Summary
Background
Endovascular therapy (EVT) was demonstrated effective in acute large vessel occlusion (LVO) with large infarction. Revealing subgroups of patients who would or would not benefit from EVT will further inform patient selection for EVT.
Methods
This post-hoc analysis of the ANGEL-ASPECT trial, a randomised controlled trial of 456 adult patients with acute anterior-circulation LVO and large infarction, defined by ASPECTS 3–5 or infarct core volume 70–100 mL, enrolled from 46 centres across China, between October 2, 2020 and May 18, 2022. Patients were randomly assigned (1:1) to receiving EVT and medical management or medical management alone. One patient withdrew consent, 455 patients were included in this post-hoc analysis and categorised into 4 subgroups by lower or higher NIHSS (< or ≥16) and smaller or larger infarct core (< or ≥70 mL). Those with lower NIHSS & smaller core, and higher NIHSS & larger core were considered clinical-radiological matched subgroups; otherwise clinical-radiological mismatched subgroups. Primary outcome was 90-day modified Rankin Scale (mRS). ANGEL-ASPECT is registered with ClinicalTrials.gov, NCT04551664.
Findings
Overall, 139 (30.5%) patients had lower NIHSS & smaller core, 106 (23.3%) higher NIHSS & larger core, 130 (28.6%) higher NIHSS & smaller core, and 80 (17.6%) lower NIHSS & larger core. There was significant ordinal shift in the 90-day mRS toward a better outcome with EVT in clinical-radiological matched subgroups: lower NIHSS & smaller core (generalised OR, 1.76; 95% CI, 1.18–2.62; p = 0.01) and higher NIHSS & larger core (1.64; 1.06–2.54; 0.01); but not in the two clinical-radiological mismatched subgroups.
Interpretation
Our findings suggested that in patients with anterior-circulation LVO and large infarction, EVT was associated with improved 90-day functional outcomes in those with matched clinical and radiological severities, but not in those with mismatched clinical and radiological severities. Simultaneous consideration of stroke severity and infarct core volume may inform patient selection for EVT.
Funding
Unrestricted grants from industry [Covidien Healthcare International Trading (Shanghai), Johnson & Johnson MedTech, Genesis MedTech (Shanghai), and Shanghai HeartCare Medical Technology].
Keywords: Endovascular therapy, Acute ischaemic stroke, Large vessel occlusion, Large infarct core, Post hoc analysis
Research in context.
Evidence before this study
We conducted a literature search of PubMed using the search term “(endovascular therapy or endovascular treatment or thrombectomy) AND (large core or large infarct∗) AND (match or mismatch or clinical-radiological match or clinical-radiological mismatch)” for the period of database inception up to Jan 15, 2024, without any language restrictions. We have also manually searched publications relevant to the six completed randomised controlled trials (RESCUE–Japan LIMIT, SELECT-2, ANGEL-ASPECT, TESLA, TENSION and LASTE) reporting the efficacy of endovascular therapy (EVT) compared to medical management alone in patients with large infarction. We found no study that explored the efficacy of EVT versus medical management alone, stratified by the clinical severity of the stroke and the radiological severity of the infarction, in acute large vessel occlusion patients with large infarction.
Added value of this study
This post-hoc analysis of the ANGEL-ASPECT trial was the first study, to our knowledge, to investigate the efficacy and safety of EVT versus medical management alone in patients with acute large vessel occlusion and a large infarction, stratified by the clinical severity of the stroke and the radiological severity of the infarction. We found that EVT was associated with improved 90-day functional outcomes in clinical-radiological matched subgroups (53.8% of all patients), i.e., lower NIHSS & smaller core and higher NIHSS & larger core, but not in clinical-radiological mismatched subgroups (46.2% of all patients), i.e., higher NIHSS & smaller core or lower NIHSS & larger core. Moreover, there were more deaths within 90 days in those receiving EVT than medical management alone in the subgroup with lower NIHSS & larger core (one of the clinical-radiological mismatched subgroups), although not reaching statistical significance.
Implications of all the available evidence
The findings suggested that simultaneous consideration of stroke severity and infarct volume may facilitate selection of acute large vessel occlusion patients with large infarction, who could truly benefit from EVT. These findings need to be verified in studies with adequate statistical power.
Introduction
Endovascular therapy (EVT) has been recommended by current guidelines as a standard treatment for acute ischaemic stroke (AIS) due to large vessel occlusion (LVO) in the anterior circulation.1 However, patient eligibility has been limited to those with an Alberta Stroke Program Early Computed Tomographic Score (ASPECTS)2 of 6–10 or infarct core volume < 70 mL, i.e., small-to-medium infarct core, based on the eligibility criteria of previous relevant randomised controlled trials (RCTs).3 The reasons why those with a large infarct core had been excluded from previous EVT trials mostly lie in the concerns over a lack of penumbra that may not benefit with EVT over medical management alone, and possibly higher bleeding risks with EVT.4,5
Encouragingly, Five recent RCTs (RESCUE–Japan LIMIT, SELECT-2, ANGEL-ASPECT, TENSION and LASTE) have demonstrated better functional outcomes with EVT than with medical management alone, in patients with anterior-circulation LVO with a large infarction.6, 7, 8, 9, 10 Yet, the radiological eligibility criteria for large infarction and the patient characteristics (e.g., the stroke severity) were different among these trials. Moreover, there have been secondary analyses of these trials indicating specific subgroups of patients who may or may not benefit from EVT than medical management alone.11,12
A mismatch between the clinical severity of ischaemic stroke by NIH Stroke Scale (NIHSS) and the radiological severity in terms of the infarct volume is commonly seen in LVO patients. Previous studies have indicated that a clinical-radiological mismatch was associated with outcomes of LVO patients receiving EVT treatment.13 Yet, it is unknown whether EVT is safe and effective in patients with a large infarction with matched/mismatched clinical and radiological severities. Relevant evidence may inform patient selection for EVT in affected patients. In the current study, we therefore aimed to investigate the efficacy and safety of EVT in patients with anterior-circulation LVO and a large infarction further stratified by the clinical and radiological severities of the stroke, based on post hoc analysis of the Endovascular Therapy in Acute Anterior Circulation Large Vessel Occlusive Patients with a Large Infarct Core (ANGEL-ASPECT) trial.8
Methods
Study design
The ANGEL-ASPECT trial is a RCT evaluating the efficacy and safety of EVT compared to medical management alone in AIS patients with a large infarction due to anterior-circulation LVO, conducted at 46 stroke centres across China.8 This study was approved by the institutional review boards at Beijing Tiantan Hospital and each trial site, which was conducted in accordance with the principles of the Declaration of Helsinki and the International Council for Harmonisation Guidelines for Good Clinical Practice. All patients or their representatives provided written informed consent before enrollment. Detailed study protocol of ANGEL-ASPECT has been reported previously.8,14,15
Participants
In brief, patient inclusion criteria were 18–80 years of age, AIS within 24 h after stroke onset with NIHSS of 6–30, prestroke modified Rankin scale (mRS) of 0–1, LVO in initial segment of middle cerebral artery (MCA) and/or intracranial segment of internal carotid artery (ICA) on compute tomography (CT) or magnetic resonance (MR) angiography. A large infarct core was defined by ASPECTS 3–5 or infarct core volume 70–100 mL, including the following conditions: ASPECTS 3–5 on noncontrast CT within 24 h after stroke onset regardless of the infarct core volume, ASPECTS 0–2 on noncontrast CT within 24 h after stroke onset and an infarct core volume of 70–100 mL, or ASPECTS greater than 5 between 6 and 24 h after stroke onset and an infarct core volume of 70–100 mL.8,14
Randomisation and masking
From October 2, 2020 to May 18, 2022, 456 patients were enrolled in ANGEL-ASPECT and randomly assigned (1:1) to receiving EVT and medical management (EVT arm) or receiving medical management alone (medical-management arm). In this post hoc analysis, patients were stratified by the clinical severity of the stroke and the radiological severity of the infarction. A cut-off of 70 mL of the infarct core volume was used to dichotomise the radiological severity, as infarctions with a volume ≥70 mL are widely considered large lesions.16,17 A cut-off of 16 in NIHSS was used to dichotomise the clinical stroke severity, which was the median NIHSS of patients in ANGEL-ASPECT,8 and a NIHSS ≥ 16 has been used to define a severe stroke in previous studies.18 Therefore, more specifically, patients were categorised into 4 subgroups, based on a combination of NIHSS < or ≥16 (i.e., lower or higher NIHSS) and infarct core volume < or ≥70 mL (i.e., smaller or larger core).16,17 Those with lower NIHSS and smaller core, and higher NIHSS and larger core were considered clinical-radiological matched subgroups; those with higher NIHSS and smaller core, and lower NIHSS and larger core were considered clinical-radiological mismatched subgroups.
