Abstract
The coreceptors used by primary syncytium-inducing (SI) human immunodeficiency virus type 1 isolates for infection of primary macrophages were investigated. SI strains using only CXCR4 replicated equally well in macrophages with or without CCR5 and were inhibited by several different ligands for CXCR4 including SDF-1 and bicyclam derivative AMD3100. SI strains that used a broad range of coreceptors including CCR3, CCR5, CCR8, CXCR4, and BONZO infected CCR5-deficient macrophages about 10-fold less efficiently than CCR5+ macrophages. Moreover, AMD3100 blocked infection of CCR5-negative macrophages by these strains. Our results therefore demonstrate that CXCR4, as well as CCR5, is used for infection of primary macrophages but provide no evidence for the use of alternative coreceptors.
Human immunodeficiency virus type 1 (HIV-1) infects several cell types of the hematopoietic lineage, including CD4+ T cells, macrophages, and dendritic cells. Non-syncytium-inducing (NSI) strains of HIV-1 infect both peripheral blood mononuclear cells (PBMCs) and macrophages derived from blood monocytes but fail to infect most established CD4+ T-cell lines (2). Conversely, the majority of T-cell line-adapted (TCLA) syncytium-inducing (SI) strains, such as the prototype IIIB strain, infect PBMCs and T-cell lines but infect macrophages at best inefficiently (46). The extent to which primary SI isolates infect macrophages is controversial. We and other groups have found that most primary SI strains replicate efficiently in macrophages (derived from blood monocytes) (47, 49), although other reports have indicated a lack of macrophage-tropism for the majority of primary SI strains (12, 29, 45). These discrepancies may be the due to differences in virus isolation, macrophage preparation, or the stage of macrophage maturation. Indeed, macrophages derived from blood, cord blood, or the placenta have distinct sensitivities to infection by different HIV-1 strains (24).
HIV has been shown to require particular seven-transmembrane chemokine receptors in addition to CD4 for entry into cells (9, 20, 35). CCR5, a receptor for the β-chemokines RANTES, MIP-1α, and MIP-1β, is the major coreceptor for NSI strains of HIV-1 (1, 15, 22), while TCLA SI strains have been shown to use CXCR4 (25), the ligand for which is SDF-1 (5, 37). In addition, several other chemokine receptors and orphan receptors can act as efficient coreceptors for some virus strains. These include CCR2b, CCR3, CCR8, BOB (or gpr15), BONZO (also known as STRL33 or TYMSTR), and CX3CR1 (V28) (8, 16, 21, 23, 27, 31, 33, 40).
At present, the relevance of each of the identified HIV-1 coreceptors for replication in vivo is unclear. CCR5 is clearly involved in transmission, since individuals homozygous for a 32-bp deletion in their CCR5 genes (Δ32/Δ32 CCR5 individuals) are substantially protected from HIV-1 infection (14, 32, 42). In vitro, this deletion also prevents the entry of NSI strains into PBMCs and macrophages (12, 38), indicating that CCR5 is the predominant coreceptor for NSI infection of these cell types.
In vivo, HIV-1 coreceptor use is thought to broaden as the disease progresses (13). CXCR4-using SI strains often emerge late in the course of HIV infection, and this emergence may precede a more rapid decline in CD4+ T-cell numbers. Some SI strains, e.g., 89.6 (11) and 2076 (described here), can use multiple coreceptors while others, e.g., 2044 (described here) and TCLA LAI (IIIB), use only CXCR4 among the identified coreceptors. The last two strains are thought to use CXCR4 for infection of PBMCs and dendritic cells since SDF-1 is an efficient inhibitor of infection (3, 18, 37). The coreceptors exploited by SI strains for the infection of macrophages are less clear. We and others have shown previously that CXCR4 is expressed on monocytes/macrophages (17, 34, 36, 50, 51); however, it has not been considered a potential coreceptor on macrophages (17, 38, 51) due to the lack of infection by CXCR4-using TCLA strains. Yet cells expressing envelope glycoproteins derived from CXCR4-using TCLA strains fuse with macrophages derived from blood monocytes (4, 46), indicating that these cells express appropriate coreceptors for membrane fusion.
Coreceptors used by primary dual-tropic SI isolates of HIV-1.
