Abstract
Background
Hysteroscopy is an operation in which the gynaecologist examines the uterine cavity using a small telescopic instrument (hysteroscope) inserted via the vagina and the cervix. Almost 50% of hysteroscopic complications are related to difficulty with cervical entry. Potential complications include cervical tears, creation of a false passage, perforation, bleeding, or simply difficulty in entering the internal os (between the cervix and the uterus) with the hysteroscope. These complications may possibly be reduced with adequate preparation of the cervix (cervical ripening) prior to hysteroscopy. Cervical ripening agents include oral or vaginal prostaglandin, which can be synthetic (e.g misoprostol) or natural (e.g. dinoprostone) and vaginal osmotic dilators, which can be naturally occurring (e.g. laminaria) or synthetic.
Objectives
To determine whether preoperative cervical preparation facilitates cervical dilatation and reduces the complications of operative hysteroscopy in women undergoing the procedure for any condition.
Search methods
In August 2014 we searched sources including the Menstrual Disorders and Subfertility Group (MDSG) Trials Register, Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, PsycINFO, CINAHL, ClinicalTrials.gov and reference lists of relevant articles. We searched for published and unpublished studies in any language.
Selection criteria
Two review authors independently selected randomised controlled trials (RCTs) of cervical ripening agents used before operative hysteroscopy in pre‐ and postmenopausal women. Cervical ripening agents could be compared to each other, placebo or no treatment.
Data collection and analysis
Data extraction and quality assessment were conducted independently by two review authors. The primary review outcomes were effectiveness of cervical dilatation (defined as the proportion of women requiring mechanical cervical dilatation) and intraoperative complications. Secondary outcomes were mean time required to dilate the cervix, preoperative pain, cervical width, abandonment of the procedure, side effects of dilating agents and duration of surgery. We calculated odds ratios (ORs) for dichotomous outcomes and mean differences (MDs) for continuous outcomes, with 95% confidence intervals ( CIs). Data were statistically pooled where appropriate. Heterogeneity was assessed using the I2 statistic. The overall quality of the evidence was assessed using GRADE methods.
Main results
Nineteen RCTs with a total of 1870 participants were included. They compared misoprostol with no treatment or placebo, dinoprostone or osmotic dilators.
Misoprostol was more effective for cervical dilatation than placebo or no intervention, with fewer women requiring mechanical dilatation (OR 0.08, 95% CI 0.04 to 0.16, five RCTs, 441 participants, I2=0%, moderate quality evidence). This suggests that in a population in which 80% of women undergoing hysteroscopy require mechanical dilatation without use of preoperative ripening agents, use of misoprostol will reduce the need for mechanical dilatation to between 14% and 39%. Misoprostol was associated with fewer intraoperative complications (OR 0.37, 95% CI 0.18 to 0.77, 12 RCTs, 901 participants, I2=0%, moderate quality evidence). This suggests that in a population in which 3% of women undergoing hysteroscopy experience intraoperative complications without use of preoperative ripening agents, use of misoprostol will reduce the risk of complications to 2% or less.
When specific complications were considered, the misoprostol group had a lower rate of cervical laceration or tearing (OR 0.25, 95% CI 0.11 to 0.57, nine RCTS, 669 women, I2=0%, moderate quality evidence) or false track formation (OR 0.34, 95% CI 0.12 to 0.97, seven RCTs, 560 participants, I2=0%, moderate quality evidence). There was no evidence of a difference between the groups in rates of uterine perforation (0.42, 95% CI 0.13 to 1.38, seven RCTs, 455 participants, I2=0%, low quality evidence) or uterine bleeding (OR 0.51, 95% CI 0.10 to 2.49, four RCTs, 340 participants, I2=0%, low quality evidence). Some treatment side effects (mild abdominal pain, vaginal bleeding, and increased body temperature) were more common in the misoprostol group.
Compared with dinoprostone, misoprostol was associated with more effective cervical dilatation, with fewer women requiring mechanical dilatation (OR 0.58; 95% CI 0.34 to 0.98; one RCT, 310 participants, low quality evidence) and with fewer intraoperative complications (OR 0.32; 95% CI 0.12 to 0.83, one RCT, 310 participants, low quality evidence). However treatment side effects were more common in the misoprostol arm.
Compared to osmotic dilatation (laminaria), misoprostol was associated with less effective cervical dilatation, with more women in the misoprostol group requiring mechanical dilatation (OR 5.96, 95% CI 2.61 to 13.59, one RCT, 110 participants, low quality evidence). There was no evidence of a difference between misoprostol and osmotic dilators in intraoperative complication rates (OR 5.14, 95% CI 0.24 to 109.01, three RCTs, 354 participants, low quality evidence), with only two events reported altogether.
The overall quality of the evidence ranged from low to moderate. The main limitations in the evidence were imprecision and poor reporting of study methods.
Authors' conclusions
There is moderate quality evidence that use of misoprostol for preoperative ripening of the cervix before operative hysteroscopy is more effective than placebo or no treatment and is associated with fewer intraoperative complications such as lacerations and false tracks. However misoprostol is associated with more side effects, including preoperative pain and vaginal bleeding. There is low quality evidence to suggest that misoprostol has fewer intraoperative complications and is more effective than dinoprostone.
There is also low quality evidence to suggest that laminaria may be more effective than misoprostol, with uncertain effects for complication rates. However the possible benefits of laminaria need to be weighed against the inconvenience of its insertion and retention for one to two days.
Keywords: Female, Humans, Pregnancy, Cervical Ripening, Hysteroscopy, Hysteroscopy/adverse effects, Cervix Uteri, Cervix Uteri/drug effects, Cervix Uteri/injuries, Dilatation, Dilatation/methods, Dinoprostone, Dinoprostone/administration & dosage, Laminaria, Misoprostol, Misoprostol/administration & dosage, Oxytocics, Oxytocics/administration & dosage, Preoperative Care, Preoperative Care/methods, Randomized Controlled Trials as Topic
Plain language summary
Preparing the cervix with different ripening agents before operative hysteroscopy
Review question
Are cervical ripening agents effective for dilating the cervix before operative hysteroscopy and do they reduce the risk of complications during the surgery?
Background
Hysteroscopy is an operation in which the gynaecologist examines the uterine cavity using a small telescope (hysteroscope) inserted via the vagina and the cervix. Potential complications of hysteroscopy include cervical tears, formation of a false passage and uterine perforation. Cervical ripening agents are used with the aim of making it easier for the hysteroscope to pass through the cervix and reducing the risk of complications. Ripening agents include different types of prostaglandins (for example misoprostol and dinoprostone) which are administered either orally or vaginally. Osmotic agents are also used, and are administered vaginally. One osmotic agent is laminaria, a sea‐weed based product. Cochrane reviewers assessed the evidence about different ripening agents. The evidence is current to August 2014.
Study characteristics
We included 19 randomised controlled trials (1870 participants) of premenopausal and postmenopausal women undergoing hysteroscopic surgery for a variety of conditions. They compared misoprostol with placebo or no treatment, dinoprostone, and osmotic agents.
Key results
There is moderate quality evidence that misoprostol is safer and is more effective for cervical ripening than placebo or no treatment, and that is associated with fewer complications occurring during the operation, with lower rates of lacerations and false tracks. However misoprostol is associated with more side effects such as preoperative pain and vaginal bleeding.
There is low quality evidence that misoprostol may be safer and more effective than dinoprostone, and that it may be associated with fewer complications occurring during the operation. There is also low quality evidence that laminaria may be more effective than misoprostol. However, the possible benefits of laminaria need to be weighed against the inconvenience of its insertion and retention for one to two days.
Quality of the evidence
The quality of the evidence ranged from low to moderate. The main limitations in the evidence were imprecision and poor reporting of study methods. The evidence is current to August 2014.
Summary of findings
Summary of findings for the main comparison. Misoprostol compared to placebo or no treatment before operative hysteroscopy.
| Misoprostol compared to placebo or no treatment for women undergoing hysteroscopy | ||||||
| Population: Pre and post menopausal women Settings: Operative hysteroscopy Intervention: Preoperative misoprostol Comparison: Placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no treatment | Misoprostol | |||||
| Ease of dilatation: need for mechanical dilatation | 797 per 1000 | 239 per 1000 (136 to 386)5 to 23 per 1000 | OR 0.08 (0.04 to 0.16) | 441 (5 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | |
| Intraoperative complications | 29 per 1000 | 11 per 1000 (5 to 23) | OR 0.37 (0.18 to 0.77) | 901 (12 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | |
| Specific intraoperative complications ‐ Cervical laceration/tear | 25 per 1000 | 6 per 1000 (3 to 14) | OR 0.25 (0.11 to 0.57) | 669 (9 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | |
| Specific intraoperative complications ‐ False track | 40 per 1000 | 14 per 1000 (5 to 39) | OR 0.34 (0.12 to 0.97) | 560 (7 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | |
| Specific intraoperative complications ‐ Uterine perforation | 29 per 1000 | 13 per 1000 (4 to 40) | OR 0.42 (0.13 to 1.38) | 455 (7 RCTs) | ⊕⊕⊝⊝ LOW 1,2 | |
| Specific intraoperative complications ‐ Uterine bleeding | 60 per 1000 | 32 per 1000 (6 to 137) | OR 0.51 (0.10 to 2.49) | 340 (4 RCTs) | ⊕⊕⊝⊝ LOW 1,2 | |
| Side effects | 18 per 1000 | 112 per 1000 (48 to 241) | OR 2.59 (1.15 to 5.79) | 272 (4 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Inadequate reporting of methodology by all or most of the studies
2Imprecision: low event rates and wide confidence intervals compatible with no effect or with meaningful benefit from misoprostol
Summary of findings 2. Misoprostol compared to dinoprostone before operative hysteroscopy.
| Misoprostol compared to dinoprostone for health problem or population | ||||||
| Population: Pre and post menopausal women Settings: Operative hysteroscopy Intervention: Preoperative misoprostol Comparison: Preoperative dinoprostone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Dinoprostone | Misoprostol | |||||
| Ease of dilatation: need for mechanical dilatation | 804 per 1000 | 704 per 1000 (582 to 801) | OR 0.58 (0.34 to 0.98) |
310 (1 RCT) | ⊕⊕⊝⊝ LOW 1,2 | |
| Intraoperative complications | 114 per 1000 | 40 per 1000 (15 to 96) | OR 0.32 (0.12 to 0.83) | 310 (1 RCT) | ⊕⊕⊝⊝ LOW 1,2 | |
| Side effects | Total side effects not reported. Some specific side effects (abdominal pain, vaginal bleeding, diarrhoea and perception of raised temperature) were more common in the misoprostol group. All side effects were mild. | ⊕⊕⊝⊝ LOW 1,3 | ||||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Inadequate description of study methods, high risk of attrition bias
2Imprecision: wide confidence intervals compatible with meaningful benefit from misoprostol or with little or no meaningful benefit
3Imprecision: wide confidence intervals compatible with harm from misoprostol or no clinically meaningful effect
Summary of findings 3. Misoprostol compared to osmotic dilator before operative hysteroscopy.
| Misoprostol compared to osmotic dilator for health problem or population | ||||||
| Population: Pre and post menopausal women Settings: Operative hysteroscopy Intervention: Preoperative misoprostol Comparison: Preoperative osmotic dilator | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Osmotic dilator | Misoprostol | |||||
| Ease of dilatation: need for mechanical dilatation | 283 per 1000 | 702 per 1000 (507 to 843) | OR 5.96 (2.61 to 13.59) |
110 (1 RCT) | ⊕⊕⊝⊝ LOW 1,2 | |
| Intraoperative complications | 0 per 1000 | 0 per 1000 (0 to 0) | OR 5.14 (0.24 to 109.01) | 354 (3 RCTs) | ⊕⊕⊝⊝ LOW 3,4 | Only two events altogether. No events in two RCTs |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Risk of attrition bias unclear; unclear whether outcome assessment was blinded
2Imprecision: single small study with total of 55 events
3One of studies does not adequately describe methods, unclear whether outcome assessment blinded
4Imprecision: only 2 events
Background
Hysteroscopy is an operation in which the gynaecologist examines the uterine cavity using a small telescopic instrument (hysteroscope) inserted via the vagina and the cervix. Hysteroscopy allows the diagnosis and treatment of submucous fibroids, polyps, scar tissue, and uterine abnormalities. Diagnostic hysteroscopy can be done under paracervical or local anaesthesia. However, operative or therapeutic hysteroscopy is usually performed under general or spinal anaesthesia. At operative hysteroscopy uterine polyps, fibroids, adhesions, or septum can be removed and endometrial resection or ablation can be done. In order to visualize the cavity adequately, the uterine cavity should be expanded with a distending medium such as a solution of glycine.
Possible complications of hysteroscopy include uterine perforation, bleeding, infection, damage to intra‐abdominal organs, and fluid overload. In some cases fluid overload may occur, which can cause electrolytes imbalance and encephalopathy, and rarely death. The incidence of fluid overload is between 1.6% and 2.5% (Agostini A 2002a; Overton 1997). The incidence of uterine perforation is 0.014%, and infectious complications account for 0.3% to 1.6% of cases (Bradley 2002). Although rare, injury to internal organs can occur and this may require a laparotomy.
Difficulty in dilating the cervix is a complication that is infrequently discussed, despite the fact that almost 50% of hysteroscopic complications are related to difficulty with cervical entry (Bradley 2002). Potential complications include cervical tears, creation of a false passage, perforation, bleeding, or simply difficulty in entering the internal os with the hysteroscope (Bradley 2002; Cooper 1996; Loffer 1989). Adequate preparation of the cervix prior to hysteroscopy may reduce these potential complications (Bradley 2002; Ostrzenski 1994).
Description of the condition
Intrauterine pathology such as uterine polyps, fibroids, or adhesions can be treated using operative hysteroscopy. Hysteroscopic endometrial ablation or resection can also be performed by an hysteroscopic approach.
Description of the intervention
Difficulty in cervical entry and associated complications may be reduced by adequate preparation of the cervix prior to hysteroscopy. Cervical ripening agents include oral or vaginal prostaglandin, which can be synthetic (e.g. misoprostol) or natural (e.g. dinoprostone) and vaginal osmotic dilators, which can also be naturally occurring (e.g. laminaria) or synthetic.
Laminaria is made from the stem of brown sea weed (Laminaria digitata). Its dry form absorbs fluid from the cervix facilitating cervical dilatation (Ostrzenski 1994). For insertion the patient is put in the dorsal lithotomy position, a sterile Cuscos speculum is applied and the cervix is sterilized using povidone‐iodine solution. The Laminaria is removed aseptically from its package and grasped from its proximal end where the string is attached using Ring forceps. It is inserted into the cervical canal until it has passed the internal os with the string resting in the vaginal vault for easy removal (Darwish 2004). Laminaria has the disadvantage of requiring insertion and retention for one to two days.
Prostaglandin vaginal suppositories (meteno‐prost potassium or gemeprost), or intracervical sulprostone gel have been used as a cervical ripening agents before hysteroscopy (Hald 1988; Rabe 1985). Oral or vaginal misoprostol, a synthetic prostaglandin E1 analogue, has been shown to be effective in cervical ripening among both pregnant and non‐pregnant women (Nagi 1997; Oppegaard 2010; Preutthipan 2000).
How the intervention might work
Adequate preparation of the cervix prior to operative hysteroscopy may reduce the complications related to difficulty with cervical entry. Preoperative ripening agents are used to dilate the cervix before hysteroscopy.
In some cases mechanical cervical dilatation is required during surgery. In addition, preoperative use of gonadotropin releasing hormone agonist (GnRHa) will create a hypoestrogenic state (medical menopause), thin the endometrium, and facilitate visualization of the endometrial cavity (Sowter 2002). Treatment with GnRHa will also reduce the size of uterine fibroids (Girgoriadis 2012). However, similar to postmenopausal women, GnRHa treated women may have increased resistance to cervical dilatation due to the hypoestrogenic cervix (Thomas 2002a).
Why it is important to do this review
This is the first systematic review related to the research question. It is important to evaluate whether preparation of the cervix prior to operative hysteroscopy is needed and, if so, what is the best method of cervical preparation.
Objectives
To determine whether preoperative cervical preparation facilitates cervical dilatation and reduces the complications of operative hysteroscopy in women undergoing the procedure for any condition.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs). Quasi‐randomized trials were not included.
Types of participants
Pre‐ or postmenopausal women and women, with or without pretreatment with GnRHa, who underwent operative hysteroscopy with or without pretreatment with cervical ripening agents.
Types of interventions
Studies of different cervical ripening agents compared to placebo or no intervention were eligible for inclusion. Eligible comparisons included the following agents:
Misoprostol versus placebo or no treatment
Laminaria versus placebo or no Laminaria
Misoprostol versus Laminaria
Prostaglandin versus placebo or no prostaglandin
Prostaglandin versus Laminaria
Prostaglandin versus misoprostol
Prostaglandin versus other types of prostaglandins
Types of outcome measures
Primary outcomes
Effectiveness of cervical dilatation, defined as requirement for mechanical cervical dilatation
Intraoperative complications of the procedure including:
all complications*
cervical tears,
creation of a false passage
uterine perforation
bleeding.
* Where studies did not report "all complications" as a combined outcome and this could not be calculated from the data reported, we included uterine perforation in this analysis, with a footnote to highlight this.
Secondary outcomes
Time required to dilate the cervix to 9 mm.
Preoperative pain, as defined by the authors of each study.
Cervical width, defined as maximum size of dilator that passes the internal os without resistance
Abandonment of the procedure due to failure to dilate the cervix.
Side effects of the cervical dilating agents (including allergic reaction).
Duration of surgery.
Search methods for identification of studies
In August 2014 we searched for all published and unpublished RCTs meeting our inclusion criteria, without language restriction and in consultation with the Menstrual Disorders and Subfertility Group (MDSG) Trials Search Co‐ordinator. It is the intention of the review authors that a new search for RCTs will be performed every two years and the review updated accordingly. See Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5 for search strategies.
