Lei L., Wang C., Yi T., Zhao X., Wei Y., Zhou S. Tumor microenvironment: the culprit for ovarian cancer metastasis? Cancer Lett. 2016;377(2):174–182. doi: 10.1016/j.canlet.2016.04.038. [DOI] [PubMed] [Google Scholar]
- 6.Cao Y., Liu X., Lu W., Chen Y., Wu X., Li M., Wang X.A., Zhang F., Jiang L., Zhang Y., Hu Y., Xiang S., Shu Y., Bao R., Li H., Wu W., Weng H., Yen Y., Liu Y. Fibronectin promotes cell proliferation and invasion through mTOR signaling pathway activation in gallbladder cancer. Cancer Lett. 2015;360(2):141–150. doi: 10.1016/j.canlet.2015.01.041. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Garcia B., Eiró N., Marín L., González-Reyes S., González L.O., Lamelas M.L., Vizoso F.J. Expression and prognostic significance of fibronectin and matrix metalloproteases in breast cancer metastasis. Histopathology. 2014;64(4):512–522. doi: 10.1111/his.12300. [DOI] [PubMed] [Google Scholar]
- 8.Gao M., Craig D., Lequin O., Campbell I.D., Vogel V., Schulten K. Structure and functional significance of mechanically unfolded fibronectin type III1 intermediates. Proc. Natl. Acad. Sci. u S. a. 2003;100(25):14784–14789. doi: 10.1073/pnas.2334390100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Au A., Vasel M., Kraft S., Sens C., Hackl N., Marx A., Stroebel P., Hennenlotter J., Todenhöfer T., Stenzl A., Schott S., Sinn H.P., Wetterwald A., Bermejo J.L., Cecchini M.G., Nakchbandi I.A. Circulating fibronectin controls tumor growth. Neoplasia. 2013;15(8):925–938. doi: 10.1593/neo.13762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato M., Jiao Q., Honda T., Kurotani R., Toyota E., Okumura S., Takeya T., Minamisawa S., Lanier S.M., Ishikawa Y. Activator of g protein signaling 8 (AGS8) is required for hypoxia-induced apoptosis of cardiomyocytes: ROLE OF Gβγ AND CONNEXIN 43 (CX43)*. Journal of Biological Chemistry. 2009;284(45):31431–31440. doi: 10.1074/jbc.M109.014068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang T., Gao W., Lin S., Chen H., Du B., Liu Q., Lin X., Chen Q. FNDC1 Promotes the invasiveness of gastric cancer via Wnt/β-catenin signaling pathway and correlates with peritoneal metastasis and prognosis. Front. Oncol. 2020;10 doi: 10.3389/fonc.2020.590492. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Das D.K., Naidoo M., Ilboudo A., Park J.Y., Ali T., Krampis K., Robinson B.D., Osborne J.R., Ogunwobi O.O. miR-1207-3p regulates the androgen receptor in prostate cancer via FNDC1/fibronectin. Exp. Cell Res. 2016;348(2):190–200. doi: 10.1016/j.yexcr.2016.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silver D.L., Naora H., Liu J., Cheng W., Montell D.J. Activated signal transducer and activator of transcription (STAT) 3: localization in focal adhesions and function in ovarian cancer cell motility. Cancer Res. 2004;64(10):3550–3558. doi: 10.1158/0008-5472.CAN-03-3959. [DOI] [PubMed] [Google Scholar]
- 14.Ren J., Niu G., Wang X., Song T., Hu Z., Ke C. Overexpression of FNDC1 in gastric cancer and its prognostic significance. J. Cancer. 2018;9(24):4586–4595. doi: 10.7150/jca.27672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yunwen C., Shanshan G., Zhifei B., Saijun C., Hua Y. The silencing of FNDC1 inhibits the tumorigenesis of breast cancer cells via modulation of the PI3K/Akt signaling pathway. Mol. Med. Rep. 2021;23(6) doi: 10.3892/mmr.2021.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L., Liu J., Wang L., Yang X., Jiang Q., Ji F., Xu Y., Fan X., Zhou Z., Fu C. Up-regulated FNDC1 accelerates stemness and chemoradiation resistance in colorectal cancer cells. Biochem. Biophys. Res. Commun. 2022;602:84–90. doi: 10.1016/j.bbrc.2022.02.038. [DOI] [PubMed] [Google Scholar]
- 17.Hsieh Y.H., Chang Y.Y., Su I.J., Yen C.J., Liu Y.R., Liu R.J., Hsieh W.C., Tsai H.W., Wang L.H., Huang W. Hepatitis B virus pre-S2 mutant large surface protein inhibits DNA double-strand break repair and leads to genome instability in hepatocarcinogenesis. J. Pathol. 2015;236(3):337–347. doi: 10.1002/path.4531. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X., Sai B., Wang F., Wang L., Wang Y., Zheng L., Li G., Tang J., Xiang J. Hypoxic BMSC-derived exosomal miRNAs promote metastasis of lung cancer cells via STAT3-induced EMT. Mol. Cancer. 