Abstract
Prader-Willi syndrome (PWS) is a genetic neurodevelopmental disorder. Global hypothalamic dysfunction is a core feature of PWS and has been implicated as a driver of many of PWS’s phenotypic characteristics (e.g., hyperphagia-induced obesity, hypogonadism, short stature). Although the two neuropeptides (i.e., oxytocin [OXT] and arginine vasopressin [AVP]) most implicated in mammalian prosocial functioning are of hypothalamic origin, and social functioning is markedly impaired in PWS, there has been little consideration of how dysregulation of these neuropeptide signaling pathways may contribute to PWS’s social behavior impairments. The present article addresses this gap in knowledge by providing a comprehensive review of the preclinical and clinical PWS literature–spanning endogenous neuropeptide measurement to exogenous neuropeptide administration studies–to better understand the roles of OXT and AVP signaling in this population. The preponderance of evidence indicates that OXT and AVP signaling are indeed dysregulated in PWS, and that these neuropeptide pathways may provide promising targets for therapeutic intervention in a patient population that currently lacks a pharmacological strategy for its debilitating social behavior symptoms.
Keywords: Prader-Willi syndrome, Oxytocin, OXTR, Arginine vasopressin, AVPR, Antidiuretic hormone, Social functioning, Social impairment, Neuropeptides, Neurogenetic syndrome, Hypothalamic dysfunction, Magel2, NDN, SNORD116, PC1, PC2, Chromosome 15, 15q11-q13
1. Introduction
Prader-Willi Syndrome (PWS) is a genetic neurodevelopmental disorder that occurs in approximately 1 in every 10,000–30,000 births (Cassidy, 1997; Cassidy et al., 2012; Donaldson et al., 1994; Nicholls et al., 1998), and is caused by either deletion of the paternal allele of 15q11-q13 (Butler, 1990; Donlon, 1988; Knoll et al., 1989; Magenis et al., 1990; Nicholls et al., 1989b; Robinson et al., 1991), maternal uniparental disomy of chromosome 15 (Mascari et al., 1992; Nicholls et al., 1989a; Robinson et al., 1991), or defects in the chromosome 15 imprinting center (Buiting et al., 1995; Dittrich et al., 1996; Horsthemke, 1997). PWS is characterized by intellectual disability, physical attributes such as facial dysmorphism, short stature, hyperphagia and low metabolic rate with significant risk of obesity, as well as a variety of other maladaptive behaviors. Many of the features seen in PWS are thought to be due to hypothalamic dysfunction resulting in hormonal abnormalities (Correa-da-Silva et al., 2021; Tauber and Hoybye, 2021).
Social functioning is particularly impaired in PWS patients, with individuals showing pronounced deficits in social cognition and social interactions (Dimitropoulos et al., 2013; Dykens et al., 2017, 2019). Children with PWS exhibit deficits in facial emotion recognition, symbolic play, joint attention (e.g., when two individuals purposefully coordinate their mutual focus of attention on an object or an event), empathy, cooperation, and parent and peer interactions, which influence the ability to seek out social engagement and build quality relationships (Debladis et al., 2019; Dimitropoulos et al., 2013; Dykens et al., 2019; Key et al., 2013; Zyga et al., 2015; Zyga and Dimitropoulos, 2020). These social deficits are also evident early in development, significantly impact quality of life, and do not resolve over time (Dimitropoulos et al., 2019; Dimitropoulos and Schultz, 2007; Dykens et al., 2017).
The social impairments observed in PWS bear significant resemblance to those of autism spectrum disorder (ASD). Studies evaluating the incidence of ASD in children with PWS have shown a prevalence of between 12.3% and 41% for genetically confirmed PWS cases (Dykens et al., 2017; Veltman et al., 2005, 2004). Variation in ASD comorbidity incidence likely reflects differences in the methodology of ASD diagnosis, though the majority of children with PWS have some level of social skill difficulty (Dykens et al., 2017; Schwartz et al., 2021).
Efforts to better understand the biological basis of ASD’s social impairments have implicated dysregulation of the two neuropeptides (i.e., oxytocin [OXT] and arginine vasopressin [AVP]) most critically involved in mammalian social functioning (Andari et al., 2010; Oztan et al., 2018, 2020; Parker et al., 2017, 2018, 2019; Zhang et al., 2016). Intriguingly, both neuropeptides are of hypothalamic origin (Zimmerman et al., 1984), and emerging evidence suggests that abnormalities in these neuropeptide signaling pathways may likewise contribute to PWS’s social behavior impairments (Francis et al., 2014; Swaab et al., 1995; Tauber et al., 2011). The present article provides a brief primer on PWS, as well as a comprehensive review of the extant preclinical and clinical PWS literature – spanning endogenous neuropeptide measurement to exogenous neuropeptide administration studies – to better understand the roles OXT and AVP signaling may play in the social functioning of this patient population.
2. The genetic basis of PWS
The genetic locus responsible for PWS, located on a large maternally imprinted region of chromosome 15q11-q13, has been known since the 1970s and has been well described (Cassidy, 1997; Cassidy et al., 2012; Donaldson et al., 1994; Nicholls et al., 1998; Polex-Wolf et al., 2017). Approximately 65–75% of PWS cases are due to deletions on paternally inherited chromosome 15, 20–30% are due to maternal uniparental disomy, and 1–3% are due to imprinting defects (Cassidy, 1997; Cassidy et al., 2012). While the genetic locus and many of the associated genes have been explored, it has been difficult to elucidate the relationship between the genetic deficits and the clinical phenotype of PWS (Burnett et al., 2017; Polex-Wolf et al., 2017).
The 15q11-q13 locus contains both protein-coding DNA genes and non-coding RNA genes. Animal models lacking single genes in the PWS locus have been developed and studied, and insights from these models have been integrated with genetic findings from clinical populations to better understand genetic contribution to the PWS phenotype. Deletions or mutations in Magel2 are associated with hypotonia, developmental delay, hypogonadism, hyperphagia, as well as robust social deficits (e.g., impaired social recognition in genetically modified mice and ASD in people) (Bischof et al., 2007; Boccaccio et al., 1999; Fountain et al., 2017; Mercer and Wevrick, 2009; Schaaf et al., 2013). The MKRN3 gene, also found in the PWS-specific region, is associated with abnormalities in reproductive timing due to effects on gonadotropin-releasing hormone (GnRH) production (Jong et al., 1999). Deficiencies in Necdin, the product of the NDN gene, result in reduction of GnRH-producing neurons in the hypothalamus, and consequently, reproductive abnormalities (Muscatelli et al., 2000). Deletions of the SNURF/SNRPN gene complex result in hypotonia and some growth restriction (Tsai et al., 1999; Yang et al., 1998). More recent work has implicated a possible role for SNORD116, a small nucleolar RNA C/D box 116 gene cluster on chromosome 15q11.2 (Burnett et al., 2017). Deletion of this region results in hyperphagia, obesity, hypogonadism, and growth hormone deficiency (de Smith et al., 2009; Ding et al., 2008; Sahoo et al., 2008), all features common in PWS; yet, there are mixed findings pertaining to the cognitive, emotional, and social behavioral abnormalities in the Snord116 deficient mouse model (Adhikari et al., 2019; Zieba et al., 2015). In sum, while several of these models recapitulate features of PWS, it is important to note that none do so consistently. Thus, these collective research efforts have shown that no one single gene is the cause of PWS. Instead, lack of expression of multiple genes in this locus leads to the disorder’s characteristic phenotype (Polex-Wolf et al., 2017; Francis et al., 2014).
3. Possible etiologies of hypothalamic dysfunction in PWS
Global hypothalamic dysfunction has been identified as a core feature of PWS and as a causal factor in many of the phenotypic characteristics of the disorder (Correa-da-Silva et al., 2021; Tauber and Hoybye, 2021). The growth hormone deficiency, thyroid hormone deficiency, hypogonadism, and hyperphagia typical of the syndrome are thought to be hypothalamic in origin (Burman et al., 2001; Diene et al., 2010). Research using induced pluripotent stem cells has also enabled molecular investigation of PWS’s neuronal dysfunction. As a result, deficiency in two factors, nescient helix–loop–helix 2 (NHLH2) and prohormone convertase 1 (PC1), encoded by the Proprotein Convertase Substilisin/Kexin Type 1 gene (PCSK1), have been implicated in the hypothalamic dysfunction seen in PWS. Neuronal induced pluripotent stem cells (iPSCs) were derived from PWS patients with microdeletions of the noncoding RNA gene SNORD116, located in the PWS critical region. These neurons show low levels of both NHLH2 and PC1 proteins, and transcriptomic analysis revealed decreased transcript of both NHLH2 and PCSK1, with the latter being one of the most suppressed genes in these cells (Burnett et al., 2017). The SNORD116 paternal knockout mouse model also shows low levels of hypothalamic PC1, as well as decreased expression in the pancreas and stomach (Burnett et al., 2017). In addition, humans with loss-of-function mutations in PCSK1 have a phenotype of hypothalamic dysfunction similar to those with PWS (Stijnen et al., 2016). Lower levels of prohormone convertase 2 (PC2), another prohormone convertase encoded by the PCSK2 gene, were also found in neuronal iPSCs from PWS patients (Eddiry et al., 2021).
Burnett et al. (2017) suggest that deletion of SNORD116 may result in a decrease in PC1 via modulation of NHLH2. NHLH2 is a basic helix-loop-helix neuronal transcription factor that positively regulates PCSK1 transcription (Fox and Good, 2008). In silico modeling shows SNORD116-mediated regulation of NHLH2, providing a mechanism by which SNORD116 deletion would result in decreases in NHLH2. These decreases would result in decreased transcription of PCSK1, leading, in turn, to lower levels of PC1. PC1 acts to cleave the pro-domain from hormones in the brain, adrenal, and pancreas, thereby increasing their activity (Seidah, 2011). SNORD116 paternal knockout mice show increases in proinsulin, pro-growth hormone-releasing hormone (proGHRH), and proghrelin. These decreases in PC1 may thus result in physiologically relevant disturbance of prohormone processing, which, in turn, may lead to hyperphagic obesity, hyperghrelinemia growth hormone deficiency, hypogonadotropic hypogonadism, and relative hypoinsulinemia (Burnett et al., 2017).
While deficits in prohormone convertase activity may account for some neuroendocrine disruption in PWS (Pan et al., 2005; Polex-Wolf et al., 2017; Stijnen et al., 2016), previous efforts have not systematically elucidated how hypothalamic processing disruption may relate to the social abnormalities exhibited by individuals with PWS. However, research has shown that PC1 is co-expressed with pro-OXT and pro-AVP in certain groups of neurons in the supraoptic nucleus (SON) and paraventricular nucleus (PVN) of the hypothalamus (Dong et al., 1997). When OXT levels are measured in the pituitaries of mice deficient in PC1, an 80% decrease in concentration has been found (Pan et al., 2005). Additionally, in a subset of PWS patients, a small study reported a link between PC2 deficiency and a hypothalamic AVP processing defect (Gabreëls et al., 1998). Thus, prohormone convertase deficiency may alter precursor processing of OXT and AVP in the hypothalamus which, in turn, may drive the pronounced social impairments evident in PWS patients.
4. OXT and AVP biology
OXT and AVP are structurally similar nonapeptides (i.e., they differ by only two amino acids) and are thought to have evolved due to duplication of a common ancestral gene (Donaldson and Young, 2008). Both molecules are synthesized as pre-prohormones and then processed into their active mature forms through enzymatic cleavage (Brownstein et al., 1980). Principal synthesis of these neuropeptides occurs in magnocellular neurons located in the SON and PVN of the hypothalamus. These neuropeptides are released into systemic circulation through posterior pituitary axon terminals following action potentials, and act as classic hormones to regulate a variety of physiological processes at distal target sites. OXT and AVP are also secreted from the dendrites of the magnocellular neurons into extracellular fluid, and from the axonal projections of parvocellular neurons, which project to the anterior pituitary, spinal cord, and various brain regions (Brownstein et al., 1980; Castel and Morris, 1988; Ludwig and Leng, 2006; Pow and Morris, 1989). In the brain, OXT and AVP act as neuromodulators to regulate a variety of species-specific behavioral functions (Baribeau and Anagnostou, 2015).
