Abstract
Rapid ascent to high altitude imposes an acute hypoxic and acid-base challenge, with ventilatory and renal acclimatization countering these perturbations. Specifically, ventilatory acclimatization improves oxygenation, but with concomitant hypocapnia and respiratory alkalosis. A compensatory, renally mediated relative metabolic acidosis follows via bicarbonate elimination, normalizing arterial pH(a). The time course and magnitude of these integrated acclimatization processes are highly variable between individuals. Using a previously developed metric of renal reactivity (RR), indexing the change in arterial bicarbonate concentration (Δ[HCO3−]a; renal response) over the change in arterial pressure of CO2 (; renal stimulus), we aimed to characterize changes in RR magnitude following rapid ascent and residence at altitude. Resident lowlanders (n = 16) were tested at 1,045 m (day [D]0) prior to ascent, on D2 within 24 h of arrival, and D9 during residence at 3,800 m. Radial artery blood draws were obtained to measure acid-base variables: , [HCO3−]a, and pHa. Compared with D0, and [HCO3−]a were lower on D2 (P < 0.01) and D9 (P < 0.01), whereas significant changes in pHa (P = 0.072) and RR (P = 0.056) were not detected. As pHa appeared fully compensated on D2 and RR did not increase significantly from D2 to D9, these data demonstrate renal acid-base compensation within 24 h at moderate steady-state altitude. Moreover, RR was strongly and inversely correlated with ΔpHa on D2 and D9 (r ≤ −0.95; P < 0.0001), suggesting that a high-gain renal response better protects pHa. Our study highlights the differential time course, magnitude, and variability of integrated ventilatory and renal acid-base acclimatization following rapid ascent and residence at high altitude.
NEW & NOTEWORTHY We assessed the time course, magnitude, and variability of integrated ventilatory and renal acid-base acclimatization with rapid ascent and residence at 3,800 m. Despite reductions in upon ascent, pHa was normalized within 24 h of arrival at 3,800 m through renal compensation (i.e., bicarbonate elimination). Renal reactivity (RR) was unchanged between days 2 and 9, suggesting a lack of plasticity at moderate steady-state altitude. RR was strongly correlated with ΔpHa, suggesting that a high-gain renal response better protects pHa.
Keywords: acid-base, high altitude, hypoxia, renal compensation, ventilatory acclimatization
INTRODUCTION
Sojourners to high altitude (≥2,500 m) are exposed to reductions in atmospheric pressure, leading to hypobaric hypoxia and resultant hypoxemia. Initial high altitude exposure reduces arterial partial pressure of oxygen , arterial blood oxygen saturation , and arterial oxygen content , triggering a cascade of integrated physiological responses. Acute responses include the hypoxic ventilatory response (HVR), whereby increases in alveolar ventilation increases the amount of oxygen carried in the blood (1) but with concomitant respiratory alkalosis (2, 3). With chronic hypoxic exposure to high altitude, acclimatization processes are mounted in respiratory, hematological, and renal systems, improving oxygenation and countering hyperventilation-induced acid-base imbalances (1, 4–6).
Peripheral chemoreceptors (i.e., carotid bodies) initially detect hypoxemia and elicit the HVR (7, 8). The HVR increases resting ventilation in attempt to partially restore while simultaneously decreasing the arterial partial pressure of carbon dioxide causing hypocapnia and respiratory alkalosis. The resultant hypocapnia dampens both central and peripheral respiratory chemoreceptor activation, blunting ventilatory drive (e.g., 9–11). Thus, for the HVR to successfully increase oxygenation, the inhibitory effects of concomitant hypocapnia must be countered. This is accomplished by ventilatory acclimatization to chronic hypoxia (VAH; 5, 12), which increases sensitivity of the HVR through carotid body type I glomus cell hyperplasia within the first 3 days of sustained hypoxia (13), and subsequent increases in carotid body sensitivity to hypoxia (14). Further, plasticity is observed within the central nervous system, through integration of afferent signaling from the carotid bodies (15). The net result is an increase in ventilatory motor output for a given hypoxic stimulus (5, 6, 15, 16). VAH progressively increases ventilation and , which partially restores oxygenation but concurrently amplifies the initial hypocapnia and respiratory alkalosis (7, 17).
Previously, we developed a metric of steady-state chemoreflex drive (SS-CD) in contexts of acute hypoxia in a laboratory setting (18) and during incremental ascent to high altitude (16, 19). The SS-CD metric takes into account the integrated steady-state ventilatory response to prevailing respiratory chemostimuli at rest and likely captures integrated ventilatory acclimatization through the contributions of both central and peripheral chemoreceptors (16, 19). However, this SS-CD metric has yet to be characterized during rapid ascent to and residence at a single altitude over time.
With sustained exposure to hypoxia, the HVR-induced hypocapnia and respiratory alkalosis causes an acute acid-base dysregulation (e.g., 2). Two renal compensatory mechanisms arise to counteract this dysregulation: 1) minimizing renal acid ([H+]) excretion (by reducing secretion) and 2) increasing bicarbonate (HCO3−) excretion into the urine (by reducing reabsorption; 2, 20–25). These two interrelated renal tubular cellular mechanisms return arterial blood pH (pHa) to homeostatic values (pHa ~7.4) through compensatory relative metabolic acidosis (3, 25). However, this bicarbonate diuresis increases central chemoreceptor sensitivity to a given change in (e.g., breath hold) by reducing buffering capacity within the respiratory control centers where the central chemoreceptors reside (5, 20, 26). In addition, the relative metabolic acidosis sensitizes central respiratory chemoreceptors and unbrakes peripheral chemoreceptor blunting resulting from hypocapnia and respiratory alkalosis, likely contributing to ventilatory acclimatization in addition to carotid body plasticity.
To describe the gain of the renal response, we previously introduced renal reactivity (RR), an index of the relative change in arterial bicarbonate concentration (D[HCO3−]a; i.e., renal response) over the relative change in arterial pressure of CO2 (; i.e., renal stimulus), during incremental ascent to high altitude (3). We showed that RR increased with incremental ascent from 3,440 m to 3,820 m from days 3 to 5 and plateaued with further ascent (4,240 m on day 7 and 5,160 m on day 10). We also showed that at all altitudes, RR was significantly and inversely correlated with relative changes in pH (ΔpHa), with an increase in correlative magnitude (i.e., larger r value) with time spent at and further ascent to altitude, suggesting those with greater renal responses to sustained hypocapnia and acute respiratory alkalosis were better able to protect pHa.
The time course and magnitude of the integrated respiratory and renal acclimatization phenotypes are variable between individuals, particularly following rapid ascent. We aimed to assess the integration between respiratory and renal acclimatization during rapid ascent and residence at high altitude (3,800 m) over 9 days. We hypothesized that 1) steady-state chemoreflex drive would increase following ascent with ventilatory acclimatization, 2) with subsequent renal compensation, RR magnitude would increase with duration at altitude, and 3) RR would be inversely correlated with relative changes in pH (ΔpHa), improving with time spent at a single altitude.
MATERIAL AND METHODS
Ethics and Participant Recruitment
This study abided by the Canadian Government Tri-Council policy on research ethics with human participants (TCPS2), and it adhered to the standards set by the latest revision of the Declaration of Helsinki, except for registration in a database. Ethical approval was received in advance through University of Calgary Conjoint Human Research Ethics Board (Protocol REB18-0374) and Mount Royal University Human Research Ethics Board (Protocol 101879), with subsequent harmonization with the University of British Columbia Clinical Research Ethics Board Protocol (Protocol H19-01734), the North Texas Regional Institutional Research Ethics Board (Protocol 2019-110), and the University of Alberta Research Ethics Board (Protocol Pro00109336).
Healthy participants were recruited on a voluntary basis to undergo cardiorespiratory and arterial blood gas measurements during a research expedition to the Barcroft Research Station (3,800 m) in the Sierra Nevada mountains in California in August 2019. Participants were recruited via verbal communication and provided voluntary, informed, verbal, and written consent before participation in the study.
All members of the expedition consulted with their family physicians for a medical check before participating in this expedition. We prescreened and recruited 16 healthy participants for inclusion in the present study, with no self-reported medical history of cardiopulmonary, neurological, or metabolic disease. Participants were excluded if they had a body mass index >35 kg/m2, had a history of smoking, or were taking prescription medication other than hormonal birth control. Inclusion criteria included adult participants between the ages of 18–60 years who planned to stay at the research station for the entire 10-day period. Each participant completed a prescreening questionnaire and consent form for documentation.
The participant recruitment, specific study design, research questions, and data collection were planned a priori. All participants were native lowlanders who had not been exposed to high altitude (>3,000 m) for at least 1 yr before the study. No participants included in the study took carbonic anhydrase inhibitors (e.g., acetazolamide) or any corticosteroids for AMS prevention or treatment at any point during this study.
