Abstract
Asthma is a chronic, heterogeneous disease of the airways, often characterised by structural changes known collectively as airway remodelling. In response to environmental insults, including pathogens, allergens and pollutants, the epithelium can initiate remodelling via an inflammatory cascade involving a variety of mediators that have downstream effects on both structural and immune cells. These mediators include the epithelial cytokines thymic stromal lymphopoietin, interleukin (IL)-33 and IL-25, which facilitate airway remodelling through cross-talk between epithelial cells and fibroblasts, and between mast cells and airway smooth muscle cells, as well as through signalling with immune cells such as macrophages. The epithelium can also initiate airway remodelling independently of inflammation in response to the mechanical stress present during bronchoconstriction. Furthermore, genetic and epigenetic alterations to epithelial components are believed to influence remodelling. Here, we review recent advances in our understanding of the roles of the epithelium and epithelial cytokines in driving airway remodelling, facilitated by developments in genetic sequencing and imaging techniques. We also explore how new and existing therapeutics that target the epithelium and epithelial cytokines could modify airway remodelling.
Shareable abstract
Our growing understanding of how the epithelium and epithelial cytokines orchestrate airway remodelling in severe asthma is enabling the development of new therapies that may make disease remission a realistic treatment goal https://bit.ly/3UOblpT
Introduction
Asthma is a chronic, inflammatory disease of the airways, characterised by variable symptoms of wheezing, chest tightness, cough, shortness of breath and varying expiratory airflow limitation [1]. The disease is phenotypically heterogeneous, with differing clinical characteristics such as age at onset, degree of severity and response to treatment [2]. Heterogeneity in the biological and immunological mechanisms that contribute to asthma led to the conception of “endotypes” to define groups of patients with similar disease characteristics at the cellular and molecular levels, such as features of inflammation [3]. The most common inflammatory endotype is type 2 (T2)-high asthma, characterised by high levels of T2 inflammatory biomarkers including eosinophils, exhaled nitric oxide, IgE, interleukin (IL)-5 and IL-13 [4]. Patients with T2-low asthma exhibit lower levels of T2 inflammation; instead, the dominant inflammatory cell types may include neutrophils or there might be very few inflammatory cells, termed paucigranulocytic inflammation [5, 6]. Neutrophilic inflammation can coexist with eosinophilic inflammation in patients with severe asthma with a mixed phenotype [5, 7]. Endotypes and phenotypes are dynamic, and diverse environmental stimuli may induce modifications resulting in mixed molecular pathways and phenotypes that overlap over a patient's lifetime [8].
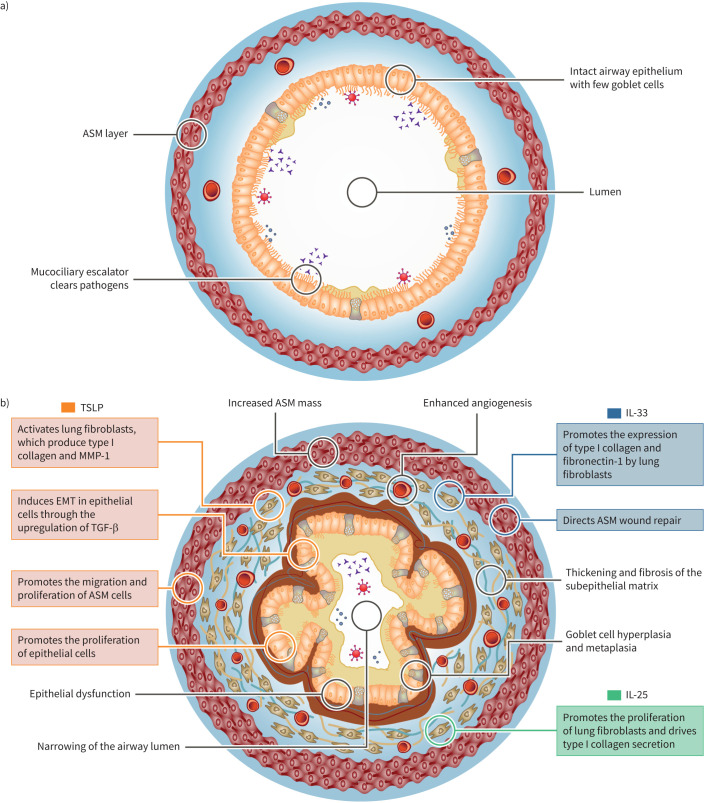
The airway epithelium plays a critical role in the pathophysiology of asthma, acting as a structural and immunological barrier to the external environment [9]. Airborne pathogens, pollutants and, in sensitised people, allergens can damage airway epithelial cells and trigger the release of epithelial cytokines that drive downstream inflammatory processes [10–12]. In the healthy state, resolution of acute inflammation can repair and restore normal airway structure and function, avoiding epithelial damage. However, in patients with asthma, aberrant immune responses and repair processes lead to recurrent or chronic inflammation and damage to the airway epithelium, which can result in structural changes in the large and small airways [13]. These structural changes, collectively referred to as airway remodelling, include epithelial dysfunction, goblet cell hyperplasia and metaplasia, thickening and fibrosis of the subepithelial matrix, increased airway smooth muscle (ASM) mass and enhanced angiogenesis. These features contribute to narrowing and stiffening of the airways, resulting clinically in airflow limitations with subsequent worsening of respiratory symptoms [14].
Airway remodelling is a near-universal feature of asthma. It is present in patients with mild disease [15] but tends to worsen with increasing disease severity [16, 17], and is associated with a higher exacerbation risk, lung function decline and disease chronicity [9, 14, 18]. Airway remodelling was once considered a secondary phenomenon, developing in late-stage asthma as a consequence of chronic inflammation. However, biopsy studies in young children indicate that airway remodelling can be an early event in asthma development, initiating before symptoms occur [19–21] and potentially even predisposing individuals to developing asthma by affecting lung development [9]. Structural changes to the airways are believed to persist through adulthood [22].
Developments in genetic sequencing and imaging techniques have advanced our understanding of the roles of the epithelium and epithelial cytokines in driving airway remodelling, taking us to the edge of a new era of scientific discovery and potential therapeutic development. Here, we review the emerging data in this growing area of research, their clinical relevance, and how new and existing therapeutics that target the epithelium and epithelial cytokines could modify airway remodelling in asthma.
How the epithelium orchestrates airway remodelling
The epithelial cells of the airways act as an initiation point for airway remodelling in asthma [14]. Epithelial cells express pattern recognition receptors, which detect pathogen-associated molecular patterns and damage-associated molecular patterns derived from pathogens, allergens and injured cells resulting from environmental insults such as pollutants and toxins [23]. The triggering of pattern recognition receptors results in the epithelial release of chemokines and cytokines (primarily epithelial cytokines and ILs) and a downstream inflammatory cascade involving various immune and structural cells [23–25]. Pro-inflammatory stimuli and cytokines also trigger epithelial inducible nitric oxide synthase expression, producing an increase in nitric oxide levels. This induces chronic inflammatory responses and nitration of proteins involved in proliferation, apoptosis or migration, triggering epithelial tissue injury [26]. Environmental insults may also induce apoptosis of the epithelium, accompanied by the release of mediators such as transforming growth factor (TGF)-β [27], which can initiate the tissue regeneration process in an attempt to restore homeostasis. However, persistent inflammation and damage to the epithelium can lead to aberrant tissue repair and pathological remodelling of the airways [9, 28, 29].
Multiple mechanical forces, including compression (cells being pushed together), stretch (of cells at the apex of the epithelial folds) and shear stress (increase in air velocity together with a reduction in airway diameter), affect the epithelium as the airway wall folds [30, 31]. A variety of experimental models have been used to show that mechanical forces can initiate airway remodelling independently of inflammation [32]. The mechanical forces present during regular respiration are balanced; however, during bronchoconstriction experienced by patients with asthma, airway epithelial cells are exposed to excessive mechanical forces [14]. This compressive stress results in mechanical stimulation, which elicits a pro-remodelling response in the absence of inflammation [33, 34]. This response causes subepithelial thickening via increased production of fibronectin and collagen III and V, and an increase in the ratio of matrix metalloproteinase (MMP)-9 to tissue inhibitor of metalloproteinase-1 [33]. Goblet cell hyperplasia and metaplasia, and subsequent mucus overproduction, may also occur as a result of epithelial compression during bronchoconstriction [34].
Airway remodelling may be self-perpetuating. Epithelial disruption can result in an abnormal epithelium-mediated immune response, potentially promoting the persistence of microbes such as bacteria and fungi in the airways [35]. Meanwhile, stiffening of the airway walls alters the local biomechanical environment. Both of these abnormalities can drive a positive feedback loop that perpetuates airway remodelling [14, 36].
Role of epithelial cytokines in airway remodelling
Three epithelial cytokines, i.e. thymic stromal lymphopoietin (TSLP), IL-33 and IL-25, known as “alarmins”, are central in asthma pathophysiology. They act as master regulators that mediate both innate and adaptive immune responses, including both T2 and non-T2 inflammation, as well as structural changes in the airways [25, 37]. Airway levels of both TSLP and IL-33 are elevated in patients with asthma compared with healthy individuals and correlate with disease severity [38–42]. IL-25 concentrations in sputum may also correlate with disease severity [43].
The three epithelial cytokines have diverse, yet often overlapping, effects on mesenchymal cells such as lung fibroblasts and ASM cells (figure 1). TSLP activates lung fibroblasts, which produce extracellular matrix (ECM) molecules such as collagen I and MMP-1 [44–46]. There is also evidence that human endothelial cells express the TSLP receptor and that TSLP induces their proliferation [47, 48]. Moreover, TSLP promotes the release of vascular endothelial growth factor (VEGF)-A from human lung macrophages [49]. IL-33 promotes the expression of collagen and fibronectin-1 in lung fibroblasts [50, 51], and IL-25 promotes lung fibroblast proliferation [52] and collagen secretion by these cells [53]. In turn, asthmatic lung fibroblasts contribute to inflammation by secreting cytokines, including TSLP and IL-33 [54, 55].
FIGURE 1.
Multiple pathogenic factors trigger structural alterations of a) healthy airways resulting in b) airway remodelling. Epithelial cytokines can play diverse, yet often overlapping, roles in airway remodelling in asthma. ASM: airway smooth muscle; EMT: epithelial-to-mesenchymal transition; IL: interleukin; MMP: matrix metalloproteinase; TGF: transforming growth factor; TSLP: thymic stromal lymphopoietin.
During epithelial-to-mesenchymal transition (EMT), epithelial cells lose their epithelial markers, migrate to the lamina propria and gain mesenchymal markers. These mesenchymal cells now synthesise ECM, which provides a framework for basal cells to replace damaged epithelium in wound healing [56]. In vitro studies have shown that TSLP- and IL-33-mediated signalling between the epithelium and fibroblast-like mesenchymal cells may drive remodelling in response to recurring injury, potentially (for TSLP) through upregulating the expression of TGF-β [14, 57, 58]. However, there is a lack of in vivo evidence regarding the role of EMT in the pathogenesis of asthma.