Data collection and imaging assessment at baseline
Baseline characteristics were obtained, including age, sex, baseline NIHSS, medical history, blood pressure, laboratory tests results, ASPECTS, hemisphere affected, LVO site, stroke etiology, infarct core volume, critically hypoperfused volume (defined by the injected tracer agent arrival time to maximum of the residue function [Tmax] exceeding 6 s),3 presence of penumbra (defined as critically hypoperfused to infarct core ratio ≥1.8 and penumbra volume ≥15 mL),3 intravenous thrombolysis, wake-up stroke, interval from onset, treatment allocation, and EVT procedural characteristics (including successful reperfusion defined by an extended Thrombolysis in the Cerebral Infarction [eTICI] score of 2b50 or greater19 and rescue therapy).
All imaging data were independently assessed at the imaging core laboratory, blinded to treatment allocation and clinical outcomes. The infarct core volume was evaluated using the RAPID software (iSchemaView), defined as the area with a relative cerebral blood flow of less than 30% of normal tissue in CT perfusion or an apparent diffusion coefficient value of less than 620 × 10−6 mm2 per second in MR imaging (MRI).20 More detailed imaging assessment methods were described previously.8
Outcomes
Consistent with the main report of ANGEL-ASPECT,8 the primary outcome in this study was the 90-day mRS score. Secondary outcomes included 90-day mRS score of 0–2, the change in infarct core volume on CT from baseline to 7 days or discharge (whichever was earlier) or on MRI from baseline to 36 h, and target-artery recanalization on CT or MR angiography at 36 h defined as a modified arterial occlusive lesion grade of 2 or 3.21 Safety outcomes included symptomatic intracranial haemorrhage (sICH) within 48 h after randomization, as defined by the Heidelberg bleeding classification (an increase of ≥4 points in NIHSS or an increase of ≥2 points in an NIHSS subcategory, with any intracranial haemorrhage [ICH] on imaging),22 any ICH within 48 h, death within 90 days, and need for decompressive craniectomy during hospitalization.
Statistical analysis
Continuous variables were presented with medians and interquartile range (IQR) and compared among the four subgroups using Kruskal–Wallis tests. Categorical variables were presented with numbers and percentages and compared among the four subgroups using χ2 or Fisher exact tests. Bonferroni correction was used to compare the baseline characteristics among the four subgroups, and two-tailed p < 0.05/4 = 0.0125 was considered of statistically significant difference.23 Standardised differences were calculated between the EVT and medical-management arms in each subgroup, with an absolute value < 0.35 considered of balance between the two arms.24
For the primary outcome in each of the 4 subgroups, the proportional-odds assumption for the ordinal logistic-regression model was not satisfied; therefore, the Wilcoxon-Mann-Whitney generalised odds ratio (OR) and 95% confidence interval (CI) were calculated in an assumption-free ordinal analysis to detect a shift in the distribution of mRS scores toward a better outcome between the EVT and medical-management arms. For the secondary and safety outcomes, we performed a general linear model to calculate mean differences with 95%CI for the change in infarct core volume, and a Cox proportional-hazards model to estimate the hazard ratio (HR) and 95% CI on death within 90 days between the two trial arms since the proportional hazards assumption was satisfied. Differences in other secondary and safety outcomes between the two arms were assessed using the Cochran–Mantel–Haenszel method, and relative risks (RR) with the 95% CI were reported. Additional analyses were conducted for the outcomes, adjusting for baseline characteristics unbalanced between EVT and medical management arms in each subgroup. Moreover, sensitivity analysis was conducted by categorizing patients into 4 subgroups, based on a combination of NIHSS < or ≥16 and infarct core volume < or ≥50 mL.
The interaction effects on all study outcomes were analysed by a Wilcoxon–Mann–Whitney method, a Cochran–Mantel–Haenszel method, a general linear model, or a Cox proportional-hazards model, which respectively included treatment options (EVT versus medical management alone) and two clinical-radiological matched versus two mismatched subgroups, and treatment options and four subgroups, as the multiplicative interaction term. Statistical analyses were performed with SAS software, version 9.4 (SAS Institute). Two-tailed p < 0.05 was considered significant.8
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Patient characteristics
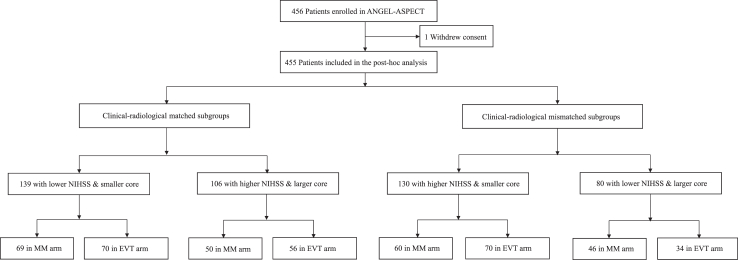
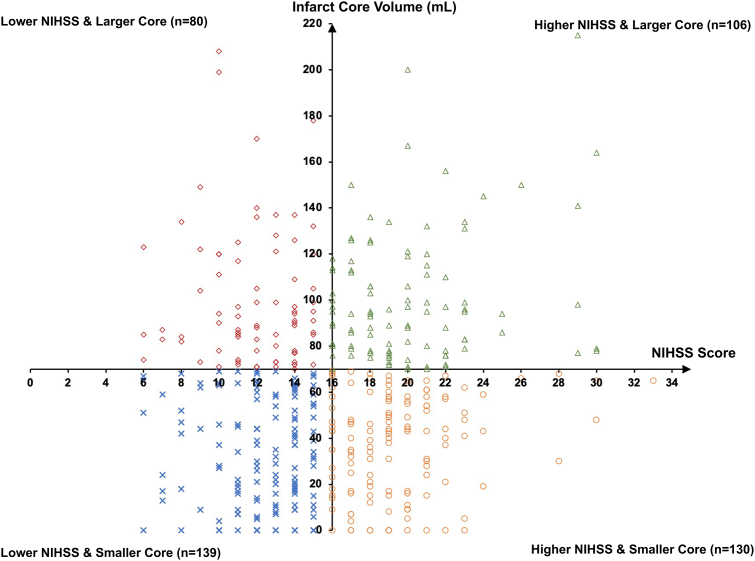
Among the 456 patients enrolled from October 2, 2020, to May 18, 2022, in ANGEL-ASPECT trial, 455 were included in this post hoc analysis, after excluding 1 who withdrew consent (Fig. 1). Of these, 139 (30.5%) had lower NIHSS (median 13, IQR 11–14) & smaller core (median 32 mL, IQR 11–55 mL); 106 (23.3%) had higher NIHSS (median 20, IQR 17–22) & larger core (median 95 mL, IQR 79–118 mL); 130 (28.6%) had higher NIHSS (median 19, IQR 17–21) & smaller core (median 42 mL, IQR 17–56 mL) and 80 (17.6%) had lower NIHSS (median 12, IQR 10–14) & larger core (median 90 mL, IQR 78–120 mL) (Fig. 1 and Table 1). Hence, 245 (53.8%) patients had matched clinical and radiological severities, and 210 (46.2%) had mismatched clinical and radiological severities. Fig. 2 shows distributions of patients by NIHSS and infarct core volume in the four subgroups in a scatter plot.
Fig. 1.
Flowchart of the study. Abbreviations: ANGEL-ASPECTS indicates Endovascular Therapy in Acute Anterior Circulation Large Vessel Occlusive Patients with a Large Infarct Core; NIHSS, National Institutes of Health Stroke Scale; MM, medical management; EVT, endovascular therapy.
Table 1.