We first assessed the range of coreceptors that were used by a panel of four dual-tropic SI isolates. 2005, 2044, 2028, and 2076 are all low-passage strains of HIV-1 belonging to clade B and have been characterized extensively (47). SL-2 and E80 are clade B NSI HIV-1 strains (47), and SF162 is a molecular clone of an NSI HIV-1 strain (7). HXB2 is a molecular clone of SI, TCLA strain HIV-1 LAI (IIIB) (39). These strains were tested for infection of feline CCC/CD4 cells transiently expressing a panel of known HIV-1 coreceptors. Two SI strains, 2005 and 2044, infect macrophages yet do not utilize CCR5 (47). These strains failed to use any coreceptor except CXCR4 efficiently (Table 1). 2076 and 2028 were capable of using a wider range of coreceptors for entry into cells, including BONZO and CCR8 as well as CCR3, CCR5, and CXCR4 (Table 1). Efficient infection of CCR5+ cells by the NSI strains SF162 and SL-2 was observed, although some infection of SF162 via BONZO and of SL-2 via BOB was also noted.
TABLE 1.
Coreceptor use by SI and NSI HIV-1 strainsa
| HIV-1 strain | Phenotype | Infectivity (FFU/ml) with coreceptorb:
|
||||||
|---|---|---|---|---|---|---|---|---|
| Mockc | CCR3 | CCR5 | CCR8 | CXCR4 | BOB | BONZO | ||
| 2005 | SI | 20 | —d | — | — | 4.5 × 104 | — | 20 |
| 2044 | SI | — | — | — | — | 8.2 × 103 | — | — |
| 2028 | SI | — | 90 | 1.7 × 102 | 30 | 1.9 × 103 | 20 | 30 |
| 2076 | SI | — | 3.8 × 102 | 1.7 × 103 | 2.0 × 102 | 3.5 × 103 | 30 | 2.4 × 102 |
| SF162 | NSI | — | — | 9.5 × 104 | 30 | 20 | — | 2.1 × 102 |
| SL-2 | NSI | — | — | 7.4 × 103 | — | — | 7.0 × 102 | — |
| HXB2 | SI | — | — | — | — | 5.0 × 103 | — | — |
CCC/CD4 cells, a cat kidney cell line expressing human CD4, resist entry by HIV-1. CCC/CD4 cells were transfected with expression vectors encoding each coreceptor as previously described (47). After 24 h, transfected CCC/CD4 cells were seeded into 48-well trays (Costar) at 6 × 104 cells per well. After 24 h, the cells were challenged with 100 μl of serial dilutions of virus for 3 h at 37°C. After 4 days, the cells were fixed and immunostained as previously described (10). The numbers of positively stained foci were estimated by light microscopy, and the numbers of focus-forming units (FFU) per milliliter were calculated.
All virus strains were negative for infectivity on CCC/CD4 cells expressing coreceptors CCR1, CCR2b, CCR4, CX3CR1, and gpr1.
Mock-transfected CCC/CD4 cells.
—, <20 FFU/ml.
Connor et al. (13) suggested that in HIV-1-infected individuals coreceptor use broadens as the disease progresses. Two of the SI strains described here were isolated from symptomatic individuals and used five of the eight reported coreceptors. Yet we also show that some SI viruses also isolated from symptomatic individuals only use CXCR4. We do not know whether such strains result from a direct switch from CCR5-using viruses or develop from strains with a broad coreceptor usage.
Infection of macrophages derived from homozygous Δ32/Δ32 CCR5 individuals.
We first investigated the role of CCR5 for macrophage infection by primary SI viruses. Macrophages were isolated from blood monocytes by plastic adherence as previously described (46). Leukocytes prepared from buffy coats were added to bacterial petri dishes containing 5% human serum (HuS) in RPMI 1640 (Gibco). After 2 h, nonadherent cells were washed off, and the remaining cells were incubated overnight in 10% HuS in RPMI 1640 (MΦ medium). Plates were again vigorously washed. The adherent cells were allowed to differentiate into macrophages in MΦ medium. After 2 days, fluorescence-activated cell sorter analysis indicated that >98% of the cells expressed monocyte/macrophage marker CD11c, as well as high levels of monocyte marker CD13 (data not shown). Seven-day-old macrophages were also positive for CD14 and had high levels of CD71 (the transferrin receptor), which is not expressed on fresh monocytes (30), while CD13 (aminopeptidase N) was significantly downregulated. These observations are consistent with monocyte differentiation into macrophages. T-cell and B-cell markers were consistently undetectable (data not shown). At the time of infection, the macrophages expressed CD4, a low level of CXCR4, and a higher level of CCR5 but no detectable CCR3. By reverse transcriptase PCR (RT-PCR) we detected mRNA for CCR5, CXCR4, BONZO, and CCR3 (weakly) but not mRNA for BOB or CCR8 (data not shown). Previously we have reported detailed analyses of the susceptibilities of such macrophage preparations to a wide range of HIV-1 strains of different phenotypes (46, 47).
