Abstract
Non-tuberculous mycobacteria (NTM) are ubiquitous environmental organisms that can cause significant disease in both immunocompromised and immunocompetent individuals. The incidence of NTM pulmonary disease (NTM-PD) is rising globally. Diagnostic challenges persist and treatment efficacy is variable. This article provides an overview of NTM-PD for clinicians. We discuss how common it is, who is at risk, how it is diagnosed and the multidisciplinary approach to its clinical management.
KEYWORDS: Non-tuberculous mycobacteria, Pulmonary disease, Epidemiology, Diagnosis, Multidisciplinary management
Background
Non-tuberculous mycobacteria (NTM), which comprise all mycobacterial species other than those that cause tuberculosis (TB) and leprosy, are ubiquitous in the environment and are found particularly in water or soil. To date, approximately 200 NTM species having been identified. In humans, they most commonly infect the lungs. This can lead to NTM pulmonary disease (NTM-PD) (Fig. 1).1 NTM infection incidence is rising both in the UK and globally.2,3 NTM-PD is particularly seen in patient populations with structural lung diseases or impaired immunity; but it can also occur in people with no apparent risk factors.4 It remains an under-recognised condition that is difficult to diagnose and challenging to treat due to the toxicity of drug therapies that often have limited efficacy. It can be associated with poor treatment outcomes in an often-older population with comorbidities.
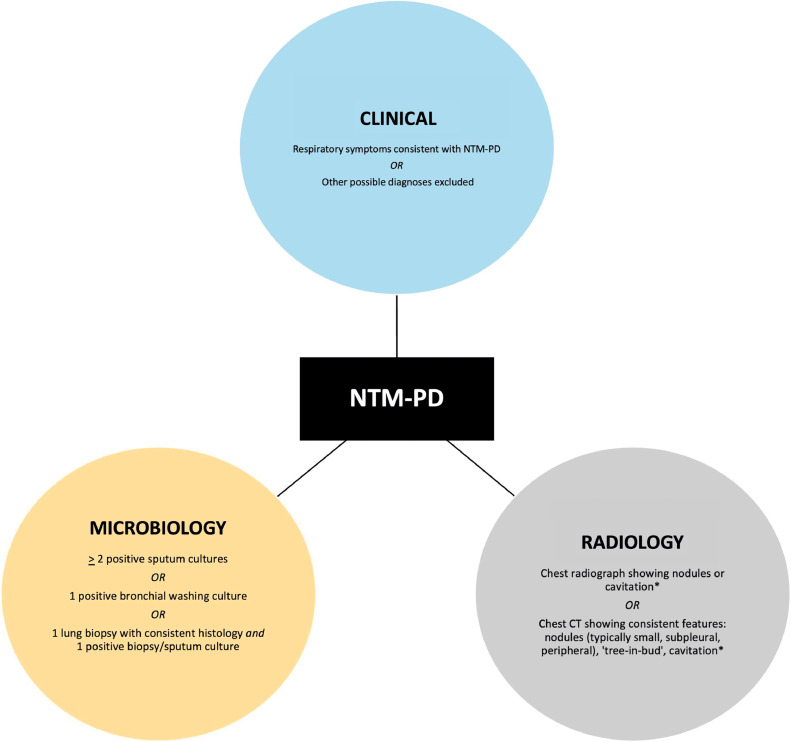
Fig 1.
Diagnostic triad for NTM-PD.1
⁎Chest CT is preferrable to a chest pain radiograph; even on CT, there may be no specific radiological features for NTM-PD.
As many people at high risk of NTM-PD are often reviewed and managed on the acute medical take or in general medicine clinics, it is important for general physicians to know about this condition and its treatment. Here, we provide a multidisciplinary overview of managing and living with NTM-PD. Using comments from patients involved in the development of this article, we consider its epidemiology, predisposing risk factors, how it is diagnosed and important aspects of its clinical management.
