Abstract
Background
Heart failure with a preserved ejection fraction (HFpEF) is common in the elderly (≥75 years) and associated with arterial stiffness. The mean age of HFpEF presentation is lower (40–55 years) in sub-Saharan Africa. No clinical study has been conducted on HFpEF in identifying and characterising this phenotype at a younger age, moreover in a South African black population where the risk of HFpEF is two times higher than in other ethnic groups. This study investigated the characteristics of HFpEF in a black South African population, the biochemical markers that predict HFpEF and cardiac structural changes in this HF phenotype.
Methods
Sixty-six participants with HFpEF and 213 controls were enrolled. All participants gave informed consent and completed a standardised questionnaire. Echocardiographic, anthropometric, central haemodynamic measurements, pulse wave velocity (PWV) and biomarker analysis were done.
Results
The mean age of HFpEF participants was 54.88 ± 13.51 years. Most of the participants (76 %) were between 20 and 64 years, while only 24 % were older. HFpEF participants were hypertensive, and more obese with increased incidence of alcohol consumption. PWV was increased in HFpEF (9.97 ± 2.78 m/s) when compared to participants without HFpEF (6.11 ± 2.18 m/s), p < 0.0001. There were no significant associations between central haemodynamic parameters, N-terminal pro B-type natriuretic peptide (NT-proBNP) (p = 0.9746), and galectin-3 (p = 0.2166). NT-proBNP, but not galectin-3, was associated with left ventricular hypertrophy (p = 0.0002) and left atrial diameter (p = 0.0005).
Conclusion
HFpEF in South Africa is predominant in obese young to middle-age individuals with arterial stiffness and who consume alcohol regularly. NT-proBNP could be used to diagnose HFpEF, however, should be interpreted with caution in populations with a high prevalence of obesity.
Keywords: Biomarkers, Haemodynamics, Cardiovascular disease, Risk factors, Heart failure with a preserved ejection fraction, Hypertension
1. Introduction
Heart failure with a preserved ejection fraction (HFpEF) is common in the elderly (≥75 years) and associated with arterial stiffness. The mean age of HFpEF presentation is lower (40–55 years) in sub-Saharan Africa [1], [2]. No clinical study has been conducted on HFpEF in identifying and characterising this phenotype at a younger age, moreover in a South African black population where the risk of HFpEF is two times higher than in other ethnic groups [3]. This study investigated biochemical markers and haemodynamic parameters that predict HFpEF and cardiac structural changes that are associated with HFpEF in a black South African population.
Heart disease, including heart failure (HF) is currently one of the leading causes of death in the United States and the Western world [4], [5]. HFpEF is characterised by a stiff left ventricle (LV) that cannot relax adequately in diastole – a condition known as diastolic dysfunction. Diastolic dysfunction may in turn result from processes including myocardial fibrosis triggered by hypertension (HT). The prevalence of HF is accepted as being between 1 % and 2 % of the general population, where HFpEF is prevalent in almost 50 % of all HF cases [6]. With such an extensive range of factors (presence of HT, diabetes mellitus (DM), valvular heart disease and myocardial infarction), it is clear that there is a relatively large population at risk, potentially in excess of 25 % of the total population [7]. There is currently limited insight into the prevalence and incidence of HF, more specifically HFpEF in sub-Saharan Africa, moreover in South Africa, although clinical characteristics and aetiologies of heart failure with a reduced ejection fraction (HFrEF) have been extensively studied previously [8], [9], [10]. Novel biomarkers for the diagnosis of HFpEF and haemodynamic measurements (pulse wave velocity and central blood pressures) in young to middle aged black South African patients have not been studied previously.
It is worth noting that as the population ages, the prevalence of HF will likely increase, exacerbated by continuing difficulties in the effective management of HT, the growing epidemic of DM type II, and the improved survival of patients with HF following an acute myocardial infarction [11], [12]. In contrast to higher income countries, the burden of HT and HT related diseases, such as HF, is currently increasing significantly in lower-income countries. This has been attributed to increased urbanisation [13], [14].
2. Methods
2.1. Study population
The study was approved by the Sefako Makgatho Health Sciences Research Ethics Committee (SMUREC/M/08/2017:PG) and was performed in compliance with the declaration of Helsinki. All participants gave informed consent before participating in the study. Potential study participants from African descent and older than 18 years, referred by both general and family physicians in primary and secondary health care settings, were screened based on a definite diagnosis of HFpEF as described in the 2016 European Society of Cardiology (ESC) guidelines for the diagnosis and treatment of acute and chronic HF. [15] Sixty-six (66) participants presenting with HFpEF signs and symptoms were enrolled between July 2017 and September 2018. The control group comprised of 213 participants without HFpEF in good health. Participants enrolled in the study underwent echocardiographic and central haemodynamic measurements on the same day that blood samples were collected for routine blood and biomarker analysis.
2.2. Questionnaire
A standardised English version of the African Project on Genes in Hypertension (APOGH) questionnaire was completed for each participant. The questionnaire was adjusted to comply with the requirements of this study. The APOGH questionnaire has previously shown to be reliable and feasible in collecting the required background information, previous family and medical history as well as determining lifestyle risk factors. Several studies of APOGH data have been published internationally using this questionnaire.
The questionnaire was administered to obtain demographic data and information on each participant’s medical history, smoking habits, intake of alcohol, use of medication, physical activity, presenting symptoms, and menopausal status. Other clinical data used was extracted from patient files (medical examination) with emphasis on the cardiovascular system. Further investigations in patients with HF included electrocardiogram and routine blood tests.
2.3. Transthoracic echocardiography
Before participating in the study, participants were diagnosed with HFpEF by a trained echocardiographer using transthoracic echocardiography. Echocardiography measurements were performed using a commercially available ultrasound system (GE Vivid 9). Participants were examined in the left lateral decubitus position using standard parasternal, short axis and apical views. Echocardiograph studies of participants were performed by an experienced senior echocardiographer and diagnosed according to the recommendations of the 2016 ESC guidelines for the diagnosis and treatment of acute and chronic HF. Left ventricular ejection fraction (LVEF) was measured using the modified biplane Simpson’s rule. Left ventricular end-diastolic diameter (LVEDd) and left ventricular end-systolic diameter (LVESd) were obtained from m-mode using parasternal short axis and parasternal long axis views. Left ventricular hypertrophy (LVH) and/or left atrial enlargement (LAE) were used as markers of structural heart disease.
