Abstract
Background:
The treatment of heavily pretreated patients with metastatic renal cell carcinoma (mRCC) represents an unmet medical need and is still challenging.
Objectives:
The primary objective was to assess the effectiveness of the lenvatinib plus everolimus combination and the secondary objective was the toxicity profile of this combination.
Design:
We conducted a longitudinal retrospective study examining mRCC patients pre-treated with one or more lines of therapy among different cancer centers in Italy.
Methods:
The study included patients who received the combination of lenvatinib plus everolimus as either a second-line treatment or beyond. We assessed progression-free survival (PFS), time to treatment failure (TTF), overall survival (OS), response rate (RR), and toxicity profile. In addition, we explored the potential relationship between treatment effectiveness and clinical and laboratory parameters.
Results:
In all, 33 patients were assessed, the median age was 60 years, 57% had an Eastern Cooperative Oncology Group performance status of 1–2 and. 63% received ⩾ 3 prior lines of therapy. 62% were ‘intermediate risk’ according to the International Metastatic Renal Cell Carcinoma Database Consortium and 30% were ‘poor risk’. The RR was 42% (no complete response), 18% stable disease. Median OS was 11.2 months (95% CI 6.8–19.9), median PFS was 6.7 months (95% CI 0.6–30.8), and median TTF was 6.7 months (95% CI 4.8–16.6). A shorter OS was significantly associated with lymph node metastases (p = 0.043, 95% CI), neutrophils/ lymphocytes ratio (NLR) ⩾ 3 (p = 0.007), hemoglobin/red cell distribution width ratio cutoff value <0.7 was significant (p = 0.03) while a shorter PFS was associated with lung (p = 0.048) and brain metastases (p = 0.023). The most frequent G1 toxicity was diarrhea (24%), G2 was fatigue (30%), and hypertension and skin toxicity (6%) for G3.
Conclusion:
Our findings suggest a clinically relevant effectiveness of lenvatinib plus everolimus combination with an acceptable toxicity profile for heavily pretreated patients with mRCC.
Keywords: everolimus, heavily, lenvatinib, mRCC, pretreated, renal cell carcinoma
Background
In Italy, 12,600 new diagnoses of renal cell carcinoma (RCC) during 2022 were made, with a 5-year overall survival (OS) of 71% for both sexes according to Associazione Italiana Registro Tumori. 1
Even though the first-line treatment of metastatic disease is consolidated with the use of tyrosine kinase inhibitors (TKIs) as monotherapy or in combination with immune checkpoint inhibitors (ICIs) immunotherapy or the option of double ICI combination, the second-line treatment (and beyond) is still controversial for this disease in the new therapeutic scenario.
Some patients are fit enough to receive multiple treatment lines,2–5 thus running out of valid therapeutic options. In this setting, the combination of lenvatinib plus everolimus represents an option that could be taken into account according to Italian AIOM (Associazione Italiana di Oncologia Medica), 1 European (ESMO guidelines), 6 and National Comprehensive Cancer Network (NCCN). 7
The rationale behind the deployment of this combination is the contemporary blockade of VEGFR (Vascular Endothelial Growth Factor Receptor), MAPK (Mitogen-Activated Protein Kinase), FGFR (Fibroblast Growth Factor Receptor) as well as mTOR signaling pathways.8,9
The safety of this combination has been studied both in a phase Ib trial in the metastatic setting 10 as well as in a randomized phase II trial (ClinicalTrials.gov, NCT01136733), 11 which led to the approval of the drug combination by the FDA in May 2016. In this study, progression-free survival (PFS) was higher in the lenvatinib plus everolimus arm with a median of 12.8 months (95% CI 7.4–17.5) than in the lenvatinib arm median of 9.0 months (95% CI 5.6–10.2), and the everolimus arm median of 5.6 months (95% CI 3.6–9.3).11,12
Another retrospective study enrolled 55 patients pretreated with ICIs or VEGFR-TKI in first line, who received as second line the lenvatinib plus everolimus combination, or only lenvatinib. 13 Median PFS was 6.2 months (95% CI 4.8–9.4), and median OS was 12.1 months (95% CI 8.8–16) in the overall population. Median OS was 11.7 months for patients treated with the combination and 12.5 for patients treated with lenvatinib monotherapy.
Interestingly, in a recent small case series, the lenvatinib plus everolimus combination was effective among patients diagnosed with metastatic RCC (mRCC) with primary resistance to first-line target therapy or immunotherapy. 14
Lastly, the safety and effectiveness of lenvatinib plus everolimus combination were evaluated in the first-line setting of patients diagnosed with RCC non-clear cell histology.15–17
When we planned the present study, there was a lack of real-world data assessing the effectiveness and safety of this drug combination among heavily pretreated mRCC patients. For this reason, we performed a retrospective study aimed at assessing the oncological outcome and the tolerability of patients with mRCC treated with lenvatinib plus everolimus combination in the second or further line.
Objectives
The primary objective was to report the results of a multi-center experience of lenvatinib plus everolimus use in Italy in the second line and beyond, assessing clinical outcomes, in terms of objective response rate (ORR), OS, PFS, and time to treatment failure (TTF), as well as its toxicity profile. Moreover, we focused on the possible correlations between these outcome measures and both clinical and hematological parameters to establish whether they could be associated with survival among the studied population.