In mammals, OXT has a single receptor (OXTR) by which it exerts its classic physiological (e.g., uterine contractions; milk let-down) and social behavioral effects, whereas AVP has three receptor subtypes [AVPR1A, AVPR1B (also known as AVPR3) and AVPR2]. All of these receptors are G protein coupled (Barberis and Tribollet, 1996), and expressed in a variety of tissues throughout the body and brain (Thibonnier et al., 2001). Like their ligands, phylogenetic analysis has shown that these receptors originated from a gene duplication event of an ancestral vertebrate chromosome (Lagman et al., 2013). AVP’s prosocial effects are predominantly mediated via central AVPR1A (Bielsky et al., 2004; Young et al., 1999), whereas its effects on stress-related anterior pituitary adrenocorticotropic hormone release and kidney-related water homeostasis are mediated through AVPR1B and AVPR2, respectively (Thibonnier et al., 2001). It should be noted that while OXT and AVP have the highest affinity for their own respective receptors, because of the high degree of structural homology between OXT and AVP, and the close evolutionary relationship between OXTR and the AVPR subtypes, there is significant “cross-talk” between each ligand and the various neuropeptide receptors (Song and Albers, 2018).
5. OXT and AVP regulation of mammalian social behavior
It is well established that both OXT and AVP regulate a wide range of species-typical mammalian social behaviors as reviewed in detail elsewhere (Baribeau and Anagnostou, 2015; Carter et al., 2008; Johnson and Young, 2017; Meyer-Lindenberg et al., 2011). OXT was first recognized as a “social” molecule over 40 years ago when it was implicated in the onset of maternal behavior in rats (Pedersen and Prange, 1979) and sheep (Kendrick et al., 1987), and subsequently, pair bond formation in female prairie voles (Williams et al., 1992). Approximately 15 years later, a “social” role for AVP was also identified: AVP was found to induce pair bonds in male prairie voles (Winslow et al., 1993), as well as paternal care in several vole species (Parker and Lee, 2001; Wang et al., 1994). Since, and of particular relevance for PWS, it has been found that reduced brain OXT or AVP signaling—either naturally occurring or experimentally induced—results in robust social behavior impairments in multiple species (Ferguson et al., 2000; Jin et al., 2007; Parker et al., 2018; Paul et al., 2016; Pedersen et al., 2006).
In addition to evidence from animal studies, research has also shown that OXT and AVP play a key role in regulating species-typical human social behavior. For example, intranasal OXT administration to neurotypical individuals improves emotion and facial recognition (Fischer-Shofty et al., 2010; Guastella et al., 2008b; Rimmele et al., 2009), enhances gaze to the eye region (Guastella et al., 2008a), promotes trust (Baumgartner et al., 2008; Kosfeld et al., 2005), and reduces behavioral and endocrine responses to social stress (Heinrichs et al., 2003; Meinlschmidt and Heim, 2007). Likewise, intranasal AVP administration to neurotypical individuals enhances various aspects of human social functioning including memory for emotional faces (Guastella et al., 2010), cooperative behavior (Brunnlieb et al., 2016; Rilling et al., 2012), and social communication ability (Thompson et al., 2004).
6. Preclinical studies of neuropeptide signaling in PWS models
Preclinical evidence for OXT and AVP’s involvement in PWS comes principally from studies of mouse models lacking single genes in the PWS locus, specifically, Necdin and Magel2. This work has focused primarily on neuropeptide measurement ranging from assessment of neuron numbers and firing rates to quantification of mRNA expression and protein concentrations. The majority of these studies reported pronounced OXT signaling disruption, with more modest support found for AVP signaling abnormalities. Findings from this research are briefly summarized below, and experimental details are provided in Table 1.
Table 1.
Preclinical endogenous neuropeptide measurement studies.
| Article title | First author & publication year |
Sample characteristics |
Substrates & measurement techniques | Relevant findings |
|---|---|---|---|---|
| “Disruption of the mouse Necdin gene results in hypothalamic and behavioral alterations reminiscent of the human Prader–Willi syndrome” | Muscatelli et al. (2000) |
Necdin mouse model Age and sex: adult males Mutant: n = 4 Wild-type: n = 4 |
Substrate: OXT Collection location: hypothalamus (PVN) Technique: IHC |
-Significant decrease of 29% in the number of OXT-producing neurons were shown in lateral portion of the PVN in mutant mice compared to wild-type mice |
| “A single postnatal injection of oxytocin rescues the lethal feeding behaviour in mouse newborns deficient for the imprinted Magel2 gene” | Schaller et al. (2010) |
Magel2 mouse model Age and sex: neonatal males and females Mutant: n = 6–14 Wild-type: n = 5–14 |
Substrates: OXT and AVP Collection location: hypothalamus (PVN), pituitary gland Techniques: EIA; IHC |
-The number of OXT- and AVP- prohormone positive cells in the PVN was similar between mutant and wild-type mice. However, there was a 1.7-fold increase in the OXT-intermediate cells but not of AVP-intermediate cells in the PVN of mutant mice compared to wild-type mice -Significant reduction in OXT (36%) and AVP (20%) concentrations was found in the hypothalamus as a whole of mutant mice compared to wild-type mice -No significant difference was detected in the pituitary gland levels of OXT and AVP between mutant mice and wild-type mice |
| “An early postnatal oxytocin treatment prevents social and learning deficits in adult mice deficient for Magel2, a gene involved in Prader-Willi syndrome and autism” | Meziane et al. (2015) |
Magel2 mouse model Age and sex: adult males Mutant: n = 4–11 Wild-type: n = 4–10 |
Substrates: OXT, AVP, OXTR Collection location: anterior commissure to the posterior hypothalamus, hypothalamus (PVN), hypophysis, amygdala, lateral septum, dorsal vagal complex Techniques: EIA; mass spectroscopy; IHC; autoradiography |
-Significant increase in OXT (34%) and AVP (21%) concentrations was detected in hypophysis, whereas only AVP (%15) concentration was increased in the hypothalamus of mutant mice compared to wild type mice -Significant increase in the total number of OXT-positive neurons (26%) in the entire PVN but no accumulation of OXT-intermediate neurons was found despite the 22% more OXT-prohormone and -intermediate neurons in the rostral PVN in mutant mice compared to wild-type mice -Significant increases in OXT immunoreactivity were found in the medial amygdala (300%), the lateral septum (172%), and the dorsal vagal complex (33%) in mutant mice compared to wild-type mice -Significant decrease in OXTR density (26%) was detected in lateral septum of mutant mice compared to wild-type mice. |
| “Inactivation of Magel2 suppresses oxytocin neurons through synaptic excitation-inhibition imbalance” | Ates et al. (2019) |
Magel2 mouse model Age and sex: adult (5–7 weeks old) males and females Mutant: n = 2–6 Wild-type: n = 2–7 |
Substrate: OXT Collection location: hypothalamus (PVN) Techniques: electrophysiology; confocal microscopy |
-Significant decrease in baseline firing rates and frequency of action potentials in OXT neurons in the PVN of mutant mice compared to wild-type mice -Significant selective reduction in excitatory drive and increase in inhibitory drive onto OXT neurons in the PVN of mutant mice compared to wild-type mice -No deficit in the number of spiny protrusions from basal OXT neuronal dendrites in the PVN of mutant mice compared to wild-type mice |
| “Colocalization of Oxtr with Prader-Willi syndrome transcripts in the trigeminal ganglion of neonatal mice” | Vaidyanathan et al. (2020) |
Magel2 mouse model Age and sex: neonatal males and females Mutant: n = 8 Wild-type: n = 12 |
Substrate: OXTR Collection location: trigeminal ganglion, lateral periodontium, rostral periodontium, tongue, olfactory epithelium, whisker pads and brainstem Techniques: chromogenic in situ hybridization; autoradiography |
-OXTR is co-expressed with Magel2 and other PWS gene transcripts in the trigeminal ganglion of wild-type mice -Trigeminal ganglion neurons express OXTR but not Magel2 in the mutant mice -Significant reduction in OXTR binding density in the lateral periodontia with intact levels in the rest of the head of mutant mice compared to wild-type mice |
| “Loss of MAGEL2 in Prader-Willi syndrome leads to decreased secretory granule and neuropeptide production” | Chen et al. (2020) |
Magel2 mouse model Age and sex: adult mice (sex unspecified) Mutant: n > 5 Wild-type: n = 5–6 |
Substrates: OXT, AVP Collection location: whole hypothalamus, plasma Techniques: Quantitative Proteomic/Mass Spectrometry, EIA |
-Significant reduction of OXT and AVP levels (~50%) in the hypothalamus of mutant mice compared to wild-type mice -Significant decrease in OXT and AVP concentrations in the plasma of mutant mice compared to wild-type mice |
| “Correction of vasopressin deficit in the lateral septum ameliorates social deficits of mouse autism model” | Borie et al. (2021) |
Magel2 mouse model Age and sex: adult males Mutant: n = 5–6 Wild-type: n = 5–6 |
Substrates: AVP, AVPR1A, AVPR1B and AVPR2 Collection location: lateral septum Techniques: IHC, autoradiography |
-Significant decrease in overall AVP receptor densities in the somatostatin neurons of the dorsal lateral septum in mutant mice compared to wild-type mice -Significant reduction in the number and length of AVP fibers in the dorsal lateral septum of mutant mice compared to wild-type mice |
AVP, arginine vasopressin; AVPR1A, arginine vasopressin receptor 1A; AVPR1B, arginine vasopressin receptor 1B; AVPR2, arginine vasopressin receptor 2; EIA, enzyme immunoassay; IHC, immunohistochemistry; OXT, oxytocin; OXTR, oxytocin receptor; PVN, paraventricular nucleus
Some of the first evidence for impaired OXT signaling came from a Necdin-deficient mouse model of PWS. Specifically, a significant decrease in the number of OXT-producing neurons in the lateral PVN was found in adult Necdin-deficient mice compared to wild-type mice (Muscatelli et al., 2000). Subsequently, investigators have studied neuropeptide biology in Magel2 mutant mice across life span development. Beginning early in life, neonatal Magel2 mutant mice exhibited a similar number of OXT-prohormone positive cells compared to wild type-littermates. However, Magel2 mutant mice showed a significant accumulation of the OXT-intermediate form in the PVN and a significant decrease in mature OXT compared to wildtype controls. This evidence suggests that transformation of OXT intermediate forms to mature OXT is impaired in the hypothalamus of mutant neonates, leading to an accumulation of intermediate forms and a reduced amount of mature OXT (Schaller et al., 2010). Early life OXT transmission abnormalities are also evident post-synaptically, as a significant reduction in OXTR density has also been observed in neonatal Magel2-deficient mice compared to wild-type mice in the lateral periodontia (a feeding-related target) (Vaidyanathan et al., 2020).
Follow up research was conducted to assess whether the observed OXT processing defect was maintained during adulthood in Magel2-deficient mice. Although some evidence suggests that there may be compensatory changes in both OXT-producing cells and OXTR density in multiple brain regions during development (Meziane et al., 2015), other work suggests that the neonatal processing defect persists, as a significant reduction of mature OXT was found in both the hypothalamus and blood of adult Magel2 mutant mice compared to adult wild-type mice (Chen et al., 2020). Convergent electrophysiological evidence likewise supports these abnormal protein findings, as a significant decrease in ex vivo OXT neuron activity with reduced synaptic excitation and increased inhibition currents in the PVN of adult Magel2-deficient mice has also been reported (Ates et al., 2019).
Somewhat parallel findings have been found for AVP in the Magel2 mutant mouse across development. Although no significant difference was found between neonatal Magel2 mutants and controls in AVP-prohormone or intermediate forms, a significant reduction of mature AVP in the hypothalamus as a whole was detected in neonatal mutants (Schaller et al., 2010). As with OXT, there also appears to be some evidence for compensatory developmental changes in mature hypothalamic AVP concentration (Meziane et al., 2015), but, again, other evidence suggests this deficit persists, as a significant reduction in mature AVP has been found in both the hypothalamus and blood of adult Magel2 mutant mice compared to adult wild-type mice (Chen et al., 2020). Further, additional findings implicate more wide-spread AVP abnormalities: A separate study reported a significant decrease in the number and length of AVP fibers and number of AVPR1A, AVPR1B, and AVPR2 in the lateral septum of adult Magel2-deficient mice which, in turn, are associated with social discrimination deficits in these adult mutants (Borie et al., 2021).
7. Preclinical studies of neuropeptide administration in PWS models
Several studies have been conducted to assess the effectiveness of OXT or AVP administration to “rescue” social functioning impairments in Magel2-deficient mice (Borie et al., 2021; Meziane et al., 2015). These studies support both OXT and AVP as potential therapeutics for ameliorating behavioral abnormalities in gene-targeted mouse models of PWS. Findings from this research are briefly summarized below, and experimental details are provided in Table 2.
Table 2.
