Abstract
BACKGROUND
We previously reported increased moderate-intensity (3–6 metabolic equivalents (METs)) physical activity (PA) reverses aging-associated vascular endothelial dysfunction, a surrogate marker of cardiovascular risk. Whether reductions in sedentary time alone contribute to this improvement is unknown.
METHODS
Data from 96 adults (aged ≥50 years) enrolled in a randomized control trial evaluating a 12-week intervention to increase PA in sedentary individuals were analyzed. Amount and intensity of activity were measured pre- and post-intervention by step count and accelerometry. Subjects were divided into 3 categories based on change in sedentary activity (<1. 5 METs): (i) ≥5% reduction in sedentary time, (ii) 0–4.99% reduction, and (iii) increase sedentary time. Vascular endothelial function was measured by brachial artery flow-mediated dilation (FMD%) pre- and post-intervention.
RESULTS
Sedentary time decreased overall ( P = 0.001), with a 101-minute decrease in category 1 ( N = 27, P < 0.001), a 42-minute decrease in category 2 ( N = 29, P = 0.003), and a 44-minute increase in category 3 ( N = 40, P = 0.02). While FMD% increased in the entire study population ( P = 0.008) over 12 weeks, no differences were observed between the categories ( P = 0.73). In category 1, FMD% improvement was associated achievement of ≥20 minutes/day of moderate intensity PA in bouts ≥ 10 minutes in length.
CONCLUSIONS
Reductions of up to 100 minutes of sedentary time per day over 12 weeks was not significantly associated with improved vascular endothelial function in older adults. FMD% was significantly higher among those with lower sedentary behavior and concomitant moderate-intensity PA of ≥20 minutes/day in bouts.
Keywords: blood pressure, endothelial function, exercise, hypertension, physical activity, sedentary time, steps, walking
Sedentary behavior is defined as “any waking behavior characterized by an energy expenditure ≤1.5 metabolic equivalents (METs) while in a sitting or reclining posture.” 1 Sedentary lifestyles are associated with increased risks of death and cardiovascular (CV) disease. 2 , 3 Prolonged sitting time is associated with obesity, CV disease, diabetes, and cancer. 4–6
Sedentary behavior’s adverse CV effects appear to be mediated by its induction of vascular endothelial dysfunction, an early contributor to atherogenesis and predictor of adverse CV events. 7 Cross-sectional studies indicate that sedentary lifestyles are associated with both impaired vascular endothelial function and adverse vascular remodeling. Interventional studies involving prolonged immobilization demonstrate sedentarism results in rapid impairment of vascular endothelial function, 8 significant resting blood flow reductions, impaired reactive hyperemia, and reduced conduit vessel diameter leading to increased resting arterial tone. 8–15 While these models have provided important insight into the physiologic remodeling of human vasculature that accompanies extreme physical inactivity, it is important to establish an understanding of how standard levels of daily sedentary behavior impact the vasculature.
Epidemiological data suggest that sedentary behavior, independent of any improvements in physical activity (PA) levels, may be a distinct risk factor for adverse CV outcomes. 16 , 17 Due to their high rates of sedentarism, adults older than 60 years may suffer disproportionately from the adverse health impact of sedentary behavior. 18 However, the quantitative association between reducing sedentary behavior and reducing CV risk remains unknown and whether improvements in metabolic regulation and vascular structure and function are associated with decreases in sedentary time remain to be explored in this at-risk population. In the context, we examined the impact of reducing sedentary time on vascular function and metabolic parameters.
METHODS
Subjects
The Medical College of Wisconsin’s Institutional Research Board approved the study protocol, and all participants were provided written informed consent. The methods, organization, and design of the randomized clinical trial from which the data for these analyses were collected have been described in detail previously. 19 Briefly, 114 sedentary older adults (aged ≥50 and ≤80 years) were recruited. The inclusion and exclusion criteria are as previously described. 19 All subjects averaged <8,000 steps/day over a 1-week screening period as measured by the Omron HJ-720ITC pedometer (Omron, Lake Forest, IL). Potential subjects were blinded to the step count limit for study inclusion. Subjects in the original trial were randomized to 1 of 3 intervention groups: control, pedometer alone, and pedometer with interactive web teaching. Of the 107 subjects who completed the study, 11 subjects did not have an adequate valid accelerometer wear time during their 12-week activity intensity assessments to allow for study inclusion (see below for inclusion/exclusion criteria specific to these data). This analysis focuses on the 96 subjects who had complete sets of accelerometry data captured at both the time of enrollment and the end of the 12-week study period. Subjects were asked not to change dietary habits during the study period.