2019;18(1):40. doi: 10.1186/s12943-019-0959-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H., Rokavec M., Jiang L., Horst D., Hermeking H. Antagonistic effects of p53 and HIF1A on microRNA-34a regulation of PPP1R11 and STAT3 and hypoxia-induced epithelial to mesenchymal transition in colorectal cancer cells. Gastroenterology. 2017;153(2):505–520. doi: 10.1053/j.gastro.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 20.Johnson D.E., O'Keefe R.A., Grandis J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018;15(4):234–248. doi: 10.1038/nrclinonc.2018.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harada D., Takigawa N., Kiura K. The Role of STAT3 in non-small cell lung cancer. Cancers. (Basel) 2014;6(2):708–722. doi: 10.3390/cancers6020708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrand A., Kowalski-Chauvel A., Bertrand C., Escrieut C., Mathieu A., Portolan G., Pradayrol L., Fourmy D., Dufresne M., Seva C. A novel mechanism for JAK2 activation by a G protein-coupled receptor, the CCK2R: implication of this signaling pathway in pancreatic tumor models. J. Biol. Chem. 2005;280(11):10710–10715. doi: 10.1074/jbc.M413309200. [DOI] [PubMed] [Google Scholar]
- 23.Li S.B., Liu Y.Y., Yuan L., Ji M.F., Zhang A., Li H.Y., Tang L.Q., Fang S.G., Zhang H., Xing S., Li M.Z., Zhong Q., Lin S.J., Liu W.L., Huang P., Zeng Y.X., Zheng Y.M., Ling Z.Q., Sui J.H., Zeng M.S. Autocrine INSL5 promotes tumor progression and glycolysis via activation of STAT5 signaling. EMBo Mol. Med. 2020;12(9):e12050. doi: 10.15252/emmm.202012050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato M., Cismowski M.J., Toyota E., Smrcka A.V., Lucchesi P.A., Chilian W.M., Lanier S.M. Identification of a receptor-independent activator of G protein signaling (AGS8) in ischemic heart and its interaction with Gbetagamma. Proc. Natl. Acad. Sci. u S. a. 2006;103(3):797–802. doi: 10.1073/pnas.0507467103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan C., Sato M., Lanier S.M., Smrcka A.V. Signaling by a non-dissociated complex of G protein βγ and α subunits stimulated by a receptor-independent activator of G protein signaling, AGS8. J. Biol. Chem. 2007;282(27):19938–19947. doi: 10.1074/jbc.M700396200. [DOI] [PubMed] [Google Scholar]
- 26.Santoni M., Miccini F., Cimadamore A., Piva F., Massari F., Cheng L., Lopez-Beltran A., Montironi R., Battelli N. An update on investigational therapies that target STAT3 for the treatment of cancer. Expert. Opin. Investig. Drugs. 2021;30(3):245–251. doi: 10.1080/13543784.2021.1891222. [DOI] [PubMed] [Google Scholar]
- 27.Thilakasiri P.S., Dmello R.S., Nero T.L., Parker M.W., Ernst M., Chand A.L. Repurposing of drugs as STAT3 inhibitors for cancer therapy. Semin. Cancer Biol. 2021;68:31–46. doi: 10.1016/j.semcancer.2019.09.022. [DOI] [PubMed] [Google Scholar]
- 28.Herbst R.S., Morgensztern D., Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553(7689):446–454. doi: 10.1038/nature25183. [DOI] [PubMed] [Google Scholar]
- 29.Geiger T.R., Peeper D.S. Metastasis mechanisms. Biochim. Biophys. Acta. 2009;1796(2):293–308. doi: 10.1016/j.bbcan.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Kalluri R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer. 2016;16(9):582–598. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 31.Yuzhalin A.E., Lim S.Y., Kutikhin A.G., Gordon-Weeks A.N. Dynamic Matrisome: ECM remodeling factors licensing cancer progression and metastasis. Biochim. Biophys. Acta Rev. Cancer. 2018;1870(2):207–228. doi: 10.1016/j.bbcan.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Anderegg U., Breitschwerdt K., Köhler M.J., Sticherling M., Haustein U.F., Simon J.C., Saalbach A. MEL4B3, a novel mRNA is induced in skin tumors and regulated by TGF-beta and pro-inflammatory cytokines. Exp. Dermatol. 2005;14(9):709–718. doi: 10.1111/j.0906-6705.2005.00349.x. [DOI] [PubMed] [Google Scholar]
- 33.Zhong M., Zhang Y., Yuan F., Peng Y., Wu J., Yuan J., Zhu W., Zhang Y. High FNDC1 expression correlates with poor prognosis in gastric cancer. Exp. Ther. Med. 2018;16(5):3847–3854. doi: 10.3892/etm.2018.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta M., Doss B., Lim C.T., Voituriez R., Ladoux B. Single cell rigidity sensing: a complex relationship between focal adhesion dynamics and large-scale actin cytoskeleton remodeling. Cell Adh. Migr. 2016;10(5):554–567. doi: 10.1080/19336918.2016.1173800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu R.Y., Zeng Y., Lei Z., Wang L., Yang H., Liu Z., Zhao J., Zhang H.T. JAK/STAT3 signaling is required for TGF-β-induced epithelial-mesenchymal transition in lung cancer cells. Int. J. Oncol. 2014;44(5):1643–1651. doi: 10.3892/ijo.2014.2310. [DOI] [PubMed] [Google Scholar]