Preclinical pharmacological neuropeptide administration studies.
| Article title | First author & publication year |
Sample characteristics | Manipulation & outcome measures | Relevant findings |
|---|---|---|---|---|
| “An early postnatal oxytocin treatment prevents social and learning deficits in adult mice deficient for Magel2, a gene involved in Prader-Willi syndrome and autism” | Meziane et al. (2015) |
Magel2 mouse model Age and sex: neonatal and adult males Mutant: n = 10–14 (OXT); n = 10–14 (NaCl) Wild type: n = 12–14 (OXT); n = 12–14 (NaCl) |
Manipulation: -Single s.c. injection of 10 mg/kg OXT or NaCl 1 h before behavioral testing (adult mutant and wild-type mice) -Daily s.c. injections of 2 μg OXT for 7 days, starting from 3 to 5 h after birth (neonate mutant and wild-type mice); behavioral testing performed in adulthood Outcome Measures: -Social recognition -Social interaction |
-Single s.c. injection of OXT reversed social recognition deficit in mutant adult mice -Daily s.c. injections of OXT during early postnatal period restored normal social recognition and interaction in adult mutant mice |
| “Correction of vasopressin deficit in the lateral septum ameliorates social deficits of mouse autism model” | Borie et al. (2021) |
Magel2 mouse model Age and sex: adult males (3–4 months old) Mutant: n = 16 (AVP); n = 14 (NaCl) Wild-type: n = 11 (Manning compound AVPR antagonist); n = 15 (NaCl) |
Manipulation: -Single injection of 30 μM AVP through cannula implanted in the dorsal LS for 9 min starting 5 min before social trial in mutant mice -Single injection of 10 nM AVPR antagonist through cannula implanted in the dorsal LS for 9 min starting 5 min before social trial in wild-type mice Outcome Measures: -Social habituation/discrimination |
-AVP injection increased social discrimination in mutant mice compared to NaCl vehicle-injected mutant controls -AVPR antagonist vs NaCl injection decreased social novelty and discrimination in wild-type mice |
AVP, arginine vasopressin; AVPR, arginine vasopressin receptor; LS, lateral septum; NaCl, sodium chloride; OXT, oxytocin; OXTR, oxytocin receptor; s.c., subcutaneous
Adult mice lacking Magel2 show social behavior and cognition deficits along with altered hypothalamic-hypophyseal OXT and AVP levels. Social recognition deficits in adult Magel2-deficient mice can be reversed by a single subcutaneous OXT injection. Moreover, Magel2-deficient mice treated with chronic subcutaneous injections of OXT as neonates showed improved social interaction and recognition abilities in adulthood (Meziane et al., 2015), providing evidence for the lasting therapeutic benefit of OXT when administered during an early “critical period” of development.
Therapeutic use of AVP on impaired social habituation and discrimination behaviors has also been studied in adult Magel2-deficient mice (Borie et al., 2021). A single AVP injection into the dorsal lateral septum (where AVP signaling is known to be impaired in this model), restored social discrimination in adult mutant mice. Moreover, a single AVPR1A antagonist injection into the dorsal lateral septum of C57BL/6 J wild-type mice decreased social novelty and discrimination (Borie et al., 2021), suggesting that septal AVP is a key factor in the pathophysiology of social deficits associated with Magel2 deficiency.
8. Clinical neuropeptide measurement studies in PWS patients
Clinical evidence for neuropeptide dysregulation in PWS patients is drawn from postmortem brain tissue studies as well as assessment of central and peripheral OXT and AVP concentrations. The majority of these findings implicate significant abnormalities in neuropeptide signaling pathways in PWS patients, with variability in the direction of effects across studies. These discordant findings may be driven, at least in part, by factors that varied across studies, including: study population, control group, biological specimen type, and neuropeptide measurement technique. Findings from this clinical research are briefly summarized below, and experimental details are provided in Table 3.
Table 3.
Clinical endogenous neuropeptide measurement studies.
| Article title | First author & publication year |
Sample characteristics | Substrates & measurement techniques | Relevant findings |
|---|---|---|---|---|
| “Alterations in the hypothalamic paraventricular nucleus and its oxytocin neurons (putative satiety cells) in Prader-Willi syndrome: a study of five cases” | Swaab et al. (1995) | Human adult study; postmortem tissue Age range: 22–64 y PWS: n = 5 Females: 3 Males: 2 Control: n = 5 Females: 3 Males: 2 (Additional 27 adult controls, i.e., 14 males and 13 females) |
Substrates: OXT and AVP Collection Location: hypothalamus (PVN) Techniques: ICC |
-Significant decrease in the volume (28%) of the PVN and the total cell numbers (38%) in the PVN was detected in the PWS cases compared to five matched controls -Significant decrease in the volume (54%) and the number (42%) of OXT neurons in the PVN was found in the PWS cases compared to five matched controls -The number of AVP neurons in the PVN was not significantly different in the PWS cases, but only the size of the nucleus was increased compared to five matched controls -Significant decrease in the number of OXT neurons and unaffected number of AVP neurons in the PVN of PWS cases remained consistent when compared to additional 27 controls |
| “Attenuation of the polypeptide 7B2, prohormone convertase PC2, and vasopressin in the hypothalamus of some Prader-Willi patients: indications for a processing defect” | Gabreëls et al. (1998) | Human adult study; postmortem tissue Age range: 19–88 y PWS: n = 7 Females: 4 Males: 3 Control: n = 11 Females: 5 Males: 6 |
Substrates: OXT and AVP Collection Location: hypothalamus (SON; PVN) Techniques: ICC |
-Normal staining intensity for OXT was found in the magnocellular neurons of the PVN and SON in all PWS cases relative to controls -The magnocellular neurons of SON and PVN in two PWS cases who were deficient for neuroendocrine polypeptide 7B2 did not show processed AVP and prohormone convertase 2 immunoreactivity, but showed normal immunoreactivity for AVP precursor relative to controls -Two other PWS cases showed moderate processed AVP immunoreactivity along with some degree of 7B2 immunoreactivity in the magnocellular neurons of SON and PVN relative to controls -Remaining three PWS cases showed normal processed AVP immunoreactivity in the magnocellular neurons of SON and PVN relative to controls |
| “Cerebrospinal fluid levels of oxytocin in Prader–Willi syndrome: a preliminary report” | Martin et al. (1998) | Human adolescent and adult study Age range: 16–28 y PWS: n = 5 Females: 3 Males: 2 Control: n = 6 Female: 6 Male: 0 |
Substrates: OXT and AVP Collection Location: cerebrospinal fluid via lumbar puncture Technique: RIA (unspecified if sample extraction was performed) |
- CSF OXT levels were significantly higher in the PWS cases compared to controls, and this finding was more prominent when males were excluded from the analysis -CSF AVP levels were not significantly different between the groups with both female and male subjects, but significantly lower in female PWS cases when excluding males from the analysis |
| “Peptides associated with hyperphagia in adults with Prader–Willi syndrome before and during GH treatment” | Höybye et al. (2003) | Human adolescent and adult study Age range: 17–32 y PWS: n = 17 Females: 8 Males: 9 |
Substrate: OXT Collection Location: peripheral blood Technique: RIA after solid phase extraction |
-Baseline plasma OXT levels were within the normal range but considered low with relevance to their obesity - Significant negative correlation was found between plasma OXT and anorexigenic hormone leptin levels in serum at baseline |
| “Whole genome microarray analysis of gene expression in Prader-Willi syndrome” | Bittel et al. (2007) | Human infant, adolescent, and adult study; included postmortem tissue Age range: 1–45 y PWS: n = 10 Females: 3 Males: 7 Control: n = 6 Females: 3 Males: 3 |
Substrate: OXTR Collection Location: lymphoblastoid cells and frontal cortex brain tissue Techniques: microarray; qPCR |
-Significant reduction in expression of OXTR in PWS cases relative to controls was found in both lymphoblastoid cells and frontal cortex |
| “Elevated plasma oxytocin levels in children with Prader–Willi syndrome compared with healthy unrelated siblings” | Johnson et al. (2016) | Human child study Age range: 5–11 y PWS: n = 23 Females: 10 Males: 13 Controls: n = 18 Females: 8 Males: 10 |
Substrate: OXT Collection location: peripheral blood Technique: multiplex sandwich immunoassays (no sample extraction) |
-Plasma OXT levels were significantly increased in the PWS cases compared to healthy unrelated siblings of children with PWS -PWS diagnosis predicted the OXT levels in regression analyses with an overall model fit -Significant positive correlation between OXT levels and age was only found in females with PWS |
AVP, arginine vasopressin; CSF, cerebrospinal fluid; GH, growth hormone; ICC, immunocytochemistry; OXT, oxytocin; OXTR, oxytocin receptor; PVN, paraventricular nucleus; PWS, Prader-Willi syndrome; qPCR, quantitative polymerase chain reaction; RIA, radioimmunoassay; SON, supraoptic nucleus
Early clinical studies suggested altered hypothalamic OXT and AVP signaling in individuals with PWS (Gabreëls et al., 1998; Swaab et al., 1995). The first evidence came from a study which investigated OXT and AVP neurons in the PVN of postmortem hypothalamic tissue from adult individuals with PWS, and age- and gender-matched controls (Swaab et al., 1995). Morphometric assessment analysis showed that PVN volume was significantly smaller, and that total PVN cell number was significantly lower, in PWS cases. Subsequent analysis revealed that these reductions were specific to OXT neurons, since no group differences were found for AVP neurons. These results were replicated when PWS cases were compared to a larger independent group of control subjects (Swaab et al., 1995).
Another study used immunocytochemistry to quantify AVP precursor, processed AVP, and processed OXT in the magnocellular neurons of the SON and PVN in postmortem brain tissue from adult PWS cases and controls (Gabreëls et al., 1998). OXT staining intensity in PWS cases was comparable to controls. However, the intensity of processed AVP immunoreactivity varied among PWS cases. This variation in processed AVP was associated with variation in neuroendocrine polypeptide 7B2, which is localized close to the PWS deletion region on chromosome 15q13–14 (Roebroek et al., 1989). In PWS cases lacking 7B2, processed PC2 and AVP immunoreactivities were not detectable. However, these PWS cases showed normal AVP precursor immunoreactivity in the SON and PVN, suggesting an AVP processing defect (Gabreëls et al., 1998).
Investigation of cerebrospinal fluid (CSF) OXT and AVP concentrations provided gender specific results in PWS cases (Martin et al., 1998). Radioimmunoassay (RIA) analysis of lumbar CSF samples showed significantly higher OXT concentrations in adolescent and adult PWS cases compared to controls without PWS. This effect was even stronger when the analysis included only females. No significant difference was found in CSF AVP concentrations between the groups when both genders were included, but CSF AVP concentrations were significantly lower in female PWS cases if male subjects were excluded from the analysis (Martin et al., 1998).
Neuropeptide dysfunction in PWS has also been evaluated by measuring blood OXT concentrations in adolescent and adult PWS cases before undergoing growth hormone treatment (Höybye et al., 2003). RIA analysis of extracted blood samples showed a normal range of baseline plasma OXT concentrations, but when compared to their obesity status, these PWS patients had low plasma OXT concentrations, suggesting that altered anorexigenic OXT concentrations may play an important role in regulation of hyperphagia-induced obesity in PWS. Unfortunately, we do not know whether low plasma OXT concentrations in this study sample were also linked to impaired social functioning, as to our knowledge this relationship was not assessed.
In another study, non-extracted plasma OXT concentrations following overnight fasting were significantly increased in children with PWS undergoing growth hormone treatment compared to age- and gender-matched control siblings of individuals with PWS (Johnson et al., 2016). In particular, these analyses showed a significant age-related increase in plasma OXT concentrations in female PWS cases. These counterintuitive findings in blood OXT concentrations could be due to ongoing growth hormone treatment at the time of blood sample collection. However, these blood samples did not undergo an extraction procedure before immunoassay, an important step in obtaining accurate OXT values (Leng and Sabatier, 2016; McCullough et al., 2013; Szeto et al., 2011), calling into question the validity of these (and other) unextracted blood sample findings in people with PWS.
Lastly, to better understand genetic alterations in several possible candidate genes that may contribute to the clinical manifestation of PWS, including OXT and OXTR, whole genome microarray and qPCR analysis were performed on lymphoblastoid cells and postmortem frontal cortex tissue from adolescent and adult PWS cases and age and gender-matched controls. PWS cases were shown to have almost undetectable levels of OXT in both tissue types (i.e., a weak probe signal on the microarray) as well as decreased OXTR gene expression levels, providing further evidence for dysfunctional OXT signaling pathways in PWS (Bittel et al., 2007).
9. Clinical studies of neuropeptide administration in PWS patients
Nine independent studies to date have tested the therapeutic effect of OXT or a related compound, carbetocin (an oxytocin analogue), on ameliorating behavioral impairments in individuals with PWS. Although all treatment trials have administered OXT or carbetocin through nasal spray, and all trials have thus far reported good safety and tolerability profiles, the therapeutic benefit of OXT in PWS has been equivocal, perhaps due to the remarkable variability in study design, length of treatment, drug dose, age group, gender, PWS genetic subtype, and outcome measures across trials. These findings are briefly summarized below, and experimental details are provided in Table 4. Interestingly, despite emerging evidence that central AVP administration “rescues” social deficits in Magel2-deficient mice (Borie et al., 2021), and intranasal AVP treatment appears to be safe, well tolerated, and improve social abilities in people with ASD (Parker et al., 2019), AVP has not yet been evaluated as a potential treatment for PWS, and thus no AVP treatment studies are reviewed here.
