Abstract
Inflammatory bowel disease (IBD) is an incurable disease characterized by remission-relapse cycles throughout its course. Both Crohn's disease (CD) and ulcerative colitis (UC), the two main forms of IBD, exhibit tendency to develop complications and substantial heterogeneity in terms of frequency and severity of relapse, thus posing great challenges to the clinical management for IBD. Current treatment strategies are effective in different ways in induction and maintenance therapies for IBD. Recent advances in studies of genetics, pharmacogenetics, proteomics and microbiome provide a strong driving force for identifying molecular markers of prognosis and treatment response, which should help clinicians manage IBD patients more effectively, and then, improve clinical outcomes and reduce treatment costs of patients. In this review, we summarize and discuss precision medicine in IBD, focusing on predictive markers of disease course and treatment response, and monitoring indices during therapeutic drug monitoring.
Keywords: disease course, inflammatory bowel disease, precision treatment, precision monitoring, treatment response
Introduction
Inflammatory bowel disease (IBD), including Crohn's disease (CD) and ulcerative colitis (UC), is characterized by chronicity, destructiveness, and a remission-relapse pattern [1]. The spectrum of disease symptoms is wide. Patients with UC typically present with diarrhea, bloody stool and tenesmus; while abdominal pain, diarrhea and weight loss are the common symptoms of CD [2]. Moreover, 6%–47% of IBD patients suffer from extra-intestinal manifestations (EIMs) involving organs or tissues like joints, eyes, skin, etc. [3]. The heterogeneous presentations of IBD make it difficult for physicians to diagnose the condition by clinical features alone. A large-scale, prospective and multicenter study including 1399 children demonstrated that diagnostic delay conferred risk for the development of complicated diseases and growth impairment in pediatric CD patients [4]. From this point, making a timely and accurate diagnosis is extremely important for IBD patients. Such precision diagnosis can be achieved by combining consideration of clinical manifestations, laboratory analysis, endoscopic examination, imaging tests, and histologic assessment. Furthermore, the severity of diseases and frequency of flare-ups vary substantially from one patient to another. Some patients may experience a mild disease course, while others may progress quickly. Remarkably, the phenotype of CD may vary and evolve over time. It can progress from non-stricturing/fistulizing behavior to stricturing and fistulizing behavior in a manner which is largely unpredictable. The one-year recurrence rate of IBD is approximately 10%–30%, despite achieving remission [5]. Although some markers have been identified to be useful in the prediction of disease flare-ups, relapses are always difficult to predict [5, 6]. IBD exhibits highly heterogeneity on all levels, and its management faces great challenges.
IBD has become a global disease with the highest prevalence in Westernized countries and the greatest growing incidence in newly industrialized countries [7]. The disease puts a heavy burden not only on patients themselves and their families, but also on health care systems [8]. Substantial evidence indicates that IBD results from the interaction of genetic/epigenetic, environmental, immunological, and microbial aspects. Large-scale genetic studies provided major insights into the etiopathogenesis of IBD, and highlighted the shared and distinct genetic risk factors in CD and UC [9, 10]. However, for most identified loci, their functions remain unknown. Progress in pharmacogenetics, proteomics and microbiome also shed light on the complicated signaling pathways of IBD. Understanding these distinct signaling pathways further provides an impetus for IBD treatment. The current therapeutic goal for IBD is “treat-to-target”, aiming at achieving mucosal healing (MH), avoiding permanent complications, and altering the natural history of IBD [11]. Thus, assessment of disease course and therapeutic response play key roles in IBD management. Selecting a targeted therapy for individual patient must be based on risk stratification by analyzing the determinants of disease course and treatment response, including clinical, genetic, epigenetic, serological and fecal markers (Fig. 1).
Figure 1.
Precision medicine in IBD. Based on risk stratification by analyzing the determinants of disease course and treatment response including clinical, genetic, epigenetic, serological and fecal markers, physicians then select a targeted therapy and apply precision monitoring for individual patient. IBD: inflammatory bowel disease.
With the “Precision Medicine Initiative” put forward in 2015, precision medicine has become a hotspot in the field of health care [12]. A large number of studies have been conducted to optimize the precision diagnosis, treatment, and monitoring of IBD. Herein, we mainly discuss how research on signaling pathways facilitates targeted therapy, and elaborate on precision treatment and precision monitoring in IBD.
Signaling pathways involved in IBD
Though the exact pathogenesis of remains unclear, it is believed that complicated mechanisms involving environmental triggers, luminal microbiota and host genetic susceptibility generate the disequilibrium between pro-inflammatory and anti-inflammatory signaling, resulting in a chronic inflammatory state in IBD patients. Amongst numerous signaling pathways implicated in IBD, pathways related to tumor necrosis factor (TNF), leukocyte trafficking, and interleukin-12 (IL-12)/interleukin-23 (IL-23) have been intensively studied [13]. Undoubtedly, an improved understanding of these signaling pathways substantially facilitates the development of targeted treatment for IBD.
TNF has two forms: transmembrane TNF (mTNF) and soluble TNF. The former mainly binds with TNF receptor I (TNFRI), and then mediates the activation of nuclear factor kappa-B (NF-κB) and caspase-8-dependent death signaling pathways, resulting in mucosal inflammation and intestinal epithelial barrier damage [14]. The latter often binds with TNF receptor II (TNFRII) and contributes to the activation of pro-survival and pro-inflammatory signaling pathways [15]. So far, available evidence indicates that TNF plays a central role in the pathogenesis of IBD. In order to block its pro-inflammatory action, researchers have developed some full-length anti-TNF monoclonal IgG1 antibodies such as infliximab, adalimumab and golimumab, and antibodies with Fab fragments such as certolizumab as well [14]. These antibodies exert anti-inflammatory effects by neutralizing mTNF and soluble TNF, reducing pro-inflammatory cytokines and cell adhesion molecules, and prompting T cell apoptosis, and inducing M2-type wound-healing macrophages [16–18]. Therefore, anti-TNF monoclonal antibodies showed outstanding therapeutic efficacy in the induction and maintenance of clinical, biochemical, and endoscopic remission in both animal models and patients with IBD [14]. It also has become a breakthrough in the precision treatment of IBD, encouraging further studies of other signaling pathways involved in IBD.
The IL-23/T helper cell 17 (Th17) pathway is critical in the pathophysiology of IBD. IL-23 consists of a p40 subunit and a p19 subunit. It is responsible for conferring pathogenicity to Th17 and producing pro-inflammatory cytokines including interleukin-17A (IL-17A), interleukin-17F (IL-17F), interleukin-22 (IL-22), TNF, C-C chemokine receptor type 6 (CCR6), chemokine ligand 20 (CCL20), and others [19]. Th17 and Th17-related cytokines are acknowledged as strong inducers of inflammation. Increased levels of Th17 cells and Th17-related cytokines in IBD patients indicate that the IL-23/Th17 pathway plays an important role in IBD [20]. The association between the IL-23/Th17 pathway and IBD has been further emphasized by Genome-wide Association Studies (GWAS). Several risk genes involved in the IL-23/Th17 pathway, such as interleukin 23 receptor (IL23R), caspase recruitment domain family member 9 (CARD9), interleukin 12B (IL12B), Janus kinase 2 (JAK2), and CCR6 have been demonstrated to be associated with susceptibility to IBD [20]. Besides Th17, IL-23 also exerted effects on another T helper cell subset. Recently, a study reported that IL-23 also drove intestinal inflammation by evoking a pathogenic phenotype in Th1-like cells [21]. This finding provides a new direction for research on IBD. IL-12 is also a heterodimeric cytokine composed of a p35 and a shared p40 subunit. IL-12 can promote Th1 cytokine-mediated immune responses that is considered to be an integral part in the pathogenesis of CD. Besides, IL-12 is also involved in the activation of natural killer (NK) cells, cytotoxic T lymphocytes (CTLs), and group 1 innate lymphoid cells (ILC1s), and the production of interferon-gamma (IFN-γ) and TNF-a [22]. Therefore, new drugs targeting IL-12/IL-23 p40 (ustekinumab), and IL-23 p19 (risankizumab and briakinumab) have showed great benefit for IBD patients [23–25]. These examples further support the idea that targeting a key molecule within a signaling pathway can be an optimal option for targeted therapy in IBD.
Migration of leukocytes from the periphery to inflamed bowel tissues, and adhesion to the intestinal vasculature are two indispensable processes in the development and progression of IBD. Leukocyte-specific integrins, including alpha 4 beta 7 (α4β7), alpha E beta 7 (αEβ7), alpha 4 beta 1 (α4β1), etc., are transmembrane glycoprotein receptors, mediating the connection between leukocytes and extracellular matrix ligands [11, 26]. The adhesive process of leukocytes to vascular endothelium can be activated by several pro-inflammatory cytokines such as TNF-α and interleukin-1 (IL-1), which are also responsible for the up-regulation of expression levels of intracellular adhesion molecules-1 (ICAM-1), mucosal adhesion cell adhesion molecule (MADCAM), and E-selectin on inflamed tissues [11]. As a gut-homing receptor, α4β7 participates in the key processes of lymphocyte homing (rolling migration and firm adhesion) by binding with MADCAM-1. Therefore, blocking the binding of α4β7 to MADCAM-1 prevents lymphocytes from homing to the gut and thus attenuates intestinal inflammation [26]. Several antibodies to integrins such as vedolizumab, etrolizumab and abrilumab have shown great improvement in clinical outcomes in IBD patients [27, 28]. Therefore, blocking the leukocyte migration and adhesion process may become a novel direction in drug discovery in IBD.
Other signaling pathways involving sphingosine 1-phosphate (S1P)/sphingosine 1-phosphate receptors (S1PRs), JAK-signal transducer and activator of transcription (STAT), and Toll-like receptor 9 (TLR9) also show promise in targeted therapy. Numerous studies have linked S1P/S1PRs to leukocyte trafficking, a pivotal process in the development of IBD. S1P/S1PRs drive intestinal inflammation and regulate intestinal immune response by mediating the egress of lymphocytes from primary and secondary lymphoid organs [29]. Thus, S1P modulators such as ozanimod and etrasimod show some beneficial effects on patients with IBD [30, 31]. Existing evidence demonstrated that JAKs mediate the phosphorylation of the STAT family and participate in the inflammatory processes of IBD [32]. Activation of JAK-STAT may cause great changes in the level and ratio of pro-inflammatory and anti-inflammatory cytokines, as well as in the balance between immune activation and tolerance [32]. Drugs inhibiting the biological activity of the JAKs such as tofacitinib, filgotinib and upadacitinib have attracted great interest. These drugs provide therapeutic options for patients who are unresponsive to or intolerant of other-class drugs [33–35]. It is noteworthy that a novel therapeutic strategy, dual therapy, a combination of a biologic with a small molecule drug, holds great promise to help refractory IBD patients achieve remission [36]. A study of 16 biologic-refractory pediatric IBD patients showed that the dual therapy (vedolizumab/ustekinumab and tofacitinib) quickly facilitated steroid-free remission in 75% of patients with little serious safety events [37]. Several other studies also drew similar conclusions [38, 39]. The synergistic effects (preventing lymphocyte homing, neutralizing pro-inflammatory cytokines, and inhibiting downstream cytokine receptor signaling pathways) of vedolizumab/infliximab/ustekinumab and tofacitinib may explain these interesting findings. Contrary to these above-mentioned signaling molecules, TLR9 shows beneficial effects on intestinal inflammation. Compared with the control group, TLR9-deficient mice with dextran sodium sulfate (DSS)-induced colitis presented more severe inflammation and delayed wound repair [40]. The protective effects of TLR9 on inflammation were further confirmed by the fact that activation of TLR9 contributed to the upregulation of mucosal IL10 and suppression of Th17 cells [41]. Correlations between mucosal TLR9 levels and severity of inflammation have also been demonstrated [41]. All these findings pave a new way to TLR9-targeted treatment in IBD. Cobitolimod, the TLR9 agonist, has been claimed to be effective in inducing clinical response in UC patients with poor response to conventional or biological treatments [42]. However, the efficacy of cobitolimod has only been evaluated in a phase II clinical trial, yet to be validated by large-sample clinical trials.
Indeed, revolutionary discoveries of different signaling pathways and major advances in IBD drug discovery have made great changes in disease management and also opened up the possibility of implementing precision treatment strategies for IBD.
Risk stratification based on clinical and molecular markers
Great heterogeneity in IBD makes it inappropriate and unreasonable for physicians to treat IBD patients with a unified therapeutic program. Disease course can substantially differ between individual patients. Some patients may undergo an aggressive disease course while others may experience a mild one. A link between a severe disease course and a poor disease outcome has been well documented [43]. Patients with an aggressive disease course need a timely and potent treatment, while a conventional step-up approach would suit a benign disease course [43]. Thus, the key of IBD management decision lies in screening out those patients with aggressive disease course at the early stage. So, doctors are advised to make risk stratification firstly according to various markers, and then select the most suitable therapy for patients [44]. Such a personalized treatment is closely correlated with better clinical outcomes, improved therapeutic efficacy and reduced risk of adverse events for IBD patients.
Clinical markers of disease course
Available data showed that patients with a diagnosis at an early age, perianal disease, complicated behaviors (structuring or penetrating lesions), and others were more likely to undergo an aggressive disease course [45]. We summarize the clinical markers in Table 1. However, onset age and disease location show some opposite effects on disease for patients with UC [46]. Different research methods and various sample sizes may explain these inconsistent results. As for the controversial factor, smoking, some studies demonstrated that it was a valuable predictor of unfavorable disease outcomes including complicated behaviors and the need for surgery, as well as the requirement for steroids/immunosuppressants and post-operative recurrence in patients with CD [47]. However, the concept that smoking cessation was linked to worse disease course for UC patients has been proposed [48]. Given that the benefits associated with smoking do not overweigh the potential risks, patients with UC are advised to give up smoking. What's more, IBD also shows strong sexual dimorphism in disease course. In comparison with male patients with CD, females frequently suffer from more severe clinical symptoms and disabilities [49]. It is noteworthy that most of these clinical markers were identified by retrospective studies, indicating a need of validating these markers in larger prospective cohort studies.
Table 1.
Risk stratification based on clinical markers.
| Markers | Roles in risk stratification | Sample number | Reference |
|---|---|---|---|
| Diagnosis at an early age | Predicted an aggressive disease course for CD | CD (1123) | [50] |
| Extensive disease | Predicted an aggressive disease course for CD and UC, and medically refractory disease for UC | CD (361); MR-UC (324), non-MR-UC (537) |
[51, 52] |
| Upper GI involvement | Predicted an aggressive disease course for CD | CD (358) | [53] |
| Ileal/ileocolonic involvement | Predicted an aggressive disease course for CD | CD (2105) | [54] |
| Perianal disease | Predicted an aggressive disease course for CD | CD (1123) | [50] |
| Complicated behaviors | Predicted an aggressive disease course for CD | CD (361) | [51] |
| Need of corticosteroids at initial presentation | Predicted an aggressive disease course for CD and UC | CD (1123) | [50] |
| Fiber intake | Decreased risk for ileocolonic CD | CD (346), UC (456) | [48] |
| Older age at disease onset | Predicted an aggressive disease course for UC | UC (601) | [46] |
| Proximal disease location | Predicted an aggressive disease course for UC | UC (601) | [46] |
| Smoking | Showed bidirectional effects (protective or destructive) on disease course for CD and UC | CD (476), UC (630), IC (81); CD (346), UC (456); UC (6754) |
[48, 55, 56] |
| Female | Predicted more severe clinical symptoms and disabilities for CD | CD (541) | [57] |
| Male | Predicted a high risk of developing CRC for UC | UC (4192), CD (3482) | [58] |
| Severe endoscopic lesions | Predicted an increased risk of penetrating behaviors and colectomy for CD | CD (102) |
[59] |
| Endoscopic MH | Predicted of lower risk of relapse, colectomy and hospitalization for CD and UC | UC (513), CD (227) | [60] |
| Coexisting with PSC | Increased risk of proximal disease extension, dysplasia, CRC and colectomy for CD and UC | UC (420); PSC-IBD (71), UC (142); IBD-neoplasia (43), IBD (102) |
[61–63] |
| Co-occurrence of psoriasis | Predicted an aggressive disease course for UC | UC (420) | [61] |
Abbreviations: CD: Crohn's disease; UC: ulcerative colitis; MR-UC: medically refractory-UC; GI: gastrointestinal; IC: indeterminate colitis; CRC: colorectal cancer; MH: mucosal healing; PSC: primary sclerosing cholangitis.
Endoscopy, a crucial tool for the assessment of mucosal inflammation and MH, is also of great importance in the prediction of disease course [64, 65]. Patients with extensive and deep ulcerations are at a higher risk of having an aggressive disease course [59]. Compared with patients exhibiting mild endoscopic lesions, the risk of colectomy was 5.43-fold higher in those with severe endoscopic lesions [59]. Conversely, endoscopic MH is associated with mild disease course [66]. Even so, endoscopic MH is not parallel to histologic remission [67]. Existing data showed that up to 40% of patients presenting with normal mucosa on endoscopy manifested mild to moderate inflammation on histopathology [67]. It is widely recognized that unresolved intestinal inflammation is associated with disease complications, colectomy, neoplasia and hospitalization, suggesting that endoscopy alone is likely inadequate to predict disease course in patients with IBD [68]. Therefore, combined analysis of endoscopic and histologic features may further reduce false negatives and increase the accuracy of prediction.
Given that IBD is an immune-mediated disease, IBD patients may present autoimmune comorbidities including primary sclerosing cholangitis (PSC), psoriasis and systemic lupus erythematosus (SLE). Co-occurrence of PSC or psoriasis contributes to a severe disease course in IBD patients [61, 69]. UC patients with PSC were more likely to suffer from progression of disease extension with a hazard ratio (HR) of 12.83 [61]. Recently, a close association between IBD and psoriasis has been reported in a Mendelian randomization study of 463,372 cases [70]. Given that autoimmune comorbidity always makes IBD management more difficult, special treatment and enhanced surveillance protocols in these patients are usually needed.
As it is known to all, IBD patients showed great heterogeneity in disease course. Different disease course often corresponds to different treatment strategies. Although lots of clinical markers have been identified to be associated with disease course, some of markers were not reliable or useful for the prediction, as the predictive accuracy is a little bit low [43]. In order to achieve adequate predictive accuracy, a prediction panel including clinical and other different class markers such as genetic, epigenetic, serological and fecal surrogates may be more helpful, and thus help physicians perform risk stratification and decide an appropriate treatment plan.
Genetic and epigenetic markers of disease course
In recent years, rapid progress has been made in the genetics of IBD. 320 risk alleles have been identified, some conferring susceptibility to IBD, while others related to disease course [6, 71]. We summarize genetic and epigenetic markers in Table 2.
Table 2.
