ABSTRACT
Background
The European Renal Association (ERA) Registry collects data on kidney replacement therapy (KRT) in patients with end-stage kidney disease (ESKD). This paper is a summary of the ERA Registry Annual Report 2021, including a comparison across treatment modalities.
Methods
Data was collected from 54 national and regional registries from 36 countries, of which 35 registries from 18 countries contributed individual patient data and 19 registries from 19 countries contributed aggregated data. Using this data, incidence and prevalence of KRT, kidney transplantation rates, survival probabilities and expected remaining lifetimes were calculated.
Result
In 2021, 533.2 million people in the general population were covered by the ERA Registry. The incidence of KRT was 145 per million population (pmp). In incident patients, 55% were 65 years or older, 64% were male, and the most common primary renal disease (PRD) was diabetes (22%). The prevalence of KRT was 1040 pmp. In prevalent patients, 47% were 65 years or older, 62% were male, and the most common PRDs were diabetes and glomerulonephritis/sclerosis (both 16%). On 31 December 2021, 56% of patients received haemodialysis, 5% received peritoneal dialysis, and 39% were living with a functioning graft. The kidney transplantation rate in 2021 was 37 pmp, a majority coming from deceased donors (66%). For patients initiating KRT between 2012–2016, 5-year survival probability was 52%. Compared to the general population, life expectancy was 65% and 68% shorter for males and females receiving dialysis, and 40% and 43% shorter for males and females living with a functioning graft.
Keywords: dialysis, ESKD, graft survival, kidney transplantation, patient survival
Graphical Abstract
Graphical Abstract.
INTRODUCTION
The European Renal Association (ERA) Registry Annual Report 2021 (Supplementary information) reports the latest data on the epidemiology of kidney replacement therapy (KRT) for patients with end-stage kidney disease (ESKD) in Europe and countries bordering the Mediterranean Sea. Data was provided by 54 national or regional renal registries from 36 countries, of which 35 registries from 18 countries contributed individual patient data and 19 registries from 19 countries contributed aggregated data (Appendix 1). The participating registries covered approximately 533.2 million people, corresponding to 61.4% of the total European population for incident results and 61.9% for prevalent results (due to a difference in available data for the Netherlands, Cyprus, and Hungary). The percentage coverage for this year was lower than the 76.8% covered in the 2020 Annual Report [1]. Compared to last year, aggregated data from Ukraine, Latvia, and Tunisia was included while data from Russia could not be included.
This paper summarizes data from 2021 on the incidence and prevalence of KRT, kidney transplantation rates, and patient and graft survival. In addition, we will also present comparisons across treatment modalities [haemodialysis (HD), peritoneal dialysis (PD), and kidney transplantation (Tx)]. More detailed results and further information on the methodology used in analyses can be found in the ERA Registry 2021 Annual Report (Supplementary information).
RESULTS
KRT incidence
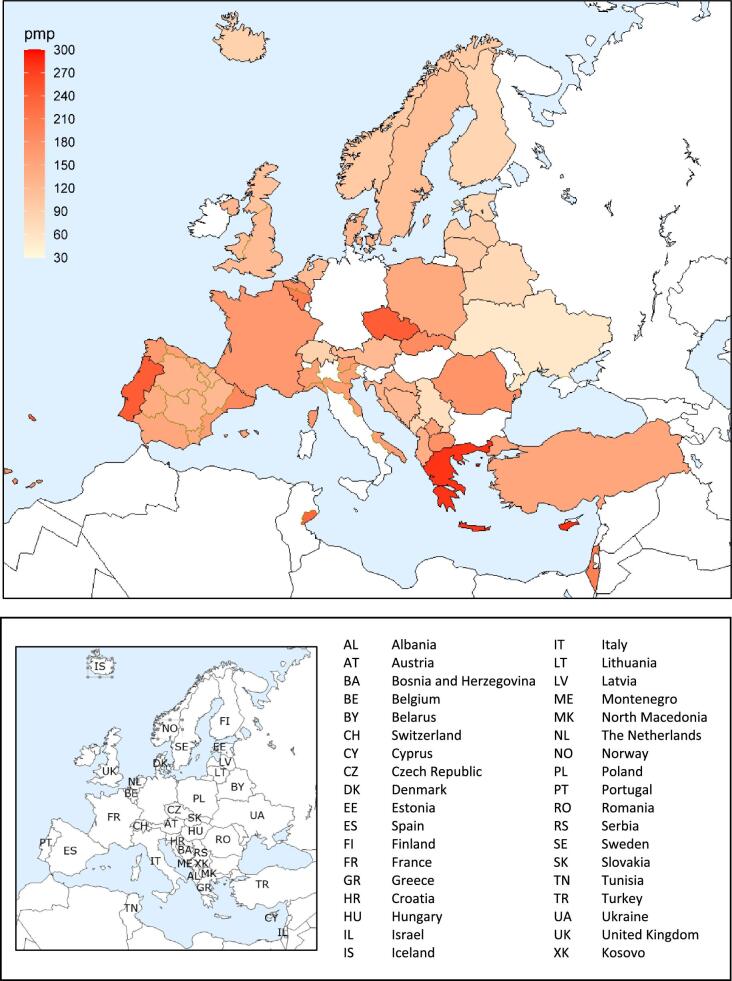
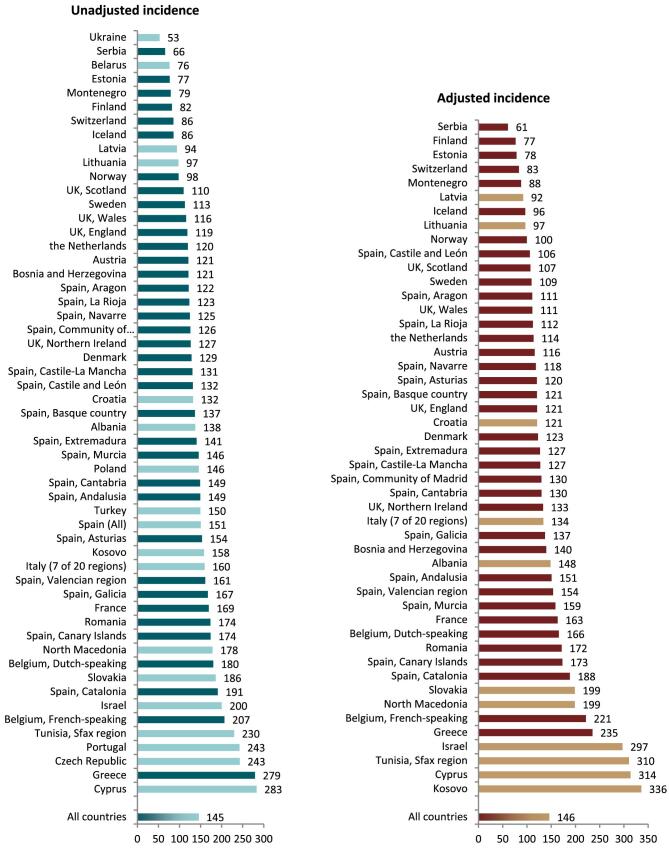
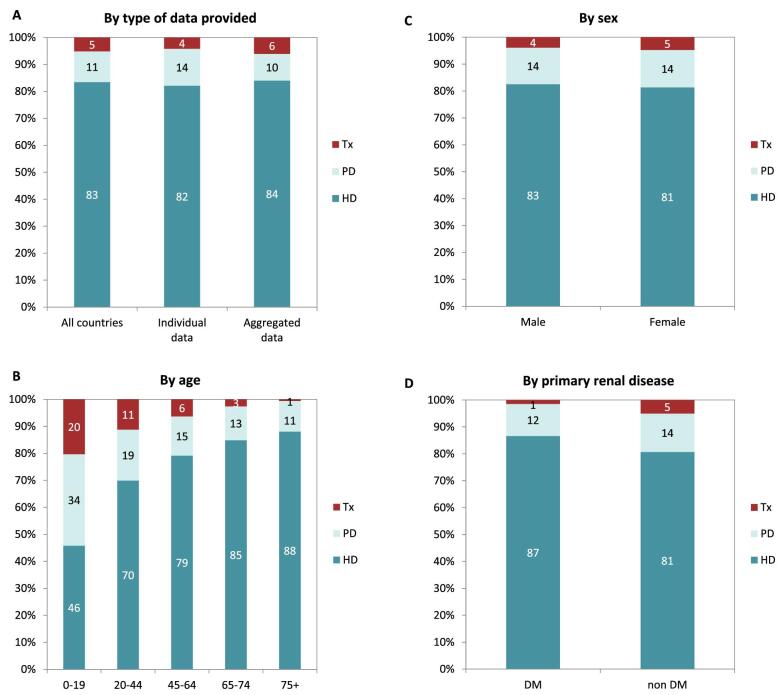
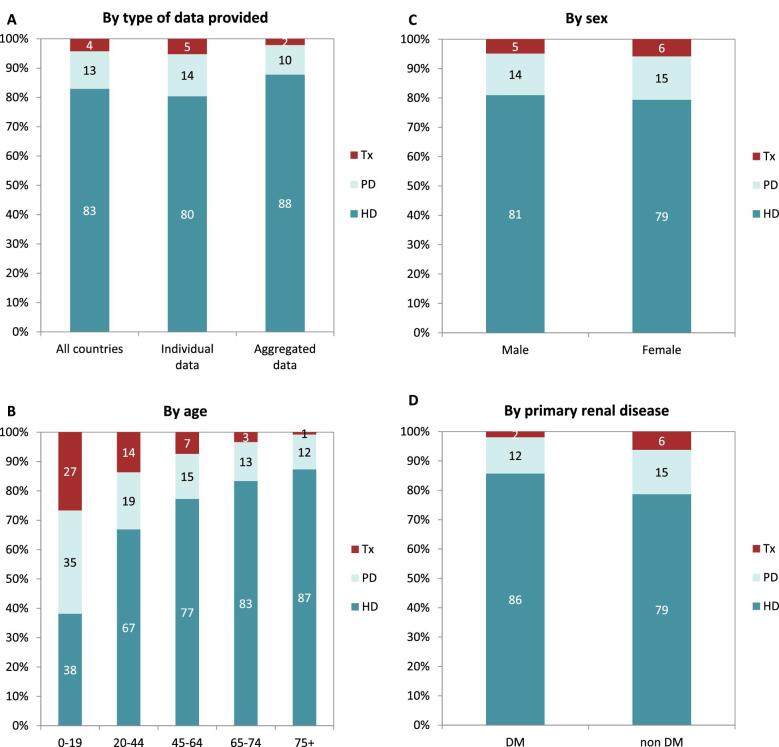
In 2021, 76 240 patients with ESKD out of a population of 525 million people initiated KRT (Table 1). This corresponds to an unadjusted KRT incidence rate of 145 per million population (pmp) or 1 in 6900 Europeans (Table 1), which was higher than the KRT incidence rate of 128 pmp in 2020 [1]. The unadjusted incidence rate ranged from 53 pmp in Ukraine (1 in 18 900 inhabitants) and 66 pmp in Serbia (1 in 15 200 inhabitants) to 279 in Greece and 283 pmp in Cyprus (approximately 1 in 3500 inhabitants, Table 1 and Figs 1 and 2). When adjusted for age and sex using the distribution of the European Union 28 countries (EU28) population [2], the overall adjusted KRT incidence rate for the available 48 countries and regions was 146 pmp or approximately 1 in 6800 Europeans (Fig. 2). The adjusted incidence rate ranged from 61 pmp in Serbia (1 in 16 400 inhabitants) to 336 pmp in Kosovo (1 in 3000 inhabitants, Fig. 2). The median age of patients starting KRT was 68.2 years, ranging from 56.0 years in Ukraine to 74.5 years in Greece (Table 1). In incident patients, 55% were aged 65 years or older, 64% were male, and the most common PRD was diabetes mellitus (DM) (22%, Fig. 3). On the first day of KRT, 83% received HD, 11% received PD, and 5% underwent pre-emptive kidney transplantation, with only minor differences between countries providing individual patient data versus aggregated data (Fig. 4). For countries providing individual patient data, initial treatment modality varied among age categories, with HD increasing in a step-wise manner from 46% HD in the age category 0–19 years to 88% HD in the age category 75 years or older (Fig. 4). On the contrary, PD and transplantation decreased from 34% and 20% in the age category 0–19 years to 11% and 1% in the age category 75 years or older, respectively (Fig. 4). The distribution of treatment modalities was similar for men and women (Fig. 4). Patients with DM as PRD received a pre-emptive kidney transplant less frequently compared to patients with a different PRD (1% versus 5%, Fig. 4). On day 91 after the start of KRT, 83% of all incident patients were receiving HD, 13% were receiving PD, and 4% had undergone kidney transplantation (Fig. 5), which is similar to previous years.