Design
This multicenter, observational, and retrospective study was conducted to investigate the use of lenvatinib plus everolimus combination in heavily pretreated patients with mRCC. The data for this study were collected from clinical records across 11 different Italian cancer centers.
The inclusion criteria required patients to have a diagnosis of non-resectable mRCC, a history of at least one previous line of systemic therapy, and treatment with lenvatinib plus everolimus as the second or subsequent line of treatment between 1 January 2017 and 31 March 2023 (with at least one administration of both drugs according to the ‘intention to treat’ principle), 18 years of age or older, available clinical records, and informed consent for alive patients.
Among laboratory parameters, we collected the following data: neutrophil/lymphocyte ratio (NLR), lactate dehydrogenase (LDH), and hemoglobin/red cell distribution width (Hb/RDW) ratio; Among clinical factors, we assessed sex, age, metastatic sites, International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) score, drug dose adjustments, and best treatment response.
Regarding the mRCC itself, we collected data on histology (clear or non-clear cell), date of initial diagnosis, date of nephrectomy (if applicable), number of previous lines of treatment, and what drugs were administered (i.e. ICI, TKI, or others), and their corresponding best responses.
Methods
For the exploratory purpose of this study, descriptive analyses were conducted to summarize the clinical features of the study population. Median and interquartile ranges were used for quantitative variables, while absolute and relative frequencies were used for categorical variables.
CT scans were reported by a general radiologist; however, in most cases, the response to treatment was assessed according to Response Evaluation Criteria in Solid Tumors (RECIST v1.1 18 ) by the treating oncologist. ORR was calculated by dividing the number of patients who achieve a predefined response [partial (PR) or complete response (CR)] by the total number of evaluable patients in the study. To calculate the disease control rate (DCR), the number of patients who achieved a CR, a PR, and stable disease (SD) after treatment was divided by the total number of evaluable patients who completed the treatment and were eligible for assessment. The DCR assessed at any time as the best response obtained by the patients from the start of the treatment until disease progression. Of note, we considered SD to be the ‘best response’ at radiological evaluation, regardless of its duration.
PFS was assessed by calculating the difference between the date of disease progression (or treatment failure) and the start date of treatment. TTF was evaluated by calculating the difference between the date when the patient experienced treatment failure or any other events that led to the discontinuation of the treatment and the date when the patient began the lenvatinib plus everolimus combination. OS was determined by evaluating the difference in months between the date of death and the start date of treatment. Censored patients’ data who did not experience any event (e.g. death or disease progression) by the end of the study or data collection period were plotted into the Kaplan–Meier curve to estimate their survival probabilities over time. The median follow-up was calculated according to the Inverse Kaplan–Meier method. 19
The study included categorical variables, like Eastern Cooperative Oncology Group performance status (ECOG PS), as frequency and percentage. Continuous variables, such as age, were reported with measures such as median, and range (minimum to maximum). For any instances of missing data, the number and percentage of missing values were also disclosed.
The Kaplan–Meier method was used to estimate PFS, TTF, and OS; the log-rank test was used to assess whether there were significant differences in survival times across key subgroups. In addition, other relevant clinical findings were summarized using graphical representations. Univariable Cox proportional hazard regression models were used to analyze the PFS and OS data.
Moreover, we collected data regarding adverse events (AEs) and their severity grading (from G1 to G4) according to Common Terminology Criteria for Adverse Events (CTCAE, version 5.0).
The reporting of this study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology statement. 20
Results
We enrolled 33 patients, and the main characteristics are summarized in Table 1. The median age of the population was 60 years, with a range from 38 to 77. In all, 27 patients were male (82%) and 6 were female (18%). The most common histology was clear cell (64%), followed by papillary (6.1%); sarcomatoid component was present in six patients (18%). The majority of patients underwent radical nephrectomy (85%) and presented at first diagnosis with metastatic disease (52%); 26 (79%) patients at the start of the first-line treatment were classified as ‘intermediate risk’ and two patients (6%) as ‘poor risk’ according to IMDC score.
Table 1.
Patients’ characteristics.
| Variable | Value |
|---|---|
| Histology | |
| Not available | 2 (6.1%) |
| Clear cell carcinoma | 21 (64%) |
| Clear cell carcinoma with sarcomatoid features | 6 (18%) |
| Papillary cell carcinoma | 2 (6.1%) |
| Cromophobe cell carcinoma | 1 (3.0%) |
| Unclassified RCC | 1 (3.0%) |
| IMDC score at baseline (before 1st line) | |
| Good risk | 5 (15%) |
| Intermediate risk | 26 (79%) |
| Poor risk | 2 (6.1%) |
| IMDC score before lenvatinib–everolimus therapy combination | |
| Good risk | 4 (12%) |
| Intermediate risk | 19 (58%) |
| Poor risk | 10 (30%) |
| ECOG PS before lenvatinib–everolimus treatment | |
| 0 | 14 (42%) |
| 1 | 13 (39%) |
| 2 | 6 (18%) |
| No. metastatic sites at the beginning of lenvatinib plus everolimus | |
| <3 | 12 (36.7%) |
| ⩾3 | 21 (63.3%) |
| Liver lesions at baseline | |
| No | 20 (61%) |
| Yes | 13 (39%) |
| Bone lesions at baseline | |
| No | 15 (45%) |
| Yes | 18 (55%) |
| Lymph node metastases at baseline | |
| No | 12 (36%) |
| Yes | 21 (64%) |
| Pancreas lesions at baseline | |
| No | 30 (91%) |
| Yes | 3 (9.1%) |
| Brain lesions at baseline | |
| No | 31 (94%) |
| Yes | 2 (6.1%) |
| Adrenal lesions at baseline | |
| No | 30 (91%) |
| Yes | 3 (9.1%) |
| Other sites lesions at baseline | |
| No | 22 (67%) |
| Yes | 11 (33%) |
| Lung lesions at baseline | |
| No | 8 (24%) |
| Yes | 25 (76%) |
ECOG PS, Eastern Cooperative Oncology Group performance status; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; NOS, not otherwise specified; RCC, renal cell carcinoma.
