Abstract
Starting from the consideration of the structure of human milk fat globule (MFG), this study aimed to investigate the effects of ultrasonic treatment on milk fat globule membrane (MFGM) and soy lecithin (SL) complexes and their role in mimicking human MFG emulsions. Ultrasonic power significantly affected the structure of the MFGM-SL complex, further promoting the unfolding of the molecular structure of the protein, and then increased solubility and surface hydrophobicity. Furthermore, the microstructure of mimicking MFG emulsions without sonication was unevenly distributed, and the average droplet diameter was large. After ultrasonic treatment, the droplets of the emulsion were more uniformly dispersed, the particle size was smaller, and the emulsification properties and stability were improved to varying degrees. Especially when the ultrasonic power was 300 W, the mimicking MFG emulsion had the highest encapsulation rate and emulsion activity index and emulsion stability index were increased by 60.88 % and 117.74 %, respectively. From the microstructure, it was observed that the spherical droplets of the mimicking MFG emulsion after appropriate ultrasonic treatment remain well separated without obvious flocculation. This study can provide a reference for the screening of milk fat globules mimicking membrane materials and the further utilization and development of ultrasound in infant formula.
Keywords: Ultrasound, Milk fat globules membrane, Soybean lecithin, Mimicking MFG emulsion, Stability, Structure
1. Introduction
Human milk is a natural emulsion that contains relatively large triacylglycerol droplets covered by a unique interfacial layer known as a milk fat globule membrane (MFGM) [1]. When breastfeeding is not available, infant formulas (IFs) become the best alternative to human milk (HM). Due to the processing or use of vegetable oils, IFs often not only lacked MFGM components, and lipid droplets interface was mainly surrounded by protein [2]. The lack of MFGM structure can affect the structure and stability of lipid droplets, which in turn affects the digestion and absorption of lipids. This can increase the risk of obesity and metabolic syndrome in infants later on in their growth [3]. Therefore, there has been increasing focus on the role of MFGM and their use in simulated human milk fat globule (MFG) emulsions in recent years. However, most of the MFGM materials used in these studies were MFGM-rich whey protein powders, which has a significantly lower content of phospholipids (PLs) (6 %–10 %) compared to PLs found in HM MFGM (60 %–65 %) [3], [4], [5]. Phospholipid (PL) has the functions of promoting fat metabolism, enhancing immunity, and improving brain and nervous system development [6]. In addition, PLs are also the backbone of biofilms; low levels of PLs will affect the formation of interfacial membranes, further affect the emulsifiers' emulsifying properties and emulsion stability [7]. The emulsification, stabilization and structural properties of mimicking MFG emulsion are essential for dairy processing and storage. Therefore, it is essential to consider not only the addition of MFGM, but also the addition of PL when preparing IFs model emulsions.
Soy lecithin (SL) is a frequently used emulsifier in IFs. It provides ample PLs, including phosphatidylcholine and phosphatidylinositol, and also possesses additional functions such as antioxidant properties, emulsification, and viscosity reduction [8]. Chen et al [9] reported that adding lecithin or MFGM to whey protein isolate improves emulsion stability by increasing the surface coverage of PL. Yu et al [4] prepared the model emulsion by mixing PL with MFGM material to create the aqueous phase, and then combining the aqueous phase with the oil phase through high-pressure homogenization. This study also proposed that adding PL to MFGM could enhance the structure of MFG. Further, ultrasound technology is a non-heat treatment technology that can enhance the nutritional value and processing characteristics of food, and has been widely used in emulsification or structural modification of food materials [10]. A recent study has noted that ultrasonic pretreated MFGM can be used as a membrane component for the preparation of mimicking MFG emulsion. This process reduces the particle size and structure of MFG, resulting in improved emulsion stability and encapsulation efficiency [11]. However, this study did not consider the effects of additional PL supplementation or different ultrasound powers. Ultrasound can be categorized into low-power and high-power based on its frequency range [12]. Low-power ultrasound is commonly used to ensure food quality and safety, while high-power ultrasound is utilized to modify the functional properties of various food products, such as enhancing the stability of emulsions [13], [14]. However, there is a lack of studies on the effects of different ultrasonic power modifications of MFGM and SL on the physicochemical properties, structure, and stability of mimicking MFG emulsions. This is not conducive to the further development of IFs or the further use of some new technologies in IFs processing.
Therefore, MFGM was combined with SL and treated with different ultrasonic powers (0–600 W) to prepare MFGM-SL complexes. The resulting MFGM-SL complex’s interaction, solubility, particle size distribution, and surface hydrophobicity were investigated. Subsequently, the ultrasound-modified MFGM-SL complex was used as the membrane material to wrap the mixed oil phase, resulting in a mimicking MFG emulsion. The microstructure, emulsification, and emulsion stability of the emulsion were evaluated. The aim of this study was to use ultrasonic treatment to improve the structural and functional properties of MFGM and SL, and to further improve the microstructure and emulsification stability of mimicking MFG emulsion. The findings of this study can provide new insights into ultrasound to improve the structure and stability of mimicking MFG emulsions.
2. Materials and methods
2.1. Chemicals
MFGM was derived from Hilmar ingredients company (CA, USA), and contained about protein (78 %), phospholipids (8 %), and lactose (small amount). Vegetable oil (soybean, palm, corn, rapeseed, coconut and sunflower oils) was obtained from the local market in Harbin (China). Fast Green (FCF) and Nile Red (NR) were purchased from Sigma-Aldrich (Beijing, China). SL was purchased from Shengtong biotechnology strength supplier (Zhejiang, China). Another reagent was analytically pure.
2.2. Preparation of MFGM–SL complex and ultrasound treatments
Dissolved MFGM and SL (1:1, w/w) in distilled water to prepare a solution at a concentration of 20 mg/mL [4], [15], and added sodium azide (0.02 %, w/w) to the solution to prevent microbial growth. The solution was stirred magnetically for 1 h (20 ± 2 °C), and the resulting solution was called MFGM-SL complex. Ultrasound conditions and methods referred to previous studies and have been slightly adjusted [16]. The DY-1200Y ultrasonic processor with a titanium probe (2 cm diameter) was used to sonicate the MFGM-SL complex at 20 kHz ultrasonic frequency. The output intensities of sonication were 0, 150, 300, 450 and 600 W, respectively, covering commonly used ultrasonic powers, and ultrasound time was set to 10 min. The probe was immersed in the MFGM-SL complex and the ultrasonic pulse system worked for 2 s and rests for 2 s. The MFGM-SL complex after sonication was stored at 4 °C for further analysis.
2.3. Fluorescence spectroscopy analysis
Fluorescence spectra were analyzed by using RF-6000 spectrofluorimeter (Shmadzu, Japan). The MFGM-SL complex was kept in a 25 °C water bath (20 min), then the MFGM-SL complex was put into the quartz cuvette. The condition for testing was set as follows, 280 nm was set as excitation wavelength (λex) with 5 nm excitation slit width, and recorded the fluorescence spectra at 298 K (320–500 nm wavelength range).
2.4. Solubility
The prepared MFGM-SL complex was lyophilized by an LGJ-10N vacuum freezing dryer (Yaxing Co., LTD, China). Next, the lyophilized MFGM-SL complex powder was dispersed in deionized water at 25 °C (1 %, w/v) and stirred (110 rpm, 30 min). The samples were then centrifuged (4000 rpm, 5 min). Take an equal volume of the supernatant and dry to constant weight in an incubator at 100 °C. The detection of solubility and calculation of solubility was based on the previous report [17].
2.5. Surface hydrophobicity
Surface hydrophobicity is based on reports by Meng et al. [18] with minor modification. 1-anilinonaphthalene-8-sulfonic acid (ANS) was dissolved in sodium phosphate buffer (pH 7.4, 0.1 mol/L) to obtain an 8 mmol/L ANS solution. The sample was diluted with 0.01 mol/L sodium phosphate buffer (pH 7.0) at a concentration of 0.05–0.25 mg/mL, 10 μL of ANS and 2 mL of sample was mixed, and reacted in a dark environment for 15 min. The surface hydrophobicity of the MFGM-SL complex was then analyzed using a Hitachi F4500 fluorescence spectrophotometer (Tokyo, Japan) at 390 nm (excitation) and 470 nm (emission). Surface hydrophobicity was the initial slope of the linear regression curve.