- 36.Hayashi H., Al Mamun A., Sakima M., Sato M. Activator of G-protein signaling 8 is involved in VEGF-mediated signal processing during angiogenesis. J. Cell Sci. 2016;129(6):1210–1222. doi: 10.1242/jcs.181883. [DOI] [PubMed] [Google Scholar]
- 37.Sato M., Blumer J.B., Simon V., Lanier S.M. Accessory proteins for G proteins: partners in signaling. Annu. Rev. Pharmacol. Toxicol. 2006;46:151–187. doi: 10.1146/annurev.pharmtox.46.120604.141115. [DOI] [PubMed] [Google Scholar]
- 38.Kimple A.J., Bosch D.E., Giguère P.M., Siderovski D.P. Regulators of G-protein signaling and their Gα substrates: promises and challenges in their use as drug discovery targets. Pharmacol. Rev. 2011;63(3):728–749. doi: 10.1124/pr.110.003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blumer J.B., Lanier S.M. Activators of G protein signaling exhibit broad functionality and define a distinct core signaling triad. Mol. Pharmacol. 2014;85(3):388–396. doi: 10.1124/mol.113.090068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sato M. Roles of accessory proteins for heterotrimeric G-protein in the development of cardiovascular diseases. Circ. J. 2013;77(10):2455–2461. doi: 10.1253/circj.cj-13-0705. [DOI] [PubMed] [Google Scholar]
- 41.Ghahremani M.H., Forget C., Albert P.R. Distinct roles for Galpha(i)2 and Gbetagamma in signaling to DNA synthesis and Galpha(i)3 in cellular transformation by dopamine D2S receptor activation in BALB/c 3T3 cells. Mol. Cell Biol. 2000;20(5):1497–1506. doi: 10.1128/mcb.20.5.1497-1506.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cimica V., Chen H.C., Iyer J.K., Reich N.C. Dynamics of the STAT3 transcription factor: nuclear import dependent on Ran and importin-β1. PLoS. One. 2011;6(5):e20188. doi: 10.1371/journal.pone.0020188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Azare J., Doane A., Leslie K., Chang Q., Berishaj M., Nnoli J., Mark K., Al-Ahmadie H., Gerald W., Hassimi M., Viale A., Stracke M., Lyden D., Bromberg J. Stat3 mediates expression of autotaxin in breast cancer. PLoS. One. 2011;6(11):e27851. doi: 10.1371/journal.pone.0027851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.You W., Tang Q., Zhang C., Wu J., Gu C., Wu Z., Li X. IL-26 promotes the proliferation and survival of human gastric cancer cells by regulating the balance of STAT1 and STAT3 activation. PLoS. One. 2013;8(5):e63588. doi: 10.1371/journal.pone.0063588. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Yin S., Yang L., Zheng Y., Zang R. MS: wip1 suppresses angiogenesis through the STAT3-VEGF signalling pathway in serous ovarian cancer. J. Ovarian. Res. 2022;15(1):56. doi: 10.1186/s13048-022-00990-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tong D., Liu Q., Liu G., Xu J., Lan W., Jiang Y., Xiao H., Zhang D., Jiang J. Metformin inhibits castration-induced EMT in prostate cancer by repressing COX2/PGE2/STAT3 axis. Cancer Lett. 2017;389:23–32. doi: 10.1016/j.canlet.2016.12.031. [DOI] [PubMed] [Google Scholar]
- 47.Liang Y., Yang N., Pan G., Jin B., Wang S., Ji W. Elevated IL-33 promotes expression of MMP2 and MMP9 via activating STAT3 in alveolar macrophages during LPS-induced acute lung injury. Cell Mol. Biol. Lett. 2018;23:52. doi: 10.1186/s11658-018-0117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuan W., Li T., Mo X., Wang X., Liu B., Wang W., Su Y., Xu L., Han W. Knockdown of CMTM3 promotes metastasis of gastric cancer via the STAT3/Twist1/EMT signaling pathway. Oncotarget. 2016;7(20):29507–29519. doi: 10.18632/oncotarget.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Egeblad M., Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer. 2002;2(3):161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 50.Yokoyama K., Kamata N., Fujimoto R., Tsutsumi S., Tomonari M., Taki M., Hosokawa H., Nagayama M. Increased invasion and matrix metalloproteinase-2 expression by Snail-induced mesenchymal transition in squamous cell carcinomas. Int. J. Oncol. 2003;22(4):891–898. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in this study are included in the article, and further inquiries can be directed to the corresponding author.