The median number of treatment lines received before levantinib plus everolimus was three, ranging from one to five. The majority of patients (42%) received at least three lines of treatment, 30% received two, 18% had four, and 3% received five. All patients except one received prior immunotherapy with ICI. All patients received at least one therapeutic line with a TKI, and four patients received prior therapy with an mTOR inhibitor in monotherapy (Supplemental Figure 1). At the beginning of lenvatinib plus everolimus, 19 patients (58%) at the start of the first-line treatment were classified as ‘intermediate risk’ and 10 patients (30%) as ‘poor risk’ according to the IMDC score.
The median values were 4410/mm3 for neutrophils (range: 3350–6020/mm3) and 1400/mm3 for lymphocytes (range: 1075–1790/mm3). The median NLR (neutrophils/lymphocytes ratio) was 3.5 (range: 2.2–5.6). Moreover, median Hb values were 12 g/dl (range 9–15 g/dl), LDH median value was 287 mU/ml (range: 75–1726 mU/ml), median corpuscular volume was 92 fl (range: 74–106 fl), and median RDW was 16% (range: 12–69%).
A total of 42% of patients had a PS of 0, 39% had a PS of 1, and 18% had a PS of 2. At lenvatinib plus everolimus combination start, most patients had lung (76%), 39% liver, 55% lymph node, 6.1% brain, and 9.1% adrenal metastases.
In all, 22 patients (67%) started with a full dose of 18 mg of lenvatinib, while dose reductions during treatment were observed in 12 cases (36%) due to AEs.
In total, 14 patients (42%) achieved PR, 6 patients (18%) obtained SD, 13 patients were primary refractory to treatment (PD 39%); no patients achieved CR, and DCR was 61% (Table 2).
Table 2.
Objective response rate.
| Variable | Value |
|---|---|
| No. lines of treatment received before lenvatinib plus everolimus | |
| 1 | 2 (6%) |
| 2 | 10 (30%) |
| 3 | 14 (42%) |
| 4 | 6 (18%) |
| 5 | 1 (3%) |
| Best overall response according to RECIST criteria | |
| PR | 14 (42%) |
| SD | 6 (18%) |
| Radiological PD | 8 (24%) |
| Clinical PD | 5 (15%) |
PD, progressive disease; PR, partial response; SD, stable disease.
Median follow-up was 10.7 months [95% CI 8.6–not reached (NR)]. Median OS was 11.2 months (95% CI 6.8–19.9) (Figure 1), median PFS was 6.7 months (95% CI 4.9–NR) (Figure 2), and median TTF was 6.7 months (95% CI 4.8–16.6) (Figure 3).
Figure 1.

Overall survival curve.
Figure 2.

Progression-free survival curve.
Figure 3.
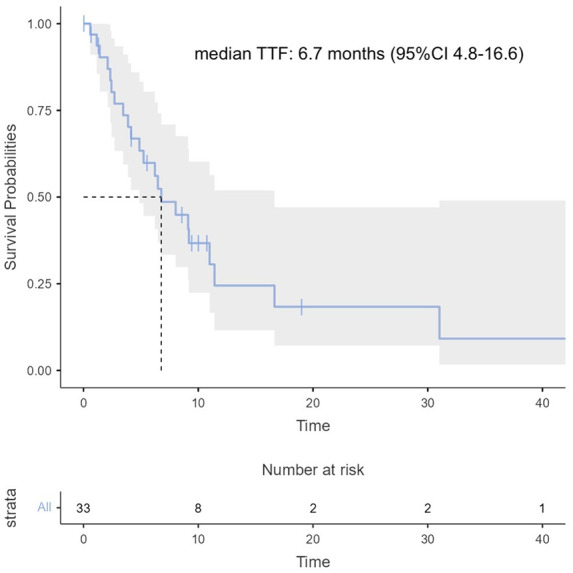
Time to treatment failure curve.
The following factors were significantly correlated with OS: presence of metastatic nodes (p = 0.043) [Figure 4(a)], NLR (p = 0.007) [Figure 4(b)], and Hb/RDW ratio (p = 0.03) [Figure 4(c)].
Figure 4.
(a) Correlation between OS and node metastases at diagnosis. (b) Correlation between NLR values and OS. (c) Correlation between Hb/RDW and OS.
Hb/RDW, red cell distribution width; NLR, neutrophils/ lymphocytes ratio; OS, overall survival.
The factors significantly associated with PFS were lung metastases (p = 0.048) and brain secondary lesions (p = 0.023) [Figure 5(a) and (b)].
