Abstract
Background:
Poor outcomes are associated with post cardiac arrest blood pressures <5th percentile for age. We aimed to study the relationship of mean arterial pressure (MAP) with favorable neurologic outcome following cardiac arrest and return of spontaneous circulation (ROSC).
Methods:
This retrospective, multi-center, observational study analyzed data from the Pediatric Resuscitation Quality Collaborative (pediRES-Q). Children (<18 years) who achieved ROSC following index in-hospital or out-of-hospital cardiac arrest and survived 6 hours were included. Lowest documented MAP within the first ≥6 hours of ROSC was percentile adjusted for age and categorized into six groups – Group I: <5th, II: 5–24th, III: 25–49th, IV: 50–74th, V: 75–94th; and VI: 95–100th percentile. Primary outcome was favorable neurologic status at hospital discharge, defined as PCPC score 1, 2, or no change from pre-arrest baseline. Multivariable logistic regression was performed to analyze the association of MAP group with favorable outcome, controlling for illness category (surgical-cardiac), initial rhythm (shockable), arrest time (weekend or overnight), age, CPR duration, and clustering by site.
Results:
787 patients were included: median [Q1,Q3] age 17.9 [4.8,90.6] months; male 58%; OHCA 21%; shockable rhythm 13%; CPR duration 7 [3,16] min; favorable neurologic outcome 54%. Median lowest documented MAP percentile for the favorable outcome group was 13 [3,43] versus 8 [1,37] for the unfavorable group. The distribution of blood pressures by MAP group was I: 37%, II: 28%, III: 13%, IV: 11%, V: 7%, and VI: 4%. Compared with patients in Group I (<5%ile), Groups II, III, and IV had higher odds of favorable outcome (aOR, 1.84 [95% CI, 1.24, 2.73]; 2.20 [95% CI, 1.32, 3.68]; 1.90 [95% CI, 1.12, 3.25]). There was no association between Groups V or VI and favorable outcome (aOR, 1.44 [95% CI, 0.75, 2.80]; 1.11 [95% CI, 0.47, 2.59]).
Conclusion:
In the first 6-hours post-ROSC, a lowest documented MAP between the 5th74th percentile for age was associated with favorable neurologic outcome compared to MAP <5th percentile for age.
Keywords: Cardiopulmonary resuscitation, Post-cardiac arrest, Pediatrics, Blood pressure, Hypotension, Outcomes
Introduction
In the United States, approximately 20,000 children suffer cardiac arrest (CA) each year. Among these, less than 50% survive to hospital discharge, with many survivors sustaining short- and long-term disability. This is primarily due to the sequelae of CA-induced brain injury, characterized by ischemia, cytotoxic cerebral edema, and the resulting dysfunctional cerebral autoregulation.1–6 The central focus of post-cardiac arrest (CA) care in the pediatric intensive care unit is to reduce secondary brain injury, a significant driver of death and long-term disability among survivors.3–5
Following cardiac arrest, hypotension in children, defined as a systolic blood pressure (SBP) <5th percentile for age, is associated with unfavorable outcomes.7–9 The American Heart Association’s (AHA) Pediatric Advanced Life Support guidelines recommend hemodynamic optimization after return of spontaneous circulation (ROSC), using intravenous fluids, inotropes, and/or vasopressors to achieve a minimum BP greater than the 5th percentile for age.10 The literature that supports this recommendation almost exclusively uses SBP data and classifies patients as hypotensive or non-hypotensive, then compares outcomes.8,9,11,12 It might be more physiologically appropriate to use mean arterial pressure (MAP), rather than SBP, considering MAP’s critical importance in maintaining cerebral blood flow. While the adverse impact of even a single hypotensive episode is clear, our understanding of the influence of BP on outcomes is limited. The optimal BP range post-CA is unclear, particularly due to the complicating factor of disrupted cerebral autoregulation, which may make the brain more vulnerable to hypoperfusion even at higher blood pressures, since after cardiac arrest, the cerebral autoregulatory range is narrowed and right-shifted.13 Maintaining a higher MAP than guideline-recommended targets may be necessary to ensure sufficient cerebral perfusion and improve neurologic outcomes.14 A large study population is needed to analyze blood pressure in a more nuanced manner, rather than simply using binary classifications (i.e. <5th percentile vs. ≥5th percentile).
Using the pediRES-Q quality improvement database we aimed to describe the association between MAP, during the first 6 hours after ROSC, and neurologic outcome at hospital discharge after pediatric cardiac arrest in a large pediatric cohort.
Methods
Design and setting
This is an observational cohort study leveraging data collected between July 2015 and June 2022 by the pediRES-Q collaborative, a global, multi-site resuscitation quality improvement network. The network consists of 60 participating sites in 17 countries across five continents (Asia, Australia, Europe, North and South America) (ClinicalTrials.gov:NCT02708134).15
Approval for the study was obtained by a central IRB and local institutional review boards (United States) and research ethics boards (Europe and Canada). The study satisfied the requirements for waiver of consent.
Population
This study included children <18 years who achieved ROSC (without ECMO) following index in-hospital (IHCA) or out-of-hospital (OHCA) CA and survived ≥6 hours.
Data collection
Variables in the pediRES-Q database included: patient characteristics, such as age, sex, race, pre-existing conditions, and illness category (medical-cardiac, medical-noncardiac, surgical-cardiac, surgical-noncardiac, trauma); pre-event characteristics, such as the presence of vascular access, endotracheal intubation, and monitoring devices; event characteristics, such as the location and timing of CA, first monitored cardiac rhythm, duration of cardiopulmonary resuscitation (CPR), defibrillation, and medications and non-drug interventions administered during CPR; and outcome data, such as Pediatric Cerebral Performance Category (PCPC) scores at hospital discharge and survival.
In the pediRES-Q database, post-ROSC hemodynamic data, ascertained either via arterial line or blood pressure cuff, was reported as minimum and maximum values for the 0–6 hour interval following ROSC. Instances where no data were documented was regarded as missing and excluded from analysis.
Exposures and outcomes
The primary exposure was the lowest documented MAP during the 0–6 hour interval post-ROSC. MAP was percentile adjusted for age based on normative data and categorized into six groups – Group I: <5th percentile, II: 5th–24th percentile, III: 25th–49th percentile, IV: 50th–74th percentile, V: 75th–94th percentile, and VI: 95–100th percentile.16
The primary outcome was survival with favorable neurologic outcome. Neurologic outcome was evaluated via the PCPC score. The PCPC is a six-point scale to characterize neurologic function: 1 = normal; 2 = mild disability; 3 = moderate disability; 4 = severe disability; 5 = coma or vegetative state; and 6 = death. Favorable outcome was defined as a PCPC of 1 or 2 at hospital discharge, or no change in PCPC score from pre-arrest baseline.8 Unfavorable outcome was defined as a discharge PCPC score of 3, 4, 5, or 6 associated with an increase in PCPC ≥1 from pre-arrest baseline.
