Abstract
Progesterone suppresses several ancient pathways in a concentration-dependent manner. Based on these characteristics, progesterone is considered a candidate anticancer drug. However, the concentration of progesterone used for therapy should be higher than the physiological concentration, which makes it difficult to develop progesterone-based anticancer drugs. We previously developed liposome-encapsulated progesterone (Lipo-P4) with enhanced anticancer effects, which strongly suppressed triple-negative breast cancer cell proliferation in humanized mice. In this study, we aimed to clarify whether Lipo-P4 effectively suppresses the proliferation of B-lineage cancer cells. We selected six B-cell lymphoma and two myeloma cell lines, and analyzed their surface markers using flow cytometry. Next, we prepared liposome-encapsulated progesterone and examined its effect on cell proliferation in these B-lineage cancer cells, three ovarian clear cell carcinoma cell lines, two prostate carcinoma cell lines, and one triple-negative breast cancer adenocarcinoma cell line. Lipo-P4 suppressed the proliferation of all cancer cell lines. All B-lineage cell lines, except for the HT line, were more susceptible than the other cell types, regardless of the expression of differentiation markers. Empty liposomes did not suppress cell proliferation. These results suggest that progesterone encapsulated in liposomes efficiently inhibits the proliferation of B-lineage cells and may become an anticancer drug candidate for B-lineage cancers.
Keywords: Progesterone, Liposome, B cell lymphoma, Myeloma
Highlights
-
•
Progesterone (P4) suppresses cancer cell proliferation dose-dependently.
-
•
This effect is stronger with liposome-encapsulated P4 (Lipo-P4).
-
•
B-lineage cells are highly susceptible to Lipo-P4.
-
•
Lipo-P4 is a good candidate drug targeting B-lineage cancer cells.
1. Introduction
Progesterone (P4) and progestins, which are analogs of P4, play crucial roles in immune regulation during pregnancy [1]. They suppress the T cell response [2], NK cell survival [3], and dendritic cell function [4] in a concentration-dependent manner. In contrast, progestins regulate proliferative signals common in cancer cells and have been considered anti-cancer drug candidates [5,6]. Progestins are useful in the treatment of endometrial and cervical cancer [7]. However, for other cancer types, such as breast cancer, issues remain in inducing significant output.
The relationship between P4 and other steroid hormones, including estrogen and glucocorticoids, has been reported [6,[8], [9], [10]]. Previously, we reported that high-dose P4 treatment for 6 h transiently suppressed T-cell proliferation; however, transient exposure did not affect T-cell survival or protect T cells from exhaustion in the presence of glucocorticoids in humanized mice [11]. Moreover, this treatment enhanced cytotoxic T-cell engraftment in vivo. However, to obtain an efficient outcome, the dose of P4 must be higher than the physiological concentration. In some studies, the effect of P4 has been reported to depend on its concentration and receptor expression [12]. To deliver a higher amount of P4 to cancer cells in vivo, we used a liposome-encapsulated form (Lipo-P4) and developed an anticancer drug candidate, Lipo-P4-aPDL1, which is a Lipo-P4 conjugated with atezolizumab [13].
To evaluate human immune conditions reconstructed under the treatment of cancer drugs, experimental animals, such as immune humanized mice, are necessary [14]. Therefore, several types of immunodeficient mice, and immunohumanized mice based on these immunodeficient mice, have been developed [[15], [16], [17], [18]]. However, the reconstruction of the individual immune environment is difficult because peripheral blood mononuclear cells (PBMCs) should be used for the reconstruction of human immunity in mice; however, PBMCs induce graft versus host disease when they are transplanted into severely immunodeficient mice. Therefore, we developed a new tumor-bearing humanized mouse system constructed by transplanting human PBMCs into a tumor-bearing NOG-hIL-4-Tg mouse system (PBL-NOG-hIL-4-Tg) to evaluate the effects of human immune drugs [13]. In the humanized NOG-hIL-4-Tg mouse system, human T and B cells were engrafted efficiently, whereas other PBL-humanized mice could not maintain human B cells [14]. The immunity of the mice did not induce graft versus host disease, but lymphocytes remained functional, and the engrafted T cells secreted both Th1 and Th2 cytokines after T-cell receptor stimulation. B cells secrete antigen-specific antibodies via immunization [19]. Lipo-P4-aPDL1 effectively suppressed the growth of the triple-negative breast cancer cell line MDA-MB231, which expresses PD-L1 when transplanted into PBL-NOG-hIL-4-Tg cells [13]. Moreover, we found that the proportion of human immune B cells engrafted in mice was significantly decreased, suggesting that Lipo-P4-aPDL1 may efficiently suppress the survival of the B-cell lineage. The anticancer effect of P4 on B-lineage cancer cells has not yet been reported and is worth examining.