2.6. Preparation of simulated milk fat globule emulsion
The emulsion was prepared by using MFGM-SL complex with different ultrasonic pretreatment as MFG membrane materials, while mixed oils phase as oil cores. A blend of different vegetable oils is used as the fat component to achieve a fatty acid composition similar to HM fat. The preparation of mixed oil phases was based on previous reports and mainly consisted of 10 % rapeseed, 10 % sunflower, 15 % soybean, 40 % palm and 25 % coconut oil [8]. The membrane material and the mixed oils phase were emulsified at 10,000 g at room temperature (2 min) by a JRJ-300-I high shear mixer in a ratio of 9:1, and then homogenized with an AFM-3 microfluidizer to obtain an MFGM-SL emulsion, namely mimicking MFG emulsion.
2.7. Emulsifying index measurements
Emulsion stability index (ESI) and emulsifying activity index (EAI) and are main emulsifying index, and were conducted based on previous reported method [8]. Precisely, SDS solution (5 mL, 0.1 g/L) was put into the emulsion (50 μL), and the absorbance values at 500 nm were recorded at 0 and 30 min, respectively, and recorded as A0 and A30. Finally, the ESI and EAI are obtained according to the calculation method below (Eq. (1) and Eq. (2)):
| (1) |
| (2) |
where N is the dilution factor, oil volume fraction and protein concentration (g/mL) are expressed as φ and ρ, respectively.
2.8. Measurement of particle size distribution and zeta potential
Zeta potential analyzer (Nano-Z, Malvern Instruments Ltd., Malvern, UK) and S3500 laser particle sizer (Microtrac Inc., Montgomery Ville, USA) were used to detect the particle size and zeta potential of different MFGM-SL complex and mimicking MFG emulsion with different ultrasonic pretreatment. Detection methods referred to previous report [4]. Briefly, dilute 0.1 mL MFGM-SL complex or mimicking MFG emulsion with ultrapure water to 50 mL to achieve 10 % shading. Finally, volume-weighted mean diameter (D4,3) of MFG is recorded. The refractive indices utilized for milk fat were 1.460 and 1.458 at 466 nm and 633 nm, respectively, while water had a refractive index of 1.33. Furthermore, 15 μL sample was diluted (400 times) with buffer (5 mM CaCl2, 50 mM NaCl, 20 mM imidazole) and zeta-potential was detected and recorded at 25 °C.
2.9. Flocculation of mimicking MFG emulsion
The flocculation of lipid droplets of the mimicking MFG emulsions were observed based on the method of Phan et al [19]. The emulsion sample was first diluted 10-fold in ultrapure water, then placed 5 μL of sample carefully on a glass slide, and covered with a coverslip. Finally, samples were observed under 40× magnification by using the Leitz Diaplan microscope (Wetzlar, Germany). Image of sample was recorded with the camera (built-in Olympus Color View) and analyzed using Olympus Belgium NV cell D software (Aartselaar, Belgium).
2.10. Microstructure observation
The Deltavision OMX SR super-resolution microsystem (GE, Boston, US) was used to analyze microstructure. The detection was based on previous methods with slight modifications [8]. Mimicking MFG emulsion (1 mL) is mixed with ultrapure water (10 mL). Fast green (FCF) dissolves in water (1 mg/mL). The proteins in the emulsion are labeled with FCF. Nile red (NR) is dissolved in ethanol (42 μg/mL) and was used to label triglycerides. 100 μL of sample is mixed with NR (20 μL) and FCF (50 μL), then the complex was reacted (1 h) at the dark environment, then the microstructure was observed.
2.11. Determination of encapsulation efficiency and stability constants
Stability constants and encapsulation efficiency (EE) of mimicking MFG emulsion are detected based on the previous method [4], [8]. Briefly, dispense the emulsion (5 mL) into dialysis bags and dialyze for 16 h in 0.005 mol/L PBS buffer (500 mL, pH 7.0). Then, 5 mL of ethanol was added into emulsions (1 mL), and sonicated (30 min, 90 W), next petroleum ether (50 mL) was added into the emulsion. Finally, absorbance was determined at 212 nm. The undialyzed emulsion was similarly treated and the absorbance was measured as A. In the detection of emulsification constants, samples (2 mL) are centrifuged (3000×g, 15 min). Remove 0.5 mL of the layer liquid and dilute with distilled water (9.5 mL). The absorbance is determined at 450 nm by using a Shimadzu UV-2600 spectrophotometer (Kyoto, Japan). The encapsulation efficiency and stability constants are calculated based on the following formula (Eq. (3) and Eq. (4)):
| (3) |
| (4) |
where A0 and A represent the absorbance of dialyzed or un-dialyzed samples at 212 nm, respectively in the determination of encapsulation efficiency. In the determination of stability constants, B and B0 represent the absorbance of samples treated with or without centrifugation at 450 nm, respectively.
2.12. Determination of oxidative stability
Freshly prepared mimicking MFG emulsion with different powers of ultrasonic was poured into a glass tube and distributed sealed for 0, 5, 10, 15, 20, 25, and 30 d (37 °C). The lipid oxidation and oxidation stability in emulsions was conducted by analyzing peroxide value (POV). The specific detection process and steps referred to the description of Li et al [20].
2.13. Extraction and identification of interfacial proteins
The extraction method of interface protein refers to previous study and has been slightly adjusted [4]. A 30 mL simulated MFG emulsion was poured into a centrifuge tube and centrifuged at 10,000g (4 °C, 30 min). The lower serological phase was removed, finally collected the upper cream phase and left it at overnight (−20 °C). Next, the cream phase was thawed in a hot bath (45 °C) and centrifuged (3500g, 10 min). After centrifugation, chloroform–methanol (2:1, v/v) was added into the lower layer. Finally, centrifuged the mixture again (3500g, 10 min) to collect the interfacial protein-containing intermediate layer.
Determination of protein distribution on the surface of MFG by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The obtained protein was dissolved in Milli-Q water (5 mg/mL). Then, sample solution (40 μL) and 5× protein loading buffer (10 μL) were mixed and boiled for 5 min. The prepared sample was then added to the gel. The electrophoresis voltage of concentrate gel (80 V) and separator gel (120 V) was set. Finally, a Coomassie distribution stain solution was applied to the stain, which was decolorized overnight until the background was clear.
2.14. Statistical analyses
The data was processed using origin 8.5 software (Northampton, MA, USA), and Tukey’s test of one-way analysis of variance was used to obtain the significant differences (p < 0.05). Each experiment was repeated at least three times and value was shown as mean ± standard deviation (SD).
3. Results and discussion
3.1. Effect of ultrasonic power on physical properties of MFGM and SL complex
3.1.1. Endogenous fluorescence spectrum
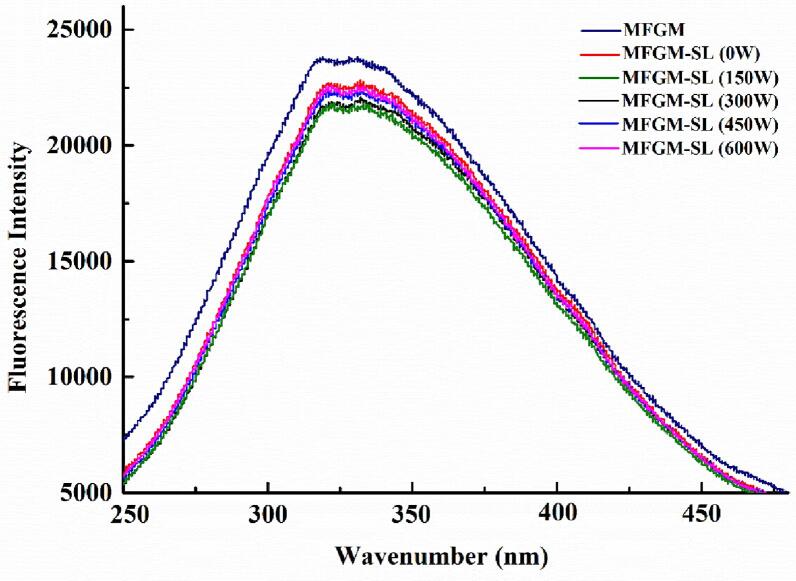
Endogenous fluorescence spectroscopy can obtain information on the binding properties of protein and small molecule. Transform in the internal structure of proteins are evaluated using changes in maximum wavelength and fluorescence intensity [21]. The endogenous fluorescence spectra of MFGM protein (mainly whey protein) and SL under different sonication were displayed in Fig. 1. Compared with the MFGM, the fluorescence intensity of the MFGM-SL complex decreased, and the fluorescence emission peak was redshifted at maximum wavelength, indicating that sonication changed the secondary structure of the protein, resulting in the expansion of the protein structure. Previous study has reported that sonication could affect whey protein-rutin interactions, which resulted in redshift at maximum wavelengths, which was consistent with our results [22]. In addition, the fluorescence intensity of the sonicated MFGM-SL complex (150 W–600 W) was lower than that of the unsonicated MFGM-SL complex, which could be due to low ultrasonic power treatment leading to quenching of fluorescence of exposed tryptophan residues [23]. These results also indicated that the ultrasonic power obvious affects the structure of the MFGM-SL complex, further promoted the molecular structure of the protein, and then affected its later physicochemical properties.
