Abstract
The dosing interval for effective recombinant adeno-associated virus (rAAV)-mediated gene therapy of cystic fibrosis lung disease remains unknown. Here, we assessed the durability of rAAV2.5T-fCFTRΔR-mediated transgene expression and neutralizing antibody (NAb) responses in lungs of adult wild-type ferrets. Within the first 3 months following rAAV2.5T-fCFTRΔR delivery to the lung, CFTRΔR transgene expression declined ∼5.6-fold and then remained stable to 5 months at ∼26% the level of endogenous CFTR. rAAV NAbs in the plasma and bronchoalveolar lavage fluid (BALF) peaked at 21 days, coinciding with peak ELISpot T cell responses to AAV capsid peptides, after which both responses declined and remained stable at 4–5 months post dosing. Administration of reporter vector rAAV2.5T-gLuc (gaussia luciferase) at 5 months following rAAV2.5T-fCFTRΔR dosing gave rise to similar levels of gLuc expression in the BALF as observed in age-matched reporter-only controls, demonstrating that residual BALF NAbs were functionally insignificant. Notably, the second vector administration led to a 2.6-fold greater ELISpot T cell response and ∼2.3-fold decline in fCFTRΔR mRNA and vector genomes derived from the initial rAAV2.5T-fCFTRΔR administration, suggesting selective destruction of transduced cells from the first vector dose. These findings provide insights into humoral and cellular immune response to rAAV that may be useful for optimizing gene therapy to the cystic fibrosis lung.
Keywords: adeno-associated virus, gene therapy, cystic fibrosis, CFTR, lung, durability, transgene, repeat administration, neutralizing antibodies, T cell responses
Graphical abstract

Engelhardt and colleagues assessed the durability of AAV2.5T vector-mediated transgene expression for at least 5 months in ferret lungs, as well as the associated immune responses induced by the vector. They also efficiently re-administered the same AAV2.5T vector with a reporter gene after a 5-month interval.
Introduction
Cystic fibrosis (CF) is a progressive, life-restrictive, autosomal recessive disease that affects the lungs, pancreas, and other organs. There are close to 40,000 children and adults living with CF in the United States, and an estimated 105,000 people have been diagnosed with CF across 94 countries (https://www.cff.org/intro-cf/about-cystic-fibrosis); CF can affect people of every racial and ethnic group.1,2 CF is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. More than 2,000 variants of this gene have been identified, of which approximately 400 are disease causing and are grouped into six classes.3 CFTR pathogenic variants impact the expression and/or function of the CFTR protein through several different mechanisms, including synthesis (mRNA and protein), maturation, trafficking, and channel activity. The absence or dysfunction of the CFTR protein results in defective chloride and bicarbonate movement across epithelia in several organs, leading to dehydration and mucus accumulation.
While CFTR modulators can rescue the function of specific CFTR mutants, ∼10% of people with CF do not benefit from the CFTR modulator therapies, due to mutations that produce little to no CFTR protein or the inability to tolerate CFTR modulators.4 Furthermore, although CFTR modulators have greatly enhanced the life expectancy of most people with CF, the drug is expensive and a lifelong burden. Therefore, the delivery of a functional CFTR gene to the CF lung holds promise as a potential genetic treatment for this disease. Indeed, gene therapy using recombinant adeno-associated virus (rAAV)-based drugs has been successful in treating certain genetic diseases, namely retinal dystrophy,5,6 hemophilia A and B,7,8,9,10 spinal muscular atrophy,11,12 and Duchenne muscular dystrophy.13
Capsid engineering by directed evolution has greatly expanded the toolbox of rAAV vectors with unique tropisms.14 rAAV2.5T is an airway tropic variant identified by directed evolution of a shuffled and mutagenized AAV2/AAV5 capsid library and panning on polarized primary human airway epithelia cultured at an air-liquid interface (HAE-ALI).15 To overcome the inherent packaging limitation of AAV for CFTR expression, we developed a vector (rAAV2.5T-fCFTRΔR) harboring a ferret CFTR minigene (CFTRΔR) in which a 156-bp sequence in the R domain was deleted.16 This vector uses a 183-bp synthetic promoter/enhancer sequence (SP183) and a small synthetic polyadenylation sequence. We have previously demonstrated that apical transduction of CF HAE-ALI cultures with rAAV2.5T-fCFTRΔR efficiently corrects CFTR-mediated chloride transport and that rAAV2.5T is capable of efficiently transducing ferret lungs in vivo.16,17 The CF ferret model manifests lung disease that resembles the pathologies found in people with CF.18,19,20,21,22 Given the availability of CF ferrets for preclinical studies, a better understanding of rAAV2.5T immunology and durability of transgene expression in ferret lungs is needed prior to embarking on studies in diseased CF animals.
rAAV contains a non-integrating vector genome (vg) that persists as an episome in the nucleus. Therefore, the lifespan of airway epithelial cells transduced by rAAV will determine the durability of transgene expression and the interval of administration required to maintain a therapeutic effect. Two major factors will impact the lifespan of rAAV transduced cells in the lung, the first of which is the normal turnover rate of epithelial cells at homeostasis. Studies using fate mapping in transgenic mouse models have suggested that at homeostasis the average half-life of a ciliated cell is 6 months in trachea and 17 months in bronchioles.23 While this is a relatively long half-life, the turnover rate is expected to be much faster in diseased states such as CF.24 Second, the cellular immune response to rAAV transduced cells facilitated by T cell recognition of capsid peptides will also impact the half-life of transgene-expressing cells. Our previous studies have demonstrated the feasibility of rAAV2.5T re-administration in neonatal ferrets without alterations in the efficiency of transduction, while rAAV2.5T re-administration in juvenile ferret lungs demonstrated significantly reduced transduction due to a maturation-dependent neutralizing antibody (NAb) response.17 However, the efficacy of rAAV2.5T re-administration to juvenile ferret lungs can be significantly improved by immunosuppressants that inhibit NAb formation during the initial vector challenge.25 While complement, humoral, and cellular responses against the AAV capsid are still a major barrier to clinical application, strategies to tackle the problem are being tested in preclinical models.26 More than 100 clinical gene therapy trials have applied prophylactic or reactive immunosuppressants, among which central nervous system (n = 18) and ocular (n = 24) trials have employed immunosuppressants despite the immune-privileged status of these organs.27
Clinical rAAV-mediated gene-replacement therapy studies for spinal muscular atrophy and Canavan disease demonstrated encouraging durable therapeutic effects over a 5- to 10-year period.28,29 Unlike these diseases, rAAV-mediated gene-replacement therapy for CF lung disease remains at a very early stage despite an increasing number of clinical studies. There is currently no research available on the durability of rAAV-mediated CFTR transgene expression following pulmonary administration in animal models. To address this gap in knowledge, we evaluated the durability vector-derived fCFTRΔR mRNA and rAAV genomes following rAAV2.5T-fCFTRΔR administration to adult ferret lung over a period of 10 days to 5 months. To evaluate the durability of NAb responses, we assessed NAb titers in the bronchoalveolar lavage fluid (BALF) and plasma over this period and evaluated the efficiency of a second administration with a reporter vector (rAAV2.5T-gLuc, gaussia luciferase) at 5 months following the initial administration of rAAV2.5T-fCFTRΔR. Cellular immune responses to rAAV capsid peptides by ELISpot and AAV capsid-binding immunoglobulin (Ig) isotype were also quantified.