Patients who lacked pre-arrest PCPC scores but had a PCPC score of 1 or 2 at discharge were considered to have favorable neurologic outcome. Those who were missing pre-arrest PCPC scores but died were classified as having unfavorable neurologic outcome. Patients who lacked baseline PCPC scores but had a discharge PCPC score of 3, 4, or 5 were excluded from the analysis since their outcome category (favorable versus unfavorable neurologic outcome) could not be established.
Statistical analysis
Descriptive statistics summarized demographic and clinical characteristics stratified by blood pressure percentile group and neurologic outcome at discharge. Continuous variables were presented as medians and interquartile ranges (IQR) and are compared between groups using the Mann-Whitney U test. Categorical variables were reported as frequencies with percentages and are compared between groups with the Chi-square or Fisher exact test as appropriate. We used multivariable logistic regression with mixed effects to estimate the association between MAP percentile category and favorable neurologic outcome, controlling for age, surgical-cardiac illness category, shockable rhythm, night/weekend arrest, CPR duration, and clustering by site as potential confounders based on a priori clinical rationale and evidence.17,18 We explored potential modifications of the effect of blood pressure on neurologic outcome via stratified subgroup analyses by 1) age category: 0–1 years; 1–8 years; and 8–18 years and 2) vasopressor use (dichotomized yes/no) during the first 6 hours after ROSC. For the subgroup analyses, MAPs ≥75th percentile were grouped together because of the small number of cases in the 75th–94th and ≥95th percentile groups. Additionally, we conducted a sensitivity analysis using a broadened classification of favorable and unfavorable neurologic outcome according to Albrecht et al., whereby favorable outcome was defined as a PCPC of 1, 2, or 3 at hospital discharge, or no change in PCPC score from pre-arrest baseline.19 Unfavorable outcome was defined as a discharge PCPC score of 4, 5, or 6 associated with an increase in PCPC ≥1 from pre-arrest baseline. P-values <0.05 were considered statistically significant. Analyses were performed using SPSS (IBM, Armonk, NY, USA).
Results
1,119 patients met inclusion criteria. 787 patients were included in the analysis (see Fig. 1). The median age was 1.5 years (IQR, 0.4–7.5). Seventy-nine percent of events occurred in-hospital, 13% had shockable rhythm, and the median CPR duration was 7 minutes (IQR, 3–16). Twenty-one percent of patients were post-operative following cardiac surgery at the time of cardiac arrest. The distribution of blood pressures by MAP group was: I = 37%; II = 28%; III = 13%; IV = 11%; V = 7%, and VI = 4%. Among the patients in group I (ie. MAP <5th percentile), 59% received vasopressor infusions within the first 6 hours post-ROSC. In groups II, III, IV, V, and VI, the proportion of patients receiving vasopressors was 47%, 37%, 32%, 26%, and 21%, respectively (see Table 1).
Fig. 1 –
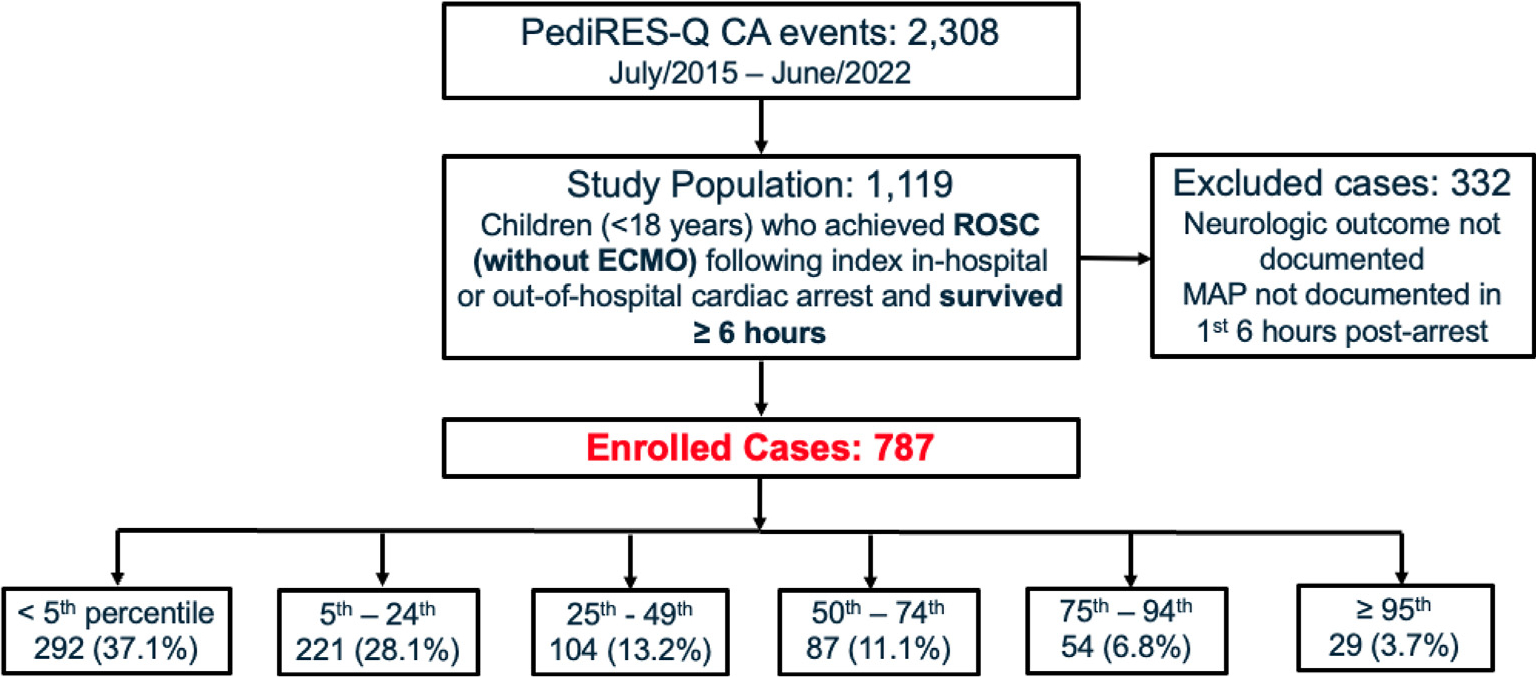
Consort diagram.
Table 1 -.