Electronic searches
We searched the following electronic sources:
Menstrual Disorders and Subfertility Group (MDSG) Trials Register
Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library,
MEDLINE
EMBASE
PsycINFO
CINAHL
ClinicalTrials.gov
The Cochrane Library at http://www.cochrane.org/index.htm for the Database of Abstracts of Reviews of Effects (DARE)
The service of the US National Institutes of Health: http://clinicaltrials.gov/ct2/home or the World Health Organization International Trials Registry Platform search portal at http://www.who.int/trialsearch/Default.aspx.
Conference abstracts on the ISI Web of Knowledge (http://isiwebofknowledge.com/).
Herbal or complimentary therapy protocols and reviews in the Chinese database as described in the MDSG Module.
OpenSigle for grey literature from Europe (http://opensigle.inist.fr/).
Searching other resources
We also searched the following sources.
Appropriate journals were handsearched. The list of journals is found in the MDSG Module. The Module is found at http://www.mrw.interscience.wiley.com/cochrane/clabout/articles/MENSTR/frame.html (we liaised with the MDSG Trials Search Coordinator to avoid duplication of handsearching).
The reference lists of relevant systematic reviews and RCTs.
Personal contact with experts or institutions.
Conference abstracts and announcements were handsearched: European Society of Human Reproduction and Embryology (ESHRE), American Society for Reproductive Medicine (ASRM) conferences
Data collection and analysis
Selection of studies
Two review authors independently selected trials in accordance with the inclusion criteria. Disagreements were resolved by consensus.
Data extraction and management
Three of the authors independently extracted all data using forms designed using Cochrane guidelines. The following information was extracted from the studies included in the review and presented in a table Characteristics of included studies.
Participants
Characteristics of the women included
Interventions
Type of intervention and control
Dose, timing, duration, and route of administration of the treatment
Outcomes
Outcomes reported
How outcomes were defined
How outcomes were measured
Timing of outcome measurement
If necessary, the authors of trials that met the eligibility criteria were contacted for additional information on trial methodology or actual trial data, or both. Differences of opinion were resolved by consensus.
Assessment of risk of bias in included studies
Two review authors independently assessed the included studies for risk of bias using the Cochrane risk of bias assessment tool (Higgins 2011) to assess: selection bias (random sequence generation and allocation concealment); performance bias (blinding of participants and personnel); detection bias (blinding of outcome assessors); attrition bias (incomplete outcome data); reporting bias (selective reporting); and other bias. Disagreements were resolved by discussion or by a third review author. We described all judgements fully and presented the conclusions in Risk of Bias tables.
Measures of treatment effect
Dichotomous data
We used the numbers of events in the control and intervention groups of each study to calculate Mantel‐Haenszel odds ratios (OR) with 95% confidence intervals (CI).
Continuous data
If all studies reported exactly the same outcomes we calculated mean differences (MDs) between treatment groups. If similar outcomes had been reported on different scales we planned to calculate the standardised mean difference (SMD) with 95% CI.
Where a study compared multiple doses or routes of misoprostol to a control group, we analysed the data as one group regardless of the misoprostol dose or route. In accordance with statistical advice, we calculated the mean by multiplying the mean for each group by the number of women per group, adding the numbers, and then dividing by the total number of women. The same approach was used for the SDs.
Where data to calculate ORs or MDs were not available, we utilised the most detailed numerical data available to facilitate similar analyses of included studies. We compared the magnitude and direction of effect reported by studies with how they were presented in the review, taking into account legitimate differences.
Unit of analysis issues
The unit of analysis in the review was per woman randomised.
Dealing with missing data
Data were analysed on an intention‐to‐treat basis as far as possible and attempts were made to obtain missing data from the original trialists. Otherwise only the available data were analysed.
If studies reported sufficient detail to calculate MDs but gave no information on the associated standard deviation (SD), the outcome was assumed to have an SD equal to the highest SD from other studies within the same analysis.
The trial authors were contacted several times to retrieve missing data. Please see the note at the end of each included study in the 'Characteristics of included studies' table for details.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. Heterogeneity (variation) of the results of different studies was examined by inspecting the scatter in the data points on the graph and the overlap in their CIs and, more formally, by checking the results of the Chi2 tests and the I2 statistics. An I2 value greater than 50% was taken to indicate substantial heterogeneity (Higgins 2003; Higgins 2011).
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, the authors aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of data. If there were ten or more studies in an analysis, we planned to use a funnel plot to explore the possibility of small study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
If the studies were sufficiently similar we pooled the data using a fixed effect model.
We reversed the direction of effect of individual studies, if required, to ensure consistency across trials. We planned to treat ordinal data as continuous data, if appropriate.
Subgroup analysis and investigation of heterogeneity
For primary outcomes, subgroup analyses were performed to determine the separate evidence in the following subgroups, where data were available:
premenopausal women
postmenopausal women
hypoestrogenic women (women who were pretreated with GnRHa, or who were postmenopausal)
Sensitivity analysis
Sensitivity analyses were performed to assess the effects of the following on primary study outcomes:
study quality: limiting analysis to studies which clearly reported acceptable methods of sequence generation and allocation concealment and which were not at high risk of bias in any domain
use of a random effects model
use of RR rather than OR
Overall quality of the body of evidence: Summary of Findings Table
A Summary of Findings Table was generated using GRADEPRO software. This table evaluated the overall quality of the body of evidence for main review outcomes, using GRADE criteria (study limitations (i.e. risk of bias), consistency of effect, imprecision, indirectness and publication bias). Judgements about evidence quality (high, moderate or low) were justified, documented, and incorporated into reporting of results for the primary outcome.
Results
Description of studies
No RCTs were found which compared the following interventions:
Laminaria versus placebo or no laminaria;
prostaglandin versus placebo or no prostaglandin (other than misoprostol);
prostaglandin versus laminaria (other than misoprostol);
prostaglandin versus other types of prostaglandins (other than misoprostol).
Comparisons included:
misoprostol versus placebo or no treatment
misoprostol versus dinoprostone (another type of prostaglandin);
misoprostol versus laminaria (natural osmotic dilator);
misoprostol versus synthetic osmotic dilators.
Results of the search
138 records were retrieved in the search, of which 29 were assessed in full text. Nineteen studies were included, nine were excluded and one awaits assessment. See Figure 1
1.
Study flow diagram.
Included studies
Study design and setting
Nineteen parallel designed randomised trials were included in the review. Two of the studies (Oppegaard 2008 a; Oppegaard 2008 b) were reported in a single trial publication, but are included separately in this review because they were run as separate trials; they include premenopausal and postmenopausal women respectively.
Participants
There were 1870 participants in total. Ten studies evaluated premenopausal women and four studies evaluated postmenopausal women. Four studies evaluated both premenopausal and postmenopausal women. Menopausal status was unclear in one study (Preen 2002). Indications for operative hysteroscopy included: uterine septae, synechiae, submucous myomas, endometrial polyps and endometrial ablation.
Interventions
1. Fifteen RCTs compared the use of misoprostol (a synthetic prostaglandin) versus placebo/no treatment
2. One RCT compared misoprostol versus dinoprostone (a natural prostaglandin)
3. Three RCTs compared misoprostol versus osmotic dilators, either synthetic or naturally occurring (laminaria)
Fernandez 2004 compared three different doses of misoprostol vaginally (200, 400 and 800 µg) to a control group. We analysed the misoprostol group data as one group regardless of the misoprostol dose, using the methods described above (see Measures of treatment effect). The final results were independent of the data from that study. Similarly, Song 2014 compared three different routes of misoprostol to a control group and the misoprostol data were analysed as one group.
Outcomes
With respect to the primary outcomes of this review, seven of the 19 studies reported need for mechanical dilatation and all 19 reported intraoperative complications. Twelve studies reported side effects but it was not always clear how many women hadany side effect. As noted above, where studies did not report "all complications" as a combined outcome and this could not be calculated from the data reported, where possible we included uterine perforation in this analysis, with a footnote to highlight this.
Two studies did not report any outcomes in a form suitable for analysis. One reported only p values (Preen 2002) and one (Thomas 2002) failed to make it clear how many women were randomised to each group or how many were analysed for each outcome. Efforts to obtain this information from the study authors were unsuccessful.
Excluded studies
Nine studies were excluded. Six were studies of diagnostic hysteroscopy, one included mixed diagnostic and operative hysteroscopy, with no distinction between the two groups, and two did not include eligible control groups.
See Characteristics of excluded studies
Study awaiting classification
One study (Chencheewachat 2012) awaits classification. It compares intravaginal isosorbide mononitrate versus placebo, in women having operative hysteroscopy.
Risk of bias in included studies
Please see the risk of bias table of the included studies in Characteristics of included studies for full details; also see Figure 2 and Figure 3.
2.
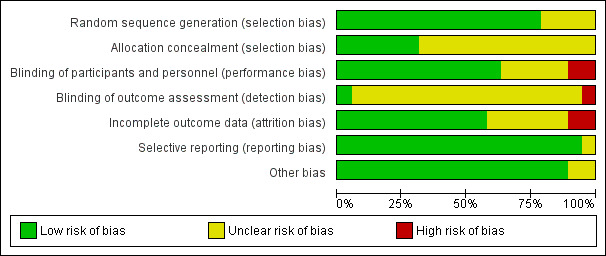
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
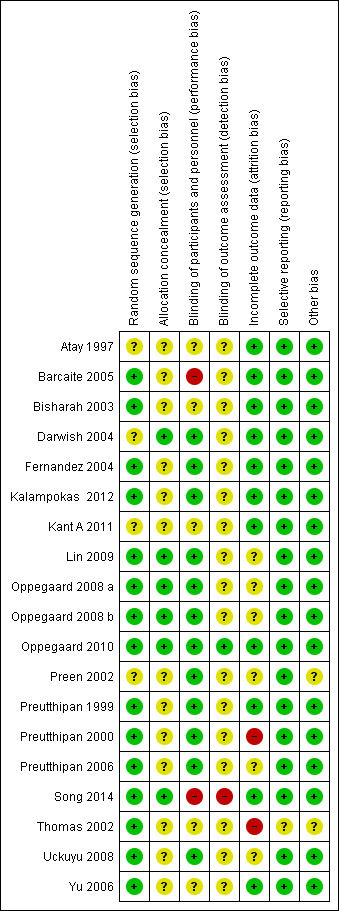
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
The methods used to generate the allocation sequence were considered adequate in 15 of the included studies, where the authors used either computer software, permuted tables, or random numbers to generate a random sequence. Accordingly, these trials were considered at low risk of bias. The methods were considered unclear in four trials where no specific method was mentioned. Overall, 15 studies were deemed to be at low risk and four studies at unclear risk of bias for random sequence generation.
Allocation concealment
A satisfactory method of allocation concealment was reported by six RCTs (Darwish 2004; Lin 2009; Oppegaard 2008 a; Oppegaard 2008 b; Oppegaard 2010; Song 2014). The rest of the RCTs were rated as unclear for allocation concealment. Overall, six studies were deemed to be at low risk and 13studies at unclear risk of bias for allocation concealment.
Blinding
Performance bias
Blinding was reported in 13 RCTs (Darwish 2004; Fernandez 2004; Kalampokas 2012; Lin 2009; Oppegaard 2008 a; Oppegaard 2008 b; Oppegaard 2010; Preen 2002; Preutthipan 1999; Preutthipan 2000; Preutthipan 2006; Uckuyu 2008; Yu 2006). Overall, 13 studies were deemed to be at low risk of bias, four studies at unclear risk, and two studies at high risk.
Detection bias
Two studies were deemed to be at low risk of detection bias (Oppegaard 2010, Thomas 2002), 16 studies at unclear risk and one (Song 2014) at high risk.
Incomplete outcome data
Eleven trials had an adequate description of follow‐up and withdrawals and were considered to be at low risk of attrition bias. One study was rated as at high risk of bias as over 10% of participants were not included in analysis (Preutthipan 2000) and one was rated as at high risk of bias because it did not clearly state how many women were randomised to each group (Thomas 2002). Six of the trials had small numbers of participants (7 to 22 women; under 10% of total participants) who were not included in the final analysis (Lin 2009; Oppegaard 2008 a; Oppegaard 2008 b; Preutthipan 2006; Uckuyu 2008) or did not clearly state how many participants were included in analysis (Preen 2002). These studies were rated as at unclear risk of attrition bias.
Selective reporting
All studies reported all prespecified outcomes including adverse effects, and were rated as at low risk of selective reporting.
Other potential sources of bias
We did not identify any other potential source of bias in 17 of the included studies, which were rated as at low risk of bias in this domain. Two studies which did not report any outcomes in a form suitable for analysis were rated as at unclear risk of bias in this domain (Preen 2002, Thomas 2002).
Effects of interventions
See: Table 1; Table 2; Table 3
1. Misoprostol versus placebo or no treatment
Fifteen RCTs compared misoprostol to placebo or no treatment. Nine (Atay 1997; Barcaite 2005; Bisharah 2003; Fernandez 2004; Kalampokas 2012; Oppegaard 2008 a; Preutthipan 1999; Preutthipan 2000; Song 2014; Uckuyu 2008) included premenopausal women and four (Barcaite 2005; Kant A 2011; Oppegaard 2008 b; Oppegaard 2010) included postmenopausal women. One included both, reporting results separately by menopausal status (Thomas 2002). Menopausal status was unclear in one study (Preen 2002).
Primary outcomes
1.1 Effectiveness of cervical dilatation
Five RCTs reported effectiveness of cervical dilatation, which we defined for the purpose of this review as requirement for mechanical cervical dilatation (Atay 1997; Barcaite 2005; Kant A 2011; Preutthipan 1999; Preutthipan 2000).
Fewer women who used misoprostol required mechanical cervical dilatation (OR 0.08; 95% CI 0.04 to 0.16; five RCTs, 441 participants, I2=0%, moderate quality evidence, Figure 4, Analysis 1.1). This suggests that in a population in which 80% of women undergoing hysteroscopy require mechanical dilatation without use of preoperative ripening agents, use of misoprostol will reduce the need for mechanical dilatation to between 14% and 37%.
4.
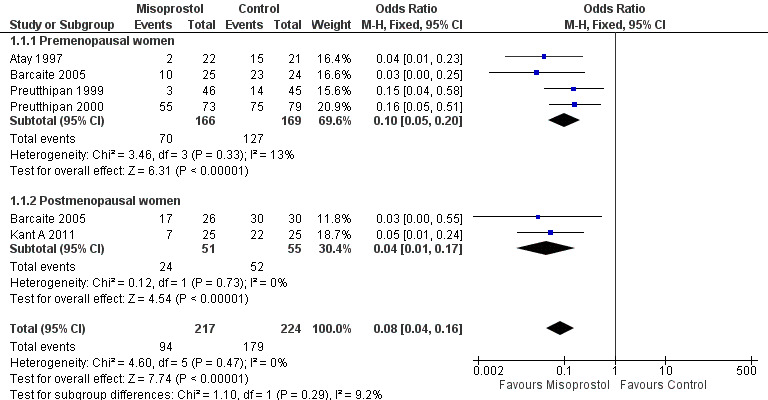
Forest plot of comparison: 1 Misoprostol versus placebo or no treatment, outcome: 1.1 Ease of dilatation: need for mechanical dilatation.
1.1. Analysis.
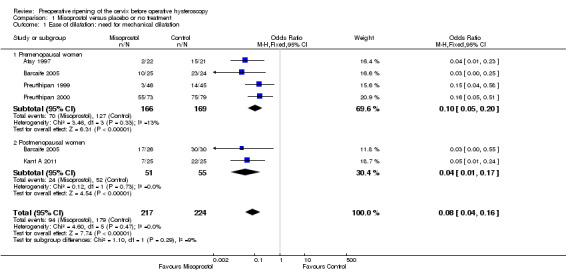
Comparison 1 Misoprostol versus placebo or no treatment, Outcome 1 Ease of dilatation: need for mechanical dilatation.
Subgroup analysis by menopausal status:
Four RCTs (Atay 1997; Barcaite 2005; Preutthipan 1999; Preutthipan 2000) reported relevant data on premenopausal women. Fewer women who used misoprostol required mechanical cervical dilatation (OR 0.10, 95% CI 0.05 to 0.20; four RCTs, 335 participants, I2=0%, Analysis 1.1).
Two RCTs (Barcaite 2005; Kant A 2011) reported relevant data on postmenopausal women. Fewer women who used misoprostol required mechanical cervical dilatation (OR 0.04, 95% CI 0.01 to 0.17106 participants, I2=9.2 %, Analysis 1.1)
1.2 Intraoperative complications of the procedure
All studies reported intraoperative complications.
Overall, intraoperative complications were less common in the misoprostol group (OR 0.37, 95% CI 0.18 to 0.77), 12 RCTs, 901 participants, I2=0%, moderate quality evidence, Figure 5, Analysis 1.2). This suggests that in a population in which 5% of women undergoing hysteroscopy experience intraoperative complications without use of preoperative ripening agents, use of misoprostol will reduce the risk of complications to between 1% and 4%.
5.
Forest plot of comparison: 1 Misoprostol versus placebo or no treatment, outcome: 1.2 Intraoperative complications.
1.2. Analysis.
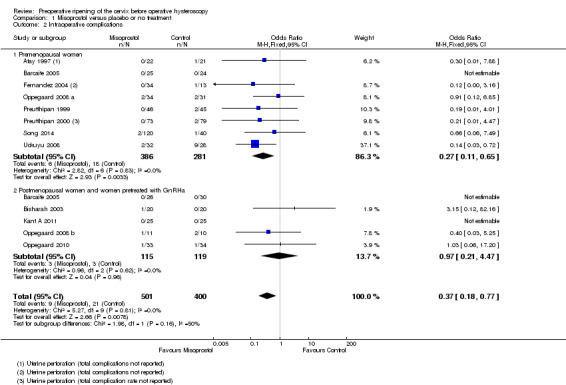
Comparison 1 Misoprostol versus placebo or no treatment, Outcome 2 Intraoperative complications.