Baseline characteristics of patients based on stroke severity and infarct core volume.
| Variablea | Lower NIHSS & smaller core (n = 139) | Higher NIHSS & larger core (n = 106) | Higher NIHSS & smaller core (n = 130) | Lower NIHSS & larger core (n = 80) | p valueb |
|---|---|---|---|---|---|
| Age, median (IQR), yr | 66 (59–71) | 69 (61–74) | 70 (63–75) | 67 (58–72) | 0.01 |
| Sex | 0.01 | ||||
| Male | 89 (64.0) | 53 (50.0) | 78 (60.0) | 59 (73.8) | |
| Female | 50 (36.0) | 53 (50.0) | 52 (40.0) | 21 (26.2) | |
| Baseline NIHSS, median (IQR) | 13 (11–14) | 20 (17–22) | 19 (17–21) | 12 (10–14) | <0.001 |
| Medical history | |||||
| Hypertension | 75 (54.0) | 67 (63.2) | 85 (65.4) | 45 (56.3) | 0.20 |
| Diabetes | 19 (13.7) | 27 (25.5) | 24 (18.5) | 13 (16.3) | 0.12 |
| Hyperlipidemia | 7 (5.0) | 6 (5.7) | 8 (6.2) | 5 (6.3) | 0.98 |
| Atrial fibrillation | 25 (18.0) | 28 (26.4) | 36 (27.7) | 15 (18.8) | 0.16 |
| Stroke | 23 (16.6) | 13 (12.3) | 24 (18.5) | 13 (16.3) | 0.63 |
| Current smoking | 43 (30.9) | 32 (30.2) | 34 (26.2) | 35 (43.8) | 0.06 |
| Systolic blood pressure, median (IQR), mmHg | 147 (133–163) | 154 (132–170) | 151 (131–168) | 148 (130–164) | 0.55 |
| Diastolic blood pressure, median (IQR), mmHg | 85 (76–97) | 87 (76–97) | 85 (76–98) | 83 (75–89) | 0.26 |
| Laboratory tests | |||||
| Blood glucose, median (IQR), mmol/L | 6.9 (5.8–8.3) | 7.8 (6.4–9.7) | 7.0 (6.2–9.1) | 7.0 (5.9–8.3) | 0.05 |
| Creatinine, median (IQR), umol/L | 65.0 (53.0–83.4) | 69.0 (55.0–79.7) | 70.2 (59.1–85.0) | 67.0 (57.8–84.1) | 0.44 |
| ASPECTS based on CT, median (IQR) | 4 (3–5) | 3 (1–4) | 3 (3–4) | 3 (2–4) | <0.001 |
| 0 | 0 (0.0) | 6 (5.7) | 0 (0.0) | 2 (2.5) | <0.001 |
| 1 | 1 (0.7) | 21 (19.8) | 1 (0.8) | 10 (12.5) | |
| 2 | 0 (0.0) | 10 (9.4) | 0 (0.0) | 11 (13.8) | |
| 3 | 63 (45.3) | 41 (38.7) | 68 (52.3) | 26 (32.5) | |
| 4 | 37 (26.6) | 21 (19.8) | 31 (23.9) | 22 (27.5) | |
| 5 | 38 (27.3) | 7 (6.6) | 30 (23.1) | 9 (11.3) | |
| Hemisphere affected | <0.001 | ||||
| Left | 35 (25.2) | 71 (67.0) | 88 (67.7) | 16 (20.0) | |
| Right | 104 (74.8) | 35 (33.0) | 42 (32.3) | 64 (80.0) | |
| Occlusion site | 0.02 | ||||
| ICA | 34 (24.5) | 45 (42.5) | 50 (38.5) | 35 (43.8) | |
| MCA-M1 | 102 (73.4) | 61 (57.6) | 79 (60.8) | 45 (56.3) | |
| MCA-M2 | 3 (2.2) | 0 (0.0) | 1 (0.8) | 0 (0.0) | |
| Stroke etiology | 0.04 | ||||
| Atherosclerosis | 37 (26.6) | 20 (18.9) | 34 (26.2) | 22 (27.5) | |
| Cardioembolic | 55 (39.6) | 56 (52.8) | 69 (53.1) | 29 (36.3) | |
| Others | 47 (33.8) | 30 (28.3) | 27 (20.8) | 29 (36.3) | |
| Infarct core volume, median (IQR), mL | 32 (11–55) | 95 (79–118) | 42 (17–56) | 90 (78–120) | <0.001 |
| Critically hypoperfused (Tmax > 6s) volume, median (IQR), mL | 162 (108–193) | 199 (165–257) | 158 (121–216) | 208 (166–247) | <0.001 |
| Critically hypoperfused to infarct core ratio ≥1.8 and penumbra volume ≥15 mL | 122 (91.7) | 60 (61.9) | 115 (91.3) | 49 (70.0) | <0.001 |
| Intravenous thrombolysis | 39 (28.1) | 28 (26.4) | 38 (29.2) | 24 (30.0) | 0.95 |
| Wake-up stroke | 47 (33.8) | 31 (29.3) | 48 (36.9) | 21 (26.3) | 0.36 |
| Onset-to-door time, median (IQR), min | 399 (209–707) | 325 (199–630) | 350 (190–559) | 311 (163–575) | 0.31 |
| Onset-to-imaging time, median (IQR), min | 466 (288–769) | 373 (236–720) | 410 (233–659) | 366 (218–655) | 0.17 |
| Onset-to-randomization time, median (IQR), min | 507 (337–803) | 427 (283–760) | 459 (293–708) | 413 (259–698) | 0.18 |
| Onset-to-puncture time, median (IQR), min | 616 (372–836) | 454 (325–735) | 428 (314–660) | 464 (297–699) | 0.13 |
| Onset-to-reperfusion time, median (IQR), min | 729 (431–956) | 531 (392–797) | 511 (409–704) | 572 (408–852) | 0.09 |
| Treatment | 0.41 | ||||
| MM | 69 (49.6) | 50 (47.2) | 60 (46.2) | 46 (57.5) | |
| EVT | 70 (50.4) | 56 (52.8) | 70 (53.9) | 34 (42.5) | |
| Successful reperfusion | 25 (35.7) | 18 (32.1) | 30 (45.5) | 12 (37.5) | 0.47 |
| Rescue therapy | 16 (22.9) | 6 (10.7) | 8 (11.9) | 4 (12.5) | 0.19 |
NIHSS indicates National Institutes of Health Stroke Scale; IQR, interquartile range; ASPECTS, Alberta Stroke Program Early Compute Tomography Score; CT, Compute Tomography; ICA, internal carotid artery; MCA-M1, middle cerebral artery M1 segment; MCA-M2, middle cerebral artery M2 segment; MM, medical management; EVT, endovascular therapy.
Values are medians (interquartile range) or numbers (%).
p value < 0.0125 was considered of statistical significance.
Fig. 2.
Scatter plots showing relatively even distributions of patients in the four subgroups by the NIHSS score and infarct core volume. Abbreviations: NIHSS indicates National Institutes of Health Stroke Scale.
Among the four subgroups, there were significant differences in age, sex, NIHSS, ASPECTS, hemisphere affected, critically hypoperfused volume and proportion of patients with critically hypoperfused to infarct core ratio ≥ 1.8 and penumbra volume ≥ 15 mL (Table 1). The 2 subgroups with higher NIHSS (with larger or smaller core) had significantly higher proportions of left-hemisphere infarctions than the 2 subgroups with lower NIHSS (Table 1). Regarding the specific ASPECTS regions, patients with higher NIHSS & smaller core had higher proportions of left internal capsule, caudate nucleus and lenticular nucleus infarctions, while patients with lower NIHSS & larger core had higher proportions of right insular ribbon and M1–M6 regions infarctions (Supplemental Table S1). No significant difference was noted with other baseline variables among the four subgroups, including the time from onset to door/imaging/randomization/puncture/reperfusion, the proportions of patients randomised to EVT or medical-management arms and the procedural characteristics. Most baseline characteristics were balanced between the EVT and medical-management arms in each of these four subgroups, except for diabetes, atrial fibrillation, ASPECTS, infarct core volume, critically hypoperfused to infarct core ratio ≥ 1.8 and penumbra volume ≥ 15 mL, and wake-up stroke in some subgroups (with an absolute value of standardised difference ≥0.35) (Table 2).
Table 2.