Figure 5.
(a) Correlation between lung metastases and PFS. (b) Correlation between brain metastases and PFS.
PFS, progression-free survival.
Regarding the toxicity profile (Table 3), the most common grade 1 treatment-related side effect was diarrhea (8 patients, 24%), followed by fatigue (4 patients, 12%) and stomatitis (3 patients, 9%). Grade 2 toxicities were mostly fatigue (10 patients, 30%), diarrhea (6 patients, 18%), and stomatitis (6 patients, 18%). In addition, hypertension (two patients, 6%) and skin toxicity (two patients, 6%) were reported in a small number of patients. It is worth noting that no grade 4 side effects were observed.
Table 3.
Drug-related toxicities.
| Treatment-related adverse events | G1 | G2 | G3 | |||
|---|---|---|---|---|---|---|
| Diarrhea | 8 | 24% | 6 | 18% | 1 | 3% |
| Fatigue | 4 | 12% | 10 | 30% | 1 | 3% |
| Stomatitis | 3 | 9% | 6 | 18% | 1 | 3% |
| Nausea | 3 | 9% | 4 | 12% | 1 | 3% |
| Vomiting | 3 | 9% | 3 | 9% | 0 | 0% |
| Hand-foot syndrome | 3 | 9% | 0 | 0% | 0 | 0% |
| Leucopenia | 2 | 6% | 0 | 0% | 0 | 0% |
| Dysgeusia | 2 | 6% | 0 | 0% | 0 | 0% |
| Hypertension | 1 | 3% | 4 | 12% | 2 | 6% |
| Epistaxis | 1 | 3% | 1 | 3% | 0 | 0% |
| Abdominal pain | 1 | 3% | 1 | 3% | 0 | 0% |
| Hypertransaminasemia | 1 | 3% | 1 | 3% | 0 | 0% |
| Dysphonia | 1 | 3% | 1 | 3% | 0 | 0% |
| Inappetence | 1 | 3% | 0 | 0% | 0 | 0% |
| Cough | 1 | 3% | 0 | 0% | 0 | 0% |
| Skin toxicity | 1 | 3% | 0 | 0% | 2 | 6% |
| Headache | 1 | 3% | 0 | 0% | 0 | 0% |
| Constipation | 1 | 3% | 0 | 0% | 0 | 0% |
| Hypertriglyceridemia | 1 | 3% | 0 | 0% | 0 | 0% |
| Flatulence | 1 | 3% | 0 | 0% | 0 | 0% |
| Paresthesia | 1 | 3% | 0 | 0% | 0 | 0% |
| Vertigo | 0 | 0% | 1 | 3% | 0 | 0% |
| Interstitial pneumonia | 0 | 0% | 1 | 3% | 1 | 3% |
| Dysphagia | 0 | 0% | 1 | 3% | 0 | 0% |
| Edema | 0 | 0% | 1 | 3% | 0 | 0% |
| Palpitation | 0 | 0% | 1 | 3% | 0 | 0% |
| Dyspnea | 0 | 0% | 0 | 0% | 1 | 3% |
Overall, 20 patients (51%) stopped the treatment for progressive disease and 1 patient (2%) suspended the combination due to intracranial hemorrhage, which was not treatment related. No treatment was suspended due to AEs.
Discussion
The need for a personalization of cancer treatments in a context of therapy optimization according to effectiveness and toxicity acceptability has been progressively improving over the last few decades, thus leading to the design and approval of many innovative target molecules, such as TKI and ICI. Nevertheless, in most cases, patients run out of viable therapeutic options, thus relying on drugs with low effectiveness and an unfavorable balance between aimed results and treatment-related side effects.
However, the combination of lenvatinib plus everolimus could prove a valid therapeutic option for heavily pretreated patients with mRCC even though the only evidence of its effectiveness for the second line is supported by a phase Ib 9 and randomized phase II trial.11,12
As such, few data are available for this combination about its effectiveness and safety in a real-world setting for third line or beyond: to our knowledge, there are only other two studies assessing the effectiveness of lenvatinib plus everolimus as advanced lines13,21 (Table 4). Our analysis sets itself apart by further studying the correlation of both clinical and laboratory values with the outcome of the treatment combination.
Table 4.
Study outcomes comparison.
