Abstract
Eosinophilic aortitis is a rare condition in animals and humans, occasionally reported associated to parasitic migration and to a poorly understood complex group of autoimmune vasculitides. Here we describe a case of eosinophilic aortitis with thoracic aortic aneurysm and rupture in a captive-born owl monkey and discuss the differential diagnoses.
Keywords: Aorta; Aotus nancymai; arteritis; autoimmune; eosinophil; granulomatosis, nonhuman primate; nonhuman primate; vasculitides
Introduction
Eosinophilic aortitis with thoracic aortic aneurysm is an extremely uncommon condition in humans and animals. In humans, it has been sporadically reported associated to a complex group of autoimmune large vessel vasculitides of unknown etiology [6, 7, 12, 17, 19]. In animals, eosinophilic aortitis without dissecting aneurysm has been reported in the mutant “mes” rat [16, 18] and associated to parasitic migration in dogs, horses, and ruminants [13]. Aortic dissecting aneurysms are relatively common in owl monkeys and tamarins but, to our knowledge, no eosinophilic aortitis has been reported in these species [4, 8]. Eosinophilic arteritis, restricted to the mesenteric arteries, was described in two owl monkeys spontaneously infected with Parastrongylus (=Angiostrongylus) costaricensis [14]. Multisystemic eosinophilia with asthma-like symptoms and multi-organ damage was reported in a captive-born Aotus vociferans [9] while eosinophilia with no untoward clinical signs has been described in A. azarae boliviensis [1]. Here we describe a case of eosinophilic aortitis with thoracic aortic aneurysm and rupture in an owl monkey. This is to our knowledge the first report of eosinophilic aortitis in a nonhuman primate.
Case Report
A 5 year-old, research naïve, captive-born, male, owl monkey (Aotus nancymai) was enrolled in an Institutional Animal Care and Use Committee-approved malaria vaccine research study at the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH). The monkey was housed and cared for in accordance with the Animal Welfare Act [2], Animal Welfare Regulations [3], and the Guide for the Care and Use of Laboratory Animals [10] in an AAALAC-accredited animal facility. The animal was pair-housed in a stainless steel 6.0-ft2 cage (Primate Products, Miami, FL) with a PVC nest box and perch. Drinking water was provided ad-libitum by means of water bottles and automatic watering system to each cage, the diet consisted of chow (Primate Diet 8794N, Harlan Teklad, Madison, WI), fruits, and treats (Bio-Serv, Frenchtown, NJ). The monkey was tested semi-annually for tuberculosis and screened for intestinal bacterial pathogens by conventional media cultures and for parasites by fecal wet mounts and flotation tests. The animal had a history of heart murmur but no other significant findings.
On morning rounds the monkey was found dead. At necropsy, the animal weighed 800 grams and was in good body condition with adequate adipose stores and hydration status. The left hemithorax contained a large blood clot occupying approximately 90% of the cavity. The right hemithorax contained approximately 10 ml of a serosanguineous fluid. The entire length of the thoracic aorta out of the heart to the aortic hiatus in the diaphragm was dilated and irregularly shaped with its widest dimension being approximately 1.3 cm. A 5 mm diameter tear was noted on the aorta approximately 1.5 cm from the base of the heart (Figure 1A). No other gross lesions were observed. The animal had adequate ingesta and digesta thoughout the gastrointestinal tract with formed feces in the descending colon. Tissue samples from the lesions and major organs were collected and fixed in 10% neutral buffered formalin, embedded in paraffin, and processed routinely for Hematoxilyn-Eosin (H&E) stain. In addition, sections of the affected aorta were processed for Brown and Hopps (B&H) Gram stain, Periodic Acid Schiff (PAS) stain, and Gomori Methenamine Silver (GMS) stain. Microscopically, the thoracic aorta had marked intimal thickening due to accumulation of mucinous ground substance, loss of the elastic lamina, separation of the tunica media by a space filled with blood and an outer band composed of abundant granulation tissue, fibroplasia, edema, mucinous deposits, and moderate infiltrate of eosinophils (Figures 1B and 1C). The outer layer contained multifocal areas of necrotic cells admixed with degenerate neutrophils. The myocardium had multiple coronary arteries and arterioles vacuolated with necrotic myocytes and hyaline change in the tunica media (Figures 1D and 1E). A few larger arteries had mild to moderate eosinophilic infiltrate surrounding them. The kidney had multifocal mild to moderate interstitial lymphoeosinophilic infiltrates, foci of interstitial fibrosis, multiple degenerate cortical tubules, and scattered ectatic tubules with attenuated epithelium filled with proteinaceous fluid or eosinophilic granular cast material (Figure 1F). No changes were noted in renal vasculature. The liver had diffuse mild to moderate hepatocellular hemosiderosis. Lungs and all other major organs were unremarkable. The special stains did not reveal a bacterial, fungal, or parasitic agent. The cause of death in this animal was severe thoracic aortic aneurysm with dissection and rupture causing peracute exsanguination into the pleural cavity. The final diagnosis was necrotizing eosinophilic aortitis with severe thoracic aortic dissecting aneurysm and rupture.