Therefore, in this study, we aimed to clarify whether Lipo-P4 suppresses the growth of B-lineage and other cancer cell lines at physiological concentrations.
2. Materials and methods
Ethical approval
The experiments involving humanized mice were conducted in accordance with the guidelines of the Declaration of Helsinki and the Japanese federal regulations required for the privacy protection of human participants. The study protocol was approved by the Tokai University (12R-002/20R211/21R277) and Central Institute for Experimental Animals (08-01) Human Research Committees. Written informed consent was obtained from all participants. All animal procedures were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals and approved by the Animal Care Committees of Tokai University School of Medicine (185016/191073/202049/213047/224039) and the Central Institute for Experimental Animals (20045). The study was conducted in compliance with the ARRIVE guidelines. As we created a healthy donor immune environment in multiple mice, the experimental design did not require blinding.
2.1. Preparation of Lipo-P4
Lipo-P4 and empty control liposomes (Lipo-Emps) were prepared as described previously [13]. Details are provided in the Supplemental Materials.
2.2. Humanized mouse
NOG-hIL-4-Tg (NOD.Cg-PrkdcscidIl2rgtm1Sug/Tg(CMV-IL4)/Jic) mice were maintained under specific pathogen-free conditions at the animal facilities of the Tokai University School of Medicine and the Central Institute for Experimental Animals. Offspring expressing human IL-4 were identified as previously described [19]. DNA was extracted from ear tissue collected at the time of genotyping. Human IL-4 protein levels were measured using a Human IL-4 ELISA Set BD OptEIA Kit (BD Biosciences, Franklin Lakes, NJ, USA). Human PBMCs (5 × 106 cells) were intravenously injected into 8-week-old NOG-hIL-4-Tg mice (hu-PBL NOG-IL-4-Tg mice). Female mice were used, while previous studies with hu-PBL NOG IL-4-Tg mice showed no influence of sex with respect to the lymphocyte engraftment [13,19]. On the same day, Lipo-anti-PD-L1-P4 was administered intraperitoneally, and this treatment continued every five days, for a total of six times. As a negative control, an equal volume of PBS was injected into hu-PBL NOG-hIL-4-Tg mice. The mice were sacrificed 28 days after PBMC transplantation, and the engrafted lymphocytes were analyzed by flow cytometry.
2.3. Cell lines
The cells used in this study are listed in Table 1, along with the culture conditions. Fetal calf serum was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Table 1.
Cell lines and conditions.
| Type | Subtype | Strain | Morphology |
Disease | Culture Medium | Number of Seeded Cells | Day of Culture | Provider |
|---|---|---|---|---|---|---|---|---|
| Tissue | ||||||||
| Leukemia | B-lymphoma | HT | B-lymphoblast | Lymphoma | RPMI1640 10%FCS | 1 × 10⁴/48 well plate | 3 | American Type Collection Center (ATCC) (CRL-2260) |
| OCI-LY3 | B-lymphoblast | Diffuse Large Cell Lymphoma (Non-Hodgkin's B cell) | RPMI1640 10%FCS | 1 × 10⁴/48 well plate | 3 | DSMZ (ACC761) | ||
| OCI-LY10 | B-lymphoblast | Diffuse Large Cell Lymphoma (Non-Hodgkin's B cell) | RPMI1640 10%FCS | 1 × 10⁴/48 well plate | 3 | kindly provided by Dr. Kawada (the Tokai University School of Medicine) | ||
