Abstract
Background:
There is a growing evidence-base underpinning implementation of person-centred outcome measures into adult palliative care. However evidence on how best to achieve this with children facing life-threatening and life-limiting conditions is limited.
Aim:
To identify the anticipated benefits, risks, barriers and facilitators to implementing person-centred outcome measures for children with life-limiting and life-threatening conditions.
Design:
Cross-sectional qualitative semi-structured interview study with key stakeholders analysed using Framework analysis informed by the adapted-Consolidated Framework for Implementation Research.
Setting/participants:
A total of n = 26 children with life-limiting or life-threatening conditions, n = 40 parents/carers, n = 13 siblings and n = 15 health and social care professionals recruited from six hospitals and three children’s hospices and n = 12 Commissioners of health services.
Results:
All participants were supportive of future implementation of person-centred outcome measures into care. Anticipated benefits included: better understanding of patient and family priorities, improved communication and collaborative working between professionals and families and standardisation in data collection and reporting. Anticipated risks included increased workload for staff and measures not being used as intended. Implementation barriers included: acceptability and usability of outcome measures by children; burden and capacity of parents/carers regarding completion; privacy concerns; and language barriers. Implementation facilitators included designing measures using language that is meaningful to children and families, ensuring potential benefits of person-centred outcome measures are communicated to encourage ‘buy-in’ and administering measures with known and trusted professional.
Conclusions:
Implementation of person-centred outcome measures offer potential benefits for children with life-limiting and life-threatening conditions. Eight recommendations are made to maximise benefits and minimise risks in implementation.
Keywords: Paediatrics, palliative care, children, implementation science, patient reported outcome measures, patient-centred outcomes research
What is already known on this topic?
Person-centred outcome measures have been shown to improve the quality of care and patient outcomes in adult palliative care when successfully implemented into routine care.
Several factors influence implementation in adult services, but they have not been identified in care for children with life-limiting and life-threatening conditions.
The views of stakeholders are key to successful development, implementation and use of outcome measures in practice.
What this paper adds?
Perceived benefits of using person-centred outcome measures include enhanced understanding of what matters to patients and families, improved communication and collaborative working and standardised data collection and reporting; perceived risks include negative impacts on care and measures not being used as intended.
Potential barriers to implementation include acceptability and usability of the measure for children, burden and capacity of patients and families to complete the measure, privacy concerns, protecting family members and language barriers; potential facilitators include explaining the benefits of person-centred outcome measures and securing ‘buy-in’, measures being implemented by known and trusted health and social care staff and the language in the measure being meaningful to children and families.
Eight recommendations are presented to minimise risks and support successful implementation of child and family-centred outcome measures for children with life-limiting and life-threatening conditions.
Implications for practice, theory or policy
The benefits of person-centred outcome measures for care should be explained to children, families and professionals to facilitate buy in and successful implementation.
Implementation strategies should be designed collaboratively with professionals to ensure implementation of person-centred outcome measures is feasible within current practice and does not impact negatively on care.
Professionals introducing and administering the measure should be known and trusted by the child and family, and should discuss usage preference and information sharing to address any privacy concerns.
Background
Person-Centred Outcome Measures (PCOMs) are standardised questionnaires that assess the effect of a health condition or treatment on the patient, and/or their family.1–3 They are usually self-completed (as with Patient Reported Outcome Measures (PROM)), or when the patient is unable, proxy-completed by a caregiver.1–3 Using PCOMs can empower patients and families to raise concerns with clinicians, and support conversations and decision-making through a shared language.2,4–6 These processes improve quality of care and patient outcomes.7,8
An estimated 21 million children and young people (hereafter ‘children’) worldwide with life-limiting and life-threatening conditions (hereafter ‘life-limiting’) could benefit from palliative care each year. 9 Whilst there is a growing body of evidence on the use and implementation of PCOMs in adult palliative care,1–3,10,11 evidence to underpin their use and implementation for children with life-limiting conditions is more limited,2,12–14 particularly outside of paediatric oncology.15–22 Whilst there are several PCOMs (including both generic tools and condition specific measures) that have been developed, validated and implemented across paediatrics,23–25 available generic tools (e.g. Paediatric Quality of Life Inventory14,26–32) do not reflect the concerns of all children with life-limiting conditions, and condition specific measures (e.g. Memorial Symptom Assessment Scale33,34) are only relevant for their specific population, and therefore not suitable for use across all children with life-limiting conditions. 35 Following development and initial validation of the Children’s Palliative care Outcome Scale: African version,36–40 development of a validated measure that can be used by all children with any life-limiting conditions outside of Africa, has been highlighted as a priority for clinical care and research.41–46
The CPOS:UK (Children’s Palliative care Outcome Scale: UK version) study aims to develop, validate and implement a novel PCOM for all children with any life-limiting condition in the UK. Five initial versions of the C-POS:UK have been developed to reflect variation in age/developmental stages of the target population and allow for proxy reporting if required47–50 following the Consensus-based Standards for the selection of health Measurement Instruments (COSMIN)52,53 and Rothrock guidance. 54 However, future implementation to be successful, implementation strategies must be informed by the views and preferences of key stakeholders: children with life-limiting conditions, their family members and professionals involved in their care.
Methods
Research questions
RQ1: What are the anticipated benefits and risks of using a PCOM in the care of children with life-limiting conditions?
RQ2: What are the potential barriers and facilitators to implementing a PCOM in in the care of children with life-limiting conditions?
Design
This cross-sectional qualitative interview study 47 is reported in accordance with the consolidated criteria for reporting qualitative studies (COREQ). 55 It sits within a sequential mixed-methods study to develop,35,47–49,56–59 validate 51 and implement60–62 a novel PCOM for children with any life-limiting illness. Qualitative interviews were conducted during the development phase with the aims of identifying priority items to include in the measure, 47 preferences for design and administration modes, 49 and potential benefits and challenges of implementing a new PCOM into routine care. The data related to implementation collected during the development phase are reported here and will inform the implementation phase.
PCOMs are complex interventions (particularly in their usage to drive assessment, care and evaluation). Consistent with the Medical Research Council’s framework for developing and evaluating complex interventions, all key stakeholder groups views were sought to inform the design of the measure and the development of an implementation strategy.1–3,10,11 This work took a child- and family-centred approach which recognises the role of parents and the family in care planning and delivery while ensuring that the child’s voice and perspective is heard and fully considered.63–65
Population
Inclusion criteria
Children: aged 5–17 years with any life-limiting condition. 66
Parents/carers: parent or carer of a child aged <18 years with any life-limiting condition. 66
Siblings: aged 5–17 years and sibling to a child <18 years with any life-limiting condition. 66
Professionals: any professional with >6 months experience caring for children <18 with any life-limiting condition. 66
Commissioners (responsible for planning, prioritising, purchasing and monitoring of services): responsible for commissioning UK paediatric palliative care services.
