Abstract
Objective
We explored whether changes in clinical parameters and inflammatory markers can facilitate early identification of positive blood culture in adult patients with COVID-19 and clinically suspected bloodstream infection (BSI).
Methods
This single-center retrospective study enrolled 20 adult patients with COVID-19 admitted to the intensive care unit who underwent blood culture for clinically suspected BSI (February 2020–November 2021). We divided patients into positive (Pos) and negative blood culture groups. Clinical parameters and inflammatory markers were obtained from medical records between blood culture collection and the first positive or negative result and compared between groups on different days.
Results
Patients in the positive culture group had significantly older age and higher D-dimer, immunoglobulin 6 (IL-6), and Sequential Organ Failure Assessment score as well as lower albumin (ALB). The area under the receiver operating characteristic curve (AUC) was 0.865 for IL-6, D-dimer and ALB on the first day after blood culture collection; the AUC was 0.979 for IL-6, IL-10, D-dimer, and C-reactive protein on the second day after blood culture collection.
Conclusion
Changes in clinical parameters and inflammatory markers after blood culture collection may facilitate early identification of positive culture in adult patients with COVID-19 and clinically suspected BSI.
Keywords: COVID-19, SARS-CoV-2 infection, bloodstream infection, early identification, positive blood culture, clinical parameter, inflammatory marker
Introduction
Coronavirus disease 2019 (COVID-19) has evolved far beyond expectations, putting unprecedented strain on the global public health care systems as severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) continues to mutate. Data from the Chinese Center for Disease Control and Prevention showed that nearly 20% of patients with COVID-19 develop severe and critical illness, which overwhelms the capacity and medical staff of intensive care units (ICUs) and causes mortality in critically ill cases of close to 50%. 1 The incidence of bacteremia in patients with COVID-19 is reportedly 1.6% to 5.6%,2,3 which is significantly higher in severely and critically ill patients owing to the barrier destruction caused by invasive interventions. Among these, intravascular catheters are frequently used in critically ill patients and are a significant contributor to the occurrence of catheter-associated bloodstream infection (BSI).4,5 In critically ill patients with COVID-19 or those requiring invasive mechanical ventilation for more than 48 hours, the incidence of BSI ranges from 14.9% to 30%.6,7 Additionally, an association between immune-suppressive treatment (e.g., tocilizumab) and a higher incidence of BSI has been found in several studies. 7 Furthermore, cumbersome personal protective equipment causes challenges to the usual patient care, which can further increase the risk of BSI. In addition, medical staff without adequate professional ICU training who enter ICU to assist in the management of patients with COVID-19 poses a further risk for the occurrence of BSI. 8
BSI is an independent predictor of poor outcomes and mortality in patients with severe influenza. A similar impact on clinical outcomes can be predicted in adult patients with COVID-19.9,10 Blood culture is essential for diagnosing BSI and guiding appropriate antibiotic use; however, in clinical practice, a long time is usually needed (more than 72 hours) before results can be obtained. Even a short delay in detection pathogen can lead to catastrophic consequences for patients with sepsis. 11 At present, clinical methods are lacking for the early identification of BSI so as to initiate appropriate treatment in a timely manner and improve clinical outcomes in adult patients with COVID-19.
In the present study, we assessed clinical parameters and inflammatory markers in patients with positive culture, between blood culture collection and the first positive result, in comparison with patients who had a negative culture. We aimed to explore whether changes in clinical parameters and inflammatory markers could be used for the early identification of positive blood culture among adult patients with COVID-19 and clinically suspected BSI. The findings of this study will provide evidence for the early diagnosis of BSI and advance initiation of corresponding treatment in adult patients with COVID-19 and clinically suspected BSI.
Methods
Study design
In this single-center retrospective study, we enrolled adult patients with COVID-19 who were admitted to the ICU in our COVID-19 treatment center and underwent blood culture owing to clinically suspected BSI between February 2020 and November 2021. Patients were divided into a positive blood culture group and negative blood culture group, according to their blood culture results. Enrolled adult patients with COVID-19 were managed by the same group of experienced intensivists and underwent similar treatment regimens according to the Diagnosis and Treatment of New Coronavirus Pneumonia (fifth, sixth and seventh editions).12–14
This study was conducted in accordance with the Declaration of Helsinki of 1975 as revised in 2013. The study protocol was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Harbin Medical University on 28 March 2022 (IRB: 2022XS14-02). Owing to the nature of this retrospective study, written informed consent in this study was waived. All patient details were de-identified, and other members of the research team were not privy to the personal information of enrolled patients beyond that required for this study. The reporting of this study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. 15
Study population
The inclusion criteria were as follows: (1) ICU admission in the COVID-19 Treatment Center of Heilongjiang Province between February 2020 and November 2021; (2) age ≥18 years; (3) patients with confirmed COVID-19; and (4) blood culture collection owing to clinically suspected BSI. The exclusion criteria were: (1) AIDS; (2) malignant tumors and leukemia patients receiving radiotherapy and/or chemotherapy within the past 6 months; (3) organ transplant within the past 6 months; (4) immunotherapy within the past 6 months; (5) chronic organ failure; (6) autoimmune disorder; (7) pregnant or breastfeeding women; (8) patients expected to die within 72 hours; and (9) incomplete medical records.
