Abstract
Spinal cord injury (SCI) can lead to the loss of motor, sensory, or autonomic function due to neuronal death. Unfortunately, the adult mammalian spinal cord has limited intrinsic regenerative capacity, making it difficult to rebuild the neural circuits necessary for functional recovery. However, recent evidence suggests that in vivo fate reprogramming of resident cells that are normally non-neurogenic can generate new neurons. This process also improves the pathological microenvironment, and the new neurons can integrate into the local neural network, resulting in better functional outcomes in SCI animal models. In this concise review, we focus on recent advances while also discussing the challenges, pitfalls, and opportunities in the field of in vivo cell fate reprogramming for spinal cord repair.
Introduction
Spinal cord injury (SCI) often results in the permanent loss of neurons and the disruption of neural circuits, which can lead to behavioral dysfunctions and impose heavy burdens on both caregivers and society. Unfortunately, to date, effective treatment options for neurological recovery after SCI do not exist due to the lack of meaningful regenerative ability in the adult mammalian spinal cord [1]. Although considerable progress has been made in the fields of cell transplantation and axonal regeneration, one of the most significant challenges in repairing SCI remains how to reconstruct the broken neural circuits to achieve functional improvements.
An emerging regeneration-based strategy involves inducing new neurons from resident glia through cell fate reprogramming in vivo [2–5]. Unlike permanent neuron loss, SCI stimulates proliferation and recruitment of various cell types, including ependymal cells, astrocytes, Nerve/glial antigen 2 (NG2) glia, fibroblasts, microglia, and macrophages, around the injury site [6]. Although none of these cells have an inherent ability to produce new neurons in vivo [7,8], they could serve as a plentiful source for cell fate reprogramming. Such reprogramming may not only produce new neurons but also modify the pathological environment for circuit reconstruction. In the following sections, we review recent publications on the adult spinal cord (Figure 1 and Table 1) and provide perspectives on this exciting research direction.
Figure 1.
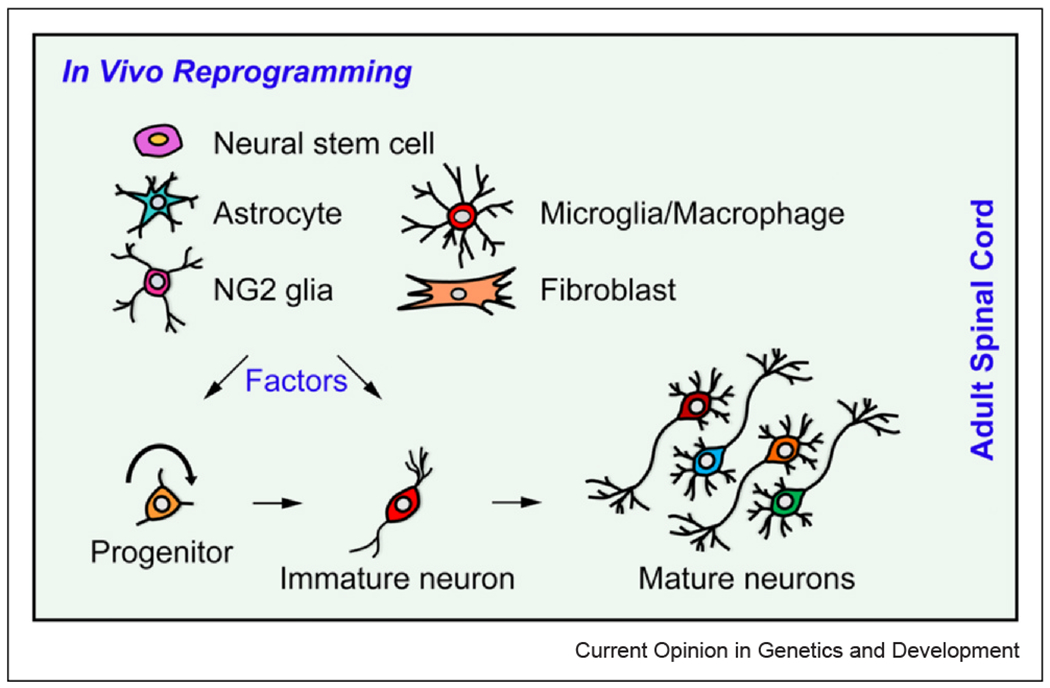
Cell fate reprogramming in the adult spinal cord. Spinal cord injury triggers activation and proliferation of various resident cells, including NSCs, astrocytes, NG2 glia, microglia/macrophages, and fibroblasts. These cells are potential sources for in vivo reprogramming. By overexpressing key factors, it is possible to convert some of these resident cells into progenitors or immature neurons, ultimately leading to the formation of mature, functional neurons in the adult mammalian spinal cord.
Table 1.