Exclusion criteria
Children: unable to communicate any views or wishes; speak a language not supported by NHS translation services; currently enrolled in another study; deemed clinically unable to give consent/assent.
Parents/carers and siblings: deemed clinically unable to give consent/assent or speak a language not supported by NHS translation services.
Setting
Six hospitals (five with specialist consultant-led paediatric palliative care teams) and three children’s hospices (one with a specialist consultant-led paediatric palliative care team, two with nurse led services) across England and Northern Ireland.
Sampling
Children and their families were purposively sampled by age and condition (i.e. cancer and non-cancer 38 ). Professionals were purposely sampled to ensure a range of different professions were represented and commissioners were purposely sampled based on geographical location. The concept of information power or pragmatic saturation 67 was used to determine the required sample size to address all of the aims of the original study.47,49,60,61 Due to the range of aims being met and the heterogeneity of the population, the dataset required diversity and depth.
Recruitment
Potential child, parent/carer and sibling participants were identified by the local clinical team. Professionals were also recruited from these sites, identified by their service manager. Commissioners were recruited through recommendations from professionals and a national children’s palliative care non-governmental organisation.
Data collection
Semi-structured interviews were conducted between April 2019 and September 2020 by LC (experienced children’s palliative care nurse, new to qualitative research), AR (new to qualitative research) and DB (experienced qualitative researcher).
Topic guides were developed collaboratively with the study steering group which includes clinicians of various professions (including doctors, nurses, social workers and other allied health professionals) working with children with life-limiting conditions, academics (including clinical academics) and bereaved parent public and patient involvement members. Further information on and examples of the topic guides are included in the Supplemental Files.
Interviewers received training and supervision on conducting interviews with children, including ‘draw and talk’ and play methods from an educational psychologist and play therapist. Participants were offered choice of location for face-to-face interviews of their clinical setting or their home (during the COVID-19 lockdown, interviews were only conducted by telephone 57 ). Interviews were audio-recorded, transcribed verbatim and pseudonymised.
Data analysis
Analysis followed the seven-step Framework method 68 ; transcription, familiarisation, coding, developing an analytical framework/codebook, applying the framework, charting into the framework matrix and interpretation. Full transcripts were coded68,69 by LC, DB, AR, HS (experienced qualitative researcher) and DH (new to qualitative research) using NVivo 12 Software. About 20% of transcripts were independently coded by two researchers for consistency and rigour. 70 Regular team (LC, DB, AR, HS and DH) meetings were held to discuss emerging codes/themes to develop and revise a codebook. 71 RH, KB and CES were consulted to resolve discrepancies. The codebook was developed through 18 revisions, applied to all transcripts and data charted into a matrix generated by HS using NVivo12, which supports comparisons across and between groups.
A second phase of deductive analysis and interpretation was performed by HS through mapping the coded, charted data in the matrix to the domains of the adapted-Consolidated Framework for Implementation Research (CFIR).72,73 This supported the identification of the anticipated benefits, risks, barriers and facilitators relating to the implementation of PCOMs into paediatric palliative care. The CFIR comprises five domains of implementation: intervention characteristics (aspects of PCOMs that might affect implementation success), outer setting (external influences on implementation of a PCOM), inner setting (characteristics of the healthcare setting implementing a PCOM), characteristics of individuals (individual beliefs, knowledge and attitudes of stakeholders towards a new PCOM and its implementation) and process (stages of the implementation process that can impact implementation success). The adapted-CFIR includes a sixth domain, ‘patient needs and resources’72,73 (the extent to which patient’s needs, as well as barriers and facilitators to meet those needs, are known and prioritised by the healthcare setting) integrating person-centredness into the implementation of complex healthcare interventions. 73 Analysis was regularly reviewed by the study steering group.
Ethical approvals and consent
Ethical approval was granted by the Bloomsbury research ethics committee and the Health Research Authority (HRA:19/LO/0033). Participants ⩾16 years old provided written informed consent. Those with parental responsibility provided written informed consent for participants <16 years. Those <16 years were given the opportunity to provide written assent.
Results
Sample characteristics
We conducted 104 interviews with 106 participants (2 parents and 2 siblings were interviewed together): 26 children, 40 parent/carers, 13 siblings, 15 professionals and 12 commissioners. Demographic characteristics are reported in Table 1.
Table 1.
Participant characteristics.
| Children (n = 26) | N or mean (range) | Parent/carers (n = 40) | N or mean (range) | Siblings (n = 13) | N or mean (range) |
|---|---|---|---|---|---|
| Age (years) | 12 (5–17) | Age (years) | 40 (21–65) | Age (years) | 9 (5–15) |
| Age of child with life-limiting condition (years) | 12 (0–17) | Age of child with life-limiting condition (years) | 10 (3–16) | ||
| Gender | 17:9 | Gender | Gender | ||
| Female:male | Female:male | 30:10 | Female:male | 7:6 | |
| Relationship to child | Relationship to child | ||||
| Mother | 29 | Sister | 7 | ||
| Father | 10 | Brother | 6 | ||
| Sibling caregiver | 1 | ||||
| Diagnosis | Diagnosis of child | Diagnosis of child | |||
| Cancer | 6 | Infectious disease | 2 | Metabolic | 1 |
| Metabolic | 1 | Cancer | 6 | Neurological | 7 |
| Neurological | 5 | Metabolic | 9 | Gastrointestinal | 2 |
| Respiratory | 1 | Neurological | 10 | Congenital | 3 |
| Gastrointestinal | 10 | Gastrointestinal | 4 | ||
| Congenital | 3 | Genitourinary | 1 | ||
| Perinatal | 1 | ||||
| Congenital | 7 | ||||
| Health and social care professionals (n = 15) | N or mean (range) | Commissioners (n = 12) | N or mean (range) | ||
| Gender | Gender | ||||
| Female:Male | 14:1 | Female:Male | 11:1 | ||
| Profession | Geographical location | ||||
| Palliative care nurse specialists | 4 | Southeast England | 4 | ||
| Children’s Community nurse | 1 | Greater London | 1 | ||
| Hospice nurse | 1 | East England | 2 | ||
| Ward sister | 1 | Northwest England | 1 | ||
| Paediatric palliative medicine consultant | 1 | Yorkshire and Humber | 4 | ||
| Haematology consultant | 1 | ||||
| General Paediatrician | 1 | ||||
| Social worker | 1 | ||||
| Chaplain | 1 | ||||
| Psychologist | 1 | ||||
| Play specialist | 1 | ||||
| Physiotherapist | 1 | ||||
Main findings
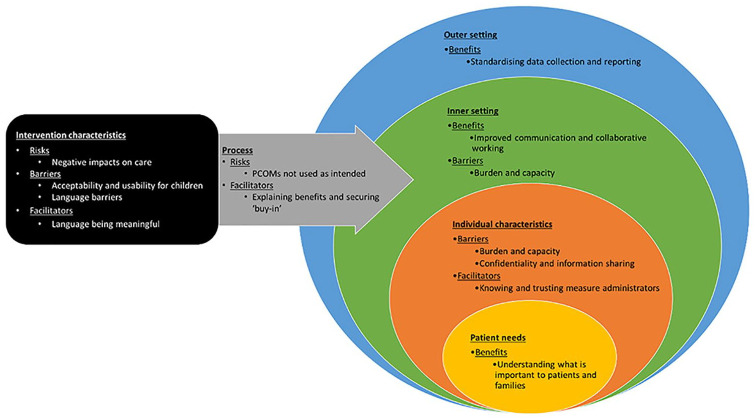
Four main themes were constructed (anticipated benefits, risks, barriers and facilitators) with 13 sub-themes mapping across all 6 adapted-CFIR domains (see Figure 1). Utilising the adapted-CFIR in this way enabled better understanding of how factors might impact implementation across multiple levels. The themes and sub-themes are described in detail below.