Both TSLP and IL-33 mediate cross-talk between ASM and mast cells, with subsequent effects on airway structure and function [40, 59]. TSLP promotes the proliferation and migration of ASM cells and the release of inflammatory cytokines, including TSLP itself, from these cells [60–62]. TSLP also acts upon mast cells, which in turn can trigger bronchoconstriction and increased ASM mass [63]. IL-33 directs ASM wound repair and drives airway hyperresponsiveness (AHR) through IL-13-based signalling between ASM and mast cells [40]. Mast cells play a key role in asthma pathophysiology and airway remodelling through the release of a plethora of cytokines (e.g. TSLP, IL-33, IL-25, IL-4, IL-13 and TGF-β) and angiogenic factors (e.g. VEGF-A), as well as other inflammatory mediators and bronchoconstrictors [64, 65]. IL-25 contributes to airway remodelling via the induction of airway angiogenesis in murine asthma models [53, 66].
Downstream immune cell actions influence airway remodelling
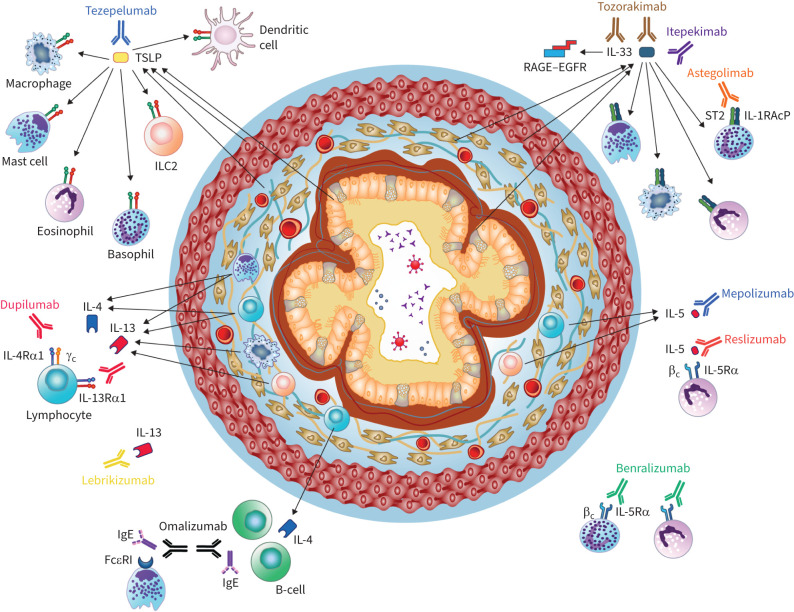
Epithelial cytokines activate several immune cells that can drive airway remodelling, including dendritic cells, T-helper type 2 (Th2) cells, group 2 innate lymphoid cells (ILC2s), eosinophils and macrophages (figure 2) [67, 68].
FIGURE 2.
Epithelial cytokines activate immune cells that drive pathogenic airway remodelling processes in patients with severe asthma. Biologic therapies for severe asthma (approved or recently in development) that target epithelial cytokines and downstream mediators may ameliorate features of airway remodelling. EGFR: epidermal growth factor receptor; IL: interleukin; ILC2: group 2 innate lymphoid cell; R: receptor; RAGE: receptor for advanced glycation end-products; ST2: serum stimulation-2; TSLP: thymic stromal lymphopoietin.
During T2 inflammatory responses, TSLP activates dendritic cells, which in turn prime naive T-helper cells to produce Th2-like cytokines such as IL-4, IL-5 and IL-13 [69]. IL-33 acts as a positive regulator of TSLP–dendritic cell signalling, initiating and maintaining Th2 cell-mediated inflammatory responses [70]. Moreover, TSLP, IL-33 and IL-25 activate ILC2s, which also produce IL-4, IL-5 and IL-13 [71–73]. The IL-5 secreted from polarised Th2 cells and ILC2s stimulates eosinophilic inflammation via effects on eosinophil recruitment, maturation and survival [74]. Activated eosinophils and mast cells release cysteinyl leukotrienes, which are potent bronchoconstrictors and induce airway remodelling through ASM cell proliferation and the release of mediators such as TGF-β, cationic proteins and cytokines [75, 76]. Both the MET gene, encoding the hepatocyte growth factor receptor, and the MMP10 gene, encoding MMP-10, are implicated in airway remodelling and cellular inflammation associated with submucosal eosinophils [77]. IL-4 and IL-13 enhance subepithelial fibrosis, mucus production via goblet cell proliferation and collagen deposition [14].
TSLP may also play a role in non-T2 inflammation-mediated airway remodelling. Dendritic cells activated by TSLP can induce the polarisation of naive T-cells towards a T-helper type 17 (Th17) phenotype [78]. The synergistic effect of dendritic cells together with Th17 cytokines promotes neutrophilic inflammation and accumulation of fibrotic matrix components that correlate with TGF-β expression [79, 80].
All three epithelial cytokines can activate human lung macrophages [25, 49]. Macrophages influence inflammatory responses in the airways through phagocytosis, cytokine and angiogenic factor production, and regulation of tissue repair in the lungs [81, 82]. Alveolar macrophages are activated by TGF-β and release MMPs that alter the ECM and airway structure [14, 49]. Two distinct populations of resident macrophages have been identified in the lungs [83–85]. One macrophage population (Lyve1loMHCIIhi) is mostly located adjacent to nerves and primarily presents antigens. The other (Lyve1hiMHCIIlo) resides alongside blood vessels; in a mouse model, Lyve1hiMHCIIlo macrophages were shown to have a critical role in suppressing inflammation and fibrosis [83–85].
Influence of genetic and epigenetic changes in the airway epithelium on remodelling
Genetic variations in the airway epithelium can initiate or worsen asthma and influence remodelling [86–88]. In the largest genome-wide association study to date in moderate-to-severe asthma, Shrine et al. [89] reported that variants in MUC5AC, GATA3 and KIAA1109 were associated with an increased susceptibility to developing moderate-to-severe asthma. MUC5AC gene variants are associated with increased mucus plugging, whereas GATA3 is a transcription factor regulating T2 immunity and allergy. The function of KIAA1109 is not fully understood [89].
Heijink et al. [90] selected 12 genes identified in asthma genetic studies that are implicated in epithelial function. These include genes related to the inflammatory environment (IL33, TSLP and IL1RL1), the response to pathogens (CDHR3), mucociliary clearance (MUC5AC, KIF3A and EFHC1), and cell homeostasis and epithelial integrity, including proliferation, migration, cell–cell adhesion, apoptosis and repair (PCDH1, SMAD3, GSDMB, ORMDL3 and PLAUR). Relevant to the latter group of genes, the 17q21 gene locus, which is linked to GSDMB and ORMDL3, is an important susceptibility locus for childhood-onset asthma [91]. Gasdermin B (GSDMB) is highly expressed in the epithelium in asthma, and increased GSDMB expression in transgenic mice leads to spontaneous remodelling and AHR in the absence of airway inflammation [92]. Orosomucoid-like protein isoform 3 (ORMDL3) is expressed in epithelial cells and regulates endoplasmic reticulum stress and sphingolipid homeostasis [93]. Transgenic mice overexpressing ORMDL3 exhibit increased ASM mass, subepithelial fibrosis and mucus [94]. Plasminogen activator urokinase receptor (PLAUR) is expressed in the epithelium and regulates the activation of urokinase plasminogen activator, triggering the plasminogen/plasmin activation cycle. PLAUR is also involved in epithelial repair, proliferation and remodelling [95]. In patients with asthma, PLAUR variants are associated with a rapid decline in lung function and with airway remodelling through effects on reticular basement membrane (RBM) thickness, collagen III deposition and basal epithelial proliferation [96].
Lastly, although its function is unclear, elevated serum levels of YKL-40, a chitin-binding glycoprotein expressed in the airway epithelium, correlate with asthma severity, airway remodelling and increased thickness of the RBM [97]. Variations in the expression of the CHI3L1 gene, which encodes YKL-40, are also associated with asthma severity, airflow obstruction and airway remodelling [98].
Epigenetic changes, such as DNA methylation or acetylation, histone modifications and microRNA modifications, in the airway epithelium are associated with asthma [10, 12, 99, 100]. The DNA methylation pattern of epithelial cells is different for children with asthma compared with healthy children or those with atopic asthma [101]. School-aged children with asthma also have differential methylation of genes relevant to epithelial barrier function, airway epithelial integrity and immune regulation [102]. Of note, overexpression of TSLP in asthmatic airway epithelial cells may be regulated by DNA demethylation [103]. Increased histone deacetylase activity could be responsible for tight junction dysfunction in asthma, and inhibiting this activity is a promising target for improved barrier integrity [104, 105]. Lastly, epigenetic “training” from repetitive activation of innate signalling pathways can induce adaptive epithelial responses [106]. Oxidative stress resulting from the innate immune response can cause conformational changes in DNA that trigger inflammation, EMT and ECM remodelling. Targeting inducible epigenetic guanine oxidation reprogramming pathways reduced airway inflammation in pre-clinical models [106].
Structural and clinical consequences of airway remodelling
The ultrastructural changes associated with airway remodelling result in altered airway geometry, including airway wall thickening and luminal narrowing, and in ventilation defects, including mucus plugging and airway obstruction. Some of these phenomena can be assessed using imaging techniques such as computed tomography (CT) [107, 108].
Goblet cell hyperplasia and submucosal gland hypertrophy lead to increased sputum production, airway narrowing due to sputum secretion and increased airway wall thickness [109]. These changes can ultimately result in the formation of mucus plugs, which are associated with severe airflow limitation and death [110, 111]. Subepithelial fibrosis resulting from increased ECM deposition leads to airway wall thickening, which correlates with asthma severity and AHR [109, 112, 113]. Increased ASM mass is associated with asthma severity [114], and ASM cells migrating towards the epithelium and subsequent increased ECM deposition within the ASM may contribute to airflow obstruction [109, 115]. Changes in airway wall microvasculature resulting from inflammatory angiogenesis can contribute to the development of airway wall oedema, leading to luminal narrowing [109]. Lastly, decreased cartilage volume and increased cartilage proteoglycan degradation in the airways can contribute to chronic airway obstruction and enable more powerful bronchoconstriction for a given degree of ASM contraction [109].
Although the qualitative assessment of chest radiographs and CT scans is part of the standard of care for asthma, other lung imaging platforms and software algorithms can quantify regional airway structure and function, as well as airway inflammation. In addition to CT imaging, new approaches such as hyperpolarised gas magnetic resonance imaging and single-photon emission CT are helping us to understand the heterogeneity of severe asthma and are likely to advance clinical management towards precision medicine [116].
Measuring the geometry of the airways provides a measure of the impact of remodelling at an individual airway level, whereas lung function tests measure remodelling at an organ level. However, the ultrastructural/cellular changes that lead to remodelling do not happen on the same timescale as the development of clinical symptoms (e.g. breathlessness). Access to and combining information from different modalities will provide insight into the mechanisms underlying airway remodelling [35].
