Abstract
Objective
To investigate the psychosocial burden in children and adolescents with juvenile rheumatic diseases during the COVID-19 pandemic.
Methods
As part of the multicentre observational KICK-COVID study linked to the National Pediatric Rheumatology Database, adolescents < 21 years and parents of children < 12 years with rheumatic diseases answered questions on perceptions of health risk (PHR) due to SARS-CoV2, stress, well-being (WHO-5) and symptoms of depression (PHQ-9) and anxiety (GAD-7). Data were collected at routine visits from June to December 2021 and assessed for association with demographic and clinical parameters, treatment and patient-reported outcomes by multivariable regression analyses.
Results
Data from 1356 individuals (69% female, 50% adolescents) were included. Median PHR on a numeric rating scale (NRS, 0–10) was 4 (IQR 2–6), median perceived stress was 3 (IQR 1–6). Adolescents reported a worse well-being with a significantly lower median WHO-5-score (60, IQR 40–76) than parents reported for their children < 12 years (80, IQR 68–84). Moderate to severe symptoms of depression and anxiety were reported by 14.3% and 12.3% of the adolescents, respectively. PHR was significantly higher in patients with systemic lupus erythematosus, methotrexate or biologic disease-modifying anti-rheumatic drug therapy than in patients without these characteristics, whereas lower WHO-5 or higher PHQ-9 or GAD-7 scores were only associated with poorer patient-reported health status and physical functioning.
Conclusion
The perception of health risk due to SARS-CoV2 infection was not paralleled by an impairment of mental health, which were, however, significantly correlated with self-rated health status and functional capacity, highlighting the importance of patient-reported outcome assessment.
Trial registration
German Clinical Trials Register (DRKS), no. DRKS00027974. Registered on 27th of January 2022.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12969-024-00979-z.
Keywords: COVID-19, Children, Adolescents, Rheumatic diseases, Risk perception, Well-being, WHO-5, PHQ-9, GAD-7
Background
The pandemic of Corona virus disease 2019 (COVID-19) caused by severe acute respiratory syndrome corona virus type 2 (SARS-CoV-2) and the measures adopted to contain it significantly changed the daily lives of children and young people, both in terms of school attendance and leisure activities. Structural changes in daily routines (e.g. no school attendance) and limited opportunities to pursue hobbies or meet friends were already stressful for healthy people in terms of their psychosocial well-being [1]. Several studies published before the COVID-19 pandemic showed that children and adolescents with chronic diseases have a higher risk of developing mental health problems compared to their healthy peers [2, 3]. In a study published in 2013, Bomba et al. [4] found higher mean Children’s Depression Inventory (CDI) scores in children and adolescents with JIA than in healthy, age- and gender-matched control subjects; in addition, 23.3% of JIA patients and 8.3% of control subjects had a total CDI score of more than 17, indicating a significant risk of depression. In the UK Juvenile Idiopathic Arthritis (JIA) Inception Cohort “Childhood Arthritis Prospective Study” (CAPS), Hanns et al. found a proportion of 14.7% of adolescent patients with JIA who reached the cut-off score of the Mood and Feelings Questionnaire (MFQ) for major depressive disorder [5].Possible stress factors added by the pandemic could include concerns about a potential impairment of medical care or an increased risk of severe COVID-19 due to the underlying disease [6]. In patients with chronic rheumatic diseases who require immunomodulatory or even immunosuppressive therapy, the extent of a health risk from SARS-CoV-2 infection was initially unclear, which may have contributed to an increased perception of risk and this in turn may be related to more depressive or anxiety symptoms. Some studies have been able to show an increased psychosocial burden during the early phase of the COVID-19 pandemic (data from 2020) in young people and children with rheumatic diseases and their parents [7, 8]. The aim of our study was to investigate the psychosocial burden on children and adolescents with rheumatic diseases during the COVID-19 pandemic and to analyze possible influencing factors. Therefore, we (i) assessed the risk and stress perception, depression and anxiety symptoms, and general well-being in children and adolescents with rheumatic diseases and (ii) analysed the associations between demographic, clinical and therapeutic parameters and the above-mentioned outcomes in an advanced phase of the COVID-19 pandemic.
Methods
Patients and data source
The KICK-COVID study was launched to investigate the impact of the COVID-19 pandemic on health care, physical and mental health, and general well-being of children and adolescents with chronic diseases in Germany [9]. The multicenter prospective observational study assessed the short- and long-term consequences of the pandemic for children and adolescents with type 1 diabetes, obesity, and rheumatic diseases and their families. Recruitment and data collection was conducted through three established pediatric registries [10–12]. For rheumatic diseases like juvenile idiopathic arthritis (JIA), systemic lupus erythematosus (SLE), juvenile dermatomyositis (JDM), this was the National Pediatric Rheumatology Database (NPRD [12]): In this long-term observational study, children and adolescents with rheumatic diseases are included after informed consent (parents or children from the age of 8 years) and followed annually. Demographic and clinical parameters are recorded by the physician like age, sex, diagnosis, antinuclear antibody (ANA) positivity, age at disease onset, date of symptom onset and diagnosis, disease activity (NRS 0–10, 0 = no disease activity, NRS ≥ 1 = active disease) and joint count (only for JIA patients), and medication, specifically therapy with synthetic or biological disease-modifying antirheumatic drugs, sDMARD or bDMARD, respectively.
In order to be able to put the risk perception in the context of the actual hazard, information on a SARS-CoV-2 infection and its clinical course was collected in the NPRD. The following question with a yes/no-answer option was added to the physician’s questionnaire: “Has the patient ever been tested positive for SARS-CoV-2?” In case of a positive test, it was asked whether symptoms were present and whether hospitalization had occurred, also each with a yes/no-answer option.
In the NPRD, in addition to the treating physicians, patients 12 years of age and older and parents of children < 12 years of age were asked to provide information about their health or their child’s health on a NRS. The following patient-reported outcomes (PRO) were recorded: global assessment of health status on a numeric rating scale (NRS 0–10, 0 = best), pain (NRS 0–10, 0 = no pain) and functional status by the German version of the Childhood Health Assessment Questionnaire. The CHAQ assesses the ability to perform certain activities in 8 areas of daily life (dressing and personal hygiene, getting up, eating and drinking, walking, personal hygiene, reaching objects, grasping, activities and domestic tasks) using four response categories (easily possible = 0 points, slightly difficult = 1 point, very difficult = 2 points, not possible = 3 points). The item with the highest score per activity area is taken into account when calculating the total score. If help from other people or aids is required to carry out activities in an area, a score of at least 2 is awarded. The total score is then divided by 8 (number of areas of activity). The CHAQ score can therefore assume a value between 0 (no restriction) and 3 (maximum restriction).For further details please see [13]).
