Abstract
Clozapine is the only approved drug for treatment-resistant schizophrenia, but the response to the drug is often inadequate. Augmentation with other antipsychotics, anticonvulsants, and antidepressants is recommended for such patients, but there is a lack of evidence regarding the most effective therapy. This network meta-analysis was conducted to evaluate the efficacy of pharmacological agents used in the augmentation strategies in patients who were partial/non-responders to clozapine. Relevant data were extracted from 30 randomized controlled trials through searches of electronic databases (MEDLINE/PubMed, Embase, Cochrane, clinical trial registries). PRISMA guidelines were followed for the extraction, management, analysis, and reporting of the data. The outcome measure in this study was a reduction in symptom severity according to total PANSS/BPRS and was reported as the standardized mean difference with a 95% credible interval. Bayesian network meta-analysis with random effects model and uninformative priors was conducted, and the ranking probability of each intervention was done. Meta-regression was done to assess the effect of duration on the reduction in symptom severity scores. Mirtazapine (−5.2 [95%CrI: −7.7, −2.7]) and memantine (−2.1 [95%CrI: −4.0, −0.19]) were more efficacious than placebo for augmentation of clozapine in partial/non-responders and were the most effective adjunctive agents as per SUCRA scores. Both drugs did not cause a significant increase in frequency of adverse events compared to placebo. There was a significant effect of duration on the reduction in symptom severity. There was no evident publication bias. Mirtazapine and memantine may prove beneficial for augmentation of clozapine in non/partial responders to monotherapy.
Keywords: Clozapine, Schizophrenia, Clozapine-resistant, Augmentation therapy
INTRODUCTION
Treatment of resistant schizophrenia remains an underachieved goal despite the availability of a number of typical and atypical antipsychotics [1,2]. Clozapine is the only antipsychotic which has been formally approved for the treatment of refractory cases, but an adequate response is evidenced in only 30−60% of patients, thus affecting the quality of life of the patients and caregivers [3,4]. Clozapine has a lower affinity for striatal D2 receptors when compared to most other antipsychotics and, even at maximally tolerated doses, occupies < 65% of these receptors; thus, it is hypothesized to lead to residual symptoms [5].
Patients who are resistant to clozapine therapy despite achieving desired plasma concentrations are termed patients with ultra-resistant schizophrenia. There are several hypotheses defining the pathophysiological pathway of non-responders or partial responders. Dysfunction of glutamatergic transmission, redox disequilibrium, and dopamine receptor supersensitivity are a few of them [6]. Thus, it is postulated that refractory patients may benefit from the modulation of complementary receptor binding pathways.
Augmentation of clozapine with other drugs has been proposed with the objective of broadening the pharmacodynamic profile of clozapine. Moreover, the drug for augmentation should be chosen so as not to compound the adverse effects such as agranulocytosis, seizures, cardiomyopathy, and adverse metabolic effects. Prescription of two or more antipsychotics seems to be the most sought-after strategy as they enhance the effect by synergistic antipsychotic potency [7]. However, antipsychotic polypharmacy may enhance adverse metabolic effects. There are a few drugs, like anticonvulsants, antidepressants, other antipsychotics, etc., that have been tried as augmentation strategies to treat persistent residual symptoms which compromise the quality of life of patients and caregivers. Most evidence on these drugs comes from case reports, and observational studies with testimony of the effectiveness remaining contradictory or inconclusive. There are a few randomized controlled trials to assess pharmacological augmentation to clozapine, but the order of preference of the drugs could not be ascertained as head-on comparisons are not available. Thus, this network meta-analysis (NMA) was planned to evaluate the efficacy of pharmacological agents used as augmentation strategies in patients who were partial responders or refractory to clozapine.
METHODS
Protocol Development and Registration
A standard network meta-analysis protocol was developed following preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) guidelines and registered in the international prospective register of ongoing systematic reviews (ROSPERO: CRD42022380302), and was submitted to the institutional ethics committee for a waiver [8]. This network meta-analysis has been conducted and reported in conformance to the PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions (PRISMA-NMA) state-ment [9].
The protocol of the meta-analysis was exempted from the full review and approved by the Institutional Ethics Committee, All India Institute of Medical Sciences (AIIMS), Bhubaneswar, as per ICMR National ethical guidelines (2017) for biomedical and health research (IEC Approval No. T/IM-NF/Pharm/22/135).
Search Strategy
MEDLINE/PubMed, Embase, Google Scholar, Cochrane clinical trial registry and the international clinical trials registry platform (ICTRP) were searched for randomized controlled trials (RCTs) on clozapine in patients with treatment-resistant schizophrenia published until December 2022. Search terms for PubMed and Embase were constructed using terms for disease, the drug clozapine and the terms for response or non-response connected with Boolean operators (“schizophrenia”[MeSH Terms] OR “schizophrenia”[All Fields] OR “schizophrenias”[All Fields] OR “schizophrenia s”[All Fields]) AND “Clozapine”[Title] AND (“response”[All Fields] OR “responses”[All Fields] OR (“non-response”[All Fields] OR “non-responses”[All Fields] OR “nonresponsive”[All Fields] OR “nonresponsive-ness”[All Fields]) OR “non-responder”[All Fields]). The list of references for the published studies was searched for possible inclusions, and the ICTRP was checked for unpublished data.
Study Selection Criteria
RCTs on pharmacological augmentation for partial or non-responders to clozapine therapy for schizophrenia were considered for inclusion. Change in symptom severity as determined by positive and negative symptom scale (PANSS) scores or its equivalent was the primary outcome measure. The studies included either PANSS or a brief psychiatric rating scale (BPRS) as an outcome measure. All studies published in peer-reviewed English language journals, irrespective of the date of publication, place of study or the ethnicity of the study population, were included for analysis. Case reports, case series and letters to the editor were excluded. Studies where “non-response”, or “partial response”, or “persistent symptoms despite clozapine therapy” were not mentioned were excluded.
Types of participants and intervention
We included studies with patients of either sex of age more than 18 years, diagnosed with schizophrenia as per the Diagnostic and Statistical Manual of Mental Disorders (DSM)-III/IV/V, and were partial responders or non-responders to clozapine therapy. Any pharmacological agent given as an add-on to clozapine therapy was considered a test intervention, and a placebo or any other pharmacological agent was taken as control.
Type of outcomes
The efficacy outcome chosen for this network meta-analysis was a change in PANSS or BPRS. The studies which reported a change in schizophrenia symptoms rating scales (PANSS or BPRS) irrespective of the study duration were included for further analysis.
Study Selection and Data Collection
The eligibility criteria for studies to be included in this network meta-analysis were laid down a priori. The selection of the studies for data extraction was made in a stepwise manner. First of all, the title and abstracts of the articles found in the database search were screened as per eligibility criteria. Next, the full text of the selected articles was retrieved and read extensively. Finally, we were left with the articles eligible for extraction of relevant data to be used in this network meta-analysis. Three reviewers (AM, RM, BRM) independently searched the databases for the selection of eligible studies, and any disagreement was resolved in consultation with the fourth reviewer (AS).
Data Extraction and Management
A predefined format was used by the reviewers (AM, RM, BRM) to extract all relevant data from all eligible studies. After data independent data extraction, they discussed their findings, and discrepancies were sorted by a discussion with another reviewer (AS). The data included study design, population studied, intervention, comparator, duration of therapy, sample size, risk of bias and effect estimates. Wherever data was available in the form of plots only, a plot digitizer was used [10]. The safety data for the interventions, which were compared to be better than the placebo, was evaluated qualitatively.
Data Analysis
The data for analysis were represented treatment arm-wise (long data) for Bayesian network meta-analysis. The analysis was performed in R studio using gemtc package in R language [11]. A network plot was created, and the geometry of the network was assessed for connections between pharmacotherapy and individual trials. Markov chain Monte Carlo simulations were used to synthesize pooled treatment effects from non-informative priors using random-effects variance consistency models. The convergence of models using various permutation-combin-ation of burn-in and inference iterations was checked by Brooks-Gelman-Rubin diagnostic tool. A potential scale reduction factor of 1.0009 was obtained, depicting optimal convergence for a combination of burn-in iteration of 5,000 and inference iterations of 100,000. Time series and density plots were used to confirm the findings of convergence. Analysis of leverage statistics and residual deviance was done to ascertain the consistency of the model. The best-fit model was evaluated by the lowest values for deviance information criteria for the Markov Chain Monte Carlo simulations. The relative effects of the pharmacological agents in comparison to the placebo were plotted. Relative effects of treatment in comparison to all the other interventions were tabulated in a relative effects table. The probability ranking of each pharmacological agent was plotted. A global test based on random effects design-by-treatment interaction model was also done to assess incoherence. The surface under cumulative ranking (SUCRA) scores were calculated to determine the probability of each treatment being the best for change in the severity scores of patients. Node-splitting analysis was done for closed triangles in the network plot. The validity of the consistency assumption was further tested by comparison between the direct and indirect evidence for the same comparison. Meta-regression was done to evaluate the effect of the duration of treatment on the relative effect estimates.
Risk of Bias Assessment
Risk of bias assessment tool 2 (RoB2) from Cochrane Collaboration was used to assess the bias in five domains (bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in measurement of the outcome, bias in selection of the reported result) [12]. The algorithm for each domain points towards a classification of low risk, some concerns and a high risk of bias. A cumulative judgement for the domains within a study leads to an overall risk-of-bias judgment. Judgement for risk of bias was evaluated independently by three reviewers (AM, RM, BRM), and points of dispute were sorted in consultation with a fourth reviewer (AS). Publication bias was assessed by comparison-adjusted funnel plot visually and Egger’s regression test statistically.
Quality of Evidence
The certainty of evidence from each comparison was rated for study design, risk of bias, indirectness, inconsistency, imprecision and publication bias for direct and indirect estimates using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) approach. Higher of the two qualities available was used for the quality rating of the NMA estimate. Wherever only direct or indirect evidence was available, the quality of the NMA estimate was based on that estimate.
RESULTS
Study Results and Study Characteristics
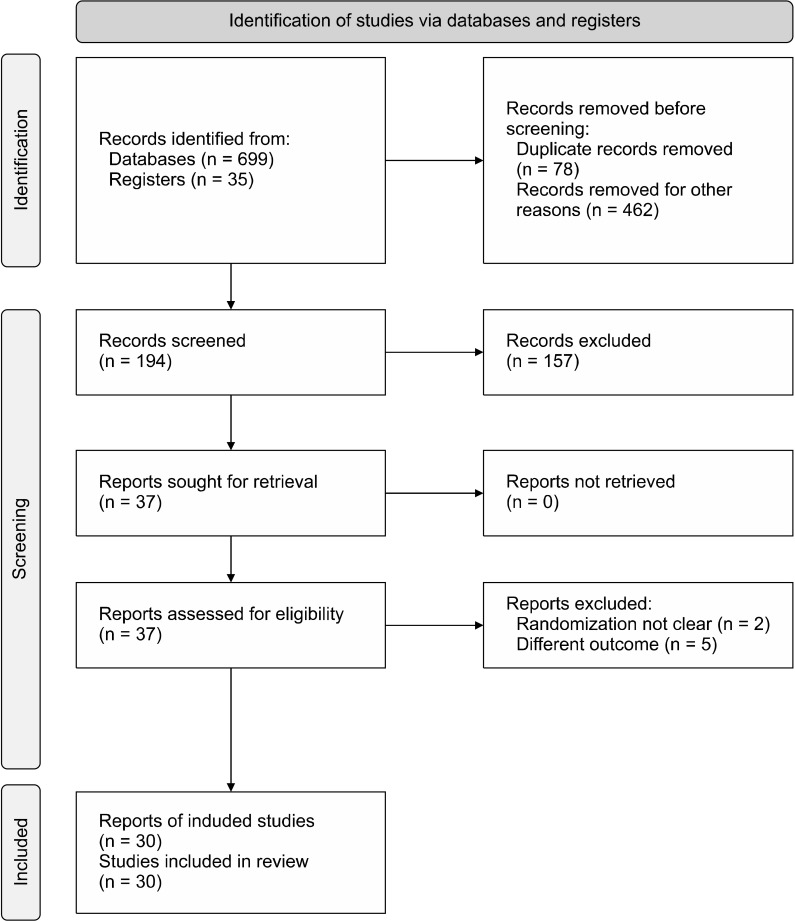
A total of 30 RCTs were included in this network meta-analysis after screening the databases as per predefined eligibility criteria [13-42]. Some of the studies which did not report improvement in total PANSS or BPRS scores were excluded [43-46]. Similarly, the studies where randomization was not performed were also excluded [47,48]. The study selection process has been represented in the PRISMA flow diagram (Fig. 1). The characteristics of individual studies have been mentioned in Table 1. Risk of bias assessment has been done using the RoB2 tool and is represented in Table 2.
Fig. 1.
PRISMA flow diagram for the study selection process.
PRISMA, preferred reporting items for systematic review and meta-analysis protocols.
Table 1.