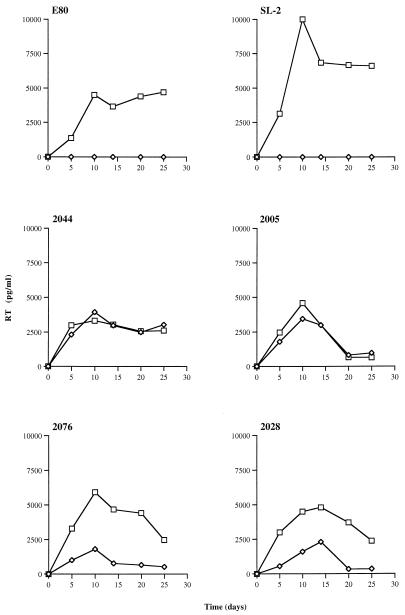
It was conceivable that the CXCR4-only strains, 2005 and 2044, were capable of infecting via CCR5 on primary cells but not on transient or stable cell lines used to assess coreceptor use (Table 1). To address this question, macrophages were prepared from donors homozygous for the 32-bp deletion in CCR5, as determined by PCR. Seven-day-old macrophages were infected with a panel of SI and NSI strains, and RT activity in the cell culture supernatant was monitored after HIV exposure (Fig. 1). Two NSI strains, SL-2 and E80, replicated to high levels in macrophages expressing wild-type CCR5 but failed to infect Δ32/Δ32 CCR5 macrophages. SI strains that use CXCR4 only (2044 and 2005; Table 1) replicated to equal levels in macrophages that lacked CCR5, while SI viruses capable of using both CCR5 and CXCR4 and other coreceptors showed a reduced tropism for macrophages from homozygous Δ32/Δ32 CCR5 donors. For the latter strains, end point infectivity titers were 10- to 20-fold lower on CCR5-deficient macrophages than on macrophages expressing CCR5, indicating that they predominantly used CCR5.
FIG. 1.
Infection of macrophages from wild-type and homozygous Δ32/Δ32 CCR5 donors by primary HIV-1 isolates. Six-day-old macrophages, plated overnight in 48-well trays (Costar) at 105 cells per well, were exposed to 100 μl of virus for 3 h at 37°C followed by extensive washing. All viruses were added at 1.5 × 105 focus-forming units (FFU)/ml except 2028 and 2005, which were added at 5.0 × 104 FFU/ml. Infection was assessed by measuring supernatant RT activity by enzyme-linked immunosorbent assay (Cavidi Tech, Uppsala, Sweden). Squares, wild-type CCR5 macrophages; diamonds, homozygous Δ32/Δ32 CCR5 macrophages. These results are representative of two experiments.
Inhibition of macrophage infectivity by ligands specific for CXCR4.