Descriptive statistics stratified by MAP percentile group.
| All Patients | < 5th | 5th - 24th | 25th - 49th | 50th - 74th | 75th - 94th | ≥ 95th | p | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Demographic (N) | 787 | 292 | 221 | 104 | 87 | 54 | 29 | |
| Age, years, median (IQR) | 1.5 (0.40, 7.6) | 1.9 (0.40, 8.5) | 0.9 (0.3, 5.6) | 0.7 (0.30, 3.42) | 1.6 (0.4, 9.1) | 4.1 (0.8, 9.4) | 5.3 (0.6, 10.8) | < 0.001 |
| Female | 328 (41.7%) | 129 (44.2%) | 87 (39.4%) | 43 (41.3%) | 29 (33.3%) | 24 (44.4%) | 16 (55.2%) | 0.300 |
| Race (N) | 787 | 292 | 221 | 104 | 87 | 54 | 29 | |
| Asian | 32 (4.1%) | 13 (4.5%) | 6 (2.7%) | 1 (1.0%) | 7 (8.0%) | 3 (5.6%) | 2 (6.9%) | 0.097 |
| Black | 127 (16.1%) | 57 (19.5%) | 36 (16.3%) | 13 (12.5%) | 13 (14.9%) | 5 (9.3%) | 3 (10.3%) | 0.337 |
| Native American | 2 (0.3%) | 1 (0.3%) | 0 (0.0%) | 1 (1.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0.654 |
| White | 381 (48.4%) | 141 (48.3%) | 114 (51.6%) | 51 (49.0%) | 40 (46.0%) | 19 (35.2%) | 16 (55.2%) | 0.366 |
| Other | 78 (9.9%) | 24 (8.2%) | 19 (8.6%) | 10 (9.6%) | 11 (12.6%) | 11 (20.4%) | 3 (10.3%) | 0.145 |
| Unknown/Not Documented | 167 (21.2%) | 56 (19.2%) | 46 (20.8%) | 28 (26.9%) | 16 (18.4%) | 16 (29.6%) | 5 (17.2%) | 0.345 |
| Height (N) | 630 | 245 | 177 | 86 | 67 | 39 | 16 | |
| Height, cm, median (IQR) | 71.0 (55.1, 113.0) | 77.0 (55.1, 121.3) | 68.0 (54.0, 101.5) | 65.0 (54.0, 91.1) | 78.0 (56.0, 129.5) | 99.0 (66.0, 121.0) | 71.0 (54.3, 129.8) | 0.007 |
| Weight (N) | 786 | 292 | 220 | 104 | 87 | 54 | 29 | |
| Weight, kg, median (IQR) | 10.3 (5.4, 25.0) | 11.5 (5.7, 26.1) | 8.5 (4.8, 21.8) | 7.8 (4.9, 15.0) | 12.5 (5.4, 30.0) | 17.6 (8.5, 30.5) | 21.7 (8.0, 38.7) | < 0.001 |
| Pre-Existing Conditions (N) | 787 | 292 | 221 | 104 | 87 | 54 | 29 | |
| Genetic/Metabolic | 234 (29.7%) | 99 (33.9%) | 66 (29.9%) | 25 (24.0%) | 23 (26.4%) | 12 (22.2%) | 9 (31.0%) | 0.309 |
| Congenital Heart | 257 (32.7%) | 93 (31.8%) | 93 (42.1%) | 40 (38.5%) | 20 (23.0%) | 8 (14.8%) | 3 (10.3%) | < 0.001 |
| Lung / Airway | 405 (51.5%) | 171 (58.6%) | 114 (51.6%) | 48 (46.2%) | 44 (50.6%) | 18 (33.3%) | 10 (34.5%) | 0.003 |
| Hematologic/Oncologic/Immune Compromise | 41 (5.2%) | 19 (6.5%) | 11 (5.0%) | 3 (2.9%) | 3 (3.4%) | 4 (7.4%) | 1 (3.4%) | 0.676 |
| Neurologic | 28 (3.6%) | 6 (2.1%) | 6 (2.7%) | 3 (2.9%) | 4 (4.6%) | 6 (11.1%) | 3 (10.3%) | 0.013 |
| Renal | 81 (10.3%) | 44 (15.1%) | 17 (7.7%) | 4 (3.8%) | 11 (12.6%) | 3 (5.6%) | 2 (6.9%) | 0.009 |
| Other Heart Disease | 79 (10.0%) | 44 (15.1%%) | 17 (7.7%) | 7 (6.7%) | 4 (4.6%) | 5 (9.3%) | 2 (6.9%) | 0.022 |
| Other | 342 (43.5%) | 143 (40.9%) | 89 (40.3%) | 41 (39.4%) | 38 (43.7%) | 19 (35.2%) | 12 (41.4%) | 0.239 |
| Illness Category (N) | 625 | 248 | 190 | 83 | 65 | 26 | 4 | |
| Medical, Cardiac | 117 (18.7%) | 42 (16.9%) | 39 (20.5%) | 17 (20.5%) | 7 (10.8%) | 8 (30.8%) | 4 (30.8%) | 0.156 |
| Medical, Non-Cardiac | 292 (46.7%) | 126 (50.8%) | 74 (38.9%) | 33 (39.8%) | 37 (56.9%) | 15 (57.7%) | 7 (53.8%) | 0.032 |
| Surgical, Cardiac | 128 (20.5%) | 46 (18.5%) | 50 (26.3%) | 20 (24.1%) | 11 (16.9%) | 1 (3.8%) | 0 (0.0%) | 0.015 |
| Surgical, Non-Cardiac | 64 (10.2%) | 23 (9.3%) | 21 (11.1%) | 10 (12.0%) | 8 (12.3%) | 2 (7.7%) | 0 (0.0%) | 0.834 |
| Trauma | 24 (3.8%) | 11 (4.4%) | 6 (3.2%) | 3 (3.6%) | 2 (3.1%) | 0 (0.0%) | 2 (15.4%) | 0.374 |
| PCPC Score Before Cardiac Arrest (N) | 689 | 249 | 190 | 94 | 77 | 51 | 28 | |
| 1 | 449 (65.2%) | 155 (62.2%) | 130 (68.4%) | 67 (71.3%) | 50 (64.9%) | 27 (52.9%) | 20 (71.4%) | 0.206 |
| 2 | 93 (13.5%) | 37 (14.9%) | 22 (11.6%) | 13 (13.8%) | 9 (11.7%) | 8 (15.7%) | 4 (14.3%) | 0.907 |
| 3 | 61 (8.9%) | 22 (8.8%) | 17 (8.9%) | 9 (9.6%) | 5 (6.5%) | 6 (11.8%) | 2 (7.1%) | 0.943 |
| 4 | 77 (11.2%) | 32 (12.9%) | 16 (8.4%) | 5 (5.3%) | 13 (16.9%) | 9 (17.6%) | 2 (7.1%) | 0.056 |
| 5 | 8 (1.2%) | 2 (0.8%) | 5 (2.6%) | 0 (0.0%) | 0 (0.0%) | 1 (2.0%) | 0 (0.0%) | 0.317 |
| 6 | 1 (0.1%) | 1 (0.4%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1.000 |