Diagnosis and classification of COVID-19
All enrolled adult patients with COVID-19 were confirmed via detection of SARS-CoV-2 nucleic acid in oropharyngeal swab, nasopharyngeal swab, or lower respiratory tract specimens. Patients were then classified according to the Diagnosis and Treatment of New Coronavirus Pneumonia (fifth, sixth and seventh editions).12–14
Blood culture collection and determination of bloodstream infection (BSI)
In our study, blood culture collection was based on clinical manifestations of BSI (e.g., high fever, chills) in adult patients with COVID-19, rather than a routine test. Standardized aseptic sampling procedures were adopted for blood culture collection to minimize the risk of contamination. Multiple blood cultures were carried out in patients with clinically suspected BSI to reduce the possibility of false negatives. Blood culture was collected from two sites at each collection (one central vein sample through a central venous catheter and one peripheral vein sample or two peripheral vein samples). Each collected specimen was divided among four 10-mL blood culture bottles. If the same pathogen was cultivated from two sites, the patient was considered to have BSI. If the pathogens cultivated from two sites were different or the culture result for one site was negative, we also considered high-risk factors, clinical symptoms, physical examination, laboratory parameters, and radiographic imaging so as to make a comprehensive judgment and reduce the possibility of false positives. A full panel of experts was jointly responsible for diagnosing BSI and adjusting each patient’s treatment regimen.
Data collection and measurement of inflammatory markers
Dedicated personnel on our research team collected demographic data at admission, as well as clinical parameters, including the severity of COVID-19, white blood cell count (WBC), neutrophil proportion (NEUT%), lymphocyte count (LYMPH), lymphocyte percentage (LYMPH%), platelet (PLT), C-reaction protein (CRP), procalcitonin (PCT), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), albumin (ALB), serum creatinine (SCr), D-dimer, fibrinogen (FIB), SOFA scores, and inflammatory markers including immunoglobulin 2 (IL-2), IL-4, IL-6, IL-10, tumor necrosis factor alpha (TNF-α), and interferon gamma (IFN-γ). These data were obtained from patients’ medical records between blood culture collection and the first positive or negative result and were then compared between the two groups on different days.
SOFA score was calculated based on clinical parameters within 24 hours after ICU admission. Serum concentrations of inflammatory markers (IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ) were quantitatively measured using cytometric bead array.
Statistical analysis
We used IBM SPSS 24.0 (IBM Corp., Armonk, NY, USA) for the statistical analyses. Continuous data with a normal distribution are presented as mean ± standard deviation, and data with a non-normal distribution are expressed as median (P25, P75). Measurement data are expressed as frequency (percentage). An independent samples t-test was used for inter-group comparisons of continuous data with a normal distribution, and the Mann–Whitney U test was applied for inter-group comparisons of data with a non-normal distribution. Fisher’s exact test was applied for comparing measurement data between the two groups. The analysis of variance and Tamhane’s T2 method were used for three-group and inter-group comparisons of continuous data with a normal distribution, and the Kruskal–Wallis method was applied for three-group and inter-group comparisons of data with a non-normal distribution, respectively. The receiver operating characteristic (ROC) curve was analyzed for predictive variables, and the sensitivity and specificity of the ROC curve were calculated. p-values <0.05 were considered to indicate statistical significance.
Results
Comparison of demographic and clinical baseline data
We retrospectively included 20 adult patients with COVID-19 who were admitted to the ICU owing to clinically suspected BSI in our center and underwent blood culture between February 2020 and November 2021. Patients were divided into a positive and negative blood culture group. Male patients accounted for 75% of the positive-culture group, and the mean age of patients with a positive blood culture was 70.50±8.47 years. In comparison with the negative-culture group, patients in the positive blood culture group exhibited significantly older age and higher D-dimer, IL-6, and SOFA score, as well as lower ALB (p = 0.001, 0.031, 0.016, 0.031, and 0.004, respectively (Table 1). No significant difference was observed for the remaining demographic and clinical baseline data.