Figure 1.
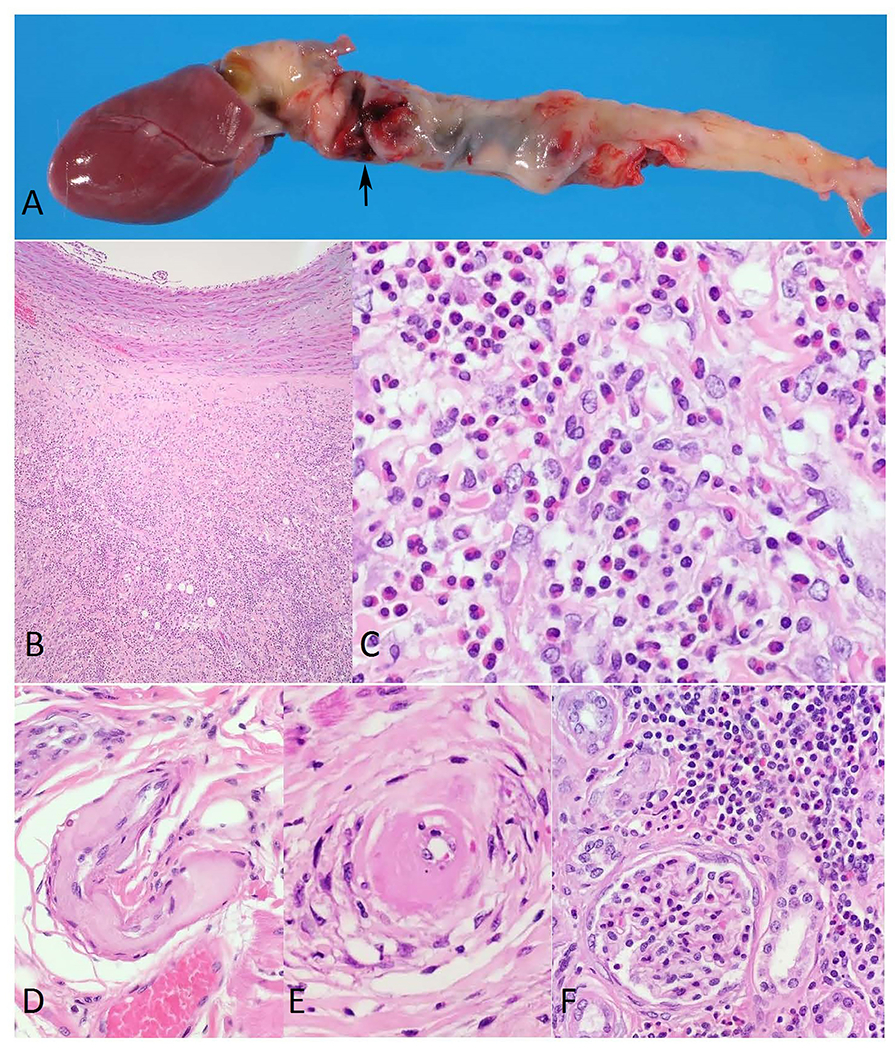
A. Owl monkey, aorta. Photograph showing entire length of the thoracic aorta out of the heart to the aortic hiatus in the diaphragm dilated and irregularly shaped with an approximately 5 mm diameter tear (arrow).
B. Owl monkey, aorta. Low power micrograph of the thoracic aorta wall showing marked intimal thickening due to accumulation of mucinous ground substance, loss of the elastic lamina, separation of the tunica media by a space filled with blood and an outer band composed of abundant granulation tissue, fibroplasia, edema, mucinous deposits, and moderate infiltrate of eosinophils. H&E stain. 40x original magnification.
C. Owl monkey, aorta. Micrograph showing at higher magnification the affected aorta infiltrated with eosinophils. H&E stain. 400x original magnification.
D. Owl monkey, heart. Micrograph of a medium-sized coronary artery with extensive fibrinoid necrosis and hyaline change affecting most of the vessel wall. H&E stain. 200x original magnification.
E. Owl monkey, heart. Micrograph of a small coronary artery with marked hyaline change surrounded by vacuolated and necrotic vascular myocytes. H&E stain. 400x original magnification.
F. Owl monkey, kidney. Micrograph showing multifocal mild to moderate interstitial lymphoeosinophilic infiltrates, foci of interstitial and periglomerular fibrosis, degenerate cortical tubules, and tubules with attenuated epithelium filled with eosinophilic granular cast material. H&E stain. 200x original magnification.