In vivo reprogramming for spinal cord repair.
| Cell sources | Delivery methods | Factors | Tracing methods | Neuron types | Functional properties | References |
|---|---|---|---|---|---|---|
| NSCs | Chitosan | NT-3 | BrdU | Undefined | Neural network, sensory and motor functional recovery | Yang et al. (2015) [14] |
| NSCs | Chitosan | NT-3 | BrdU | Undefined | Electrophysiological and motor functional recovery | Zhao et al. (2022) [17] |
| NSCs | Lentivirus | Nkx6.1 | Lentiviral RFP | Cholinergic interneurons | Reduction of inflammation and glial scar | Patel et al. (2021) [18,19] |
| NSCs | Lentivirus | Gsx1 | Lentiviral RFP | Glutamatergic, cholinergic interneurons | Reduction of astrogliosis and glial scar, enhanced serotonin neuronal activity and locomotor function | Patel et al. (2021) [18,19] |
| NSCs | Retrovirus | Neurod4 | Retroviral AcGFP1 or tauAcGFP1, BrdU | Excitatory and inhibitory neurons, motor neurons | Functional synapses, improved locomotor function | Fukuoka et al. (2021) [20] |
| Astrocytes | Lentivirus | SOX2 | Lentiviral GFP, BrdU, mGfap-Cre;R26R-tdT | GABAergic, glutamatergic interneurons | Synapses with preexisting ChAT+ motor neurons | Su et al. (2014) [25] |
| Astrocytes | Lentivirus | SOX2, shRNA–p53, and BDNF-NOG | BrdU, mGfap-Cre;R26R-tdT | Glutamatergic 80%, GABAergic, glycinergic, serotonergic, and cholinergic interneurons | Synaptic connections | Wang et al. (2016) [26] |
| Astrocytes | Retrovirus, AAV | NeuroD1 | Retroviral GFP, AAV-expressed mCherry | Glutamatergic interneurons | Repetitive action potentials and spontaneous synaptic responses | Puls et al. (2020) [27] |
| Astrocytes | AAV | Neurog2 | AAV-expressed mCherry | Glutamatergic and GABAergic interneurons | Response to afferent inputs from dorsal root ganglion | Liu et al. (2021) [28] |
| Astrocytes | AAV | Ngn2+Isl1 | AAV-expressed GFP, hGFAP-CreERT2;R26R-tdT | Motor neurons | Electrophysiological activities | Zhou et al. (2021) [29] |
| Astrocytes | Lentivirus | shNOTCH1, NOTCH inhibitor | BrdU, lentiviral GFP | GABAergic interneurons | N/A | Tan et al. (2022) [32] |
| NG2 glia | Lentivirus | SOX2, p75-2 | BrdU, lentiviral GFP, Pdgfra-CreER™;R26R-YFP, Ascl1-CreERT2;R26R-tdT | Glutamatergic, GABAergic, and glycinergic interneurons | Synaptic connections, scarring reduction, and improved functional recovery | Tai et al. (2021) [37] |
| Proliferating cells | Retrovirus | Ngn2, EGF, FGF2, and BDNF | Retroviral GFP, BrdU | GABAergic interneurons | N/A | Ohori et al. (2006) [38] |
Neurons from resident neural stem cells
While spinal cord ependymal cells have shown neural stem cell (NSC) properties in culture [9], they primarily generate glial cells that form scars, rather than neurons, after an injury [10]. Additionally, there is still debate about the extent to which ependymal cell-derived NSCs can contribute to the formation of new cells around the lesion core after SCI [11–13]. Despite this controversy, a study by Yang et al. found that implantation of neurotrophin-3 (NT3)-loaded chitosan biomaterial into completely transected rat spinal cords promoted activation, migration, and neuronal differentiation of endogenous NSCs [14]. The implanted biomaterial contained newly produced neurons, identified by BrdU labeling of proliferating cells and neuronal markers such as Tuj1. These NSC-derived neurons formed functional networks with preexisting neurons, connecting damaged ascending and descending axons and improving sensory, motor, and bladder functions [14–16]. For chronic SCI, it appears that removing cystic tissues and solid scars is essential for neurogenesis and regeneration induced by the NT3-chitosan [17]. Thus, biomaterial-elicited neurogenesis from resident NSCs could provide a much-needed therapeutic strategy for patients with severe SCI.
Patel et al. reported that endogenous NSCs can be activated by ectopic expression of key transcription factors such as Nkx6.1 or Gsx1, both after acute and chronic SCI [18,19]. The studies also found that ectopic Nkx6.1 or Gsx1 expression reduced reactive astrogliosis and glial scarring, and enhanced the generation of propriospinal interneurons, including glutamatergic and cholinergic neurons. These neurons were identified by virus-expressed red fluorescent protein under the cytomegalovirus promoter [18,19]. Although Nkx6.1 expression failed to show functional consequences, Gsx1 was found to improve the activity of serotonergic neurons and locomotor function after SCI. Additionally, molecular analyses revealed factor-dependent gene expression changes related to stem cell activity, reactive gliosis, and neuroinflammation [18,19].
