Abstract
Objective
In recent years, frailty has been reported to be an important predictive factor associated with worse outcomes in neurosurgical patients. The purpose of the present systematic review was to analyze the impact of frailty on outcomes of chronic subdural hematoma (cSDH) patients.
Methods
We performed a systematic review of literature using the PubMed, Cochrane library, Wiley online library, and Web of Science databases following PRISMA guidelines of studies evaluating the effect of frailty on outcomes of cSDH published until January 31, 2023.
Results
A comprehensive literature search of databases yielded a total of 471 studies. Six studies with 4085 patients were included in our final qualitative systematic review. We found that frailty was associated with inferior outcomes (including mortality, complications, recurrence, and discharge disposition) in cSDH patients. Despite varying frailty scales/indices used across studies, negative outcomes occurred more frequently in patients that were frail than those who were not.
Conclusions
While the small number of available studies, and heterogenous methodology and reporting parameters precluded us from conducting a pooled analysis, the results of the present systematic review identify frailty as a robust predictor of worse outcomes in cSDH patients. Future studies with a larger sample size and consistent frailty scales/indices are warranted to strengthen the available evidence. The results of this work suggest a strong case for using frailty as a pre-operative risk stratification measure in cSDH patients.
Keywords: Frailty, Chronic subdural hematoma, Outcomes, Mortality, Complications
1. Introduction
Chronic subdural hematomas (cSDH) are prevalent in the general population, especially among the elderly.1, 2, 3 Their acute counterparts usually occur due to tearing of the bridging veins from trauma or age-related atrophy of brain parenchyma. However, the exact mechanism of the cSDH remains unclear. In about half of the cases, no specific history of trauma can be identified.1,4 In cSDH patients, various factors have been reported to play a role in prognosis including advanced age, previous anticoagulant/antithrombotic use, history of falls, male gender, severity of injury, and other comorbid conditions such as hypertension and chronic kidney disease which led to increased hospital length of stay (LOS) and costs.1,3,5, 6, 7, 8, 9
In recent years, frailty has been reported to be an important predictive factor associated with worse outcomes in neurosurgical patients.10,11 Frailty is defined as an age-related syndrome characterized by weakened physiologic vigor and an increased propensity of dependency and death.12,13 The precise pathophysiology of frailty remains largely unknown, but hypothesized mechanisms include dysregulation of hormones and cytokines in the aging body, progressive accumulation of disease-related insults to various organ systems, and lifelong wear and tear.12,13 Frailty is a comprehensive term that can potentially assess patient's functionality by incorporating comorbid systemic conditions including congestive heart failure, chronic obstructive pulmonary disease (COPD), functional dependency, diabetes mellitus, and hypertension.11, 12, 13, 14 In the present review, we sought to systematically review literature related to the impact of frailty on outcomes of cSDH.
2. Methods
We performed a systematic review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.15,16 We conducted a comprehensive literature search of studies evaluating the effect of frailty on outcomes of cSDH published from inception until January 31, 2023. The following databases were searched: PubMed, Cochrane library, Wiley online library, and Web of Science (Science and Social Science Citation Index), employing the following MeSH terms: “frail” OR “frailty” AND “chronic subdural hematoma” OR “chronic subdural hemorrhage” OR “chronic subdural hematoma” OR “chronic subdural hemorrhage”.