Table 1.
Comparison of demographic and clinical baseline data between the two groups.
| Positive blood culture group | Negative blood culture group | Fisher/t/z | p | |
|---|---|---|---|---|
| Sex (male/female) | 9/3 | 3/5 | – | 0.167 |
| Hypertension (yes/no) | 7/5 | 3/5 | – | 0.650 |
| Diabetes (yes/no) | 4/8 | 3/5 | – | >0.99 |
| Cerebral infarction (yes/no) | 4/8 | 1/7 | – | 0.603 |
| Severity of COVID-19 (severe/other) | 7/5 | 6/2 | – | 0.642 |
| Age (years) | 70.50 ± 8.47 | 53.13 ± 12.09 | 3.79 | 0.001 |
| Number of comorbidities | 2.16 ± 1.59 | 1.50 ± 1.85 | 0.86 | 0.400 |
| WBC (×109/L) | 8.39 ± 4.05 | 9.16 ± 5.93 | −0.35 | 0.734 |
| NEUT% (%) | 83.45 ± 7.85 | 84.10 ± 6.05 | −0.20 | 0.845 |
| LYMPH (×109/L) | 0.55 (0.37, 0.86) | 0.60 (0.40, 0.96) | −0.12 | 0.910 |
| LYMPH% (%) | 9.19 ± 5.44 | 8.54 ± 3.64 | 0.30 | 0.770 |
| PLT (×109/L) | 168.67 ± 76.01 | 197.38 ± 79.59 | −0.81 | 0.427 |
| CRP (mg/L) | 165.25 ± 68.96 | 95.49 ± 81.84 | 2.06 | 0.054 |
| PCT (ng/mL) | 0.24 (0.11, 2.58) | 0.49 (0.21, 1.57) | −0.84 | 0.442 |
| ALT (U/L) | 44.53 (34.19, 77.63) | 32.84 (23.27, 46.15) | −1.62 | 0.115 |
| AST (U/L) | 58.15 ± 35.24 | 44.98 ± 19.82 | 0.96 | 0.352 |
| TBIL (μmol/L) | 19.31 ± 8.91 | 15.85 ± 6.87 | 0.93 | 0.367 |
| ALB (g/L) | 30.69 ± 3.84 | 36.22 ± 3.44 | −3.28 | 0.004 |
| SCr (μmol/L) | 63.11 (44.83 88.28) | 63.87 (28.64, 78.53) | −0.62 | 0.571 |
| D-dimer (μg/L) | 3.41 (2.39, 4.57) | 1.32 (1.05, 3.22) | −2.12 | 0.031 |
| FIB (g/L) | 6.02 ± 1.35 | 5.44 ± 0.94 | 1.04 | 0.311 |
| IL-2 (pg/mL) | 1.37 (0.83, 1.42) | 0.92 (0.78, 1.25) | −1.35 | 0.181 |
| IL-4 (pg/mL) | 0.82 (0.75, 1.06) | 0.92 (0.62, 1.18) | −0.23 | 0.851 |
| IL-6 (pg/mL) | 179.50 (98.14, 543.82) | 43.02 (17.81, 82.11) | −2.39 | 0.016 |
| IL-10 (pg/mL) | 4.53 (2.23, 7.40) | 5.75 (3.93, 9.57) | −1.04 | 0.305 |
| TNF-α (pg/mL) | 1.36 ± 0.39 | 1.01 ± 0.40 | 1.92 | 0.071 |
| IFN-γ (pg/mL) | 1.35 (0.92, 1.97) | 2.00 (1.04, 3.44) | −0.97 | 0.343 |
| SOFA score | 5.33 ± 1.23 | 3.88 ± 1.55 | 2.34 | 0.031 |
Values are n (%), mean ± standard deviation (SD), or median (P25, P75).
WBC, white blood cell count; NEUT%, neutrophil proportion; LYMPH, lymphocyte count; LYMPH%, lymphocyte percentage; PLT, platelet; CRP, C-reaction protein; PCT, procalcitonin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBIL, total bilirubin; ALB, albumin; SCr, serum creatinine; FIB, fibrinogen; SOFA, Sequential Organ Failure Assessment; IL, immunoglobulin; TNF, tumor necrosis factor; IFN, interferon.
Comparison of clinical parameters and inflammatory markers after blood culture collection
As shown in Table 2, significantly higher CRP, PCT, D-dimer, and IL-6, as well as lower LYMPH% and ALB were observed in patients with positive culture on the first day after blood culture collection (p = 0.014, 0.016, 0.020, 0.025, 0.005, and 0.033, respectively). As shown in Table 3, we found significantly higher CRP, D-dimer, IL-6, and IL-10 in patients with positive culture on the second day after blood culture collection (p = 0.029, 0.010, 0.047, and 0.007, respectively).
Table 2.
Comparison of clinical parameters and inflammatory markers on the first day after blood culture collection.
| Positive blood culture group | Negative blood culture group | t/z | p | |
|---|---|---|---|---|
| WBC (×109/L) | 8.39 ± 2.67 | 6.95 ± 2.58 | 1.21 | 0.244 |
| NEUT% (%) | 81.54 ± 7.14 | 77.84 ± 12.26 | 0.86 | 0.403 |
| LYMPH (×109/L) | 0.77 ± 0.38 | 1.39 ± 0.77 | −2.10 | 0.064 |
| LYMPH% (%) | 9.38 ± 4.34 | 16.46 ± 5.49 | −3.22 | 0.005 |
| PLT (×109/L) | 160 (138.75, 266.25) | 176 (159, 234.25) | −0.70 | 0.521 |
| CRP (mg/L) | 158.62 ± 79.30 | 74.60 ± 43.76 | 2.718 | 0.014 |
| PCT (ng/mL) | 2.90 (1.24, 30.49) | 0.35 (0.06, 1.28) | −2.39 | 0.016 |
| ALT (U/L) | 47.11 ± 29.98 | 40.27 ± 14.88 | 0.68 | 0.509 |
| AST (U/L) | 54.51 ± 25.31 | 47.68 ± 18.83 | 0.65 | 0.524 |
| TBIL (μmol/L) | 19.38 ± 9.28 | 16.74 ± 10.79 | 0.58 | 0.566 |
| ALB (g/L) | 29.74 ± 4.29 | 33.61 ± 2.41 | −2.30 | 0.033 |
| SCr (μmol/L) | 62.17 (48.55, 100.38) | 57.13 (41.76, 85.62) | −1.16 | 0.270 |
| D-dimer (μg/L) | 4.13 (1.95, 12.11) | 1.15 (1.02, 1.71) | −2.32 | 0.020 |
| FIB (g/L) | 5.09 ± 1.79 | 5.30 ± 1.26 | −0.29 | 0.777 |
| IL-2 (pg/mL) | 1.20 ± 0.54 | 0.90 ± 0.53 | 1.24 | 0.232 |
| IL-4 (pg/mL) | 1.02 ± 0.33 | 0.78 ± 0.38 | 1.49 | 0.153 |
| IL-6 (pg/mL) | 131.74 (76.39, 375.13) | 45.46 (18.09, 64.40) | −2.24 | 0.025 |
| IL-10 (pg/mL) | 5.01 (2.85, 13.45) | 2.58 (1.71, 5.43) | −1.70 | 0.098 |
| TNF-α (pg/mL) | 1.41 ± 0.62 | 1.21 ± 0.59 | 0.72 | 0.483 |
| IFN-γ (pg/mL) | 1.31 (0.71, 2.81) | 2.00 (0.56, 2.88) | −0.31 | 0.792 |
Values are mean ± standard deviation, or median (P25, P75).