Study Visit Procedures (Prior to and following the 12 week intervention period): General Procedures and Measures of Vascular Form and Function: All subjects fasted overnight prior to their study visits. Anthropomorphics, heart rate, and blood pressure were measured and blood samples taken for biomarker analyses as previously described. 19 Endothelial function by brachial artery reactivity (flow-mediated dilation, FMD%) and carotid pulse wave velocity and augmentation index by digital tonometry equipment and software (Sphygmocor Mx; Atcor Medical, Chicago, IL) were also measured as previously described. 19 Insulin resistance was calculated using both the homeostatic model of assessment-insulin resistance [HOMA-IR = fasting glucose (mg/dl) × fasting insulin (μU/ml)/405] and the quantitative insulin sensitivity check test [QUICKI = 1/([log (fasting insulin μU/ml) + log (fasting glucose mg/dl)]]. In subjects without contraindication to nitroglycerin, nitroglycerin-mediated dilatation (NMD%) by ultrasound of the brachial artery was measured to determine endothelium-independent vasodilation. Baseline and peak hyperemic flow velocity were also recorded during brachial artery reactivity testing to calculate resting and hyperemic shear stress in the brachial artery as previously described. 20 If an individual did not have quality waveforms as determined by digital criteria at both weeks 1 and 12, the vascular stiffness measurements were not included in the analyses. The numbers of subjects that had quality waveforms for analyses are explicitly listed in the relevant tables.
Physical Activity Data: Step count and accelerometry data were collected using the Omron HJ-720ITC and ActiGraph GT3X (ActiGraph GTX3; ActiGraph, Pensacola, FL), respectively, for 1 week at the time of enrollment and during the final week of the 12-week intervention period. Each minute of accelerometer data was coded as sedentary activities (0–100 counts, ≤1.5 METs intensity), light activity (101–1951 counts, 1.51–3 METs), moderate-intensity activity (1,952–5,924 counts, 3.01–6 METs), or vigorous activity (>5,925 counts, >6 METs). 21 Further, a bout of PA was defined as at least 10 consecutive minutes of at least moderate-intensity PA (3–6 METs, vigorous PA; 6 METs).
Accelerometer data were screened according to the following quality control procedures: (i) Any block of time equal to or greater than 60 minutes where the accelerometer recorded 0 counts was considered time when the monitor was not worn and was removed from analysis, (ii) to constitute a valid day of accelerometer wear, participants had to have a minimum of 600 minutes of valid wear time, and (iii) only participants who had at least 4 days of valid accelerometer data were included in analysis. 22
Statistical analysis
Statistical analyses were performed using SigmaStat 12.0 (Systat Software Inc., San Jose, CA) and SPSS 21.0 (IBM, Armonk, NY). Our primary vascular outcome variable was FMD%. Secondary vascular outcomes include carotid pulse wave velocity, augmentation index, aortic baseline brachial diameter, baseline peak shear, hyperemic peak shear, and nitroglycerin-mediated dilation of the brachial artery. Subjects were divided into categories, based on change in percent of sedentary activity (<1.5 METs) over the study period: (i) 5% or greater reduction in sedentary time, (ii) 0–4.99% reduction, and (iii) increase in sedentary time. These categories were chosen to have reasonable numbers of subjects in each group with one group of individuals with a substantial decrease in sedentary time. Vascular outcome data were secondarily analyzed by strict tertiles of percent change in sedentary activity from baseline, where the tertile ranges are the following: >1.25% decrease (tertile 1), 1.25% decrease to 4.04% increase (tertile 2), and >4.05% increase in sedentary time. The baseline characteristics were compared using one-way analysis of variance or χ2 as appropriate ( Figure 1 ) Anthropomorphic measurements, plasma biomarkers, measurements of endothelial function and vascular stiffness, step-count, and time spent in differing levels of PA intensity were compared using general linear models with time (measurements pre- and post-12-week intervention period) as the within-subjects factor and between the 3 sedentary time change categories as the between-subjects factor. Category-by-time interactions were analyzed for all outcomes. Post hoc testing was performed using Tukey’s honest significant difference test as appropriate. To determine whether concomitant PA modified the impact of changing sedentary time, we repeated the general linear models for brachial FMD% by whether or not the individuals achieved a ≥5% reduction in sedentary time and by whether or not they concomitantly achieved the goal of ≥20 minutes/day of moderate-intensity PA (3–6 METs) in bouts of at least 10 minute of continuous activity PA. This PA goal was shown to impact brachial FMD% favorably in our previously reported clinical trial. 19
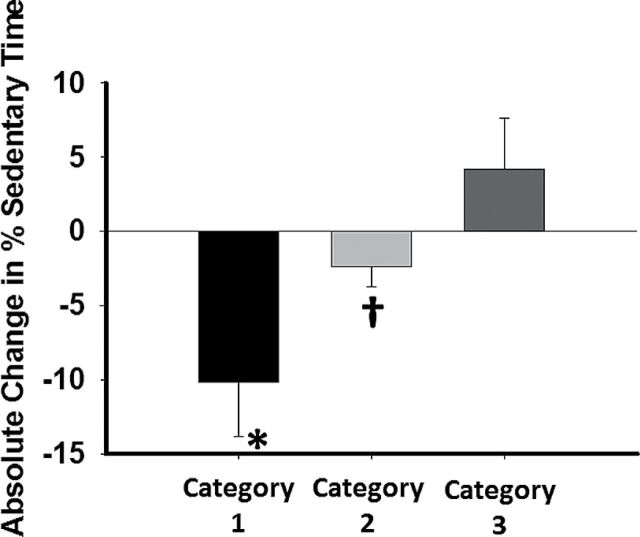
Figure 1.
Percentage change in overall sedentary time by category. P < 0.001 overall by analysis of variance (ANOVA), * P < 0.001 for category 1 vs. both categories 2 and 3. †P < 0.001 vs. category 3.