Inclusion criteria were peer-reviewed studies in the English language reporting frailty and cSDH outcomes in patients > than 18 years of age. Exclusion criteria included studies assessing the impact of frailty on acute SDH, and individual case reports or narrative reviews. The reference lists of the selected articles were also searched for articles potentially eligible for study inclusion. Using a standardized data abstraction form, two reviewers (BP and JV) independently reviewed and extracted relevant data. Disagreements during review were explored and resolved, either through discussion or by a third investigator (DA-C). Data pertaining to study design, sample size, patient demographics, time to follow up, frailty measures/indices, mortality, LOS, and other outcomes were extracted. The quality of included studies was also assessed using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement checklist.17
3. Results
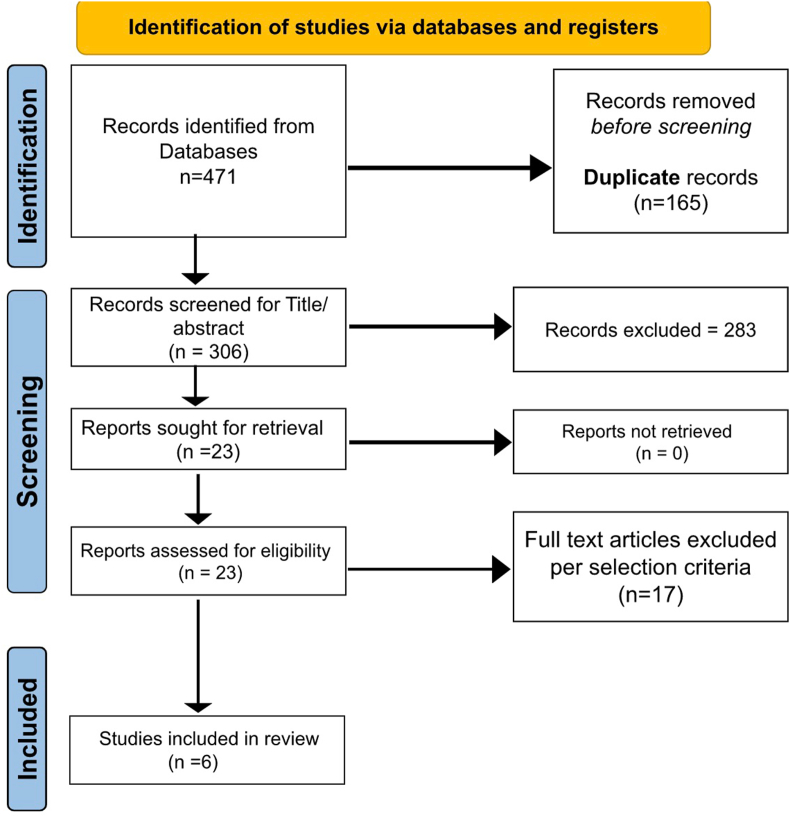
Our initial search identified 471 studies. We excluded a total of 448 studies after duplicate removal and thorough title and abstract screening. After full-text screening according to inclusion and exclusion criteria, Six studies (n = 4085, cSDH patients) were eligible for study inclusion (Fig. 1).18, 19, 20, 21, 22, 23 Table 1 depicts key findings of the studies assessing impact of frailty on outcomes in cSDH patients.
Fig. 1.
Study selection flow diagram per PRISMA guidelines.
Table 1.
Summary of studies evaluating impact of frailty on cSDH outcomes.
| Author, year | Study outline | Study design/characteristics | Frailty measures used | Findings |
|---|---|---|---|---|
| Shizimu et al, 201818 | Evaluation of frailty as an index for prediction of outcomes following surgical intervention via single burr hole drainage for cSDH | Retrospective, single center. n = 252 Follow up: 3 years |
CFS & CCI | Frail patients were more likely to have higher mRS at 3 months, lower discharge rates and median hospital days than those without frailty. Unfavorable prognosis was reported to be 100% in patients with frailty and 12% in patients without frailty |
| Kesserwan et al, 202019 | Evaluation of frailty as an index for outcomes prediction following surgical intervention via twist-drill craniostomy for cSDH. | Retrospective single-center n = 217 Follow up till discharge from hospital or in-hospital mortality |
mFI-11 & CFS | Frailty was correlated with greater dependence at discharge and lower functional improvement. CFS showed greater correlation to functional dependence at discharge than mFI-11. |
| McIntyre et al, 202020 | Comparison of frailty as an index for measuring outcomes following cSDH as compared to initial GCS. | Retrospective single center n = 109 Follow up till discharge from hospital or in-hospital mortality |
mFI-5, mFI-11, and CCI | Frail patients with higher mFI-11 and mFI-5 scores were found to have significantly lower Glasgow coma score (GCS) at discharge and significant reduction in rates of discharge home. |
| Zhou et al, 202021 | Evaluation of different factors, including frailty, to develop a prognostic prediction model for unfavorable outcomes in patients with antithrombotic-related cSDH in patients with recent AMI | Retrospective multi-center n = 553 Follow up at least 6 months. |
CFS | Patients with unfavorable outcomes were associated with frailty |
| Sastry et al, 202122 | Evaluation of pre-operative frailty as an index for outcomes following craniotomy for evacuation of atraumatic cSDH. | Retrospective cohort NSQIP database study from 2005 to 2018. n = 1647 Follow-up for 30-days |
mFI-5 | Pre-operative frailty was correlated with increased occurrence of postoperative complications, discharge to destination other than home, and 30-day mortality. |
| Blaauw et al, 202223 | Evaluation of frailty as an index for mortality rate in patients with cSDH. | Retrospective cohort n = 1307 median follow up 56 months |
CCI | Within the cohort of CSDH patients, all six frailty indicators were associated with death Among CSDH patients frequent falling, inability to live independently, inability to perform daily self-care, and number of medications used were independently associated with mortality. |