Risk stratification based on genetic and epigenetic markers.
| Markers | Roles in risk stratification | Sample number | Reference |
|---|---|---|---|
| NOD2 | Predicted of stricturing/penetrating phenotype, ileal involvement and colectomy for CD | CD (316), UC (408), HC (205); CD (107) |
[72, 73] |
| IRGM | Predicted of colectomy, stricturing phenotype, ileal involvement and perianal disease for CD | CD (263), UC (206), HC (245) | [74] |
| TNFSF15 | Predicted of colectomy, stricturing phenotype and perianal fistula for CD, and medically refractory disease for UC | CD (906); MR-UC (324), non-MR-UC (537) |
[52, 75] |
| IL23R | Predicted of stricturing/penetrating phenotype and ileocolonic involvement for CD | CD (1528) | [76] |
| PRDM1 | Predicted of penetrating phenotype for CD | CD (1528) | [76] |
| IL12B | Predicted of medically refractory disease for UC | MR-UC (324), non-MR-UC (537) | [52] |
| HLA-DRB1*0103 | Predicted of extensive disease for UC | UC (466), HC (2099) | [77] |
| NFKBIL1 | Predicted of extensive disease and more severe disease for UC | UC (155), HC (298) | [78] |
| PAR2 (hypermethylation) | Predicted of extensive disease, steroid-dependent and steroid-refractory disease for UC | UC (84) | [79] |
| MDR1 (hypermethylation) | Predicted of extensive disease and earlier onset of disease for UC | UC (83) | [80] |
| RPS6KA2 (hypomethylation) | Predicted of stricturing/penetrating phenotype for CD, and extensive disease for UC | CD (121), UC (119), HC (191) | [81] |
| miR-29 family (low mucosa expression) | Predicted of stricturing phenotype for CD | CD (13) | [82] |
| miR-19-3p family (low serum expression) | Predicted of stricturing phenotype for CD | CD (108) | [83] |
| miR-200 family (low mucosa expression) | Predicted of stricturing phenotype for CD | CD (20), HC (16) | [84] |
| miR-31-5p, miR-215 and miR-223-3p (high mucosa expression) | Predicted of stricturing/penetrating phenotype for CD | CD (21), NIBD (14) | [85] |
| miR-149-5p and miR-203 (low mucosa expression) | Predicted of stricturing/penetrating phenotype for CD | CD (21), NIBD (14) | [85] |
Abbreviations: NOD2: nucleotide binding oligomerization domain containing 2; CD: Crohn's disease; UC: ulcerative colitis; HC: healthy control; IRGM: immunity related GTPase M; TNFSF15: TNF superfamily member 15; MR-UC: medically refractory-UC; IL23R: interleukin 23 receptor; PRDM1: positive regulatory domain 1; IL12B: interleukin 12B; HLA-DRB1*0103: major histocompatibility complex, class II, DR beta 1, 0103; NFKBIL1: NFKB inhibitor like 1; PAR2: protease-activated receptor2; MDR1: multi-drug resistance gene 1; RPS6KA2: ribosomal protein S6 kinase A2; NIBD: non-IBD.
The gene Nucleotide binding oligomerization domain containing 2 (NOD2) was the first susceptibility gene of CD, and three risk single nucleotide polymorphisms (SNPs) (R702W, G908R, and L1007finsC) have been studied extensively. A large-scale, multicenter study revealed that the three NOD2 SNPs were significantly associated with an aggressive disease course [86]. NOD2 risk SNPs conferred a 58% increase in the risk for colectomy [86]. In addition, risk genes including immunity related GTPase M (IRGM), TNF superfamily member 15 (TNFSF15), IL23R, etc. were also reported to be predictive markers of an aggressive disease course [6]. Although genetic markers are stable and heritable, they may only explain a small portion of variability. It has been shown that epigenetic markers (such as DNA methylation and non-coding RNAs) also shape the disease course of IBD patients [6, 87]. Tahara et al. claimed that higher methylation levels of protease-activated receptor2 (PAR2) and multi-drug resistance gene 1 (MDR1) were correlated with total colitis phenotypes, and the former was also identified as a potential marker in the prediction of refractory phenotypes of UC [79, 80]. In 2018, a Cambridge research team further observed that gut segment-specific DNA methylation profiles might be used as a clinically useful tool for predicting the requirement for biologics and the time to third treatment escalation [88]. Similarly, cell-specific DNA methylation signatures are also correlated with disease severity and colectomy in patients with UC [89]. One predictive model incorporating three methylation markers can predict treatment escalation with an HR of 5.19 [87]. From this point, DNA methylation markers are crucial in the evaluation of disease course. Furthermore, several studies also suggested that miRNAs are differentially expressed in IBD patients. Expression levels of the miR-29 family, miR-19–3p family and miR-200 family were significantly decreased in patients with stricturing disease, in comparison with those with inflammatory phenotypes [90, 91]. In contrast, some other miRNAs are associated with complicated phenotypes [85]. One prospective study proposed that the expression level of miR-215 increased 4.8-fold when the disease behavior progressed from inflammatory phenotype to penetrating phenotype. In this regard, miRNAs may provide important clues in the assessment of disease course in IBD patients.
For patients with a higher risk of undergoing complicated disease and surgery, physicians are advised to make an aggressive therapeutic approach, aiming at improving disease outcomes. Although genetic and epigenetic markers show their potential role in the prediction of disease course and risk stratification, there are still some limitations. Firstly, although genetic markers are stable and heritable, their value is ethnicity-specific. Some risk loci are reliable markers in predicting disease course in one ethnic population, but may be absent in some other ethnicities, and showed no predictive value in this respect. Secondly, given that DNA methylation patterns are cell-specific, the epigenome differs substantially between sampling sites, which might result in dubious conclusions and limit their clinical application [92]. Thirdly, the association between a genetic/epigenetic marker and disease course is not always robust, therefore leaving uncertainty in its predictive value for disease course. Fourthly, the functional relevance of DNA methylation and miRNAs to intestinal stricturing/penetrating remains largely unknown [91]. Therefore, exploring DNA methylation and miRNA downstream targets is urgently required. Most importantly, considering that IBD results from the complex interplay between different contributors, a reliable disease course prediction must be based on the combined assessment of serological and fecal markers, in addition to clinical, genetic and epigenetic ones. Moreover, identified markers also should be validated and replicated in other ethnic groups, thereby generalizing them in clinical practice.
Serological markers of disease course
Existing and emerging serum markers have been studied extensively in IBD, thus providing valuable information into the prediction of disease course. Different kinds of antibodies against microbial components, neutrophils, and exocrine pancreas such as anti-Saccharomyces cerevisiae (ASCA), anti-outer membrane protein C (anti-OmpC), anti-neutrophil cytoplasmic antibodies (ANCA) and anti-glycoprotein 2 (anti-GP2) have been found in the serum of IBD patients. They are more likely to be detected in IBD patients in comparison with healthy controls, suggesting a possibility of differentiating IBD and controls by them [6]. More importantly, there is substantial evidence demonstrating that seropositivity to these antibodies is associated with disease course in IBD patients. We summarize the serological markers in Table 3.
Table 3.
Risk stratification based on serological markers.
| Markers | Roles in risk stratification | Sample number | Reference |
|---|---|---|---|
| ASCA | Predicted of small bowel surgery, stricturing/penetrating phenotype, ileocolonic disease and perianal lesion for CD | CD (303); CD (252), UC (53), HC (43); CD (169), UC (102) |
[93–95] |
| AMCA | Predicted of surgery and stricturing/penetrating phenotype for CD, and severe disease course for UC | CD (103), CD-ITB (10), ITB (9), HC (68); CD (913), UC (272), HC (200) NIBD (113); CD (107), UC (88) |
[96–98] |
| ACCA | Predicted of steroid dependency and severe disease course for CD and UC | CD (107), UC (88) | [98] |
| pANCA | Predicted of low risk of developing stricturing/penetrating phenotype and receiving surgery for CD, and severe disease course for UC | CD (913), UC (272), HC (200) NIBD (113); CC (17), UC (143), IBDU (146) |
[97, 99] |
| Anti-Fla2 | Predicted of stricturing phenotype for CD | CD (252), UC (53), HC (43) | [94] |
| Anti-Fla-X | Predicted of stricturing phenotype for CD | CD (252), UC (53), HC (43) | [94] |
| Anti-CBir1 | Predicted of ileal disease, surgery and stricturing/penetrating phenotype for CD | CD (796) | [100] |
| Anti-GP2 | Predicted of stricturing phenotype and perianal disease for CD | CD (169), UC (102) | [95] |
| anti-OmpC | Predicted of small bowel surgery and stricturing/penetrating phenotype for CD | CD (303); CD (796) |
[93, 100] |
| anti-I2 | Predicted of small bowel disease, surgery, stricturing/penetrating phenotype and long disease duration for CD | CD (303); CD (196); CD (142) |
[93, 101, 102] |
| CRP (high baseline level) | Predicted of intestinal surgery for CD, and the need of immunosuppressant treatment for CD and UC | CD (957); CD (313), UC (111), IBDU (41); CD (162) |
[103–105] |
| Albumin (low baseline level) | Predicted of surgery and severe postoperative complications for CD, and the need for biologics and colectomy for UC | CD (957); UC (710); UC (97), CD (87), IBDU (6) |
[103, 106, 107] |
Abbreviations: ASCA: anti-Saccharomyces cerevisiae; CD: Crohn's disease; UC: ulcerative colitis; AMCA: anti-mannobioside carbohydrate IgG antibodies; ITB: intestinal tuberculosis; NIBD: non-IBD; ACCA: anti-chitobioside carbohydrate IgA; pANCA: perinuclear anti-neutrophil cytoplasmic antibodies; CC: crohn's colitis; IBDU: inflammatory bowel disease-unclassified; anti-GP2: anti-glycoprotein 2; anti-OmpC: anti-outer membrane protein C; anti-I2: anti-bacterial sequence I2; CRP: c-reactive protein.
With regard to CD, several studies have identified an association between serological antibodies such as ASCA, anti-OmpC and anti-bacterial sequence I2 (anti-I2) and complicated disease and small bowel surgery [93, 94]. Furthermore, serum responses to flagellin and GP2 also help identify patients with complicated disease [94, 95]. A prospective study further suggested that increasing seropositivity to ASCA, anti-CBir1, and anti-OmpC was predictive for a faster disease progression. When patients with these three positive antibodies, they progress to penetrating and/or stricturing disease with an HR of 6.0, and receive CD-related surgery with an HR of 6.6 [100]. This is in line with the perspective of Schoepfer et al. that the risk of suffering from complicated disease and surgery was increased in patients with an increasing number of antibodies [94]. As for UC, pANCA + and ANCA-IgG levels were claimed to be associated with severe disease course [99, 108]. A French study also reported that combined analysis of anti-mannobioside carbohydrate IgG antibodies (AMCA) and anti-chitobioside carbohydrate IgA (ACCA) could correctly identify UC patients with severe disease course with an area under curve (AUC) of 0.67 [98].
Of course, other conventional serological markers including C-reactive protein (CRP) and albumin are also claimed to be associated with disease course in IBD [103, 106]. Combined analysis of more serum antibodies might increase the prediction accuracy to some extent, but we should also keep in mind that there is dissimilarity between association and predictivity. Only a small part of studies explored the predictive role of serum antibody markers in IBD patients. Most studies simply retrospectively analyzed associations between markers and disease course, while didn't investigate the predictive values of these markers in a prospective cohort. Besides, although predictive panels of different-class markers performed better in the disease course prediction, medical cost is another factor should be taken into account [109]. This indicated a need to do a cost-effectiveness analysis and develop a cost-effective panel for IBD patients. It is worth noting that the above serological surrogates also present in other diseases such as intestinal tuberculosis, irritable bowel syndrome (IBS), celiac disease and even healthy controls, which might render it suboptimal in the prediction of disease course and discrimination of disease subtypes [110]. Now that different studies set various thresholds of serum antibodies in different cohorts, this might bring additional hurdles to explain these test results, thereby limiting the clinical application in other cohorts. So, it is absolutely a critical need to validate these results in larger, external and prospective cohorts.
Fecal markers of disease course
It has increasingly become apparent that fecal microbiome plays a critical role in the development and progression of IBD. Explorations in fecal microbiome not only cast insight into the complex pathogenesis of IBD, but also give a new perspective and way to evaluate of disease course. We summarize the fecal markers in Table 4.
Table 4.
Risk stratification based on fecal markers.
| Markers | Roles in risk stratification | Sample number | Reference |
|---|---|---|---|
| Ruminococcus (high baseline level) | Predicted of stricturing phenotype for CD | CD (913) | [111] |
| Collinsella (high baseline level) | Predicted of penetrating phenotype for CD | CD (913) | [111] |
| Veillonella (low baseline level) | Predicted of penetrating phenotype for CD, and severe disease course for UC | CD (913); UC (48), HC (48) |
[111, 112] |
| Rothia (low baseline level) | Predicted of stricturing phenotype for CD | CD (913) | [111] |
| Bacteroides (low baseline level) | Protected from severe disease course for UC | UC (48), HC (48) | [112] |
| F. prausnitzii (low baseline level) | Predicted of severe disease course for UC | UC (48), HC (48) | [112] |
| Proteobacteria (high baseline level) | Predicted of severe disease course for CD and UC | CD (72), UC (51), HC (73) | [113] |
| FC (high level) | Predicted of colectomy and pouchitis for UC, and postoperative recurrence for CD and UC | UC (90); CD (135); UC (60) |
[114–116] |
| FL (high level) | Predicted of pouchitis for UC | UC (60) | [116] |
| Fecal BAFF (high level) | Predicted of severe disease course for UC | CD (44), UC (49), IBS (27), HC (26) | [117] |
| Fecal NGAL (high level) | Predicted of severe disease course for CD and UC | UC (43), CD (30), IEC (21), IBS (21), HC (23) | [118] |
Abbreviations: CD: Crohn's disease; UC: ulcerative colitis; HC: healthy control; F. prausnitzii: faecalibacterium prausnitzii; HC: healthy control; FC: fecal calprotectin; FL: fecal lactoferrin; BAFF: B cell-activating factor of the TNF family; IBS: irritable bowel syndrome; IEC: infectious enterocolitis; NGAL: neutrophil gelatinase-associated lipocalin
In 2017, the RISK study clearly demonstrated that gut microbiota was significantly associated with disease phenotypes [111]. Ruminococcus and Collinsella are enriched in patients with stricturing/penetrating behaviors. While, the levels of Rothia and Veillonella are deceased in complicated disease [111]. As for patients with UC, different kinds of species of microbes were also claimed to be associated with severe disease course [112]. These findings provide additional information about the discriminant power of fecal bacteria between different disease phenotypes and courses. One year later, a Chinese study team also made a similar conclusion that different kinds of gut microbiota conferred risk to different phenotypes [113]. Most importantly, this study further revealed consistent microbial alteration patterns between Chinese and Western IBD patients, suggesting the possibility of using microbial markers to classify IBD patients across different ethnicities [113]. Although microbiota markers showed great potential in risk prediction, they haven't been broadly applicable in clinical practice. The following factors should be considered before application. Firstly, it is an established fact that diet, smoking, drugs, etc. markedly influence the composition and diversity of the microbiome [43, 119]. Some studies didn't take these confounding variables into consideration, which might affect the reliability and accuracy of results. Secondly, microbiota can indeed add value to the prediction of disease course, but it is not specific to IBD. Other diseases such as infective enteritis, celiac disease and IBS can also influence its form and diversity. Further work is warranted to elucidate its specific association with IBD. Thirdly, the functional consequences of most microbiota are unclear. So, conducting a metabolomics study is definitely needed. Fourthly, microbial shift in stool samples is not parallel with that in tissue samples [120]. Gevers et al. claimed that microbial imbalance was less seen in stool samples, but more often in tissue samples [120]. Therefore, additional efforts are required to further study the microbial community network in different intestinal segments. Combined analyzing microbiota markers in stool and tissues may be more reliable, but tissue samples must be collected by invasive endoscopy, which might increase medical expenses and expose patients to additional risks caused by endoscopy. Based on the above, exploring more reliable and cost-effective fecal markers is in desperate need.
Besides the fecal microbiome, fecal calprotectin (FC) is now widely used as a reliable and noninvasive marker in assessing disease activity and differentiating IBD [121]. Patients with increased FC are at a higher risk of receiving colectomy and having postoperative recurrence [114, 115]. FC was superior to CRP and Crohn's Disease Activity Index (CDAI) in the reflection of the presence and severity of recurrence [115]. As for UC patients, several studies claimed that higher levels of FC were more often presented in patients with pouchitis [116]. It is important to note that the levels of FC were elevated two months before the confirmed diagnosis of pouchitis [116]. Based on these findings, FC might be a prominent marker in the prediction of postoperative recurrence. Other fecal markers including fecal lactoferrin (FL), fecal B cell-activating factor of the TNF family (BAFF), fecal neutrophil gelatinase-associated lipocalin (NGAL) also show their potential role in the evaluation of disease course [117, 118, 122]. However, as mentioned above, we should pay attention to the difference between association and prediction. So, the predictive value should be validated further.
Combined predictive models
Analyzing one class of markers alone cannot ensure an accurate prediction of disease course, combined analysis of different-class of markers such as clinical, genetic, epigenetic, serological and fecal surrogates may facilitate the prediction process. The RISK study developed a competing-risk model consisting of age, race, disease location, serologic markers and extracellular matrix gene profiling. It could predict complicated disease in CD patients with an AUC of 0.72 [111]. Similarly, another web-based system dynamic model incorporating disease location, serologic markers, NOD2 polymorphisms, and an interaction term between perianal disease and ASCA could correctly identify a high-risk population (developing strictures/fistulas, or receiving surgery over a three-year period) with a high concordance index [109]. More recently, a promising model including six genetic SNPs, ileal location, and three specific antibodies can predict intestinal surgery and/or complicated disease at 5 years with an AUC of 0.84 [123]. In general, the combined predictive model outperforms the single predictive model in helping physicians perform risk stratification and decide an appropriate treatment plan. But the cost of examinations and genetic heterogeneity should be taken into consideration when interpreting these results.
Available data indicate that IBD patients with an aggressive disease course are more likely to undergo frequent flares, disease complications, treatment refractory, bowel surgeries and frequent hospitalization [124]. Some severe patients even present stricturing and/or fistulizing disease and have to get abdominal surgery at the time of diagnosis. The intestinal surgery rate is as many as 80% and 30% for CD and UC, respectively [125]. Undoubtedly, physicians should do risk stratification before embarking on treatment. Any one-size-fits-all therapeutic approach is improper [43]. Given that bowel damage is progressive, accumulative and nearly irreversible, any delayed and inadequate treatment may accelerate disease progression, especially in those severe IBD patients. Early and progressive therapeutics can mitigate the disabling disease course and even alter the natural history of IBD. Therefore, patients with an aggressive disease course need a timely and potent treatment. A combined therapy of biologics and immunomodulators (even small molecule inhibitors) is recommended for these patients. For severe perianal fistulizing CD, early surgical treatments including abscess drainage, abscess setons, fistulotomy, and ligation of the intersphincteric fistula tract are also recommended [126]. With respect to intestinal stricturing/fistulizing CD, the anti-TNF biologic and ileocolic resection is the optimal pharmacotherapy and surgical treatment choice, respectively [127]. For acute severe ulcerative colitis (ASUC), besides corticosteroids and anti-TNF biologic salvage therapy, timely colectomy should be taken into consideration. While, a conventional step-up approach would suit those mild IBD patients. Conventional treatments including 5-aminosalicylate (5-ASA), corticosteroids, immunomodulators and others are recommended [43]. This personalized treatment not only reduces unnecessary expenses, but also decreases the unnecessary risk of adverse events including myelotoxicity, opportunistic infection and lymphoma for patients with an indolent disease course. Moreover, it also markedly improves clinical outcomes for patients with an aggressive disease course [90, 128]. Even so, the challenge remains to select the most suitable drugs for each individual patient, given that different patients show significant differences in drug metabolism and treatment response. Thus, physicians are advised to make an individualized therapeutic regimen based on the clinical characteristics and molecular markers for each patient.
Precision treatment with key medications
Despite many drugs showing promising potential in the treatment of IBD, unfortunately, the pharmacokinetics and pharmacogenetics vary between different patients with IBD. Some patients respond well to them with no adverse events, while others have lower response rates with serious adverse reactions. Therefore, adequate curative effects should be balanced with adverse events associated with their use before treatment. Here, this review will only discuss these well-studied drugs for IBD, namely thiopurines (azathioprine and 6-mercaptopurine), infliximab, adalimumab, vedolizumab, and ustekinumab (Table 5).
Table 5.