The assessments developed for the KICK-COVID study were collected as an add-on module to the patient/parent questionnaire alongside the other items in the NPRD. The institutions participating in the NPRD were asked to distribute the questionnaires to patients with rheumatic diseases 12 years or older or the parents of patients < 12 years of age during routine examinations. Completion of the KICK-COVID study questions was on a voluntary basis. For the analysis presented here, a subset of the questions (3 questions, see below) as well as scores on well-being, depressive as well as anxiety symptoms were evaluated. The data used were collected between June 2021 (start of implementation of the KICK-COVID questions in the NPRD) and December 2021, corresponding to the interim period (summer plateau 2021 [calendar week (CW) 24/2021-30/2021) and the fourth wave (CW 31/2021-51/2021; variant of concern (VOC) delta) of the SARS-CoV2 pandemic in Germany [14].
Questions and questionnaires of the KICK-COVID study add-on module
Perception of health risk, stress and loneliness during the COVID-19 pandemic
The adolescents were asked how dangerous they consider a SARS-CoV2 infection to be for themselves.
For children with rheumatic diseases < 12 years of age, parents were asked how dangerous they consider a SARS-CoV2 infection to be for their child.
Perception of stress and loneliness were investigated by the question how stressed and how lonely young people with rheumatic diseases currently felt, respectively.
For children < 12 years, parents were asked to answer how stressed and how lonely their child currently felt, respectively.
The items were answered on a self-constructed 11-point numeric rating scale from 0 (“not dangerous/stressed/lonely at all”, respectively) to 10 (“totally dangerous/stressed/lonely”, respectively).
World Health Organization Five Well-Being Index (WHO-5)
The German version of the WHO-5 with 5 items was used to measure the general well-being of the participants in the last two weeks before their routine visits to the pediatric rheumatologist [15]. Responses were recorded using a 6-point Likert scale ranging from 0 “not at all” to 5 “always”. The WHO-5 raw score is calculated by summing up the numbers of the five answers and thus ranges from 0 (lack of well-being) to 25 (maximum well-being) and translated into a percentage scale from 0 to 100 by multiplying raw scores by 4, where 100 reflects best possible well-being. A WHO-5 score below 52 (raw score 13) indicates poor well-being and should lead to further psychological exploration [16]. The WHO-5 was filled out by the adolescents and for the children < 12 years by their parents on their behalf.
The WHO-5 has an internal consistency of Cronbach`s α = 0.89 to Cronbach`s α = 0.92 [15].
Patient Health Questionnaire-9 (PHQ-9)
The PHQ-9 consists of 9 questions related to symptoms of depression and was developed as a screening instrument. It assesses how often the respondent has shown depressive symptoms within the last 2 weeks [17] and has been validated for adolescents [18]. The answer categories are as follows: 0 (“Not at all”), 1 (“On some days”), 2 (“On more than half of the days”) and 3 (“Almost every day”). Accordingly, the sum of the scores is between 0 and 27, which are categorised as follows: 1–4: minimal depressive symptoms; 5–9: mild depressive symptoms; 10–14: moderate depressive symptoms; 15–27: severe depressive symptoms [17]. The PHQ-9 has an internal consistency of Cronbach`s α = 0.91 [19].
The PHQ-9 was only completed by patients ≥ 12 years.
Generalized anxiety disorder Scale-7 (GAD-7)
The GAD-7 consists of 7 questions that depict the core symptoms of a generalised anxiety disorder and measures the extent of generalised anxiety symptoms within the last 2 weeks [20]. It has also been validated for the use in adolescents [21].
There are the following response options: “not at all”, “on some days”, “on more than half of the days” and “almost every day”. These are assigned the numerical values 0 to 3, resulting in a sum of scores from 0 to 21, which are categorised as follows: 0–4: minimal anxiety symptoms, 5–9: mild anxiety symptoms, 10–14: moderate anxiety symptoms, 15–21: severe anxiety symptoms [20]. The GAD-7 has an internal consistency of Cronbach`s α = 0.79 to Cronbach`s α = 0.91 [22, 23]. The GAD-7 was only completed by patients ≥ 12 years.
Statistics
Descriptive statistics were used to describe the distribution of categorical and continuously distributed parameters. Outcome parameters (perceived risk, stress, loneliness, WHO-5, PHQ-9, GAD-7) were compared by Mann-Whitney U-Test or Chi2-test between groups defined by disease parameters, sex, treatment and patient-reported health status. Multivariable linear regression analysis was performed in order to determine the association between the outcomes perceived health risk, stress, loneliness, WHO-5 score, PHQ-9 score and GAD-7 score with demographic, clinical, therapeutic and physician- as well as patient-reported outcome parameters (H). Standardized beta coefficients were calculated after fitting the linear regression models. As this was an exploratory study we did not adjust for multiple testing. All statistical analyses were performed with SAS 9.3 (SAS Institute Inc.).
Results
Of 7278 patients documented from June to December 2021 by 49 centers participating in the NPRD, data from 1356 patients with KICK-COVID add-on module questionnaires (response rate 18.6%) from 674 adolescents < 21 years of age and 682 parents of children < 12 years of age with rheumatic diseases (JIA, SLE, JDM) could be analyzed. Demographic and clinical parameters, treatments and outcomes of the study cohort are reported in Table 1:
Table 1.