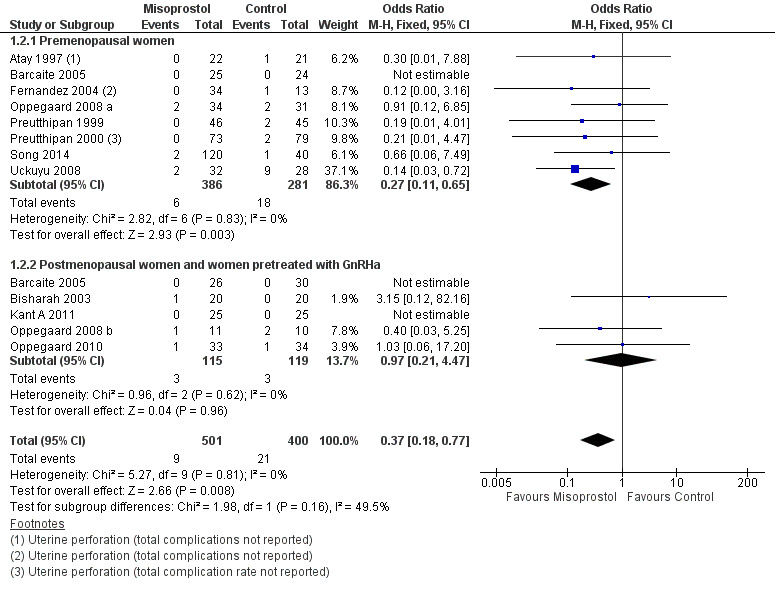
When specific types of complication were considered separately, misoprostol decreased the risk of cervical lacerations and tears (OR 0.25, 95% CI 0.11 to 0.57, nine RCTS, 669 women, I2=0%, moderate quality evidence) and false track formation (OR 0.34, 95% CI 0.12 to 0.97, seven RCTs, 560 participants, I2=0%, moderate quality evidence). There was no evidence of a difference between the groups in rates of uterine perforation (OR 0.42, 95% CI 0.13 to 1.38, seven RCTs, 455 participants, I2=0%, low quality evidence) or uterine bleeding (OR 0.51, 95% CI 0.10 to 2.49, four RCTs, 340 participants, I2=0%, low quality evidence). See Analysis 1.3.
1.3. Analysis.
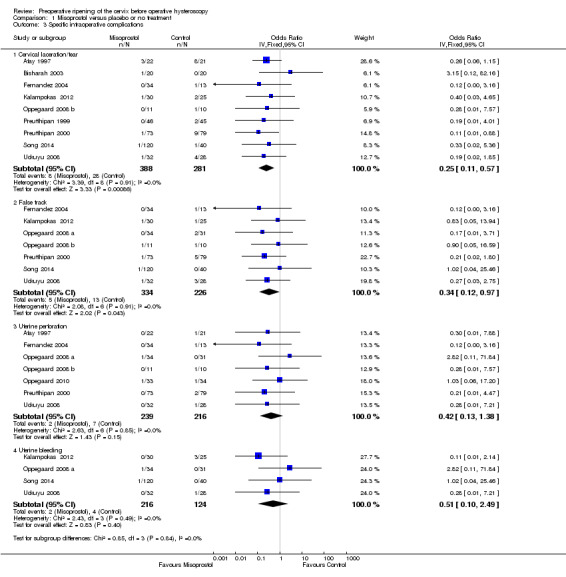
Comparison 1 Misoprostol versus placebo or no treatment, Outcome 3 Specific intraoperative complications.
Preen 2002 reported one cervical laceration and one false track among the 23 women in the misoprostol group, but did not report whether there were any complications in the placebo group. Thomas 2002 reported four cervical lacerations and two perforations in each group, with no significant difference between the groups; however these data were not included in our analysis because it was unclear how many women were in each group.
Subgroup analysis by menopausal status
Eight RCTs (Atay 1997; Barcaite 2005; Fernandez 2004; Oppegaard 2008 a; Preutthipan 1999; Preutthipan 2000; Song 2014; Uckuyu 2008) reported the overall complication rate in premenopausal women. Fewer women in the misoprostol group had complications (OR 0.27, 95% CI 0.11 to 0.65), eight RCTs, 667 participants, I2=0%)
Five RCTs (Barcaite 2005;Bisharah 2003Kant A 2011; Oppegaard 2008 b; Oppegaard 2010) reported the overall complication rate in postmenopausal women and hypoestrogenic women( pretreated with GnRHa). Only five events occurred in these studies and there was no evidence of a difference between the groups (OR 0.97, 95% CI 0.21 to 4.47) , five RCTs, 234 participants, I2=0%) Analysis 1.2
Secondary outcomes
1.3 Time required to dilate the cervix to 9 mm
The time required to dilate the cervix to perform the operative hysteroscopy was reported in nine studies (Bisharah 2003; Fernandez 2004; Kant A 2011; Oppegaard 2008 a; Oppegaard 2008 b; Oppegaard 2010; Preen 2002; Song 2014; Thomas 2002), including a total of 700 women. Seven of these studies reported data suitable for analysis but they were not pooled as there was high heterogeneity (I2=69%) and differences between the studies in the direction of effect. Two studies (Kant A 2011; Song 2014) found a benefit in the misoprostol group, while the other studies found no evidence of a difference between the groups (Analysis 1.4). Two studies reported data unsuitable for analysis, but noted that there was no difference between the groups (Preen 2002: p=0.830, 46 women; Thomas 2002: p=0.18, 204 women).
1.4. Analysis.
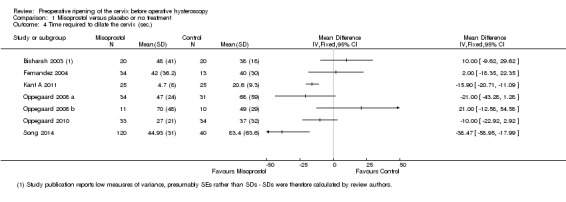
Comparison 1 Misoprostol versus placebo or no treatment, Outcome 4 Time required to dilate the cervix (sec.).
1.4 Preoperative pain
Preoperative pain scores were reported in four studies (Fernandez 2004; Oppegaard 2008 a; Oppegaard 2008 b; Oppegaard 2010). The studies used a visual analogue scale score (VAS) for pain tolerance evaluation, with a range from 0 (no pain) to 10 (unbearable pain). The pooled MD showed a difference between the two groups, favouring the placebo or no treatment group (MD 1.25; 95% CI 0.77 to 1.72; I2=29%, Analysis 1.5).
1.5. Analysis.
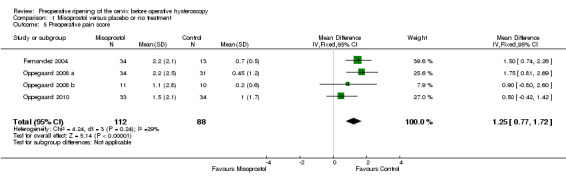
Comparison 1 Misoprostol versus placebo or no treatment, Outcome 5 Preoperative pain score.
1.5 Cervical width
Cervical width, defined as the largest dilator that passes the internal os without resistance (cervical width), was reported in 12 trials with a total of 913participants (Barcaite 2005;Bisharah 2003;Fernandez 2004; Kalampokas 2012; Kant A 2011; Oppegaard 2008 a; Oppegaard 2008 b; Oppegaard 2010; Preutthipan 1999; Preutthipan 2000; Uckuyu 2008; Song 2014). The findings of these trials varied widely, with summary effects ranging from a mean difference of ‐1.5 to + 3.5 mm. The data were not pooled as this resulted in very high heterogeneity (I2=97%). However nine of the 12 individual RCTS favoured misoprostol. Analysis 1.6
1.6. Analysis.
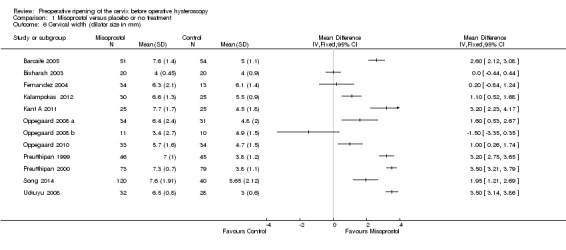
Comparison 1 Misoprostol versus placebo or no treatment, Outcome 6 Cervical width (dilator size in mm).
1.6 Abandonment of the procedure due to failure to dilate the cervix
Failure to dilate the cervix was reported in 10 RCTs (Atay 1997; Barcaite 2005; Fernandez 2004; Kalampokas 2012; Kant A 2011; Oppegaard 2008 a; Oppegaard 2008 b; Preutthipan 2000 ; Preutthipan 1999; Uckuyu 2008).
In nine studies there were no events in either group. In one study (Uckuyu 2008) failure to dilate the cervix was reported in 1/32 (3%) in the misoprostol group and 7/28 (25%) in the placebo group (OR 0.09, 95% CI 0.01 to 0.82). Analysis 1.7
1.7. Analysis.
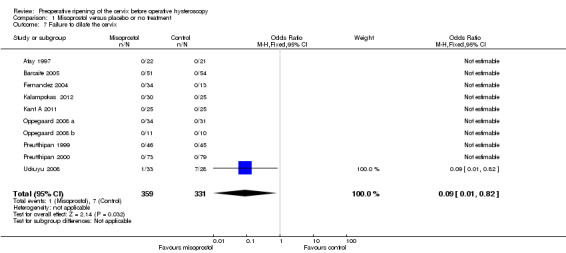
Comparison 1 Misoprostol versus placebo or no treatment, Outcome 7 Failure to dilate the cervix.
1.7 Side effects
The number of women who had side effects was reported in seven RCTs (Barcaite 2005; Bisharah 2003; Oppegaard 2010; Preen 2002; Song 2014; Thomas 2002; Uckuyu 2008).
When side effects were considered overall, they were more common in the misoprostol group (OR 2.59; 95% CI 1.15 to 5.79; four RCTs, 272 women, I2=0%, Analysis 1.8); 24/136 (19%) in the misoprostol group and 12/136 (9%) of the control group experienced side effects.
1.8. Analysis.
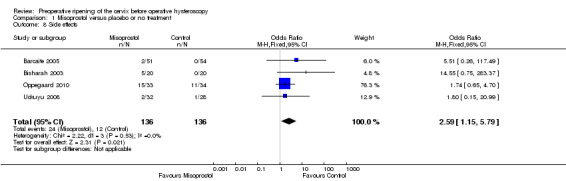
Comparison 1 Misoprostol versus placebo or no treatment, Outcome 8 Side effects.
When specific side effects were considered, compared to placebo misoprostol was associated with a higher incidence of mild abdominal pain (OR 8.48, 95% CI 3.77 to 19.04, fiveRCTs, 548 participants, I2=0%), vaginal bleeding (OR 7.09, 95% CI 2.83 to 17.78, six RCTs, 532 participants, I2=0%), and increased body temperature (OR 5.25, 95% CI 1.28 to 21.44, two RCTs, 243 participants, I2=0%). There was no conclusive evidence of a difference between the groups in rates of nausea (OR 2.41, 95% CI 0.66 to 8.75, five RCTs, 489 participants, I2=0%), diarrhoea (OR 5.66, 95% CI 0.96 to 33.16, five RCTs, 489participants, I2=0%) or shivering (OR 0.91, 95% CI 0.09 to 9.18, two RCTs 86 participants, I2=0%). See Analysis 1.9.
1.9. Analysis.
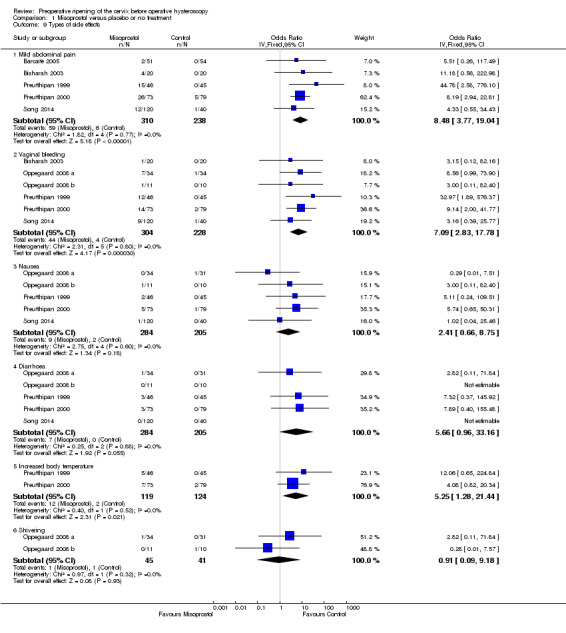
Comparison 1 Misoprostol versus placebo or no treatment, Outcome 9 Types of side effects.
Preen 2002 did not report data suitable for analysis, but noted that there was no evidence of a difference between the groups in postoperative pain rating (p=0.880, 46 women) and that rates of discomfort and overall side effects appeared to be similar in both groups.
Thomas 2002 reported higher rates of overall side‐effects, abdominal pain and vaginal bleeding in the misoprostol group (all p<0.0001), and no significant difference in rates of nausea (p<0.07). These data were not included in our analysis because it was unclear how many women were in each group.
1.8 Duration of surgery.
Duration of surgery was reported in four RCTs, which included a total of 463 participants (Barcaite 2005; Preen 2002; Song 2014; Preutthipan 2000 ). Findings were inconsistent. Two studies found no difference between the groups, while the other reported that the duration of surgery was shorter in the misoprostol group. Three of these studies reported data suitable for analysis but they were not pooled due to high heterogeneity I2=93%). The fourth study (Preen 2002, n=46) failed to report data suitable for analysis.See Analysis 1.10.
1.10. Analysis.
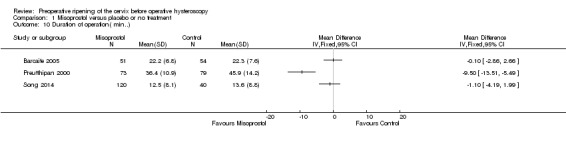
Comparison 1 Misoprostol versus placebo or no treatment, Outcome 10 Duration of operation( min..).
2. Misoprostol versus dinoprostone
A single RCT (Preutthipan 2006) compared misoprostol to dinoprostone (another prostaglandin).
Primary outcomes
2.1 Effectiveness of cervical dilatation
Misoprostol decreased the number of women requiring mechanical cervical dilation, compared to dinoprostone (OR 0.58; 95% CI 0.34 to 0.98; one RCT, 310 participants, low quality evidence, Figure 6, Analysis 2.1); 107/152 (70%) of the women in the misoprostol arm required cervical dilation versus 127/158 (80%) in the dinoprostone arm.
2.1. Analysis.
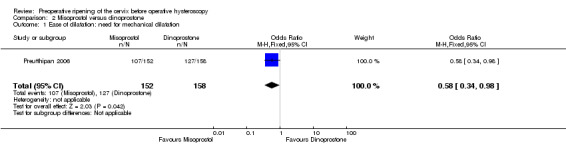
Comparison 2 Misoprostol versus dinoprostone, Outcome 1 Ease of dilatation: need for mechanical dilatation.
2.2 Intraoperative complications
Misoprostol was associated with fewer intraoperative complications than dinoprostone (OR 0.32; 95% CI 0.12 to 0.83, one RCT, 310 participants, low quality evidence, Analysis 2.2). A total of 6/152 (4%) of the women in the misoprostol arm had intraoperative complications versus 18/158 (11%) in the dinoprostone arm.
2.2. Analysis.
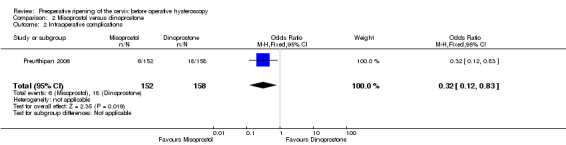
Comparison 2 Misoprostol versus dinoprostone, Outcome 2 Intraoperative complications.
Secondary outcomes
2.3 Time required to dilate the cervix to 9 mm
The time required to dilate the cervix was shorter in the misoprostol group (MD ‐4.60 seconds; 95% CI ‐8.61 to ‐0.59, one RCT, 310 participants, Analysis 2.4). Women in the misoprostol arm required 39 ± 18.8 seconds to dilate the cervix versus 43.6 ± 17.1 seconds in the dinoprostone arm.
2.4. Analysis.
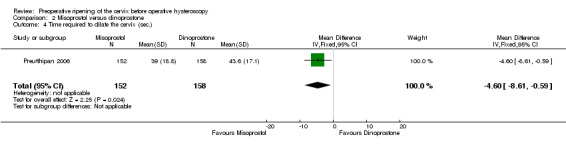
Comparison 2 Misoprostol versus dinoprostone, Outcome 4 Time required to dilate the cervix (sec.).
2.4 Pain
This outcome was not reported.
2.5 Cervical width
The mean cervical width was greater in women who used preoperative misoprostol than in those using dinoprostone (MD 0.40 mm; 95% CI 0.21 to 0.59, one RCT, 310 participants, Analysis 2.5).
2.5. Analysis.
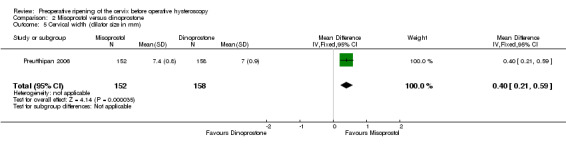
Comparison 2 Misoprostol versus dinoprostone, Outcome 5 Cervical width (dilator size in mm).
2.6 Abandonment of the procedure due to failure to dilate the cervix
This outcome was not reported
2.7 Side effects of the cervical dilating agents
The total number of women experiencing any side effect was not reported.
With regard to specific complications, women in the misoprostol group had a higher incidence of abdominal pain (OR 2.07, 95% CI 1.25 to 3.42), vaginal bleeding (OR 2.14, 95% CI 1.24 to 3.69) and perception of increased body temperature (OR 6.09, 95% CI 1.33 to 27.93). There was no evidence of a difference between the groups in the incidence of headache (OR 1.38, 95% CI 0.59 to 3.26), nausea (OR 1.61 95% CI 0.64 to 4.05), vomiting (OR 1.31, 95% CI 0.34 to 4.97) or diarrhoea (OR 2.87, 95% CI 0.75 to 11.03) Analysis 2.3. All side effects were mild.