| Interventions In-Place Prior to Cardiac Arrest (N) | 787 | 292 | 221 | 104 | 87 | 54 | 29 | |
| Assisted/Mechanical Ventilation | 336 (42.7%) | 141 (48.3%) | 100 (45.2%) | 43 (41.3%) | 30 (34.5%) | 16 (29.6%) | 6 (20.7%) | 0.006 |
| Conscious/Procedural Sedation | 110 (14.0%) | 43 (14.7%) | 35 (15.8%) | 15 (14.4%) | 10 (11.5%) | 5 (9.3%) | 2 (6.9%) | 0.705 |
| Dialysis | 18 (2.3%) | 12 (4.1%) | 1 (0.5%) | 2 (1.9%) | 2 (2.3%) | 1 (1.9%) | 0 (0.0%) | 0.120 |
| ECG | 399 (50.7%) | 160 (54.8%) | 127 (57.5%) | 53 (51.0%) | 37 (42.5%) | 14 (25.9%) | 8 (27.6%) | < 0.001 |
| Implantable Cardiac Defibrillator (ICD) | 2 (0.3%) | 0 (0.0%) | 0 (0.0%) | 2 (1.5%) | 0 (0.0%) | 1 (1.6%) | 1 (3.4%) | 0.011 |
| Arterial Line | 207 (26.3%) | 75 (25.7%) | 78 (35.3%) | 25 (24.0%) | 18 (20.7%) | 8 (14.8%) | 3 (10.3%) | 0.002 |
| Endotracheal Tube/Tracheostomy | 377 (47.9%) | 154 (52.7%) | 121 (54.8%) | 45 (43.3%) | 36 (41.4%) | 15 (27.8%) | 6 (20.7%) | < 0.001 |
| IV/IO Infusion of Antiarrhythmics | 59 (7.5%) | 27 (9.2%) | 15 (6.8%) | 4 (3.8%) | 7 (8.0%) | 4 (7.4%) | 2 (6.9%) | 0.621 |
| Pulse Oximetry | 525 (66.7%) | 218 (74.7%) | 162 (73.3%) | 63 (60.6%) | 55 (63.2%) | 18 (33.3%) | 9 (31.0%) | < 0.001 |
| Supplemental Oxygen | 376 (47.8%) | 160 (54.8%) | 120 (54.3%) | 45 (43.3%) | 34 (39.1%) | 12 (22.2%) | 5 (17.2%) | < 0.001 |
| Other | 62 (7.9%) | 22 (7.5%) | 23 (10.4%) | 11 (10.6%) | 6 (6.9%) | 0 (0.0%) | 0 (0.0%) | 0.040 |
| Characteristics of CPR Event Location (N) | 787 | 292 | 221 | 104 | 87 | 54 | 29 | |
| In-Hospital | 625 (79.4%) | 248 (84.9%) | 190 (86.0%) | 83 (79.8%) | 65 (74.7%) | 26 (48.1%) | 13 (44.8%) | < 0.001 |
| Out-of-Hospital, Witnessed | 73 (9.3%) | 19 (6.5%) | 13 (5.9%) | 9 (8.7%) | 10 (11.5%) | 16 (29.6%) | 6 (20.7%) | < 0.001 |
| Out-of-Hospital, Not Witnessed | 88 (11.2%) | 25 (8.6%) | 18 (8.1%) | 11 (10.6%) | 12 (13.8%) | 12 (22.2%) | 10 (34.5%) | < 0.001 |
| Location of In-Hospital Arrests (N) | 625 | 248 | 190 | 83 | 65 | 26 | 13 | |
| ED | 32 (5.1%) | 15 (6.0%) | 2 (1.1%) | 6 (7.2%) | 3 (4.6%) | 5 (19.2%) | 1 (7.7%) | 0.002 |
| PICU | 366 (58.6%) | 155 (62.5%) | 111 (58.4%) | 42 (50.6%) | 40 (61.5%) | 12 (46.2%) | 6 (46.2%) | 0.265 |
| CICU | 115 (18.4%) | 47 (19.0%) | 39 (20.5%) | 15 (18.1%) | 9 (13.8%) | 3 (11.5%) | 2 (15.4%) | 0.841 |
| NICU | 15 (2.4%) | 8 (3.2%) | 2 (1.1%) | 4 (4.8%) | 1 (1.5%) | 0 (0.0%) | 0 (0.0%) | 0.419 |
| PACU | 3 (0.5%) | 2 (0.8%) | 1 (0.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0% | 0 (0.0%) | 1.000 |
| Other | 94 (15.0%) | 35 (18.4%) | 16 (19.3%) | 12 (18.5%) | 6 (23.1%) | 4 (30.8%) | 94 (15.0%) | 0.003 |
| Characteristics of CPR Event Time (N) | 692 | 270 | 201 | 90 | 72 | 38 | 21 | |
| Weekend/Night Arrest | 339 (49.0%) | 137 (50.7%) | 90 (44.8%) | 44 (48.9%) | 35 (48.6%) | 22 (57.9%) | 11 (52.4%) | 0.693 |
| Duration of Resuscitation (N) | 779 | 287 | 221 | 103 | 87 | 52 | 29 | |
| Duration, Minutes, Median (IQR) | 7.0 (3.0, 16.0) | 7.0 (4.0, 17.0) | 6.0 (3.0, 12.5) | 6.0 (3.0, 13.0) | 7.0 (3.0, 18.0) | 15.0 (5.0, 23.8) | 10.0 (5.0, 20.5) | 0.002 |
| Defibrillation Status (N) | 787 | 292 | 221 | 104 | 87 | 54 | 29 | |
| Any Defibrillation | 100 (12.7%) | 37 (12.7%) | 28 (12.7%) | 13 (12.5%) | 15 (17.2%) | 6 (11.1%) | 1 (3.4%) | 0.578 |
| Shockable Rhythm | 100 (12.7%) | 32 (11.0%) | 37 (16.7%) | 11 (10.6%) | 12 (13.8%) | 6 (11.1%) | 2 (6.9%) | 0.406 |
| Medications Administered During CPR Event (N) | 787 | 292 | 221 | 104 | 87 | 54 | 29 | |
| None | 21 (2.7%) | 5 (1.7%) | 7 (3.2%) | 3 (2.9%) | 2 (2.3%) | 3 (5.6%) | 1 (3.4%) | 0.514 |
| Amiodarone | 20 (2.5%) | 6 (2.1%) | 8 (3.6%) | 3 (2.9%) | 1 (1.1%) | 1 (1.9%) | 1 (3.4%) | 0.775 |
| Atropine | 63 (8.0%) | 27 (9.2%) | 23 (10.4%) | 7 (6.7%) | 3 (3.4%) | 1 (1.9%) | 2 (6.9%) | 0.159 |
| Calcium | 177 (22.5%) | 80 (27.4%) | 55 (24.9%) | 15 (14.4%) | 15 (17.2%) | 8 (14.8%) | 4 (13.8%) | 0.022 |
| Epinephrine - 1 Dose | 206 (26.2%) | 83 (28.4%) | 62 (28.1%) | 29 (27.9%) | 20 (23.0%) | 8 (14.8%) | 4 (13.8%) | 0.174 |