All the included studies were retrospective and published from 2018 onwards. Three were single-center studies18, 19, 20 and three were multicenter studies,21,22 one of which was performed using patient data extracted from on the National Surgical Quality Improvement Program (NSQIP) database22 and the other one was performed using the Dutch CBS database.23 Two studies used clinical frailty scale (CFS) alone as a measure of frailty,18,21 one study employed modified frailty index-5 (mFI-5) alone,22 one study used both mFI-5 and modified frailty index-11 (mFI-11),20 and another study used mFI-11 and CFS as frailty measures.19 One study23 employed Charlson Comorbidity Index (CCI) alone while two studies employed it in addition to other frailty scales/indices.18,20 The duration of outcomes assessment varied widely between studies including, 6 months,21 3 months,18 and 30 days22; two studies used disposition i.e., discharge from hospital or in-hospital mortality as outcomes measurement time point.19,20 Four studies used modified Rankin score (mRS) at follow-up endpoint as a measure of disability.18,19,21,23
In all studies, frailty was associated with inferior outcomes. In the study by Kesserwan and colleagues,19 frailty, measured by either mFI-11 or CFS was significantly associated with dependence at discharge, lower rate of functional improvement, higher rates of altered mental status, and death. It was also reported that frailty measured by the CFS had a stronger association with functional dependence than frailty measured by mFI-11, as well as inclusion of frailty measured by CFS into their predictive model was shown to improve accuracy.19 In the study by Mcyntire and colleagues,20 frail patients with higher mFI-11 and mFI-5 scores were found to have significantly lower Glasgow coma score (GCS) at discharge and a significant reduction in rates of discharge home. In the NSQIP 14 year analysis (2005–2018) of 1647 cSDH patients, Sastry and colleagues22 reported that rates of major complications, discharge destination other than home, 30-day readmission, and 30-day mortality were increased progressively with increasing frailty. In the study by Shizimu and colleagues,18 higher mRS scores and lower discharge rates to home were associated with frailty, however, in multivariate logistic regression analysis, mRS scores at 3 months after initial surgery were not significantly associated with frailty. Nonetheless, the authors also developed two models to assess prognosis in patients with cSDH, one including frailty measured by CFS and another without frailty.18 The model that included frailty was more accurately correlated with an unfavorable prognosis.18 Zhou et al21 tried to identify a prognostic model to predict 6-month outcomes of treatment with anti-thrombotic therapy in patients with acute myocardial infarction (AMI) at risk of antithrombotic-related cSDH. To do so, they divided the population into two groups, unfavorable outcomes (anyone who experienced one or more of the following: major adverse cardiovascular events, recurrence of cSDH, or mRS score of 2–6) and favorable outcomes.21 The presence of frailty was determined to be significantly associated with higher odds of unfavorable outcomes.21 In the most recent study by Blaauw et al,23 association of mortality in cSDH patients was established with frailty, defined in terms of cognitive problems, frequent falling, unable to live independently, unable to perform daily self-care, use of benzodiazepines or psychotropic drugs, and number of medications. They performed a retrospective case control study and found that in patients with cSDH all the 6 frailty indicators decreased the survival. A full model with these indicators was suggestive of the independent association of frequent falling, unable to live independently, unable to perform daily self-care and number of medications used with mortality. while a multivariate analysis revealed higher CCI scores and older age to be predictors for mortality. However, MGS score had no statistically significant association.