Study characteristics of the included studies
| Sl. No | Trial and location | Study design | Participants | Interventions & number of participants | Duration of therapy | Outcomes | Notes/remarks |
|---|---|---|---|---|---|---|---|
| 1. | Anil Yağcioğlu et al. 2005 [13], Turkey | Randomized, double-blind, placebo-controlled trial |
Diagnosed with Schizophrenia according to DSM-IV; Persistent symptoms despite 2 antipsychotic trial prior to clozapine and treated with clozapine monotherapy for at least 6 months and had inadequate response | Clozapine + Risperidone = 16 Clozapine + Placebo = 14 |
6 weeks | PANSS CGI-S UKU side effect rating scale CDSS GAF |
No significant difference found between the groups |
| 2. | Assion et al. 2008 [14], Germany | Randomized, double-blind, placebo-controlled trial | Diagnosed with Schizophrenia according to DSM-IV; Persistent symptoms despite an adequate trial of clozapine for at least 3 months | Clozapine + Amisulpride = 6 Clozapine + Placebo = 3 |
6 weeks | BPRS GAF MADRS CGI |
Improvement in global outcomes with amisulpride |
| 3. | Barbui et al. 2011 [15], Italy | Parallel-group, randomized controlled trial | Diagnosed with Schizophrenia according to DSM-IV; Failed at least to 2 antipsychotic trial prior to clozapine and treated with clozapine monotherapy for at least 6 months and unsatisfactory response |
Clozapine + Haloperidol = 53 Clozapine + Aripiprazole = 53 |
3 months | BPRS LUNSERS |
Augmentation with haloperidol is not significantly different aripiprazole |
| 4. | Barnes et al. 2017 [16], United Kingdom | Randomized double-blind, parallel-group, placebo-controlled trial | Persistent symptoms despite treatment with clozapine for at least 12 weeks | Clozapine + Amisulpride = 35 Clozapine + Placebo = 33 |
12 weeks | PANSS CDSS SOFAS |
There was no sig-nificant differ-ence between amisulpride and placebo groups |
| 5. | Chang et al. 2008 [17], Republic of Korea | Randomized double-blind, parallel-group, placebo-controlled trial | Diagnosed with Schizophrenia according to DSM-IV; Failed at least to 2 antipsychotic trial prior to clozapine and treated with clozapine monotherapy for at least 1 year and had shown unsatisfactory response | Clozapine + Aripiprazole = 29 Clozapine + Placebo = 32 |
8 weeks | BPRS SANS YBOCS MADRS UKU side effect rating scale |
A favourable effect in negative symptoms was observed with aripiprazole |
| 6. | de Lucena et al. 2009 [18], Brazil | Randomized double-blind, parallel-group, placebo-controlled trial | Diagnosed with Schizophrenia according to DSM-IV; On clozapine treatment over last 10 years with partial remission | Clozapine + Memantine = 10 Clozapine + Placebo = 11 |
12 weeks | BPRS CGI SAS MMSE |
Memantine was associated with improvement in refractory schizophrenia |
| 7. | Doruk et al. 2008 [19], Turkey | Randomized parallel-group, placebo-controlled trial | Diagnosed with Schizophrenia according to DSM-IV; Failed at least to 2 antipsychotic trial prior to clozapine and treated with clozapine monotherapy for at least 6 months and had shown unsatisfactory response | Clozapine + Ginkgo biloba = 20 Clozapine + Placebo = 22 |
12 weeks | BPRS SAPS SANS |
Ginkgo biloba was effective in decreasing nega-tive symptoms but not overall symptoms |
| 8. | Freudenreich et al. 2007 [20], USA | Randomized parallel-group, double-blind, placebo-controlled trial | Diagnosed with Schizophrenia according to DSM-IV; Failed at least to 2 antipsychotic trial prior to clozapine and treated with clozapine monotherapy for at least 6 months with unsatisfactory response | Clozapine + Risperisone = 11 Clozapine + Placebo = 13 |
8 weeks | PANSS SANS AIMS |
Risperidone did not show any significant benefit over placebo |
| 9. | Friedman et al. 2011 [21], USA | Randomized double-blind, parallel-group, placebo- controlled trial | Diagnosed with Schizophrenia according to DSM-IV; Treatment unresponsive to an adequate trial of clozapine therapy | Clozapine + Pimozide = 25 Clozapine + Placebo = 28 |
12 weeks | PANSS CGI Safety evaluation |
No evidence of benefit from pimozide augmentation |
| 10. | Genç et al. 2007 [22], Turkey | Single-blind, randomized controlled trial | Diagnosed with Schizophrenia according to DSM-IV; Unsatisfactory response despite adequate trial of clozapine for at least 12 weeks | Clozapine + Amisulpride = 27 Clozapine + Quetiapine = 23 |
8 weeks | BPRS SAPS SANS CGI UKU side effect rating scale |
Amisulpride appears to be effective and well tolerated |
| 11. | Gunduz-Bruce et al. 2013 [23], USA | Randomized double-blind, parallel-group, placebo-controlled trial | Diagnosed with Schizophrenia according to DSM-IV; Partial responders to an adequate trial of clozapine | Clozapine + Pimozide = 14 Clozapine + Placebo = 14 |
12 weeks | BPRS SANS CGI AIMS |
Pimozide augmentation did not prove to be an effective strategy |
| 12. | Honer et al. 2006 [24], Canada | Randomized double-blind, parallel-group, placebo-controlled trial | Diagnosed with Schizophrenia according to DSM-IV; Failed at least to 2 antipsychotic trial prior to clozapine and treated with clozapine monotherapy for at least 12 weeks and did not show satisfactory response | Clozapine + Risperidone = 34 Clozapine + Placebo = 34 |
8 weeks | PANSS CGI Verbal working memory |
Risperidone did not improve symptoms in patients |
| 13. | Josiassen et al. 2005 [25], USA | Randomized double-blind, parallel-group, placebo-controlled trial | Diagnosed with Schizophrenia according to DSM-IV; Failed at least to 2 antipsychotic trial prior to clozapine and treated with clozapine monotherapy for at least 3 months and did not show satisfactory response | Clozapine + Risperidone = 20 Clozapine + Placebo = 20 |
12 weeks | BPRS CGI SANS SAS |
Risperidone improved overall symptoms |
| 14. | Kelly et al. 2015 [26], USA |
Randomized, double-blind, placebo-controlled trial | Diagnosed with Schizophrenia according to DSM-IV; Persistent symptoms despite an adequate trial of clozapine for at least 6 months | Clozapine + Minocycline = 27 Clozapine + Placebo = 23 |
10 weeks | BPRS CGI |
No significant difference between minocycline and placebo groups |
| 15. | Lane et al. 2006 [27], Taiwan | Randomized, double-blind, placebo-controlled trial | Diagnosed with Schizophrenia according to DSM-IV; Failed at least to 2 antipsychotic trial prior to clozapine and treated with clozapine monotherapy for at least 3 months and had shown inadequate response | Clozapine + Sarcosine = 10 Clozapine + Placebo = 10 |
6 weeks | PANSS | No improvement with the addition of sarcosine in patients with schizophrenia |
| 16. | Lin et al. 2018 [28], Taiwan | Randomized double-blind, parallel-group, placebo-controlled trial | Diagnosed with Schizophrenia according to DSM-IV; Failed at least to 2 antipsychotic trial prior to clozapine and treated with clozapine monotherapy for at least 6 months and had shown no response | Clozapine + Sodium benzoate = 20 Clozapine + Placebo = 20 |
6 weeks | PANSS SANS QoLS GAF HAMD-17 Cognitive function |
Sodium benzoate improves symptomatology in patients with Clozapine resistance |
| 17. | Lu et al. 2018 [29], Taiwan | Randomized double-blind, parallel-group, placebo- controlled trial | Diagnosed with Schizophrenia according to DSM-IV; Failed at least to 2 antipsychotic trial prior to clozapine and treated with clozapine monotherapy | Clozapine + Fluvoxamine = 43 Clozapine + Placebo = 42 |
12 weeks | PANSS MADRS Pharmaco-kinetics |
Significant reduction in PANSS scores |
| 18. | Mico et al. 2011 [30], Italy | Randomized double-blind, parallel-group, placebo-controlled trial | Diagnosed with Schizophrenia according to DSM-IV; Unsatisfactory response despite an adequate trial of clozapine for at least 1 year | Clozapine + Duloxetine = 20 Clozapine + Placebo = 20 |
16 weeks | PANSS BPRS CDSS Safety evaluation |
Duloxetine showed a beneficial effect |
| 19. | Muscatello et al. 2011 [31], Italy | Randomized double-blind, parallel-group, placebo- controlled trial | Diagnosed with Schizophrenia according to DSM-IV; Unsatisfactory response despite adequate trial of clozapine | Clozapine + Topiramate = 19 Clozapine + Placebo = 24 | 24 weeks | BPRS CDSS SANS SAPS |
Topiramate was scarcely beneficial |
| 20. | Muscatello et al. 2011 [32], Italy | Randomized double-blind, parallel-group, placebo-controlled trial | Diagnosed with Schizophrenia according to DSM-IV; Unsatisfactory response despite adequate trial of clozapine | Clozapine + Aripiprazole = 14 Clozapine + Placebo = 17 |
24 weeks | BPRS SAPS SANS CDSS Safety evaluation |
Aripiprazole may be beneficial to patients partially responsive to clozapine |
| 21. | Muscatello et al. 2014 [33], Italy | Randomized double-blind, parallel-group, placebo-controlled trial | Diagnosed with Schizophrenia according to DSM-IV; Persistent symptoms despite adequate trial of clozapine for at least 1 year | Clozapine + Ziprasidone = 20 Clozapine + Placebo = 20 |
16 weeks | PANSS BPRS CDSS Safety evaluation |
Ziprasidone was effective on negative and cognitive symptoms |
| 22. | Shiloh et al. 1997 [34], Israel | Randomized, double-blind, placebo-controlled trial |
Diagnosed with Schizophrenia according to DSM-IV; Persistent symptoms despite 3 antipsychotic trials prior to clozapine and treated with clozapine monotherapy for at least 12 months and had partial response | Clozapine + Sulpiride = 16 Clozapine + Placebo = 12 |
10 weeks | BPRS SAPS SANS HAM-D |
Sulpiride augmentation shows beneficial effect in partial responders |
| 23. | Tiihonen et al. 2003 [35], Finland | Randomized double-blind, placebo-controlled crossover trial | Diagnosed with Schizophrenia according to DSM-IV; Unsatisfactory response despite adequate trial of clozapine for at least 6 months | Clozapine + Lamotrigine = 29 Clozapine + Placebo = 30 |
14 weeks | PANSS UKU side effect rating scale |
Beneficial effect on both positive and general psy-chopathological symptoms |
| 24. | Vayısoğlu et al. 2013 [36], Turkey | Randomized, double-blind, placebo-controlled trial | Diagnosed with Schizophrenia according to DSM-IV; Persistent symptoms despite an adequate trial of clozapine for at least 1 year |
Clozapine + Lamotrigine = 17 Clozapine + Placebo = 17 |
12 weeks | PANSS CDS CGI-S UKU side effect rating scale |
No benefit of lamotrigine over placebo |
| 25. | Weiner et al. 2010 [37], USA | Randomized double-blind, parallel-group, placebo-controlled trial | Diagnosed with Schizophrenia according to DSM-IV; Unsatisfactory response despite adequate trial of clozapine for at least 6 months | Clozapine + Risperidone = 30 Clozapine + Placebo = 34 |
16 weeks | BPRS SANS CGI |
Adjunctive risperidone may have modest benefit |
| 26. | Zhu et al. 2022 [38], China | Randomized double-blind, parallel-group, placebo-controlled trial | Diagnosed with Schizophrenia according to DSM-IV; Failed at least to 2 antipsychotic trial prior to clozapine and treated with clozapine monotherapy for at least 3 months and did not show satisfactory response | Clozapine + Amisulpride = 40 Clozapine + Placebo = 40 |
12 weeks | PANSS SANS CGI-S CGI-I TESS |
Amisulpride augmentation improves symptoms |
| 27. | Zink et al. 2009 [39], Germany | Parallel-group Randomized controlled trial | Diagnosed with Schizophrenia according to DSM-IV; Failed at least to 2 antipsychotic trial prior to clozapine and treated with clozapine monotherapy for at least 3 months and had inadequate response | Clozapine + Risperidone = 12 Clozapine + Ziprasidone = 12 |
6 weeks | PANSS HAMD SANS CGI |
Significant psychopatho-logical improvements observed in both the groups |
| 28. | Zoccali et al. 2004 [40], Italy | Randomized, double-blind, placebo-controlled trial |
Diagnosed with Schizophrenia according to DSM-IV; Persistent symptoms despite an adequate trial of clozapine for at least 1 year | Clozapine + Mirtazapine = 10 Clozapine + Placebo = 10 |
8 weeks | BPRS SANS SAPS HAM-D |
Suggest a potential role for mirtazapine as an augmentation strategy in schizophrenia |
| 29. | Zoccali et al. 2007 [41], Italy | Randomized double-blind, parallel-group, placebo- controlled trial | Diagnosed with Schizophrenia according to DSM-IV; Persistent symptoms despite adequate trial of clozapine for at least 1 year | Clozapine + Lamotrigine = 26 Clozapine + Placebo = 25 |
24 weeks | SANS SAPS BPRS CDSS Stroop test |
Lamotrigine showed a beneficial effect |
| 30. | Evins et al. 2000 [42], USA | Randomized double-blind, parallel-group, placebo-controlled trial | Diagnosed with Schizophrenia according to DSM-III; Persistent symptoms despite adequate trial of clozapine for at least 4 weeks | Clozapine + Glycine = 14 Clozapine + Placebo = 13 |
8 weeks | SANS PANSS BPRS |
No significant effect of Glycine |
PANSS, positive and negative syndrome scale; BPRS, brief psychiatric rating scale; CGI, clinical global impression scale; SAPS, scale for assessment of positive symptoms; SANS, scale for assessment of negative symptoms; MADRS, Montgomery–Åsberg depression rating scale; HAM-D, Hamilton rating scale for depression; GAF, global functioning assessment; AIMS, abnormal involuntary movement scale; QoLS, quality of life scale; SAS, Simpson-Angus scale; CDSS, Calgary depression scale; LUNSERS, Liverpool University neuroleptic side effect rating scale; UKU side effect rating scale, Udvalg for Kliniske Undersøgelser side effect rating scale; TESS, treatment emergent symptom scale; CDS, cognitive difficulties scale; MMSE, mini‐mental state examination; YBOCS, Yale–Brown obsessive compulsive scale; SOFAS, social and occupational functioning assessment scale; DSM, diagnostic and statistical manual of mental disorders.