Identification of airway remodelling phenotypes in asthma
Bronchial biopsy and post-mortem tissue section studies show differences in airway structural characteristics between patients with different asthma phenotypes. Patients with severe asthma and high levels of eosinophilic inflammation have greater RBM thickness than those with nearly absent eosinophils [117]. In a subsequent study, no difference in RBM thickness was found between patients with early-onset (childhood) and late-onset (adulthood) asthma, despite those with adult-onset asthma having inferior forced expiratory volume in 1 s (FEV1) and forced vital capacity and a shorter duration of disease [118]. Among patients with late-onset asthma, those with eosinophilic inflammation had greater RBM thickening than those without eosinophilic inflammation. Patients with paucigranulocytic asthma had increased thicknesses of the ASM layer and RBM compared with healthy individuals [119]. Meanwhile, patients with granulocytic asthma also had increased airway wall thickness and narrowing of the airway lumen due to ASM shortening and mucus obstruction [119]. This finding suggested that some components of remodelling are dependent on inflammation whereas others are not. In the paucigranulocytic endotype, airway remodelling is thought to occur in a manner “uncoupled” from inflammation, potentially through mechanotransduction-related pathways [6].
Attempts have also been made to identify airway remodelling phenotypes using quantitative CT imaging [120, 121]. Cluster analysis of wall and lumen volumes of the large airways in an adult asthma cohort identified three phenotypes, all of which demonstrated air trapping: one cluster with increased airway wall and lumen volumes and another cluster with luminal narrowing, both with poor lung function, and a third cluster lacking airway remodelling that had clinically mild disease [121]. A second analysis of remodelling across the small and large airways in a cohort of patients with severe asthma identified three phenotypes: one characterised by large-to-medium bronchial wall thickening, mucus plugging and bronchiectasis, associated with systemic eosinophilic inflammation; one characterised by small airway remodelling and fixed airflow obstruction, associated with male sex, smoking and more frequent use of controller medication; and one lacking both eosinophilic inflammation and airway remodelling [120].
Successful identification of remodelling phenotypes may enable the development of targeted approaches to treat traits specifically related to airway remodelling, with the aim of preventing lung function decline. The combination of clinical, inflammatory and remodelling phenotypes within a patient could determine the optimal personalised approach to their treatment [22].
Targeting the epithelium and epithelial cytokines to modify airway remodelling
Various therapies for asthma target the epithelium either directly or indirectly and, as well as improving clinical symptoms, may ameliorate features of airway remodelling and lung function decline as measured by FEV1 and other spirometric parameters.
Glucocorticoids
First-line therapy for asthma typically includes inhaled corticosteroids (ICS), targeting glucocorticoid receptors that are expressed almost ubiquitously, including in epithelial cells. Maintenance use of ICS (≥1 year) is associated with modest improvements in FEV1 [122]. Glucocorticoids mitigate chronic inflammation, which indirectly contributes to airway remodelling. However, evidence for a direct beneficial impact of ICS or oral corticosteroids on airway remodelling has been contradictory. High-dose ICS reduced both submucosal vascularity and RBM thickness in patients with mild-to-moderate asthma [64]. Glucocorticoid treatment also restored the integrity of epithelial cell monolayers through the redistribution of tight junction proteins [123]. Furthermore, studies in epithelial cells suggest that glucocorticoid exposure may reduce goblet cell hyperplasia [124, 125], but ICS treatment in patients with mild asthma did not confirm this [126]. Glucocorticoid treatment may in fact contribute to airway remodelling, potentially by inducing caspase-mediated epithelial cell apoptosis [127].
Biologics targeting T2 inflammation
Biologic therapies may be prescribed as add-on maintenance treatments to improve disease control in patients with moderate-to-severe asthma [1]. Monoclonal antibodies (mAbs) that target IgE or Th2 cytokines (IL-5, IL-4 and IL-13) and their receptors have secondary effects on the epithelium through their actions on these immune cell mediators (figure 2 and table 1) [128, 129]. The possible effects of these biologics on airway remodelling have been reviewed in detail elsewhere [128–130] and are summarised briefly here.
TABLE 1.
| Biologic | Target | GINA eligibility criteria (in addition to having severe asthma) | Biological effects | Effects on airway remodelling | Expected clinical outcomes |
| Omalizumab | IgE | Severe exacerbations within past year, sensitisation to inhaled allergens, total serum IgE and weight within local dosing range | ↓ Circulating total IgE Downregulation of FcεRI receptors on basophils, mast cells and dendritic cells |
↓ RBM thickness ↓ Airway wall thickness on CT ↓ Fibronectin deposition Prevents IgE-mediated ECM deposition in vitro |
↑ Lung function (FEV1) ↓ Severe exacerbations ↑ Health-related QoL ↑ Symptom control ↓ OCS (possible benefit) |
| Mepolizumab | IL-5 | Severe exacerbations within past year, BEC ≥150 or ≥300 cells·µL−1 (locally specified) | Blockage of IL-5/IL-5R binding | ↓ Airway eosinophils and TGF-β1+ eosinophils ↓ Tenascin, lumican and procollagen III expression ↓ RBM thickness ↓ Airway wall thickness on CT ↓ ASM mass |
↑ Lung function (FEV1) ↓↓ Severe exacerbations ↑ QoL ↑ Symptom control ↓ OCS |
| Reslizumab | IL-5 | Severe exacerbations within past year, BEC ≥150 or ≥300 cells·µL−1 (locally specified) | Blockage of IL-5/IL-5R binding | Not reported | ↑ Lung function (FEV1) ↓↓ Severe exacerbations ↑ QoL ↑ Symptom control |
| Benralizumab | IL-5Rα | Severe exacerbations within past year, BEC ≥150 or ≥300 cells·µL−1 (locally specified) | ↓ Eosinophils and basophils via antibody-dependent cell-mediated cytotoxicity | ↓ Airway eosinophils ↓ ASM mass |
↑ Lung function (FEV1) ↓↓ Severe exacerbations ↑ Health-related QoL ↑ Symptom control ↓ OCS |
| Dupilumab | IL-4Rα | Severe exacerbations within past year, BEC ≥150 and ≤1500 cells·µL−1, or FENO ≥25 ppb, or maintenance OCS | Blockage of IL-4/IL-4Rα binding Blockage of IL-13/IL-4Rα binding |
Prevents eosinophil infiltration into lung tissue in a mouse model of asthma | ↑ Lung function (FEV1) ↓↓ Severe exacerbations ↑ Health-related QoL ↑ Symptom control ↓ OCS |
| Tezepelumab | TSLP | Severe exacerbations within past year | Blockage of TSLP/TSLPR binding | ↓ Airway eosinophils ↓ AHR to mannitol ↓ Airway inflammation ↓ TGF-β1 ↑ CT scan-determined lumen area |
↑ Lung function (FEV1) ↓↓ Severe exacerbations ↑ Health-related QoL ↑ Symptom control ↓ OCS (possible benefit) |
AHR: airway hyperresponsiveness; ASM: airway smooth muscle; BEC: blood eosinophil count; CT: computed tomography; ECM: extracellular matrix; FENO: fractional exhaled nitric oxide; FEV1: forced expiratory volume in 1 s; GINA: Global Initiative for Asthma; IL: interleukin; OCS: oral corticosteroids; QoL: quality of life; R: receptor; RBM: reticular basement membrane; TGF: transforming growth factor; TSLP: thymic stromal lymphopoietin.
Omalizumab binds to free IgE, inhibiting its binding to the high-affinity IgE receptor FcεRI on mast cells, basophils and dendritic cells [131]. Although omalizumab did not improve FEV1 in randomised controlled trials (RCTs) [132, 133], there is some evidence for FEV1 improvement and reduction in severe exacerbations with omalizumab in real-world settings [134, 135]. Omalizumab reduced RBM thickness [136, 137] and fibronectin deposits in asthmatic airways [138] and prevented ASM remodelling in vitro [139].
Mepolizumab and reslizumab bind to IL-5, preventing it from binding to the IL-5 receptor α subunit (IL-5Rα) on eosinophils, thereby reducing eosinophil activation and maturation [140, 141]. Benralizumab has a similar mechanism of action, targeting IL-5Rα [142]. These biologics improved FEV1 in RCTs in patients with severe eosinophilic asthma [143–147] and in real-world settings [148–150]. Mepolizumab treatment reduced levels of the ECM proteins tenascin, lumican and procollagen III and airway eosinophils expressing TGF-β1 in the bronchial biopsies of patients with mild atopic asthma, as well as TGF-β1 levels in bronchoalveolar lavage [151]. In patients with refractory eosinophilic asthma, a reduction in CT-measured airway wall area was observed with mepolizumab [152]. Preliminary results from the MESILICO study showed that mepolizumab significantly reduced basement membrane thickness, ASM area, the extent of epithelial damage and tissue eosinophil numbers in patients with late-onset, severe eosinophilic asthma and fixed airflow obstruction [153]. Benralizumab reduced both eosinophil numbers in the bronchial lamina propria and ASM mass in patients with severe eosinophilic asthma, with no significant change in myofibroblast numbers. The effects of benralizumab on ASM mass were attributed to an indirect effect mediated by the depletion of TGF-β1+ eosinophils [154]. The effects of reslizumab on airway remodelling have not yet been reported.
Dupilumab binds to the IL-4 receptor α subunit found on lymphocytes, including B- and T-cells, as well as on epithelial cells, blocking both IL-4 and IL-13 signalling [155]. This may be expected to reduce mucus production, goblet cell hyperplasia, subepithelial fibrosis and collagen deposition [14, 156]. In patients with severe asthma, dupilumab improved FEV1 and other lung function measures both in RCTs [157, 158] and a real-world study [159]. An ongoing RCT is evaluating the effects of dupilumab on lung function and airway remodelling using functional respiratory imaging, a novel technology that uses high-resolution CT scans to quantify airway structure and function [160].
Although not currently an approved therapy for asthma, treatment with the anti-IL-13 mAb lebrikizumab reduced subepithelial collagen thickness in patients with uncontrolled asthma, providing further evidence of a role for IL-13 in airway remodelling [161].
In a proof-of-concept study, combined vaccination against IL-4 and IL-13 showed both prophylactic and therapeutic efficacy in a mouse model of asthma [162]. This opens the path for clinical development of a vaccine against asthma that would be a cost-effective alternative to therapeutic antibodies.