Results
Duration of transgene expression in ferret lungs following a single dose of rAAV2.5T-fCFTRΔR
To investigate the durability of rAAV2.5T-fCFTRΔR-mediated CFTR expression in ferret lungs, we established a real-time RT-qPCR assay to discriminate the vector-transcribed fCFTRΔR mRNA and endogenous fCFTR mRNA using specific primer/probe sets (Table 1). rAAV2.5T-fCFTRΔR, combined with doxorubicin, was intratracheally delivered to adult ferrets (∼5–8 months old). After sacrifice, whole lungs were snap frozen in liquid nitrogen and pulverized to allow for evaluation of whole-lung expression at time points ranging from 10 days to 5 months following transduction (Figure 1A). Vector-derived and endogenous fCFTR mRNA was quantified by RT-qPCR and normalized to expression of the GAPDH housekeeping gene (Figure 1B). The most significant decline in vector-derived fCFTRΔR mRNA occurred within the 3 months following vector administration, with stabilization of expression between 3 and 5 months (Figure 1B). To reference transgene-derived to endogenous fCFTR, we compared the ratio of vector-derived fCFTRΔR to endogenous fCFTR mRNA (Figure 1C). fCFTRΔR expression was 2.6- to 1.9-fold higher than that of the endogenous fCFTR at early time points (10 and 21 days, respectively) after administration (Figure 1C). Notably, at these time points when transgene-derived fCFTRΔR mRNA level was the highest, endogenous fCFTR expression was suppressed compared to naive controls (day 0) (Figure 1B). Endogenous fCFTR levels returned to normal levels by 1 month post transduction, and the ratio of transgene-derived fCFTR mRNA remained stable from 3 to 5 months, averaging ∼26% of endogenous fCFTR mRNA levels (Figure 1C).
Table 1.
Primers and probes for measurement of mRNA and DNA copy number by TaqMan qPCR
| Primer name | Sequence | Target template |
|---|---|---|
| Fw-tran.endo | CAAGTCTCGCTCTCAAATTGC | AAV2.5T-fCFTRΔR gene and ferret endogenous CFTR gene |
| Rev tran | GGAACATTTATGCTGCTCTC | AAV2.5T-fCFTRΔR gene |
| Prb-tran.endo | ACCTCTTCTTCCGTCTCCTCCTTCA | AAV2.5T-fCFTRΔR gene and ferret endogenous CFTR gene |
| Rev endo | CAGCCCATGCCAATATCTGG | ferret endogenous CFTR gene |
| Fw-GAPDH | CAACTTTGGCATTGTGGAGG | ferret GAPDH gene |
| Prb-GAPDH | CAGTGATGGCATGGACGGTGG | ferret GAPDH gene |
| Rev-GAPDH | CAGTGGAAGCAGGGATGATG | ferret GAPDH gene |
Fw-tran.endo, forward primer for transgene and endogenous gene; Rev tran, reverse primer for transgene; Rev endo, reverse primer for endogenous gene; Prb-tran.endo, probe for transgene and endogenous gene.
Figure 1.
Durability of rAAV2.5T-fCFTRΔR transduction in adult ferret lung
(A) Six groups of adult ferrets received rAAV2.5T-fCFTRΔR (1 × 1013 DRP/kg body weight) via intratracheal atomization. The lungs, plasma, bronchoalveolar lavage fluid (BALF), and splenocytes were harvested at the indicated time points. (B) Copies of transgene-derived fCFTRΔR and endogenous fCFTR mRNA normalized to GAPDH and quantified by qPCR with plasmid copy number standard curves. Six technical replicates were measured by independent sampling of the pulverized lung tissue from each animal, and values were averaged. (C) Ratio of the copy number of transgene-derived fCFTRΔR to endogenous fCFTR mRNA (i.e., fCFTRΔR/fCFTR). (D and E) (D) Number of rAAV2.5T-fCFTRΔR vector genome (vg) copies in 100 ng of total cellular DNA and (E) per cell following normalization to the genomic DNA content in a single ferret cell. Six technical replicates were measured from 100 ng of total cellular DNA generated from independent sampling of the pulverized lung tissue from each animal and values averaged. (F) Total endogenous fCFTR DNA copies found in 100 ng of lung tissue DNA. Three technical replicates were measured by independent sampling of the pulverized lung tissue from each animal and values averaged. (G) Endogenous fCFTR DNA copies per lung cell. Based on the endogenous genomic fCFTR copies from 100 ng of lung genomic DNA, a single diploid ferret cell is estimated to contain 5.78 pg of DNA, which is very close to the cellular content of a diploid human cell (∼6 pg/cell). Results show the mean ± SEM. Statistical significance was analyzed by one-way ANOVA followed by Tukey’s multiple comparisons test using original data in (B) and (C) and using log2(Y + 1) transformed data in (D) and (E). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; ns, not significant. n = 5–8 animals in each group as shown in (A).
Quantification of rAAV vg copies in lung lysates showed the highest level at 10 days post transduction (∼103 copies of vg per 100 pg of genomic DNA or ∼60 vg/cell) (Figure 1D). This calculation assumes that a single ferret cell contains a similar amount of genomic DNA as a human cell (∼6 pg). The largest drop in vg occurred between 10 and 21 days post transduction (30-fold) and remained relatively stable out to 5 months, with only a further 2.7-fold decline. Since the size of the ferret genome is not known, we also calculated the number of endogenous fCFTR copies in genomic DNA samples from ferrets receiving vector (Figure 1F) and used this to recalculate the number of diploid genomes in 100 pg of DNA. This averaged 17.3 cells per 100 pg of genomic DNA or 5.78 pg of DNA in a single ferret cell. Using these calculations we estimate 63.6 vg/cell at 10 days and ∼0.79 vg/cell at 5 months post transduction (Figure 1E), where the number of endogenous fCFTR DNA copies per cell averaged 2 ± 0.08 across all samples tested (Figure 1G).