Table 2.
Risk of bias table for included studies
| Sl. No | Trial | Randomization process |
Deviation from intended intervention | Missing outcome data | Measurement of outcome data | Selection of reported result | Overall judgment |
|---|---|---|---|---|---|---|---|
| 1. | Anil Yağcioğlu et al. 2005 [13] | Some concerns | Low | High | Low | Low | Some concerns |
| 2. | Assion et al. 2008 [14] | Low | Low | Low | Low | Low | Low |
| 3. | Barbui et al. 2011 [15] | Low | Low | Low | Low | Low | Low |
| 4. | Barnes et al. 2017 [16] | Low | Low | Low | Low | Some concerns | Some concerns |
| 5. | Chang et al. 2008 [17] | Low | Low | Low | Low | Low | Low |
| 6. | de Lucena et al. 2009 [18] | Some concerns | Low | Low | Low | Low | Some concerns |
| 7. | Doruk et al. 2008 [19] | Some concerns | Low | Low | Low | Low | Some concerns |
| 8. | Freudenreich et al. 2007 [20] | Low | Low | Low | Low | Low | Low |
| 9. | Friedman et al. 2011 [21] | Some concerns | Low | Low | Low | Low | Some concerns |
| 10. | Genç et al. 2007 [22] | Some concerns | High | Low | Low | Low | High |
| 11. | Gunduz-Bruce et al. 2013 [23] | Some concerns | Low | Low | Low | Low | Some concerns |
| 12. | Honer et al. 2006 [24] | Low | Low | Low | Low | Low | Low |
| 13. | Josiassen et al. 2005 [25] | Some concerns | Low | Low | Low | Low | Some concerns |
| 14. | Kelly et al. 2015 [26] | Low | Low | Low | Low | Low | Low |
| 15. | Lane et al. 2006 [27] | Low | Low | Low | Low | Low | Low |
| 16. | Lin et al. 2018 [28] | Low | Low | Low | Low | Low | Low |
| 17. | Lu et al. 2018 [29] | Low | Low | Low | Low | Low | Low |
| 18. | Mico et al. 2011 [30] | Low | Low | Low | Low | Low | Low |
| 19. | Muscatello et al. 2011 [31] | Low | Low | Some concerns | Low | Low | Some concerns |
| 20. | Muscatello et al. 2011 [32] | Low | Some concerns | Low | Low | Low | Some concerns |
| 21. | Muscatello et al. 2014 [33] | Low | Low | Low | Low | Low | Low |
| 22. | Shiloh et al. 1997 [34] | High | Low | Low | Low | Low | High |
| 23. | Tiihonen et al. 2003 [35] | Low | Low | Low | Low | Low | Low |
| 24. | Vayısoğlu et al. 2013 [36] | Low | Low | Low | Low | Low | Low |
| 25. | Weiner et al. 2010 [37] | Some concerns | Low | Low | Low | Low | Some concerns |
| 26. | Zhu et al. 2022 [38] | Low | Low | Low | Low | Low | Low |
| 27. | Zink et al. 2009 [39] | Some concerns | Low | Low | High | Low | High |
| 28. | Zoccali et al. 2004 [40] | Some concerns | Some concerns | Low | Low | Low | Some concerns |
| 29. | Zoccali et al. 2007 [41] | Low | Low | Low | Low | Low | Low |
| 30. | Evins et al. 2000 [42] | Low | Low | Low | Low | Low | Low |
Summary of Network
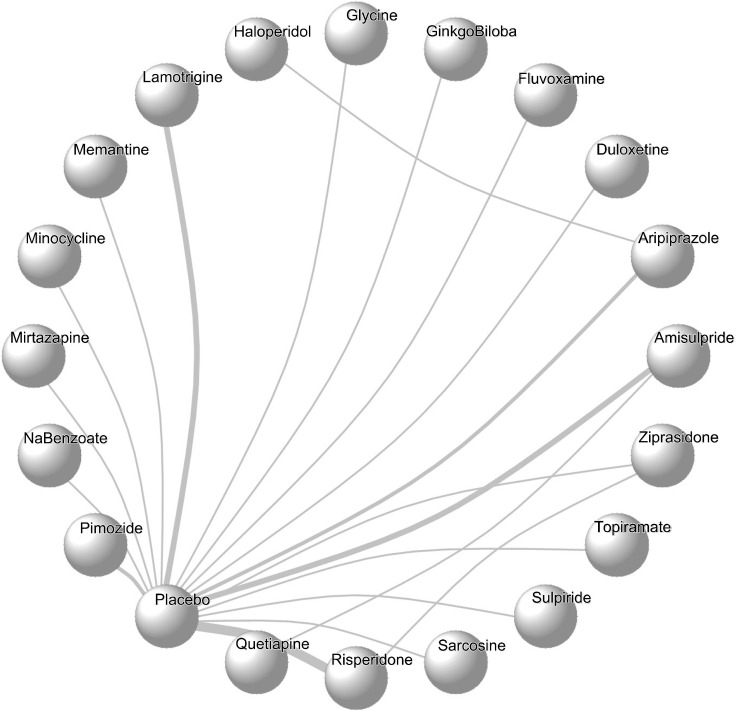
In this network meta-analysis, 30 studies, including 1,335 patients who were non-responders or partial responders to clozapine therapy, were included. A total of 19 interventions have been analyzed cumulatively in the above-mentioned studies. All the studies were two-arm studies except Assion 2008, where two doses of amisulpride were compared against a placebo. However, we have used the higher dose (600 mg/day) for the purpose of this analysis. Network geometry has been plotted and represented in Figure 2.
Fig. 2.
Network plot of all possible augmentation agents to clozapine.
Analysis of comparison of all possible interventions
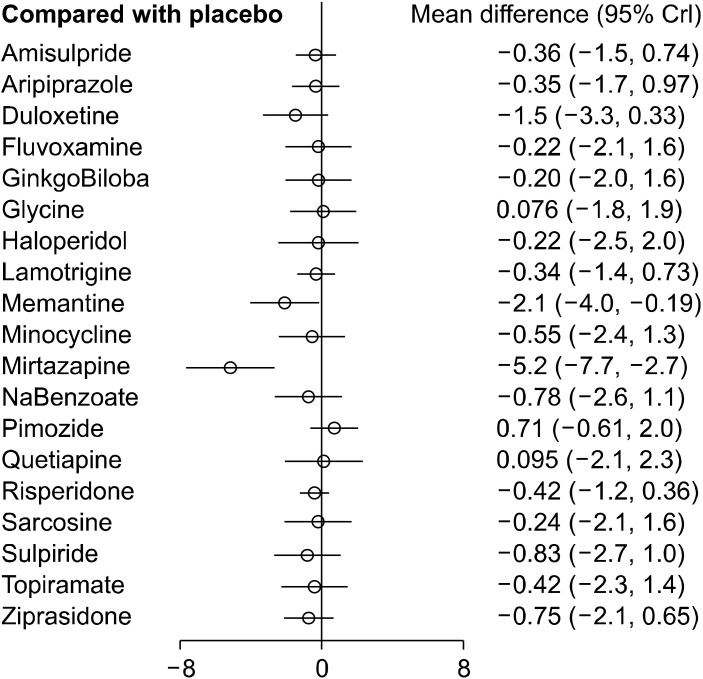
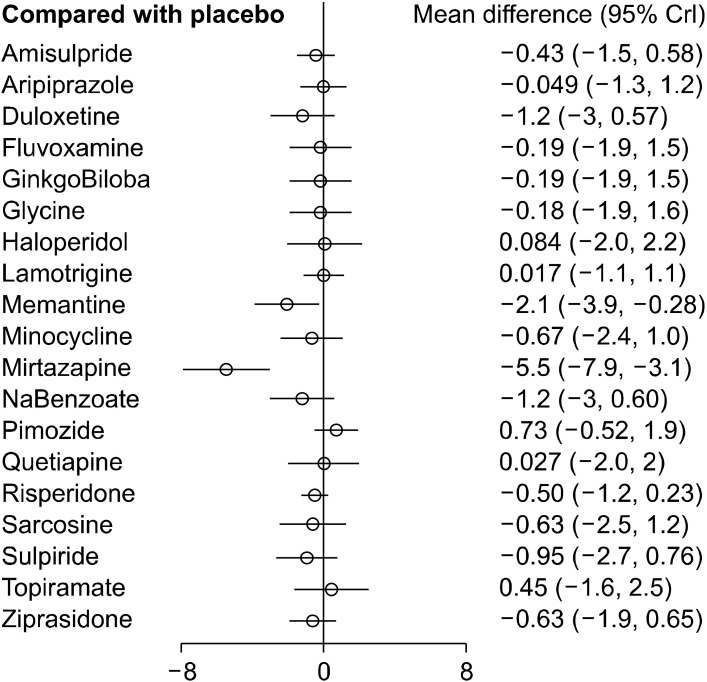
The consistency model was built, which compared all possible pharmacological agents used in the augmentation of clozapine therapy in patients with schizophrenia. Global test based on a random-effects design-by-treatment interaction model yielded a χ2 = 1.297 (p = 0.255), meaning no concerns for incoherence in the NMA. The standardized mean difference and 95% credible interval (95%CrI) of augmentation agent(s) have been determined with respect to all the other agents and have been represented in the relative effects table (Table 3). A relative effect plot was constructed for the effect estimate of all interventions with respect to the placebo (Fig. 3). Mirtazapine (−5.2 [95%CrI: −7.7, −2.7]) and memantine (−2.1 [95%CrI: −4.0, −0.19]) were observed to be more efficacious in the reduction of symptom severity scores when combined with clozapine in non/partial responders to clozapine when compared with placebo. None of the other interventions showed any significant difference in the improvement of the severity of symptoms in comparison to the placebo.
Table 3.