RESULTS
Baseline characteristics
There were no significant differences between the categories of sedentary time change with respect to all baseline characteristics ( Table 1 ). Of those in category 1, 6 individuals were in the control group in the clinic trial, 13 in the pedometer only group, and 8 in the pedometer plus interactive website group. For category 2, the subject breakdown by original randomization was 8, 10, and 11 subjects, respectively. For category 3, the breakdown was 21, 9, and 10, respectively.
Table 1.
Baseline demographics and characteristics by category
| 5% or greater reduction in sedentary time ( N = 27) | 0–4.99% reduction ( N = 29) | Increase in sedentary time ( N = 40) | P -value | |
|---|---|---|---|---|
| Female sex% | 74.0 (20) | 51.7 (15) | 65.0 (26) | 0.22 |
| Current smoker% | 7.4 (2) | 6.8 (2) | 10.0 (4) | 0.76 |
| History of diabetes% | 0 (0) | 0 (0) | 2.5 (1) | 0.49 |
| History of hypertension% | 33.3 (9) | 31.0 (9) | 30.0 (12) | 0.96 |
| Caucasian% | 92.6 (25) | 93.1 (27) | 100.0 (40) | 0.38 |
| Age (years) | 62.22±5.7 | 62.8±6.6 | 62.3±7.3 | 0.94 |
| Weight (kg) | 82.0±17.4 | 88.3±16.8 | 82.3±20.7 | 0.35 |
| Body mass index (kg/m 2 ) | 29.4±5.4 | 30.0±6.1 | 29.4±6.3 | 0.94 |
| Waist circumference (cm) | 100.3±14.7 | 101.2±11.8 | 100.7±15.4 | 0.97 |
| Glucose (mg/dl) | 88.2±8.2 | 89.0±7.6 | 94.8±23.9 | 0.21 |
| Insulin (µU/l) | 12.7±6.5 | 14.4±7.5 | 15.8±9.0 | 0.32 |
| Total cholesterol (mg/dl) | 131±35 | 136±30 | 129±35 | 0.69 |
| Triglycerides | 89±42 | 72±27 | 83±26 | 0.12 |
| HDL cholesterol (mg/dl) | 43±18 | 46±10 | 40±16 | 0.29 |
| LDL cholesterol (mg/dl) | 71±34 | 77±31 | 71±37 | 0.74 |
| hsCRP (mg/dl) | 2.9±3.3 | 2.9±2.1 | 4.1±5.1 | 0.34 |
| Systolic blood pressure | 129±16 | 131±12 | 130±14 | 0.93 |
| Diastolic blood pressure | 68±8 | 71±7 | 68±8 | 0.15 |
| Heart rate (bpm) | 65±8 | 65±11 | 61±9 | 0.17 |
| QUICKI | 0.3±0.0 | 0.3±0.0 | 0.3±0.0 | 0.24 |
| HOMA-IR | 2.8±1.4 | 3.2±1.8 | 3.9±2.7 | 0.12 |
| Trial randomization group | ||||
| Control ( N ) | 6 | 8 | 21 | 0.06 |
| Pedometer alone ( N ) | 13 | 10 | 9 | |
| Pedometer + website ( N ) | 8 | 10 | 11 |
Abbreviations: HDL, high-density lipoprotein; HOMA-IR, homeostatic model of assessment-insulin resistance; hsCRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; QUICKI, quantitative insulin sensitivity check test.
Changes in baseline characteristics by sedentary time change category
Over the study period, weight ( P = 0.02), body mass index ( P = 0.001), waist circumference ( P = 0.02), and heart rate ( P = 0.03) decreased for the entire cohort. However, there were no significant differences over time based on sedentary time change category for weight and waist circumference. Body mass index showed a significant decrease over time based on category, with a significant decrease occurring only in category 1 (category 1: −1.1±2.0, category 2: -0.2±0.7 and category 3: −0.2±1.4 ( P = 0.03)). There were no significant differences between categories at 12 weeks for blood pressure, fasting glucose, insulin sensitivity, lipid profile, or C-reactive protein ( Table 2 ).
Table 2.