Biologics targeting epithelial cytokines
Targeting the epithelium more directly by inhibiting the epithelial cytokines TSLP, IL-33 and IL-25 may be a promising approach to improving airway remodelling (figure 2). Tezepelumab, a human mAb that binds TSLP, has recently been approved for the treatment of patients with severe asthma, with no phenotype or biomarker limitations (table 1) [163]. In phase 2 and 3 RCTs in patients with severe, uncontrolled asthma, there were rapid and sustained improvements in FEV1 and other lung function measures with tezepelumab compared with placebo [164, 165]. Tezepelumab also reduced serum levels of MMP-3 and MMP-10 [166]. In an exploratory, mechanistic study in patients with moderate-to-severe asthma, tezepelumab significantly reduced submucosal eosinophil counts in bronchial biopsies compared with placebo, irrespective of baseline blood eosinophil count [167]. There were no significant differences between treatment groups in RBM thickness and epithelial integrity, although there were greater increases in the CT-measured lumen area across airway generations with tezepelumab than with placebo [167]. The increases in lumen area were potentially related to reductions in occlusive mucus plugs, which correlated with improvements in FEV1 and eosinophilic inflammation [168]; this was the first demonstration that an asthma biologic could reduce mucus plugs. AHR to mannitol was also reduced with tezepelumab compared with placebo in CASCADE [167], a finding confirmed in an independent study [169], indicating that tezepelumab has effects independent of T2 inflammation. Further evidence for the benefits of TSLP blockade on remodelling comes from the use of anti-TSLP antibodies in animal studies. Blocking TSLP reduced airway inflammation and hyperresponsiveness, together with TGF-β1 levels and airway remodelling, in a mouse model of allergic asthma [170] and attenuated airway inflammation and remodelling in asthmatic rats [171]. Furthermore, TSLP blockade suppressed airway remodelling in a mouse model of asthma via reduced MMP, TGF-β and connective tissue growth factor levels [172].
Several biologic therapies targeting IL-33 signalling are, or have recently been, in development for asthma [173, 174]. Itepekimab is an anti-IL-33 mAb that improved FEV1 compared with placebo after 12 weeks of treatment in patients with moderate-to-severe asthma [173]. By contrast, astegolimab, a human mAb that inhibits the IL-33 receptor serum stimulation-2 (ST2), did not significantly improve FEV1 after 54 weeks of treatment in patients with severe asthma, although exacerbations were reduced [174]. However, neither itepekimab nor astegolimab are currently being pursued in asthma [175, 176]. Lastly, tozorakimab is a novel dual-pharmacology mAb that inhibits IL-33 activity via both the ST2 and receptor for advanced glycation end-products–epidermal growth factor receptor complex signalling pathways [177]. Tozorakimab reduced biomarkers of inflammation, including serum IL-5, IL-13 and blood eosinophils, in a phase 1 study [178] and is being evaluated in a phase 2 study in patients with asthma (ClinicalTrials.gov: NCT04570657). Although the impact of these mAbs on airway remodelling has not yet been assessed in clinical studies, IL-33 blockade prevented exacerbations in a mouse model of chronic airway inflammation by blunting persistent inflammation and remodelling [179]. Furthermore, in a mouse model of asthma, ST2 knockout reduced airway inflammation, Th2 cytokine expression and fibrosis-related protein deposition, all of which were further reduced by additional blockade with anti-TSLP and anti-IL-25 antibodies [180].
No biologics targeting IL-25 or its receptor are approved or known to be in late-stage development for the treatment of asthma. However, in a mouse model of allergen-induced airway remodelling, IL-25 blockade reduced airway eosinophils and levels of Th2 cytokines, and abrogated peribronchial collagen deposition, ASM hyperplasia and airway hyperreactivity [53].
Future perspectives
Airway remodelling is a composite term used to describe the changes observed at the cell-to-tissue level within the airways of patients with asthma. Although imaging and physiology are highly predictive of the presence of remodelling, they do not provide insight into the underlying mechanisms. Our understanding of how airway remodelling mechanisms impact asthma development is limited by the lack of consensus on which airway remodelling parameters to study and a lack of appropriate tissue samples or longitudinal sampling approaches for this slowly progressing process [181]. Other fundamental limitations in airway remodelling research include a lack of relevant model systems and difficulty in accurately measuring critical indices (including biomarkers) that encompass genetic, molecular, biochemical, anatomical and functional aspects of airway remodelling. Research is also limited by the costs of the sensitive and specific techniques necessary to identify and quantify longitudinal changes in airway remodelling [181].
Multidisciplinary efforts are needed to identify accessible and valid biomarkers of airway remodelling induction, maintenance, progression and responsiveness to therapy [181, 182]. The most relevant biomarkers are likely to be those linked to pathways that can be successfully targeted by interventions and have consequent benefits at an organ level, with associated improvements in asthma control and exacerbations. Integrating data from multiple disease aspects (structural and inflammatory cell interactions, genetic background, environmental exposure to allergens, pollutants and pathogens, as well as omics) can also be used to develop statistical models of the future risk of developing frequent exacerbations or lung function decline, or the likelihood of responding to therapy [35].
To date, asthma therapies have shown limited effects on airway remodelling [181]. However, the effects of newer therapies, such as those directed against TSLP and IL-33, are yet to be fully elucidated. Nevertheless, it is important to improve the targeting of biological treatments to the most suitable patients. The 3TR (Taxonomy, Treatment, Target and Remission) consortium, involving European academic researchers, patients and the pharmaceutical industry, has set out a plan to increase the clinical impact of targeted immune-mediated therapies for asthma and other immune diseases [183]. Their key aim is to define outcome measures for documenting the therapeutic response to enable comparisons across different studies. The 3TR consortium will then study biomarkers and immunological mechanisms related to different responder profiles and will use advanced omics and systems biology analyses to identify biomarkers for predicting response. Their goal is to uncover future treatment targets and to move towards more ambitious treatment goals for patients with asthma using precision medicine [183].
Conclusion
Evidence shows that airway remodelling can result from the combination of two major components. The inflammatory component is associated with persistent airway infiltration and activation of a wide spectrum of cells of the innate and adaptive immune systems [88, 129]. The structural component is characterised by changes involving goblet cell metaplasia, ASM hypertrophy/hyperplasia, RBM thickening, increased sensory nerve endings and increased angiogenesis [64, 129, 184, 185]. The combination of inflammatory and structural changes in airway remodelling provides the basis of what is canonically referred to as fixed airway obstruction. T2 inflammation, involving eosinophils, mast cells, ILC2s, Th2 cells and basophils, contributes to airway remodelling in most patients with severe asthma [128, 186, 187]. The contribution of non-T2 inflammation to airway remodelling, presumably involving mast cells, macrophages and neutrophils [49, 63], remains to be fully clarified [186, 188, 189].
Increasing evidence indicates that biological therapies targeting IgE, IL-5/IL-5Rα, TSLP and IL-33/ST2 can improve not only clinical symptoms but also certain features of airway remodelling in asthma [128, 129]. With continuing development of new therapies, the achievement of asthma remission and the prevention of airway remodelling could become realistic possibilities in the treatment of severe asthma.
Shareable PDF
Acknowledgements
All authors contributed to manuscript preparation and approved the final manuscript before submitting for publication. Medical writing and editing support, including preparation of the draft manuscript under the direction and guidance of the authors, incorporating author feedback and manuscript submission, was provided by Zoe Kelly, Tove Anderson and Richard Claes of PharmaGenesis London (London, UK), and was funded by AstraZeneca and Amgen Inc.
Footnotes
Conflict of interest: G. Varricchi has received grants from AstraZeneca. C.E. Brightling has received grants and consultancy fees from 4D Pharma, AstraZeneca, Chiesi, Genentech, GSK, Mologic, Novartis, Regeneron Pharmaceuticals, Roche and Sanofi. C. Grainge has received grants from AstraZeneca and industry-sponsored grants from Boehringer Ingelheim and Cyclopharma. B.N. Lambrecht has received grants from the European Research Council, Ghent University, Research Foundation Flanders and the Flanders Institute of Biotechnology, and has received consulting fees from Argenx, AstraZeneca, GSK and Sanofi. P. Chanez has received consultancy fees from ALK, Almirall, AstraZeneca, Boehringer Ingelheim, Boston Scientific, Centocor, Chiesi, GSK, Johnson & Johnson, MSD, Novartis, Sanofi, SNCF and Teva Pharmaceuticals, has received industry-sponsored grants from ALK, AstraZeneca, Boehringer Ingelheim, Boston Scientific, Centocor, Chiesi, GSK, Novartis, Roche and Teva Pharmaceuticals, and is the president of the scientific committee for Fondation du Souffle.