Figure 1.
Themes and sub-themes mapped to adapted-CFIR domains.
Theme 1: Anticipated benefits of using PCOMs
Understanding what is important to patients and families
All participants were supportive of the implementation of PCOMs into paediatric palliative care. One of the most frequently reported anticipated benefits for all stakeholder groups was the ability to enhance clinicians’ understanding about what is important to children and their families. Children actively wanted professionals to know and ask about the symptoms and concerns that matter to them
‘If they knew what I thought was important, and what I liked, and who I was as a person, then I think that would help a lot more’
Young Person aged 17 with cancer diagnosis
A further anticipated benefit was better identification of, and improved ability to meet, the needs of children and their families
‘I think if you can have a tool that is universal [. . .] but is flexible enough that it really draws out what’s important for the child and family, I think we will be better as professionals at meeting those needs’
Clinical Nurse Specialist
Although rarely discussed by parents in interviews, compared to children or professionals, one parent did recognise the benefit a PCOM could bring in terms of better understanding the needs of their children in the specific context of the family
‘I think people have to understand what your priorities are to really be able to, as a family and in looking after your child, the whole picture to be able to meet and support you in the best way’
Mother of 3-year-old with neurological diagnosis
Improved communication and collaborative working
One of the most commonly anticipated benefits among parent/carers and professionals was improvements to joined-up working and communication across teams and services
‘everyone can be on the same page about what is important for the child and this family. So. . . the wider disciplinary team, because they might be under cardiology but actually they might need to keep in mind that actually this is what’s important, so they are not doing things that are unnecessary and they [the family] don’t want to happen’
Trainee Clinical Nurse Specialist
Using PCOMs to share information with the wider care team about symptoms and concerns was seen as helpful by children and their families. Parents/carers felt it would reduce repetition in communication with different professionals
‘I think erm. . .erm everybody that’s in his care [should have access], that have an involvement in his. . .because at least then they’re all seeing the same information and you’re not having to repeat yourself’
Mother of 12 year old with congenital diagnosis
Standardising data collection and reporting
The benefit of standardised data collection and reporting was particularly important to commissioners. They discussed being able to use outcomes data to ensure that services they commissioned (and resource allocation) were best serving and meeting the needs of children and families
‘one of the main incentives I can see is around whether we can secure additional funding and investment by demonstrating the outcomes because at the moment I think it feels like everybody who works in paediatric palliative care knows that it makes a really huge difference. And we agree but it’s really difficult to demonstrate’
Commissioner
‘any information that tells you about the needs of the services that you are commissioning is incredibly helpful. [. . .] I think it's all very useful as it helps you plan your services’
Commissioner
Theme 2: Anticipated risks in using PCOMs
Negative impacts on care
Whilst most participants felt that using PCOMs in routine care would have a positive impact on the care provided, two parent/carers raised concerns around the additional workload and the potential for this to negatively impact on provision of care
‘Resources are always stretched and people are, you know the whole team are always busy, especially if they’re understaffed. And you don’t want to sort of create more work, more admin if that makes sense cos it’s going to affect their ability to care’
Father of 1 year old with infectious disease
PCOMs not used as intended
Parents of children with neurological conditions raised concerns that completion of PCOMs could become a ‘tick box exercise’, which would just be another thing to do but with no real benefit to their child and family
‘Yeah, it’s all. . .always to tick their boxes, that’s what it is. Like especially like with the social side of things, they have to tick’
Mother of 15 year old with neurological condition
Similar concerns were echoed by a nurse regarding teenagers whether implementing a PCOM would truly enable active participation in their own care, and avoiding tokenistic inclusion
‘like [teenagers] they’re actually taking part rather than they’re actually just being asked loads of questions’
Nurse
Theme 3: Potential barriers to implementing PCOMs
Acceptability and usability for children
Participants raised concerns around accessibility of a measure for children of different ages/cognitive abilities. This was emphasised as important to consider in the design of a measure to maximise acceptability and usability
‘it will depend on the cognitive ability of the child [. . .] even if the child has a limited cognitive ability there are some basic questions you could ask them about, you know how. . .how they’re feeling and how. . .what their experiences have been. . .umm obviously you know. . .umm if they’ve got greater cognitive ability you can ask them more in-depth’
Commissioner
‘maybe not this one [points to example of an outcome measure] because what if you don’t know what mild pain is or the words’
Child aged 15 with gastrointestinal diagnosis
Burden and capacity
Many professionals and commissioners noted that families were already being asked to complete several forms and expressed concern that they may not want to complete an additional measure. They also reported that children or their parents may not have the time or capacity, be that due to physical symptoms or emotional distress
‘that maybe could be a little bit onerous sometimes when people have you know, perhaps pain or in emotional distress, they’re not really wanting particularly to fill in a bit of paper’
Social worker and family therapist
‘Cause they won’t want to fill something in that’s a million pages because they just don’t have the time’
Trainee Clinical Nurse Specialist
Capacity was also raised as a concern primarily by commissioners with regards to the feasibility of implementing and using PCOMs in practice in relation to professionals’ workload
‘it’s difficult because it takes time and space and effort and energy and in a system which is so stretched, actually that’s not always a priority’
Commissioner
However, one commissioner noted that PCOMs were already used routinely in adult palliative care and therefore suggested it was possible that a PCOM could similarly fit into existing clinical workflows in the paediatric palliative care context
‘I mean the community nurses could use it. Certainly, the adult one is used by district nurses. It’s used in inpatient hospice, it’s used [in] inpatient hospitals’
Commissioner
Confidentiality and information sharing
Children (including siblings), primarily with cancer, congenital or gastrointestinal conditions, raised concerns regarding the sharing of information elicited through completion of a PCOM. Some were concerned about who the information might be shared with, often not wanting them to be shared beyond their parents or the professionals in the room. Some parent/carers and professionals also endorsed this view
‘I just kind of want whoever asks me I just want to be like, just tell them or the people in the room. . . I don’t want it to go anywhere’
Child aged 14 with Congenital Condition
‘I think some people wouldn’t want everybody to know their business’
Mother of 12 year old with cancer diagnosis
The importance of retaining control of the information, to protect family members, was also mentioned
‘you know being asked taken aside and asked independently is quite nice, it just feels more personal, it doesn't worry the others as much’
Sibling carer of 17 year old with cancer diagnosis
A similar potential barrier to using PCOMs was identified in relation to what parent/carers would allow professionals to share or discuss with the child
