Abstract
Non-tuberculous mycobacteria (NTM) infections predominantly present as pulmonary disease. Although relatively rare, 20–30 % originate from extrapulmonary sites resulting in a wide range of clinical syndromes. Immunocompromised individuals are particularly susceptible. Clinical manifestations include skin and soft-tissue infections, lymphadenitis, musculoskeletal infections and disseminated disease. Diagnosing extrapulmonary NTM is challenging, and management is complex, often involving multiple radiological and microbiological investigations, long courses of combination antibiotic regimens and may require adjuvant surgical interventions. We highlight both the importance of involving NTM experts at an early stage and the role of a multidisciplinary approach in the diagnosis and management of these infections.
Keywords: Non-tuberculous mycobacteria infection, Localised, Disseminated, Extrapulmonary
Key points.
-
•
Cosmetic procedures or surgical procedures including prosthetic implants can cause extrapulmonary non-tuberculous mycobacteria (NTM) disease.
-
•
For localised extrapulmonary NTM disease, treatment is not usually urgent. Ensure adequate samples are obtained for microbiology (acid fast bacilli smear, culture and molecular testing) and histology before starting treatment.
-
•
Always ask for NTM expert advice before commencing treatment.
-
•
Treatment for NTM extrapulmonary disease may require two or more antibiotics.
-
•
In cases of disseminated disease further, investigation is required to look for immunosuppression including an HIV test.
Alt-text: Unlabelled box
1. Introduction
Non-tuberculous mycobacteria (NTM) are environmental opportunistic pathogens distributed widely in soil and water. The prevalence of NTM isolates is increasing in the UK and globally.1,2,3 This may be attributable to an increased at-risk population (secondary to increased organ transplantation, and the use of immunosuppressive therapies, in particular biologics), alongside increased awareness and improved diagnostics. Despite this rising prevalence, NTM disease remains relatively rare and poorly understood.4,5
Broadly, NTM are classified into slow growing mycobacteria (SGM) and rapid growing mycobacteria (RGM). This is based on the time taken to form a colony on solid culture media. RGM usually grow within 7 days, whereas SGM often need up to 14 days. Their ability to cause disease is dependent on the site of inoculation and the ability of the host immune system to clear them. NTM infection primarily manifests as pulmonary disease, although can affect any organ.
Previous data indicate that 20–30% of NTM infections originate from extrapulmonary sites.1,2,3,6,7 Extra-pulmonary disease is usually defined as a culture-confirmed NTM infection in the presence of clinical features suggestive of skin, soft tissue (wound or abscess) or lymphatic tissue disease, although it may also be found in the spine or joints. NTM may be isolated from body fluids such as urine, blood or cerebrospinal fluid. When culture is negative, molecular tests and/or histopathology are useful diagnostic tools in the presence of an appropriate clinical and epidemiological context.
Predominant NTM species vary according to country and geographical region. In recent years, outbreaks of extrapulmonary NTM (EP-NTM) have contributed to an increased awareness of EP-NTM related diseases and their associated high rates of morbidity and mortality. A notable example is a global outbreak from 2011–2016 of prosthetic valve endocarditis secondary to Mycobacterium chimaera (M. chimaera), which was later traced to contamination of water heater-cooler units used during open-heart surgery.8
In this article, we aim to describe the clinical risk factors for EP-NTM, relevant diagnostic tests, pharmacological treatments and non-pharmacological interventions. National and international management guidelines specific to EP-NTM remain limited owing to few prospective clinical trials.9 Therefore, expert clinical opinion and knowledge from relevant multidisciplinary NTM teams and advisory groups should be sought when there is a suspicion of EP-NTM disease.
1.1. Clinical presentation and risk factors
EP-NTM disease often affects a younger population but can occur at any age. There is a similar distribution between males and females.10 Patients with EP-NTM disease are most likely to present to outpatient settings with recurrent or long-standing infections that have failed to improve after initial standard antimicrobial treatment. Due to reduced awareness and the wide variety of presenting symptoms, patients with EP-NTM may have had multiple encounters with varying health care professionals across primary care, community services and outpatient settings.