Durability of humoral and cellular immune responses following a single administration of rAAV2.5T
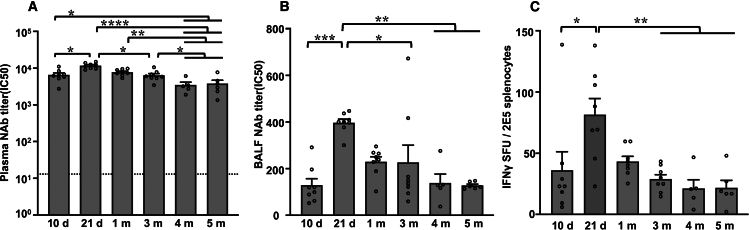
We examined the titers of rAAV2.5T NAb in plasma and BALF from rAAV2.5T-fCFTRΔR treated ferrets over a 5-month period. Titers of NAb peaked at 21 days post dosing, declined ∼3-fold by 4 months, and remained stable thereafter (Figures 2A and 2B). The ELISpot assay was used to assess the interferon-γ (IFN-γ)-mediated cellular immune responses and demonstrated similar trends as observed for the NAb response (Figure 2C). Assessment of capsid-binding Igs over the 5-month period following rAAV2.5T-fCFTRΔR transduction demonstrated a gradual rise in the levels of plasma and BALF capsid-binding IgG that stabilized at 3–5 months (Figures 3A and 3D). By contrast, both plasma and BALF capsid-binding IgM and IgA levels peaked at 10 days post dosing and significantly declined thereafter (Figures 3B, 3C, 3E, and 3F). Overall, the time-dependent increases in capsid-binding IgG inversely correlated with the decline in fCFTRΔR transgene mRNA expression.
Figure 2.
Humoral and cellular immune responses following rAAV2.5T-fCFTRΔR administration to the ferret lung
Neutralizing antibody (NAb) IC50 titers measured by rAAV2.5T-fLuc preincubation with serially diluted (A) plasma or (B) BALF prior to incubation on A549 cells and assessment of firefly luciferase expression. (C) ELISpot measurement of IFN-γ levels from 2 × 105 splenocytes following challenge with AAV2.5T capsid peptides. The dashed line in (A) indicates the average plasma NAb titer of all naive ferrets in the study prior to rAAV2.5T-fCFTRΔR transduction (10.21 ± 0.83). Results show the mean ± SEM. Statistical significance was analyzed by one-way ANOVA followed by Tukey’s multiple comparisons test using log2(Y + 1) transformed data. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. n = 5–8 animals in each group as shown in Figure 1A.
Figure 3.
Capsid-binding immunoglobulin levels in plasma and BALF following rAAV2.5T-fCFTRΔR administration to the ferret lung
AAV capsid-binding Ig titers were assessed by ELISA using purified vector as the capture antigen. (A) Plasma IgG levels (diluted 4,000-fold). (B) Plasma IgM levels (diluted to 1:2,000). (C) Plasma IgA levels (diluted to 1:20). (D) BALF IgG levels (diluted 50-fold). (E and F) BALF (E) IgM level and (F) IgA level were assessed using undiluted samples. Results represent the mean ± SEM. Data were analyzed by one-way ANOVA followed by Tukey’s multiple comparisons test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; ns, not significant. n = 5–8 animals in each group as shown in Figure 1A.
Efficient repeat administration of rAAV2.5T at a 5-month interval despite persistent capsid-binding IgG and NAbs in the BALF
In our previous report, we demonstrated that repeat administration of rAAV2.5T to the lungs of juvenile ferrets at a 1-month interval resulted in an 11-fold decline in transduction efficiency as compared to animals receiving only reporter vector.25 This decrement in transduction following repeat administration correlated with elevated NAb titers in the BALF. Although BALF NAb titers at 5 months post vector administration declined ∼3-fold from peak 21-day levels (Figure 2B), it remained unclear whether this level of immunity would be sufficient to block transduction following a second administration of rAAV2.5T. To this end, we assessed the efficiency of re-administering rAAV2.5T at 5 months using a reporter vector of rAAV2.5T-gLuc that enabled precise quantification of transgene expression by measuring secreted gLuc in the blood and BALF (Figure 4A). Age-matched naive ferrets that received a single dose of rAAV2.5T-gLuc were used as controls, and animals in both groups were evaluated at 2 weeks following reporter vector administration.
Figure 4.
Transduction efficiency following repeat administration of rAAV2.5T
(A) Schematic of the three experimental groups of ferrets used to evaluate repeat administration of rAAV2.5T. The repeat group received rAAV2.5T-fCFTRΔR (1 × 1013 DRP/kg body weight), were administered rAAV2.5T-gLuc (1 × 1013 DRP/kg body weight) 5 months later, and were euthanized 14 days later (5m.14d). The single group was only transduced with AAV2.5T-gLuc, while the control group was only transduced with rAAV2.5T-fCFTRΔR. (B) Secreted gLuc activity in BALF at 14 days after rAAV2.5-gLuc transduction. (C) gLuc activity in plasma within the first 14 days following rAAV2.5T-gLuc transduction. Results represent the mean ± SEM. Statistical significance was analyzed by Mann-Whitney test in (B) and using one-way ANOVA followed by Tukey’s multiple comparisons test in (C). ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; ns, not significant. n = 4 or 6 animals in each group.
Comparison of gLuc expression between the single-dose group and repeat-dose group at 14 days post rAAV2.5T-gLuc vector administration revealed similar levels of gLuc activity in the BALF (Figure 4B). While these data suggested that the repeat administration of rAAV2.5T at a 5-month interval was effective, the time course of the gLuc activity in plasma revealed that the expression kinetics of the two groups were quite different. For example, plasma gLuc expression from the single-dose group significantly declined 1,770-fold between 5 and 14 days post vector administration (p < 0.0001), whereas gLuc expression in the repeat-dose group remained relatively stable over this period (Figure 4C).
Repeat administration of rAAV2.5T promotes clearance of vector genomes and fCFTRΔR transgene mRNA expression from a previous exposure to vector 5 months earlier
The discordant BALF and plasma gLuc expression profile following rAAV2.5T single dose and repeat dose suggested that transduction of cellular targets capable of secreting gLuc apically into the BALF and basolaterally into the blood may be altered by prior exposure to rAAV2.5T vector. We hypothesized that an altered immune response in ferrets previously exposed to rAAV2.5T-fCFTRΔR might be responsible for these differences, and thus we compared both NAb titers and T cell responses between the repeat-dose and single-dose groups following transduction with rAAV2.5T-gLuc. As expected, pre-existing plasma NAb titers were significantly higher in the repeat-dose group (day 0) as compared to the single-dose group ferrets (Figure 5A). This correlated with a 23.6-fold lower (p < 0.001) level of plasma gLuc at 5 days post transduction (Figure 4C). Repeat-dosed ferrets retained significantly higher levels of plasma NAb at all time points examined post transduction (5, 10, and 14 days) (Figure 5A). Notably, at day 14, BALF NAb titers were not significantly different between single-dose and repeat-dose groups (Figure 5B). However, ELISpot IFN-γ T cell responses to capsid peptides were significantly higher (p < 0.05) in the repeat-dose group compared to either the 10-month-old age-matched group with a single dose of rAAV2.5T-gLuc reporter vector (Figure 5C) or the group receiving a single dose of rAAV2.5-fCFTRΔR after a 5-month follow-up period (Figure 2C).