Clinical and molecular markers in prediction of treatment response and adverse events to thiopurines and biologics.
| Markers | |||||
|---|---|---|---|---|---|
| Drugs | Good response | Poor response | Adverse events | Sample number | References |
| Thiopurines | N/A | N/A | TPMT; NUDT15 (myelosuppression) | CD (41); CD (253) |
[129, 130] |
| Infliximab | Concurrent immunomodulator treatment; male; non-stricturing/penetrating phenotype; IL23R (risk-increasing variants); FasL-843 (CC/CT); ADAM17 (rs10929587, TT); SLCO1C1 (rs3794271, CC); high CRP; high DEF5; high ECP; high F. prausnitzii; high Bifidobacteriales; high Clostridium | Smoking; older age at first dose; IL23R (risk-decreasing variants); caspase-9 93 (CC/CT); HLA-DQA1*05 (A>G); ATG16L1 (rs2241880, AA); PHACTR3 (rs6100556, TT); CXCL12 (rs10508884, CC/CT); p-ANCA+/ASCA-; high baseline WBC; high CATH; high FC | STAT3 (rs744166) | CD (169); CD (210); UC (90); CD (287); CD (131); CD (226); UC (56); CD (17), UC (6), IBDU (6); CD (36), UC (26) JIA (18), HC (8); CD (106); CD (1610); CD (152), UC (110); CD (240), UC (93), IC (7); UC (191); CD (35); CD (5) |
[131–144] |
| Adalimumab | ATG16L1 (rs10210302, CT/TT); TLR2 (rs3804099, TC/CC); TLR4 (rs5030728, GA/AA); TNFRSF1A (rs4149570, TT); MIF (rs755622, GG); TNFa (rs361525, GG); IRGM (rs13361189, TT); high CRP*; high mTNF+ cells; high Barnesiell; high Anaerostipes; high Tyzzerella; high Lachnoclostridium; high Lachnospiraceae_unclassified; high F. prausnitzii | Male; Smoking; family history of IBD; infliximab failure; EIMs; perianal disease; high CDAI; high BMI; FasL (rs763110, CC genotype); TNF-308 (rs1800629, AA/GA); IL17A (rs2275913, AA/AG); low fT3/fT4; high CD25; high IL-5; high Escherichia-Shigella | N/A | UC (56); CD (1610); CD (102); CD (483); CD (482), UC (256); CD (102); UC (64); CD (25); CD (115); CD (121); CD (997); UC (56) |
[140, 144–152] |
| Vedolizumab | Mayo score<9; CDAI score≤330; younger patients; longer disease duration*; Concurrent thiopurine treatment; high BLOC1S1; high TLCD1; high TMEM223; high α4β7*; high Roseburia inulinivorans; high Burkholderiales | Smoking history; anti-TNF failure; active perianal lesions; concomitant steroid use; HBI score>10; high CRP*; low albumin; high PHLDA1; high OSBPL11; high CXCL3; high α4β1; high αEβ7; low α4β7 receptor saturation; absent α4β7 expressing cells | N/A | CD (161), UC (111) CD (1115); UC (620); CD (18), UC (19); CD (15), UC/IC (11); CD (19), UC (17); CD (42), UC (43); CD (212); CD (128), UC (117); CD (173), UC (121); CD (967), HC (148); CD (5) |
[153–164] |
| Ustekinumab | Colonic/ileocolonic disease; concurrent immunomodulator treatment; CRP≥10mg/L; high TNF; high TBX21; high IL-23A; high IL-6; high FOXP3; high OSM; high OSMR; high F. prausnitzii; high Bacteroides; high clostridium citroniae; high Agathobaculum butyriciproducens; high Phascolarctobacterium faecium; low FC | HBI>7; structuring disease; perianal disease; intestinal resection history; current corticosteroid use; anti-TNF failure; Mayo score>6; high BMI; high albumin | N/A | CD (167); CD (104); UC (133); CD (102); CD (28), UC (28), NIBD (11); CD (306); CD (108), UC (77); CD (439); CD (116); CD (1369) |
[23, 165–171] |
Abbreviations: TPMT: thiopurine s-methyltransferase; NUDT15: nudix hydrolase 15; CD: Crohn's disease; STAT3: signal transducer and activator of transcription 3; IL23R: interleukin 23 receptor; UC: ulcerative colitis; FasL-843: Fas ligand-843; ADAM17: ADAM metallopeptidase domain 17; SLCO1C1: solute carrier organic anion transporter family member 1C1; ATG16L1: autophagy related 16 like 1; CRP: c-reactive protein; DEF5: defensin 5; PHACTR3: phosphatase and actin regulator 3; IBDU: inflammatory bowel disease unclassified; CXCL12: C-X-C motif chemokine ligand 12; ECP: eosinophil cationic protein; F. prausnitzii: faecalibacterium prausnitzii; p-ANCA: perinuclear anti-neutrophil cytoplasmic antibodies; ASCA: anti-Saccharomyces cerevisiae; WBC: white blood cell; CATH: cathelicidin antimicrobial peptide; FC: fecal calprotectin; FL: fecal lactoferrin; JIA: juvenile idiopathic arthritis; TLR2: Toll like receptor 2; TLR4: Toll like receptor 4; EIMs: extra-intestinal manifestations; TNFRSF1A: TNF receptor superfamily member 1A; MIF: macrophage migration inhibitory factor; TNFa: tumor necrosis factor alpha; BMI: body mass index; IL17A: interleukin 17A; fT3/fT4: free triiodothyronine-to-thyroxin; CDAI: crohn's disease activity index; HBI: harvey-bradshaw index; BLOC1S1: biogenesis of lysosomal organelles complex 1 subunit 1; IC: indeterminate colitis; TLCD1: TLC domain containing 1; TMEM223: transmembrane protein 223; α4β7: alpha 4 beta 7; PHLDA1: pleckstrin homology like domain family a member 1; OSBPL11: oxysterol binding protein like 11; CXCL3: C-X-C motif chemokine ligand 3; α4β1: alpha 4 beta 1; αEβ7: alpha E beta 7; TBX21: t-box transcription factor 21; NIBD: non-IBD; IL-23A: interleukin 23A; IL-6: interleukin 6; FOXP3: forkhead box p3; OSM: oncostatin M; OSMR: oncostatin M receptor. * controversial.
Thiopurines
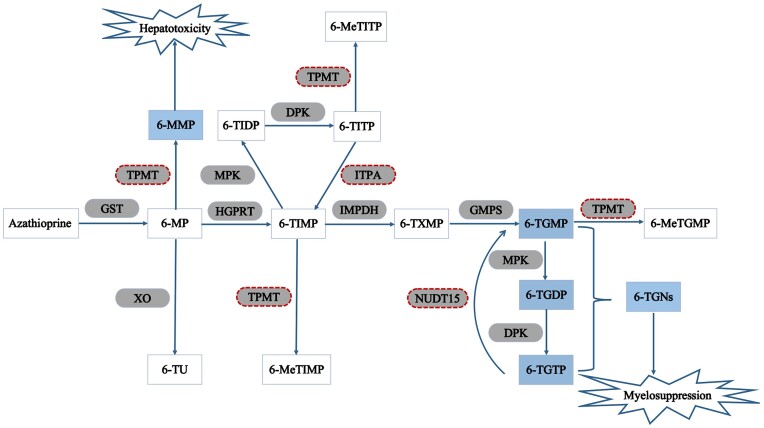
Thiopurines, conventional immunosuppressants, have extremely complicated metabolic pathways. Taking azathioprine for example, azathioprine changes into 6-mercaptopurine after absorption by the GI tract. 6-mercaptopurine can then be metabolized through three competing pathways: conversion into 6-thioinosine monophosphate (6-TIMP) by hypoxanthine phosphoribosyltransferase (HGPRT); methylation by TPMT into 6-MMP that is responsible for hepatotoxicity; and conversion into 6-thiouric acid (6-TU) by xanthine oxidase (XO). 6-TIMP can then be successively metabolized into 6-thioxanthosine monophosphate (6-TXMP) and 6-thioguanine nucleotides (6-TGNs) by inosine-5-monophosphate dehydrogenase (IMPDH) and guanidine-5-monophosphate synthetase (GMPS), respectively(Fig. 2) [172]. Thiopurines play a well-established role in the induction and maintenance of remission, facilitation of MH, and prevention of postoperative recurrence for IBD patients. Such good therapeutic effects are directly related to their metabolites 6-TGNs, which are also responsible for the common side effect, myelosuppression [172]. Similarly, an increased concentration of another metabolite 6-methylmercaptopurine (6-MMP) is involved in hepatotoxicity [173]. Available data indicated that thiopurines had to be reduced or discontinued due to adverse effects in about 34%-35% of patients [174].
Figure 2.
The metabolic pathways of azathioprine. Azathioprine changes into 6-mercaptopurine after absorption by the GI tract. 6-mercaptopurine can then be metabolized through three competing pathways: conversion into 6-TIMP by HGPRT; methylation by TPMT into 6-MMP; and conversion into 6-TU by XO. 6-TIMP can then be successively metabolized into 6-TXMP and 6-TGNs by IMPDH and GMPS, respectively. GST: glutathione s-transferase; 6-MP: 6-mercaptopurine; TPMT: thiopurine s-methyltransferase; 6-MMP: 6-methylmercaptopurine; XO: xanthine oxidase; 6-TU: 6-thiouric acid; HGPRT: hypoxanthine–guanine phosphoribosyl transferase; 6-TIMP: 6-thioinosine monophosphate; MPK: monophosphate kinase; 6-TIDP: 6-thioinosine diphosphate; DPK: diphosphate kinase; 6-TITP: 6-thioinosine triphosphate; 6-MeTITP: 6-methylthioinosine triphosphate; ITPA: inosine triphosphate pyrophosphatase; IMPDH: inosine monophosphate dehydrogenase; 6-TXMP: 6-thioxanthosine 5’-monophosphate; GMPS: guanosine monophosphate synthetase; 6-TGMP: 6-thioguanine monophosphate; 6-TGDP: 6-thioguanine diphosphate; 6-TGTP: 6-thioguanine triphosphate; NUDT15: nudix hydrolase 15; 6-TGNs: 6-thioguanine nucleotides; 6-MeTGMP: 6-methythioguanine monophosphate.
Thiopurine S-methyltransferase (TPMT), and nudix hydrolase 15 (NUDT15) gene variants can influence the activities of important enzymes implicated in the metabolism of thiopurines. Therefore, pharmacogenetics analyses may add value to treatment decisions and individualized treatment. One population frequency analysis of TPMT alleles showed that TPMT*3A is the most common allele in Caucasians, while Asian and African populations often present with TPMT*3C [175]. In the Caucasian population, approximately 11% of individuals are heterozygous carriers with intermediate TPMT activity, and only 0.3% are homozygous for TPMT variants with low/absent TPMT activity [173]. Thus, patients with TPMT variants are prone to develop myelosuppression when compared with those with wild-type genotypes [129]. Notably, the TPMT variant allele frequency is significantly lower in Asians than that in European populations [130]. The low frequency of TPMT variants in Asians limits the clinical value of predicting thiopurine-induced myelosuppression. It is also noteworthy that TPMT variants cannot explain the overall myelosuppression, suggesting other contributing factors should be explored further. Sutiman et al. reported that NUDT15 (p. Arg139Cys) conferred a 22.9-fold increased risk of leukopenia in Asian IBD patients [176]. Another European case-control study drew a similar conclusion and strongly recommended to detect NUDT15 polymorphisms before initiation of thiopurine treatment [177]. Given that NUDT15 genetic variants are more common in Asians, NUDT15 polymorphisms are claimed to be the better genetic surrogate for the prediction of thiopurine-induced myelotoxicity compared with TPMT genetic variants in Asians [130]. However, the correlation between adverse events of thiopurines and inosine triphosphate pyrophosphatase (ITPA) polymorphisms is fairly controversial [178, 179]. Further studies should be taken to elaborate on their correlations and guide treatment decisions. The question then arises whether TPMT/NUDT15 genetic testing should be systematically indicated in all patients who are going to receive thiopurines, considering that this testing is not cheap. In recent years, we proposed to foster value-based healthcare, a strategy to increase the quality and value of healthcare services by promoting the shift from volume-based payments to outcomes-based payments. So, several studies have done cost-effectiveness analyses of pretreatment screening TPMT/NUDT15 polymorphisms. The final results prove it as a cost-beneficial strategy [180, 181]. Therefore, prospective screening for TPMT and NUDT15 should be considered in principle before starting thiopurine therapy in various races.
Besides genetic markers, the roles of gut microbiota in predicting thiopurine treatment response should also be noted. Available data demonstrated that gut microbiota affect thiopurine biotransformation by releasing microbial enzymes [182]. Liu et al. found that Bacteroides vulgatus could encode thiopurine metabolic enzymes including GST, HGPRT, GMPS and IMPDH [183]. Besides the above enzymes, this study also suggested that Escherichia coli further possessed another critical enzyme, XO [183]. Several other gut bacteria including Enterococcus faecalis, Bacteroides fragilis, and Pseudomonas aeruginosa were also claimed to be responsible for azathioprine metabolism [184]. Based on these findings, we can conclude that gut microbiota might be a promising and novel tool for personalized thiopurine treatment of IBD. However, little study prospectively evaluates the predictive performance of baseline microbiota in thiopurine response. More studies are needed to fill this gap.
Dose reduction or even exclusion of thiopurines should be taken into account for patients with mutant genotypes. Recently, a Chinese research team conducted a randomized clinical trial and demonstrated that NUDT15 C415T-based dose optimization before treatment mitigated the risk of developing leucopenia in CD patients [185]. The predictive roles of these risk genes have been well confirmed in clinical practice. However, some subjects with wild-type genotypes still suffered from severe adverse events, indicating that other factors such as environmental, microbiota, other genetic predictors, etc. may account for the remaining toxicity. Further work is warranted to explore potential predictors and their interactions with thiopurine-induced adverse events, in order to achieve a precision selection of appropriate medication for individual patients. Other immunomodulators used for IBD including methotrexate, ciclosporin and tacrolimus are also effective in achieving steroid-free remission. Predictive markers of treatment response and adverse events have not been fully investigated, therefore more studies are needed to identify predictors for these medications.
Albeit immunomodulators are widely used in clinical practice and show acceptable efficacy in the treatment of IBD, many discontinue these treatments partly due to toxicity, intolerance, unfavorable response rate, and inconvenience of application. Even among those who continue receiving immunomodulators, a great number of patients fail to improve the aggressive disease course and poor prognosis. Therefore, appealing biological agents such as infliximab, adalimumab, vedolizumab, and ustekinumab have been added to the treatment options for those with a moderate to severe disease course. Adequate therapeutic effects make them highly acceptable for patients and physicians, while there are also plenty of primary non-responders and secondary non-responders to biological agents. Additionally, high cost, increased risk of opportunistic infections and malignant tumor, and inconvenience of parenteral application limit their routine clinical use. Current evidence suggests that clinical features, genetic surrogates, and some other predictive molecular markers can assist physicians in distinguishing responders from non-responders with good accuracy. Therefore, physicians should assess disease status carefully and make an individualized treatment plan based on existing markers, in order to minimize the risk of adverse events, maximize treatment effects, reduce medical costs, and improve the quality of life of patients to the most extent.
Infliximab
Infliximab, a chimeric monoclonal IgG1 antibody against TNF-α, shows excellent therapeutic effects in CD and UC, especially in those with moderate to severe disease and medically refractory disease [186]. However, nearly 40% of patients do not show an early response, and 23%–46% develop a secondary loss of response over time [187]. Some different classes of factors are of great value in predicting the initial and sustained response to infliximab, which can assist physicians in determining individualized therapy for individual patients.
Among these various predictive factors of response to infliximab, genetic predisposing factors are the most studied. Jürgens et al. concluded that homozygous carriers for IBD risk-increasing IL23R variants were more prone to respond to infliximab than those who are homozygous for IBD risk-decreasing IL23R variants (74.1% vs. 34.6%) [133]. In addition to alleles of IL23R, a favorable treatment response is also linked to the Fas ligand (FasL)-843 CC/CT genotype in CD [134]. Besides, a significant association between HLA-DQA1*05 and poor response in patients with IBD has been found in some studies [141, 188]. HLA-DQA1*05 carriers are at a higher risk of developing antidrug antibodies (ADAs) and losing therapeutic response [141]. However, Laserna-Mendieta et al. drew a negative result that HLA-DQA1*05 didn't affect infliximab response [135]. Different standards of treatment response may explain this opposite conclusion. In another study, an apoptotic pharmacogenetic index (API) based on genetic and clinical data for the prediction of response rates has been developed. Higher API scores implied a higher response rate to infliximab in patients with CD [189]. Other genes such as autophagy related 16 like 1 (ATG16L1), C-X-C motif chemokine ligand 12 (CXCL12), FasL-670, etc. were also claimed to be valuable markers of predicting treatment response [134, 142]. Besides single genetic predictor, gene expression signatures also add value to precision prediction. By combined expression analysis of five identified genes, the responders can be distinguished from non-responders in UC patients with a sensitivity and a specificity of 95% and 85%, respectively [190]. The predictive value of gene expression profiles in CD patients has also been investigated. Arijs et al. reported that differentially expressed gene profiles were capable of predicting response to infliximab with an overall accuracy of 100% based on microarray analysis [191]. For those patients carrying risk haplotypes, concomitant immunomodulator treatment or switch therapy may be the next step of treatment. Based on these findings, we can make a conclusion that pharmacogenetics paves a novel way to the prediction of treatment response. If possible, prior-to-treatment screening for risk genetic markers should be considered in routine clinical practice.
Aside from genotype testing, other clinical and serological markers may provide additional information on the prediction of infliximab response. Previous studies claimed that long disease duration, smoking and others were associated with poor response to infliximab, while concurrent immunomodulator treatment and non-stricturing/penetrating phenotype were possible predictors of favorable response rates [131, 132]. However, some patients with clinical risk factors show adequate response to infliximab and even gain MH, indicating that relying on clinical factors alone cannot guarantee an accurate prediction. Studies in serological, histologic, and fecal markers might provide more valuable and reliable information. Studies suggested that a high baseline CRP was associated with a better response rate, while p-ANCA+/ASCA- was a hopeful predictor for lower response rates to infliximab [133, 136]. Moreover, serum and mucosal proteomic profiling can also add value to a more precise prediction [137]. Pre-treatment serum infliximab-modulated immune profiling including oncostatin-M (OSM), TNFSF14 and others was demonstrated to be helpful in the prediction of clinical response [192]. Caution needs to be exercised when interpreting these results, because some results were gained from a single-center study with a small sample. Moreover, these candidate proteomic markers have not been further validated, resulting in little clinical utility.
Gut microbiota is a key factor in the pathophysiology of IBD [193]. Available data strongly support that fecal surrogates can not only assist physicians in differential diagnosis and assessment of disease activity, but also serve as clinically useful predictors of therapeutic response to infliximab. Analysis of the composition, abundance, and diversity of intestinal microbiome before and after the infliximab therapy may provide some clues about treatment response. A study demonstrated that six groups of fecal bacteria might be promising predictive markers of therapeutic response to infliximab [139]. In accordance with it, responders presented lower dysbiosis indexes and a higher number of faecalibacterium prausnitzii (F. prausnitzii) and Bifidobacteriales when compared to non-responders, suggesting that F. prausnitzii and Bifidobacteriales could be candidate markers of predicting therapeutic response of infliximab [137, 138]. Moreover, in virtue of non-invasiveness, intestinal-specificity and stability, fecal proteins such as FC and FL are also claimed to be potential markers for prediction. Although these fecal markers were reported to be associated with response rates, divergent results should also be noted [194]. Moreover, most of these findings are at the candidate discovery stage of the biomarker pipeline, more efforts are needed to qualify and verify these candidate predictors in larger populations. Therefore, larger, prospective, and independent studies should be carried out to clarify their roles in predicting treatment response, and thus achieve precise prediction and avoid exposure of non-responders to infliximab.
One class of predictors is insufficient for an accurate prediction, therefore combined analysis of different-class markers may further improve the accuracy of prediction and assist in making individualized treatment regimens. This is a matter of prime importance when making a therapeutic plan. Recently, a combined panel of genetic and clinical surrogates showed an increased accuracy in the prediction of primary nonresponse, compared with a clinical-only panel (AUC 0.87 vs. 0.57) [195]. Dubinsky and colleagues combined analyzed genetic effects, clinical markers, and serological surrogates, and built a predictive model comprising of three “pharmacogenetic” loci, a known locus, p-ANCA positivity and diagnosis of UC in pediatric patients [196]. When the risk factors were more than two, the relative risk of non-response became 15-fold higher than those who had only two or fewer risk factors, with an AUC of 0.98. Similarly, Zhou et al. claimed that combined analysis of Clostridiales abundance, FC levels and CDAI could discriminate infliximab responders from non-responders with an accuracy of 93.8% [113]. Indeed, these findings provide a possibility for physicians to use a predictive model for the prediction of infliximab response, although they must be confirmed independently, on a larger scale, in a prospective cohort and also studied in an adult cohort.