| Epinephrine - 2–4 Doses | 298 (37.9%) | 124 (42.5%) | 86 (38.9%) | 33 (31.7%) | 27 (31.0%) | 19 (35.2%) | 9 (31.0%) | 0.232 |
| Epinephrine - 5 + Doses | 124 (15.8%) | 50 (17.1%) | 27 (12.2%) | 15 (14.4%) | 17 (19.5%) | 10 (18.5%) | 5 (17.2%) | 0.514 |
| Fluid Bolus | 163 (20.7%) | 68 (23.3%) | 42 (19.0%) | 14 (13.5%) | 21 (24.1%) | 11 (20.4%) | 7 (24.1%) | 0.334 |
| Inhaled Nitric Oxide | 15 (1.9%) | 8 (2.7%) | 4 (1.8%) | 2 (1.9%) | 0 (0.0%) | 1 (1.9%) | 0 (0.0%) | 0.750 |
| Lidocaine | 13 (1.7%) | 5 (1.7%) | 6 (2.7%) | 0 (0.0%) | 0 (0.0%) | 1 (1.9%) | 1 (3.4%) | 0.287 |
| Magnesium Sulfate | 26 (3.3%) | 10 (3.4%) | 9 (4.1%) | 1 (1.0%) | 2 (2.3%) | 2 (3.7%) | 2 (6.9%) | 0.500 |
| Other Vasopressors | 25 (3.2%) | 12 (4.1%) | 7 (3.2%) | 1 (1.0%) | 2 (2.3%) | 1 (1.9%) | 2 (6.9%) | 0.483 |
| Sodium Bicarbonate | 200 (25.4%) | 88 (30.1%) | 54 (24.4%) | 22 (21.2%) | 20 (23.0%) | 10 (18.5%) | 6 (20.7%) | 0.260 |
| Vasopressin | 10 (1.3%) | 4 (1.4%) | 1 (0.0%) | 0 (0.0%) | 2 (2.3%) | 1 (1.9%) | 2 (6.9%) | 0.063 |
| Other | 238 (30.2%) | 78 (26.7%) | 59 (26.7%) | 34 (32.7%) | 32 (36.8%) | 20 (37.0%) | 15 (51.7%) | 0.027 |
| Non-Drug Interventions During CPR Event (N) | 787 | 292 | 221 | 104 | 87 | 54 | 29 | |
| None | 401 (51.0%) | 152 (52.1%) | 121 (54.8%) | 53 (51.0%) | 44 (50.6%) | 20 (37.0%) | 11 (37.9%) | 0.182 |
| Chest Tube | 26 (3.3%) | 12 (4.1%) | 8 (3.6%) | 2 (1.9%) | 0 (0.0%) | 1 (1.9%) | 3 (10.3%) | 0.103 |
| Needle Thoracostomy | 14 (1.8%) | 8 (2.7%) | 4 (1.8%) | 0 (0.0%) | 1 (1.1%) | 0 (0.0%) | 1 (3.4%) | 0.400 |
| Transcutaneous Pacemaker | 8 (1.0%) | 6 (2.1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (1.9%) | 1 (3.4%) | 0.054 |
| Transvenous Pacemaker | 10 (1.3%) | 3 (1.0%) | 5 (2.3%) | 0 (0.0%) | 2 (2.3%) | 0 (0.0%) | 0 (0.0%) | 0.491 |
| Pericardiocentesis | 2 (0.3%) | 0 (0.0%) | 2 (0.9%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0.474 |
| Intubation | 264 (33.5%) | 92 (31.5%) | 65 (29.4%) | 37 (35.6%) | 30 (34.5%) | 26 (48.1%) | 14 (48.3%) | 0.063 |
| Other | 129 (16.4%) | 44 (15.1%) | 38(17.2%) | 14 (13.5%) | 17 (19.5%) | 11 (20.4%) | 5 (17.2%) | 0.762 |
| Post-ROSC Vasopressor Utilization (N) | 787 | 292 | 221 | 104 | 87 | 54 | 29 | |
| Vasopressor Use | 361 (45.9%) | 172 (58.9%) | 103 (46.6%) | 38 (36.5%) | 28 (32.2%) | 14 (25.9%) | 6 (20.7%) | < 0.001 |
| Vasopressor Score, median (IQR) | 0.0 (0.0, 15.0) | 5.0 (0.0, 28.7) | 0.0 (0.0, 14.5) | 0.0 (0.0, 8.4) | 0.0 (0.0, 4.0) | 0.0 (0.0, 1.3) | 0.0 (0.0, 0.0) | < 0.001 |
| PCPC Score At Hospital Discharge (N) | 787 | 292 | 221 | 104 | 87 | 54 | 29 | |
| 1 | 253 (32.1%) | 68 (23.3%) | 84 (38.0%) | 48 (46.2%) | 31 (35.6%) | 15 (27.8%) | 7 (24.1%) | |
| 2 | 105 (13.3%) | 36 (12.3%) | 37 (16.7%) | 14 (13.5%) | 12 (13.8%) | 5 (9.3%) | 1 (3.4%) | |
| 3 | 52 (6.6%) | 22 (7.5%) | 11 (5.0%) | 7 (6.7%) | 5 (5.7%) | 3 (5.6%) | 4 (13.8%) | |
| 4 | 53 (6.7%) | 20 (6.8%) | 12 (5.4%) | 5 (4.8%) | 8 (9.2%) | 7 (13.0%) | 1 (3.4%) | |
| 5 | 3 (0.4%) | 0 (0.0%) | 2 (0.9%) | 0 (0.0%) | 1 (1.1%) | 0 (0.0%) | 0 (0.0%) | |
| Deaths | 321 (40.8%) | 146 (50.0%) | 75 (33.9%) | 30 (28.8%) | 30 (34.5%) | 24 (44.4%) | 16 (55.2%) | |
| Neurologic Outcome (N) | 787 | 292 | 221 | 104 | 87 | 54 | 29 | |
| Favorable | 424 (53.8%) | 128 (43.8%) | 137 (61.9%) | 69 (66.3%) | 52 (59.8%) | 26 (48.1%) | 12 (41.4%) | |
Four hundred twenty-four patients (54%) had favorable outcome at hospital discharge (Supplementary Table 1). These patients received shorter durations of CPR; fewer doses of epinephrine and sodium bicarbonate intra-arrest; more frequently received a fluid bolus during arrest; and had lower VIS scores in the first 6 hours post-CA. The median MAP percentile for the favorable vs unfavorable outcome groups was 13 (IQR, 3–43) versus 8 (IQR, 1–37) (p = 0.441). There was an inverted U-shaped relationship between MAP category and favorable outcome (Fig. 2). The greatest proportion of patients with favorable outcome was observed with MAP between the 25th–49th percentile (66%), followed by the 5th–24th percentile (62%) and the 50th–74th percentile (60%) (Table 2).