2.4. Anthropometric and standard laboratory measurements
Anthropometric measurements included the participant’s height, body weight, waist and hip circumference using standard approaches. Participants were identified as being overweight if their body mass index (BMI) was ≥25 kg/m2 and obese if the BMI was ≥30 kg/m2 according to the European guidelines for obesity management in adults. [16] Kidney, liver, and thyroid function were measured through standard laboratory tests. A lipid profile and full blood count with random glucose and percentage glycated haemoglobin (HbA1c) were included. DM or an abnormal blood glucose level was defined as the use of oral hypoglycaemic drugs or the use of insulin or an HbA1c value of >6.1 %.
2.5. Conventional blood pressure (BP) measurements
BP measurements were obtained, according to standard guidelines, with a manual sphygmomanometer. BP measurements were measured five consecutive times after the participant rested in a supine position for five minutes. The BP cuff was deflated whereby Korotkov phases I and V were employed to identify systolic and diastolic BP respectively. The five BP readings were averaged and used for analysis. HT was defined as the use of antihypertensive treatment or a BP ≥140/90 mmHg.
2.6. Arterial stiffness and central haemodynamic parameters
Pulse wave velocity (PWV) was determined using applanation tonometry and PulsePen software. After participants had rested for 15 min in the supine position, arterial waveforms were determined at the carotid, radial and femoral pulse by applanation tonometry using a tonometer (PulsePen) interfaced with a computer employing PulsePen, version 6.21 software (AtCor Medical Pty. Ltd., West Ryde, New South Wales, Australia). Recordings where the systolic or diastolic variability of consecutive waveforms exceeded 5 %, or where the amplitude of the pulse wave signal was less than 80 mV, were discarded. The pulse wave was calibrated by manual BP measurements (auscultation) taken immediately before the recordings. From a validated inbuilt transfer function, an aortic waveform was generated from which central systolic blood pressure (SBPc), central diastolic blood pressure (DBPc) and central mean arterial blood pressure (MAPc) were derived. Central pulse pressure (PPc) was calculated as the difference between SBPc and DBPc and MAPc calculated as DBPc + 1/3(PPc).
To determine aortic PWV, distances from the suprasternal notch to the carotid sampling site (distance A) and from the suprasternal notch to the femoral artery (distance B) were measured. PWV distance was calculated as distance B minus distance A. Pulse transit time, calculated as the mean time difference between sites A and B were determined from the average of 12 consecutive beats. Aortic PWV was calculated as the ratio of the distance in metres to the transit time in seconds. To determine central BP, the radial waveform was recorded at the left arm over an eight second period and the pulse wave calibrated by auscultatory measurement of the brachial BP immediately before the recordings. From an inbuilt transfer function an aortic waveform was generated from which SBPc, DBPc and MAPc were derived. The magnitude of the forward and reflected waves was determined with wave separation analysis using the triangular flow wave method. The ratio between the reflected and forward waves was employed as an index of arterial stiffness.
2.7. Biomarker analysis
Blood samples of participants were centrifuged and immediately stored at −80 °C. Plasma concentrations of NT pro-BNP were measured using an enzyme-linked immunosorbent assay (Cloud Clone Corp, USA, SEA485Hu) with a lower detection limit of 17 pg/mL and intra-assay and interassay coefficients of variation ranging from less than 10 % and less than 12 % respectively. Plasma galectin-3 levels were measured using an enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, USA, DGAL 30) with a mean lower detection limit of 0.016 ng/mL and inter-assay and interassay coefficients of variation ranging from 3.67 % and 6.1 %, respectively.
2.8. Statistical analysis
SAS software package version 9.4 (SAS Institute Inc., Cary, NC) was used for data management and analysis. Data were expressed as mean ± the standard deviation (SD), median (interquartile range) (IQR) or proportions as a percentage. Unadjusted means and proportions were compared by the large-sample z test and the χ2-test. An independent t-test was performed to compare those with and those without HFpEF. A Wilcoxon signed rank test was performed in those instances where variables did not follow a normal distribution. Multivariate unadjusted and adjusted regression analysis was also performed to determine independent relationships. Confounders that were included in the regression analysis were identified from univariate analysis.
3. Results
3.1. Demographic and anthropometric characteristics
Table 1 gives the demographic and anthropometric characteristics of the study participants. More participants from middle age (45–64 years) [17] and more females (56 %), of whom 61 % were postmenopausal, compared to males (44 %) presented with HFpEF. Specifically (not shown in Table 1), the prevalence of HFpEF was 32 % in young patients (18–44 years), 44 % in middle age and 24 % in the elderly (≥65 years). In addition, a high proportion of participants were overweight or obese (BMI ≥25 kg/m2) with obesity statistically significant pronounced in HFpEF participants (86 %).
Table 1.
Demographic and anthropometric characteristics of study participants.
| PARAMETER | CONTROLS | PARTICIPANTS WITH HFpEF |
|---|---|---|
| Sample number (n) | 213 | 66 |
| % Female | 57.75 | 56.06 |
| % Postmenopausal females | 70.27 | 60.66 |
| Age (years) | 52.81(24.81) | 56.00(21.00) |
| % ≤60 years | 64 | 62 |
| Height (m) | 1.60(0.12) | 1.62(0.14) |
| Weight (kg) | 77.00(24.00) | 81.40(30.40)* |
| BMI (kg/m2) | 29.00(10.90) | 32.16(10.13)** |
| Waist circumference (cm) | 93.55 ± 17.14 | 97.48 ± 16.76 |
| Hip circumference (cm) | 107.00(19.00) | 108.70(20.80) |
| Waist:Hip | 0.88(0.15) | 0.88(0.13) |
| % Overweight/Obese | 69.48 | 85.71** |
| % Smokers | 17.84 | 13.85 |
| % Alcohol | 16.90 | 33.85** |
| % With HT | 55.39 | 69.23 |
| % Treated for HT | 34.74 | 83.08*** |
| % With DM I/II | 13.62 | 19.70 |
Data is presented as mean ± SD, median(IQR) or proportions. *p < 0.05, **p < 0.01, ***p < 0.001 Participants without HFpEF vs participants with HFpEF. HFpEF, heart failure with a preserved ejection fraction; BMI, body mass index; WHR, waist to hip ratio; HT, hypertension; DM I/II, diabetes mellitus I/II; SD, standard deviation; IQR, interquartile range).