Epidemiology and risk factors
NTM belonging to Mycobacterium avium complex (MAC), which comprises M avium, M intracellulare and M chimaera, are the most frequently isolated in the UK.5 M abscessus is often seen in east Asia; in the UK, it is typically associated with significant immunocompromise or cystic fibrosis (CF).5,6 Other common respiratory NTM species are M kansasii, M malmoense and M xenopi. NTM-PD is not a notifiable disease in the UK or in most jurisdictions and therefore understanding its epidemiology relies on local and regional surveillance mechanisms.4 Between 2007 and 2012, the incidence of NTM culture positive isolates increased in England, Wales and Northern Ireland from 5.6/100,000 to 7.6/100,000; among those with pulmonary infections, incidence increased from 4.0/100,000 to 6.1/100,000.2 In Scotland, NTM infection incidence rose from 3.4/100,000 to 6.5/100,000 between 2011 and 2019.7 The 5-year all-cause mortality for MAC lung disease ranges between 10 and 48%.8
NTM are often isolated from soil and water, and exposure to water supplies contaminated with NTM has been associated with an increased risk of developing NTM disease. NTM have been isolated in taps, shower heads, swimming pools and hot tubs.9 Epidemiological studies have shown that high levels of humidity are associated with increased pulmonary NTM infection.10 While NTM infections are generally not considered to be transmissible between people, some M abscessus clones may be communicable between certain at-risk individuals via fomites or aerosols.11 Studies on potential transmission mechanisms are ongoing.
Host risk factors that predispose to NTM-PD can be divided into underlying structural lung diseases (including certain genetic disorders), impaired immunity and other associated conditions. Some people with NTM-PD have one or more of a characteristic set of features including chest wall or spinal abnormalities, mitral valve prolapse and cystic fibrosis transmembrane conductance regulator protein dysfunction.12 The most common comorbid risks are chronic obstructive pulmonary disease (COPD) and bronchiectasis. Hence there should be a low threshold to suspect NTM-PD in these patients (Table 1).13
Table 1.
Host risk factors for NTM-PD.
| Host risk factor | Examples |
|---|---|
| Structural lung disease |
|
| Impaired immunity |
|
| Other associated conditions |
|
Typically a risk factor for disseminated NTM infection.
Diagnosis
The diagnosis of NTM-PD is contingent on a triad of clinical, microbiological and radiological criteria being satisfied (Fig. 1).1 This is important when differentiating between incidental airway presence of NTM and significant pulmonary disease.
Clinical features
‘Many patients may be symptomatic for years before being tested for NTM.’ (Patient expert)