Study Protocol and Ascent Profile
Baseline measurements were conducted at 1,045 m (Calgary, Canada; day 0; D0) over a week. Participants were then flown to, and spent one night (less than 12 h) at 610 m (Las Vegas, Nevada) before driving up to 3,800 m (Barcroft Research Station, California) the following day over 5–6 h for a study on acute ascent and sustained altitude exposure. Participants resided at 3,800 m for 10 days and nights to study acclimatization to altitude. Early exposure and late exposure arterial blood gas measurements were obtained on days 2 (D2; within 24 h of arrival), and day 9 (D9), respectively.
Measurements
Daily ancillary cardiorespiratory measures.
Every morning at high altitude and once at low altitude between 06:00–09:00 local time, fasted cardiorespiratory measures were taken to characterize steady-state physiological responses. Participants self-reported their acute mountain sickness (AMS) scores using the standard Lake Louise Questionnaire (27). Hemoglobin concentration [Hb] was obtained via finger capillary blood sample using sterile lancets (AccuChek, Softclix) with standard practice and universal precautions, measured via hemoglobinometer (Hemocue Hemoglobin System, Hb201+ with microcuvettes; ängelholm, Sweden). All cardiorespiratory measures were obtained at rest in the supine position with eyes closed and white noise played through headphones to limit external distraction. Three brachial blood pressure measurements were obtained via an automated blood pressure monitor (model BP786n; Omron, San Ramon, CA) then averaged. A peripheral pulse oximeter (Masimo SET Rad-5, Danderyd, Sweden) was placed on the participant’s left middle finger for measurement of peripheral oxygen saturation (SpO2; %) and heart rate (HR; beats/min). Respiratory measures were obtained using a personal mouthpiece, bacteriological filter, and nose clip. We instrumented participants with a portable capnograph (EMMA, Masimo, Danderyd, Sweden) for measurement of end-tidal PCO2 (; mmHg) and breathing frequency (fB; breaths/min), and a respirometer (nSpire Haoscale, Colorado) for ventilation (; L/min). Once instrumentation was complete, the participants laid down with their eyes closed and after approximately 3 min, we recorded and archived their values. Steady-state chemoreflex drive (SS-CD) was calculated as previously described (16, 18, 19). First, we calculated a steady-state stimulus index (SI; /SpO2; mmHg/%), which represents the prevailing central and peripheral chemoreceptor stimulus. We then divided by the SI to calculate SS-CD each day to characterize ventilatory acclimatization over time at 3,800 m.
Arterial blood gas and electrolytes.
Arterial blood samples were obtained in Calgary (1,045 m) and on days 2 and 9 of the expedition at 3,800 m during the day (08:00–15:00). Arterial blood samples were acquired percutaneously from the radial artery using a preheparinized, self-filling arterial blood syringe (PICO50, Radiometer) while the participants were resting in the supine position. Standard procedures involved topical sterilization and local anesthesia (i.e., Emla topical cream; 2.5% lidocaine/2.5% prilocaine cream) with universal precautions, including a modified Allen’s test to ensure redundant circulation of the hand. Samples were then immediately analyzed using a cartridge-based system for measurement/calculation of (mmHg), (%), (mmHg), [HCO3−]a (mM), pHa, hematocrit (%), hemoglobin concentration (g/L), and [creatinine] (μM), using a portable analyzer and cartridges (Abbottt iSTAT, CG4+ and CHEM8+ cartridges; Abbott, Mississauga, Ontario, Canada). All samples were subject to thermal correction and atmospheric pressure calibration.
Data analysis.
Atmospheric pressure for each altitude was calculated from https://baillielab.net/critical_care/air_pressure/, and partial pressure of inspired oxygen () was calculated as:
| (1) |
where 47 mmHg is water vapor pressure at normal body temperature (37°C) and 0.21 is the fraction of inspired oxygen.
Strong ion difference (SID; mEq/L) was calculated according to the traditional Stewart approach (28).
| (2) |
Serum osmolality (mOsmol/kgH2O) was calculated from the recommended formula in (29).
| (3) |
Arterial oxygen content () was calculated as:
| (4) |
where [Hb] = hemoglobin (g/dL); = arterial oxygen saturation (%; or SpO2); and = partial pressure of arterial oxygen (mmHg). 1.36 is the binding capacity of oxygen to hemoglobin, and 0.003 is the fraction of free oxygen dissolved in blood (at 37°C). In cases where we did not have (Table 1), was estimated without it (using only [Hb] and SpO2), given the negligible contribution of when [Hb] is normal or high.
Table 1.
Daily ancillary measures of cardiorespiratory and hematological variables before and during rapid ascent to and residence: days (D)0 (1,045 m) and D1–D9 (at 3,800 m)
| Variable | Calgary | Barcroft Lab | |||||||
|---|---|---|---|---|---|---|---|---|---|
| (D0) | (D2) | (D3) | (D4) | (D5) | (D6) | (D7) | (D8) | (D9) | |
| Altitude, m | 1,045 | 3,800 | |||||||
| , mmHg | 665 | 487 | |||||||
| , mmHg | 130 | 92 | |||||||
| Cardiorespiratory Variables | |||||||||
| HR, min−1 | 65.5 ± 12.4 | 75.5 ± 9.5* | 69.1 ± 11.0 | 72.3 ± 12.0 | 72.3 ± 12.9 | 69.5 ± 11.1 | 70.3 ± 9.0 | 69.1 ± 11.7 | 70.9 ± 9.2 |
| MAP, mmHg | 90.3 ± 11.9 | 89.7 ± 12.8 | 87.5 ± 11.5 | 84.2 ± 11.5* | 86.3 ± 11.2 | 86.3 ± 9.8 | 84.4 ± 8.9* | 84.5 ± 9.4* | 87.0 ± 11.0 |
| , L/min | 8.6 ± 2.8 | 11.0 ± 3.8 | 10.4 ± 1.9 | 10.0 ± 2.4 | 9.5 ± 1.4 | 9.6 ± 1.9 | 10.0 ± 1.6 | 9.9 ± 2.8 | 10.1 ± 1.9 |
| , mmHg | 36.9 ± 3.1 | 30.8 ± 4.1* | 30.3 ± 2.9* | 29.3 ± 3.6* | 28.9 ± 1.6* | 28.3 ± 2.7* | 28.8 ± 2.7* | 26.3 ± 3.0*‡ | 28.2 ± 2.5*‡ |
| , % | 97.5 ± 1.6 | 87.5 ± 4.2* | 88.9 ± 3.0* | 88.3 ± 2.2* | 87.9 ± 2.9* | 89.6 ± 2.3* | 89.2 ± 2.7* | 89.4 ± 2.2* | 90.6 ± 2.9* |
| SS-CD, a.u. | 22.8 ± 7.2 | 31.9 ± 11.9* | 30.5 ± 5.7* | 30.4 ± 8.2* | 28.8 ± 4.2* | 30.8 ± 8.6* | 31.3 ± 5.3* | 34.3 ± 11.9* | 32.8 ± 7.7* |
| [Hb], g/L | 149.9 ± 12.2 | 153.0 ± 11.7 | 151.2 ± 10.5a | 154.1 ± 15.4 | 162.2 ± 12.6* | 168.5 ± 15.8* | 166.5 ± 13.7* | 166.4 ± 10.3* | 162.7 ± 14.8* |
| , mg/dL | 19.9 ± 1.5 | 18.2 ± 1.4* | 18.3 ± 1.4*a | 18.5 ± 2.0* | 19.4 ± 1.7‡ | 20.5 ± 1.9 | 20.2 ± 1.7 | 20.2 ± 1.2 | 20.0 ± 1.6 |
| AMS (Median, [Range]) | 0 [0,0] | 1 [0,8]* | 1 [0,4]a | 0 [0,2]‡a | 0 [0,1] | 0 [0,2] | 0 [0,2] | 0 [0,2] | 0 [0,2]b |
AMS, acute mountain sickness scores; , arterial oxygen content (calculated using SpO2 and without );[Hb], concentration of hemoglobin; HR, heart rate; MAP, mean arterial pressure; , partial pressure of arterial oxygen; PATM, atmospheric pressure; , partial pressure of end-tidal carbon dioxide; , partial pressure of inspired oxygen; SpO2, peripheral oxygen saturation; SS-CD, steady-state chemoreflex drive; , expiratory ventilation.
Significant difference from baseline (1,045 m), P < 0.05.
Significant difference from previous day, P < 0.05. Unless otherwise stated, n = 15.
n = 14.
n = 13.
Relative delta values were calculated with respect to baseline (1,045 m) using the following equation:
| (5) |
where x represents any variable.
RR was calculated for both nights at altitude (D2 and D9) following the formula reported in (3):
| (6) |
where is arterial bicarbonate (mM; i.e., renal response) and (mmHg; i.e., renal stimulus) is partial pressure of arterial carbon dioxide.
Statistical analysis.