| Study reference | Study type | n | Disease setting | Prognostic distribution | ORR, n (%) | mPFS (months) | OS (months) |
|---|---|---|---|---|---|---|---|
| Motzer et al. (2015–2016) | Phase II trial | 51 | Second line | MSKCC ‘good’: 12 (24%) MSKCC ‘intermediate’: 32 (64%) MSKCC ‘poor’: 10 (20%) |
22 (43%) | 14.6 months (95% CI 5.9–20.1) [2015] and 12.8 months (95% CI 7.4–17.5) [2016] | 25.5 months (95% CI 20.8–25.5) |
| Wiele et al. (2021) | Retrospective | 55 | Fourth line: 4 (7.3%) Fifth line: 22 (40%) Sixth line: 11 (20%) Seventh line: 9 (16.4%) ⩾ Eighth line: 9 (16.4%) |
IMDC ‘good’: 6 (10.9%) IMDC ‘intermediate’: 42 (76.4%) IMDC ‘poor’: 7 (12.7%) |
12 (21.8%) | 6.2 months (95% CI 4.8–9.4) | 12.1 months (95% CI 4.3–NA) |
| Vogelzang et al. (2021) | Retrospective | 79 | Third line: 5 (6.3%) Fourth line: 18 (22.8%) Fifth line: 25 (31.6%) Sixth line: 16 (20.3%) ⩾ Seventh line: 15 (19%) |
IMDC ‘good’: 15 (19%) IMDC ‘intermediate’: 42 (53.2%) IMDC ‘poor’: 10 (12.7%) IMDC NA: 12 (15.1%) |
34 (55.7%) | 6.1 months (95% CI 4.4–9.0) | 14.8 months (95% CI 10.2–23.9) |
| Present study (2024) | Retrospective | 33 | Second line: 2 (6%) Third line: 10 (30%) Fourth line: 14 (42%) Fifth line: 6 (18%) Sixth line: 1 (3%) |
IMDC ‘good’: 4 (12%) IMDC ‘intermediate’: 19 (58%) IMDC ‘poor’: 10 (30%) |
14 (42%) | 6.7 months (95% CI 4.9–not reached) | 11.2 months (95% CI 6.8–19.9) |
IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; mPFS, median progression-free survival; MSKCC, Memorial Sloan-Kettering Cancer Center (MSKCC/Motzer); ORR, objective response rate; OS, overall survival.
Our research findings reinforce the well-established prognostic significance of NLR in mRCC, even in the context of lenvatinib plus everolimus combination for advanced cases. NLR serves as a well-defined inflammatory index and has already demonstrated its prognostic value in kidney cancer, particularly in early treatment stages.22,23
In addition, the Hb/RDW ratio, an inflammatory index primarily considered for cardiovascular and metabolic diseases,24,25 also showed promise as a prognostic indicator for kidney cancers, especially in the specified setting. Although it is not extensively studied as a prognostic index, our study unveiled its potential significance in predicting outcomes for kidney cancer patients.
Interestingly, a recent exploratory retrospective analysis of the phase II study 26 found that five biomarkers were associated with either PFS or OS and they could potentially predict patients’ benefit from the lenvatinib plus everolimus combination. The lab values assessed in our study were easier to collect from a clinical standpoint and were found to be correlated with OS for both NLR ⩾ 3 and Hb/RDW ratio ⩾ 0.7.
Moreover, our study revealed another significant parameter correlated with OS, namely the presence of lymph node metastases. This well-known unfavorable prognostic factor has been consistently associated with poorer outcomes in renal cell carcinoma patients. 27 Regarding factors related to a worse PFS, our findings corroborate the negative prognostic impact of both lung and brain metastases. 28 These results emphasize the importance of considering these metastatic sites when assessing the potential disease progression in patients.
Interestingly, our patient population had similar demographical characteristics to those reported in other clinical trials and retrospective analysis9–11 (Table 4) even though our study had a more limited number of patients.
Even though our study population comprised a higher proportion of ‘poor risk’ patients compared to other studies, ORR, mPFS, and mOS remained consistent with previous retrospective analyses that focused on heavily pretreated individuals. Specifically, the ORR ranged from 21% to 55%, mPFS ranged from 6.1 to 6.7 months, and mOS ranged from 11.2 to 12.1 months.
Regarding the toxicity profile, Vogelzang et al. reported that 37% of patients experienced a dose reduction in lenvatinib, and 11% had a dose reduction in everolimus. Our study aligns with these findings, as we observed dose reductions of either lenvatinib or everolimus during treatment in 12 cases (36%). Lastly, despite our results supporting a relatively safe toxicity profile of the drug combination, a network meta-analysis 29 pointed out that lenvatinib plus everolimus is associated with more renal severe side effects than other VEGF-TKI.
Several limitations warrant consideration in our investigation. First, it is important to acknowledge that our study is retrospective in nature, which may introduce inherent biases and constraints in data collection and interpretation. Second, the study’s sample size is relatively small, which has restricted the feasibility of conducting a robust multivariable analysis.
Moreover, a relatively high percentage of patients with ECOG PS equal to 0 in this setting reflects a possible positive selection (i.e. patients needed to be able to tolerate a combination of TKI + mTOR in an advanced setting).
Conclusion
In summary, our research findings have validated the efficacy and tolerability of the lenvatinib and everolimus combination as a viable option for heavily pretreated mRCC patients. It is encouraging that future research, encompassing a larger and more diverse patient dataset, could enhance the statistical significance of the correlations we observed.
To further support the role of this combination in the advanced disease setting, randomized prospective studies are essential. These trials will provide more robust evidence and further validate the potential benefits of using lenvatinib and everolimus in treating advanced mRCC.