WBC, white blood cell count; NEUT%, neutrophil proportion; LYMPH, lymphocyte count; LYMPH%, lymphocyte percentage; PLT, platelet; CRP, C-reaction protein; PCT, procalcitonin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBIL, total bilirubin; ALB, albumin; SCr, serum creatinine; FIB, fibrinogen; IL, immunoglobulin; TNF, tumor necrosis factor; IFN, interferon.
Table 3.
Comparison of clinical parameters and inflammatory markers on the second day after blood culture collection.
| Positive blood culture group | Negative blood culture group | t/z | p | |
|---|---|---|---|---|
| WBC (×109/L) | 8.69 ± 3.78 | 8.28 ± 3.34 | 0.25 | 0.806 |
| NEUT% (%) | 82.93 ± 9.67 | 76.31 ± 9.18 | 1.53 | 0.144 |
| LYMPH (×109/L) | 0.61 (0.32, 0.98) | 0.78 (0.70, 1.35) | −1.43 | 0.157 |
| LYMPH% (%) | 8.57 ± 5.53 | 13.35 ± 5.89 | −1.85 | 0.081 |
| PLT (×109/L) | 157.58 ± 71.81 | 198.25 ± 54.68 | −1.36 | 0.192 |
| CRP (mg/L) | 201.49 ± 56.95 | 107.44 ± 119.66 | 2.37 | 0.029 |
| PCT (ng/mL) | 1.77 (0.40, 0.52) | 0.31 (0.18, 1.86) | −1.62 | 0.115 |
| ALT (U/L) | 44.65 ± 31.31 | 38.56 ± 15.68 | 0.51 | 0.619 |
| AST (U/L) | 49.17 ± 28.94 | 40.90 ± 16.17 | 0.73 | 0.474 |
| TBIL (μmol/L) | 19.08 (13.75, 33.70) | 13.05 (8.50, 23.72) | −1.47 | 0.157 |
| ALB (g/L) | 29.80 ± 3.84 | 31.54 ± 4.66 | −0.91 | 0.374 |
| SCr (μmol/L) | 65.99 (55.80, 101.41) | 54.45 (26.49, 79.74) | 1.78 | 0.092 |
| D-dimer (μg/L) | 4.57 (3.24, 9.06) | 1.48 (1.29, 3.00) | −2.55 | 0.010 |
| FIB (g/L) | 5.98 ± 1.53 | 6.51 ± 1.74 | −0.71 | 0.490 |
| IL-2 (pg/mL) | 1.30 (1.13, 1.92) | 0.99 (0.69, 1.69) | −1.24 | 0.238 |
| IL-4 (pg/mL) | 1.17 ± 0.34 | 1.05 ± 0.38 | 0.75 | 0.462 |
| IL-6 (pg/mL) | 128.14 (56.48, 279.29) | 26.12 (11.29, 209.99) | −2.01 | 0.047 |
| IL-10 (pg/mL) | 9.16 (6.61, 13.45) | 3.58 (2.03, 5.40) | −2.62 | 0.007 |
| TNF-α (pg/mL) | 1.60 (1.22, 1.85) | 1.04 (0.95, 1.41) | −1.62 | 0.115 |
| IFN-γ (pg/mL) | 1.64 (0.92, 7.00) | 1.19 (1.10, 7.01) | −0.39 | 0.734 |
Values are mean ± standard deviation, or median (P25, P75).
WBC, white blood cell count; NEUT%, neutrophil proportion; LYMPH, lymphocyte count; LYMPH%, lymphocyte percentage; PLT, platelet; CRP, C-reaction protein; PCT, procalcitonin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBIL, total bilirubin; ALB, albumin; SCr, serum creatinine; FIB, fibrinogen; IL, immunoglobulin; TNF, tumor necrosis factor; IFN, interferon.
On the first day after blood culture collection, LYMPH% in patients with negative culture and PCT in patients with positive culture were significantly higher than the baseline, as shown in Table 4.
Table 4.
Comparison of clinical parameters and inflammatory markers between the two groups on different days.
| Group | Baseline | First day after blood culture collection | Second day after blood culture collection | F/z | p | |
|---|---|---|---|---|---|---|
| LYMPH% (%) | Positive blood culture group | 9.19 ± 5.44 | 9.38 ± 4.34 | 8.57 ± 5.53 | 0.082 | 0.922 |
| Negative blood culture group | 8.54 ± 3.64 | 16.46 ± 5.49 a | 13.35 ± 5.89 | 4.89 | 0.018 | |
| CRP (mg/L) | Positive blood culture group | 165.25 ± 68.96 | 158.62 ± 79.30 | 201.49 ± 56.95 | 1.342 | 0.275 |
| Negative blood culture group | 95.49 ± 81.84 | 74.60 ± 43.76 | 107.44 ± 119.66 | 0.29 | 0.752 | |
| ALB (g/L) | Positive blood culture group | 30.69 ± 3.84 | 29.74 ± 4.29 | 29.80 ± 3.84 | 0.603 | 0.553 |
| Negative blood culture group | 36.22 ± 3.44 | 33.61 ± 2.41 | 31.54 ± 4.66 | 0.25 | 0.784 | |
| D-dimer (μg/L) | Positive blood culture group | 3.41 (2.39, 4.57) | 4.13 (1.95, 12.11) | 4.57 (3.24, 9.06) | 0.67 | 0.716 |
| Negative blood culture group | 1.32 (1.05, 3.22) | 1.15 (1.02, 1.71) | 1.48 (1.29, 3.00) | 1.86 | 0.395 | |
| PCT (ng/mL) | Positive blood culture group | 0.24 (0.11, 2.58) | 2.90 (1.24, 30.49)a | 1.77 (0.40, 0.52) | 6.67 | 0.036 |
| Negative blood culture group | 0.49 (0.21, 1.57) | 0.35 (0.06, 1.28) | 0.31 (0.18, 1.86) | 0.21 | 0.900 | |
| IL-6 (pg/mL) | Positive blood culture group | 179.50 (98.14, 543.82) | 131.74 (76.39, 375.13) | 128.14 (56.48, 279.29) | 0.46 | 0.793 |
| Negative blood culture group | 43.02 (17.81, 82.11) | 45.46 (18.09, 64.40) | 26.12 (11.29, 209.99) | 0.24 | 0.887 | |
| IL-10 (pg/mL) | Positive blood culture group | 4.53 (2.23, 7.40) | 5.01 (2.85, 13.45) | 9.16 (6.61, 13.45) | 3.858 | 0.145 |
| Negative blood culture group | 5.75 (3.93, 9.57) | 2.58 (1.71, 5.43) | 3.58 (2.03, 5.40) | 1.81 | 0.406 |
Significant difference compared with the baseline.