Table 1:
Summary: data on the unadjusted incidence of KRT in 2021 on day 1 by country or region, the mean and median age at the start of KRT, and the incidence of KRT in patients with DM as PRD.
| Incidence of KRT in 2021 on day 1 | |||||||
|---|---|---|---|---|---|---|---|
| Country/region | General population covered by the registry in thousands | All (n) | All (pmp) | Mean age (years) | Median age (years) | DM (n) | DM (pmp) |
| Albania | 2784 | 383 | 138 | 63.6 | 67.0 | 89 | 32 |
| Austriaa | 8825 | 1068 | 121 | 65.7 | 68.4 | 256 | 29 |
| Belarusb | 9256 | 707 | 76 | 147 | 16 | ||
| Belgium, Dutch-speakingc | 6687 | 1205 | 180 | 70.3 | 73.7 | 249 | 37 |
| Belgium, French-speakingc | 4899 | 1013 | 207 | 67.6 | 69.9 | 169 | 34 |
| Bosnia and Herzegovina | 3531 | 428 | 121 | 63.4 | 65.3 | 109 | 31 |
| Croatiad | 3306 | 437 | 132 | 68.8 | 70.0 | 119 | 36 |
| Cyprus | 905 | 256 | 283 | 69.0 | 72.0 | 112 | 124 |
| Czech Republicd | 10 307 | 2505 | 243 | ||||
| Denmark | 5857 | 753 | 129 | 64.2 | 68.4 | 220 | 38 |
| Estonia | 1331 | 102 | 77 | 62.2 | 65.6 | 17 | 13 |
| Finland | 5541 | 455 | 82 | 61.3 | 66.2 | 146 | 26 |
| France | 67 356 | 11 416 | 169 | 67.7 | 71.0 | 2650 | 39 |
| Greece | 10 569 | 2952 | 279 | 72.1 | 74.5 | 660 | 62 |
| Iceland | 373 | 32 | 86 | 57.7 | 67.7 | 7 | 19 |
| Israel | 9372 | 1877 | 200 | 66.5 | 69.8 | 797 | 85 |
| Italy (7 of 20 regions) | 20 658 | 3299 | 160 | 68.8 | 71.8 | 409 | 20 |
| Kosovo | 1688 | 267 | 158 | 62.1 | 64.0 | 91 | 54 |
| Latvia | 1704 | 160 | 94 | 58.6 | 61.0 | 29 | 17 |
| Lithuania | 2811 | 274 | 97 | 60.4 | 62.6 | 46 | 16 |
| Montenegroc | 619 | 49 | 79 | 62.3 | 63.0 | 13 | 21 |
| North Macedonia | 2069 | 369 | 178 | 63.4 | 65.0 | 84 | 41 |
| Norway | 5403 | 530 | 98 | 64.1 | 67.3 | 87 | 16 |
| Poland | 38 162 | 5570 | 146 | 1428 | 37 | ||
| Portugale | 10 348 | 2510 | 243 | 820 | 79 | ||
| Romania | 19 122 | 3319 | 174 | 62.2 | 64.8 | 334 | 17 |
| Serbia | 6493 | 428 | 66 | 59.9 | 64.0 | 68 | 10 |
| Slovakiad | 4452 | 828 | 186 | 63.5 | 66.0 | 285 | 64 |
| Spain (All) | 47 385 | 7135 | 151 | 64.0 | 68.5 | 1766 | 37 |
| Spain, Andalusia | 8511 | 1271 | 149 | 64.8 | 67.8 | 311 | 37 |
| Spain, Aragon | 1323 | 161 | 122 | 65.7 | 68.0 | 56 | 42 |
| Spain, Asturias | 1010 | 155 | 154 | 67.9 | 70.1 | 38 | 38 |
| Spain, Basque country | 2181 | 298 | 137 | 65.9 | 69.4 | 80 | 37 |
| Spain, Canary Islands | 2248 | 391 | 174 | 64.7 | 67.5 | 128 | 57 |
| Spain, Cantabriac | 584 | 87 | 149 | 66.9 | 70.1 | 21 | 36 |
| Spain, Castile and León | 2383 | 314 | 132 | 67.6 | 70.5 | 68 | 29 |
| Spain, Castile-La Manchac | 2049 | 268 | 131 | 67.1 | 68.6 | 74 | 36 |
| Spain, Catalonia | 7763 | 1481 | 191 | 66.6 | 69.6 | 320 | 41 |
| Spain, Community of Madrid | 6751 | 850 | 126 | 64.3 | 66.9 | 236 | 35 |
| Spain, Extremadura | 1060 | 149 | 141 | 66.3 | 69.0 | 35 | 33 |
| Spain, Galicia | 2694 | 451 | 167 | 67.4 | 70.6 | 113 | 42 |
| Spain, La Rioja | 316 | 39 | 123 | 64.0 | 64.1 | 12 | 38 |
| Spain, Murcia | 1518 | 221 | 146 | 64.3 | 67.3 | 55 | 36 |
| Spain, Navarrec | 658 | 82 | 125 | 62.8 | 64.9 | 22 | 33 |
| Spain, Valencian region | 5058 | 816 | 161 | 66.1 | 69.3 | 163 | 32 |
| Sweden | 10 416 | 1175 | 113 | 64.1 | 68.7 | 297 | 29 |
| Switzerland | 8452 | 725 | 86 | 65.7 | 69.2 | 136 | 16 |
| the Netherlands | 16 130 | 1934 | 120 | 63.3 | 66.8 | 404 | 25 |
| Tunisia, Sfax regiond | 1023 | 235 | 230 | 59.8 | 62.0 | 81 | 79 |
| Turkeyf | 84 680 | 12 661 | 150 | 2275 | 50 | ||
| Ukraineb | 26 632 | 1411 | 53 | 54.0 | 56.0 | 289 | 11 |
| UK, England | 55 349 | 6570 | 119 | 60.7 | 63.3 | 1692 | 31 |
| UK, Northern Ireland | 1905 | 241 | 127 | 64.3 | 66.7 | 52 | 27 |
| UK, Scotland | 5480 | 602 | 110 | 60.4 | 63.0 | 172 | 31 |
| UK, Wales | 3105 | 359 | 116 | 60.2 | 63.4 | 112 | 36 |
| All countries | 524 985 | 76 240 | 145 | 65.0 | 68.2 | 16 717 | 36 |
DM, diabetes mellitus; PRD, primary renal disease.
When cells are left empty, the data are unavailable and could not be used for the calculation of the summary data.
aThe incidence is underestimated by approximately 1% due to one haemodialysis centre not submitting data.
bPatients younger than 18 years of age are not reported.
cPatients younger than 20 years of age are not reported.
dData includes dialysis patients only.
eData on primary renal disease are available for dialysis patients only (N = 2495, 99.1% of total).
fData on DM are extrapolated from data of 6774 patients (53.3% of total).
Figure 1:
Incidence per million population of KRT in 2021 on day 1 by country or region, unadjusted.
Figure 2:
Incidence per million population of KRT in 2021 on day 1 by country or region, unadjusted (left panel) and adjusted (right panel). Registries providing individual patient data are shown as dark bars and registries providing aggregated data as light bars. See Appendix 1 for a list of countries and regions providing individual patient or aggregated data. Adjustment was performed by standardizing the incidence to the age and sex distribution of the EU28 population.
Figure 3:
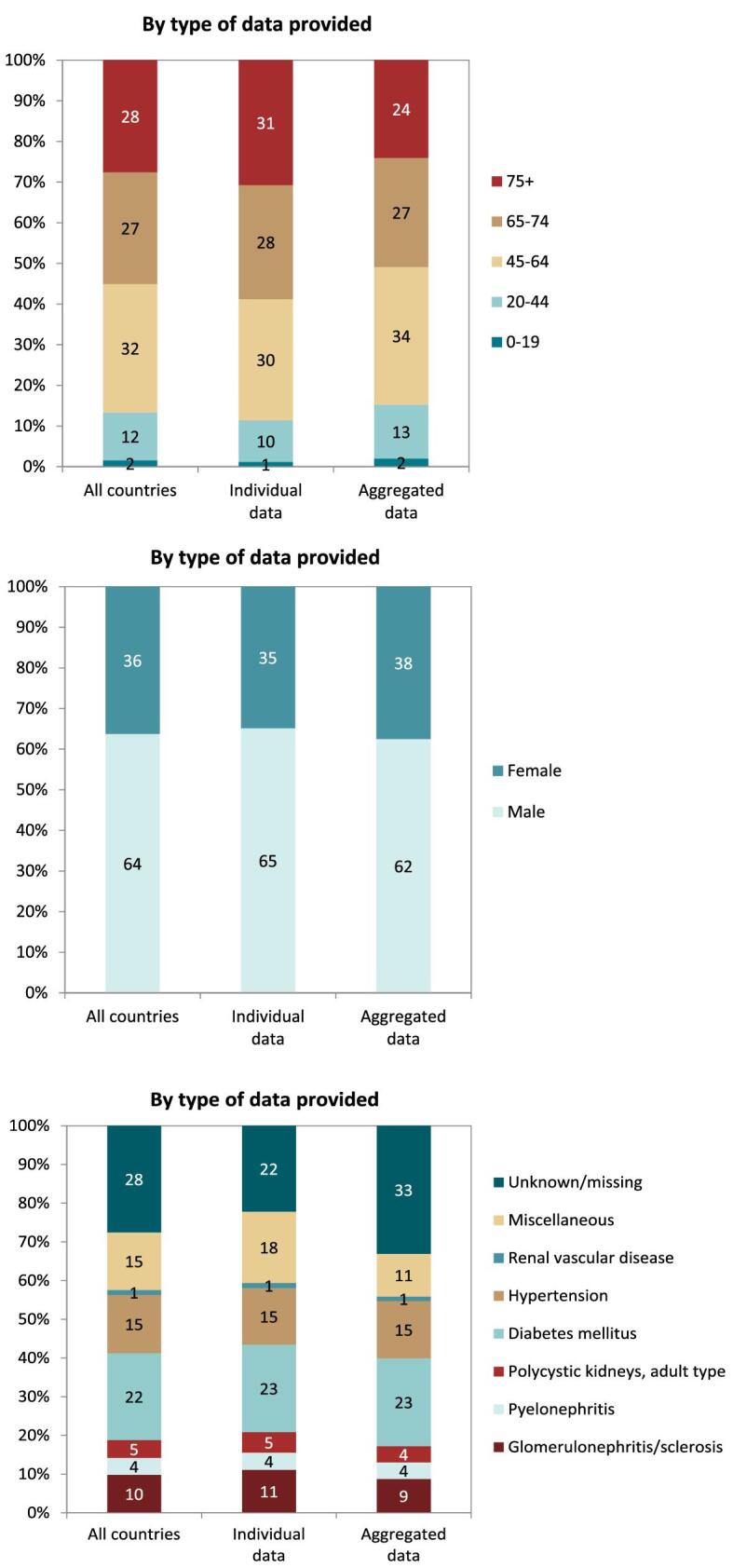
Distribution of (A) age, (B) sex, and (C) primary renal disease by type of data provided for incident patients accepted for KRT in 2021 on day 1, unadjusted.
Figure 4:
Distribution of treatment modality by (A) type of data provided, (B) age, (C) sex, and (D) primary renal disease (DM and non-DM) for incident patients accepted for KRT in 2021 on day 1, unadjusted. Panels (B), (C), and (D) are only based on the data from registries providing individual patient data. See Appendix 1 for a list of countries and regions providing individual patient or aggregated data. HD: haemodialysis; PD: peritoneal dialysis; Tx: transplant.
Figure 5:
Distribution of treatment modality by (A) type of data provided, (B) age, (C) sex, and (D) primary renal disease (DM and non-DM) for incident patients accepted for KRT in 2021 on day 91, unadjusted. Panels (B), (C), and (D) are only based on the data from registries providing individual patient data. See Appendix 1 for a list of countries and regions providing individual patient or aggregated data. HD: haemodialysis; PD: peritoneal dialysis; Tx: transplant.