4. Discussion
To the best of our knowledge, this study is the first to systematically synthesize the literature related to the impact of frailty on outcomes of cSDH patients. Previously, frailty has been reported to be an accurate predictor of outcomes in critical care and surgical patients.24, 25, 26, 27, 28, 29, 30, 31 The concept of frailty as predictor of outcomes has been applied in the neurosurgical community in recent years.10,11 In fact, recent studies, have shown frailty predicts poor outcomes in patients undergoing spine surgery10,32 patients with traumatic spinal cord injury (SCI),13 and brain tumors.33,34 Our findings add to the growing evidence that frailty is an effective predictor of poor outcomes for cSDH patients. Despite varying methods of the determination of frailty, negative outcomes occurred more frequently in patients that were frail than those who underwent the same surgical procedures but were not frail.
4.1. Frailty scales
Multiple frailty scales were used by the studies in this review to quantify the frailty of their patients. These scales analyzed the presence of various comorbidities as methods of stratifying the frailty of neurosurgical patients, with different comorbidities carrying greater weight in causing frailty. The mFI-5 and mFI-11 have both been established as efficacious predictors of mortality, postoperative complication, and unplanned 30-day readmission. Additionally, both scales were noted as correlating with one another at greater than 90%.31 The mFI-5 utilized functional status, diabetes, congestive heart failure, chronic obstructive pulmonary disease, and hypertension as variables, and mFI-11 included the same variables as well as including pneumonia, myocardial infarction, previous cardiac surgery, impaired sensorium, stroke both with or without deficits, and revascularization or amputation. With the inclusion of stroke and delirium, the mFI-11 is more tailored to the prediction of neurosurgical frailty. These scales were both deemed as valid quantifications for frailty within the NSQIP database.31
The CFS included clinical judgment as the basis for its quantification of frailty. The score ranges from 1 to 9, with 1 being very fit and 9 being terminally ill. The use of CFS has been established as correlating with existing frailty scales at 94%. CFS was also found to accurately predict mortality, complications, length of stay, falls, cognition, and function.35
The CCI utilizes the presence of various pre existing conditions and history of medical events on a stratified scale for quantifying frailty, with more detrimental diagnoses carrying a greater weight. Among the most impactful comorbidities were age, liver disease, metastatic solid tumor, and AIDS. The CCI was recently validated using ICD-10 codes as an accurate predictor of 12-month mortality.
4.2. Frailty and cSDH-related mortality
Frailty has been identified to be associated with increased odds of mortality in surgical patients.10,11,29,30,34 In the studies included in our systematic review, three evaluated mortality as a primary outcome for cSDH.20,22,23 In the first study out of these three, patients identified as frail using CCI were found to have higher morality only if treated with surgical intervention.20 As expected, GCS was found to predict mortality of cSDH patients regardless of treatment modality.20 In the second one, patients identified as frail using mFI-5 preoperatively were found to be more likely to die within 30 days than non-frail patients.22 Increased mFI-5 score was associated with increased prevalence of mortality, with over a quarter of highly frail patients passing within 30 days as compared to less than 10% of non-frail patients and 12.81% of the total sample.22 In the third study, frail patients identified using the six self chosen indicators had higher mortality rates as compared to non frail patients with cSDH.23
4.3. Frailty and surgical intervention for cSDH
One of the six studies looked at the correlation between frailty and the need for surgical intervention versus nonoperative treatment.20 The study found no significant difference between treatment modalities and outcomes, however frailty as defined by CCI was a predictor for mortality and discharge location in the surgical treatment group.20 Another study showed that patients undergoing surgery had higher survival rates than the ones having conservative or no treatment at all.23
To this end, it may be more efficacious to treat high-frail patients with conservative measures, as surgery may be a potential risk factor for adverse outcome in frail patients, nevertheless, clinical judgment should always take place in making treatment decisions and weighing possible risks and benefits in individual patients. The mFI-5 and mFI-11 showed no significance in outcome prediction in either group.20 Further study of CCI as a predictor of negative outcomes in surgical treatment of cSDH is warranted. In addition, the impact of frailty on outcomes of patients treated with traditional surgical treatment (burr hole craniostomy or craniotomy) versus the newer minimally invasive neurointerventional procedure, middle meningeal artery (MMA) embolization, remains to be evaluated.