Values are mean ± standard deviation, or median (P25, P75).
LYMPH%, lymphocyte percentage; PCT, procalcitonin; CRP, C-reaction protein; ALB, albumin; IL, immunoglobulin.
Receiver operating characteristic curve in adult patients with COVID-19 and clinically suspected BSI
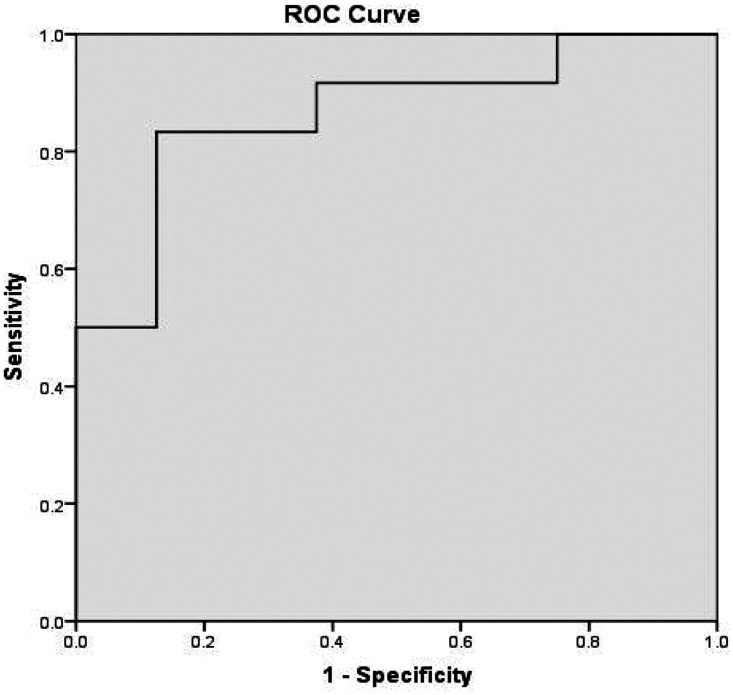
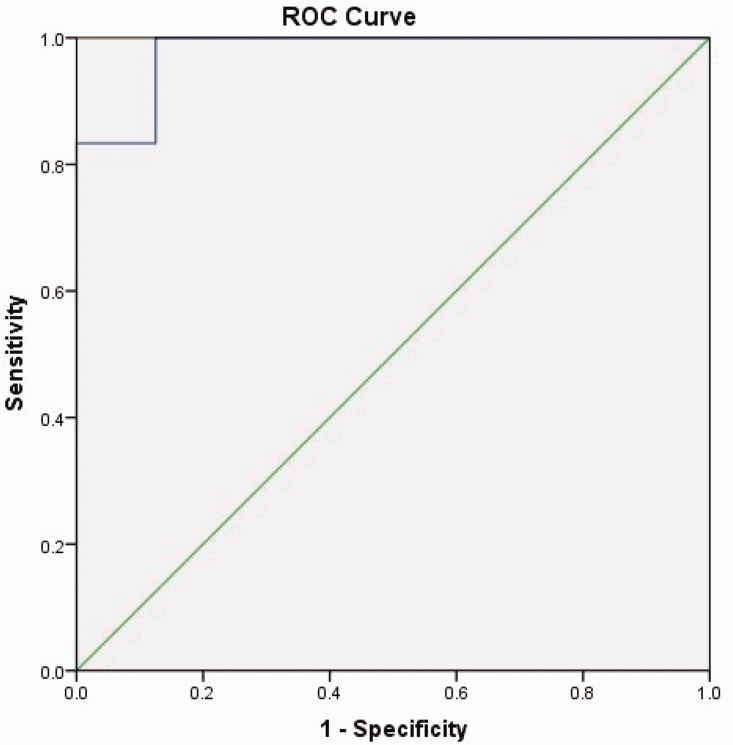
To predict positive culture, we calculated the area under the ROC curve for IL-6, D-dimer, and ALB related to adult patients with COVID-19 and clinically suspected BSI on the first day after blood culture collection, which had a value of 0.865. The sensitivity and specificity of the ROC curve were 83.3% and 87.5%, respectively (Table 5 and Figure 1). The area under the ROC curve for IL-6, IL-10, D-dimer, and CRP on the second day after blood culture collection had a value of 0.979. The sensitivity and specificity of the ROC curve were 100% and 87.5%, respectively (Table 6 and Figure 2).