Figure 5.
Humoral and cellular immune response following repeat administration of rAAV2.5T
Schematic of rAAV2.5T dosing in single, repeat, and control ferrets is as shown in Figure 4A. (A and B) Neutralizing antibody (NAb) titers in (A) plasma and (B) BALF from single and repeat groups. The dotted line in (B) indicates the average NAb titer of plasma from the control group of ferrets at 5 months post rAAV2.5T-fCFTRΔR administration. (C) ELISpot measurement of IFN-γ levels from 2 × 105 splenocytes following challenge with AAV2.5T capsid peptides. The dotted line in (B) indicates the average NAb titer of plasma from the control group of ferrets at 5 months post rAAV2.5T-fCFTRΔR administration. (D) Copies of transgene-derived fCFTRΔR and endogenous fCFTR mRNA normalized to GAPDH from lung tissue harvested at 5 months after a single administration of AAV2.5T-fCFTRΔR (control) or at 14 days after repeat administration with AAV2.5T-gLuc in ferrets transduced with AAV2.5T-fCFTRΔR 5 months earlier (repeat). (E) Copy number ratio of transgene-derived fCFTRΔR mRNA to the endogenous fCFTR mRNA. (F) Number of AAV vg in 100 ng of lung cellular DNA from control and repeat groups tested. Results represent the mean ± SEM. Statistical significance was analyzed by Mann-Whitney test using log2(Y + 1) transformed data in (A) and non-transformed data in (B–F). ∗p < 0.05, ∗∗p < 0.01. n = 4–6 animals in each group.
To evaluate whether the enhanced T cell response to a second dose of rAAV2.5T might alter the persistence of rAAV2.5T-fCFTRΔR transduced cells from vector administered 5 months earlier, we compared the levels of fCFTRΔR mRNA and vg between two groups of ferrets: (1) those that received rAAV2.5T-fCFTRΔR and then 5 months later received rAAV2.5T-gLuc with analysis at 14 days after the second vector administration (repeat group, Figure 4A); and (2) those that received only rAAV2.5T-fCFTRΔR with analysis at 5 months after vector administration (control group, Figure 4A). The results demonstrated that fCFTRΔR mRNA expression was significantly lower (2.4-fold) in the repeat-dose group as compared to the control group (Figure 5D), and this was mirrored by a 2.9-fold lower ratio of transgene-derived to endogenous fCFTR mRNA in the repeat-dose group (Figure 5E). rAAV2.5T-fCFTRΔR vg in the lung were also significantly lower (2.2-fold) in the repeat-dose group as compared to the control group (Figure 5F). Given that rAAV2.5T-fCFTRΔR vg and fCFTRΔR mRNA expression remained stable between 3 and 5 months following single vector administration, the rapid decline in vg and fCFTRΔR mRNA at 14 days after rAAV2.5T-gLuc transduction suggests that boosted immunity led to partial clearance of rAAV2.5T-fCFTRΔR transduced cells following re-administration of rAAV2.5T vector. Notably, the second dose of rAAV2.5T encoding gLuc did not alter endogenous fCFTR expression levels (Figure 5D), suggesting that the lower endogenous fCFTR expression initially observed following rAAV2.5T-fCFTRΔR transduction (Figure 1B) was not likely to be due to a non-specific response to viral vector transduction.
Repeat administration of rAAV2.5T to the ferret lung alters the capsid-binding Ig isotypes found in BALF and plasma
We further assessed the capsid-binding Ig isotypes in plasma and BALF following single and repeat dosing with rAAV2.5T-fCFTRΔR and/or rAAV2.5T-gLuc (Figure 4A). Plasma IgG and IgA titers were significantly higher, whereas IgM titers were lower, in the repeat-dose group as compared to the single-dose group, at nearly all time points within the 14-day monitoring period (Figures 6A–6C). However, capsid-binding IgG in the plasma was the predominant isotype. Notably, there was no difference in BALF IgG levels between the two groups (Figure 6D), but BALF IgM levels were significantly lower (2.3-fold) and IgA levels were significantly higher (3.3-fold) in the repeat-dose group as compared to the single-dose group (Figures 6E and 6F). This is consistent with an Ig switch from IgM to IgA following repeat exposure to rAAV2.5T at a 5-month interval (Figures 6B, 6C, 6E, and 6F).
Figure 6.
Capsid-binding immunoglobulin levels in plasma and BALF following repeat administration of rAAV2.5T to ferret lung
Schematic of rAAV2.5T administration in single and repeat groups of ferrets is as shown in Figure 4A. AAV capsid-binding Ig titers were assessed by ELISA using purified vector as the capture antigen. (A) Plasma IgG levels (diluted 4,000-fold). (B) Plasma IgM levels (diluted to 1:2,000). (C) Plasma IgA levels (diluted to 1:20). (D) BALF IgG levels (diluted 50-fold). (E and F) (E) BALF IgM levels and (F) IgA levels assessed using undiluted samples. Results represent the mean ± SEM. Data were analyzed by Mann-Whitney test. ∗p < 0.05, ∗∗p < 0.01. n = 4 or 6 animals in each group.
Discussion
Innate and adaptive immune responses pose potential challenges for CF lung-directed gene therapy that could limit the durability of transgene expression in the airway. The limited lifespan of most airway luminal cell types also suggests that repeat administration will likely be required for a sustained therapeutic effect. It is clear that the efficiency of rAAV2.5T transduction in the ferret lung declines with short-interval (1-month) repeat administration due to NAb.25 However, there is a lack knowledge about the durability of both rAAV-mediated CFTR transgene expression and NAb responses that could limit efficacy following repeat vector administration to the lung over longer intervals. This study in ferrets was specifically designed to address these critical questions. To study the durability of transgene expression, adult ferrets were transduced with an rAAV2.5T vector harboring the ferret CFTR minigene (fCFTRΔR) to limit host immunity to only the vector capsid. A second administration of rAAV at a 5-month interval used a reporter vector (rAAV2.5T-gLuc) to enable quantitative monitoring of secreted gLuc over a 14-day period.