4.4. Frailty and cSDH complications and recurrence
Major complications were not uniformly defined across the identified studies, including but not limited to adverse cardiovascular events, recurrence, and readmission within 30 days of discharge. In the NSQIP data, preoperative frailty as identified by mFI-5 correlated with increased occurrence of postoperative complications, with patients identified with medium frailty (mFI-5 of 0.4) having increased risk of major complications.22 Patients identified as frail using CFS were also found to have increased probability of unfavorable outcome, including recurrence of cSDH within a 6-month follow-up period or major adverse cardiovascular event.18,21 The finding of frailty predicting poor outcomes within 30 days after cSDH is in congruence with previously reported impact of frailty on 30-day outcomes for other neurosurgical patients.34 Further studies are warranted to explore this with uniform definitions of “major complications” as well as to identify if frailty is indicative of specific complications. In addition, future studies should seek to analyze recurrence and readmission as individual variables rather than combined with postoperative complications as unfavorable outcomes.
4.5. Frailty and cSDH outcome assessment using mRS
The mRS was used to determine cognitive function and dependence of patients undergoing surgical intervention for cSDH in four of six studies.18,19,21,23 Frailty as measured by mFI-11 and CFS was associated with increased mRS at discharge, indicating an increased dependence and disability.18,19 CFS was determined to be the better predictor of the two scales.19 In addition, patients who were identified as frail were more likely to maintain increased mRS at follow up points at 3 months18 and 6 months21 after discharge, while non-frail patients were more likely to have decreased mRS. Nonetheless, no correlation was studied between the severity of frailty and elevated mRS. Further studies with larger sample sizes are needed to further explore this relationship, and the comparative predictive ability of different frailty scales.
4.6. Frailty and cSDH patients’ discharge disposition
Discharge following treatment to a location other than the patients’ home was analyzed in 3 out of 6 studies.18,20,22 Frailty as measured by CCI was associated with discharge to a location other than home in patients treated surgically as well as patients treated with non-surgical intervention.20 Also, preoperative frailty measured by mFI-5 was associated with discharge to locations other than home.22 In another study, patients deemed frail by CFS were also less likely to be discharged home than non-fail patients.18 Patients identified as frail, employing different frailty scales, were deemed less likely to be discharged home, indicating frailty as a predictor of discharge location to a place other than home in cSDH patients. Future multicenter studies with larger sample sizes are warranted to further strengthen these findings.
Frailty scales have inherent variability due to their differing methods of quantifying frailty, however in their validation they are compared to one another and in the case of mFI-5, mFI-11, CFS, and CCI, have been proven to elicit the same results as one another.31,35 Additionally, these scales utilize similar variables in their quantification of frailty, and their differences lie in the emphasis placed on each variable, except for CFS that uses clinical judgment. For instance, CHF, COPD, and diabetes were common between mFI-5, mFI-11, and CCI. mFI-11 and CCI both also include history of stroke and MI. In that vein, CCI and mFI-11 prove to be more relevant in the prediction of adverse outcomes in cSDH patients considering the inclusion of previous brain infarct. Loss of previously viable neurological tissue may be a mechanism for limited recovery following intervention measures for cSDH. Future research should compare outcome directly between patients with history of cerebral infarct and overall frailty scores to determine if stroke is the main reason for adverse outcome. Additionally, common variables of COPD, CHF, and diabetes may contribute to impaired tissue recovery via decreased delivery of key nutrients to the brain. Limited delivery of oxygen and glucose to brain tissue is a possible mechanism for decreased functional outcomes in patients with high frailty following cSDH. Additional study should examine management of these conditions and outcome in comparison to frailty.