‘We also need permission from parents on what to give them and that then has an impact on what we can hand out, depending ‘cause each child has very different information based on what the parents allow us to tell them’
Nurse
‘Some children [. . .] Their parents don’t even share what their care is or what’s happening’
Mother 12 year old with cancer diagnosis
Language barriers
Language barriers when the child and family did not speak English fluently was a concern primarily raised by professionals. As translators were not always available, professionals found communicating and assessing children’s needs challenging
‘So language is a really big problem. [. . .] when we’re having big discussions we can bring in our translators but we don’t have the ability to do that all the time. So that is difficult [. . .] we have had kids in the past where we have used faces and emotions where we have had more like what we are feeling to help them communicate as well. I think that’s really important especially for some of the overseas patients who are maybe like 6 or 7 but they don’t speak English. But it’s very, usually in a basic way’
Ward Sister
One parent also discussed difficulties in communicating their child’s symptoms and concerns with professionals as English was not their first language
‘English is not my first language, and I tell them what I can see but it’s so hard to explain’
Mother of 1 year old with congenital condition
Theme 4: Potential facilitators for implementing PCOMs
Explaining the benefits and securing ‘buy-in’
A key facilitator to successful implementation is educating or explaining potential benefits PCOMs may afford in practice. The importance of PCOMs being rigorously developed and evidence based was key to this explanation
‘a lot of the time I think people in all kinds of organisations, but certainly in commissioning organisations, we have to explain that to people who don’t necessarily know as much about it as we do in a way that’s compelling erm and having a set of measures that are evidenced based and are used across more than just our services it would be really helpful to evidence that what we are doing is making a difference’
Commissioner
The importance of explaining and demonstrating the benefits of PCOMs to teenagers in particular, to gain their buy-in, was echoed by children and professionals
‘How it’s benefiting them. Especially the teenagers. If you know, you’re asking them and then they’re asking and being asked again and they go well what am I getting out of it?’
Nurse
‘sometimes there are days when I’m feeling like oh yes its fine, it’s all alright [to complete a questionnaire] and sometimes I’m feeling like, yeah it’s making me feel not okay, it’s making me feel uncomfortable [. . .] but at the same time it’s helpful and you know it’s for a good cause’
Child aged 14 with cancer diagnosis
Knowing and trusting measure administrators
Drawing on existing rapport and trusted relationships was central to engaging children and families in using PCOMs. When children did not know or trust professionals, they felt less comfortable sharing their symptoms and concerns
‘I think it would depend on who you sort of had that rapport with. Yeah, I don’t know. I mean for us it would probably be the nurses on the ward cos they just know us better’
Mother of 12 year old with cancer diagnosis
‘the nurses often don’t really know me and that’s why I’m often a bit like. . . weird around them’
Child aged 12 with cancer diagnosis
Some children however, were less concerned about knowing and trusting the person administering the PCOM, and instead felt that it was more important that the person cared and would be able to help them
‘I would, just someone who just has like compassion. Like erm. . .someone who’s not there just to get info, but someone who’s there actually to help the child and that’s. . .that’s what matters’
Child aged 17 with gastrointestinal diagnosis
Language being meaningful
The language used in PCOMs was highlighted as an important design consideration to ensure acceptability and usability. Professionals suggested that using language that is meaningful, or mirrors words used by the child and family themselves, may reduce barriers to use
‘I think it needs to be. . .I think it needs to be written in a language that is meaningful to families, more than to us. I think we can. . .it’s got to be easily accessible’
Clinical Nurse Specialist
Discussion
Main findings
This study provides novel evidence on anticipated risks and benefits of, and barriers and facilitators to, the implementation of PCOMs into the care of children with life-limiting conditions and their families. Stakeholders recognised and welcomed the ability of PCOMs to improve understanding of child and family priorities, improve collaborative working and assist the standardisation of assessment outcome reporting. 6 The greatest anticipated value of implementing PCOMS was in helping professionals better understand what was important to children and families, and helping commissioners ensure that services were meeting their needs.46,47
Feasibility of implementing and using PCOMs in routine practice is important to consider, particularly given concerns that additional workload could compromise care. Reported acceptability and implementability the C-POS: African Version suggests that a collaboratively-developed implementation strategy could facilitate the successful implementation of a similar measure into care for children with life-limiting conditions in the UK. 40 PCOMs are used routinely across adult palliative care settings (including inpatient, acute and community/home settings) suggesting that a PCOM may be similarly incorporated into paediatric settings without negatively impacting clinical workflows or compromising care.74,75 Nevertheless, it is important to ensure that PCOMs are used in a meaningful way whereby PCOMs actively support children to participate in their own care and shared decision-making and to avoid it becoming tokenistic whereby children are just asked to complete a PCOM but with no additional discission, involvement or feedback. 6 Given the expressed preference by children for measure completion to be integrated within conversations with their healthcare team, 49 there may be impacts to clinical workflow beyond those identified in the adult care situation.
Barriers relating to measure design, staff time, skills and gatekeeping were anticipated; some of which can be overcome by robust design and psychometric testing. 49 Concerns about respondent burden highlight the importance of designing measures that are quick to complete, to increase acceptability for patients and their families.48,49 Concerns relating to privacy and information sharing may be barriers to implementation and are often found to be linked to family members’ desire to protect each other.23,76 Shared-decision-making with parents about how and with whom PCOMs are completed 77 along with more open communication about use and benefit of measures, may mitigate this potential barrier.
Understanding family dynamics was also important in terms of how and with whom measures should be completed. This must be managed sensitively by professionals for successful PCOM implementation in the paediatric setting. Children often want someone to talk to about their problems when completing a measure 49 so measures may need to be completed in dialogue, and with a professional who has a relationship with the child and family. Potential barriers related to parents withholding information to protect their children, 78 is a unique finding in relation to barriers that have been identified previously in adult palliative care settings 79 or across paediatric settings more broadly, 62 and similarly calls for consideration in how PCOMs are administered. Whether children and their families complete it together or separately must be considered as it may impact on the mode of administration and thus implementation and use of the measure in practice. 6
As identified in previous work80,81 and noted in this study, for many children with life-limiting conditions and their families the lingua franca is not necessarily their primary language. Therefore, once developed and validated, it is also important that PCOMs are translated. This will ensure that PCOMs can support all children with life-limiting conditions and their families, irrespective of primary language or language proficiency and not introduce inequities in care, and potential disparities in outcomes.