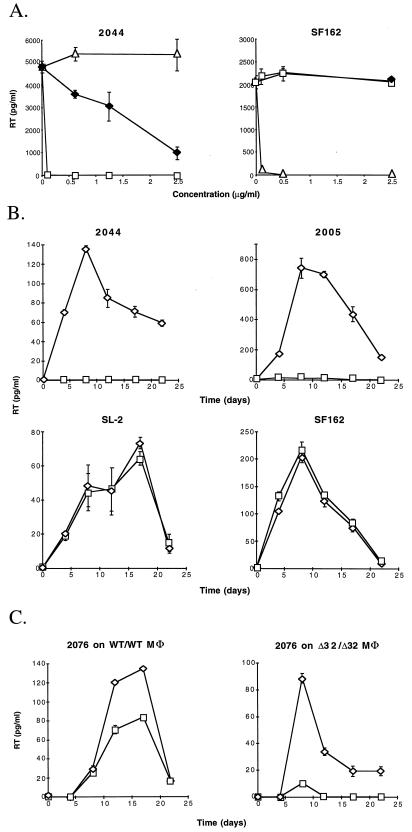
To investigate whether CXCR4 is the major coreceptor used by 2005 and 2044 to infect macrophages, we tested if CXCR4 ligands could inhibit infection. SDF-1, the natural ligand for CXCR4, blocks infection of CXCR4+ cells by TCLA HIV-1 strains (5, 37). Bicyclam derivative AMD3100 has also been shown to inhibit HIV-1 infection specifically through CXCR4 (19, 43). Figure 2A shows that SDF-1 but not AOP-RANTES (a potent inhibitor of infection through CCR5) inhibited 2044, although relatively inefficiently, giving a reduction of greater than 75% at 2,500 ng/ml (Fig. 2A). In comparison to SDF-1, AMD3100 was much more efficient at inhibiting replication by 2044, as assessed by determining the RT activity in the supernatant. Almost total reduction at 100 ng/ml was noted (Fig. 2A). As expected, SF162 (R5) replication in macrophages was inhibited by AOP-RANTES but not by AMD3100 (Fig. 2A). In a time course experiment (Fig. 2B), AMD3100 was used at a single dose (100 ng/ml), and this concentration was replenished every 3 days. Figure 2B shows that in this situation macrophage infection by both 2044 and 2005 was greatly inhibited. Again, AMD3100 had no effect on SF162 or SL-2 replication. These results prove conclusively that SI viruses 2044 and 2005 used CXCR4 predominantly for macrophage infection. In addition, monoclonal antibodies (MAbs) specific for CXCR4 also showed inhibition of 2044, but not SF162, infection of macrophages (data not shown). Three MAbs, 23, 24, and 27 (a kind gift from R&D Systems), specific for CXCR4 all reduced 2044 infection by over 95% at 10 μg/ml, while CCR5-specific MAb 33 (R&D Systems) had no effect, even at 25 μg/ml.
FIG. 2.
(A) Inhibition of 2044 and SF162 by increasing concentrations of chemokine receptor ligands. Wild-type CCR5 macrophages were prepared as described for Fig. 1. A recombinant form of SDF-1 (Met-SDF-1) with its N-terminal methionine retained was used throughout. One hundred microliters of medium, chemokine, or AMD3100 was added at double the required final concentration, and the mixture was incubated at 37°C for 30 min. Then, 100 μl of virus at 1.5 × 105 focus-forming units (FFU)/ml was added. After 3 h at 37°C, the cells were washed four times and fresh medium containing the relevant inhibitor at the required concentration was added. RT levels were determined at the peak of infection, on day 11. Squares, AMD3100; filled diamonds, SDF-1; triangles, AOP-RANTES. (B) Time course of inhibition of primary HIV-1 strains by AMD3100. A total of 2 × 103 FFU of 2044, 2005, SF162, and SL-2 per ml was used to infect wild-type CCR5 macrophages in the presence (squares) or absence (diamonds) of 100 ng of AMD3100 per ml. RT activity was measured every 3 to 5 days, and the medium was replaced. Squares, AMD3100; diamonds, medium alone. (C) Inhibition of multi-coreceptor-using strain 2076 by AMD3100. A total of 2 × 103 FFU of 2076 per ml was used to infect both wild-type and homozygous Δ32/Δ32 CCR5 macrophages. Squares, AMD3100; diamonds, medium alone. All results presented in the three panels are representative of three independent experiments.
We next tested the effect of AMD3100 on macrophage infection by 2076, which can use a broad range of coreceptors. Inhibition was tested on both Δ32/Δ32 CCR5 and wild-type CCR5 (WT/WT CCR5) macrophages. Figure 2C shows that on WT/WT CCR5 macrophages, less than 40% inhibition of RT production was observed in the presence of AMD31000. In contrast, nearly complete inhibition on Δ32/Δ32 CCR5 macrophages was observed. Thus, although 2076 can utilize a broad range of coreceptors, infection of macrophages is dependent predominantly on CCR5 and CXCR4.