Generally, a lower percentage of participants presented with DM Type I/II in participants without HFpEF (14 %) when compared to those with HFpEF (20 %). On average a low proportion of participants in both groups reported either regular smoking or regular consumption of alcohol. However, the percentage alcohol consumption was statistically significantly greater in those with HFpEF compared to those without HFpEF. The preferred type of alcohol included beer, cider, and wine. A greater proportion of HFpEF participants were hypertensive compared to the control group (69.23 % vs 55.39 %). The percentage of participants with HFpEF receiving antihypertensive treatment was also significantly higher than the participants without HFpEF (83 % vs 35 %).
3.2. Clinical, haemodynamic and laboratory characteristics
Participants generally presented with symptoms of breathlessness, tiredness, fatigue, and signs of peripheral oedema such as swelling of the ankles regardless of age. The following common comorbid conditions associated with HFpEF were observed in South African HFpEF participants: HT, DM Type I/II, obesity, obstructive sleep apnoea, chronic kidney disease, chronic obstructive pulmonary disease, and pulmonary HT. None of the participants with HFpEF reported coronary artery disease. The clinical, haemodynamic and laboratory characteristics are shown in Table 2. The PPp was significantly higher in HFpEF participants (p < 0.0001) when compared to participants without HFpEF. The PPp for both groups were above the normal range (40 mmHg) [18]. In the participants with HFpEF with a mean age of 54.88 ± 13.51 years, a high carotid to femoral PWV of 9.97 ± 2.78 m/s was observed when compared to those without HFpEF (6.11 ± 2.18 m/s).
Table 2.
Clinical characteristics of study participants.
| PARAMETER | CONTROLS | PARTICIPANTS WITH HFpEF |
|---|---|---|
| Peripheral haemodynamic measurements | ||
| SBP/DBP (mmHg) | 130(24.00)/84(14.00) | 136(18.00)***/83(11.80) |
| PP (mmHg) | 42(18.00) | 50(15.60)*** |
| HR (bpm) | 63(16.00) | 70(12.00)** |
| Central haemodynamic measurements | ||
| Carotid-Femoral PWV (m/s) | 5.59(2.33) | 9.35(3.60)*** |
| SBP/DBP (mmHg) | 130(26.00)/83(15.20) | 125(24.00)/82(12.50) |
| PP (mmHg) | 33(17.00) | 39(19.50)*** |
| HR (bpm) | 63(16.00) | 68(18.00)** |
| Laboratory characteristics | ||
| Total cholesterol (mmol/L) | 4.85 ± 0.96 | 4.77 ± 0.95 |
| Galectin-3 (pg/mL) | 7.95(3.33) | 8.93(3.04)** |
| NT-proBNP (pg/mL) | – | 15.56(104.10) |
Data is presented as mean ± SD or median (IQR). *p < 0.05, **p < 0.01, ***p < 0.001 Participants without HFpEF vs participants with HFpEF. HFpEF, heart failure with a preserved ejection fraction; SBP, systolic blood pressure; DBP, diastolic blood pressure; PP, pulse pressure; HR, heart rate; PWV, pulse wave velocity; NT-proBNP, N-terminal pro B-type natriuretic peptide; SD, standard deviation; IQR, interquartile range.
In addition, statistically significant differences between participants without and with HF were observed in peripheral haemodynamic measures (SBP, PP and heart rate (HR)) with p-values < 0.001, < 0.0001 and 0.008 respectively. Carotid to femoral PWV (p < 0.0001) and PPc (p = 0.0002) showed statistically significant differences between the two groups. There were no statistically significant differences between peripheral DBP, SBPc and DBPc.
Regarding biomarkers of HF, a significantly higher galectin-3 level (p = 0.0026) in the HFpEF group was noted compared to the control group. Galectin-3 concentrations in both groups were still below the upper limit cut-off point of 17.7 pg/mL [19]. The NT-proBNP level was not measured in the participants without HFpEF as those participants were not presenting with signs and symptoms of HF. Importantly, the level of NT-proBNP of participants with HFpEF was lower than the 125 pg/mL cut-off given by the ESC guidelines for the diagnosis and management of HFpEF. Normal blood creatinine values were measured in HFpEF patients (95.56 ± 91.64 µmol/L) excluding renal disease as a comorbidity.
The clinical cut-off points were determined for PWV, PPp, PPc, galectin-3 as 7,5 m/s, 39,4 mmHg, 23,5 mmHg and 7,6 pg/mL respectively as shown in Fig. 8. PWV showed the highest sensitivity and specificity, followed by galectin-3.
Fig. 8.
ROC curves for PWV, PPp, PPc, and galectin-3 to determine clinical cut-off points. The optimal cut-off was determined by maximising the Youden index as maximum = sensitivity + specificity − 1. ROC, receiver operating curve; PWV, pulse wave velocity; PPp, peripheral pulse pressure; PPc, central pulse pressure.