Table 4.
Clinical pharmacological neuropeptide administration studies.
| Article title | First author & publication year |
Sample characteristics |
Study design | Manipulation & outcome measures |
Relevant findings |
|---|---|---|---|---|---|
| “Oxytocin may be useful to increase trust in others and decrease disruptive behaviours in patients with Prader-Willi syndrome: a randomised placebo-controlled trial in 24 patients” |
Tauber et al. (2011) | Human adult study Age range: 18–44 y Median age: 28.5 y PWS: n = 24 Females: 16 Males: 8 |
Double-blind, randomized, placebo-controlled | Manipulation: single intranasal dose of 24 IU OXT or placebo Outcomes: -Study specific behavioral measures -Sally-Anne Test -Reading the Mind in the Eyes Test |
-Significant improvements were found in the two days following single intranasal dose of OXT on 3 items in the caregiver reported behavior grid: increase in trust, decrease in sadness tendencies, and a decrease in disruptive behaviors compared to placebo group -No improvement was found on validated social skills measures -Intranasal OXT was well tolerated without any adverse events |
| “A double-blind randomized controlled trial of oxytocin nasal spray in Prader Willi syndrome” | Einfeld et al. (2014) | Human adolescent and adult study Age range: 12–30 y Mean age: 17.8 y PWS: n = 30 Females: 10 Males: 20 |
Double-blind, randomized, placebo-controlled, cross-over |
Manipulation: twice daily intranasal OXT or placebo (a washout period of 2 weeks before cross over to the alternative treatment) administration in the following doses for 8 weeks: -The first 11 PWS participants: 24 IU OXT (16 years and over) and 18 IU OXT (13–15 years) -Remaining 18 PWS participants: 40 IU OXT (16 years and over) and 32 IU OXT (13–15 years) Outcomes: -3 items on the Developmental Behavior Checklist (hyperphagia/pica, temper outbursts and weight) |
-No significant improvement was found for any outcome measures or treatment groups -Significant increase in temper outbursts was found in the OXT group compared to the placebo group -The increase in temper outbursts was more significant in the high dose of OXT group than the overall group, but was not significant in the lower dose of OXT group -No side effects were reported |
| “Dyssynchrony and perinatal psychopathology impact of child disease on parents-child interactions, the paradigm of Prader Willi syndrom” | Viaux-Savelon et al. (2016) (Note: same study/population as Tauber et al., 2017 below) | Human infant study Age range: 0.8–5.7 m Mean age: 3.9 m PWS: n = 18 Females: 8 Males: 10 |
Unblinded dose escalation with 3 steps over 7 days | Manipulation: intranasal dose of 4 IU OXT every other day, daily, or twice daily for one week Outcomes: -Alarm Distress Baby Scale -Coding Interactive Behavior Scale |
-Significant improvements on 4 out of the 8 Alarm Distress Baby Scale items: increase in eye contact, facial expression, general level of activity, and relationships were found after intranasal OXT treatment compared to baseline - Significant improvements on 4 out of 7 Coding Interactive Behavior Scale composites: increase in parental sensitivity, dyadic reciprocity, child social engagement and child state were found after intranasal OXT treatment compared to baseline -No adverse events were reported |
| “Promising effects of oxytocin on social and food-related behavior in young children with Prader-Willi syndrome: a randomized, double-blind, controlled crossover trial” | Kuppens et al. (2016) | Human child study Age range: 6–14 y Mean age: 9.3 y PWS: n = 25 Females: 11 Males: 14 |
Double-blind, randomized, placebo-controlled, cross-over | Manipulation: twice daily intranasal dose of 24–48 IU OXT or placebo for 4 weeks followed by no washout period to cross over to the alternative treatment for further 4 weeks Outcomes: -Food intake -Hyperphagia Questionnaire -Oxytocin Study Questionnaire (focused on changes in emotions, social, and eating behavior, as well as possible side effects) |
-When all participants were included in the analysis, no significant effect of OXT was found on any outcome measures -When participants were divided into groups based on age (older vs. younger than 11 years), the younger participants showed significant improvements in social behavior, food-related behavior, anger, sadness, and conflicts during OXT treatment compared to placebo -Lower serum oxytocin levels after OXT treatment were associated with positive effects on social behavior in the younger children. This effect was not found in the older children. (Note: blood samples were not extracted). -No side effects or adverse events were reported |
| “Oxytocin treatment in children with Prader-Willi syndrome: A double-blind, placebo-controlled, crossover study” | Miller et al. (2017) | Human child study Age range: 5–11 y Mean age: 8.2 y PWS: n = 24 Females: 15 Males: 9 |
Double-blind, randomized, placebo-controlled, cross-over |
Manipulation: daily intranasal dose of 16 IU OXT or placebo for 5 days followed by a minimum of 4-week washout period to cross over to the alternative treatment for further 5 days Outcomes: -Aberrant Behavior Checklist -Social Responsiveness Scale -Repetitive Behavior Scale-Revised -Hyperphagia Questionnaire -Clinical Global Impression Scale |
- No significant improvements in any of the measures but trend towards significance was reported in all of the measures following intranasal OXT treatment -Intranasal OXT treatment was shown to be safe and well tolerated with a few adverse events (i.e., nasal irritation and irritability) |
| “The use of oxytocin to improve feeding and social skills in infants with Prader–Willi syndrome” | Tauber et al. (2017) (Note: same study/population as Viaux-Savelon et al., 2016 above) | Human infant study Age range: 0.8–5.7 m Mean age: 3.9 m PWS: n = 18 Females: 8 Males: 10 |
Unblinded dose escalation with 3 steps over 7 days | Manipulation: intranasal dose of 4 IU OXT every other day, daily, or twice daily for one week Outcomes: -Neonatal Oral-Motor Assessment -Clinical Global Impression -Alarm Distress Baby Scale - Coding Interactive Behavior Scale |
-Significant improvements in sucking, swallowing, Clinical Global Impression scores, social withdrawal behavior and mother-infant interactions were reported following intranasal OXT treatment compared to baseline - Intranasal OXT was well tolerated without any adverse events |
| “Intranasal carbetocin reduces hyperphagia in individuals with Prader-Willi syndrome” | Dykens et al. (2018) | Human adolescent study Age range: 10–18 y Mean age: 13.7 y PWS: n = 37 Females: 23 Males: 14 |
Double-blind, randomized, placebo-controlled parallel trial between Subjects | Manipulation: thrice daily intranasal dose of 9.6 mg carbetocin or placebo for two weeks Outcomes: -Hyperphagia in PWS Questionnaire-Responsiveness -Children’s Yale-Brown Obsessive- Compulsive Scale -Clinical Global Impression-Improvement Scale -Reiss Profile food domain |
-Significant improvements in hyperphagic symptoms, food-related behaviors and emotions, compulsivity, and overall functioning were found following intranasal carbetocin treatment compared to placebo -Intranasal carbetocin was well tolerated |
| “Oxytocin in young children with Prader-Willi syndrome: Results of a randomized, double-blind, placebo-controlled, crossover trial investigating 3 months of oxytocin” | Damen et al. (2021) | Human child study Age range: 3–11 y Mean age: 7.5 y PWS: n = 26 Females: 13 Males: 13 |
Double-blind, randomized, placebo-controlled, cross-over | Manipulation: twice daily intranasal dose of 16–40 IU OXT or placebo for 3 months followed by a 1-month washout period to cross over to the alternative treatment for further 3 months Outcomes: -Oxytocin Study Questionnaire (focused on changes in emotions, social, and eating behavior, as well as possible side effects) -Hyperphagia Questionnaire -Repetitive Behavior Scale-Revised -Social Responsiveness Scale |
-No significant improvement in hyperphagia or social behavior was found following OXT treatment in the total group -Oxytocin Study Questionnaire scores improved significantly only in boys but not in girls following OXT treatment compared to placebo -Significant improvements in hyperphagia and social behavior were found in children with PWS who had paternal deletion on chromosome 15 -Intranasal OXT was well tolerated without significant side effects |
| “Intranasal oxytocin versus placebo for hyperphagia and repetitive behaviors in children with Prader-Willi Syndrome: A randomized controlled pilot trial” | Hollander et al. (2021) | Human child and adolescent study Age range: 5–18 y Mean age: 8.87 y PWS: n = 23 Females: n = 5 Males: n = 18 |
Double-blind, randomized, placebo-controlled parallel trial | Manipulation: daily intranasal dose of 16 IU OXT or placebo for 8 weeks Outcomes: -Hyperphagia Questionnaire -Repetitive Behavior Scale-Revised -World Health Organization Quality of Life Scale-BREF -Caregiver Strain Questionnaire |
-No significant improvement in hyperphagia or repetitive behaviors was found following OXT treatment but there were significant reductions in these domains in the placebo group -Intranasal OXT was well tolerated with only one adverse event (i.e., nocturia) |
IU, international units; OXT, oxytocin; PWS, Prader-Willi syndrome
9.1. Oxytocin administration and behavioral functioning
Single dose OXT vs. placebo administration was found to improve trust, sadness, and disruptive behaviors, but not social skills, in adults with PWS (Tauber et al., 2011). These promising single dose OXT data paved the way for investigating the effects of longer-term OXT treatment in adolescents and adults with PWS. However, 8-week OXT treatment showed no significant benefit on compulsivity, social behaviors, or hyperphagia, but instead resulted in increased temper outbursts, particularly at higher doses of OXT (Einfeld et al., 2014). The authors of this study speculated that the increased temper outburst behaviors observed at higher OXT doses could be due to AVP receptor occupancy, since AVP receptor activation has been shown to increase negative emotional arousal in rodents under some circumstances (Albers, 2012). However, it is worth noting that individuals with ASD also frequently exhibit temper outburst behaviors (Adler et al., 2015; Research Units on Pediatric Psychopharmacology Autism Network, 2005), but increased temper outburst behaviors were not previously associated with higher doses of OXT administration in a large sample which included at least some similar aged individuals with ASD (Sikich et al., 2021). Additionally, a meta-analysis of intranasal OXT treatment trial findings found OXT to be safe and well tolerated in people with ASD (Cai et al., 2018). Thus, whether the observed PWS response to OXT treatment is disease-specific (i.e., high-dose OXT induces temper outburst behaviors in people with PWS but not in those with ASD), a false positive due to chance inclusion of non-representative individuals in the trial’s small sample, or due to age-related developmental effects rather than dose per se (see below), is unknown.
Subsequent OXT treatment trials have been conducted in children with PWS, with varying results, reviewed here by duration of treatment. Five-day OXT treatment in children with PWS showed no significant change in behavior, but trends were observed for improvement in hyperphagia, socialization, anxiety, and repetitive behaviors (Miller et al., 2017). Following a 4-week OXT treatment, age-specific effects were detected: Children under 11 years showed significant improvements in social and food-related behaviors, as well as improvements in anger, sadness, and conflicts, whereas children over 11 years of age showed decreased happiness and increased anger and sadness (Kuppens et al., 2016). It remains unclear why older children did not benefit from OXT treatment. Perhaps lack of efficacy was due to puberty-related changes in the OXT system that may prevent older children from responding to OXT treatment.
Another study examined the effects of daily 8-week OXT vs. placebo treatment on hyperphagia and repetitive behaviors in children and adolescents with PWS. This trial reported modest significant improvement in hyperphagia and repetitive behaviors following placebo but not OXT treatment (Hollander et al., 2021). Although both male and female children and adolescents were included in this trial, randomization was not gender-balanced and the study was not adequately powered to examine PWS genetic subtype, age, or gender effects. These findings highlight the need for larger, well-powered studies to better understand the effects of OXT treatment in individuals with PWS.
Finally, in the longest duration pediatric OXT clinical trial in PWS to date, boys with PWS treated daily with OXT for a three month period showed significant improvements in social and eating behaviors compared to placebo (Damen et al., 2021). These effects were particularly pronounced in children with the paternal deletion vs the maternal uniparental disomy subtype of PWS. These genetic subtype findings were surprising, as maternal uniparental disomy of chromosome 15 has been more tightly linked to ASD (and its attendant social impairments) than has paternal deletion of 15q11-q13 (Veltman et al., 2005).