All continuous data are represented as mean ± SD in table format unless otherwise stated. Data were assessed for normality and variance using the Shapiro–Wilk and the Brown–Forsythe test. One-factor repeated measures ANOVAs were used to assess changes in daily ancillary cardiorespiratory and hematological variables, and arterial blood oxygenation, acid-base and electrolyte metrics. Where significant F-ratios were detected, Student–Newman–Keuls post hoc tests were used for multiple pair-wise comparisons between testing days. Student’s two-tailed t tests were used to evaluate the difference between delta changes from baseline on days 2 and 9 for RR and delta pHa. A Pearson product moment correlation was used to assess RR against the change in pHa from baseline. A Cohen’s d statistic was used to calculate effect size for the t test comparing RR between D2 and D9. In all cases, statistical significance was assumed at P < 0.05 (SigmaPlot v10, Systat Software, Inc., San Jose, CA).
RESULTS
In the final analysis, we included 16 participants where we had repeated measures on all metrics (5 female, 11 male, BMI = 26.0 ± 3.8 kg/m2; Age = 31.3 ± 11.7 yr).
Daily cardiorespiratory measures and AMS scores from D0 to D9 are presented in Table 1. In addition, comprehensive arterial blood gas and electrolyte values from D0, D2, and D9 are presented in Table 2.
Table 2.
Arterial blood gas and electrolyte data during rapid ascent to and residence at 3,800 m on days (D)0, (D)2, and (D)9
| Variable, Means ± SD | Calgary (D0) | Barcroft Lab (D2) | Barcroft Lab (D9) |
|---|---|---|---|
| Altitude, m | 1,045 | 3,800 | |
| , mmHg | 665 | 487 | |
| , mmHg | 130 | 92 | |
| Oxygenation and Acid-Base/Electrolyte Variables | |||
| , mmHg | 80.8 ± 8.8 | 50.5 ± 5.2* | 53.8 ± 4.1*‡ |
| , % | 95.8 ± 1.2 | 85.8 ± 3.8* | 88.3 ± 1.9*‡ |
| [Hb], g/L | 148.4 ± 12.0 | 149.1 ± 9.4 | 158.3 ± 9.8*‡ |
| Hct, % | 43.7 ± 3.5 | 43.9 ± 2.8 | 46.6 ± 2.9*‡ |
| , mL/dL | 19.6 ± 1.5 | 17.5 ± 1.3* | 19.2 ± 1.4‡ |
| , mmHg | 38.4 ± 3.0 | 34.3 ± 2.8* | 30.5 ± 1.8*‡ |
| , mM | 24.4 ± 1.8 | 22.5 ± 2.2* | 19.8 ± 1.4*‡ |
| pHa | 7.412 ± 0.014 | 7.424 ± 0.017 | 7.420 ± 0.013 |
| RR, | 0.46 ± 0.28 | 0.59 ± 0.14 | |
| SID, mEq/L | 41.0 ± 2.2a | 39.4 ± 1.7* | 37.5 ± 1.5*‡ |
| Base Excess, mM | −0.13 ± 1.8 | −1.8 ±2.5* | −4.7 ± 1.5*‡ |
| Anion Gap, mM | 15.8 ± 1.8 | 16.3 ± 1.3 | 16.5 ± 1.1 |
| Osmolality, mOsmol/kgH2O | 283.2 ± 3.2 | 283.8 ± 1.8 | 282.3 ± 2.5 |
| Lactate, mM | 0.97 ± 0.5a | 0.98 ± 0.5 | 0.74 ± 0.3 |
| Creatinine, μM | 73.3 ± 12.4 | 77.3 ± 12.4 | 81.6 ± 14.3* |
, arterial oxygen content; [Hb], concentration of hemoglobin; , concentration of arterial bicarbonate; Hct, hematocrit; , partial pressure of arterial carbon dioxide; , partial pressure of arterial oxygen; PATM, atmospheric pressure; pHa, arterial pH; , partial pressure of inspired oxygen; RR, renal reactivity; , arterial oxygen saturation; SID, strong ion difference.
Significant difference from baseline (1,045 m), P < 0.05.
Significant difference from previous day, P < 0.05. Unless otherwise stated, n = 16.
n = 15.
Figure 1 illustrates metrics of oxygenation with rapid ascent (D2) and residence (D9) at altitude. and were lower on D2 and D9 compared with D0 (P < 0.01; Fig. 1, A and B) but were higher on D9 than D2 (P < 0.01; Fig. 1B). [Hb] was greater on D9 compared with D0 and D2 (P < 0.01; Fig. 1C). decreased from D0 to D2 (P < 0.01) but then was partially corrected from D2 to D9 (P < 0.01) such that D0 and D9 were not different (Fig. 1D).
Figure 1.
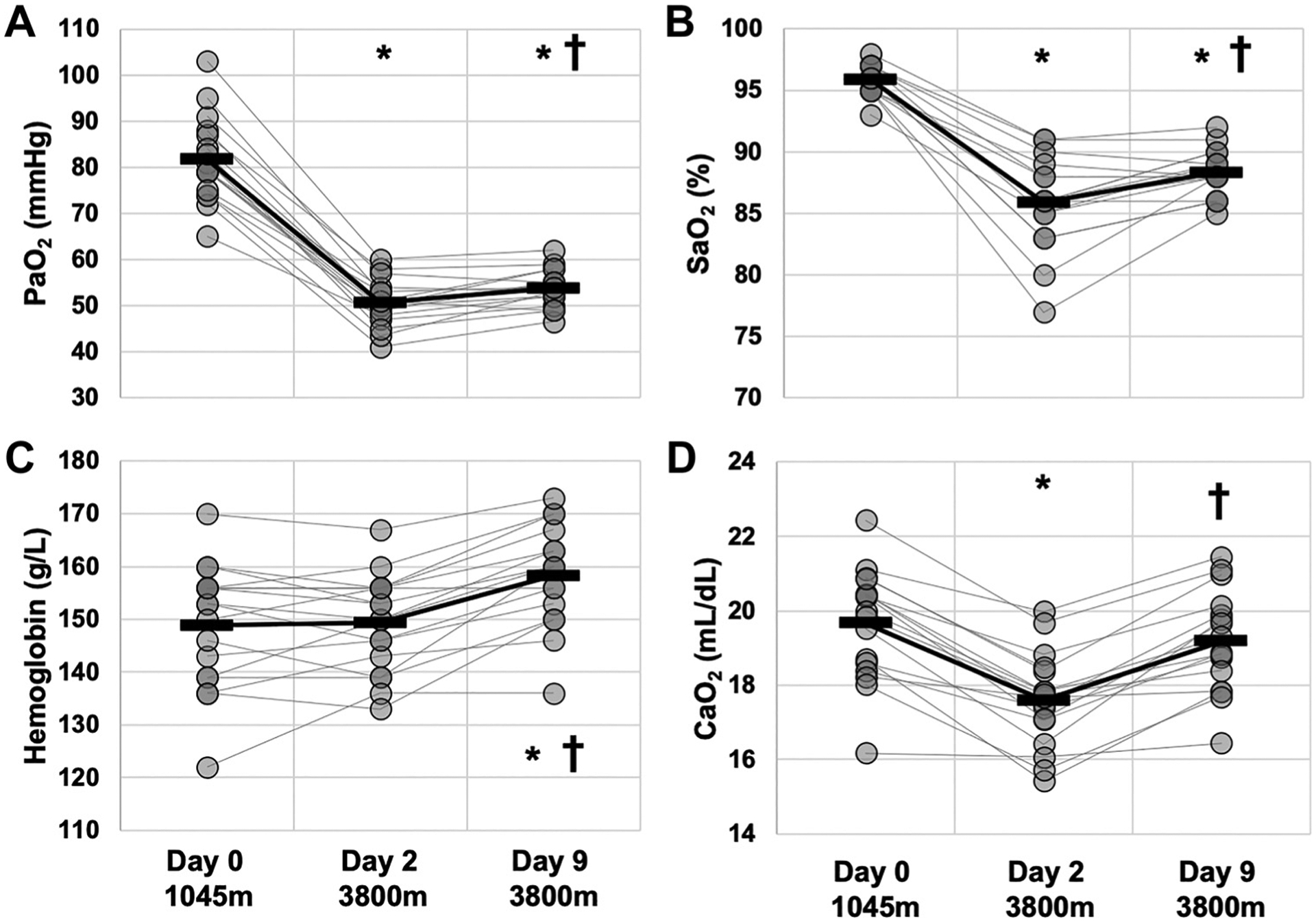
Oxygenation during rapid ascent and residence at 3,800 m. Data were obtained via arterial blood gas/electrolyte measures at 1,045 m (day 0), prior to ascent, and at 3,800 m on days 2 (within 24 h of arrival by rapid ascent) and 9. A: , pressure of arterial oxygen. B: , arterial oxygen saturation. C: [Hb], hemoglobin concentration D: , arterial oxygen content. *Different than day 0. †Different than previous day. Presented as means ± SD. n = 16.
Figure 2 illustrates metrics of acid-base homeostasis with rapid ascent (D2) and residence (D9) at altitude. and [HCO3−]a were lower on D2 compared with D0 (P < 0.01), and lower still on D9 (P < 0.01; Fig. 2, A and B). pHa was not different on D2 or D9 from D0 (P = 0.072; Fig. 2C).
Figure 2.