Adalimumab
Adalimumab is one full recombinant human IgG1 antibody against TNF-α and shows great effectiveness in induction and maintenance of remission in CD and UC patients [197, 198]. It is also used as a second-line therapy for moderate to severe active patients and those nonresponse or intolerance to infliximab [199]. Similar to infliximab, a great number of patients do not respond to adalimumab. About one-third of CD patients fail to respond to adalimumab in one-year follow-up [200]. More importantly, even among primary responders, 18.2% of patients suffer from secondary adalimumab failure, and 37% of cases need dose escalation [149]. Therefore, discriminating responders from non-responders prior to initial therapy becomes particularly important.
Some genetic markers might aid physicians in predicting the therapeutic response to adalimumab. Koder et al. suggested that patients with ATG16L1 (rs10210302) CT/TT genotype were more likely to achieve biological response, compared to those with CC genotype (OR: 9.44) [144]. Moreover, other candidate predictive markers including Toll like receptor 2 (TLR2), TNF receptor superfamily member 1A (TNFRSF1A), FasL etc. were also claimed to be associated with adalimumab response [142, 145, 150]. However, it is noteworthy that different standards of treatment response are set in various studies. Some investigated the clinical response rates, whereas others explored the difference in endoscopic remission and histologic remission between responders and non-responders. Besides, these identified genetic variants show relatively small effect sizes on composite disease response scores [194]. So, more risk SNPs with large effect sizes are needed to be explored. What's more, genetic heterogeneity across ethnicities also should be noted.
In addition to genetic predictors, predictive roles of clinical, serological and fecal markers have also been identified. Available data showed that demographic and disease characteristics including smoking, primary failure to infliximab, EIMs and others are correlated with a loss of response and dose escalation [140, 149]. With respect to CRP, contradictory results have been found. Some studies suggested an association between low baseline CRP and good treatment response, while other studies claimed that high baseline levels of CRP were associated with a better therapeutic response [194, 197, 201]. Such inconsistency can be explained by the fact that CRP is not only associated with inflammatory phenotypes, but also predictive of more severe disease [194]. Besides the conventional inflammatory protein, a team from Switzerland further investigated the predictive role of T-cells from peripheral blood mononuclear cells (PBMCs). A serological predictive panel comprising T-cell surface receptor (CD25) and related cytokine markers (IL-5) was generated, which performed effectively with an acceptable accuracy of 91% [152]. Recent advances in endoscopy also provide a possibility for physicians to predict treatment response. in vivo molecular imaging by confocal laser endomicroscopy (CLE) and fluorescent antibodies to TNF revealed that the patients with increased baseline levels of mTNF + cells had significantly higher short-term response rates than those with decreased numbers of mTNF + cells [147]. This result could be explained by the fact that high levels of mTNF + cells indicate high numbers of targets for anti-TNF biologics. Therefore, the response rates increase. This finding does hold promise for endoscopy-based treatment prediction. Fecal markers also show promising potential in the prediction of therapeutic response to adalimumab. The abundance of protective microbiota including Barnesiella, Anaerostipes, Tyzzerella, etc. was increased in responders. Conversely, a decrease in pathogenic bacteria Escherichia-Shigella was found in adalimumab-responsive patients [148]. From this point, these changed fecal microbiota are capable of predicting the treatment response to adalimumab. It is important to note that human gut microbiome is highly dynamic and personalized, but most microbiome studies concentrate on a single time point and certain patients (small sample size and specific ethnic group). Longitudinal studies of the long-term change of microbiome in responders and non-responders across different ethnicities are therefore required.
It should be noted that a single marker seems to be inadequate for the prediction of treatment response. So, Gorenjak et al. used machine learning support vector machines algorithm, and developed a prediction model consisting of the expression and genotype data of four potential genes [202]. This model showed a surprisingly high accuracy of 100% in predicting adalimumab response. More recently, Busquets et al. developed an algorithm comprising four microbial markers and used it to differentiate responders from non-responders, with a favorable sensitivity and specificity (93.33% and 100%) [203]. Furthermore, Bouhnik et al. assigned a value to different variables (clinical, laboratory, and imaging parameters) and constructed a prognostic score to aid precise prediction [204]. A higher prognostic score represents a high possibility of adalimumab response at week 24.
Given that adalimumab and infliximab are both anti-TNF-α agents, most predictors used in infliximab therapy might also be used in adalimumab treatment. However, an important issue deserves our close attention. For those with a loss of response to infliximab, the response rate to adalimumab varies significantly between different individuals. Some show astonishing response rates, while others are still non-responders. There may be various underlying factors influencing the responsiveness to adalimumab and infliximab respectively. Therefore, comparative studies are required to identify specific predictors of infliximab and adalimumab with the aim of improving the accuracy of prediction and avoiding the failure of second-line anti-TNF therapy of adalimumab.
Vedolizumab
Vedolizumab is a humanized, more selective, monoclonal antibody against gut-homing a4β7 integrin [154]. Well-known, three-phase, randomized controlled trials (GEMINI) demonstrated its adequate efficacy in induction and maintenance of remission for patients with CD and UC [154, 205]. Similar to infliximab and adalimumab, vedolizumab is not always an effective treatment. Available data suggested that the clinical response rates at week 14 after vedolizumab therapy are 49%–64% in CD and 43%–57% in UC, respectively [162, 206, 207]. However, even in these initial responders, approximately 20% of patients become secondary non-responders and stop vedolizumab due to lack or loss of effectiveness [208, 209]. Hence, identifying predictors of treatment response to vedolizumab holds the key to precision treatment.
Existing evidence demonstrated that clinical features and serological biomarkers, as well as fecal surrogates and pharmacological parameters, are correlated with the therapeutic response of vedolizumab in IBD. The association between baseline disease activity and clinical remission rates has been confirmed in several studies. GEMINI 1 and 2 trials showed that patients with baseline Mayo score < 9 and CDAI score ≤ 330 had higher clinical remission rates at week 6 and week 54 [207]. Other clinical characteristics such as smoking history, anti-TNF failure, active perianal lesions, etc. are also predictors of unfavorable response rates [154, 160, 161]. Different opinions regarding the association between disease course and vedolizumab response have been expressed. Patients with longer disease duration are more prone to lose response to vedolizumab [154, 207]. However, the contradictory finding was seen in another study [155]. The former can be explained by the fact that patients with longer disease duration are prone to have a severe disease course and to be treated with anti-TNF before, thus, they are at risk of losing response to vedolizumab. However, longer disease duration also results in very chronic inflammation and T cell exhaustion, indicating a good prognosis in chronic autoimmune disease [155, 210]. These findings again need further replication studies to validate their predictive roles in vedolizumab response.
Conventional serum markers may further assist physicians in evaluating the disease state and selecting the most appropriate patients. Current evidence shows that high baseline CRP and low baseline albumin are associated with poor response [155, 163]. However, whether CRP served as a positive or negative predictor of therapeutic response remains to be determined [194, 206]. Underlying factors including different outcome definition, different observation time, and confounding variables might contribute to these paradoxical findings. Recently, vedolizumab responders were claimed to have higher baseline expression of transmembrane protein 223 (TMEM223) in PBMC Treg cells in comparison with those non-responders. On the contrary, a high expression level of CXCL3 was suggested to be a negative marker of adequate response to vedolizumab [156]. Besides PBMC, transcriptional profiles of mucosal Treg cells also provided additional information about discrimination between vedolizumab responders and non-responders [156].
Besides traditional inflammatory markers, specific changes in integrin expression profiles are also associated with treatment response. Schneider et al. demonstrated that the baseline frequencies of α4β7-expressing T cells were statistically lower in clinical responders than that in non-responders [211]. However, other studies drew the opposite conclusion that high baseline α4β7 expression levels of T, B and NK cells predicted good therapeutic response [157, 158]. During vedolizumab therapy, an increased expression of α4β7 integrin was associated with good clinical presentation, while increased levels of α4β1 and αEβ7 indicated bad outcomes [158]. Such contrasting conclusions provide an impetus for further studies to clarify the relationship between baseline α4β7 integrin levels and vedolizumab response. In addition, α4β7 receptor saturation was also identified as a candidate predictive biomarker. Non-responders often present lower α4β7 receptor saturation rates at trough than responders, and the saturation rates are reduced over time [157]. In 2017, Rath et al. used CLE to detect α4β7 expressing cells in colonic mucosa, and further suggested that absent α4β7 expressing cells might lead to poor therapeutic response to vedolizumab [164]. These results certainly open a new approach for identifying patients who will benefit most from vedolizumab and add value to personalized vedolizumab therapy. However, this study only included five patients with CD, highlighting the need to conduct studies with a larger sample size and validating its predictive role in the UC patients.
As mentioned previously, a central role of gut microbiota has been confirmed in the pathophysiology of IBD. A recent study assessed its relationship with vedolizumab response. CD patients in remission (at week 14) had a higher baseline α-diversity and more abundant Roseburia inulinivorans and Burkholderiales species, compared with the patients with high disease activity [159]. Thirteen microbial pathways including branched chain amino acid (BCAA) synthesis were markedly enriched in quiescent CD patients, compared with non-remission patients. With the help of gut microbiota, physicians might predict the vedolizumab response more accurately and make a personalized therapeutic regimen according to individual microbiota characteristics of each patient.
However, the one class of markers alone performs imperfectly in predicting vedolizumab response. Therefore, researchers successfully developed a mixed model consisting of clinical data, microbial taxonomy and pathway relative abundance to predict treatment response with an AUC of 0.776, which outperformed each individual model established in their study [159]. Furthermore, another two scoring systems consisting of various clinical and serological markers have also been established and validated in patients with CD and UC [155, 212]. Dulai et al. assigned different values to various variables (medication history, surgery, disease behavior, albumin and CRP) and developed a vedolizumab response scoring system [212]. It performed effectively in the prediction of clinical remission, and MH with an AUC of 0.67 and 0.72, respectively [212]. As for UC, another scoring system consisting of different parameters including medication history, disease duration, endoscopic activity and albumin was constructed. When the score is below 26 points, patients are less likely to achieve corticosteroid-free remission at week 26 (the sensitivity and specificity is 93% and 15%, respectively) [155]. It should be noted that there are few data specifically investigating the effects of genetic variants, FL and serum antibodies such as ASCA and p-ANCA on the vedolizumab response prediction. Further studies are needed to clarify the relationships clearly.
Ustekinumab
Ustekinumab is a fully human monoclonal IgG 1k antibody to the p40 subunit of IL-12 and IL-23 [213]. Ustekinumab binds the common p40 subunit, blocks the biological activity of IL-12 and IL-23, and finally stops the inflammatory cascade [214]. Well-established UNITI trials demonstrated that ustekinumab is a more effective treatment than placebo in induction and maintenance therapy for patients with CD [23]. It has also been approved to treat moderate to severe UC patients in the UNIFI study [24]. Similar to the above biological agents, there were also lots of primary non-responders and secondary non-responders to ustekinumab. Moreover, some patients suffer from unacceptable side effects during the course of treatment. Therefore, exploring predictors of ustekinumab response and applying them to clinical practice become an essential part of the treatment work in IBD.
Initial studies revealed that patients with higher disease activity exhibited poorer response in the long term [165, 166]. CD patients with Harvey-Bradshaw index (HBI)>7 at induction have a lower likelihood of achieving clinical response at follow-up [165]. Disease locations and phenotypes may also provide clinically useful information for the prediction process. Structuring disease is a negative predictor of good clinical response, while patients with colonic/ileocolonic disease are more prone to have clinical response at 6 months [165]. Other clinical characteristics including female, previous anti-TNF failure and others may also help physicians to predict ustekinumab response [207].
As for genetic, serological, and fecal predictors, few studies investigated the associations with ustekinumab response in patients with IBD. Most studies focused on psoriasis. Several genetic studies claimed that SNPs in IL-12B and TNFAIP3 (TNF alpha induced protein 3) influence therapeutic response in psoriasis patients [215, 216]. However, genetic studies on IBD patients are still limited. A Japanese study team analyzed the mucosal gene expression pattern and found that the baseline expression levels of IL-23A, TNF, FOXP3 and others differed between ustekinumab responders and non-responders [168]. This opens the possibility of using mucosal gene expression patterns to predict therapeutic response in IBD. As for serological data, a previous study suggested that the response rates were higher in CD patients with baseline CRP ≥ 10mg/L than that in those with CRP < 10 mg/L [167]. Overall, little is known about the effects of other serum inflammatory markers and antibodies such as ESR, ANCA and ASCA on the response rates of ustekinumab. Recently, the low baseline FC level was claimed to be a valuable predictor of good response to ustekinumab [171]. With respect to intestinal microbiota, the CERTIFI study suggested that baseline microbial signatures could predict disease remission with acceptable accuracy [169]. The baseline Faecalibacterium and Bacteroides were significantly higher in patients in remission than that in non-remission patients six weeks after ustekinumab induction [169]. Thus, a random forest prediction model including several clinical and microbiota markers has been developed. It can successfully predict clinical remission and clinical response with an AUC of 0.844 and 0.733, respectively [169]. These findings suggested that baseline microbial metacommunity could help physicians identifypatients who will benefit most from specific treatment.
Based on the above findings, Ustekinumab Clinical Decision Support Tool (UST-CDST) has been developed. The UST-CDST is calculated using five markers including anti-TNF-α exposure, bowel surgery, fistulizing disease, smoking and albumin level. Then, Park et al. assessed the predictive performance of UST-CDST in 130 patients with CD, and demonstrated it highly effective in predicting clinical remission at week 20 [217]. On the whole, exploration and analysis of predictors of ustekinumab do add value to personalized therapy, but available predictors need to be validated in independent and larger cohorts. More novel, accurate and feasible predictors are also required to be identified.
Indeed, biological agents become the mainstay in the treatment of IBD. They effectively help IBD patients in achieving disease remission and prevent patients from abdominal surgery and hospitalization. However, response rates are extremely different in individuals. To those primary non-responders, biologics not only expose patients to unnecessary risk of infection, allergy and even death, but also delay effective treatment and increase medical expense. Therefore, precise prediction of treatment response to biologics before giving treatment has been a pressing matter in the management of IBD. Additional new predictors with favorable sensitivity and specificity, and comprehensive panels or models of different-class predictors are required to guide the treatment.
Precision monitoring of key medications
Therapeutic drug monitoring (TDM) is the most important aspect in the field of precision monitoring. Once patients start treatment, rigorous monitoring of treatment response becomes an integral part in the management of IBD. Variations of pharmacodynamics, pharmacokinetics and pharmacogenetics between different patients lead to further study into the relationships between drug metabolites, serum drug concentrations, anti–drug antibodies, and clinical outcomes. Thiopurines and biological agents including infliximab, adalimumab, vedolizumab and ustekinumab are the most studied in the treatment of IBD.
Thiopurines
Thiopurines have extremely complicated metabolic pathways. As aforementioned, 6-TGNs are the therapeutic metabolites, and also responsible for myelosuppression [172]. Therefore, during thiopurine treatment, monitoring thiopurine metabolites become an essential part, which may assist in selecting appropriate therapeutic doses, achieving better therapeutic effectiveness, and reducing the possibility of adverse events.
Among these kinds of metabolites, measurements of 6-TGN and 6-MMP levels are applied in routine clinical practice. Several studies reported that the 6-TGN cut-off level of 230 pmol/8 × 108 red blood cells (RBCs) was associated with clinical remission [218]. Combined analysis of prior-treatment TPMT activity and post-treatment 6-TGN levels can further assist physicians in monitoring treatment response of thiopurines. Kwan and colleagues proposed that TPMT activity below 30.5 U combined with a 6-TGN level above 230 pmol/8 × 108 RBCs was significantly correlated with clinical response [219]. Another commonly used monitoring parameter is 6-MMP. Combined assessment of 6-TGN and 6-MMP further helps physicians distinguish clinical response, resistance and nonadherence, and thus guide dose and therapeutic program adjustment [220]. However, the monitoring of 6-TGN and 6-MMP levels shows an unfavorable sensitivity of 62% and a specificity of 72% for clinical response [221]. Due to different study designs, sample sizes and included groups, as well as different assays and instruments used to detect metabolite concentrations, various threshold values have been set in different studies. This caused some difficulties for physicians to make explanations for 6-TGN and 6-MMP values and monitor therapeutic effects.
As aforementioned, thiopurine metabolites are also in close association with adverse events secondary to thiopurine treatment. Thus, it is possible to minimize the risk of side effects of thiopurines by measuring 6-TGN and 6-MMP concentrations. Patients with 6-MMP levels above 5700 pmol/8 × 108 RBCs have an increased 3-fold risk of hepatotoxicity than those with lower 6-MMP levels, whereas 6-TGN steady-state levels above 490 pmol/8 × 108 RBCs are found to be significantly correlated with leukopenia [222]. The TOPIC study also revealed that not only increased 6-TGN concentrations (213 pmol/8 × 108 RBCs), but also elevated 6-MMP levels (3525 pmol/8 × 108 RBCs) were in association with leukopenia with an OR of 6.2 and 5.9, respectively [173]. Given that patients with mutant genotypes of TPMT, NUDT15 and ITPA presented higher 6-TGN levels in comparison with wild-types, the optimal cut-off value of 6-TGN should be considered to be reduced in those with mutant genotypes [223]. So, measurements of 6-MMP and 6-TGN concentrations have been recommended as an effective strategy to maximize therapeutic efficacy and minimize adverse events. A target 6-TGN level between 230 and 450 pmol/8 × 108 RBCs is recommended by the American Gastroenterological Association Institute for IBD patients with thiopurine monotherapy [224]. Dose escalation or therapy switch should be considered when the 6-TGN concentration is below 230 pmol/8 × 108 RBCs, while dose reduction should be suggested once the 6-TGN concentration is above 450 pmol/8 × 108 RBCs. It is noteworthy that some patients with very high concentrations of 6-MMP and 6-TGN do not develop hepatotoxicity and leukopenia, while patients with relatively lower levels of 6-MMP and 6-TGN may still suffer from these adverse events. In this regard, thiopurine metabolite measurement cannot replace serial monitoring of liver enzymes and complete blood counts, but may provide useful supplemental information to therapeutic monitoring.
Infliximab
Infliximab is a highly effective treatment in both CD and UC patients, however about 20%–40% of patients become secondary non-responders over time [225]. The rationale for the lack or loss of response is complex. Multiple factors including molecular structures, pharmacodynamics, pharmacokinetics and pharmacogenetics result in different response rates. A good many non-responders show inadequate serum drug concentrations, which are associated with increased clearance by either development of ADAs or mechanisms other than immunogenicity [220]. ADAs neutralize infliximab effects by binding to it and forming an immune complex, then cleared by the reticuloendothelial system. Smoking, a diagnosis of rheumatoid arthritis, high disease activity, treatment interval of more than 11 weeks, neutrophil CD64 ratio > 6 and starting infliximab dose < 7.5 mg/kg are claimed to be risk factors for ADAs [226, 227]. Many studies demonstrated that serum drug concentrations and ADAs are significantly correlated with clinical efficacy. The landmark study into the correlation was pioneered by Baert and colleagues [228]. They reported that a serum infliximab concentration of 12.0 μg/ml or more at week four was associated with a longer duration of clinical response. In the same study, the concentration of ADAs (8.0 ug/ml) was claimed to be inversely correlated with the duration of response to infliximab. The following studies also confirmed the correlation in UC patients [229]. Besides clinical response, increased IFX trough levels are also associated with MH, improved radiologic outcomes and a better disease course, as well as reduced hospitalizations and surgeries [225, 230]. Therefore, monitoring of serum infliximab concentrations and ADAs during infliximab treatment is particularly important in the management of IBD.