KRT prevalence
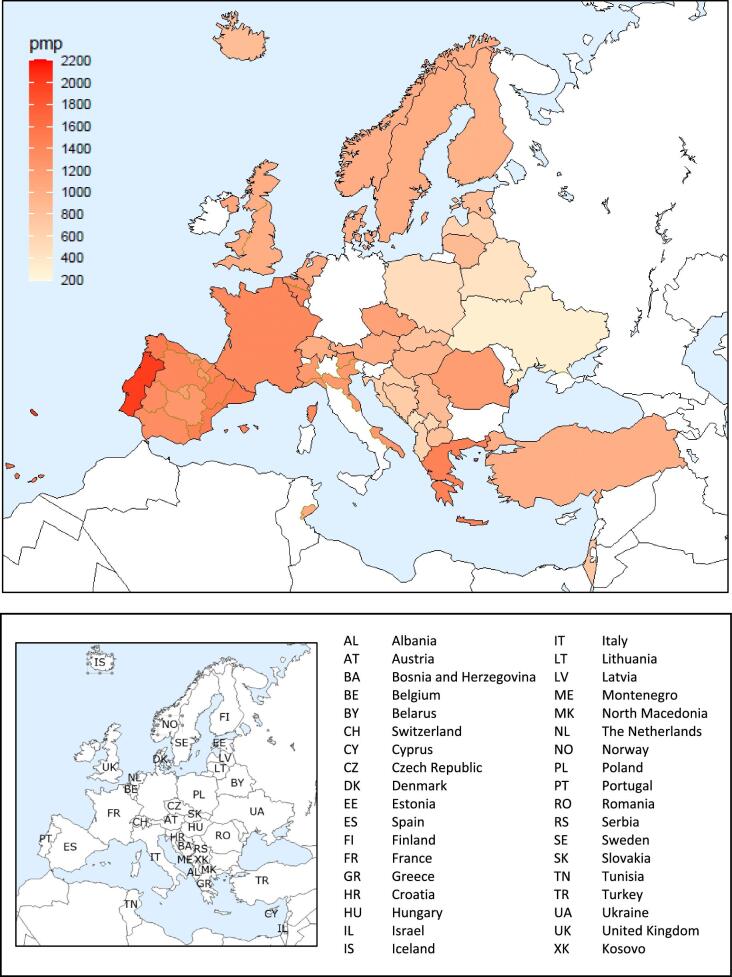
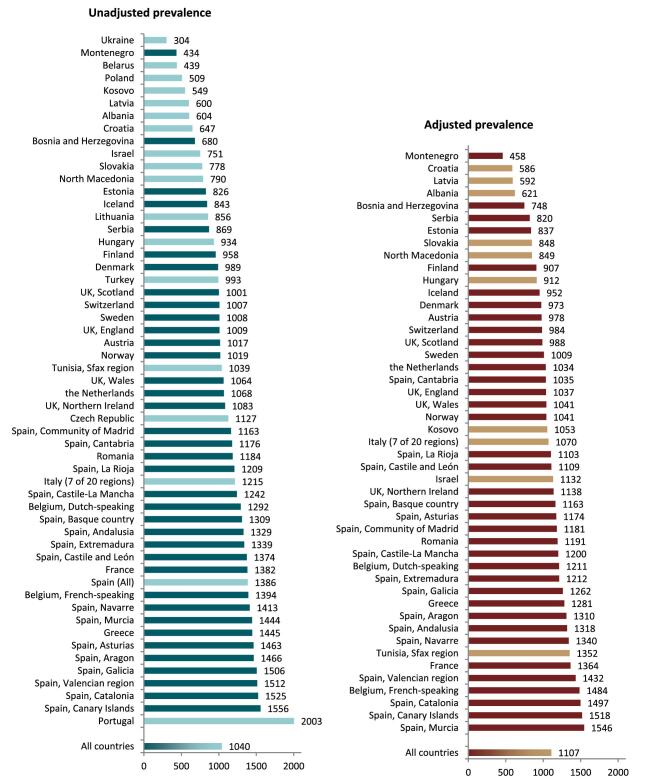
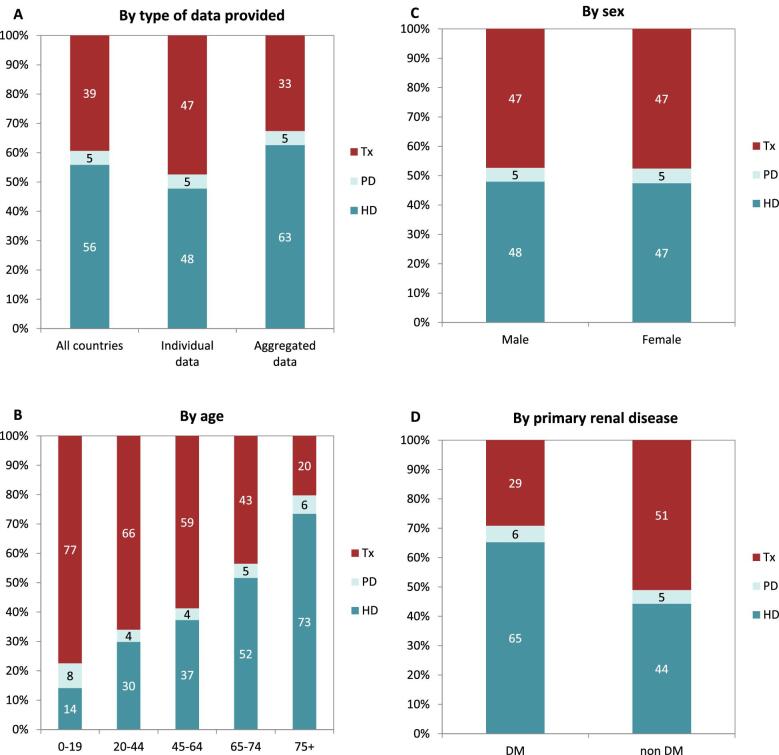
On 31 December 2021, 554 797 patients with ESKD were receiving KRT, corresponding to a KRT prevalence of 1040 pmp or 1 in 950 Europeans (Table 2). The unadjusted prevalence ranged from 304 pmp in Ukraine (1 in 3300 inhabitants) and 434 pmp in Montenegro (1 in 2300 inhabitants) to 1556 pmp in Canary Islands, Spain (1 in 650 inhabitants) and 2003 pmp in Portugal (1 in 500 inhabitants, Table 2 and Figs 6 and 7). When adjusted for age and sex using the EU28 distribution [2], the overall adjusted KRT prevalence rate for the available 47 countries and regions was 1107 pmp or approximately 1 in 900 Europeans (Fig. 7). The adjusted prevalence ranged from 458 pmp in Montenegro (1 in 2200 inhabitants) and 586 pmp in Croatia (1 in 1700 inhabitants) to 1518 pmp in the Canary Islands, Spain and 1546 pmp in Murcia, Spain (approximately 1 in 650 inhabitants, Fig. 7). The median age of prevalent patients was 64 years, ranging from 54 years in Ukraine to 70 years in Croatia and Israel, although the latter two countries only provided data on dialysis patients (Table 2). In prevalent patients, 47% were aged 65 years or older, 62% were male, and the most common PRDs were DM and glomerulonephritis/sclerosis (both 16%, Fig. 8). While there was almost no difference in the distribution of age and sex between countries providing individual patient versus aggregated data, the distribution of PRD varied by type of data provided, which was likely due to the higher proportion of missing or unknown PRDs for countries providing aggregated data (Fig. 8). Additionally, there was a larger proportion of patients living with a functioning graft in countries providing individual data (47%) compared to countries providing aggregated data (33%, Fig. 9). For countries providing individual patient data, the distribution of treatment modalities was almost equal for men and women (47–48% received HD; 5% received PD; 47% received Tx, Fig. 9). However, the distribution of treatment modalities across age groups varied widely, with the largest contrast between the youngest and oldest age groups. In paediatric patients aged 0–19 years, 77% of patients were living with a functioning graft while in patients aged 75 years and older, only 20% were living with a functioning graft (Fig. 9). Lastly, only 29% of patients with DM as PRD were living with a functioning graft compared to 51% of patients with a different PRD (Fig. 9).
Table 2:
Summary: data on the unadjusted prevalence of KRT on 31 December 2021 by country or region, the mean and median age on 31 December 2021, and the prevalence of KRT in patients with DM as PRD.
| Prevalent patients on KRT in 2021 | |||||||
|---|---|---|---|---|---|---|---|
| Country/region | General population covered by the registry in thousands | All (n) | All (pmp) | Mean age (years) | Median age (years) | DM (n) | DM (pmp) |
| Albania | 2784 | 1680 | 604 | 57.4 | 61.0 | 380 | 137 |
| Austriaa | 8825 | 8977 | 1017 | 62.2 | 63.8 | 1695 | 192 |
| Belarusb | 9256 | 4063 | 439 | 496 | 54 | ||
| Belgium, Dutch-speakingc | 6687 | 8640 | 1292 | 66.5 | 68.4 | 1393 | 208 |
| Belgium, French-speakingc | 4899 | 6829 | 1394 | 65.7 | 67.5 | 1182 | 241 |
| Bosnia and Herzegovina | 3531 | 2400 | 680 | 59.7 | 61.5 | 419 | 119 |
| Croatiad | 3306 | 2140 | 647 | 66.9 | 70.0 | 520 | 157 |
| Czech Republic | 10 307 | 11 613 | 1127 | ||||
| Denmark | 5857 | 5794 | 989 | 59.5 | 61.0 | 991 | 169 |
| Estonia | 1331 | 1100 | 826 | 59.8 | 61.0 | 203 | 153 |
| Finland | 5541 | 5309 | 958 | 60.2 | 62.8 | 1307 | 236 |
| France | 67 356 | 93 098 | 1382 | 63.5 | 65.8 | 15 388 | 228 |
| Greece | 10 569 | 15 277 | 1445 | 66.2 | 68.2 | 2752 | 260 |
| Hungary | 9731 | 9091 | 934 | 62.0 | 64.0 | ||
| Iceland | 373 | 314 | 843 | 58.5 | 59.9 | 35 | 94 |
| Israeld | 9372 | 7042 | 751 | 67.8 | 70.0 | 3245 | 346 |
| Italy (7 of 20 regions) | 20 658 | 25 100 | 1215 | 62.9 | 64.9 | 2512 | 122 |
| Kosovob | 1688 | 927 | 549 | 60.7 | 62.0 | 237 | 140 |
| Latvia | 1704 | 1023 | 600 | 55.8 | 58.0 | 111 | 65 |
| Lithuania | 2811 | 2405 | 856 | ||||
| Montenegroc | 619 | 269 | 434 | 59.8 | 62.5 | 48 | 78 |
| North Macedonia | 2069 | 1634 | 790 | 59.4 | 61.0 | 270 | 131 |
| Norway | 5403 | 5503 | 1019 | 60.4 | 62.3 | 738 | 137 |
| Polandd | 38 162 | 19 416 | 509 | 5391 | 141 | ||
| Portugale | 10 348 | 20 731 | 2003 | 68.3 | 3721 | 553 | |
| Romania | 19 122 | 22 650 | 1184 | 63.8 | 65.7 | 2078 | 109 |
| Serbia | 6493 | 5644 | 869 | 61.6 | 63.9 | 993 | 153 |
| Slovakiad | 4452 | 3463 | 778 | 64.9 | 67.0 | 1082 | 243 |
| Spain (All) | 47 385 | 65 678 | 1386 | 61.3 | 64.8 | 10 796 | 228 |
| Spain, Andalusia | 8511 | 11 312 | 1329 | 61.8 | 63.0 | 1898 | 223 |
| Spain, Aragon | 1323 | 1939 | 1466 | 65.7 | 67.3 | 355 | 268 |
| Spain, Asturias | 1010 | 1477 | 1463 | 64.9 | 66.5 | 266 | 263 |
| Spain, Basque country | 2181 | 2856 | 1309 | 62.6 | 64.7 | 383 | 176 |
| Spain, Canary Islands | 2248 | 3499 | 1556 | 63.0 | 64.2 | 916 | 407 |
| Spain, Cantabriac | 584 | 687 | 1176 | 63.9 | 65.0 | 115 | 197 |
| Spain, Castile and Leónc | 2383 | 3274 | 1374 | 66.3 | 67.4 | 540 | 227 |
| Spain, Castile-La Manchac | 2049 | 2545 | 1242 | 64.1 | 64.8 | 449 | 219 |
| Spain, Catalonia | 7763 | 11 841 | 1525 | 63.6 | 65.4 | 1822 | 235 |
| Spain, Community of Madrid | 6751 | 7850 | 1163 | 62.3 | 63.8 | 1366 | 202 |
| Spain, Extremadura | 1060 | 1419 | 1339 | 63.8 | 64.5 | 235 | 222 |
| Spain, Galicia | 2694 | 4057 | 1506 | 64.1 | 65.5 | 709 | 263 |
| Spain, La Rioja | 316 | 382 | 1209 | 62.4 | 63.0 | 55 | 174 |
| Spain, Murcia | 1518 | 2192 | 1444 | 62.9 | 63.9 | 352 | 232 |
| Spain, Navarrec | 658 | 930 | 1413 | 63.9 | 65.5 | 159 | 241 |
| Spain, Valencian region | 5058 | 7650 | 1512 | 63.8 | 65.6 | 1125 | 222 |
| Sweden | 10 416 | 10 499 | 1008 | 60.5 | 62.5 | 1782 | 171 |
| Switzerland | 8452 | 8512 | 1007 | 62.9 | 65.0 | 1224 | 145 |
| the Netherlands | 16 832 | 17 975 | 1068 | 61.3 | 63.3 | 2378 | 141 |
| Tunisia, Sfax regiond | 1023 | 1063 | 1039 | 57.5 | 59.0 | 247 | 241 |
| Turkeyf | 84 680 | 84 128 | 993 | 5842 | 340 | ||
| Ukraineb | 26 632 | 8086 | 304 | 52.8 | 54.0 | 1304 | 49 |
| UK, England | 55 349 | 55 870 | 1009 | 58.3 | 59.6 | 10 032 | 181 |
| UK, Northern Ireland | 1905 | 2062 | 1083 | 58.8 | 59.8 | 293 | 154 |
| UK, Scotland | 5480 | 5488 | 1001 | 57.1 | 59.0 | 954 | 174 |
| UK, Wales | 3105 | 3304 | 1064 | 58.4 | 59.7 | 611 | 197 |
| All countries | 534 512 | 554 797 | 1040 | 62.2 | 64.0 | 82 650 | 186 |
DM, diabetes mellitus; PRD, primary renal disease.