Finally, recognition of the benefits of using PCOMs in routine paediatric palliative care is a particularly important facilitator for implementation. Research in adult palliative care 79 and non-palliative paediatric settings 62 suggests that recognition of potential benefits of PCOMs in practice often facilitates implementation through addressing barriers across multiple adapted-CFIR domains. Thus, the potential benefits of PCOMs should be emphasised with all stakeholders as part of implementation to encourage uptake and to ensure the benefits are realised for this population.
What this study adds?
Whilst several barriers and facilitators for implementing PCOMs into paediatric palliative care have been identified in other paediatric settings, 62 this work highlights barriers and facilitators that are specific to paediatric palliative care. We make eight recommendations for implementation and sustained use of new PCOMs into paediatric palliative care; displayed in Table 2 alongside how they have been informed by the findings of this study.
Table 2.
Recommendations for implementation and sustained use of new person-centred outcome measures into care for children and young people with life-limiting and life-threatening conditions and their families.
| Recommendation | Sub-themes informing recommendations |
|---|---|
| 1. Children and families must be involved in the development of PCOMs to ensure that measure characteristics do not act as barriers to implementation | • Acceptability and usability for children • Language being meaningful |
| 2. Strategies for implementation should be designed collaboratively with professionals to ensure they are optimal | • Negative impacts on care • PCOMs not used as intended • Burden and capacity |
| 3. The benefits of PCOMs for care should be explained to families and professionals to facilitate implementation | • Explaining the benefits and securing ‘buy-in’ • Understanding what is important to patients and families • Improved communication and collaborative working • Standardising data collection and reporting |
| 4. The professional administering the measure should be known and trusted by the child and family | • Knowing and trusting measure administrators |
| 5. Completion of the measure should be a collaborative dialogue, in which the child, family and healthcare professional are fully involved where possible and appropriate | • Understanding what is important to patients and families • PCOMs not used as intended |
| 6. Professionals should respond appropriately to issues raised through completion of the measure and ensure children and families are involved and informed of any changes in care as a result | • Understanding what is important to patients and families • PCOMs not used as intended |
| 7. Discussions should be held with children and families to address privacy concerns, find out who they are comfortable with their information being shared with and to explain use and benefits of information-sharing in relation to PCOMs | • Confidentiality and information sharing • Explaining the benefits and securing ‘buy-in’ • Knowing and trusting measure administrators |
| 8. Once robustly and scientifically developed and validated, PCOMs should be translated to locally relevant languages, to increase usability for families where the local language is not their primary language | • Language barriers • Language being meaningful |
Strengths and weaknesses
Using framework analysis facilitated the structured management of a large dataset, and the matrix function allowed for comparison between groups. 68 This enabled the identification of factors that were specific to or common across the different participant groups, diagnoses or other participant or contextual factors. The use of the adapted-CFIR in the interpretation stage will enable the development of a theoretically informed strategy to support implementation of PCOMs in paediatric palliative care practice, shaped by key stakeholder perspectives.10,11,82,83
Due to the lack of existing measures in practice, 35 the implementation of PCOMs was presented in a hypothetical way to interview participants. The original interview topic guides also did not include prompts related to specific implementation factors as described by the adapted-CFIR. 73 Thus, further research is needed to understand what barriers may occur during implementation in practice in order to develop further strategies to facilitate implementation. Furthermore, this study was conducted in the UK through a western cultural lens and therefore the findings may not be as generalisable to other contexts.
Conclusion
Understanding the perspectives of the different stakeholder groups within the paediatric palliative care setting has supported the identification of several context specific strategies and development of eight recommendations to support implementation of new PCOMs. Next steps will include the development of an implementation strategy specific to the UK paediatric palliative care context, and exploratory work to further understand how implementing a novel outcome measure would work in practice.
Supplemental Material
Supplemental material, sj-pdf-1-pmj-10.1177_02692163241234797 for What are the anticipated benefits, risks, barriers and facilitators to implementing person-centred outcome measures into routine care for children and young people with life-limiting and life-threatening conditions? A qualitative interview study with key stakeholders by Hannah May Scott, Lucy Coombes, Debbie Braybrook, Daney Harðardóttir, Anna Roach, Katherine Bristowe, Myra Bluebond-Langner, Lorna K Fraser, Julia Downing, Bobbie Farsides, Fliss EM Murtagh, Clare Ellis-Smith and Richard Harding in Palliative Medicine
Acknowledgments
We thank the European Research Council and the NIHR Applied Research Collaboration South London (NIHR ARC South London) at King’s College Hospital NHS Foundation Trust for the financial support needed to undertake this study.
Footnotes
On behalf of C-POS authors: The Children’s Palliative care Outcome Scale (C-POS) Study Steering Group members are: AK Anderson, Jo Bayly, Lydia Bate (PPI), Myra Bluebond-Langner, Debbie Box, Katherine Bristowe, Rachel Burman, Lizzie Chambers, Lucy Coombes, Alan Craft, Fin Craig, Aislinn Delaney, Jonathan Downie, Julia Downing, Bobbie Farsides, Sara Fovargue, Lorna Fraser, Jane Green (PPI), Jay Halbert, Julie Hall-Carmichael, Irene Higginson, Michelle Hills, Mevhibe Hocaoglu, Vanessa Holme, Gill Hughes, Jo Laddie, Angela Logun (PPI), Eve Malam, Steve Marshall, Linda Maynard, Andrina McCormack, Catriona McKeating, Lis Meates, Fliss Murtagh, Eve Namisango, Veronica Neefjes, Cheryl Norman, Sue Picton, Christina Ramsenthaler, Anna Roach, Ellen Smith, Frances Waite, Michelle Ward and Mark Whiting.
Author contributions: All authors: conception and design of the work. LC, DB and AR: data collection. LC, DB, AR, DH and HS: data analysis. All authors: interpretation of data. HS draft of paper. All authors: critical review and revision of article.
Data management and sharing: No data are available. Not applicable.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The C-POS study is supported by the European Research Council’s Horizon 2020 programme [Grant ID: 772635]; this article reflects only the author’s views, and the European Research Council is not liable for any use that may be made of the information contained therein. The C-POS study is supported by the National Institute for Health and Care Research (NIHR) Applied Research Collaboration South London (NIHR ARC South London) at King’s College Hospital NHS Foundation Trust. The views expressed are those of the author[s] and not necessarily those of the NIHR or the Department of Health and Social Care. Hannah Scott, King’s College London, is supported by the National Institute for Health and Care Research (NIHR) Applied Research Collaboration South London (NIHR ARC South London) [Grant ID: NIHR-INF-2158] at King’s College Hospital NHS Foundation Trust. The views expressed are those of the author[s] and not necessarily those of the NIHR or the Department of Health and Social Care. Professor Fliss Murtagh is a UK National Institute for Health and Care Research (NIHR) Senior Investigator. The views expressed in this article are those of the author(s) and not necessarily those of the NIHR, or the Department of Health and Social Care. Professor Myra Bluebond-Langner’s post is supported by funding from The True Colours Trust. All research at Great Ormond Street Hospital NHS Foundation Trust is made possible by the NIHR Great Ormond Street Hospital Biomedical Research Centre. The funding bodies above did not have any role in the design of the study, collection, analysis, interpretation of data or writing of the manuscript.