Relative effects table
| Amisulpride | 0.01 (−1.68, 1.73) |
−1.13 (−3.27, 1.02) |
0.14 (−1.99, 2.31) |
0.15 (−1.97, 2.30) |
0.43 (−1.70, 2.61) |
0.13 (−2.34, 2.67) |
0.02 (−1.49, 1.58) |
−1.74 (−3.95, 0.46) |
−0.19 (−2.32, 2.00) |
−4.83 (−7.53, −2.08) |
−0.42 (−2.55, 1.77) |
1.06 (−0.65, 2.82) |
0.36 (−0.73, 1.48) |
0.45 (−1.40, 2.30) |
−0.06 (−1.41, 1.29) |
0.13 (−2.05, 2.29) |
−0.46 (−2.62, 1.70) |
−0.06 (−2.20, 2.11) |
−0.38 (−2.14, 1.38) |
| Aripiprazole | −1.14 (−3.43, 1.10) |
0.13 (−2.15, 2.40) |
0.14 (−2.12, 2.43) |
0.42 (−1.86, 2.68) |
0.12 (−1.71, 1.99) |
0.01 (−1.67, 1.71) |
−1.75 (−4.10, 0.55) |
−0.20 (−2.46, 2.06) |
−4.84 (−7.66, −2.04) |
−0.42 (−2.71, 1.85) |
1.05 (−0.79, 2.93) |
0.35 (−0.97, 1.65) |
0.44 (−2.08, 2.97) |
−0.06 (−1.60, 1.43) |
0.11 (−2.17, 2.41) |
−0.48 (−2.77, 1.81) |
−0.06 (−2.33, 2.18) |
−0.40 (−2.31, 1.50) |
|
| Duloxetine | 1.28 (−1.31, 3.86) |
1.30 (−1.31, 3.91) |
1.58 (−1.05, 4.18) |
1.27 (−1.59, 4.19) |
1.16 (−0.95, 3.27) |
−0.61 (−3.25, 2.05) |
0.95 (−1.66, 3.56) |
−3.69 (−6.80, −0.61) |
0.72 (−1.90, 3.33) |
2.19 (−0.04, 4.50) |
1.49 (−0.33, 3.34) |
1.59 (−1.24, 4.40) |
1.07 (−0.90, 3.06) |
1.26 (−1.36, 3.94) |
0.67 (−1.94, 3.29) |
1.07 (−1.52, 3.68) |
0.74 (−1.55, 3.08) |
||
| Fluvoxamine | 0.01 (−2.56, 2.61) |
0.29 (−2.31, 2.90) | 0.00 (−2.90, 2.89) |
−0.12 (−2.23, 2.02) | −1.90 (−4.54, 0.76) | −0.34 (−2.92, 2.27) |
−4.97 (−8.05, −1.86) |
−0.56 (−3.17, 2.07) |
0.92 (−1.32, 3.19) |
0.21 (−1.63, 2.06) |
0.31 (−2.51, 3.12) |
−0.20 (−2.18, 1.78) |
−0.01 (−2.64, 2.59) |
−0.61 (−3.21, 2.01) |
−0.20 (−2.83, 2.41) |
−0.52 (−2.85, 1.78) |
|||
| Ginkgo Biloba | 0.27 (−2.31, 2.89) |
−0.02 (−2.93, 2.91) |
−0.13 (−2.25, 1.98) |
−1.90 (−4.56, 0.76) |
−0.35 (−2.94, 2.24) |
−4.99 (−8.10, −1.87) |
−0.57 (−3.18, 2.05) |
0.91 (−1.35, 3.16) |
0.20 (−1.63, 2.04) |
0.29 (−2.53, 3.11) |
−0.22 (−2.23, 1.78) |
−0.03 (−2.68, 2.59) |
−0.62 (−3.24, 1.97) |
−0.22 (−2.81, 2.36) |
−0.53 (−2.83, 1.75) |
||||
| Glycine | −0.29 (−3.21, 2.62) |
−0.41 (−2.52, 1.73) |
−2.18 (−4.85, 0.48) |
−0.63 (−3.21, 1.98) |
−5.27 (−8.31, −2.15) |
−0.85 (−3.48, 1.75) |
0.62 (−1.62, 2.92) |
−0.07 (−1.89, 1.78) |
0.02 (−2.85, 2.82) |
−0.49 (−2.49, 1.52) |
−0.30 (−2.95, 2.32) |
−0.90 (−3.49, 1.73) |
−0.49 (−3.10, 2.12) |
−0.82 (−3.14, 1.49) |
|||||
| Haloperidol | −0.11 (−2.60, 2.37) |
−1.88 (−4.86, 1.10) | −0.33 (−3.23, 2.60) |
−4.96 (−8.33, −1.59) |
−0.56 (−3.50, 2.36) |
0.92 (−1.67, 3.54) |
0.22 (−2.04, 2.46) |
0.31 (−2.83, 3.41) |
−0.19 (−2.60, 2.16) |
−0.01 (−2.93, 2.89) |
−0.60 (−3.54, 2.33) |
−0.19 (−3.15, 2.69) |
−0.52 (−3.19, 2.11) |
||||||
| Lamotrigine | −1.77 (−3.96, 0.43) |
−0.21 (−2.34, 1.90) |
−4.85 (−7.52, −2.13) |
−0.44 (−2.58, 1.71) |
1.03 (−0.63, 2.75) |
0.33 (−0.73, 1.39) |
0.43 (−2.01, 2.82) | −0.08 (−1.40, 1.22) |
0.10 (−2.05, 2.27) |
−0.48 (−2.63, 1.64) |
−0.08 (−2.24, 2.03) |
−0.41 (−2.17, 1.34) |
|||||||
| Memantine | 1.55 (−1.09, 4.21) |
−3.08 (−6.24, 0.05) |
1.32 (−1.36, 4.01) |
2.81 (0.49, 5.16) | 2.10 (0.18, 4.03) | 2.20 (−0.71, 5.10) |
1.68 (−0.39, 3.79) |
1.87 (−0.81, 4.51) |
1.28 (−1.42, 3.94) |
1.68 (−0.98, 4.32) |
1.36 (−1.00, 3.73) |
||||||||
| Minocycline | −4.64 (−7.71, −1.52) |
−0.23 (−2.82, 2.38) |
1.25 (−0.98, 3.51) |
0.54 (−1.27, 2.39) |
0.64 (−2.20, 3.49) |
0.12 (−1.86, 2.13) |
0.31 (−2.33, 2.96) |
−0.28 (−2.85, 2.32) |
0.12 (−2.49, 2.72) |
−0.20 (−2.53, 2.12) |
|||||||||
| Mirtazapine | 4.42 (1.27, 7.47) | 5.89 (3.08, 8.72) | 5.19 (2.69, 7.66) | 5.28 (1.96, 8.57) | 4.76 (2.14, 7.35) | 4.96 (1.82, 8.08) | 4.36 (1.23, 7.42) | 4.77 (1.65, 7.82) | 4.44 (1.58, 7.26) | ||||||||||
| NaBenzoate | 1.48 (−0.781, 3.75) |
0.78 (−1.07, 2.62) |
0.87 (−2.02, 3.71) |
0.35 (−1.68, 2.34) |
0.54 (−2.10, 3.16) |
−0.05 (−2.67, 2.57) |
0.36 (−2.27, 2.99) |
0.02 (−2.30, 2.346) |
|||||||||||
| Pimozide | −0.70 (−2.03, 0.60) | −0.60 (−3.20, 1.91) | −1.13 (−2.67, 0.40) | −0.94 (−3.24, 1.35) | −1.53 (−3.81, 0.74) | −1.13 (−3.42, 1.14) | −1.45 (−3.38, 0.44) | ||||||||||||
| Placebo | 0.09 (−2.09, 2.27) | −0.42 (−1.21, 0.35) | −0.23 (−2.11, 1.63) | −0.83 (−2.69, 1.01) | −0.42 (−2.26, 1.41) | −0.74 (−2.13, 0.64) | |||||||||||||
| Quetiapine | −0.51 (−2.83, 1.78) | −0.32 (−3.16, 2.52) | −0.92 (−3.77, 1.97) | −0.52 (−3.41, 2.36) | −0.84 (−3.41, 1.73) | ||||||||||||||
| Risperidone | 0.18 (−1.86, 2.22) | −0.40 (−2.41, 1.60) | 0.00 (−2.01, 2.00) | −0.32 (−1.70, 1.07) | |||||||||||||||
| Sarcosine | −0.59 (−3.22, 2.05) | −0.18 (−2.85, 2.46) | −0.51 (−2.87, 1.83) | ||||||||||||||||
| Sulpiride | 0.40 (−2.19, 3.05) | 0.08 (−2.23, 2.40) | |||||||||||||||||
| Topiramate | −0.32 (−2.62, 2.00) | ||||||||||||||||||
| Ziprasidone |
Values are presented as standardized mean difference (95% credible interval).
Comparison of column-specific intervention with row-specific interventions.
Fig. 3.
Relative effects plots. Comparative efficacy of all possible augmentation agents in comparison to placebo in the network meta-analysis.
CrI, credible intervals.
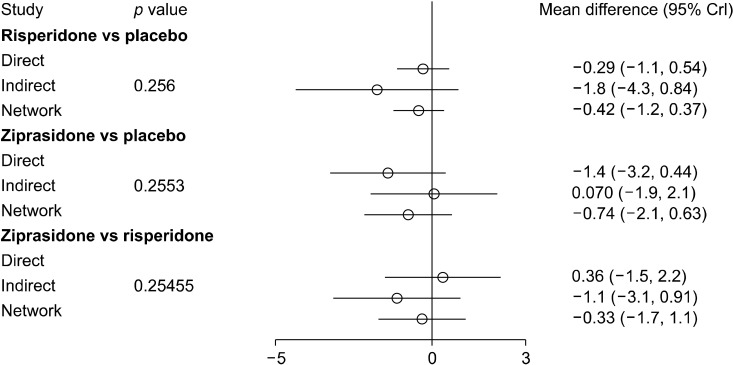
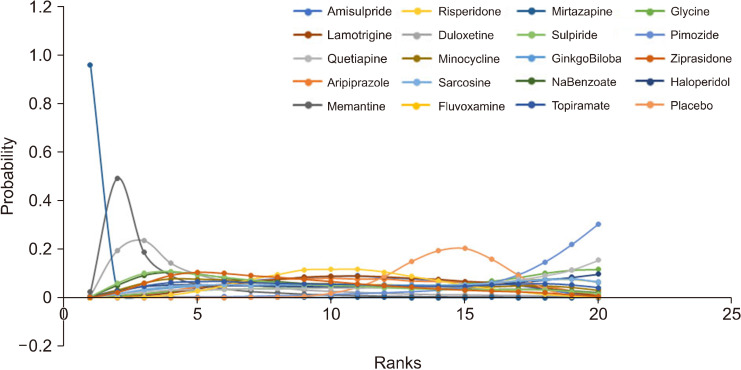
Node-splitting analysis was done for closed triangles in the network plot. The pvalue for inconsistency in the networks was > 0.05, and thus the direct evidence seems to be consistent with the indirect evidence. The summary plot for node-split analysis is represented in Figure 4. A matrix for the probability of each intervention for every rank was created and plotted; the rank probability matrix has been plotted in Figure 5.
Fig. 4.
Node split analysis of all possible interventions forming a closed triangle in the network meta-analysis.
CrI, credible intervals.
Fig. 5.
The rank probability of all augmentation strategies.
SUCRA, surface under cumulative ranking.
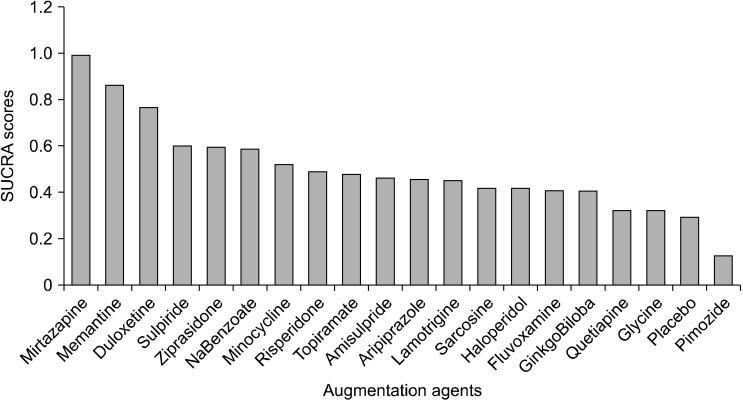
According to the rank probability matrix, the highest probability for the first rank was for mirtazapine, followed by memantine and duloxetine, and for the second rank, the probability was highest for memantine, and duloxetine had the highest probability for third rank. Mirtazapine was observed to have the highest SUCRA score (0.99), followed by memantine (0.86) and duloxetine (0.77). SUCRA score has been represented in a bar plot in Figure 6.
Fig. 6.
SUCRA scores of all aug-mentation strategies.
SUCRA, surface under cumulative ranking.
Meta-regression analysis
Meta-regression was done to assess the effect of duration on the reduction in the severity of symptoms in patients. The analysis showed that there was a significant improvement in the severity of symptoms with increasing duration of therapy (slope: −0.74 [95%CrI: −1.039, −0.438]). The estimates at the centring value of 11.73 weeks for the duration of therapy have been represented in the relative effect plot in Figure 7.
Fig. 7.
Relative effects plot for meta-regression. Effect of duration on efficacy outcome in network meta-analysis.
CrI, credible intervals.
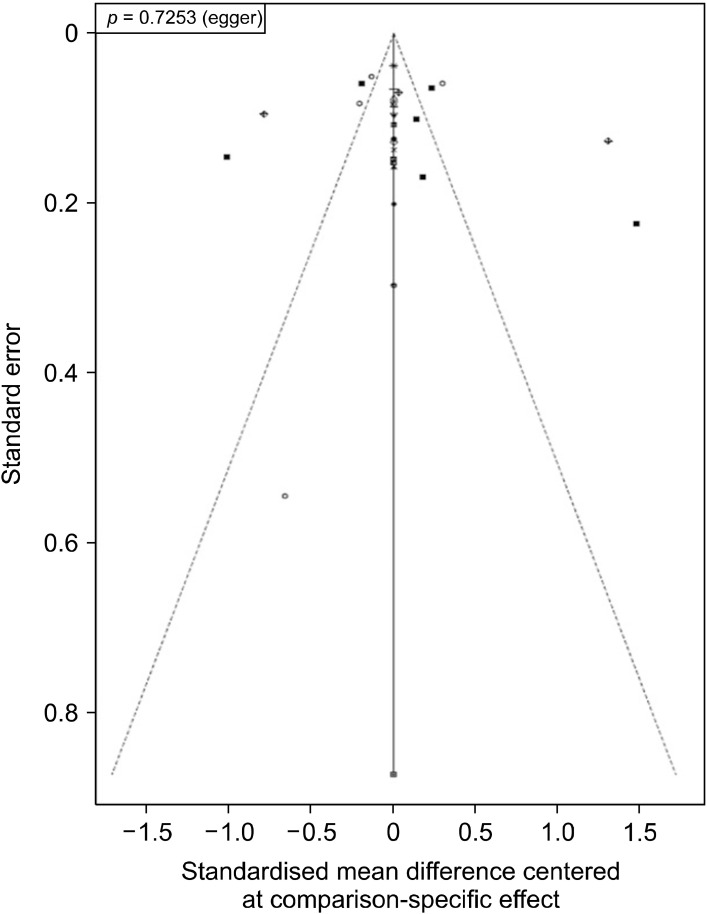
Publication bias
Comparison-adjusted funnel plot was created by plotting standard error against mean difference centred at comparison-specific effect. The plot appeared symmetrical in visual inspection (Fig. 8). This was further corroborated by Egger’s test (p = 0.725), which was not found to be significant. Thus, we conclude that there was no evident publication bias in this network meta-analysis.
Fig. 8.
Comparison adjusted Funnel plot for assessing publication bias.
Quality of evidence
Certainty of evidence for all possible comparisons used as augmentation therapy was determined. The evidence was rated as very low to moderate in quality (Table 4). NMA results sorted based on the quality of evidence for a decrease in scores for severity of symptoms for augmentation agents to clozapine when compared with placebo has been tabulated in Table 5.
Table 4.
