Abstract
Despite California’s dependence on hired farm labor, scarce research has been conducted on the respiratory health of hired farm workers. Agricultural exposures to inorganic and organic dusts can adversely affect an individual’s respiratory health and differ by farm type and job task. The purpose of the present analysis was to examine associations between agricultural work exposures and pulmonary function among 450 California farm workers. Data were collected as part of the Mexican Immigration to California: Agricultural Safety and Acculturation (MICASA) study, a prospective cohort study examining occupational risk factors and health of hired farm worker families in Mendota, California. Time-weighted self-reported average (TWSRA) dust scores were calculated from assessments of past-12-month agricultural work history. Other dust exposure indicator variables included months worked in agriculture in the past 12 months and years worked in agriculture. Multiple linear regression modeled FEV1 (forced expiratory volume in 1 second), FEF25–75% (forced midexpiratory flow rate), FVC (forced vital capacity), FEV6, FEV1/FVC, and FEV1/FEV6 separately. Seventy-six percent of participants had worked in agriculture in the past year. In models conducted for crops and tasks separately, high TWSRA dust score was associated with better FEV6. Crop and task models showed associations between greater months worked in agriculture in the past year and better FEV1, FEF25–75%, and FEV6. Both models also found greater years worked in agriculture to be associated with worse FEV1/FEV6. Results were generally in the opposite direction as expected given past research but not uncommon. Future research should investigate relationships between pulmonary function and agricultural dust exposure over a lifetime and changes in pulmonary function over time.
Keywords: Agriculture, Hispanic Americans, respiratory function tests
INTRODUCTION
The agriculture industry in California is the nation’s largest and most diverse.1 Approximately 70% of the industry’s sales are non–animal-related products such as field crops, vegetables and melons, fruits and nuts, and nursery and floriculture. California’s Fresno County, home to the rural town of Mendota, is the most agriculturally productive county in the United States.2 It is estimated that between 372,600 and 648,000 persons are employed in California’s agricultural work force. Between 63% and 95% of workers are born outside of the United States and almost all self-identify as Latino or Hispanic.3,4
Despite the importance of hired farm labor to California’s agriculture, scare research has been conducted on the respiratory health of hired farm workers.5 The majority of investigations regarding agricultural work and respiratory symptoms have focused on farm operators and primarily on farms with live-stock.5–10 Agricultural work exposures to inorganic dusts from soil5,11 and organic dusts containing microorganisms or allergens5,12–39 can adversely affect an individual’s respiratory health and differ by farm type and job task.10,40–44 In the dry climate of California, exposure to agricultural dust has been found to be associated with persistent wheeze and chronic bronchitis.10 Additionally, mineral dust has been implicated in cases of small airway disease and pneumoconiosis, although its clinical significance is unknown.44
Spirometry, a component of pulmonary function testing, is an invaluable screening tool of respiratory function.45 Testing maneuvers are highly reproducible when performed adequately and can detect respiratory disease in its early stages, but spirometric measures don’t always correlate with clinically relevant outcomes and minimal clinically important differences have not been clearly defined for all respiratory conditions.46–50
The purpose of the present analysis was to examine associations between agricultural work exposures and pulmonary function among California farm workers. Our objective was to assess the associations between self-reported dust scores in the past 12 months, months worked in agriculture in the past 12 months, and years worked in agriculture and worse pulmonary function.
METHODS
Data were collected as part of the Mexican Immigration to California: Agricultural Safety and Acculturation (MICASA) study,51 a prospective cohort study examining occupational risk factors and health of hired farm worker families residing in Mendota, California. Households were sampled from randomly selected census blocks, and those containing at least one hired farm worker were contacted for recruitment into the study. Methods of sampling and recruitment for the MICASA study have been described in detail elsewhere.51
Participants consisted of men and women, aged 22 to 70 years, who self-identified as Mexican or Central American. Those who completed the follow-up interview, conducted between August 2008 and February 2010, and spirometry, conducted between February 2009 and June 2010, were included in the present analysis. In total, 640 participants completed the follow-up interview and 472 were screened for pulmonary function testing. Of these, 5 were screened out of spirometry for medical reasons. Seventeen participants who did not reproduce an acceptable maneuver according to American Thoracic Society (ATS) criteria were excluded.52
Most interviews were conducted in the participant’s home and assessed demographic characteristics, cigarette smoking,53 and agricultural work history exposures. Spirometry was conducted according to standards established by ATS and the European Respiratory Society45,54 by certified55 spirometry technicians using New Diagnostic Designs EasyOne spirometers (Andover, Massachusetts).
Questions regarding dust exposure and agricultural work history exposures were adapted from the California Agricultural Workers Health Survey.56 Time-weighted self-reported average (TWSRA) dust scores were calculated for dust exposure in the past year. Calculation of a participant’s overall TWSRA dust score began by multiplying the number of weeks a participant worked in each crop type and job task combination by the average number of days worked per week. Next, the number of days worked for each crop type and job task combination was multiplied by its corresponding self-rated dust score. These values were summed over all time periods in the 12-month work history grid and divided by the number of days worked in agriculture in the past year. Participants with TWSRA dust scores above the mean were categorized as high TWSRA dust scores. Other dust exposure indicator variables included months worked in agriculture in the past 12 months and years worked in agriculture. Pesticide exposure was neither measured objectively nor assessed by self-report among participants enrolled in the broader MICASA study.