Changes in baseline characteristics by category
| 5% or greater reduction in sedentary time ( N = 27) | 0–4.99% reduction ( N = 29) | Increase in sedentary time ( N = 40) | P -values (time effect) | P -values (time × group interaction) | |
|---|---|---|---|---|---|
| Weight (kg) | −1.3±3.6 | −0.13±1.6 | −1.2±4.5 | 0.02 | 0.37 |
| Body mass index (kg/m 2 ) | −1.1±2.0 | −0.2±0.7 | −0.2±1.4 | 0.001 | 0.03 |
| Waist circumference (cm) | −1.7±3.7 | −1.2±3.1 | −0.6±6.0 | 0.02 | 0.63 |
| Glucose (mg/dl) | 0.9±6.0 | −0.3±6.0 | 0.3±9.4 | 0.7 | 0.86 |
| Insulin (µU/l) | −0.5±4.0 | −0.3±4.1 | −0.3±5.0 | 0.46 | 0.97 |
| Total cholesterol (mg/dl) | −2±36 | 0±32 | 8±39 | 0.66 | 0.53 |
| Triglycerides | −5±26 | 3±22 | 8±26 | 0.5 | 0.15 |
| HDL cholesterol (mg/dl) | 4±18 | 5±14 | 0±14 | 0.1 | 0.38 |
| LDL cholesterol (mg/dl) | −5±40.0 | −6±33 | 7±37 | 0.74 | 0.35 |
| hsCRP (mg/dl) | 0.8±2.4 | 1.1±3.9 | −0.1±2.5 | 0.07 | 0.29 |
| Systolic blood pressure | 0±11 | −1±11 | −4±11 | 0.11 | 0.31 |
| Diastolic blood pressure | 1±7 | 0±6 | 0±5 | 0.82 | 0.69 |
| Heart rate (bpm) | −3±6 | −1±6 | −1.0±5 | 0.03 | 0.05 |
| QUICKI | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.55 | 0.83 |
| HOMA-IR | −0.1±1.0 | 0.0±1.0 | 0.0±1.3 | 0.72 | 0.99 |
Abbreviations: HDL, high-density lipoprotein; HOMA-IR, homeostatic model of assessment-insulin resistance; hsCRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; QUICKI, quantitative insulin sensitivity check test.
Changes in sedentary time and step count by category
The full set of accelerometry data by sedentary time category are reported in Table 3 . At baseline, category 2 had significantly more sedentary time than category 3 but otherwise there were no differences ( P = 0.02 overall and 0.02 for category 2 vs. category 3). After 12 weeks, the average time spent at sedentary activity levels significantly decreased in categories 1 and 2 and increased in category 3 [ P = 0.001 for overall time × group interaction; category 1: 653±121 to 552±100 minutes ( P < 0.001), category 2: 699±92 to 657±70 ( P = 0.003), and category 3: 629±93 to 673±120 ( P = 0.02)]. The percentage of sedentary time dropped by 10.2±3.6% in category 1 and 2.4±1.3% in category 2, whereas increasing by 4.2±3.4% in category 3 ( P < 0.001 overall, P < 0.001 for all post hoc comparisons between categories).
Table 3.
Step count and physical activity data by category
| 5% or greater reduction in sedentary time | 0–4.99% reduction | Increase in sedentary time | P -values (time effect) | P -values (time × group interaction) | ||||
|---|---|---|---|---|---|---|---|---|
| N = 27 (step counts) | N = 28 (step count) | N = 38 (step count) | ||||||
| N = 27 (accelerometry) | N = 29 (accelerometry) | N = 40 (accelerometry) | ||||||
| Baseline | Week 12 | Baseline | Week 12 | Baseline | Week 12 | |||
| Average step count | 5,072±1,759 | 10,150±3,736 | 5,132±1,799 | 7,891±3,446 | 5,317±1,617 | 5,610±3,017 | <0.001 | <0.001 |
| Average minutes observed/ day | 925.2±128.1 | 911.3±110.2 | 953.5±89.7 | 926.7±72.6 | 912.4±87.4 | 919.5±135.6 | 0.35 | 0.47 |
| Average minutes: sedentary (≤1.5 METs) | 653±121 | 552±100 | 699±92 | 657±70 | 629±93 | 673±120 | 0.001 | <0.001 |
| Average light activity minutes (1.5–3 METs) | 252±63 | 310±64 | 232±63 | 230±63 | 267±69 | 228±60 | 0.18 | <0.001 |
| Average moderate- intensity activity minutes (3–6 METs) | 20±12 | 49±27 | 22±15 | 40±26 | 16±10 | 18±19 | <0.001 | <0.001 |
| Average moderate intensity in bouts | 26±30 | 40±31 | 26±28 | 43±30 | 19±26 | 22±29 | 0.19 | 0.002 |
| Percentage of total time spent sedentary | 70.1±6.7 | 60.3±6.7 | 73.3±6.5 | 71.0±6.1 | 68.9±7.4 | 73.1±6.6 | <0.001 | <0.001 |
Abbreviation: MET, metabolic equivalent.
Changes in brachial artery endothelial function and vascular stiffness by change in sedentary time
Full data on the vascular changes over the study period by sedentary time change category are presented in Table 4 . Brachial FMD% improved for the entire study population (5.7±2.7% to 6.9±3.6% P = 0.006). However, this increase did not differ based on sedentary time category ( P = 0.73, category 1: 5.4±2.9 to 7.0±3.6%, category 2: 5.7±2.9 to 6.9±4.3%, category 3: 6.0±2.5 to 6.7±3.6%). Analyzing the entire group as continuous data, there were no correlations between change in FMD% and change in sedentary time over the entire cohort ( r = −0.04, P = 0.70), change in body mass index ( r = −0.03, P = 0.77), change in waist circumference ( r = 0.10, P = 0.30), or change in heart rate ( r = −0.03, P = 0.77). Adjustment for sex did not alter our results ( P = 0.51). Organizing the data into 3 equal tertiles did not alter our findings ( P = 0.86, tertile 1: 5.6±2.4 to 6.6±3.2%, tertile 2: 6.1±3.0 to 7.1±3.9%, category 3: 5.4±2.8 to 6.9±3.5%).
Table 4.