Demographic and clinical parameter, treatment and outcome of children and adolescents in the study sample
| Parameter | Patients ≥ 12 years | Patients < 12 years |
|---|---|---|
| N | 674 | 682 |
| Female subjects, n (%) | 456 (67.7) | 483 (70.8) |
| Age, years, mean (SD) | 15.2 (1.9) | 7.2 (2.9) |
| Age at disease onset, years, mean (SD) | 8.8 (4.9) | 4.3 (2.8) |
| Disease duration, years, mean (SD) | 6.4 (4.9) | 2.9 (2.7) |
| Diagnoses, n (%) | ||
| JIA | 6201 | 6592 |
| Systemic arthritis | 24 (3.6) | 34 [5] |
| Oligoarthritis, extended | 73 (10.8) | 64 (9.4) |
| Oligoarthritis, persistent | 214 (31.8) | 355 (52) |
| Psoriatic arthritis | 44 (6.5) | 24 (3.5) |
| Enthesitis-related arthritis | 85 (12.6) | 19 (2.8) |
| Polyarthritis, seropositive | 19 (2.8) | 11 (1.6) |
| Polyarthritis, seronegative | 127 (18.8) | 124 (18.2) |
| Other arthritis | 26 (3.9) | 21 (3.1) |
| Systemic lupus erythematosus | 42 (6.2) | 6 (0.9) |
| Juvenile dermatomyositis | 12 (1.8) | 17 (2.5) |
| ANA positive/tested, n (%) | 341/556; (61) | 37/524; (71) |
| Therapy, n (%)* | ||
| DMARD | 352 (55.5) | 346 (55) |
| sDMARD | 262 (41.3) | 290 (46.1) |
| bDMARD | 195 (30.8) | 124 (19.7) |
| Systemic glucocorticoids | 35 (6.2) | 24 (4.8) |
| Outcome parameter | ||
| PhGA disease activity, NRS, mean (SD) | 1.3 (1.8) | 1.5 (2.2) |
| Joint count, mean (SD)# | 1.1 (2.9) | 1.2 (3.3) |
| Functional status, CHAQ (0–3), mean (SD) | 0.2 (0.5) | 0.3 (0.5) |
| PGA, NRS, mean (SD) | 2.3 (2.5) | 2.1 (2.5) |
JIA: juvenile idiopathic arthritis; ANA: antinuclear antibodies; DMARD: disease modifying antirheumatic drugs; sDMARD: synthetic (conventional and targeted) disease modifying antirheumatic drugs; bDMARD: biologic disease modifying antirheumatic drugs; PhGA: physician`s global assessment of disease activity; PGA: Patient`s global assessment of health status *Percentages according to available valid information on the medication; #only determined in JIA patients, n = 1279. 1There was a diagnosis of oligoarthritis without indication of persistent or extended in 5 cases, a diagnosis of polyarthritis without information on rheumatoid factor in 2 cases, and a diagnosis of JIA without indication of category in 1 case, which therefore could not be assigned. 2There was a diagnosis of oligoarthritis without indication of persistent or extended in 4 cases, a diagnosis of polyarthritis without information on rheumatoid factor in 1 case, and a diagnosis of JIA without indication of category in 2 cases
The distribution of diagnoses as well as the demographic, clinical, therapeutic and outcome parameters of the patients in our study cohort are largely comparable to the parameters of all patients documented in the NPRD from June to December 2021 (Additional file, Supplementary Table 2).
Questionnaires were completed broadly in equal numbers across the months from June 2021 to December 2021 (10–15% of the total per month), with July 2021 and October 2021 having proportionally the highest number of documentations (Table 2).
Table 2.
Distribution of questionnaires in 2021, SARS Cov2 test results and overall incidence in Germany
| Month of questionnaire administration | June 2021 | July 2021 | August 2021 | September 2021 | October2021 | November 2021 | December 2021 |
|---|---|---|---|---|---|---|---|
| Questionnaires, n (%) | 115 [10] | 211 [18] | 149 [13] | 189 [16] | 226 [19] | 171 [14] | 120 [10] |
| Patients, who reported to have ever been tested positive, n (%) | 4 (3.5) | 10 (4.7) | 6 (4.0) | 12 (6.3) | 11 (4.9) | 7 (4.1) | 9 (7.5) |
| SARS-CoV2 incidence# | 17 | 6 | 36 | 82 | 74 | 303 | 389 |
#infections per 100.000 inhabitants in Germany in the middle of the month [24]
Between the beginning of June 2021 and the end of October 2021, the measure index in Germany, that served as a measure of the type and number of restraints or restrictions on public life for infection control reasons, ranged between 40 and 30 (0 = no measure, 100 = most stringent measures), i.e. in the medium-low range, and then rose to an average of about 45 in November and to about 55 in December [25]
In the overall 1309 physician questionnaires, information on testing for SARS-CoV2 was provided for 1181 patients: 59 patients were reported to had ever been tested positive for SARS-CoV2 (5%) of whom 30 patients had developed symptoms (51%) and 2 patients had been hospitalised for COVID-19 (3%).
Perception of health risk, stress and loneliness, well-being, symptoms of depression and anxiety
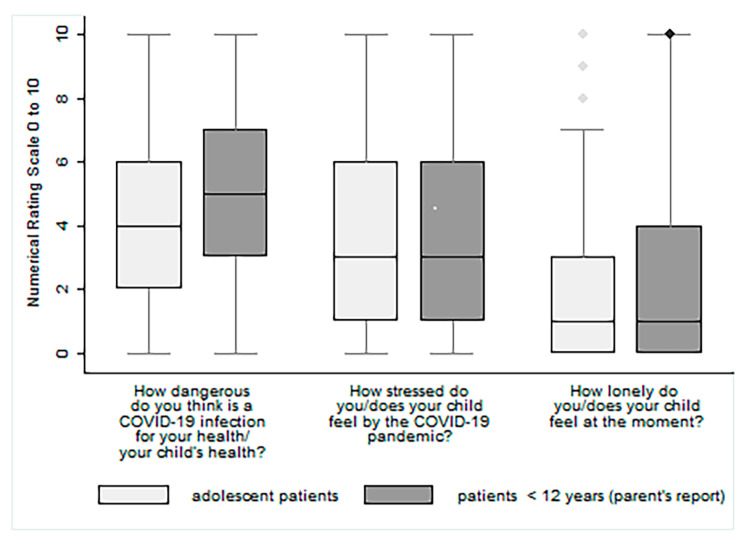
Adolescents and parents of children < 12 years of age with rheumatic diseases rated the danger of a SARS-CoV2 infection to their own or their children`s health with a median value of 4 and 5, respectively (Fig. 1).
Fig. 1.
Box plots of answers to health-risk perception, stress and loneliness on a self-constructed 11-point NRS
Legend: Shown are median values with interquartile ranges, stratified by age groups; 0: “not dangerous/stressed/lonely at all”; 10: “totally dangerous/stressed/lonely”
The perception of stress related to the COVID-19 pandemic was reported by both adolescents and parents reporting for their children with a median value of 3, and the responses showed a wide range (IQR 1–6). The feeling of loneliness was rated by both groups with a median value of 1 (IQR 0–3, and IQR 0–4, respectively).
Overall, the WHO-5 score was lower in adolescents (mean 57.38 ± 22.97, median 60, IQR 40–76) than in children under 12 years of age (73.87 ± 19.11, median 80, IQR 68–84). A conspicuous WHO 5 score < 52 (raw score < 13) was reported by 36.5% of adolescent patients, compared with 11.5% of children younger than 12 years by their parents.
The mean PHQ-9 score of the adolescents was 4.5 ± 4.7 (median 3, IQR 1–7), the mean GAD-7 score was 4.2 ± 4.2 (median 3, IQR 1–6). A PHQ-9 score ≥ 10 was found in 14.2% and a GAD-7 score ≥ 10 in 12.3% of the adolescent patients.
Association of outcome parameters (WHO-5, PHQ-9, GAD-7, perceived risk, stress and loneliness) with age, sex, disease activity, treatment, physician- and patient-reported outcome parameters
Univariable analysis
Among adolescents, female patients were found to have significantly lower WHO-5 scores, higher PHQ-9 scores and GAD-7 scores as well as a higher perception of SARS-CoV2-related health risk and loneliness compared to male patients. Among children < 12 years of age (parent-reported), girls had significantly higher mean WHO-5 scores than boys.