Baseline characteristics of patients between the medical-management and endovascular therapy arms in each of the four subgroups stratified by NIHSS and infarct-core volume.
| Variablea | Clinical-radiological matched subgroups |
Clinical-radiological mismatched subgroups |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower NIHSS & smaller core (n = 139) |
Higher NIHSS & larger core (n = 106) |
Higher NIHSS & smaller core (n = 130) |
Lower NIHSS & larger core (n = 80) |
|||||||||
| MM arm (n = 69) | EVT arm (n-70) | SDb | MM arm (n = 50) | EVT arm (n = 56) | SDb | MM arm (n = 60) | EVT arm (n = 70) | SDb | MM arm (n = 46) | EVT arm (n = 34) | SDb | |
| Age, median (IQR), yr | 65 (59–71) | 67 (59–71) | 0.11 | 69 (59–74) | 70 (63–75) | 0.12 | 68 (59–75) | 70 (64–75) | 0.18 | 68 (57–73) | 64 (59–72) | −0.02 |
| Sex | −0.10 | −0.23 | −0.19 | −0.12 | ||||||||
| Male | 42 (60.9) | 47 (67.1) | 28 (56.0) | 25 (44.6) | 39 (65.0) | 39 (55.7) | 35 (76.1) | 24 (70.6) | ||||
| Female | 27 (39.1) | 23 (32.9) | 22 (44.0) | 31 (55.4) | 21 (35.0) | 31 (44.3) | 11 (23.9) | 10 (29.4) | ||||
| Baseline NIHSS, median (IQR) | 12 (11–14) | 13 (11–14) | 0.05 | 19 (17–21) | 20 (18–23) | 0.29 | 19 (17–21) | 19 (17–21) | 0.07 | 12 (10–14) | 13 (10–14) | 0.03 |
| Medical history | ||||||||||||
| Hypertension | 39 (56.5) | 36 (51.4) | −0.10 | 35 (70.0) | 32 (57.1) | −0.27 | 37 (61.7) | 48 (68.6) | 0.15 | 25 (54.4) | 20 (58.8) | 0.09 |
| Diabetes | 12 (17.4) | 7 (10.0) | −0.22 | 15 (30.0) | 12 (21.4) | −0.20 | 6 (10.0) | 18 (25.7) | 0.42 | 7 (15.2) | 6 (17.7) | 0.07 |
| Hyperlipidemia | 3 (4.4) | 4 (5.7) | 0.06 | 3 (6.0) | 3 (5.4) | −0.03 | 5 (8.3) | 3 (4.3) | −0.17 | 2 (4.4) | 3 (8.8) | 0.18 |
| Atrial fibrillation | 8 (11.6) | 17 (24.3) | 0.36 | 15 (30.0) | 13 (23.2) | −0.15 | 14 (23.3) | 22 (31.4) | 0.18 | 10 (21.7) | 5 (14.7) | −0.18 |
| Stroke | 11 (15.9) | 12 (17.1) | 0.03 | 6 (12.0) | 7 (12.5) | 0.02 | 13 (21.7) | 11 (15.7) | −0.15 | 6 (13.0) | 7 (20.6) | 0.20 |
| Current smoking | 20 (29.0) | 23 (32.9) | 0.08 | 18 (36.0) | 14 (25.0) | −0.24 | 20 (33.3) | 14 (20.0) | −0.31 | 19 (41.3) | 16 (47.1) | 0.12 |
| Systolic blood pressure, median (IQR), mmHg | 151 (141–166) | 144 (130–162) | −0.29 | 156 (131–170) | 145 (134–170) | −0.02 | 153 (134–169) | 150 (131–168) | −0.04 | 149 (134–164) | 144 (123–163) | 0.10 |
| Diastolic blood pressure, median (IQR), mmHg | 90 (80–97) | 84 (73–95) | −0.25 | 87 (76–97) | 88 (76–97) | 0.12 | 87 (80–99) | 84 (70–96) | −0.31 | 83 (75–89) | 83 (75–89) | 0.01 |
| Laboratory tests | ||||||||||||
| Blood glucose, median (IQR), mmol/L | 6.9 (5.7–8.5) | 7.0 (5.9–8.2) | −0.10 | 7.9 (6.6–11.2) | 7.7 (6.1–9.2) | −0.31 | 6.7 (5.9–8.9) | 7.1 (6.3–9.1) | 0.14 | 6.7 (5.7–7.9) | 7.2 (6.0–8.6) | 0.05 |
| Creatinine, median (IQR), umol/L | 63.0 (48.5–79.4) | 69.4 (58.6–88.5) | 0.26 | 65.5 (54.5–82.0) | 69.9 (55.0–78.0) | −0.09 | 69.4 (58.0–81.7) | 72.0 (59.2–90.0) | 0.23 | 67.0 (60.0–80.5) | 67.6 (54.1–86.9) | 0.02 |
| ASPECTS based on CT, median (IQR) | 4 (3–5) | 4 (3–4) | −0.13 | 3 (2–4) | 3 (1–3) | −0.27 | 3 (3–4) | 4 (3–4) | 0.08 | 3 (2–4) | 3 (2–4) | 0.15 |
| ASPECTS | 0.28 | 0.57 | 0.31 | 0.65 | ||||||||
| 0 | 0 (0.0) | 0 (0.0) | 1 (2.0) | 5 (8.9) | 0 (0.0) | 0 (0.0) | 1 (2.2) | 1 (2.9) | ||||
| 1 | 0 (0.0) | 1 (1.4) | 11 (22.0) | 10 (17.9) | 0 (0.0) | 1 (1.4) | 9 (19.6) | 1 (2.9) | ||||
| 2 | 0 (0.0) | 0 (0.0) | 4 (8.0) | 6 (10.7) | 0 (0.0) | 0 (0.0) | 4 (8.7) | 7 (20.6) | ||||
| 3 | 32 (46.4) | 31 (44.3) | 18 (36.0) | 23 (41.1) | 35 (58.3) | 33 (47.1) | 15 (32.6) | 11 (32.4) | ||||
| 4 | 15 (21.7) | 22 (31.4) | 10 (20.0) | 11 (19.6) | 11 (18.3) | 20 (28.6) | 11 (23.9) | 11 (32.4) | ||||
| 5 | 22 (31.9) | 16 (22.9) | 6 (12.0) | 1 (1.8) | 14 (23.3) | 16 (22.9) | 6 (13.0) | 3 (8.8) | ||||
| Hemisphere affected | −0.02 | −0.04 | 0.03 | 0.10 | ||||||||
| Right | 52 (75.4) | 52 (74.3) | 17 (34.0) | 18 (32.1) | 19 (31.7) | 23 (32.9) | 36 (78.3) | 28 (82.4) | ||||
| Left | 17 (24.6) | 18 (25.7) | 33 (66.0) | 38 (67.9) | 41 (68.3) | 47 (67.1) | 10 (21.7) | 6 (17.7) | ||||
| Occlusion site | 0.15 | 0.09 | 0.16 | −0.30 | ||||||||
| ICA | 16 (23.2) | 18 (25.7) | 20 (40.0) | 25 (44.6) | 22 (36.7) | 28 (40.0) | 23 (50.0) | 12 (35.3) | ||||
| MCA-M1 | 51 (73.9) | 51 (72.9) | 30 (60.0) | 31 (55.4) | 38 (63.3) | 41 (58.6) | 23 (50.0) | 22 (64.7) | ||||
| MCA-M2 | 2 (2.9) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.4) | 0 (0.0) | 0 (0.0) | ||||
| Stroke etiology | 0.24 | 0.19 | 0.08 | 0.29 | ||||||||
| Atherosclerosis | 17 (24.6) | 20 (28.6) | 10 (20.0) | 10 (17.9) | 15 (25.0) | 19 (27.1) | 10 (21.7) | 12 (35.3) | ||||
| Cardioembolic | 25 (36.2) | 30 (42.9) | 24 (48.0) | 32 (57.1) | 33 (55.0) | 36 (51.4) | 18 (39.1) | 11 (32.4) | ||||
| Others | 27 (39.1) | 20 (28.6) | 16 (32.0) | 14 (25.0) | 12 (20.0) | 15 (21.4) | 18 (39.1) | 11 (32.4) | ||||
| Infarct core volume, median (IQR), mL | 32 (10–57) | 31 (13–54) | −0.02 | 97 (77–125) | 91 (80–111) | −0.38 | 40 (15–55) | 43 (19–56) | 0.14 | 88 (77–105) | 97 (78–121) | −0.05 |
| Critically hypoperfused (Tmax > 6s) volume, median (IQR), mL | 170 (108–209) | 156 (107–188) | 0.05 | 195 (143–248) | 210 (179–267) | 0.22 | 162 (121–220) | 155 (122–216) | 0.07 | 204 (152–237) | 216 (166–270) | 0.10 |
| Critically hypoperfused to infarct core ratio ≥1.8 and penumbra volume ≥15 mL | 58 (87.9) | 64 (95.5) | 0.28 | 21 (51.2) | 39 (69.6) | 0.38 | 54 (93.1) | 61 (89.7) | −0.12 | 29 (76.3) | 20 (62.5) | −0.30 |
| Intravenous thrombolysis | 22 (31.9) | 17 (24.3) | −0.17 | 10 (20.0) | 18 (32.1) | 0.28 | 17 (28.3) | 21 (30.0) | 0.04 | 14 (30.4) | 10 (29.4) | 0.02 |
| Wake-up stroke | 20 (29.0) | 27 (38.6) | 0.20 | 17 (34.0) | 14 (25.0) | −0.20 | 26 (43.3) | 22 (31.4) | −0.25 | 15 (32.6) | 6 (17.7) | −0.35 |
| Onset-to-door time, median (IQR), min | 344 (198–662) | 444 (225–720) | 0.26 | 315 (164–633) | 325 (211–518) | −0.03 | 419 (201–682) | 313 (177–492) | −0.25 | 301 (181–608) | 325 (119–562) | 0.05 |
| Onset-to-imaging time, median (IQR), min | 412 (291–766) | 514 (283–769) | 0.19 | 387 (231–742) | 367 (241–570) | −0.11 | 453 (241–750) | 355 (233–648) | −0.23 | 357 (214–679) | 374 (239–592) | 0.01 |
| Onset-to-randomization time, median (IQR), min | 463 (335–818) | 562 (346–791) | 0.17 | 435 (261–777) | 401 (285–644) | −0.09 | 540 (303–781) | 410 (289–681) | −0.22 | 395 (253–745) | 419 (264–607) | −0.03 |
NIHSS indicates National Institutes of Health Stroke Scale; MM, medical management; EVT, endovascular therapy; SD, standardised differences; IQR, interquartile range; ASPECTS, Alberta Stroke Program Early Compute Tomography Score; CT, Compute Tomography; ICA, internal carotid artery; MCA-M1, middle cerebral artery M1 segment; MCA-M2, middle cerebral artery M2 segment; Tmax, time to maximum of the residue function.