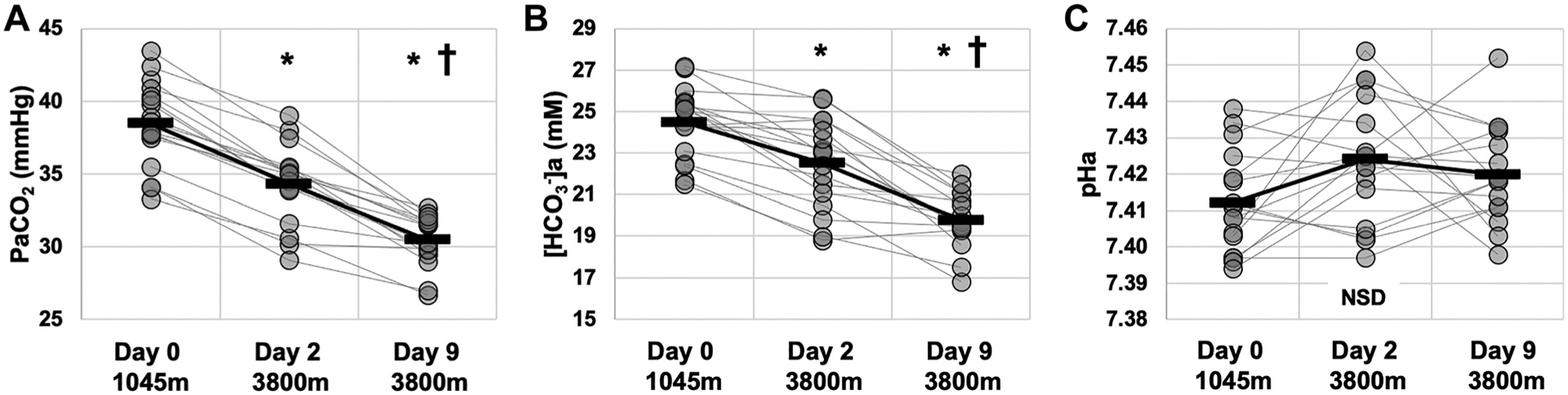
Carbon dioxide and acid-base variables during rapid ascent to and residence at 3,800 m. Data were obtained via arterial blood gas/electrolyte measures at 1,045 m (day 0), prior to ascent, and at 3,800 m on days 2 (within 24 h of arrival by rapid ascent) and 9. partial pressure of arterial carbon dioxide. a, arterial bicarbonate concentration. C: pHa, arterial pH. *Different than day 0. †Different than previous day. Presented as means ± SD. n = 16. NSD, nonsignificant difference.
Figure 3 illustrates renal reactivity (RR) and the change (Δ) in pHa on D2 and D9. RR was not significantly increased from D2 to D9 (P = 0.056; effect size 0.61; Fig. 3A). ΔpHa was also unchanged between D2 and D9 (P = 0.4; Fig. 3B). The magnitude of RR was negatively correlated with ΔpHa on D2 (r = −0.95, P < 0.0001; Fig. 3C) and D9 (r = −0.98, P < 0.0001; Fig. 3D).
Figure 3.
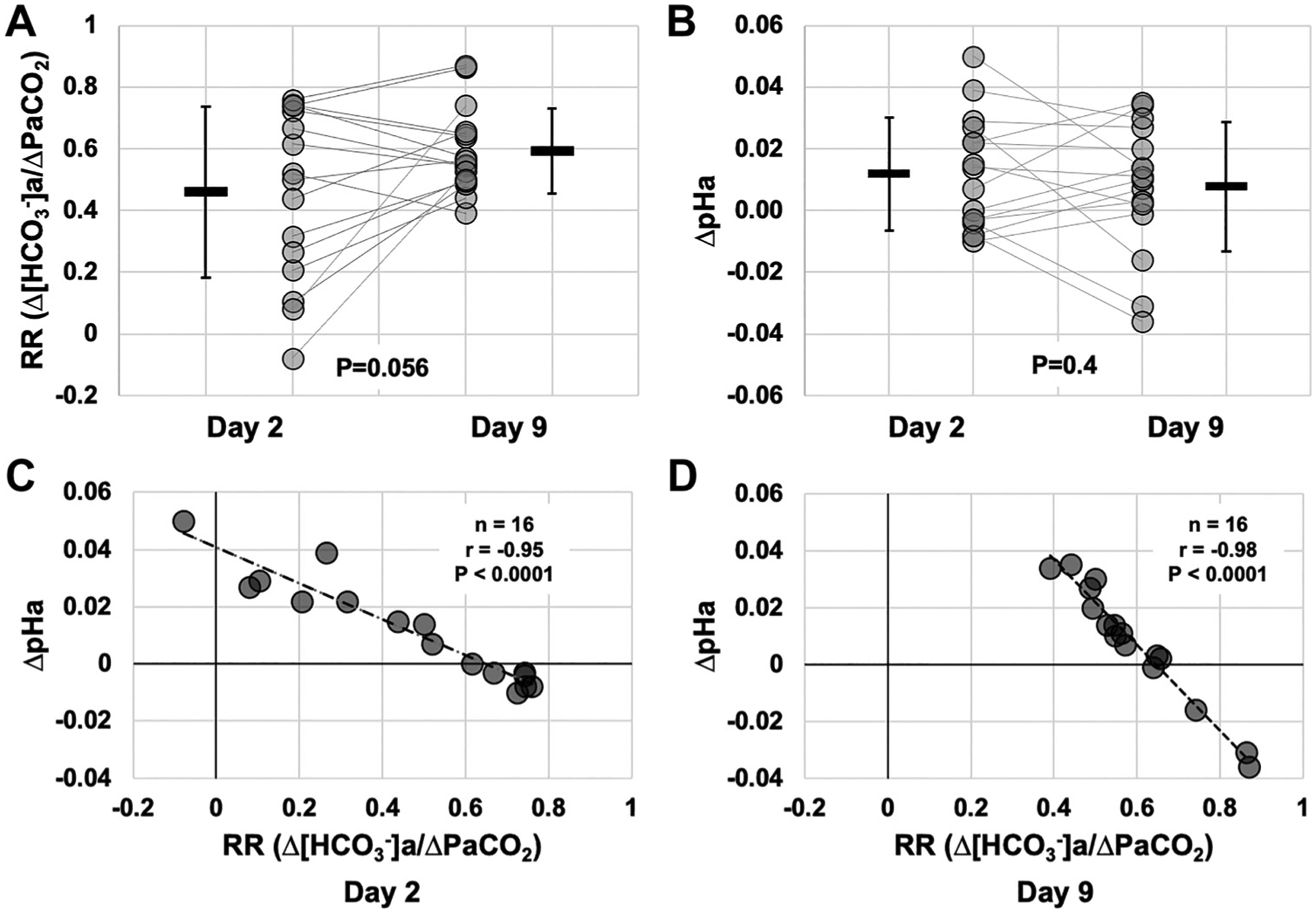
Renal reactivity and changes in pHa during rapid ascent and residence at 3,800 m. Data were calculated from , and pHa variables reported in Fig. 2. A: renal reactivity (RR) calculated on days 2 (within 24 h of arrival by rapid ascent) and 9 at 3,800 m, compared with D0 (1,045 m; P = 0.056). B: delta pHa calculated on days 2 and 9 at 3,800 m, compared with D0 (1,045 m; P = 0.4). C: correlation between RR vs. pHa at day 2 at 3,800 m. D: correlation between RR vs. pHa at day 9 at 3,800 m. For correlations (C and D), r, P, and n values are presented on each graph. n = 16. a,, arterial bicarbonate concentration; , partial pressure of arterial carbon dioxide; pHa, arterial pH.
Figure 4 illustrates Davenport diagrams on D0, D2, and D9 (Fig. 4, A–C). Although these plots are qualitative, they illustrate the integrated changes in (right hand y-axis), pHa (x-axis), and [HCO3−]a (left hand y-axis; same data as Fig. 2). Qualitatively, it is evident that and [HCO3−]a values are incrementally lower with time at altitude (D2 and D9) compared with D0, and that pHa was relatively well-maintained throughout, despite what appears to be an initial alkalinization within 24 h of arrival on D2. Mean data in Fig. 4D illustrate the integrated disturbances and compensation over time.
Figure 4.