To our knowledge, this is the first report of the CFTR transgene expression being sustained for at least 5 months in a large animal model. rAAV2.5T-mediated fCFTRΔR transgene expression exceeded the level of endogenous CFTR mRNA (200%–250%) within the first 21 days after administration but declined thereafter before stabilizing at 3–5 months at an average level of 26% that of endogenous CFTR. It has been suggested that CF people with heterozygous CFTR gene-splicing mutations (3272-26A>G/F508del genotype) produce ∼5% normal CFTR transcripts and have more mild symptoms as compared to people with homozygous F508del mutations.30 Other in vitro studies in CF polarized airway epithelia have suggested that gene replacement in as little as 6%–10%31 or 25%32 of cells can complement CFTR-mediated chloride currents to wild-type levels. Our studies at 5 months post administration of rAAV2.5T-fCFTRΔR demonstrated transgene expression at 19% of endogenous CFTR (Figure 1C). While this may provide a pharmacologically relevant level of CFTR expression, it is also a level we considered to be within the limits requiring re-dosing of the vector.
Notably, the drop in vector-derived mRNA between 10 and 21 days was 1.4-fold (Figure 1B, left), while there was a 15-fold drop in lung vector genomes (Figure 1D) over the same time period. One explanation for this discrepancy involves enhanced clearance of ineffective viral vector particles by one of the following mechanisms. The most plausible mechanism may be clearance and degradation of viral vector particles by alveolar macrophages and dendritic cell of the lung. Activation of the cellular immune response, which peaked at 21 days (Figure 2C), could also be functionally linked to the clearance of ineffective vector particles taken up by macrophages, dendritic cells, natural killer cells, monocytes, and neutrophils in the lung. We also know from our previous ferret study that ∼20% of AAV2.5T escapes the lung and vg can be found in the liver at 14 days post transduction without transgene expression.17 Each of these mechanisms could have contributed to the more rapid decline in vg as compared to transgene mRNA.
Repeat administration with rAAV2.5T-gLuc at 5 months following rAAV2.5T-fCFTRΔR administration was equally as effective as single-dose administration of rAAV2.5T-gLuc to naive animals when assessing gLuc levels in BALF (Figure 4B), despite persistent NAbs (Figure 2B) and capsid-binding antibodies (BAbs) (Figures 3D–3F) in the BALF. This result demonstrates the feasibility of efficient repeat dosing to the ferret lung at a 5-month dose interval. However, the kinetics of gLuc expression in plasma of the repeat-dose group, which remained stable between 5 and 14 days, was very different from that of the single-dose group, which declined >1,700-fold between 5 and 14 days (Figure 4C). While the mechanism responsible for these differences remains unclear, we reason that an altered immune response between the two groups impacts both the transduction and clearance of transduced lung epithelial cells capable of secreting gLuc into the blood. We have previously shown that only a small fraction of rAAV2.5T-gLuc vector escapes the lung and is taken up by the liver; while vg were found in the liver at 14 days following rAAV2.5T-gLuc lung delivery to ferrets, there was no expression of gLuc in other organs including the liver.17 These findings suggest that a lung-intrinsic mechanism is responsible for the durability and differential secretion of gLuc into the blood between single-dosed and repeat-dosed animals.
Previous studies with rAAV8 have shown that non-neutralizing capsid BAbs can enhance vector uptake and transduction of the liver in mice.33 This study also demonstrated that BAbs altered the biodistribution profile of vg, enhancing uptake in muscle while reducing uptake in spleen and lymph nodes.33 Given that in our study AAV2.5T capsid-binding IgM and IgA levels in the BALF were significantly different between single-dose and repeat-dose groups (Figures 6E and 6F), it is possible that BAbs impacted the cellular distribution of AAV2.5T-gLuc transduction in the lung of the repeat-dose group to favor transduction of epithelial cells that lack host-immune-mediated clearance and/or have differing capacities to secrete gLuc apically into the BALF or basolaterally into the blood.
Cellular immune responses to prior exposure to rAAV2.5T also likely impacted outcomes in this study. The enhanced IFN-γ T cell responses to capsid peptide challenge were significantly elevated in animals receiving two doses of rAAV2.5T (Figure 5C). This could partially explain the rapid clearance of rAAV2.5-fCFTRΔR transduced cells following the second exposure to rAAV2.5T-gLuc, as indexed by vg and vector-derived fCFTRΔR mRNA (Figures 5D–5F). While the mechanism of selective clearance of rAAV2.5-fCFTRΔR, but not rAAV2.5T-gLuc, transduced epithelial cells remains unclear, it could involve immune memory by virally transduced epithelial cells.34,35 While viral immune cell memory by resident B cells and T cells in the lung and other organs has been well established,36,37,38 much less is known about the mechanisms of epithelial cell memory in viral infection and inflammation in the lung. This emerging field of inflammatory memory (or trained immunity) is thought to involve epigenetic reprogramming of stress-response genes to an open chromatin state that allows for rapid changes in the expression of genes that enhance fitness of cells or the organism to repeated environmental stresses or pathogen exposure.34,35 For example, interleukin-6-pSTAT3-mediated epigenetic reprogramming has been shown to be involve inflammatory memory of intestinal and pancreatic epithelia.34 While epithelial inflammatory memory has yet to be extensively studied in epithelia of the lung, its presence in other epithelial barrier organs such as the intestine and skin35 support a potential role in the lung in the context of gene therapy. While epithelial inflammatory memory primes cells to respond to infection in an antigen agnostic manner, networks that drive epithelial-to-immune communication can intersect to drive cooperative responses to infection.35,36 Thus, further research is needed to understand such mechanisms and to develop approaches that minimize their involvement in repeat administration of rAAV to the lung.
In summary, we find that fCFTRΔR transgene expression following a single intratracheal administration of rAAV2.5-fCFTRΔR to the adult ferret lung declines rapidly within the first 3 months and then remains relatively stable between 3 and 5 months, averaging 26% that of endogenous fCFTR. Repeat administration of rAAV2.5T at a 5-month interval was as efficient as a single administration of vector despite residual NAbs in the BALF. However, re-exposure to rAAV2.5T may have led to enhanced clearance of cells in the lung transduced 5 months earlier, suggesting that inflammatory memory may be a barrier to repeat administration. These findings support a need for immunomodulation in applications of rAAV gene therapy to the lung and highlight areas of research needed to understand immune mechanisms that impact the durability of transduced cells in the lung.