In addition, physician-reported active disease (NRS > 1) and at least one active joint (joint count ≥ 1) were significantly associated with a lower WHO-5 score. No significant association of WHO-5 score, PHQ-9 score or GAD-7 score (in adolescents) was found for therapy (no DMARD versus DMARD). However, the DMARD-treated patient groups reported significantly higher health risk perception than the patients without DMARD therapy.
Patient-reported impaired health status (PGA NRS > 1) and functioning (CHAQ > 0) were associated not only with a significantly lower WHO-5 score, higher PHQ-9 score and GAD-7 score (in adolescents) but also with higher perception of health risk, stress and loneliness. (Additional file, Supplementary Table 3) [26].
Multivariable analysis (data of adolescent patients)
Risk perception among adolescents was significantly associated with each of the following parameters: diagnosis of SLE, treatment with methotrexate, bDMARD therapy, PGA and physical functioning. Perceptions of stress and loneliness in adolescents were associated with female gender and again with patient’s assessment of health status and functioning (Table 3).
Table 3.
Association of demographic, clinical, therapeutic parameters and PROs with health risk perception, stress and loneliness
| How dangerous… | How stressed… | How lonely… | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| beta | 95% CI | p value | betaST | beta | 95% CI | p value | betaST | beta | 95% CI | p value | betaST | |
| Female | 0.20 | -0.27; 0.66 | 0.403 | 0.03 | 0.13 | -0.40; 0.66 | 0.625 | 0.02 | 0.66 | 0.22; 1.10 | 0.003 | 0.12 |
| Age | -0.02 | -0.13; 0.10 | 0.742 | -0,01 | 0.09 | -0.04; 0.22 | 0.174 | 0.06 | 0.00 | -0.11; 0.10 | 0.935 | 0.01 |
| JIA | (ref) | (ref) | (ref) | |||||||||
| SLE | 1.84 | 1.00; 2.68 | < 0.001 | 0.18 | 0.49 | -0.49; 1.46 | 0.328 | 0.04 | 0.02 | -0.78; 0.82 | 0.953 | 0.01 |
| JDM | 0.37 | -1.25; 2.00 | 0.651 | 0.02 | 0.02 | -1.86; 1.91 | 0.980 | 0.01 | -1.13 | -2.68; 0.42 | 0.151 | -0.06 |
| PhGA | -0.14 | -0.27; 0.00 | 0.054 | -0.08 | -0.03 | -0.19; 0.13 | 0.732 | -0.02 | 0.05 | -0.08; 0.18 | 0.453 | 0.03 |
| MTX | 0.92 | 0.44; 1.40 | < 0.001 | 0.16 | 0.45 | -0.10; 1.00 | 0.109 | 0.07 | 0.16 | -0.29; 0.61 | 0.498 | 0.03 |
| bDMARD | 0.54 | 0.06; 1.01 | 0.027 | 0.09 | -0.16 | -0.71; 0.39 | 0.567 | -0.02 | -0.10 | -0.55; 0.35 | 0.651 | -0.02 |
| PGA | 0.17 | 0.07; 0.27 | 0.001 | 0.15 | 0.22 | 0.11; 0.34 | < 0.001 | 0.18 | 0.10 | 0.01; 0.19 | 0.045 | 0.09 |
| CHAQ | 0.85 | 0.30; 1.40 | 0.002 | 0.14 | 0.75 | 0.13; 1.38 | 0.019 | 0.11 | 1.04 | 0.52; 1.55 | < 0.001 | 0.19 |
betaST: standardized beta coefficient; JIA: Juvenile idiopathic arthritis; SLE: Systemic lupus erythematosus; JDM: Juvenile dermatomyositis; PhGA: Physician`s global assessment of disease activity; MTX: Methotrexate; bDMARD: Biologic disease modifying antirheumatic drug; PGA: Patient`s global assessment of health status; CHAQ: Child health assessment questionnaire
Results of the multivariable analysis of these parameters in patients < 12 years of age (parents’ reports) are available in the supplement.
In addition, in adolescents there were significant associations of a lower WHO-5 score and a higher PHQ-9 score and GAD-7 score with female sex, PGA and physical functioning, but not with diagnosis (JIA, SLE, JDM) or treatment. For the PHQ-9, there was a significant association with the age of the patients (Table 4).
Table 4.
Association of demographic, clinical and therapeutic parameters and PROs with WHO-5, PHQ-9 and GAD-7 scores
| WHO-5 score | PHQ-9 score | GAD-7 score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| beta | 95% CI | p value | betaST | beta | 95% CI | p value | betaST | beta | 95% CI | p value | betaST | |
| Female | -4.17 | -8.06; -0.28 | 0.036 | -0.08 | 1.65 | 0.87; 2.43 | < 0.001 | 0.17 | 1.52 | 0.79; 2.24 | < 0.001 | 0.17 |
| Age | -0.94 | -1.89; 0.01 | 0.052 | -0.08 | 0.20 | 0.01; 0.40 | 0.037 | 0.08 | 0.14 | -0.04; 0.31 | 0.134 | 0.06 |
| JIA | (ref) | (ref) | (ref) | |||||||||
| SLE | 3.31 | -3.88; 10.50 | 0.367 | 0.04 | -0.41 | -1.87; 1.05 | 0.580 | -0.02 | -0.21 | -1.54; 1.13 | 0.761 | -0.01 |
| JDM | 5.13 | -7.77; 18.04 | 0.435 | 0.03 | -0.64 | -3.41; 2.13 | 0.650 | -0.02 | -0.25 | -2.84; 2.35 | 0.853 | -0.01 |
| PhGA | -0.78 | -1.95; 0.40 | 0.196 | -0.06 | -0.11 | -0.36; 0.13 | 0.375 | -0.04 | 0.00 | -0.23; 0.22 | 0.984 | -0.01 |
| MTX | -2.04 | -6.01; 1.94 | 0.315 | -0.04 | 0.50 | -0.30; 1.29 | 0.223 | 0.05 | 0.64 | -0.10; 1.38 | 0.088 | 0.07 |
| bDMARD | 1.76 | -2.24; 5.76 | 0.389 | 0.03 | 0.16 | -0.64; 0.96 | 0.692 | 0.02 | -0.08 | -0.82; 0.66 | 0.826 | -0.01 |
| PGA | -3.05 | -3.91; -2.19 | < 0.001 | -0.32 | 0.58 | 0.41; 0.75 | < 0.001 | 0.30 | 0.43 | 0.27; 0.58 | < 0.001 | 0.24 |
| CHAQ | -5.33 | -9.91; -0.75 | 0.023 | -0.10 | 1.68 | 0.72; 2.64 | 0.001 | 0.16 | 1.65 | 0.81; 2.50 | < 0.001 | 0.18 |
betaST: standardized beta coefficient; JIA: Juvenile idiopathic arthritis; SLE: Systemic lupus erythematosus; JDM: Juvenile dermatomyositis; PhGA: Physician`s global assessment of disease activity; MTX: Methotrexate; bDMARD: Biologic disease modifying antirheumatic drug; PGA: Patient`s global assessment of health status; CHAQ: Child health questionnaire
Discussion
In our study, 1 in 7 adolescents with a rheumatic disease reported moderate to severe depressive symptoms, and 1 in 8 adolescent patients described moderate to severe anxiety symptoms. In addition, somewhat more than a third of the adolescents reported significantly reduced general well-being. A significantly lower WHO-5 score in both age groups and significantly higher PHQ-9 and GAD-7 scores in adolescents were associated with poorer assessment of general health and functioning by patients or their parents. In contrast, there was no significant association between physician-assessed disease activity, number of active joints, treatment or education and these scores. Adolescents with a diagnosis of SLE or MTX or bDMARD therapy reported a significantly higher perception of health risk due to SARS-CoV2 infection, whereas this diagnosis or these therapies were not associated with higher levels of depressive or anxiety symptoms or lower well-being.