Certainty of evidence for comparisons of all included interventions
| Comparison | Direct evidence | Indirect evidence | Network meta–analysis | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| Mean diff (95% CI) | Quality of evidence | Mean diff (95% CrI) | Quality of evidence | Mean diff (95% CrI) | Quality of evidence | |||
| Aripiprazole – Amisulpiride | NA | – | 0.01 (−1.68, 1.73) | Low | 0.01 (−1.68, 1.73) | Low | ||
| Duloxetine – Amisulpiride | NA | – | −1.13 (−3.27, 1.02) | Low | −1.13 (−3.27, 1.02) | Low | ||
| Fluvoxamine – Amisulpiride | NA | – | 0.14 (−1.99, 2.31) | Low | 0.14 (−1.99, 2.31) | Low | ||
| GinkgoBiloba – Amisulpiride | NA | – | 0.15 (−1.97, 2.30) | Low | 0.15 (−1.97, 2.30) | Low | ||
| Glycine – Amisulpiride | NA | – | 0.43 (−1.70, 2.61) | Low | 0.43 (−1.70, 2.61) | Low | ||
| Haloperidol – Amisulpiride | NA | – | 0.13 (−2.34, 2.67) | Low | 0.13 (−2.34, 2.67) | Low | ||
| Lamotrigine – Amisulpiride | NA | – | 0.02 (−1.49, 1.58) | Low | 0.02 (−1.49, 1.58) | Low | ||
| Memantine – Amisulpiride | NA | – | −1.74 (−3.95, 0.46) | Low | −1.74 (−3.95, 0.46) | Low | ||
| Minocycline – Amisulpiride | NA | – | −0.19 (−2.32, 2.00) | Low | −0.19 (−2.32, 2.00) | Low | ||
| Mirtazapine – Amisulpiride | NA | – | −4.83 (−7.53, −2.08) | Low | −4.83 (−7.53, −2.08) | Low | ||
| NaBenzoate – Amisulpiride | NA | – | −0.42 (−2.55, 1.77) | Low | −0.42 (−2.55, 1.77) | Low | ||
| Pimozide – Amisulpiride | NA | – | 1.06 (−0.65, 2.82) | Very low | 1.06 (−0.65, 2.82) | Very low | ||
| Placebo – Amisulpiride | 0.36 (−0.73, 1.48) | Low | NE | – | 0.36 (−0.73, 1.48) | Low | ||
| Quetiapine – Amisulpiride | 0.45 (−1.40, 2.30) | Low | NE | – | 0.45 (−1.40, 2.30) | Low | ||
| Risperidone – Amisulpiride | NA | – | −0.06 (−1.41, 1.29) | Very low | −0.06 (−1.41, 1.29) | Very low | ||
| Sarcosine – Amisulpiride | NA | – | 0.13 (−2.05, 2.29) | Low | 0.13 (−2.05, 2.29) | Low | ||
| Sulpiride – Amisulpiride | NA | – | −0.46 (−2.62, 1.70) | Low | −0.46 (−2.62, 1.70) | Low | ||
| Topiramate – Amisulpiride | NA | – | −0.06 (−2.20, 2.11) | Low | −0.06 (−2.20, 2.11) | Low | ||
| Ziprasidone – Amisulpiride | NA | – | −0.38 (−2.14, 1.38) | Low | −0.38 (−2.14, 1.38) | Low | ||
| Duloxetine – Aripiprazole | NA | – | −1.14 (−3.43, 1.10) | Low | −1.14 (−3.43, 1.10) | Low | ||
| Fluvoxamine – Aripiprazole | NA | – | 0.13 (−2.15, 2.40) | Low | 0.13 (−2.15, 2.40) | Low | ||
| GinkgoBiloba – Aripiprazole | NA | – | 0.14 (−2.12, 2.43) | Low | 0.14 (−2.12, 2.43) | Low | ||
| Glycine – Aripiprazole | NA | – | 0.42 (−1.86, 2.68) | Low | 0.42 (−1.86, 2.68) | Low | ||
| Haloperidol – Aripiprazole | 0.12 (−1.71, 1.99) | Moderate | NE | – | 0.12 (−1.71, 1.99) | Moderate | ||
| Lamotrigine – Aripiprazole | NA | – | 0.01 (−1.67, 1.71) | Low | 0.01 (−1.67, 1.71) | Low | ||
| Memantine – Aripiprazole | NA | – | −1.75 (−4.10, 0.55) | Low | −1.75 (−4.10, 0.55) | Low | ||
| Minocycline – Aripiprazole | NA | – | −0.20 (−2.46, 2.06) | Low | −0.20 (−2.46, 2.06) | Low | ||
| Mirtazapine – Aripiprazole | NA | – | −4.84 (−7.66, −2.04) | Low | −4.84 (−7.66, −2.04) | Low | ||
| NaBenzoate – Aripiprazole | NA | – | −0.42 (−2.71, 1.85) | Low | −0.42 (−2.71, 1.85) | Low | ||
| Pimozide – Aripiprazole | NA | – | 1.05 (−0.79, 2.93) | Low | 1.05 (−0.79, 2.93) | Low | ||
| Placebo – Aripiprazole | 0.35 (−0.97, 1.65) | Moderate | NE | – | 0.35 (−0.97, 1.65) | Moderate | ||
| Quetiapine – Aripiprazole | NA | – | 0.44 (−2.08, 2.97) | Low | 0.44 (−2.08, 2.97) | Low | ||
| Risperidone – Aripiprazole | NA | – | −0.06 (−1.60, 1.43) | Low | −0.06 (−1.60, 1.43) | Low | ||
| Sarcosine – Aripiprazole | NA | – | 0.11 (−2.17, 2.41) | Low | 0.11 (−2.17, 2.41) | Low | ||
| Sulpiride – Aripiprazole | NA | – | −0.48 (−2.77, 1.81) | Low | −0.48 (−2.77, 1.81) | Low | ||
| Topiramate – Aripiprazole | NA | – | −0.06 (−2.33, 2.18) | Low | −0.06 (−2.33, 2.18) | Low | ||
| Ziprasidone – Aripiprazole | NA | – | −0.40 (−2.31, 1.50) | Low | −0.40 (−2.31, 1.50) | Low | ||
| Fluvoxamine – Duloxetine | NA | – | 1.28 (−1.31, 3.86) | Low | 1.28 (−1.31, 3.86) | Low | ||
| GinkgoBiloba – Duloxetine | NA | – | 1.30 (−1.31, 3.91) | Low | 1.30 (−1.31, 3.91) | Low | ||
| Glycine – Duloxetine | NA | – | 1.58 (−1.05, 4.18) | Low | 1.58 (−1.05, 4.18) | Low | ||
| Haloperidol – Duloxetine | NA | – | 1.27 (−1.59, 4.19) | Low | 1.27 (−1.59, 4.19) | Low | ||
| Lamotrigine – Duloxetine | NA | – | 1.16 (−0.95, 3.27) | Low | 1.16 (−0.95, 3.27) | Low | ||
| Memantine – Duloxetine | NA | – | −0.61 (−3.25, 2.05) | Low | −0.61 (−3.25, 2.05) | Low | ||
| Minocycline – Duloxetine | NA | – | 0.95 (−1.66, 3.56) | Low | 0.95 (−1.66, 3.56) | Low | ||
| Mirtazapine – Duloxetine | NA | – | −3.69 (−6.80, −0.61) | Low | −3.69 (−6.80, −0.61) | Low | ||
| NaBenzoate – Duloxetine | NA | – | 0.72 (−1.90, 3.33) | Low | 0.72 (−1.90, 3.33) | Low | ||
| Pimozide – Duloxetine | NA | – | 2.19 (−0.04, 4.50) | Moderate | 2.19 (−0.04, 4.50) | Moderate | ||
| Placebo – Duloxetine | 1.49 (−0.33, 3.34) | High | NE | – | 1.49 (−0.33, 3.34) | High | ||
| Quetiapine – Duloxetine | NA | – | 1.59 (−1.24, 4.40) | Low | 1.59 (−1.24, 4.40) | Low | ||
| Risperidone – Duloxetine | NA | – | 1.07 (−0.90, 3.06) | Low | 1.07 (−0.90, 3.06) | Low | ||
| Sarcosine – Duloxetine | NA | – | 1.26 (−1.36, 3.94) | Low | 1.26 (−1.36, 3.94) | Low | ||
| Sulpiride – Duloxetine | NA | – | 0.67 (−1.94, 3.29) | Low | 0.67 (−1.94, 3.29) | Low | ||
| Topiramate – Duloxetine | NA | – | 1.07 (−1.52, 3.68) | Low | 1.07 (−1.52, 3.68) | Low | ||
| Ziprasidone – Duloxetine | NA | – | 0.74 (−1.55, 3.08) | Low | 0.74 (−1.55, 3.08) | Low | ||
| GinkgoBiloba–Fluvoxamine | NA | – | 0.01 (−2.56, 2.61) | Low | 0.01 (−2.56, 2.61) | Low | ||
| Glycine – Fluvoxamine | NA | – | 0.29 (−2.31, 2.90) | Low | 0.29 (−2.31, 2.90) | Low | ||
| Haloperidol – Fluvoxamine | NA | – | 0.00 (−2.90, 2.89) | Low | 0.00 (−2.90, 2.89) | Low | ||
| Lamotrigine – Fluvoxamine | NA | – | −0.12 (−2.23, 2.02) | Low | −0.12 (−2.23, 2.02) | Low | ||
| Memantine – Fluvoxamine | NA | – | −1.90 (−4.54, 0.76) | Moderate | −1.90 (−4.54, 0.76) | Moderate | ||
| Minocycline – Fluvoxamine | NA | – | −0.34 (−2.92, 2.27) | Low | −0.34 (−2.92, 2.27) | Low | ||
| Mirtazapine – Fluvoxamine | NA | – | −4.97 (−8.05, −1.86) | Low | −4.97 (−8.05, −1.86) | Low | ||
| NaBenzoate – Fluvoxamine | NA | – | −0.56 (−3.17, 2.07) | Low | −0.56 (−3.17, 2.07) | Low | ||
| Pimozide – Fluvoxamine | NA | – | 0.92 (−1.32, 3.19) | Low | 0.92 (−1.32, 3.19) | Low | ||
| Placebo – Fluvoxamine | 0.21 (−1.63, 2.06) | Moderate | NE | – | 0.21 (−1.63, 2.06) | Moderate | ||
| Quetiapine – Fluvoxamine | NA | – | 0.31 (−2.51, 3.12) | Low | 0.31 (−2.51, 3.12) | Low | ||
| Risperidone – Fluvoxamine | NA | – | −0.20 (−2.18, 1.78) | Very low | −0.20 (−2.18, 1.78) | Very low | ||
| Sarcosine – Fluvoxamine | NA | – | −0.01 (−2.64, 2.59) | Low | −0.01 (−2.64, 2.59) | Low | ||
| Sulpiride – Fluvoxamine | NA | – | −0.61 (−3.21, 2.01) | Low | −0.61 (−3.21, 2.01) | Low | ||
| Topiramate – Fluvoxamine | NA | – | −0.20 (−2.83, 2.41) | Low | −0.20 (−2.83, 2.41) | Low | ||
| Ziprasidone – Fluvoxamine | NA | – | −0.52 (−2.85, 1.78) | Low | −0.52 (−2.85, 1.78) | Low | ||
| Glycine – GinkgoBiloba | NA | – | 0.27 (−2.31, 2.89) | Low | 0.27 (−2.31, 2.89) | Low | ||
| Haloperidol – GinkgoBiloba | NA | – | −0.02 (−2.93, 2.91) | Low | −0.02 (−2.93, 2.91) | Low | ||
| Lamotrigine – GinkgoBiloba | NA | – | −0.13 (−2.25, 1.98) | Low | −0.13 (−2.25, 1.98) | Low | ||
| Memantine – GinkgoBiloba | NA | – | −1.90 (−4.56, 0.76) | Low | −1.90 (−4.56, 0.76) | Low | ||
| Minocycline – GinkgoBiloba | NA | – | −0.35 (−2.94, 2.24) | Very low | −0.35 (−2.94, 2.24) | Very low | ||
| Mirtazapine – GinkgoBiloba | NA | – | −4.99 (−8.10, −1.87) | Low | −4.99 (−8.10, −1.87) | Low | ||
| NaBenzoate – GinkgoBiloba | NA | – | −0.57 (−3.18, 2.05) | Low | −0.57 (−3.18, 2.05) | Low | ||
| Pimozide – GinkgoBiloba | NA | – | 0.91 (−1.35, 3.16) | Very low | 0.91 (−1.35, 3.16) | Very low | ||
| Placebo – GinkgoBiloba | 0.20 (−1.63, 2.04) | Low | NE | – | 0.20 (−1.63, 2.04) | Low | ||
| Quetiapine – GinkgoBiloba | NA | – | 0.29 (−2.53, 3.11) | Low | 0.29 (−2.53, 3.11) | Low | ||
| Risperidone – GinkgoBiloba | NA | – | −0.22 (−2.23, 1.78) | Very low | −0.22 (−2.23, 1.78) | Very low | ||
| Sarcosine – GinkgoBiloba | NA | – | −0.03 (−2.68, 2.59) | Low | −0.03 (−2.68, 2.59) | Low | ||
| Sulpiride – GinkgoBiloba | NA | – | −0.62 (−3.24, 1.97) | Low | −0.62 (−3.24, 1.97) | Low | ||
| Topiramate – GinkgoBiloba | NA | – | −0.22 (−2.81, 2.36) | Very low | −0.22 (−2.81, 2.36) | Very low | ||
| Ziprasidone – GinkgoBiloba | NA | – | −0.53 (−2.83, 1.75) | Low | −0.53 (−2.83, 1.75) | Low | ||
| Haloperidol – Glycine | NA | – | −0.29 (−3.21, 2.62) | Low | −0.29 (−3.21, 2.62) | Low | ||
| Lamotrigine – Glycine | NA | – | −0.41 (−2.52, 1.73) | Low | −0.41 (−2.52, 1.73) | Low | ||
| Memantine – Glycine | NA | – | −2.18 (−4.85, 0.48) | Low | −2.18 (−4.85, 0.48) | Low | ||
| Minocycline – Glycine | NA | – | −0.63 (−3.21, 1.98) | Low | −0.63 (−3.21, 1.98) | Low | ||
| Mirtazapine – Glycine | NA | – | −5.27 (−8.31, −2.15) | Low | −5.27 (−8.31, −2.15) | Low | ||
| NaBenzoate – Glycine | NA | – | −0.85 (−3.48, 1.75) | Low | −0.85 (−3.48, 1.75) | Low | ||
| Pimozide – Glycine | NA | – | 0.62 (−1.62, 2.92) | Low | 0.62 (−1.62, 2.92) | Low | ||
| Placebo – Glycine | −0.07 (−1.89, 1.78) | Moderate | NE | – | −0.07 (−1.89, 1.78) | Moderate | ||
| Quetiapine – Glycine | NA | – | 0.02 (−2.85, 2.82) | Low | 0.02 (−2.85, 2.82) | Low | ||