Additional work has been conducted examining the effect of OXT on feeding and social behaviors in infants with PWS using unblinded, differing dosing schedules (Tauber et al., 2017; Viaux-Savelon et al., 2016). Namely, escalated doses of OXT administration over a 7-day period normalized sucking, and improved swallowing, mother-infant interactions, and social skills, regardless of OXT dose. Long-term observational data implicated improvement on muscle tone and motor coordination, as OXT-treated infants were able to crawl as toddlers compared to untreated age-matched controls with PWS. However, long-term effects of this short-term OXT administration on feeding and social behaviors remain unclear. Furthermore, the study was not placebo-controlled and clinicians who rated behaviors were not blind to the intervention. Additional studies in infants with double-blind, randomized, placebo-controlled study designs investigating short- and long-terms effects are needed to more fully ascertain the therapeutic efficacy of OXT.
9.2. Carbetocin administration and behavioral functioning
The mixed findings from OXT treatment trials in PWS may speak to OXT’s lack of specificity. Carbetocin is an analog of OXT and has receptor-selectivity for OXT receptors over AVP receptors which may overcome potential unfavorable effects of OXT at higher doses. A recent double-blind, randomized, placebo-controlled study examined the therapeutic benefit of carbetocin in adolescents with PWS (Dykens et al., 2018). Three times daily carbetocin administration for 14 days reduced hyperphagia and obsessive-compulsive behaviors as well as improved overall behavioral symptoms. A larger multi-center, double-blind, randomized, placebo-controlled phase 3 study has also evaluated the efficacy, safety, and tolerability of intranasal carbetocin in participants with PWS (https://clinicaltrials.gov/ct2/show/NCT03649477). Numeric trends toward improvement in Children’s Yale-Brown Obsessive--Compulsive Scale (CY-BOCS) and PWS Anxiety and Distress Questionnaire (PADQ) scores were observed in each dose arm, but did not reach statistical significance versus placebo. Unfortunately, this study was terminated early due to the global SARS-CoV-2 pandemic, and no additional data are currently available. During this studyTauber et and adolescents with PWS (https://www.levotx.com/news/care-pw-s_top-line_results/). These data indicate that longer studies to confirm safety and efficacy of carbetocin in individuTauber et als with PWS across all ages are warranted.
10. Summary and implications
The goal of the present effort was to review the extant literature on OXT and AVP signaling and neuropeptide administration in animal models and in individuals with PWS. Across both preclinical and clinical studies, pronounced and wide-ranging dysfunction in OXT and/or AVP signaling pathways was evident, impacting cellular production, neural transmission, and/or receptor availability (Fig. 1; Tables 1 and 3). The majority of preclinical research to date has been conducted in a single PWS animal model involving a single gene, Magel2; perhaps this is why the preclinical PWS “story” is fairly straightforward at present: Magel2 mutant mice exhibit pronounced central neuropeptide signaling disruption that likely arises from a prohormone processing defect early in life, and which in all likelihood persists into adulthood. In PWS patients, although neuropeptide dysregulation is robustly evident, the directionality and severity of this dysfunction is not consistent across studies, likely due, at least in part, to differences in subjects’ age, sex, and PWS genetic subtype, as well as the type of biological specimen collected and the biological measurement techniques employed.
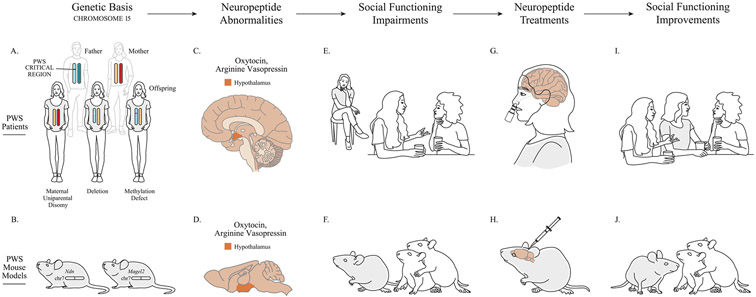
Fig. 1.
Hypothalamic neuropeptides and social functioning in Prader-Willi syndrome (PWS). A. PWS is caused by genetic changes within the PWS critical region on chromosome 15 (15q11-q13). The top section of panel A represents an unaffected father (with the PWS critical region indicated on chromosome 15) and an unaffected mother. The bottom section of panel A depicts the specific changes to chromosome 15 that cause PWS in offspring (shaded in gray): maternal uniparental disomy of chromosome 15 (i.e., both copies of chromosome 15 are inherited from the mother), paternal deletion of 15q11-q13, or an imprinting (methylation) defect of paternal 15q11-q13. B. Mice carrying targeted gene mutations of PWS (shaded in gray) in Ndn and Magel2 on chromosome 7 (chr7) recapitulate many core characteristics of PWS. C. and D. Prohormone convertase deficiency (not pictured) is hypothesized to alter precursor processing of the hypothalamic neuropeptides, oxytocin and arginine vasopressin, in both PWS patients and mouse models of PWS. E. and F. The resulting neuropeptide signaling abnormalities, in turn, are associated with social functioning deficits in both PWS patients and mouse models. G. and I. Intranasal oxytocin or carbetocin (an oxytocin analogue) treatment improves social functioning in at least some individuals with PWS. H. and J. Oxytocin or arginine vasopressin administration improves social functioning in mouse models of PWS. (The effect of arginine vasopressin administration on social functioning has not been tested in PWS patients.).
Preclinically, OXT (Meziane et al., 2015) and AVP (Borie et al., 2021) administration were each shown to reverse behavioral deficits in a PWS mouse model (Fig. 1; Table 2). Although far more pharmacological research has been conducted in people with PWS than in mouse models, clinical trial outcomes have been highly variable, and likely impacted by subjects’ age, sex, and PWS genetic subtype, as well as by the study design, drug dose, treatment length, and power to detect meaningful effects. Nevertheless, despite this study-to-study variability, several OXT clinical trials reported promising efficacy on behavioral endpoints, particularly in patients at younger ages, and particularly in males (Fig. 1; Table 4).
11. Limitations and directions for future research
Findings from the studies reviewed here point to numerous avenues for future research. First, although multiple mouse models lacking single genes in the PWS locus have been developed (Kummerfeld et al., 2021), the vast majority of research has focused on eating behavior (a topic discussed in detail elsewhere (Griggs et al., 2015) and one beyond the scope of this social behavior focused review). The work that has been conducted on neuropeptide dysregulation and social impairment in PWS has been restricted nearly exclusively to a single genetic model (i.e., Magel2). Social behavior-focused research using existing mouse genetic models can thus readily be conducted to gain a more nuanced understanding of the unique contribution of each PWS-associated gene to neuropeptide dysregulation and its role(s) in social impairment. Second, these mouse models can also be deployed to gain a better mechanistic understanding of the therapeutic actions of OXT and AVP on social behavior, and the PWS phenotype more generally, as specific receptor targets for each of these medications remain poorly understood in humans (Rae et al., 2022). Third, although preclinical research efforts have established a credible link between neuropeptide dysregulation and social impairment in PWS, mechanistic research is still needed to better understand the relationship between prohormone convertases and OXT and AVP signaling, particularly given the large role prohormone processing deficits may play in other neuroendocrine features of PWS (Burnett et al., 2017). Fourth, it is likely that neuropeptide dysregulation differs across the PWS population as a whole. This possibility points to the importance of systematically measuring OXT and AVP concentrations in individuals with PWS compared to matched controls, using gold-standard neuropeptide measurement techniques (Tabak et al., 2022), to better understand how and to what extent neuropeptide signaling may be disrupted in this clinical population. Moreover, it is also imperative to determine whether individual differences in neuropeptide levels relate to social symptom severity, and how certain factors, such as age, sex, and PWS genetic subtype may impact OXT and AVP concentrations. Fifth, once this biological information is better synthesized, researchers will be poised to conduct biologically informed (and possibly biomarker stratified) precision treatment trials to better and more rigorously test OXT’s therapeutic potential in PWS. A similar precision medicine approach has already been applied to individuals with idiopathic ASD (Parker et al., 2017) and to fragile-X syndrome patients (Hall et al., 2012), the latter who as a population have been hypothesized to have OXT signaling deficits (Francis et al., 2014). Sixth, a precision medicine approach to studying and treating PWS would also increase the power to detect effects of OXT or AVP administration in the small study samples which are common in rare disease research efforts. Indeed, the low population prevalence of PWS typically limits the ability of single sites to recruit large study samples, and standard research funding caps limit the number of multi-site projects which can support large-scale recruitment efforts. Cross-over trial designs enable a repeated measures approach to statistical analysis, and, in theory, can be useful for testing medications in smaller study samples. However, cross-over designs are vulnerable to learning effects across treatment arms when a participant is repeatedly exposed to test stimuli (which is particularly problematic if these stimuli are used to evaluate treatment efficacy). Cross-over designs are also vulnerable to carry-over effects that can occur when active treatment is administered prior to placebo. Standard placebo-controlled parallel designs guard against these concerns, but typically require larger study samples. One potential way to mitigate these concerns is to employ Sequential Parallel Comparison Design (SPCD) (Doros et al., 2013; Tamura and Huang, 2007) in PWS medication trials. SPCD is an innovative and statistically powerful approach to double-blind randomized placebo-controlled trial design. SPCD enhances signal detection by minimizing the effect of placebo responses and can be used successfully in the context of smaller study samples. Seventh, carefully designed research studies are also needed to ascertain when during development OXT treatment is most beneficial, and whether there is a “critical period” for its administration (DeMayo et al., 2019). Eighth, it is also crucial to determine whether OXT treatment is most efficacious when administered intermittently in the context of social skills training (which would be the case if its primary mechanism of action is to increase social salience) (Ford and Young, 2022; Peled-Avron et al., 2020) or whether OXT treatment is most effective as a “replacement” strategy for a neuroendocrine deficiency, not unlike other hypothalamic-pituitary hormones in PWS which require routine, standard-of-care replacement therapy (Tauber and Diene, 2021). Finally, the safety and efficacy of AVP treatment for social impairments in individuals with PWS warrants investigation, particularly in light of the fact that AVP appears to be deficient in at least some PWS patients, and AVP has been shown to “rescue” social functioning in Magel2-deficient mice with impaired AVP signaling (Borie et al., 2021). However, as with OXT, there is a need to develop a therapy with appropriate receptor specificity, and demonstrated target engagement in this patient population.
12. Conclusion
Compelling evidence from both animal models and individuals with PWS implicates pronounced dysregulation of brain OXT and AVP signaling pathways and subsequent effects on social behavior. Preclinical research has begun to forge links between neuropeptide dysregulation and social functional deficits, although the process by which this dysregulation occurs in PWS awaits mechanistic investigation. Clinical research to date points to similar links, but further research is still needed to better and systematically characterize how OXT and AVP biology relate to social impairment and how this dysregulation may be affected by other factors, such as age, sex, and genetic subtype in individuals with PWS. Nevertheless, the preponderance of biological evidence, and accumulating clinical trial results, suggest that these neuropeptide pathways may provide promising targets for therapeutic intervention in a patient population that currently lacks a pharmacological strategy for its debilitating social behavior symptoms.
Acknowledgements
This research was supported by the NIH Sleep and Genetics Multi-Institution T32 Postdoctoral Fellow Training Program (HL110952) and Stanford’s Department of Psychiatry and Behavioral Sciences.