Only just under 5% of all patients reported that they had ever been tested positive for SARS-CoV2. Since at the time of our survey, Germany’s schools were regularly testing pupils for SARS-CoV2 nationwide, this information appears valid. According to the data collected here, only half of the patients who tested positive in our cohort had symptoms. We do not have information on the severity of COVID-19 in this cohort, however only 2 patients (3%) were hospitalised for COVID-19, suggesting a mostly mild disease course. According to a data collection originating from the early phase of the COVID-19 pandemic in Germany, SARS-CoV-2 infection in children and adolescents with rheumatic diseases under various medications was usually mild and had a good outcome in the majority of cases [27]. In the KICK-COVID study, adolescents as well as parents of children < 12 years of age indicated the risk of such an infection for their health/their child`s health in the middle range. Thus, there is some indication that the perception of health risk due to a SARS-CoV2 infection in our study population is higher than the actual health risk of such an infection. However, using data from the registry for COVID-19 in adults with rheumatic diseases, it has been shown that indeed certain immunosuppressive therapies (glucocorticoids, rituximab, Janus kinase inhibitors) increase the risk for a severe course of SARS-CoV2 infection [28]. Therefore, it seems important to inform parents, children and adolescents with rheumatic diseases about the COVID-19 related risks of an immunomodulatory or immunosuppressive therapy in order to reduce fears on the one hand and to encourage reasonable precautions on the other hand.
In our study, 14.2% and 12.3% of adolescent patients had moderate to severe symptoms of depression or anxiety, respectively. In the type 1 diabetes cohort of the KICK-COVID study, 74 patients (11.9%) reported moderate to severe anxiety symptoms, and the reported level of depressive symptoms in this cohort was moderate to severe in 88 (14.1%) participants [29], so there seem to be no relevant differences in the rate of these psychological comorbidities between these two cohorts despite different underlying chronic conditions. Futhermore, Kamrath et al. [29] showed that anxiety and depressive symptoms of type 1 diabetes patients during the COVID-19 pandemic were comparable to the assessments in these patients before the pandemic.
A meta-analysis of psychiatric comorbidities in patients with an immune-mediated disease diagnosed in childhood [30] revealed a prevalence of anxiety disorders of 13% (95% confidence interval: 12–15%) and of depression of 20% (95% confidence interval: 15–26%) for rheumatic diseases based on ICD-10 code or psychiatrist assessment. However, the included pre-pandemic studies differed considerably with regard to various parameters such as age and gender distribution as well as type and duration of illness. In the British JIA inception cohort Childhood Arthritis Prospective Study (CAPS), Hanns et al investigated mental comorbidities using the Mood and Feelings Questionnaire (MFQ): In 102 adolescent patients with JIA and short disease duration (mean 5 months), 14.7% exceeded the cut-off value (≥ 27) for major depressive disorder [5]. Fair et al. found similar results for children and adolescents with longer established JIA (mean duration of illness 4.7 years) using the Patient-Reported Outcome Measurement Information System (PROMIS) pediatric depressive symptoms and anxiety t-scores: Fourteen (17%) and 13 patients (16%) had moderate depressive or anxious symptoms, respectively, and 1 patient (1%) had severe depressive or anxious symptoms, respectively [31]. Overall, we can conclude that the proportion of adolescents with depressive or anxious symptoms in our study was similar to that described before the COVID-19 pandemic in the literature cited above for adolescents with JIA, who accounted for the majority of adolescent patients (92%) in our cohort. Furthermore, we found higher levels of anxiety and depression and lower levels of social well-being in female adolescents than in male adolescents. This is a long-standing observation that has been made in both healthy adolescents [32–34] and adolescents with rheumatic diseases [5] before the pandemic, and therefore our finding may not be attributed to pandemic-related stress factors, which are perceived differently by female adolescents than by male adolescents.The WHO-5 score as a measure of general well-being was on average lower in adolescents (median 60, IQR 40–76) than in children under 12 years of age (parent`s report, median 80, IQR 68–84) in our study. In the type 1 diabetes cohort of the KICK-COVID study (688 adolescents, mean age 15.6 ± 2.1, 45.6% female), the median score of the WHO-5 questionnaire was 56 (IQR 44–72), which was lower than in a pre-COVID-19 survey of a comparable cohort from Germany [29]. Furthermore, in our cohort of patients with rheumatic diseases, 36.5% of adolescent patients and 11.5% of children under 12 years of age (as reported by parents) had a WHO-5 score of < 52, a threshold that should lead to further psychological assessment [15]. In a study published in 2018, Balasz et al. examined the characteristics and correlates of physical disorders, self-rated health, subjective well-being and anxiety in 11,230 adolescents aged 14–16 years from 11 countries [35]. 15% of the participants answered “Yes” to the question: ”Do you suffer from a chronic illness?” In these individuals, the mean WHO-5 score was 59.5 ± 18.7 (adolescents in our study: 57.4 ± 23) and significantly lower than the WHO-5 score of healthy individuals (63.5 ± 20.6). In addition, Balasz reported more pronounced differences in the mean WHO-5 score between those with a chronic disease with resulting functional limitations in everyday life versus individuals with a chronic disease without resulting limitations, who had a WHO-5 score comparable to healthy individuals. Finally, the largest difference in the WHO-5 score was seen in the study by Balasz et al. between individuals who assessed their health status as poor or very poor (WHO-5 score 44 ± 21.6) versus subjects with a self-reported fair, good or very good state of health (WHO-5 score 63.4 ± 18.7). Our study revealed similar results: A significantly lower WHO-5 score, a higher PHQ-9 score and a higher GAD-7 score was found in patients with a poorer assessment of health status by the patient him- or herself and reported functional limitations. In the multivariable analysis, physician-assessed disease activity was not correlated with the PHQ-9 score, the GAD-7 score or the WHO-5 score. Already before the COVID-19 pandemic (data collection in 2019), Fair et al. found comparable results with depressive symptoms being correlated with the subjectively perceived functional impairment in everyday life (CHAQ score), pain and stress, but not with gender, JIA category, illness duration or activity (cJADAS-10) [31]. Hanns et al. described a significant association of symptoms of anxiety disorder and depression in JIA patients with functional limitations, pain and physician’s global assessment of disease activity, but not with inflammatory markers or number of active joints, based on data analysed before 2020 [36]. These data may suggest that the same parameters are associated with impaired well-being, depressive or anxious symptoms before and during the pandemic.