Supplemental Material
Supplemental material, sj-docx-1-tau-10.1177_17562872241244574 for Assessing the effectiveness and safety of lenvatinib and everolimus in advanced renal cell carcinoma: insights from the RELIEVE study’s analysis of heavily pretreated patients by Sebastiano Buti, Alessandro Olivari, Cristina Masini, Davide Bimbatti, Donata Sartori, Paola Ermacora, Carlo Cattrini, Maria Giuseppa Vitale, Ernesto Rossi, Claudia Mucciarini, Mimma Rizzo, Michele Sisani, Matteo Santoni, Giandomenico Roviello, Veronica Mollica, Vincenza Conteduca, Francesco Grillone, Marika Cinausero, Giuseppe Prati, Francesco Atzori, Marco Stellato, Francesco Massari and Melissa Bersanelli in Therapeutic Advances in Urology
Supplemental material, sj-pdf-1-tau-10.1177_17562872241244574 for Assessing the effectiveness and safety of lenvatinib and everolimus in advanced renal cell carcinoma: insights from the RELIEVE study’s analysis of heavily pretreated patients by Sebastiano Buti, Alessandro Olivari, Cristina Masini, Davide Bimbatti, Donata Sartori, Paola Ermacora, Carlo Cattrini, Maria Giuseppa Vitale, Ernesto Rossi, Claudia Mucciarini, Mimma Rizzo, Michele Sisani, Matteo Santoni, Giandomenico Roviello, Veronica Mollica, Vincenza Conteduca, Francesco Grillone, Marika Cinausero, Giuseppe Prati, Francesco Atzori, Marco Stellato, Francesco Massari and Melissa Bersanelli in Therapeutic Advances in Urology
Acknowledgments
None.
Footnotes
ORCID iDs: Sebastiano Buti  https://orcid.org/0000-0003-0876-0226
https://orcid.org/0000-0003-0876-0226
Alessandro Olivari  https://orcid.org/0009-0000-2138-905X
https://orcid.org/0009-0000-2138-905X
Cristina Masini  https://orcid.org/0000-0002-9926-8458
https://orcid.org/0000-0002-9926-8458
Carlo Cattrini  https://orcid.org/0000-0003-4785-9480
https://orcid.org/0000-0003-4785-9480
Mimma Rizzo  https://orcid.org/0000-0001-7743-741X
https://orcid.org/0000-0001-7743-741X
Francesco Grillone  https://orcid.org/0009-0009-6010-7604
https://orcid.org/0009-0009-6010-7604
Melissa Bersanelli  https://orcid.org/0000-0002-6527-6281
https://orcid.org/0000-0002-6527-6281
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Sebastiano Buti, Department of Medicine and Surgery, University Hospital of Parma, Parma, Italy Oncology Unit, University Hospital of Parma, Parma, Italy.
Alessandro Olivari, Department of Medicine and Surgery, University Hospital of Parma, 14 Gramsci Street, Parma, 43125, Italy Oncology Unit, University Hospital of Parma, Parma, Italy.
Cristina Masini, Oncology Unit, Clinical Cancer Centre AUSL-IRCCS di Reggio, Emilia, Italy.
Davide Bimbatti, Oncology Unit 1, Department of Oncology, Istituto Oncologico Veneto IOV – IRCCS, Padova, Italy.
Donata Sartori, Oncologia Dolo-Mirano, AULSS3 Veneziana, Venezia, Italy.
Paola Ermacora, Department of Oncology, ASUFC Santa Maria Della Misericordia, Udine, Italy.
Carlo Cattrini, Azienda Ospedaliero-Universitaria ‘Maggiore della Carità’ – Università del Piemonte Orientale, Novara, Italy.
Maria Giuseppa Vitale, Department of Oncology and Hematology, University Hospital of Modena, Modena, Italy.
Ernesto Rossi, Medical Oncology, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy.
Claudia Mucciarini, Medical Oncology Unit, AUSL Modena, Ramazzini Hospital, Carpi, Italy.
Mimma Rizzo, Azienda Ospedaliera Universitaria Consorziale, Policlinico di Bari, Bari, Italy.
Michele Sisani, U.O.C. Oncologia Medica, USL Toscana sudest, Arezzo, Italy.
Matteo Santoni, UOC Oncologia, Ospedale Generale Provinciale di Macerata, Macerata, Italy.
Giandomenico Roviello, Department of Health Sciences, Section of Clinical Pharmacology and Oncology, University of Florence, Firenze, Italy.
Veronica Mollica, Medical Oncology, IRCCS - Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy.
Vincenza Conteduca, Department of Medical and Surgical Sciences, Unit of Medical Oncology and Biomolecular Therapy, University of Foggia, Policlinico Riuniti, Foggia, Italy.
Francesco Grillone, Medical Oncology Unit, Azienda Ospedaliera Universitaria “Mater-Domini” Policlinico di Catanzaro, Catanzaro, Italy.
Marika Cinausero, Department of Medicine (DAME), University of Udine, Udine, Italy.
Giuseppe Prati, Azienda Unità Sanitaria Locale – IRCCS di Reggio Emilia, Reggio Emilia, Italy.
Francesco Atzori, Unità di Oncologia Medica, Azienda Ospedaliero Universitaria di Cagliari, Cagliari, Italy.
Marco Stellato, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Francesco Massari, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy Medical Oncology, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy.
Melissa Bersanelli, University Hospital of Parma, Parma, Italy.
Declarations
Ethics approval and consent to participate: The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the University Hospital of Parma, protocol code 634/2022/OSS/AOUPR-‘RELIEVE’ n 0000732 date of approval: 19th October 2022. Due to the utilization of preexisting data and records in this project, the study information was examined in a way that prevented direct identification of the research participants. Informed consent for each living patient at the time of study conduction. Informed consent for participation has been waived by the Institutional Review Board of the University Hospital of Parma only for deceased patients. This decision is in accordance with ethical considerations pertaining to retrospective analyses involving deceased subjects at the time of study approval.