Discussion
Aortic aneurysms are not uncommon in owl monkeys [4], however, in this case the pathology findings resemble features of polyarteritis nodosa, granulomatosis with polyangiitis (formerly known as Wegener’s granulomatosis), eosinophilic granulomatosis with polyangiitis (formerly known as Churg-Strauss disease), giant cell arteritis, and immunoglobulin G4-related sclerosing disease, all poorly understood human diseases, with the exemption of polyarteritis nodosa which occasionally affects animals [13]. These diseases are part of a complex group of autoimmune vasculitides characterized by eosinophil infiltrates damaging small, medium and, occasionally, large blood vessels [6, 7, 11, 12, 15, 17, 19, 20]. Aotus azarae boliviensis persistently have one order of magnitude more eosinophils in the peripheral blood than Aotus nancymai but the condition does not appear to cause any untoward clinical signs in this species [1]. On the other hand, a fatal case of hypereosinophilic syndrome with severe respiratory distress and multiple organ infiltration with eosinophils was described in a captive-born Aotus vociferans [9]. The factors separating benign and pathologic manifestations of hypereosinophilia in Aotus species are unknown. Recently, a fatal case of granulomatous arteritis affecting multiple organs resembling the human disease giant cell arteritis was described in a grey mouse lemur [5]. However, in the lemur, the lungs were mainly affected sparing the aorta and the inflammatory infiltrate consisted mostly of giant cells with moderate numbers of eosinophils while in the current case report no giant cells were observed and the lungs were not affected.
The vacuolated and necrotic myocytes and hyaline change in the tunica media of the coronary arteries and arterioles in the present case report resemble lesions found in polyarteritis nodosa, aging, and malignant arterial hypertension [15]. However, the lesions were not accompanied by inflammatory cells nor affected other organs as is common in polyarteritis nodosa, the animal was only 5 years old which corresponds to a young adult in this species making aging an unlikely cause, and no vascular lesions where noted in lungs or kidneys as would be expected with malignant arterial hypertension [15]. Parasitic aortitis was ruled out as a possible diagnosis since no parasites or lesions suggestive of parasitic migration were found in the gastrointestinal tract, liver, lungs, heart, or other organs. Granulomatosis with polyangiitis, eosinophilic granulomatosis with polyangiitis, and giant cell arteritis are usually accompanied by giant cells which were not found in the present case [11, 20]. Other possible causes for hypereosinophilia like, drug-related hypersensitivity, exposure to experimental vaccines or substances, or experimental parasitic infection, were ruled out since none were administered to this animal preceding his death. Unfortunately, no blood or serum samples were available from this animal to check for possible current or past systemic eosinophilia, presence of antinuclear antibodies, antineutrophil cytoplasmic antibodies, or elevated serum IgG4 levels to better characterize the lesions and any similarities it may have with the human diseases [6, 7, 12].
A weakened aorta, due to inflammation and dissecting aneurysm, most likely caused the aortic rupture and death of the animal [15]. This is to our knowledge the first report of eosinophilic aortitis with severe aortic dissecting aneurysm and rupture in a nonhuman primate.
Acknowledgements
This study was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases, Comparative Medicine Branch and the Laboratory of Malaria Immunology and Vaccinology. We thank Dr. Matthew Starost for pathology support. This case report was presented as an abstract at the 45th Association of Primate Veterinarians Annual Workshop. Lost Pines, TX, USA. Oct 19-22, 2017.
References
- 1.Albert DL, Brodie SJ, Sasseville VG, Ringler DJ. Peripheral blood eosinophilia in owl monkeys is associated with increased eosinophilopoeisis yet depressed recruitment kinetics to the Chemokine RANTES. Blood 1996; 88(5):1718–1724. [PubMed] [Google Scholar]
- 2.Animal Welfare Act as Amended. 2013. 7 USC § 2131-2159.
- 3.Animal Welfare Regulations. 2013. 9 CFR § 3.75-3.92.