In view of the close relationship between clinical efficacy and serum infliximab concentrations, TDM can be used to manage patients with a secondary loss of response to infliximab. Physicians can make therapy adjustments such as dose intensification, dose reduction, dose interval shortening, adding concomitant immunomodulator, or therapy switch (other anti-TNF agents or other-class biological agents) according to concentrations of infliximab and ADAs. In comparison with the empiric management of secondary non-responders, the TDM-tailored therapeutic algorithms show improved outcomes and cost-effectiveness [225]. The TAXIT study concluded that trough-level-based infliximab therapy outperforms system-based therapy in preventing flares during maintenance treatment. This study also indicated that TDM-based therapy can be proactively applied prior to loss of response [231]. Importantly, different disease phenotypes may show different optimal trough concentrations of infliximab. For example, trough levels of 10 ug/ml or more are recommended for patients with fistulizing phenotypes, while for patients with luminal CD, the recommended range is 3–7μg/ml [224]. From this point, target drug concentrations are not universal.
It should be noted that about 16%–39% of patients receiving scheduled infusion of infliximab have undetectable drug concentrations without the development of antibodies [232]. Antibody-positive subjects show similar rates of clinical remission and endoscopic improvement to antibody-negative patients, which limits its clinical utility in guiding physicians to optimize therapy outcomes [229, 232]. Moreover, studies also found that similar serum drug concentrations resulted in different effectiveness of infliximab between IBD patients, and a large number of non-responders had very high circulating drug trough levels [233]. These findings indicate that other inflammatory mediators other than TNF-α may be implicated in the ongoing inflammatory activity, and other contributing factors such as body weight, gender and unhealthy lifestyles may influence therapeutic effectiveness. Thus, monitoring drug concentrations and ADAs alone is not adequate enough for precisely monitoring therapeutic effects. Algorithms consisting of different contributing factors such as body weight, gender, disease activity, disease extent, albumin levels, CRP concentrations, etc. are needed to be explored.
Adalimumab
Adalimumab is another anti-TNF agent widely used in clinical practice. Lacking of or losing response to adalimumab is also relatively frequent in IBD patients. Undetectable concentrations of adalimumab and the development of ADAs partly account for the unfavorable response rates. Several studies demonstrated that IBD patients greatly benefit from higher adalimumab drug concentrations in clinical, endoscopic and histological remission [234]. In an American study, a cut-off value of 7.5 μg/mL and 7.8 μg/mL of adalimumab was best associated with endoscopic healing and histological remission, respectively [234]. Similarly, another exposure–response relationship study suggested that a cut-off value of 8.14 ug/ml correctly distinguished patients with MH from those without MH, with a sensitivity of 91.4% [235]. With respect to ADAs, the random adalimumab concentrations are notably lower in those with detectable ADAs. As a result, histological and endoscopic remission rates are lower [234]. These findings reflected that monitoring serum adalimumab concentrations and ADAs during the treatment is of great importance in disease management.
Given the vital roles of serum adalimumab concentrations and ADAs, TDM is of great help in guiding clinical decision making. For example, secondary non-responders with low adalimumab trough levels and lacking of ADAs conformation benefit most from adalimumab escalation. However, switching to other-class biologics should be considered in patients with low concentrations of adalimumab and detectable ADAs. However, one aspect should be taken into consideration is that no defined threshold has been established for guidance of therapeutic interventions. One pilot study of 78 children with CD investigated the association between proactive TDM and clinical remission. They set the treatment target of adalimumab level as 5 μg/ml. As a result, the proportion of corticosteroid-free clinical remission in the proactive TDM group and the reactive TDM group was 82% and 48%, respectively. Although most of patients in the proactive TDM group achieved clinical remission, about 87% of subjects underwent adalimumab escalation [236]. From this point, the optimal concentration target would be higher than 5 μg/ml. Thus, some studies then recommend a target range of 7.2–12.0 μg/ml [237, 238]. Moreover, currently available assay techniques used for the detection and quantification of serum drug levels and ADAs include the enzyme-linked immunosorbent assay (ELISA), fluid-phase radioimmunoassay, and homogeneous mobility shift assay (HMSA) [233]. The lack of a gold standard assay limited its routine clinical use. Different studies used different assay techniques and proposed different threshold values, which caused great difficulties for disease management in daily clinical practice. Therefore, it is definitely a pressing need to establish a gold standard or optimal assay technique, and set up a universally acknowledged threshold of adalimumab to assist in optimizing dosing regimens, therefore maximizing the effectiveness and minimizing the adverse events of adalimumab in clinical practice.
Vedolizumab
Vedolizumab shows a unique function that it specifically suppresses gut inflammation without systemic immunosuppression [163]. Moreover, it also presents a better safety profile with minor infusion reaction and serious infection than anti-TNF agents, because it is a more specific antibody against gut-homing α4β7 integrin [154]. Published data demonstrated that higher vedolizumab serum concentrations are associated with higher remission rates and better clinical response in both UC and CD patients [239]. The GEMINI trials demonstrated that the median trough concentrations at week 6 were higher in remitters than that in non-remitters [163]. In 2019, Osterman et al. proposed that a cut-off value of 37.1 μg/ml at week 6 and 12.7 ug/ml at steady state was associated with clinical remission [240]. More recently, the target trough concentration of 32.0 μg/ml at week 6 was claimed to be correlated with week 52 clinical remission [239]. However, other studies suggested a lower target concentration for endoscopic remission (10ug/ml) and MH (18ug/ml) [225, 241]. A cut-off value of 20.0 ug/ml at week 22 was suggested to be a predictor of achieving endoscopic remission in another study [242]. It is clear that higher trough concentrations are correlated with better outcomes. So, in view of this, monitoring serum drug levels may add value to dosing regimens in patients with insufficient response to vedolizumab.
Several factors have been reported to have an effect on drug concentrations or clearance rates. A population pharmacokinetic analysis demonstrated similar clearance rates in CD and UC patients, while for patients with extremely lower albumin and higher body weight, the clearance rates would increase [243]. Given that only 3.7%–4.1% of patients develop transient ADAs and 0.4%–1.0% of patients have persistently positive ADAs in GEMINI trials, the relationship between ADAs and clinical efficacy is still uncertain [154, 205]. A randomized, double-blind, placebo-controlled study suggested similar clinical remission rates in patients with ADAs and those without ADAs (12% vs. 14%) [244]. While in the GEMINI trials, the development of ADAs was associated with a significant decrease in serum drug concentrations, and the latter was confirmed to be correlated with clinical effectiveness [154, 205]. This is in line with the results of a population pharmacokinetic analysis that vedolizumab linear clearance in those with positive ADAs was estimated to be 12% higher than that in patients with negative ADAs [243]. Therefore, more efforts are needed to elucidate the effects of ADAs on the clinical outcomes, and then optimize disease management.
With regard to these patients with concomitant immunosuppressive treatment, special attention should be paid. Available data suggested that concomitant immunomodulator is associated with decreased immunogenicity of vedolizumab, while it has no clinical effect on the pharmacokinetics of vedolizumab [163, 243]. This did not correspond to the finding in anti-TNF agents that concomitant immunosuppressant therapy is not only correlated with decreased immunogenicity, but also associated with increased clearance [228]. There might be additional modes of action of vedolizumab and some underlying factors accounting for the difference. Exploring these contributing factors and other mechanisms of action might further assist physicians in determining therapeutic strategies in patients with insufficient responses. It is important to note that the evidence for proactive/reactive TDM of vedolizumab is relatively limited. Whether TDM of vedolizumab is cost-effective also remains to be elucidated.
Ustekinumab
Ustekinumab is a new biological agent used for patients with moderate-to-severe CD and UC [23, 24]. Available data into the relationship between trough ustekinumab concentrations and treatment outcomes are relatively limited. In accordance with the above biologics, higher trough concentrations of ustekinumab indicate higher response rates. Ustekinumab target threshold of 3.7 ug/ml at week 8 was proved to be correlated with clinical response, while a trough concentration of 4.5 ug/ml at week 26 was claimed to be associated with endoscopic improvement and lower CRP levels, as well as trends toward FC normalization and endoscopic remission [245, 246]. The IM-UNITI trial proposed that the target trough concentration of ustekinumab at week 24 was 1 ug/mL, which was best associated with clinical remission [247]. More recently, a target ustekinumab concentration of 2.11 μg/mL at week 16 was claimed to be correlated with fistula healing in CD [248]. Caution needs to be exercised when explaining these results, because different disease phenotypes, measurement time points, and desired outcomes of interest have been set in studies. As a result, different target trough concentrations of ustekinumab have been proposed.
Contrary to anti-TNF biologics, immunogenicity has less of an effect on the response rates to ustekinumab [213]. About 2.3% of patients were reported to develop ADAs during treatment in the IM-UNITI trial, while in the CERTIFI trial, only 0.7% of patients had positive results for ADAs at week 36 [23, 213]. Such a low prevalence of ADAs is not powerful enough to explain reasons for the tloss of response. Moreover, the positive effect of concomitant immunosuppressive therapy on the prevention of ADAs development seen in anti-TNF-α treatment may not be relevant to ustekinumab [249]. Therefore, further exploration of other factors influencing therapeutic response should be a research priority. Although dose optimization results in higher response rates in several other studies, whether patients with low trough ustekinumab concentrations will benefit from dose escalation is unclear. So, proactive/reactive TDM studies are needed to fill this gap. Moreover, definite thresholds and therapeutic drug concentration intervals are also required to be defined and validated in large, independent cohorts.
It is an indisputable fact that TDM plays a vital role in the monitoring and management of IBD. Based on pharmacodynamic, pharmacokinetic and pharmacogenetic properties of drugs, therapeutic targets will be achieved more easily and final outcomes of IBD patients will be improved. Indeed, measurements of drug metabolites, drug concentrations and ADAs significantly optimize IBD therapy, but it is still insufficient to achieve precise monitoring. Combined analysis of other clinical, serological, histologic and fecal factors along with TDM might further improve the precision of monitoring. Although TDM shows its great advantages in monitoring treatment response, deficiencies such as invasiveness, inconvenience and costliness make it unacceptable for some patients. Exploring markers directly or indirectly reflecting drug concentrations in saliva, sweat and feces, as well as noninvasive tests with acceptable price, sensitivity and specificity is in urgent demand. What's more, the time delay between sample collection and sample results should also be taken into consideration. Current studies mostly elucidate the influence of TDM on clinical and endoscopic outcomes. The relationship between TDM and optimal therapeutic targets such as MH, deep remission, and disease course change remains to be established in the following studies. Of note, various studies claimed an association between drug levels and disease remission. Whether this relationship is causal (high drug levels cause disease remission) or consequential (disease remission/decreased disease activity causes reduced drug clearance/high drug levels) remains to be fully clarified. Moreover, TDM for infliximab has been widely used in clinic, while TDM for new biologics such as adalimumab, vedolizumab and ustekinumab has been limited partly due to incomplete analytic techniques, undefined thresholds, and unclear pharmacokinetics. Therefore, more efforts should be put into the investigation of standard assay techniques, optimal thresholds, and exact metabolic mechanisms. With the unceasing efforts, TDM will play an increasingly key role in precision monitoring in patients with IBD.
Future precision medicine in IBD
Medical therapy does play a critical role in the treatment of patients with IBD, and biological drugs such as infliximab, adalimumab, vedolizumab and ustekinumab targeting different signaling pathways have brought a revolutionary influence on the treatment of IBD. To achieve the goal of precision treatment, studies regarding new therapeutic agents, optimal therapeutic targets, different disease patterns, and patients’ choices are in desperate need. With the increasing understanding of the pathogenesis of IBD, new pathophysiology has been found. Exploration of novel medicine targeting new targets with excellent therapeutic effects may further promote the development of precise treatment. Some new medicines targeting different targets such as JAK3, interleukins, chemokine receptor, cell adhesion molecule and protein kinase are developed. In recent years, IBD has been added to the expanding disease indications for some ‘old’ medicines that have already been applied in other immune-mediated diseases such as RA, SLE and psoriasis. This paved the way for the new use of old medicine. Therefore, exploration of the same signaling pathways implicated in IBD and other diseases may add value to the precise treatment of IBD. Combination therapy of immunosuppressants and biological agents obtained favorable therapeutic efficacy, which provides a possibility of application of various drugs targeting different pathways for IBD treatment.
Given that IBD is a progressive disease, patients with IBD present different pathophysiological characteristics in different disease stages. Therefore, physicians are advised to select different therapeutic targets in different disease stages during the entire disease course. Precision and individualized therapy will be the future medical model. Although numerous markers have been identified for precision treatment and precision monitoring in IBD, however, these available markers need external and prospective validation. Well-designed, large-scale, and well-paired phase II or phase III trials may provide more information about clinical translation. Moreover, clarifying that these identified markers merely reflect inflammation (correlation) or are part of the pathogenesis of IBD (causation) is also required. Compared studies on the above markers between unaffected siblings of IBD patients and those affected siblings will facilitate the identification of the exact roles of available markers in IBD. Besides, it is also important to determine the various roles of markers in different disease stages and the functional impacts on disease onset and progression. More importantly, intestinal damage of IBD is a progressive process, which impels doctors to carry out early and effective interventions before bowel damage. Thus, defining the terminology of the preclinical phase and exploring preclinical markers will be needed. Prospectively collecting preclinical samples and closely following up ‘at-risk’ family cohorts hold great promise to help precision prevention and change the natural disease course of IBD. Moreover, more importance should be attached to the environmental risk markers including prenatal and perinatal factors, drug exposure, diet and physical exercise, and imaging (such as magnetic resonance imaging and ultrasound) characteristics. Explaining the contributions of environmental and imaging risk markers in the preclinical stage might provide crucial insights into the disease pathogenesis and precision prediction of disease onset and development. What's more, considering different healthcare systems and financial structures around the world, more multidimensional prediction and monitoring tools integrating multi-omics data should be developed. Thus, an interdisciplinary collaboration between medical scientists, bioinformaticians, economists and manufacturers is encouraged. By achieving these endeavors, we are getting closer and closer to the goal of precision medicine in IBD.
Acknowledgements
This study was supported in part by the Sichuan International Science and Technology Innovation Cooperation/Hong Kong/Macao/Taiwan Science and Technology Innovation Cooperation Project (Grant No. 2021YFH0189), the Sichuan International Science Foundation Project (Grant No. 2022NSFSC1363), and the project for disciplines of excellence–Clinical Research Incubation Project, West China Hospital, Sichuan University (Grant No. 2021HXFH065). Fig. 1. is created on BioRender.com.
Contributor Information
Zhen Zeng, Department of Gastroenterology, West China Hospital, Sichuan University, Chengdu 610041, China; Centre for Inflammatory Bowel Disease, West China Hospital, Sichuan University, Chengdu 610041, China; Lab of Inflammatory Bowel Disease, Frontiers Science Center for Disease-Related Molecular Network, West China Hospital, Sichuan University, Chengdu 610041, China.
Mingshan Jiang, Department of Gastroenterology, West China Hospital, Sichuan University, Chengdu 610041, China; Centre for Inflammatory Bowel Disease, West China Hospital, Sichuan University, Chengdu 610041, China; Lab of Inflammatory Bowel Disease, Frontiers Science Center for Disease-Related Molecular Network, West China Hospital, Sichuan University, Chengdu 610041, China.
Xi Li, Lab of Inflammatory Bowel Disease, Frontiers Science Center for Disease-Related Molecular Network, West China Hospital, Sichuan University, Chengdu 610041, China; General Practice Ward/International Medical Center Ward, General Practice Medical Center, West China Hospital, Sichuan University, Chengdu 610041, China.
Jing Yuan, Department of Gastroenterology, West China Hospital, Sichuan University, Chengdu 610041, China; Centre for Inflammatory Bowel Disease, West China Hospital, Sichuan University, Chengdu 610041, China; Lab of Inflammatory Bowel Disease, Frontiers Science Center for Disease-Related Molecular Network, West China Hospital, Sichuan University, Chengdu 610041, China.
Hu Zhang, Department of Gastroenterology, West China Hospital, Sichuan University, Chengdu 610041, China; Centre for Inflammatory Bowel Disease, West China Hospital, Sichuan University, Chengdu 610041, China; Lab of Inflammatory Bowel Disease, Frontiers Science Center for Disease-Related Molecular Network, West China Hospital, Sichuan University, Chengdu 610041, China.
Conflict of interest statement
None declared. In addition, as an Editorial Board Member of Precision Clinical Medicine, the corresponding author Hu Zhang was blinded from reviewing and making decisions on this manuscript.
Author contributions
Writing—original draft preparation, Z.Z.; writing—review and editing, Z.Z. M. J. X.L. and J.Y.; supervision—H.Z. All authors have read and agreed to the published version of the manuscript.