Crop-specific and task-specific TWSRA dust scores were calculated using the same procedure to calculate the overall TWSRA dust score. Time periods in the past year were identified by crop and task. For time periods that specified more than one crop or more than one task, the number of days worked in that time period was divided by the number of crops or number of tasks specified and each crop or each task received an equal number of days worked for that time period. Among the crops reported were melons, tomatoes, grapes, nuts, cotton, lettuce, asparagus, onion, pomegranates, and other less frequently reported crops. Reported tasks included packing/sorting, hoeing/weeding, pruning/cutting, irrigation, picking, cleaning, planting, machine driving, supervising, and other less common tasks.
For the present analysis, the following spirometric measures were modeled separately as primary outcomes: FEV1 (forced expiratory volume in 1 second), FEF25–75% (forced midexpiratory flow rate), FVC (forced vital capacity), FEV6, FEV1/FVC, and FEV1/FEV6. Their reproducibility was assessed following ATS recommendations.57 Participants with variability up to 8% on spirometric measures were included because their estimates remained stable during sensitivity analyses.58–61
Multiple linear regression was used to model observed values of FEV1, FEF25–75%, FVC, FEV6, FEV1/FVC, and FEV1/FEV6 in separate models. All models were adjusted for age, sex, height squared,62 and current smoking because of their influence on pulmonary function. Estimates modeling FEV6 had lower standard errors than those modeling FVC; therefore, estimates and standard errors for FVC and FEV1/FVC were excluded from the results. FEV6 can be used as a more reproducible surrogate for FVC, since it is a less physically demanding measure and is therefore easier to complete for individuals with respiratory conditions.45 All analyses were performed using Statistical Analysis Software, version 9.2 (SAS Institute, Cary, NC).
RESULTS
A total of 450 participants, who completed the follow-up interview and spirometry, were included in analyses. Of these, 76% had worked in agriculture in the past year (Table 1). Participants who worked in agriculture in the past year did not differ from their counterparts by age but were more likely to be male and less likely to be born in the United States. Fifty-three percent of those who worked in agriculture in the past year were men (P < .0001). There were no differences by agricultural work status on years lived in the United States, household income, and proportion of participants reporting fair or poor health.
TABLE 1.
Description of Study Cohort at Spirometry Testing
| Characteristic | Total (N = 450) | Worked in agriculture in past year (n = 340) | Didn’t work in agriculture in past yeara (n = 110) |
|---|---|---|---|
| Age, % (n) | |||
| 30 years old or younger | 18 (82) | 18 (62) | 18 (20) |
| 31–40 years old | 32 (145) | 33 (112) | 30 (33) |
| 41–50 years old | 33 (147) | 33 (112) | 32 (35) |
| 51 years old or older | 17 (76) | 16 (54) | 20 (22) |
| Male, % (n) | 43 (192) | 53 (179) | 12 (13)*** |
| Married/living with partner, % (n) | 95 (427) | 94 (321) | 96 (106) |
| Country of birth, % (n)b | |||
| United States | 3 (13) | 2 (6) | 6 (7)** |
| Mexico | 67 (290) | 64 (208) | 75 (82) |
| El Salvador | 28 (121) | 31 (101) | 18 (20) |
| Other Central American | 3 (11) | 3 (10) | 1 (1) |
| Years lived in the United States, mean ± SDb | 15.3 ± 9.4 | 15.1 ± 9.2 | 15.6 ± 10.1 |
| Highest grade completed in school, % (n) | |||
| No school | 6 (25) | 7 (24) | 1 (1)* |
| Primary or less | 58 (262) | 57 (192) | 64 (70) |
| Greater than primary | 36 (162) | 36 (123) | 35 (39) |
| Household income <$30,000, % (n) | 74 (311) | 73 (233) | 78 (78) |
| Current smoking, % (n) | 6 (25) | 6 (19) | 5 (6) |
| Smoking pack years, mean ± SD | 7.4 ± 8.6 | 6.5 ± 7.6 | 12.3 ± 12.4 |
Includes those who worked in other occupations or didn’t work in the past year.
Data assessed at the baseline interview, conducted between January 2006 and May 2007.
P < .05 for the comparison between those who worked and didn’t work in agriculture in the past year.
P < .01 for the comparison between those who worked and didn’t work in agriculture in the past year.
P < .001 for the comparison between those who worked and didn’t work in agriculture in the past year.
Among participants who worked in agriculture in the past year, women worked significantly less and had different work experience than men. Women worked an average of 10.6 years in agriculture compared with 17.4 years for men (P < .0001) (Table 2). In the past year, women worked fewer months in agriculture than men (5.0 months compared with 7.3 months, respectively; P < .0001).
TABLE 2.
Twelve-Month Agricultural Work History
| Work history | Total (N = 340) | Men (n = 180) | Women (n = 160) |
|---|---|---|---|
| Months worked in agriculture in past year, mean ± SD | 6.2 ± 2.6 | 7.3 ± 2.3 | 5.0 ± 2.3*** |
| Years worked in agriculture, mean ± SDa | 14.2 ± 9.2 | 17.4 ± 9.7 | 10.6 ± 7.1*** |
Data assessed at the baseline interview, conducted between January 2006 and May 2007.
P < .001 for the comparison between men and women
Based upon TWSRA dust scores for each crop and task the least dusty crops were asparagus (TWSRA dust score = 2.4) and lettuce (TWSRA dust score = 3.2) (Figure 1). Most crops had TWSRA dust scores that fell between those of pomegranates (TWSRA dust score = 5.7) and cotton (TWSRA dust score = 7.4). Little variability existed among TWSRA dust scores for tasks; hoeing/weeding being the highest (TWSRA dust score = 7.2) and pruning/cutting being the lowest (TWSRA dust score = 5.4).