- 4.Baer JF, Gibson SV, Weller RE, Buschbom RL, Leathers CW. Naturally occurring aortic aneurysms in owl monkeys (Aotus spp.). Lab Anim Sci 1992; 42(5):463–466. [PubMed] [Google Scholar]
- 5.Cichon N, Lampe K, Bremmer F, Becker T, Mätz-Rensing K. Unique case of granulomatous arteritis in a grey mouse lemur (Microcebus murinus) – first case description. Primate Biol 2017; 4(1):71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danlos FX, Rossi GM, Blockmans D, Emmi G, Kronbichler A, Durupt S, Maynard C, Luca L, Garrouste C, Lioger B, Mourot-Cottet R, Dhote R, Arlet JB, Hanslik T, Rouvier P, Ebbo M, Puéchal X, Nochy D, Carlotti A, Mouthon L, Guillevin L, Vaglio A, Terrier B; French Vasculitis Study Group. Antineutrophil cytoplasmic antibody-associated vasculitides and IgG4-related disease: A new overlap syndrome. Autoimmun Rev. 2017; 16(10):1036–1043. [DOI] [PubMed] [Google Scholar]
- 7.Fujii K, Hidaka Y. Churg-Strauss syndrome complicated by chronic periaortitis: a case report and review of the literature. Intern Med 2012; 51(1):109–112. [DOI] [PubMed] [Google Scholar]
- 8.Gozalo AS, Ragland DR, StClaire MC, Elkins WR, Michaud CR. Intracardiac thrombosis and aortic dissecting aneurysms in mustached tamarins (Saguinus mystax) with cardiomyopathy. Comp Med 2011; 61(2):176–181. [PMC free article] [PubMed] [Google Scholar]
- 9.Gozalo A, Rosenberg H, Elkins W, Montoya E, Weller R. Multisystemic eosinophilia resembling hypereosinophilic syndrome in a colony-bred owl monkey (Aotus vociferans). J Am Assoc Lab Anim Sci 2009; 48(3):303–306. [PMC free article] [PubMed] [Google Scholar]
- 10.Institute for Laboratory Animal Research. Guide for the Care and Use of Laboratory Animals, 8th ed. Washington (DC): The National Academies Press; 2011. 220 p. [Google Scholar]
- 11.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen EC, Hoffman GS, Jayne DR, Kallenberg CG, Lamprecht P, Langford CA, Luqmani RA, Mahr AD, Matteson EL, Merkel PA, Ozen S, Pusey CD, Rasmussen N, Rees AJ, Scott DG, Specks U, Stone JH, Takahashi K, Watts RA. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013; 65(1):1–11. [DOI] [PubMed] [Google Scholar]
- 12.Kasashima S, Zen Y, Kawashima A, Endo M, Matsumoto Y, Kasashima F, Ohtake H, Nakanuma Y. A clinicopathologic study of immunoglobulin G4-related sclerosing disease of the thoracic aorta. J Vasc Surg 2010; 52(6):1587–1595. [DOI] [PubMed] [Google Scholar]
- 13.Maxie MG and Robinson WF. Cardiovascular System. In: M. Grant Maxie, ed. Jubb Kennedy, and Palmer’s Pathology of Domestic Animals, Vol 3, 5th Edition. Philadelphia: Elsevier-Saunders; 2007:87–92. [Google Scholar]
- 14.Miller CL, Kinsella JM, Garner MM, Evans S, Gullett PA, Schmidt RE. Endemic infections of Parastrongylus (=Angiostrongylus) costaricensis in two species of nonhuman primates, raccoons, and an opossum from Miami, Florida. J Parasitol 2006; 92(2):406–408. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell RN. Blood Vessels. In: Kumar V, Abbas AK, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease, 9th Edition. Philadelphia: Elsevier-Saunders; 2015:483–522. [Google Scholar]
- 16.Muto S, Hayashi M, Matsushita N, Momose Y, Shibata N, Umemura T, Matsumoto K. Systemic and eosinophilic lesions in rats with spontaneous eosinophilia (mes rats). Vet Pathol 2001; 38(3):346–350. [DOI] [PubMed] [Google Scholar]
- 17.Ohta N, Waki T, Fukase S, Suzuki Y, Kurakami K, Aoyagi M, Kakehata S. Aortic aneurysm rupture as a rare complication of granulomatosis with polyangiitis: a case report. J Med Case Rep 2013; 7:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sano K, Kobayashi M, Sakaguchi N, Ito M, Hotchi M, Matsumoto K. A rat model of hypereosinophilic syndrome. Pathol Int 2001; 51(2):82–88. [DOI] [PubMed] [Google Scholar]
- 19.Segal OR, Gibbs JS, Sheppard MN. Eosinophilic aortitis and valvitis requiring aortic valve replacement. Heart 2001; 86(3):245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stone JR, Bruneval P, Angelini A, Bartoloni G, Basso C, Batoroeva L, Buja LM, Butany J, d’Amati G, Fallon JT, Gittenberger-de Groot AC, Gouveia RH, Halushka MK, Kelly KL, Kholova I, Leone O, Litovsky SH, Maleszewski JJ, Miller DV, Mitchell RN, Preston SD, Pucci A, Radio SJ, Rodriguez ER, Sheppard MN, Suvarna SK, Tan CD, Thiene G, van der Wal AC, Veinot JP. Consensus statement on surgical pathology of the aorta from the Society for Cardiovascular Pathology and the Association for European Cardiovascular Pathology: I. Inflammatory diseases. Cardiovasc Pathol 2015;24(5):267–278. [DOI] [PubMed] [Google Scholar]