| RI-1 | B-lymphoblast | B cell lymphoma (Non-Hodgkin's B cell) (ABC-like) | RPMI1640 10%FCS | 1 × 10⁴/48 well plate | 3 | DSMZ (ACC585) | ||
| Raji | B-lymphoblast | Burkitt's Lymphoma | RPMI1640 10%FCS | 1 × 10⁴/48 well plate | 3 | American Type Collection Center (ATCC) (CCL-86) | ||
| Toledo | B-lymphoblast | Diffuse Large Cell Lymphoma (Non-Hodgkin's B cell) | RPMI1640 10%FCS | 1 × 10⁴/48 well plate | 9 | American Type Collection Center (ATCC) (CRL-2631) | ||
| Myeloma | RPMI8226 | B-lymphoblast | Myeloma | RPMI1640 10%FCS | 1 × 10⁴/48 well plate | 3 | the Japanese Collection of Research Bioresources cell bank (Osaka, Japan) | |
| U266 | B-lymphoblast | Myeloma | RPMI1640 10%FCS | 1 × 10⁴/48 well plate | 5 | kindly provided by Dr. Shinsuke Iida (Nagoya City University, Aichi, Japan) | ||
| T lymphoma | Jurkat | T-lymphoblast | Acute T cell leukemia | RPMI1640 10%FCS | 1 × 10⁴/48 well plate | 3 | American Type Collection Center (ATCC) (TIB-152) | |
| Adeno-carcinoma | Ovarian Clear Cell Carcinoma | ES-2 | Ovary | Clear Cell Carcinoma | McCOy’s5A 10%FCS | 1 × 10⁴/48 well plate | 5 | kindly provided by Dr. Mikio Mikami (the Tokai University School of Medicine) |
| HUOCAⅡ | Ovary | Clear Cell Carcinoma | HAM’S F12 10%FCS | 1 × 10⁴/48 well plate | 5 | kindly provided by Dr. Mikio Mikami (the Tokai University School of Medicine) | ||
| RMG-1 | Ovary | Clear Cell Carcinoma | HAM’S F12 10%FCS | 1 × 10⁴/48 well plate | 3 | kindly provided by Dr. Mikio Mikami (the Tokai University School of Medicine) | ||
| Prostate Carcinoma | LNCap | Prostate | Carcinoma | RPMI1640 10%FCS | 1 × 10⁴/48 well plate | 6 | kindly provided by Dr. Susumu Takekoshi (the Tokai University School of Medicine) | |
| PC-3 | Prostate | Adeno-carcinoma | HAM’S F12 10%FCS | 1 × 10⁴/48 well plate | 6 | kindly provided by Dr. Susumu Takekoshi (the Tokai University School of Medicine) | ||
| TN Breast Cancer Carcinoma | MDA-MB-231 | Breast Mammary Gland | Adeno-carcinoma | L-15 15%FCS | 1 × 10⁴/48 well plate | 3 | European Collection of Authenticated Cell Cultures |
Each cell line was cultured in the presence of 0–200 μM P4, 2–20 μM Lipo-P4, or Lipo-Emp (the amount was equivalent to 20 μM Lipo-P4). The cells were cultured under the conditions listed in Table 1, and the cell number was counted.
2.4. Flow cytometry
Fluorochrome-conjugated anti-human monoclonal antibodies were used to analyze the B-cell lines and human immune cells. The antibodies used in this study are listed in Table S1. The cells were incubated with appropriate dilutions of fluorescently labeled mouse anti-human monoclonal antibodies for 15 min at 4 °C, washed with PBS containing 1 % (w/v) bovine serum albumin (Sigma-Aldrich), and analyzed using a FACS Fortessa or Verse flow cytometer (BD Biosciences). For each analysis, a live gate with white blood cells or lymphocytes was used, based on human CD45 expression. Data were analyzed using the FlowJo software (PRID:SCR_008520; BD Biosciences).
2.5. Statistics
Significant differences between groups were calculated using one-way analysis of variance or two-sided Student's t-tests in Microsoft Excel 2021 (Microsoft Corporation, WA, USA).