| Risperidone – Glycine | NA | – | −0.49 (−2.49, 1.52) | Low | −0.49 (−2.49, 1.52) | Low | ||
| Sarcosine – Glycine | NA | – | −0.30 (−2.95, 2.32) | Low | −0.30 (−2.95, 2.32) | Low | ||
| Sulpiride – Glycine | NA | – | −0.90 (−3.49, 1.73) | Low | −0.90 (−3.49, 1.73) | Low | ||
| Topiramate – Glycine | NA | – | −0.49 (−3.10, 2.12) | Low | −0.49 (−3.10, 2.12) | Low | ||
| Ziprasidone – Glycine | NA | – | −0.82 (−3.14, 1.49) | Low | −0.82 (−3.14, 1.49) | Low | ||
| Lamotrigine – Haloperidol | NA | – | −0.11 (−2.60, 2.37) | Low | −0.11 (−2.60, 2.37) | Low | ||
| Memantine – Haloperidol | NA | – | −1.88 (−4.86, 1.10) | Low | −1.88 (−4.86, 1.10) | Low | ||
| Minocycline – Haloperidol | NA | – | −0.33 (−3.23, 2.60) | Low | −0.33 (−3.23, 2.60) | Low | ||
| Mirtazapine – Haloperidol | NA | – | −4.96 (−8.33, −1.59) | Low | −4.96 (−8.33, −1.59) | Low | ||
| NaBenzoate – Haloperidol | NA | – | −0.56 (−3.50, 2.36) | Low | −0.56 (−3.50, 2.36) | Low | ||
| Pimozide – Haloperidol | NA | – | 0.92 (−1.67, 3.54) | Low | 0.92 (−1.67, 3.54) | Low | ||
| Placebo – Haloperidol | NA | – | 0.22 (−2.04, 2.46) | Low | 0.22 (−2.04, 2.46) | Low | ||
| Quetiapine – Haloperidol | NA | – | 0.31 (−2.83, 3.41) | Low | 0.31 (−2.83, 3.41) | Low | ||
| Risperidone – Haloperidol | NA | – | −0.19 (−2.60, 2.16) | Very low | −0.19 (−2.60, 2.16) | Very low | ||
| Sarcosine – Haloperidol | NA | – | −0.01 (−2.93, 2.89) | Low | −0.01 (−2.93, 2.89) | Low | ||
| Sulpiride – Haloperidol | NA | – | −0.60 (−3.54, 2.33) | Low | −0.60 (−3.54, 2.33) | Low | ||
| Topiramate – Haloperidol | NA | – | −0.19 (−3.15, 2.69) | Low | −0.19 (−3.15, 2.69) | Low | ||
| Ziprasidone – Haloperidol | NA | – | −0.52 (−3.19, 2.11) | Low | −0.52 (−3.19, 2.11) | Low | ||
| Memantine – Lamotrigine | NA | – | −1.77 (−3.96, 0.43) | Low | −1.77 (−3.96, 0.43) | Low | ||
| Minocycline – Lamotrigine | NA | – | −0.21 (−2.34, 1.90) | Low | −0.21 (−2.34, 1.90) | Low | ||
| Mirtazapine – Lamotrigine | NA | – | −4.85 (−7.52, −2.13) | Low | −4.85 (−7.52, −2.13) | Low | ||
| NaBenzoate – Lamotrigine | NA | – | −0.44 (−2.58, 1.71) | Low | −0.44 (−2.58, 1.71) | Low | ||
| Pimozide – Lamotrigine | NA | – | 1.03 (−0.63, 2.75) | Very low | 1.03 (−0.63, 2.75) | Very low | ||
| Placebo – Lamotrigine | 0.33 (−0.73, 1.39) | Moderate | NE | – | 0.33 (−0.73, 1.39) | Moderate | ||
| Quetiapine – Lamotrigine | NA | – | 0.43 (−2.01, 2.82) | Low | 0.43 (−2.01, 2.82) | Low | ||
| Risperidone – Lamotrigine | NA | – | −0.08 (−1.40, 1.22) | Very low | −0.08 (−1.40, 1.22) | Very low | ||
| Sarcosine – Lamotrigine | NA | – | 0.10 (−2.05, 2.27) | Low | 0.10 (−2.05, 2.27) | Low | ||
| Sulpiride – Lamotrigine | NA | – | −0.48 (−2.63, 1.64) | Low | −0.48 (−2.63, 1.64) | Low | ||
| Topiramate – Lamotrigine | NA | – | −0.08 (−2.24, 2.03) | Low | −0.08 (−2.24, 2.03) | Low | ||
| Ziprasidone – Lamotrigine | NA | – | −0.41 (−2.17, 1.34) | Low | −0.41 (−2.17, 1.34) | Low | ||
| Minocycline – Memantine | NA | – | 1.55 (−1.09, 4.21) | Low | 1.55 (−1.09, 4.21) | Low | ||
| Mirtazapine – Memantine | NA | – | −3.08 (−6.24, 0.05) | Very low | −3.08 (−6.24, 0.05) | Very low | ||
| NaBenzoate – Memantine | NA | – | 1.32 (−1.36, 4.01) | Low | 1.32 (−1.36, 4.01) | Low | ||
| Pimozide – Memantine | NA | – | 2.81 (0.49, 5.16) | Very low | 2.81 (0.49, 5.16) | Very low | ||
| Placebo – Memantine | 2.10 (0.18, 4.03) | Moderate | NE | – | 2.10 (0.18, 4.03) | Moderate | ||
| Quetiapine – Memantine | NA | – | 2.20 (−0.71, 5.10) | Low | 2.20 (−0.71, 5.10) | Low | ||
| Risperidone – Memantine | NA | – | 1.68 (−0.39, 3.79) | Low | 1.68 (−0.39, 3.79) | Low | ||
| Sarcosine – Memantine | NA | – | 1.87 (−0.81, 4.51) | Low | 1.87 (−0.81, 4.51) | Low | ||
| Sulpiride – Memantine | NA | – | 1.28 (−1.42, 3.94) | Low | 1.28 (−1.42, 3.94) | Low | ||
| Topiramate – Memantine | NA | – | 1.68 (−0.98, 4.32) | Low | 1.68 (−0.98, 4.32) | Low | ||
| Ziprasidone – Memantine | NA | – | 1.36 (−1.00, 3.73) | Low | 1.36 (−1.00, 3.73) | Low | ||
| Mirtazapine – Minocycline | NA | – | −4.64 (−7.71, −1.52) | Low | −4.64 (−7.71, −1.52) | Low | ||
| NaBenzoate – Minocycline | NA | – | −0.23 (−2.82, 2.38) | Low | −0.23 (−2.82, 2.38) | Low | ||
| Pimozide – Minocycline | NA | – | 1.25 (−0.98, 3.51) | Low | 1.25 (−0.98, 3.51) | Low | ||
| Placebo – Minocycline | 0.54 (−1.27, 2.39) | Moderate | NE | – | 0.54 (−1.27, 2.39) | Moderate | ||
| Quetiapine – Minocycline | NA | – | 0.64 (−2.20, 3.49) | Low | 0.64 (−2.20, 3.49) | Low | ||
| Risperidone – Minocycline | NA | – | 0.12 (−1.86, 2.13) | Very low | 0.12 (−1.86, 2.13) | Very low | ||
| Sarcosine – Minocycline | NA | – | 0.31 (−2.33, 2.96) | Low | 0.31 (−2.33, 2.96) | Low | ||
| Sulpiride – Minocycline | NA | – | −0.28 (−2.85, 2.32) | Low | −0.28 (−2.85, 2.32) | Low | ||
| Topiramate – Minocycline | NA | – | 0.12 (−2.49, 2.72) | Low | 0.12 (−2.49, 2.72) | Low | ||
| Ziprasidone – Minocycline | NA | – | −0.20 (−2.53, 2.12) | Low | −0.20 (−2.53, 2.12) | Low | ||
| NaBenzoate – Mirtazapine | NA | – | 4.42 (1.27, 7.47) | Low | 4.42 (1.27, 7.47) | Low | ||
| Pimozide – Mirtazapine | NA | – | 5.89 (3.08, 8.72) | Moderate | 5.89 (3.08, 8.72) | Moderate | ||
| Placebo – Mirtazapine | 5.19 (2.69, 7.66) | Moderate | NE | – | 5.19 (2.69, 7.66) | Moderate | ||
| Quetiapine – Mirtazapine | NA | – | 5.28 (1.96, 8.57) | Moderate | 5.28 (1.96, 8.57) | Moderate | ||
| Risperidone – Mirtazapine | NA | – | 4.76 (2.14, 7.35) | Low | 4.76 (2.14, 7.35) | Low | ||
| Sarcosine – Mirtazapine | NA | – | 4.96 (1.82, 8.08) | Moderate | 4.96 (1.82, 8.08) | Moderate | ||
| Sulpiride – Mirtazapine | NA | – | 4.36 (1.23, 7.42) | Low | 4.36 (1.23, 7.42) | Low | ||
| Topiramate – Mirtazapine | NA | – | 4.77 (1.65, 7.82) | Low | 4.77 (1.65, 7.82) | Low | ||
| Ziprasidone – Mirtazapine | NA | – | 4.44 (1.58, 7.26) | Moderate | 4.44 (1.58, 7.26) | Moderate | ||
| Pimozide – NaBenzoate | NA | – | 1.48 (−0.781, 3.75) | Low | 1.48 (−0.781, 3.75) | Low | ||
| Placebo – NaBenzoate | 0.78 (−1.07, 2.62) | Low | NE | – | 0.78 (−1.07, 2.62) | Low | ||
| Quetiapine – NaBenzoate | NA | – | 0.87 (−2.02, 3.71) | Low | 0.87 (−2.02, 3.71) | Low | ||
| Risperidone – NaBenzoate | NA | – | 0.35 (−1.68, 2.34) | Very low | 0.35 (−1.68, 2.34) | Very low | ||
| Sarcosine – NaBenzoate | NA | – | 0.54 (−2.10, 3.16) | Low | 0.54 (−2.10, 3.16) | Low | ||
| Sulpiride – NaBenzoate | NA | – | −0.05 (−2.67, 2.57) | Low | −0.05 (−2.67, 2.57) | Low | ||
| Topiramate – NaBenzoate | NA | – | 0.36 (−2.27, 2.99) | Low | 0.36 (−2.27, 2.99) | Low | ||
| Ziprasidone – NaBenzoate | NA | – | 0.02 (−2.30, 2.346) | Low | 0.02 (−2.30, 2.346) | Low | ||
| Placebo – Pimozide | −0.70 (−2.03, 0.60) | Moderate | NE | – | −0.70 (−2.03, 0.60) | Moderate | ||
| Quetiapine – Pimozide | NA | – | −0.60 (−3.20, 1.91) | Low | −0.60 (−3.20, 1.91) | Low | ||
| Risperidone – Pimozide | NA | – | −1.13 (−2.67, 0.40) | Very low | −1.13 (−2.67, 0.40) | Very low | ||
| Sarcosine – Pimozide | NA | – | −0.94 (−3.24, 1.35) | Low | −0.94 (−3.24, 1.35) | Low | ||
| Sulpiride – Pimozide | NA | – | −1.53 (−3.81, 0.74) | Low | −1.53 (−3.81, 0.74) | Low | ||
| Topiramate – Pimozide | NA | – | −1.13 (−3.42, 1.14) | Very low | −1.13 (−3.42, 1.14) | Very low | ||
| Ziprasidone – Pimozide | NA | – | −1.45 (−3.38, 0.44) | Low | −1.45 (−3.38, 0.44) | Low | ||
| Quetiapine – Placebo | NA | – | 0.09 (−2.09, 2.27) | Low | 0.09 (−2.09, 2.27) | Low | ||
| Risperidone – Placebo | −0.29 (−1.1, 0.54) | Low | −1.8 (−4.3, 0.84) | Very low | −0.42 (−1.21, 0.35) | Low | ||
| Sarcosine – Placebo | −0.23 (−2.11, 1.63) | Moderate | NE | – | −0.23 (−2.11, 1.63) | Moderate | ||
| Sulpiride – Placebo | −0.83 (−2.69, 1.01) | Moderate | NE | – | −0.83 (−2.69, 1.01) | Moderate | ||
| Topiramate – Placebo | −0.42 (−2.26, 1.41) | Low | NE | – | −0.42 (−2.26, 1.41) | Low | ||
| Ziprasidone – Placebo | −0.74 (−2.13, 0.64) | Moderate | −0.07 (−1.9, 2.1) | Low | −0.74 (−2.13, 0.64) | Moderate | ||
| Risperidone – Quetiapine | NA | – | −0.51 (−2.83, 1.78) | Low | −0.51 (−2.83, 1.78) | Low | ||
| Sarcosine – Quetiapine | NA | – | −0.32 (−3.16, 2.52) | Low | −0.32 (−3.16, 2.52) | Low | ||
| Sulpiride – Quetiapine | NA | – | −0.92 (−3.77, 1.97) | Low | −0.92 (−3.77, 1.97) | Low | ||
| Topiramate – Quetiapine | NA | – | −0.52 (−3.41, 2.36) | Low | −0.52 (−3.41, 2.36) | Low | ||
| Ziprasidone – Quetiapine | NA | – | −0.84 (−3.41, 1.73) | Low | −0.84 (−3.41, 1.73) | Low | ||
| Sarcosine – Risperidone | NA | – | 0.18 (−1.86, 2.22) | Low | 0.18 (−1.86, 2.22) | Low | ||
| Sulpiride – Risperidone | NA | – | −0.40 (−2.41, 1.60) | Low | −0.40 (−2.41, 1.60) | Low | ||
| Topiramate – Risperidone | NA | – | 0.00 (−2.01, 2.00) | Very low | 0.00 (−2.01, 2.00) | Very low | ||
| Ziprasidone – Risperidone | 0.36 (−1.5, 2.2) | Low | −1.1 (−3.1, 0.91) | Very low | −0.33 (−1.70, 1.07) | Low | ||
| Sulpiride – Sarcosine | NA | – | −0.59 (−3.22, 2.05) | Low | −0.59 (−3.22, 2.05) | Low | ||
| Topiramate – Sarcosine | NA | – | −0.18 (−2.85, 2.46) | Low | −0.18 (−2.85, 2.46) | Low | ||
| Ziprasidone – Sarcosine | NA | – | −0.51 (−2.87, 1.83) | Low | −0.51 (−2.87, 1.83) | Low | ||
| Topiramate – Sulpiride | NA | – | 0.40 (−2.19, 3.05) | Low | 0.40 (−2.19, 3.05) | Low | ||
| Ziprasidone – Sulpiride | NA | – | 0.08 (−2.23, 2.40) | Low | 0.08 (−2.23, 2.40) | Low | ||
| Ziprasidone – Topiramate | NA | – | −0.32 (−2.62, 2.00) | Low | −0.32 (−2.62, 2.00) | Low | ||
CI, confidence interval; CrI, credible intervals; NE, not estimable; NA, not applicable.