The clinical presentation of NTM-PD is similar to that of several other respiratory conditions, including lung cancer and TB, as well as pre-existing lung disease. Symptoms may include a persistent cough, sputum production, haemoptysis, breathlessness, fever, night sweats, unintentional weight loss and significant fatigue.1 It is important to look for clues such as a person reporting an increased frequency of lower respiratory tract infections despite antibiotics. This is most relevant in those with COPD or bronchiectasis where symptoms may be indistinguishable and the persistence or duration of symptoms becomes key.
Microbiology
Specific microbiological criteria must be met for NTM-PD to be diagnosed. Three separate sputum samples should be collected, ideally on different days and in the early morning if possible. Acquiring samples by bronchoscopy is in most settings more of an undertaking than using sputum to diagnose NTM-PD. It should be considered if sputum is either unavailable or negative (including induced sputum samples, where available) and there is a high level of clinical suspicion.14 Microbiological confirmation is important because different underlying NTM species are associated with varying clinical courses and require appropriately tailored antibiotic regimens. Slow-growing NTM can take up to 6–8 weeks to culture and include MAC, M kansasii, M malmoense and M xenopi. Rapid-growing NTM usually culture within 7 days and include M abscessus, M chelonae and M fortuitum. Alongside culture-based techniques, speciation can be achieved using line probe assays, matrix-assisted laser desorption ionization–time-of-flight mass spectrometry or gene sequencing.15, 16, 17, 18
Drug susceptibility testing (DST) is important when choosing antimicrobial therapy, though in vitro DST results are frequently inconsistent with in vivo effectiveness and discussion with an expert in NTM infection is advised.19 As a minimum, MAC should be tested against clarithromycin (which will also give information on azithromycin sensitivities) and amikacin; M abscessus against clarithromycin, amikacin and cefoxitin; and M kansasii against rifampicin.1
Radiology
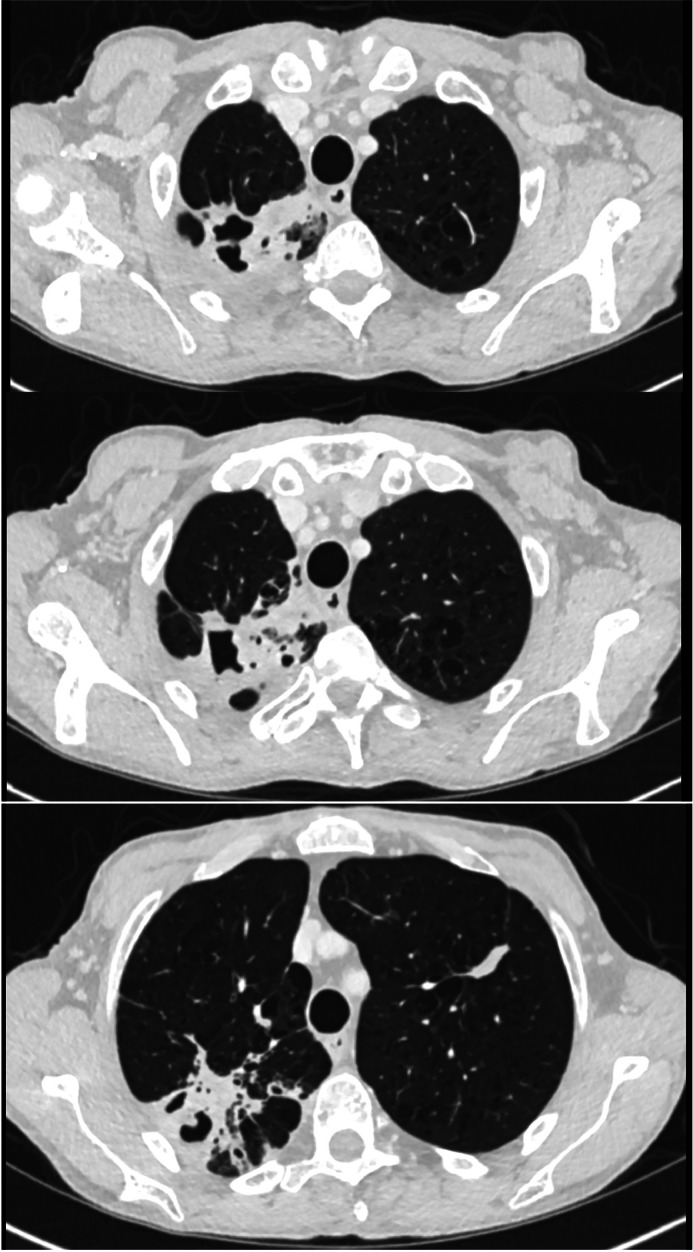
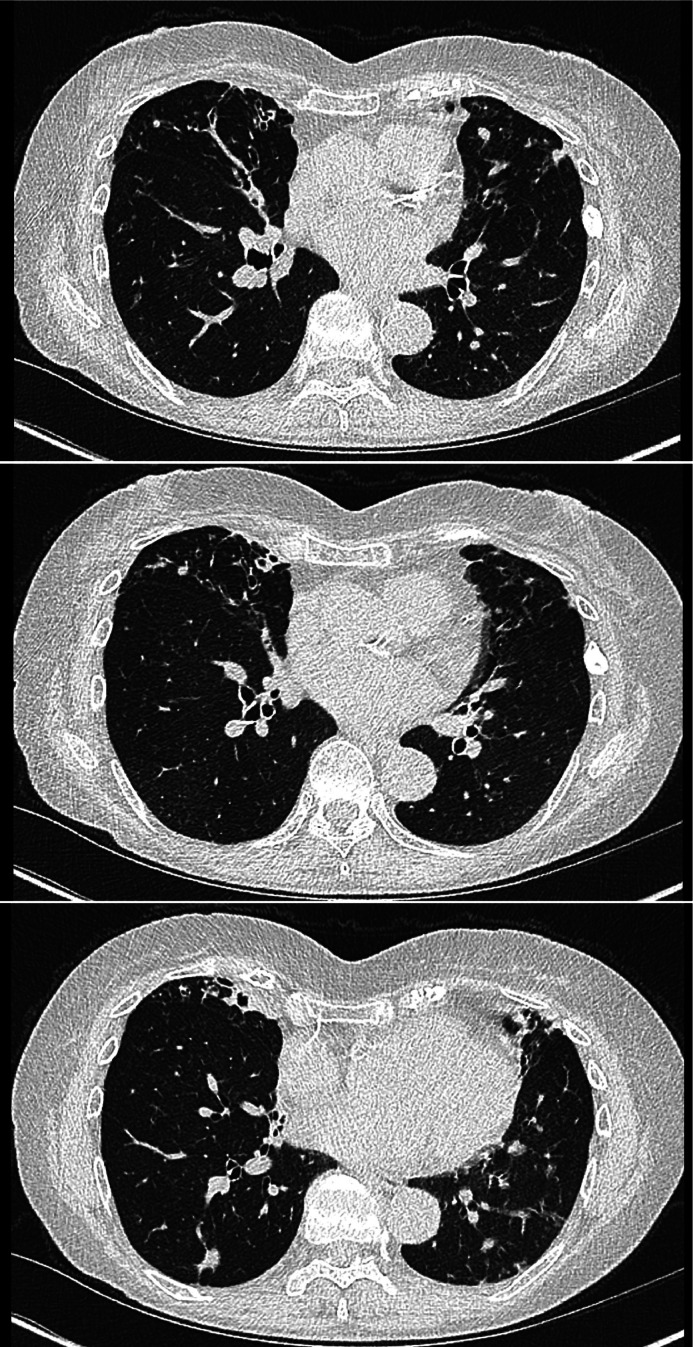
All patients in whom NTM-PD is suspected should have a baseline chest radiograph and preferably high-resolution chest CT imaging. There are two broad radiological patterns observed in NTM-PD: fibrocavitary disease, characterised by multiple, thin-walled cavities, usually in the upper lobes of the lungs (Fig. 2); and nodular bronchiectatic disease, with nodules, bronchiectasis and bronchial wall thickening (Fig. 3).20 The latter is commonly seen in individuals with no known pre-existing lung disease, but can often be mixed with cavitating nodular change.21
Fig 2.