3.3. Echocardiography data
The echocardiographic data of participants with and without HFpEF is presented in Table 3. A LVEF of above 50 % was noted in both groups. Surprisingly the HFpEF group presented with a significantly higher EF (69.00(6.00) % vs 66.87(12.69) %). An increased fractional shortening percentage (FS) (39.66(12.51) %) and LVEDd (4.70(0.84) cm) was observed in participants without HFpEF, with a higher interventricular septum thickness diameter in diastole (IVSd) (1.30(0.20) cm) and left ventricular posterior wall diameter at end diastole (LVPWd) (1.20(0.10) cm) in the HFpEF group. A large difference between the two groups in the LV mass (LVM), relative wall thickness (RWT) and LV mass index (LVMI) was noted, with an LVM of 22.38 ± 70.33 g in those with HFpEF compared to 142.91 ± 54.38 g in those without HFpEF. The diameters of the LA (3.74 ± 0.54 cm vs 2.66 ± 0.48 cm) as well as the diameter of the aorta in diastole (2.62 ± 0.46 cm vs 2.52 ± 0.44 cm), were higher in HFpEF participants compared to the control group. Furthermore, the ratios of the LA diameter to the diameter of the aorta in diastole (LA/Ao) (1.39(0.44) vs 1.05(0.23) and LA/LV (0.82 ± 0.12 vs 0.56 ± 0.10) ratio measurements were increased in HFpEF participants compared to the participants without HFpEF. All echocardiographic parameters showed a statistically significant difference between participants without HFpEF and participants with HFpEF except for LVEd, LVPWs and diameter of the aorta in diastole. The IVSd, LVPWd, LVM, RWT, LVMI, LA, LA/Ao and ratio of LA and LV (LA/LV) parameters were significantly increased (p < 0.0001) in participants with HFpEF compared to the control group.
Table 3.
Echocardiography data of study participants.
| PARAMETER | CONTROLS | PARTICIPANTS WITH HFpEF |
|---|---|---|
| LVEF (%) | 66.87(12.69) | 69.00(6.00)* |
| FS (%) | 39.66(12.51) | 36.50(36.80)** |
| LVEDd (cm) | 4.70(0.84) | 4.50(0.80) |
| IVSd (cm) | 0.88(0.21) | 1.30(0.20)*** |
| LVPWd (cm) | 0.87(0.18) | 1.20(0.10)*** |
| LVPWs (cm) | 1.20(0.29) | 1.35(0.44) |
| LVM (g) | 136.96(72.44) | 224.33(77.03)*** |
| RWT | 0.38(0.08) | 0.55(0.12)*** |
| LVMI (g/m2) | 37.24(18.03) | 62.13(27.66)*** |
| LA diameter (cm) | 2.66 ± 0.48 | 3.74 ± 0.54*** |
| Ao (cm) | 2.52 ± 0.44 | 2.62 ± 0.46 |
| LA/Ao | 1.05(0.23) | 1.39(0.44)*** |
| LA/LV | 0.56 ± 0.10 | 0.82 ± 0.12*** |
| E (mm/s) | 66.40(31.00) | 0.63(0.27)** |
| A (mm/s) | 61.70(29.70) | 0.73(0.26)* |
| E/A | 1.08(0.54) | 0.82(0.19)*** |
| e′ Septal (mm/s) | 0.09(0.05) | 0.07(0.04)* |
| a′ Septal (mm/s) | 0.08(0.03) | 0.10(0.05)*** |
| E/e’ | 7.68(3.93) | 8.67(6.03) |
Data is presented as mean ± SD or median (IQR). *p < 0.05, **p < 0.01, ***p < 0.001 Participants without HFpEF vs participants with HFpEF. LVMI indexed to height (2.7). HFpEF, heart failure with a preserved ejection fraction; LVEF, left ventricular ejection fraction; FS, fractional shortening; LVEDd, left ventricular end-diastolic diameter; IVSd, interventricular septum thickness diameter in diastole; LVPWd, left ventricular posterior wall diameter at end diastole; LVPWs, left ventricular posterior wall diameter at end systole; LVM, left ventricular mass; RWT, relative wall thickness; LVMI, left ventricular mass index; LA, left atrium/atrial; Ao, diameter of aorta in diastole; E, peak velocity blood flow from left ventricular relaxation in early diastole; A, peak velocity flow in late diastole caused by atrial contraction; e’, peak septal e’ velocity; a’, peak septal a’ velocity; E/e’, ratio of early mitral inflow velocity to early diastolic mitral annulus velocity ratio; SD, standard deviation; IQR, interquartile range.
3.4. Association between central haemodynamic measurements, biomarker concentrations and echocardiography parameters
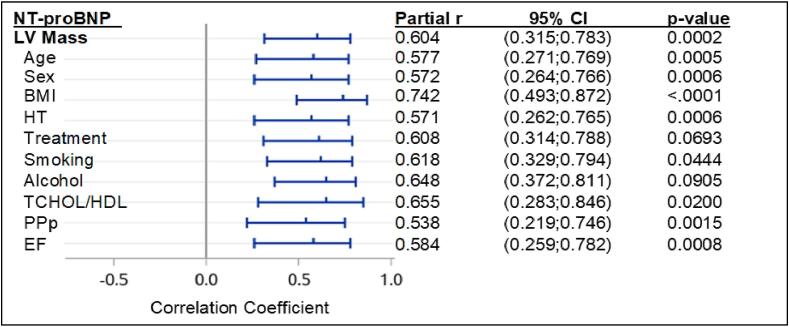
The strongest relationships were shown between peripheral pulse pressure (PPp) and PPc, NT-proBNP and LA diameter, LA diameter and LA/LV and galectin-3 and NT-proBNP (p < 0.001) as indicated in Table 1 (Supplementary material). Statistically significant relationships were observed between PWV and PPp (Table 1 (Supplementary material)), NT-proBNP and PPp (Fig. 1, Fig. 4), LA diameter and PWV (Fig. 7), NT-proBNP and PWV, LVMI and PWV, LVMI and LA diameter, LA/Ao and LA diameter, NT-proBNP and LA/LV and galectin-3 and NT-proBNP (Table 2 (Supplementary material)). Furthermore, only a trend effect was noted between LA/Ao and NT-proBNP (p = 0.065). Fig. 1, Fig. 2, Fig. 3 shows the linear regression analysis for NT-proBNP and echocardiography parameters as well as PWV and echocardiography parameters. A strong relationship was observed between NT-proBNP and LA diameter (p = 0.0005) (Fig. 1). A statistically significant regression relationship was seen between biomarkers of HF (NT-proBNP and galectin-3) and LA diameter above 3.5 cm in diastole (Fig. 2). NT-proBNP was independently associated with both LVM and LA diameter after adjustments for physiological and cardiovascular risk factors (Fig. 5, Fig. 6).