References
- 1. Zeng Z, Mukherjee A, Varghese AP et al. Roles of G protein-coupled receptors in inflammatory bowel disease. World J Gastroenterol. 2020;26:1242–61. 10.3748/wjg.v26.i12.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abraham C, Cho JH Inflammatory bowel disease. N Engl J Med. 2009;361:2066–78. 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ott C, Schölmerich J Extraintestinal manifestations and complications in IBD. Nat Rev Gastroenterol Hepatol. 2013;10:585–95. 10.1038/nrgastro.2013.117. [DOI] [PubMed] [Google Scholar]
- 4. Ricciuto A, Mack DR, Huynh HQ et al. Diagnostic delay is associated with complicated disease and growth impairment in paediatric Crohn's Disease. J Crohns Colitis. 2021;15: 419–31. 10.1093/ecco-jcc/jjaa197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Borren NZ, Plichta D, Joshi AD et al. Multi-“omics” profiling in patients with quiescent inflammatory bowel disease identifies biomarkers predicting relapse. Inflamm Bowel Dis. 2020; 26:1524–32. 10.1093/ibd/izaa183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang H, Zeng Z, Mukherjee A et al. Molecular diagnosis and classification of inflammatory bowel disease. Expert Rev Mol Diagn. 2018;18:867–86. 10.1080/14737159.2018.1516549. [DOI] [PubMed] [Google Scholar]
- 7. Kaplan GG, Ng SC Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology. 2017;152:313–21. 10.1053/j.gastro.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 8. Chen K, Shen S, Chen Y et al. A proteomic and RNA-seq transcriptomic dataset of capsaicin-aggravated mouse chronic colitis model. Sci Data. 2022;9:549. 10.1038/s41597-022-01637-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Lange KM, Moutsianas L, Lee JC et al. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat Genet. 2017;49: 256–61. 10.1038/ng.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sazonovs A, Stevens CR, Venkataraman GR et al. Large-scale sequencing identifies multiple genes and rare variants associated with Crohn's disease susceptibility. Nat Genet. 2022;54:1275–83. 10.1038/s41588-022-01156-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Argollo M, Fiorino G, Hindryckx P et al. Novel therapeutic targets for inflammatory bowel disease. J Autoimmun. 2017;85:103–16. 10.1016/j.jaut.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 12. Collins FS, Varmus H A new initiative on precision medicine. N Engl J Med. 2015;372:793–5. 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen H, Wu X, Xu C et al. Dichotomous roles of neutrophils in modulating pathogenic and repair processes of inflammatory bowel diseases. Precis Clin Med. 2021;4:246–57. 10.1093/pcmedi/pbab025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Levin AD, Wildenberg ME, van den Brink GR Mechanism of action of anti-TNF therapy in inflammatory bowel disease. J Crohns Colitis. 2016;10:989–97. 10.1093/ecco-jcc/jjw053. [DOI] [PubMed] [Google Scholar]
- 15. Kalliolias GD, Ivashkiv LB TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol. 2016;12:49–62. 10.1038/nrrheum.2015.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Biancheri P, Brezski RJ, Di Sabatino A et al. Proteolytic cleavage and loss of function of biologic agents that neutralize tumor necrosis factor in the mucosa of patients with inflammatory bowel disease. Gastroenterology. 2015;149:1564–74. 10.1053/j.gastro.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 17. Atreya R, Zimmer M, Bartsch B et al. Antibodies against tumor necrosis factor (TNF) induce T-cell apoptosis in patients with inflammatory bowel diseases via TNF receptor 2 and intestinal CD14⁺ macrophages. Gastroenterology. 2011;141:2026–38. 10.1053/j.gastro.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 18. Vos AC, Wildenberg ME, Duijvestein M et al. Anti-tumor necrosis factor-α antibodies induce regulatory macrophages in an Fc region-dependent manner. Gastroenterology. 2011;140:221–30. 10.1053/j.gastro.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 19. Miossec P, Kolls JK Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11:763–76. 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- 20. Bishu S, Hou G Th17 Cells in inflammatory bowel disease: an update for the clinician. Inflamm Bowel Dis. 2020;26:653–61. 10.1093/ibd/izz316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pawlak M, DeTomaso D, Schnell A et al. Induction of a colitogenic phenotype in Th1-like cells depends on interleukin-23 receptor signaling. Immunity. 2022;55:1663–79. 10.1016/j.immuni.2022.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moschen AR, Tilg H, Raine T IL-12, IL-23 and IL-17 in IBD: immunobiology and therapeutic targeting. Nat Rev Gastroenterol Hepatol. 2019;16:185–96. 10.1038/s41575-018-0084-8. [DOI] [PubMed] [Google Scholar]
- 23. Feagan BG, Sandborn WJ, Gasink C et al. Ustekinumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2016;375: 1946–60. 10.1056/NEJMoa1602773. [DOI] [PubMed] [Google Scholar]
- 24. Sands BE, Sandborn WJ, Panaccione R et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019;381:1201–14. 10.1056/NEJMoa1900750. [DOI] [PubMed] [Google Scholar]
- 25. Verstockt B, Salas A, Sands BE et al. IL-12 and IL-23 pathway inhibition in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2023;20:433–46. 10.1038/s41575-023-00768-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Slack RJ, Macdonald SJF, Roper JA et al. Emerging therapeutic opportunities for integrin inhibitors. Nat Rev Drug Discov. 2022;21:60–78. 10.1038/s41573-021-00284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vermeire S, O'Byrne S, Keir M et al. Etrolizumab as induction therapy for ulcerative colitis: a randomised, controlled, phase 2 trial. Lancet. 2014;384:309–18. 10.1016/s0140-6736(14)60661-9. [DOI] [PubMed] [Google Scholar]
- 28. Sandborn WJ, Cyrille M, Hansen MB et al. Efficacy and safety of Abrilumab in a randomized, placebo-controlled trial for moderate-to-severe ulcerative colitis. Gastroenterology. 2019;156:946–57. 10.1053/j.gastro.2018.11.035. [DOI] [PubMed] [Google Scholar]
- 29. Verstockt B, Vetrano S, Salas A et al. Sphingosine 1-phosphate modulation and immune cell trafficking in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2022;19:351–66. 10.1038/s41575-021-00574-7. [DOI] [PubMed] [Google Scholar]
- 30. Feagan BG, Sandborn WJ, Danese S et al. Ozanimod induction therapy for patients with moderate to severe Crohn's disease: a single-arm, phase 2, prospective observer-blinded endpoint study. Lancet Gastroenterol Hepatol. 2020;5:819–28. 10.1016/s2468-1253(20)30188-6. [DOI] [PubMed] [Google Scholar]
- 31. Sandborn WJ, Peyrin-Biroulet L, Zhang J et al. Efficacy and safety of etrasimod in a phase 2 randomized trial of patients with ulcerative colitis. Gastroenterology. 2020;158:550–61. 10.1053/j.gastro.2019.10.035. [DOI] [PubMed] [Google Scholar]
- 32. Salas A, Hernandez-Rocha C, Duijvestein M et al. JAK-STAT pathway targeting for the treatment of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17:323–37. 10.1038/s41575-020-0273-0. [DOI] [PubMed] [Google Scholar]
- 33. Dai C, Jiang M, Sun MJ Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;377:496. 10.1056/NEJMc1707500. [DOI] [PubMed] [Google Scholar]
- 34. Vermeire S, Schreiber S, Petryka R et al. Clinical remission in patients with moderate-to-severe Crohn's disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet. 2017;389:266–75. 10.1016/s0140-6736(16)32537-5. [DOI] [PubMed] [Google Scholar]
- 35. Sandborn WJ, Feagan BG, Loftus EVJ et al. Efficacy and safety of Upadacitinib in a randomized trial of patients with Crohn's disease. Gastroenterology. 2020;158:2123–38. 10.1053/j.gastro.2020.01.047. [DOI] [PubMed] [Google Scholar]
- 36. Balderramo D Role of the combination of biologics and/or small molecules in the treatment of patients with inflammatory bowel disease. World J Gastroenterol. 2022;28:6743–51. 10.3748/wjg.v28.i47.6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dolinger MT, Spencer EA, Lai J et al. Dual Biologic and small molecule therapy for the treatment of refractory pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2021;27:1210–4. 10.1093/ibd/izaa277. [DOI] [PubMed] [Google Scholar]
- 38. Alayo QA, Khatiwada A, Patel A et al. Effectiveness and safety of combining Tofacitinib with a biologic in patients with refractory inflammatory bowel diseases. Inflamm Bowel Dis. 2021;27:1698–702. 10.1093/ibd/izab112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee SD, Singla A, Harper J et al. Safety and efficacy of Tofacitinib in combination with biologic therapy for refractory Crohn's disease. Inflamm Bowel Dis. 2022;28:309–13. 10.1093/ibd/izab176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rose 2nd WA, Sakamoto K, Leifer CA TLR9 is important for protection against intestinal damage and for intestinal repair. Sci Rep. 2012;2:574. 10.1038/srep00574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schmitt H, Ulmschneider J, Billmeier U et al. The TLR9 agonist cobitolimod induces IL10-producing wound healing macrophages and regulatory T cells in ulcerative colitis. J Crohns Colitis. 2020;14:508–24. 10.1093/ecco-jcc/jjz170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Atreya R, Peyrin-Biroulet L, Klymenko A et al. Cobitolimod for moderate-to-severe, left-sided ulcerative colitis (CONDUCT): a phase 2b randomised, double-blind, placebo-controlled, dose-ranging induction trial. Lancet Gastroenterol Hepatol. 2020;5:1063–75. 10.1016/s2468-1253(20)30301-0. [DOI] [PubMed] [Google Scholar]
- 43. Noor NM, Verstockt B, Parkes M et al. Personalised medicine in Crohn's disease. Lancet Gastroenterol Hepatol. 2020;5:80–92. 10.1016/s2468-1253(19)30340-1. [DOI] [PubMed] [Google Scholar]
- 44. Fiocchi C, Dragoni G, Iliopoulos D et al. Results of the seventh scientific workshop of ECCO: precision medicine in IBD-what, why, and how. J Crohns Colitis. 2021;15:1410–30. 10.1093/ecco-jcc/jjab051. [DOI] [PubMed] [Google Scholar]
- 45. Flamant M, Roblin X Inflammatory bowel disease: towards a personalized medicine. Therap Adv Gastroenterol. 2018;11:1756283–17745029. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Waterman M, Knight J, Dinani A et al. Predictors of outcome in ulcerative colitis. Inflamm Bowel Dis. 2015;21:2097–105. 10.1097/mib.0000000000000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Torres J, Caprioli F, Katsanos KH et al. Predicting outcomes to optimize disease management in inflammatory bowel diseases. J Crohns Colitis. 2016;10:1385–94. 10.1093/ecco-jcc/jjw116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lopes EW, Lochhead P, Burke KE et al. Risk factors for incident inflammatory bowel disease according to disease phenotype. Clin Gastroenterol Hepatol. 2022;20:2347–57. 10.1016/j.cgh.2022.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Goodman WA, Erkkila IP, Pizarro TT Sex matters: impact on pathogenesis, presentation and treatment of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17:740–54. 10.1038/s41575-020-0354-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Beaugerie L, Seksik P, Nion-Larmurier I et al. Predictors of Crohn's disease. Gastroenterology. 2006;130:650–6. 10.1053/j.gastro.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 51. Loly C, Belaiche J, Louis E Predictors of severe Crohn's disease. Scand J Gastroenterol. 2008;43:948–54. 10.1080/00365520801957149. [DOI] [PubMed] [Google Scholar]
- 52. Haritunians T, Taylor KD, Targan SR et al. Genetic predictors of medically refractory ulcerative colitis. Inflamm Bowel Dis. 2010;16:1830–40. 10.1002/ibd.21293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wolters FL, Russel MG, Sijbrandij J et al. Phenotype at diagnosis predicts recurrence rates in Crohn's disease. Gut. 2006;55: 1124–30. 10.1136/gut.2005.084061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lazarev M, Huang C, Bitton A et al. Relationship between proximal Crohn's disease location and disease behavior and surgery: a cross-sectional study of the IBD Genetics Consortium. Am J Gastroenterol. 2013;108:106–12. 10.1038/ajg.2012.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Romberg-Camps MJ, Dagnelie PC, Kester AD et al. Influence of phenotype at diagnosis and of other potential prognostic factors on the course of inflammatory bowel disease. Am J Gastroenterol. 2009;104:371–83. 10.1038/ajg.2008.38. [DOI] [PubMed] [Google Scholar]
- 56. Blackwell J, Saxena S, Alexakis C et al. The impact of smoking and smoking cessation on disease outcomes in ulcerative colitis: a nationwide population-based study. Aliment Pharmacol Ther. 2019;50:556–67. 10.1111/apt.15390. [DOI] [PubMed] [Google Scholar]
- 57. Wagtmans MJ, Verspaget HW, Lamers CB et al. Gender-related differences in the clinical course of Crohn's disease. Am J Gastroenterol. 2001;96:1541–6. 10.1111/j.1572-0241.2001.03755.x. [DOI] [PubMed] [Google Scholar]
- 58. Söderlund S, Granath F, Broström O et al. Inflammatory bowel disease confers a lower risk of colorectal cancer to females than to males. Gastroenterology. 2010;138:1697–703. 10.1053/j.gastro.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 59. Allez M, Lemann M, Bonnet J et al. Long term outcome of patients with active Crohn's disease exhibiting extensive and deep ulcerations at colonoscopy. Am J Gastroenterol. 2002;97:947–53. 10.1111/j.1572-0241.2002.05614.x. [DOI] [PubMed] [Google Scholar]
- 60. Frøslie KF, Jahnsen J, Moum BA et al. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007;133:412–22. 10.1053/j.gastro.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 61. Etchevers MJ, Aceituno M, García-Bosch O et al. Risk factors and characteristics of extent progression in ulcerative colitis. Inflamm Bowel Dis. 2009;15:1320–5. 10.1002/ibd.20897. [DOI] [PubMed] [Google Scholar]
- 62. Loftus EVJ, Harewood GC, Loftus CG et al. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005;54:91–6. 10.1136/gut.2004.046615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jess T, Loftus EVJ, Velayos FS et al. Risk factors for colorectal neoplasia in inflammatory bowel disease: a nested case-control study from Copenhagen county, Denmark and Olmsted county, Minnesota. Am J Gastroenterol. 2007;102:829–36. 10.1111/j.1572-0241.2007.01070.x. [DOI] [PubMed] [Google Scholar]
- 64. Jiang J, Xie Q, Cheng Z et al. AI based colorectal disease detection using real-time screening colonoscopy. Precis Clin Med. 2021;4:109–18. 10.1093/pcmedi/pbab013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhang J, Jia X, Guo Y et al. Patient-directed vs. fixed-volume PEG for colonoscopy preparation: a randomized controlled trial. Precis Clin Med. 2022;5:pbac009. 10.1093/pcmedi/pbac009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Allez M, Lemann M Role of endoscopy in predicting the disease course in inflammatory bowel disease. World J Gastroenterol. 2010;16:2626–32. 10.3748/wjg.v16.i21.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bessissow T, Lemmens B, Ferrante M et al. Prognostic value of serologic and histologic markers on clinical relapse in ulcerative colitis patients with mucosal healing. Am J Gastroenterol. 2012;107:1684–92. 10.1038/ajg.2012.301. [DOI] [PubMed] [Google Scholar]
- 68. Li K, Friedman JR, Chan D et al. Effects of Ustekinumab on histologic disease activity in patients with Crohn's disease. Gastroenterology. 2019;157:1019–31. 10.1053/j.gastro.2019.06.037. [DOI] [PubMed] [Google Scholar]
- 69. Bezzio C, Della Corte C, Vernero M et al. Inflammatory bowel disease and immune-mediated inflammatory diseases: looking at the less frequent associations. Therap Adv Gastroenterol. 2022;15:17562848221115312. 10.1177/17562848221115312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Freuer D, Linseisen J, Meisinger C Association between inflammatory bowel disease and both psoriasis and psoriatic arthritis: a bidirectional 2-sample mendelian randomization study. JAMA Dermatol. 2022;158:1262–8. 10.1001/jamadermatol.2022.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Liu Z, Liu R, Gao H et al. Genetic architecture of the inflammatory bowel diseases across East Asian and European ancestries. Nat Genet. 2023;55:796–806. 10.1038/s41588-023-01384-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Annese V, Lombardi G, Perri F et al. Variants of CARD15 are associated with an aggressive clinical course of Crohn's disease—an IG-IBD study. Am J Gastroenterol. 2005;100:84–92. 10.1111/j.1572-0241.2005.40705.x. [DOI] [PubMed] [Google Scholar]
- 73. Alvarez-Lobos M, Arostegui JI, Sans M et al. Crohn's disease patients carrying Nod2/CARD15 gene variants have an increased and early need for first surgery due to stricturing disease and higher rate of surgical recurrence. Ann Surg. 2005;242:693–700. 10.1097/01.sla.0000186173.14696.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rufini S, Ciccacci C, Di Fusco D et al. Autophagy and inflammatory bowel disease: Association between variants of the autophagy-related IRGM gene and susceptibility to Crohn's disease. Dig Liver Dis. 2015;47:744–50. 10.1016/j.dld.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 75. Yang DH, Yang SK, Song K et al. TNFSF15 is an independent predictor for the development of Crohn's disease-related complications in Koreans. J Crohns Colitis. 2014;8:1315–26. 10.1016/j.crohns.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 76. Cleynen I, González JR, Figueroa C et al. Genetic factors conferring an increased susceptibility to develop Crohn's disease also influence disease phenotype: results from the IBDchip European Project. Gut. 2013;62:1556–65. 10.1136/gutjnl-2011-300777. [DOI] [PubMed] [Google Scholar]
- 77. Venkateswaran S, Prince J, Cutler DJ et al. Enhanced contribution of HLA in pediatric onset ulcerative colitis. Inflamm Bowel Dis. 2018;24:829–38. 10.1093/ibd/izx084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. de la Concha EG, Fernandez-Arquero M, Lopez-Nava G et al. Susceptibility to severe ulcerative colitis is associated with polymorphism in the central MHC gene IKBL. Gastroenterology. 2000;119:1491–5. 10.1053/gast.2000.20258. [DOI] [PubMed] [Google Scholar]
- 79. Tahara T, Shibata T, Nakamura M et al. Promoter methylation of protease-activated receptor (PAR2) is associated with severe clinical phenotypes of ulcerative colitis (UC). Clin Exp Med. 2009;9:125–30. 10.1007/s10238-008-0025-x. [DOI] [PubMed] [Google Scholar]
- 80. Tahara T, Shibata T, Nakamura M et al. Effect of MDR1 gene promoter methylation in patients with ulcerative colitis. Int J Mol Med. 2009;23:521–7. 10.3892/ijmm_00000160. [DOI] [PubMed] [Google Scholar]
- 81. Ventham NT, Kennedy NA, Adams AT et al. Integrative epigenome-wide analysis demonstrates that DNA methylation may mediate genetic risk in inflammatory bowel disease. Nat Commun. 2016;7:13507. 10.1038/ncomms13507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Nijhuis A, Biancheri P, Lewis A et al. In Crohn's disease fibrosis-reduced expression of the miR-29 family enhances collagen expression in intestinal fibroblasts. Clin Sci (Lond). 2014;127:341–50. 10.1042/cs20140048. [DOI] [PubMed] [Google Scholar]
- 83. Lewis A, Mehta S, Hanna LN et al. Low serum levels of microRNA-19 are associated with a stricturing Crohn's disease phenotype. Inflamm Bowel Dis. 2015;21:1926–34. 10.1097/mib.0000000000000443. [DOI] [PubMed] [Google Scholar]
- 84. Chen Y, Ge W, Xu L et al. miR-200b is involved in intestinal fibrosis of Crohn's disease. Int J Mol Med. 2012;29:601–6. 10.3892/ijmm.2012.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Peck BC, Weiser M, Lee SE et al. MicroRNAs classify different disease behavior phenotypes of Crohn's disease and may have prognostic utility. Inflamm Bowel Dis. 2015;21:2178–87. 10.1097/mib.0000000000000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Adler J, Rangwalla SC, Dwamena BA et al. The prognostic power of the NOD2 genotype for complicated Crohn's disease: a meta-analysis. Am J Gastroenterol. 2011;106:699–712. 10.1038/ajg.2011.19. [DOI] [PubMed] [Google Scholar]