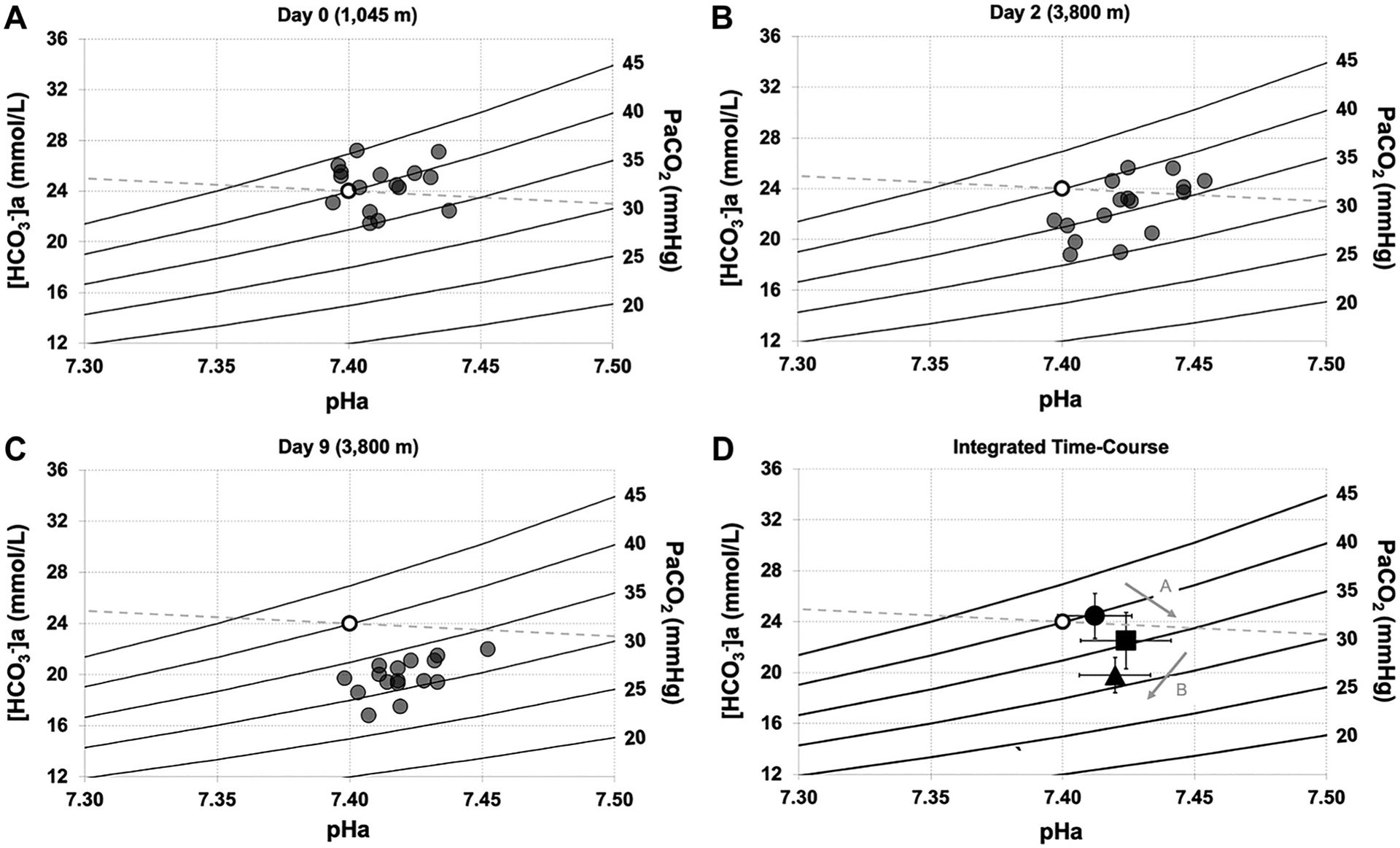
Davenport diagrams during rapid ascent to and residence at 3,800 m. Individual data demonstrating relationships between , , and pHa at 1,045 m on day 0 (A), 3,800 m on day 2 (B; within 24 h of arrival by rapid ascent), and 3,800 m on day 9 (C). D: mean data demonstrating dynamic relationship of stimulus-response relationships between acid-base variables and time at 3,800 m. Open circle represents reference value. For A, B, and C, filled circles represent individual data. For D, filled circle represents 1,045 m (day 0), filled square represents 3,800 m (day 2), filled triangle represents 3,800 m (day 9), and arrows denote the group directional changes over time. Error bars represent standard deviation. n = 16. , arterial bicarbonate concentration; , partial pressure of arterial carbon dioxide; pHa, arterial pH.
DISCUSSION
We aimed to characterize the time course and magnitude of integrated ventilatory and renal acclimatization following rapid ascent to and residence at 3,800 m for nine days. The principal findings were 1) compared with low altitude (D0), and were lower on D2 and D9, 2) pHa was unchanged at D2 or D9 due to renal compensation, 3) RR did not increase significantly from D2 to D9 (P = 0.056), suggesting that renal acclimatization was in full effect within 24 h of rapid ascent, and 4) there was a strong, negative correlation between RR and ΔpHa from baseline on both D2 and D9. Our findings highlight the differential time course, magnitude, and variability of integrated ventilatory and renal acclimatization following rapid ascent to and residence at high altitude.
Ventilatory Acclimatization
Previous studies have assessed ventilatory acclimatization using transient peak HVR tests before and after ascent (e.g., 8, 30). However, we argue these methods are confounded due in part to the lack of equivalency between the HVR response at sea level with the HVR at altitude, given 1) is lower at high altitude, resulting from the hypoxic ventilatory response in the steady state, 2) continues to be reduced over time resulting from ventilatory acclimatization, and 3) renally mediated bicarbonate elimination and relative metabolic acidosis sensitizes both central chemoreceptors and unbrakes the hypocapnia-mediated blunting of peripheral chemoreceptors, given the stimulus interaction between O2 and CO2/[H+] at the carotid body (10, 31). In addition, a transient peak response test may not capture the steady-state ventilatory strategy given the prevailing chemostimuli acting on both chemoreceptor compartments. Thus, we recently developed an index of steady-state chemoreflex drive (18), which we suggest captures ventilatory acclimatization in the steady-state during ascent (16, 19). Specifically, we showed that SS-CD increased with incremental ascent to high altitude (16, 19), and thus captured ventilatory acclimatization during rest with ascent to (16, 19) and descent from 5,160 m (16). In the present study, we assessed SS-CD during rapid ascent to and residence at 3,800 m for 9 days. We showed that both and SS-CD increased upon immediate ascent to 3,800 m, and both further increased by 9 days of residence at this altitude. These data suggest that an immediate HVR was elicited with rapid ascent and that ventilatory acclimatization was responsible for further hypocapnia over time at 3,800 m (Tables 1 and 2, Fig. 2). These data further validate the utility of the SS-CD metric in high altitude fieldwork contexts in assessing an integrated central and peripheral chemoreflex for resting steady state while residing at altitude. In addition, they illustrate respiratory-induced alkalosis with hypoxic exposure, which is a stimulus for renal compensation.
Renal Acclimatization and Acid-Base Regulation
Previous studies that assessed longitudinal acid-base regulation at altitude investigated , and pHa following rapid ascent to and residence at ~4,300 m over 7 days (32) and 10 of 11 days (20). These investigators found that pHa compensation was incomplete, due in part to disproportionate reductions in both and [HCO3−]a. Participants were still alkalotic at day 7 (pH = 7.45; 32) and day 10 of 11 (pH = 7.449; 20). Although Forster et al. (20) tested a limited sample size (n = 5), the consistency of the reported acid-base variables for these two studies suggests that pHa remained alkalotic following 10 of 11 days of high altitude exposure. In contrast, Zouboules et al. (3) found during incremental ascent to high altitude that although there was alkalosis at 3,440 m following 3 days of ascent, pHa was normalized to baseline values at 3,820 m on day 5 and 4,240 m on day 7. However, with further ascent, a larger prevailing hypocapnic stimulus relative to bicarbonate compensation was observed, as participants were alkalotic again at 5,160 m on day 10. Similar relative alkalosis when ascending from 3,647 m to 4,554 m has been demonstrated in a small cohort (n = 5; 33). Conversely, pHa was normalized within 24 h upon arrival at 3,800 m in the present study, suggesting that pH maintenance is largely driven by the capacity of the renal system to detect and respond to dynamic changes in during incremental and/or sustained residence-style ascent profiles.
Studies of chronic hypercapnia suggest that the severity of the hypercapnic stimulus determines whether the kidneys are able to facilitate a full compensatory metabolic alkalosis. Under conditions of mild chronic hypercapnia (1.5% inspired), renal compensatory mechanisms fully corrected pHa (34), whereas more severe chronic hypercapnia (3% and 6% inspired) elicited only a partial renal compensation (35–37). In fact, in a severe hypercapnic environment (6% CO2 inspired), pHa was not fully compensated, even after 30 days of exposure (35). Further, when inspiring 3% CO2, urine bicarbonate excretion was nonexistent until 6 of 7 days after returning to breathing room air, suggesting maximal bicarbonate retention was employed to combat the respiratory acidosis (37). When viewed together, these data suggest that there is a threshold for the efficacy of renal responses to mitigate challenges. Similarly, in the context of high-altitude hypoxia and hypocapnia, there may be a threshold of hypocapnic stimulus below which full renal compensation cannot be achieved, likely at an altitude between 3,800 m and 4,300 m.