Endothelial function and vascular compliance data by category
| 5% or greater reduction in sedentary time | 0–4.99% reduction | Increase in sedentary time | P -values (time effect) | P -values (time × group interaction) | ||||
|---|---|---|---|---|---|---|---|---|
| ( N = 27) | ( N = 27) | ( N = 39) | ||||||
| Baseline | Week 12 | Baseline | Week 12 | Baseline | Week 12 | |||
| Baseline brachial diameter (mm) | 3.86±0.74 | 3.80±0.81 | 3.91±0.72 | 3.92±0.74 | 3.66±0.75 | 3.68±0.76 | 0.76 | 0.63 |
| Baseline peak shear (dynes/ cm 2 ) | 43±14 | 41±14 | 42±12 | 39±12 | 44±14 | 43±13 | 0.09 | 0.9 |
| Hyperemic peak shear (dynes/ cm 2 ) a | 71±20 | 75±36 | 74±24 | 71±20 | 78±21 | 73±24 | 0.53 | 0.35 |
| Nitroglycerin- mediated dilation(%) b | 19.9±6.1 | 19.1±6.3 | 21.0±8.0 | 17.90±7.6 | 22.1±5.7 | 19.7±5.9 | 0.05 | 0.66 |
| Carotid- femoral pulse wave velocity (cm/sec) c | 9.6±2.3 | 9.1±2.5 | 9.9±1.6 | 9.9±1.5 | 9.3±2.1 | 9.2±1.9 | 0.31 | 0.56 |
| Augmentation index d | 28.3±8.0 | 25.48±7.1 | 24.30±8.5 | 25.2±17.0 | 28.4±10.0 | 25.8±9.6 | 0.19 | 0.34 |
| Aortic systolic blood pressure d | 120±13 | 121±15 | 120±10 | 120±13 | 119±13 | 118±14 | 0.81 | 0.63 |
| Aortic diastolic blood pressure d | 69±8 | 69±9 | 73±7 | 72±8 | 68±7 | 69±8 | 0.91 | 0.71 |
a N = 26, 27, and 38 for category groups 1, 2, and 3, respectively. bN = 21, 18, and 25 for category groups 1, 2, and 3, respectively. cN = 24, 23, and 23 for category groups 1, 2, and 3, respectively. dN = 27, 27, and 36 for category groups 1, 2, and 3, respectively.
There were no significant changes in brachial artery diameter, baseline and peak hyperemic shear, carotid pulse wave velocity, augmentation index, or nitroglycerin-mediated vasodilation over the study period within or between categories ( Table 4 ). Similar analyses by tertile of activity change were also not significant (data not shown).
Changes in brachial FMD% in association to achieving physical activity goals
When we analyze the data stratified by the original study’s intervention group, there were no significant differences in FMD% in those that were in the control group ( P = 0.10), in the pedometer alone group ( P = 0.52), or in the pedometer + website group ( P = 0.31) when compared across sedentary time categories. In the 31 subjects who achieved ≥20 minutes of moderate-intensity PA in bouts of at least 10 minutes in duration, increasing FMD% tended toward but was not significantly associated with reducing sedentary time by ≥ 5% ( P = 0.12). For those achieving both metrics ( N = 12), FMD% significantly increased from 4.1±2.5% to 8.2±4.2% ( P = 0.01). For those achieving only the moderate-intensity PA goal but not the ≥5% reduction in sedentary time ( N = 19), FMD% increased from 6.0±3.3% to 7.7±3.6% ( P = 0.04). For those who reduced sedentary time by ≥ 5% but did not increase moderate-intensity PA in bouts to ≥20 minutes/day in continuous bouts of ≥10 minutes, no significant change in FMD% was observed ( N = 15, 6.4±2.9% to 6.0±2.9%, P = 0.72).
Multivariable analyses of the entire data set for the associations of change in FMD with changes in sedentary time, moderate-intensity bout time, and the interaction of the two (product term) did not show interaction between the change in sedentary time and change in moderate-intensity PA in bouts ( R = 0.16, P = 0.54 for the entire model; standardized β = 0.10, P = 0.48 for the interaction term; standardized β = −0.11, P = 0.37 for sedentary time change; and standardized β = 0.08, P = 0.54 for moderate PA in bout time change). However, when the analysis included only those who achieved ≥20 minutes of moderate-intensity PA in bouts of at least 10 minutes in duration, model prediction improved ( R = 0.44, P = 0.12 for the entire model; standardized β = −0.34, P = 0.43 for the interaction term; β = 0.65, P = 0.09 for the change in sedentary time) although did not reach statistical significance. The addition of body mass index change to the model did not alter the predictive value of either model.
DISCUSSION
Our data demonstrate that in healthy, free-living adults aged ≥50 years, reducing sedentary time by ~100 minutes per day is not associated with vascular endothelial function in the absence of a concomitant increase in moderate-intensity PA performed in bouts as previously described. 19 Further, this level of reduction in sedentary time may not be associated with vascular stiffness or insulin sensitivity. Previous work showed the vascular and metabolic impact of short-term forced near complete immobilization. 8 The current study extends this by demonstrating more modest yet significant reductions in sedentary time in free-living healthy older adults may not have a favorable impact on vascular function or insulin sensitivity. Further, our data provide a new context through which to view cross-sectional epidemiological data on the association between sedentary time and cardiometabolic risk. Specifically, a singular focus on reducing sedentary time alone may not be enough to impact vascular and metabolic health favorably in older adults.