Fig. 1.
Endogenous fluorescence spectrum changes of MFGM-SL complex.
3.1.2. Solubility
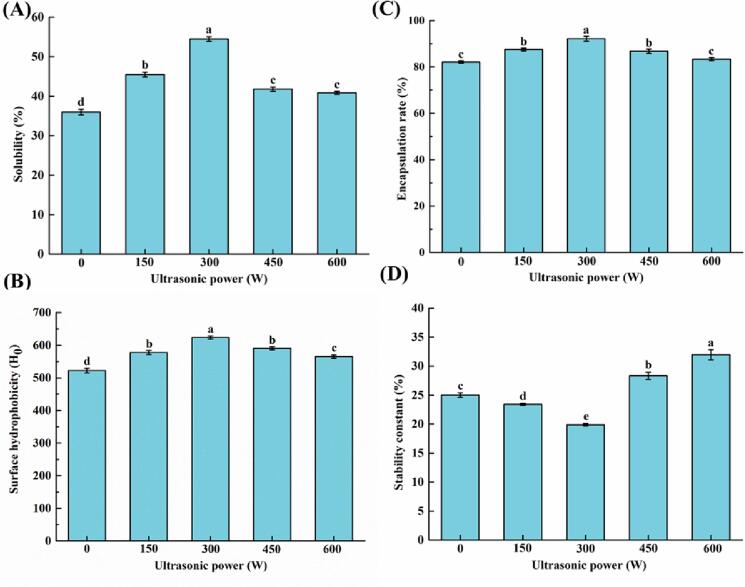
Solubility can reflect protein denaturation and aggregation, so it affects other functional properties, such as emulsifying properties [24]. Furthermore, solubility is also a key indicator of infant formula. The solubility of proteins in the MFGM-SL complex after different sonication was shown in Fig. 2A. The solubility of MFGM-SL complex improved after sonication, and reached highest when the ultrasonic power was 300 W, about 54.46 %. The result indicated that increasing the ultrasonic power could improve the solubility of proteins, which may be related to their decreased protein particle size and increased surface hydrophobicity, thereby increasing protein-water interaction to some extent [25]. Previous study showed that the solubility of MFGM after sonication was greatly improved compared with no treatment [11]. Under ultrasound action, the protein molecules in MFGM partially unfold, increasing solubility and easier interaction with lecithin [26].
Fig. 2.
Solubility (A) and surface hydrophobicity (B) of MFGM-SL complex at different ultrasonic powers. Effects of ultrasonic powers on the encapsulation efficiency (C) and stability constants (D) of mimicking MFG emulsions. Values were expressed as mean ± SD, different letters represented significant differences (P < 0.05).
3.1.3. Surface hydrophobicity analysis
Surface hydrophobicity (H0) was one of the vital surface-related properties of proteins. H0 indicated the number of hydrophobic groups of protein molecule surface [27]. In Fig. 2B, as the ultrasonic power increases, more proteins in the MFGM were dissolved in water. After MFGM protein in complex with lecithin, the hydrophobic groups of MFGM protein were more exposed to the surface of the molecule, resulting in increased surface hydrophobic interactions. Hydrophobicity was greatest at an ultrasonic power of 300 W. The increase in hydrophobicity favored the adsorption of hydrophobic groups of milk proteins and phospholipids on the surface of small oil droplets, thereby preventing or slowing down the accumulation of oil droplets during emulsion formation. Furthermore, the surface hydrophobicity of the sample decreased as the ultrasonic power increased, which may be due to more extension of non-covalent bonds due to partially unfolded protein molecules, thus protecting hydrophobic regions in the protein [28].
3.1.4. Particle size and zeta potential
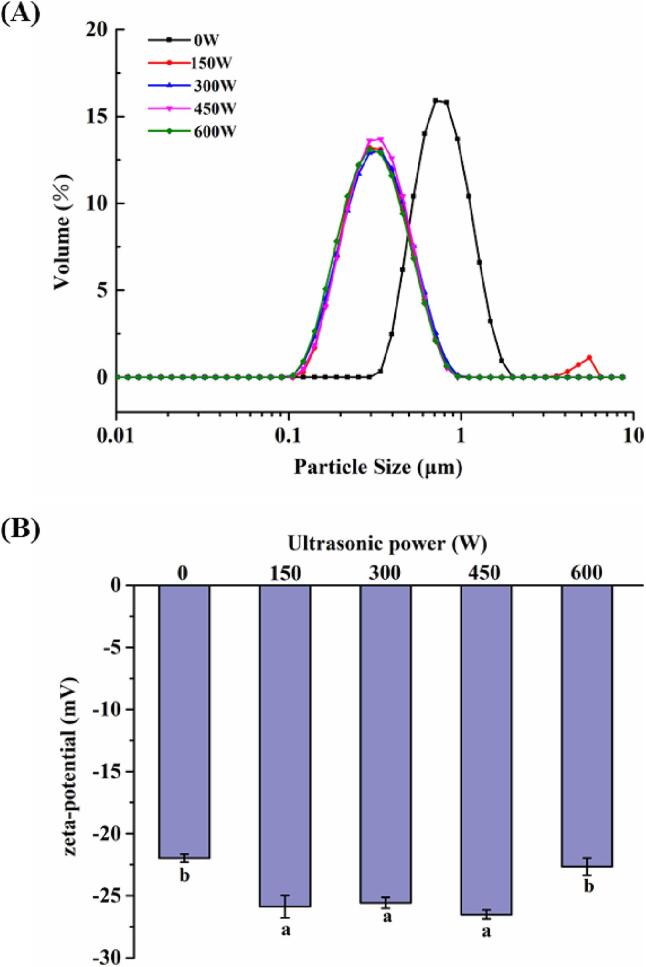
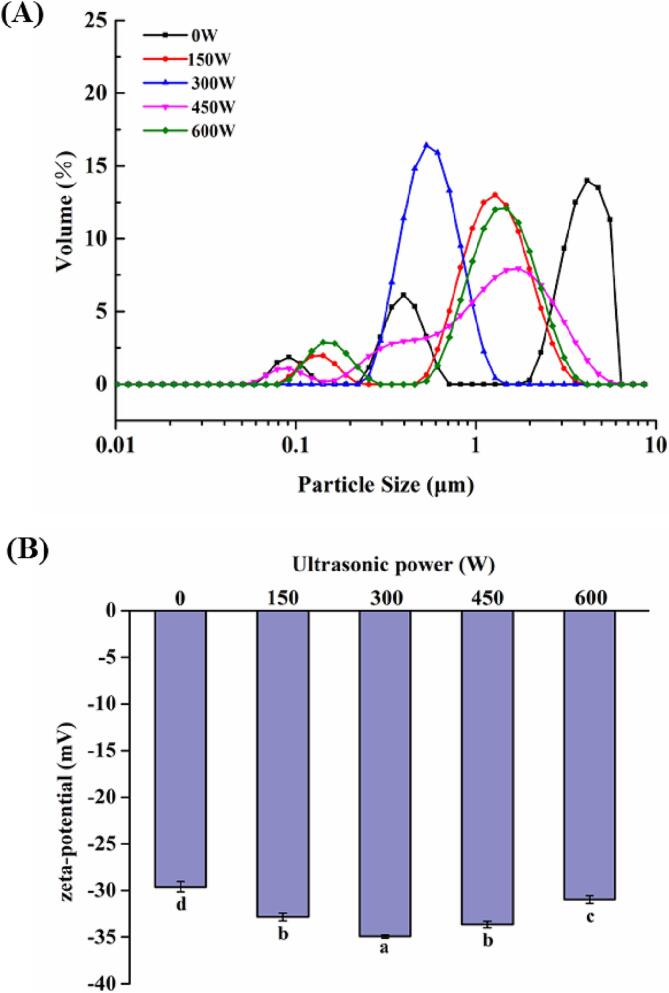
The effect of different ultrasonic powers on zeta potential and particle size of MFGM-SL complex was studied. The particle size distribution was shown in Fig. 3A, and the corresponding D4,3 was shown in Table 1. The particle size of the unsonicated MFGM-SL complex reached 0.68 ± 0.034 μm, while the MFGM-SL complex after sonication was significantly reduced (P < 0.05). The phenomenon of ultrasonic cavitation could lead to turbulence and high shear forces, which further reduce the particle size [29]. Turbulence and high shear force broke up milk protein and phospholipid aggregates into smaller aggregates. In addition, phospholipids were small molecule emulsifiers and had a certain viscosity, and existed as large aggregates in water before sonication, and were violently agitated and broken into smaller aggregates after ultrasonic cavitation. However, there were no significant differences in particle size of MFGM-SL complex under different ultrasonic power treatments.