Serial cross-sectional chest CT images from an individual with cavitary NTM-PD secondary to Mycobacterium avium complex. Note the co-existent emphysema.
Fig 3.
Serial cross-sectional chest CT images from an individual with nodular NTM-PD secondary to Mycobacterium avium complex. Note the associated middle lobe bronchiectasis.
Additional considerations
Specialist tests for immune deficiency should be considered in people with a history of recurrent pulmonary or extrapulmonary infections or in those with a known family history of immunodeficiency.22 All patients with bronchiectasis should have immunoglobulins measured. Furthermore, there is a potential link between NTM-PD and Aspergillus pulmonary infections.23,24 It is therefore prudent to consider checking sputum fungal cultures and fungal serology to exclude concomitant or sequential fungal pulmonary infections.25
Management
‘People with NTM-PD should be encouraged to ask questions and seek clarification about all aspects of their diagnosis and treatment.’ (Patient expert)
The decision to start treatment should be based on national guidelines and involve a multidisciplinary team with experience of delivering high-quality, standardised care for NTM infection (Table 2).26 The benefits, risks and potential outcomes of commencing treatment should be discussed with patients.
Table 2.
Possible multidisciplinary team members managing NTM-PD (listed in alphabetical order).
| • Clinical immunologists |
| • Dietitians |
| • Microbiologists |
| • Nurses |
| • Pharmacists |
| • Physicians with expertise in NTM-PD |
| • Physiotherapists |
| • Psychologists |
| • Radiologists |
Pharmacological treatment
‘Patients should be informed of the potential need for oral, nebulised or intravenous medications and understand that the efficacy of different regimens may vary. Treatment regimens should be commenced following clear and detailed discussions between patients and their clinical team.’ (Patient expert)
As current drug therapies are associated with significant tolerance and toxicity issues, the decision to commence treatment should consider the NTM species, severity of disease, risks of progression, comorbidities and patient choice.1 The goals of treatment should be agreed at the outset.
Recommendations are available for treating the most common NTM species that cause NTM-PD: MAC, M kansasii, M malmoense, M xenopi and M abscessus (Table 3). Treatment is usually continued for at least 12 months after achieving culture conversion.1,14 Nebulised liposomal amikacin has recently been approved in the UK as an add-on treatment for refractory MAC lung disease, which is where the patient remains culture positive despite treatment.27 Although treatment response varies, overall about two-thirds of patients with MAC will have a sustained improvement following therapy. However, up to half may then relapse again (often with a different organism to the original infection). Treatment of M abscessus lung disease is particularly complex and practice is not standardised. Antibiotic choices are guided by DST, patient tolerance and MDT discussion. The optimised regimen has not yet been determined (Table 3).
Table 3.