Research ethics and patient consent: Ethical approval for the study was granted by the Bloomsbury research ethics committee (HRA:19/LO/0033). All participants ⩾16 years old provided written informed consent. Those with parental responsibility provided written informed consent for participants <16 years. Those <16 years provided written assent.
ORCID iDs: Hannah May Scott  https://orcid.org/0000-0003-1243-3500
https://orcid.org/0000-0003-1243-3500
Debbie Braybrook  https://orcid.org/0000-0001-9253-4955
https://orcid.org/0000-0001-9253-4955
Anna Roach  https://orcid.org/0000-0002-0635-0429
https://orcid.org/0000-0002-0635-0429
Katherine Bristowe  https://orcid.org/0000-0003-1809-217X
https://orcid.org/0000-0003-1809-217X
Myra Bluebond-Langner  https://orcid.org/0000-0001-9281-5431
https://orcid.org/0000-0001-9281-5431
Lorna K Fraser  https://orcid.org/0000-0002-1360-4191
https://orcid.org/0000-0002-1360-4191
Fliss EM Murtagh  https://orcid.org/0000-0003-1289-3726
https://orcid.org/0000-0003-1289-3726
Clare Ellis-Smith  https://orcid.org/0000-0003-3453-3203
https://orcid.org/0000-0003-3453-3203
Richard Harding  https://orcid.org/0000-0001-9653-8689
https://orcid.org/0000-0001-9653-8689
Supplemental material: Supplemental material for this article is available online.
References
- 1. Bradshaw A, Santarelli M, Khamis AM, et al. Implementing person-centred outcome measures (PCOMs) into routine palliative care: a protocol for a mixed-methods process evaluation of The RESOLVE PCOM Implementation Strategy. BMJ Open 2021; 11: e051904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bele S, Chugh A, Mohamed B, et al. Patient-reported outcome measures in routine pediatric clinical care: a systematic review. Front Pediatr 2020; 8: 364–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Antunes B, Harding R, Higginson IJ. Implementing patient-reported outcome measures in palliative care clinical practice: a systematic review of facilitators and barriers. Palliat Med 2014; 28: 158–175. [DOI] [PubMed] [Google Scholar]
- 4. Greenhalgh J, Gooding K, Gibbons E, et al. How do patient reported outcome measures (PROMs) support clinician-patient communication and patient care? A realist synthesis. J Patient Rep Outcomes 2018; 2: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Etkind SN, Daveson BA, Kwok W, et al. Capture, transfer, and feedback of patient-centered outcomes data in palliative care populations: does it make a difference? A systematic review. J Pain Symptom Manage 2015; 49: 611–624. [DOI] [PubMed] [Google Scholar]
- 6. Brock K, Wolfe J, Ullrich C. From the child’s word to clinical intervention: novel, new, and innovative approaches to symptoms in pediatric palliative care. Children 2018; 5: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Olde Rikkert MGM, Van Der Wees PJ, Schoon Y, et al. Using patient reported outcomes measures to promote integrated care. Int J Integr Care 2018; 18: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Øvretveit J, Zubkoff L, Nelson EC, et al. Using patient-reported outcome measurement to improve patient care. Int J Qual Health Care 2017; 29: 874–879. [DOI] [PubMed] [Google Scholar]
- 9. Connor SR, Downing J, Marston J. Estimating the global need for palliative care for children: a cross-sectional analysis. J Pain Symptom Manage 2017; 53: 171–177. [DOI] [PubMed] [Google Scholar]
- 10. Skivington K, Matthews L, Simpson SA, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ 2021: n2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Greenhalgh J. The applications of PROs in clinical practice: what are they, do they work, and why? Qual Life Res 2009; 18: 115–123. [DOI] [PubMed] [Google Scholar]
- 12. Huang I-C, Revicki DA, Schwartz CE. Measuring pediatric patient-reported outcomes: good progress but a long way to go. Qual Life Res 2014; 23: 747–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Engelen V, Haverman L, Koopman H, et al. Development and implementation of a patient reported outcome intervention (QLIC-ON PROfile) in clinical paediatric oncology practice. Patient Educ Couns 2010; 81: 235–244. [DOI] [PubMed] [Google Scholar]
- 14. Huang I-C, Shenkman EA, Madden VL, et al. Measuring quality of life in pediatric palliative care: challenges and potential solutions. Palliat Med 2010; 24: 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wolfe J, Orellana L, Cook EF, et al. Improving the care of children with advanced cancer by using an electronic patient-reported feedback intervention: results from the PediQUEST randomized controlled trial. J Clin Oncol 2014; 32: 1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wolfe J, Orellana L, Ullrich C, et al. Symptoms and distress in children with advanced cancer: prospective patient-reported outcomes from the PediQUEST study. J Clin Oncol 2015; 33: 1928–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hinds PS, Brandon J, Allen C, et al. Patient-reported outcomes in end-of-life research in pediatric oncology. J Pediatr Psychol 2007; 32: 1079–1088. [DOI] [PubMed] [Google Scholar]
- 18. Hinds PS, Nuss SL, Ruccione KS, et al. PROMIS pediatric measures in pediatric oncology: valid and clinically feasible indicators of patient-reported outcomes. Pediatr Blood Cancer 2013; 60: 402–408. [DOI] [PubMed] [Google Scholar]
- 19. Howell D, Molloy S, Wilkinson K, et al. Patient-reported outcomes in routine cancer clinical practice: a scoping review of use, impact on health outcomes, and implementation factors. Ann Oncol 2015; 26: 1846–1858. [DOI] [PubMed] [Google Scholar]
- 20. Meryk A, Kropshofer G, Hetzer B, et al. Implementation of daily patient-reported outcome measurements to support children with cancer. Pediatr Blood Cancer 2021: e29279. [DOI] [PubMed] [Google Scholar]
- 21. Nguyen H, Butow P, Dhillon H, et al. A review of the barriers to using Patient-Reported Outcomes (PROs) and Patient-Reported Outcome Measures (PROMs) in routine cancer care. J Med Radiat Sci 2021; 68: 186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Varni JW, Burwinkle TM, Katz ER, et al. The PedsQL in pediatric cancer: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales, Multidimensional Fatigue Scale, and Cancer Module. Cancer 2002; 94: 2090–2106. [DOI] [PubMed] [Google Scholar]
- 23. Scott HM, Braybrook D, Harðardóttir D, et al. Implementation of child-centred outcome measures in routine paediatric healthcare practice: a systematic review. Health Qual Life Outcomes 2023; 21: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Horan MR, Sim J-A, Krull KR, et al. A review of patient-reported outcome measures in childhood cancer. Children 2022; 9: 1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dussel V, Orellana L, Holder R, et al. A multisite randomized controlled trial of an early palliative care intervention in children with advanced cancer: the PediQUEST Response Study Protocol. PLOS ONE 2022; 17: e0277212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care 1999; 37: 126–139. [DOI] [PubMed] [Google Scholar]