When cells are left empty, the data are unavailable and could not be used for the calculation of the summary data.
aThe prevalence is underestimated by approximately 1% due to one haemodialysis centre not submitting data.
bPatients younger than 18 years of age are not reported.
cPatients younger than 20 years of age are not reported.
dData on prevalence includes dialysis patients only.
eData on DM are extrapolated from data of 13 473 patients (65.0% of total).
fData on DM are extrapolated from data of 17 074 patients (20.2% of total).
Figure 6:
Prevalence per million population of KRT on 31 December 2021 by country or region, unadjusted.
Figure 7:
Prevalence per million population of KRT on 31 December 2021 by country or region, unadjusted (left panel) and adjusted (right panel). Registries providing individual patient data are shown as dark bars and registries providing aggregated data as light bars. See Appendix 1 for a list of countries and regions providing individual patient or aggregated data. Adjustment was performed by standardizing the incidence to the age and sex distribution of the EU28 population.
Figure 8:
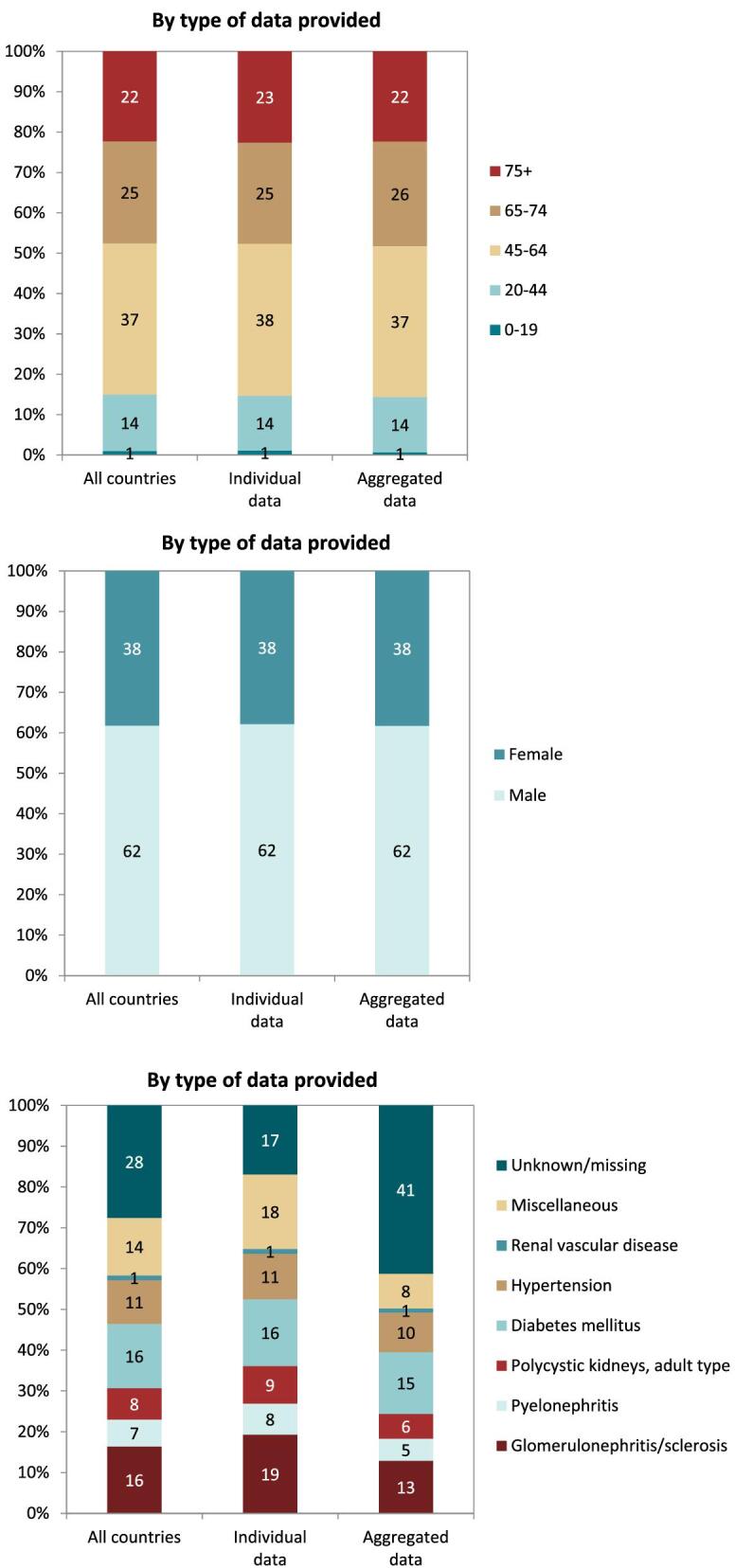
Distribution of (A) age, (B) sex, and (C) primary renal disease by type of data provided for prevalent patients on KRT on 31 December 2021, unadjusted. See Appendix 1 for a list of countries and regions providing individual patient or aggregated data.
Figure 9:
Distribution of treatment modality by (A) type of data provided, (B) age, (C) sex, and (D) primary renal disease (DM and non-DM) for prevalent patients on KRT on 31 December 2021, unadjusted. Panels (B), (C), and (D) are only based on the data from registries providing individual patient data. See Appendix 1 for a list of countries and regions providing individual patient or aggregated data. HD: haemodialysis; PD: peritoneal dialysis; Tx: transplant.
Kidney transplantation
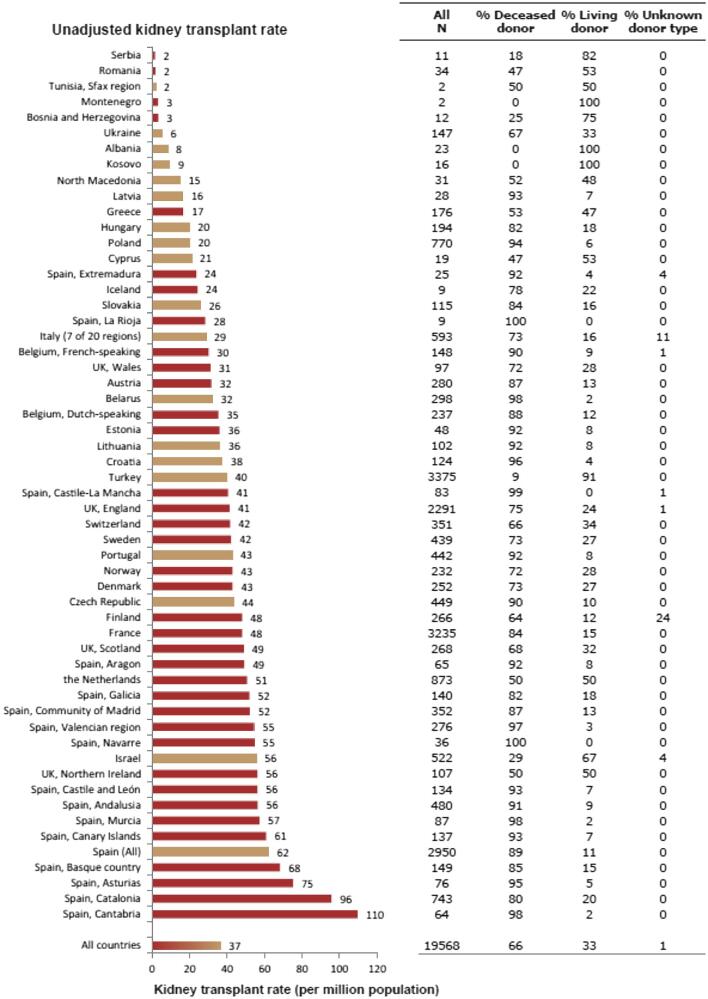
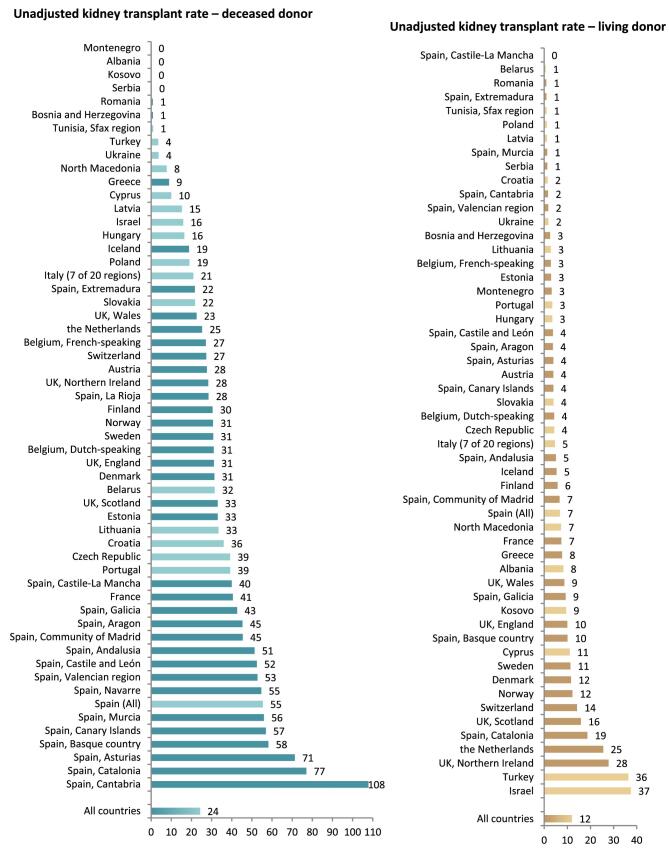
In 2021, 19 568 kidney transplantations were performed. The unadjusted kidney transplantation rate was 37 pmp or 1 in 27 000 Europeans (Fig. 10), which was higher than in 2020 (28 pmp) [1] but comparable to data from 2019 (35 pmp) [3]. The unadjusted kidney transplantation rate ranged from 2 pmp in Serbia, Romania, and Tunisia (1 in 500 000 inhabitants) to 96 pmp in Spain, Catalonia (1 in 10 400 inhabitants) and 110 pmp in Spain, Cantabria (1 in 9 100 inhabitants, Fig. 10). A majority (66%) of kidney transplantations were from deceased donors (DD), while 33% were from living donors (LD), and 1% had an unknown donor type (Fig. 10). Two regions in Spain (La Rioja and Navarre) had all kidney transplants come from deceased donors while Montenegro, Albania, and Kosovo had all kidney transplants come from living donors (Fig. 10). The overall unadjusted DD kidney transplantation rate was twice that from LD (DD: 24 pmp or 1 in 41 700 inhabitants versus LD: 12 pmp or 1 in 83 300 inhabitants), and was highest in Spain, Cantabria (108 pmp or 1 in 9 300 inhabitants, Fig. 11). The highest rate of LD kidney transplantations was in Israel (37 pmp or 1 in 27 000 inhabitants, Fig. 11). In countries providing individual patient data, 78% of kidney transplants came from DD compared to 58% in countries providing aggregated data (Fig. 12).