Values are medians (interquartile range) or numbers (%).
SD < 0.35 was considered of balance between the two arms.
Outcomes
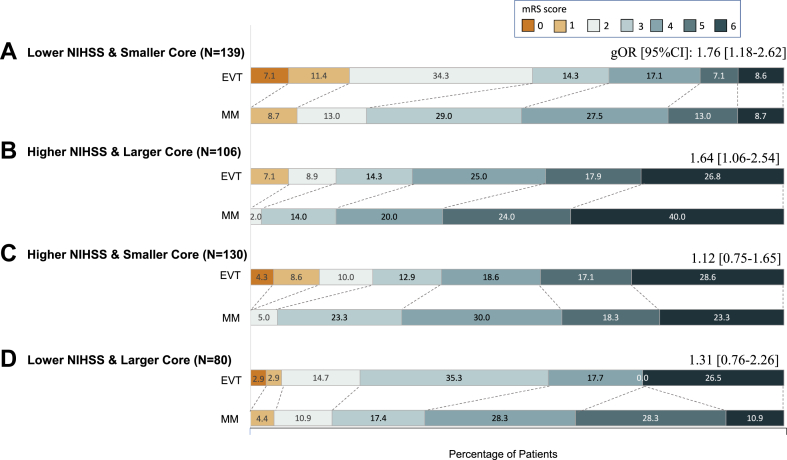
Outcome data are presented in Table 3, Fig. 3 and Supplemental Fig. S1. There was significant ordinal shift in the 90-day mRS distribution toward a better outcome in EVT versus medical management in the clinical-radiological matched subgroups: lower NIHSS & smaller core (generalised OR, 1.76; 95% CI, 1.18–2.62; p = 0.01) and higher NIHSS & larger core (generalised OR, 1.64; 95% CI, 1.06–2.54; p = 0.01); but not in the clinical-radiological mismatched subgroups: higher NIHSS & smaller core and lower NIHSS & larger core. Yet, there was no statistically significant interaction of the two clinical-radiological matched and the two mismatched subgroups, nor across the four subgroups, over the treatment effects of EVT versus medical management on the primary outcome (p = 0.13 and 0.38, respectively; Table 3 and Supplemental Fig. S1).
Table 3.
Efficacy and safety outcomes in the 4 subgroups classified by NIHSS and infarct core volume.
| Clinical-radiological matched subgroups |
Clinical-radiological mismatched subgroups |
p value for interaction |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower NIHSS & smaller core (n = 139) |
Higher NIHSS & larger core (n = 106) |
Higher NIHSS & smaller core (n = 130) |
Lower NIHSS & larger core (n = 80) |
Matched versus Mismatched subgroups | 4 subgroups | |||||||||||||
| MM (n = 69)a | EVT (n = 70)a | Treatment effect (95% CI)b | p value | MM (n = 50)a | EVT (n = 56)a | Treatment effect (95% CI)b | p value | MM (n = 60)a | EVT (n = 70)a | Treatment effect (95% CI)b | p value | MM (n = 46)a | EVT (n = 34)a | Treatment Effect (95% CI)b | p value | |||
| Primary outcome | ||||||||||||||||||
| 90-day mRSc | 3 (3–4) | 2 (2–4) | 1.76 (1.18–2.62) | 0.01 | 5 (4–6) | 4 (3–6) | 1.64 (1.06–2.54) | 0.01 | 4 (3–5) | 4 (3–6) | 1.12 (0.75–1.65) | 0.24 | 4 (3–5) | 3 (3–6) | 1.31 (0.76–2.26) | 0.37 | 0.13 | 0.38 |
| Secondary outcomes | ||||||||||||||||||
| 90-day mRS of 0–2c | 15 (21.7) | 37 (52.9) | 2.43 (1.48–4.01) | <0.001 | 1 (2.0) | 9 (16.1) | 8.04 (1.05–61.21) | 0.01 | 3 (5.0) | 16 (22.9) | 4.57 (1.40–14.94) | 0.01 | 7 (15.2) | 7 (20.6) | 1.35 (0.52–3.50) | 0.54 | 0.56 | 0.29 |
| Change in infarct core volume, mLd | 56.7 (26.6–115.6) | 53.9 (24.6–111.4) | 12.68 (−13.89 to 39.26) | 0.35 | 120.1 (71.9–170.7) | 72.6 (27.9–151.1) | −25.44 (−60.35 to 9.47) | 0.16 | 98.3 (29.6–165.7) | 56.8 (32.0–150.6) | −4.54 (−39.78 to 30.70) | 0.80 | 100.4 (61.7–176.9) | 77.2 (30.2–144.5) | −18.16 (−59.42 to 23.11) | 0.38 | 0.85 | 0.74 |
| Target-artery recanalization at 36 he | 26 (42.6) | 53 (84.1) | 1.97 (1.44–2.69) | <0.001 | 9 (23.1) | 40 (90.9) | 3.94 (2.20–7.04) | <0.001 | 23 (47.9) | 55 (87.3) | 1.82 (1.34–2.48) | <0.001 | 9 (25.0) | 21 (77.8) | 3.11 (1.71–5.67) | <0.001 | 0.51 | 0.22 |
| Safety outcomes | ||||||||||||||||||
| sICH within 48 hf | 2 (2.9) | 6 (8.6) | 2.96 (0.62–14.15) | 0.15 | 2 (4.0) | 3 (5.4) | 1.34 (0.23–7.69) | 0.74 | 1 (1.7) | 3 (4.3) | 2.57 (0.27–24.08) | 0.39 | 1 (2.2) | 2 (5.9) | 2.71 (0.26–28.63) | 0.39 | 0.87 | 0.92 |
| Any ICH within 48 h | 14 (20.3) | 36 (51.4) | 2.53 (1.51–4.26) | <0.001 | 6 (12.0) | 23 (41.1) | 3.42 (1.51–7.72) | <0.001 | 12 (20.0) | 39 (55.7) | 2.79 (1.61–4.82) | <0.001 | 7 (15.2) | 15 (44.1) | 2.90 (1.33–6.32) | 0.01 | 0.78 | 0.98 |
| Death within 90 d | 6 (8.7) | 6 (8.6) | 0.98 (0.32–3.03) | 0.97 | 20 (40.0) | 15 (26.8) | 0.62 (0.32–1.21) | 0.16 | 14 (23.3) | 20 (28.6) | 1.23 (0.62–2.44) | 0.55 | 5 (10.9) | 9 (26.5) | 2.69 (0.90–8.02) | 0.08 | 0.06 | 0.13 |
| Decompressive hemicraniectomy during hospitalization | 1 (1.5) | 2 (2.9) | 1.97 (0.18–21.24) | 0.57 | 3 (6.0) | 5 (8.9) | 1.49 (0.37–5.91) | 0.57 | 1 (1.7) | 4 (5.7) | 3.43 (0.39–29.85) | 0.23 | 3 (6.5) | 6 (17.7) | 2.71 (0.73–10.06) | 0.12 | 0.59 | 0.90 |
NIHSS indicates National Institutes of Health Stroke Scale; MM, medical management; EVT, endovascular therapy; CI, confidence interval; mRS, modified Rankin Scale; sICH, symptomatic intracranial haemorrhage; ICH, intracranial haemorrhage.