FIGURE 1.
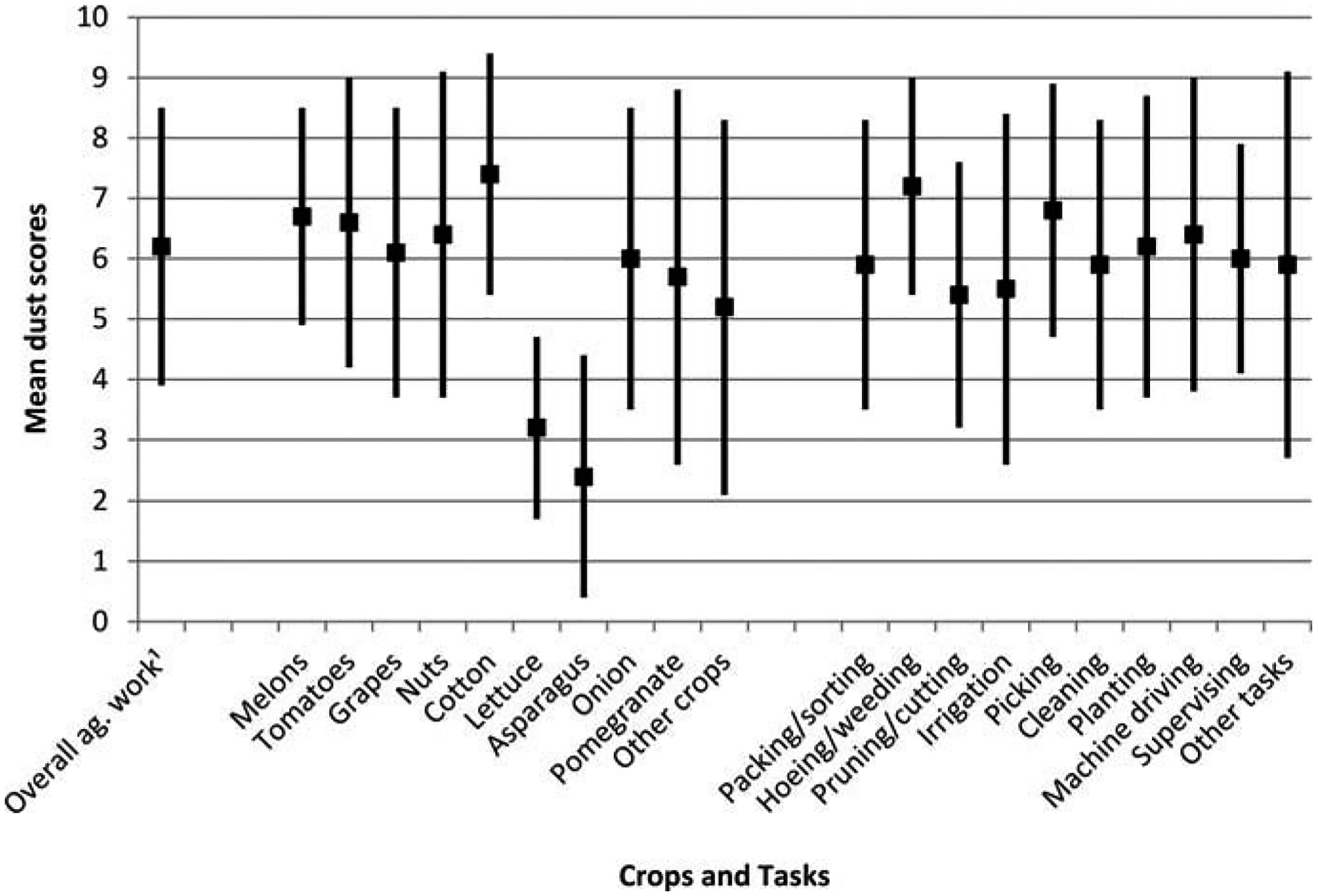
Mean (± SD) self-reported dust scores by crop and task (N = 450). Self-reported scores of participants in a given crop type or job task were time-weighted then averaged.
1ag. = agricultural; dust score across all crop types and job tasks.
All multiple linear regression models were adjusted for age, sex, height squared, and current smoking (Table 3). Models assessing high TWSRA dust score, months worked in agriculture in the past year, and years worked in agriculture were analyzed for crops (Model 1) and tasks (Model 2) separately. Although nonsignificant in both crops and tasks models, workers with high TWSRA dust scores in the past year had worse FEV1/FEV6. However, workers with high TWSRA dust scores also had better FEV6 in both models. Assessment of months worked in agriculture in the past year for crops and tasks showed that individuals who worked more months had significantly better FEV1, FEF25–75%, and FEV6 but nonsignificantly worse FEV1/FEV6. Years worked in agriculture produced nonsignificant results in the same direction as months worked in agriculture in the past year and TWSRA dust score in both models with regards to FEV1 and FEV6, but longer time in agriculture was significantly associated with worse FEV1/FEV6 and nonsignificantly associated with worse FEF25–75%.
TABLE 3.