Adolescents’ health risk perception due to a SARS-CoV2 infection was associated with a diagnosis of SLE, treatment with methotrexate as well as bDMARD therapy, but not with WHO-5 score, PHQ-9 score or GAD-7 score in patients with this diagnosis or treatment. Perceptions of stress and loneliness were significantly higher in both adolescents and patients < 12 years of age (parental report) who reported poorer health and functional impairment, but no association was found with diagnosis, physician assessment of disease activity or treatment. It can be speculated that these patients may have fewer coping resources and therefore experience greater psychosocial stress during a pandemic with the changed circumstances of daily life. On the other hand, those who feel stressed during the pandemic may also have more difficulty managing their condition, for example in terms of adhering to medication or implementing physiotherapy. PROs, particularly PGA and physical functioning assessed with the CHAQ, appear to be important correlates of perceptions of stressors and strain as well as well-being during the pandemic, confirming the data of Peng et al. [7], whereas an increased perception of risk in certain patient groups did not translate into poorer mental health.
A limitation of our study is the lack of a matched pre-pandemic control group. Furthermore, selection bias cannot be excluded. Because this is a paper-and-pencil survey, patients must have been on-site for a medical consultation with their treating rheumatologist to fill in the KICK-COVID questionnaires. Therefore, those who visited a doctor more frequently than others, for example because of a more active condition, more severe pain or functional limitations, may have been more likely to participate in KICK-COVID. On the other hand, it might have been those who were significantly impaired who did not show up for regular follow-up visits because they considered themselves to be more at risk for COVID-19 infection and its consequences and therefore avoided places such as physicians’ offices or hospitals/outpatient clinics to reduce the risk of infection there. In addition, it is possible that the willingness to answer the questions on the KICK-COVID study varied in the different patient groups, which could have led to biases. The fact that the distribution of diagnoses and the demographic, clinical, therapeutic and outcome parameters of the patients in our study cohort are largely comparable to the parameters of all patients enrolled in the NPRD during the analyzed study period is a strength of our study, as is the large number of patients. Furthermore, by combining clinical, demographic and treatment data from the NPRD with results from the KICK-COVID add-on module, a wide range of possible associations could be investigated.
Conclusions
Our data suggest that although a good third of adolescents reported significantly reduced general well-being, there does not appear to be increased mental health comorbidity in our cohort, since depressive and anxiety symptoms were reported at about the same level as in pre-pandemic studies of adolescents with rheumatic diseases. The perception of health risk due to SARS-CoV2 infection is not paralleled by an impairment of mental health and well-being in children and adolescents with rheumatic diseases, at least in the advanced pandemic situation in the second half of 2021. As before the pandemic, depressive symptoms as well as anxiety symptoms and well-being were associated with the perceived health situation and functioning related to the chronic disease, both in children and adolescents. This underlines the importance of patient-reported outcomes to uncover potential needs and provide targeted support services to improve both physical and mental health in the routine care of chronically ill children and adolescents.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Additional file 1: Supplementary Table 1: Demographic and clinical parameters, treatment and outcome of children and adolescents with systemic lupus erythematosus or juvenile dermatomyositis in the study sample. Supplementary Table 2: Demographic and clinical parameter, treatment and outcome of patients who participated in KICK-COVID and those included in the NPRD without participation in KICK-COVID. Supplementary Table 3: Association of outcome parameters (perceived risk, stress, loneliness, WHO-5, PHQ-9, GAD-7) with disease parameters, sex, treatment and patient-reported health status stratified by age group
Acknowledgements
We thank all patients and their parents, as well as all physicians and nurses who participated in the KICK-COVID study as part of the NPRD: Gerd Horneff, Sankt Augustin; Maria Haller, Gundelfingen; Catharina Schütz, Dresden; Philipp von Bismarck, Kiel; Michael Rühlmann, Göttingen; Markus Hufnagel, Freiburg; Betina Rogalski, Püttlingen; Annette Holl-Wieden, Würzburg; Tim Niehues, Krefeld; Kirsten Mönkemöller, Köln; Bernd-Ulrich Keck, Schwäbisch-Halle; Maria Faßhauer, Dresden; Benjamin Reinbeck, Neuss, vormals Mönchengladbach; Annette Jansson, München; Thomas Lutz, Heidelberg; Rainer Berendes, Landshut; Tilmann Kallinich, Berlin; Hermann Girschick, Berlin; Thomas Berger, Datteln; Georg Heubner, Dresden; Jan Maier, Leinfelden; Ales Janda, Ulm; Prasad Oommen, Düsseldorf; Georg Leipold, Regensburg; Jasmin Kümmerle-Deschner, Tübingen; Antje Nimtz-Talaska, Frankfurt/Oder; Christiane Reiser, Bregenz; Peggy Rühmer, Plauen; Ralf Seul, Witten; Tobias Krickau, Erlangen; Nils Onken, Lüneburg; Sonja Higgins, Pulheim, vormals Hürth; Regina Hühn, Halle; Nadja Hofmann, Bamburg; Fabian Speth, Hamburg; Christoph Rietschel, Frankfurt/M; Jürgen Quietzsch, Lichtenstein; Ute Derichs, Mainz; Jörn Lorenz Gröbel, Paderborn; Regina Borchers, Augsburg; Rahel Jähing, Borna. We would like to thank Nadine Grösch for her excellent data preparation and methodological support in the data analysis.