Data are presented as number (percentage) of patients for categorical values and median (IQR) for continuous or ordinal variables.
The treatment effect is reported for the primary outcome as a generalised odds ratio with the 95% confidence interval for the ordinal shift in the distribution of scores on the modified Rankin scale toward a better outcome; for change in the infarct core volume, as the mean difference with the 95% confidence interval; for death, as a hazard ratio with the 95% confidence interval; and for other outcomes, as the relative risk with the 95% confidence intervals. The widths of the confidence intervals for the secondary outcomes were not adjusted for multiple comparisons and may not be used for hypothesis testing.
Scores on the modified Rankin scale range from 0 to 6, with higher scores indicating greater disability.
Change in infarct core volume was measured from baseline imaging (CT perfusion or diffusion-weighted imaging) to noncontrast CT at 7 days or at discharge (whichever is earlier) or to magnetic resonance imaging (MRI) at 36 h. Six patients (three in each trial group) could not be assessed because of poor follow-up image quality, serious illness, or death.
Target-artery recanalization was defined as a modified arterial occlusive lesion grade of 2 or 3, as assessed on CT angiography (CTA) or magnetic resonance angiography (MRA) at 36 h (with a window of ±12 h). Data on the follow-up CTA or MRA were not available for 74 patients (33 in the endovascular therapy arm and 41 in the medical-management arm) because of serious illness or death.
Symptomatic intracranial haemorrhage was defined according to the Heidelberg bleeding classification (an increase in the NIHSS score of ≥4 points or an increase in the score for an NIHSS subcategory of ≥2 points with any intracranial haemorrhage on imaging).
Fig. 3.
Distributions of modified Rankin Scale at 90 days in the four subgroups stratified by NIHSS and infarct core volume. A generalised odds ratio (gOR) and 95% confidence interval (95%CI) in each subgroup is provided in the figure to present a shift in the distribution of mRS scores toward a better outcome between the endovascular therapy and medical-management arms. A gOR >1 favored the endovascular therapy arm over the medical-management arm. Abbreviations: mRS indicates modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; EVT, endovascular therapy; MM, medical management; gOR, generalised odds ratio; CI, confidence interval.
For the secondary outcomes, patients receiving EVT was more likely to have a 90-day mRS of 0–2 than those receiving medical management alone, in the two clinical-radiological matched subgroups (52.9% versus 21.7%; RR, 2.43; 95% CI, 1.48–4.01; p < 0.001; and 16.1% versus 2.0%; RR, 8.04; 95% CI, 1.05–61.21; p = 0.01, respectively) and in the subgroup with higher NIHSS & smaller core (22.9% versus 5.0%; RR, 4.57; 95% CI, 1.40–14.94; p = 0.01). Change in infarct core volume from baseline to early follow-up (36 h, 7 days or discharge) were similar between those receiving EVT and medical management alone in each of the four subgroups. The target-artery recanalization rate at 36 h was significantly higher in EVT patients than those receiving medical management alone in each of the four subgroups (all RR > 1, p < 0.05). There was no statistically significant interaction of the two clinical-radiological matched and the two mismatched subgroups, nor across the four subgroups, over the treatment effects of EVT versus medical management on any of the secondary outcomes (Table 3).
For the safety outcomes, any ICH was more frequently seen in the EVT arm than the medical-management arm in each of the four subgroups (all RR > 1, p < 0.05). There were more sICH and decompressive hemicraniectomy during hospitalization, in the EVT arm than the medical-management arm in each of the four subgroups, but none of the differences was statistically significant (all p > 0.05). There were more deaths within 90 days in the EVT arm than the medical-management arm in the subgroup of lower NIHSS and larger core (not reaching statistical significance; p = 0.08); death within 90 days was not significantly different between the two arms in other subgroups (p > 0.05). For the interaction analyses, there was no statistically significant interaction of the two clinical-radiological matched versus two mismatched subgroups over the treatment effects of EVT versus medical management on death (p = 0.06), or for other safety outcomes (Table 3).
After adjusting for baseline characteristics unbalanced between EVT and medical management arms in each subgroup, the results for the outcomes (Supplemental Table S2) were similar with the findings above.
Sensitivity analyses
The sensitivity analysis, by categorizing patients into 4 subgroups by NIHSS < or ≥16 and infarct core volume < or ≥50 mL, showed similar results (Supplemental Table S3) with the analyses above using a cut-off of 70 mL to dichotomise the infarct core volume.
Discussion
The ANGEL-ASPECT trial demonstrated better functional outcomes with EVT than medical management alone in anterior-circulation LVO patients with a large infarction defined by ASPECTS 3–5 or infarct core volume 70–100 mL. In this post hoc analysis, we further stratified the patients into 4 subgroups by the “consistency” of the clinical and radiological severities, respectively scaled by NIHSS (< or ≥16) and infarct core volume (< or ≥70 mL). We found that patients with lower NIHSS & smaller core or higher NIHSS & larger core, i.e., the clinical-radiological matched subgroups, had improved functional outcomes with EVT compared with medical management alone. However, this study did not provide evidence of the effectiveness of EVT over medical management alone in subgroups with mismatched clinical and radiological severities (higher NIHSS & smaller core or lower NIHSS & larger core). Moreover, there were more deaths within 90 days in those receiving EVT than medical management alone in the subgroup with lower NIHSS & larger core.
Many multicentre trials investigating EVT in LVO patients used ASPECTS in the imaging eligibility criteria to assess the infarct size, as it is easy to conduct.6, 7, 8 However, ASPECTS, either read manually, or automatically with advances in imaging assessment techniques in recent years, could be prone to errors or discrepancies due to different display settings and other factors, particularly when assessed in noncontrast CT.2 In addition, ASPECTS may not accurately reflect the infarct volume, with regions of different sizes or different functional significance weighted the same in the scale, e.g., 1 point for each of the subcortical regions (caudate, internal capsule, lentiform and insular ribbon) and cortical regions in MCA territory.17,25 Therefore, in this post hoc analysis, we used the quantitatively measured infarct core volume rather than ASPECTS for the infarct size, to more precisely classify the clinical-radiological matched and mismatched subgroups.
This study supported the benefit of EVT over medical management alone in anterior-circulation LVO patients with a large infarction with matched clinical-radiological severities. It is intuitive that the subgroup of patients with lower NIHSS (median 13) and a smaller infarct core (median 32 mL) would benefit from EVT than medical management alone, who resembled patients in previous trials with a small-to-medium sized infarction.26,27 These patients were probably recruited to ANGEL-ASPECT based solely on ASPECTS (meeting one of the imaging eligibility criteria), which again reflected possible differences in ASPECTS and the infarct volume as discussed above. The other clinical-radiological matched subgroup with higher NIHSS (median 20) and larger infarct core (median 95 mL) also had improved functional outcomes with EVT over medical management alone. These are the most “severe” patients, as shown by the higher 90-day median mRS and very low rate of functional independence in both the EVT and medical management groups (16.1% versus 2.0%). Even so, the benefit of EVT in improving the functional outcomes remained, largely driven by the in general very poor functional outcome (median mRS 5) and the minimal chance of achieving mRS 0–2 at 90 days (2.0%) if treated with medical management alone. On the other hand, around 62% of patients with higher NIHSS & larger core had a presence of penumbra (defined by critically hypoperfused to infarct core ratio ≥1.8 and penumbra volume ≥15 mL), which might be another reason why these “most severe” patients could benefit from EVT.
However, this study did not provide evidence on the effectiveness of EVT over medical management alone in subgroups of patients with mismatched clinical and radiological severities (higher NIHSS & smaller core, or lower NIHSS & larger core). A considerable proportion of patients with higher NIHSS (median 19) & smaller core (median 42 mL) had ischaemic lesions in the left subcortical nucleus regions with important neurological functions, hence the severe neurological deficits despite a relatively small infarct core.28,29 As these regions are often bereft of collateral supply, there may not be much room for reperfusion therapy to salvage brain tissue in these regions, which therefore may not improve the functional outcomes.30 Considering the higher chance of achieving mRS 0–2 (22.9% versus 5.0%) with EVT but meanwhile a higher risk of any ICH (55.7% versus 20.0%) with EVT over medical management alone, further studies are needed to verify the safety and efficacy of EVT in this subgroup. For patients with lower NIHSS & larger core, a considerable proportion had the infarctions in the less-functional cortical regions in the right hemisphere. Therefore, restoring blood flow in these regions might have little effect on neurological function recovery, which, however, could increase the risk of any ICH (44.1% versus 15.2%) and death (26.5% versus 10.9%).