Twelve-Month Agricultural Work History Percent-Time Regression Coefficients by Crop and Task (N = 340)a
| Work history | FEV1 | FEF25–75% | FEV6 | FEV1/FEV6 | ||||
|---|---|---|---|---|---|---|---|---|
| Estimate (SE) | P value | Estimate (SE) | P value | Estimate (SE) | P value | Estimate (SE) | P value | |
| Model 1: Crops | ||||||||
| High TWSRA dust score in past yearb | .13 (.09) | .16 | .08 (.15) | .57 | .22 (.10) | .04 | −.008 (.006) | .14 |
| Months worked in agriculture in past year | .08 (.01) | <.001 | .11 (.03) | <.001 | .11 (.02) | <.001 | −.00003 (.001) | .97 |
| Years worked in agriculture | .006 (.003) | .08 | −.004 (.005) | .42 | .009 (.005) | .05 | -.0005 (.0002) | .03 |
| Model 2: Tasks | ||||||||
| High TWSRA dust score in past yearb | .14 (.09) | .12 | .02 (.16) | .89 | .22 (.10) | .02 | −.008 (.006) | .20 |
| Months worked in agriculture in past year | .08 (.01) | <.001 | .11 (.03) | <.001 | .11 (.02) | <.001 | −.00001 (.001) | .99 |
| Years worked in agriculture | .006 (.003) | .07 | −.005 (.005) | .39 | .009 (.004) | .05 | -.0005 (.0002) | .04 |
Adjusted for age, sex, height squared, and current smoking.
TWSRA = time-weighted self-reported average.
Bold font indicates statistical significance.
DISCUSSION
Analytical findings were relatively consistent across all three measures of agricultural work history exposure but unexpected given past research. High TWSRA dust score in the past year was associated with better FEV6, more months worked in agriculture in the past year was associated with better FEV1, FEF25–75%, and FEV6, and more years worked in agriculture was associated with worse FEV1/FEV6.
Surprisingly, there were few differences between individuals who worked in agriculture in the past 12 months compared with those who didn’t. Demographically, those who worked in agriculture in the past year were more likely to be men and less likely to be US-born. This is consistent with what we know about farm workers in California. The National Agricultural Workers Survey estimated that 73% of farm workers in California were men and 5% were born in the United States.3
Agricultural dust exposure in California’s Central Valley differs from most traditional agricultural work.43,44,63 Therefore, occupational exposure concentrations have been quantified within the broader MICASA study.64 Moran and colleagues64 found that personal dust exposure levels were higher for almond workers than melon or tomato workers; which shared similar exposure concentrations. Although we didn’t observe TWSRA dust scores to be higher for nuts, TWSRA dust scores were comparable between melons and tomatoes. They also reported that exposure concentrations were slightly lower for workers tasked with packing/sorting compared with harvesting and weeding/sweeping. These results support our findings of higher TWSRA dust scores for picking and hoeing/weeding tasks than packing/sorting. Moran et al. also assessed the correlation between actual dust concentrations and self-reported assessments of dust exposure. They noted that as time working in a crop increased, participants were less able to accurately predict dust exposure. This may explain why TWSRA dust scores did not always agree with the objective measures of dust exposure in the MICASA study.
In regression models that assessed TWSRA dust scores in the past year, the lack of association and positive association between dust exposure and pulmonary function were unexpected findings. Previous research among farm workers in California has linked particulate matter with persistent wheeze and chronic bronchitis and implicated mineral dust in cases of small airway disease and pneumoconiosis.10,44 We also expected months worked in agriculture in the past year and years worked in agriculture to be associated with worse pulmonary function, although years worked in agriculture was associated with worse FEV1/FEV6. Prior analyses of MICASA farm worker cohort data found the number of years worked in agriculture to be associated with chronic cough and asthma.65 Among farm workers in California’s Central Valley, researchers observed that working >8 months per year in agriculture was associated with coughing most days at work while also observing pulmonary function to be better than predicted.66 It is not uncommon in the literature for agricultural work to be associated with respiratory symptoms but not worse pulmonary function. Cross-sectional studies have linked work and dust exposure in animal confinement and herbal tea farming to respiratory symptoms, such as chronic bronchitis, wheezing, shortness of breath, and chronic cough, but not decrements in pulmonary function.67–71
It has been suggested that cross-sectional investigations may not detect associations between exposure and worse pulmonary function because only the mean level of function in a group of workers is analyzed, therefore covering up an association that may exist among a minority of workers susceptible to the effects of exposure.72–74 This would explain why Bigham65 found years worked in agriculture to be associated with respiratory symptoms among MICASA farm workers but we didn’t observe an association between TWSRA dust scores in the past year, months worked in agriculture in the past year, or years worked in agriculture and worse pulmonary function with the exception of years worked in agriculture and worse FEV1/FEV6.
Ideally, measured exposure concentrations in agricultural settings are used to assess the amount of dust present in the work environment. Unfortunately, assessing exposure using objective measurements was not possible in the present analysis. Nieuwenhuijsen et al. determined that self-reported measures (scores) of dust by farm workers were reliable predictors of personal dust exposure when averaged independent of age, the number of years worked in agriculture, level of education, the presence of respiratory symptoms, and the language of the questionnaire, providing confidence in the reliability of these assessments.75
The present study benefited from the design of the MICASA study. First, established relationships with participants encouraged their continued participation as the study progressed. The study’s presence in the community prior to the follow-up interview promoted the trust of participants, some of whom likely reside in the United States without legal authorization. Second, work history and exposures were assessed prior to pulmonary function ensuring that exposures of interest preceded spirometry outcomes. Third, use of dust exposure and agricultural work history exposure questions from the California Agricultural Workers Health Survey allowed participants to specify agricultural work by week for up to 12 months. Previous studies of respiratory health among farm workers identified only one job type, which may not reflect true patterns of work and exposure over the course of a year.76,77
A limitation of the present analysis was the potential for a “healthy worker” bias. Results suggested that participants who worked in agriculture longer in the past year may have been healthier. This is an issue that plagues occupational studies with varying magnitude, and little consensus has been reached on how to control it.78–80 Past research has described the existence of the “healthy worker” bias among farmers81–85 as well as hired farm workers66,86–90 and warned researchers to anticipate it when assessing their respiratory health.91 Another limitation of this study is its limited sample size given the enormity of California’s agricultural work force. Although data from a representative sample of farm workers in California’s Central Valley were analyzed, caution should be exercised when generalizing these results to farm workers working in other areas of the state.