Table 5.
AUC for IL-6, D-dimer and ALB related to adult patients with COVID-19 and clinically suspected BSI on the first day after blood culture collection.
| AUC | SE a | Asymptotic, p-value b | Asymptotic 95% CI |
|
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| 0.865 | 0.084 | 0.007 | 0.699 | 1.000 |
Under the non-parametric assumption.
Null hypothesis: true area = 0.5.
AUC, area under the receiver operating characteristic curve; IL, immunoglobulin; ALB, albumin; BSI, bloodstream infection; SE, standard error; CI, confidence interval.
Figure 1.
Receiver operating characteristic (ROC) curve of immunoglobulin 6 (IL-6), D-dimer, and albumin (ALB) related to adult patients with COVID-19 and clinically suspected bloodstream infection on the first day after blood culture collection. BSI, bloodstream infection; SE, standard error; CI, confidence interval.
Table 6.
AUC for IL-6, IL-10, D-dimer and CRP related to adult patients with COVID-19 and clinically suspected BSI on the second day after blood culture collection.
| AUC | SE a | Asymptotic, p-value b | Asymptotic 95% CI |
|
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| 0.979 | 0.027 | <0.001 | 0.927 | 1.000 |
Under the non-parametric assumption.
Null hypothesis: true area = 0.5.
AUC, area under the receiver operating characteristic curve; IL, immunoglobulin; CRP, C-reactive protein; BSI, bloodstream infection; SE, standard error; CI, confidence interval.
Figure 2.
Receiver operating characteristic (ROC) curve of IL-6, IL-10, D-dimer, and CRP related to adult patients with COVID-19 and clinically suspected bloodstream infection on the second day after blood culture collection. IL-6, immunoglobulin 6; CRP, C-reactive protein; BSI, bloodstream infection; SE, standard error; CI, confidence interval.
Discussion
COVID-19 can involve viral sepsis caused by SARS-CoV-2 infection; thus, virus-induced immune-suppressive responses could reduce granulocyte infiltrates and cause sustained desensitization of lung sentinel cells to Toll-like receptor ligands, thereby enhancing susceptibility to co-infection and secondary infection.16,17 The proportion of patients with COVID-19 who have co-infection or secondary infection with at least one additional pathogen varies widely from 3.6% to 17.8%.18–21 Two recent meta-analyses including 3448 and 3834 patients found that approximately 7% of hospitalized patients with COVID-19 had a bacterial co-infection, with a significantly higher proportion among critical patients, patients in the ICU, and older people.20–26 Given that hundreds of millions of people have been infected with SARS-CoV-2, this very large group would include many with abnormal clinical parameters, receiving antibiotics, and a worsening clinical condition with prolonged hospital or ICU stay, as well as significant mortality, and this is especially true among older patients.18,27,28 Blood is the most common source of sterile specimens, and BSI is one of the most common co-infections/secondary infections in patients with COVID-19, with an incidence of 5.3% to 9.8%. This rate is significantly increased in critically ill patients with COVID-19 or those admitted to the ICU for more than 48 hours, reaching 40% to 67.4%.4,29,30 Furthermore, the incidence of BSI also rises with prolonged duration of ICU stay. 31
Approximately 7.1% of patients with COVID-19 are reported to have positive blood cultures, but more than half of cultures are identified as contaminated. 32 In the ICU setting, a significant increase in blood culture contamination has been reported during the COVID-19 pandemic.33,34 False-negative results mean that patients are not identified in time and the optimal opportunity for clinical intervention is missed. False-positive results can lead to unnecessary antibiotic use and an increased risk of adverse drug events, antibiotic-associated infections, antimicrobial resistance, and colonization with resistant pathogens, as well as higher hospital costs. As a novel universal method to detect all pathogens in clinical samples, metagenomic next-generation sequencing can improve the pathogen detection rate but is time-consuming. 35 The problem of how to obtain accurate blood culture results as early as possible using common clinical parameters and inflammatory markers must be urgently solved.
Acute-phase reactants, such as CRP, PCT, and various cytokines, are associated with co-infections and secondary infections in patients with COVID-19. Among these, IL-6 is a recognized biomarker for early detection of COVID-19 progression and is associated with adverse clinical outcomes.36,37 However, a cytokine storm may only be part of pathogenesis of COVID-19, which may also be involved in inflammation, immunity, coagulation, and multiple organ functions. 38 This view is further supported by the significant differences in D-dimer, ALB, and LYMPH% in our comparison of clinical parameters after blood culture collection between the groups.
In the positive blood culture group, the significance of older age further confirmed that advanced age is a high-risk factor and valuable predictor of increased mortality in adult patients with COVID-19. 10 SOFA score was significantly different between the two groups, in a comparison of clinical baseline data, suggesting that the occurrence of BSI is closely related to disease severity, consistent with previous findings. 31 Although the sensitivity of the ROC curve for IL-6, IL-10, D-dimer, and CRP increased to 100% on the second day after blood culture collection, but its clinical timeliness was reduced, thereby affecting the timeliness of initiating relevant interventions and consequently, patient prognosis. Early identification of positive blood culture in adult patients with COVID-19 who have clinically suspected BSI requires a balance between accuracy and timeliness. The sensitivity and specificity of the ROC curve for IL-6, D-dimer, and ALB on the first day after blood culture collection were 83.3% and 87.5%, respectively, which was a relatively satisfactory result in terms of accuracy and timeliness. The above result suggested that decreased ALB combined increased D-dimer and IL-6 on the first day after blood culture collection can predict a high probability of positive culture in adult patients with COVID-19 and clinically suspected BSI.