Fig. 1.
Linear regression relationships between haemodynamic, echocardiography and biomarker parameters in HFpEF patients (n = 66). *p < 0.05, **p < 0.01, ***p < 0.001. NT-proBNP, N-terminal pro B-type natriuretic peptide; PPp, peripheral pulse pressure; LV, left ventricle/ventricular; LA, left atrium/atrial.
Fig. 4.
Partial correlation coefficients (r) and 95% confidence intervals for the relationship between NT-proBNP and PPp with adjustments for physiological and cardiovascular risk factors. P-values are for significant independent relationships. NT-proBNP, N-terminal pro B-type natriuretic peptide; PPp, peripheral pulse pressure; BMI, body mass index; HT, hypertension; TCHOL/HDL, total cholesterol/high-density lipoprotein; LA, left atrium/atrial; CI, confidence interval.
Fig. 7.
Partial correlation coefficients (r) and 95% confidence intervals for the relationship between PWV and LA diameter with adjustments for physiological and cardiovascular risk factors. P-values are for significant independent relationships. PWV, pulse wave velocity; LA, left atrium/atrial; BMI, body mass index; DM I/II, diabetes mellitus type I/II; HT, hypertension; TCHOL/HDL, total cholesterol/high-density lipoprotein; CI, confidence interval.
Fig. 2.
Linear regression relationships between biomarkers of HF and LA diameter (≥3.5 cm). *p < 0.05, **p < 0.01, ***p < 0.001. LA; left atrium/atrial; NT-proBNP, N-terminal pro B-type natriuretic peptide.
Fig. 3.
Linear regression relationship between PWV carotid to femoral and LA diameter in participants with HF. *p < 0.05, **p < 0.01, ***p < 0.001. PWV; pulse wave velocity; LA, left atrium/atrial.
Fig. 5.
Partial correlation coefficients (r) and 95% confidence intervals for the relationship between NT-proBNP and LVM with adjustments for physiological and cardiovascular risk factors. P-values are for significant independent relationships. NT-proBNP, N-terminal pro B-type natriuretic peptide; LV, left ventricle/ventricular; BMI, body mass index; HT, hypertension; TCHOL/HDL, total cholesterol/high-density lipoprotein; PPp, peripheral pulse pressure; EF, ejection fraction; CI, confidence interval.
Fig. 6.
Partial correlation coefficients (r) and 95% confidence intervals for the relationship between NT-proBNP and echocardiography parameters with adjustments for physiological and cardiovascular risk factors. P-values are for significant independent relationships. NT-proBNP, N-terminal pro B-type natriuretic peptide; LA/LV, ratio of left atrium/atria diameter to left ventricle/ventricular diameter; BMI, body mass index; DM I/II, diabetes mellitus type I/II; HT, hypertension; TCHOL/HDL, total cholesterol/high-density lipoprotein; PPp, peripheral pulse pressure; CI, confidence interval.
4. Discussion
The main findings of this study are as follows: Firstly, the mean age for the prevalence or onset of HFpEF in black South African patients is 54.88 ± 13.51 years with 76 % of HFpEF participants being of young to middle-age, while only 24 % were older (>65 years). Secondly, arterial stiffness as assessed by PWV was high in participants with HFpEF when compared to participants without this pathology. However, there were no significant associations between central haemodynamic parameters, NT-proBNP and galectin-3. Lastly, NT-proBNP, but not galectin-3, was significantly associated with LVH, LA diameter and LA/LV, in both univariate and multivariate analysis, suggesting the favourable use of NT-proBNP in the diagnosis of HFpEF, particularly in patients with both structural heart disease as marked by LVH and LAE, and those with LV diastolic dysfunction.
This study shows that in a community with a high prevalence of HT and obesity, HFpEF occurs more frequently at a younger age as opposed to the elderly. These participants presents with stiff arteries (PWV of 9.97 ± 2.78 m/s), a feature that is not common in developed countries (6.5 ± 1.6 m/s) [11], [20], [21]. Urbanisation is a likely cause of elevated BP in black South Africans that ultimately lead to HT, which can be attributed to accompanying dietary, sedentary lifestyle, and behavioural changes. Indeed, elevated arterial stiffness increases myocardial afterload, which ultimately leads to impaired LV relaxation, increased LV filling pressure and oxygen consumption stress, which are precursors to exercise intolerance and ultimately HFpEF [11].
The study further confirms the presentation of concentric remodelled LV in this age group which has only been reported previously in the elderly [22], probably due to longstanding HT and obesity. Evidence of abnormal LV relaxation and increased LV stiffness was confirmed by increased FS which affects early ventricular filling.
In comparing and contrasting the findings with other studies conducted in different populations or regions, especially regarding the age of onset, prevalence, and associated factors of HFpEF, the mean age for HFpEF in black South Africans in this study is low. Generally, the mean age of HF presentation is considerably lower (40–55 years) in sub-Saharan Africa [1], [2] and similarly these studies were conducted in black individuals in Nigeria and Ghana. Findings are in contrast with a later disease onset reported in the studies conducted in American and European countries [23], [24], [25], [26], [27], [28], [29]. The differences in the prevalence and onset of HFpEF observed in black South Africans can be attributed to the high prevalence and early onset of HT at an early age when compared to the prevalence of 35 % observed in the United States [30]. Collaboration with laboratories that specialise in molecular mechanisms can help to explore the endothelial dysfunction, and genetic makeup in these patients. Addressing the link between early onset of disease and risk factors is crucial to unravel the pathophysiological mechanisms of HFpEF in black patients in South Africa, prevention, and the clinical management thereof.