Previous human intervention studies commonly employ extreme reductions in step count, short-term imposed bed rest, or dry water immersion models to test the CV and metabolic impact of extreme inactivity. 8 , 23 Collectively, these data demonstrate the deleterious impact of near around-the-clock inactivity, bed rest, or anti-gravity suspension adversely impact vascular endothelial function and insulin sensitivity. 9–13 , 24 ,25 In bed rest models, the vascular findings are most striking in the lower extremities, the limbs with the greatest limitations in free movement. 10 While establishing sedentary behavior in these models adversely impacts vascular function and insulin sensitivity, the extreme amount and types of inactivity in these studies leave unanswered whether the much less extreme and commonly encountered sedentary behaviors of free living, ambulatory adults comprised commonly by large amounts of sitting similarly impacts cardiometabolic risk. 23 Our data offer an important addition to this literature by demonstrating that, in isolation, a reduction in free-living sedentary activity by 10%, approximately 100 minutes/day, may not lead to an appreciable improvement in either vascular endothelial function or insulin sensitivity in older adults. Whether larger reductions in free-living sedentary time or similar reductions over a longer period of observation would result in a favorable impact remains unknown, although cross-sectional data do suggest a possible favorable impact of longer-term changes. 26 These questions merit further investigation in future studies.
A unique aspect of this investigation is its focus on alterations of sedentary time in adults ≥50 years old in association with changes in vascular structure and function and insulin sensitivity. Previously mentioned intervention studies have primarily focused on younger, healthy adults, 9–13 , 24 ,25 with one study performed in a pediatric population showing no association between seasonal changes in sedentary behavior and conduit vessel endothelial function. 27 To our knowledge, our data are the first of its kind in older adults and appear generally consistent with the data from the aforementioned pediatric study with respect to the vascular impact of changes in sedentary time in ambulatory individuals. Whether more extreme alterations in sedentary activity alter vascular form and physiology and insulin sensitivity in older adults remains unknown.
Epidemiological data examining the association between sedentary behavior and cardiometabolic risk suggest individuals engaged in a greater amount of sedentary behaviors are at higher risk to develop diabetes and CV disease. 3 Activities such as prolonged TV watching are associated with increased CV risk and insulin resistance. 5 , 6 These data suggest increased sedentary/sitting time, in some cases independent of the level of moderate to vigorous intensity activity, 28 , 29 is associated with increased cardiometabolic risk. 2–4 , 28 –30 Breaking up continuous sedentary time with intermittent breaks is associated with reduced CV risk and its potential favorable impact is biologically plausible, 2 , 31 , 32 but the length of break and activity intensity necessary for risk reduction remain unknown. 30 In contrast to these epidemiological data, we found no significant association between changes in sedentary time and cardiometabolic risk markers in the absence of a significant increase in moderate-intensity PA. Differences between findings may relate to differences in study design, populations studied, and differences in capturing overall sedentary and active time. Our study specifically quantified the amount and intensity of activity of our study participants via objective accelerometry, an important strength of our design.
Our study has several limitations. Our study cohort represented relatively healthy adults group of older adults. It is possible that a reduction in sedentary time of the magnitude seen in category 1 in our study may have a different effect in older adults with a greater burden of prevalent CV risk factors and disease. As mentioned in the methods, 11 subjects did not have adequate accelerometer data at their 12-week visit. There were no significant differences between this population of subjects and the 96 subjects in this report in relationship to subject baseline demographics (data not shown). Post hoc power analysis suggests that this study has 80% power to detect and effect size of 0.33. This suggests that our study could miss a small-to-moderate change in FMD% related to the changes in sedentary time observed but not a moderate to large effect. Balanced against these limitations are the novelties of the data with respect to the population studied (older adults) and the focus on “real world” changes in sedentary time associated with vascular function, vascular structure, and insulin sensitivity.
CLINICAL PERSPECTIVES
In summary, our data suggest that reductions in sedentary time up to 100 minutes/day over a 12-week period may not be associated with improvements in vascular form, vascular function, or insulin sensitivity in relatively healthy older adults. In combination with our prior work, 19 these data suggest that the intensity and duration of activity replacing sedentary time may influence how reductions in sedentary time impact the vasculature and metabolic risk factors. These data have implications for the design of clinical interventions to reduce sedentary time in older adults, suggesting reductions in sedentary time may need to exceed 100 minutes and should also include plans to measure the duration, intensity, and architecture of the PA that replaces previous sedentary time. Further studies will be necessary to determine the ideal dosing of activity in older adults to mitigate cardiometabolic risk.
DISCLOSURE
M.E.W. receives grant support from the NIH (K23HL089326 and HL081587), the Doris Duke Foundation, and Merck, Sharp, & Dohme Corp. S.J.S. receives funding from the NIH (HL091019) and from a VA Merit Award (1/01RX000555). K.D. and T.B.S. have been funded by T32HL007792.
AUTHOR CONTRIBUTIONS
T.B.S. collected study data, performed study procedures, analyzed study data, wrote the first draft of the manuscript, and provided key editorial comments to the draft revisions. K.D., K.H., D.K., and A.C. collected study data, performed study procedures, and provided key editorial comments to the draft revisions. M.E.W. and S.J.S. designed the study protocol, secured funding, collected study data, analyzed study data, and provided key editorial comments to the draft revisions.