There are some limitations in the present study. First, the results and conclusion of our study may be influenced by false positive and false negative blood culture results with respect to BSI. However, this study included comprehensive assessment by an expert panel, and multiple blood cultures to reduce the possibility of false results. Additionally, the credibility and generalizability of our conclusions may be weakened owing to the nature of this single-center retrospective study. Lastly, we enrolled a small number of adult patients with COVID-19 and clinically suspected BSI. Our findings should be further validated in multi-center large-sample studies.
Conclusion
Changes in clinical parameters and inflammatory markers after blood culture collection can be used for early identification of positive culture in adult patients with COVID-19 and clinically suspected BSI. This approach can assist intensivists in the early diagnosis of BSI and initiation of appropriate treatments to improve clinical outcomes. To our knowledge, our study is the first to explore the possibility of early identification of positive blood culture using clinical parameters and inflammatory markers in adult patients with COVID-19 and clinically suspected BSI. Our findings should be further validated in future multi-center large-sample studies. Greater emphasis should be placed on removing unnecessary catheters, restoring intact barriers, and strengthening patient care procedures to minimize the threat of BSI.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605241238134 for Changes in clinical parameters and inflammatory markers after blood culture collection facilitate early identification of positive culture in adult patients with COVID-19 and clinically suspected bloodstream infection by Nana Li, Long Zhang, Yang Gao, Qiqi Lai, Yujia Tang, Xue Du, Pengfei Chen, Chuangshi Yue, Mingyan Zhao, Kaijiang Yu and Kai Kang in Journal of International Medical Research
Acknowledgements
The authors are grateful to all colleagues who worked with them in the COVID-19 Treatment Center of Heilongjiang Province, and all those who provided selfless advice and help for this article. We pay tribute to the medical staff who lost their lives in the national fight against the COVID-19 epidemic.
Author contributions: Nana Li, Long Zhang, Yang Gao, Kai Kang, and Kaijiang Yu took part in the literature search; conception and study design; statistical analysis; analysis and discussion of the results; and manuscript preparation, editing, and review. Qiqi Lai, Yujia Tang, Xue Du, Pengfei Chen, Chuangshi Yue, and Mingyan Zhao provided assistance for the literature search, conception, data acquisition and collation, statistical analysis, analysis and discussion of the results, and manuscript preparation. All authors read and approved the final manuscript.
The authors declare that there is no conflict of interest.
Funding: This study was supported by the National Natural Science Foundation of China (no. 82372172), the Key Research and Development Plan Project of Heilongjiang Province (no.GA23C007), the Heilongjiang Province Postdoctoral Start-up Fund (no. LBH-Q20037), the Research Project of Heilongjiang Provincial Health Commission (no. 20231717010461), the Special Fund for Clinical Research of Wu Jie-Ping Medical Foundation (no. 320.6750.2022-02-16), and the Scientific Research Innovation Fund of The First Affiliated Hospital of Harbin Medical University (no. 2021M08).
ORCID iDs: Yang Gao https://orcid.org/0000-0002-0612-0818
Data availability statement
The raw data supporting the conclusions of this article are available upon request.
References
- 1.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020; 323: 1239–1242. [DOI] [PubMed] [Google Scholar]
- 2.Sepulveda J, Westblade LF, Whittier S, et al. Bacteremia and Blood Culture Utilization during COVID-19 Surge in New York City. J Clin Microbiol 2020; 58: e00875–e00820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goyal P, Choi JJ, Pinheiro LC, et al. Clinical Characteristics of Covid-19 in New York City. N Engl J Med 2020; 382: 2372–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cultrera R, Barozzi A, Libanore M, et al. Co-Infections in Critically Ill Patients with or without COVID-19: A Comparison of Clinical Microbial Culture Findings. Int J Environ Res Public Health 2021; 18: 4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mormeneo Bayo S, Palacián Ruíz MP, Moreno Hijazo M, et al. Bacteremia during COVID-19 pandemic in a tertiary hospital in Spain. Enferm Infecc Microbiol Clin (Engl Ed) 2021; 40: 183–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gragueb-Chatti I, Lopez A, Hamidi D, et al. Impact of dexamethasone on the incidence of ventilator-associated pneumonia and blood stream infections in COVID-19 patients requiring invasive mechanical ventilation: a multicenter retrospective study. Ann Intensive Care 2021; 11: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buetti N, Ruckly S, De Montmollin E, et al. COVID-19 increased the risk of ICU-acquired bloodstream infections: a case-cohort study from the multicentric OUTCOMEREA network. Intensive Care Med 2021; 47: 180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeh CY, Wang FD, Chuang YC, et al. Clinical outcomes and prognostic factors of patients with severe influenza receiving intravenous peramivir salvage therapy in intensive care units. J Microbiol Immunol Infect 2018; 51: 697–704. [DOI] [PubMed] [Google Scholar]