Time Course, Magnitude, and Variability in Renal Responses
Our data suggest that there was an immediate HVR with ascent, which reduced , as expected (Tables 1 and 2 and Fig. 3A). However, we did not expect to find that pHa was fully compensated for within ~24 h upon arrival (day 2 of exposure; see Fig. 3C), due to bicarbonate excretion (Fig. 3B). This highlights a remarkably rapid renal compensation within our sample population. This may not be surprising however, as rapid compensatory metabolic alkalosis (36) and acidosis (21) have been previously shown to begin to occur within 24 h of exposure to respiratory perturbations. In any case, as evidenced by our scatter plots on day 2 (Fig. 3), there appears to be distinct ventilatory and renal phenotypes along a continuum within our group of participants, based on the differential and integrated ventilatory and renal responses within the first 24 h of exposure to high altitude. In one group, there was minimal HVR, and thus no renal hypocapnic stimulus, and pH remained unchanged. In yet another group, there was a robust HVR and concomitant hypocapnia, with no early renal compensation. Lastly, in another group, there was a robust HVR and concomitant hypocapnia, with early renal compensation. On balance, early HVR, hypocapnia and subsequent early renal compensation dominated the statistical analysis in our study of 16 participants, bringing mean group pHa statistically back to baseline values within 24 h of arrival to high altitude. Unfortunately, with only three time points (i.e., low altitude, day 2 and day 9 at 3,800 m) and 16 participants, our resolution is low to confirm the existence of three distinct phenotypes that participants move through with variable time courses and magnitudes with time spent at altitude. Future studies should aim to explore these potentially distinct 24-h postascent phenotypes more systematically to determine if they play a role in both short and longer term acclimatization. In addition, the fact that 1) we were unable to maintain strict water and electrolyte ingestion in this field study (e.g., 38, 39) and 2) there was heterogeneity in BMI, age, and ovarian hormone status (e.g., 40, 41) in our participant pool, these factors that may introduce additional variability, and should be better controlled for in future studies to address phenotypical variability in renal acid-base regulation at altitude.
The Davenport diagrams (Fig. 4) represent a graphical demonstration of the Henderson–Hasselbalch relationships. They depict primary acid-base disturbances and their corresponding secondary compensations, including isopleths (stimuli), pHa (as the controlled variable), [HCO3−]a (response), and the non-[HCO3−]a buffer slope (42). Here, we plotted Davenport diagrams on D0 (1,045 m), D2 within 24 h of arrival at 3,800 m, and D9 at 3,800 m. As expected, we observed that on D2, both and [HCO3−]a are reduced, with pHa slightly, but not statistically elevated. On D9, both and [HCO3−]a are reduced further and pHa appears to be fully corrected to baseline values. The superimposed mean data in Fig. 4D shows the trajectory of disturbance-compensation relationship from D0, to D2, to D9. To our knowledge, this is the first time Davenport diagrams have been used to demonstrate acid-base variables with rapid ascent and residence to high altitude. This further expands our understanding of acid-base disturbances and their respective renal compensations during ascent, particularly the extent of variability between a large group of participants.
Considerations and Reconciliation with Previous Reports
In a previous study from our group, Zouboules et al. (3) developed and characterized a novel index of renal reactivity (RR) during incremental ascent to high altitude (5,160 m) whereby the change in [bicarbonate] (i.e., renal response) was indexed against the change in (i.e., renal stimulus). We demonstrated plasticity in renal responses with incremental ascent, where RR increased with increasing altitude from 3,440 m (day 3) to 3,820 m (day 5) but plateaued thereafter. This plasticity may have been a consequence of incremental increases in altitude, which increased the HVR-mediated hypocapnic stimuli to the renal tubules and stimulated larger renal responses over time, until the full response was realized. In the current study, we employed a rapid ascent and steady-state model, where participants ascended from 610 m to 3,800 m over 5–6 h and resided at this altitude for 10 days before descending. Thus, the present study differed from (3) in two important ways. First, we did not increase the magnitude of the hypoxic stimulus over the approximately same time course. In fact, as evidenced by the increase in , SpO2, [Hb], and between days 2 and 9 in our study, our ascent and steady-state residence model eliminated the confounder of progressive, incremental hypoxic stimulation with further ascent that occurred in (3). Second, given the VAH observed between days 2 and 9, as evidenced by the increased SS-CD and continued decreases in and , the hypocapnic stimulus progressively increased over the duration of our study (from ~33 to 30 mmHg). However, RR did not increase significantly from day 2 (0.46 ± 0.28) to day 9 (0.59 ± 0.14; P = 0.056), suggesting that there were concomitant, proportionate decreases in both and [HCO3−]a, without affecting RR. In comparison, Forster et al. (20) had participants reside at 4,300 m for 10 days. A calculation from their mean data suggests similar RR values, from ~0.45 on day 2 to ~0.6 on day 10, similar to the present study. This is further corroborated by Limmer et al. (33) during incremental ascent where a calculated mean RR of ~0.64 on day 3 (3,425 m) increased to ~0.71 on day 7 (4,554 m). These values from Limmer et al. appear to be slightly higher than other studies highlighted for two reasons. First, RR was calculated on day 3 of ascent, allowing time for more relative bicarbonate excretion to occur. Second, baseline measurements were obtained at 100 m, thereby giving comparatively larger baseline bicarbonate values (~25.4 mM) and thus a potentially larger bicarbonate excretion capacity. These results contrast with the findings of Steele et al. (32) where an increase in RR from day 1 (0.098) to day 7 (0.54) was demonstrated. These considerations illustrate the biphasic nature of RR as ventilatory and renal compensations differ temporally. The low RR on day 1 for Steele et al. can be attributed to a predominance of Phenotype B (HVR-mediated hypocapnia, but with no appreciable renal response) when the measures were taken only 12 h after ascent. As the acute HVR precedes the renal acclimatization process, there will be a point in time (e.g., <12 h postascent) where there will be relatively minor changes in bicarbonate but concurrently large changes in elicited by the HVR. Under these conditions, the numerator remains small (i.e., no change in bicarbonate), whereas the denominator will increase dramatically (i.e., large change in ), resulting in a very low RR. Thus, RR should be interpreted taking into context the temporal differences between both HVR-mediated changes in and the subsequent renal compensation. By contrast, in (33), a larger RR was observed by day 3 as the full renal response is realized, and participants moved toward Phenotype C, where a HVR-mediated hypocapnia had elicited a renal compensation.
An alternative explanation for the plasticity noted in (3) may have been due to progressive exposure to superimposed hypoxia and hypocapnia. Interestingly, in the present study, the increase in hypocapnic stimulus was larger (Fig. 2) and oxygenation was improved (Fig. 1) compared with the period RR plasticity was observed in (3). There, was not decreased from day 3 (30.7 mmHg) to day 5 (30.3 mmHg), and participants were hypoxic, which suggests that a dose-dependent interaction may exist between sustained hypoxia and hypocapnia in driving renal plasticity. There is some evidence to support the hypothesis that sustained hypoxia interacts with hypocapnia to enhance renal compensation. In (22), participants voluntarily hyperventilated for 26 h in either room air or in a hypobaric hypoxia chamber simulating 3,100 m with roughly equivalent between the two trials over time. The hypoxic-hypocapnic trial (n = 8) appeared to show more complete pHa compensation over time compared with the normoxic-hypocapnic trial, although the authors report that these observed differences in pHa were not significant. However, a calculation from their mean data between groups at the 9-h time point suggests that RR was higher by ~45% in the hypoxic-hypocapnic trial compared with the hypocapnic trial alone (0.29 vs. 0.20, respectively). This potential difference between renal responses to sustained hypocapnia between hypoxia and normoxia in the same individuals hints that the comparable, iso-hypoxic stimulus between day 2 and 9 in the present study may have attenuated the magnitude of RR compared with the incremental ascent model (3). There, and were maintained at ~50 mmHg and 85%, respectively, between days 3 and 5, and RR appeared to show plasticity, whereas in the present study, the continual rise in , [Hb], and (Tables 1 and 2; Fig. 1) over 7 days may have attenuated plasticity in RR, despite sustained hypocapnic stimulus due to VAH (Fig. 3).
With respect to the effects of systemic hypoxia on the renal system, a previous report suggests that the HVR magnitude was related to diuresis and natriuresis in response to sustained (6 h) of steady-state hypoxia (43). Indeed, anatomical substrates are already known to exist whereby both extrinsic and intrinsic systems may account for the effects of hypoxia on the renal tubules. First, renal sympathetic nerves innervate not only the renal vasculature but also the renal tubular network (44), and hypoxia enhances sympathetic outflow (45–47), likely through carotid body activation (48). Second, there is a known peritubular capillary system involved in oxygen sensing and hematological acclimatization through local hypoxia-inducible factor-1α (HIF-1α) expression and subsequent erythropoietin release (e.g., 49, 50). Thus, the intriguing possibility exists that hypoxia interacts with hypocapnia to augment renal acid-base acclimatization through bicarbonate elimination and acid retention, potentially through upregulation of membrane transporters in intercalated cells (23, 51–54). This hypothesis remains to be fully elucidated and requires further experimental attention in both animal and human models.