Although at least eight seven-transmembrane coreceptors have been identified, in vivo only CCR5 and CXCR4 have so far been linked to transmission (14, 32, 42) and/or pathogenesis (28). CXCR4 is important in pathogenesis in some patients, as emergence of CXCR4-using SI viruses precedes a more rapid decline in CD4+ T cells in over one-half of the individuals progressing to AIDS (48). Since CXCR4 is expressed on distinct cell populations compared to CCR5 (6), it is possible that emerging SI strains target a subset of T cells crucial for CD4+ T-cell homeostasis. Whether other coreceptors play roles in in vivo infection or are linked to colonization of particular tissues or organs or to a particular disease state is currently not clear.
For infection of primary CD4+ cell cultures in vitro, only three (CCR5, CCR3, and CXCR4) of the eight coreceptors described have so far been implicated. CCR5 is expressed on CD45RO+ CD45RA− memory T cells, macrophages, dendritic cells, Langerhans cells, and probably microglial cells in the brain. All these cell types are targets for NSI, CCR5-using strains in vivo and can be infected by them in vitro. Moreover, lymphocytes and macrophages derived from Δ32/Δ32 CCR5 individuals are resistant to infection by CCR5-using strains (12, 38). These observations provide powerful evidence that CCR5 is an active and crucial coreceptor for HIV-1 replication in vivo.
A role for CCR3 in infection of microglia cells was suggested by He et al. (26), who demonstrated that infection with NSI strains was blocked by both CCR3 and CCR5 ligands, eotaxin and MIP-1β, respectively. CCR3 is also expressed on T helper 2 T lymphocytes (41); however, its role in their infection is not yet defined. CXCR4 on cultured CD4+ T cells and on dendritic and Langerhans cells is used by primary and TCLA SI strains that predominantly use this coreceptor (3, 18). Although CCR5 is a major coreceptor for infection of macrophages, the role of other coreceptors is less clear. Macrophages cultured in vitro, like other primary cell types, may express several potential coreceptors, and it is therefore difficult to assess the relative contribution of each coreceptor for infection. Homozygous Δ32/Δ32 CCR5 individuals are an excellent source of CCR5-deficient macrophages; however, the effects of this CCR5 “knockout” on the expression of other coreceptors has not yet been addressed. Blocking infection with ligands specific for particular coreceptors provides good evidence of their involvement in virus entry, yet the possibility of indirect mechanisms affecting the function of other coreceptors cannot be ruled out completely. In our studies we showed that three completely different ligands for CXCR4 inhibited macrophage infection by CXCR4-using viruses. Moreover, one of these, AMD3100, fails to signal via CXCR4 and is thus unlikely to modulate the cell surface expression of other coreceptors (44). Our results therefore confirm and extend those of Yi et al., who reported that a single multi-coreceptor-using strain of HIV-1 (89.6) can use CXCR4 on Δ32/Δ32 CCR5 macrophages (50).
There is much current effort to develop therapeutic reagents targeted to coreceptors. CCR5 seems an excellent candidate to target, since individuals homozygous for a 32-bp deletion are usually healthy. However, if HIV strains readily use alternative coreceptors in vivo, then therapeutics aimed at CCR5 will be ineffective. At least for primary macrophages prepared from blood monocytes, our study shows that CCR5 and CXCR4 are the predominant coreceptors used for infection by both NSI and SI strains of HIV-1. No evidence was found for the use of alternative coreceptors for HIV-1 entry.
Acknowledgments
We thank Monica Tsang at R&D Systems for generously providing CCR5 and CXCR4 monoclonal antibodies and Robin Weiss for continued support, advice, and critical comments on the manuscript. We also thank Ian Titley for help with FACS analysis.
Our work is supported by an MRC program grant and partly by an EC Biomed II grant.