Fig. 2 –
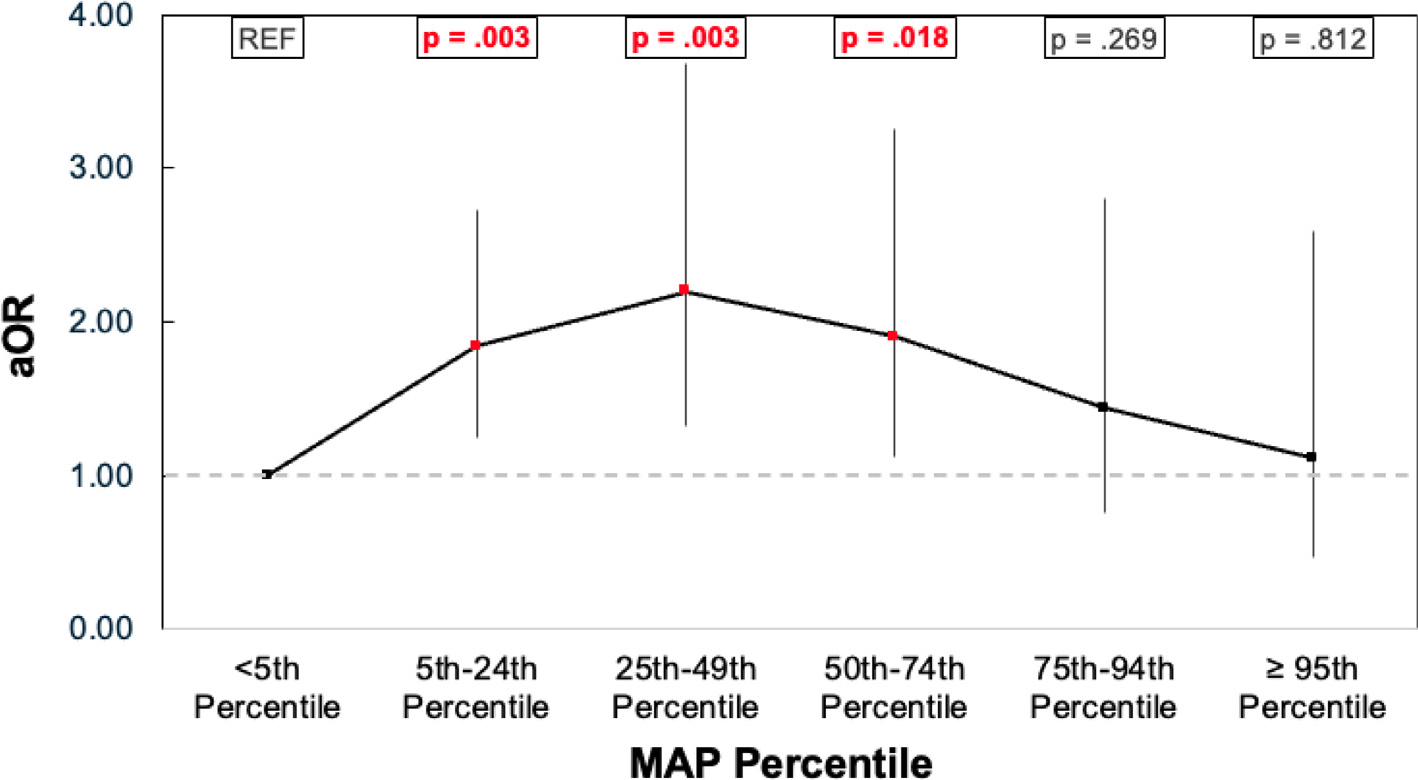
Adjusted odds of favorable outcome associated with MAP percentile group.
Table 2 -.
Odds of favorable neurologic outcome associated with different percentiles.
| Percentile | No. of Children | Favorable Outcome (%) | OR (95% CI) Favorable Outcome | AOR (95% CI) Favorable Outcome |
|---|---|---|---|---|
|
| ||||
| <5th | 292 | 128 (43.8%) | REF | REF |
| 5–24th | 221 | 137 (61.9%) | 2.09 (1.46, 2.99) p < 0.001 | 1.84 (1.24, 2.73) p = 0.003 |
| 25–49th | 104 | 69 (66.3%) | 2.53 (1.58, 4.03) p < 0.001 | 2.20 (1.32, 3.68) p = 0.003 |
| 50–74th | 87 | 52 (59.8%) | 1.90 (1.17, 3.10) p = 0.010 | 1.90 (1.12, 3.250) p =0.018 |
| 75–94th | 54 | 26 (48.1%) | 1.19 (0.67, 2.13) p = 0.558 | 1.45 (0.75, 2.80) p = 0.269 |
| ≥95th | 29 | 12 (41.4%) | 0.90 (0.42, 1.96) p = 0.799 | 1.11 (0.47, 2.60) p =0.812 |
Adjusted for: 1) age, 2) illness category (surgical-cardiac), 3) initial shockable rhythm, 4) time (weekend/night), 5) CPR duration 6) clustering by site.
In the multivariable logistic regression analysis, compared with group I (MAP <5th percentile), those in groups II, III, IV had greater odds of favorable outcome (aOR, 1.84 [95% CI, 1.24, 2.73]; 2.20 [95% CI, 1.32, 3.68]; 1.90 [95% CI, 1.12, 3.25], respectively). However, groups V and VI did not have significantly greater odds of favorable outcome than group I (aOR, 1.44 [95% CI, 0.75, 2.80]; 1.11 [95% CI, 0.47, 2.59]) (Fig. 2).
For the planned subgroup analyses by age and post-ROSC vasopressor use, point estimates for the association between MAP percentile category and favorable outcome were consistent with the primary analysis (see Supplementary Data, Tables 2–6). The sensitivity analysis using a more inclusive definition of favorable neurologic outcome showed similar results to the primary analysis (see Supplementary Data, Fig. 1).
Discussion
In this study of children who achieved ROSC following IHCA or OHCA, a clear inverted U-shaped association emerged between lowest recorded MAP in the first 6 hours post-ROSC and favorable neurologic outcome at hospital discharge. The analysis revealed three distinct trends across the range of MAP percentiles (Fig. 2): 1) less favorable outcomes when MAP was below than the 5th percentile, 2) increasing likelihood of favorable outcomes when MAP was between the 5th to 74th percentile, and 3) diminishing probability of favorable outcomes when MAP was ≥75th percentile. Among children with MAP between the 5th to 74th percentile, 63% had favorable outcome compared to 44% who had MAP <5th or ≥75th percentiles, reinforcing that proactively maintaining blood pressure within an optimal range is an important therapeutic goal.
Our analysis supports targeting a higher MAP threshold than the 5th percentile for age, specifically the 5th–74th percentile. The AHA Pediatric Advanced Life Support Guidelines explicitly recommend intervening to maintain blood pressure greater than the 5th percentile for age.9,20,21 However, in our analysis, among the 37% of patients with documented hypotension, only 59% received vasopressor infusions within 6 hours of ROSC, highlighting that a significant proportion of these high-risk patients are potentially undertreated.