4.7. Limitations
We acknowledge several limitations in this study. Given the paucity of data surrounding cSDH and frailty, our study is limited in the number of included studies. Heterogeneity has previously been identified as a limiting factor of frailty research in neurosurgery.11 Our study was also limited in this regard given the heterogeneity of frailty measurements assessed. Between the five studies, four distinct frailty scales/indices were used to assess outcomes. A standardized measurement of frailty across studies is warranted to better assess the available evidence and generalize the findings. However, we believe that these measures of frailty do have merit in that they classify the coexistence of high-impact comorbidities as an indication of frailty. Given their widespread use in the literature, we agree that in the case of cSDH patients there is relevance in their analytical power. Moreover, there was inconsistency regarding the quantification of adverse outcomes, with only three studies using mRS as a measure of outcome. Additionally, the studies were heterogeneous in location, sample size, single versus multi center, and type of hospital. Patient follow-up was also highly variable among the studies which is an important limitation when considering patient outcome as the primary variable. Lastly, all studies were retrospective, therefore, it is possible there may have been selection bias in patients included in the studies. Finally, statistical analysis, such as pooled analysis, was not performed due to heterogeneous methodology and reporting parameters.
4.8. Concluding remarks
The results of this work identify frailty as a robust predictor of worse outcomes in cSDH patients. Future studies with larger sample size are warranted to strengthen the available evidence. The most important clinical message from the present systematic review is the improvement in preoperative risk stratification and subsequent preoperative patient counseling regarding the risks and benefits of proposed surgical intervention in cSDH patients. Cerebrovascular surgeons can use the knowledge that frailty is an important variable in predicting outcomes in cSDH patients to more accurately judge whether a patient would do well with a surgical intervention. While in emergent cases of cSDH, the clinical judgment of the providers will have to remain the driving force behind decision making, however in certain cases, frailty may be used to inform patients and families of the risks and benefits of treatment options. In conclusion, our findings suggest providers should include frailty in risk stratification when making clinical decisions regarding treatment modalities in cSDH patients.
Funding
The authors did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Bhavya Pahwa: Writing – original draft, Writing – review & editing. Syed Faraz Kazim: Conceptualization. John Vellek: Writing – original draft. Daniel J. Alvarez-Crespo: Writing – original draft. Smit Shah: Data curation. Omar Tarawneh: Writing – original draft. Alis J. Dicpinigaitis: Conceptualization, Data curation. Ramesh Grandhi: Conceptualization. William T. Couldwell: Conceptualization. Meic H. Schmidt: Supervision. Christian A. Bowers: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None.