CONCLUSION
The present analysis has illustrated the challenges of assessing the respiratory health of hired farm workers. Recommendations for future research include investigating relationships between dust exposure over a lifetime of agricultural work and pulmonary function, assessing changes in pulmonary function over time among hired farm workers, and using objective measures of dust exposure. Findings can be used as an example of the challenges when conducting research on the respiratory health of hired farm workers.
ACKNOWLEDGMENTS
Appreciation and gratitude goes to the MICASA study participants, local field staff (Gloria Andrade, Alex Cervantes, Ana Cervantes, and Giselle Garcia), and the Western Center for Agricultural Health and Safety.
FUNDING
Funding for the MICASA study was provided by the National Institute for Occupational Safety and Health (R01OH009293). The present analysis was supported in part by the National Cancer Institute (NCI) of the National Institutes of Health (R25CA113710).
Contributor Information
Erik J. Rodriquez, Center for Tobacco Control Research and Education, University of California, San Francisco, San Francisco, California, USA.
Maria T. Stoecklin-Marois, Center for Healthcare Policy and Research, University of California, Davis, Davis, California, USA.
Deborah H. Bennett, Department of Public Health Sciences, University of California, Davis, Davis, California, USA.
Daniel J. Tancredi, Department of Pediatrics, University of California Davis School of Medicine, Sacramento, California, USA.
Marc B. Schenker, Department of Public Health Sciences, University of California, Davis, Davis, California, USA.
REFERENCES
- 1.National Agricultural Statistics Service. California Agricultural Statistics, Crop Year 2010. Sacramento, CA: US Department of Agriculture; 2011. [Google Scholar]
- 2.California Department of Food and Agriculture. California Agricultural Highlights 2010. Sacramento, CA: California Department of Food and Agriculture; 2010. [Google Scholar]
- 3.Aguirre International. The California Farm Labor Force: Overview and Trends From the National Agricultural Workers Survey. Burlingame, CA: Aguirre International; 2005. [Google Scholar]
- 4.State of California Employment Development Department. California’s Agricultural Employment 2008. Sacramento, CA: State of California Employment Development Department; 2008. [Google Scholar]
- 5.Schenker MB, Christiani D, Cormier Y. Respiratory health hazards in agriculture. Am J Respir Crit Care Med. 1998; 158(5 Pt 2): S1–S76. [DOI] [PubMed] [Google Scholar]
- 6.Garcia JG, Matheny Dresser KS, Zerr AD. Respiratory health of Hispanic migrant farm workers in Indiana. Am J Ind Med. 1996;29:23–32. [DOI] [PubMed] [Google Scholar]
- 7.Schenker M. Exposures and health effects from inorganic agricultural dusts. Environ Health Perspect. 2000;108(Suppl 4):661–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linaker C, Smedley J. Respiratory illness in agricultural workers. Occup Med (Lond). 2002;52:451–459. [DOI] [PubMed] [Google Scholar]
- 9.Eduard W, Douwes J, Omenaas E, Heederik D. Do farming exposures cause or prevent asthma? Results from a study of adult Norwegian farmers. Thorax. 2004;59:381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schenker MB, Farrar JA, Mitchell DC, et al. Agricultural dust exposure and respiratory symptoms among California farm operators. J Occup Environ Med. 2005;47:1157–1166. [DOI] [PubMed] [Google Scholar]
- 11.American Thoracic Society. Adverse effects of crystalline silica exposure. American Thoracic Society Committee of the Scientific Assembly on Environmental and Occupational Health. Am J Respir Crit Care Med. 1997;155:761–768. [DOI] [PubMed] [Google Scholar]
- 12.Buck WB, Cote LM. Trichothecene Mycotoxins. New York: Marcel Dekker; 1991. [Google Scholar]
- 13.Croft WA, Jarvis BB, Yatawara CS. Airborne outbreak of trichothecene toxicosis. Atmos Environ. 1986;20:549–552. [Google Scholar]
- 14.Haliburton JD, Buck WB. Equine leucoencephalomalacia: an historical review. Curr Top Vet Med Anim Sci. 1986;33:75–79. [Google Scholar]
- 15.Harrison LR, Colvin BM, Greene JT, Newman LE, Cole JR Jr. Pulmonary edema and hydrothorax in swine produced by fumonisin B1, a toxic metabolite of Fusarium moniliforme. J Vet Diagn Invest. 1990;2:217–221. [DOI] [PubMed] [Google Scholar]
- 16.Ross PF, Nelson PE, Richard JL, et al. Production of fumonisins by Fusarium moniliforme and Fusarium proliferatum isolates associated with equine leukoencephalomalacia and a pulmonary edema syndrome in swine. Appl Environ Microbiol. 1990;56:3225–3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson TM, Ross PF, Rice LG, et al. Fumonisin B1 levels associated with an epizootic of equine leukoencephalomalacia. J Vet Diagn Invest. 1990;2:213–216. [DOI] [PubMed] [Google Scholar]