Fig. 3.
Effects of different ultrasonic powers on particle size distribution (A) and zeta potential (B) of MFGM-SL complex. Values were expressed as mean ± SD, different letters represented significant differences (P < 0.05).
Table 1.
Volume-weighted mean diameter (D4,3) of MFGM-SL complex and mimicking MFG emulsion with different ultrasonic power treatments.
| Ultrasonic power (W) | MFGM-SL complex (μm) | Mimicking MFG emulsion (μm) |
|---|---|---|
| 0 | 0.68 ± 0.034a | 1.02 ± 0.10a |
| 150 | 0.31 ± 0.003b | 0.83 ± 0.05b |
| 300 | 0.29 ± 0.004b | 0.58 ± 0.04c |
| 450 | 0.28 ± 0.006b | 0.76 ± 0.02b |
| 600 | 0.27 ± 0.002b | 0.77 ± 0.02b |
Notes: Different letters in the column indicate significant (p < 0.05) differences among the samples. MFGM, milk fat globule membrane; SL, soy lecithin; MFG, milk fat globule.
The potential change of the MFGM-SL complex was shown in Fig. 3B. The absolute value of zeta potential increased, indicating that the electrostatic repulsion between molecules increased in the MFGM-SL complex, and the solution tends to be stable. Hydrophobic interactions between proteins and phospholipids could open up protein conformations, exposed more negatively charged residues, further increased electrostatic repulsion between particles and absolute value of the potential [30]. In addition, increased electrostatic repulsion could destroy proteins aggregation and also increase emulsion stability. The increase in negative charge might be due to that the phosphate group in phospholipids was negatively charged [31].
3.2. Effect of ultrasonic power on physical properties on mimicking MFG emulsion
3.2.1. Emulsifying properties
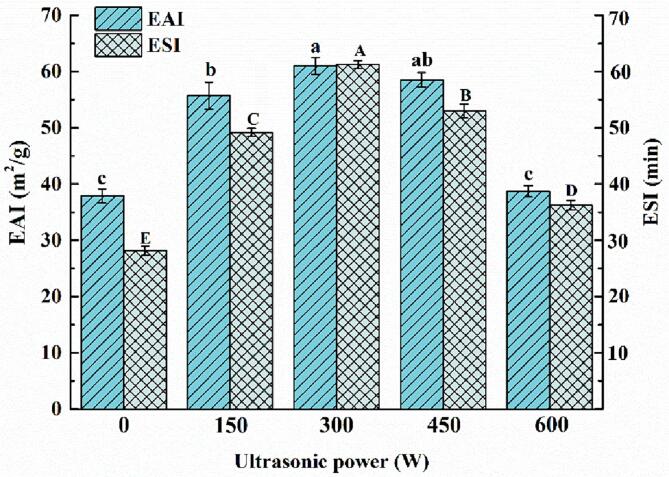
The emulsifying reflects the capacity of the emulsifier to reduce the interfacial tension and form and stabilize the emulsifier by adsorbing on the O/W interface [20]. The emulsifying properties of emulsions were often characterized by the emulsion stability index (ESI) and emulsion activity Index (EAI). EAI indicated the ability of a substance to stabilize the O/W interface area per unit mass, while ESI indicated the ability of a substance to keep the stability of an emulsion over a period of time [32]. EAI and ESI of mimicking MFG emulsion were shown in Fig. 4. Compared with unsonicated emulsions, the ESI and EAI values of sonicated mimicking MFG emulsion increased with the increase of ultrasound power in the range of 0–300 W. The mimicking MFG emulsion treated with 300 W showed the best emulsification performance, ESI and EAI increased by 117.74 % and 60.88 %, respectively, which might be due to the increased solubility of the protein by sonication. Xue et al [24] also reported that sonicated buckwheat protein has better solubility and emulsification properties. In addition, turbulence and shear forces resulting from cavitation led to protein dispersion and significant reductions in complex size. The reduction in the size of the complex allows more of the MFGM-SL complex to be adsorbed at the O/W interface, which is also conducive to improving the stability of the emulsion [33]. EAI and ESI were low at 600 W ultrasound power, which might be due to that excessive power that might break the structure of the protein and formed protein aggregate, further reducing the emulsifying properties of the protein, these results were consistent with the study of Li et al [16].
Fig. 4.
Emulsifying activity index (EAI) and emulsifying stability index (ESI) of mimicking MFG emulsions at different ultrasonic powers. Different capital letters (A-E) and lowercase letters (a-c) indicated significant differences of EAI and ESI between groups (p < 0.05), respectively.
3.2.2. Particle size and zeta potential of mimicking MFG emulsion
Particle size was one of the key parameters, which could reflect the functional and physicochemical properties of emulsions [34]. The particle size distribution and average particle size were shown in Fig. 5A and Table 1. The particle size decreased with the increase of ultrasonic power, and the distribution was more uniform, reaching a minimum of 0.58 ± 0.04 μm at a power of 300 W. During sonication, due to the energy generated by cavitation, the complex droplets are stirred vigorously, eventually particle size is reduced [35]. However, the particle size of the mimicking MFG emulsion increased when the ultrasonic power was 450 W and 600 W, which might be due to the formation and collapse of cavitation bubbles to generate strong shock waves and jets, resulting in a significant increase in particle size, which in turn led to a tendency for the emulsion to clustering [36]. Sui et al [37] investigate the trend between sonication and particle size of emulsion, and found that after sonication, the emulsion particle size decreases to a minimum at medium power but increases at higher power values. The particle size of the mimicking MFG emulsion prepared in this study was significantly smaller than that of the HM particle size (4.56 μm) reported previously [38]. Variations in the composition and structure, including particle size, of MFG may impact infant growth and metabolic by affecting lipid digestion [39], [40]. However, it has been shown in previous research that the composition and structure of fat globule interfaces have a greater effect on lipid digestion than particle size [41]. Therefore, this study aims to use ultrasonicated MFGM-SL complex to modify the interfacial structure of MFG. This modification may further improve lipid digestion and subsequent nutritional function. This will be investigated further in future studies.
Fig. 5.
Effects of different ultrasonic powers on particle size distribution (A) and zeta potential (B) of mimicking MFG emulsion. Values were expressed as mean ± SD, different letters represented significant differences (P < 0.05).
Zeta potential is an important indicator for evaluating the stability of emulsions, mainly used to observe the degree of electrostatic repulsion or attraction between adjacent examples. Emulsions with low absolute zeta potential tended to flocculate and coagulate, while those with high absolute zeta potential were electrically stable. The zeta potential of mimicking MFG emulsions was shown in Fig. 5B. Compared to untreated emulsions, the absolute zeta potential after sonication was significantly increased, reaching a maximum at 300 W. Increasing the zeta potential could increase the electrostatic repulsion of proteins, further inhibit the aggregation and promote the stability of the emulsion, which was also one of the reasons for improving the emulsification performance (Fig. 4) [35].