Clinical presentation of EP-NTM disease is dependent on the site(s) of infection. A spectrum of conditions exists ranging from localised disease secondary to inoculation, lymphadenitis, infections affecting skin, soft tissue or musculoskeletal (MSK) system and disseminated disease (Table 1).
Table 1.
Extrapulmonary non-tuberculous mycobacterial (EP-NTM) disease – risk factors and clinical manifestations.
| Risk factor | Causative organism | Clinical manifestations |
|---|---|---|
| Inoculation | ||
| Fish tank +/- swimming pool granuloma | M. marinum | A slow growing, inflamed, erythematous nodule or plaque. Often painful and ulcerates over time. Associated with cellulitis |
| Buruli ulcer | M. ulcerans | Initially painless nodule that develops into large ulcers with a discoloured white/yellow base. Most commonly affects upper or lower limbs. |
| Lymphadenitis | M. avium complexa | Lymph node enlargement, most often cervical (usually affects children) |
| Skin and soft tissue infections | ||
| Keratitis, choroiditis, endophthalmitis | M. fortuitum M. chelonae M. abscessus | Erythematous and nodular lesions to the skin, epiphora, purulent discharge and proptosis |
| Cosmetic surgery | Wound dehiscence; erythema and induration; nodule and abscess formation | |
| Post-traumatic wound infections | ||
| Musculoskeletal infections | ||
| Tenosynovitis | M. avium complex* | Gradual and insidious onset of enlargement of the tendon and synovium with persistent slow progression. Rarely complicated by involvement of underlying muscles, bone structures and/or joint spaces |
| Osteomyelitis |
M. abscessus M. fortuitum M. chelonae |
Usually observed following trauma or joint replacement. Most commonly associated with disseminated NTM infection(s) and secondary to immunosuppression. Vertebral involvement – bone pain, neuropathic pain and neurological symptoms |
| Iatrogenic and post-procedural infections | ||
| Joint replacement |
M. abscessus, M. fortuitum M. chelonae |
Gradual onset of joint infection with swelling, erythema and fevers |
| Prosthetic heart valve |
M. chimaera M. fortuitum M. chelonae M. abscessus |
Infective endocarditis – fever, weight loss, malaise, anaemia, pancytopenia, valve insufficiency on echocardiogram |
| Cardiac surgery | M. chimaera | Overlying sternal wound infection with wound dehiscence; erythema and induration; nodule and abscess formation |
| Peritoneal dialysis-associated infection |
M. fortuitum M. chelonae M. abscessus |
Peritonitis – abdominal pain, nausea, vomiting, pseudo bowel obstruction. Rarely complicated by loculated ascites and formation of abdominal adhesions |
| Disseminated disease | ||
| HIV infection | M. avium complexa | Disseminated disease will usually present as persistent nonspecific symptoms including fever, night sweats, malaise and weight loss. Associated with respiratory (dry cough, dyspnoea) and/or abdominal (pain, diarrhoea, malabsorption) symptoms. Patients often have an associated pancytopenia and abnormal liver function tests indicating dissemination to bone marrow, liver and other organ systems |
| Post solid organ transplantation | ||
M. avium complex refers to a group of multiple NTM species which includes M. avium, M. intracellulare and M. chimaera
Specific examples of EP-NTM disease secondary to inoculation include M. marinum infection following trauma to the skin in contaminated swimming pools or bodies of water containing tropical fish and M. ulcerans infection, which can cause chronic, indolent necrotic skin ulcers known as ‘Buruli ulcers’. Whilst the latter has typically been rare outside of the African continent and in high-income settings, it has been reported with increasing incidence in Australia and attributed to changing climate.11
Cervical lymphadenitis is characteristically observed in immunocompetent children below the age of 5 years, and rarely affects adults unless immunosuppressed. This is usually a benign condition which is cured following surgical resection without the need for antibiotics. The most common NTMs causing lymphadenitis are M. avium, M. haemophilum, M. intracellulare and M. malmoense.