- 9.Evans IVR, Phillips GS, Alpern ER, et al. Association Between the New York Sepsis Care Mandate and In-Hospital Mortality for Pediatric Sepsis. JAMA 2018; 320: 358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cusumano JA, Dupper AC, Malik Y, et al. Staphylococcus aureus Bacteremia in Patients Infected With COVID-19: A Case Series. Open Forum Infect Dis 2020; 7: ofaa518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen S, Zhu Q, Xiao Y, et al. Clinical and etiological analysis of co-infections and secondary infections in COVID-19 patients: An observational study. Clin Respir J 2021; 15: 815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng J, Li R, Yin H, et al. A case report of a pregnant woman infected with coronavirus disease 2019 pneumonia. Medicine (Baltimore) 2020; 99(30): e21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu M, Lyu Y, Zhao W, et al. Case analysis of novel coronavirus pneumonia in the Second Hospital of Wuhan Iron and Steel Company, Qingshan District, Wuhan, China. Ann Palliat Med 2020; 9(4): 2193–2202. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Zhang C, Wu Z, et al. The Mechanism and Clinical Outcome of patients with Corona Virus Disease 2019 Whose Nucleic Acid Test has changed from negative to positive, and the therapeutic efficacy of Favipiravir: A structured summary of a study protocol for a randomised controlled trial. Trials 2020; 21: 488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Von Elm E, Altman DG, Egger M, STROBE Initiative et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 16.Navarini AA, Recher M, Lang KS, et al. Increased susceptibility to bacterial superinfection as a consequence of innate antiviral responses. Proc Natl Acad Sci U S A 2006; 103: 15535–15539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Didierlaurent A, Goulding J, Patel S, et al. Sustained desensitization to bacterial Toll-like receptor ligands after resolution of respiratory influenza infection. J Exp Med 2008; 205: 323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Vidal C, Sanjuan G, Moreno-García E, COVID-19 Researchers Group et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect 2021; 27: 83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nori P, Cowman K, Chen V, et al. Bacterial and fungal coinfections in COVID-19 patients hospitalized during the New York City pandemic surge. Infect Control Hosp Epidemiol 2021; 42: 84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect 2020; 26: 1622–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H, Zhang Y, Wu J, et al. Risks and features of secondary infections in severe and critical ill COVID-19 patients. Emerg Microbes Infect 2020; 9: 1958–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lansbury L, Lim B, Baskaran V, et al. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect 2020; 81: 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu Y, Yang Q, Xu M, et al. Secondary Bacterial Infections in Critical Ill Patients With Coronavirus Disease 2019. Open Forum Infect Dis 2020; 7: ofaa220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, He W, Yu X, et al. Coronavirus disease 2019 in elderly patients: Characteristics and prognostic factors based on 4-week follow-up. J Infect 2020; 80: 639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Contou D, Claudinon A, Pajot O, et al. Bacterial and viral co-infections in patients with severe SARS-CoV-2 pneumonia admitted to a French ICU. Ann Intensive Care 2020; 10: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Póvoa HCC, Chianca GC, Iorio NLPP. COVID-19: An Alert to Ventilator-Associated Bacterial Pneumonia. Infect Dis Ther 2020; 9: 417–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu CP, Adhi F, Highland K. Recognition and management of respiratory co-infection and secondary bacterial pneumonia in patients with COVID-19. Cleve Clin J Med 2020; 87: 659–663. [DOI] [PubMed] [Google Scholar]
- 28.Giacobbe DR, Battaglini D, Ball L, et al. Bloodstream infections in critically ill patients with COVID-19. Eur J Clin Invest 2020; 50: e13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughes S, Troise O, Donaldson H, et al. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin Microbiol Infect 2020; 26: 1395–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Søgaard KK, Baettig V, Osthoff M, et al. Community-acquired and hospital-acquired respiratory tract infection and bloodstream infection in patients hospitalized with COVID-19 pneumonia. J Intensive Care 2021; 9: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonazzetti C, Morena V, Giacomelli A, et al. Unexpectedly High Frequency of Enterococcal Bloodstream Infections in Coronavirus Disease 2019 Patients Admitted to an Italian ICU: An Observational Study. Crit Care Med 2021; 49: e31–e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engsbro AL, Israelsen SB, Pedersen M, et al. Predominance of hospital-acquired bloodstream infection in patients with Covid-19 pneumonia. Infect Dis (Lond) 2020; 52: 919–922. [DOI] [PubMed] [Google Scholar]
- 33.Ohki R, Fukui Y, Morishita N, et al. Increase of blood culture contamination during COVID-19 pandemic. A retrospective descriptive study. Am J Infect Control 2021; 49: 1359–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu D, Ininbergs K, Hedman K, et al. Low prevalence of bloodstream infection and high blood culture contamination rates in patients with COVID-19. PLoS One 2020; 15: e0242533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldberg B, Sichtig H, Geyer C, et al. Making the Leap from Research Laboratory to Clinic: Challenges and Opportunities for Next-Generation Sequencing in Infectious Disease Diagnostics. mBio 2015; 6: e01888–e01815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang C, Fei D, Li X, et al. IL-6 may be a good biomarker for earlier detection of COVID-19 progression. Intensive Care Med 2020; 46: 1475–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coomes EA, Haghbayan H. Interleukin-6 in Covid-19: A systematic review and meta-analysis. Rev Med Virol 2020; 30: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao Y, Wang C, Kang K, et al. Cytokine Storm May Not Be the Chief Culprit for the Deterioration of COVID-19. Viral Immunol 2021; 34: 336–341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605241238134 for Changes in clinical parameters and inflammatory markers after blood culture collection facilitate early identification of positive culture in adult patients with COVID-19 and clinically suspected bloodstream infection by Nana Li, Long Zhang, Yang Gao, Qiqi Lai, Yujia Tang, Xue Du, Pengfei Chen, Chuangshi Yue, Mingyan Zhao, Kaijiang Yu and Kai Kang in Journal of International Medical Research
Data Availability Statement
The raw data supporting the conclusions of this article are available upon request.