- 87. Kalla R, Adams AT, Nowak JK et al. Analysis of systemic epigenetic alterations in inflammatory bowel disease: defining geographical, genetic and immune-inflammatory influences on the circulating methylome. J Crohns Colitis. 2023;17:170–84. 10.1093/ecco-jcc/jjac127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Howell KJ, Kraiczy J, Nayak KM et al. DNA methylation and transcription patterns in intestinal epithelial cells from pediatric patients with inflammatory bowel diseases differentiate disease subtypes and associate with outcome. Gastroenterology. 2018;154:585–98. 10.1053/j.gastro.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Venkateswaran S, Somineni HK, Matthews JD et al. Longitudinal DNA methylation profiling of the rectal mucosa identifies cell-specific signatures of disease status, severity and clinical outcomes in ulcerative colitis cell-specific DNA methylation signatures of UC. Clin Epigenetics. 2023;15:50. 10.1186/s13148-023-01462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zeng Z, Mukherjee A, Zhang H From genetics to epigenetics, roles of epigenetics in inflammatory bowel disease. Front Genet. 2019;10:1017. 10.3389/fgene.2019.01017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lewis A, Nijhuis A, Mehta S et al. Intestinal fibrosis in Crohn's disease: role of microRNAs as fibrogenic modulators, serum biomarkers, and therapeutic targets. Inflamm Bowel Dis. 2015;21:1141–50. 10.1097/mib.0000000000000298. [DOI] [PubMed] [Google Scholar]
- 92. Cooke J, Zhang H, Greger L et al. Mucosal genome-wide methylation changes in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2128–37. 10.1002/ibd.22942. [DOI] [PubMed] [Google Scholar]
- 93. Mow WS, Vasiliauskas EA, Lin Y-C et al. Association of antibody responses to microbial antigens and complications of small bowel Crohn's disease. Gastroenterology. 2004;126:414–24. 10.1053/j.gastro.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 94. Schoepfer AM, Schaffer T, Mueller S et al. Phenotypic associations of Crohn's disease with antibodies to flagellins A4-Fla2 and Fla-X, ASCA, p-ANCA, PAB, and NOD2 mutations in a Swiss Cohort. Inflamm Bowel Dis. 2009;15:1358–67. 10.1002/ibd.20892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bogdanos DP, Roggenbuck D, Reinhold D et al. Pancreatic-specific autoantibodies to glycoprotein 2 mirror disease location and behaviour in younger patients with Crohn's disease. BMC Gastroenterol. 2012;12:102. 10.1186/1471-230x-12-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Jiang M, Zeng Z, Chen K et al. Enterogenous microbiotic markers in the differential diagnosis of crohn's disease and intestinal tuberculosis. Front Immunol. 2022;13:820891. 10.3389/fimmu.2022.820891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ferrante M, Henckaerts L, Joossens M et al. New serological markers in inflammatory bowel disease are associated with complicated disease behaviour. Gut. 2007;56:1394–403. 10.1136/gut.2006.108043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Paul S, Boschetti G, Rinaudo-Gaujous M et al. Association of anti-glycan antibodies and inflammatory bowel disease course. J Crohns Colitis. 2015;9:445–51. 10.1093/ecco-jcc/jjv063. [DOI] [PubMed] [Google Scholar]
- 99. Birimberg-Schwartz L, Wilson DC, Kolho KL et al. pANCA and ASCA in children with IBD-unclassified, Crohn's colitis, and ulcerative colitis-a longitudinal report from the IBD porto group of ESPGHAN. Inflamm Bowel Dis. 2016;22:1908–14. 10.1097/mib.0000000000000784. [DOI] [PubMed] [Google Scholar]
- 100. Dubinsky MC, Kugathasan S, Mei L et al. Increased immune reactivity predicts aggressive complicating Crohn's disease in children. Clin Gastroenterol Hepatol. 2008;6:1105–11. 10.1016/j.cgh.2008.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Dubinsky MC, Lin YC, Dutridge D et al. Serum immune responses predict rapid disease progression among children with Crohn's disease: immune responses predict disease progression. Am J Gastroenterol. 2006;101:360–7. 10.1111/j.1572-0241.2006.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Arnott ID, Landers CJ, Nimmo EJ et al. Sero-reactivity to microbial components in Crohn's disease is associated with disease severity and progression, but not NOD2/CARD15 genotype. Am J Gastroenterol. 2004;99:2376–84. 10.1111/j.1572-0241.2004.40417.x. [DOI] [PubMed] [Google Scholar]
- 103. Bossuyt P, Debeuckelaere C, Ferrante M et al. The operative risk and natural history after the diagnosis of ileal penetrating Crohn's disease. Eur J Gastroenterol Hepatol. 2018;30:539–45. 10.1097/meg.0000000000001091. [DOI] [PubMed] [Google Scholar]
- 104. Henderson P, Kennedy NA, Van Limbergen JE et al. Serum C-reactive protein and CRP genotype in pediatric inflammatory bowel disease: influence on phenotype, natural history, and response to therapy. Inflamm Bowel Dis. 2015;21:596–605. 10.1097/mib.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 105. Kruis W, Katalinic A, Klugmann T et al. Predictive factors for an uncomplicated long-term course of Crohn's disease: a retrospective analysis. J Crohns Colitis. 2013;7:e263–70. 10.1016/j.crohns.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 106. Khan N, Patel D, Shah Y et al. Albumin as a prognostic marker for ulcerative colitis. World J Gastroenterol. 2017;23:8008–16. 10.3748/wjg.v23.i45.8008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ziade F, Rungoe C, Kallemose T et al. Biochemical markers, genotype, and inflammation in pediatric inflammatory bowel disease: a danish population-based study. Dig Dis. 2019;37:140–6. 10.1159/000494215. [DOI] [PubMed] [Google Scholar]
- 108. Pang Y, Ruan H, Wu D et al. Assessment of clinical activity and severity using serum ANCA and ASCA antibodies in patients with ulcerative colitis. Allergy Asthma Clin Immunol. 2020;16:37. 10.1186/s13223-020-00433-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Siegel CA, Horton H, Siegel LS et al. A validated web-based tool to display individualised Crohn's disease predicted outcomes based on clinical, serologic and genetic variables. Aliment Pharmacol Ther. 2016;43:262–71. 10.1111/apt.13460. [DOI] [PubMed] [Google Scholar]
- 110. Prideaux L, De Cruz P, Ng SC et al. Serological antibodies in inflammatory bowel disease: a systematic review. Inflamm Bowel Dis. 2012;18:1340–55. 10.1002/ibd.21903. [DOI] [PubMed] [Google Scholar]
- 111. Kugathasan S, Denson LA, Walters TD et al. Prediction of complicated disease course for children newly diagnosed with Crohn's disease: a multicentre inception cohort study. Lancet. 2017;389:1710–8. 10.1016/s0140-6736(17)30317-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Basha OM, Hafez RA, Salem SM et al. Impact of gut microbiome alteration in ulcerative colitis patients on disease severity and outcome. Clin Exp Med. 2022;23:1763–72. 10.1007/s10238-022-00917-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zhou Y, Xu ZZ, He Y et al. Gut microbiota offers universal biomarkers across ethnicity in inflammatory bowel disease diagnosis and Infliximab response prediction. mSystems. 2018;3:e00188–17. 10.1128/mSystems.00188-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ho GT, Lee HM, Brydon G et al. Fecal calprotectin predicts the clinical course of acute severe ulcerative colitis. Am J Gastroenterol. 2009;104:673–8. 10.1038/ajg.2008.119. [DOI] [PubMed] [Google Scholar]
- 115. Wright EK, Kamm MA, De Cruz P et al. Measurement of fecal calprotectin improves monitoring and detection of recurrence of Crohn's disease after surgery. Gastroenterology. 2015;148:938–47. 10.1053/j.gastro.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 116. Yamamoto T, Shimoyama T, Bamba T et al. Consecutive monitoring of fecal calprotectin and lactoferrin for the early diagnosis and prediction of pouchitis after restorative proctocolectomy for ulcerative colitis. Am J Gastroenterol. 2015;110:881–7. 10.1038/ajg.2015.129. [DOI] [PubMed] [Google Scholar]
- 117. Fu Y, Wang L, Xie C et al. Comparison of non-invasive biomarkers faecal BAFF, calprotectin and FOBT in discriminating IBS from IBD and evaluation of intestinal inflammation. Sci Rep. 2017;7:2669. 10.1038/s41598-017-02835-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Thorsvik S, Damås JK, Granlund AV et al. Fecal neutrophil gelatinase-associated lipocalin as a biomarker for inflammatory bowel disease. J Gastroenterol Hepatol. 2017;32:128–35. 10.1111/jgh.13598. [DOI] [PubMed] [Google Scholar]
- 119. Dang Y, Ma C, Chen K et al. The effects of a high-fat diet on inflammatory bowel disease. Biomolecules. 2023;13:905. 10.3390/biom13060905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Gevers D, Kugathasan S, Denson LA et al. The treatment-naive microbiome in new-onset Crohn's disease. Cell Host Microbe. 2014;15:382–92. 10.1016/j.chom.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Singh S, Ananthakrishnan AN, Nguyen NH et al. AGA clinical practice guideline on the role of biomarkers for the management of ulcerative colitis. Gastroenterology. 2023;164:344–72. 10.1053/j.gastro.2022.12.007. [DOI] [PubMed] [Google Scholar]
- 122. Chen R, Li L, Tie Y et al. Trajectory of fecal lactoferrin for predicting prognosis in ulcerative colitis. Precis Clin Med. 2023;6:pbad022. 10.1093/pcmedi/pbad022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Sarter H, Savoye G, Marot G et al. A novel 8-predictors signature to predict complicated disease course in pediatric-onset crohn's disease: a population-based study. Inflamm Bowel Dis. 2023;29:1793–804. 10.1093/ibd/izad090. [DOI] [PubMed] [Google Scholar]
- 124. Lamb CA, Saifuddin A, Powell N et al. The future of precision medicine to predict outcomes and control tissue remodeling in inflammatory bowel disease. Gastroenterology. 2022;162:1525–42. 10.1053/j.gastro.2021.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Cosnes J, Gower-Rousseau C, Seksik P et al. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–94. 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 126. Panés J, Rimola J Perianal fistulizing Crohn's disease: pathogenesis, diagnosis and therapy. Nat Rev Gastroenterol Hepatol. 2017;14:652–64. 10.1038/nrgastro.2017.104. [DOI] [PubMed] [Google Scholar]
- 127. Agrawal M, Spencer EA, Colombel JF et al. Approach to the management of recently diagnosed inflammatory bowel disease patients: a user's guide for adult and pediatric gastroenterologists. Gastroenterology. 2021;161:47–65. 10.1053/j.gastro.2021.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Gomollon F, Dignass A, Annese V et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn's disease 2016: Part 1: diagnosis and medical management. J Crohns Colitis. 2017;11:3–25. 10.1093/ecco-jcc/jjw168. [DOI] [PubMed] [Google Scholar]
- 129. Colombel JF, Ferrari N, Debuysere H et al. Genotypic analysis of thiopurine S-methyltransferase in patients with Crohn's disease and severe myelosuppression during azathioprine therapy. Gastroenterology. 2000;118:1025–30. 10.1016/s0016-5085(00)70354-4. [DOI] [PubMed] [Google Scholar]
- 130. Zhu X, Wang XD, Chao K et al. NUDT15 polymorphisms are better than thiopurine S-methyltransferase as predictor of risk for thiopurine-induced leukopenia in Chinese patients with Crohn's disease. Aliment Pharmacol Ther. 2016;44:967–75. 10.1111/apt.13796. [DOI] [PubMed] [Google Scholar]
- 131. Colombel JF, Sandborn WJ, Reinisch W et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362:1383–95. 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 132. Sprakes MB, Ford AC, Warren L et al. Efficacy, tolerability, and predictors of response to infliximab therapy for Crohn's disease: a large single centre experience. J Crohns Colitis. 2012;6:143–53. 10.1016/j.crohns.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 133. Jürgens M, Laubender RP, Hartl F et al. Disease activity, ANCA, and IL23R genotype status determine early response to infliximab in patients with ulcerative colitis. Am J Gastroenterol. 2010;105:1811–9. 10.1038/ajg.2010.95. [DOI] [PubMed] [Google Scholar]
- 134. Hlavaty T, Pierik M, Henckaerts L et al. Polymorphisms in apoptosis genes predict response to infliximab therapy in luminal and fistulizing Crohn's disease. Aliment Pharmacol Ther. 2005;22:613–26. 10.1111/j.1365-2036.2005.02635.x. [DOI] [PubMed] [Google Scholar]
- 135. Laserna-Mendieta EJ, Salvador-Martín S, Arias A et al. Single nucleotide polymorphisms in ADAM17, IL23R and SLCO1C1 genes protect against infliximab failure in adults with Crohn's disease. Biomed Pharmacother. 2023;159:114225. 10.1016/j.biopha.2023.114225. [DOI] [PubMed] [Google Scholar]
- 136. Louis E, Vermeire S, Rutgeerts P et al. A positive response to infliximab in Crohn disease: association with a higher systemic inflammation before treatment but not with -308 TNF gene polymorphism. Scand J Gastroenterol. 2002;37:818–24. 10.1080/gas.37.7.818.824. [DOI] [PubMed] [Google Scholar]
- 137. Magnusson MK, Strid H, Sapnara M et al. Anti-TNF therapy response in patients with ulcerative colitis is associated with colonic antimicrobial peptide expression and microbiota composition. J Crohns Colitis. 2016;10:943–52. 10.1093/ecco-jcc/jjw051. [DOI] [PubMed] [Google Scholar]
- 138. Höyhtyä M, Korpela K, Saqib S et al. Quantitative fecal microbiota profiles relate to therapy response during induction with tumor necrosis factor α antagonist Infliximab in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2023;29:116–24. 10.1093/ibd/izac182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Kolho KL, Korpela K, Jaakkola T et al. Fecal microbiota in pediatric inflammatory bowel disease and its relation to inflammation. Am J Gastroenterol. 2015;110:921–30. 10.1038/ajg.2015.149. [DOI] [PubMed] [Google Scholar]
- 140. Kennedy NA, Heap GA, Green HD et al. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn's disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol. 2019;4:341–53. 10.1016/s2468-1253(19)30012-3. [DOI] [PubMed] [Google Scholar]
- 141. Wilson A, Peel C, Wang Q et al. HLADQA1*05 genotype predicts anti-drug antibody formation and loss of response during infliximab therapy for inflammatory bowel disease. Aliment Pharmacol Ther. 2020;51:356–63. 10.1111/apt.15563. [DOI] [PubMed] [Google Scholar]
- 142. Zapata-Cobo P, Salvador-Martín S, Velasco M et al. Polymorphisms indicating risk of inflammatory bowel disease or antigenicity to anti-TNF drugs as biomarkers of response in children. Pharmacol Res. 2023;194:106859. 10.1016/j.phrs.2023.106859. [DOI] [PubMed] [Google Scholar]
- 143. Beltrán B, Iborra M, Sáez-González E et al. Fecal calprotectin pretreatment and induction infliximab levels for prediction of primary nonresponse to Infliximab therapy in Crohn's disease. Dig Dis. 2019;37:108–15. 10.1159/000492626. [DOI] [PubMed] [Google Scholar]
- 144. Koder S, Repnik K, Ferkolj I et al. Genetic polymorphism in ATG16L1 gene influences the response to adalimumab in Crohn's disease patients. Pharmacogenomics. 2015;16:191–204. 10.2217/pgs.14.172. [DOI] [PubMed] [Google Scholar]
- 145. Bank S, Andersen PS, Burisch J et al. Associations between functional polymorphisms in the NFκB signaling pathway and response to anti-TNF treatment in Danish patients with inflammatory bowel disease. Pharmacogenomics J. 2014;14:526–34. 10.1038/tpj.2014.19. [DOI] [PubMed] [Google Scholar]
- 146. Jezernik G, Gorenjak M, Potočnik U MIF Variant rs755622 is associated with severe crohn's disease and better response to anti-TNF Adalimumab therapy. Genes (Basel). 2023;14:452. 10.3390/genes14020452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Atreya R, Neumann H, Neufert C et al. In vivo imaging using fluorescent antibodies to tumor necrosis factor predicts therapeutic response in Crohn's disease. Nat Med. 2014;20:313–8. 10.1038/nm.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Chen L, Lu Z, Kang D et al. Distinct alterations of fecal microbiota refer to the efficacy of adalimumab in Crohn's disease. Front Pharmacol. 2022;13:913720. 10.3389/fphar.2022.913720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Billioud V, Sandborn WJ, Peyrin-Biroulet L Loss of response and need for adalimumab dose intensification in Crohn's disease: a systematic review. Am J Gastroenterol. 2011;106:674–84. 10.1038/ajg.2011.60. [DOI] [PubMed] [Google Scholar]
- 150. Netz U, Carter JV, Eichenberger MR et al. Genetic polymorphisms predict response to anti-tumor necrosis factor treatment in Crohn's disease. World J Gastroenterol. 2017;23:4958–67. 10.3748/wjg.v23.i27.4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Lin S, Chanchlani N, Carbery I et al. Understanding anti-TNF treatment failure: does serum triiodothyronine-to-thyroxine (T3/T4) ratio predict therapeutic outcome to anti-TNF therapies in biologic-naïve patients with active luminal Crohn's disease?. Aliment Pharmacol Ther. 2022;56:783–93. 10.1111/apt.17089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Magnusson MK, Strid H, Isaksson S et al. Cultured blood T-cell responses predict anti-TNF therapy response in patients with ulcerative colitis. Aliment Pharmacol Ther. 2015;41:1149–61. 10.1111/apt.13192. [DOI] [PubMed] [Google Scholar]
- 153. Amiot A, Serrero M, Peyrin-Biroulet L et al. One-year effectiveness and safety of vedolizumab therapy for inflammatory bowel disease: a prospective multicentre cohort study. Aliment Pharmacol Ther. 2017;46:310–21. 10.1111/apt.14167. [DOI] [PubMed] [Google Scholar]
- 154. Sandborn WJ, Feagan BG, Rutgeerts P et al. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2013;369:711–21. 10.1056/NEJMoa1215739. [DOI] [PubMed] [Google Scholar]
- 155. Dulai PS, Singh S, Vande Casteele N et al. Development and validation of clinical scoring tool to predict outcomes of treatment with Vedolizumab in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2020;18:2952–61. 10.1016/j.cgh.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Abreu MT, Davies JM, Quintero MA et al. Transcriptional behavior of regulatory T Cells predicts IBD patient responses to Vedolizumab therapy. Inflamm Bowel Dis. 2022;28:1800–12. 10.1093/ibd/izac151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Boden EK, Shows DM, Chiorean MV et al. Identification of candidate biomarkers associated with response to Vedolizumab in inflammatory bowel disease. Dig Dis Sci. 2018;63:2419–29. 10.1007/s10620-018-4924-8. [DOI] [PubMed] [Google Scholar]
- 158. Fuchs F, Schillinger D, Atreya R et al. Clinical response to Vedolizumab in ulcerative colitis patients is associated with changes in integrin expression profiles. Front Immunol. 2017;8:764. 10.3389/fimmu.2017.00764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Ananthakrishnan AN, Luo C, Yajnik V et al. Gut microbiome function predicts response to anti-integrin biologic therapy in inflammatory bowel diseases. Cell Host Microbe. 2017;21:603–10. 10.1016/j.chom.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Dulai PS, Singh S, Jiang X et al. The real-world effectiveness and safety of Vedolizumab for moderate-severe Crohn's disease: results from the US VICTORY Consortium. Am J Gastroenterol. 2016;111:1147–55. 10.1038/ajg.2016.236. [DOI] [PubMed] [Google Scholar]
- 161. Mader O, Juillerat P, Biedermann L et al. Factors influencing the outcome of vedolizumab treatment: Real-life data with objective outcome measurements. United European Gastroenterol J. 2021;9: 398–406. 10.1177/2050640620965106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Amiot A, Grimaud JC, Peyrin-Biroulet L et al. Effectiveness and safety of Vedolizumab induction therapy for patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2016;14:1593–601. 10.1016/j.cgh.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 163. Rosario M, French JL, Dirks NL et al. Exposure-efficacy relationships for Vedolizumab induction therapy in patients with ulcerative colitis or Crohn's disease. J Crohns Colitis. 2017;11:921–29. 10.1093/ecco-jcc/jjx021. [DOI] [PubMed] [Google Scholar]
- 164. Rath T, Bojarski C, Neurath MF et al. Molecular imaging of mucosal α4β7 integrin expression with the fluorescent anti-adhesion antibody vedolizumab in Crohn's disease. Gastrointest Endosc. 2017;86:406–8. 10.1016/j.gie.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 165. Ma C, Fedorak RN, Kaplan GG et al. Clinical, endoscopic and radiographic outcomes with ustekinumab in medically-refractory Crohn's disease: real world experience from a multicentre cohort. Aliment Pharmacol Ther. 2017;45:1232–43. 10.1111/apt.14016. [DOI] [PubMed] [Google Scholar]
- 166. Ma C, Fedorak RN, Kaplan GG et al. Long-term maintenance of clinical, endoscopic, and radiographic response to Ustekinumab in moderate-to-severe Crohn's disease: Real-world experience from a multicenter cohort study. Inflamm Bowel Dis. 2017;23:833–9. 10.1097/mib.0000000000001074. [DOI] [PubMed] [Google Scholar]
- 167. Toedter GP, Blank M, Lang Y et al. Relationship of C-reactive protein with clinical response after therapy with ustekinumab in Crohn's disease. Am J Gastroenterol. 2009;104:2768–73. 10.1038/ajg.2009.454. [DOI] [PubMed] [Google Scholar]
- 168. Nishioka K, Ogino H, Chinen T et al. Mucosal IL23A expression predicts the response to Ustekinumab in inflammatory bowel disease. J Gastroenterol. 2021;56:976–87. 10.1007/s00535-021-01819-7. [DOI] [PubMed] [Google Scholar]