Figure 10:
Kidney transplantations performed in 2021 counts and per million population by country or region, unadjusted. Registries providing individual patient data are shown as red bars and registries providing aggregated data as orange bars. See Appendix 1 for a list of countries and regions providing individual patient or aggregated data.
Figure 11:
Kidney transplantations performed in 2021 per million population by donor type and by country or region, unadjusted. Registries providing individual patient data are shown as dark bars and registries providing aggregated data as light bars. See Appendix 1 for a list of countries and regions providing individual patient or aggregated data.
Figure 12:
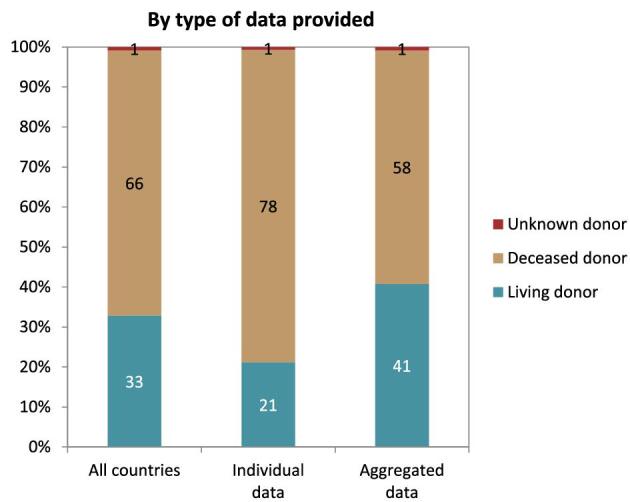
Donor type distribution for kidney transplantations performed in 2021 by type of data provided, unadjusted. See Appendix 1 for a list of countries and regions providing individual patient or aggregated data.
Survival probability of patients receiving KRT
For patients initiating KRT between 2012 and 2016, the unadjusted 5-year patient survival probability was 51.7% [95% confidence interval (95% CI) 51.4–51.9, Table 3]. In patients starting dialysis, the unadjusted 5-year survival probabilities were 41.4% (95% CI 41.1–41.7), while in patients receiving a first kidney transplant, 5-year survival was 85.8% (95% CI 85.4–86.1) for DD and 94.2% (95% CI 93.8–94.6) for LD (Table 3). The unadjusted 5-year probability of graft survival was higher in LD kidney transplantations (88.3%; 95% CI 87.7–88.8) compared to DD kidney transplantations (77.0%; 95% CI 76.6–77.5, Table 3). Similar trends were observed when analyses were adjusted using fixed values for age, sex, and PRD (Table 3).
Table 3:
One-, two-, and five-year survival probabilities by treatment modality and cohort from day 1 of the start of KRT, dialysis, or from the day of kidney transplantation.
| Survival probabilities as % (95% confidence intervals) | |||||
|---|---|---|---|---|---|
| Cohort: 2012–2016 | Cohort: 2015–2019 | ||||
| Survival type | 1 year | 2 year | 5 year | 1 year | 2 year |
| Patient survival on KRT | |||||
| Unadjusted | 85.1 (84.9–85.3) | 75.1 (74.9–75.3) | 51.7 (51.4–51.9) | 85.7 (85.6–85.9) | 75.7 (75.5–75.9) |
| Adjusteda | 87.9 (87.7–88.0) | 78.9 (78.7–79.1) | 54.1 (53.8–54.3) | 88.3 (88.2–88.5) | 79.2 (79.0–79.4) |
| Patient survival on dialysis | |||||
| Unadjusted | 84.0 (83.8–84.2) | 72.4 (72.2–72.6) | 41.4 (41.1–41.7) | 84.7 (84.5–84.8) | 73.0 (72.8–73.2) |
| Adjusteda | 86.2 (86.1–86.4) | 75.9 (75.7–76.1) | 46.6 (46.3–46.9) | 87.0 (86.9–87.2) | 76.6 (76.5–77.0) |
| Patient survival after a first kidney transplantation (deceased donor) | |||||
| Unadjusted | 96.2 (96.0–96.4) | 94.1 (93.9–94.3) | 85.8 (85.4–86.1) | 96.4 (96.2–96.5) | 93.9 (93.7–94.1) |
| Adjustedb | 98.0 (97.9–98.2) | 96.9 (96.8–97.1) | 92.1 (91.8–92.4) | 98.1 (98.0–98.2) | 96.8 (96.7–97.0) |
| Graft survival after a first kidney transplantation (deceased donor) | |||||
| Unadjusted | 91.1 (90.8–91.4) | 87.9 (87.6–88.2) | 77.0 (76.6–77.5) | 91.4 (91.1–91.7) | 88.0 (87.7–88.3) |
| Adjustedb | 93.1 (92.8–93.3) | 90.5 (90.2–90.8) | 81.4 (81.0–81.9) | 93.4 (93.2–93.6) | 90.7 (90.4–91.0) |
| Patient survival after a first kidney transplantation (living donor) | |||||
| Unadjusted | 98.9 (98.7–99.0) | 98.0 (97.7–98.2) | 94.2 (93.8–94.6) | 98.9 (98.6–99.0) | 97.9 (97.7–98.2) |
| Adjustedb | 99.1 (98.9–99.2) | 98.3 (98.1–98.6) | 95.1 (94.7–95.5) | 99.1 (98.9–99.3) | 98.4 (98.1–98.6) |
| Graft survival after a first kidney transplantation (living donor) | |||||
| Unadjusted | 96.7 (96.3–97.0) | 95.1 (94.7–95.4) | 88.3 (87.7–88.8) | 96.8 (96.5–97.1) | 95.2 (94.8–95.6) |
| Adjustedb | 96.5 (96.2–96.9) | 94.9 (94.5–95.3) | 87.7 (87.1–88.3) | 96.6 (96.3–97.0) | 95.0 (94.6–95.4) |
aAnalyses were adjusted using fixed values: age (67 years), sex (63% male), and PRD (24% diabetes mellitus, 19% hypertension/renal vascular disease, 11% glomerulonephritis, and 46% other causes)
bAnalyses were adjusted using fixed values: age (50 years), sex (63% men), and PRD (14% diabetes mellitus, 10% hypertension/renal vascular disease, 23% glomerulonephritis, and 53% other causes)
This table is based on data from the following registries providing individual patient data: Austria, Belgium (Dutch-speaking), Belgium (French-speaking), Bosnia and Herzegovina, Denmark, Estonia, Finland, France, Greece, Iceland, Norway, Spain (Andalusia), Spain (Aragon), Spain (Asturias), Spain (Basque country), Spain (Cantabria), Spain (Castile and León), Spain (Castile-La Mancha), Spain (Catalonia), Spain (Community of Madrid), Spain (Extremadura), Spain (Galicia), Spain (Murcia), Spain (Navarre), Spain (Valencian Region), Sweden, the Netherlands, United Kingdom (England/Northern Ireland/Wales) and United Kingdom (Scotland).
Expected remaining lifetime
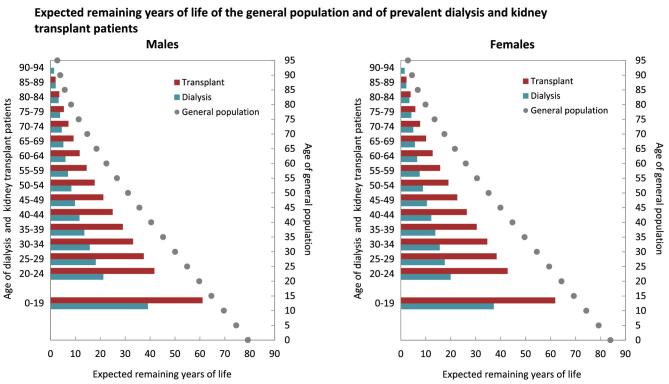
In the period from 2017 to 2021, life expectancy for all age groups combined was approximately 65% and 68% shorter for males and females on dialysis, and 40% and 43% shorter for males and females living with a functioning graft when compared to males and females in the general population (Fig. 13). In males aged 20 to 24 years, life expectancy was on average 36 years (63%) shorter if receiving dialysis, and 16 years (27% shorter) if living with a functioning graft when compared to males from the general population. In females aged 20 to 24 years, this was 42 years (68%) shorter on dialysis and 19 years (31%) shorter if living with a functioning graft. However, as age increased, the absolute gap in expected remaining lifetime between patients on dialysis and patients living with a functioning graft decreased. In males aged 70 to 74 years, the difference in expected remaining lifetime was 9 years (65%) if on dialysis and 6 years (45%) if living with a functioning graft, and in females this was 11 years (68%) if on dialysis and 8 years (50%) if living with a functioning graft.
Figure 13:
Expected remaining years of life in the general population and for prevalent dialysis and kidney transplant patients (cohort 2017–2021), by sex. This figure is based on data from the following registries providing individual patient data: Austria, Belgium (Dutch-speaking), Belgium (French-speaking), Bosnia and Herzegovina, Denmark, Estonia, Finland, France, Greece, Iceland, Norway, Spain (Andalusia), Spain (Aragon), Spain (Asturias), Spain (Basque country), Spain (Cantabria), Spain (Castille and León), Spain (Castille-La Mancha), Spain (Catalonia), Spain (Community of Madrid), Spain (Extremadura), Spain (Galicia), Spain (Murcia), Spain (Navarre), Spain (Valencian Region), Sweden, the Netherlands, United Kingdom (England/Northern Ireland/Wales), and United Kingdom (Scotland).