2.3. Analysis.
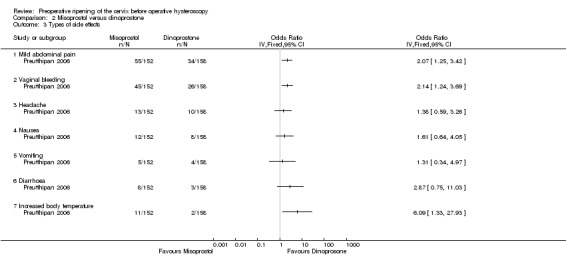
Comparison 2 Misoprostol versus dinoprostone, Outcome 3 Types of side effects.
2.8 Duration of surgery.
The duration of surgery was longer in the misoprostol group (MD 3.20 minutes; 95% CI 1.16 to 5.24, one RCT, 310 participants, Analysis 2.6). The duration of operative hysteroscopy in women using misoprostol was 39.4 ± 9.1 minutes versus 36.2 ± 9.2 minutes in women using dinoprostone.
2.6. Analysis.
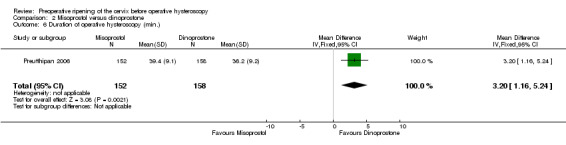
Comparison 2 Misoprostol versus dinoprostone, Outcome 6 Duration of operative hysteroscopy (min.).
3. Misoprostol versus osmotic dilators (natural and synthetic)
Three RCTs (Darwish 2004,Lin 2009,Yu 2006) compared misoprostol to osmotic dilators. Two of them (Darwish 2004; Lin 2009) compared misoprostol to a natural osmotic dilator (Laminaria), while the remaining RCT (Yu 2006) compared misoprostol to a synthetic osmotic dilator.
Primary outcomes
3.1 Effectiveness of cervical dilatation
Only Darwish 2004, which compared misoprostol to laminaria, reported this outcome. Women in the misoprostol group were more likely to require mechanical dilatation (OR 5.96, 95% CI 2.61 to 13.59, one RCT, 110 participants, low quality evidence, Figure 7, Analysis 3.1). Cervical dilatation was required by 15/53 (43%) women using laminaria and by 40/57 (70%) of those using misoprostol.
3.1. Analysis.
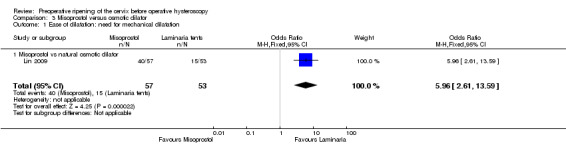
Comparison 3 Misoprostol versus osmotic dilator, Outcome 1 Ease of dilatation: need for mechanical dilatation.
3.2 Intraoperative complications of the procedure
All three studies (Darwish 2004,Lin 2009,Yu 2006) reported on intraoperative complications. None of the women in either Lin 2009 or Yu 2006 had any intraoperative complications, while in Darwish 2004 two women in the misoprostol group had intraoperative complications; there was no evidence of a difference between the groups (OR 5.14, 95% CI 0.24 to 109.01, one RCT, 310 participants, Analysis 3.2).
3.2. Analysis.
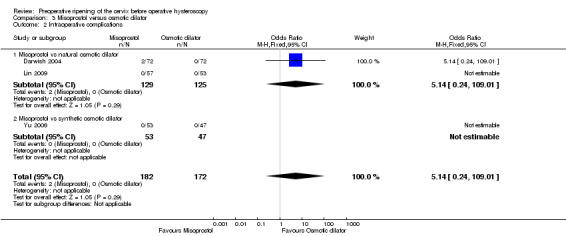
Comparison 3 Misoprostol versus osmotic dilator, Outcome 2 Intraoperative complications.
Secondary outcomes
3.3 Time required to dilate the cervix to 9 mm
Only Darwish 2004, which compared misoprostol to laminaria, reported on cervical dilation time. There was no evidence of a difference between the groups (OR 1.00 second, 95% CI ‐9.41 to 11.41, one RCT, 310 participants, Analysis 3.3).
3.3. Analysis.
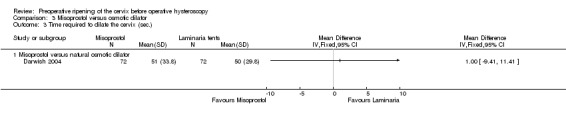
Comparison 3 Misoprostol versus osmotic dilator, Outcome 3 Time required to dilate the cervix (sec.).
3.4 Pain
This outcome was not reported.
3.5 Cervical width
All three studies (Darwish 2004,Lin 2009,Yu 2006) reported on cervical width. They included 354 participants.
Findings were inconsistent, and these studies were not pooled due to very high heterogeneity (I2=95%). Two studies Lin 2009,Yu 2006) reported a benefit for osmotic dilators, while the third Darwish 2004 found no evidence of a difference between the groups. (Analysis 3.4)
3.4. Analysis.
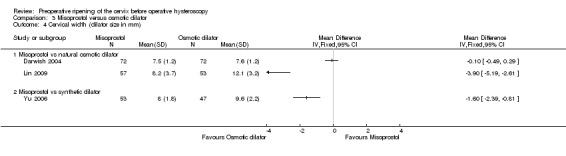
Comparison 3 Misoprostol versus osmotic dilator, Outcome 4 Cervical width (dilator size in mm).
3.6 Abandonment of the procedure due to failure to dilate the cervix
This outcome was not reported.
3.7 Side effects of the cervical dilating agents
This outcome was not reported.
3.8 Duration of surgery.
This outcome was not reported.
Subgroup and sensitivity analyses
There were insufficient data to conduct our planned subgroup analysis of hypoestrogenic women. Other subgroup analyses are reported above.
Sensitivity analysis by quality
Only three studies (Darwish 2004; Oppegaard 2008 b; Oppegaard 2010) met our criteria for lower risk of bias (i.e. clear reporting of acceptable methods of sequence generation and allocation concealment and not at high risk of bias in any domain).This sensitivity analysis did not substantially affect our findings for primary outcomes, except that there was no difference in complication rate between misoprostol and placebo or no treatment, when analysis was restricted to studies at lower risk of bias.
Sensitivity analysis by choice of statistical model
Use of a random effects model did not substantially affect our findings for primary outcomes.
Sensitivity analysis by choice of effect estimate
Use of risk ratios did not substantially affect our findings for primary outcomes.
Assessment of heterogeneity
Heterogeneity was fairly low or absent for our primary outcomes (0% to 33%). However there was high heterogeneity (69% to 97%) for several continuous outcomes, for which there was no clear explanation. Data for these outcomes were not pooled.
Assessment of publication bias
A funnel plot was constructed for the analysis of intraoperative complications in the comparison of misoprostol versus placebo or no treatment. There was no strong suggestion of publication bias. See Figure 6.
6.
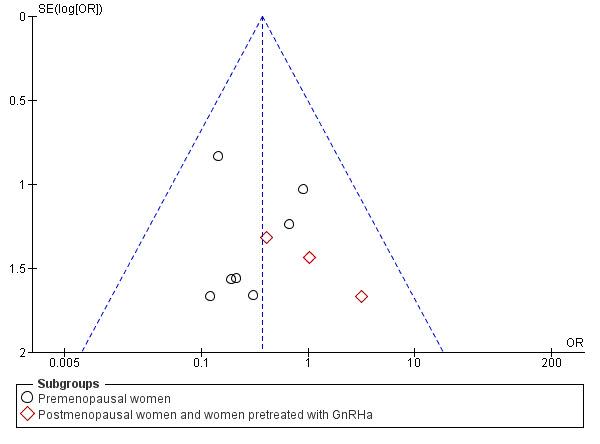
Funnel plot of comparison: 1 Misoprostol versus placebo or no treatment, outcome: 1.2 Intraoperative complications.
Discussion
Summary of main results
Misoprostol, a prostaglandin E1 analogue, was superior to placebo in facilitating cervical dilatation, with fewer women requiring mechanical dilatation. It was also less likely to cause intraoperative complications. With regard to specific complications, cervical lacerations and false tracks were less common in the misoprostol group. There were too few data on uterine perforation or uterine bleeding to show whether there is any difference between the groups for these outcomes. Findings on cervical width were too heterogeneous to pool, but most studies reported a shorter time in the misoprostol group. Some side effects (preoperative pain, mild abdominal pain, vaginal bleeding, and increased body temperature) were more common in the misoprostol group. Findings on time to dilate the cervix and duration of surgery were inconclusive. In most studies abandonment of the procedure did not occur in either group.
Only one study compared misoprostol with dinoprostone. The findings favoured misoprostol for effectiveness of dilatation, risk of intraoperative complications, cervical width and time required to dilate the cervix. However the duration of surgery was about 3 minutes longer and side effects were more common in the misoprostol arm. Our other review outcomes were not reported in this study.
Misoprostol was compared with osmotic dilators, either natural (laminaria) or synthetic. Only one study reported effectiveness of dilatation (defined as need for mechanical dilatation). It found that women in the misoprostol group were more likely to require mechanical dilatation than women in the laminaria group. There was no evidence of a difference between the groups in the rate of intraoperative complications or time taken to dilate the cervix, and findings for cervical width were inconsistent. Other review outcomes were not reported. Clinicians would need to balance the possible benefit of laminaria for cervical dilation and the inconvenience of its insertion and retention for one to two days.
In summary, this review suggests that misoprostol (a synthetic prostaglandin) is preferable to placebo for cervical ripening before operative hysteroscopy. It facilitates cervical dilation and decreases the risk of cervical tears and the formation of a false passage. However, it is associated with treatment side effects including mild abdominal pain, nausea, diarrhoea, and vaginal bleeding. Osmotic dilators are an option for women with contraindications to prostaglandins.
Overall completeness and applicability of evidence
Seven of the 19 included studies reported our primary effectiveness outcome (effectiveness of cervical dilatation), though some of our secondary measures of effectiveness were limited by heterogeneity between the studies which precluded statistical pooling. All studies reported our primary safety outcome (intraoperative complications) and most studies reported on side effects associated with treatment.
The evidence appears to be applicable to women undergoing operative hysteroscopy for any condition, as the indications for operative hysteroscopy in the included studies included: uterine septae, synechiae, submucous myomas, endometrial polyps and endometrial ablation. More evidence is needed on variables such as the specific doses, intervals, and routes of administration used for these agents.
Quality of the evidence
The quality of evidence for comparisons in this review varied from low to moderate. The main limitations in the evidence were imprecision, poor reporting of study methods and statistical heterogeneity which precluded statistical pooling for most of the continuous outcomes. Two of the studies failed to provide data suitable for analysis.
Potential biases in the review process
We conducted this systematic review according to a protocol developed following the recommendations of The Cochrane Collaboration; the protocol was published before conducting the full review. We systematically searched a number of databases and reference lists for randomised clinical trials, which should have reduced the selection bias to a minimum. Two authors selected trials and extracted data in duplicate, independent of each other. We conducted analyses according to the different interventions.
Where a study compared multiple doses or routes of misoprostol to a control group, we analysed the data as one group regardless of the misoprostol dose or route. In accordance with statistical advice, we calculated the mean by multiplying the mean for each group by the number of women per group, adding the numbers, and then dividing by the total number of women. The same approach was used for the SDs. We acknowledge that this approach provides an estimate only, as there is will be no way to obtain the accurate data.
Agreements and disagreements with other studies or reviews
Our review is generally in agreement with other systematic reviews.
Gkrozou F 2011 in their systematic review evaluated the use of vaginal misoprostol before diagnostic or operative hysteroscopy. Misoprostol reduced the need for cervical dilatation in the total population of pre‐ and postmenopausal women. In the subgroup of operative hysteroscopy the need for dilatation and the duration of the procedure were also reduced. Side effects occurred more frequently in the misoprostol group than in the placebo group.
Polyzos 2012 reported in his systematic review on the use of misoprostol prior to hysteroscopy (diagnostic or operative) that misoprostol appears to facilitate an easier and uncomplicated procedure in premenopausal women but not postmenopausal women.
Authors' conclusions
Implications for practice.
There is moderate quality evidence that use of misoprostol for preoperative ripening of the cervix before operative hysteroscopy is more effective than placebo or no treatment and is associated with fewer intraoperative complications such as lacerations and false tracks. However misoprostol is associated with more side effects, including preoperative pain and vaginal bleeding.
There is low quality evidence to suggest that misoprostol has fewer intraoperative complications and is more effective than dinoprostone.
There is low quality evidence to suggest that laminaria may be more effective than misoprostol, with uncertain effects for complication rates. However the possible benefits of laminaria need to be weighed against the inconvenience of its insertion and retention for one to two days.
Clinicians should inform women of the common side effects of misoprostol, including mild abdominal pain and vaginal bleeding, and provide them with appropriate preoperative analgesia.
Implications for research.
Further research is needed on the optimal dose and interval of misoprostol, route of administration, patient satisfaction, and adverse effects. Studies should be stratified by menopausal status. Only large randomised trials will have the power to detect clinically important and patient‐oriented outcomes.
Acknowledgements
We thank all the women and investigators who were involved in the clinical trials mentioned in this review. We thank the staff of the Cochrane Menstrual Disorders and Subfertility Group Editorial Team for their contribution and excellent collaboration. We appreciate the support of the National and Gulf Center for Evidence Health Care Practice (NGCEBHP). We acknowledge the advice of statisticians M. Hassan Murad, MD, MPH and Zhen Wang, PhD (Mayo Clinic, Rochester, MN, USA) for advice on combining the intervention groups in Fernandez 2004.