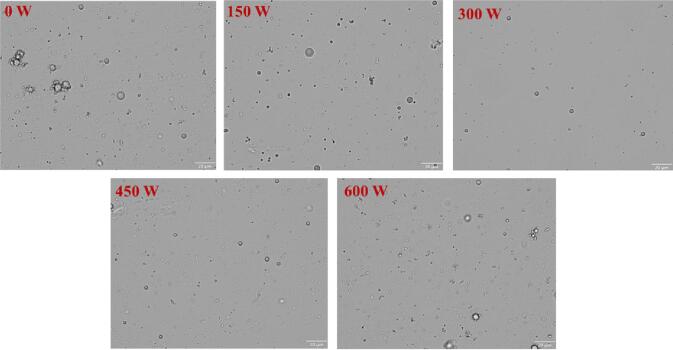
3.2.3. Droplets flocculation of mimicking MFG emulsion
A continuous phase consisting of water and other soluble substances could not be observed by light microscopy in milk and dairy products. However, the dispersed phase consists of milk proteins, MFG and lactose could be observed [42]. The effect of ultrasonic powers on the flocculation of lipid droplets was shown in Fig. 6. The microstructure distribution of mimicking MFG emulsion without sonication was uneven, the flocculation phenomenon of lip-shaped droplets was obvious, and the average droplet diameter was large. As the ultrasonic power increased (<300 W), the droplet size decreased, and the spherical droplets remained well separated, with no obvious flocculation and a more uniform distribution. This might be due to the reduction of the particle size of the mimicking MFG emulsion due to the cavitation of ultrasound, so that the distribution of MFGM-SL complex at the oil-liquid interface was rapid and uniform, and the oil droplet coating rate was high [43]. When the ultrasonic power was further increased to 600 W, the morphology of the mimicking MFG emulsion droplet changed significantly, which might be due to the network structure formed by the inter-particle junction, further resulting in an uneven distribution of lipid droplets in the emulsion system. Furthermore, homogeneously dispersed droplet reaggregation might be due to the thermal effects of sonication, resulting in larger particle sizes, consistent with previous observations [33].
Fig. 6.
The microscopic flocculation of mimicking MFG emulsions at different ultrasonic powers.
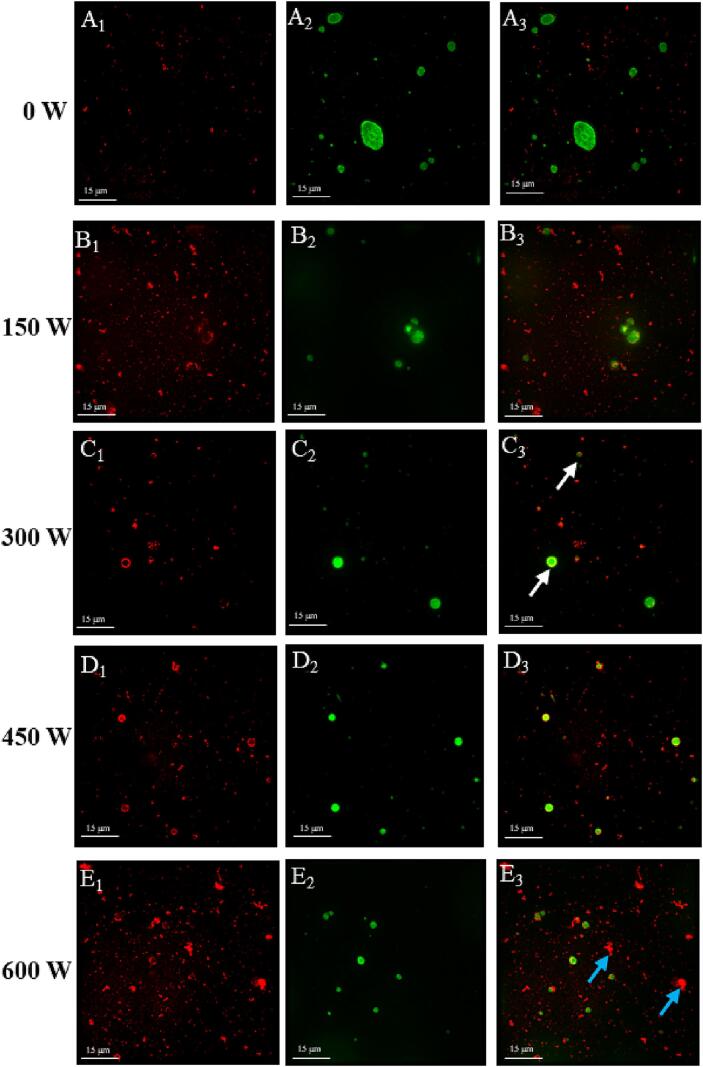
3.2.4. Microstructure observation
Microstructure of mimicking MFG emulsion by ultra-high-resolution microscopy was shown in Fig. 7. Triglycerides and proteins in the emulsion are labeled green and red by fluorescent markers, respectively, and the red fluorescent complex and green fluorescent complex overlap in the same location as a yellow-fluorescent composite layer. The unsonicated mimicking MFG emulsion had the largest oil droplets. Compared to unsonicated mimicking MFG emulsions, sonication improved the dispersion and avoided aggregation of emulsions. When the ultrasonic power increased from 0 W to 300 W, droplet sizes were obviously reduced. At an ultrasonic power of 300 W, the sample showed a more structured matrix, and the fat droplets were wrapped by protein aggregation (Fig. 7C, white arrow), which indicated that 300 W sonication had higher emulsification stability. The stable interface structure of MFGM was heterogeneous and consisted mainly of MFGM fragment particles, interspersed with free phospholipids and proteins. After ultrasound treatment of MFGM and SL, the phospholipid distribution content in MFGM ingredients was higher and more uniform, and the interphase protein part was replaced by lecithin, which improved the stability of the emulsion [4]. Livney et al [44] also found that phospholipids could compete with protein and occupied more of the oil surface more efficiently, which was related to phospholipids having a smaller molecular weight and higher amphiphilicity [45]. When the ultrasonic power was changed from 450 W to 600 W, the interfacial structure of the oil droplets wrapped in the protein was disrupted, further forming insoluble protein aggregation (Fig. 7E, blue arrow). This resulted in lower emulsification stability, which was consistent with our previous results (Fig. 4). Sui et al [37] also observed that more proteins were aggregated as the ultrasound power increases, the stability of the emulsion was further destabilized.
Fig. 7.
Microstructure of mimicking MFG emulsions at different ultrasonic powers. (The A1-E1 indicates FCF-labeled proteins; A2-E2 indicates Nile Red-labeled triglyceride; A3-E3 indicates protein- triglyceride overlap in yellow. A-E represent samples with different ultrasonic power treatments of 0 W, 150 W, 300 W, 450 W, and 600 W, respectively. Scale bar = 15 μm). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2.5. Encapsulation rate and stability constants
As an important parameter of the success of various encapsulation methods, encapsulation rate was defined as the percentage of biologically active components encapsulated in the internal aqueous phase [46]. In addition, through the external mechanical force, the stability constant was determined to investigate the stability of the emulsion, the smaller the stability constant, the higher the stability. In Fig. 2C and D, the encapsulation rate without sonication and after sonication treatment was higher than 80 %. When not sonicated, the encapsulation rate of mimicking MFG emulsion was low, while emulsion had a high stability constant. As the ultrasonic power increased, the encapsulation rate of the emulsion increased, while stability constant declined. In the ultrasonic power reached 300 W, the encapsulation rate reached the highest, about 92.15 ± 1.07 %, while the stability constant reached 19.89 ± 0.20 %. These results indicated that a relatively stable system might be obtained. The cavitation effect formed by ultrasound can make the lipid droplets smaller and more dispersed. Thus, sonication enhanced the physical characteristic of proteins and improved the emulsion stability. Chung et al [47]] used either sodium caseinate and soy lecithin to prepare O/W emulsions with high physical stability, and concluded that the increase in stability may form a complex between protein and phospholipids, and can better cover the surface of the emulsion fat droplet, thereby increasing the thickness of the interface layer. However, as the ultrasonic power continued to increase, the encapsulation rate was lower and stability was poor.