There is a wide range of MAP percentiles, between the 5th to 74th percentile, associated with favorable outcome. Above the 75th percentile, the probability of favorable outcome declined. This finding is relevant because current clinical practice is focused on avoiding hypotension and few clinicians would consider blood pressure above the 75th percentile post-ROSC a cause for concern or intervention. In fact, there is limited evidence indicating a connection between high MAP/hypertension and poor outcomes, so the finding that the “optimal” MAP range concludes/terminates at the 75th percentile is intriguing.22 It is possible that very high MAP post-CA manifests in patients with severe anoxic brain injury and early cerebral edema. Indeed, patients with elevated MAP had longer duration of CPR (Table 1), likely predisposing them to more severe neurological injury.22 Alternatively, the strain on the cardiovascular system imposed by high MAP might initiate a cardio-depressive feedback loop that leads to decreased cardiac output and cerebral perfusion, and as a consequence, poor neurologic outcome.22,23 Equally plausible, in the setting of impaired cerebral autoregulation, higher MAPs can result in an excessive and potentially harmful increase in blood flow to the brain, known as hyperemia.14
The relationship between post-arrest MAP and neurologic outcome is complex. To illustrate this, several patient characteristics were associated with MAP category. As expected, congenital heart disease patients were overly represented in the lower MAP percentile groups (see Table 1), likely related to impaired cardiac output and decreased cardiac reserve associated with their underlying condition. Age of patients increased with higher MAP percentile groups. This pattern could be indicative of older children having more mature autonomic nervous systems and a stronger sympathetic response to the physiologic stress associated with cardiac arrest. Alternatively, younger patients were more likely to be post-operative following cardiac surgery at the time of CA, which implies they had sicker hearts to begin with compared to older patients. Their cardiac dysfunction may have made it more challenging to maintain normal blood pressures after CA. Optimal blood pressure management is further complicated by post-arrest cerebral physiology and pathophysiology: degree of ischemic brain injury, cerebral metabolic requirements, and range of preserved cerebral autoregulation.14 This may explain why randomized controlled trials in adults, such as the recent BOX trial, demonstrated no difference in neurological outcomes between patients treated to higher versus low MAP thresholds.16,24–29 Overall, these observations are consistent with the idea that age-related physiological development, underlying medical conditions, and cerebral physiology can influence blood pressure patterns in the context of post-cardiac arrest care, highlighting the need for tailored management strategies to optimize outcomes for different patient populations.
This study has several limitations. We only assessed the single lowest MAP recorded in the first 6 hours post-ROSC. It is unclear if this measurement alone represents BP during the entire first 6 hours. The pediRES-Q database did not indicate duration of the lowest MAP. Previous analyses demonstrate prolonged periods of hypotension are more detrimental than short episodes of hypotension.7,21,25 In addition, our assessment of neurologic outcome was performed by PCPC at hospital discharge. Longer term outcomes are desirable, but beyond the scope of this analysis.30 Longitudinal data suggests that even for patients with favorable outcome at hospital discharge, the PCPC score captured at this time point may not fully reflect longer-term neurocognitive and neurobehavioral function.31
Conclusion
In the first 6-hours after pediatric cardiac arrest, a lowest documented MAP between the 5th to 74th percentile was associated with favorable neurologic outcome. This finding underscores the significance of effectively managing post-arrest blood pressure. Further study is merited to ascertain whether personalized MAP goals within this range, driven by patients’ unique physiology, improves pediatric cardiac arrest outcomes.
Supplementary Material
Acknowledgements
We would like to thank the clinicians and staff at all pediRES-Q sites for their indispensable time and dedication to this collaborative effort.
Funding
The pediRES-Q is supported by an unrestricted research grant to the Children’s Hospital of Philadelphia from ZOLL Medical. The sponsor had no role in the design, interpretation, writing, editing, or submission of the manuscript.
Footnotes
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: The authors declared no financial conflicts of interest. (1) Dana Niles disclosed that the Children’s Hospital of Philadelphia receives support from an unrestricted research grant from ZOLL Medical Corporation. The remaining authors have no disclosures to report. (2) Matthew Kirschen received NIH funding to his institution. (3) Vinay Nadkarni MD received unrestricted research funding to his institution form the National Institutes of Health, Agency for Healthcare Research and Quality, Zoll Medical, Nihon-Kohden Inc., and Volunteers on Scientific Advisory Committees for the American heart Association, Citizen CPR Foundation, INSPIRE simulation network, and Citizen CPR. Dr. Nadkarni is a member of Resuscitation’s Editorial Board. Dr. Nadkarni is the President of the Society of Critical Care Medicine 2022 to 2023. The content reflects his own personal work and is not intended to represent the views of the Society of Critical Care Medicine.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.resuscitation.2023.110066.
CRediT authorship contribution statement
A Ushpol: Conceptualization, Methodology, Formal analysis, Writing – original draft. S Je: Data curation, Methodology, Writing – review & editing, Project administration. D Niles: Conceptualization, Data curation, Methodology, Project administration. T Majmudar: Data curation. M Kirschen: Writing – review & editing, Supervision. J del Castillo: Writing – review & editing. C Buysse: Writing – review & editing, Supervision. A Topjian: Writing – review & editing, Supervision. V Nadkarni: Conceptualization, Writing – review & editing, Project administration, Supervision. S Gangadharan: Conceptualization, Writing – review & editing, Supervision.