Conclusions
We characterized the time course, magnitude, and variability of integrated ventilatory and renal acclimatization following rapid ascent to and residence at 3,800 m for 9 days. We found that compared with D0 (i.e., baseline), and [HCO3−]a were lower on D2 and D9, and that pHa was unchanged at D2 or D9 due to renal compensation, suggesting a rapid time course of renal compensation (within 24 h). We also found that RR did not increase from D2 to D9. This indicates a short time course of renal acclimatization followed by maintenance of pHa with progressive hypocapnia following rapid ascent and a subsequent 9-day residence at steady-state altitude (3,800 m). Similar to a previous study during incremental ascent, there was a strong negative correlation between RR and ΔpHa from baseline on both D2 and D9, which suggests that carbon dioxide and bicarbonate are the dominant buffer system for acid-base homeostasis at high altitude, and those with larger renal compensation better protect pHa in the context of sustained progressive hypocapnia. Our findings highlight the differential time course, magnitude, and variability of ventilatory and renal acclimatization following rapid ascent to and residence at high altitude and suggest future work investigating the capacity for renal acclimatization and the role of renal-specific hypoxia on acid-base homeostasis.
ACKNOWLEDGMENTS
We are grateful to our participants for their time and effort in participating in our study. We gratefully acknowledge the staff at the White Mountain Research Station for their support on this expedition.
GRANTS
Financial support for this study was provided by the Alberta Government Student Temporary Employment Program, Natural Sciences and Engineering Research Council of Canada (NSERC) Undergraduate Student Research Assistantships. In addition, funding was also provided by the Natural Sciences and Engineering Research Council of Canada Discovery grants (C. D. Steinback: RGPIN 06637; T. A. Day: RGPIN 04915; R. J. A. Wilson: RGPIN-03941). G. E. Foster is a Michael Smith Foundation for Health Research Scholar. J. K. Leacy was funded by the Department of Physiology, University College Cork. C. D. Steinback is funded by a Heart and Stroke Foundation of Canada Joint National and Alberta New Investigator Award (Steinback). N. G. Jendzjowsky was funded by an NSERC BRAIN CREATE Program and a Parker B. Francis Foundation Postdoctoral Fellowship. C. A. Rickards was funded by an American Heart Association Grant-in-Aid (17GRNT33671110). Additional funding for the expedition was obtained from a University of Calgary University Research Grants Committee (URGC) grant.
Footnotes
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Goldfarb-Rumyantzev AS, Alper SL. Short-term responses of the kidney to high altitude in mountain climbers. Nephrol Dial Transplant 29: 497–506, 2014. doi: 10.1093/ndt/gft051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krapf R, Beeler I, Hertner D, Hulter HN. Chronic respiratory alkalosis. N Engl J Med 324: 1394–1401, 1991. doi: 10.1056/NEJM199105163242003. [DOI] [PubMed] [Google Scholar]
- 3.Zouboules SM, Lafave HC, O’Halloran KD, Brutsaert TD, Nysten HE, Nysten CE, Steinback CD, Sherpa MT, Day TA. Renal reactivity: acid-base compensation during incremental ascent to high altitude. J Physiol 596: 6191–6203, 2018. doi: 10.1113/JP276973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beidleman BA, Staab JE, Muza SR, Sawka MN. Quantitative model of hematologic and plasma volume responses after ascent and acclimation to moderate to high altitudes. Am J Physiol Regul Integr Comp Physiol 312: R265–R272, 2017. doi: 10.1152/ajpregu.00225.2016. [DOI] [PubMed] [Google Scholar]
- 5.Dempsey JA, Powell FL, Bisgard GE, Blain GM, Poulin MJ, Smith CA. Role of chemoreception in cardiorespiratory acclimatization to, and deacclimatization from, hypoxia. J Appl Physiol (1985) 116: 858–866, 2014. doi: 10.1152/japplphysiol.01126.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powell FL. The influence of chronic hypoxia upon chemoreception. Respir Physiol Neurobiol 157: 154–161, 2007. doi: 10.1016/j.resp.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dempsey JA, Forster HV. Mediation of ventilatory adaptations. Physiol Rev 62: 262–346, 1982. doi: 10.1152/physrev.1982.62.1.262. [DOI] [PubMed] [Google Scholar]
- 8.Teppema LJ, Dahan A. The ventilatory response to hypoxia in mammals: mechanisms, measurement, and analysis. Physiol Rev 90: 675–754, 2010. doi: 10.1152/physrev.00012.2009. [DOI] [PubMed] [Google Scholar]
- 9.Daristotle L, Berssenbrugge AD, Bisgard GE. Hypoxic-hypercapnic ventilatory interaction at the carotid body of awake goats. Respir Physiol 70: 63–72, 1987. doi: 10.1016/s0034-5687(87)80032-4. [DOI] [PubMed] [Google Scholar]
- 10.Lahiri S, DeLaney RG. Relationship between carotid chemoreceptor activity and ventilation in the cat. Respir Physiol 24: 267–286, 1975. doi: 10.1016/0034-5687(75)90018-3. [DOI] [PubMed] [Google Scholar]
- 11.Steinback CD, Poulin MJ. Ventilatory responses to isocapnic and poikilocapnic hypoxia in humans. Respir Physiol Neurobiol 155: 104–113, 2007. doi: 10.1016/j.resp.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Ainslie PN, Lucas SJE, Burgess KR. Breathing and sleep at high altitude. Respir Physiol Neurobiol 188: 233–256, 2013. doi: 10.1016/j.resp.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z-Y, Olson EB, Bjorling DE, Mitchell GS, Bisgard GE. Sustained hypoxia-induced proliferation of carotid body type I cells in rats. J Appl Physiol (1985) 104: 803–808, 2008. doi: 10.1152/japplphysiol.00393.2007. [DOI] [PubMed] [Google Scholar]
- 14.Vizek M, Pickett CK, Weil JV. Increased carotid body hypoxic sensitivity during acclimatization to hypobaric hypoxia. J Appl Physiol (1985) 63: 2403–2410, 1987. doi: 10.1152/jappl.1987.63.6.2403. [DOI] [PubMed] [Google Scholar]
- 15.Moya EA, Go A, Kim CB, Fu Z, Simonson TS, Powell FL. Neuronal HIF-1α in the nucleus tractus solitarius contributes to ventilatory acclimatization to hypoxia. J Physiol 598: 2021–2034, 2020. doi: 10.1113/JP279331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leacy JK, Linares AM, Zouboules SM, Rampuri ZH, Bird JD, Herrington BA, Mann LM, Soriano JE, Thrall SF, Kalker A, Brutsaert TD, O’Halloran KD, Sherpa MT, Day TA. Cardiorespiratory hysteresis during incremental high altitude ascent-descent quantifies the magnitude of ventilatory acclimatization. Exp Physiol 106: 139–150, 2021. doi: 10.1113/EP088488. [DOI] [PubMed] [Google Scholar]
- 17.Robbins PA. Role of the peripheral chemoreflex in the early stages of ventilatory acclimatization to altitude. Respir Physiol Neurobiol 158: 237–242, 2007. doi: 10.1016/j.resp.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Pfoh JR, Steinback CD, Vanden Berg ER, Bruce CD, Day TA. Assessing chemoreflexes and oxygenation in the context of acute hypoxia: implications for field studies. Respir Physiol Neurobiol 246: 67–75, 2017. doi: 10.1016/j.resp.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Bruce CD, Saran G, Pfoh JR, Leacy JK, Zouboules SM, Mann CR, Peltonen JDB, Linares AM, Chiew AE, O’Halloran K, D Sherpa MT, Day TA. What is the point of the peak? Assessing steady-state respiratory chemoreflex drive in high altitude field studies. In: Arterial Chemoreceptors. Advances in Experimental Medicine and Biology, Gauda E, Monteiro M, Prabhakar N, Wyatt C, Schultz H. (eds). Cham, Switzerland: Springer, 2018, pp 13–23. [DOI] [PubMed] [Google Scholar]
- 20.Forster HV, Dempsey JA, Chosy LW. Incomplete compensation of CSF [H +] in man during acclimatization to high altitude (48300 M). J Appl Physiol 38: 1067–1072, 1975. doi: 10.1152/jappl.1975.38.6.1067. [DOI] [PubMed] [Google Scholar]
- 21.Ge R-L, Babb TG, Sivieri M, Resaland GK, Karlsen T, Stray-Gundersen J, Levine BD. Urine acid–base compensation at simulated moderate altitude. High Alt Med Biol 7: 64–71, 2006. doi: 10.1089/ham.2006.7.64. [DOI] [PubMed] [Google Scholar]
- 22.Gledhill N, Beirne GJ, Dempsey JA. Renal response to short-term hypocapnia in man. Kidney Int 8: 376–384, 1975. doi: 10.1038/ki.1975.130. [DOI] [PubMed] [Google Scholar]