- 169. Doherty MK, Ding T, Koumpouras C et al. Fecal microbiota signatures are associated with response to Ustekinumab therapy among Crohn's disease patients. mBio. 2018;9:e02120–17. 10.1128/mBio.02120-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Lee JWJ, Plichta D, Hogstrom L et al. Multi-omics reveal microbial determinants impacting responses to biologic therapies in inflammatory bowel disease. Cell Host Microbe. 2021;29:1294–304. 10.1016/j.chom.2021.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Chaparro M, Baston-Rey I, Fernández Salgado E et al. Using interpretable machine learning to identify baseline predictive factors of remission and drug durability in Crohn's disease patients on Ustekinumab. J Clin Med. 2022;11:4518. 10.3390/jcm11154518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Wright S, Sanders DS, Lobo AJ et al. Clinical significance of azathioprine active metabolite concentrations in inflammatory bowel disease. Gut. 2004;53:1123–8. 10.1136/gut.2003.032896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Wong DR, Coenen MJ, Vermeulen SH et al. Early assessment of thiopurine metabolites identifies patients at risk of thiopurine-induced leukopenia in inflammatory bowel disease. J Crohns Colitis. 2017;11:175–84. 10.1093/ecco-jcc/jjw130. [DOI] [PubMed] [Google Scholar]
- 174. Zhang F, Gao X, Chen M et al. Should thiopurine methyltransferase genotypes and phenotypes be measured before thiopurine therapy in patients with inflammatory bowel disease?. Ther Drug Monit. 2012;34:695–701. 10.1097/FTD.0b013e3182731925. [DOI] [PubMed] [Google Scholar]
- 175. Winter JW, Gaffney D, Shapiro D et al. Assessment of thiopurine methyltransferase enzyme activity is superior to genotype in predicting myelosuppression following azathioprine therapy in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2007;25:1069–77. 10.1111/j.1365-2036.2007.03301.x. [DOI] [PubMed] [Google Scholar]
- 176. Sutiman N, Chen S, Ling KL et al. Predictive role of NUDT15 variants on thiopurine-induced myelotoxicity in Asian inflammatory bowel disease patients. Pharmacogenomics. 2018;19:31–43. 10.2217/pgs-2017-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Walker GJ, Harrison JW, Heap GA et al. Association of genetic variants in NUDT15 with thiopurine-induced myelosuppression in patients with inflammatory bowel disease. JAMA. 2019;321:773–85. 10.1001/jama.2019.0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Van Dieren JM, Hansen BE, Kuipers EJ et al. Meta-analysis: Inosine triphosphate pyrophosphatase polymorphisms and thiopurine toxicity in the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2007;26:643–52. 10.1111/j.1365-2036.2007.03412.x. [DOI] [PubMed] [Google Scholar]
- 179. Pavlovic S, Kotur N, Stankovic B et al. Clinical application of thiopurine pharmacogenomics in pediatrics. Curr Drug Metab. 2020;21:53–62. 10.2174/1389200221666200303113456. [DOI] [PubMed] [Google Scholar]
- 180. Gisbert JP, Gomollon F Thiopurine-induced myelotoxicity in patients with inflammatory bowel disease: a review. Am J Gastroenterol. 2008;103:1783–800. 10.1111/j.1572-0241.2008.01848.x. [DOI] [PubMed] [Google Scholar]
- 181. Zarca K, Chansavang A, Loriot MA et al. Cost-effectiveness analysis of pretreatment screening for NUDT15 defective alleles. Pharmacogenet Genomics. 2020;30:175–83. 10.1097/fpc.0000000000000410. [DOI] [PubMed] [Google Scholar]
- 182. Lazarević S, Đanic M, Al-Salami H et al. Gut microbiota metabolism of Azathioprine: A new hallmark for personalized drug-targeted therapy of chronic inflammatory bowel disease. Front Pharmacol. 2022;13:879170. 10.3389/fphar.2022.879170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Liu F, Ma R, Riordan SM et al. Azathioprine, mercaptopurine, and 5-aminosalicylic acid affect the growth of IBD-associated campylobacter species and other enteric microbes. Front Microbiol. 2017;8:527. 10.3389/fmicb.2017.00527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Franzin M, Stefančič K, Lucafò M et al. Microbiota and drug response in inflammatory bowel disease. Pathogens. 2021;10:211. 10.3390/pathogens10020211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Chao K, Huang Y, Zhu X et al. Randomised clinical trial: dose optimising strategy by NUDT15 genotyping reduces leucopenia during thiopurine treatment of Crohn's disease. Aliment Pharmacol Ther. 2021;54:1124–33. 10.1111/apt.16600. [DOI] [PubMed] [Google Scholar]
- 186. Järnerot G, Hertervig E, Friis-Liby I et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology. 2005;128:1805–11. 10.1053/j.gastro.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 187. Ben-Horin S, Kopylov U, Chowers Y Optimizing anti-TNF treatments in inflammatory bowel disease. Autoimmun Rev. 2014;13:24–30. 10.1016/j.autrev.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 188. Sazonovs A, Kennedy NA, Moutsianas L et al. HLA-DQA1*05 carriage associated with development of anti-drug antibodies to infliximab and adalimumab in patients with Crohn's disease. Gastroenterology. 2020;158:189–99. 10.1053/j.gastro.2019.09.041. [DOI] [PubMed] [Google Scholar]
- 189. Hlavaty T, Ferrante M, Henckaerts L et al. Predictive model for the outcome of infliximab therapy in Crohn's disease based on apoptotic pharmacogenetic index and clinical predictors. Inflamm Bowel Dis. 2007;13:372–9. 10.1002/ibd.20024. [DOI] [PubMed] [Google Scholar]
- 190. Arijs I, Li K, Toedter G et al. Mucosal gene signatures to predict response to infliximab in patients with ulcerative colitis. Gut. 2009;58:1612–9. 10.1136/gut.2009.178665. [DOI] [PubMed] [Google Scholar]
- 191. Arijs I, Quintens R, Van Lommel L et al. Predictive value of epithelial gene expression profiles for response to infliximab in Crohn's disease. Inflamm Bowel Dis. 2010;16:2090–8. 10.1002/ibd.21301. [DOI] [PubMed] [Google Scholar]
- 192. Jongsma MME, Costes LMM, Tindemans I et al. Serum immune profiling in pediatric Crohn's disease demonstrates stronger immune modulation with first-line infliximab than conventional therapy and pre-treatment profiles predict clinical response to both treatments. J Crohns Colitis. 2023;17:1262–77. 10.1093/ecco-jcc/jjad049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Yang W, Yu T, Cong Y CD4(+) T cell metabolism, gut microbiota, and autoimmune diseases: implication in precision medicine of autoimmune diseases. Precis Clin Med. 2022;5:pbac018. 10.1093/pcmedi/pbac018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Gisbert JP, Chaparro M Predictors of primary response to biologic treatment [anti-TNF, Vedolizumab, and Ustekinumab] in patients with inflammatory bowel disease: from basic science to clinical practice. J Crohns Colitis. 2020;14:694–709. 10.1093/ecco-jcc/jjz195. [DOI] [PubMed] [Google Scholar]
- 195. Burke KE, Khalili H, Garber JJ et al. Genetic markers predict primary nonresponse and durable response to anti-tumor necrosis factor therapy in ulcerative colitis. Inflamm Bowel Dis. 2018;24:1840–8. 10.1093/ibd/izy083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Dubinsky MC, Mei L, Friedman M et al. Genome wide association (GWA) predictors of anti-TNFalpha therapeutic responsiveness in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:1357–66. 10.1002/ibd.21174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Colombel JF, Sandborn WJ, Rutgeerts P et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007;132:52–65. 10.1053/j.gastro.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 198. Sandborn WJ, van Assche G, Reinisch W et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142:257–65. 10.1053/j.gastro.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 199. Hinojosa J, Gomollón F, García S et al. Efficacy and safety of short-term adalimumab treatment in patients with active Crohn's disease who lost response or showed intolerance to infliximab: a prospective, open-label, multicentre trial. Aliment Pharmacol Ther. 2007;25:409–18. 10.1111/j.1365-2036.2006.03232.x. [DOI] [PubMed] [Google Scholar]
- 200. Qiu Y, Chen BL, Mao R et al. Systematic review with meta-analysis: loss of response and requirement of anti-TNFα dose intensification in Crohn's disease. J Gastroenterol. 2017;52:535–54. 10.1007/s00535-017-1324-3. [DOI] [PubMed] [Google Scholar]
- 201. Iborra M, Pérez-Gisbert J, Bosca-Watts MM et al. Effectiveness of adalimumab for the treatment of ulcerative colitis in clinical practice: comparison between anti-tumour necrosis factor-naïve and non-naïve patients. J Gastroenterol. 2017;52:788–99. 10.1007/s00535-016-1274-1. [DOI] [PubMed] [Google Scholar]
- 202. Gorenjak M, Repnik K, Jezernik G et al. Genetic prediction profile for adalimumab response in Slovenian Crohn's disease patients. Z Gastroenterol. 2019;57:1218–25. 10.1055/a-0981-6516. [DOI] [PubMed] [Google Scholar]
- 203. Busquets D, Oliver L, Amoedo J et al. RAID prediction: pilot study of fecal microbial signature with capacity to predict response to anti-TNF treatment. Inflamm Bowel Dis. 2021;27: S63–6. 10.1093/ibd/izab273. [DOI] [PubMed] [Google Scholar]
- 204. Bouhnik Y, Carbonnel F, Laharie D et al. Efficacy of adalimumab in patients with Crohn's disease and symptomatic small bowel stricture: a multicentre, prospective, observational cohort (CREOLE) study. Gut. 2018;67:53–60. 10.1136/gutjnl-2016-312581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Feagan BG, Rutgeerts P, Sands BE et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699–710. 10.1056/NEJMoa1215734. [DOI] [PubMed] [Google Scholar]
- 206. Shelton E, Allegretti JR, Stevens B et al. Efficacy of Vedolizumab as induction therapy in refractory IBD Patients: A multicenter cohort. Inflamm Bowel Dis. 2015;21:2879–85. 10.1097/mib.0000000000000561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Barré A, Colombel JF, Ungaro R Review article: predictors of response to vedolizumab and ustekinumab in inflammatory bowel disease. Aliment Pharmacol Ther. 2018;47:896–905. 10.1111/apt.14550. [DOI] [PubMed] [Google Scholar]
- 208. Vermeire S, Loftus EVJ, Colombel JF et al. Long-term efficacy of Vedolizumab for Crohn's disease. J Crohns Colitis. 2017;11:412–24. 10.1093/ecco-jcc/jjw176. [DOI] [PubMed] [Google Scholar]
- 209. Loftus EVJ, Colombel JF, Feagan BG et al. Long-term efficacy of Vedolizumab for ulcerative colitis. J Crohns Colitis. 2017;11:400–11. 10.1093/ecco-jcc/jjw177. [DOI] [PubMed] [Google Scholar]
- 210. McKinney EF, Lee JC, Jayne DR et al. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature. 2015;523:612–6. 10.1038/nature14468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Schneider I, Allner C, Mühl L et al. Expression and function of α4β7 integrin predict the success of vedolizumab treatment in inflammatory bowel disease. Transl Res. 2023;253:8–15. 10.1016/j.trsl.2022.10.003. [DOI] [PubMed] [Google Scholar]
- 212. Dulai PS, Boland BS, Singh S et al. Development and validation of a scoring system to predict outcomes of Vedolizumab treatment in patients with Crohn's disease. Gastroenterology. 2018;155:687–95. 10.1053/j.gastro.2018.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Sandborn WJ, Gasink C, Gao LL et al. Ustekinumab induction and maintenance therapy in refractory Crohn's disease. N Engl J Med. 2012;367:1519–28. 10.1056/NEJMoa1203572. [DOI] [PubMed] [Google Scholar]
- 214. Benson JM, Peritt D, Scallon BJ et al. Discovery and mechanism of ustekinumab: a human monoclonal antibody targeting interleukin-12 and interleukin-23 for treatment of immune-mediated disorders. MAbs. 2011;3:535–45. 10.4161/mabs.3.6.17815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Galluzzo M, Boca AN, Botti E et al. IL12B (p40) gene polymorphisms contribute to Ustekinumab response prediction in psoriasis. Dermatology. 2016;232:230–6. 10.1159/000441719. [DOI] [PubMed] [Google Scholar]
- 216. van den Reek J, Coenen MJH, van de L'Isle Arias M et al. Polymorphisms in CD84, IL12B and TNFAIP3 are associated with response to biologics in patients with psoriasis. Br J Dermatol. 2017;176:1288–96. 10.1111/bjd.15005. [DOI] [PubMed] [Google Scholar]
- 217. Park J, Chun J, Yoon H et al. Feasibility of a clinical decision support tool for Ustekinumab to predict clinical remission and relapse in patients with Crohn's disease: a multicenter observational study. Inflamm Bowel Dis. 2023;29:548–54. 10.1093/ibd/izac105. [DOI] [PubMed] [Google Scholar]
- 218. Mottet C, Schoepfer AM, Juillerat P et al. Experts opinion on the practical use of Azathioprine and 6-Mercaptopurine in inflammatory bowel disease. Inflamm Bowel Dis. 2016;22:2733–47. 10.1097/mib.0000000000000923. [DOI] [PubMed] [Google Scholar]
- 219. Kwan LY, Devlin SM, Mirocha JM et al. Thiopurine methyltransferase activity combined with 6-thioguanine metabolite levels predicts clinical response to thiopurines in patients with inflammatory bowel disease. Dig Liver Dis. 2008;40:425–32. 10.1016/j.dld.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 220. Yang S-K Personalizing IBD therapy: The Asian perspective. Dig Dis. 2016;34:165–74. 10.1159/000443134. [DOI] [PubMed] [Google Scholar]
- 221. Waljee AK, Joyce JC, Wang S et al. Algorithms outperform metabolite tests in predicting response of patients with inflammatory bowel disease to thiopurines. Clin Gastroenterol Hepatol. 2010;8:143–50. 10.1016/j.cgh.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 222. Dubinsky MC, Lamothe S, Yang HY et al. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology. 2000;118:705–13. 10.1016/s0016-5085(00)70140-5. [DOI] [PubMed] [Google Scholar]
- 223. Luo X, Yan S, Jin L et al. Inosine triphosphate pyrophosphatase and NUDT15 are good predictors of clinical outcomes in thiopurine-treated chinese patients with inflammatory bowel disease. Ther Drug Monit. 2022;44:391–5. 10.1097/ftd.0000000000000965. [DOI] [PubMed] [Google Scholar]
- 224. Feuerstein JD, Nguyen GC, Kupfer SS et al. American gastroenterological association institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology. 2017;153:827–34. 10.1053/j.gastro.2017.07.032. [DOI] [PubMed] [Google Scholar]
- 225. Argollo M, Kotze PG, Kakkadasam P et al. Optimizing biologic therapy in IBD: how essential is therapeutic drug monitoring?. Nat Rev Gastroenterol Hepatol. 2020;17:702–10. 10.1038/s41575-020-0352-2. [DOI] [PubMed] [Google Scholar]
- 226. Brun MK, Goll GL, Jørgensen KK et al. Risk factors for anti-drug antibody formation to infliximab: Secondary analyses of a randomised controlled trial. J Intern Med. 2022;292:477–91. 10.1111/joim.13495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Colman RJ, Xiong Y, Mizuno T et al. Antibodies-to-infliximab accelerate clearance while dose intensification reverses immunogenicity and recaptures clinical response in paediatric Crohn's disease. Aliment Pharmacol Ther. 2022;55:593–603. 10.1111/apt.16733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Su CG, Lichtenstein GR Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. Gastroenterology. 2003;125:1544–6. 10.1016/j.gastro.2003.05.009. [DOI] [PubMed] [Google Scholar]
- 229. Seow CH, Newman A, Irwin SP et al. Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut. 2010;59:49–54. 10.1136/gut.2009.183095. [DOI] [PubMed] [Google Scholar]
- 230. De Gregorio M, Lee T, Krishnaprasad K et al. Higher anti-tumor necrosis factor-α levels correlate with improved radiologic outcomes in Crohn's perianal fistulas. Clin Gastroenterol Hepatol. 2022;20:1306–14. 10.1016/j.cgh.2021.07.053. [DOI] [PubMed] [Google Scholar]
- 231. Vande Casteele N, Ferrante M, Van Assche G et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015;148:1320–9. 10.1053/j.gastro.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 232. Hanauer SB, Wagner CL, Bala M et al. Incidence and importance of antibody responses to infliximab after maintenance or episodic treatment in Crohn's disease. Clin Gastroenterol Hepatol. 2004;2:542–53. 10.1016/s1542-3565(04)00238-1. [DOI] [PubMed] [Google Scholar]
- 233. Steenholdt C, Bendtzen K, Brynskov J et al. Optimizing treatment with TNF inhibitors in inflammatory bowel disease by monitoring drug levels and antidrug antibodies. Inflamm Bowel Dis. 2016;22:1999–2015. 10.1097/mib.0000000000000772. [DOI] [PubMed] [Google Scholar]
- 234. Yarur AJ, Jain A, Hauenstein SI et al. Higher Adalimumab levels are associated with histologic and endoscopic remission in patients with Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2016;22:409–15. 10.1097/mib.0000000000000689. [DOI] [PubMed] [Google Scholar]
- 235. Zittan E, Kabakchiev B, Milgrom R et al. Higher Adalimumab drug levels are associated with mucosal healing in patients with Crohn's disease. J Crohns Colitis. 2016;10:510–5. 10.1093/ecco-jcc/jjw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Assa A, Matar M, Turner D et al. Proactive monitoring of Adalimumab trough concentration associated with increased clinical remission in children with Crohn's disease compared with reactive monitoring. Gastroenterology. 2019;157:985–96. 10.1053/j.gastro.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 237. Chaparro M, Barreiro-de Acosta M, Echarri A et al. Correlation between anti-TNF serum levels and endoscopic inflammation in inflammatory bowel disease patients. Dig Dis Sci. 2019;64:846–54. 10.1007/s10620-018-5362-3. [DOI] [PubMed] [Google Scholar]
- 238. Juncadella A, Papamichael K, Vaughn BP et al. Maintenance Adalimumab concentrations are associated with biochemical, endoscopic, and histologic remission in inflammatory bowel disease. Dig Dis Sci. 2018;63:3067–73. 10.1007/s10620-018-5202-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Vande Casteele N, Sandborn WJ, Feagan BG et al. Real-world multicentre observational study including population pharmacokinetic modelling to evaluate the exposure-response relationship of vedolizumab in inflammatory bowel disease: ERELATE Study. Aliment Pharmacol Ther. 2022;56:463–76. 10.1111/apt.16937. [DOI] [PubMed] [Google Scholar]
- 240. Osterman MT, Rosario M, Lasch K et al. Vedolizumab exposure levels and clinical outcomes in ulcerative colitis: determining the potential for dose optimisation. Aliment Pharmacol Ther. 2019;49:408–18. 10.1111/apt.15113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Yacoub W, Williet N, Pouillon L et al. Early vedolizumab trough levels predict mucosal healing in inflammatory bowel disease: a multicentre prospective observational study. Aliment Pharmacol Ther. 2018;47:906–12. 10.1111/apt.14548. [DOI] [PubMed] [Google Scholar]
- 242. Hanzel J, Dreesen E, Vermeire S et al. Pharmacokinetic-pharmacodynamic model of vedolizumab for targeting endoscopic remission in patients with Crohn disease: posthoc analysis of the LOVE-CD study. Inflamm Bowel Dis. 2022;28:689–99. 10.1093/ibd/izab143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Rosario M, Dirks NL, Gastonguay MR et al. Population pharmacokinetics-pharmacodynamics of vedolizumab in patients with ulcerative colitis and Crohn's disease. Aliment Pharmacol Ther. 2015;42:188–202. 10.1111/apt.13243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Feagan BG, Greenberg GR, Wild G et al. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N Engl J Med. 2005;352:2499–507. 10.1056/NEJMoa042982. [DOI] [PubMed] [Google Scholar]
- 245. Battat R, Kopylov U, Bessissow T et al. Association between Ustekinumab trough concentrations and clinical, biomarker, and endoscopic outcomes in patients with Crohn's disease. Clin Gastroenterol Hepatol. 2017;15:1427–34. 10.1016/j.cgh.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 246. Adedokun OJ, Xu Z, Marano C et al. Ustekinumab pharmacokinetics and exposure response in a phase 3 randomized trial of patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2020;18:2244–55. 10.1016/j.cgh.2019.11.059. [DOI] [PubMed] [Google Scholar]
- 247. Adedokun OJ, Xu Z, Gasink C et al. Pharmacokinetics and exposure response relationships of ustekinumab in patients with Crohn's disease. Gastroenterology. 2018;154:1660–71. 10.1053/j.gastro.2018.01.043. [DOI] [PubMed] [Google Scholar]
- 248. Yao J, Zhang H, Su T et al. Ustekinumab promotes radiological fistula healing in perianal fistulizing Crohn's disease: a retrospective real-world analysis. J Clin Med. 2023;12:939. 10.3390/jcm12030939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Sandborn WJ, Rebuck R, Wang Y et al. Five-year efficacy and safety of Ustekinumab treatment in Crohn's disease: The IM-UNITI trial. Clin Gastroenterol Hepatol. 2022;20:578–90. 10.1016/j.cgh.2021.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]