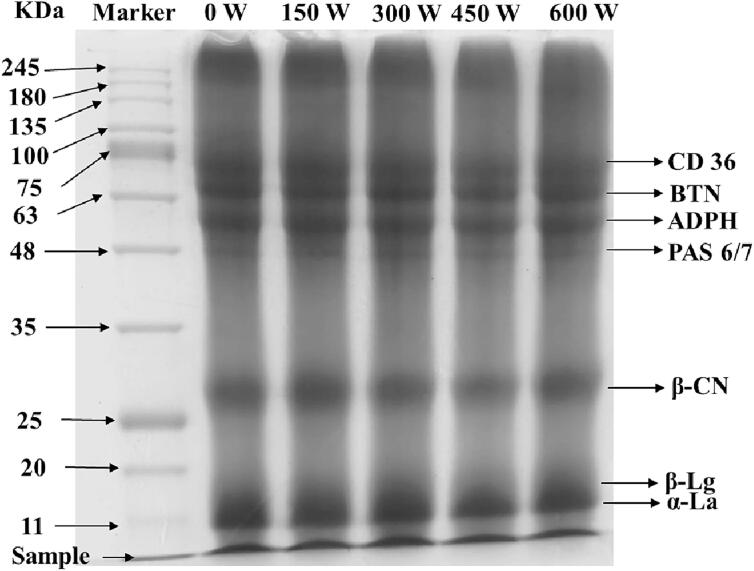
3.2.6. Interfacial protein composition
Sodium lauryl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was used to analyze whether different ultrasonic power treatments would affect the molecular weight of proteins, resulting in molecular weight aggregation or degradation. The MFGM ingredients contain casein and whey protein, as well as a small amount of MFGM protein. As shown in Fig. 8, the interfacial protein type in the mimicking MFG emulsion was basically the same as those in the MFGM ingredients. In addition, there were no obvious difference at molecular weight (La, Lg, CN) between samples with and without sonication. O'Sullivan et al [48] also found no differential effect of ultrasonic on the protein content of milk protein isolate and whey protein isolate. Applications of ultrasound in test sound intensity range did not have obvious effects on the primary structure of the protein, which might be related to ultrasound-induced changes in protein structure that might be caused by hydrophobic interactions (non-covalent) rather than peptide cleavage [49]. In addition, the main protein in the MFGM was cluster of differentiation 36 (CD36), butyrophilin (BTN), adipophilin (ADPH), and periodic acid Schiff glycoprotein 6/7 (PAS6/7), with molecular weights of 77 kDa, 67 kDa, 52 kDa, and 48 kDa, respectively. Which were similar to the composition of the MFGM studied in previous study [41]. There was no difference in MFGM protein composition after sonication. MFGM protein components have beneficial effects on immune regulation and intestinal development [50].
Fig. 8.
Interfacial protein composition of mimicking MFG emulsion. CD36, differentiation 36; BNT, butyrophilin; ADPH, adipophilin; PAS 6/7, periodic acid Schiff glycoprotein 6/7; β-CN, β-casein; β-Lg, β-lactoglobulin; α-La: α-lactalbumin.
3.2.7. Oxidative stability
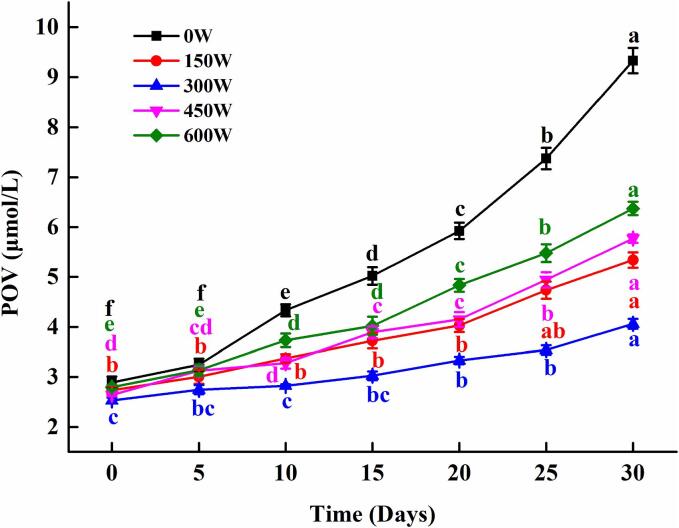
Peroxide value (POV) is a common indicator of the degree of oxidation of fat. In our studies, the POV values of different mimicking MFG emulsion on days 0, 5, 10, 15, 20, 25, and 30 were investigated, and the results were shown in Fig. 9. Within 30 d, POV values of all mimicking MFG emulsion increased as increasing storage time. Furthermore, the POV value of the emulsion after ultrasonic treatment during storage for 10–30 d compared to the untreated emulsion (p < 0.05). These results indicated that ultrasonic inhibited the increase of hydroperoxides of mimicking MFG emulsion and increased the oxidative stability. At the ultrasonic power of 300 W, the suppression effect was the best, and the POV value was the lowest after 30 days of storage, which was 3.96 ± 0.09 μmol/L. This might be due to the fact that the mimicking MFG emulsion with 300 W sonicated had the smallest particles and the highest adsorbed protein on the surface of the fat droplets, and we knew that the reduction in particles might improve the vulnerability of the emulsion to oxygen reactions [15], [51]. Which were consistent with previous particle size results (Table 1). The reduction in particles might lead to an increase in adsorbed proteins, which could scavenge free radicals more quickly, thereby inhibiting lipid oxidation and improving oxidative stability [52]. Nakaya et al [53] studied the effect of particles size on oxidation stability and found that emulsions with large oil droplets oxidize faster than emulsions with small oil droplets. With the increase of ultrasonic power (>450 W), the POV value increased, and the POV value at 600 W reached 6.37 ± 0.13 μmol/L, which might be due to the strong cavitation effects high and sound field produced by sonication, which in turn benefit the formation of highly reactive radicals (hydroxyl or hydrogen radicals) and the synthesis of hydrogen peroxide, and finally oxidation [54]. In addition, the low POV value, microstructure also showed uniform fat distribution and less aggregation. Therefore, moderate ultrasonic treatment could reduce the POV value during the storage of emulsion and improve the oxidation stability.
Fig. 9.
The oxidation stability of mimicking MFG emulsion at different ultrasonic powers during storage. Values were expressed as mean ± SD, different letters represented significant differences (P < 0.05).
4. Conclusions
Ultrasonic power significantly affects the structure of the MFGM-SL complex, further promoted the unfolding of the molecular structure of the protein, and then increased solubility and surface hydrophobicity. Furthermore, the ultrasound-modified MFGM-SL complex was further used as a membrane material to obtain a mimicking MFG emulsion. Our study indicated that compared to the emulsion without ultrasound, absolute zeta potential of the sonicated mimicking MFG emulsions were significantly increased (P < 0.05). At ultrasound power up to 300 W, the mimicking MFG emulsion had the highest encapsulation rate (92.15 ± 1.07 %) and emulsion activity Index (EAI) and emulsion stability index (ESI) were increased by 60.88 % and 117.74 %, respectively. Microstructure results showed that appropriate ultrasonic power treatment to simulate MFG emulsion reduced droplet size and avoided emulsion aggregation. Especially when the ultrasonic power was 300 W, the spherical droplets were well separated, the flocculation phenomenon was not obvious, and the distribution was more uniform. However, excessive ultrasonic power (>450 W) would disrupt the spherical structure of proteins, further forming insoluble proteins aggregation and reducing the emulsifying characteristic of proteins. In general, appropriate ultrasonic power treatment could obtain mimicking MFG emulsions with good encapsulation rate, emulsification and stability performance. This study can provide a reference for the further development and utilization of infant formula that simulates the structure of human MFG.
CRediT authorship contribution statement
Qian Ma: Writing – review & editing, Writing – original draft, Methodology, Formal analysis, Conceptualization. Tao Zhou: Resources, Investigation. Zhong Wang: Data curation. Yanjie Zhao: Investigation. Xiaodong Li: Supervision, Project administration, Funding acquisition. Lu Liu: Supervision, Validation, Writing – review & editing. Xiuxiu Zhang: Visualization. Kouadio Jean Eric-Parfait Kouame: Visualization. Shuo Chen: Software.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The present work was financially supported by National Center of Technology Innovation for Dairy (2022-Open Project-7), Collaborative Innovation Achievement Project of Universities in Heilongjiang Province (LJGXCG2022-026) and “Academic backbone” Project of Northeast Agricultural University.