Obesity, mainly waist circumference, contributes to the pathogenic pathway of HFpEF through endothelium dysfunction and systemic inflammation [31]. In this regard, markers of endothelial function such as such as endothelin-1 and nitric oxide, can provide insight into the health of the blood vessels and the risk of cardiovascular disease in our participants. Although decline in oestrogen levels during menopause could lead to increased reactive oxygen species production, potentially contributing to a systemic inflammatory state which could lead to endothelial dysfunction and myocardial fibrosis, it is unlikely that menopausal stage played a major role in arterial stiffness of these HFpEF patients as the prevalence of menopause was similar in those with and without HFpEF.
The average level of NT-proBNP in the HFpEF participant group was below the suggested cut-off point of 125 pg/mL given by the ESC [15]. This finding concurs with several other studies that reported low levels of circulating NT-proBNP in obese HF patients despite having increased LV filling pressures [32], [33], [34], [35]. Increased levels of NT-proBNP reflect the attempt of the body to restore homeostasis in a response to increased LV filling pressures [36]. BNP and NT-proBNP are nonetheless depressed in obesity despite higher LV end-diastolic pressures [34]. The increased circulating levels of NT-proBNP is therefore secondary to LV dysfunction and independent of adipose tissue functioning. Hence, NT-proBNP is involved in very different and independent physiological processes that include both the cardiovascular system and the function of adipose tissue [35]. Increased NT-proBNP clearance by adipose tissue in HFpEF patients and decreased NT-proBNP production due to ‘cardiac cachexia’ have also been proposed as possible mechanisms [35], but the reduced production or secretion of NT-proBNP in the atrial or ventricular myocardium in HFpEF remains to be explored [37]. This was also observed in a study done on 30 stable, mostly obese middle-aged HFpEF patients [32]. In contrast, analysis from the TOPCAT trial showed that among obese HFpEF patients with elevated natriuretic peptides levels, such as NT-proBNP, identify a higher risk phenotype with a significantly increased incidence of both mortality and HF hospitalisation [38]. In this regard, caution should be taken when diagnosing HFpEF in obese individuals. The diagnosis should therefore be confirmed by the E/A or E/e’ ratio, cardiac structure, and function through echocardiography. Treatment or management of HFpEF in black obese individuals in other regions of the continent and in developed countries should include weight loss and target obesity induced pathologies.
Lastly, the use of clinical cut-off points for PWV, PPp, PPc and galectin-3 could be further explored as potential markers for the diagnosis and management of HFpEF patients in this population. PWV, a marker of arterial stiffness, showed the highest sensitivity and specificity followed by galectin-3, a marker of fibrosis and remodelling.
5. Limitations and recommendations
Our study has several limitations. This study was conducted at multiple research sites, however the sample size for HFpEF participants could be increased for the results to be more representative at population level. Increasing the sample size will also allow sex specific differences in HFpEF participants and the interaction of modifiable risk factors and common comorbidities leading to early onset of this HF phenotype in this ethnic group to be explored. A bigger sample size and longitudinal follow-ups (minimum of 6-months) are recommended to explore the individual and cumulative effects of risk factors on the progression of disease. In that case, data can be extrapolated to represent the ethnic group or population, thus provide more accurate results.
Another shortcoming of the study is that most of the participants were already on treatment for HT. Treatment included vasodilators, beta-blockers, diuretics, calcium channel blockers, and angiotensin converting enzyme inhibitors. These medications were taken chronically and might have affected the vascular and cardiac parameters discussed in this study, leading to a lack of associations between central haemodynamic parameters, NT-proBNP and galectin-3. The degree of arterial stiffness, the pumping ability of the heart, are among the other parameters that also might have been affected by treatment. Further research is warranted to probe the interaction of modifiable risk factors and common comorbidities on cardiac structure and function before and after treatment for HT, moreover in studies involving a black population or patients.
Larger studies of patients with LVH due to HT, but without clinically apparent HFpEF, are needed to clarify the role of NT-proBNP in the diagnosis of LVH in the routine follow-up of patients with HT, and in the monitoring of the regression of LVH with treatment for HT.
6. Conclusion
HFpEF is more prevalent in a young to middle-aged black South African community sample with increased arterial stiffness as assessed by high PWV and longstanding HT. NT pro-BNP, but not galectin-3, is independently associated with both LA diameter and LVH, hence NT pro-BNP could be used for the diagnosis of HFpEF in populations with high prevalence of obesity and HT.
7. Study Registration Number (Ethics)
The study was approved by the Sefako Makgatho Health Sciences Research Ethics Committee (SMUREC/M/08/2017:PG).
Sources of funding
This study was supported by the National Research Foundation (NRF) (Grant number: 107368) and the Department of Physiology, Sefako Makgatho Health Sciences University.