ACKNOWLEDGMENTS
This study was supported by a T. Franklin Williams Scholars Award provided by Atlantic Philanthropies, the American Heart Association (10GRNT3880044), the John A. Hartford Foundation, and the Association of Specialty Physicians (PI: M.E.W.).
Contributor Information
Tisha B. Suboc, Department of Medicine, Division of Cardiovascular Medicine, Medical College of Wisconsin , Milwaukee, Wisconsin , USA
Daniel Knabel, Graduate School of Biomedical Sciences, Medical College of Wisconsin , Milwaukee , Wisconsin , USA.
Scott J. Strath, Department of Kinesiology, University of Wisconsin–Milwaukee , Milwaukee , Wisconsin , USA
Kodlipet Dharmashankar, Department of Medicine, Division of Cardiovascular Medicine, Medical College of Wisconsin , Milwaukee, Wisconsin , USA.
Allison Coulliard, Department of Medicine, Division of Cardiovascular Medicine, Medical College of Wisconsin , Milwaukee, Wisconsin , USA.
Mobin Malik, Department of Medicine, Division of Cardiovascular Medicine, Medical College of Wisconsin , Milwaukee, Wisconsin , USA.
Kristoph Haak, Department of Orthopaedic Surgery, University of Missouri–Kansas City , Kansas City , Missouri , USA.
Michael E. Widlansky, Department of Medicine, Division of Cardiovascular Medicine, Medical College of Wisconsin , Milwaukee, Wisconsin , USA Department of Pharmacology, Medical College of Wisconsin , Milwaukee, Wisconsin , USA.
REFERENCES
- 1. Sedentary Behaviour Research Network . Letter to the Editor: Stanardized use of the term “sedentary” and “sedentary behaviours. Appl Physiol Nutr Metab 2012; 37:540–542. [DOI] [PubMed] [Google Scholar]
- 2. Healy GN Matthews CE Dunstan DW Winkler EA Owen N . Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003-06 . Eur Heart J 2011. ; 32 : 590 – 597 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wilmot EG Edwardson CL Achana FA Davies MJ Gorely T Gray LJ Khunti K Yates T Biddle SJ . Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis . Diabetologia 2012. ; 55 : 2895 – 2905 . [DOI] [PubMed] [Google Scholar]
- 4. Katzmarzyk PT Church TS Craig CL Bouchard C . Sitting time and mortality from all causes, cardiovascular disease, and cancer . Med Sci Sports Exerc 2009. ; 41 : 998 – 1005 . [DOI] [PubMed] [Google Scholar]
- 5. Grøntved A Hu FB . Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a meta-analysis . JAMA 2011. ; 305 : 2448 – 2455 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hu FB Li TY Colditz GA Willett WC Manson JE . Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women . JAMA 2003. ; 289 : 1785 – 1791 . [DOI] [PubMed] [Google Scholar]
- 7. Widlansky ME Gokce N Keaney JF Jr Vita JA . The clinical implications of endothelial dysfunction . J Am Coll Cardiol 2003. ; 42 : 1149 – 1160 . [DOI] [PubMed] [Google Scholar]
- 8. Widlansky ME . The danger of sedenterism: endothelium at risk . Am J Physiol Heart Circ Physiol 2010. ; 299 : H243 – H244 . [DOI] [PubMed] [Google Scholar]
- 9. Hesse C Siedler H Luntz SP Arendt BM Goerlich R Fricker R Heer M Haefeli WE . Modulation of endothelial and smooth muscle function by bed rest and hypoenergetic, low-fat nutrition . J Appl Physiol (1985) 2005. ; 99 : 2196 – 2203 . [DOI] [PubMed] [Google Scholar]
- 10. Hamburg NM McMackin CJ Huang AL Shenouda SM Widlansky ME Schulz E Gokce N Ruderman NB Keaney JF Jr Vita JA . Physical inactivity rapidly induces insulin resistance and microvascular dysfunction in healthy volunteers . Arterioscler Thromb Vasc Biol 2007. ; 27 : 2650 – 2656 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bleeker MW De Groot PC Rongen GA Rittweger J Felsenberg D Smits P Hopman MT . Vascular adaptation to deconditioning and the effect of an exercise countermeasure: results of the Berlin Bed Rest study . J Appl Physiol (1985) 2005. ; 99 : 1293 – 1300 . [DOI] [PubMed] [Google Scholar]
- 12. Demiot C Dignat-George F Fortrat J-O Sabatier F Gharib C Larina I Gauquelin-Koch G Hughson R Custaud M-A . WISE 2005: chronic bed rest impairs microcirculatory endothelium in women . Am J Physiol Heart Circ Physiol 2007. ; 293 : H3159 – H3164 . [DOI] [PubMed] [Google Scholar]
- 13. Navasiolava NM Dignat-George F Sabatier F Larina IM Demiot C Fortrat J-O Gauquelin-Koch G Kozlovskaya IB Custaud M-A . Enforced physical inactivity increases endothelial microparticle levels in healthy volunteers . Am J Physiol Heart Circ Physiol 2010. ; 299 : H248 – H256 . [DOI] [PubMed] [Google Scholar]