Support statement: This work was supported by AstraZeneca and Amgen Inc. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Global Initiative for Asthma (GINA) . Global Strategy for Asthma Management and Prevention. 2022. Available from: http://ginasthma.org/
- 2.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med 2012; 18: 716–725. doi: 10.1038/nm.2678 [DOI] [PubMed] [Google Scholar]
- 3.Kuruvilla ME, Lee F, Lee GB. Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin Rev Allergy Immunol 2019; 56: 219–233. doi: 10.1007/s12016-018-8712-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fahy JV. Type 2 inflammation in asthma – present in most, absent in many. Nat Rev Immunol 2015; 15: 57–65. doi: 10.1038/nri3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore WC, Hastie AT, Li X, et al. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol 2014; 133: 1557–1563. doi: 10.1016/j.jaci.2013.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tliba O, Panettieri RA Jr. Paucigranulocytic asthma: uncoupling of airway obstruction from inflammation. J Allergy Clin Immunol 2019; 143: 1287–1294. doi: 10.1016/j.jaci.2018.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bullone M, Carriero V, Bertolini F, et al. Elevated serum IgE, oral corticosteroid dependence and IL-17/22 expression in highly neutrophilic asthma. Eur Respir J 2019; 54: 1900068. doi: 10.1183/13993003.00068-2019 [DOI] [PubMed] [Google Scholar]
- 8.Ricciardolo FLM, Guida G, Bertolini F, et al. Phenotype overlap in the natural history of asthma. Eur Respir Rev 2023; 32: 220201. doi: 10.1183/16000617.0201-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fehrenbach H, Wagner C, Wegmann M. Airway remodeling in asthma: what really matters. Cell Tissue Res 2017; 367: 551–569. doi: 10.1007/s00441-016-2566-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noureddine N, Chalubinski M, Wawrzyniak P. The role of defective epithelial barriers in allergic lung disease and asthma development. J Asthma Allergy 2022; 15: 487–504. doi: 10.2147/JAA.S324080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonser LR, Erle DJ. The airway epithelium in asthma. Adv Immunol 2019; 142: 1–34. doi: 10.1016/bs.ai.2019.05.001 [DOI] [PubMed] [Google Scholar]
- 12.Hellings PW, Steelant B. Epithelial barriers in allergy and asthma. J Allergy Clin Immunol 2020; 145: 1499–1509. doi: 10.1016/j.jaci.2020.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levy BD, Vachier I, Serhan CN. Resolution of inflammation in asthma. Clin Chest Med 2012; 33: 559–570. doi: 10.1016/j.ccm.2012.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hough KP, Curtiss ML, Blain TJ, et al. Airway remodeling in asthma. Front Med 2020; 7: 191. doi: 10.3389/fmed.2020.00191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson SJ, Rigden HM, Ward JA, et al. The relationship between eosinophilia and airway remodelling in mild asthma. Clin Exp Allergy 2013; 43: 1342–1350. doi: 10.1111/cea.12156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonsignore MR, Profita M, Gagliardo R, et al. Advances in asthma pathophysiology: stepping forward from the Maurizio Vignola experience. Eur Respir Rev 2015; 24: 30. doi: 10.1183/09059180.10011114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bourdin A, Neveu D, Vachier I, et al. Specificity of basement membrane thickening in severe asthma. J Allergy Clin Immunol 2007; 119: 1367–1374. doi: 10.1016/j.jaci.2007.01.055 [DOI] [PubMed] [Google Scholar]
- 18.Krings JG, Goss CW, Lew D, et al. Quantitative CT metrics are associated with longitudinal lung function decline and future asthma exacerbations: results from SARP-3. J Allergy Clin Immunol 2021; 148: 752–762. doi: 10.1016/j.jaci.2021.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saglani S, Payne DN, Zhu J, et al. Early detection of airway wall remodeling and eosinophilic inflammation in preschool wheezers. Am J Respir Crit Care Med 2007; 176: 858–864. doi: 10.1164/rccm.200702-212OC [DOI] [PubMed] [Google Scholar]
- 20.Lezmi G, Gosset P, Deschildre A, et al. Airway remodeling in preschool children with severe recurrent wheeze. Am J Respir Crit Care Med 2015; 192: 164–171. doi: 10.1164/rccm.201411-1958OC [DOI] [PubMed] [Google Scholar]
- 21.Pohunek P, Warner JO, Turzíková J, et al. Markers of eosinophilic inflammation and tissue re-modelling in children before clinically diagnosed bronchial asthma. Pediatr Allergy Immunol 2005; 16: 43–51. doi: 10.1111/j.1399-3038.2005.00239.x [DOI] [PubMed] [Google Scholar]
- 22.Saglani S, Lloyd CM. Novel concepts in airway inflammation and remodelling in asthma. Eur Respir J 2015; 46: 1796. doi: 10.1183/13993003.01196-2014 [DOI] [PubMed] [Google Scholar]
- 23.Li D, Wu M. Pattern recognition receptors in health and diseases. Signal Transduct Target Ther 2021; 6: 291. doi: 10.1038/s41392-021-00687-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato A, Favoreto S Jr, Avila PC, et al. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol 2007; 179: 1080–1087. doi: 10.4049/jimmunol.179.2.1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whetstone CE, Ranjbar M, Omer H, et al. The role of airway epithelial cell alarmins in asthma. Cells 2022; 11: 1105. doi: 10.3390/cells11071105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bayarri MA, Milara J, Estornut C, et al. Nitric oxide system and bronchial epithelium: more than a barrier. Front Physiol 2021; 12: 687381. doi: 10.3389/fphys.2021.687381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makinde T, Murphy RF, Agrawal DK. The regulatory role of TGF-beta in airway remodeling in asthma. Immunol Cell Biol 2007; 85: 348–356. doi: 10.1038/sj.icb.7100044 [DOI] [PubMed] [Google Scholar]
- 28.Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol 2010; 298: L715–L731. doi: 10.1152/ajplung.00361.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Y, Jia M, Ou Y, et al. Mechanisms and biomarkers of airway epithelial cell damage in asthma: a review. Clin Respir J 2021; 15: 1027–1045. doi: 10.1111/crj.13407 [DOI] [PubMed] [Google Scholar]
- 30.Wiggs BR, Hrousis CA, Drazen JM, et al. On the mechanism of mucosal folding in normal and asthmatic airways. J Appl Physiol 1997; 83: 1814–1821. doi: 10.1152/jappl.1997.83.6.1814 [DOI] [PubMed] [Google Scholar]
- 31.Wirtz HR, Dobbs LG. The effects of mechanical forces on lung functions. Respir Physiol 2000; 119: 1–17. doi: 10.1016/S0034-5687(99)00092-4 [DOI] [PubMed] [Google Scholar]
- 32.Veerati PC, Mitchel JA, Reid AT, et al. Airway mechanical compression: its role in asthma pathogenesis and progression. Eur Respir Rev 2020; 29: 190123. doi: 10.1183/16000617.0123-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tschumperlin DJ, Drazen JM. Mechanical stimuli to airway remodeling. Am J Respir Crit Care Med 2001; 164: S90–S94. doi: 10.1164/ajrccm.164.supplement_2.2106060 [DOI] [PubMed] [Google Scholar]
- 34.Grainge CL, Lau LC, Ward JA, et al. Effect of bronchoconstriction on airway remodeling in asthma. N Engl J Med 2011; 364: 2006–2015. doi: 10.1056/NEJMoa1014350 [DOI] [PubMed] [Google Scholar]
- 35.Brightling CE, Gupta S, Gonem S, et al. Lung damage and airway remodelling in severe asthma. Clin Exp Allergy 2012; 42: 638–649. doi: 10.1111/j.1365-2222.2011.03917.x [DOI] [PubMed] [Google Scholar]
- 36.Joseph C, Tatler AL. Pathobiology of airway remodeling in asthma: the emerging role of integrins. J Asthma Allergy 2022; 15: 595–610. doi: 10.2147/JAA.S267222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Porsbjerg CM, Sverrild A, Lloyd CM, et al. Anti-alarmins in asthma: targeting the airway epithelium with next-generation biologics. Eur Respir J 2020; 56: 2000260. doi: 10.1183/13993003.00260-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shikotra A, Choy DF, Ohri CM, et al. Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J Allergy Clin Immunol 2012; 129: 104–111. doi: 10.1016/j.jaci.2011.08.031 [DOI] [PubMed] [Google Scholar]
- 39.Ying S, O'Connor B, Ratoff J, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol 2005; 174: 8183–8190. doi: 10.4049/jimmunol.174.12.8183 [DOI] [PubMed] [Google Scholar]
- 40.Kaur D, Gomez E, Doe C, et al. IL-33 drives airway hyper-responsiveness through IL-13-mediated mast cell: airway smooth muscle crosstalk. Allergy 2015; 70: 556–567. doi: 10.1111/all.12593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Momen T, Ahanchian H, Reisi M, et al. Comparison of interleukin-33 serum levels in asthmatic patients with a control group and relation with the severity of the disease. Int J Prev Med 2017; 8: 65. doi: 10.4103/ijpvm.IJPVM_179_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prefontaine D, Lajoie-Kadoch S, Foley S, et al. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J Immunol 2009; 183: 5094–5103. doi: 10.4049/jimmunol.0802387 [DOI] [PubMed] [Google Scholar]
- 43.Paplinska-Goryca M, Grabczak EM, Dabrowska M, et al. Sputum interleukin-25 correlates with asthma severity: a preliminary study. Postepy Dermatol Alergol 2018; 35: 462–469. doi: 10.5114/ada.2017.71428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cao L, Liu F, Liu Y, et al. TSLP promotes asthmatic airway remodeling via p38-STAT3 signaling pathway in human lung fibroblast. Exp Lung Res 2018; 44: 288–301. doi: 10.1080/01902148.2018.1536175 [DOI] [PubMed] [Google Scholar]
- 45.Jin A, Tang X, Zhai W, et al. TSLP-induced collagen type-I synthesis through STAT3 and PRMT1 is sensitive to calcitriol in human lung fibroblasts. Biochim Biophys Acta Mol Cell Res 2021; 1868: 119083. doi: 10.1016/j.bbamcr.2021.119083 [DOI] [PubMed] [Google Scholar]
- 46.Wu J, Liu F, Zhao J, et al. Thymic stromal lymphopoietin promotes asthmatic airway remodelling in human lung fibroblast cells through STAT3 signalling pathway. Cell Biochem Funct 2013; 31: 496–503. doi: 10.1002/cbf.2926 [DOI] [PubMed] [Google Scholar]
- 47.Xie F, Meng YH, Liu LB, et al. Cervical carcinoma cells stimulate the angiogenesis through TSLP promoting growth and activation of vascular endothelial cells. Am J Reprod Immunol 2013; 70: 69–79. doi: 10.1111/aji.12104 [DOI] [PubMed] [Google Scholar]
- 48.Zhang B, Wei CY, Chang KK, et al. TSLP promotes angiogenesis of human umbilical vein endothelial cells by strengthening the crosstalk between cervical cancer cells and eosinophils. Oncol Lett 2017; 14: 7483–7488. doi: 10.3892/ol.2017.7121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Braile M, Fiorelli A, Sorriento D, et al. Human lung-resident macrophages express and are targets of thymic stromal lymphopoietin in the tumor microenvironment. Cells 2021; 10: 2012. doi: 10.3390/cells10082012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo Z, Wu J, Zhao J, et al. IL-33 promotes airway remodeling and is a marker of asthma disease severity. J Asthma 2014; 51: 863–869. doi: 10.3109/02770903.2014.921196 [DOI] [PubMed] [Google Scholar]