Abbreviations
- COVID-19
Corona Virus Disease 19
- PHR
Perception of Health Risk
- WHO
World Health Organization
- PHQ-9
Patient Health Questionnaire 9
- GAD-7
Generalized anxiety disorder 7
- NRS
Numeric Rating Scale
- IQR
Interquartile Range
- PRO
Patient Reported Outcomes
- DRKS
Deutsches Register für klinische Studien (German Clinical Trials Register)
- SARS-CoV-2
Severe acute respiratory syndrome corona virus type 2
- JIA
Juvenile idiopathic arthritis
- SLE
Systemic lupus erythematosus
- JDM
Juvenile idiopathic arthritis
- NPRD
National Pediatric Rheumatology Database
- ANA
Antinuclear antibodies
- sDMARD
Synthetic disease modifying antirheumatic drug
- bDMARD
Biologic disease modifying antirheumatic drug
- CHAQ
Childhood Health Assessment Questionnaire
- VOC
Variant of concern
- PGA
Patient’s global assessment of health status
- PhGA
Physician’s global assessment of disease activity
- CAPS
Childhood Arthritis Prospective Study
- PROMIS
Patient-Reported Outcome Measurement Information System
- cJADAS-10
Clinical Juvenile Arthritis Disease Activity Score
Author contributions
KM, CS, MN, JG, PW, RH, SL, CK developed the study concept and design. MN and NG were responsible for the administrative and technical support. The funding was obtained by KM, PW and RH. Data were acquired by DW, JPH, FD, RT, AH, FWH and KM. Data were analyzed and interpreted by NG, JK, CS, KM, MJP, JG and PW. CS and KM drafted the manuscript, all other authors revised it critically for important intellectual content, agreed to be accountable for all aspects of the work and approved the final version of the manuscript.
Funding
This study was conducted as part of the collaborative project “A prospective analysis of the long-term impact of the COVID-19 pandemic on the well-being and health care of children with a chronic high-risk disease and their families,” funded by the German Research Foundation. (Grant No: HO 3228/2 − 1; MI 2760/1–1; WA 1143/18 − 1).
The National Pediatric Rheumatology Database has been funded in the year 2021 by GSK and Novartis.
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The KICK-COVID study was approved by the ethics committee of the University of Potsdam (request number 62/2020) and conducted according the Declaration of Helsinki as well as the EU General Data Protection Regulation (GDPR). The NPRD was approved by the ethics committee of the Charité—Universitätsmedizin Berlin (EA1/044/07).
Children and adolescents with rheumatic diseases are included in the study after informed consent of the parents (for children under 8 years of age) or the children and adolescents themselves.
Consent for publication
Not applicable.
Competing interests
CS: none; NG: none; MJP: none; MN: none; JK: none; JG: none; DW: none; JPH: none; FD: speaker`s fees from Novartis and Pfizer; RT: none, AH: none; FWH: none, SL: none, CK: none; RWH: none, PW: none; KM: received honoraria from Amgen, Pfizer, Novartis and medac.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Racine N, McArthur BA, Cooke JE, Eirich R, Zhu J, Madigan S. Global prevalence of depressive and anxiety symptoms in children and adolescents during COVID-19: a Meta-analysis. JAMA Pediatr. 2021;175(11):1142–50. doi: 10.1001/jamapediatrics.2021.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinquart M, Shen Y. Depressive symptoms in children and adolescents with chronic physical illness: an updated meta-analysis. J Pediatr Psychol. 2011;36(4):375–84. doi: 10.1093/jpepsy/jsq104. [DOI] [PubMed] [Google Scholar]
- 3.Pinquart M, Shen Y. Behavior problems in children and adolescents with chronic physical illness: a meta-analysis. J Pediatr Psychol. 2011;36(9):1003–16. doi: 10.1093/jpepsy/jsr042. [DOI] [PubMed] [Google Scholar]
- 4.Bomba M, Meini A, Molinaro A, et al. Body experiences, emotional competence, and psychosocial functioning in juvenile idiopathic arthritis. Rheumatol Int. 2013;33(8):2045–52. doi: 10.1007/s00296-013-2685-4. [DOI] [PubMed] [Google Scholar]
- 5.Hanns L, Cordingley L, Galloway J, Norton S, Carvalho LA, Christie D, et al. Depressive symptoms, pain and disability for adolescent patients with juvenile idiopathic arthritis: results from the Childhood Arthritis prospective study. Rheumatology (Oxford) 2018;57(8):1381–9. doi: 10.1093/rheumatology/key088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warschburger P, Kamrath C, Lanzinger S, Sengler C, Wiegand S, Goldel JM, et al. A prospective analysis of the long-term impact of the COVID-19 pandemic on well-being and health care among children with a chronic condition and their families: a study protocol of the KICK-COVID study. BMC Pediatr. 2023;23(1):130. doi: 10.1186/s12887-023-03912-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng J, Mehta P, Khatun A, Wu WH, Hennelly L, Doolan G, et al. Self-perceived disease activity was the strongest predictor of COVID-19 pandemic-related concerns in young people with autoimmune rheumatic diseases, irrespective of their gender, with females reporting higher concerns. Rheumatol Adv Pract. 2022;6(2):rkac031. doi: 10.1093/rap/rkac031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durcan G, Barut K, Haslak F, Doktur H, Yildiz M, Adrovic A, et al. Psychosocial and clinical effects of the COVID-19 pandemic in patients with childhood rheumatic diseases and their parents. Rheumatol Int. 2021;41(3):575–83. doi: 10.1007/s00296-021-04790-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warschburger P, Petersen AC, von Rezori RE, Buchallik F, Baumeister H, Holl RW, et al. A prospective investigation of developmental trajectories of psychosocial adjustment in adolescents facing a chronic condition - study protocol of an observational, multi-center study. BMC Pediatr. 2021;21(1):404. doi: 10.1186/s12887-021-02869-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bohn B, Karges B, Vogel C, Otto KP, Marg W, Hofer SE, et al. 20 years of Pediatric Benchmarking in Germany and Austria: age-dependent analysis of Longitudinal Follow-Up in 63,967 children and adolescents with type 1 diabetes. PLoS ONE. 2016;11(8):e0160971. doi: 10.1371/journal.pone.0160971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reinehr T, Wabitsch M, Andler W, Beyer P, Bottner A, Chen-Stute A, et al. Medical care of obese children and adolescents. APV: a standardised multicentre documentation derived to study initial presentation and cardiovascular risk factors in patients transferred to specialised treatment institutions. Eur J Pediatrics. 2004;163(6):308–12. doi: 10.1007/s00431-004-1421-1. [DOI] [PubMed] [Google Scholar]
- 12.Minden K, Niewerth M, Listing J, Zink A, Rheumatologists TGSGP. Health care provision in pediatric rheumatology in Germany - National Rheumatologic Database. J Rheumatol. 2002;29(3):622–8. [PubMed] [Google Scholar]
- 13.I Foeldvari 1, The German version of the Childhood Health Assessment Questionnaire (CHAQ) and the Child Health Questionnaire (CHQ), Ruperto N, Dressler F, Häfner R, Küster RM, Michels H, Minden K, Schauer-Petrowskaja C, Bullinger M, Landgraf JM. H I Huppertz; Paediatric Rheumatology International Trials Organisation. Clin Exp Rheumatol 2001 Jul-Aug;19(4 Suppl 23):S71-5. [PubMed]
- 14.Schilling J, Buda S, Tolksdorf K. Zweite Aktualisierung Der „Retrospektiven Phaseneinteilung Der COVID-19- pandemie in Deutschland. Epidemiologisches Bull. 2022(10):3–5.