CRediT authorship contribution statement
M van Hoogland-van Heerden: Methodology, Investigation, Formal analysis, Data curation, Conceptualization, Project administration, Visualization, Writing – original draft, Writing – review & editing. LH Böhmer: Supervision, Writing – review & editing. O Heyneke: Data curation. T Lechaba: Data curation. L Scott: Project administration. G Norton: Resources, Funding acquisition, Conceptualization, Validation. A Woodiwiss: Conceptualization, Formal analysis, Funding acquisition, Resources, Validation, Writing – review & editing. P Mntla: Validation, Resources, Conceptualization. OHI Majane: Writing – review & editing, Validation, Supervision, Resources, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study would not have been possible without the voluntary collaboration of all the study participants, the Department of Cardiology at the Dr George Mukhari Academic Hospital, and collaborators from the University of the Witwatersrand.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2024.101408.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Sliwa K., Wilkinson D., Hansen C., Ntyintyane L., Tibazarwa K., Becker A., Stewart S. Spectrum of heart disease and risk factors in a black urban population in South Africa (the heart of Soweto study): a cohort study. Lancet. 2008;371:915–922. doi: 10.1016/S0140-6736(08)60417-1. [DOI] [PubMed] [Google Scholar]
- 2.Ogah O.S., Adegbite G.D., Akinyemi R.O., Adesina J.O., Alabi A.A., Udofia O.I., Ogundipe R.F., Osinfade J.K.L. Spectrum of heart diseases in a new cardiac service in Nigeria: an echocardiographic study of 1441 subjects in Abeokuta. BMC. Res. Notes. 2008;1:98. doi: 10.1186/1756-0500-1-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bozkurt B., Ahmad T., Alexander K.M., Baker W.L., Bosak K., Breathett K., Fonarow G.C., Heidenreich P., Ho J.E., Hsich E., Ibrahim N.E., Jones L.M., Khan S.S., Khazanie P., Koelling T., Krumholz H.M., Khush K.K., Lee C., Morris A.A., Page R.L., II, Pandey A., Piano M.R., Stehlik J., Stevenson L.W., Teerlink J.R., Vaduganathan M., Ziaeian B. Heart failure epidemiology and outcomes statistics: a report of the heart failure society of America. J. Card. Fail. 2023;29:1412–1451. doi: 10.1016/j.cardfail.2023.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaur R., Isherwood A., Ayodele L., Hughes M. The global burden of chronic heart failure. Eur. Heart J. 2017;38:S504. [Google Scholar]
- 5.Vaduganathan M., Solomon S.D. Expanding the global borders of heart failure: the SHOP and PEOPLE studies. Eur. Heart J. 2018;39:1781–1783. doi: 10.1093/eurheartj/ehy152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Urbanek K., Cappetta D., Coppini R., Ciuffreda L.P., Esposito G., Rossi F., Berrino L., De Angelis A. Treatment of HFpEF: an unresolved enigma. Vasc. Pharmacol. 2018;103:68. [Google Scholar]
- 7.McDonald K., Wilkinson M. Evolving use of natriuretic peptides as part of strategies for heart failure prevention. Clin. Chem. 2017;63:66–72. doi: 10.1373/clinchem.2016.255075. [DOI] [PubMed] [Google Scholar]
- 8.Makubi A., Hage C., Lwakatare J., Kisenge P., Makani J., Rydén L., Lund L.H. Contemporary aetiology, clinical characteristics and prognosis of adults with heart failure observed in a tertiary hospital in Tanzania: the prospective Tanzania heart failure (TaHeF) study. Heart. 2014;100:1235–1241. doi: 10.1136/heartjnl-2014-305599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ntusi N.B.A., Mayosi B.M. Epidemiology of heart failure in sub-Saharan Africa. Expert Rev. Cardiovasc. Ther. 2009;7:169–180. doi: 10.1586/14779072.7.2.169. [DOI] [PubMed] [Google Scholar]
- 10.Bloomfield G.S., Barasa F.A., Doll J.A., Velazquez E.J. Heart failure in sub-Saharan Africa. Curr. Cardiol. Rev. 2013;9:157–173. doi: 10.2174/1573403X11309020008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lüers C., Trippel T.D., Seeländer S., Wachter R., Hasenfuss G., Lindhorst R., Bobenko A., Nolte K., Pieske B., Edelmann F. Arterial stiffness and elevated left ventricular filling pressure in patients at risk for the development or a previous diagnosis of HF - a subgroup analysis from the DIAST-CHF study. J. Am. Soc. Hypertens. 2017;11:303–313. doi: 10.1016/j.jash.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Pfeffer M.A., Shah A.M., Borlaug B.A. Heart failure with preserved ejection fraction in perspective. Circ. Res. 2019;124:1598–1617. doi: 10.1161/CIRCRESAHA.119.313572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh R., Suh I., Singh V., Chaithiraphan S., Laothavorn P., Sy R.G., Babilonia N.A., Rahman A.R., Sheik S., Tomlinson B., Sarraf-Zadisan N. Hypertension and stroke in Asia: prevalence, control and strategies in developing countries from prevention. J. Hum. Hypertens. 2000;14:749–763. doi: 10.1038/sj.jhh.1001057. [DOI] [PubMed] [Google Scholar]
- 14.Tromp J., Claggett B.L., Liu J., Jackson A.M., Jhund P.S., Køber L., Widimský J., Boytsov S.A., Chopra V.K., Anand I.S., Ge J., Chen C.-H., Maggioni A.P., Martinez F., Packer M., Pfeffer M.A., Pieske B., Redfield M.M., Rouleau J.L., Van Veldhuisen D.J., Zannad F., Zile M.R., Rizkala A.R., Inubushi-Molessa A., Lefkowitz M.P., Shi V.C., McMurray J.J.V., Solomon S.D., Lam C.S.P. Global differences in heart failure with preserved ejection fraction. Circ. Heart Fail. 2021;14:e007901. doi: 10.1161/CIRCHEARTFAILURE.120.007901. [DOI] [PubMed] [Google Scholar]
- 15.Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G.F., Coats A.J.S., Falk V., González-Juanatey J.R., Harjola V.-P., Jankowska E.A., Jessup M., Linde C., Nihoyannopoulos P., Parissis J.T., Pieske B., Riley J.P., Rosano G.M.C., Ruilope L.M., Ruschitzka F., Rutten F.H., van der Meer P., Group ESCSD ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution o. Eur. Heart J. 2016;2016(37):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 16.Yumuk V., Tsigos C., Fried M., Schindler K., Busetto L., Micic D., Toplak H. European guidelines for obesity management in adults. Obes. Facts. 2015;8:402–424. doi: 10.1159/000442721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Statistics South Africa. Mortality and causes of death in South Africa, 2016, Findings from death notification. Pretoria (Gauteng), 2016.