3. Results
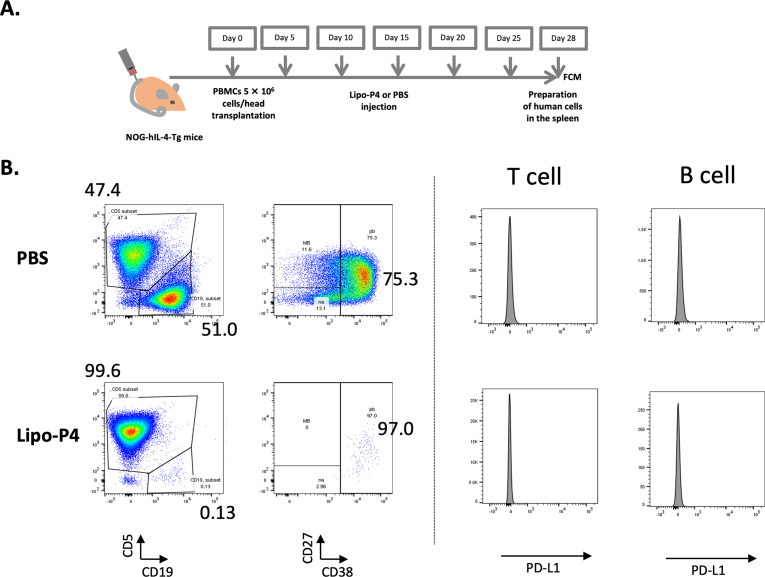
Previously, we showed that the number of engrafted B cells decreased after Lipo-P4 treatment in humanized tumor-bearing mice. As shown in Fig. 1, CD5+ human T cells and CD19+ human B cells were engrafted into the spleens of NOG-hIL-4-Tg mice 4 weeks after PBMC transplantation. Most B cells became antibody-secreting, plasmablast-like cells. In mice injected with Lipo-P4, which is targeted to PD-L1-expressing immune cells, the proportion of B cells decreased, although PD-L1 was not significantly expressed compared to T cells (Fig. 1B). Moreover, the proportion of plasmablasts in B cells did not substantially change, as we previously reported in tumor-bearing PBL-NOG-hIL-4-Tg mice [13]. This suggests that P4 specifically suppresses the differentiation of plasmablasts.
Fig. 1.
Effect of Lipo-P4 on B-cell engraftment in humanized mice. (A) Protocol for Lipo-P4 evaluation using PBL-NOG-hIL-4-Tg. (B) Typical FACS patterns of human CD45+ leukocytes in the humanized mouse spleen (n = 4). The numbers shown in each panel are the percentages of the gated cells. Left two panels: expression of CD5 and CD19 in the human CD45+ cell-gated cells. Middle two panels: expression of CD27 and CD38 in the human CD19+ cell-gated cells shown in the left panels. Human CD19 (B cells), CD5 (T cells and B1 cells), CD27 (memory and plasmablast B cells), and CD38 (plasmablast and plasma cells). Right four panels: expression of PD-L1 in CD5+ T cells (left panels) and CD5− B cells (right panels).
Therefore, we attempted to determine the stage of B cells in which their fate is affected by Lipo-P4. To examine whether cell proliferation was inhibited at the plasmablast differentiation stage, we selected various lymphoma and myeloma cell lines and examined whether the proliferation of each cell line was inhibited. For this purpose, we examined the surface markers of B-lymphoma and myeloma cells, as listed in Table 1. As shown in Table S2, the B-lymphoma and myeloma cell lines expressed different surface marker profiles. The B-lymphoma cell lines expressed CD45 and CD19, whereas the myeloma cell lines did not express CD45 but expressed CD138. None of the cell lines expressed CD5, and the expression of CD27 and CD38 varied among cell lines.
Next, we compared the effect of P4 on suppressing the proliferation of B-lymphoma and myeloma cell lines. In a previous report, we clarified that, in cell lines such as JEG-3, BeWo (placental choriocarcinoma), MDA-MB-231 (triple-negative breast cancer), HEK293 (a kidney cell line), and Karpus707H (myeloma), Lipo-P4 had an approximately ten times stronger anticancer effects than P4 alone [13]. Therefore, we examined whether the effect of Lipo-P4 on B-lineage cell lines was stronger than that of P4 alone. To avoid any effects caused by the liposome itself, we prepared Lipo-Emp, which was used as a negative control. As shown in Fig. S1, in OCI-LY3, a non-Hodgkin diffuse large-cell lymphoma cell line, cell proliferation was suppressed at all time points. We also selected non-targeting Lipo-P4 to avoid a decrease in the targeting efficiency of cell lines that did not express PD-L1. Therefore, we compared the cell number on the 3rd day and found that Lipo-P4 almost completely suppressed cell growth at a dose of 20 μM, which is equivalent to the P4 concentration in the placental intervillous blood. In contrast, Lipo-Emp did not suppress cell proliferation. All cell lines listed in Table 1 showed the same kinetics, and we determined the best time point to compare the effects of P4, Lipo-P4, and Lipo-Emp. The result for OCI-LY3 suggests that P4 efficiently suppressed OCI-LY3 proliferation in a dose-dependent manner, and the effect was enhanced if P4 was encapsulated in the liposome.