Materials and methods
Production of rAAV2.5T vector
rAAV2.5T vectors expressing ferret CFTRΔR (rAAV2.5T-SP183-fCFTRΔR, herein called rAAV2.5-fCFTRΔR), firefly luciferase (rAAV2.5T-SP183-fLuc, herein called rAAV2.5-fLuc), and gaussia luciferase (rAAV2.5T-SP183-gLuc, herein called rAAV2.5-gLuc) utilized a short 183-bp promoter (SP183) and were prepared as previously described.17 In brief, HEK293 cells were transfected with the AAV trans-plasmid (pAV2.5Trep/cap), adenovirus helper plasmid (pAd4.1), and the AAV proviral plasmid listed above containing the AAV2 inverted terminal repeat sequences. rAAV vectors in the cell lysate from transfected HEK293 cells were purified on an iodixanol cushion by ultracentrifugation followed by two cesium chloride density gradients. Titers were determined as DNase-I-resistant particles (DRPs) by TaqMan qPCR with primer and probe sets specific to each transgene. The purity of each vector stock was confirmed by SDS-PAGE and silver staining.
Vector administration to ferret lungs
All animal experimentation was performed according to protocols approved by the institutional animal care and use committee of the University of Iowa. Five- to 8-month-old adult ferrets (n = 4–8 per group) were anesthetized with a mixture of ketamine and xylazine injected subcutaneously and then intratracheally administered rAAV2.5-fCFTRΔR at a dose of 1 × 1013 DRP/kg body weight (BW) with a MADgic sprayer atomization device (Teleflex.com, model MAD720). The rAAV2.5T vector inoculum contained doxorubicin at a concentration of 200 μM, and the administered volume was normalized to ferret BW at 2 mL/kg BW. Ferrets were euthanized at 10 and 21 days and 1, 3, 4, and 5 months post delivery of rAAV2.5T-fCFTRΔR, and BALF from the lung was first collected using a weight-normalized volume of PBS, after which the entire lung block was snap frozen for CFTR mRNA and vector DNA. Blood and splenocytes were also collected as previously described.25 A second administration of rAAV2.5-gLuc vector to the lung was delivered (1 × 1013 DRP/kg BW) at 5 months after rAAV2.5-fCFTRΔR vector dosing, and the ferrets were euthanized at 14 days later. Plasma was collected at 0, 5, 10, and 14 days following rAAV2.5-gLuc administration, and BALF was collected at 14 days, and both plasma and BALF were used for titration of NAbs and measurement of gaussia luciferase activity.
TaqMan qPCR quantification of CFTR mRNA and vector genomic DNA
Transgene-derived (fCFTRΔR) and endogenous ferret CFTR mRNA was quantified by TaqMan qPCR using GAPDH as a housekeeping reference gene; the primers and probe sets are listed in Table 1. The methods for quantifying mRNA copies in transduced lung tissue used a previously described approach that minimized regional variations in delivered vector.25 The entire snap-frozen lung was cryo-ground to powder at a particle size of <1 mm3 using a tissue grinder (BioCryo, Biospec, model BCTG120) with supplemental dry ice added to the chamber during grinding. Extraction of total RNA from lung tissue samples (six technical replicates per sample tested and averaged, 80 ± 5 mg/test) was performed using TRIzol according to the manufacturer’s protocol. RNA samples were digested with DNase (Turbo DNase, Ambion) to remove rAAV genomes and genomic DNA. cDNA synthesis was performed on 100 ng of total RNA using the ABI High-Capacity cDNA Reverse Transcription Kit (ABI: 4368814) and pd(N)6 random primers according to the manufacturer’s protocol. For each sample tested, a control was performed in the absence of reverse transcriptase to verify the absence of vector genomic DNA contamination. Plasmid copy number standard curves were generated each time the assay was performed.
The real-time qPCR assays were performed using iTaq Universal probe Supermix (Bio Rad). Amplification reactions contained 12.5 μL of 2× Supermix, 1 μL of cDNA, 2.5 pmol/μL of each specific primer, 2.5 pmol/μL of probe, and added water to total volume of 25 μL per reaction. PCR conditions were 95°C for 3 min, followed by 40 cycles of 95°C for 15 s and 62.5°C for 40 s.
rAAV2.5-fCFTRΔR vg with ferret lung genomic DNA was isolated from cryo-ground preclinical models lung powder using standard methods with TRIzol. The same primer/probe sets (Table 1) and TaqMan qPCR methods were used to quantify DNA and RNA copy number. Each DNA copy number assay was performed with three technical replicate samples containing 100 ng of purified DNA, and the values were averaged.
Measurement of gLuc activity in plasma and BALF
On days 0, 5, 10, and 14 after administration of the AAV2.5T-gLuc vector, blood was harvested from the jugular vein of anesthetized ferrets and collected into heparinized tubes for plasma isolation. Ferrets were euthanized with Euthasol (Virbac AH) at 14 days post vector administration, and BALF was collected by lavage of the trachea/lung cassette using 5 mL of PBS per 300 g BW. The gLuc activity in the plasma and BALF were immediately measured after harvest with a Renilla Luciferase Assay Kit (Promega) using the manufacturer’s methods.
Titration of rAAV2.5T NAbs and capsid BAbs levels in plasma and BALF
The level of NAb in the plasma and BALF was quantified using a micro-neutralization assay based on the transduction of A549 cells using rAAV2.5T-fLuc incubated with serially diluted plasma or BALF as previously described.17,25 Firefly luciferase activity in cell lysates was measured using a Firefly Luciferase Assay Kit (Promega). The titers of rAAV2.5T NAb in each plasma or BALF sample was calculated as the IC50 using Prism software (GraphPad). The levels of total capsid-binding IgG, IgM, and IgA in the plasma and BALF were determined by ELISA using rAAV5 as the capture antigen as previously described.17,25 rAAV5 was used because the VP2 and the VP3 capsid proteins of rAAV2.5T are derived from rAAV5 with a single A581T mutation. We have previously shown that the use of rAAV5 or rAAV2.5T vector as the capture antigen gives similar quantification of anti-rAAV2.5T capsid BAbs by ELISA.17
ELISpot assay of T cell immune response against the AAV2.5T capsid
The T cell responses to the rAAV2.5T capsid were measured using a ferret IFN-γ-ELISpot assay as previously described.25 In brief, splenocytes were thawed, washed, and counted. Phorbol 12-myristate 13-acetate (10 ng/mL, Sigma) and ionomycin (0.2 mg/mL, Sigma) were used as a positive control for splenocyte responses, and medium alone (i.e., no stimulation) was used as a negative control. Controls and peptide antigens (1 mg/mL) were added to each well (n = 2 technical replicates) containing splenocytes (2 × 105/well). The number of spot-forming units (SFU) per well was determined using an Immunospot plate reader (Astor; Mabtech, Cincinnati, OH, USA), and results were expressed as SFU/2 × 105 cells after subtracting the SFU from the medium-alone negative control. Four rAAV2.5T capsid peptides previously shown to demonstrate the highest level of stimulation25 were pooled to stimulate ferret splenocytes.