Treatment regimens for NTM-PD (adapted from British Thoracic Society NTM-PD guidelines from 2017).1
| NTM species | Treatment regimen for associated pulmonary disease | Comments |
|---|---|---|
| Mycobacterium avium complex (MAC) | Rifampicin + Ethambutol + Either clarithromycin or azithromycin |
|
| M kansasii | Rifampicin + Ethambutol + Either clarithromycin or azithromycin or isoniazid (with pyridoxine) |
|
| M malmoense | Rifampicin + Ethambutol + Either clarithromycin or azithromycin |
|
| M xenopi | Rifampicin + Ethambutol + Either clarithromycin or azithromycin + Either moxifloxacin or isoniazid |
|
| M abscessus |
Initial phase Amikacin (IV) + Tigecycline (IV) + Imipenem (IV)b + Either clarithromycin or azithromycin Continuation phase Amikacin (NEB) + Either clarithromycin or azithromycin + One to three of the following: clofazimine, linezolid, minocycline, moxifloxacin, co-trimoxazole |
|
Route of drug administration is oral unless otherwise stated. DST = drug susceptibility testing. IV = intravenous. NEB = nebulised.
Severe disease includes cavitary disease and/or smear positive sputum.
Medication given if tolerated.
The complex drug regimens pose a significant challenge for many patients due to overlapping toxicities (Table 4) and potential drug–drug interactions with their pre-existing medications. Pharmacists are well placed to advise patients how to take their treatment and the importance of good adherence to regimens, as well as identifying and managing any adverse effects. Monitoring for toxicity through blood tests, ECG and audiometry should be performed regularly according to national recommendations and the potential toxicity profiles of the prescribed drugs.1,14 TB drug monographs is a helpful resource for clinicians in this regard.28
Table 4.
Common side effects and adverse drug reactions during the treatment of NTM-PD.
| Drug | Side effects and adverse drug reactions | Approach to symptoms and suggested management |
|---|---|---|
| Rifampicin | Orange-red discolouration of urine and other body secretions |
|
| Rifampicin Ethambutol Azithromycin Clarithromycin Tigecycline Clofazimine Co-trimoxazole Imipenem Linezolid Minocycline Moxifloxacin |
Gastrointestinal (nausea, vomiting and diarrhoea) |
|
| Isoniazid Linezolid |
Peripheral neuropathy (PN) |
|
| Rifampicin | Flu-like symptoms |
|
| Ethambutol Linezolid |
Optic neuritis |
|
| Moxifloxacin | Musculoskeletal |
|
| Rifampicin Isoniazid Moxifloxacin Linezolid Tigecycline |
Hepatotoxicity |
|
| Amikacin | Nephrotoxicity |
|
| Amikacin Azithromycin |
Ototoxicity |
|
| Azithromycin Clarithromycin Moxifloxacin Clofazimine (possible) |
Cardiovascular |
|
| Rifampicin Macrolides Clofazimine Co-trimoxazole Minocycline Imipenem Tigecycline Moxifloxacin |
Rash |
|
| Clofazimine | Skin discolouration |
|
PN = peripheral neuropathy; ULN = upper limit of normal
Clinical response to treatment should be assessed regularly by using sputum culture every four to 12 weeks until culture conversion and by evaluating symptoms, quality of life measures and radiological parameters. People who remain culture positive despite 6 months of NTM treatment are more likely to have poor outcomes and may require extended treatment. Surgical intervention may be warranted in specific clinical contexts, such as massive haemoptysis.
Respiratory physiotherapy
‘Prompt review by respiratory physiotherapists is paramount.’ (Patient expert)
Respiratory physiotherapy is an integral part of the daily management of NTM-PD. Persistent detection of NTM in sputum is associated with worse outcomes29 and physiotherapy interventions have been shown to mitigate this in people with bronchiectasis and CF.30,31 The aims of physiotherapy are to maintain ventilation in all parts of the lungs; postpone progression of pulmonary disease; stimulate establishment and retention of normal physical capacity; and avoid pain and musculoskeletal complications due to pulmonary or bone disease. The wider role of the physiotherapist in NTM-PD can minimise the consequences of repeated respiratory exacerbations by educating patients in airway clearance techniques and through the use of adjunctive and nebulised therapies.30 Physiotherapists adapt and optimise treatment regimens for individual patients in line with their age and disease progression. They offer education and support for people living with NTM-PD enabling them to self-manage their respiratory symptoms.
Nutritional aspects
‘Access to dietitians for advice about nutrition is important.’ (Patient expert)
Low body mass index (BMI) and malnutrition are recognised risk factors for the development and progression of NTM-PD.32,33 Current research is notably lacking in identifying whether weight restoration can positively impact outcomes in this context. In the broader landscape of respiratory diseases, a low BMI is consistently correlated with reduced lung function and compromised immune responses.34,35 In cases of frequent or severe respiratory infections, malnutrition tends to follow, leading to higher mortality rates, increased hospitalisation and reduced quality of life. Moreover, treatment modalities that produce gastrointestinal side effects can further exacerbate malnutrition through anorexia, weight loss or restrictive dietary patterns. In light of the paucity of specific research in this area, it is prudent to advocate for universal malnutrition assessment using the internationally recognised Malnutrition Universal Screening Tool (MUST).36 Robust referral systems to dietitians should be established, mirroring practices in analogous respiratory conditions, until further research can inform more targeted support in this area.