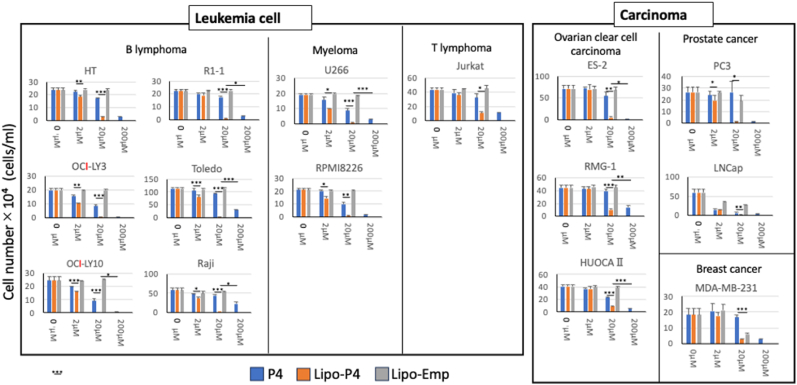
Next, we examined the effects of these reagents on other B-lymphoma and myeloma cell lines. We also examined a T-cell line and various carcinoma cell lines for comparison with the B-cell lines. Information regarding the cancer cell lines is shown in Table 1. As shown in Fig. 2, all cell lines examined showed a significant dose-dependent decrease in the number of cells in the presence of P4. Moreover, Lipo-P4 suppressed cell proliferation more efficiently than P4. In some cell lines (R1-1, Toledo, Raji, and U266), a more than ten times greater effect was observed.
Fig. 2.
Differences in the effects of P4 on cancer-cell proliferation. Left panels: leukemia cell lines (B-cell lymphoma, myeloma, and T-cell lymphoma); right panels: carcinoma cell lines (ovarian clear cell carcinoma, prostate cancer, and triple-negative breast cancer). Data are expressed as the mean ± standard deviation (SD) (n = 3). Student's t-test was performed, and significance is shown as *.
In contrast, Lipo-Emp did not suppress the proliferation of most of the cell lines examined. Among them, LNCap, a prostate cancer cell line, and MDA-MB-231, a triple-negative breast cancer cell line, showed significant decreases in cell numbers. However, the cell number in these cell lines tended to be higher than that in the Lipo-P4-treated group, suggesting that some suppressive effects of liposomes may exist in these cell lines.
These results suggest that P4 effectively suppressed the proliferation of most of the cancer cell lines examined when encapsulated in liposomes. Liposomes did not affect the proliferation of many cell lines; however, a suppressive effect was observed in some cell lines.
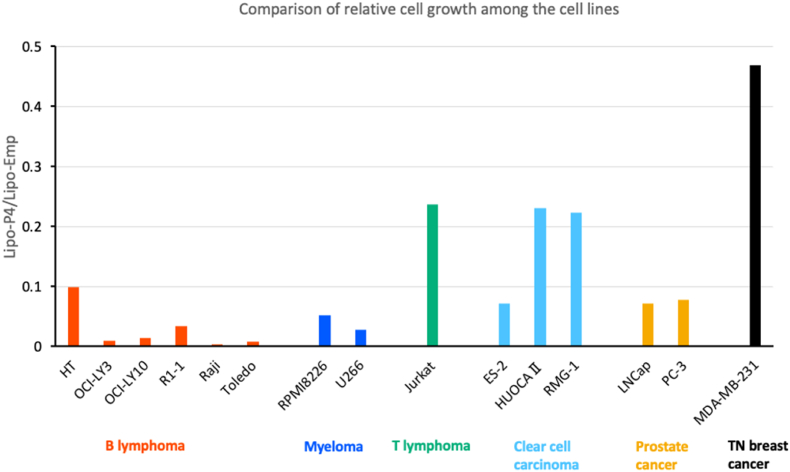
Because the suppression of proliferation varies among cell lines, we compared the relative suppression effect of Lipo-P4 based on the cell line subtype. Relative growth was calculated as Lipo-P4/Lipo-Emp because the scores showed the effects of P4 without the effect of the liposomes. As shown in Fig. 3, the relative growth was the lowest in the B-lymphoma cell lines, except for the HT and myeloma cell lines. Compared with these B-lineage leukemia cell lines, carcinoma cell lines showed higher relative growth, suggesting that the effect was the highest in B-lineage leukemia. Although LNCap cells showed low proliferation, the Lipo-Emp score was also low, which caused the relative growth to decrease. These results suggest that the effects of Lipo-P4 are the highest for B-cell lymphoma and myeloma among the cancer subtypes.