- 27. Varni JW, Burwinkle TM, Seid M, et al. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr 2003; 3: 329–341. [DOI] [PubMed] [Google Scholar]
- 28. Varni JW, Limbers CA, Burwinkle TM. How young can children reliably and validly self-report their health-related quality of life?: An analysis of 8,591 children across age subgroups with the PedsQL™ 4.0 Generic Core Scales. Health Qual Life Outcomes 2007; 5: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Varni JW, Limbers CA, Newman DA. Factorial Invariance of the PedsQL™ 4.0 generic core scales child self-report across gender: a multigroup confirmatory factor analysis with 11,356 children ages 5 to 18. Appl Res Qual Life 2008; 3: 137–148. [Google Scholar]
- 30. Varni JW, Limbers CA, Newman DA. Using factor analysis to confirm the validity of children's self-reported health-related quality of life across different modes of administration. Clin Trials 2009; 6: 185–195. [DOI] [PubMed] [Google Scholar]
- 31. Amin L, Rosenbaum P, Barr R, et al. Rasch analysis of the PedsQL: an increased understanding of the properties of a rating scale. J Clin Epidemiol 2012; 65: 1117–1123. [DOI] [PubMed] [Google Scholar]
- 32. Varni JW, Limbers CA, Burwinkle TM. Parent proxy-report of their children's health-related quality of life: an analysis of 13,878 parents’ reliability and validity across age subgroups using the PedsQL™ 4.0 Generic Core Scales. Health Qual Life Outcomes 2007; 5: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Collins JJ, Byrnes ME, Dunkel IJ, et al. The measurement of symptoms in children with cancer. J Pain Symptom Manage 2000; 19: 363–377. [DOI] [PubMed] [Google Scholar]
- 34. Collins JJ, Devine TD, Dick GS, et al. The measurement of symptoms in young children with cancer. J Pain Symptom Manage 2002; 23: 10–16. [DOI] [PubMed] [Google Scholar]
- 35. Coombes LH, Wiseman T, Lucas G, et al. Health-related quality-of-life outcome measures in paediatric palliative care: a systematic review of psychometric properties and feasibility of use. Palliat Med 2016; 30: 935–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Downing J, Atieno M, Powell RA, et al. Development of a palliative care outcome measure for children in sub-Saharan Africa: findings from early phase instrument development. Eur J Palliat Care 2012; 19: 4. [Google Scholar]
- 37. Downing J, Namisango E, Harding R. Outcome measurement in paediatric palliative care: lessons from the past and future developments. Ann Palliat Med 2018; 7: S151–S163. [DOI] [PubMed] [Google Scholar]
- 38. Namisango E, Bristowe K, Allsop MJ, et al. Symptoms and concerns among children and young people with life-limiting and life-threatening conditions: a systematic review highlighting meaningful health outcomes. Patient 2019; 12: 15–55. [DOI] [PubMed] [Google Scholar]
- 39. Namisango E, Bristowe K, Murtagh FE, et al. Towards person-centred quality care for children with life-limiting and life-threatening illness: Self-reported symptoms, concerns and priority outcomes from a multi-country qualitative study. Palliat Med 2020; 34: 319–335. [DOI] [PubMed] [Google Scholar]
- 40. Namisango E, Bristowe K, Murtagh FE, et al. Face and content validity, acceptability, feasibility, and implementability of a novel outcome measure for children with life-limiting or life-threatening illness in three sub-Saharan African countries. Palliat Med 2022; 36: 1140–1153. [DOI] [PubMed] [Google Scholar]
- 41. Friedel M, Aujoulat I, Dubois A-C, et al. Instruments to measure outcomes in pediatric palliative care: a systematic review. Pediatrics 2019; 143: e20182379. [DOI] [PubMed] [Google Scholar]
- 42. Medical Research Council. Patient- Reported Outcome Measures (PROMs): Identifying UK research priorities. London: MRC, 2009. [Google Scholar]
- 43. Booth A, Maddison J, Wright K, et al. Research prioritisation exercises related to the care of children and young people with life-limiting conditions, their parents and all those who care for them: A systematic scoping review. Palliat Med 2018; 32: 1552–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Baker JN, Levine DR, Hinds PS, et al. Research priorities in pediatric palliative care. J Pediatr 2015; 167: 467–470.e463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Downing J, Knapp C, Muckaden MA, et al. Priorities for global research into children’s palliative care: results of an International Delphi Study. BMC Palliat Care 2015; 14: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Harding R, Chambers L, Bluebond-Langner M. Advancing the science of outcome measurement in paediatric palliative care. Int J Palliat Nurs 2019; 25: 72–79. [DOI] [PubMed] [Google Scholar]
- 47. Coombes L, Braybrook D, Roach A, et al. Achieving child-centred care for children and young people with life-limiting and life-threatening conditions: a qualitative interview study. Eur J Pediatr 2022; 181: 3739–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Coombes L, Bristowe K, Ellis-Smith C, et al. Enhancing validity, reliability and participation in self-reported health outcome measurement for children and young people: a systematic review of recall period, response scale format, and administration modality. Qual Life Res 2021; 30: 1803–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Coombes L, Harðardóttir D, Braybrook D, et al. Design and administration of patient-centred outcome measures: the perspectives of children and young people with life-limiting or life-threatening conditions and their family members. Patient 2023; 16: 473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Coombes L, Harðardóttir D, Braybrook D, et al. Achieving consensus on priority items for paediatric palliative care outcome measurement: Results from a modified Delphi survey, engagement with a children’s research involvement group and expert item generation. Palliat Med 2023; 37: 1509–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Coombes L, Braybrook D, Scott H, et al. OA11:01: comprehensibility, comprehensiveness and acceptability of a novel paediatric palliative care outcome measure: a cognitive interview study with children and families. In: 12th world research congress of the European Association for Palliative Care, 18th-20th May; Online; 2022; Palliative Medicine, 36(1_suppl): 34. [Google Scholar]
- 52. Mokkink LB, Terwee CB, Patrick DL, et al. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol 2010; 63: 737–745. [DOI] [PubMed] [Google Scholar]
- 53. Terwee CB, Prinsen CAC, Chiarotto A, et al. COSMIN methodology for evaluating the content validity of patient-reported outcome measures: a Delphi study. Qual Life Res 2018; 27: 1159–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rothrock NE, Kaiser KA, Cella D. Developing a valid patient-reported outcome measure. Clin Pharmacol Ther 2011; 90: 737–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007; 19: 349–357. [DOI] [PubMed] [Google Scholar]