REFERENCES
- 1.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 2.Asjo B, Morfeldt Manson L, Albert J, Biberfeld G, Karlsson A, Lidman K, Fenyo E M. Replicative capacity of human immunodeficiency virus from patients with varying severity of HIV infection. Lancet. 1986;ii:660–662. [PubMed] [Google Scholar]
- 3.Ayehunie S, Garcia Zepeda E A, Hoxie J A, Horuk R, Kupper T S, Luster A D, Ruprecht R M. Human immunodeficiency virus-1 entry into purified blood dendritic cells through CC and CXC chemokine coreceptors. Blood. 1997;90:1379–1386. [PubMed] [Google Scholar]
- 4.Bazan M, Alkhatib G, Broder C C, Berger E A. Patterns of CCR5, CXCR4, and CCR3 usage by envelope glycoproteins from human immunodeficiency virus type 1 primary isolates. J Virol. 1998;72:4485–4491. doi: 10.1128/jvi.72.5.4485-4491.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bleul C C, Farzan M, Choe H, Parolin C, Clark Lewis I, Sodroski J, Springer T A. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 6.Bleul C C, Wu L, Hoxie J A, Springer T A, Mackay C R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng Mayer C, Weiss C, Seto D, Levy J A. Isolates of human immunodeficiency virus type 1 from the brain may constitute a special group of the AIDS virus. Proc Natl Acad Sci USA. 1989;86:8575–8579. doi: 10.1073/pnas.86.21.8575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 9.Clapham P R. HIV and chemokines: ligands sharing cell-surface receptors. Trends Cell Biol. 1997;7:264–268. doi: 10.1016/S0962-8924(97)01075-1. [DOI] [PubMed] [Google Scholar]
- 10.Clapham P R, Blanc D, Weiss R A. Specific cell surface requirements for the infection of CD4-positive cells by human immunodeficiency virus types 1 and 2 and by simian immunodeficiency virus. Virology. 1991;181:703–715. doi: 10.1016/0042-6822(91)90904-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collman R, Balliet J W, Gregory S A, Friedman H, Kolson D L, Nathanson N, Srinivasan A. An infectious molecular clone of an unusual macrophage-tropic and highly cytopathic strain of human immunodeficiency virus type 1. J Virol. 1992;66:7517–7521. doi: 10.1128/jvi.66.12.7517-7521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connor R I, Paxton W A, Sheridan K E, Koup R A. Macrophages and CD4+ T lymphocytes from two multiply exposed, uninfected individuals resist infection with primary non-syncytium-inducing isolates of human immunodeficiency virus type 1. J Virol. 1996;70:8758–8764. doi: 10.1128/jvi.70.12.8758-8764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dean M, Carrington M, Winkler C, Huttley G A, Smith M W, Allikmets R, Goedert J J, Buchbinder S P, Vittinghoff E, Gomperts E, Donfield S, Vlahov D, Kaslow R, Saah A, Rinaldo C, Detels R, O’Brien S J. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 15.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 16.Deng H K, Unutmaz D, KewalRamani V N, Littman D R. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 17.Di Marzio P, Tse J, Landau N R. Chemokine receptor regulation and HIV type 1 tropism in monocyte-macrophages. AIDS Res Hum Retroviruses. 1998;14:129–138. doi: 10.1089/aid.1998.14.129. [DOI] [PubMed] [Google Scholar]
- 18.Dittmar M T, Simmons G, Hibbitts S, O’Hare M, Louisirirotchanakul S, Beddows S, Weber J, Clapham P R, Weiss R A. Langerhans cell tropism of human immunodeficiency virus type 1 subtype A through F isolates derived from different transmission groups. J Virol. 1997;71:8008–8013. doi: 10.1128/jvi.71.10.8008-8013.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donzella G A, Schols D, Lin S W, Este J A, Nagashima K A, Maddon P J, Allaway G P, Sakmar T P, Henson G, De Clercq E, Moore J P. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat Med. 1998;4:72–77. doi: 10.1038/nm0198-072. [DOI] [PubMed] [Google Scholar]
- 20.Doranz B J, Berson J F, Rucker J, Doms R W. Chemokine receptors as fusion cofactors for human immunodeficiency virus type 1 (HIV-1) Immunol Res. 1997;16:15–28. doi: 10.1007/BF02786321. [DOI] [PubMed] [Google Scholar]