Appendices
Appendix 1. MDSG search string
MDSG search string HAF1151 02.04.09
Keywords CONTAINS "hysteroscopy" or "hysteroscopy pain" or "hysteroscopy pain ‐surgical" or "hysteroscopy, techniques" or "hysterscope " or "office hysteroscopy" or "operative hysteroscopy" or Title CONTAINS "hysteroscopy" or "hysteroscopy pain" or "hysteroscopy pain ‐surgical" or "hysteroscopy, techniques" or "hysterscope " or "office hysteroscopy" or "operative hysteroscopy"
AND
Keywords CONTAINS "cervical dilatation" or "cervical priming" or "cervical relaxation" or "cervical ripening" or "Laminaria" or "misoprostol" or "Prostaglandin*" or "dilation of cervix" or "priming" or Title CONTAINS "cervical dilatation" or "cervical priming" or "cervical relaxation" or "cervical ripening" or "Laminaria" or "misoprostol" or "Prostaglandin*" or "dilation of cervix" or "priming"
Appendix 2. CENTRAL search
Database: EBM Reviews ‐ Cochrane Central Register of Controlled Trials inception to August 2014 1 exp hysteroscopy/ (265) 2 hysteroscop$.tw. (485) 3 or/1‐2 (511) 4 exp Cervical Ripening/ (238) 5 (Cervi$ adj3 Ripe$).tw. (776) 6 (cervi$ adj3 prim$).tw. (321) 7 (cervi$ adj3 dilat$).tw. (736) 8 (cervi$ adj3 soften$).tw. (71) 9 exp Misoprostol/ (1003) 10 Misoprostol.tw. (1665) 11 exp Laminaria/ (50) 12 laminaria.tw. (90) 13 (Dilateria or Kombu).tw. (0) 14 exp Prostaglandins/ (4450) 15 Prostaglandin$.tw. (4678) 16 (cervi$ adj3 agent$).tw. (127) 17 or/4‐16 (8228) 18 3 and 17 (71) 19 randomized controlled trial.pt. (347677) 20 controlled clinical trial.pt. (84452) 21 randomized.ab. (197013) 22 placebo.tw. (131772) 23 clinical trials as topic.sh. (33055) 24 randomly.ab. (101373) 25 trial.ti. (121577) 26 (crossover or cross‐over or cross over).tw. (45909) 27 or/19‐26 (547268) 28 exp animals/ not humans.sh. (4) 29 27 not 28 (547267) 30 18 and 29 (65)
Appendix 3. MEDLINE search
Database: Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to August 2014> 1 exp hysteroscopy/ (3594) 2 hysteroscop$.tw. (4827) 3 or/1‐2 (5615) 4 exp Cervical Ripening/ (790) 5 (Cervi$ adj3 Ripe$).tw. (1565) 6 (cervi$ adj3 prim$).tw. (2399) 7 (cervi$ adj3 dilat$).tw. (2983) 8 (cervi$ adj3 soften$).tw. (214) 9 exp Misoprostol/ (3363) 10 Misoprostol.tw. (3765) 11 exp Laminaria/ (483) 12 laminaria.tw. (855) 13 (Dilateria or Kombu).tw. (26) 14 exp Prostaglandins/ (93501) 15 Prostaglandin$.tw. (88398) 16 (cervi$ adj3 agent$).tw. (592) 17 or/4‐16 (129044) 18 3 and 17 (232) 19 randomized controlled trial.pt. (386058) 20 controlled clinical trial.pt. (89678) 21 randomized.ab. (306238) 22 placebo.tw. (163050) 23 clinical trials as topic.sh. (172069) 24 randomly.ab. (220457) 25 trial.ti. (132096) 26 (crossover or cross‐over or cross over).tw. (62294) 27 or/19‐26 (951578) 28 exp animals/ not humans.sh. (3996345) 29 27 not 28 (877040) 30 18 and 29 (77)
Appendix 4. EMBASE search
Database: Embase <1980 to 2014 Week 33> 1 exp hysteroscopy/ (7727) 2 hysteroscop$.tw. (7507) 3 or/1‐2 (9336) 4 (Cervi$ adj3 Ripe$).tw. (1906) 5 (cervi$ adj3 prim$).tw. (2868) 6 (cervi$ adj3 dilat$).tw. (3327) 7 (cervi$ adj3 soften$).tw. (204) 8 exp Misoprostol/ (9167) 9 Misoprostol.tw. (4878) 10 exp Laminaria/ (664) 11 laminaria.tw. (828) 12 (Dilateria or Kombu).tw. (34) 13 Prostaglandin$.tw. (90598) 14 (cervi$ adj3 agent$).tw. (713) 15 exp uterine cervix ripening/ (1818) 16 exp Prostaglandin/ (121209) 17 or/4‐16 (150343) 18 3 and 17 (419) 19 Clinical Trial/ (833175) 20 Randomized Controlled Trial/ (347685) 21 exp randomization/ (62979) 22 Single Blind Procedure/ (18684) 23 Double Blind Procedure/ (114887) 24 Crossover Procedure/ (39813) 25 Placebo/ (244133) 26 Randomi?ed controlled trial$.tw. (101777) 27 Rct.tw. (14473) 28 random allocation.tw. (1324) 29 randomly allocated.tw. (20543) 30 allocated randomly.tw. (1939) 31 (allocated adj2 random).tw. (715) 32 Single blind$.tw. (14487) 33 Double blind$.tw. (142389) 34 ((treble or triple) adj blind$).tw. (379) 35 placebo$.tw. (200386) 36 prospective study/ (258597) 37 or/19‐36 (1375448) 38 case study/ (27309) 39 case report.tw. (261663) 40 abstract report/ or letter/ (898356) 41 or/38‐40 (1181592) 42 37 not 41 (1337586) 43 42 and 18 (133)
Appendix 5. PsycINFO search
Database: PsycINFO 1 hysteroscop$.tw. (9) 2 (Cervi$ adj3 Ripe$).tw. (4) 3 (cervi$ adj3 prim$).tw. (36) 4 (cervi$ adj3 dilat$).tw. (37) 5 (cervi$ adj3 soften$).tw. (1) 6 Misoprostol.tw. (45) 7 Laminaria.tw. (3) 8 (Dilateria or Kombu).tw. (0) 9 exp Prostaglandins/ (518) 10 Prostaglandin$.tw. (1156) 11 (cervi$ adj3 agent$).tw. (4) 12 or/2‐11 (1300) 13 1 and 12 (0)
Data and analyses
Comparison 1. Misoprostol versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Ease of dilatation: need for mechanical dilatation | 5 | 441 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.04, 0.16] |
| 1.1 Premenopausal women | 4 | 335 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.05, 0.20] |
| 1.2 Postmenopausal women | 2 | 106 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.01, 0.17] |
| 2 Intraoperative complications | 12 | 901 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.18, 0.77] |
| 2.1 Premenopausal women | 8 | 667 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.11, 0.65] |
| 2.2 Postmenopausal women and women pretreated with GnRHa | 5 | 234 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.21, 4.47] |
| 3 Specific intraoperative complications | 11 | Odds Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Cervical laceration/tear | 9 | 669 | Odds Ratio (IV, Fixed, 95% CI) | 0.25 [0.11, 0.57] |
| 3.2 False track | 7 | 560 | Odds Ratio (IV, Fixed, 95% CI) | 0.34 [0.12, 0.97] |
| 3.3 Uterine perforation | 7 | 455 | Odds Ratio (IV, Fixed, 95% CI) | 0.42 [0.13, 1.38] |
| 3.4 Uterine bleeding | 4 | 340 | Odds Ratio (IV, Fixed, 95% CI) | 0.51 [0.10, 2.49] |
| 4 Time required to dilate the cervix (sec.) | 7 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Preoperative pain score | 4 | 200 | Mean Difference (IV, Fixed, 95% CI) | 1.25 [0.77, 1.72] |
| 6 Cervical width (dilator size in mm) | 12 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Failure to dilate the cervix | 10 | 690 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 0.82] |
| 8 Side effects | 4 | 272 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.59 [1.15, 5.79] |
| 9 Types of side effects | 7 | Odds Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Mild abdominal pain | 5 | 548 | Odds Ratio (IV, Fixed, 95% CI) | 8.48 [3.77, 19.04] |
| 9.2 Vaginal bleeding | 6 | 532 | Odds Ratio (IV, Fixed, 95% CI) | 7.09 [2.83, 17.78] |
| 9.3 Nausea | 5 | 489 | Odds Ratio (IV, Fixed, 95% CI) | 2.41 [0.66, 8.75] |
| 9.4 Diarrhoea | 5 | 489 | Odds Ratio (IV, Fixed, 95% CI) | 5.66 [0.96, 33.16] |
| 9.5 Increased body temperature | 2 | 243 | Odds Ratio (IV, Fixed, 95% CI) | 5.25 [1.28, 21.44] |
| 9.6 Shivering | 2 | 86 | Odds Ratio (IV, Fixed, 95% CI) | 0.91 [0.09, 9.18] |
| 10 Duration of operation( min..) | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 2. Misoprostol versus dinoprostone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Ease of dilatation: need for mechanical dilatation | 1 | 310 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.34, 0.98] |
| 2 Intraoperative complications | 1 | 310 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.12, 0.83] |
| 3 Types of side effects | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Mild abdominal pain | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Vaginal bleeding | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Headache | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Nausea | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Vomiting | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Diarrhoea | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 Increased body temperature | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Time required to dilate the cervix (sec.) | 1 | 310 | Mean Difference (IV, Fixed, 95% CI) | ‐4.60 [‐8.61, ‐0.59] |
| 5 Cervical width (dilator size in mm) | 1 | 310 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [0.21, 0.59] |
| 6 Duration of operative hysteroscopy (min.) | 1 | 310 | Mean Difference (IV, Fixed, 95% CI) | 3.20 [1.16, 5.24] |
Comparison 3. Misoprostol versus osmotic dilator.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Ease of dilatation: need for mechanical dilatation | 1 | 110 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.96 [2.61, 13.59] |
| 1.1 Misoprostol vs natural osmotic dilator | 1 | 110 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.96 [2.61, 13.59] |
| 2 Intraoperative complications | 3 | 354 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.14 [0.24, 109.01] |
| 2.1 Misoprostol vs natural osmotic dilator | 2 | 254 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.14 [0.24, 109.01] |
| 2.2 Misoprostol vs synthetic osmotic dilator | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Time required to dilate the cervix (sec.) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Misoprostol versus natural osmotic dilator | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Cervical width (dilator size in mm) | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Misoprostol vs natural osmotic dilator | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Misoprostol vs synthetic dilator | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Atay 1997.
| Methods | Randomized, placebo‐controlled clinical trial | |
| Participants | Patients undergoing hysteroscopy, for simultaneous diagnostic and operative indications such as uterine septae, synechiae, submucous myomas, endometrial polyps and lost intrauterine devices, were included into the study. They studied a total of 43 women scheduled for hysteroscopy at Guhane school of medicine Ankara, Turkey | |
| Interventions | 43 patients undergoing hysteroscopy as an operative office procedure were randomised to misoprostol (n = 22) or placebo (n = 21) groups. Indications for hysteroscopy were similar in both groups and included: uterine septae, synechiae, submucous myomas, endometrial polyps and removal of intrauterine devices. In the misoprostol group, the number of patients having undergone previous delivery was nine (41%) and it was eight (38%) in the placebo group. The median age for misoprostol group was 26.2 years with range of(17‐36), while in placebo group was 27.1 years with range of (18‐38). Misoprostol 400 mcg, was administered to the posterior fornix 4 h before hysteroscopic intervention. The same protocol was performed for the placebo (control) group. A pain score was provided by using a comparison with the patients worst menstrual pain. The patient was asked to score the pain of dilatation, noted that the worst menstrual pain was scored 10, moderate pain as 5, and no pain as zero. |
|
| Outcomes | Dilatation time, pain score, cervical bleeding and laceration, and uterine perforation. | |
| Notes | The procedure was done as an office hysteroscopy, but Intravenous analgesia was used in addition to cervical block which possible in case of operative procedure. For operative hysteroscopy in this study, the 7 mm operative hysteroscopic sheath was used.The cervix was dilated to 7‐8 mm in all patients even if it was started as diagnostic. Author contacted for: Method of randomisation, Method of treatment allocation, Was blinding used, and if so who was blinded, intention‐to‐treat analysis, Power Calculation, Source of funding, Declaration of interest, mean +/‐ SD ( or cervical width, dilation time, and operation time), and no.of women who had chills as a side effect, with no response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 43 women were included in the study and all were included in the analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, including complications |
| Other bias | Low risk | no other sources of bias can be identified |
Barcaite 2005.
| Methods | Prospective randomised controlled trial. | |
| Participants | From June to December 2004, they studied a total of 105 women scheduled for hysteroscopy at the department of obstetrics and gynaecology of Kaunas University hospital of Medicine, Kaunas, Lithuania. The inclusion criteria were being perimenopausal or postmenopausal women, having definite indication for hysteroscopy and being in good health. Allergy to prostaglandin and lesions of the endocervical canal were considered as exclusion criteria. | |
| Interventions | 105 women were assigned to 2 groups using a computer‐generated randomisation table. In the study group (n = 51) 400 micrograms of misoprostol was inserted in the posterior vaginal fornix at least 12 h before hysteroscopy. No patient received any cervical ripening agent prior to surgery in the control group (n = 54). The hysteroscopy was performed under general anaesthesia by 2 investigators. Cervical dilation was performed using successively larger Hegar dilators until resistance was met. Cervical width was reported as 1 size smaller as the final Hegar dilator used. Further, cervix was dilated to Hegar No. 8.5 for diagnostic purposes and minor operative procedures, and to Hegar No. 10 for hysteroscopic resection of fibroids. An 8mm hysteroscope was used and the uterine cavity distended with normal saline solution. The 2 groups were similar with respect to age, gravidity and parity, number of menopausal women, and number of years after menopause. None of the menopausal women were taking hormone therapy. The number of women who had previous cervical dilation and cervical surgery and the indications for hysteroscopy were also similar in the 2 groups. |
|
| Outcomes | The primary outcome measure was the number of women who required cervical dilation. The secondary outcomes were the cervical width (measured by the largest size of Hegar dilator that could be inserted without resistance), total operative time, complications, and adverse effects of misoprostol | |
| Notes | Diagnostic procedures were included, but the cervix in these cases was dilated under general anaesthesia to Hegar No. 8.5 mm using 8 mm hysteroscope. Such dilatation use for operative hysteroscopic procedures. Author contacted for: Was blinding used, and if so who was blinded, intention to treat analysis, Source of funding, Declaration of interest, mean +/‐ SD dilation time. The author responded as: the blinding was not used, Intention to treat analysis was used. No source of funding and no conflict of interest. They did not record dilatation time. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated randomization table was used |
| Allocation concealment (selection bias) | Unclear risk | No information supplied |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding, based in author's response |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up all 105 included patients were included in the analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, including complications |
| Other bias | Low risk | no other sources of bias can be identified |
Bisharah 2003.
| Methods | Prospective randomized placebo controlled study. | |
| Participants | Forty nulliparous reproductive age women at University of teaching centre, Royal Victoria Hospital,who required operative hysteroscopy were considered eligible for the study. | |
| Interventions | Forty nulliparous reproductive age women who received injection of leuprolide acetate 3.75 mg, 4 weeks before operative hysteroscopy were randomly allocated by computer generated random table. 20 of them received sublingual misoprostol 100 mug and the other 20 received placebo administered 12 hours before operative hysteroscopy. The hysteroscopy performed under general anaesthesia by the senior author.stop watch was used to evaluate the length of the time required to dilate the cervix to 9 mm, the 2 (difficult), or 3 (very difficult).Indication for hysteroscopy was similar in the two groups. | |
| Outcomes | The time to dilate the cervix to 9 mm, complications, and adverse effects of misoprostol | |
| Notes | The author was contacted for : Method of treatment allocation; Was blinding used, and if so who was blinded, intention to treat analysis, Power Calculation, Source of funding, Declaration of interest, Duration of operation and number of women required cervical dilatation, with no response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly allocated by computer‐generated random table" |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | No mention of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss of follow‐up, all randomised women were analysed |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, including complications |
| Other bias | Low risk | No other sources of bias can be identified |
Darwish 2004.
| Methods | Randomized controlled trial. The allocation concealment was by means of sealed envelopes. | |
| Participants | The study was carried at the Gynecologic Endoscopy Unit, Assiut University Hospital from February 2002 to July 2003. It comprised 144 patients recruited from the Gynaecology, Infertility, and Family Planning Clinics with different indications for operative hysteroscopy Inclusion criteria included nulliparous or multiparous women with primary or secondary cervical stenosis (defined as difficult or failed cervical sounding in the office) who were scheduled for operative hysteroscopy. Selected cases had a full history, thorough general and pelvic examinations, and transvaginal ultrasonography to determine the nature, site and extent of intrauterine lesions. | |
| Interventions | 144 patients were included in this double blind randomised study. The evaluator included as: Group A (72 cases) received 200 mcg misoprostol in to the posterior fornix 8 h prior to surgery. Patients in group B (72 cases) received a single laminaria (Med Gyn Products, Inc., USA) as fine as 2mm inserted in to the cervical canal. There was no difference between the groups for age and parity, indications and type of surgery. Primary or secondary infertility were the main indications in 38 (52.8%) and 38 (52.71%) patients in both groups respectively due to a suspected intrauterine cause as diagnosed by transvaginal scan (TVS) or hysterosalpingography (HSG). In the operating room, the degree of initial cervical dilatation was assessed by introducing Hegar dilators under general anaesthesia.It was defined as the maximal calibre dilator that passed without resistance in a descending order, starting with the largest size dilator. |
|
| Outcomes | The duration of subsequent cervical dilatation until reaching 10mm, and feasibility of the procedure, were recorded. Cervical canal dilatation complications (false passage or perforation) were reported. At the end of the procedure, doctor assessment in the form of feasibility of the hysteroscopic operation was reported, and patient impression in the form of insertion difficulties, convenience and fear of either method. All operations were done by only three members of the Endoscopic Unit with a comparable level of experience. | |
| Notes | Author contacted for: Allocation concealment, intention to treat analysis, Source of funding, Declaration of interest, number of women required cervical dilatation, and side effects of both, with no response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The randomisation was done by means of sealed envelopes" |
| Allocation concealment (selection bias) | Low risk | The allocation concealment was by means of sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It was a double‐blind randomised study in that the evaluator (first author) masked the key from the researcher (third author) to avoid bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss of follow‐up, all included women were analysed |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, including complications |
| Other bias | Low risk | no other sources of bias can be identified |
Fernandez 2004.
| Methods | Randomized placebo‐controlled study. | |
| Participants | 48 women of reproductive age who required operative hysteroscopy for submucous myoma or polyps and considered medically fit were included. The exclusion criteria were: contraindication to prostaglandins (asthma, glaucoma, hypertension), history of cervical surgery or of cervical incompetence, menopausal women, and treatment with GnRH agonists. | |
| Interventions | Between January 1 and March 30, 2001, 48 women of reproductive age who required operative hysteroscopy for submucous myoma (n= 36) or polyps (n= 12) were randomly allocated by computer‐generated randomisation table to receive either four placebo tablets or three placebo tablets and 200 mcg misoprostol or two placebo tablets and 400 or 800 mcg misoprostol (Cytotec Laboratories Searle, France), given vaginally 4 h before surgery. The placebo tablets were identical to misoprostol in appearance. Four hours before the procedure, the dry tablets were placed by a nurse in the posterior vaginal fornix. Surgeons and operating theatre nurses who removed the tablets which were not totally disintegrated after 4 h were blinded to patient allocation. The study was set in one centre. Patients who were considered medically fit were scheduled for operative hysteroscopy under general anaesthesia with a 10 mm hysteroscope during the follicular phase of their cycles. Surgery was performed by two investigators to reduce individual variability. Pain tolerance evaluated by a visual analogue scale (VAS) and side‐ effects were noted by the surgical nurses before the procedure. | |
| Outcomes | The primary outcome measure was cervical width, which was assessed by the subjective force required to enter the cervical os without resistance with successive Hegar dilators from 3‐8 mm. Secondary outcome measurements included the subjective ease of cervical dilatation, the time required for dilatation up to Hegar 10, preoperative pain, and the adverse effects and complications of the procedure (cervical injuries, uterine perforation, false passage, bleeding). |
|
| Notes | Author contacted for: Method of treatment allocation. Source of funding, Declaration of interest. number of women required cervical dilatation, side effects of both placebo and misoprostol, and duration of surgery for both groups, with no response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Surgeons and operating theatre nurses who removed the tablets which were not totally disintegrated after 4 h were blinded to patient allocation |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information supplied. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One patient had to withdraw from the study because she was found during surgery to be pregnant. The data for this patient were included in the analysis, in compliance with the principle of intention‐to‐treat analysis. Similarly, one patient received a different treatment than that to which she was randomly allocated. Indeed, she received placebo tablets instead of 800 mcg of misoprostol. So, the analysis considered her to be in the group to which she was allocated, that is, group 1. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, including complications |
| Other bias | Low risk | no other sources of bias can be identified |
Kalampokas 2012.
| Methods | Randomized placebo‐controlled study. | |
| Participants | 55 patients of reproductive age, who have undergone a cesarean section at least once, from January 2008 to December 2010 with an indication for diagnostic hysteroscopy for menstrual problems or during investigation for some intra‐uterine lesion. They were classified into two groups by a computer generated randomizations table, group I (misoprostol study group) and group II (control group). Study’s protocol was approved by the ethical committee of our hospital. Age of the patient, number of caesarean sections, time from last cesarean operation and complications during cervical dilatation were also recorded. Patients who delivered vaginally, or had undergone any other transcervical or transabdominal uterine and cervical intervention, such as loop electrosurgical procedures and spontaneous abortions, were not included in the study. Main indications (as far as are concerned intrauterine lesions) for hysteroscopy were endometrial polyps, submucosal myomas and endometrial hyperplasia, mainly diagnosed at a routine visit or during diagnostic process. Hysteroscopy was, by routine, performed on patients during the follicular phase of their cycles. | |
| Interventions | In group I (n = 30), a 200 microgram of misoprostol tablet (Cytotec, Cipla Limited, Athens, Greece) was inserted in the posterior fornix 12 h before hysteroscopy, by a medical doctor of our team. In group II (n = 25), hysteroscopy was performed without misoprostol tablet or other placebo drugs. Both patient and surgeon were unaware of the classification of the groups (study and control). All operations were performed by the same surgeon to avoid possible discrepancies between different surgeons. A 12 mm sheath diameter resectoscope with a 15° oblique lens (Karl Storz) was used. The uterine cavity was expanded with a NaCl 0.9 % solution. Cervical width was measured by a Hegar bougie. After operative procedure, patients were hospitalised for 6 h before being sent home. The groups were similar regarding age, number of previous caesarean section operations and number of months since the last caesarean operation. | |
| Outcomes | The outcome included cervical width detected with Hegar dilators and complication rate. | |
| Notes | Author contacted for: Method of treatment allocation. Intention‐to‐treat analysis, Source of funding, Declaration of interest, number of women required cervical dilatation, time required for cervical dilatation,failure to dilate the cervix, duration for surgery, preoperative pain score and side effects of both. Author's response ‐ The method of treatment a location was a computer‐generated random table. ‐No intention‐to‐treat analysis. ‐Funding was provided by their institution. ‐No participant doctor in the study had any conflict of interest. ‐No record on the duration of the procedure ‐No record failure to dilate all operations were uneventful ‐No record on the duration of the procedure. ‐No record on preoperative pain score , as all operations were performed under general anaesthesia. ‐Side effects were 0 for both groups. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated randomisation table |
| Allocation concealment (selection bias) | Unclear risk | Not clear |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both patient and surgeon were unaware of the classification of the groups (study and control) |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information supplied. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were included in the analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, including complications |
| Other bias | Low risk | no other sources of bias can be identified |
Kant A 2011.