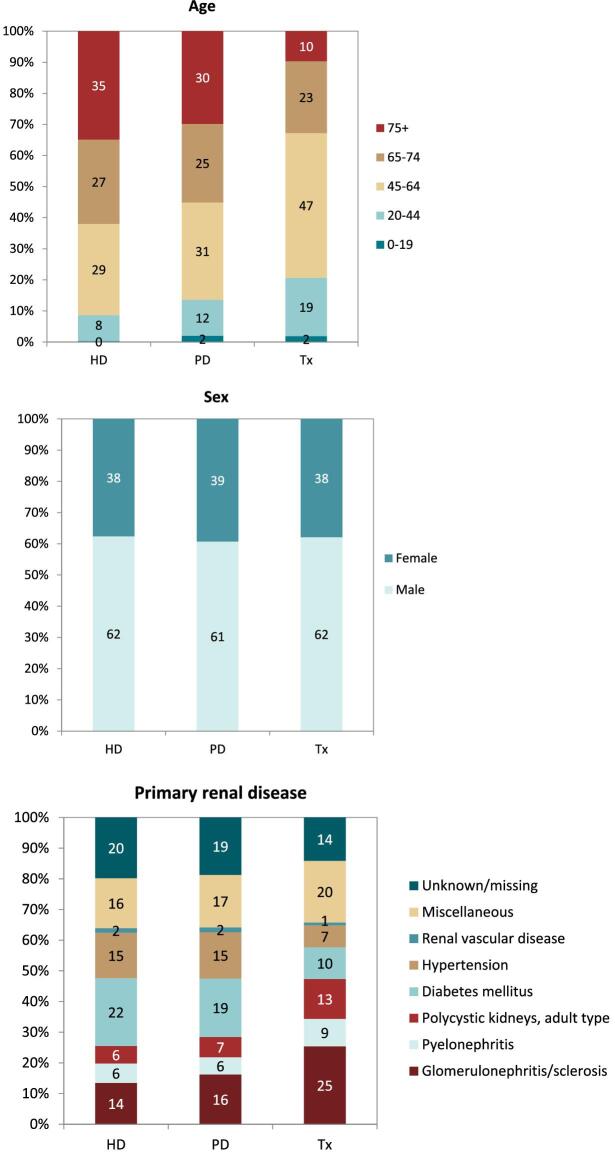
Comparisons by treatment modality
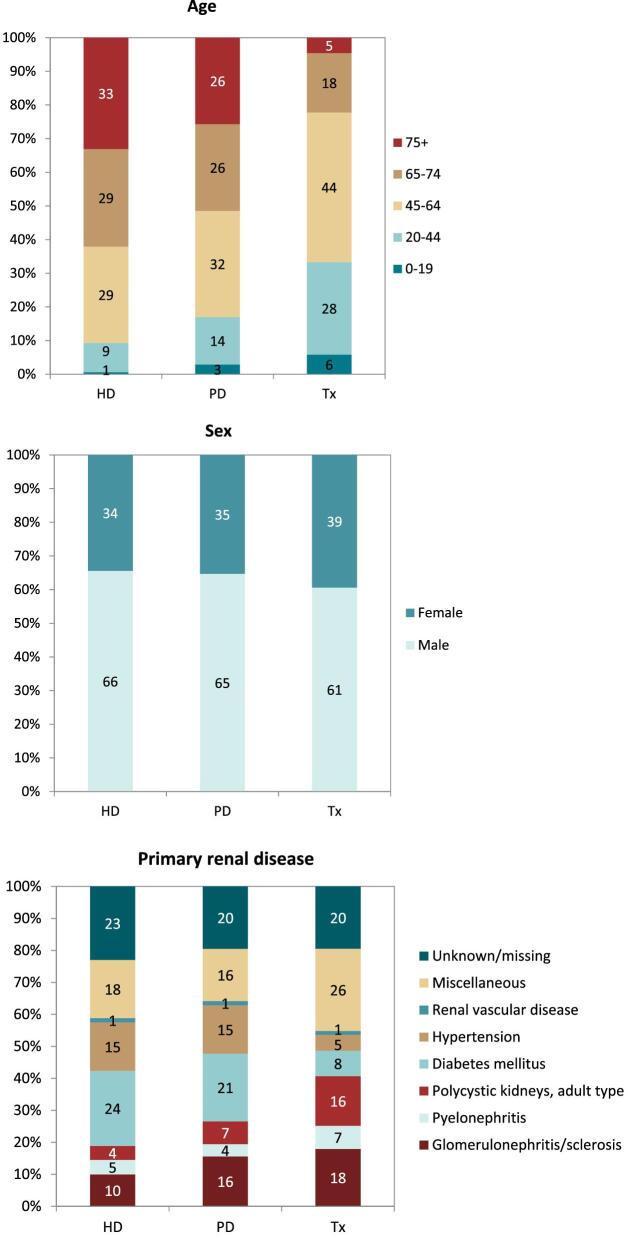
In this year's annual report, additional comparisons across treatment modalities are presented. In 2021, the rate of pre-emptive kidney transplantation was low (8 pmp or 1 in 125 000 Europeans) compared to PD (16 pmp or 1 in 62 500 Europeans) and HD (121 pmp or 1 in 8300 Europeans, Fig. 14). The percentage of pre-emptive kidney transplantations in incident patients ranged from 0% to 16% and was highest in Turkey, Northern Ireland, and the Netherlands (Fig. 15). In countries providing individual patient data, the distribution of age varied across treatment modalities. Among patients starting KRT with HD, a greater proportion was aged 65 years or older (62%) when compared to PD (52%) and pre-emptive transplantation (23%, Fig. 16). Among pre-emptively transplanted patients, 44% of patients were 45–64 years of age (Fig. 16). The distribution of sex across initial treatment modality was similar, but there were substantial differences in the distribution of PRD. Most notably, among patients initiating KRT with dialysis, the proportion of patients with hypertension or DM as PRD was triple that of pre-emptively transplanted patients (hypertension: HD and PD 15%; Tx 5%; and DM: HD 24%; PD 21%; Tx 8%, Fig. 16). Alternatively, in pre-emptively transplanted patients, the proportion of patients with polycystic kidneys and glomerulonephritis/sclerosis as PRD was higher (polycystic kidney disease: HD 4%; PD 7%; Tx 16%; and glomerulonephritis/sclerosis: HD 10%; PD 16%; Tx 18%, Fig. 16).
Figure 14:
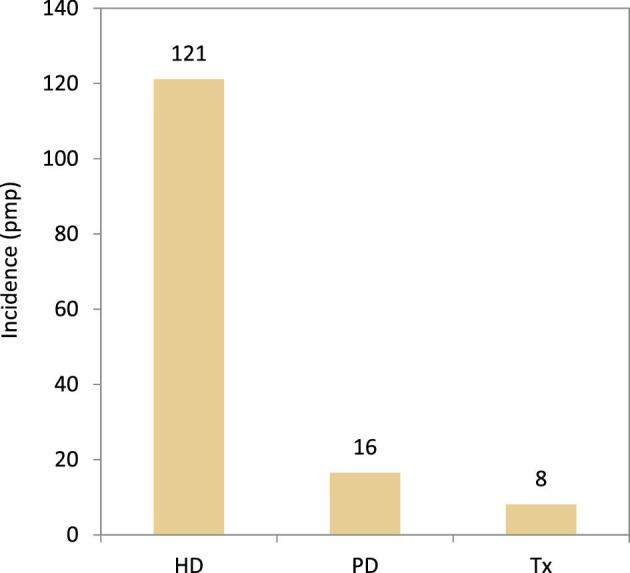
Incidence per million population of KRT in 2021 on day 1 by treatment modality, unadjusted. HD: haemodialysis; PD: peritoneal dialysis; Tx: transplant.
Figure 15:
Percentage of pre-emptive kidney transplantations for incident patients accepted for KRT in 2021 on day 1 by country or region, unadjusted.
Figure 16:
Distribution of (A) age, (B) sex, and (C) primary renal disease by treatment modality for incident patients accepted for KRT in 2021 on day 1, unadjusted. This figure is only based on data from registries providing individual patient data. HD: haemodialysis; PD: peritoneal dialysis; Tx: transplant.
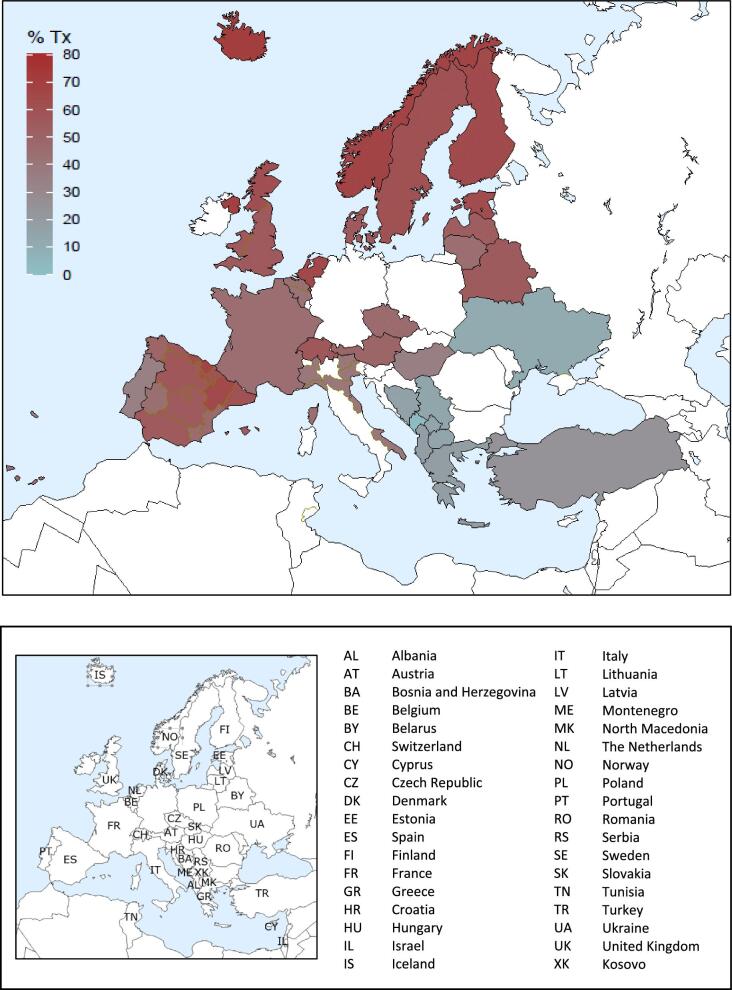
On 31 December 2021, HD was the most prevalent treatment modality for patients on KRT (580 pmp or 1 in 1700 Europeans), followed by transplantation (471 pmp or 1 in 2100 Europeans) and PD (50 pmp or 1 in 20 000 Europeans, Fig. 17). The highest prevalence of kidney transplantation was found in Norway, Northern Ireland, and several Spanish regions (Fig. 18). In countries providing individual patient data, patients aged 65 years and older comprised 62% of all HD patients, 55% of all PD patients, and 33% of all transplanted patients (Fig. 19). There was no difference in the distribution of sex across treatment modalities (Fig. 19). However, similar to the trends observed in incident patients, there were considerable differences in the distribution of PRD across dialysis patients and transplanted patients (Fig. 19). Among patients receiving dialysis, the proportion of hypertension or DM as PRD was double that of patients living with a functioning graft (hypertension: HD and PD 15%; Tx 7%; and DM: HD 22%; PD 19%; Tx 10%, Fig. 19). In contrast, the proportion of patients with polycystic kidneys and glomerulonephritis/sclerosis as PRD was considerably higher in transplanted patients (polycystic kidney disease: HD 6%; PD 7%; Tx 13%; and glomerulonephritis/sclerosis: HD 14%; PD 16%; Tx 25%, Fig. 19).
Figure 17:
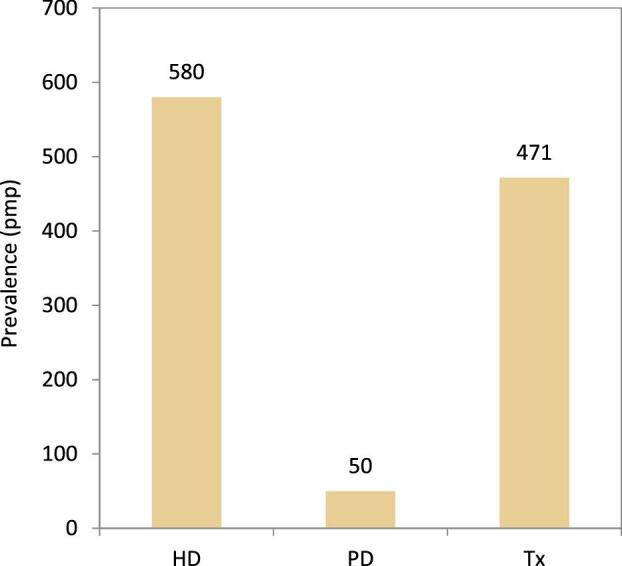
Prevalence per million population of KRT on 31 December 2021 by treatment modality, unadjusted. HD: haemodialysis; PD: peritoneal dialysis; Tx: transplant.
Figure 18:
Percentage of kidney transplantations for prevalent patients on KRT on 31 December 2021, unadjusted.
Figure 19:
Distribution of (A) age, (B) sex, and (C) primary renal disease by treatment modality for prevalent patients on KRT on 31 December 2021, unadjusted. This figure is only based on data from registries providing individual patient data. HD: haemodialysis; PD: peritoneal dialysis; Tx: transplant.
Using data on patients starting dialysis during the period 2012–2016, patients receiving PD on day 91 had a comparable survival probability to those receiving HD (Fig. 20), with a 5-year unadjusted patient survival of 41.4% (95% CI 41.1–41.7) in HD patients and 42.4% (95% CI 41.6–43.2) in PD patients. In patients receiving a first kidney transplant, both the unadjusted patient and graft survival was higher in patients receiving a kidney from a LD compared to patients receiving a kidney from a DD (Figs 21 and 22).