Consent for publication: Not applicable.
Author contributions: Sebastiano Buti: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Supervision; Validation; Writing – review & editing.
Alessandro Olivari: Data curation; Investigation; Validation; Visualization; Writing – original draft.
Cristina Masini: Data curation; Validation.
Davide Bimbatti: Data curation; Validation.
Donata Sartori: Data curation; Validation.
Paola Ermacora: Data curation; Validation.
Carlo Cattrini: Data curation; Validation.
Maria Giuseppa Vitale: Data curation; Validation.
Ernesto Rossi: Data curation; Validation.
Claudia Mucciarini: Data curation.
Mimma Rizzo: Data curation; Validation.
Michele Sisani: Data curation; Validation.
Matteo Santoni: Data curation; Validation.
Giandomenico Roviello: Data curation; Validation.
Veronica Mollica: Data curation; Validation.
Vincenza Conteduca: Data curation; Validation.
Francesco Grillone: Data curation; Validation.
Marika Cinausero: Data curation; Validation.
Giuseppe Prati: Data curation; Validation.
Francesco Atzori: Data curation; Validation.
Marco Stellato: Data curation; Validation.
Francesco Massari: Data curation; Validation.
Melissa Bersanelli: Data curation; Validation.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
V.C. has served as a consultant/advisory board member for Janssen, Astellas, Merck, AstraZeneca, Amgen, and Bayer and has received speaker honoraria or travel support from Astellas, Janssen, Ipsen, Bayer, and Sanofi. E.R. had a role as consultant for Bristol Meyers Squibb, MSD, Novartis, Pierre Fabre, Immunocore, and Pfizer.
Availability of data and materials: The datasets generated and analyzed during the current study are available upon specific and well-justified requests from qualified researchers or scientists. All the data provided has been appropriately anonymized to protect patient confidentiality. The data is stored in an Excel spreadsheet format. Requests for data access should be directed to Dr Olivari Alessandro at alessandro.olivari@unipr.it. Requests will be evaluated on a case-by-case basis to ensure compliance with ethical standards and privacy regulations.
References
- 1. Associazione Italiana di Oncologia Medica, 2022 – “I numeri del cancro in Italia” , https://www.registri-tumori.it (accessed 6 October 2023).
- 2. Tannir NM, Pal SK, Atkins MB. Second-line treatment landscape for renal cell carcinoma: a comprehensive review. Oncologist 2018; 23: 540–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shah AY, Kotecha RR, Lemke EA, et al. Outcomes of patients with metastatic clear-cell renal cell carcinoma treated with second-line VEGFR-TKI after first-line immune checkpoint inhibitors. Eur J Cancer 2019; 114: 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Campbell MT, Bilen MA, Shah AY, et al. Cabozantinib for the treatment of patients with metastatic non-clear cell renal cell carcinoma: a retrospective analysis. Eur J Cancer 2018; 104: 188–194. [DOI] [PubMed] [Google Scholar]
- 5. Deuker M, Chun FKH, Karakiewicz PI. Second-line tyrosine kinase inhibitor-therapy after immunotherapy-failure. Curr Opin Support Palliat Care 2020; 14: 276–285. [DOI] [PubMed] [Google Scholar]
- 6. Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2019; 30: 706–720. [DOI] [PubMed] [Google Scholar]
- 7. Motzer RJ, Memorial Sloan Kettering Cancer Center, Jonasch E, et al. NCCN Guidelines Version 1.2024 kidney cancer continue NCCN Guidelines Panel Disclosures, https://www.nccn.org/home/member-(2023, accessed 6 October 2023).
- 8. Leonetti A, Leonardi F, Bersanelli M, et al. Clinical use of lenvatinib in combination with everolimus for the treatment of advanced renal cell carcinoma. Ther Clin Risk Manag 2017; 13: 799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matsubara N, Naito Y, Nakano K, et al. Lenvatinib in combination with everolimus in patients with advanced or metastatic renal cell carcinoma: a phase 1 study. Int J Urol 2018; 25: 922–928. [DOI] [PubMed] [Google Scholar]
- 10. Molina AM, Hutson TE, Larkin J, et al. A phase 1b clinical trial of the multi-targeted tyrosine kinase inhibitor lenvatinib (E7080) in combination with everolimus for treatment of metastatic renal cell carcinoma (RCC). Cancer Chemother Pharmacol 2014; 73: 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Motzer RJ, Hutson TE, Glen H, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol 2015; 16: 1473–1482. [DOI] [PubMed] [Google Scholar]
- 12. Motzer RJ, Hutson TE, Ren M, et al. Independent assessment of lenvatinib plus everolimus in patients with metastatic renal cell carcinoma. Lancet Oncol 2016; 17: e4–e5. [DOI] [PubMed] [Google Scholar]
- 13. Wiele AJ, Bathala TK, Hahn AW, et al. Lenvatinib with or without everolimus in patients with metastatic renal cell carcinoma after immune checkpoint inhibitors and vascular endothelial growth factor receptor-tyrosine kinase inhibitor therapies. Oncologist 2021; 26: 476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hamieh L, Beck RL, Le VH, et al. The efficacy of lenvatinib plus everolimus in patients with metastatic renal cell carcinoma exhibiting primary resistance to front-line targeted therapy or immunotherapy. Clin Genitourin Cancer 2020; 18: 252–257.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hutson TE, Michaelson MD, Kuzel TM, et al. A single-arm, multicenter, phase 2 study of lenvatinib plus everolimus in patients with advanced non-clear cell renal cell carcinoma. Eur Urol 2021; 80: 162–170. [DOI] [PubMed] [Google Scholar]
- 16. Zielli T, Gnetti L, Buti S. Activity of lenvatinib plus everolimus combination in a heavily pretreated patient with papillary renal cell carcinoma: a case report. Tumori 2020; 106: NP79–NP83. [DOI] [PubMed] [Google Scholar]
- 17. Schwartz C, Pfanzelter N, Kuzel TM. The efficacy of lenvatinib and everolimus in chromophobe-type non–clear-cell renal cell carcinoma: a case report and literature review. Clin Genitourin Cancer 2017; 15: e903–e906. [DOI] [PubMed] [Google Scholar]
- 18. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 19. Shuster JJ. Median follow-up in clinical trials. J Clin Oncol 1991; 9: 191–192. [DOI] [PubMed] [Google Scholar]
- 20. STROBE Initiative. (2023). STROBE checklist v4.0 for cross-sectional studies. Retrieved from https://www.strobe-statement.org/checklists/ (accessed 2 February 2024).