Nursing considerations
‘People living with NTM-PD should be directed towards support and resources, including NTM Patient Care UK (www.ntmpatientcare.uk).’ (Patient expert)
Most NTM services are in need of specific NTM specialist nurse provisions and rely on ad hoc support from TB, infectious disease and respiratory clinical nurse specialists.37 Given the current absence of a standard of care model for NTM-PD patients, the role of nurse specialists has been articulated using the Royal College of Nursing case management tool for people with TB.38 This defines the level of case management required and provides a standard care framework for every patient.39 In light of the chronicity and complexity of NTM-PD, the relationship between the patient and their nurse specialist may be particularly important as nurses are typically the first point of contact and well-placed to provide long-term support, education and treatment supervision. Liaising with psychology services should be considered if appropriate.
Summary
NTM-PD is a complex lung condition. Recognition of the disease (and hence when it is diagnosed following symptom onset) can be considerably delayed. Even then, it may be difficult to treat. It should be considered in people with a relevant clinical history, especially in COPD or bronchiectasis. Improving the accuracy and timeliness of diagnosis while enhancing clinical outcomes in those living with NTM-PD are important clinical priorities. Achieving these will be contingent upon clinicians recognising those at risk, understanding the variable presentation of the disease and arranging timely investigations. Respiratory or infectious disease specialist input should be sought to discuss potential cases. Multidisciplinary management is essential to ensure that care remains holistic and patient-centred, particularly when long-term drug treatment is initiated.26
Key points.
-
•
Non-tuberculous mycobacterial pulmonary disease (NTM-PD) is becoming more common and may present with non-specific respiratory or systemic symptoms in individuals with or without underlying risk factors.
-
•
Diagnosis is contingent upon clinical, microbiological and radiological criteria being met.
-
•
People in whom NTM-PD is suspected or diagnosed should be reviewed and managed by clinicians with appropriate expertise and experience.
-
•
A multidisciplinary approach to management is required, comprising tailored pharmacological intervention, respiratory physiotherapy, dietetic input and nursing support.
-
•
Individuals diagnosed with NTM-PD should be provided with access to relevant support services and signposted to appropriate information resources about their ongoing care.
Alt-text: Unlabelled box
Declaration of competing interest
All authors are members of NTM Network UK, which is a network of healthcare professionals, researchers and patients from across the UK who have an interest in infections caused by non-tuberculous mycobacteria. TGDC has received non-financial support from Napp and GSK for attendance at ERS conference; TGDC's employer has received payment for his participation in advisory boards or for providing teaching sessions from AstraZeneca, Chiesi, GSK, Novartis, Boehringer Ingelheim, and Insmed, outside the submitted work. CC received non-financial support from GSK for attendance at ERS; CC's employer has received financial support from AstraZeneca through her grant application; CC received payment for her participation in advisory boards or providing teaching from AstraZeneca, Chiesi, GSK and Insmed, outside the submitted work. RH has received consultancy fees from Insmed, outside the submitted work.
Funding
Kartik Kumar was supported by the National Institute for Health and Care Research (NIHR) Imperial Biomedical Research Centre (BRC). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. Kartik Kumar was also supported by the Lee Family endowment to the Faculty of Medicine at Imperial College London.