References
- Adhikari A, Copping NA, Onaga B, Pride MC, Coulson RL, Yang M, Yasui DH, LaSalle JM, Silverman JL, 2019. Cognitive deficits in the Snord116 deletion mouse model for Prader-Willi syndrome. Neurobiol. Learn Mem 165, 106874 10.1016/j.nlm.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler BA, Wink LK, Early M, Shaffer R, Minshawi N, McDougle CJ, Erickson CA, 2015. Drug-refractory aggression, self-injurious behavior, and severe tantrums in autism spectrum disorders: a chart review study. Autism 19, 102–106. 10.1177/1362361314524641. [DOI] [PubMed] [Google Scholar]
- Albers HE, 2012. The regulation of social recognition, social communication and aggression: vasopressin in the social behavior neural network. Horm. Behav 61, 283–292. 10.1016/j.yhbeh.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Andari E, Duhamel J-R, Zalla T, Herbrecht E, Leboyer M, Sirigu A, 2010. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc. Natl. Acad. Sci. USA 107, 4389–4394. 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ates T, Oncul M, Dilsiz P, Topcu IC, Civas CC, Alp MI, Aklan I, Oz EA, Yavuz Y, Yilmaz B, 2019. Inactivation of Magel2 suppresses oxytocin neurons through synaptic excitation-inhibition imbalance. Neurobiol. Dis 121, 58–64. [DOI] [PubMed] [Google Scholar]
- Barberis C, Tribollet E, 1996. Vasopressin and oxytocin receptors in the central nervous system. Crit. Rev. Neurobiol 10, 119–154. 10.1615/critrevneurobiol.v10.i1.60. [DOI] [PubMed] [Google Scholar]
- Baribeau DA, Anagnostou E, 2015. Oxytocin and vasopressin: linking pituitary neuropeptides and their receptors to social neurocircuits. Front Neurosci. 9, 335. 10.3389/fnins.2015.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E, 2008. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron 58, 639–650. 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu S-B, Szegda KL, Westphal H, Young LJ, 2004. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology 29, 483–493. 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- Bischof JM, Stewart CL, Wevrick R, 2007. Inactivation of the mouse Magel2 gene results in growth abnormalities similar to Prader-Willi syndrome. Hum. Mol. Genet 16, 2713–2719. 10.1093/hmg/ddm225. [DOI] [PubMed] [Google Scholar]
- Bittel DC, Kibiryeva N, Sell SM, Strong TV, Butler MG, 2007. Whole genome microarray analysis of gene expression in Prader–Willi syndrome. Am. J. Med. Genet. Part A 143, 430–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccaccio I, Glatt-Deeley H, Watrin F, Roëckel N, Lalande M, Muscatelli F, 1999. The human MAGEL2 gene and its mouse homologue are paternally expressed and mapped to the Prader-Willi region. Hum. Mol. Genet 8, 2497–2505. [DOI] [PubMed] [Google Scholar]
- Borie AM, Dromard Y, Guillon G, Olma A, Manning M, Muscatelli F, Desarménien MG, Jeanneteau F, 2021. Correction of vasopressin deficit in the lateral septum ameliorates social deficits of mouse autism model. J. Clin. Invest 131, 144450. 10.1172/JCI144450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstein MJ, Russell JT, Gainer H, 1980. Synthesis, transport, and release of posterior pituitary hormones. Science 207, 373–378. 10.1126/science.6153132. [DOI] [PubMed] [Google Scholar]
- Brunnlieb C, Nave G, Camerer CF, Schosser S, Vogt B, Münte TF, Heldmann M, 2016. Vasopressin increases human risky cooperative behavior. Proc. Natl. Acad. Sci 113, 2051–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buiting K, Saitoh S, Gross S, Dittrich B, Schwartz S, Nicholls RD, Horsthemke B, 1995. Inherited microdeletions in the Angelman and Prader-Willi syndromes define an imprinting centre on human chromosome 15. Nat. Genet 9, 395–400. 10.1038/ng0495-395. [DOI] [PubMed] [Google Scholar]
- Burman P, Ritzén EM, Lindgren AC, 2001. Endocrine dysfunction in Prader-Willi syndrome: a review with special reference to GH. Endocr. Rev 22, 787–799. 10.1210/edrv.22.6.0447. [DOI] [PubMed] [Google Scholar]
- Burnett LC, LeDuc CA, Sulsona CR, Paull D, Rausch R, Eddiry S, Carli JFM, Morabito MV, Skowronski AA, Hubner G, Zimmer M, Wang L, Day R, Levy B, Fennoy I, Dubern B, Poitou C, Clement K, Butler MG, Rosenbaum M, Salles JP, Tauber M, Driscoll DJ, Egli D, Leibel RL, 2017. Deficiency in prohormone convertase PC1 impairs prohormone processing in Prader-Willi syndrome. J. Clin. Invest 127, 293–305. 10.1172/JCI88648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, 1990. Prader-Willi syndrome: current understanding of cause and diagnosis. Am. J. Med Genet 35, 319–332. 10.1002/ajmg.1320350306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Feng L, Yap KZ, 2018. Systematic review and meta-analysis of reported adverse events of long-term intranasal oxytocin treatment for autism spectrum disorder. Psychiatry Clin. Neurosci 72, 140–151. 10.1111/pcn.12627. [DOI] [PubMed] [Google Scholar]
- Carter CS, Grippo AJ, Pournajafi-Nazarloo H, Ruscio MG, Porges SW, 2008. Oxytocin, vasopressin and sociality. Prog. brain Res 170, 331–336. [DOI] [PubMed] [Google Scholar]
- Cassidy SB, 1997. Prader-Willi syndrome. J. Med. Genet 34, 917–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy SB, Schwartz S, Miller JL, Driscoll DJ, 2012. Prader-willi syndrome. Genet. Med 14, 10–26. [DOI] [PubMed] [Google Scholar]
- Castel M, Morris JF, 1988. The neurophysin-containing innervation of the forebrain of the mouse. Neuroscience 24, 937–966. 10.1016/0306-4522(88)90078-4. [DOI] [PubMed] [Google Scholar]
- Chen H, Victor AK, Klein J, Tacer KF, Tai DJ, de Esch C, Nuttle A, Temirov J, Burnett LC, Rosenbaum M, Zhang Y, Ding L, Moresco JJ, Diedrich JK, Yates JR, Tillman HS, Leibel RL, Talkowski ME, Billadeau DD, Reiter LT, Potts PR, 2020. Loss of MAGEL2 in Prader-Willi syndrome leads to decreased secretory granule and neuropeptide production. JCI Insight 5, 138576. 10.1172/jci.insight.138576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa-da-Silva F, Fliers E, Swaab DF, Yi C-X, 2021. Hypothalamic neuropeptides and neurocircuitries in Prader Willi syndrome. J. Neuroendocr 33, e12994 10.1111/jne.12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damen L, Grootjen LN, Juriaans AF, Donze SH, Huisman TM, Visser JA, Delhanty PJD, Hokken-Koelega ACS, 2021. Oxytocin in young children with Prader-Willi syndrome: results of a randomized, double-blind, placebo-controlled, crossover trial investigating 3 months of oxytocin. Clin. Endocrinol 94, 774–785. 10.1111/cen.14387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debladis J, Valette M, Strenilkov K, Mantoulan C, Thuilleaux D, Laurier V, Molinas C, Barone P, Tauber M, 2019. Face processing and exploration of social signals in Prader-Willi syndrome: a genetic signature. Orphanet J. Rare Dis 14, 262. 10.1186/s13023-019-1221-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMayo MM, Young LJ, Hickie IB, Song YJC, Guastella AJ, 2019. Circuits for social learning: a unified model and application to Autism Spectrum Disorder. Neurosci. Biobehav Rev 107, 388–398. 10.1016/j.neubiorev.2019.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diene G, Mimoun E, Feigerlova E, Caula S, Molinas C, Grandjean H, Tauber M, 2010. Endocrine disorders in children with Prader-Willi syndrome–data from 142 children of the French database. Horm. Res Paediatr 74, 121–128. 10.1159/000313377. [DOI] [PubMed] [Google Scholar]
- Dimitropoulos A, Schultz RT, 2007. Autistic-like symptomatology in Prader-Willi syndrome: a review of recent findings. Curr. Psychiatry Rep 9, 159–164. [DOI] [PubMed] [Google Scholar]
- Dimitropoulos A, Ho A, Feldman B, 2013. Social responsiveness and competence in Prader-Willi syndrome: direct comparison to autism spectrum disorder. J. Autism Dev. Disord 43, 103–113. 10.1007/s10803-012-1547-3. [DOI] [PubMed] [Google Scholar]
- Dimitropoulos A, Zyga O, Russ SW, 2019. Early social cognitive ability in preschoolers with prader-willi syndrome and autism spectrum disorder. J. Autism Dev. Disord 49, 4441–4454. 10.1007/s10803-019-04152-4. [DOI] [PubMed] [Google Scholar]
- Ding F, Li HH, Zhang S, Solomon NM, Camper SA, Cohen P, Francke U, 2008. SnoRNA Snord116 (Pwcr1/MBII-85) deletion causes growth deficiency and hyperphagia in mice. PLoS One 3, e1709. 10.1371/journal.pone.0001709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittrich B, Buiting K, Korn B, Rickard S, Buxton J, Saitoh S, Nicholls RD, Poustka A, Winterpacht A, Zabel B, Horsthemke B, 1996. Imprint switching on human chromosome 15 may involve alternative transcripts of the SNRPN gene. Nat. Genet 14, 163–170. 10.1038/ng1096-163. [DOI] [PubMed] [Google Scholar]
- Donaldson MD, Chu CE, Cooke A, Wilson A, Greene SA, Stephenson JB, 1994. The Prader-Willi syndrome. Arch. Dis. Child 70, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ, 2008. Oxytocin, vasopressin, and the neurogenetics of sociality. Science 322, 900–904. [DOI] [PubMed] [Google Scholar]
- Dong W, Seidel B, Marcinkiewicz M, Chrétien M, Seidah NG, Day R, 1997. Cellular localization of the prohormone convertases in the hypothalamic paraventricular and supraoptic nuclei: selective regulation of PC1 in corticotrophin-releasing hormone parvocellular neurons mediated by glucocorticoids. J. Neurosci 17, 563. 10.1523/JNEUROSCI.17-02-00563.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlon TA, 1988. Similar molecular deletions on chromosome 15q11.2 are encountered in both the Prader-Willi and Angelman syndromes. Hum. Genet 80, 322–328. 10.1007/BF00273644. [DOI] [PubMed] [Google Scholar]
- Doros G, Pencina M, Rybin D, Meisner A, Fava M, 2013. A repeated measures model for analysis of continuous outcomes in sequential parallel comparison design studies. Stat. Med 32, 2767–2789. 10.1002/sim.5728. [DOI] [PubMed] [Google Scholar]
- Dykens EM, Roof E, Hunt-Hawkins H, Dankner N, Lee EB, Shivers CM, Daniell C, Kim S-J, 2017. Diagnoses and characteristics of autism spectrum disorders in children with Prader-Willi syndrome. J. Neurodev. Disord 9, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykens EM, Miller J, Angulo M, Roof E, Reidy M, Hatoum HT, Willey R, Bolton G, Korner P, 2018. Intranasal carbetocin reduces hyperphagia in individuals with Prader-Willi syndrome. JCI Insight 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykens EM, Roof E, Hunt-Hawkins H, Daniell C, Jurgensmeyer S, 2019. Profiles and trajectories of impaired social cognition in people with Prader-Willi syndrome. PLoS One 14, e0223162. 10.1371/journal.pone.0223162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddiry S, Diene G, Molinas C, Salles J, Auriol FC, Gennero I, Bieth E, Skryabin BV, Rozhdestvensky TS, Burnett LC, Leibel RL, Tauber M, Salles JP, 2021. SNORD116 and growth hormone therapy impact IGFBP7 in Prader–Willi syndrome. Genet. Med 23, 1664–1672. 10.1038/s41436-021-01185-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einfeld SL, Smith E, McGregor IS, Steinbeck K, Taffe J, Rice LJ, Horstead SK, Rogers N, Hodge MA, Guastella AJ, 2014. A double-blind randomized controlled trial of oxytocin nasal spray in Prader Willi syndrome. Am. J. Med. Genet. Part A 164, 2232–2239. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT, 2000. Social amnesia in mice lacking the oxytocin gene. Nat. Genet 25, 284–288. 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- Fischer-Shofty M, Shamay-Tsoory SG, Harari H, Levkovitz Y, 2010. The effect of intranasal administration of oxytocin on fear recognition. Neuropsychologia 48, 179–184. [DOI] [PubMed] [Google Scholar]