Table 5.
NMA results sorted based on GRADE certainty of evidence for the comparisons of active augmentation strategies versus placebo for Schizophrenia refractory/partially responding to clozapine
| Outcome | Certainty of evidence | Classification | Intervention | MD (95% CrI) |
|---|---|---|---|---|
| Reduction in PANSS/BPRS | High (high and moderate) | Amongst the most effective | Mirtazapine | −5.2 (−7.7, −2.7) |
| Memantine | −2.1 (−4.0, −0.19) | |||
| Amongst the least effective | Glycine | −0.07 (−1.89, 1.78) | ||
| Low (low and very low) | May be more effective than placebo | None | ||
NMA, network meta-analysis; PANSS, positive and negative syndrome scale; BPRS, brief psychiatric rating scale; CI, confidence interval; CrI, credible intervals; MD, mean difference.
Safety Evaluation
The safety concerns and safety evaluation parameters were different in each study. There was only one study each per comparison of mirtazapine and memantine to placebo. These studies used different tools to report the adverse event as per the drug-specific safety profile, and thus quantitative analysis was not feasible. A few patients in the mirtazapine group experienced mild drowsiness and weight gain. Three patients receiving a placebo and one receiving memantine complained of dizziness and nausea, while there was no significant difference in extrapyramidal adverse effects and weight gain. All the drugs evaluated in the articles included in this meta-analysis were well tolerated and did not show any significant increase in adverse events when compared to the placebo. The effect could not be pooled in the absence of a uniform tool across the studies.
DISCUSSION
This network meta-analysis summarizes the effect of interventions employed in 30 RCTs on the reduction in symptom severity scores in patients with schizophrenia who were partial/non-responders to clozapine therapy and were started on pharmacological augmentation. Aug-mentation of clozapine with mirtazapine and memantine proves to be the most efficacious in patients as per the consistency model of this NMA. It was observed that improvement in symptoms was directly influenced positively by the duration of therapy. The agents were well tolerated with a good safety profile when compared to the placebo.
These agents act through different mechanisms and may provide potential therapeutic options based on the patient’s characteristics. It seems possible that a combination of clozapine and mirtazapine exerts a potentiating synergistic action on multiple receptor subtypes and on the neurotransmission system involved in the aetiopathogenesis of resistant symptoms in schizophrenia [40]. Chronic treatment with clozapine has the property of elevating the expression of mGlu5 receptors, which improves glutamatergic transmission by modelling the N-methyl-D-aspartate (NMDA) glutamatergic system [49]. Memantine is a low-affinity uncompetitive NMDA receptor antagonist which blocks activity specifically at pathological receptors. The drug may play an important role in the improvement of symptoms in this glutamatergic environment when administered in combination with clozapine [50]. Duloxetine is a potent inhibitor of the reuptake of serotonin and noradrenaline and shows balanced affinity and high selectivity for 5HT transporters [30]. The drug may possess pharmacodynamic synergism with clozapine, and thus a beneficial effect is observed in augmentation therapy. Sulpiride augmentation modulates the interactions between serotonin and dopamine neurotransmitters to achieve a moderate 5HT/D2 ratio, which may be possible for enhanced clinical efficacy when given for adequate duration [34]. Cariprazine, a newer atypical antipsychotic drug, may offer a better tolerated and more acceptable treatment option for partial/non-responders to clozapine, however, presently, no published radomized controlled trial available [51].
This network meta-analysis seemed essential to draw conclusions for therapeutic decisions from all available relevant evidence. As the source of data for result synthesis depends on both direct and indirect evidence, the relative effects obtained for each one of the interventions with respect to the other is interpretable, more robust and reliable. Clozapine is the only approved drug for treatment-resistant schizophrenia, and with only one-third to one-half of the patients responding to the drug, evaluating adjunctive therapy to provide an effective alternative to patients is crucial. None of the antipsychotic augmentation strategies with clozapine outperformed the controls, according to a meta-analysis by Correll et al. [52]. In another study by Galling et al. [53] it was observed that regardless of whether studies used clozapine or not, there is no proof that antipsychotic augmentation above monotherapy had any further benefits. Thus, augmentation agents involving modulation of another neurotransmission systems like glutamate and serotonin as observed in the results of this meta-analysis would be beneficial. This can be reiterated here that negative and cognitive domains of schizophrenia are the most resistant domains of psychopathology to be addressed with the adjunctive therapies, which could be better managed by targeting serotonin and glutamatergic systems [54].
A network meta-analysis by Yeh et al. [55] in patients with clozapine-resistant schizophrenia found that mirtazapine, duloxetine, and memantine were the most efficacious pharmacological augmentation agents, while duloxetine was not found to be more efficacious than placebo. However, the above-cited study was conducted using a frequentist approach which does not provide ample flexibility in analysis. Markov Chain Monte Carlo simulations used in Bayesian analysis allow realistic models to be fitted in complex datasets. It gives a more principled way of easily combining prior knowledge with available data within the realm of a solid decision and theoretical framework, which is not possible with a frequentist approach. Secondly, our NMA includes certainty of the evidence for the comparisons between the treatment, which would help in building confidence in the therapeutic agent.
The demographics of the patients included in our network meta-analysis were like a meta-analysis conducted on ultra-resistant patients [56]. Tiihonen et al. [57] conducted a meta-analysis including four studies comparing lamotrigine with a placebo and found lamotrigine has higher efficacy than a placebo in clozapine-resistant schizo-phrenia. However, on further exploring the articles included in this meta-analysis, the inclusion does not seem to be restricted to clozapine resistance. The review authors have focussed on an add-on lamotrigine therapy but they have included studies with patients on other conventional and atypical antipsychotics apart from clozapine. This may be the reason for contradicting findings between this meta-analysis and this NMA showing contrasting results as our NMA concludes NMA, as our results conclude lamotrigine to be no better than placebo. Similarly, a pairwise meta-analysis by Siskind et al. [58] showed interventions like aripiprazole, fluoxetine, and sodium valproate to be most effective, but the inclusion criteria were not confined according to response to clozapine i.e., patients receiving clozapine for the first time were included along with partial responders and clozapine refractory patients. This meta-analysis did not confine inclusion criteria to patients with schizophrenia but in addition included schizoaffective disorder and psychosis spectrum disorders as well. In addition, the two studies mentioned above are pairwise meta-analyses whereas our study is a network meta-analysis that ranks treatments based on their SUCRA scores along with the calculation of the relative effect of each intervention tested against all others.
The inclusion of more severe patients in medication trials and the increased likelihood of bias in psychotherapy research were the two main differences discovered between the two categories of studies according to a report by Bighelli et al. [59]. These variations suggest that before considering a network meta-analysis, study and patient characteristics should be carefully taken into account. Thus, we did not include psychological interventions in this analysis.
In our NMA, ranking results and relative effects estimates indicate towards same pharmacological agents for improvement in symptoms in comparison to placebo. This allows us to interpret results with more confidence. Furthermore, there was coherence between the direct and indirect evidence synthesized in this NMA, thus fulfilling the criteria of transitivity assumption.
There are certain limitations to this analysis. Safety reporting equipment was different across the studies, and thus we could not do a quantitative analysis of the safety data. Second, the number of studies per comparison is limited to one for most of the comparisons, and the number of participants per study is very small for most of the studies. However, using the Bayesian approach using non-informative priors in this situation makes the interpretation of results reliable and straightforward, which is the main strength of our study.
In conclusion, our findings indicate that mirtazapine and memantine are the two best augmentation agents in schizophrenia patients who are partial/non-responders to clozapine therapy. However, studies with large sample sizes are warranted to enhance the generalizability of the findings.
Footnotes
Funding
None.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Literature search: Archana Mishra. Study screening and selection: Archana Mishra, Rituparna Maiti, Biswa Ranjan Mishra, Anand Srinivasan. Data extraction and management: Archana Mishra, Rituparna Maiti, Biswa Ranjan Mishra, Anand Srinivasan. Data analysis and interpretation of data: Archana Mishra. Drafting of the manuscript: Archana Mishra, Rituparna Maiti. Concept and design: Rituparna Maiti, Anand Srinivasan. Critical revision of the manuscript for important intellectual content: Biswa Ranjan Mishra, Anand Srinivasan. Final approval of the manuscript: Archana Mishra, Rituparna Maiti, Biswa Ranjan Mishra, Anand Srinivasan.
References
- 1.Lao KSJ, Tam AWY, Wong ICK, Besag FMC, Man KKC, Chui CSL, et al. Prescribing trends and indications of antipsychotic medication in Hong Kong from 2004 to 2014: general and vulnerable patient groups. Pharmacoepidemiol Drug Saf. 2017;26:1387–1394. doi: 10.1002/pds.4244. [DOI] [PubMed] [Google Scholar]
- 2.Kane JM, Correll CU. The role of clozapine in treatment-resistant schizophrenia. JAMA Psychiatry. 2016;73:187–188. doi: 10.1001/jamapsychiatry.2015.2966. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman JA, Safferman AZ, Pollack S, Szymanski S, Johns C, Howard A, et al. Clinical effects of clozapine in chronic schizophrenia: response to treatment and predictors of outcome. Am J Psychiatry. 1994;151:1744–1752. doi: 10.1176/ajp.151.12.1744. [DOI] [PubMed] [Google Scholar]
- 4.Meltzer HY. Treatment-resistant schizophrenia--the role of clozapine. Curr Med Res Opin. 1997;14:1–20. doi: 10.1185/03007999709113338. [DOI] [PubMed] [Google Scholar]
- 5.Kapur S, Zipursky RB, Remington G. Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry. 1999;156:286–293. doi: 10.1176/ajp.156.2.286. [DOI] [PubMed] [Google Scholar]
- 6.Davis J, Moylan S, Harvey BH, Maes M, Berk M. Neuropro-gression in schizophrenia: pathways underpinning clinical staging and therapeutic corollaries. Aust N Z J Psychiatry. 2014;48:512–529. doi: 10.1177/0004867414533012. [DOI] [PubMed] [Google Scholar]
- 7.Sernyak MJ, Rosenheck R. Clinicians' reasons for antipsychotic coprescribing. J Clin Psychiatry. 2004;65:1597–1600. doi: 10.4088/JCP.v65n1203. [DOI] [PubMed] [Google Scholar]
- 8.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 10.PlotDigitizer [Internet] Porbital; [cited at 2023 May 21]. https://plotdigitizer.com/ [Google Scholar]
- 11.van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, Welton NJ. Gemtc: network meta-analysis using bayesian methods [Internet] 2023. [cited at 2023 May 21]. https://CRAN.R-project.org/package=gemtc.