Statistical analysis
The experimental data are presented as mean ± SEM, and Prism 9 (GraphPad Software) was used for the data analyses. The statistical significance between multiple groups was determined using one-way ANOVA followed by the Tukey test (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 in figures). Certain data was log2(Y + 1) transformed prior to ANOVA and the Tukey test because of the large range in values. Comparisons of two groups used the Mann-Whitney test.
Data and code availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Acknowledgments
This work was supported by grants from the National Institutes of Health (HL152960, DK054759, and 75N92019C00010 to J.F.E.) and a sponsored research agreement (SRA) from Spirovant Sciences. We would also like to acknowledge Lillian Falese for her support with the ELISpot.
Author contributions
Conceptualization, Y.T., Z.Y., and J.F.E.; investigation, Y.T., M.E., J.L., S.F., P.W., and Z.F.; methodology, Y.T. and Z.Y.; validation, Y.T., M.E., S.F., J.L., P.W., and Z.F.; formal analysis, Y.T. and J.F.E.; writing – original draft, Y.T.; writing – review & editing, K.J.E., M.P.L., M.D.S., R.K., Z.Y., and J.F.E.; project administration, K.J.E., M.P.L., and R.K.; supervision, J.F.E.; funding acquisition, J.F.E.
Declaration of interests
This work was funded by an SRA from Spirovant Sciences, Inc. Z.Y. and J.F.E. are consultants for Spirovant Sciences, Inc. K.E., M.P.L., M.S., and R.K. are employees of Spirovant Sciences, Inc.
Contributor Information
Ziying Yan, Email: ziying-yan@uiowa.edu.
John F. Engelhardt, Email: john-engelhardt@uiowa.edu.
References
- 1.Lopes-Pacheco M. CFTR Modulators: The Changing Face of Cystic Fibrosis in the Era of Precision Medicine. Front. Pharmacol. 2019;10:1662. doi: 10.3389/fphar.2019.01662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma N., Cutting G.R. The genetics and genomics of cystic fibrosis. J. Cyst. Fibros. 2020;19(Suppl 1):S5–S9. doi: 10.1016/j.jcf.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bareil C., Bergougnoux A. CFTR gene variants, epidemiology and molecular pathology. Arch. Pediatr. 2020;27(Suppl 1):eS8–eS12. doi: 10.1016/S0929-693X(20)30044-0. [DOI] [PubMed] [Google Scholar]
- 4.Saluzzo F., Riberi L., Messore B., Loré N.I., Esposito I., Bignamini E., De Rose V. CFTR Modulator Therapies: Potential Impact on Airway Infections in Cystic Fibrosis. Cells. 2022;11:1243. doi: 10.3390/cells11071243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bordet T., Behar-Cohen F. Ocular gene therapies in clinical practice: viral vectors and nonviral alternatives. Drug Discov. Today. 2019;24:1685–1693. doi: 10.1016/j.drudis.2019.05.038. [DOI] [PubMed] [Google Scholar]
- 6.Bennett J., Wellman J., Marshall K.A., McCague S., Ashtari M., DiStefano-Pappas J., Elci O.U., Chung D.C., Sun J., Wright J.F., et al. Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: a follow-on phase 1 trial. Lancet. 2016;388:661–672. doi: 10.1016/S0140-6736(16)30371-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozelo M.C., Mahlangu J., Pasi K.J., Giermasz A., Leavitt A.D., Laffan M., Symington E., Quon D.V., Wang J.D., Peerlinck K., et al. Valoctocogene Roxaparvovec Gene Therapy for Hemophilia A. N. Engl. J. Med. 2022;386:1013–1025. doi: 10.1056/NEJMoa2113708. [DOI] [PubMed] [Google Scholar]
- 8.Pipe S.W., Leebeek F.W.G., Recht M., Key N.S., Castaman G., Miesbach W., Lattimore S., Peerlinck K., Van der Valk P., Coppens M., et al. Gene Therapy with Etranacogene Dezaparvovec for Hemophilia B. N. Engl. J. Med. 2023;388:706–718. doi: 10.1056/NEJMoa2211644. [DOI] [PubMed] [Google Scholar]
- 9.FDA . 2022. FDA Approves First Gene Therapy to Treat Adults with Hemophilia B. [Google Scholar]
- 10.FDA . 2023. FDA Approves First Gene Therapy for Adults with Severe Hemophilia A. [Google Scholar]
- 11.Mendell J.R., Al-Zaidy S., Shell R., Arnold W.D., Rodino-Klapac L.R., Prior T.W., Lowes L., Alfano L., Berry K., Church K., et al. Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy. N. Engl. J. Med. 2017;377:1713–1722. doi: 10.1056/NEJMoa1706198. [DOI] [PubMed] [Google Scholar]
- 12.FDA . 2019. FDA Approves Innovative Gene Therapy to Treat Pediatric Patients with Spinal Muscular Atrophy, a Rare Disease and Leading Genetic Cause of Infant Mortality. [Google Scholar]
- 13.FDA . 2023. FDA Approves First Gene Therapy for Treatment of Certain Patients with Duchenne Muscular Dystrophy. [Google Scholar]
- 14.Gonzalez T.J., Mitchell-Dick A., Blondel L.O., Fanous M.M., Hull J.A., Oh D.K., Moller-Tank S., Castellanos Rivera R.M., Piedrahita J.A., Asokan A. Structure-guided AAV capsid evolution strategies for enhanced CNS gene delivery. Nat. Protoc. 2023;18:3413–3459. doi: 10.1038/s41596-023-00875-y. [DOI] [PubMed] [Google Scholar]
- 15.Excoffon K.J.D.A., Koerber J.T., Dickey D.D., Murtha M., Keshavjee S., Kaspar B.K., Zabner J., Schaffer D.V. Directed evolution of adeno-associated virus to an infectious respiratory virus. Proc. Natl. Acad. Sci. USA. 2009;106:3865–3870. doi: 10.1073/pnas.0813365106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan Z., Sun X., Feng Z., Li G., Fisher J.T., Stewart Z.A., Engelhardt J.F. Optimization of Recombinant Adeno-Associated Virus-Mediated Expression for Large Transgenes, Using a Synthetic Promoter and Tandem Array Enhancers. Hum. Gene Ther. 2015;26:334–346. doi: 10.1089/hum.2015.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang Y., Yan Z., Lin S., Huntemann E.D., Feng Z., Park S.Y., Sun X., Yuen E., Engelhardt J.F. Repeat Dosing of AAV2.5T to Ferret Lungs Elicits an Antibody Response That Diminishes Transduction in an Age-Dependent Manner. Mol. Ther. Methods Clin. Dev. 2020;19:186–200. doi: 10.1016/j.omtm.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan F., Gasser G.N., Lemire E., Montoro D.T., Jagadeesh K., Zhang Y., Duan Y., Ievlev V., Wells K.L., Rotti P.G., et al. Transgenic ferret models define pulmonary ionocyte diversity and function. Nature. 