- 51.Saglani S, Lui S, Ullmann N, et al. IL-33 promotes airway remodeling in pediatric patients with severe steroid-resistant asthma. J Allergy Clin Immunol 2013; 132: 676–685. doi: 10.1016/j.jaci.2013.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu X, Luo S, Li B, et al. IL-25 contributes to lung fibrosis by directly acting on alveolar epithelial cells and fibroblasts. Exp Biol Med 2019; 244: 770–780. doi: 10.1177/1535370219843827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gregory LG, Jones CP, Walker SA, et al. IL-25 drives remodelling in allergic airways disease induced by house dust mite. Thorax 2013; 68: 82–90. doi: 10.1136/thoraxjnl-2012-202003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Drake LY, Koloko Ngassie ML, Roos BB, et al. Asthmatic lung fibroblasts promote type 2 immune responses via endoplasmic reticulum stress response dependent thymic stromal lymphopoietin secretion. Front Physiol 2023; 14: 1064822. doi: 10.3389/fphys.2023.1064822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saikumar Jayalatha AK, Hesse L, Ketelaar ME, et al. The central role of IL-33/IL-1RL1 pathway in asthma: from pathogenesis to intervention. Pharmacol Ther 2021; 225: 107847. doi: 10.1016/j.pharmthera.2021.107847 [DOI] [PubMed] [Google Scholar]
- 56.Raby KL, Michaeloudes C, Tonkin J, et al. Mechanisms of airway epithelial injury and abnormal repair in asthma and COPD. Front Immunol 2023; 14: 1201658. doi: 10.3389/fimmu.2023.1201658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tan QY, Cheng ZS. TGFβ1-Smad signaling pathway participates in interleukin-33 induced epithelial-to-mesenchymal transition of A549 cells. Cell Physiol Biochem 2018; 50: 757–767. doi: 10.1159/000494241 [DOI] [PubMed] [Google Scholar]
- 58.Cai LM, Zhou YQ, Yang LF, et al. Thymic stromal lymphopoietin induced early stage of epithelial-mesenchymal transition in human bronchial epithelial cells through upregulation of transforming growth factor beta 1. Exp Lung Res 2019; 45: 221–235. doi: 10.1080/01902148.2019.1646841 [DOI] [PubMed] [Google Scholar]
- 59.Gauvreau GM, Sehmi R, Ambrose CS, et al. Thymic stromal lymphopoietin: its role and potential as a therapeutic target in asthma. Expert Opin Ther Targets 2020; 24: 777–792. doi: 10.1080/14728222.2020.1783242 [DOI] [PubMed] [Google Scholar]
- 60.Redhu NS, Shan L, Movassagh H, et al. Thymic stromal lymphopoietin induces migration in human airway smooth muscle cells. Sci Rep 2013; 3: 2301. doi: 10.1038/srep02301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shan L, Redhu NS, Saleh A, et al. Thymic stromal lymphopoietin receptor-mediated IL-6 and CC/CXC chemokines expression in human airway smooth muscle cells: role of MAPKs (ERK1/2, p38, and JNK) and STAT3 pathways. J Immunol 2010; 184: 7134–7143. doi: 10.4049/jimmunol.0902515 [DOI] [PubMed] [Google Scholar]
- 62.Kaur D, Doe C, Woodman L, et al. Mast cell-airway smooth muscle crosstalk: the role of thymic stromal lymphopoietin. Chest 2012; 142: 76–85. doi: 10.1378/chest.11-1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poto R, Criscuolo G, Marone G, et al. Human lung mast cells: therapeutic implications in asthma. Int J Mol Sci 2022; 23: 14466. doi: 10.3390/ijms232214466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chetta A, Zanini A, Foresi A, et al. Vascular component of airway remodeling in asthma is reduced by high dose of fluticasone. Am J Respir Crit Care Med 2003; 167: 751–757. doi: 10.1164/rccm.200207-710OC [DOI] [PubMed] [Google Scholar]
- 65.Ali Komi ED, Bjermer L. Mast cell-mediated orchestration of the immune responses in human allergic asthma: current insights. Clin Rev Allergy Immunol 2019; 56: 234–247. doi: 10.1007/s12016-018-8720-1 [DOI] [PubMed] [Google Scholar]
- 66.Yao X, Wang W, Li Y, et al. IL-25 induces airways angiogenesis and expression of multiple angiogenic factors in a murine asthma model. Respir Res 2015; 16: 39. doi: 10.1186/s12931-015-0197-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Afferni C, Buccione C, Andreone S, et al. The pleiotropic immunomodulatory functions of IL-33 and its implications in tumor immunity. Front Immunol 2018; 9: 2601. doi: 10.3389/fimmu.2018.02601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Varricchi G, Pecoraro A, Marone G, et al. Thymic stromal lymphopoietin isoforms, inflammatory disorders, and cancer. Front Immunol 2018; 9: 1595. doi: 10.3389/fimmu.2018.01595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Soumelis V, Reche PA, Kanzler H, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol 2002; 3: 673–680. doi: 10.1038/ni805 [DOI] [PubMed] [Google Scholar]
- 70.Murakami-Satsutani N, Ito T, Nakanishi T, et al. IL-33 promotes the induction and maintenance of Th2 immune responses by enhancing the function of OX40 ligand. Allergol Int 2014; 63: 443–455. doi: 10.2332/allergolint.13-OA-0672 [DOI] [PubMed] [Google Scholar]
- 71.Barlow JL, Peel S, Fox J, et al. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J Allergy Clin Immunol 2013; 132: 933–941. doi: 10.1016/j.jaci.2013.05.012 [DOI] [PubMed] [Google Scholar]
- 72.Bartemes KR, Kephart GM, Fox SJ, et al. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol 2014; 134: 671–678. doi: 10.1016/j.jaci.2014.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Camelo A, Rosignoli G, Ohne Y, et al. IL-33, IL-25, and TSLP induce a distinct phenotypic and activation profile in human type 2 innate lymphoid cells. Blood Adv 2017; 1: 577–589. doi: 10.1182/bloodadvances.2016002352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Karlen S, De Boer ML, Lipscombe RJ, et al. Biological and molecular characteristics of interleukin-5 and its receptor. Int Rev Immunol 1998; 16: 227–247. doi: 10.3109/08830189809042996 [DOI] [PubMed] [Google Scholar]
- 75.Possa SS, Leick EA, Prado CM, et al. Eosinophilic inflammation in allergic asthma. Front Pharmacol 2013; 4: 46. doi: 10.3389/fphar.2013.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Halwani R, Vazquez-Tello A, Sumi Y, et al. Eosinophils induce airway smooth muscle cell proliferation. J Clin Immunol 2013; 33: 595–604. doi: 10.1007/s10875-012-9836-3 [DOI] [PubMed] [Google Scholar]
- 77.Kuo CS, Pavlidis S, Zhu J, et al. Contribution of airway eosinophils in airway wall remodeling in asthma: role of MMP-10 and MET. Allergy 2019; 74: 1102–1112. doi: 10.1111/all.13727 [DOI] [PubMed] [Google Scholar]
- 78.Tanaka J, Watanabe N, Kido M, et al. Human TSLP and TLR3 ligands promote differentiation of Th17 cells with a central memory phenotype under Th2-polarizing conditions. Clin Exp Allergy 2009; 39: 89–100. doi: 10.1111/j.1365-2222.2008.03151.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peters M, Kohler-Bachmann S, Lenz-Habijan T, et al. Influence of an allergen-specific Th17 response on remodeling of the airways. Am J Respir Cell Mol Biol 2016; 54: 350–358. doi: 10.1165/rcmb.2014-0429OC [DOI] [PubMed] [Google Scholar]
- 80.Gao H, Ying S, Dai Y. Pathological roles of neutrophil-mediated inflammation in asthma and its potential for therapy as a target. J Immunol Res 2017; 2017: 3743048. doi: 10.1155/2017/3743048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van der Veen TA, de Groot LE, Melgert BN. The different faces of the macrophage in asthma. Curr Opin Pulm Med 2020; 26: 62. doi: 10.1097/MCP.0000000000000647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Balhara J, Gounni AS. The alveolar macrophages in asthma: a double-edged sword. Mucosal Immunol 2012; 5: 605–609. doi: 10.1038/mi.2012.74 [DOI] [PubMed] [Google Scholar]
- 83.Chakarov S, Lim HY, Tan L, et al. Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science 2019; 363: eaau0964. doi: 10.1126/science.aau0964 [DOI] [PubMed] [Google Scholar]
- 84.Mould KJ, Jackson ND, Henson PM, et al. Single cell RNA sequencing identifies unique inflammatory airspace macrophage subsets. JCI Insight 2019; 4: e126556. doi: 10.1172/jci.insight.126556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ural BB, Yeung ST, Damani-Yokota P, et al. Identification of a nerve-associated, lung-resident interstitial macrophage subset with distinct localization and immunoregulatory properties. Sci Immunol 2020; 5: eaax8756. doi: 10.1126/sciimmunol.aax8756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hsieh A, Assadinia N, Hackett TL. Airway remodeling heterogeneity in asthma and its relationship to disease outcomes. Front Physiol 2023; 14: 1113100. doi: 10.3389/fphys.2023.1113100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moheimani F, Hsu AC, Reid AT, et al. The genetic and epigenetic landscapes of the epithelium in asthma. Respir Res 2016; 17: 119. doi: 10.1186/s12931-016-0434-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Holgate ST. Pathogenesis of asthma. Clin Exp Allergy 2008; 38: 872–897. doi: 10.1111/j.1365-2222.2008.02971.x [DOI] [PubMed] [Google Scholar]
- 89.Shrine N, Portelli MA, John C, et al. Moderate-to-severe asthma in individuals of European ancestry: a genome-wide association study. Lancet Respir Med 2019; 7: 20–34. doi: 10.1016/S2213-2600(18)30389-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Heijink IH, Kuchibhotla VNS, Roffel MP, et al. Epithelial cell dysfunction, a major driver of asthma development. Allergy 2020; 75: 1902–1917. doi: 10.1111/all.14421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moffatt MF, Kabesch M, Liang L, et al. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature 2007; 448: 470–473. doi: 10.1038/nature06014 [DOI] [PubMed] [Google Scholar]
- 92.Das S, Miller M, Beppu AK, et al. GSDMB induces an asthma phenotype characterized by increased airway responsiveness and remodeling without lung inflammation. Proc Natl Acad Sci USA 2016; 113: 13132–13137. doi: 10.1073/pnas.1610433113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.James B, Milstien S, Spiegel S. ORMDL3 and allergic asthma: from physiology to pathology. J Allergy Clin Immunol 2019; 144: 634–640. doi: 10.1016/j.jaci.2019.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miller M, Rosenthal P, Beppu A, et al. ORMDL3 transgenic mice have increased airway remodeling and airway responsiveness characteristic of asthma. J Immunol 2014; 192: 3475–3487. doi: 10.4049/jimmunol.1303047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ierodiakonou D, Portelli MA, Postma DS, et al. Urokinase plasminogen activator receptor polymorphisms and airway remodelling in asthma. Eur Respir J 2016; 47: 1568–1571. doi: 10.1183/13993003.01571-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hur GY, Broide DH. Genes and pathways regulating decline in lung function and airway remodeling in asthma. Allergy Asthma Immunol Res 2019; 11: 604–621. doi: 10.4168/aair.2019.11.5.604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chupp GL, Lee CG, Jarjour N, et al. A chitinase-like protein in the lung and circulation of patients with severe asthma. N Engl J Med 2007; 357: 2016–2027. doi: 10.1056/NEJMoa073600 [DOI] [PubMed] [Google Scholar]