References
- 1.Haworth CS, Banks J, Capstick T, et al. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD) Thorax. 2017;72:ii1–ii64. doi: 10.1136/thoraxjnl-2017-210927. [DOI] [PubMed] [Google Scholar]
- 2.Shah NM, Davidson JA, Anderson LF, et al. Pulmonary Mycobacterium avium-intracellulare is the main driver of the rise in non-tuberculous mycobacteria incidence in England, Wales and Northern Ireland, 2007–2012. BMC Infect Dis. 2016;16:195. doi: 10.1186/s12879-016-1521-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prevots DR, Marshall JE, Wagner D, Morimoto K. Global epidemiology of nontuberculous mycobacterial pulmonary disease: a review. Clin Chest Med. 2023;44:675–721. doi: 10.1016/j.ccm.2023.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar K, Loebinger MR. Nontuberculous mycobacterial pulmonary disease: clinical epidemiologic features, risk factors, and diagnosis: the nontuberculous mycobacterial series. Chest. 2022;161:637–646. doi: 10.1016/j.chest.2021.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Hoefsloot W, van Ingen J, Andrejak C, et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. Eur Respir J. 2013;42:1604–1613. doi: 10.1183/09031936.00149212. [DOI] [PubMed] [Google Scholar]
- 6.Martiniano SL, Nick JA, Daley CL. Nontuberculous mycobacterial infections in cystic fibrosis. Clin Chest Med. 2022;43:697–716. doi: 10.1016/j.ccm.2022.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Jarchow-MacDonald A, Smith M, et al. Changing incidence and characteristics of nontuberculous mycobacterial infections in Scotland and comparison with Mycobacterium tuberculosis complex incidence (2011 to 2019) Open Forum Infect Dis. 2023;10:ofac665. doi: 10.1093/ofid/ofac665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diel R, Lipman M, Hoefsloot W. High mortality in patients with Mycobacterium avium complex lung disease: a systematic review. BMC Infect Dis. 2018;18:206. doi: 10.1186/s12879-018-3113-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falkinham JO., 3rd Nontuberculous mycobacteria from household plumbing of patients with nontuberculous mycobacteria disease. Emerg Infect Dis. 2011;17:419–424. doi: 10.3201/eid1703.101510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adjemian J, Olivier KN, Seitz AE, et al. Spatial clusters of nontuberculous mycobacterial lung disease in the United States. Am J Respir Crit Care Med. 2012;186:553–558. doi: 10.1164/rccm.201205-0913OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bryant JM, Grogono DM, Rodriguez-Rincon D, et al. Emergence and spread of a human-transmissible multidrug-resistant nontuberculous mycobacterium. Science. 2016;354:751–757. doi: 10.1126/science.aaf8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim RD, Greenberg DE, Ehrmantraut ME, et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med. 2008;178:1066–1074. doi: 10.1164/rccm.200805-686OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loebinger MR, Quint JK, van der Laan R, et al. Risk factors for nontuberculous mycobacterial pulmonary disease: a systematic literature review and meta-analysis. Chest. 2023;164:1115–1124. doi: 10.1016/j.chest.2023.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Daley CL, Iaccarino JM, Lange C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Clin Infect Dis. 2020;71:905–913. doi: 10.1093/cid/ciaa1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez-Temporal D, Alcaide F, Mareković I, et al. Multicentre study on the reproducibility of MALDI-TOF MS for nontuberculous mycobacteria identification. Sci Rep. 2022;12:1237. doi: 10.1038/s41598-022-05315-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cowman SA, James P, Wilson R, et al. Profiling mycobacterial communities in pulmonary nontuberculous mycobacterial disease. PLoS One. 2018;13 doi: 10.1371/journal.pone.0208018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solanki P, Lipman M, McHugh TD, Satta G. Whole genome sequencing and prediction of antimicrobial susceptibilities in non-tuberculous mycobacteria. Front Microbiol. 2022;13 doi: 10.3389/fmicb.2022.1044515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar K, Kon OM. Personalised medicine for tuberculosis and non-tuberculous mycobacterial pulmonary disease. Microorganisms. 2021;9:2220. doi: 10.3390/microorganisms9112220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar K, Daley CL, Griffith DE, Loebinger MR. Management of Mycobacterium avium complex and Mycobacterium abscessus pulmonary disease: therapeutic advances and emerging treatments. Eur Respir Rev. 2022;31 doi: 10.1183/16000617.0212-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erasmus JJ, McAdams HP, Farrell MA, Patz EF. Pulmonary nontuberculous mycobacterial infection: radiologic manifestations. Radiographics. 1999;19:1487–1505. doi: 10.1148/radiographics.19.6.g99no101487. [DOI] [PubMed] [Google Scholar]