Skin and soft tissue EP-NTM infections often occur following traumatic injury, healthcare associated procedures or cosmetic procedures. Iatrogenic and/or nosocomial infections have been reported secondary to surgical site infections including post-laparoscopic surgery for peritoneal dialysis catheter insertion, abdominal wall abscesses from liposuction, and central intravenous line-associated blood stream infections. Prostheses including heart valves and musculoskeletal joints are also implicated. Outbreaks associated with acupuncture needle use and contaminated ultrasonography (US) gel have been reported in medical settings.12,13 In addition, EP-NTM infections have been attributed to cosmetic procedures including tattooing, piercing, mesotherapy, hydrotherapy, pedicures, injection of dermal fillers, botulinum toxin and augmentation surgery.14
Focal MSK infections range from infections of tendon sheaths, bursa, joints and bones, to those with spinal involvement. Other rare but observed sites of EP-NTM include cerebral, ocular, oral, breast and bloodstream infections.15
Disseminated EP-NTM disease is defined as an infection in two non-contiguous sterile sites or a positive result for mycobacteria from blood or bone marrow culture.3 This occurs primarily in immunocompromised individuals, for example those with advanced HIV infection or on long-term immunosuppression following solid organ transplantation (notably renal, cardiac, lung, liver transplants).16 Other risk factors include haematological malignancies, biologic agents, chemotherapy, and chronic corticosteroid use.
1.2. Diagnosis of EP-NTM disease
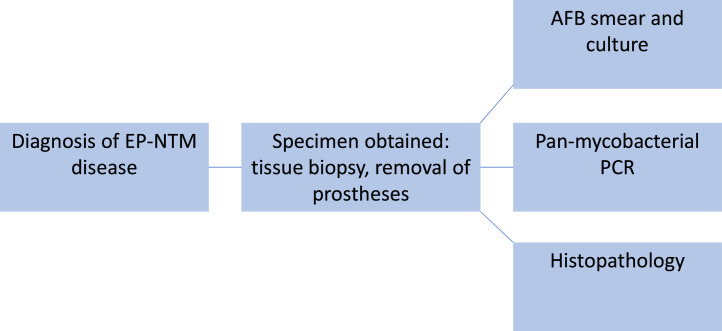
Diagnosis of EP-NTM is complex as the organisms may be fastidious, slow growing and require specialised culture media. It is crucial to make an aetiological diagnosis before starting empirical treatment, since drug susceptibility tests (DSTs) greatly vary depending on the NTM species. A three-pronged approach including mycobacterial culture, molecular studies when available, and histopathology can facilitate rapid and accurate diagnosis (Fig. 1). Diagnosing lymphadenitis microbiologically requires either excisional biopsy or fine needle aspiration for microscopy, mycobacterial culture and/or PCR.
Fig. 1.
Flow diagram of diagnostic strategy for extrapulmonary nontuberculous mycobacterial infections.
When NTM skin infections are suspected, skin biopsies for culture and histology are often sufficient to make a diagnosis. In some cases molecular testing may be required and offers a way to determine drug susceptibilities.17 In disseminated disease, mycobacterial blood cultures, urine cultures, and stool cultures in combination with bone marrow aspirate may be helpful.
The most common SGM NTM species causing extra-pulmonary disease in the UK are M. marinum group and M. malmoense. The most common RGM species are M. chelonae complex, M. abscessus complex, M. mucogenicum and M. fortuitum group.10
Radiological findings may be variable and non-specific depending on the site of infection. For suspected skin and soft tissue NTM infections, imaging may not be routinely required unless a localised abscess or deep tissue infection involving adjacent bone is suspected. In these cases, modalities including ultrasonography, computed tomography (CT) and magnetic resonance imaging (MRI) may be used. NTM lymphadenitis may present as enlarged lymph nodes with central necrosis similar to TB lymphadenitis. MSK involvement that has progressed to osteomyelitis and/or involvement of the vertebra may be apparent on MRI of the spine or affected bones and joint. Positron Emission Tomography (PET)-CT may be helpful especially in suspected disseminated disease. Imaging may be indicated for monitoring response to treatment, particularly when abscesses are inaccessible, or drainage is not possible.