- 23.Hamm LL, Nakhoul N, Hering-Smith KS. Acid-base homeostasis. Clin J Am Soc Nephrol 10: 2232–2242, 2015. doi: 10.2215/CJN07400715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Höhne C, Boemke W, Schleyer N, Francis RC, Krebs MO, Kaczmarczyk G. Low sodium intake does not impair renal compensation of hypoxia-induced respiratory alkalosis. J Appl Physiol 92: 2097–2104, 2002. doi: 10.1152/japplphysiol.00719.2001. [DOI] [PubMed] [Google Scholar]
- 25.Swenson ER. Hypoxia and its acid–base consequences: from mountains to malignancy. In: Hypoxia. Heidelberg, Germany: Springer, 2016, pp. 301–323. [DOI] [PubMed] [Google Scholar]
- 26.Fan J-L, Burgess KR, Basnyat R, Thomas KN, Peebles KC, Lucas SJE, Lucas RAI, Donnelly J, Cotter JD, Ainslie PN. Influence of high altitude on cerebrovascular and ventilatory responsiveness to CO2. J Physiol 588: 539–549, 2010. doi: 10.1113/jphysiol.2009.184051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roach RC, Hackett PH, Oelz O, Bärtsch P, Luks AM, MacInnis MJ, Baillie JK; Lake Louise AMS Score Consensus Committee. The 2018 Lake Louise Acute Mountain Sickness Score. High Alt Med Biol 19: 4–6, 2018. doi: 10.1089/ham.2017.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stewart PA. Modern quantitative acid–base chemistry. Can J Physiol Pharmacol 61: 1444–1461, 1983. doi: 10.1139/y83-207. [DOI] [PubMed] [Google Scholar]
- 29.Martín-Calderón JL, Bustos F, Tuesta-Reina LR, Varona JM, Caballero L, Solano F. Choice of the best equation for plasma osmolality calculation: comparison of fourteen formulae. Clin Biochem 48: 529–533, 2015. doi: 10.1016/j.clinbiochem.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Powell FL, Dwinell MR, Aaron EA. Measuring ventilatory acclimatization to hypoxia: comparative aspects. Respir Physiol 122: 271–284, 2000. doi: 10.1016/s0034-5687(00)00165-1. [DOI] [PubMed] [Google Scholar]
- 31.Fitzgerald RS, Dehghani GA. Neural responses of the cat carotid and aortic bodies to hypercapnia and hypoxia. J Appl Physiol Respir Environ Exerc Physiol 52: 596–601, 1982. doi: 10.1152/jappl.1982.52.3.596. [DOI] [PubMed] [Google Scholar]
- 32.Steele AR, Tymko MM, Meah VL, Simpson Ll Gasho C, Dawkins Tg Villafuerte Fc Ainslie Pn Stembridge M, Moore JP. Global REACH 2018: renal oxygen delivery is maintained during early acclimatization to 4330 m. Am J Physiol Renal Physiol 319: F1081–F1089, 2020. doi: 10.1152/ajprenal.00372.2020. [DOI] [PubMed] [Google Scholar]
- 33.Limmer M, de Marées M, Platen P. Effects of daily ingestion of sodium bicarbonate on acid-base status and anaerobic performance during an altitude sojourn at high altitude: a randomized controlled trial. J Int Soc Sports Nutr 17: 14, 2020. doi: 10.1186/s12970-020-00351-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schaefer KE, Hastings BJ, Carey CR, Nichols G. Respiratory acclimatization to carbon dioxide. J Appl Physiol 18: 1071–1078, 1963. doi: 10.1152/jappl.1963.18.6.1071. [DOI] [PubMed] [Google Scholar]
- 35.Burgraff NJ, Neumueller SE, Buchholz K, Langer IIT, Hodges MR, Pan L, Forster HV. Ventilatory and integrated physiological responses to chronic hypercapnia in goats. J Physiol 596: 5343–5363, 2018. doi: 10.1113/JP276666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crosby A, Talbot NP, Balanos GM, Donoghue S, Fatemian M, Robbins PA. Respiratory effects in humans of a 5-day elevation of end-tidal PCO2 by 8 Torr. J Appl Physiol 95: 1947–1954, 2003. doi: 10.1152/japplphysiol.00548.2003. [DOI] [PubMed] [Google Scholar]
- 37.Schaefer KE. Atmung und Saure-Basengleichgewicht bei lang-dauerndem Aufenthalt in 3% CO2. [Breathing and acid-base equilibrium in the case of long-term stay in 3% CO2]. Pflugers Arch 251: 689–715, 1949. doi: 10.1007/BF00361640. [DOI] [Google Scholar]
- 38.Biollaz J, Buclin T, Hildebrandt W, Décosterd LA, Nussberger J, Swenson ER, Bärtsch P. No renal dysfunction or salt and water retention in acute mountain sickness at 4,559 m among young resting males after passive ascent. J Appl Physiol (1985) 130: 226–236, 2021. doi: 10.1152/japplphysiol.00382.2020. [DOI] [PubMed] [Google Scholar]
- 39.Gougoux A, Kaehny WD, Cohen JJ. Renal adaptation to chronic hypocapnia: dietary constraints in achieving H+ retention. Am J Physiol 229: 1330–1337, 1975. doi: 10.1152/ajplegacy.1975.229.5.1330. [DOI] [PubMed] [Google Scholar]
- 40.Dick IM, Prince RL. The effect of estrogen on renal phosphorus handling in the rat. Am J Nephrol 21: 323–330, 2001. doi: 10.1159/000046269. [DOI] [PubMed] [Google Scholar]
- 41.Gohar EY, Kasztan M, Becker BK, Speed JS, Pollock DM. Ovariectomy uncovers purinergic receptor activation of endothelin-dependent natriuresis. Am J Physiol Renal Physiol 313: F361–F369, 2017. doi: 10.1152/ajprenal.00098.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davenport HW. The ABC of Acid-Base Chemistry: The Elements of Physiological Blood-Gas Chemistry for Medical Students and Physicians. Chicago, IL: University of Chicago Press, 1974. [Google Scholar]
- 43.Swenson ER, Duncan TB, Goldberg SV, Ramirez G, Ahmad S, Schoene RB. Diuretic effect of acute hypoxia in humans: relationship to hypoxic ventilatory responsiveness and renal hormones. J Appl Physiol (1985) 78: 377–383, 1995. [7759405] doi: 10.1152/jappl.1995.78.2.377. [DOI] [PubMed] [Google Scholar]
- 44.Johns EJ, Kopp UC, DiBona GF. Neural control of renal function. Compr Physiol 1: 731–767, 2011. doi: 10.1002/cphy.c100043. [DOI] [PubMed] [Google Scholar]
- 45.Keir DA, Duffin J, Millar PJ, Floras JS. Simultaneous assessment of central and peripheral chemoreflex regulation of muscle sympathetic nerve activity and ventilation in healthy young men. J Physiol 597: 3281–3296, 2019. doi: 10.1113/JP277691. [DOI] [PubMed] [Google Scholar]
- 46.Prasad B, Morgan BJ, Gupta A, Pegelow DF, Teodorescu M, Dopp JM, Dempsey JA. The need for specificity in quantifying neurocirculatory vs. respiratory effects of eucapnic hypoxia and transient hyperoxia. J Physiol 598: 4803–4819, 2020. doi: 10.1113/JP280515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steinback CD, Salzer D, Medeiros PJ, Kowalchuk J, Shoemaker JK. Hypercapnic vs. hypoxic control of cardiovascular, cardiovagal, and sympathetic function. Am J Physiol Integr Comp Physiol 296: R402–R410, 2009. doi: 10.1152/ajpregu.90772.2008. [DOI] [PubMed] [Google Scholar]
- 48.Dempsey JA, Morgan BJ. Humans in hypoxia: a conspiracy of maladaptation?!. Physiology (Bethesda) 30: 304–316, 2015. doi: 10.1152/physiol.00007.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haase VH. Hypoxic regulation of erythropoiesis and iron metabolism. Am J Physiol Renal Physiol 299: F1–F13, 2010. doi: 10.1152/ajprenal.00174.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosenberger C, Mandriota S, Jürgensen JS, Wiesener MS, Hörstrup JH, Frei U, Ratcliffe PJ, Maxwell PH, Bachmann S, Eckardt K-U. Expression of hypoxia-inducible factor-1α and-2α in hypoxic and ischemic rat kidneys. J Am Soc Nephrol 13: 1721–1732, 2002. doi: 10.1097/01.asn.0000017223.49823.2a. [DOI] [PubMed] [Google Scholar]
- 51.Al-Awqati Q Terminal differentiation of intercalated cells: the role of hensin. Annu Rev Physiol 65: 567–583, 2003. doi: 10.1146/annurev.physiol.65.092101.142645. [DOI] [PubMed] [Google Scholar]
- 52.Roy A, Al-Bataineh MM, Pastor-Soler NM. Collecting duct intercalated cell function and regulation. Clin J Am Soc Nephrol 10: 305–324, 2015. doi: 10.2215/CJN.08880914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwartz GJ, Tsuruoka S, Vijayakumar S, Petrovic S, Mian A, Al-Awqati Q. Acid incubation reverses the polarity of intercalated cell transporters, an effect mediated by hensin. J Clin Invest 109: 89–99, 2002. doi: 10.1172/JCI13292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Welsh-Bacic D, Nowik M, Kaissling B, Wagner CA. Proliferation of acid-secretory cells in the kidney during adaptive remodelling of the collecting duct. PLoS One 6: e25240, 2011. doi: 10.1371/journal.pone.0025240. [DOI] [PMC free article] [PubMed] [Google Scholar]