Fig. 3.
Comparison of the relative cell growth among cell lines. The relative cell growth score was calculated using Lipo-P4/Lipo-EMP. The results of each cell line were categorized and are shown in different colors (red, B lymphoma; orange; myeloma, Green; T lymphoma; pale blue, clear cell carcinoma; pale green, prostate cancer; dark blue, triple-negative (TN) breast cancer). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
In this study, we showed that the proliferation of all the cell lines examined was significantly suppressed in the presence of high-dose P4 and Lipo-P4. This indicates that suppression may be common in cancer cells. The physiological concentration of P4 was sufficient to suppress growth when P4 was delivered in a liposome-like form because the concentration (20 μM) was similar to that in the placenta.
Progesterone and progestins induce G0/G1 cell cycle arrest and mitochondria-dependent apoptosis [20]. Therefore, it is plausible that high concentrations of P4 inhibit the proliferation of cancer cell lines. In contrast, in our previous study, P4 treatment decreased B-cell engraftment, which was confirmed in this study [13]. Almost all human B cells engrafted into NOG-hIL-4-Tg mice were plasmablasts. Therefore, we hypothesized that plasmablasts and plasma cells are affected by P4, but that naïve and developing B cells might survive. However, in our study, the proliferation of all B-cell lymphoma lines was suppressed, suggesting that this suppression was not specific to plasma cell differentiation. In contrast, based on the effects of P4 on proliferative signaling pathways, P4-sensitive B cells may be proliferating B cells. This is because P4 can downregulate Bcl-2 expression in various cancer cell lines, although the extent of apoptosis depends on its concentration [21]. Moreover, P4 and progestins regulate various pathways related to cell proliferation and apoptosis [22]. These pathways include the PI3K/AKT, Ras/Raf/MEK/ERK, and WNT/β-catenin signaling pathways. Therefore, the suppression of cell growth may be induced through the regulation of these pathways. For example, multiple myeloma cells [23] and B-lineage acute lymphoblastic leukemia cells [24] activate the PI3K/AKT pathway. In contrast, low concentrations of P4 enhance cancer progression [25]. These results suggest that progesterone signaling involves complicated dose-dependent signaling cascades, and the regulation of these signaling molecules by different progesterone receptors (PRs) or their strength is pivotal to induce specific outcomes.
As the expression of PRs is reportedly necessary to induce suppressive activity [12], they might have been expressed in the cells we examined. PRs include nuclear and membrane PRs. Although nuclear PRs are expressed in a limited number of cancer cell lineages, membrane PRs are widely expressed in many cancer cell lines [26]. B cells have also been reported to express PRs [27,28]; however, enhanced expression of PRs has not been reported in B cells or B-lineage cancer cell lines. Therefore, in B-lineage cancer cells, unidentified receptors may be expressed and involved in this function. To understand the specific effects of P4 among the many points of action, exploring drug delivery systems with modified forms and doses of P4 could be important.
Because Lipo-P4 is not toxic to T and myeloid cells engrafted into humanized mice [13], suggesting that normal lymphoid cells other than B cells are not significantly affected, it could be a powerful drug candidate for B-lineage cancer cells. The anticancer effect might not be limited to B-lineage cancer cells because the cell growth of other cancer cell lines was affected as well, while some of those cell lines were also affected by Lipo-Emp. Among them, MDA-MB231 was sensitive to only P4-Lipo in the humanized mouse system, and Lipo-Emp did not significantly suppress tumor growth [13]. Therefore, high amounts of liposomes might be toxic to some cell lines, and this toxicity should be carefully examined further. As we have not identified the receptors and molecular mechanisms involved in the enhancement effects of Lipo-P4, this should be addressed in the near future.
5. Conclusion
P4 suppressed the proliferation of cancer cell lines in a dose-dependent manner. This effect was greater with the liposome-encapsulated form of P4. B-lineage cells are highly susceptible to Lipo-P4. Thus, Lipo-P4 is a good candidate as a cancer drug targeting B-lineage cancer cells.