1.3. Management of extrapulmonary NTM disease
Optimal management of a patient with EP-NTM requires a well-coordinated multidisciplinary team approach. This may include a range of professionals such as those in infectious diseases, respiratory medicine, microbiology, plastic surgery or orthopaedic surgery as applicable, and dermatology. Histopathologists with interest in infectious diseases can provide valuable support.
1.4. Treatment of extrapulmonary NTM infections
Pharmacological management may be avoided in some instances with source control in the form of debridement surgery. Mycobacteria involved in EP-NTM disease may have resistance to multiple antibiotics and adverse effects induced by these antibiotics may make treatment challenging.
Due to the paucity of prospective, randomised controlled clinical trials, choice of medication is often guided by evidence from treatment of pulmonary NTM infections, clinical experience and expert opinion. Susceptibility testing has a role, but discussion with the Microbiology Reference Laboratory is crucial to ensure the right DST is performed to aid therapy. DSTs are not always predictive of treatment outcomes, nor always feasible due to slow or failed growth of NTM species and/or degradation of antibiotics in culture. Whole genome sequencing may be useful to identify gene mutations that predict resistance and susceptibility to some antibiotics. The treating clinician should use in vitro susceptibility tests with an appreciation of their limitations and with the awareness that, unlike with TB, some NTM disease may not be eradicated in a given patient with therapy based on in vitro susceptibility results.
Duration of therapy is often between 6–18 months, however there are no existing guidelines on duration of therapy for EP-NTM infections. Treatment is often guided by clinical experience, expert opinion and clinical progress (Tables 2 and 3). Antibiotic regimens may be altered during the course of treatment due to the frequent occurrence of adverse effects. Treatment for EP NTM infections should only be started by multidisciplinary teams with experience in managing patients with EP-NTM.
Table 2.
Recommended treatment regimens for mild and severe disease.
| NTM Isolate | Organs affected | Recommended treatment regimen for severe disease | Recommended treatment regimen for mild disease |
|---|---|---|---|
| M. avium complex | Skin, soft tissue, tendons, joints, bone Disseminated infection |
Induction phase Intravenous amikacin + rifampicin + ethambutol + azithromycin/clarithromycin Continuation phase Rifampicin + ethambutol + azithromycin/clarithromycin |
Rifampicin + ethambutol + azithromycin/clarithromycin |
| M. abscessus | Osteomyelitis Peritoneal dialysis Catheter infections |
Induction phase Intravenous amikacin + cefoxitin/imipenem + tigecycline +azithromycin/clarithromycin Continuation phase Azithromycin, clofazimine, linezolid and consider quinolones |
Azithromycin, clofazimine, linezolid and consider quinolones |
| M. fortuitum | Cosmetic surgery |
Induction phase Intravenous amikacin + quinolone + doxycycline, consider linezolid Continuation phase Fluoroquinolone + doxycycline |
Fluoroquinolone + doxycycline |
| M. chelonae | Skin infections |
Induction phase Azithromycin/clarithromycin + tobramycin ± imipenem Continuation phase Azithromycin/clarithromycin + clofazimine (or doxycycline, linezolid or fluoroquinolones) |
Azithromycin/clarithromycin + clofazimine, doxycycline, linezolid, clofazimine, fluoroquinolones |
| M. marinum | Fishtank granulomaa |
Induction phase (usually not required as localised disease) Azithromycin, clarithromycin + rifampicin + ethambutol Continuation phase Azithromycin/clarithromycin + rifampicin + ethambutol |
Azithromycin/clarithromycin + rifampicin + ethambutol |
Based on expert opinion and adapted from 1999 Joint Tuberculosis Committee British Thoracic Society and American Thoracic Society/International Diseases Society of America guidelines.10,18 Treatment for EP NTM infections should only be started by multidisciplinary teams with experience in managing patients with EP-NTM.