References
- 1.Adhiyaman V., Asghar M., Ganeshram K.N., Bhowmick B.K. Chronic subdural haematoma in the elderly. Postgrad Med J. 2002;78(916):71–75. doi: 10.1136/pmj.78.916.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balser D., Farooq S., Mehmood T., Reyes M., Samadani U. Actual and projected incidence rates for chronic subdural hematomas in United States Veterans Administration and civilian populations. J Neurosurg. 2015;123(5):1209–1215. doi: 10.3171/2014.9.JNS141550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee L., Ker J., Ng H.Y., et al. Outcomes of chronic subdural hematoma drainage in nonagenarians and centenarians: a multicenter study. J Neurosurg. 2016;124(2):546–551. doi: 10.3171/2014.12.JNS142053. [DOI] [PubMed] [Google Scholar]
- 4.Jafari N., Gesner L., Koziol J.M., Rotoli G., Hubschmann O.R. The pathogenesis of chronic subdural hematomas: a study on the formation of chronic subdural hematomas and analysis of computed tomography findings. World Neurosurg. 2017;107:376–381. doi: 10.1016/j.wneu.2017.07.108. [DOI] [PubMed] [Google Scholar]
- 5.Aspegren O.P., Åstrand R., Lundgren M.I., Romner B. Anticoagulation therapy a risk factor for the development of chronic subdural hematoma. Clin Neurol Neurosurg. 2013;115(7):981–984. doi: 10.1016/j.clineuro.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Bacigaluppi S., Guastalli F., Bragazzi N.L., Balestrino A., Bruzzi P., Zona G. Prognostic factors in chronic subdural hematoma: results from a monocentric consecutive surgical series of 605 patients. J Neurosurg Sci. 2021;65(1):14–23. doi: 10.23736/S0390-5616.17.04103-0. [DOI] [PubMed] [Google Scholar]
- 7.Han M.H., Ryu J.I., Kim C.H., Kim J.M., Cheong J.H., Yi H.J. Predictive factors for recurrence and clinical outcomes in patients with chronic subdural hematoma. J Neurosurg. 2017;127(5):1117–1125. doi: 10.3171/2016.8.JNS16867. [DOI] [PubMed] [Google Scholar]
- 8.Kanat A., Kayaci S., Yazar U., Kazdal H., Terzi Y. Chronic subdural hematoma in adults: why does it occur more often in males than females? Influence of patient's sexual gender on occurrence. J Neurosurg Sci. 2010;54(3):99–103. [PubMed] [Google Scholar]
- 9.Rovlias A., Theodoropoulos S., Papoutsakis D. Chronic subdural hematoma: surgical management and outcome in 986 cases: a classification and regression tree approach. Surg Neurol Int. 2015;6:127. doi: 10.4103/2152-7806.161788. Published 2015 Jul 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan V., Wilson J.R.F., Ravinsky R., et al. Frailty adversely affects outcomes of patients undergoing spine surgery: a systematic review. Spine J. 2021;21(6):988–1000. doi: 10.1016/j.spinee.2021.01.028. [DOI] [PubMed] [Google Scholar]
- 11.Pazniokas J., Gandhi C., Theriault B., et al. The immense heterogeneity of frailty in neurosurgery: a systematic literature review. Neurosurg Rev. 2021;44(1):189–201. doi: 10.1007/s10143-020-01241-2. [DOI] [PubMed] [Google Scholar]
- 12.Morley J.E., Vellas B., van Kan G.A., et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xue Q.L. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27(1):1–15. doi: 10.1016/j.cger.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shahrestani S., Lehrich B.M., Tafreshi A.R., et al. The role of frailty in geriatric cranial neurosurgery for primary central nervous system neoplasms. Neurosurg Focus. 2020;49(4):E15. doi: 10.3171/2020.7.FOCUS20426. [DOI] [PubMed] [Google Scholar]
- 15.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. 2009;3(3):e123–e130. [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D., Shamseer L., Clarke M., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. Published 2015 Jan 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Elm E., Altman D.G., Egger M., et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu K., Sadatomo T., Hara T., Onishi S., Yuki K., Kurisu K. Importance of frailty evaluation in the prediction of the prognosis of patients with chronic subdural hematoma. Geriatr Gerontol Int. 2018;18(8):1173–1176. doi: 10.1111/ggi.13436. [DOI] [PubMed] [Google Scholar]
- 19.Kesserwan M., Bergin B., Trivedi A., et al. Assessment of frailty in predicting surgical outcomes in patients with chronic subdural hematomas: retrospective chart review. World Neurosurg. 2021;146:e168–e174. doi: 10.1016/j.wneu.2020.10.061. [DOI] [PubMed] [Google Scholar]