| Methods | Randomized controlled trial. | |
| Participants | Fifty postmenopausal women, who underwent hysteroscopy, between March 2010 and January 2011, were included in the study. The women requiring hysteroscopy randomised to into two groups: pretreatment with misoprostol group 25 women ( study group) and no pretreatment group 25 women (control group). A full medical, obstetrical, and gynaecological history was taken followed by physical examination. | |
| Interventions | The study group was given 200 micrograms of misoprostol to be inserted in the vagina at least 12 hours before the procedure and the control group did not receive any cervical priming agent. All hysteroscopies were carried out under general anaesthesia. Before hysteroscopy the dilatation of cervix was assessed with the number of Hegar's dilator passed without resistance (pre‐procedural dilatation). If sufficient cervical dilatation did not occur then dilatation was done using successively larger Hegar's dilators. Using a stop watch, the length of time required to dilate the cervix to 8 mm was noted. Indication for hysteroscopy in all women was postmenopausal bleeding. | |
| Outcomes | Preprocedural cervical width, number of women requiring additional dilatation, time required for dilatation and also the intraoperative complications. | |
| Notes | Author contacted for: Method of treatment allocation. Intention to treat analysis, Source of funding, Declaration of interest, failure to dilute the cervix, duration for surgery, preoperative pain score and side effects of both, and no answer. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized controlled trial; method not reported |
| Allocation concealment (selection bias) | Unclear risk | Not clear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not clear |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All study subjects were included in the analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, including complications |
| Other bias | Low risk | no other sources of bias can be identified |
Lin 2009.
| Methods | Randomized, controlled study. Patients were randomly allocated to receive a laminaria tent (n=59) or misoprostol (n=58) using sealed envelopes and a computer‐generated random‐number allocation table. A study nurse assigned the patients to treatment protocols. | |
| Participants | From March 2005 to January 2007. All premenopausal women who were to undergo operative hysteroscopy were recruited for the study. Exclusion criteria were contraindication to prostaglandins including asthma, glaucoma, and hypertension. | |
| Interventions | In the laminaria group (n=59), patients received a single laminaria tent (Mizutani Laminana Inc., Nagoya, Japan) about 3 mm in diameter 12 hours before operative hysteroscopy. In the misoprostol group (n=58), patients were given 400 microgram of misoprostol (Orally Cytotec; Pharmacia Corp, Chicago, Illinois) 12 and 24 hours before operative hysteroscopy. No patients were given any analgesics to compare the pain intensity associated with cervical priming. All operative hysteroscopies were performed by the same surgeon (YR. Lin). Patients received intravenous general anaesthesia with propofol, or in those with submucous leiomyoma, endotracheal general anaesthesia. After the patients were anaesthetized, residents in training prepared the patients, including disinfection and draping, and the surgeon was summoned to perform the hysteroscopy. The hysteroscopist was blinded to the priming method. Hegar dilators (Atom Medical Co., Tokyo, Japan.) were introduced through the cervical os starting with No. I and followed by increasingly larger dilators until resistance was encountered. Postpriming cervical width was defined as the smallest dilator that passed with resistance. Operative hysteroscopies were performed using a 22F resectoscope (Karl Storz GmbH, Tuttlingen, Germany) with a sheath diameter of 7 mm. A 5% glucose solution was used for uterine distention and irrigation. No complications occurred during cervical dilation or hysteroscopic surgeries. Patients were given a questionnaire to record any adverse effects including pain after cervical priming. Pain intensity was assessed using a visual analogue scale (VAS). | |
| Outcomes | Primary outcomes were post‐priming cervical width and need for cervical dilation, and secondary outcomes were adverse effects of the priming methods. | |
| Notes | A total of 117 patients were included in the study. In 6 patients randomised to the laminaria group (10.2%), laminaria tent insertion failed because of cervical stenosis. All of these patients were nulliparous. In the misoprostol group, 1 patient discontinued the second dosage of misoprostol because of unbearable pain. Therefore, data for analysis were available for 53 patients in the laminaria group and 57 in the misoprostol group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly allocated using sealed envelopes and a computer‐generated random‐number allocation table |
| Allocation concealment (selection bias) | Low risk | A study nurse assigned the patients to treatment protocols |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The hysteroscopist was blinded to the priming method |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | A total of 117 patients were included in the study In 6 patients randomised to the laminaria group (10.2%), laminaria tent insertion failed because of cervical stenosis. All of these patients were nulliparous. In the misoprostol group, 1 patient discontinued the second dosage of misoprostol because of unbearable pain. Therefore, data for analysis were available for 53 patients in the laminaria group and 57 in the misoprostol group |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, including complications |
| Other bias | Low risk | no other sources of bias can be identified |
Oppegaard 2008 a.
| Methods | Randomised, double‐blind, placebo‐controlled sequential trial (premenopausal study) The randomisation was performed with permuted blocks, using the randomisation plan generator. The randomisation procedure was third party concealed randomisation, performed by the hospital pharmacist. Placebo misoprostol tablets are difficult to make; therefore, gelatine capsules with an identical appearance were manufactured by the hospital pharmacist. The hospital pharmacist prepared numbered, opaque, sealed plastic containers for intervention agents. |
|
| Participants | 69 Premenopausal women referred to outpatient operative hysteroscopy at Ullevâl University Hospital between 1 August 2006 and 20 April 2007. Women who had a medical indication for operative hysteroscopy and who had given informed consent were eligible for study recruitment. Exclusion criteria were as follows: women who were unable to communicate in Norwegian, women without an indication for hysteroscopy, women who were medically unfit for surgery and women with a known allergy to misoprostol. | |
| Interventions | 84 women referred to outpatient operative hysteroscopy at Ullevâl University Hospital between 1 August 2006 and 20 April 2007.During the 8 months the study was carried out, 82% of the total number of women referred to operative hysteroscopy participated in the study. 11% declined the offer and 7% excluded based on the exclusion. Two separate studies (Oppegaard a for premenopausal and Oppegaard b for postmenopausal), but identical, studies were conducted in parallel, based on the women's menopausal status. 69 Premphase women were randomised to either misoprostol or placebo (34 in misoprostol group and 35 in placebo group). Each participant received either 1000 micrograms of misoprostol or placebo, which they self‐ inserted vaginally at least 12 hours before operative hysteroscopy. Those involved in administering the intervention and the women were blinded to the treatment received. The procedures were done under general intravenous anaesthetic (propofol/fentanyl/alfentanil). Six experienced senior gynaecologists (with 5‐20 years experience in operative hysteroscopy) performed the operative hysteroscopies during the study period .Before the operative hysteroscopy, the operator measured the preoperative degree of cervical dilatation by passing Hegar dilators through the cervix in ascending order starting with a size of 4 mm. The size of the largest dilator passed into the inner cervical ostium without subjective resistance felt by the operator was recorded as the preoperative degree of dilatation. If there was initial resistance with Hegar dilator of size 4 mm, then dilators of size 3 or 2 mm were tried. If there was resistance with Hegar dilator of size 2 mm, the result was recorded as 0 mm. After the cervical canal was dilated to a Hegar dilator of size 10 or 11 mm, a rigid resectoscope equipped with a Hopkins 12° rigid fibre optic was passed into the uterine cavity. A sodium chloride 9% solution was infused for uterine irrigation. A bipolar diathermal current of 280 watts (pure cut) was routinely used for resection of pathological uterine masses (myomas, polyps, uterine septae, etc.) and endometrium. The two treatment groups were comparable regarding baseline clinical preoperative characteristics, and the indications for operative hysteroscopy and the operative procedure. |
|
| Outcomes | Preoperative cervical dilatation (primary outcome). Secondary end points were as follows: the number of women who achieve satisfactory cervical priming (cervical dilatation 5 mm); 5 mm was chosen as satisfactory, as this would permit insertion of a diagnostic hysteroscope without further dilatation. A preoperative cervical dilatation of 5 mm would also make it much easier to further dilate the cervix with Hegar dilators if necessary (for insertion of an operative resectoscope of 10‐11 mm), decreasing the risk of creating a false passage, acceptability of self‐administration of vaginal capsules at home, the number of dilatations judged as easy or difficult by the operator, and the frequency of complications, as registered by the nurses preoperatively and the operators intraoperatively. |
|
| Notes | 4 patients were not analysed for the outcomes in the placebo group, the reason for this is either did not attend the surgery, Asherman's syndrome, postponed operation or no indication for hysteroscopy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed with permuted blocks, using the randomisation plan generator |
| Allocation concealment (selection bias) | Low risk | The randomisation procedure was third party concealed randomisation, performed by the hospital pharmacist and the hospital pharmacist prepared numbered, opaque, sealed plastic containers labelled Misoprostol 0.5 mg/Placebo, 2 vaginal capsules |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Those administering the intervention and the women were blinded to the treatment received |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4 patients were not analysed for the outcomes in the placebo group |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, including complications |
| Other bias | Low risk | no other sources of bias can be identified |
Oppegaard 2008 b.
| Methods | Randomized, double‐blind, placebo‐controlled sequential trial (postmenopausal study) The randomisation was performed with permuted blocks, using the randomisation plan generator. The randomisation procedure was third party concealed randomisation, performed by the hospital pharmacist. Placebo misoprostol tablets are difficult to make; therefore, gelatine capsules with an identical appearance were manufactured by the hospital pharmacist. The hospital pharmacist prepared numbered, opaque, sealed plastic containers for intervention agents. |
|
| Participants | 24 postmenopausal women referred to outpatient operative hysteroscopy at Ullevâl University Hospital between 1 August 2006 and 20 April 2007. Women who had a medical indication for operative hysteroscopy and who had given informed consent were eligible for study recruitment. Exclusion criteria were as follows: women who were unable to communicate in Norwegian, women without an indication for hysteroscopy, women who were medically unfit for surgery and women with a known allergy to misoprostol. | |
| Interventions | 29 women referred to outpatient operative hysteroscopy at Ullevâl University Hospital between 1 August 2006 and 20 April 2007. Two separate (Oppegaard 2008 afor premenopausal andOppegaard 2008 bfor postmenopausal), but identical, studies were conducted in parallel, based on the women's menopausal status. 24 postmenopausal women were randomised to either misoprostol or placebo. Each participant received either 1000 micrograms of misoprostol or placebo, which they self‐ inserted vaginally at least 12 hours before operative hysteroscopy. Those involved in administering the intervention and the women were blinded to the treatment received. Each study participant opened a numbered container at home, containing either misoprostol or lactosum monohydricum in capsules. The women were instructed to insert the capsules as deep as possible vaginally after voiding urine at approximately 9 pm the evening before the operation.On admission to the operating theatre, nurses recorded symptoms and comments from the women on the case report form. The procedures were done under general intravenous anaesthetic (propofol/fentanyl/alfentanil). Six experienced senior gynaecologists (with 5‐20 years experience in operative hysteroscopy) performed the operative hysteroscopies during the study period .Before the operative hysteroscopy, the operator measured the preoperative degree of cervical dilatation by passing Hegar dilators through the cervix in ascending order starting with a size of 4 mm. The size of the largest dilator passed into the inner cervical ostium without subjective resistance felt by the operator was recorded as the preoperative degree of dilatation. If there was initial resistance with Hegar dilator of size 4 mm, then dilators of size 3 or 2 mm were tried. If there was resistance with Hegar dilator of size 2 mm, the result was recorded as 0 mm. After the cervical canal was dilated to a Hegar dilator of size 10 or 11 mm, a rigid resectoscope equipped with a Hopkins 12° rigid fibre optic was passed into the uterine cavity. A sodium chloride 9% solution was infused for uterine irrigation. A bipolar diathermal current of 280 watts (pure cut) was routinely used for resection of pathological uterine masses (myomas, polyps, uterine septae, etc.) and endometrium. The two treatment groups were comparable regarding basal clinical preoperative characteristics, and the indications for operative hysteroscopy and the operative procedure. |
|
| Outcomes | Preoperative cervical dilatation (primary outcome). Secondary end points were as follows: the number of women who achieve satisfactory cervical priming (cervical dilatation 5 mm); 5 mm was chosen as satisfactory, as this would permit insertion of a diagnostic hysteroscope without further dilatation. A preoperative cervical dilatation of 5 mm would also make it much easier to further dilate the cervix with Hegar dilators if necessary (for insertion of an operative resectoscope of 10‐11 mm), decreasing the risk of creating a false passage, acceptability of self‐administration of vaginal capsules at home, the number of dilatations judged as easy or difficult by the operator, and the frequency of complications, as registered by the nurses preoperatively and the operators intraoperatively. |
|
| Notes | 2 patients were not analysed for the outcomes one in each group; reason for this is that they did not attend the surgery. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed with permuted blocks, using the randomisation plan generator |
| Allocation concealment (selection bias) | Low risk | The randomisation procedure was third party concealed randomisation, performed by the hospital pharmacist and the hospital pharmacist prepared numbered, opaque, sealed plastic containers labelled Misoprostol 0.5 mg/Placebo, 2 vaginal capsules |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Administering the intervention and the women were blinded to the treatment received |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 patients were not analysed for the outcomes one in each group reason for this is did not attend the surgery; thus 92% of randomised participants were included in analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, including complications |
| Other bias | Low risk | no other sources of bias can be identified |
Oppegaard 2010.
| Methods | Randomised double‐blind placebo‐controlled sequential trial. Participants were randomly assigned at a ratio of 1:1 to 1000 Microgram of vaginal misoprostol or placebo, according to a randomised list of three permuted blocks on a double‐blind basis at the outpatient consultation. The hospital pharmacist manufactured the study drug and placebo capsules, which were delivered in numbered, opaque, sealed plastic containers. Both patient and examiner were blind to randomisation. |
|
| Participants | Postmenopausal women referred for day‐care operative hysteroscopy at Norwegian university teaching hospital. The inclusion criteria: All postmenopausal (>1 year since last menstruation) women who are referred to day‐care hysteroscopy with a medical indication for hysteroscopy, and who have given informed consent, were eligible for study recruitment. Exclusion criteria: women who do not wish to participate, women who are medically unfit for hysteroscopy, women who are medically unfit for participation in any clinical trial, women who do not have a medical indication for hysteroscopy, women who have previously had, or currently have breast or gynaecological cancer, women who have a medical contraindication for locally applied estradiol or misoprostol, the current use of hormone therapy or unable to communicate in Norwegian. Between January 2008 and April 2009, 72 women were enrolled and randomly assigned to treatment .The study was stopped on 13 May 2009 as a result of a significant difference between the misoprostol and placebo groups on the primary efficacy outcome. 75 women were excluded (52 on hormone therapy,15 history of breast cancer in mamma, 3 no indication for operative hysteroscopy, 2 previous D&C in the last three months, one suspected uterine cancer , one cervical dysplasia, and one could not speak Norwegain), 79 women were eligible for randomisation, 7 of them did not wish to participate in the study, 72 women were randomised (36 women in each group). 5 women withdrew their consent (2 in the placebo group and 3 in the misoprostol group), so the analysis was done for 33 women in the misoprostol group and 34 in the placebo group. |
|
| Interventions | Before a cervical smear and endometrial biopsy were taken as part of the routine outpatient examination, cervical dilatation was measured by passing Hegar dilators through the cervix in ascending order, starting with a Hegar dilator of size 2 mm. Women participating in the study started daily treatment with 25‐Microgram estradiol vaginal tablets for 2 weeks before operative hysteroscopy. The study participants were instructed to insert the misoprostol/placebo capsules vaginally, as deep as possible, after voiding urine at approximately 21.00 hours on the evening before the operation. Those involved in administering the intervention and the women were blinded to the treatment received. Each study participant opened a numbered container at home, containing either misoprostol or lactosum monohydricum in capsules. On admission to the operating theatre, nurses recorded symptoms and comments from the women on the case report form. The procedures were done under general intravenous anaesthetic (propofol/fentanyl/alfentanil). Six experienced senior gynaecologists (with 5‐20 years experience in operative hysteroscopy) performed the operative hysteroscopies during the study period .Before the operative hysteroscopy, the operator measured the preoperative degree of cervical dilatation by passing Hegar dilators through the cervix in ascending order starting with a size of 2 mm. The size of the largest dilator passed into the inner cervical ostium without subjective resistance felt by the operator was recorded as the preoperative degree of dilatation. The size of the largest huger dilator passed through the internal os without resistance was recorded as the preoperative degree of dilatation. A rigid resectoscope equipped with a Hopkins 12° rigid fibre optic was passed into the uterine cavity. A sodium chloride 9% solution was infused for uterine irrigation. A bipolar diathermal current of 280 watts (pure cut) was routinely used for resection of pathological uterine masses (myomas, polyps, uterine septae, etc.) and endometrium. The two treatment groups were comparable regarding basal clinical preoperative characteristics, and the indications for operative hysteroscopy and the operative procedure. |
|
| Outcomes | The primary outcome is the preoperative baseline cervical dilatation in the two treatment groups. The secondary outcomes included the number of dilatation judged as 'easy' or 'difficult' by the operator, the time used to dilate the cervix, and the difference between baseline cervical dilatation at recruitment and pre‐operative dilatation at hysteroscopy. Further data recorded where the symptoms and side effects experienced between the insertion of the capsules and the operations, as well as the three frequency of side effects and complications during hysteroscopy and during the 14‐day follow‐up period, the acceptability of the treatment by the woman and the histological result. | |
| Notes | Funded by research grants. No pharmaceutical funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomly assigned at a ratio of 1:1 to 1000 lg of vaginal misoprostol or placebo, according to a randomised list of three permuted blocks" |
| Allocation concealment (selection bias) | Low risk | "The hospital pharmacist manufactured the study drug and placebo capsules, which were delivered in numbered containers". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Those involved in administering the intervention and the women will be blinded to the treatment received" |
| Blinding of outcome assessment (detection bias) | Low risk | Primary outcome assessed by operator, who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 72 women were randomised (36 women in each group). 5 women withdrew their consent (2 in the placebo group and 3 in the misoprostol group), so the analysis was done for 33 women in the misoprostol group and 34 in the placebo group: thus 93% of women randomised were analysed. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, including complications |
| Other bias | Low risk | no other sources of bias can be identified |
Preen 2002.