Funding
This work was supported by MEXT KAKENHI (22220007), an AMED Translational Research Grant (A259TS, A321TS; to YK), the Tokai University School of Medicine Project Research (to YK), and a Tokai University Grant-in-Aid (to YK).
CRediT authorship contribution statement
Toshiro Seki: Writing – original draft, Investigation. Rikio Suzuki: Writing – original draft, Resources, Investigation, Conceptualization. Shino Ohshima: Data curation. Yoshiyuki Manabe: Resources, Methodology. Shion Onoue: Data curation. Yuki Hoshino: Data curation. Atsushi Yasuda: Resources, Data curation. Ryoji Ito: Resources, Methodology. Hiroshi Kawada: Writing – original draft, Supervision. Hitoshi Ishimoto: Writing – review & editing, Supervision. Takashi Shiina: Writing – review & editing, Supervision. Yoshie Kametani: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Methodology, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank the members of the Teaching and Research Support Center at Tokai University School of Medicine for their technical skills. We thank Yumiko Nakagawa for her excellent animal care skills.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2024.101710.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Hoffmann J.P., Liu J.A., Seddu K., Klein S.L. Sex hormone signaling and regulation of immune function. Immunity. 2023;56:2472–2491. doi: 10.1016/j.immuni.2023.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Hierweger A.M., Engler J.B., Friese M.A., Reichardt H.M., Lydon J., DeMayo F., et al. Progesterone modulates the T-cell response via glucocorticoid receptor-dependent pathways. Am. J. Reprod. Immunol. 2019;81 doi: 10.1111/aji.13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arruvito L., Giulianelli S., Flores A.C., Paladino N., Barboza M., Lanari C., et al. NK cells expressing a progesterone receptor are susceptible to progesterone-induced apoptosis. J. Immunol. 2008;180:5746–5753. doi: 10.4049/jimmunol.180.8.5746. [DOI] [PubMed] [Google Scholar]
- 4.Butts C.L., Bowers E., Horn J.C., Shukair S.A., Belyavskaya E., Tonelli L., et al. Inhibitory effects of progesterone differ in dendritic cells from female and male rodents. Gend. Med. 2008;5:434–447. doi: 10.1016/j.genm.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim J.J., Kurita T., Bulun S.E. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr. Rev. 2013;34:130–162. doi: 10.1210/er.2012-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Modugno F., Laskey R., Smith A., Andersen C., Haluska P., Oesterreich S. Hormone response in ovarian cancer: time to reconsider as a clinical target? Endocr. Relat. Cancer. 2012;19:R255–R279. doi: 10.1530/ERC-12-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fedotcheva T. Clinical use of progestins and their mechanisms of action: present and future (review), Sovrem. Tekhnologii. Méd. 2021;13:93–106. doi: 10.17691/stm2021.13.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawana K., Kawana Y., Schust D. Female steroid hormones use signal transducers and activators of transcription protein-mediated pathways to modulate the expression of T-bet in epithelial cells: a mechanism for local immune regulation in the human reproductive tract. Mol. Endocriol. 2005;19:2047–2059. doi: 10.1210/me.2004-0489. [DOI] [PubMed] [Google Scholar]
- 9.Kojima J., Ono M., Kuji N., Nishi H. Human chorionic villous differentiation and placental development. Int. J. Mol. Sci. 2022;23:8003. doi: 10.3390/ijms23148003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solano M., Arck P. Steroids, pregnancy and fetal development. Front. Immunol. 2020;10:3017. doi: 10.3389/fimmu.2019.03017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kashiwagi H., Seki T., Oshima S., Ohno Y., Shimizu T., Yamada S., et al. High-progesterone environment preserves T cell competency by evading glucocorticoid effects on immune regulation. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.1000728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu C., Zhang Z., Yu Y., Liu Y., Zhao F., Yin L., et al. Inhibiting the PI3K/Akt pathway reversed progestin resistance in endometrial cancer. Cancer Sci. 2011;102:557–564. doi: 10.1111/j.1349-7006.2010.01829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kametani Y., Ito R., Ohshima S., Manabe Y., Ohno Y., Shimizu T., et al. Construction of the systemic anticancer immune environment in tumour-bearing humanized mouse by using liposome-encapsulated anti-programmed death ligand 1 antibody-conjugated progesterone. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1173728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kametani Y., Ohno Y., Ohshima S., Tsuda B., Yasuda A., Seki T., et al. Humanized nice as an effective evaluation system for peptide vaccines and immune checkpoint inhibitors. Int. J. Mol. Sci. 2019;20:6337. doi: 10.3390/ijms20246337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Covassin L., Jangalwe S., Jouvet N., Laning J., Burzenski L., Shultz L.D., et al. Human immune system development and survival of non-obese diabetic (NOD)-scid IL2rgamma(null) (NSG) mice engrafted with human thymus and autologous haematopoietic stem cells. Clin. Exp. Immunol. 2013;174:372–388. doi: 10.1111/cei.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito M., Hiramatsu H., Kobayashi K., Suzue K., Kawahata M., Hioki K., et al. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 17.Ito R., Takahashi T., Katano I., Ito M. Current advances in humanized mouse models. Cell. Mol. Immunol. 2012;9:208–214. doi: 10.1038/cmi.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kollet O., Peled A., Byk T., Ben-Hur H., Greiner D., Shultz L., et al. beta2 microglobulin-deficient (B2m(null)) NOD/SCID mice are excellent recipients for studying human stem cell function. Blood. 2000;95:3102–3105. doi: 10.1182/blood.V95.10.3102. [DOI] [PubMed] [Google Scholar]
- 19.Kametani Y., Katano I., Miyamoto A., Kikuchi Y., Ito R., Muguruma Y., et al. NOG-hIL-4-Tg, a new humanized mouse model for producing tumor antigen-specific IgG antibody by peptide vaccination. PLoS One. 2017;12 doi: 10.1371/journal.pone.0179239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang X., Zhong R., He X., Deng Q., Peng X., Li J., et al. Investigations on the mechanism of progesterone in inhibiting endometrial cancer cell cycle and viability via regulation of long noncoding RNA NEAT1/microRNA-146b-5p mediated Wnt/β-catenin signaling. IUBMB Life. 2019;71:223–234. doi: 10.1002/iub.1959. [DOI] [PubMed] [Google Scholar]
- 21.Walsh N.C., Kenney L.L., Jangalwe S., Aryee K.E., Greiner D.L., Brehm M.A., et al. Humanized mouse models of clinical disease. Annu. Rev. Pathol. 2017;12:187–215. doi: 10.1146/annurev-pathol-052016-100332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fedotcheva T., Fedotcheva N., Shimanovsky N. Progestins as anticancer drugs and chemosensitizers, new targets and applications. Pharmaceutics. 2021;13:1616. doi: 10.3390/pharmaceutics13101616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akhtar S., Ali T.A., Faiyaz A., Khan O.S., Raza S.S., Kulinski M., et al. Cytokine-mediated dysregulation of signaling pathways in the pathogenesis of multiple myeloma. Int. J. Mol. Sci. 2020;21:5002. doi: 10.3390/ijms21145002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simioni C., Martelli A.M., Zauli G., Vitale M., McCubrey J.A., Capitani S., et al. Targeting the phosphatidylinositol 3-kinase/Akt/mechanistic target of rapamycin signaling pathway in B-lineage acute lymphoblastic leukemia: an update. J. Cell. Physiol. 2018;233:6440–6454. doi: 10.1002/jcp.26539. [DOI] [PubMed] [Google Scholar]
- 25.Ho S.M. Estrogen, progesterone and epithelial ovarian cancer. Reprod. Biol. Endocrinol. 2003;1:73. doi: 10.1186/1477-7827-1-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas P. Membrane progesterone receptors (mPRs, PAQRs): review of structural and signaling characteristics. Cells. 2022;11:1785. doi: 10.3390/cells11111785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ndiaye K., Poole D., Walusimbi S., Cannon M., Toyokawa K., Maalouf S., et al. Progesterone effects on lymphocytes may be mediated by membrane progesterone receptors. J. Reprod. Immunol. 2012;95:15–26. doi: 10.1016/j.jri.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Campe K.-N., Redlich A., Zenclussen A., Busse M. An increased proportion of progesterone receptor A in peripheral B cells from women who ultimately underwent spontaneous preterm birth. J. Reprod. Immunol. 2002;154 doi: 10.1016/j.jri.2022.103756. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.