- Ford CL, Young LJ, 2022. Refining oxytocin therapy for autism: context is key. Nat. Rev. Neurol 18, 67–68. 10.1038/s41582-021-00602-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain MD, Tao H, Chen C-A, Yin J, Schaaf CP, 2017. Magel2 knockout mice manifest altered social phenotypes and a deficit in preference for social novelty. Genes Brain Behav. 16, 592–600. 10.1111/gbb.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox DL, Good DJ, 2008. Nescient helix-loop-helix 2 interacts with signal transducer and activator of transcription 3 to regulate transcription of prohormone convertase 1/3. Mol. Endocrinol 22, 1438–1448. 10.1210/me.2008-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis SM, Sagar A, Levin-Decanini T, Liu W, Carter CS, Jacob S, 2014. Oxytocin and vasopressin systems in genetic syndromes and neurodevelopmental disorders. Brain Res. 1580, 199–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabreëls BTF, Swaab DF, De Kleijn DPV, Seidah NG, Van de Loo J-W, Van de Ven WJM, Martens GJM, Van Leeuwen FW, 1998. Attenuation of the polypeptide 7B2, prohormone convertase PC2, and vasopressin in the hypothalamus of some Prader-Willi patients: indications for a processing defect. J. Clin. Endocrinol. Metab 83, 591–599. [DOI] [PubMed] [Google Scholar]
- Griggs JL, Sinnayah P, Mathai ML, 2015. Prader-Willi syndrome: from genetics to behaviour, with special focus on appetite treatments. Neurosci. Biobehav Rev 59, 155–172. 10.1016/j.neubiorev.2015.10.003. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Dadds MR, 2008a. Oxytocin increases gaze to the eye region of human faces. Biol. Psychiatry 63, 3–5. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Mathews F, 2008b. Oxytocin enhances the encoding of positive social memories in humans. Biol. Psychiatry 64, 256–258. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Kenyon AR, Alvares GA, Carson DS, Hickie IB, 2010. Intranasal arginine vasopressin enhances the encoding of happy and angry faces in humans. Biol. Psychiatry 67, 1220–1222. [DOI] [PubMed] [Google Scholar]
- Hall SS, Lightbody AA, McCarthy BE, Parker KJ, Reiss AL, 2012. Effects of intranasal oxytocin on social anxiety in males with fragile X syndrome. Psychoneuroendocrinology 37, 509–518. 10.1016/j.psyneuen.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U, 2003. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol. Psychiatry 54, 1389–1398. [DOI] [PubMed] [Google Scholar]
- Hollander E, Levine KG, Ferretti CJ, Freeman K, Doernberg E, Desilva N, Taylor BP, 2021. Intranasal oxytocin versus placebo for hyperphagia and repetitive behaviors in children with Prader-Willi Syndrome: a randomized controlled pilot trial. J. Psychiatr. Res 137, 643–651. 10.1016/j.jpsychires.2020.11.006. [DOI] [PubMed] [Google Scholar]
- Horsthemke B., 1997. Structure and function of the human chromosome 15 imprinting center. J. Cell Physiol 173, 237–241 https://doi.org/10.1002. [DOI] [PubMed] [Google Scholar]
- Höybye C, Barkeling B, Espelund U, Petersson M, Thorén M, 2003. Peptides associated with hyperphagia in adults with Prader–Willi syndrome before and during GH treatment. Growth Horm. IGF Res 13, 322–327. [DOI] [PubMed] [Google Scholar]
- Jin D, Liu H-X, Hirai H, Torashima T, Nagai T, Lopatina O, Shnayder NA, Yamada K, Noda M, Seike T, Fujita K, Takasawa S, Yokoyama S, Koizumi K, Shiraishi Y, Tanaka S, Hashii M, Yoshihara T, Higashida K, Islam MS, Yamada N, Hayashi K, Noguchi N, Kato I, Okamoto H, Matsushima A, Salmina A, Munesue T, Shimizu N, Mochida S, Asano M, Higashida H, 2007. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature 446, 41–45. 10.1038/nature05526. [DOI] [PubMed] [Google Scholar]
- Johnson L, Manzardo AM, Miller JL, Driscoll DJ, Butler MG, 2016. Elevated plasma oxytocin levels in children with Prader–Willi syndrome compared with healthy unrelated siblings. Am. J. Med. Genet. Part A 170, 594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV, Young LJ, 2017. Oxytocin and vasopressin neural networks: Implications for social behavioral diversity and translational neuroscience. Neurosci. Biobehav Rev 76, 87–98. 10.1016/j.neubiorev.2017.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong MT, Gray TA, Ji Y, Glenn CC, Saitoh S, Driscoll DJ, Nicholls RD, 1999. A novel imprinted gene, encoding a RING zinc-finger protein, and overlapping antisense transcript in the Prader-Willi syndrome critical region. Hum. Mol. Genet 8, 783–793. 10.1093/hmg/8.5.783. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Keverne EB, Baldwin BA, 1987. Intracerebroventricular oxytocin stimulates maternal behaviour in the sheep. Neuroendocrinology 46, 56–61. 10.1159/000124796. [DOI] [PubMed] [Google Scholar]
- Key AP, Jones D, Dykens EM, 2013. Social and emotional processing in Prader-Willi syndrome: genetic subtype differences. J. Neurodev. Disord 5, 7. 10.1186/1866-1955-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll JH, Nicholls RD, Magenis RE, Graham JM, Lalande M, Latt SA, 1989. Angelman and Prader-Willi syndromes share a common chromosome 15 deletion but differ in parental origin of the deletion. Am. J. Med. Genet 32, 285–290. 10.1002/ajmg.1320320235. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E, 2005. Oxytocin increases trust in humans. Nature 435, 673–676. [DOI] [PubMed] [Google Scholar]
- Kummerfeld D-M, Raabe CA, Brosius J, Mo D, Skryabin BV, Rozhdestvensky TS, 2021. A comprehensive review of genetically engineered mouse models for prader-willi syndrome research. Int. J. Mol. Sci 22, 3613. 10.3390/ijms22073613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppens RJ, Donze SH, Hokken-Koelega ACS, 2016. Promising effects of oxytocin on social and food-related behaviour in young children with Prader–Willi syndrome: a randomized, double-blind, controlled crossover trial. Clin. Endocrinol 85, 979–987. [DOI] [PubMed] [Google Scholar]
- Lagman D, Ocampo Daza D, Widmark J, Abalo XM, Sundström G, Larhammar D, 2013. The vertebrate ancestral repertoire of visual opsins, transducin alpha subunits and oxytocin/vasopressin receptors was established by duplication of their shared genomic region in the two rounds of early vertebrate genome duplications. BMC Evol. Biol 13, 238. 10.1186/1471-2148-13-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G, Sabatier N, 2016. Measuring oxytocin and vasopressin: bioassays, immunoassays and random numbers. J. Neuroendocrinol 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig M, Leng G, 2006. Dendritic peptide release and peptide-dependent behaviours. Nat. Rev. Neurosci 7, 126–136. 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Magenis RE, Toth-Fejel S, Allen LJ, Black M, Brown MG, Budden S, Cohen R, Friedman JM, Kalousek D, Zonana J, 1990. Comparison of the 15q deletions in Prader-Willi and Angelman syndromes: specific regions, extent of deletions, parental origin, and clinical consequences. Am. J. Med. Genet 35, 333–349. 10.1002/ajmg.1320350307. [DOI] [PubMed] [Google Scholar]
- Martin A, State M, Anderson GM, Kaye WM, Hanchett JM, McConaha CW, North WG, Leckman JF, 1998. Cerebrospinal fluid levels of oxytocin in Prader–Willi syndrome: a preliminary report. Biol. Psychiatry 44, 1349–1352. [DOI] [PubMed] [Google Scholar]
- Mascari MJ, Gottlieb W, Rogan PK, Butler MG, Waller DA, Armour JA, Jeffreys AJ, Ladda RL, Nicholls RD, 1992. The frequency of uniparental disomy in Prader-Willi syndrome. Implications for molecular diagnosis. New Engl. J. Med 326, 1599–1607. 10.1056/NEJM199206113262404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough ME, Churchland PS, Mendez AJ, 2013. Problems with measuring peripheral oxytocin: can the data on oxytocin and human behavior be trusted? Neurosci. Biobehav. Rev 37, 1485–1492. [DOI] [PubMed] [Google Scholar]
- Meinlschmidt G, Heim C, 2007. Sensitivity to intranasal oxytocin in adult men with early parental separation. Biol. Psychiatry 61, 1109–1111. 10.1016/j.biopsych.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Mercer RE, Wevrick R, 2009. Loss of magel2, a candidate gene for features of Prader-Willi syndrome, impairs reproductive function in mice. PLoS One 4, e4291. 10.1371/journal.pone.0004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M, 2011. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat. Rev. Neurosci 12, 524–538. [DOI] [PubMed] [Google Scholar]
- Meziane H, Schaller F, Bauer S, Villard C, Matarazzo V, Riet F, Guillon G, Lafitte D, Desarmenien MG, Tauber M, 2015. An early postnatal oxytocin treatment prevents social and learning deficits in adult mice deficient for Magel2, a gene involved in Prader-Willi syndrome and autism. Biol. Psychiatry 78, 85–94. [DOI] [PubMed] [Google Scholar]
- Miller JL, Tamura R, Butler MG, Kimonis V, Sulsona C, Gold J-A, Driscoll DJ, 2017. Oxytocin treatment in children with Prader–Willi syndrome: a double-blind, placebo-controlled, crossover study. Am. J. Med. Genet. Part A 173, 1243–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatelli F, Abrous DN, Massacrier A, Boccaccio I, Moal ML, Cau P, Cremer H, 2000. Disruption of the mouse Necdin gene results in hypothalamic and behavioral alterations reminiscent of the human Prader–Willi syndrome. Hum. Mol. Genet 9, 3101–3110. 10.1093/hmg/9.20.3101. [DOI] [PubMed] [Google Scholar]
- Nicholls RD, Knoll JH, Butler MG, Karam S, Lalande M, 1989a. Genetic imprinting suggested by maternal heterodisomy in nondeletion Prader-Willi syndrome. Nature 342, 281–285. 10.1038/342281a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls RD, Knoll JH, Glatt K, Hersh JH, Brewster TD, Graham JM, Wurster-Hill D, Wharton R, Latt SA, 1989b. Restriction fragment length polymorphisms within proximal 15q and their use in molecular cytogenetics and the Prader-Willi syndrome. Am. J. Med. Genet 33, 66–77. 10.1002/ajmg.1320330109. [DOI] [PubMed] [Google Scholar]
- Nicholls RD, Saitoh S, Horsthemke B, 1998. Imprinting in Prader–Willi and Angelman syndromes. Trends Genet. 14, 194–200. [DOI] [PubMed] [Google Scholar]
- Oztan O, Garner JP, Partap S, Sherr EH, Hardan AY, Farmer C, Thurm A, Swedo SE, Parker KJ, 2018. Cerebrospinal fluid vasopressin and symptom severity in children with autism. Ann. Neurol 84, 611–615. 10.1002/ana.25314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oztan O, Garner JP, Constantino JN, Parker KJ, 2020. Neonatal CSF vasopressin concentration predicts later medical record diagnoses of autism spectrum disorder. Proc. Natl. Acad. Sci. U. S. A 117, 10609–10613. 10.1073/pnas.1919050117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Nanno D, Che F-Y, Zhu X, Salton SR, Steiner DF, Fricker LD, Devi LA, 2005. Neuropeptide processing profile in mice lacking prohormone convertase-1. Biochemistry 44, 4939–4948. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Lee TM, 2001. Central vasopressin administration regulates the onset of facultative paternal behavior in Microtus pennsylvanicus (meadow voles). Horm. Behav 39, 285–294. 10.1006/hbeh.2001.1655. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Oztan O, Libove RA, Sumiyoshi RD, Jackson LP, Karhson DS, Summers JE, Hinman KE, Motonaga KS, Phillips JM, Carson DS, Garner JP, Hardan AY, 2017. Intranasal oxytocin treatment for social deficits and biomarkers of response in children with autism. Proc. Natl. Acad. Sci. U. S. A 114, 8119–8124. 10.1073/pnas.1705521114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Garner JP, Oztan O, Tarara ER, Li J, Sclafani V, Del Rosso LA, Chun K, Berquist SW, Chez MG, Partap S, Hardan AY, Sherr EH, Capitanio JP, 2018. Arginine vasopressin in cerebrospinal fluid is a marker of sociality in nonhuman primates. Sci. Transl. Med 10 10.1126/scitranslmed.aam9100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Oztan O, Libove RA, Mohsin N, Karhson DS, Sumiyoshi RD, Summers JE, Hinman KE, Motonaga KS, Phillips JM, Carson DS, Fung LK, Garner JP, Hardan AY, 2019. A randomized placebo-controlled pilot trial shows that intranasal vasopressin improves social deficits in children with autism. Sci. Transl. Med 11. 10.1126/scitranslmed.aau7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul MJ, Peters NV, Holder MK, Kim AM, Whylings J, Terranova JI, de Vries GJ, 2016. Atypical social development in vasopressin-deficient brattleboro rats. eNeuro 3. 10.1523/ENEURO.0150-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Prange AJ, 1979. Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc. Natl. Acad. Sci. USA 76, 6661–6665. 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Vadlamudi SV, Boccia ML, Amico JA, 2006. Maternal behavior deficits in nulliparous oxytocin knockout mice. Genes Brain Behav. 5, 274–281. 10.1111/j.1601-183X.2005.00162.x. [DOI] [PubMed] [Google Scholar]