Contributor Information
Xiaodong Li, Email: hrblxd@163.com.
Lu Liu, Email: liulu89824@163.com.
References
- 1.Liang L., Zhang X., Wang X., Jin Q., Mcclements D.J. Influence of dairy emulsifier type and lipid droplet size on gastrointestinal fate of model emulsions: in vitro digestion study. J. Agric. Food Chem. 2018;66:9761–9769. doi: 10.1021/acs.jafc.8b02959. [DOI] [PubMed] [Google Scholar]
- 2.Garcia C., Antona C., Robert B., Lopez C., Armand M. The size and interfacial composition of milk fat globules are key factors controlling triglycerides bioavailability in simulated human gastro-duodenal digestion. Food Hydrocoll. 2014;35:494–504. [Google Scholar]
- 3.Ma Q., Zhang X., Li X., Liu L., Liu S., Hao D., Bora A.F.M., Kouame K.J.E., Xu Y., Liu W. Novel trends and challenges in fat modification of next-generation infant formula: Considering the structure of milk fat globules to improve lipid digestion and metabolism of infants. Food Res. Int. 2023;174 doi: 10.1016/j.foodres.2023.113574. [DOI] [PubMed] [Google Scholar]
- 4.Yu X., Zhao Y., Sun M., Liu L., Li X., Zhang X., Sun Y., Bora A.F.M., Li C., Leng Y. Effects of egg yolk lecithin/milk fat globule membrane material ratio on the structure and stability of oil-in-water emulsions. LWT-Food Sci Technol. 2022;168 [Google Scholar]
- 5.Liu Y., Liu Y., Liu Q., Zhao J.Y., Qian W.C., Liu B., Yang B.Y., Chen L.J. Comparison of phospholipid composition and microstructure of milk fat globules contained in human milk and infant formulae. Food Chem. 2023;415 doi: 10.1016/j.foodchem.2023.135762. [DOI] [PubMed] [Google Scholar]
- 6.Zheng L., Fleith M., Giuffrida F., Barry V., O'Neill N.S. Dietary polar lipids and cognitive development: A narrative review. Adv. Nutr. 2019;10:1163–1176. doi: 10.1093/advances/nmz051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold G., Schade E., Schneider Y., Friedrichs J., Babick F., Werner C., Rohm H. Influence of individual phospholipids on the physical properties of oil-based suspensions. J. Am. Oil Chem. Soc. 2013;91:71–77. [Google Scholar]
- 8.Ma Q., Ma S., Zhao Y., Sun M., Li X., Liu L., Zhang X., Sun Y., Bora A.F.M., Tian S. Interaction between whey protein and soy lecithin and its influence on physicochemical properties and in vitro digestibility of emulsion: A consideration for mimicking milk fat globule. Food Res. Int. 2023;163 doi: 10.1016/j.foodres.2022.112181. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y., Ge H., Zheng Y., Zhang H., Li Y., Su X.R., Worawan P., Tan C.P., Cheng L.Z. Phospholipid–protein structured membrane for microencapsulation of DHA oil and evaluation of its in vitro digestibility: Inspired by milk fat globule membrane. J. Agric. Food Chem. 2020;68:6190–6201. doi: 10.1021/acs.jafc.0c01250. [DOI] [PubMed] [Google Scholar]
- 10.Wang X., Zhang L., Chen L., Wang Y., Okonkwo C.E., Yagoub A.E.G.A., Wahia H., Zhou C. Application of ultrasound and its real-time monitoring of the acoustic field during processing of tofu: Parameter optimization, protein modification, and potential mechanism. Compr. Rev. Food Sci. Food Saf. 2023;2023:1–26. doi: 10.1111/1541-4337.13161. [DOI] [PubMed] [Google Scholar]
- 11.Sun Y., Yu X., Hussain M., Li X., Liu L., Liu Y., Ma S., Kouame K.-J.-E.-P., Li C., Leng Y. Influence of milk fat globule membrane and milk protein concentrate treated by ultrasound on the structural and emulsifying stability of mimicking human fat emulsions. Ultrason. Sonochem. 2022;82 doi: 10.1016/j.ultsonch.2021.105881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Awad T., Moharram H., Shaltout O., Asker D., Youssef M. Applications of ultrasound in analysis, processing and quality control of food: A review. Food Res. Int. 2012;48:410–427. [Google Scholar]
- 13.Chemat F., Khan M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011;18:813–835. doi: 10.1016/j.ultsonch.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 14.Kaltsa O., Gatsi I., Yanniotis S., Mandala I. Influence of ultrasonication parameters on physical characteristics of olive oil model emulsions containing xanthan. Food Bioprocess. Technol. 2014;7:2038–2049. [Google Scholar]
- 15.Ma Q., Sun M., Zhao Y., Chen S., Li X., Liu L., Zhang X., Wang Y., Kouame K.-J.-E.-P., Yu X. Improving lipid digestion by modulating interfacial structure of fat globule based on milk fat globule membrane and different phospholipids. Food Hydrocoll. 2024;150 [Google Scholar]
- 16.Li N., Wang T., Yang X., Qu J., Wang N., Wang L., Yu D., Han C. Effect of high-intensity ultrasonic treatment on the emulsion of hemp seed oil stabilized with hemp seed protein. Ultrason. Sonochem. 2022;86 doi: 10.1016/j.ultsonch.2022.106021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vargas S.A., Delgado-Macuil R.J., Ruiz-Espinosa H., Rojas-López M., Amador-Espejo G.G. High-intensity ultrasound pretreatment influence on whey protein isolate and its use on complex coacervation with kappa carrageenan: Evaluation of selected functional properties. Ultrason. Sonochem. 2021;70 doi: 10.1016/j.ultsonch.2020.105340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng Y., Liang Z., Zhang C., Hao S., Han H., Du P., Li A., Shao H., Li C., Liu L. Ultrasonic modification of whey protein isolate: Implications for the structural and functional properties. LWT-Food Sci. Technol. 2021;152 [Google Scholar]
- 19.Phan T.T.Q., Le T.T., Van der Meeren P., Dewettinck K. Comparison of emulsifying properties of milk fat globule membrane materials isolated from different dairy by-products. J. Dairy Sci. 2014;97:799–4810. doi: 10.3168/jds.2014-8030. [DOI] [PubMed] [Google Scholar]
- 20.Li D., Zhao Y., Wang X., Tang H., Wu N., Wu F., Yu D., Elfalleh W. Effects of (+)-catechin on a rice bran protein oil-in-water emulsion: Droplet size, zeta-potential, emulsifying properties, and rheological behavior. Food Hydrocoll. 2020;98 [Google Scholar]
- 21.Shen L., Tang C.H. Microfluidization as a potential technique to modify surface properties of soy protein isolate. Food Res. Int. 2012;48:108–118. [Google Scholar]
- 22.Guo N., Ye S., Zhou G., Zhang Y., Zhang F., Xu J., Pan S., Zhu G., Wang Z. Effect of ultrasound treatment on interactions of whey protein isolate with rutin. Ultrason. Sonochem. 2023;95 doi: 10.1016/j.ultsonch.2023.106387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao H., Ma L., Li T., Sun D., Hou J., Li A., Jiang Z. Impact of ultrasonic power on the structure and emulsifying properties of whey protein isolate under various pH conditions. Process Biochem. 2019;81:113–122. [Google Scholar]
- 24.Xue F., Wu Z., Tong J., Zheng J., Li C. Effect of combination of high-intensity ultrasound treatment and dextran glycosylation on structural and interfacial properties of buckwheat protein isolates. Biosci. Biotechnol. Biochem. 2017;81:1891–1898. doi: 10.1080/09168451.2017.1361805. [DOI] [PubMed] [Google Scholar]
- 25.Nazari B., Mohammadifar M.A., Shojaee-Aliabadi S., Feizollahi E., Mirmoghtadaie L. Effect of ultrasound treatments on functional properties and structure of millet protein concentrate. Ultrason. Sonochem. 2018;41:382–388. doi: 10.1016/j.ultsonch.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Jambrak A.R., Lelas V., Mason T.J., Krešić G., Badanjak M. Physical properties of ultrasound treated soy proteins. J. Food Eng. 2009;93:386–393. [Google Scholar]