In cases that have not responded to macrolide monotherapy.
Table 3.
Drugs used in the treatment of adult EP-NTM diseases and common adverse effects.
| Drug | Dosage | Drug monitoring (Baseline screening and follow up)* | Common adverse effects |
|---|---|---|---|
| Amikacin | 15 mg/kg once daily (max 1 g) intravenous Age >59 years: 10 mg/kg once daily (max 750 mg) intravenous Consider adjusted body weight in obese |
|
|
| Azithromycin | 250–500 mg once daily (oral) |
|
Dermatological: rash, pruritus Gastrointestinal: nausea, vomiting, diarrhoea, dyspepsia, change in taste, abdominal pain, flatulence General: fatigue Metabolism: anorexia Musculoskeletal and connective tissue: arthralgia Neurological: dizziness, headache, paraesthesia Ophthalmic: visual impairment Ototoxicity: deafness Other: increased concentration of other drugs |
| Bedaquiline | 400 mg daily for the first 2 weeks, followed by 200 mg three times per week (oral) (at least 48 h between doses) |
|
Musculoskeletal and connective tissue disorders: arthralgia, myalgia Cardiovascular: QTc prolongation Gastrointestinal: nausea, vomiting, diarrhoea Neurological: headache, dizziness Respiratory: haemoptysis |
| Clofazimine | 200 mg once daily (oral) loading for 2 months. Continue with 100 mg OD |
|
Dermatological: pigmentation from pink to brownish-black in 75–100 % of the patients within a few weeks of treatment; ichthyosis and dryness; rash and pruritus. Pigmentation resolves after cessation of treatment Gastrointestinal: abdominal and epigastric pain, diarrhoea, nausea, vomiting, gastrointestinal intolerance Ocular: diminished vision, conjunctival and corneal pigmentation due to clofazimine crystal deposits; dryness; burning; itching; irritation Other: discoloration of urine, faeces, sputum, sweat; elevated blood sugar; elevated erythrocyte sedimentation rate (ESR) |
| Doxycycline | 100 mg twice daily (oral) | Routine monitoring |
Dermatological: photosensitivity, rash Gastrointestinal: nausea, vomiting Neurological: headache, myasthenia gravis may be increased and SLE worsened Immunological: hypersensitivity, angioedema |
| Ethambutol | 15 mg/kg once daily (Max dose 1.2 g) Obesity: where actual body weight (ABW) is >20 % above ideal body weight (IBW), use IBW |
|
Endocrine: hyperuricaemia, Gastrointestinal: nausea and vomiting Ocular: optic neuritis |
| Imipenem | 1000 mg two to three times daily (intravenous) Consider 500 mg two to three times daily (intravenous) for small, frail or elderly patients |
Routine monitoring of liver function tests |
Dermatologic: rash Gastrointestinal: diarrhoea, vomiting, nausea Haematologic: eosinophilia Hepatic: increases in serum transaminases, increases in serum alkaline phosphatase Vascular: thrombophlebitis |
| Moxifloxacin | 400 mg once daily, depending on weight |
|
Gastrointestinal: diarrhoea, nausea, and vomiting Skin: photosensitivity, rash Hepatic: transient increases in LFTs Other: be aware of possible drug interactions MHRA important safety information: increased risk of tendon damage, aortic aneurysm, heart valve regurgitation, psychiatric reactions. Please see MHRA alert for further information |
| Linezolid | 600 mg once daily (oral or intravenous) |