- 14. Thijssen DH Maiorana AJ O’Driscoll G Cable NT Hopman MT Green DJ . Impact of inactivity and exercise on the vasculature in humans . Eur J Appl Physiol 2010. ; 108 : 845 – 875 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Duijnhoven NT Green DJ Felsenberg D Belavy DL Hopman MT Thijssen DH . Impact of bed rest on conduit artery remodeling: effect of exercise countermeasures . Hypertension 2010. ; 56 : 240 – 246 . [DOI] [PubMed] [Google Scholar]
- 16. Owen N Sugiyama T Eakin EE Gardiner PA Tremblay MS Sallis JF . Adults’ sedentary behavior determinants and interventions . Am J Prev Med 2011. ; 41 : 189 – 196 . [DOI] [PubMed] [Google Scholar]
- 17. Thorp AA Owen N Neuhaus M Dunstan DW . Sedentary behaviors and subsequent health outcomes in adults a systematic review of longitudinal studies, 1996-2011 . Am J Prev Med 2011. ; 41 : 207 – 215 . [DOI] [PubMed] [Google Scholar]
- 18. Matthews CE Chen KY Freedson PS Buchowski MS Beech BM Pate RR Troiano RP . Amount of time spent in sedentary behaviors in the United States, 2003-2004 . Am J Epidemiol 2008. ; 167 : 875 – 881 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Suboc TB Strath SJ Dharmashankar K Coulliard A Miller N Wang J Tanner MJ Widlansky ME . Relative Importance of Step Count, Intensity, and Duration on Physical Activity’s Impact on Vascular Structure and Function in Previously Sedentary Older Adults . J Am Heart Assoc 2014. ; 3 : e000702 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kizhakekuttu TJ Gutterman DD Phillips SA Jurva JW Arthur EI Das E Widlansky ME . Measuring FMD in the brachial artery: how important is QRS gating? J Appl Physiol (1985) 2010. ; 109 : 959 – 965 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Freedson PS Melanson E Sirard J . Calibration of the Computer Science and Applications, Inc. accelerometer . Med Sci Sports Exerc 1998. ; 30 : 777 – 781 . [DOI] [PubMed] [Google Scholar]
- 22. Thompson AM Campagna PD Durant M Murphy RJ Rehman LA Wadsworth LA . Are overweight students in Grades 3, 7, and 11 less physically active than their healthy weight counterparts? Int J Pediatr Obes 2009. ; 4 : 28 – 35 . [DOI] [PubMed] [Google Scholar]
- 23. Thosar SS Johnson BD Johnston JD Wallace JP . Sitting and endothelial dysfunction: The role of shear stress . Med Sci Monit 2012. ; 18 : RA173 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shoemaker JK Hogeman CS Silber DH Gray K Herr M Sinoway LI . Head-down-tilt bed rest alters forearm vasodilator and vasoconstrictor responses . J Appl Physiol (1985) 1998. ; 84 : 1756 – 1762 . [DOI] [PubMed] [Google Scholar]
- 25. Krogh-Madsen R Thyfault JP Broholm C Mortensen OH Olsen RH Mounier R Plomgaard P van Hall G Booth FW Pedersen BK . A 2-wk reduction of ambulatory activity attenuates peripheral insulin sensitivity . J Appl Physiol (1985) 2010. ; 108 : 1034 – 1040 . [DOI] [PubMed] [Google Scholar]
- 26. Huynh QL Blizzard CL Sharman JE Magnussen CG Dwyer T Venn AJ . The cross-sectional association of sitting time with carotid artery stiffness in young adults . BMJ Open 2014. ; 4 : e004384 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hopkins N Stratton G Ridgers ND Graves LE Cable NT Green DJ . Lack of relationship between sedentary behaviour and vascular function in children . Eur J Appl Physiol 2012. ; 112 : 617 – 622 . [DOI] [PubMed] [Google Scholar]
- 28. Wijndaele K Duvigneaud N Matton L Duquet W Delecluse C Thomis M Beunen G Lefevre J Philippaerts RM . Sedentary behaviour, physical activity and a continuous metabolic syndrome risk score in adults . Eur J Clin Nutr 2009. ; 63 : 421 – 429 . [DOI] [PubMed] [Google Scholar]
- 29. Helmerhorst HJ Wijndaele K Brage S Wareham NJ Ekelund U . Objectively measured sedentary time may predict insulin resistance independent of moderate- and vigorous-intensity physical activity . Diabetes 2009. ; 58 : 1776 – 1779 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Owen N Healy GN Matthews CE Dunstan DW . Too much sitting: the population health science of sedentary behavior . Exerc Sport Sci Rev 2010. ; 38 : 105 – 113 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Healy GN Dunstan DW Salmon J Cerin E Shaw JE Zimmet PZ Owen N . Breaks in sedentary time: beneficial associations with metabolic risk . Diabetes Care 2008. ; 31 : 661 – 666 . [DOI] [PubMed] [Google Scholar]
- 32. Hamilton MT Hamilton DG Zderic TW . Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease . Diabetes 2007. ; 56 : 2655 – 2667 . [DOI] [PubMed] [Google Scholar]