- 21. Vogelzang NJ, Monnette AM, Wang Y, et al. Real-world clinical effectiveness of lenvatinib/everolimus in a heavily pretreated advanced/metastatic renal cell carcinoma population in the US Community Oncology Setting. Clin Genitourin Cancer 2021; 19: 531–539. [DOI] [PubMed] [Google Scholar]
- 22. Hu K, Lou L, Ye J, et al. Prognostic role of the neutrophil-lymphocyte ratio in renal cell carcinoma: a meta-analysis. BMJ Open 2015; 5: e006404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen X, Meng F, Jiang R. Neutrophil-to-lymphocyte ratio as a prognostic biomarker for patients with metastatic renal cell carcinoma treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Front Oncol; 11. Epub ahead of print 11 November 2021. DOI: 10.3389/fonc.2021.746976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hong J, Hu X, Liu W, et al. Impact of red cell distribution width and red cell distribution width/albumin ratio on all-cause mortality in patients with type 2 diabetes and foot ulcers: a retrospective cohort study. Cardiovasc Diabetol 2022; 21: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rahamim E, Zwas DR, Keren A, et al. The ratio of hemoglobin to red cell distribution width: a strong predictor of clinical outcome in patients with heart Failure. J Clin Med 2022; 11: 886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee CH, Motzer RJ, Glen H, et al. Correlative serum biomarker analyses in the phase 2 trial of lenvatinib-plus-everolimus in patients with metastatic renal cell carcinoma. Br J Cancer 2021; 124: 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kroeger N, Pantuck AJ, Wells JC, et al. Characterizing the impact of lymph node metastases on the survival outcome for metastatic renal cell carcinoma patients treated with targeted therapies. Eur Urol 2015; 68: 506–515. [DOI] [PubMed] [Google Scholar]
- 28. Wang J, Zhang N, Giordano SH, et al. Impact of site-specific metastases and clinical complications on outcomes of patients with metastatic renal cell carcinoma (mRCC). J Clin Oncol 2022; 40: e16527–e16527. [Google Scholar]
- 29. Xie R, Wu J, Shang B, et al. Optimizing targeted drug selection in combination therapy for patients with advanced or metastatic renal cell carcinoma: a systematic review and network meta-analysis of safety. Cancer Med. Epub ahead of print 1 March 2022. DOI: 10.1002/cam4.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tau-10.1177_17562872241244574 for Assessing the effectiveness and safety of lenvatinib and everolimus in advanced renal cell carcinoma: insights from the RELIEVE study’s analysis of heavily pretreated patients by Sebastiano Buti, Alessandro Olivari, Cristina Masini, Davide Bimbatti, Donata Sartori, Paola Ermacora, Carlo Cattrini, Maria Giuseppa Vitale, Ernesto Rossi, Claudia Mucciarini, Mimma Rizzo, Michele Sisani, Matteo Santoni, Giandomenico Roviello, Veronica Mollica, Vincenza Conteduca, Francesco Grillone, Marika Cinausero, Giuseppe Prati, Francesco Atzori, Marco Stellato, Francesco Massari and Melissa Bersanelli in Therapeutic Advances in Urology
Supplemental material, sj-pdf-1-tau-10.1177_17562872241244574 for Assessing the effectiveness and safety of lenvatinib and everolimus in advanced renal cell carcinoma: insights from the RELIEVE study’s analysis of heavily pretreated patients by Sebastiano Buti, Alessandro Olivari, Cristina Masini, Davide Bimbatti, Donata Sartori, Paola Ermacora, Carlo Cattrini, Maria Giuseppa Vitale, Ernesto Rossi, Claudia Mucciarini, Mimma Rizzo, Michele Sisani, Matteo Santoni, Giandomenico Roviello, Veronica Mollica, Vincenza Conteduca, Francesco Grillone, Marika Cinausero, Giuseppe Prati, Francesco Atzori, Marco Stellato, Francesco Massari and Melissa Bersanelli in Therapeutic Advances in Urology