- 56. Coombes L, Ellis-Smith C, Gao W, et al. I-31: Modified delphi survey to ascertain stakeholder consensus on priority outcomes for inclusion in the children’s palliative outcome scale (C-POS). In: 17th world congress of the EAPC 2021, 6th – 8th October; Online; 2021, Palliative Medicine, 35(1_suppl): 139–140. [Google Scholar]
- 57. Scott HM, Coombes L, Braybrook D, et al. COVID-19: impact on pediatric palliative care. J Pain Symptom Manage 2022; 64: e1–e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Braybrook D, Coombes L, Roach A, et al. The meaning of ‘normality’ among children and young people with life-limiting or life threatening conditions. In: 5th Maruzza international congress on paediatric palliative care, 26 May; Rome; 2022, Online:https://www.childrenpalliativecarecongress.org/congress-2022/oral-abstract-presentations/ [accessed 24/02/2024]. [Google Scholar]
- 59. Scott HM, Coombes L, Braybrook D, et al. Spiritual, religious, and existential concerns of children and young people with life-limiting and life-threatening conditions: a qualitative interview study. Palliat Med 2023; 37: 856–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Scott H, Coombes L, Braybrook D, et al. O38 Challenges to implementing person-centred outcome measures into routine paediatric palliative care. In: 5th UK Implementation Science Conference 2022; 14th 15th July; online; 2022, Implementation Science, 18(Suppl 1):20. [Google Scholar]
- 61. Scott H, Coombes L, Braybrook D, et al. P02:37: Challenges and incentives for integrating person-centred outcome measures into routine paediatric palliative care: health, social care and commissioner perspectives. In: 12th world research congress of the European association for palliative care. 18th-20th May; Online; 2022; Palliative Medicine, 36(1_suppl): p.56. [Google Scholar]
- 62. Scott HM, Braybrook D, Ellis-Smith C, et al. 230 Implementing child-centred outcome measures into routine practice: a systematic review. Arch Dis Childhood 2023; 108: A319–A319. [Google Scholar]
- 63. Hinds PS, Menard JC, Jacobs SS. The child’s voice in pediatric palliative and end-of-life care. Progress Palliat Care 2012; 20: 337–342. [Google Scholar]
- 64. Gerlach A, Varcoe C. Orienting child- and family-centered care toward equity. J Child Health Care 2021; 25: 457–467. [DOI] [PubMed] [Google Scholar]
- 65. Committee on Hospital Care and Institute for Patient-and Family-Centered Care. Patient- and family-centered care and the pediatrician’s role. Pediatrics 2012; 129: 394–404. [DOI] [PubMed] [Google Scholar]
- 66. Chambers L. A Guide to Children’s Palliative Care: Supporting babies, children and young people with life-limiting and life-threatening conditions and their families. Bristol: Together for Short Lives, 2018. [Google Scholar]
- 67. Low J. A Pragmatic definition of the concept of theoretical saturation. Sociol Focus 2019; 52: 131–139. [Google Scholar]
- 68. Gale NK, Heath G, Cameron E, et al. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013; 13: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rivas C. Coding and analysing qualitative data. In: Seale C. (ed.) Researching society and culture. 3rd ed. London: Sage, 2012. [Google Scholar]
- 70. Church S, Dunn M, Prokopy L. Benefits to qualitative data quality with multiple coders: two case studies in multi-coder data analysis. J Rural Soc Sci 2019; 34: 2.37559698 [Google Scholar]
- 71. Roberts K, Dowell A, Nie J-B. Attempting rigour and replicability in thematic analysis of qualitative research data; a case study of codebook development. BMC Med Res Methodol 2019; 19: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Damschroder LJ, Aron DC, Keith RE, et al. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 2009; 4: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Safaeinili N, Brown-Johnson C, Shaw JG, et al. CFIR simplified: Pragmatic application of and adaptations to the Consolidated Framework for Implementation Research (CFIR) for evaluation of a patient-centered care transformation within a learning health system. Learn Health Syst 2020; 4: 10201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lind S, Sandberg J, Brytting T, et al. Implementation of the integrated palliative care outcome scale in acute care settings – a feasibility study. Palliat Support Care 2018; 16: 698–705. [DOI] [PubMed] [Google Scholar]
- 75. Högberg C, Alvariza A, Beck I. Patients’ experiences of using the Integrated Palliative care Outcome Scale for a person-centered care: a qualitative study in the specialized palliative home-care context. Nurs Inq 2019; 26: e12297. [DOI] [PubMed] [Google Scholar]
- 76. Rost M, Mihailov E. In the name of the family? Against parents’ refusal to disclose prognostic information to children. Med Health Care Philos 2021; 24: 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mitchell S, Bennett K, Morris A, et al. Achieving beneficial outcomes for children with life-limiting and life-threatening conditions receiving palliative care and their families: a realist review. Palliat Med 2020; 34: 387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Aldridge J, Shimmon K, Miller M, et al. ‘I can’t tell my child they are dying’. Helping parents have conversations with their child. Arch Dis Child Educ Pract Ed 2017; 102: 182–187. [DOI] [PubMed] [Google Scholar]
- 79. Bausewein C, Schildmann E, Rosenbruch J, et al. Starting from scratch: implementing outcome measurement in clinical practice. Ann Palliat Med 2018; 7: S253–S261. [DOI] [PubMed] [Google Scholar]
- 80. Dreier LA, Zernikow B, Wager J. Quantifying the language barrier—a total survey of parents’ spoken languages and local language skills as perceived by different professions in pediatric palliative care. Children 2020; 7: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Brock KE, Steineck A, Twist CJ. Trends in end-of-life care in pediatric hematology, oncology, and stem cell transplant patients. Pediatr Blood Cancer 2016; 63: 516–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Stover AM, Haverman L, Van Oers HA, et al. Using an implementation science approach to implement and evaluate patient-reported outcome measures (PROM) initiatives in routine care settings. Qual Life Res 2021; 30: 3015–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Nilsen P. Making sense of implementation theories, models and frameworks. Implement Sci 2015; 10: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-pmj-10.1177_02692163241234797 for What are the anticipated benefits, risks, barriers and facilitators to implementing person-centred outcome measures into routine care for children and young people with life-limiting and life-threatening conditions? A qualitative interview study with key stakeholders by Hannah May Scott, Lucy Coombes, Debbie Braybrook, Daney Harðardóttir, Anna Roach, Katherine Bristowe, Myra Bluebond-Langner, Lorna K Fraser, Julia Downing, Bobbie Farsides, Fliss EM Murtagh, Clare Ellis-Smith and Richard Harding in Palliative Medicine