- 18.Marasas WF, Kriek NP, Fincham JE, van Rensburg SJ. Primary liver cancer and oesophageal basal cell hyperplasia in rats caused by Fusarium moniliforme. Int J Cancer. 1984;34:383–387. [DOI] [PubMed] [Google Scholar]
- 19.Gelderblom WC, Jaskiewicz K, Marasas WF, et al. Fumonisins—novel mycotoxins with cancer-promoting activity produced by Fusarium moniliforme. Appl Environ Microbiol. 1988;54:1806–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Toxicology Program. Sixth Annual Report on Carcinogens. Rockville, MD: US Department of Health and Human Services, Public Health Service; 1991. [Google Scholar]
- 21.Hayes RB, van Nieuwenhuize JP, Raatgever JW, ten Kate FJ. Aflatoxin exposures in the industrial setting: an epidemiological study of mortality. Food Chem Toxicol. 1984;22:39–43. [DOI] [PubMed] [Google Scholar]
- 22.Sorenson WG, Gerberick GF, Lewis DM, Castranova V. Toxicity of mycotoxins for the rat pulmonary macrophage in vitro. Environ Health Perspect. 1986;66:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dutch Expert Committee on Occupational Standards. Health Based Recommended Occupational Exposure Limit for Endotoxins. Rijswijk, The Netherlands: Health Council of The Netherlands; 1997. [Google Scholar]
- 24.Douwes J, Doekes G, Montijn R, Heederik D, Brunekreef B. An immunoassay for the measurement of (1→3)-beta-D-glucans in the indoor environment. Mediators Inflamm. 1997;6:257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rylander R, Peterson Y. Second Glucan Inhalation Toxicity Workshop. ICOH Committee on Organic Dusts Report. Rijswijk, The Netherlands: International Commission on Occupational Health; 1993. [Google Scholar]
- 26.Ingram CG, Jeffrey IG, Symington IS, Cuthbert OD. Bronchial provocation studies in farmers allergic to storage mites. Lancet. 1979;2:1330–1332. [DOI] [PubMed] [Google Scholar]
- 27.Blainey AD, Topping MD, Ollier S, Davies RJ. Respiratory symptoms in arable farmworkers: role of storage mites. Thorax. 1988;43:697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iversen M, Korsgaard J, Hallas T, Dahl R. Mite allergy and exposure to storage mites and house dust mites in farmers. Clin Exp Allergy. 1990;20:211–219. [DOI] [PubMed] [Google Scholar]
- 29.van Hage-Hamsten M, Johansson SG, Zetterstrom O. Predominance of mite allergy over allergy to pollens and animal danders in a farming population. Clin Allergy. 1987;17:417–423. [DOI] [PubMed] [Google Scholar]
- 30.Rautalahti M, Terho EO, Vohlonen I, Husman K. Atopic sensitization of dairy farmers to work-related and common allergens. Eur J Respir Dis Suppl. 1987;152:155–164. [PubMed] [Google Scholar]
- 31.Terho EO, Vohlonen I, Husman K, Rautalahti M, Tukiainen H, Viander M. Sensitization to storage mites and other work-related and common allergens among Finnish dairy farmers. Eur J Respir Dis Suppl. 1987;152: 165–174. [PubMed] [Google Scholar]
- 32.Rautiainen M, Ruoppi P, Jagerroos H, Nuutinen J, Mantyjarvi R, Virtanen T. Nasal sensitization of dairy farmers to bovine epithelial and urinary antigens. Rhinology. 1992;30:121–127. [PubMed] [Google Scholar]
- 33.Ylonen J, Mantyjarvi R, Taivainen A, Virtanen T. IgG and IgE antibody responses to cow dander and urine in farmers with cow-induced asthma. Clin Exp Allergy. 1992;22:83–90. [DOI] [PubMed] [Google Scholar]
- 34.Virtanen T, Vilhunen P, Husman K, Mantyjarvi R. Sensitization of dairy farmers to bovine antigens and effects of exposure on specific IgG and IgE titers. Int Arch Allergy Appl Immunol. 1988;87:171–177. [DOI] [PubMed] [Google Scholar]
- 35.Baldo BA, Wrigley CW. IgE antibodies to wheat flour components. Studies with sera from subjects with baker’s asthma or coeliac condition. Clin Allergy. 1978;8:109–124. [DOI] [PubMed] [Google Scholar]
- 36.Prichard MG, Ryan G, Walsh BJ, Musk AW. Skin test and RAST responses to wheat and common allergens and respiratory disease in bakers. Clin Allergy. 1985;15:203–210. [DOI] [PubMed] [Google Scholar]
- 37.Sutton R, Skerritt JH, Baldo BA, Wrigley CW. The diversity of allergens involved in bakers’ asthma. Clin Allergy. 1984;14:93–107. [DOI] [PubMed] [Google Scholar]
- 38.Valdivieso R, Pola J, Zapata C, et al. Farm animal feeders: another group affected by cereal flour asthma. Allergy. 1988;43:406–410. [DOI] [PubMed] [Google Scholar]
- 39.Iversen M, Dahl R. Specific antigens in dust from swine confinement buildings. Am J Ind Med. 1994;25:49–51. [DOI] [PubMed] [Google Scholar]
- 40.Nieuwenhuijsen MJ, Schenker MB. Determinants of personal dust exposure during field crop operations in California agriculture. Am Ind Hyg Assoc J. 1998;59:9–13. [DOI] [PubMed] [Google Scholar]
- 41.Nieuwenhuijsen MJ, Kruize H, Schenker MB. Exposure to dust and its particle size distribution in California agriculture. Am Ind Hyg Assoc J. 1998;59:34–38. [DOI] [PubMed] [Google Scholar]