- Peled-Avron L, Abu-Akel A, Shamay-Tsoory S, 2020. Exogenous effects of oxytocin in five psychiatric disorders: a systematic review, meta-analyses and a personalized approach through the lens of the social salience hypothesis. Neurosci. Biobehav. Rev 114, 70–95. 10.1016/j.neubiorev.2020.04.023. [DOI] [PubMed] [Google Scholar]
- Polex-Wolf J, Yeo GS, O’Rahilly S, 2017. Impaired prohormone processing: a grand unified theory for features of Prader-Willi syndrome? The. J. Clin. Investig 127, 98–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pow DV, Morris JF, 1989. Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience 32, 435–439. 10.1016/0306-4522(89)90091-2. [DOI] [PubMed] [Google Scholar]
- Rae M, Lemos Duarte M, Gomes I, Camarini R, Devi LA, 2022. Oxytocin and vasopressin: signalling, behavioural modulation and potential therapeutic effects. Br. J. Pharm 179, 1544–1564. 10.1111/bph.15481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Research Units on Pediatric Psychopharmacology Autism Network, 2005. Risperidone treatment of autistic disorder: longer-term benefits and blinded discontinuation after 6 months. Am. J. Psychiatry 162, 1361–1369. 10.1176/appi.ajp.162.7.1361. [DOI] [PubMed] [Google Scholar]
- Rilling JK, DeMarco AC, Hackett PD, Thompson R, Ditzen B, Patel R, Pagnoni G, 2012. Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology 37, 447–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmele U, Hediger K, Heinrichs M, Klaver P, 2009. Oxytocin makes a face in memory familiar. J. Neurosci 29, 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson WP, Bottani A, Xie YG, Balakrishman J, Binkert F, Mächler M, Prader A, Schinzel A, 1991. Molecular, cytogenetic, and clinical investigations of Prader-Willi syndrome patients. Am. J. Hum. Genet 49, 1219–1234. [PMC free article] [PubMed] [Google Scholar]
- Roebroek AJ, Dehaen MR, van Bokhoven A, Martens GJ, Marÿnen P, van den Berghe H, Van de Ven WJ, 1989. Regional mapping of the human gene encoding the novel pituitary polypeptide 7B2 to chromosome 15q13—q14 by in situ hybridization. Cytogenet Cell Genet. 50, 158–160. 10.1159/000132749. [DOI] [PubMed] [Google Scholar]
- Sahoo T, del Gaudio D, German JR, Shinawi M, Peters SU, Person RE, Garnica A, Cheung SW, Beaudet AL, 2008. Prader-Willi phenotype caused by paternal deficiency for the HBII-85C/D box small nucleolar RNA cluster. Nat. Genet 40, 719–721. 10.1038/ng.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf CP, Gonzalez-Garay ML, Xia F, Potocki L, Gripp KW, Zhang B, Peters BA, McElwain MA, Drmanac R, Beaudet AL, Caskey CT, Yang Y, 2013. Truncating mutations of MAGEL2 cause Prader-Willi phenotypes and autism. Nat. Genet 45, 1405–1408. 10.1038/ng.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller F, Watrin F, Sturny R, Massacrier A, Szepetowski P, Muscatelli F, 2010. A single postnatal injection of oxytocin rescues the lethal feeding behaviour in mouse newborns deficient for the imprinted Magel2 gene. Hum. Mol. Genet 19, 4895–4905. [DOI] [PubMed] [Google Scholar]
- Schwartz L, Caixàs A, Dimitropoulos A, Dykens E, Duis J, Einfeld S, Gallagher L, Holland A, Rice L, Roof E, Salehi P, Strong T, Taylor B, Woodcock K, 2021. Behavioral features in Prader-Willi syndrome (PWS): consensus paper from the International PWS Clinical Trial Consortium. J. Neurodev. Disord 13, 25. 10.1186/s11689-021-09373-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidah NG, 2011. The proprotein convertases, 20 years later. Methods Mol. Biol 768, 23–57. 10.1007/978-1-61779-204-5_3. [DOI] [PubMed] [Google Scholar]
- Sikich L, Kolevzon A, King BH, McDougle CJ, Sanders KB, Kim S-J, Spanos M, Chandrasekhar T, Trelles MDP, Rockhill CM, Palumbo ML, Witters Cundiff A, Montgomery A, Siper P, Minjarez M, Nowinski LA, Marler S, Shuffrey LC, Alderman C, Weissman J, Zappone B, Mullett JE, Crosson H, Hong N, Siecinski SK, Giamberardino SN, Luo S, She L, Bhapkar M, Dean R, Scheer A, Johnson JL, Gregory SG, Veenstra-VanderWeele J, 2021. Intranasal oxytocin in children and adolescents with autism spectrum disorder. New Engl. J. Med 385, 1462–1473. 10.1056/NEJMoa2103583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Smith AJ, Purmann C, Walters RG, Ellis RJ, Holder SE, Van Haelst MM, Brady AF, Fairbrother UL, Dattani M, Keogh JM, Henning E, Yeo GSH, O’Rahilly S, Froguel P, Farooqi IS, Blakemore AIF, 2009. A deletion of the HBII-85 class of small nucleolar RNAs (snoRNAs) is associated with hyperphagia, obesity and hypogonadism. Hum. Mol. Genet 18, 3257–3265. 10.1093/hmg/ddp263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Albers HE, 2018. Cross-talk among oxytocin and arginine-vasopressin receptors: relevance for basic and clinical studies of the brain and periphery. Front Neuroendocr. 51, 14–24. 10.1016/j.yfrne.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stijnen P, Ramos-Molina B, O’Rahilly S, Creemers JWM, 2016. PCSK1 mutations and human endocrinopathies: from obesity to gastrointestinal disorders. Endocr. Rev 37, 347–371. 10.1210/er.2015-1117. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Purba JS, Hofman MA, 1995. Alterations in the hypothalamic paraventricular nucleus and its oxytocin neurons (putative satiety cells) in Prader-Willi syndrome: a study of five cases. J. Clin. Endocrinol. Metab 80, 573–579. [DOI] [PubMed] [Google Scholar]
- Szeto A, McCabe PM, Nation DA, Tabak BA, Rossetti MA, McCullough ME, Schneiderman N, Mendez AJ, 2011. Evaluation of enzyme immunoassay and radioimmunoassay methods for the measurement of plasma oxytocin. Psychosom. Med 73, 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak BA, Leng G, Szeto A, Parker KJ, Verbalis JG, Ziegler TE, Lee MR, Neumann ID, Mendez AJ, 2022. Advances in human oxytocin measurement: challenges and proposed solutions. Mol. Psychiatry 10.1038/s41380-022-01719-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura RN, Huang X, 2007. An examination of the efficiency of the sequential parallel design in psychiatric clinical trials. Clin. Trials 4, 309–317. 10.1177/1740774507081217. [DOI] [PubMed] [Google Scholar]
- Tauber M, Diene G, 2021. Prader-Willi syndrome: Hormone therapies. Handb. Clin. Neurol 181, 351–367. 10.1016/B978-0-12-820683-6.00026-9. [DOI] [PubMed] [Google Scholar]
- Tauber M, Hoybye C, 2021. Endocrine disorders in Prader-Willi syndrome: a model to understand and treat hypothalamic dysfunction. Lancet Diabetes Endocrinol. 9, 235–246. 10.1016/S2213-8587(21)00002-4. [DOI] [PubMed] [Google Scholar]
- Tauber M, Mantoulan C, Copet P, Jauregui J, Demeer G, Diene G, Rogé B, Laurier V, Ehlinger V, Arnaud C, 2011. Oxytocin may be useful to increase trust in others and decrease disruptive behaviours in patients with Prader-Willi syndrome: a randomised placebo-controlled trial in 24 patients. Orphanet J. Rare Dis 6, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber M, Boulanouar K, Diene G, Çabal-Berthoumieu S, Ehlinger V, Fichaux-Bourin P, Molinas C, Faye S, Valette M, Pourrinet J, 2017. The use of oxytocin to improve feeding and social skills in infants with Prader–Willi syndrome. Pediatrics 139. [DOI] [PubMed] [Google Scholar]
- Thibonnier M, Coles P, Thibonnier A, Shoham M, 2001. The basic and clinical pharmacology of nonpeptide vasopressin receptor antagonists. Annu. Rev. Pharmacol. Toxicol 41, 175–202. [DOI] [PubMed] [Google Scholar]
- Thompson R, Gupta S, Miller K, Mills S, Orr S, 2004. The effects of vasopressin on human facial responses related to social communication. Psychoneuroendocrinology 29, 35–48. [DOI] [PubMed] [Google Scholar]
- Tsai T-F, Jiang Y, Bressler J, Armstrong D, Beaudet AL, 1999. Paternal deletion from snrpn to Ube3a in the mouse causes hypotonia, growth retardation and partial lethality and provides evidence for a gene contributing to prader-willi syndrome. Hum. Mol. Genet 8, 1357–1364. 10.1093/hmg/8.8.1357. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan R, Schaller F, Muscatelli F, Hammock EA, 2020. Colocalization of Oxtr with Prader-Willi Syndrome transcripts in the trigeminal ganglion of neonatal mice. Hum. Mol. Genet [DOI] [PubMed] [Google Scholar]
- Veltman MWM, Thompson RJ, Roberts SE, Thomas NS, Whittington J, Bolton PF, 2004. Prader-Willi syndrome–a study comparing deletion and uniparental disomy cases with reference to autism spectrum disorders. Eur. Child Adolesc. Psychiatry 13, 42–50. 10.1007/s00787-004-0354-6. [DOI] [PubMed] [Google Scholar]
- Veltman MWM, Craig EE, Bolton PF, 2005. Autism spectrum disorders in Prader-Willi and Angelman syndromes: a systematic review. Psychiatr. Genet 15, 243–254. 10.1097/00041444-200512000-00006. [DOI] [PubMed] [Google Scholar]
- Viaux-Savelon S, Rosenblum O, Guedeney A, Diene G, Çabal-Berthoumieu S, Fichaux-Bourin P, Molinas C, Faye S, Valette M, Bascoul C, 2016. Dyssynchrony and perinatal psychopathology impact of child disease on parents-child interactions, the paradigm of Prader Willi syndrom. J. Physiol 110, 427–433. [DOI] [PubMed] [Google Scholar]
- Wang Z, Ferris CF, De Vries GJ, 1994. Role of septal vasopressin innervation in paternal behavior in prairie voles (Microtus ochrogaster). Proc. Natl. Acad. Sci. USA 91, 400–404. 10.1073/pnas.91.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JR, Carter CS, Insel T, 1992. Partner preference development in female prairie voles is facilitated by mating or the central infusion of oxytocin. Ann. N. Y Acad. Sci 652, 487–489. 10.1111/j.1749-6632.1992.tb34393.x. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR, 1993. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature 365, 545–548. 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Yang T, Adamson TE, Resnick JL, Leff S, Wevrick R, Francke U, Jenkins NA, Copeland NG, Brannan CI, 1998. A mouse model for Prader-Willi syndrome imprinting-centre mutations. Nat. Genet 19, 25–31. 10.1038/ng0598-25. [DOI] [PubMed] [Google Scholar]
- Young LJ, Nilsen R, Waymire KG, MacGregor GR, Insel TR, 1999. Increased affiliative response to vasopressin in mice expressing the V1a receptor from a monogamous vole. Nature 400, 766–768. 10.1038/23475. [DOI] [PubMed] [Google Scholar]
- Zhang H-F, Dai Y-C, Wu J, Jia M-X, Zhang J-S, Shou X-J, Han S-P, Zhang R, Han J-S, 2016. Plasma oxytocin and arginine-vasopressin levels in children with autism spectrum disorder in china: associations with symptoms. Neurosci. Bull 32, 423–432. 10.1007/s12264-016-0046-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieba J, Low JK, Purtell L, Qi Y, Campbell L, Herzog H, Karl T, 2015. Behavioural characteristics of the Prader–Willi syndrome related biallelic Snord116 mouse model. Neuropeptides 53, 71–77. [DOI] [PubMed] [Google Scholar]
- Zimmerman EA, Nilaver G, Hou-Yu A, Silverman AJ, 1984. Vasopressinergic and oxytocinergic pathways in the central nervous system. Fed. Proc 43, 91–96. [PubMed] [Google Scholar]
- Zyga O, Dimitropoulos A, 2020. Preliminary characterization of parent-child interaction in preschoolers with prader-willi syndrome: the relationship between engagement and parental stress. Am. J. Intellect. Dev. Disabil 125, 76–84. 10.1352/1944-7558-125.1.76. [DOI] [PubMed] [Google Scholar]
- Zyga O, Russ S, Ievers-Landis CE, Dimitropoulos A, 2015. Assessment of pretend play in Prader–Willi syndrome: a direct comparison to autism spectrum disorder. J. Autism Dev. Disord 45, 975–987. [DOI] [PubMed] [Google Scholar]
Web references
- AnonLevo Therapeutics Announces Top-line Results from Phase 3 CARE-PWS Study of LV-101 (Intranasal Carbetocin) for the Treatment of Prader-Willi Syndrome, 2020. Levo Therapeutics. URL ⟨https://www.levotx.com/news/care-pws_top-line_results/.⟩ (accessed 5.16.22). [Google Scholar]
- AnonLevo Therapeutics, Inc., 2021. Phase 3, Randomized, Double-Blind, Placebo-Controlled, 8-week Clinical Study to Assess the Efficacy, Safety, and Tolerability, of Intranasal Carbetocin (LV-101) in Prader-Willi Syndrome (PWS) With Long Term Follow-Up (CARE-PWS) (⟨https://clinicaltrials.gov/ct2/show/NCT03649477⟩ Clinical trial registration No. NCT03649477). clinicaltrials.gov. (accessed 5.16.22).