- 18.Safar M.E., St S.L., Safavian A.L., Pannier B.M., London G.M. Pulse pressure in sustained essential hypertension: a haemodynamic study. J. Hypertens. 1987;5:213–218. doi: 10.1097/00004872-198704000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Lok D.J.A., Van Der Meer P., de la Porte P.W.B-A., Lipsic E., Van Wijngaarden J., Hillege H.L., van Veldhuisen D.J. Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: data from the DEAL-HF study. Clin. Res. Cardiol. 2010;99:323–328. doi: 10.1007/s00392-010-0125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferreira A.V.L., Viana M.C., Mill J.G., Asmar R.G., Cunha R.S. Racial differences in aortic stiffness in normotensive and hypertensive adults. J. Hypertens. 1999;17:631–637. doi: 10.1097/00004872-199917050-00006. [DOI] [PubMed] [Google Scholar]
- 21.Chaturvedi N., Bulpitt C.J., Leggetter S., Schiff R., Nihoyannopoulos P., Strain W.D., Shore A.C., Rajkumar C. Ethnic differences in vascular stiffness and relations to hypertensive target organ damage. J. Hypertens. 2004;22:1731–1737. doi: 10.1097/00004872-200409000-00017. [DOI] [PubMed] [Google Scholar]
- 22.Shimizu G., Hirota Y., Kita Y., Kawamura K., Saito T., Gaasch W.H. Left ventricular midwall mechanics in systemic arterial hypertension. Myocardial function is depressed in pressure-overload hypertrophy. Circulation. 1991;83:1676–1684. doi: 10.1161/01.cir.83.5.1676. [DOI] [PubMed] [Google Scholar]
- 23.Kaila K., Haykowsky M.J., Thompson R.B., Paterson D.I. Heart failure with preserved ejection fraction in the elderly: scope of the problem. Heart Fail. Rev. 2012;17:555–562. doi: 10.1007/s10741-011-9273-z. [DOI] [PubMed] [Google Scholar]
- 24.Lee D.S., Gona P., Vasan R.S., Larson M.G., Benjamin E.J., Wang T.J., Tu J.V., Levy D. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: Insights from the Framingham Heart Study of the National Heart, Lung, and Blood Institute. Circulation. 2009;119:3070–3077. doi: 10.1161/CIRCULATIONAHA.108.815944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldberg R.J., Ciampa J., Lessard D., Meyer T.E., Spencer F.A. Long-term survival after heart failure: a contemporary population-based perspective. Arch. Intern. Med. 2007;167:490–496. doi: 10.1001/archinte.167.5.490. [DOI] [PubMed] [Google Scholar]
- 26.Onwuanyi A., Hodges D., Avancha A., Weiss L., Rabinowitz D., Shea S., Francis C.K. Hypertensive vascular disease as a cause of death in blacks versus whites: autopsy findings in 587 adults. Hypertension. 1998;31:1070–1076. doi: 10.1161/01.hyp.31.5.1070. [DOI] [PubMed] [Google Scholar]
- 27.Thomas S., Booth J., Dai C., Li X., Allen N., Calhoun D., Carson A. Cumulative incidence of hypertension by 55 years of age in blacks and whites: the CARDIA study. J. Am. Heart Assoc. 2018;7:e007988. doi: 10.1161/JAHA.117.007988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spence J.D., Rayner B.L. Hypertension in blacks: individualised therapy based on renin/aldosterone phenotyping. Hypertension. 2018;72:263–269. doi: 10.1161/HYPERTENSIONAHA.118.11064. [DOI] [PubMed] [Google Scholar]
- 29.Lacruz M.E., Kluttig A., Hartwig S., Löer M., Tiller D., Greiser K.H., Werdan K., Haerting J. Prevalence and incidence of hypertension in the general adult population: results of the CARLA-cohort study. Medicine (Baltimore) 2015;94:e952–e. doi: 10.1097/MD.0000000000000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hajjar I., Kotchen J.M., Kotchen T.A. Hypertension: trends in prevalence, incidence, and control. Annu Rev Public Heal. 2006;27:465–490. doi: 10.1146/annurev.publhealth.27.021405.102132. [DOI] [PubMed] [Google Scholar]
- 31.Shah A.M., Pfeffer M.A. The many faces of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2012;9:555–556. doi: 10.1038/nrcardio.2012.123. [DOI] [PubMed] [Google Scholar]
- 32.Buckley L.F., Canada J.M., Del Buono M.G., Carbone S., Trankle C.R., Billingsley H., Kadariya D., Arena R., Van Tassell B.W., Abbate A. Low NT-proBNP levels in overweight and obese patients do not rule out a diagnosis of heart failure with preserved ejection fraction. ESC Heart Fail. 2018;5:372–378. doi: 10.1002/ehf2.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park S.J., Cho K.I., Jung S.J., Choi S.W., Choi J.W., Lee D.W., Lee H.G., Kim T.I. N-terminal pro-b-type natriuretic peptide in overweight and obese patients with and without diabetes: an analysis based on body mass index and left ventricular geometry. Korean Circ J. 2009;39:538–544. doi: 10.4070/kcj.2009.39.12.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor J.A., Christenson R.H., Rao K., Jorge M., Gottlieb S.S. B-type natriuretic peptide and N-terminal pro b-type natriuretic peptide are depressed in obesity despite higher left ventricular end diastolic pressures. Am. Heart J. 2006;152:1071–1076. doi: 10.1016/j.ahj.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 35.Khalid U., Wruck L.M., Quibrera P.M., Bozkurt B., Nambi V., Virani S.S., Jneid H., Agarwal S., Chang P.P., Loehr L., Basra S.S., Rosamond W., Ballantyne C.M., Deswal A. BNP and obesity in acute decompensated heart failure with preserved versus reduced ejection fraction: the atherosclerosis risk in communities surveillance study. Int. J. Cardiol. 2017;233:61–66. doi: 10.1016/j.ijcard.2017.01.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weber M., Hamm C. Role of B-type natriuretic peptide (BNP) and NT-proBNP in clinical routine. Heart. 2006;92:843–849. doi: 10.1136/hrt.2005.071233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKie P.M., Schirger J.A., Costello-Boerrigter L.C., Benike S.L., Harstad L.K., Bailey K.R., Hodge D.O., Redfield M.M., Simari R.D., Burnett J.C. Impaired natriuretic and renal endocrine response to acute volume expansion in pre-clinical systolic and diastolic dysfunction. J. Am. Coll. Cardiol. 2011;58:2095–2103. doi: 10.1016/j.jacc.2011.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelly J.P., Mentz R.J., Mebazaa A., Voors A.A., Butler J., Roessig L., Fiuzat M., Zannad F., Pitt B., O’Connor C.M., Lam C.S.P. Patient selection in heart failure with preserved ejection fraction clinical trials. J. Am. Coll. Cardiol. 2015;65:1668–1682. doi: 10.1016/j.jacc.2015.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.