- 21.Cowman SA, Jacob J, Obaidee S, et al. Latent class analysis to define radiological subgroups in pulmonary nontuberculous mycobacterial disease. BMC Pulm Med. 2018;18:145. doi: 10.1186/s12890-018-0675-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lake MA, Ambrose LR, Lipman MC, Lowe DM. Why me, why now?” Using clinical immunology and epidemiology to explain who gets nontuberculous mycobacterial infection. BMC Med. 2016;14:54. doi: 10.1186/s12916-016-0606-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunst H, Wickremasinghe M, Wells A, Wilson R. Nontuberculous mycobacterial disease and Aspergillus-related lung disease in bronchiectasis. Eur Respir J. 2006;28:352–357. doi: 10.1183/09031936.06.00139005. [DOI] [PubMed] [Google Scholar]
- 24.Zoumot Z, Boutou AK, Gill SS, et al. Mycobacterium avium complex infection in non-cystic fibrosis bronchiectasis. Respirology. 2014;19:714–722. doi: 10.1111/resp.12287. [DOI] [PubMed] [Google Scholar]
- 25.Kumar K, Loebinger MR. Concomitant or sequential pulmonary infection with non-tuberculous mycobacteria and Aspergillus. Int J Tuberc Lung Dis. 2023;27:797–802. doi: 10.5588/ijtld.23.0215. [DOI] [PubMed] [Google Scholar]
- 26.Malhotra AM, Bryant S, Kunst H, Haworth CS, Lipman M. Management of nontuberculous mycobacteria-pulmonary disease: Results from the first UK survey of clinical practice. J Infect. 2023;87:64–67. doi: 10.1016/j.jinf.2023.04.009. [DOI] [PubMed] [Google Scholar]
- 27.NHS England. Clinical commissioning policy: Nebulised liposomal amikacin for the treatment of non-tuberculous mycobacterial pulmonary disease caused by mycobacterium avium complex refractory to current treatment options (adults and post pubescent children). NHSE, 2022. www.england.nhs.uk/publication/clinical-commissioning-policy-nebulised-liposomal-amikacin/[Accessed 1 December 2023].
- 28.Potter JL, Capstick T, Ricketts WM, Whitehead N, Kon OM. TB drug monographs. www.tbdrugmonographs.co.uk/[Accessed 1 December 2023].
- 29.Lin CY, Huang HY, Hsieh MH, et al. Impacts of nontuberculous mycobacteria isolates in non-cystic fibrosis bronchiectasis: a 16-year cohort study in Taiwan. Front Microbiol. 2022;13 doi: 10.3389/fmicb.2022.868435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill AT, Sullivan AL, Chalmers JD, et al. British Thoracic Society Guideline for bronchiectasis in adults. Thorax. 2019;74:1–69. doi: 10.1136/thoraxjnl-2018-212463. [DOI] [PubMed] [Google Scholar]
- 31.Floto RA, Olivier KN, Saiman L, et al. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis. Thorax. 2016;71(Suppl 1):i1–22. doi: 10.1136/thoraxjnl-2015-207360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gochi M, Takayanagi N, Kanauchi T, et al. Retrospective study of the predictors of mortality and radiographic deterioration in 782 patients with nodular/bronchiectatic Mycobacterium avium complex lung disease. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2015-008058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song JH, Kim BS, Kwak N. Impact of body mass index on development of nontuberculous mycobacterial pulmonary disease. Eur Respir J. 2021;57 doi: 10.1183/13993003.00454-2020. [DOI] [PubMed] [Google Scholar]
- 34.Do JG, Park CH, Lee YT, Yoon KJ. Association between underweight and pulmonary function in 282,135 healthy adults: A cross-sectional study in Korean population. Sci Rep. 2019;9:14308. doi: 10.1038/s41598-019-50488-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dobner J, Kaser S. Body mass index and the risk of infection - from underweight to obesity. Clin Microbiol Infect. 2018;24:24–28. doi: 10.1016/j.cmi.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 36.British Association for Parenteral and Enteral Nutrition. Malnutrition Universal Screening Tool. BAPEN, 2023. www.bapen.org.uk/pdfs/must/must_full.pdf [Accessed 1 December 2023].
- 37.Lipman M, Cleverley J, Fardon T, et al. Current and future management of non-tuberculous mycobacterial pulmonary disease (NTM-PD) in the UK. BMJ Open Respir Res. 2020;7 doi: 10.1136/bmjresp-2020-000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Royal College of Nursing . RCN; 2022. A case management tool for TB prevention and control in the UK.www.rcn.org.uk/-/media/Royal-College-Of-Nursing/Documents/Publications/2023/May/010-230.pdf [Accessed 1 December 2023] [Google Scholar]
- 39.Whitehead N. Managing nontuberculous mycobacterium pulmonary disease. Nursing Times, 8 March 2021. www.nursingtimes.net/clinical-archive/respiratory-clinical-archive/managing-nontuberculous-mycobacterium-pulmonary-disease-08-03-2021/.