|
Gastrointestinal: diarrhoea, nausea, vomiting Neurological: headache, dizziness, peripheral neuropathy Haematological: Bone marrow suppression Hepatic: transient increases in liver function tests Dermatological: urticaria, rash |
| Rifampicin | <50 kg: 450 mg once a day (oral or intravenous) > 50 kg: 600 mg once a day (oral or intravenous |
|
General: flu-like syndrome Gastrointestinal: nausea, vomiting Haematologic: thrombocytopenia with or without purpura (usually associated with intermittent therapy). Reversible after discontinuing rifampicin Hepatic: transient increases in LFTs Neurological: headache, dizziness Other: reddish discolouration of urine, sweat, sputum, tears. Multiple drug interactions. |
| Tobramycin | 4.5–7 mg/kg once a day (intravenous) |
|
Gastrointestinal: appetite decreased, diarrhoea, nausea, taste altered, oropharyngeal pain, vomiting Neurological: dizziness, headache Dermatological: skin reactions Respiratory: bronchospasm, chest discomfort, cough aphonia, dysphonia haemoptysis Nephrotoxicity: accumulation if renal impairment Ototoxicity and vestibular toxicity: irreversible vestibulocochlear nerve damage leading to hearing loss, and/or loss of balance Other: fever |
The majority of drugs listed are currently used off-license in the treatment of NTM in the UK. The adult doses stated are based on normal hepatic and renal function. Treatment for EP NTM infections should only be started by multidisciplinary teams with experience in managing patients with EP-NTM.
Routine toxicity monitoring tests (full blood count [FBC], urea and electrolytes [U&Es], liver function tests [LFTs]) should be performed at baseline and intermittently throughout antibiotic treatment. More specific monitoring, if required, is outlined above.
Surgical options can play a key part in management of EP-NTM and procedures vary from debridement, excision and drainage. In cases of bacteraemia, removal of devices, as well as anti-mycobacterial therapy, is indicated for a minimum of 2–3 months. Relapse of bacteraemia has been reported.19
Unlike with TB, patients with EP-NTM do not necessarily need to be isolated as person-to-person transmission of NTM is rare. Transmission, especially of pulmonary NTM, has however been reported.20 Currently, NTMs are non-notifiable to public health services in the UK, they are notifiable in some other parts of the world.19 This facilitates and enables public health services to detect outbreaks for example cutaneous infections arising from point-sources including cosmetic surgery and tattoo parlours. Whether NTM should be notifiable has led to much debate in the UK, not least due to the interesting dynamic with other notifiable mycobacterial diseases, predominantly TB, where person-to-person transmission predominates.
2. Conclusion
EP-NTM disease varies from localised to disseminated infection. Diagnosis is challenging and may require multiple biopsies and cultures to confirm a diagnosis, as well as the input of several specialties. Treatment is complex and often requires a prolonged and multi-drug regimen following expert opinion. Adverse effects are common, and patients require ongoing monitoring.
Footnotes
10.7861/clinmed.2023-0519.