- 15.Brähler E, Mühlan H, Albani C, Schmidt S. Teststatistische Prüfung und Normierung Der Deutschen Versionen Des EUROHIS-QOL Lebensqualität-Index Und Des WHO-5 wohlbefindens-Index. Diagnostica. 2007;53(2):83–96. doi: 10.1026/0012-1924.53.2.83. [DOI] [Google Scholar]
- 16.WHO. Info Package: Mastering Depression in Primary Care. World Health Organization, Regional Office for Europe, Psychiatric Research Unit, Frederiksborg General Hospital; 1998.
- 17.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richardson LP, McCauley E, Grossman DC, McCarty CA, Richards J, Russo JE, et al. Evaluation of the Patient Health Questionnaire-9 item for detecting major depression among adolescents. Pediatrics. 2010;126(6):1117–23. doi: 10.1542/peds.2010-0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erbe D, Eichert H-C, Rietz C, Ebert D. Interformat reliability of the patient health questionnaire: validation of the computerized version of the PHQ-9. Internet Interv. 2016;5:1–4. doi: 10.1016/j.invent.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–7. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 21.Mossman SA, Luft MJ, Schroeder HK, Varney ST, Fleck DE, Barzman DH, et al. The generalized anxiety disorder 7-item scale in adolescents with generalized anxiety disorder: Signal detection and validation. Ann Clin Psychiatry. 2017;29(4):227–A234. [PMC free article] [PubMed] [Google Scholar]
- 22.Dear, Titov, Sunderland, McMillan, Anderson, Lorian, et al. Psychometric comparison of the generalized anxiety disorder scale-7 and the Penn State worry questionnaire for measuring response during treatment of generalised anxiety disorder. Cogn Behav Ther. 2011;40:216–27. doi: 10.1080/16506073.2011.582138. [DOI] [PubMed] [Google Scholar]
- 23.Hinz A, Klein AM, Brähler E, Glaesmer H, Luck T, Riedel-Heller SG, Wirkner K, Hilbert A. Psychometric evaluation of the generalized anxiety disorder screener GAD-7, based on a large German general population sample. J Affect Disord. 2017;210:338–44. doi: 10.1016/j.jad.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Resource for SARS-CoV2 incidence. https://de.statista.com/statistik/daten/studie/1192085/umfrage/coronainfektionen-covid-19-in-den-letzten-sieben-tagen-in-deutschland
- 25.Resource for measure index. https://www.corona-daten-deutschland.de/dataset/6297e9d4-b86b-4b82-b1c8-651d8b144932/resource/027e7b91-9fdc-4a82-b6d0-f877a74a543/download/dokumentation_massnahmenindex.pdf
- 26.Cohen J. Statistical Power Analysis for the behavioral sciences. New York, NY: Routledge Academic; 1988.
- 27.Sengler C, Eulert S, Minden K, Niewerth M, Horneff G, Kuemmerle-Deschner J et al. Clinical manifestations and outcome of SARS-CoV-2 infections in children and adolescents with rheumatic musculoskeletal diseases: data from the National Pediatric Rheumatology Database in Germany. RMD Open. 2021;7(2). [DOI] [PMC free article] [PubMed]
- 28.Regierer AC, Hasseli R, Schafer M, Hoyer BF, Krause A, Lorenz HM et al. TNFi is associated with positive outcome, but JAKi and Rituximab are associated with negative outcome of SARS-CoV-2 infection in patients with RMD. RMD Open. 2021;7(3). [DOI] [PMC free article] [PubMed]
- 29.Kamrath C, Tittel SR, Buchal G, Brämswig S, Preiss E, Göldel JM, Wiegand S, Minden K, Warschburger P, Stahl-Pehe A, Holl RW, Lanzinger S. Psychosocial Burden During the COVID-19 Pandemic in Adolescents With Type 1 Diabetes in Germany and Its Association With Metabolic Control. J Adolesc Health. 2024 Feb 5:S1054-139X(23)01000-5. 10.1016/j.jadohealth.2023.12.004 [DOI] [PubMed]
- 30.Jansson S, Malham M, Wewer V, Rask CU. Psychiatric comorbidity in childhood onset immune-mediated diseases-A systematic review and meta-analysis. Acta Paediatr. 2022;111(3):490–9. doi: 10.1111/apa.16246. [DOI] [PubMed] [Google Scholar]
- 31.Fair DC, Nocton JJ, Panepinto JA, Yan K, Zhang J, Rodriguez M, Olson J. Anxiety and depressive symptoms in Juvenile Idiopathic Arthritis Correlate with Pain and stress using PROMIS measures. J Rheumatol. 2022;49(1):74–80. doi: 10.3899/jrheum.210101. [DOI] [PubMed] [Google Scholar]
- 32.Nolen-Hoeksema S, Girgus JS. The emergence of gender differences in depression during adolescence. Psychol Bull. 1994;115:424–43. doi: 10.1037/0033-2909.115.3.424. [DOI] [PubMed] [Google Scholar]
- 33.Campbell O, Bann D, Patalay P. (2021) The gender gap in adolescent mental health: a cross-national investigation of 566,829 adolescents across 73 countries. SSM-Population Health:100742. [DOI] [PMC free article] [PubMed]
- 34.Yoon Y, Eisenstadt M, Lere ya ST, et al. Gender difference in the change of adolescents’ mental health and subjective wellbeing trajectories. Eur Child Adolesc Psychiatry. 2023;32:1569–78. doi: 10.1007/s00787-022-01961-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balazs J, Miklosi M, Kereszteny A, Hoven CW, Carli V, Wasserman C et al. Comorbidity of physical and anxiety symptoms in adolescent: functional impairment, self-rated Health and Subjective Well-Being. Int J Environ Res Public Health. 2018;15(8). [DOI] [PMC free article] [PubMed]
- 36.Hanns L, Radziszewska A, Suffield L, Josephs F, Chaplin H, Peckham H, et al. Association of anxiety with Pain and disability but not with increased measures of inflammation in adolescent patients with juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2020;72(9):1266–74. doi: 10.1002/acr.24006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Table 1: Demographic and clinical parameters, treatment and outcome of children and adolescents with systemic lupus erythematosus or juvenile dermatomyositis in the study sample. Supplementary Table 2: Demographic and clinical parameter, treatment and outcome of patients who participated in KICK-COVID and those included in the NPRD without participation in KICK-COVID. Supplementary Table 3: Association of outcome parameters (perceived risk, stress, loneliness, WHO-5, PHQ-9, GAD-7) with disease parameters, sex, treatment and patient-reported health status stratified by age group
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.