Our study had several limitations. First, although ANGEL-ASPECT aimed to test EVT in patients with a “large infarction”, a considerable proportion of patients enrolled had an infarct core <70 mL, as ASPECTS 3–5 alone was used as the imaging inclusion criterion for some patients. A similar issue existed in other trials testing EVT in those with a “large infarction”.6,7 However, this was inevitable when these trials aimed to include the maximum number of patients with a large core for whom EVT is not recommended by current guidelines with Level 1 evidence.14 Second, there was no established thresholds to define match or mismatch between clinical and radiological severities with NIHSS and infarct core volume. It was defined differently in previous studies with different patient populations, e.g., NIHSS ≥10 & infarct core volume <20 mL in an intravenous thrombolysis study31; NIHSS ≥10 & infarct core volume <21 mL in patients older than 80 years, or NIHSS ≥10 & infarct core volume <31 mL in patients younger than 80 years, or NIHSS ≥20 & infarct core volume 31–51 mL in patients younger than 80 years in an EVT trial.32 Further studies are needed to establish such thresholds or criteria, to more accurately identify patients who may or may not benefit from EVT in future trials. Third, the sample sizes of the subgroups were relatively small. Therefore, the analyses were underpowered (82%, 61%, 8% and 18% power for the primary outcome, respectively in the 4 subgroups), and we were unable to estimate a treatment effect with enough precision to make definitive conclusions, particularly in the clinical-radiological mismatched subgroups. Further, despite the different findings in these subgroups, there was no statistically significant interaction of NIHSS and infarct core volume with the treatment effects of EVT versus medical management on the outcomes, either between the clinical-radiological match and mismatch groups, or across the four subgroups. Hence, our findings need to be verified in studies with adequate statistical power, for instance, by patient-level meta-analysis with recent RCTs6, 7, 8, 9, 10,33 on EVT in LVO with a large infarction. Such subsequent analyses could also validate the current findings (from Chinese patients) in other populations.
In patients with anterior-circulation large vessel occlusion and large infarction defined by ASPECTS 0–5 and/or infarct core volume 70–100 mL in the ANGEL-ASPECT trial, we found EVT associated with improved 90-day functional outcomes in those with matched clinical and radiological severities, i.e., NIHSS < 16 & infarct core <70 mL or NIHSS ≥16 & infarct core ≥70 mL. Yet, the current analyses did not provide evidence over the benefit of EVT in patients with mismatched clinical and radiological severities, i.e., NIHSS ≥16 & infarct core <70 mL or NIHSS < 16 & infarct core ≥ 70 mL. In addition, EVT may be associated with a higher risk of any ICH and death within 90 days in the subgroup with NIHSS <16 & infarct core ≥70 mL. With limited sample sizes of the subgroups and lack of significant interaction between the clinical-radiological matched and mismatched subgroups on the outcomes, the “neutral” findings in the clinical-radiological mismatched subgroups should be interpreted with caution, particularly for the subgroup with NIHSS ≥ 16 & infarct core < 70 mL who are treated with EVT on a daily basis. While future studies are needed to verify the findings, simultaneous consideration of the stroke severity and infarct core volume may facilitate selection of acute LVO patients with large infarctions, who could truly benefit from EVT.
Contributors
XL, ZM and LL had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: LZ, XN, YP, XL, LL. Acquisition, analysis, or interpretation of data: LZ, MW, XL, WD, ZZ, YW, JL, GM, XH. Drafting of the manuscript: LZ, XL. Critical revision of the manuscript for important intellectual content: TNN, MW, ZY, TWL, ZM, LL. Statistical analysis: LZ, MW, YP. Obtained funding: ZM, LL. Supervision: TWL, XL, ZM, LL. ZM and LL had access to the database and LL had final responsibility for the decision to submit for publication. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Data sharing statement
Data are available to researchers on request for purposes of reproducing the results or replicating the procedure by directly contacting the corresponding author.
Declaration of interests
We declare no competing interests.
Acknowledgements
This study was supported by unrestricted grants from Covidien Healthcare International Trading (Shanghai), Johnson & Johnson MedTech, Genesis MedTech (Shanghai), and Shanghai HeartCare Medical Technology. The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. We would like to thank all investigators for their efforts in conducting the ANGEL-ASPECT trial.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102595.
Contributor Information
Xinyi Leng, Email: xinyi_leng@cuhk.edu.hk.
Zhongrong Miao, Email: zhongrongm@163.com.
Liping Liu, Email: lipingsister@gmail.com.
ANGEL-ASPECT Investigators:
Yongjun Wang, Yilong Wang, Liping Liu, David S. Liebeskind, Zhongrong Miao, Zeguang Ren, Vitor Mendes Pereira, Xunming Ji, Qiang Dong, Anding Xu, Xinfeng Liu, Qingwu Yang, Jing Jing (Chair), Zhe Zhang, Yingkui Zhang, Wei Wu, Dapeng Sun, Zhongqi Qi, Shuo Li, Zhenqiang Liu, Zequan Yu, Jingyu Zhang, Fangguang Chen, Kangyue Li, Kai Zhang, Mingkai Hu, Jianmin Liu (Chair), Chen Yao, Kangning Chen, Kun Fang (Chair), Bo Song, Yi Dong, Zhongrong Miao, Xiaochuan Huo, Gaoting Ma, Guangxiong Yuan, Hongxing Han, Wenhuo Chen, Ming Wei, Jiangang Zhang, Zhiming Zhou, Xiaoxi Yao, Guoqing Wang, Weigen Song, Xueli Cai, Guangxian Nan, Di Li, Yizhou Wang, Wentong Ling, Chuwei Cai, Changming Wen, En Wang, Liyong Zhang, Changchun Jiang, Yajie Liu, Geng Liao, Xiaohui Chen, Tianxiao Li, Shudong Liu, Jinglun Li, Yaxuan Sun, Na Xu, Zong’en Gao, Dongsheng Ju, Cunfeng Song, Jinggang Xuan, Feng Zhou, Qing Shi, Jun Luo, Yan Liu, Zaiyu Guo, Tong Li, Hongbo Zheng, Linzhi Dai, Junfeng Zhao, Liqiang Gui, Xiaokun Geng, Yufeng Tang, Congguo Yin, Hua Yang, Xiaochuan Huo, Gaoting Ma, Ruiyang An, Yuying Sun, Yanan Wu, Chunlai Yu, Shuangcheng Zheng, Yuesong Pan, Aoming Jin, Xianglong Xiang, Mengxing Wang, Hongyi Yan, Yuanling He, Chunyang Li, Weixia Kong, Yuhuan Chen, Chenhao Guo, Fengjie Ji, Pengshan Ji, Lei Liu, Xinghua Lu, Guangkuo Luo, Nanjing Wang, Yu Zhang, Bo Liu, Jian Yang, Jingjing Deng, Juan Wang, Wanru Wang, Hang Yu, Le Cui, Wenwen Liu, Ziyong Wang, Xia Zhao, Zhou Zhou, Zhongrong Miao, Guangxiong Yuan, Hongxing Han, Wenhuo Chen, Ming Wei, Jiangang Zhang, Zhiming Zhou, Xiaoxi Yao, Guoqing Wang, Weigen Song, Xueli Cai, Guangxian Nan, Di Li, Alvin Yi-Chou Wang, Wentong Ling, Chuwei Cai, Changming Wen, En Wang, Liyong Zhang, Changchun Jiang, Yajie Liu, Geng Liao, Xiaohui Chen, Tianxiao Li, Shudong Liu, Jinglun Li, Yaxuan Sun, Na Xu, Zong’en Gao, Dongsheng Ju, Cunfeng Song, Jinggang Xuan, Feng Zhou, Qing Shi, Jun Luo, Yan Liu, Zaiyu Guo, Tong Li, Hongbo Zheng, Linzhi Dai, Junfeng Zhao, Liqiang Gui, Xiaokun Geng, Yufeng Tang, Congguo Yin, and Hua Yang
Appendix ASupplementary data
References
- 1.Powers W.J., Rabinstein A.A., Ackerson T., et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for Healthcare professionals from the American heart association/American stroke association. Stroke. 2019;50:e344–e418. doi: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 2.Pexman J.H., Barber P.A., Hill M.D., et al. Use of the Alberta stroke Program early CT score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR Am J Neuroradiol. 2001;22:1534–1542. [PMC free article] [PubMed] [Google Scholar]