- 42.Wu JD, Nieuwenhuijsen MJ, Samuels SJ, Lee K, Schenker MB. Identification of agricultural tasks important to cumulative exposures to inhalable and respirable dust in California. AIHA J (Fairfax, Va). 2003;64: 830–836. [DOI] [PubMed] [Google Scholar]
- 43.Nieuwenhuijsen MJ, Noderer KS, Schenker MB, Vallyathan V, Olenchock S. Personal exposure to dust, endotoxin and crystalline silica in California agriculture. Ann Occup Hyg. 1999;43:35–42. [PubMed] [Google Scholar]
- 44.Schenker MB, Pinkerton KE, Mitchell D, Vallyathan V, Elvine-Kreis B, Green FH. Pneumoconiosis from agricultural dust exposure among young California farmworkers. Environ Health Perspect. 2009;117: 988–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005; 26:319–38. [DOI] [PubMed] [Google Scholar]
- 46.Rabe KF. Roflumilast for chronic obstructive pulmonary disease—author’s reply. Lancet. 2005;366: 1846–1847. [DOI] [PubMed] [Google Scholar]
- 47.Wise RA. The value of forced expiratory volume in 1 second decline in the assessment of chronic obstructive pulmonary disease progression. Am J Med. 2006;119(10 Suppl 1):4–11. [DOI] [PubMed] [Google Scholar]
- 48.Akamatsu K, Yamagata T, Kida Y, Tanaka H, Ueda H, Ichinose M. Poor sensitivity of symptoms in early detection of COPD. COPD. 2008;5:269–273. [DOI] [PubMed] [Google Scholar]
- 49.Cazzola M, MacNee W, Martinez FJ, et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J. 2008;31:416–469. [DOI] [PubMed] [Google Scholar]
- 50.Glaab T, Vogelmeier C, Buhl R. Outcome measures in chronic obstructive pulmonary disease (COPD): strengths and limitations. Respir Res. 2010;11:79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stoecklin-Marois MT, Hennessy-Burt TE, Schenker MB. Engaging a hard-to-reach population in research: sampling and recruitment of hired farm workers in the MICASA study. J Agric Saf Health. 2011; 17:291–302. [DOI] [PubMed] [Google Scholar]
- 52.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–68. [DOI] [PubMed] [Google Scholar]
- 53.Ferris BG. ATS-DLD-78 recommended adult questionnaire. Am Rev Respir Dis. 1978;118:1–120. [PubMed] [Google Scholar]
- 54.Miller MR, Crapo R, Hankinson J, et al. General considerations for lung function testing. Eur Respir J. 2005;26:153–161. [DOI] [PubMed] [Google Scholar]
- 55.Palmer Associates. NIOSH Approved Spirometry, Training Course #051, Training Manual© 06.04.2008. Capitola, CA: Palmer Associates; 2008. [Google Scholar]
- 56.Villarejo D, McCurdy SA. The California Agricultural Workers Health Survey. J Agric Saf Health. 2008;14:135–146. [DOI] [PubMed] [Google Scholar]
- 57.American Thoracic Society. Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–1136. [DOI] [PubMed] [Google Scholar]
- 58.Eisen EA. Standardizing spirometry: problems and prospects. Occup Med. 1987;2:213–225. [PubMed] [Google Scholar]
- 59.Eisen EA, Dockery DW, Speizer FE, Fay ME, Ferris BG Jr. The association between health status and the performance of excessively variable spirometry tests in a population-based study in six U.S. cities. Am Rev Respir Dis. 1987;136:1371–1376. [DOI] [PubMed] [Google Scholar]
- 60.Eisen EA, Oliver LC, Christiani DC, Robins JM, Wegman DH. Effects of spirometry standards in two occupational cohorts. Am Rev Respir Dis. 1985;132:120–124. [DOI] [PubMed] [Google Scholar]
- 61.Eisen EA, Robins JM, Greaves IA, Wegman DH. Selection effects of repeatability criteria applied to lung spirometry. Am J Epidemiol. 1984;120:734–742. [DOI] [PubMed] [Google Scholar]
- 62.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. [DOI] [PubMed] [Google Scholar]
- 63.Lee K, Lawson RJ, Olenchock SA, et al. Personal exposures to inorganic and organic dust in manual harvest of California citrus and table grapes. J Occup Environ Hyg. 2004;1:505–514. [DOI] [PubMed] [Google Scholar]
- 64.Moran RE, Bennett DH, Garcia J, Schenker MB. Occupational exposure to particulate matter from three agricultural crops in California. Int J Hyg Environ Health. 2014;217:226–230. [DOI] [PubMed] [Google Scholar]
- 65.Bigham CW. The Effects of Acculturation, Immigration, and Agricultural Workplace Exposures on Respiratory Health in a Cross-sectional Study of Central California Farm Workers. Davis, CA: University of California, Davis; 2009. [Google Scholar]
- 66.Gamsky TE, Schenker MB, McCurdy SA, Samuels SJ. Smoking, respiratory symptoms, and pulmonary function among a population of Hispanic farmworkers. Chest. 1992;101:1361–1368. [DOI] [PubMed] [Google Scholar]
- 67.Castellan RM, Boehlecke BA, Petersen MR, Thedell TD, Merchant JA. Pulmonary function and symptoms in herbal tea workers. Chest. 1981; 79(4 Suppl):81S–85S. [DOI] [PubMed] [Google Scholar]