Figure 20:
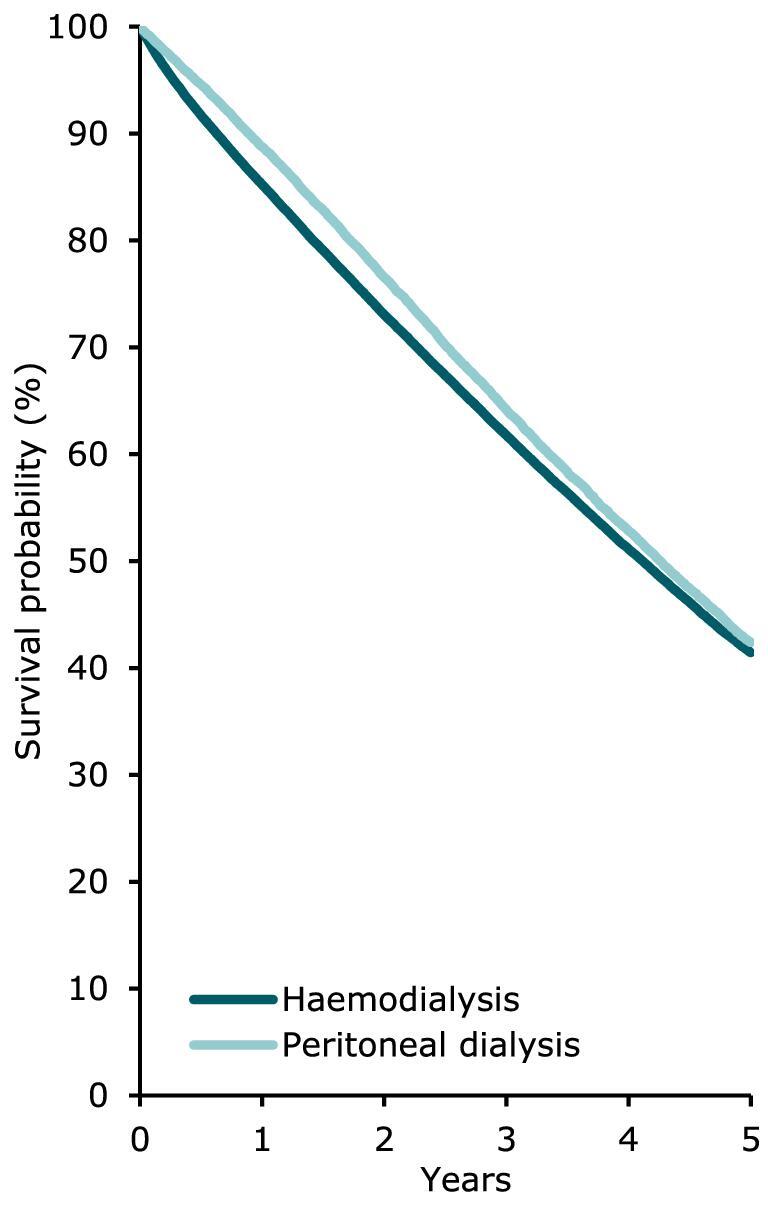
Patient survival by modality (HD or PD) for incident dialysis patients accepted for KRT in 2021 on day 91 (cohort 2012–2016), unadjusted. This figure is based on data from registries providing individual patient data.
Figure 21:
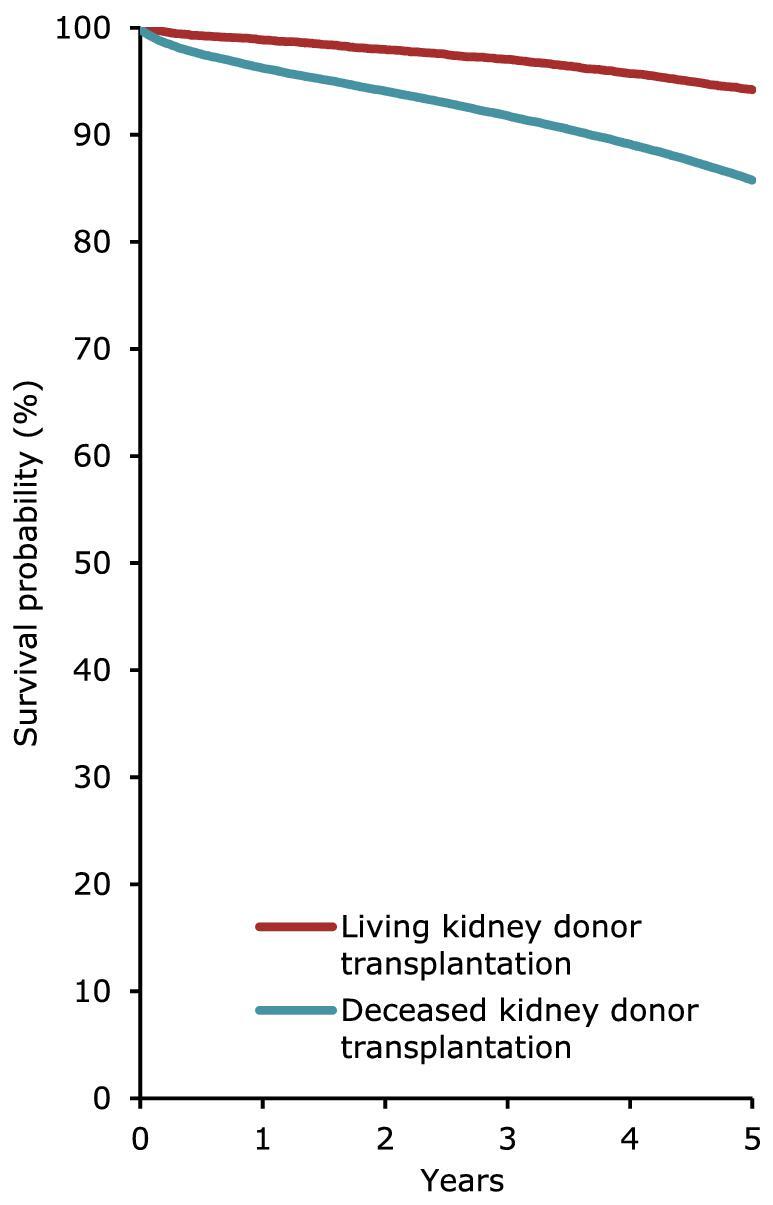
Patient survival in first-time transplant recipients by donor type (deceased or living) from day of transplantation (cohort 2012–2016), unadjusted. This figure is based on data from registries providing individual patient data.
Figure 22:
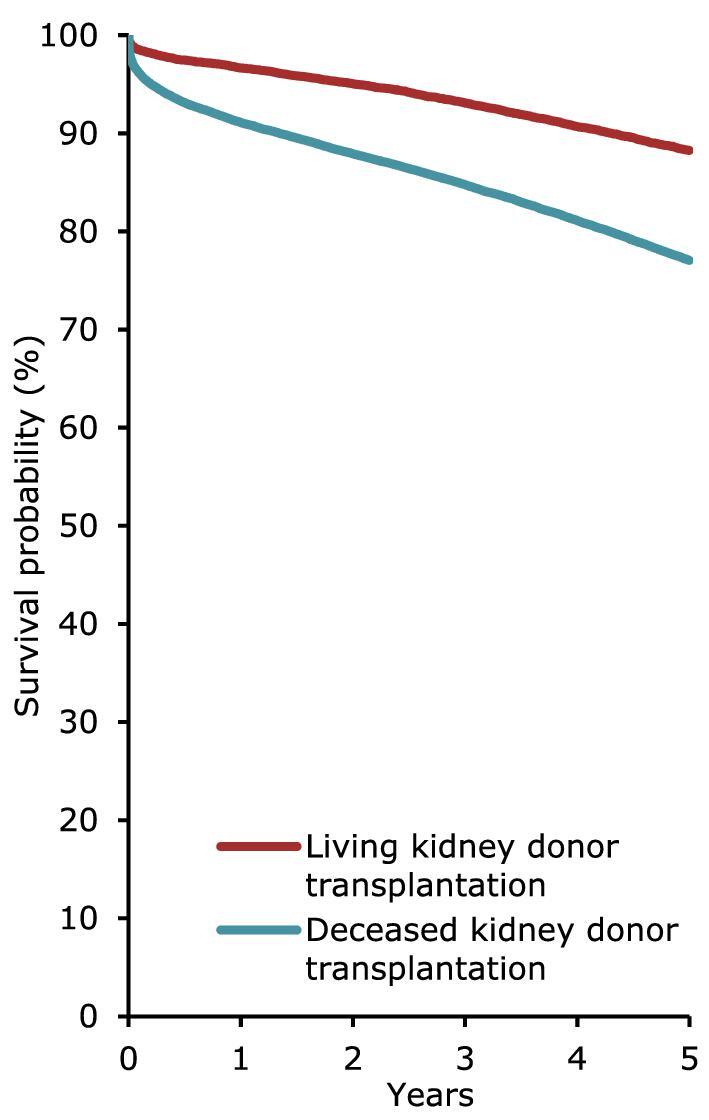
Graft survival in first-time kidney transplant recipients by donor type (deceased or living) from day of transplant (cohort 2012–2016), unadjusted. This figure is based on data from registries providing individual patient data.
AFFILIATED REGISTRIES
We would like to thank the patients and the staff of the dialysis and transplant units for contributing the data via their national and regional renal registries. Furthermore, we gratefully acknowledge the following registries and persons for their contribution of the data: Albanian Renal Registry (M. Rroji and E. Likaj); Austrian Dialysis and Transplant Registry [OEDTR] (D. Kaiser-Feistmantl, L. Buchwinkler, and G. Mayer); Belarus Renal Registry (K.S. Kamisarau and A. Kalachyk); Dutch speaking Belgian Society of Nephrology [NBVN] (L. Heylen and V. De Meyer); French speaking Belgian Society of Nephrology [GNFB] (JM. des Grottes and F. Collart); Renal Registry Bosnia and Herzegovina (B. Jakovljevic and Z. Kelava); Croatian Renal Registry (D. Katicic and K. Altabas); Cyprus Renal Registry (S. Glyki, E. Magiroudi, and A. Savvidou); Czech Republic: Registry of Dialysis Patients [RDP] (F. Lopot, I. Rychlík, and L. Francová); Danish Nephrology Registry [DNS]; Estonian Society of Nephrology (Ü. Pechter and K. Lilienthal); Finnish Registry for Kidney Diseases; France: The Epidemiology and Information Network in Nephrology [REIN] (C. Couchoud); Hellenic Renal Registry (G. Moustakas); Hungarian Renal Registry (I. Kulcsar, L. Wagner, and L. Rosivall); Icelandic End-Stage Renal Disease Registry (R. Palsson); Montenegro Renal Registry (M. Ratkovic and F. Tomović); Israel National Registry of Renal Replacement Therapy (L. Keinan-Boker and R. Dichtiar); Italian Registry of Dialysis and Transplantation [RIDT] (M. Nordio, M. Postorino, and A. Limido); Kosovo Renal Registry (S. Selmani); Latvian Renal Registry (V Kuzema, A Popova, and A Pētersons); Lithuanian Renal Registry (I. Nedzelskiene and V. Vainauskas); North Macedonian Renal Registry (I. Rambabova Bushljetikj); Norwegian Renal Registry (A.V. Reisæter); Renal Registry of Poland (P. Jagodzinski and R. Gellert); Portuguese Renal Registry (A Galvão and A. Ferreira); Romanian Renal Registry [RRR] (G. Mircescu, L. Garneata, and E. Podgoreanu); Renal Registry in Serbia (M Lausevic and all dialysis units in Serbia); Slovakian Renal Registry (I. Lajdová, V. Spustová, and A. Oksa); Spain Renal Registry (B. Mahillo Durán); Swedish Renal Registry [SRR] (K.G. Prütz, M. Evans, T. Lundgren, H. Rydell, and M. Segelmark); Swiss Dialysis Registry (P. Ambühl); Dutch Renal Registry [RENINE] (P. Verschoor and L. Heuveling); Tunisian (Sfax region) Renal Registry (D. Zalila); Registry of the Nephrology, Dialysis and Transplantation in Turkey [TSNNR] (K. Ateş and G. Süleymanlar); Ukrainian Renal Data System [URDS] (M. Kolesnyk); UK Renal Registry (all the staff of the UK Renal Registry and of the renal units submitting data); Scottish Renal Registry [SRR] (all of the Scottish renal units); and the regional registries of Andalusia [SICATA], Aragon, Asturias (M.R. Camblor, J.R. Quirós, and RERCA working group), Basque country [UNIPAR] (Á. Magaz, J. Aranzabal, M. Rodrigo, and I. Moina), Canary Islands (C. García Cantón and D. Marrero Miranda), Cantabria (J.C. Ruiz San Millán), Castile and León (P. Ucio Mingo, M. Prieto Velasco, and M.Á. Palencia García), Castile-La Mancha (G. Gutiérrez Ávila and I. Moreno Alía), Catalonia [RMRC] (J. Tort, J. Comas, and M. Vázquez), Community of Madrid (M.I. Aparicio de Madre and F. Tornero Molina), Extremadura (all the renal units (Nephrology and Dialysis)), Galacia (E. Bouzas-Caamaño), La Rioja (E. Huarte Loza, M. Artamendi Larrañaga, and H. Hernández Vargas), Murcia (I. Marín Sánchez), Navarre (J. Manrique Escola), and Valencian region (O. Zurriaga).