REFERENCES
- 1.Holmberg MJ, Ross CE, Fitzmaurice G, et al. Annual incidence of adult and pediatric in-hospital cardiac arrest in the United States. Circ Cardiovasc Qual Outcomes 2019;12:e005580. [PMC free article] [PubMed] [Google Scholar]
- 2.Kirschen MP, Majmudar T, Beaulieu F, et al. Deviations from NIRS-derived optimal blood pressure are associated with worse outcomes after pediatric cardiac arrest. Resuscitation 2021;168:110–8. 10.1016/j.resuscitation.2021.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkins DL, Everson-Stewart S, Sears GK, et al. Epidemiology and outcomes from out-of-hospital cardiac arrest in children: the ROC epistry-cardiac arrest. Circulation 2009;119:1484–91. 10.1161/CIRCULATIONAHA.108.802678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jayaram N, McNally B, Tang F, Chan PS. Survival after out-of-hospital cardiac arrest in children. J Am Heart Assoc Cardiovasc Cerebrovasc Dis 2015;4:e002122. 10.1161/JAHA.115.002122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fink EL, Prince DK, Kaltman JR, et al. Unchanged pediatric out-of-hospital cardiac arrest incidence and survival rates with regional variation in North America. Resuscitation 2016;107:121–8. 10.1016/j.resuscitation.2016.07.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Topjian AA, de Caen A, Wainwright MS, et al. Pediatric post-cardiac arrest care: a scientific statement from the American Heart Association. Circulation 2019;140:e194–233. 10.1161/CIR.0000000000000697. [DOI] [PubMed] [Google Scholar]
- 7.Laverriere EK, Polansky M, French B, Nadkarni VM, Berg RA, Topjian AA. Association of duration of hypotension with survival after pediatric cardiac arrest. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc 2020;21:143–9. 10.1097/PCC.0000000000002119. [DOI] [PubMed] [Google Scholar]
- 8.Topjian AA, French B, Sutton RM, et al. Early postresuscitation hypotension is associated with increased mortality following pediatric cardiac arrest. Crit Care Med 2014;42:1518–23. 10.1097/CCM.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Topjian AA, Telford R, Holubkov R, et al. Association of early postresuscitation hypotension with survival to discharge after targeted temperature management for pediatric out-of-hospital cardiac arrest: secondary analysis of a randomized clinical trial. JAMA Pediatr 2018;172:143–53. 10.1001/jamapediatrics.2017.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Topjian AA, Raymond TT, Atkins D, et al. Part 4: pediatric basic and advanced life support: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2020;142:S469–523. 10.1161/CIR.0000000000000901. [DOI] [PubMed] [Google Scholar]
- 11.Trzeciak S, Jones AE, Kilgannon JH, et al. Significance of arterial hypotension after resuscitation from cardiac arrest*. Crit Care Med 2009;37:2895. 10.1097/CCM.0b013e3181b01d8c. [DOI] [PubMed] [Google Scholar]
- 12.Kilgannon JH, Roberts BW, Reihl LR, et al. Early arterial hypotension is common in the post-cardiac arrest syndrome and associated with increased in-hospital mortality. Resuscitation 2008;79:410–6. 10.1016/j.resuscitation.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van den Brule JMD, van der Hoeven JG, Hoedemaekers CWE. Cerebral perfusion and cerebral autoregulation after cardiac arrest. BioMed Res Int 2018;2018:4143636. 10.1155/2018/4143636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slovis JC, Bach A, Beaulieu F, Zuckerberg G, Topjian A, Kirschen MP. Neuromonitoring after pediatric cardiac arrest: cerebral physiology and injury stratification. Neurocrit Care 2023. 10.1007/s12028-023-01685-6. Published online April 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niles DE, Duval-Arnould J, Skellett S, et al. Characterization of pediatric in-hospital cardiopulmonary resuscitation quality metrics across an international resuscitation collaborative. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc 2018;19:421–32. 10.1097/PCC.0000000000001520. [DOI] [PubMed] [Google Scholar]
- 16.Roberts JS, Yanay O, Barry D. Age-based percentiles of measured mean arterial pressure in pediatric patients in a hospital setting. Pediatr Crit Care Med 2020;21:e759. 10.1097/PCC.0000000000002495. [DOI] [PubMed] [Google Scholar]
- 17.Esangbedo I, Yu P, Raymond T, et al. Pediatric in-hospital CPR quality at night and on weekends. Resuscitation 2020;146:56–63. 10.1016/j.resuscitation.2019.10.039. [DOI] [PubMed] [Google Scholar]
- 18.The ICU-RESUS and Eunice Kennedy Shriver National Institute of Child Health, Human Development Collaborative Pediatric Critical Care Research Network Investigator Groups. Effect of physiologic point-of-care cardiopulmonary resuscitation training on survival with favorable neurologic outcome in cardiac arrest in pediatric ICUs: a randomized clinical trial. JAMA 2022;327:934–45. 10.1001/jama.2022.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albrecht M, Dulfer K, Hunfeld M, de Jonge RCJ, Buysse CMP. What is the true meaning of a “good” pediatric cerebral performance category score at hospital discharge in pediatric cardiac arrest survivors? Crit Care Med 2023;51:e134–5. 10.1097/CCM.0000000000005857. [DOI] [PubMed] [Google Scholar]
- 20.Topjian AA, Telford R, Holubkov R, et al. The association of early post-resuscitation hypotension with discharge survival following targeted temperature management for pediatric in-hospital cardiac arrest. Resuscitation 2019;141:24–34. 10.1016/j.resuscitation.2019.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiu YK, Lui CT, Tsui KL. Impact of hypotension after return of spontaneous circulation on survival in patients of out-of-hospital cardiac arrest. Am J Emerg Med 2018;36:79–83. 10.1016/j.ajem.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 22.McGuigan PJ, Giallongo E, Blackwood B, et al. The effect of blood pressure on mortality following out-of-hospital cardiac arrest: a retrospective cohort study of the United Kingdom Intensive Care National Audit and Research Centre database. Crit Care 2023;27:4. 10.1186/s13054-022-04289-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ameloot K, Meex I, Genbrugge C, et al. Hemodynamic targets during therapeutic hypothermia after cardiac arrest: a prospective observational study. Resuscitation 2015;91:56–62. 10.1016/j.resuscitation.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Bhate TD, McDonald B, Sekhon MS, Griesdale DEG. Association between blood pressure and outcomes in patients after cardiac arrest: a systematic review. Resuscitation 2015;97:1–6. 10.1016/j.resuscitation.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 25.Russo JJ, James TE, Hibbert B, et al. Impact of mean arterial pressure on clinical outcomes in comatose survivors of out-of-hospital cardiac arrest: insights from the University of Ottawa Heart Institute Regional Cardiac Arrest Registry (CAPITAL-CARe). Resuscitation 2017;113:27–32. 10.1016/j.resuscitation.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Ameloot K, De Deyne C, Eertmans W, et al. Early goal-directed haemodynamic optimization of cerebral oxygenation in comatose survivors after cardiac arrest: the neuroprotect post-cardiac arrest trial. Eur Heart J 2019;40:1804–14. 10.1093/eurheartj/ehz120. [DOI] [PubMed] [Google Scholar]
- 27.Jakkula P, Pettila V, Skrifvars MB, et al. Targeting low-normal or high-normal mean arterial pressure after cardiac arrest and resuscitation: a randomised pilot trial. Intensive Care Med 2018;44:2091–101. 10.1007/s00134-018-5446-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kjaergaard J, Møller JE, Schmidt H, et al. Blood-pressure targets in comatose survivors of cardiac arrest. N Engl J Med 2022;387:1456–66. 10.1056/NEJMoa2208687. [DOI] [PubMed] [Google Scholar]
- 29.Niemelä V, Siddiqui F, Ameloot K, et al. Higher versus lower blood pressure targets after cardiac arrest: systematic review with individual patient data meta-analysis. Resuscitation 2023;189. 10.1016/j.resuscitation.2023.109862109862. [DOI] [PubMed] [Google Scholar]
- 30.Topjian AA, Scholefield BR, Pinto NP, et al. P-COSCA (Pediatric Core Outcome Set for Cardiac Arrest) in children: an advisory statement from the international liaison committee on resuscitation. Resuscitation 2021;162:351–64. 10.1016/j.resuscitation.2021.01.023. [DOI] [PubMed] [Google Scholar]
- 31.Hickson MR, Winters M, Thomas NH, et al. Long-term function, quality of life and healthcare utilization among survivors of pediatric out-of-hospital cardiac arrest. Resuscitation 2023:109768. 10.1016/j.resuscitation.2023.109768. Published online March 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