- 27.Wang Q., Pan M.H., Chiou Y.S., Li Z., Ding B. Surface characteristics and emulsifying properties of whey protein/nanoliposome complexes. Food Chem. 2022;384 doi: 10.1016/j.foodchem.2022.132510. [DOI] [PubMed] [Google Scholar]
- 28.Constantino A.B.T., Garcia-Rojas E.E. Modifications of physicochemical and functional properties of amaranth protein isolate (Amaranthus cruentus BRS Alegria) treated with high-intensity ultrasound. J. Cereal Sci. 2020;95 [Google Scholar]
- 29.Bourlieu C., Ménard O., De La Chevasnerie A., Sams L., Rousseau F., Madec M.N., Robert B., Deglaire A., Pezennec S., Bouhallab S. The structure of infant formulas impacts their lipolysis, proteolysis and disintegration during in vitro gastric digestion. Food Chem. 2015;182:224–235. doi: 10.1016/j.foodchem.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Kwaambwa H.M., Rennie A.R. Interactions of surfactants with a water treatment protein from Moringa oleifera seeds in solution studied by zeta-potential and light scattering measurements. Biopolymer. 2012;97:209–218. doi: 10.1002/bip.22014. [DOI] [PubMed] [Google Scholar]
- 31.Zou H., Zhao N., Li S., Sun S., Dong X., Yu C. Physicochemical and emulsifying properties of mussel water-soluble proteins as affected by lecithin concentration. Int. J. Biol. Macromol. 2020;163:180–189. doi: 10.1016/j.ijbiomac.2020.06.225. [DOI] [PubMed] [Google Scholar]
- 32.Lam R.S., Nickerson M.T. The effect of pH and temperature pre-treatments on the physicochemical and emulsifying properties of whey protein isolate, LWT-Food. Sci. Technol. 2015;60:427–434. [Google Scholar]
- 33.Wang T., Chen X., Wang W., Wang L., Jiang L., Yu D., Xie F. Effect of ultrasound on the properties of rice bran protein and its chlorogenic acid complex. Ultrason. Sonochem. 2021;79 doi: 10.1016/j.ultsonch.2021.105758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McClements D.J. Principles of ultrasonic droplet size determination in emulsions. Langmuir. 1996;12:3454–3461. [Google Scholar]
- 35.Wang T., Wang N., Li N., Ji X., Zhang H., Yu D., Wang L. Effect of high-intensity ultrasound on the physicochemical properties, microstructure, and stability of soy protein isolate-pectin emulsion. Ultrason. Sonochem. 2022;82 doi: 10.1016/j.ultsonch.2021.105871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teng F., He M., Xu J., Chen F., Wu C., Wang Z., Li Y. Effect of ultrasonication on the stability and storage of a soy protein isolate-phosphatidylcholine nanoemulsions. Sci. Rep. 2020;10:1–9. doi: 10.1038/s41598-020-70462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sui X., Bi S., Qi B., Wang Z., Zhang M., Li Y., Jiang L. Impact of ultrasonic treatment on an emulsion system stabilized with soybean protein isolate and lecithin: Its emulsifying property and emulsion stability. Food Hydrocoll. 2017;63:727–734. [Google Scholar]
- 38.Pan Y., Liu L., Tian S.F., Li X.D., Hussain M., Li C.M., Zhang L.H., Zhang Q.M., Leng Y.B., Jiang S.Y. Comparative analysis of interfacial composition and structure of fat globules in human milk and infant formulas. Food Hydrocoll. 2022;124 [Google Scholar]
- 39.Hageman J.H.J., Danielsen M., Nieuwenhuizen A.G., Feitsma A.L., Dalsgaard T.K. Comparison of bovine milk fat and vegetable fat for infant formula: Implications for infant health. Int. Dairy J. 2019;92:37–49. [Google Scholar]
- 40.Liu L., Pan Y., Zhang X.X., Zhang Y., Li X.D. Effect of particle size and interface composition on the lipid digestion of droplets covered with membrane phospholipids. J. Agric. Food Chem. 2021;69:159–169. doi: 10.1021/acs.jafc.0c04945. [DOI] [PubMed] [Google Scholar]
- 41.Yu X., Zhou W., Jia Z., Liu L., Li X., Zhang X., Cheng J., Ma C., Sun L., Jiao Y. Interfacial composition in infant formulas powder modulate lipid digestion in simulated in-vitro infant gastrointestinal digestion. Food Res. Int. 2023;165 doi: 10.1016/j.foodres.2023.112553. [DOI] [PubMed] [Google Scholar]
- 42.Salve A.R., Pegu K., Arya S.S. Comparative assessment of high-intensity ultrasound and hydrodynamic cavitation processing on physico-chemical properties and microbial inactivation of peanut milk. Ultrason. Sonochem. 2019;59 doi: 10.1016/j.ultsonch.2019.104728. [DOI] [PubMed] [Google Scholar]
- 43.Wang W., Wang R., Yao J., Luo S., Wang X., Zhang N., Wang L., Zhu X. Effect of ultrasonic power on the emulsion stability of rice bran protein-chlorogenic acid emulsion. Ultrason. Sonochem. 2022;84 doi: 10.1016/j.ultsonch.2022.105959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Livney Y.D., Ruimy E., Aiqian M.Y., Zhu X., Singh H. A milkfat globule membrane-inspired approach for encapsulation of emulsion oil droplets. Food Hydrocoll. 2017;65:121–129. [Google Scholar]
- 45.McClements D.J., Jafari S.M. Improving emulsion formation, stability and performance using mixed emulsifiers: A review. Adv. Colloid Interface Sci. 2018;251:55–79. doi: 10.1016/j.cis.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 46.Sobti B., Kamal-Eldin A., Rasul S., Alnuaimi M.S.K., Alnuaimi K.J.J., Alhassani A.A.K., Almheiri M.M., Nazir A. Encapsulation properties of mentha piperita leaf extracts prepared using an ultrasound-assisted double emulsion method. Foods. 2023;12 doi: 10.3390/foods12091838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chung C., Koo C.K., Sher A., Fu J.T.R., Rousset P., McClements D.J. Modulation of caseinate-stabilized model oil-in-water emulsions with soy lecithin. Food Res. Int. 2019;122:361–370. doi: 10.1016/j.foodres.2019.04.032. [DOI] [PubMed] [Google Scholar]
- 48.O'Sullivan J., Arellano M., Pichot R., Norton I. The effect of ultrasound treatment on the structural, physical and emulsifying properties of dairy proteins. Food Hydrocoll. 2014;42:386–396. [Google Scholar]
- 49.Nyuydze C., Martínez-Monteagudo S.I. Role of soy lecithin on emulsion stability of dairy beverages treated by ultrasound. Int. J. Dairy Technol. 2021;74:84–94. [Google Scholar]
- 50.Brink L.R., Lönnerdal B. Milk fat globule membrane: The role of its various components in infant health and development. J. Nutr. Biochem. 2020;85 doi: 10.1016/j.jnutbio.2020.108465. [DOI] [PubMed] [Google Scholar]
- 51.Qayum A., Li M., Shi R., Bilawal A., Gantumur M.A., Hussain M., Ishfaq M., Shah S.W.A., Jiang Z., Hou J. Laccase cross-linking of sonicated α-Lactalbumin improves physical and oxidative stability of CLA oil in water emulsion. Ultrason. Sonochem. 2021;71 doi: 10.1016/j.ultsonch.2020.105365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu J.N., Zheng H., Chen X.X., Li X., Xu Y., Xu M.F. Synergetic effects of whey protein isolate and naringin on physical and oxidative stability of oil-in-water emulsions. Food Hydrocoll. 2020;101 [Google Scholar]
- 53.Nakaya K., Ushio H., Matsukawa S., Shimizu M., Ohshima T. Effects of droplet size on the oxidative stability of oil-in-water emulsions. Lipids. 2005;40:501–507. doi: 10.1007/s11745-005-1410-4. [DOI] [PubMed] [Google Scholar]
- 54.Fu X., Belwal T., Cravotto G., Luo Z. Sono-physical and sono-chemical effects of ultrasound: Primary applications in extraction and freezing operations and influence on food components. Ultrason. Sonochem. 2020;60 doi: 10.1016/j.ultsonch.2019.104726. [DOI] [PubMed] [Google Scholar]