| Methods | Prospective randomised double‐blind placebo‐controlled trial. | |
| Participants | All women scheduled for planned operative hysteroscopy at their University based Obstetrics and Gynecology residency training program were included for study. | |
| Interventions | Forty‐six women were enrolled in the study and randomised to two groups (active medication and placebo) containing 23 subjects each. Patients were randomised to receive an opaque coded envelope that contained capsules of either misoprostol (400 micrograms) or placebo. Group selection was determined by random permuted blocks. Patients were instructed to ingest the capsules 12 hours prior to the planned procedure. The groups were similar in age, number of prior vaginal deliveries, and menopausal status. | |
| Outcomes | Time required for cervical dilation and hysteroscopy, first dilator used that encountered resistance, subjective ease of the procedure, patient pain, side‐effects, and the incidence of complications. | |
| Notes | The result was recorded as P value only ( see below please). The author was contacted but no response. The groups did not differ for time of dilation (P = 0.830), time of hysteroscopy (P = 0.243), dilator with first resistance (P = 0.402), ease of the procedure (P = 0.302) or pain rating after surgery (P = 0.880). Discomfort and side effects were similar in both groups. One cervical laceration and one false track were found in the misoprostol group. There were no uterine perforations. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not fully described: "Patients were randomized to receive an opaque coded envelope that contained capsules of either misoprostol(400 micrograms) or placebo. Group selection was determined by random permuted blocks" |
| Allocation concealment (selection bias) | Unclear risk | Method not fully described: "Patients were randomized to receive an opaque coded envelope that contained capsules of either misoprostol(400 micrograms) or placebo." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Described as double blinded but does not clearly state whether outcomes assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Does not state how many women were inlcuded in analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, including complications |
| Other bias | Unclear risk | Abstract available only, no statistical data reported, other than p values |
Preutthipan 1999.
| Methods | Randomized controlled double blind trial. Randomization done by resident in training who picked random numbers to assign the subjects to receive either 200 mcg of misoprostol (n=46) or an identical looking placebo (lactose filler, n=45). |
|
| Participants | From October 1997 through September 1998, 91 women who underwent routine investigation for infertility were recruited for a randomized study at Ramathibodi hospital. These patients were suspected of having intrauterine abnormalities that needed further definitive diagnosis and treatment by hysteroscopy. Women who were suspected of being in early pregnancy or of having genital tract infections were excluded. | |
| Interventions | All subjects were outpatients. The drug or placebo was placed in the posterior vaginal fornix 9‐10 hours before the procedure. In the operating room, with the women in lithotomy position, a number 1 Hegar dilator was inserted through the internal os, followed by successively larger Hegar dilator until resistance was met ( cervical width). For diagnostic purposes if the endocervical canal was tight, the cervix was dilated to Hegar dilator number 6. If operative sheath was required for targeted biopsy or another minor procedure, the cervix was dilated to Hegar dilator number 8. For hysteroscopic resection the cervix was dilated for Hegar dilator number 9. All women were nulliparous , most of them suffer from primary infertility.The mean age of the women in misoprostol group was 29.6±5.3 and31.2±5 years. The indications for hysteroscopy were similar in the two groups. The hysteroscopy was usually performed in the follicular phase of the menstrual cycle.They used rigid 4mm hysteroscope with 30 degree forward oblique lens ans a 5.5 mm diagnostic sheath. The women were placed under general anesthesia.In most cases the uterine cavity was distended with CO2, when the visualization was inadequate because of excessive bleeding or when an operative procedure was needed, the distention media changed to 1.5% glycine solution. |
|
| Outcomes | Cervical width, number of women who required cervical dilatation, duration of hysteroscopy form the insertion of the hysteroscope till the completion of hysteroscopic examination, and side effects. | |
| Notes | Diagnostic and operative procedures are included because 90.1% of cases the cervix was dilated till 8mm or 9mm Hegar dilator, such dilatation use for operative hysteroscopic procedures. 9.9% (5 patients in misoprostol and 4 patients in placebo group) did not required operative procedure nor dilatation beyond 6 mm Hegar dilator, the % is fairly equal in both groups. Author was contacted for: Method of treatment allocation, Was blinding used, and if so who was blinded, intention‐to‐treat analysis. Power Calculation, Source of funding, Declaration of interest. mean +/‐ duration to dilate the cervix and duration of surgery for both groups, with no response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was done by a resident‐in‐training who picked random numbers to assign the subjects to receive either 200 pg of misoprostol or an identical‐looking placebo (lactose filler)." |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double‐blinded. "Subjects received either 200 mcg of misoprostol or an identical looking placebo". |
| Blinding of outcome assessment (detection bias) | Unclear risk | Described as double‐blinded but does not clearly state whether outcomes assessment was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss of follow‐up, all included women were analysed |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, including complications |
| Other bias | Low risk | no other sources of bias can be identified |
Preutthipan 2000.
| Methods | A randomised controlled double‐blind trial. Randomization was done by random numbers to assign the subjects to receive either 200 mcg of misoprostol or an identical looking placebo. |
|
| Participants | From October 1998 through December 1999, 174 women who underwent routine investigation for infertility were recruited for a randomised study at Ramathibodi hospital, Bangkok, Thailand. These patients were suspected of having intrauterine abnormalities that needed further definitive diagnosis and treatment by hysteroscopy. Patients with intrauterine pathology were treated with operative hysteroscopy after the diagnostic procedure. Women who were suspected of being in early pregnancy or of having genital tract infections and normal hysterosalpingogram were excluded. | |
| Interventions | The study was performed on an outpatients basis. The drug was placed in the posterior vaginal fornix 9‐10 hours before the procedure. In the operating room, with the women in lithotomy position, a number 1 Hegar dilator was inserted through the internal os, followed by successively larger Hegar dilator until resistance was met (cervical width). For diagnostic purposes ,if the endocervical canal was tight , the cervix was dilated to Hegar dilator number 6.The time taken to this stage was noted. After the completion of the diagnostic procedure, women with intrauterine pathology proceeded for operative hysteroscopy. For the operative procedure, if the endocervical canal was tight, the cervix was dilated to Hegar number 9 and the time taken was noted. The hysteroscopy was mostly performed in the follicular phase of the menstrual cycle under general anaesthesia. Diagnostic procedure was done using 5.5 mm diagnostic sheath, while operative procedures was done using either 7 mm operative sheath or a resectoscope with an outer sheath 9mm in diameter. Operation was done by the same operator.The patients's age, body weight and number of women with previous cervical dilatation were similar in the two groups. |
|
| Outcomes | Cervical width, number of women who required cervical dilatation, duration of cervical dilatation, duration of operative hysteroscopy, intraoperative complications and side effects | |
| Notes | 174 patients were recruited and randomised for the study, 22 of them were excluded, 3 were in early pregnancy, 2 had genital tract infections and 13 had normal hysteroscopic findings. All 152 nulliparous patients had operative procedures ( Based on author response) where either 7 mm operative sheath or resectoscope with an outer sheath 9 mm in diameter were used. Author contacted for : Method of treatment allocation, Was blinding used, and if so who was blinded, intention to treat analysis, Power Calculation, Source of funding, Declaration of interest. and duration to dilate the cervix and duration of surgery for both groups, with no response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was done using a random number |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was performed in double‐blind manner |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | They excluded 22 patients after randomisation and not included in the analysis (13%) |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, including complications |
| Other bias | Low risk | no other sources of bias can be identified |
Preutthipan 2006.
| Methods | A prospective randomised controlled double‐blind trial. Randomization was performed by a resident in training by picking a random number to assign the patients to receive either 200 mcg of misoprostol or 3 mg of dinoprostone. |
|
| Participants | The study was done between January 2000 through December 2004; 329 women who had completed routine investigation for infertility were recruited for a prospective randomised study at Ramathibodi Hospital, Bangkok, Thailand. These patients were those with suspected intrauterine abnormalities that needed further definite diagnosis and treatment by hysteroscopy. Patients with suspected early pregnancy or having genital tract infection were excluded from this study. | |
| Interventions | The study was conducted as an outpatient protocol. Patients to receive either 200 mcg of misoprostol or 3 mg of dinoprostone placed digitally in the posterior fornix of the vagina 9‐10 hours before operative hysteroscopy. In the theatre room, with the patient in lithotomy position, Hegar's dilator number 1 was first introduced through the internal os followed by subsequent larger Hegar dilators until resistance was met. The cervical width was assessed by the size of the Hegar's dilator. For diagnostic purposes, if the endocervical canal was believed to be tight, the cervix was dilated to Hegar number 6. If operative hysteroscopy was required, the cervix needed to be dilated to Hegar number 8. For hysteroscopic resection, the cervix was then dilated to Hegar number 9. Hysteroscopy was timed mostly in the proliferative phase of the menstrual cycle under general anaesthesia, using propofol as total IV anaesthesia. Diagnostic hysteroscopy was performed with a standard rigid hysteroscope with a 5.5‐mm diagnostic sheath using carbon dioxide running on an electronic Hamou hysteroflator as a distending medium. Operative procedures were performed using either an operative hysteroscope with a 7‐mm operative sheath or a resectoscope with an outer sheath of 9 mm diameter. Sterile 1.5% glycine solution was used for uterine distention and irrigation. All of the recruited patients were nulliparous. Most of them suffered from primary infertility. The two treatment groups were similar with respect to age, body weight, previous cervical dilatation. |
|
| Outcomes | The outcome assessed included cervical width at hysteroscopy, number of patients requiring cervical dilatation, the combined time of cervical dilatation up to Hegar numbers 6 and 8‐9 before inserting the operative instrument, the duration of the operative hysteroscopy, the cervicouterine complication related to cervical dilatation and hysteroscopic surgery, and the associated side effects. Any side effects such as nausea, vomiting, diarrhoea, headache, feeling an increase in body temperature, lower abdominal pain, and vaginal bleeding were also recorded. | |
| Notes | 329 patients were recruited for the study, 19 of them were excluded, 8 were in early pregnancy, 6 had genital tract infections and 5 had normal hysteroscopic findings. All 310(94%) nulliparous patients had operative procedures (Based on author response) Author contacted for: Method of treatment allocation, Was blinding used, and if so who was blinded, intention‐to‐treat analysis, Power Calculation, Source of funding, Declaration of interest. , and duration to dilate the cervix and duration of surgery for both groups, with no response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was done using a random number table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind manner |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | They excluded 19 patients after randomisation and not included in the analysis (6%) |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, including complications |
| Other bias | Low risk | no other sources of bias can be identified |
Song 2014.
| Methods | Randomized controlled trial | |
| Participants | Between April 2013 and February 2014 , women who were suspected as having an intrauterine pathology were included in the study. Inclusion criteria were as follows: women who were of reproductive age and were not pregnant at the time of presentation. Exclusion criteria included any evidence of a contraindication to prostaglandins. | |
| Interventions | Subjects were randomly assigned to the oral, sublingual, vaginal, or control group at a 1:1:1: ratio .160 subjects underwent randomization.Groups of 40 subjects each were randomly assigned to the oral group,sublingual group,vaginal group (in each case 400 micrograms of misoprostol),or control group.The four groups were comparable in age,body mass index, marital status, gravidity, parity, history of vaginal or cesarean section delivery,and indication for operative hysteroscopy. | |
| Outcomes | The primary outcome measure was the preoperative cervical width at the time of surgery and patient preference for the administration route of misoprostol. The cervical width was assessed by performing cervical dilation,Secondary outcome measurements included the time required for dilation,the subjective ease of cervical dilation recorded by a surgeon on a 5‐point Likert scale,patient, acceptability of the self‐administration of medications preoperatively at home on a 5‐point Likert scale,self‐reported misoprostol‐associated adverse effects before the procedure and complications arising within 4 weeks after surgery. | |
| Notes | Intervention group data combined, as described in Methods section. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were randomly assigned to the oral, sublingual, vaginal, or control group at a 1:1:1:1 ratio using a random permuted‐block algorithm via an interactive Web‐based response system (www.randomization.com)" |
| Allocation concealment (selection bias) | Low risk | "Sequentially numbered, sealed, opaque envelopes were prepared by the study nurse (not directly involved in the study), and each contained a folded slip of paper with the treatment route (orally, sublingually, vaginally, or no medication) written on it. When the subjects agreed to participate... the envelopes were opened by the study nurse, and the randomization took place |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded, not placebo‐controlled |
| Blinding of outcome assessment (detection bias) | High risk | Not blinded, not placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised women included in analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, including complications |
| Other bias | Low risk | No other potential bias identifed. |
Thomas 2002.
| Methods | Prospective randomised placebo‐controlled trial. | |
| Participants | From January 2000 to March 2001, they studied a total of 204 women scheduled for hysteroscopy at the Mt Sinai Hospital and women's College Hospital, both of which are tertiary hospitals in Toronto, Ontario, Canada. Any patient who was considered medically fit and scheduled for an operative hysteroscopy were considered eligible for the study. Exclusion criteria included any difficulty with oral administration of misoprostol. | |
| Interventions | A total of 204 women were assigned to 2 groups using a computer‐generated randomizations table. In the study group (n = 102) 400 micrograms of misoprostol was given orally 12 and 24 hours before the surgery .The placebo group (n=102), the capsules were identical to misoprostol in appearance and dosing schedule. Seven surgeons, all experienced in the use of the hysteroscope, performed all the procedures. GnRH was used in 24 patients in misoprostol group and in 26 in placebo group. The age, parity, number of women premenopausal and postmenopausal and previous cervical surgery were similar in the two groups. | |
| Outcomes | The primary outcome measure was the cervical width (measured by the largest size of Hegar dilator that could be inserted without resistance). The secondary outcome measurements were time required for dilatation, subjective assessment of the ease of dilatation on a 5 points like scale, operative complication and adverse effects. | |
| Notes | 23 patients did not complete the protocol, 13 in the treatment group and 10 in placebo group. The reasons for not completing the protocol included surgery was cancelled in 6, adverse effects in 7, forgot to take medication 8 and others 2. Author contacted for: Method of treatment allocation, Was blinding used, and if so who was blinded, intention‐to‐treat analysis, Power Calculation, Source of funding, Declaration of interest. mean +/‐ SD for cervical width, duration of surgery, number of women required cervical dilatation and number of women who had side effects in both groups, with no response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated number table |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Surgeons, patients, and the outcome assessors were blinded to patient allocation.The placebo capsules were identical to misoprostol in appearance and dosing schedule." |
| Blinding of outcome assessment (detection bias) | Unclear risk | "Surgeons, patients, and the outcome assessors were blinded to patient allocation. The placebo capsules were identical to misoprostol in appearance and dosing schedule." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Numbers randomised to each group unclear, numbers reported in each group also unclear |
| Selective reporting (reporting bias) | Unclear risk | All prespecified outcomes reported, including complications |
| Other bias | Unclear risk | Data unsuitable for analysis due to poor reporting of study conduct |
Uckuyu 2008.
| Methods | A randomised placebo‐controlled trial. Eligible patients were assigned to 2 groups with a computer‐generated randomization table. |
|
| Participants | Patients of reproductive age referred to the Konya Investigation and Practice Center of Baskent University from September 2003 through September 2007, who had undergone a cesarean section at least once and were scheduled for operative hysteroscopy for various medical conditions were included in the study. Patients who had delivered vaginally, those who had undergone any transcervical or trans‐ abdominal uterine and cervical intervention other than cesarean section, such as hysteroscopy, cervical cryotherapy, cervical biopsies, loop electrosurgical excision procedures, spontaneous abortions, previous dilation, and previous elective abortions were excluded. The patients with a contraindication to prostaglandins were omitted. | |
| Interventions | A total of 60 patients eligible were assigned to 2 groups. In the study group (n = 32), misoprostol 400 mcg was inserted in the posterior vaginal fornix twice, 6 and 12 hours before the procedure. In the control group (n =28) a hexetidine vaginal pill as placebo was administered in the posterior vaginal fornix twice, 6 and 12 hours before the hysteroscopy. Indications for hysteroscopy were endometrial polyp, submucous myoma, uterus septum, and uterine synechia. All patients were hospitalised 1 day before surgery. The written treatment scheme followed a randomised allocation that was prepared by an independent statistician, with computer‐generated random numbers. Misoprostol and placebo were given by the attending physician, according to the route indicated in the randomisation envelope. The patient and surgeon performing the surgery were unaware of which group (study/control) these patients were from. To decrease individual differences, the operation was performed by 2 surgeons. Each surgeon was given an equal number of patients. Spinal anaesthetic was administered to patients during the operation. Hysteroscopy was performed on patients during the follicular phase of their cycles.