- 12.Higgins JP, Altman DG, Gøtzsche PC, Moher D, Jüni P, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anil Yağcioğlu AE, Kivircik Akdede BB, Turgut TI, Yazici MK, Tümüklü M, Alptekin K, et al. A double-blind controlled study of adjunctive treatment with risperidone in schizophrenic patients partially responsive to clozapine: efficacy and safety. J Clin Psychiatry. 2005;66:63–72. doi: 10.4088/JCP.v66n0109. [DOI] [PubMed] [Google Scholar]
- 14.Assion HJ, Reinbold H, Lemanski S, Basilowski M, Juckel G. Amisulpride augmentation in patients with schizophrenia partially responsive or unresponsive to clozapine. A randomized, double-blind, placebo-controlled trial. Pharmacopsychiatry. 2008;41:24–28. doi: 10.1055/s-2007-993209. [DOI] [PubMed] [Google Scholar]
- 15.Barbui C, Accordini S, Nosè M, Stroup S, Purgato M, Girlanda F, et al. Aripiprazole versus haloperidol in combination with clozapine for treatment-resistant schizophrenia in routine clinical care: A randomized, controlled trial. J Clin Psycho-pharmacol. 2011;31:266–273. doi: 10.1097/JCP.0b013e318219cba3. [DOI] [PubMed] [Google Scholar]
- 16.Barnes TR, Leeson VC, Paton C, Marston L, Davies L, Whittaker W, et al. Amisulpride augmentation in clozapine-unrespon-sive schizophrenia (AMICUS): a double-blind, placebo-controlled, randomised trial of clinical effectiveness and cost-effectiveness. Health Technol Assess. 2017;21:1–56. doi: 10.3310/hta21490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang JS, Ahn YM, Park HJ, Lee KY, Kim SH, Kang UG, et al. Aripiprazole augmentation in clozapine-treated patients with refractory schizophrenia: an 8-week, randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69:720–731. doi: 10.4088/JCP.v69n0505. [DOI] [PubMed] [Google Scholar]
- 18.de Lucena D, Fernandes BS, Berk M, Dodd S, Medeiros DW, Pedrini M, et al. Improvement of negative and positive symptoms in treatment-refractory schizophrenia: a double-blind, randomized, placebo-controlled trial with memantine as add-on therapy to clozapine. J Clin Psychiatry. 2009;70:1416–1423. doi: 10.4088/JCP.08m04935gry. Erratum in: J Clin Psychiatry 2011;72:1157. [DOI] [PubMed] [Google Scholar]
- 19.Doruk A, Uzun O, Ozşahin A. A placebo-controlled study of extract of ginkgo biloba added to clozapine in patients with treatment-resistant schizophrenia. Int Clin Psychopharmacol. 2008;23:223–227. doi: 10.1097/YIC.0b013e3282fcff2f. [DOI] [PubMed] [Google Scholar]
- 20.Freudenreich O, Henderson DC, Walsh JP, Culhane MA, Goff DC. Risperidone augmentation for schizophrenia partially responsive to clozapine: a double-blind, placebo-controlled trial. Schizophr Res. 2007;92:90–94. doi: 10.1016/j.schres.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 21.Friedman JI, Lindenmayer JP, Alcantara F, Bowler S, Parak M, White L, et al. Pimozide augmentation of clozapine inpatients with schizophrenia and schizoaffective disorder unrespon-sive to clozapine monotherapy. Neuropsychopharmacology. 2011;36:1289–1295. doi: 10.1038/npp.2011.14. Erratum in: Neuropsycho-pharmacology 2011;36:1317. Erratum in: Neuropsychophar-macology 2011;36:2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Genç Y, Taner E, Candansayar S. Comparison of clozapine-amisulpride and clozapine-quetiapine combinations for patients with schizophrenia who are partially responsive to clozapine: a single-blind randomized study. Adv Ther. 2007;24:1–13. doi: 10.1007/BF02849987. [DOI] [PubMed] [Google Scholar]
- 23.Gunduz-Bruce H, Oliver S, Gueorguieva R, Forselius-Bielen K, D'Souza DC, Zimolo Z, et al. Efficacy of pimozide augmentation for clozapine partial responders with schizophrenia. Schizophr Res. 2013;143:344–347. doi: 10.1016/j.schres.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Honer WG, Thornton AE, Chen EY, Chan RC, Wong JO, Bergmann A, et al. Clozapine alone versus clozapine and risperidone with refractory schizophrenia. N Engl J Med. 2006;354:472–482. doi: 10.1056/NEJMoa053222. [DOI] [PubMed] [Google Scholar]
- 25.Josiassen RC, Joseph A, Kohegyi E, Stokes S, Dadvand M, Paing WW, et al. Clozapine augmented with risperidone in the treatment of schizophrenia: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2005;162:130–136. doi: 10.1176/appi.ajp.162.1.130. [DOI] [PubMed] [Google Scholar]
- 26.Kelly DL, Sullivan KM, McEvoy JP, McMahon RP, Wehring HJ, Gold JM, et al. Adjunctive minocycline in clozapine-treated schizophrenia patients with persistent symptoms. J Clin Psychopharmacol. 2015;35:374–381. doi: 10.1097/JCP.0000000000000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lane HY, Huang CL, Wu PL, Liu YC, Chang YC, Lin PY, et al. Glycine transporter I inhibitor, N-methylglycine (sarcosine), added to clozapine for the treatment of schizophrenia. Biol Psychiatry. 2006;60:645–649. doi: 10.1016/j.biopsych.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Lin CH, Lin CH, Chang YC, Huang YJ, Chen PW, Yang HT, et al. Sodium benzoate, a D-amino acid oxidase inhibitor, added to clozapine for the treatment of schizophrenia: a randomized, double-blind, placebo-controlled trial. Biol Psychiatry. 2018;84:422–432. doi: 10.1016/j.biopsych.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Lu ML, Chen TT, Kuo PH, Hsu CC, Chen CH. Effects of adjunctive fluvoxamine on metabolic parameters and psychopathology in clozapine-treated patients with schizophrenia: a 12-week, randomized, double-blind, placebo-controlled study. Schizophr Res. 2018;193:126–133. doi: 10.1016/j.schres.2017.06.030. [DOI] [PubMed] [Google Scholar]
- 30.Mico' U, Bruno A, Pandolfo G, Maria Romeo V, Mallamace D, D'Arrigo C, et al. Duloxetine as adjunctive treatment to clozapine in patients with schizophrenia: a randomized, placebo-controlled trial. Int Clin Psychopharmacol. 2011;26:303–310. doi: 10.1097/YIC.0b013e32834bbc0d. [DOI] [PubMed] [Google Scholar]
- 31.Muscatello MR, Bruno A, Pandolfo G, Micò U, Bellinghieri PM, Scimeca G, et al. Topiramate augmentation of clozapine in schizophrenia: a double-blind, placebo-controlled study. J Psychopharmacol. 2011;25:667–674. doi: 10.1177/0269881110372548. [DOI] [PubMed] [Google Scholar]
- 32.Muscatello MR, Bruno A, Pandolfo G, Micò U, Scimeca G, Di Nardo F, et al. Effect of aripiprazole augmentation of clozapine in schizophrenia: a double-blind, placebo-controlled study. Schizophr Res. 2011;127:93–99. doi: 10.1016/j.schres.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 33.Muscatello MR, Pandolfo G, Micò U, Lamberti Castronuovo E, Abenavoli E, Scimeca G, et al. Augmentation of clozapine with ziprasidone in refractory schizophrenia: a double-blind, placebo-controlled study. J Clin Psychopharmacol. 2014;34:129–133. doi: 10.1097/JCP.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 34.Shiloh R, Zemishlany Z, Aizenberg D, Radwan M, Schwartz B, Dorfman-Etrog P, et al. Sulpiride augmentation in people with schizophrenia partially responsive to clozapine. A double-blind, placebo-controlled study. Br J Psychiatry. 1997;171:569–573. doi: 10.1192/bjp.171.6.569. [DOI] [PubMed] [Google Scholar]
- 35.Tiihonen J, Hallikainen T, Ryynänen OP, Repo-Tiihonen E, Kotilainen I, Eronen M, et al. Lamotrigine in treatment-resistant schizophrenia: a randomized placebo-controlled crossover trial. Biol Psychiatry. 2003;54:1241–1248. doi: 10.1016/S0006-3223(03)00524-9. [DOI] [PubMed] [Google Scholar]
- 36.Vayısoğlu S, Anıl Yağcıoğlu AE, Yağcıoğlu S, Karahan S, Karcı O, Gürel SC, et al. Lamotrigine augmentation in patients with schizophrenia who show partial response to clozapine treatment. Schizophr Res. 2013;143:207–214. doi: 10.1016/j.schres.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 37.Weiner E, Conley RR, Ball MP, Feldman S, Gold JM, Kelly DL, et al. Adjunctive risperidone for partially responsive people with schizophrenia treated with clozapine. Neuropsycho-pharmacology. 2010;35:2274–2283. doi: 10.1038/npp.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu MH, Liu ZJ, Hu QY, Yang JY, Jin Y, Zhu N, et al. Amisulpride augmentation therapy improves cognitive performance and psychopathology in clozapine-resistant treatment-refractory schizophrenia: a 12-week randomized, double-blind, placebo-controlled trial. Mil Med Res. 2022;9:59. doi: 10.1186/s40779-022-00420-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zink M, Kuwilsky A, Krumm B, Dressing H. Efficacy and tolerability of ziprasidone versus risperidone as augmentation in patients partially responsive to clozapine: a randomised controlled clinical trial. J Psychopharmacol. 2009;23:305–314. doi: 10.1177/0269881108089593. [DOI] [PubMed] [Google Scholar]
- 40.Zoccali R, Muscatello MR, Cedro C, Neri P, La Torre D, Spina E, et al. The effect of mirtazapine augmentation of clozapine in the treatment of negative symptoms of schizophrenia: a double-blind, placebo-controlled study. Int Clin Psychophar-macol. 2004;19:71–76. doi: 10.1097/00004850-200403000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Zoccali R, Muscatello MR, Bruno A, Cambria R, Micò U, Spina E, et al. The effect of lamotrigine augmentation of clozapine in a sample of treatment-resistant schizophrenic patients: a double-blind, placebo-controlled study. Schizophr Res. 2007;93:109–116. doi: 10.1016/j.schres.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 42.Evins AE, Fitzgerald SM, Wine L, Rosselli R, Goff DC. Placebo-controlled trial of glycine added to clozapine in schizo-phrenia. Am J Psychiatry. 2000;157:826–828. doi: 10.1176/appi.ajp.157.5.826. [DOI] [PubMed] [Google Scholar]
- 43.Nielsen J, Emborg C, Gydesen S, Dybbro J, Aagaard J, Haderup K, et al. Augmenting clozapine with sertindole: a double-blind, randomized, placebo-controlled study. J Clin Psycho-pharmacol. 2012;32:173–178. doi: 10.1097/JCP.0b013e318248dfb8. [DOI] [PubMed] [Google Scholar]
- 44.Buchanan RW, Kirkpatrick B, Bryant N, Ball P, Breier A. Fluoxetine augmentation of clozapine treatment in patients with schizophrenia. Am J Psychiatry. 1996;153:1625–1627. doi: 10.1176/ajp.153.12.1625. [DOI] [PubMed] [Google Scholar]
- 45.Fleischhacker WW, Heikkinen ME, Landsberg W, Olié JP, Dewaele P, McQuade RD, et al. Effects of adjunctive treatment with aripiprazole on body weight and clinical efficacy in schizophrenia patients treated with clozapine: a randomized, double-blind, placebo-controlled trial. Int J Neuropsycho-pharmacol. 2010;13:1115–1125. doi: 10.1017/S1461145710000490. [DOI] [PubMed] [Google Scholar]
- 46.Tsai GE, Yang P, Chung LC, Tsai IC, Tsai CW, Coyle JT. D-serine added to clozapine for the treatment of schizophrenia. Am J Psychiatry. 1999;156:1822–1825. doi: 10.1176/ajp.156.11.1822. [DOI] [PubMed] [Google Scholar]
- 47.Goff DC, Henderson DC, Evins AE, Amico E. A placebo-controlled crossover trial of D-cycloserine added to clozapine in patients with schizophrenia. Biol Psychiatry. 1999;45:512–514. doi: 10.1016/S0006-3223(98)00367-9. [DOI] [PubMed] [Google Scholar]
- 48.Goff DC, Leahy L, Berman I, Posever T, Herz L, Leon AC, et al. A placebo-controlled pilot study of the ampakine CX516 added to clozapine in schizophrenia. J Clin Psychopharmacol. 2001;21:484–487. doi: 10.1097/00004714-200110000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Gray L, van den Buuse M, Scarr E, Dean B, Hannan AJ. Clozapine reverses schizophrenia-related behaviours in the metabotropic glutamate receptor 5 knockout mouse: association with N-methyl-D-aspartic acid receptor up-regulation. Int J Neuropsychopharmacol. 2009;12:45–60. doi: 10.1017/S1461145708009085. [DOI] [PubMed] [Google Scholar]
- 50.Lieberman JA, Papadakis K, Csernansky J, Litman R, Volavka J, Jia XD, et al. A randomized, placebo-controlled study of memantine as adjunctive treatment in patients with schizo-phrenia. Neuropsychopharmacology. 2009;34:1322–1329. doi: 10.1038/npp.2008.200. [DOI] [PubMed] [Google Scholar]
- 51.Montgomery A, Rogowska M, Dratcu L. Cariprazine - an alternative treatment for clozapine-resistant schizophrenia? Clin Psychopharmacol Neurosci. 2023;21:202–206. doi: 10.9758/cpn.2023.21.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Correll CU, Rubio JM, Inczedy-Farkas G, Birnbaum ML, Kane JM, Leucht S. Efficacy of 42 pharmacologic cotreatment strategies added to antipsychotic monotherapy in schizophrenia: systematic overview and quality appraisal of the meta-analytic evidence. JAMA Psychiatry. 2017;74:675–684. doi: 10.1001/jamapsychiatry.2017.0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Galling B, Roldán A, Hagi K, Rietschel L, Walyzada F, Zheng W, et al. Antipsychotic augmentation vs. monotherapy in schizophrenia: systematic review, meta-analysis and meta-regression analysis. World Psychiatry. 2017;16:77–89. doi: 10.1002/wps.20387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nucifora FC, Jr, Woznica E, Lee BJ, Cascella N, Sawa A. Treatment resistant schizophrenia: clinical, biological, and therapeutic perspectives. Neurobiol Dis. 2019;131:104257. doi: 10.1016/j.nbd.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yeh TC, Correll CU, Yang FC, Chen MH, Tseng PT, Hsu CW, et al. Pharmacological and nonpharmacological augmentation treatments for clozapine-resistant schizophrenia: a systematic review and network meta-analysis with normalized entropy assessment. Asian J Psychiatr. 2023;79:103375. doi: 10.1016/j.ajp.2022.103375. [DOI] [PubMed] [Google Scholar]
- 56.Campana M, Falkai P, Siskind D, Hasan A, Wagner E. Charac-teristics and definitions of ultra-treatment-resistant schizophrenia - A systematic review and meta-analysis. Schizophr Res. 2021;228:218–226. doi: 10.1016/j.schres.2020.12.002. [DOI] [PubMed] [Google Scholar]
- 57.Tiihonen J, Wahlbeck K, Kiviniemi V. The efficacy of lamotrigine in clozapine-resistant schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2009;109:10–14. doi: 10.1016/j.schres.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 58.Siskind DJ, Lee M, Ravindran A, Zhang Q, Ma E, Motamarri B, et al. Augmentation strategies for clozapine refractory schizophrenia: a systematic review and meta-analysis. Aust N Z J Psychiatry. 2018;52:751–767. doi: 10.1177/0004867418772351. [DOI] [PubMed] [Google Scholar]
- 59.Bighelli I, Leucht C, Huhn M, Reitmeir C, Schwermann F, Wallis S, et al. Are randomized controlled trials on pharmacotherapy and psychotherapy for positive symptoms of schizophrenia comparable? A systematic review of patient and study characteristics. Schizophr Bull. 2020;46:496–504. doi: 10.1093/schbul/sbz090. [DOI] [PMC free article] [PubMed] [Google Scholar]