ERA REGISTRY COMMITTEE MEMBERS
C. Wanner, Germany (ERA President); A. Ortiz, Spain (Chairman); P. Ambühl, Switzerland; M. Arici, Turkey; M. Arnol, Slovenia; S. Bakkaloglu, Turkey; P.M. Ferraro, Italy; J. Helve, Finland; J.E. Sánchez-Alvarez, Spain; M. Segelmark, Sweden; S.S. Sørensen, Denmark; E. Vidal, Italy.
ERA REGISTRY OFFICE STAFF
V.S. Stel (Managing Director), M.E. Astley, B.A. Boerstra, M. Bonthuis, R. Boenink, I.H. van den Brand, N.C. Chesnaye, R. Cornet, K.J. Jager, A. Kramer, and A.J. Weerstra.
Supplementary Material
ACKNOWLEDGEMENTS
The ERA Registry would like to thank the patients and staff of all the dialysis and transplant units who have contributed data via their national and regional renal registries. In addition, we would like to thank the persons and organizations listed in the paragraph ‘affiliated registries’ for their contribution to the work of the ERA Registry.
Appendix 1
Countries or regions providing individual patient data to the ERA Registry
Austria, Belgium (Dutch-speaking), Belgium (French-speaking), Bosnia and Herzegovina, Denmark, Estonia, Finland, France, Greece, Iceland, Montenegro, Norway, Romania, Serbia, Spain (Andalusia), Spain (Aragon), Spain (Asturias), Spain (Basque country), Spain (Canary Islands), Spain (Cantabria), Spain (Castile and León), Spain (Castile-La Mancha), Spain (Catalonia), Spain (Community of Madrid), Spain (Extremadura), Spain (Galicia), Spain (La Rioja), Spain (Murcia), Spain (Navarre), Spain (Valencian region), Sweden, Switzerland, the Netherlands, United Kingdom (England/Northern Ireland/Wales), and United Kingdom (Scotland).
Countries or regions providing aggregated data to the ERA Registry
Albania, Belarus, Croatia, Cyprus, Czech Republic, Hungary, Israel, Italy, Kosovo, Latvia, Lithuania, North Macedonia, Poland, Portugal, Slovakia, Spain, Tunisia (Sfax region), Turkey, and Ukraine.
Countries part of the European Union (EU28) population as of 1 July 2013 (used as a reference population)
Austria, Belgium, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, the Netherlands, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden, and United Kingdom.
Contributor Information
Brittany A Boerstra, Department of Medical Informatics, University of Amsterdam, ERA Registry, Amsterdam UMC, Amsterdam, The Netherlands; Amsterdam Public Health, Health Behaviours & Chronic Diseases, Amsterdam, The Netherlands; Amsterdam Public Health, Ageing & Later Life, Amsterdam, The Netherlands.
Rianne Boenink, Department of Medical Informatics, University of Amsterdam, ERA Registry, Amsterdam UMC, Amsterdam, The Netherlands; Amsterdam Public Health, Ageing & Later Life, Amsterdam, The Netherlands; Amsterdam Public Health, Quality of Care, Amsterdam, The Netherlands.
Megan E Astley, Department of Medical Informatics, University of Amsterdam, ERA Registry, Amsterdam UMC, Amsterdam, The Netherlands; Amsterdam Public Health, Health Behaviours & Chronic Diseases, Amsterdam, The Netherlands; Amsterdam Public Health, Global Health, Amsterdam, The Netherlands.
Marjolein Bonthuis, Department of Medical Informatics, University of Amsterdam, ERA Registry, Amsterdam UMC, Amsterdam, The Netherlands; Amsterdam Public Health, Quality of Care, Amsterdam, The Netherlands; Amsterdam Public Health, Methodology, Amsterdam, The Netherlands.
Samar Abd ElHafeez, Department of Medical Informatics, University of Amsterdam, ERA Registry, Amsterdam UMC, Amsterdam, The Netherlands; Epidemiology Department, High Institute of Public Health, Alexandria University, Alexandria, Egypt.
Federico Arribas Monzón, Department of Aragon Health, General Direction of Health Care and Planning, Zaragoza, Spain.
Anders Åsberg, The Norwegian Renal Registry, Department of Transplantation Medicine, Oslo University Hospital – Rikshospitalet, Oslo, Norway.
Pazit Beckerman, Institute of Nephrology and Hypertension, Sheba Medical Center, Ramat Gan, Israel; Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
Samira Bell, Scottish Renal Registry, Public Health Scotland, Glasgow, UK; Division of Population Health & Genomics, University of Dundee, Ninewells Hospital, Dundee, UK.
Aleix Cases Amenós, Nephrology Unit, Hospital Clínic de Barcelona, Barcelona, Spain.
Pablo Castro de la Nuez, SICATA: Information System of the Autonomous Coordination of Transplants of Andalusia, Seville, Andalusia, Spain.
Marc A G J ten Dam, Nefrodata Dutch Renal Registry, Utrecht, The Netherlands.
Alicja Debska-Slizien, Department of Nephrology, Transplantology and Internal Medicine, Gdańsk Medical University, Gdańsk, Poland.
Nikola Gjorgjievski, Clinic of Nephrology, Skopje, North Macedonia; Faculty of Medicine, University ‘SS Cyril and Methodius’ Skopje, Skopje, North Macedonia.
Rebecca Giudotti, City Hospital Zurich, Zurich, Switzerland.
Jaakko Helve, Finnish Registry for Kidney Diseases, Helsinki, Finland; Abdominal Center Nephrology, University of Helsinki and Helsinki University Hospital, Helsinki, Finland.
Kristine Hommel, Department of Nephrology, Holbaek Hospital, Holbaek, Denmark.
Alma Idrizi, Service of Nephrology, UHC ‘Mother Teresa’, Tirana, Albania.
Ólafur S Indriðason, Division of Nephrology, Landspitali – The National University Hospital of Iceland, Reykjavik, Iceland.
Faiçal Jarraya, Research Lab LR19ES11 and Nephrology, Faculty of Medicine, Sfax University, Sfax, Tunisia.
Julia Kerschbaum, Austrian Dialysis and Transplant Registry, Department of Internal Medicine IV – Nephrology and Hypertension, Medical University Innsbruck, Innsbruck, Austria.
Kirill S Komissarov, Minsk Scientific and Practical Center for Surgery, Transplantology and Hematology, Minsk, Belarus.
Nadiia Kozliuk, Ukrainian Renal Data System (URDS), Ukraine.
Milica Kravljaca, Clinic of Nephrology, Clinical Center of Serbia, Belgrade, Serbia.
Mathilde Lassalle, REIN registry (Renal Epidemiology and Information Network), Agence de la Biomédecine, Saint-Denis La Plaine, France.
Johan M De Meester, Dutch-speaking Belgian Renal Registry (NBVN), Edegem, Belgium.
Mai Ots-Rosenberg, Tartu University, Faculty of Medicine, Department of Internal Medicine, Tartu, Estonia; Tartu University Hospital, Department of Internal Medicine, Tartu, Estonia.
Zoe Plummer, UK Kidney Association, Bristol, UK.
Danilo Radunovic, Clinic for Nephrology, Clinical Center of Montenegro, Podgorica, Montenegro.
Olena Razvazhaieva, Ukrainian Renal Data System (URDS), Ukraine.
Halima Resic, Society for Nephrology, Dialysis, and Transplantation of Bosnia and Herzegovina, Sarajevo, Bosnia and Herzegovina.
Olga Lucía Rodríguez Arévalo, Registry of Kidney Patients of the Valencian Community, General Directorate of Public Health, Ministry of Health, Valencia, Spain; Health and Well-being Technologies Program, Polytechnic University of Valencia, Valencia, Spain.
Carmen Santiuste de Pablos, Murcia Renal Registry, Department of Epidemiology, Murcia Regional Health Council, IMIB-Arrixaca, Murcia, Spain; CIBER Epidemiología y Salud Pública (CIBERESP), Madrid, Spain.
Nurhan Seyahi, Istanbul University-Cerrahpasa, Cerrahpasa Medical Faculty, Department of Nephrology, Istanbul, Turkey.
María Fernanda Slon-Roblero, Hospital Universitario de Navarra, Pamplona, Navarra, Spain.
Maria Stendahl, Swedish Renal Registry, Dept of Internal Medicine, Jonkoping Hospital, Jonkoping, Sweden.
Miloreta Tolaj-Avdiu, Nephrology Clinic, University Clinical Centre of Kosovo, Prishtina, Kosovo.
Sara Trujillo-Alemán, Health Quality Assessment and Information System Service, Dirección General de Programas Asistenciales, Servicio Canario de la Salud, Canary Islands, Spain.
Ieva Ziedina, Center of Nephrology, Pauls Stradins Clinical University Hospital, Riga, Latvia; Department of Internal Medicine, Riga Stradins University, Riga, Latvia; Latvian Association of Nephrology, Riga, Latvia.
Edita Ziginskiene, Lithuanian Nephrology, Dialysis and Transplantation Association, Kaunas, Lithuania; Nephrology Department, Medical Academy, Lithuanian University of Health Sciences, Kaunas, Lithuania.
Alberto Ortiz, Department of Nephrology and Hypertension, IIS-Fundacion Jimenez Diaz UAM, Madrid, Spain; Department of Medicine, Universidad Autonoma de Madrid, Madrid, Spain.
Kitty J Jager, Department of Medical Informatics, University of Amsterdam, ERA Registry, Amsterdam UMC, Amsterdam, The Netherlands; Amsterdam Public Health, Ageing & Later Life, Amsterdam, The Netherlands; Amsterdam Public Health, Quality of Care, Amsterdam, The Netherlands.
Vianda S Stel, Department of Medical Informatics, University of Amsterdam, ERA Registry, Amsterdam UMC, Amsterdam, The Netherlands; Amsterdam Public Health, Ageing & Later Life, Amsterdam, The Netherlands; Amsterdam Public Health, Quality of Care, Amsterdam, The Netherlands.
Anneke Kramer, Department of Medical Informatics, University of Amsterdam, ERA Registry, Amsterdam UMC, Amsterdam, The Netherlands; Amsterdam Public Health, Methodology, Amsterdam, The Netherlands.
FUNDING
The ERA Registry is funded by the European Renal Association (ERA). This article was written by B.A. Boerstra et al. on behalf of the ERA Registry, which is an official body of the ERA. P.B. reports payments from AstraZeneca and Takeda. S.B. reports consulting fees from GSK, Bayer, and AstraZeneca. A.C.A. reports payments from Diaverum Spain. F.J. reports payments from AstraZeneca, Boehringer Ingelheim, Servier, and Merck; and support for attending meetings and/or travel from Servier, AstraZeneca, Pfizer, and Fresenius. J.M. reports receiving support for attending meetings and/or travel from CSL Vifor. M.F.S.-R. reports receiving consulting fees from Baxter, Fresenius, and Nipro; payments from Baxter and Fresenius; and support for attending meetings and/or travel from Vifor, Fresenius, and Palex. I.Z. reports consulting fees from Astellas, Pharma, and Bayer; and payments from AstraZeneca, Bayer, Behringer Ingelheim, Norameda, and Swixx Biopharma. A.O. reports receiving grants from Sanofi; and consultancy or speaker fees or travel support from Advicciene, Astellas Pharma, AstraZeneca, Amicus, Amgen, Boehringer Ingelheim, Fresenius Medical Care, GSK, Bayer, Sanofi-Genzyme, Menarini, Mundipharma, Kyowa Kirin, Lilly, Alexion, Freeline, Idorsia, Chiesi, Otsuka, Novo-Nordisk, Sysmex, and Vifor Fresenius Medical Care Renal Pharma. K.J.J. reports receiving funds from ERA during the conduct of the study and grants from ESPN. V.S.S. reports receiving funds from ERA.
DATA AVAILABILITY STATEMENT
The data underlying this article have been published in the ERA Registry Annual Report 2021 (Supplementary information).
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1.Astley ME, Boenink R, Abd ElHafeez Set al. . The ERA Registry Annual Report 2020: a summary. Clin Kidney J 2023;16:1330–54. 10.1093/ckj/sfad087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eurostat: www.ec.europa.eu/eurostat/data/database (28 August 2023, date last accessed ).
- 3.Boenink R, Astley ME, Huijben JAet al. . The ERA Registry Annual Report 2019: summary and age comparisons. Clin Kidney J 2021;15:452–72. 10.1093/ckj/sfab273 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article have been published in the ERA Registry Annual Report 2021 (Supplementary information).