- 3.Albers G.W., Marks M.P., Kemp S., et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708–718. doi: 10.1056/NEJMoa1713973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phan K., Saleh S., Dmytriw A.A., et al. Influence of ASPECTS and endovascular thrombectomy in acute ischemic stroke: a meta-analysis. J Neurointerv Surg. 2019;11:664–669. doi: 10.1136/neurintsurg-2018-014250. [DOI] [PubMed] [Google Scholar]
- 5.Schwarz G., Agostoni E.C., Saliou G., et al. Perfusion imaging mismatch profiles in the early thrombectomy window: a single-center analysis. Stroke. 2023;54:1182–1191. doi: 10.1161/STROKEAHA.122.041981. [DOI] [PubMed] [Google Scholar]
- 6.Yoshimura S., Sakai N., Yamagami H., et al. Endovascular therapy for acute stroke with a large ischemic region. N Engl J Med. 2022;386:1303–1313. doi: 10.1056/NEJMoa2118191. [DOI] [PubMed] [Google Scholar]
- 7.Sarraj A., Hassan A.E., Abraham M.G., et al. Trial of endovascular thrombectomy for large ischemic strokes. N Engl J Med. 2023;388:1259–1271. doi: 10.1056/NEJMoa2214403. [DOI] [PubMed] [Google Scholar]
- 8.Huo X., Ma G., Tong X., et al. Trial of endovascular therapy for acute ischemic stroke with large infarct. N Engl J Med. 2023;388:1272–1283. doi: 10.1056/NEJMoa2213379. [DOI] [PubMed] [Google Scholar]
- 9.Bendszus M., Fiehler J., Subtil F., et al. Endovascular thrombectomy for acute ischaemic stroke with established large infarct: multicentre, open-label, randomised trial. Lancet. 2023;402:1753–1763. doi: 10.1016/S0140-6736(23)02032-9. [DOI] [PubMed] [Google Scholar]
- 10.Vincent C.B.L., Tudor J., Julien L., Caroline A., on behalf of LASTE Investigators . 2023. Evaluation of mechanical thrombectomy in LARGE STROKE (ASPECT 0-5) with T or M1 occlusion < 7 hours LSW. (Conference presentation SLICE wordwide 2023; Montpellier, France). [Google Scholar]
- 11.Uchida K., Shindo S., Yoshimura S., et al. Association between Alberta stroke Program early computed tomography score and efficacy and safety outcomes with endovascular therapy in patients with stroke from large-vessel occlusion: a secondary analysis of the recovery by endovascular salvage for cerebral ultra-acute embolism-Japan large ischemic core trial (RESCUE-Japan LIMIT) JAMA Neurol. 2022;79:1260–1266. doi: 10.1001/jamaneurol.2022.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Namitome S., Uchida K., Shindo S., et al. Number of passes of endovascular therapy for stroke with a large ischemic core: secondary analysis of RESCUE-Japan LIMIT. Stroke. 2023;54:1985–1992. doi: 10.1161/STROKEAHA.123.042552. [DOI] [PubMed] [Google Scholar]
- 13.Kim B.J., Lee Y., Kwon B., et al. Clinical-diffusion mismatch is associated with early neurological improvement after late-window endovascular treatment. Cerebrovasc Dis. 2022;51:331–337. doi: 10.1159/000519310. [DOI] [PubMed] [Google Scholar]
- 14.Ren Z., Huo X., Ma G., et al. Selection criteria for large core trials: rationale for the ANGEL-ASPECT study design. J Neurointerv Surg. 2022;14:107–110. doi: 10.1136/neurintsurg-2021-017798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huo X., Ma G., Zhang X., et al. Endovascular therapy in acute anterior circulation large vessel occlusive patients with a large infarct core (ANGEL-ASPECT): protocol of a multicentre randomised trial. Stroke Vasc Neurol. 2023;8:169–174. doi: 10.1136/svn-2022-001865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xing P., Zhou X., Shen F., et al. Imaging mismatch between Alberta Stroke Program Early CT Score and perfusion imaging may be a good variable for endovascular treatment. Eur Radiol. 2023;33:2629–2637. doi: 10.1007/s00330-022-09273-6. [DOI] [PubMed] [Google Scholar]
- 17.Demeestere J., Garcia-Esperon C., Garcia-Bermejo P., et al. Evaluation of hyperacute infarct volume using ASPECTS and brain CT perfusion core volume. Neurology. 2017;88:2248–2253. doi: 10.1212/WNL.0000000000004028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koton S., Pike J.R., Johansen M., et al. Association of ischemic stroke incidence, severity, and recurrence with dementia in the atherosclerosis risk in communities cohort study. JAMA Neurol. 2022;79:271–280. doi: 10.1001/jamaneurol.2021.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaidat O.O., Yoo A.J., Khatri P., et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44:2650–2663. doi: 10.1161/STROKEAHA.113.001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell B.C.V., Majoie C., Albers G.W., et al. Penumbral imaging and functional outcome in patients with anterior circulation ischaemic stroke treated with endovascular thrombectomy versus medical therapy: a meta-analysis of individual patient-level data. Lancet Neurol. 2019;18:46–55. doi: 10.1016/S1474-4422(18)30314-4. [DOI] [PubMed] [Google Scholar]
- 21.Millan M., Remollo S., Quesada H., et al. Vessel patency at 24 hours and its relationship with clinical outcomes and infarct volume in REVASCAT trial (randomized trial of revascularization with solitaire FR device versus best medical therapy in the treatment of acute stroke due to anterior circulation large vessel occlusion presenting within eight hours of symptom onset) Stroke. 2017;48:983–989. doi: 10.1161/STROKEAHA.116.015455. [DOI] [PubMed] [Google Scholar]
- 22.von Kummer R., Broderick J.P., Campbell B.C., et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46:2981–2986. doi: 10.1161/STROKEAHA.115.010049. [DOI] [PubMed] [Google Scholar]
- 23.FCaP Schulz. Multiple correlations and Bonferroni’s correction. Biol Psychiatry. 1998;44:775–777. doi: 10.1016/s0006-3223(98)00043-2. [DOI] [PubMed] [Google Scholar]
- 24.Schober P., Mascha E.J., Vetter T.R. Statistics from A (agreement) to Z (z score): a guide to interpreting common measures of association, agreement, diagnostic accuracy, effect size, heterogeneity, and reliability in medical research. Anesth Analg. 2021;133:1633–1641. doi: 10.1213/ANE.0000000000005773. [DOI] [PubMed] [Google Scholar]
- 25.Yoshimoto T., Inoue M., Yamagami H., et al. Use of diffusion-weighted imaging-alberta stroke Program early computed tomography score (DWI-ASPECTS) and ischemic core volume to determine the malignant profile in acute stroke. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.012558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell B.C., Mitchell P.J., Kleinig T.J., et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 27.Jovin T.G., Chamorro A., Cobo E., et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–2306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 28.Hedna V.S., Bodhit A.N., Ansari S., et al. Hemispheric differences in ischemic stroke: is left-hemisphere stroke more common? J Clin Neurol. 2013;9:97–102. doi: 10.3988/jcn.2013.9.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seyedsaadat S.M., Neuhaus A.A., Nicholson P.J., et al. Differential contribution of ASPECTS regions to clinical outcome after thrombectomy for acute ischemic stroke. AJNR Am J Neuroradiol. 2021;42:1104–1108. doi: 10.3174/ajnr.A7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee J.S., Bang O.Y. Collateral status and outcomes after thrombectomy. Transl Stroke Res. 2023;14:22–37. doi: 10.1007/s12975-022-01046-z. [DOI] [PubMed] [Google Scholar]
- 31.Qureshi A.I., Chaudhry S.A., Sivagnanam K., et al. Clinical-radiological severity mismatch phenomenon: patients with severe neurological deficits without matching infarction on computed tomographic scan. J Neuroimaging. 2013;23:21–27. doi: 10.1111/j.1552-6569.2012.00737.x. [DOI] [PubMed] [Google Scholar]
- 32.Nogueira R.G., Jadhav A.P., Haussen D.C., et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–21. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 33.Zaidat O.O.Y.A., on behalf of TESLA Investigators . 2023. TESLA trial: primary results. (Conference presentation European stroke organisation conference 2023; Munich, Germany). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.