References
- 1.Jarchow-MacDonald A, Smith M, Seagar AL, et al. Changing incidence and characteristics of nontuberculous mycobacterial infections in Scotland and comparison with Mycobacterium tuberculosis complex incidence (2011 to 2019) Open Forum Infect Dis. 2022;10:ofac665. doi: 10.1093/ofid/ofac665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shih DC, Cassidy PM, Perkins KM, et al. Extrapulmonary nontuberculous mycobacterial disease surveillance – Oregon, 2014–2016. MMWR Morb Mortal Wkly Rep. 2018;67:854–857. doi: 10.15585/mmwr.mm6731a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobayashi T, Nishijima T, Teruya K, et al. High mortality of disseminated non-tuberculous mycobacterial infection in HIV-infected patients in the antiretroviral therapy era. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0151682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dahl VN, Mølhave M, Fløe A, et al. Global trends of pulmonary infections with nontuberculous mycobacteria: a systematic review. Int J Infect Dis. 2022;125:120–131. doi: 10.1016/j.ijid.2022.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed I, Tiberi S, Farooqi J, et al. Non-tuberculous mycobacterial infections – a neglected and emerging problem. Int J Infect Dis. 2020;92:S46–S50. doi: 10.1016/j.ijid.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 6.Hermansen TS, Ravn P, Svensson E, Lillebaek T. Nontuberculous mycobacteria in Denmark, incidence and clinical importance during the last quarter-century. Sci Rep. 2017;7:6696. doi: 10.1038/s41598-017-06931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simons S, Van Ingen J, Hsueh PR, et al. Nontuberculous mycobacteria in respiratory tract infections, eastern Asia. Emerg Infect Dis. 2011;17:343–349. doi: 10.3201/eid1703100604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wetzstein N, et al. Clinical characteristics and outcome of Mycobacterium chimaera infections after cardiac surgery: systematic review and meta-analysis of 180 heater-cooler unit associated cases. Clin Microbiol Infect. 2023;29:1008–1014. doi: 10.1016/j.cmi.2023.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Martiniano SL, Caceres SM, Poch K, et al. Prospective evaluation of nontuberculous mycobacteria disease in cystic fibrosis: The design of the PREDICT study. J Cyst Fibros. 2023;2023 doi: 10.1016/j.jcf.2023.08.007. S1569–1993(23)00876-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 11.Blasdell KR, McNamara B, O'Brien DP, et al. Environmental risk factors associated with the presence of Mycobacterium ulcerans in Victoria, Australia. PLoS ONE. 2022;17 doi: 10.1371/journal.pone.0274627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang P, Walsh S, Murray C, et al. Outbreak of acupuncture-associated cutaneous Mycobacterium abscessus infections. J Cutan Med Surg. 2006;10:166–169. doi: 10.2310/7750.2006.00041. [DOI] [PubMed] [Google Scholar]
- 13.Cheng A, Sheng WH, Huang YC, et al. Prolonged postprocedural outbreak of Mycobacterium massiliense infections associated with ultrasound transmission gel. Clin Microbiol Infect. 2016;22 doi: 10.1016/j.cmi.2015.11.021. 382-e1. [DOI] [PubMed] [Google Scholar]
- 14.Daniau C, Lecorche E, Mougari F, et al. Association of healthcare and aesthetic procedures with infections caused by nontuberculous mycobacteria, France, 2012‒2020. Emerg Infect Dis. 2022;28:518. doi: 10.3201/eid2803.211791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ricotta EE, Adjemian J, Blakney RA, et al. Extrapulmonary nontuberculous mycobacteria infections in hospitalized patients, United States, 2009–2014. Emerg Infect Dis. 2021;27:845. doi: 10.3201/eid2703.201087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abad CL, Razonable RR. Non-tuberculous mycobacterial infections in solid organ transplant recipients: an update. J Clin Tuberc Other Mycobact Dis. 2016;4:1–8. doi: 10.1016/j.jctube.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cowman S, Burns K, Benson S, et al. The antimicrobial susceptibility of non-tuberculous mycobacteria. J Infect. 2016;72:324–331. doi: 10.1016/j.jinf.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 18.Subcommittee of the Joint Tuberculosis Committee of the British Thoracic Society*. Management of opportunist mycobacterial infections: Joint Tuberculosis Committee guidelines 1999. Thorax. 2000;55:210–218. doi: 10.1136/thorax.55.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dabaja-Younis H, Damouni-Shalabi R, Ganem-Zoubi N, et al. Nontuberculous mycobacteria blood stream infection in pediatric and adult patients: 15 years surveillance. Pediatr Infect Dis J. 2022;41:e216–e219. doi: 10.1097/INF.0000000000003473. [DOI] [PubMed] [Google Scholar]
- 20.Yan J, Kevat A, Martinez E, et al. Investigating transmission of Mycobacterium abscessus amongst children in an Australian cystic fibrosis centre. J Cyst Fibros. 2020;19:219–224. doi: 10.1016/j.jcf.2019.02.011. [DOI] [PubMed] [Google Scholar]