- 20.McIntyre M.K., Rawanduzy C., Afridi A., et al. The effect of frailty versus initial Glasgow coma score in predicting outcomes following chronic subdural hemorrhage: a preliminary analysis. Cureus. 2020;12(8) doi: 10.7759/cureus.10048. Published 2020 Aug 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou P., Wan J., Ran F., et al. Development and validation of a prognostic prediction model for antithrombotic-related chronic subdural hematoma in patients with recent acute myocardial infarction. Cardiovasc Diagn Ther. 2020;10(6):1770–1784. doi: 10.21037/cdt-20-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sastry R.A., Pertsch N., Tang O., Shao B., Toms S.A., Weil R.J. Frailty and outcomes after craniotomy or craniectomy for atraumatic chronic subdural hematoma. World Neurosurg. 2021;145:e242–e251. doi: 10.1016/j.wneu.2020.10.022. [DOI] [PubMed] [Google Scholar]
- 23.Blaauw J., Jacobs B., Hertog H.M.D., et al. Mortality after chronic subdural hematoma is associated with frailty. Acta Neurochir. 2022;164(12):3133–3141. doi: 10.1007/s00701-022-05373-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arya S., Kim S.I., Duwayri Y., et al. Frailty increases the risk of 30-day mortality, morbidity, and failure to rescue after elective abdominal aortic aneurysm repair independent of age and comorbidities. J Vasc Surg. 2015;61(2):324–331. doi: 10.1016/j.jvs.2014.08.115. [DOI] [PubMed] [Google Scholar]
- 25.Brummel N.E., Girard T.D., Pandharipande P.P., et al. Prevalence and course of frailty in survivors of critical illness. Crit Care Med. 2020;48(10):1419–1426. doi: 10.1097/CCM.0000000000004444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hewitt J., Long S., Carter B., Bach S., McCarthy K., Clegg A. The prevalence of frailty and its association with clinical outcomes in general surgery: a systematic review and meta-analysis. Age Ageing. 2018;47(6):793–800. doi: 10.1093/ageing/afy110. [DOI] [PubMed] [Google Scholar]
- 27.Karam J., Tsiouris A., Shepard A., Velanovich V., Rubinfeld I. Simplified frailty index to predict adverse outcomes and mortality in vascular surgery patients. Ann Vasc Surg. 2013;27(7):904–908. doi: 10.1016/j.avsg.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 28.Makary M.A., Segev D.L., Pronovost P.J., et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210(6):901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 29.Panayi A.C., Orkaby A.R., Sakthivel D., et al. Impact of frailty on outcomes in surgical patients: a systematic review and meta-analysis. Am J Surg. 2019;218(2):393–400. doi: 10.1016/j.amjsurg.2018.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shinall MC Jr, Youk A., Massarweh N.N., et al. Association of preoperative frailty and operative stress with mortality after elective vs emergency surgery. JAMA Netw Open. 2020;3(7) doi: 10.1001/jamanetworkopen.2020.10358. Published 2020 Jul 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Subramaniam S., Aalberg J.J., Soriano R.P., Divino C.M. New 5-factor modified frailty index using American college of surgeons NSQIP data. J Am Coll Surg. 2018;226(2):173–181.e8. doi: 10.1016/j.jamcollsurg.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Flexman A.M., Street J., Charest-Morin R. The impact of frailty and sarcopenia on patient outcomes after complex spine surgery. Curr Opin Anaesthesiol. 2019;32(5):609–615. doi: 10.1097/ACO.0000000000000759. [DOI] [PubMed] [Google Scholar]
- 33.Sastry R.A., Pertsch N.J., Tang O., Shao B., Toms S.A., Weil R.J. Frailty and outcomes after craniotomy for brain tumor. J Clin Neurosci. 2020;81:95–100. doi: 10.1016/j.jocn.2020.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Youngerman B.E., Neugut A.I., Yang J., Hershman D.L., Wright J.D., Bruce J.N. The modified frailty index and 30-day adverse events in oncologic neurosurgery. J Neuro Oncol. 2018;136(1):197–206. doi: 10.1007/s11060-017-2644-0. [DOI] [PubMed] [Google Scholar]
- 35.Church S., Rogers E., Rockwood K., Theou O. A scoping review of the clinical frailty scale. BMC Geriatr. 2020;20(1):393. doi: 10.1186/s12877-020-01801-7. Published 2020 Oct 7. [DOI] [PMC free article] [PubMed] [Google Scholar]