- 21.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 22.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 23.Farzan M, Choe H, Martin K, Marcon L, Hofmann W, Karlsson G, Sun Y, Barrett P, Marchand N, Sullivan N, Gerard N, Gerard C, Sodroski J. Two orphan seven-transmembrane segment receptors which are expressed in CD4-positive cells support simian immunodeficiency virus infection. J Exp Med. 1997;186:405–411. doi: 10.1084/jem.186.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fear W R, Kesson A M, Naif H, Lynch G W, Cunningham A L. Differential tropism and chemokine receptor expression of human immunodeficiency virus type 1 in neonatal monocytes, monocyte-derived macrophages, and placental macrophages. J Virol. 1998;72:1334–1344. doi: 10.1128/jvi.72.2.1334-1344.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 26.He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay C R, Sodroski J, Gabuzda D. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 27.Horuk R, Hesselgesser R, Zhou Y, Faulds D, Halks-Miller M, Harvey S, Taub D, Samson M, Parmentier M, Rucker J, Doranz B J, Doms R W. The CC chemokine I-309 inhibits CCR8-dependent infection by diverse HIV-1 strains. J Biol Chem. 1998;273:386–391. doi: 10.1074/jbc.273.1.386. [DOI] [PubMed] [Google Scholar]
- 28.Huang Y, Paxton W A, Wolinsky S M, Neumann A U, Zhang L, He T, Kang S, Ceradini D, Jin Z, Yazdanbakhsh K, Kunstman K, Erickson D, Dragon E, Landau N R, Phair J, Ho D D, Koup R A. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med. 1996;2:1240–1243. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- 29.Karita E, Nkengasong J N, Willems B, Vanham G, Fransen K, Heyndrickx L, Janssens W, Piot P, van der Groen G. Macrophage-tropism of HIV-1 isolates of different genetic subtypes. AIDS. 1997;11:1303–1304. doi: 10.1097/00002030-199710001-00010. [DOI] [PubMed] [Google Scholar]
- 30.Kreutz M, Andreesen R, Krause S W, Szabo A, Ritz E, Reichel H. 1,25-Dihydroxyvitamin D3 production and vitamin D3 receptor expression are developmentally regulated during differentiation of human monocytes into macrophages. Blood. 1993;82:1300–1307. [PubMed] [Google Scholar]
- 31.Liao F, Alkhatib G, Peden K W, Sharma G, Berger E A, Farber J M. STRL33, a novel chemokine receptor-like protein, functions as a fusion cofactor for both macrophage-tropic and T cell line-tropic HIV-1. J Exp Med. 1997;185:2015–2023. doi: 10.1084/jem.185.11.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu R, Paxton W A, Choe S, Ceradini D, Martin S R, Horuk R, MacDonald M E, Stuhlmann H, Koup R A, Landau N R. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 33.Loetscher M, Amara A, Oberlin E, Brass E, Legler D F, Loetscher P, D’Apuzzo M, Meese E, Rousset D, Virelizier J-L, Baggiolini M, Arenzana-Seisdedos F, Moser B. TYMSTR, a putative chemokine receptor selectively expressed in activated T cells, exhibits HIV-1 coreceptor function. Curr Biol. 1997;7:652–660. doi: 10.1016/s0960-9822(06)00292-2. [DOI] [PubMed] [Google Scholar]
- 34.McKnight A, Wilkinson D, Simmons G, Talbot S, Picard L, Ahuja M, Marsh M, Hoxie J A, Clapham P R. Inhibition of human immunodeficiency virus fusion by a monoclonal antibody to a coreceptor (CXCR4) is both cell type and virus strain dependent. J Virol. 1997;71:1692–1696. doi: 10.1128/jvi.71.2.1692-1696.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore J P, Trkola A, Dragic T. Co-receptors for HIV-1 entry. Curr Opin Immunol. 1997;9:551–562. doi: 10.1016/s0952-7915(97)80110-0. [DOI] [PubMed] [Google Scholar]
- 36.Naif H M, Li S, Alali M, Sloane A, Wu L, Kelly M, Lynch G, Lloyd A, Cunningham A L. CCR5 expression correlates with susceptibility of maturing monocytes to human immunodeficiency virus type 1 infection. J Virol. 1998;72:830–836. doi: 10.1128/jvi.72.1.830-836.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier J L, Arenzana Seisdedos F, Schwartz O, Heard J M, Clark Lewis I, Legler D F, Loetscher M, Baggiolini M, Moser B. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 38.Rana S, Besson G, Cook D G, Rucker J, Smyth R J, Yi Y, Turner J D, Guo H-H, Du J-G, Peiper S C, Lavi E, Samson M, Libert F, Liesnard C, Vassart G, Doms R W, Parmentier M, Collman R G. Role of CCR5 in infection of primary macrophages and lymphocytes by macrophage-tropic strains of human immunodeficiency virus: resistance to patient-derived and prototype isolates resulting from the Δccr5 mutation. J Virol. 1997;71:3219–3227. doi: 10.1128/jvi.71.4.3219-3227.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ratner L, Fisher A, Jagodzinski L L, Mitsuya H, Liou R S, Gallo R C, Wong Staal F. Complete nucleotide sequences of functional clones of the AIDS virus. AIDS Res Hum Retroviruses. 1987;3:57–69. doi: 10.1089/aid.1987.3.57. [DOI] [PubMed] [Google Scholar]