- 98.Gomez JL, Crisafi GM, Holm CT, et al. Genetic variation in chitinase 3-like 1 (CHI3L1) contributes to asthma severity and airway expression of YKL-40. J Allergy Clin Immunol 2015; 136: 51–58. doi: 10.1016/j.jaci.2014.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sheikhpour M, Maleki M, Ebrahimi Vargoorani M, et al. A review of epigenetic changes in asthma: methylation and acetylation. Clin Epigenetics 2021; 13: 65. doi: 10.1186/s13148-021-01049-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jakwerth CA, Ordovas-Montanes J, Blank S, et al. Role of respiratory epithelial cells in allergic diseases. Cells 2022; 11: 1387. doi: 10.3390/cells11091387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stefanowicz D, Hackett TL, Garmaroudi FS, et al. DNA methylation profiles of airway epithelial cells and PBMCs from healthy, atopic and asthmatic children. PLoS One 2012; 7: e44213. doi: 10.1371/journal.pone.0044213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Forno E, Wang T, Qi C, et al. DNA methylation in nasal epithelium, atopy, and atopic asthma in children: a genome-wide study. Lancet Respir Med 2019; 7: 336–346. doi: 10.1016/S2213-2600(18)30466-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li YL, Xing XQ, Xiao Y, et al. Correlation between DNA methylation and thymic stromal lymphopoietin expression in asthmatic airway epithelial cells. Genes Genomics 2020; 42: 1399–1406. doi: 10.1007/s13258-020-01000-z [DOI] [PubMed] [Google Scholar]
- 104.Steelant B, Wawrzyniak P, Martens K, et al. Blocking histone deacetylase activity as a novel target for epithelial barrier defects in patients with allergic rhinitis. J Allergy Clin Immunol 2019; 144: 1242–1253. doi: 10.1016/j.jaci.2019.04.027 [DOI] [PubMed] [Google Scholar]
- 105.Wawrzyniak P, Wawrzyniak M, Wanke K, et al. Regulation of bronchial epithelial barrier integrity by type 2 cytokines and histone deacetylases in asthmatic patients. J Allergy Clin Immunol 2017; 139: 93–103. doi: 10.1016/j.jaci.2016.03.050 [DOI] [PubMed] [Google Scholar]
- 106.Brasier AR, Boldogh I. Targeting inducible epigenetic reprogramming pathways in chronic airway remodeling. Drugs Context 2019; 8: 3. doi: 10.7573/dic.2019-8-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Berair R, Hartley R, Mistry V, et al. Associations in asthma between quantitative computed tomography and bronchial biopsy-derived airway remodelling. Eur Respir J 2017; 49: 1601507. doi: 10.1183/13993003.01507-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hartley RA, Barker BL, Newby C, et al. Relationship between lung function and quantitative computed tomographic parameters of airway remodeling, air trapping, and emphysema in patients with asthma and chronic obstructive pulmonary disease: a single-center study. J Allergy Clin Immunol 2016; 137: 1413–1422. doi: 10.1016/j.jaci.2016.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bergeron C, Tulic MK, Hamid Q. Airway remodelling in asthma: from benchside to clinical practice. Can Respir J 2010; 17: e85–e93. doi: 10.1155/2010/318029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tang M, Elicker BM, Henry T, et al. Mucus plugs persist in asthma, and changes in mucus plugs associate with changes in airflow over time. Am J Respir Crit Care Med 2022; 205: 1036–1045. doi: 10.1164/rccm.202110-2265OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dunican EM, Watchorn DC, Fahy JV. Autopsy and imaging studies of mucus in asthma. Lessons learned about disease mechanisms and the role of mucus in airflow obstruction. Ann Am Thorac Soc 2018; 15: Suppl. 3, S184–S191. doi: 10.1513/AnnalsATS.201807-485AW [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chetta A, Foresi A, Del Donno M, et al. Airways remodeling is a distinctive feature of asthma and is related to severity of disease. Chest 1997; 111: 852–857. doi: 10.1378/chest.111.4.852 [DOI] [PubMed] [Google Scholar]
- 113.Westergren-Thorsson G, Chakir J, Lafreniere-Allard MJ, et al. Correlation between airway responsiveness and proteoglycan production by bronchial fibroblasts from normal and asthmatic subjects. Int J Biochem Cell Biol 2002; 34: 1256–1267. doi: 10.1016/S1357-2725(02)00058-4 [DOI] [PubMed] [Google Scholar]
- 114.Benayoun L, Druilhe A, Dombret MC, et al. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med 2003; 167: 1360–1368. doi: 10.1164/rccm.200209-1030OC [DOI] [PubMed] [Google Scholar]
- 115.Jones RL, Noble PB, Elliot JG, et al. Airflow obstruction is associated with increased smooth muscle extracellular matrix. Eur Respir J 2016; 47: 1855–1857. doi: 10.1183/13993003.01709-2015 [DOI] [PubMed] [Google Scholar]
- 116.Siddiqui S, Castro M, Brightling CE. Imaging. In: Chung KF, Israel E, Gibson P, ed. Severe Asthma (ERS Monograph). Sheffield, European Respiratory Society, 2019; pp. 113–131. [Google Scholar]
- 117.Wenzel SE, Schwartz LB, Langmack EL, et al. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med 1999; 160: 1001–1008. doi: 10.1164/ajrccm.160.3.9812110 [DOI] [PubMed] [Google Scholar]
- 118.Miranda C, Busacker A, Balzar S, et al. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol 2004; 113: 101–108. doi: 10.1016/j.jaci.2003.10.041 [DOI] [PubMed] [Google Scholar]
- 119.Elliot JG, Noble PB, Mauad T, et al. Inflammation-dependent and independent airway remodelling in asthma. Respirology 2018; 23: 1138–1145. doi: 10.1111/resp.13360 [DOI] [PubMed] [Google Scholar]
- 120.Kim S, Lee CH, Jin KN, et al. Severe asthma phenotypes classified by site of airway involvement and remodeling via chest CT scan. J Investig Allergol Clin Immunol 2018; 28: 312–320. doi: 10.18176/jiaci.0265 [DOI] [PubMed] [Google Scholar]
- 121.Gupta S, Hartley R, Khan UT, et al. Quantitative computed tomography-derived clusters: redefining airway remodeling in asthmatic patients. J Allergy Clin Immunol 2014; 133: 729–738. doi: 10.1016/j.jaci.2013.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tan DJ, Bui DS, Dai X, et al. Does the use of inhaled corticosteroids in asthma benefit lung function in the long-term? A systematic review and meta-analysis. Eur Respir Rev 2021; 30: 200185. doi: 10.1183/16000617.0185-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sekiyama A, Gon Y, Terakado M, et al. Glucocorticoids enhance airway epithelial barrier integrity. Int Immunopharmacol 2012; 12: 350–357. doi: 10.1016/j.intimp.2011.12.006 [DOI] [PubMed] [Google Scholar]
- 124.Lu W, Lillehoj EP, Kim KC. Effects of dexamethasone on Muc5ac mucin production by primary airway goblet cells. Am J Physiol Lung Cell Mol Physiol 2005; 288: L52–L60. doi: 10.1152/ajplung.00104.2004 [DOI] [PubMed] [Google Scholar]
- 125.Lachowicz-Scroggins ME, Finkbeiner WE, Gordon ED, et al. Corticosteroid and long-acting β-agonist therapy reduces epithelial goblet cell metaplasia. Clin Exp Allergy 2017; 47: 1534–1545. doi: 10.1111/cea.13015 [DOI] [PubMed] [Google Scholar]
- 126.Groneberg D, Eynott P, Lim S, et al. Expression of respiratory mucins in fatal status asthmaticus and mild asthma. Histopathology 2002; 40: 367–373. doi: 10.1046/j.1365-2559.2002.01378.x [DOI] [PubMed] [Google Scholar]
- 127.White SR, Dorscheid DR. Corticosteroid-induced apoptosis of airway epithelium: a potential mechanism for chronic airway epithelial damage in asthma. Chest 2002; 122: 278S–284S. doi: 10.1378/chest.122.6_suppl.278S [DOI] [PubMed] [Google Scholar]
- 128.Kardas G, Kuna P, Panek M. Biological therapies of severe asthma and their possible effects on airway remodeling. Front Immunol 2020; 11: 1134. doi: 10.3389/fimmu.2020.01134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Varricchi G, Ferri S, Pepys J, et al. Biologics and airway remodeling in severe asthma. Allergy 2022; 77: 3538–3552. doi: 10.1111/all.15473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shah PA, Brightling C. Biologics for severe asthma – which, when and why? Respirology 2023; 28: 709–721. doi: 10.1111/resp.14520 [DOI] [PubMed] [Google Scholar]
- 131.US Food and Drug Administration . ZOLAIR® (omalizumab) prescribing information. 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2003/omalgen062003LB.pdf Date last accessed: 15 May 2023.
- 132.Normansell R, Walker S, Milan SJ, et al. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev 2014; 13: CD003559. doi: 10.1002/14651858.CD003559.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hanania NA, Alpan O, Hamilos DL, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med 2011; 154: 573–582. doi: 10.7326/0003-4819-154-9-201105030-00002 [DOI] [PubMed] [Google Scholar]
- 134.Casale TB, Luskin AT, Busse W, et al. Omalizumab effectiveness by biomarker status in patients with asthma: evidence from PROSPERO, a prospective real-world study. J Allergy Clin Immunol Pract 2019; 7: 156–164. doi: 10.1016/j.jaip.2018.04.043 [DOI] [PubMed] [Google Scholar]
- 135.Hew M, Gillman A, Sutherland M, et al. Real-life effectiveness of omalizumab in severe allergic asthma above the recommended dosing range criteria. Clin Exp Allergy 2016; 46: 1407–1415. doi: 10.1111/cea.12774 [DOI] [PubMed] [Google Scholar]
- 136.Riccio AM, Dal Negro RW, Micheletto C, et al. Omalizumab modulates bronchial reticular basement membrane thickness and eosinophil infiltration in severe persistent allergic asthma patients. Int J Immunopathol Pharmacol 2012; 25: 475–484. doi: 10.1177/039463201202500217 [DOI] [PubMed] [Google Scholar]
- 137.Hoshino M, Ohtawa J. Effects of adding omalizumab, an anti-immunoglobulin E antibody, on airway wall thickening in asthma. Respiration 2012; 83: 520–528. doi: 10.1159/000334701 [DOI] [PubMed] [Google Scholar]
- 138.Zastrzezynska W, Przybyszowski M, Bazan-Socha S, et al. Omalizumab may decrease the thickness of the reticular basement membrane and fibronectin deposit in the bronchial mucosa of severe allergic asthmatics. J Asthma 2020; 57: 468–477. doi: 10.1080/02770903.2019.1585872 [DOI] [PubMed] [Google Scholar]
- 139.Roth M, Zhao F, Zhong J, et al. Serum IgE induced airway smooth muscle cell remodeling is independent of allergens and is prevented by omalizumab. PLoS One 2015; 10: e0136549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.US Food and Drug Administration . NUCALA® (mepolizumab) prescribing information. 2015. www.accessdata.fda.gov/drugsatfda_docs/label/2015/125526Orig1s000Lbl.pdf Date last accessed: 24 February 2024.
- 141.US Food and Drug Administration . CINQAIR® (reslizumab) prescribing information. 2016. www.accessdata.fda.gov/drugsatfda_docs/label/2016/761033lbl.pdf Date last accessed: 24 February 2024.
- 142.US Food and Drug Administration . FASENRA™ (benralizumab) prescribing information. 2017. www.accessdata.fda.gov/drugsatfda_docs/label/2017/761070s000lbl.pdf Date last accessed: 24 February 2024.