- 68.Donham KJ, Zavala DC, Merchant JA. Respiratory symptoms and lung function among workers in swine confinement buildings: a cross-sectional epidemiological study. Arch Environ Health. 1984;39:96–101. [DOI] [PubMed] [Google Scholar]
- 69.Holness DL, O’Blenis EL, Sass-Kortsak A, Pilger C, Nethercott JR. Respiratory effects and dust exposures in hog confinement farming. Am J Ind Med. 1987; 11:571–580. [DOI] [PubMed] [Google Scholar]
- 70.Iversen M, Pedersen B. Relation between respiratory symptoms, type of farming, and lung function disorders in farmers. Thorax. 1990;45:919–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Choudat D, Goehen M, Korobaeff M, Boulet A, Dewitte JD, Martin MH. Respiratory symptoms and bronchial reactivity among pig and dairy farmers. Scand J Work Environ Health. 1994;20:48–54. [DOI] [PubMed] [Google Scholar]
- 72.Hurley JF, Soutar CA. Can exposure to coalmine dust cause a severe impairment of lung function? Br J Ind Med. 1986;43:150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Becklake MR, Irwig L, Kielkowski D, Webster I, de Beer M, Landau S. The predictors of emphysema in South African gold miners. Am Rev Respir Dis. 1987;135:1234–1241. [DOI] [PubMed] [Google Scholar]
- 74.Garshick E, Schenker MB. Occupation and chronic airflow limitation. In: Hensley MJ, Saunders NA, eds. Clinical Epidemiology of Chronic Obstructive Pulmonary Disease. New York: Marcel Dekker; 1989:227–258. [Google Scholar]
- 75.Nieuwenhuijsen MJ, Noderer KS, Schenker MB. The relation between subjective dust exposure estimates and quantitative dust exposure measurements in California agriculture. Am J Ind Med. 1997;32:355–363. [DOI] [PubMed] [Google Scholar]
- 76.Larson RK, Barman ML. A longitudinal study of pulmonary function in cotton gin workers in the San Joaquin Valley. Chest. 1989;96:819–823. [DOI] [PubMed] [Google Scholar]
- 77.Gamsky TE, McCurdy SA, Samuels SJ, Schenker MB. Reduced FVC among California grape workers. Am Rev Respir Dis. 1992; 145(2 Pt 1): 257–262. [DOI] [PubMed] [Google Scholar]
- 78.Meijers JM, Swaen GM, Volovics A, Lucas LJ, van Vliet K. Occupational cohort studies: the influence of design characteristics on the healthy worker effect. Int J Epidemiol. 1989;18:970–975. [DOI] [PubMed] [Google Scholar]
- 79.Arrighi HM, Hertz-Picciotto I. The evolving concept of the healthy worker survivor effect. Epidemiology. 1994;5:189–196. [DOI] [PubMed] [Google Scholar]
- 80.Li CY, Sung FC. A review of the healthy worker effect in occupational epidemiology. Occup Med (Lond). 1999;49:225–229. [DOI] [PubMed] [Google Scholar]
- 81.Chenard L, Senthilselvan A, Grover VK, et al. Lung function and farm size predict healthy worker effect in swine farmers. Chest. 2007;131:245–254. [DOI] [PubMed] [Google Scholar]
- 82.Iversen M, Dahl R, Korsgaard J, Hallas T, Jensen EJ. Respiratory symptoms in Danish farmers: an epidemiological study of risk factors. Thorax. 1988;43:872–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Braback L, Hjern A, Rasmussen F. Selective migration contributes to a healthy worker effect in the farming population. J Clin Epidemiol. 2006;59:102–103; author reply 103. [DOI] [PubMed] [Google Scholar]
- 84.Vogelzang PF, van der Gulden JW, Tielen MJ, Folgering H, van Schayck CP. Health-based selection for asthma, but not for chronic bronchitis, in pig farmers: an evidence-based hypothesis. Eur Respir J. 1999; 13:187–189. [DOI] [PubMed] [Google Scholar]
- 85.Eduard W, Omenaas E, Bakke PS, Douwes J, Heederik D. Atopic and non-atopic asthma in a farming and a general population. Am J Ind Med. 2004;46:396–399. [DOI] [PubMed] [Google Scholar]
- 86.Zock JP, Heederik D, Doekes G. Evaluation of chronic respiratory effects in the potato processing industry: indications of a healthy worker effect? Occup Environ Med. 1998;55:823–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Smid T, Heederik D, Houba R, Quanjer PH. Dust-and endotoxin-related respiratory effects in the animal feed industry. Am Rev Respir Dis. 1992;146:1474–1479. [DOI] [PubMed] [Google Scholar]
- 88.Broder I, Corey P, Davies G, et al. Longitudinal study of grain elevator and control workers with demonstration of healthy worker effect. J Occup Med. 1985;27:873–880. [PubMed] [Google Scholar]
- 89.Zejda JE, Pahwa P, Dosman JA. Decline in spirometric variables in grain workers from start of employment: differential effect of duration of follow up. Br J Ind Med. 1992;49:576–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dosman JA, McDuffie HH, Pahwa P. Atopic status as a factor in job decision making in grain workers. J Occup Med. 1991;33:1007–1010. [PubMed] [Google Scholar]
- 91.Le Moual N, Kauffmann F, Eisen EA, Kennedy SM. The healthy worker effect in asthma: work may cause asthma, but asthma may also influence work. Am J Respir Crit Care Med. 2008;177:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]