2023;621:857–867. doi: 10.1038/s41586-023-06549-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun X., Olivier A.K., Liang B., Yi Y., Sui H., Evans T.I.A., Zhang Y., Zhou W., Tyler S.R., Fisher J.T., et al. Lung phenotype of juvenile and adult cystic fibrosis transmembrane conductance regulator-knockout ferrets. Am. J. Respir. Cell Mol. Biol. 2014;50:502–512. doi: 10.1165/rcmb.2013-0261OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He N., Liu X., Vegter A.R., Evans T.I.A., Gray J.S., Guo J., Moll S.R., Guo L.J., Luo M., Ma N., et al. Ferret models of alpha-1 antitrypsin deficiency develop lung and liver disease. JCI Insight. 2022;7 doi: 10.1172/jci.insight.143004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan Z., Stewart Z.A., Sinn P.L., Olsen J.C., Hu J., McCray P.B., Jr., Engelhardt J.F. Ferret and pig models of cystic fibrosis: prospects and promise for gene therapy. Hum. Gene Ther. Clin. Dev. 2015;26:38–49. doi: 10.1089/humc.2014.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Semaniakou A., Croll R.P., Chappe V. Animal Models in the Pathophysiology of Cystic Fibrosis. Front. Pharmacol. 2018;9:1475. doi: 10.3389/fphar.2018.01475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rawlins E.L., Hogan B.L.M. Ciliated epithelial cell lifespan in the mouse trachea and lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2008;295:L231–L234. doi: 10.1152/ajplung.90209.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leigh M.W., Kylander J.E., Yankaskas J.R., Boucher R.C. Cell proliferation in bronchial epithelium and submucosal glands of cystic fibrosis patients. Am. J. Respir. Cell Mol. Biol. 1995;12:605–612. doi: 10.1165/ajrcmb.12.6.7766425. [DOI] [PubMed] [Google Scholar]
- 25.Tang Y., Fakhari S., Huntemann E.D., Feng Z., Wu P., Feng W.Y., Lei J., Yuan F., Excoffon K.J., Wang K., et al. Immunosuppression reduces rAAV2.5T neutralizing antibodies that limit efficacy following repeat dosing to ferret lungs. Mol. Ther. Methods Clin. Dev. 2023;29:70–80. doi: 10.1016/j.omtm.2023.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verdera H.C., Kuranda K., Mingozzi F. AAV Vector Immunogenicity in Humans: A Long Journey to Successful Gene Transfer. Mol. Ther. 2020;28:723–746. doi: 10.1016/j.ymthe.2019.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen W., Liu S., Ou L. rAAV immunogenicity, toxicity, and durability in 255 clinical trials: A meta-analysis. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.1001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leone P., Shera D., McPhee S.W.J., Francis J.S., Kolodny E.H., Bilaniuk L.T., Wang D.J., Assadi M., Goldfarb O., Goldman H.W., et al. Long-term follow-up after gene therapy for Canavan disease. Sci. Transl. Med. 2012;4 doi: 10.1126/scitranslmed.3003454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendell J.R., Al-Zaidy S.A., Lehman K.J., McColly M., Lowes L.P., Alfano L.N., Reash N.F., Iammarino M.A., Church K.R., Kleyn A., et al. Five-Year Extension Results of the Phase 1 START Trial of Onasemnogene Abeparvovec in Spinal Muscular Atrophy. JAMA Neurol. 2021;78:834–841. doi: 10.1001/jamaneurol.2021.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramalho A.S., Beck S., Meyer M., Penque D., Cutting G.R., Amaral M.D. Five percent of normal cystic fibrosis transmembrane conductance regulator mRNA ameliorates the severity of pulmonary disease in cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 2002;27:619–627. doi: 10.1165/rcmb.2001-0004OC. [DOI] [PubMed] [Google Scholar]
- 31.Johnson L.G., Olsen J.C., Sarkadi B., Moore K.L., Swanstrom R., Boucher R.C. Efficiency of gene transfer for restoration of normal airway epithelial function in cystic fibrosis. Nat. Genet. 1992;2:21–25. doi: 10.1038/ng0992-21. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L., Button B., Gabriel S.E., Burkett S., Yan Y., Skiadopoulos M.H., Dang Y.L., Vogel L.N., McKay T., Mengos A., et al. CFTR delivery to 25% of surface epithelial cells restores normal rates of mucus transport to human cystic fibrosis airway epithelium. PLoS Biol. 2009;7 doi: 10.1371/journal.pbio.1000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fitzpatrick Z., Leborgne C., Barbon E., Masat E., Ronzitti G., van Wittenberghe L., Vignaud A., Collaud F., Charles S., Simon Sola M., et al. Influence of Pre-existing Anti-capsid Neutralizing and Binding Antibodies on AAV Vector Transduction. Mol. Ther. Methods Clin. Dev. 2018;9:119–129. doi: 10.1016/j.omtm.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naik S., Fuchs E. Inflammatory memory and tissue adaptation in sickness and in health. Nature. 2022;607:249–255. doi: 10.1038/s41586-022-04919-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ordovas-Montanes J., Beyaz S., Rakoff-Nahoum S., Shalek A.K. Distribution and storage of inflammatory memory in barrier tissues. Nat. Rev. Immunol. 2020;20:308–320. doi: 10.1038/s41577-019-0263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mettelman R.C., Allen E.K., Thomas P.G. Mucosal immune responses to infection and vaccination in the respiratory tract. Immunity. 2022;55:749–780. doi: 10.1016/j.immuni.2022.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takamura S., Kato S., Motozono C., Shimaoka T., Ueha S., Matsuo K., Miyauchi K., Masumoto T., Katsushima A., Nakayama T., et al. Interstitial-resident memory CD8(+) T cells sustain frontline epithelial memory in the lung. J. Exp. Med. 2019;216:2736–2747. doi: 10.1084/jem.20190557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arjomandnejad M., Dasgupta I., Flotte T.R., Keeler A.M. Immunogenicity of Recombinant Adeno-Associated Virus (AAV) Vectors for Gene Transfer. BioDrugs. 2023;37:311–329. doi: 10.1007/s40259-023-00585-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.