A rigid resectoscope with a 10‐mm outer sheath diameter and a 30‐degree forward‐oblique lens was used. The uterine cavity was expanded with a solution of 1.5% glycine at an insuffiation pressure of100 to 150 mm Hg, with careful monitoring of the balance of fluids. Patients remained in the hospital after surgery for a minimum of 8 hours. To determine the cervical width at baseline, a no. 10 Hegar bougie was first applied to the cervical canal, and progressively smaller sizes were used. A bougie one size smaller than the Hegar bougie that enabled passage was recorded as the cervical width. The groups were similar with regard to age, number of previous cesarean section operations, and number of months since the last caesarean operation. The indications for hysteroscopy and intraoperative findings were similar in both groups. |
|
| Outcomes | The primary end point was the highest cervical canal width detected with the largest Hegar bougie that could be passed without resistance. Secondary end points were the rates of surgical complications, including failure of cervical dilation, cervical tear, uterine perforation, the creation of a false tract, bleeding, and adverse drug effects. | |
| Notes | Author contacted for: Method of treatment allocation, Was blinding used, and if so who was blinded, intention‐to‐treat analysis, Power Calculation, Source of funding, Declaration of interest, mean +/‐ SD ( dilation time, and operation time), and no. of women who required cervical dilatation, with no response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The patient and surgeon performing the surgery were unaware of which group (study/control) these patients were from |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, including complications |
| Other bias | Low risk | no other sources of bias can be identified |
Yu 2006.
| Methods | A prospective, randomised controlled trial. All patients were assigned to one of two groups by a computer‐generated series of random numbers. |
|
| Participants | From August 2001 to Jan 2002, 100 patients from Fuxing Hospital, Beijing, China,scheduled for hysteroscopic operations (with a 9‐mm hysteroscope) were recruited into the study. | |
| Interventions | In group 1 (the misoprostol group n=53), 400 mcg misoprostol was inserted into the posterior fornix of the vagina 12 h prior to the operation. In group 2 (the osmotic dilator group n=47), a cervical osmotic dilator (disposable, synthetic hygroscopic polyacrylonitrile rod‐shaped dilators, with a diameter of 6 mm and a sectional expanding rate of 100% ) was inserted into the cervical canal until it passed the internal os. 12 h before the surgical procedure. The osmotic dilator was removed immediately before the patient was transferred to theatre. The surgeons therefore had no knowledge of what the patient received (misoprostol or osmotic dilator) prior to the surgery. All the hysteroscopic surgeries were performed in the early proliferative phase of the menstrual cycle with an Olympus 9‐mm hysteroscope under epidural anaesthesia). There were no differences between the two groups with regard to age. number of previous miscarriages, parity and types of hysteroscopic procedures performed. | |
| Outcomes | The primary outcome measure in this study was cervical dilatation, which was assessed by the size of the Hegar dilator entering the cervix without resistance, up to a maximum of 11 mm. The largest number of Hegar dilators that could be inserted into the cervix without resistance, called spontaneous dilatation, was recorded. The secondary outcome measure was subjective assessments of the ease of dilatation recorded by the surgeon when inserting a 9‐mm Hegar dilator into the cervix. Adverse effects experienced, including preoperative pain, preoperative vaginal bleeding and any other complications, were recorded for each group. | |
| Notes | Author was contacted for: Method of treatment allocation, Was blinding used, and if so who was blinded, intention‐to‐treat analysis, Power Calculation, Source of funding, Declaration of interest , mean +/‐ SD ( for cervical width, dilation time, the cervix and operation time), and no.of women who had chills as a side effect, with no response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated series of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Surgeons were blind, as the osmotic dilator was removed immediately before the patient was transferred to theatre |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss of follow‐up, all included women were analysed |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, including complications |
| Other bias | Low risk | no other sources of bias can be identified |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Esin 2013 | No eligible control intervention: in this RCT, sublingual misoprostol is compared with lidocaine spray (which is not a cervical ripening agent) |
| Fung 2002 | It is diagnostic hysteroscopy not operative hysteroscopy |
| Hald 1988 | It is for outpatient hysteroscopy |
| Hassa 2013 | Surgery is diagnostic not operative hysteroscopy |
| Moiety 2012 | It is for mixed diagnostic and operative hysteroscopy, cases of each not distinguished |
| Ngai 1997 | It is for diagnostic not operative hysteroscopy |
| Ngai 2001 | It is diagnostic not operative hysteroscopy |
| Sofoudis 2012 | Surgery is diagnostic not operative hysteroscopy |
| Tanha 2013 | This RCT compares different routes of misoprostol (sublingual and vaginal). There is no placebo or no‐intervention control group |
Characteristics of studies awaiting assessment [ordered by study ID]
Chencheewachat 2012.
| Methods | Randomised double blinded trial |
| Participants | Women scheduled for operative hysteroscopy |
| Interventions | Intravaginal isosorbide mononitrate versus placebo |
| Outcomes | Cervical width, operative complications, adverse effects |
| Notes | Intervention is inhaled. Review authors to assess whether intervention is eligible for next review update |
Differences between protocol and review
Since the protocol was published in 2006 there have been numerous methodological advances. The review now reflects the current methodologies employed by The Cochrane Collaboration.
in the primary outcomes the power required to perform procedure was removed.
-
In the full review, we reported the following outcomes in addition to those listed in the protocol.
Women requiring cervical dilatation (our primary outcome)
Duration of surgery
In the protocol our primary outcome was cervical width. After peer review, we decided to use need for mechanical dilatation as our primary effectiveness outcome, as this was deemed more clinically relevant.
We will consider expanding eligibility to other intervention agents in future updates.
Contributions of authors
Haya Al‐Fozan: conceptualised and wrote the initial version of the protocol, updated it in the light of the co‐reviewers' changes and comments, and wrote the final version. Updated the protocol, assessment of trials and quality analysis, data collection, analysis, and wrote the initial draft of the review. Checked literature search, wrote the final version of the review.
Belal Firwana: run in analysis and helped in writing the initial draft of the review. Samar Hassan: updated and commented on the protocol and review. Hanan Kadri: updated and commented on the protocol and review. Togas Tulandi: commented on the protocol, evaluated the review and revised the language.
Sources of support
Internal sources
-
IVF and Reproductive Endocrinology Unit , KAMC,Riyadh AND National and Gulf center for Evidence Based Health Practice(NGCEBHP), Saudi Arabia.
Free time with free workshops/courses in relation to systematic review and meta analysis
External sources
None, Other.
Declarations of interest
None
New
References
References to studies included in this review
Atay 1997 {published data only}
- Atay V, Duru NK, Pabuccu R, Ergun A, Tokac G, Aydin BA. Vaginal misoprostol for cervical dilatation before operative office hysteroscopy. Gynaecological Endoscopy 1997;6:47‐9. [Google Scholar]
Barcaite 2005 {published data only}
- Barcaite E, Bartusevicius A, Railaite DR, Nadisauskiene R. Vaginal misoprostol for cervical priming before hysteroscopy in perimenopausal and postmenopausal women. International Journal of Gynaecology and Obstetrics 2005;91:141‐5. [DOI] [PubMed] [Google Scholar]
Bisharah 2003 {published data only}
- Bisharah M, Al‐Fozan H, Tulandi T. A randomized trial of sublingual misoprostol for cervical priming before hysteroscopy. The Journal of American Association of Gynecologic Laparoscopists 2003;10:390‐1. [DOI] [PubMed] [Google Scholar]
Darwish 2004 {published data only}
- Darwish AM, Ahmad AM, Mohammad AM. Cervical priming prior to operative hysteroscopy: a randomized comparison of laminaria versus misoprostol. Human Reproduction 2004;19:2391‐4. [DOI] [PubMed] [Google Scholar]
Fernandez 2004 {published data only}
- Fernandez H, Alby JD, Tournoux C, Chauveaud‐Lambling A, et al. Vaginal misoprostol for cervical ripening before operative hysteroscopy in pre‐menopausal women:a double ‐blind,placebo‐controlled trial with three dose regimens. Human Reproduction 2004;19:1618‐21. [DOI] [PubMed] [Google Scholar]
Kalampokas 2012 {published data only}
- Kalampokas E, Sofoudis C, Antonogeorgos G, Panoulis K, et al. A randomised controlled trial for cervical priming using vaginal misoprostol prior to hysteroscopy in women who have only undergone cesarean section. Archives of Gynecology and Obstetrics 2012;286(4):2374‐7. [DOI] [PubMed] [Google Scholar]
Kant A 2011 {published data only}
- Kant A, Divyakumar, Priyambada U. A randomized trial of vaginal misoprostol for cervical priming before hysteroscopy in postmenopausal women. Journal of Mid Life Health 2011;2:25‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 2009 {published data only}
- Lin YH, Hwang JL, Seow KM, Huang LW, Chen HJ, Hsieh BC. Laminaria tent vs misoprostol for cervical priming before hysteroscopy: Randomized study. Journal of Minimally Invasive Gynecology 2009;16:708‐12. [DOI] [PubMed] [Google Scholar]
Oppegaard 2008 a {published data only}
- Oppegaard KS, Nesheim BI, Istre O, Qvigstad E. Comparison of self‐administered vaginal misoprostol versus placebo for cervical ripening prior to operative hysteroscopy using a sequential trial design. BJOG 2008;115:663‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oppegaard 2008 b {published data only}
- Oppegaard KS, Nesheim B‐I, Istre O, Qvigstadc E. Comparison of self‐administered vaginal misoprostol versus placebo for cervical ripening prior to operative hysteroscopy using a sequential trial design. BJOG 2008;115:663‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oppegaard 2010 {published data only}
- Oppegaard KS, Lieng M, Berg A, Istre O, Qvigstad E, Nesheim BI. A combination of misoprostol and estradiol for preoperative cervical ripening in postmenopausal women: a randomised controlled trial. BJOG 2010;117:53‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Preen 2002 {published data only}
- Preen AE, Jeffrey L, Keeby MC, et al. Oral misoprostol prior to operative hysteroscopy does not enhance cervical dilation or improve operative time: A prospective randomized, double blind, placebo‐controlled trial. Fertility and Sterility 2002;78:S79. [Google Scholar]
Preutthipan 1999 {published data only}
- Preutthipan S, Herabutya Y. A randomized controlled trial of vaginal misoprostol for cervical priming before hysteroscopy. Obstetrics and Gynecology 1999;94:427‐30. [DOI] [PubMed] [Google Scholar]
Preutthipan 2000 {published data only}
- Preutthipan S, Herabutya Y. Vaginal misoprostol for cervical priming before operative hysteroscopy: a randomized controlled trial. Obstetrics and Gynecology 2000;96:890‐4. [DOI] [PubMed] [Google Scholar]
Preutthipan 2006 {published data only}
- Preutthipan S, Herabutya Y. A randomized comparison of vaginal misoprostol and dinoprostone for cervical priming in nulliparous women before operative hysteroscopy. Fertility and Sterility 2006;86:990‐4. [DOI] [PubMed] [Google Scholar]
Song 2014 {published data only}
- Song T, Kim MK, Kim ML, Jung YW, Yoon BS, Seong SJ. Effectiveness of different routes of misoprostol administration before operative hysteroscopy: a randomized, controlled trial.. Fertil Steril. 2014;102:519‐24.. [DOI] [PubMed] [Google Scholar]
Thomas 2002 {published data only}
- Thomas JA, Leyland N, Durand N, Windrim RC. The use of oral misoprostol as a cervical ripening agent in operative hysteroscopy: a double‐blind, placebo‐controlled trial. American Journal of Obstetrics and Gynecology 2002;186:876‐9.i. [DOI] [PubMed] [Google Scholar]
Uckuyu 2008 {published data only}
- Uckuyu A, Ozcimen EE, Sevinc FC, Zeyneloglu HB. Efficacy of vaginal misoprostol before hysteroscopy for cervical priming in patients who have undergone cesarean section and no vaginal deliveries. Journal of Minimally Invasive Gynecology 2008;15:472‐5. [DOI] [PubMed] [Google Scholar]
Yu 2006 {published data only}
- YU D, Li T‐C, Xia E, Huang X. Aprospective randomized controlled trial comparing vaginal misoprostol and osmotic dilator in achieving cervical ripening before operative hysteroscopy. Gynecological Surgery 2006;3:186‐9. [Google Scholar]
References to studies excluded from this review
Esin 2013 {unpublished data only}
Fung 2002 {published data only}
- Fung TM, Lam MH, Wong SF, Ho LC. A randomized placebo‐controlled trial of vaginal misoprostol for cervical priming before hysteroscopy in post menopausal women. BJOG 2002;109:561‐5. [DOI] [PubMed] [Google Scholar]
Hald 1988 {published data only}
- Hald F, Kristoffersen SE, Gregersen E. Prostaglandin vaginal suppositories in non pregnant women requiring cervical dilatation prior to hysteroscopy. Acta Obstetricia et Gynecologica Scandinavica 1988;67:219‐22. [DOI] [PubMed] [Google Scholar]
Hassa 2013 {published data only}
- Hassa H, Aydin Y, Oge T, Cicek K. Effectiveness of vaginal misoprostol and rectal nonsteroida anti‐inflammatory drug in vaginoscopic diagnostic outpatien hysteroscopy in primarily infertile women: double‐blind, randomized, controlled trial. JMIG 2013;20(6):880‐5. [DOI] [PubMed] [Google Scholar]
Moiety 2012 {published data only}
- Moiety FMS, Azzam A. Prostaglandins prior to hysteroscopy. Gynecological Surgery 2012;9:169‐73. [Google Scholar]
Ngai 1997 {published data only}
- Ngai SW, Chan YM, Liu KL, Ho PC. Oral misoprostol for cervical priming in non‐pregnant women. Human Reproduction 1997;12:2373‐5. [DOI] [PubMed] [Google Scholar]
Ngai 2001 {published data only}
- Ngai SW, Chan YM, Ho PC. The use of misoprostol prior to hysteroscopy in post menopausal women. Human Reproduction 2001;16:1486‐8. [DOI] [PubMed] [Google Scholar]
Sofoudis 2012 {unpublished data only}
- Sofoudis C, Bakas P, Creatsa M, Kalampokas M, Grigoriou V, Vlahos N. A doble randomized cntrolled trial of oral misoprostol before diagnostic hysteroscopy. Gynecol Surg. 2012; Vol. 9(Suppl 1):S1‐S137.
Tanha 2013 {published data only}
- Tanha FD, Salimi S, Ghajarzadeh M. Sublingual versus vaginal misoprostol for cervical ripening before hysteroscopy: a randomized clinical trial. General Gynecology 2013;287:937‐40. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Chencheewachat 2012 {unpublished data only}
- Chencheewachat C, Lertvikool S, Sukprasert M, Chanrachakul B. The efficacy of nitric oxide donor on cervical ripening prior to operative hysteroscopy: a randomized, double blinded, controlled trial. Reproductive Sciences. 2012; Vol. 19, No 3(Supplement).
Additional references
Agostini A 2002a
- Agostini A, Cravello L, Bretelle F, Shojai R, Roger V, Blanc B. Risk of uterine perforation during hysteroscopic surgery. The Journal of the American Association of Gynecologic Laparoscopists 2002a;9:264‐7. [DOI] [PubMed] [Google Scholar]
Bradley 2002
- Bradley LD. Complications in hysteroscopy: prevention, treatment and legal risk. Current Opinion in Obstetrics & Gynecology 2002;14:409‐15. [DOI] [PubMed] [Google Scholar]
Cooper 1996
- Cooper KG, Pinion SB, Bhattacharya S, Parkin DE. The effects of the gonadotrophin releasing hormone analogue (goserelin) and prostaglandin E1 (misoprostol) on cervical resistance prior to transcervical resection of the endometrium. British Journal Obstetrics and Gynaecology 1996;103:375‐8. [DOI] [PubMed] [Google Scholar]
Girgoriadis 2012
- Grigoriadis C, Papaconstantinou E, Mellou A, Hassiakos D, Liapis A, Kondi‐Pafiti A. Clinicopathological changes of uterine leiomyomas after GnRH agonist therapy. Clinical and Experimental Obstetrics & Gynecology 2012;39:191‐4. [PubMed] [Google Scholar]
Gkrozou F 2011
- Gkrozou F1, Koliopoulos G, Vrekoussis T, Valasoulis G, Lavasidis L, Navrozoglou I, Paschopoulos M. A systematic review and meta‐analysis of randomized studies comparing misoprostol versus placebo for cervical ripening prior to hysteroscopy. Eur J Obstet Gynecol Reprod Biol. 2011;158:17‐23. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Loffer 1989
- Loffer. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstetrics and Gynecology 1989;73:16‐20. [PubMed] [Google Scholar]
Nagi 1997
- Ngai SW, Chan YM, Liu KL, Ho PC. Oral misoprostol for cervical priming in non‐pregnant women. Human Reproduction 1997;12:2373‐5. [DOI] [PubMed] [Google Scholar]
Ostrzenski 1994
- Ostrzenski A. Resectoscopic cervical trauma minimized by inserting Laminaria digitata preoperatively. International Journal of Fertility and Menopausal Studies 1994;39:111‐3. [PubMed] [Google Scholar]
Overton 1997
- Overton C, Hargreaves J, Maresh M. A national survey of the complications of endometrial destruction for menstrual disorders: the MISTLETOE study. British Journal of Obstetrics and Gynaecology 1997;104:135‐9. [DOI] [PubMed] [Google Scholar]
Polyzos 2012
- Polyzos NP, Zavos A, Valachis A, et al. Misoprostol prior to hysteroscopy in premenopausal and post‐menopausal women. A systematic review and meta‐analysis. Human Reproduction Update 2012;18:393‐404. [DOI] [PubMed] [Google Scholar]
Rabe 1985
- Rabe T, Willinger G, Kiesel L, Runnebaum B, Kubili F, Mosche R, et al. Study of 16,16'‐dimethyl‐trans‐delta 2 prostaglandin E1 methyl ester vaginal suppository for cervical dilatation in premenopausal and postmenopausal women. Advances in Contraception 1985;1:91‐102. [DOI] [PubMed] [Google Scholar]
Sowter 2002
- Sowter MC, Lethaby A, Singla AA. Pre‐operative endometrial thinning agents before endometrial destruction for heavy menstrual bleeding. Cochrane Database of Systematic Reviews 2002;3:CD001124. [DOI] [PubMed] [Google Scholar]