- 40.Reeves J D, McKnight A, Potempa S, Simmons G, Gray P W, Power C A, Wells T, Weiss R A, Talbot S J. CD4-independent infection by HIV-2 (ROD/B): use of the 7-transmembrane receptors CXCR-4, CCR-3, and V28 for entry. Virology. 1997;231:130–134. doi: 10.1006/viro.1997.8508. [DOI] [PubMed] [Google Scholar]
- 41.Sallusto F, Mackay C R, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–2007. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- 42.Samson M, Libert F, Doranz B J, Rucker J, Liesnard C, Farber C M, Saragosti S, Lapoumeroulie C, Cognaux J, Forceille C, Muyldermans G, Verhofstede C, Burtonboy G, Georges M, Imai T, Rana S, Yi Y, Smyth R J, Collman R G, Doms R W, Vassart G, Parmentier M. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 43.Schols D, Este J A, Henson G, De Clercq E. Bicyclams, a class of potent anti-HIV agents, are targeted at the HIV coreceptor fusin/CXCR-4. Antivir Res. 1997;35:147–156. doi: 10.1016/s0166-3542(97)00025-9. [DOI] [PubMed] [Google Scholar]
- 44.Schols D, Struyf S, Van Damme J, Este J A, Henson G, De Clercq E. Inhibition of T-tropic HIV strains by selective antagonization of the chemokine receptor CXCR4. J Exp Med. 1997;186:1383–1388. doi: 10.1084/jem.186.8.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schuitemaker H, Kootstra N A, de Goede R E, de Wolf F, Miedema F, Tersmette M. Monocytotropic human immunodeficiency virus type 1 (HIV-1) variants detectable in all stages of HIV-1 infection lack T-cell line tropism and syncytium-inducing ability in primary T-cell culture. J Virol. 1991;65:356–363. doi: 10.1128/jvi.65.1.356-363.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simmons G, McKnight A, Takeuchi Y, Hoshino H, Clapham P R. Cell-to-cell fusion, but not virus entry in macrophages by T-cell line tropic HIV-1 strains: a V3 loop-determined restriction. Virology. 1995;209:696–700. doi: 10.1006/viro.1995.1307. [DOI] [PubMed] [Google Scholar]
- 47.Simmons G, Wilkinson D, Reeves J D, Dittmar M T, Beddows S, Weber J, Carnegie G, Desselberger U, Gray P W, Weiss R A, Clapham P R. Primary, syncytium-inducing human immunodeficiency virus type 1 isolates are dual-tropic and most can use either Lestr or CCR5 as coreceptors for virus entry. J Virol. 1996;70:8355–8360. doi: 10.1128/jvi.70.12.8355-8360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tersmette M, de Goede R E, Al B J, Winkel I N, Gruters R A, Cuypers H T, Huisman H G, Miedema F. Differential syncytium-inducing capacity of human immunodeficiency virus isolates: frequent detection of syncytium-inducing isolates in patients with acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. J Virol. 1988;62:2026–2032. doi: 10.1128/jvi.62.6.2026-2032.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valentin A, Albert J, Fenyo E M, Asjo B. Dual tropism for macrophages and lymphocytes is a common feature of primary human immunodeficiency virus type 1 and 2 isolates. J Virol. 1994;68:6684–6689. doi: 10.1128/jvi.68.10.6684-6689.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yi Y, Rana S, Turner J D, Gaddis N, Collman R G. CXCR-4 is expressed by primary macrophages and supports CCR5-independent infection by dual-tropic but not T-tropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:772–777. doi: 10.1128/jvi.72.1.772-777.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zaitseva M, Blauvelt A, Lee S, Lapham C K, Klaus-Kovtun V, Mostowski H, Manischewitz J, Golding H. Expression and function of CCR5 and CXCR4 on human Langerhans cells and macrophages: implications for HIV primary infection. Nat Med. 1997;3:1369–1375. doi: 10.1038/nm1297-1369. [DOI] [PubMed] [Google Scholar]