- 143.Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med 2014; 371: 1198–1207. doi: 10.1056/NEJMoa1403290 [DOI] [PubMed] [Google Scholar]
- 144.Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet 2012; 380: 651–659. doi: 10.1016/S0140-6736(12)60988-X [DOI] [PubMed] [Google Scholar]
- 145.Bjermer L, Lemiere C, Maspero J, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest 2016; 150: 789–798. doi: 10.1016/j.chest.2016.03.032 [DOI] [PubMed] [Google Scholar]
- 146.Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med 2015; 3: 355–366. doi: 10.1016/S2213-2600(15)00042-9 [DOI] [PubMed] [Google Scholar]
- 147.Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016; 388: 2115–2127. doi: 10.1016/S0140-6736(16)31324-1 [DOI] [PubMed] [Google Scholar]
- 148.Pelaia C, Crimi C, Pelaia G, et al. Real-life evaluation of mepolizumab efficacy in patients with severe eosinophilic asthma, according to atopic trait and allergic phenotype. Clin Exp Allergy 2020; 50: 780–788. doi: 10.1111/cea.13613 [DOI] [PubMed] [Google Scholar]
- 149.Bagnasco D, Caminati M, Menzella F, et al. One year of mepolizumab. Efficacy and safety in real-life in Italy. Pulm Pharmacol Ther 2019; 58: 101836. doi: 10.1016/j.pupt.2019.101836 [DOI] [PubMed] [Google Scholar]
- 150.Wechsler ME, Peters SP, Hill TD, et al. Clinical outcomes and health-care resource use associated with reslizumab treatment in adults with severe eosinophilic asthma in real-world practice. Chest 2021; 159: 1734–1746. doi: 10.1016/j.chest.2020.11.060 [DOI] [PubMed] [Google Scholar]
- 151.Flood-Page P, Menzies-Gow A, Phipps S, et al. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest 2003; 112: 1029–1036. doi: 10.1172/JCI17974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med 2009; 360: 973–984. doi: 10.1056/NEJMoa0808991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Domvri K, Tsiouprou I, Bakakos P, et al. Effect of mepolizumab on airways remodeling in patients with late-onset severe eosinophilic asthma and fixed obstruction (preliminary data of the MESILICO study). Eur Respir J 2023; 62: Suppl. 67, OA3152. doi: 10.1183/13993003.congress-2023.OA3152 [DOI] [Google Scholar]
- 154.Chachi L, Diver S, Kaul H, et al. Computational modelling prediction and clinical validation of impact of benralizumab on airway smooth muscle mass in asthma. Eur Respir J 2019; 54: 1900930. doi: 10.1183/13993003.00930-2019 [DOI] [PubMed] [Google Scholar]
- 155.US Food and Drug Administration . DUPIXENT® (dupilumab) prescribing information. 2017. www.accessdata.fda.gov/drugsatfda_docs/label/2017/761055lbl.pdf Date last accessed: 24 February 2024.
- 156.Pelaia C, Pelaia G, Crimi C, et al. Biological therapy of severe asthma with dupilumab, a dual receptor antagonist of interleukins 4 and 13. Vaccines 2022; 10: 974. doi: 10.3390/vaccines10060974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med 2018; 378: 2486–2496. doi: 10.1056/NEJMoa1804092 [DOI] [PubMed] [Google Scholar]
- 158.Wenzel S, Castro M, Corren J, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet 2016; 388: 31–44. doi: 10.1016/S0140-6736(16)30307-5 [DOI] [PubMed] [Google Scholar]
- 159.Renner A, Marth K, Patocka K, et al. Dupilumab rapidly improves asthma control in predominantly anti-IL5/IL5R pretreated Austrian real-life severe asthmatics. Immun Inflamm Dis 2021; 9: 624–627. doi: 10.1002/iid3.434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.De Prado Gomez L, Castro M, Papi A, et al. Randomized, double-blind, placebo-controlled study to evaluate the effect of dupilumab on airway remodeling in patients with uncontrolled, moderate-to-severe asthma: the VESTIGE study. Chest 2021; 160: Suppl. 1, A1492–A1495. doi: 10.1016/j.chest.2021.07.1367 [DOI] [Google Scholar]
- 161.Austin CD, Gonzalez Edick M, Ferrando RE, et al. A randomized, placebo-controlled trial evaluating effects of lebrikizumab on airway eosinophilic inflammation and remodelling in uncontrolled asthma (CLAVIER). Clin Exp Allergy 2020; 50: 1342–1351. doi: 10.1111/cea.13731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Conde E, Bertrand R, Balbino B, et al. Dual vaccination against IL-4 and IL-13 protects against chronic allergic asthma in mice. Nat Commun 2021; 12: 2574. doi: 10.1038/s41467-021-22834-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.US Food and Drug Administration . TEZSPIRE™ (tezepelumab) prescribing information. 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2021/761224s000lbl.pdf Date last accessed: 1 August 2023.
- 164.Corren J, Parnes JR, Wang L, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med 2017; 377: 936–946. doi: 10.1056/NEJMoa1704064 [DOI] [PubMed] [Google Scholar]
- 165.Menzies-Gow A, Corren J, Bourdin A, et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl J Med 2021; 384: 1800–1809. doi: 10.1056/NEJMoa2034975 [DOI] [PubMed] [Google Scholar]
- 166.Pham T, Cook B, Parnes JR, et al. Tezepelumab reduces biomarkers of airway remodeling, MMP-10 and MMP-3: exploratory results from the phase 3 NAVIGATOR study. Am J Respir Crit Care Med 2022; 205: A2359. doi: 10.1164/ajrccm-conference.2022.205.1_MeetingAbstracts.A2359 [DOI] [Google Scholar]
- 167.Diver S, Khalfaoui L, Emson C, et al. Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate-to-severe uncontrolled asthma (CASCADE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med 2021; 9: 1299–1312. doi: 10.1016/S2213-2600(21)00226-5 [DOI] [PubMed] [Google Scholar]
- 168.Nordenmark LH, Hellqvist Å, Emson C, et al. Tezepelumab and mucus plugs in patients with moderate-to-severe asthma. NEJM Evidence 2023; 2: EVIDoa2300135. doi: 10.1056/EVIDoa2300135 [DOI] [PubMed] [Google Scholar]
- 169.Sverrild A, Hansen S, Hvidtfeldt M, et al. The effect of tezepelumab on airway hyperresponsiveness to mannitol in asthma (UPSTREAM). Eur Respir J 2022; 59: 2101296. doi: 10.1183/13993003.01296-2021 [DOI] [PubMed] [Google Scholar]
- 170.Chen ZG, Zhang TT, Li HT, et al. Neutralization of TSLP inhibits airway remodeling in a murine model of allergic asthma induced by chronic exposure to house dust mite. PLoS One 2013; 8: e51268. doi: 10.1371/journal.pone.0051268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cheng Z, Wang X, Dai LL, et al. Thymic stromal lymphopoietin signaling pathway inhibition attenuates airway inflammation and remodeling in rats with asthma. Cell Physiol Biochem 2018; 47: 1482–1496. doi: 10.1159/000490865 [DOI] [PubMed] [Google Scholar]
- 172.Lin SC, Chou HC, Chen CM, et al. Anti-thymic stromal lymphopoietin antibody suppresses airway remodeling in asthma through reduction of MMP and CTGF. Pediatr Res 2019; 86: 181–187. doi: 10.1038/s41390-018-0239-x [DOI] [PubMed] [Google Scholar]
- 173.Wechsler ME, Ruddy MK, Pavord ID, et al. Efficacy and safety of itepekimab in patients with moderate-to-severe asthma. N Engl J Med 2021; 385: 1656–1668. doi: 10.1056/NEJMoa2024257 [DOI] [PubMed] [Google Scholar]
- 174.Kelsen SG, Agache IO, Soong W, et al. Astegolimab (anti-ST2) efficacy and safety in adults with severe asthma: a randomized clinical trial. J Allergy Clin Immunol 2021; 148: 790–798. doi: 10.1016/j.jaci.2021.03.044 [DOI] [PubMed] [Google Scholar]
- 175.Genentech . Astegolimab. 2024. www.gene.com/medical-professionals/pipeline/astegolimab Date last accessed: 10 January 2024.
- 176.Sanofi . Sanofi delivered close to double-digit Q4 2020 business EPS(1) growth at CER. 2021. www.sanofi.com/assets/dotcom/pressreleases/2021/2021-02-05-06-30-00-2170436-en.pdf Date last accessed: 10 January 2024.
- 177.England E, Rees DG, Scott IC, et al. Tozorakimab (MEDI3506): an anti-IL-33 antibody that inhibits IL-33 signalling via ST2 and RAGE/EGFR to reduce inflammation and epithelial dysfunction. Sci Rep 2023; 13: 9825. doi: 10.1038/s41598-023-36642-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Scott I, Killick H, Newcombe P, et al. Proof of mechanism for anti-interleukin-33 antibody tozorakimab in a phase 1 study in healthy adults and patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2022; 205: A2397. doi: 10.1164/ajrccm-conference.2022.205.1_MeetingAbstracts.A2397 [DOI] [Google Scholar]
- 179.Allinne J, Lim WK, Scott G, et al. IL-33 blockade prevents exacerbations in a model of chronic airway inflammation by blunting persistent inflammation and remodeling. Eur Respir J 2019; 54: Suppl. 63, PA3876. doi: 10.1183/13993003.congress-2019.PA3876 [DOI] [Google Scholar]
- 180.An G, Wang W, Zhang X, et al. Combined blockade of IL-25, IL-33 and TSLP mediates amplified inhibition of airway inflammation and remodelling in a murine model of asthma. Respirology 2020; 25: 603–612. doi: 10.1111/resp.13711 [DOI] [PubMed] [Google Scholar]
- 181.Prakash YS, Halayko AJ, Gosens R, et al. An official American Thoracic Society research statement: current challenges facing research and therapeutic advances in airway remodeling. Am J Respir Crit Care Med 2017; 195: e4–e19. doi: 10.1164/rccm.201611-2248ST [DOI] [PubMed] [Google Scholar]
- 182.Zhang J, Dong L. Status and prospects: personalized treatment and biomarker for airway remodeling in asthma. J Thorac Dis 2020; 12: 6090–6101. doi: 10.21037/jtd-20-1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Porsbjerg C, Maitland-van der Zee AH, Brusselle G, et al. 3TR: a pan-European cross-disease research consortium aimed at improving personalised biological treatment of asthma and COPD. Eur Respir J 2021; 58: 2102168. doi: 10.1183/13993003.02168-2021 [DOI] [PubMed] [Google Scholar]
- 184.Hoshino M, Takahashi M, Aoike N. Expression of vascular endothelial growth factor, basic fibroblast growth factor, and angiogenin immunoreactivity in asthmatic airways and its relationship to angiogenesis. J Allergy Clin Immunol 2001; 107: 295–301. doi: 10.1067/mai.2001.111928 [DOI] [PubMed] [Google Scholar]
- 185.Liu G, Philp AM, Corte T, et al. Therapeutic targets in lung tissue remodelling and fibrosis. Pharmacol Ther 2021; 225: 107839. doi: 10.1016/j.pharmthera.2021.107839 [DOI] [PubMed] [Google Scholar]
- 186.Al Heialy S, Ramakrishnan RK, Hamid Q. Recent advances in the immunopathogenesis of severe asthma. J Allergy Clin Immunol 2022; 149: 455–465. doi: 10.1016/j.jaci.2021.12.765 [DOI] [PubMed] [Google Scholar]
- 187.Poto R, Loffredo S, Marone G, et al. Basophils beyond allergic and parasitic diseases. Front Immunol 2023; 14: 1190034. doi: 10.3389/fimmu.2023.1190034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Nair P, Surette MG, Virchow JC. Neutrophilic asthma: misconception or misnomer? Lancet Respir Med 2021; 9: 441–443. doi: 10.1016/S2213-2600(21)00023-0 [DOI] [PubMed] [Google Scholar]
- 189.Schiffer M, Peters K, Peters M. Comparison of airway remodeling in two different endotypes of allergic asthma. Int Arch Allergy Immunol 2022; 183: 714–725. doi: 10.1159/000522189 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This one-page PDF can be shared freely online.
Shareable PDF ERJ-01619-2023.Shareable (276KB, pdf)