In a similar study, Fukuoka et al. used lymphocytic choriomeningitis virus-pseudotyped retroviruses with a tropism for NSCs and reported that the introduction of ectopic Neurod4 could activate NSCs after SCI [20]. This led to the promotion of their differentiation into both inhibitory and excitatory neurons, which projected to motor neurons. The study also found that immature and mature neurons were present, as detected by co-expression of the viral AcGFP1 with doublecortin (DCX) and neuronal nuclei, respectively, surrounding the lesion core. Additionally, ectopic Neurod4 suppressed reactive astrogliosis marked by glial fibrillary acidic protein (GFAP) expression and greatly enhanced recovery of locomotion, as indicated by improved behavioral scores [20].
Neurons from resident astrocytes
In response to SCI, astrocytes undergo a reactive process, proliferate, and constitute a major component of the glial scar surrounding the lesion core [21,22]. They represent an excellent source of cells for fate conversion in vivo. Through in vivo screening efforts, Niu et al. identified SOX2 as a stem cell factor capable of converting resident astrocytes into proliferative neural progenitors, which subsequently generate mature neurons in the adult mouse brain [23,24]. This finding was further examined in the context of injured adult mouse spinal cord [25]. Genetic lineage tracing and BrdU-labeling studies confirmed that astrocytes are the cell origin of new neurons. Marker expression studies indicated that these new neurons form synaptic connections with nearby endogenous motor neurons. Furthermore, Wang et al. found that SOX2-mediated neuronal reprogramming of astrocytes could be further enhanced by downregulating the p53–p21 pathway, as it normally inhibits the expansion of astrocyte-derived progenitors [26].
Several studies have also reported the conversion of resident astrocytes to neurons in the adult murine spinal cord by controlling key factors. For instance, Puls et al. reported that ectopic expression of Neurod1 efficiently converted reactive astrocytes into neurons in the dorsal horn of injured mouse spinal cord [27]. These converted neurons expressed molecular markers specific to local neuronal subtypes and functionally integrated into the neural networks. This conversion was also reported in a contusion SCI model, suggesting a potential therapeutic approach for SCI repair [27]. In another study, Liu et al. overexpressed Neurog2 (also known as Ngn2) in the adult mouse spinal cord with or without SCI and directly converted resident astrocytes into glutamatergic and GABAergic neurons [28]. These neurons were identified by the virus-expressed mCherry and exhibited mature neuron-like electrophysiological properties while receiving inputs from dorsal root ganglion. Similarly, Zhou et al. reported that the combination of Ngn2 and Isl1 efficiently converted resident spinal astrocytes into motor neurons that projected precisely into the sciatic nerves [29].
The NOTCH signaling pathway has recently been identified as a crucial regulator of astrocyte fate. Its downregulation is both necessary and sufficient for adult resident astrocytes in the mouse brain to convert into neurons [30,31]. Through analyzing the signaling pathways involved in Ascl1- and Neurog2-mediated reprogramming in culture, Tan et al. discovered that NOTCH1 acts as a suppressor of astrocyte-to-neuron conversion [32]. Moreover, shRNA-mediated knockdown of NOTCH1 in endogenous reactive astrocytes in the injured adult spinal cord resulted in the emergence of new neurons, as evidenced by BrdU incorporation and DCX expression. Excitingly, intraperitoneal injections of N-[N-(3, 5-difluorophenacetyl)-1-alanyl]-s-phenylglycinet-butyl ester, a chemical inhibitor of the NOTCH signaling pathway, also induced neurogenesis in the vicinity of the lesion site following SCI [32]. These newly formed neurons were mainly GABAergic inhibitory neurons expressing the presynaptic marker synapsin-1, indicating potential synaptic connections. This chemical approach could be highly relevant for clinical translation.
Neurons from resident NG2 glia
NG2 glia, which are also known as oligodendrocyte progenitor cells, are known to proliferate and become a major component of the glial scar under pathological conditions such as SCI. Although there are reports indicating their potential to differentiate into other cell types such as astrocytes [33], their contribution to neurons is minimal [8,34–36]. However, recent research by Tai et al. using genetic lineage tracings has shown that resident NG2 glia can express markers of immature neurons such as DCX in response to various SCI types. Despite this, they failed to become mature neurons even in the presence of supplied neurotrophic factor brain derived neurotrophic factor (BDNF)-noggin (NOG) [37]. Nevertheless, the properties of NG2 glia in response to SCI make them an ideal cell source for in vivo reprogramming. In fact, Ohori et al. reported that retrovirus could transduce proliferating cells expressing NG2, OLIG2, and NKX2.2, and that retroviral expression of Ngn2 in combination with growth factors such as EGF and FGF2 promoted the generation of GABAergic neurons in adult rats after SCI. However, few of these neurons survived beyond 56 days, even when BDNF was supplied [38].
Through cell-type-specific genetic deletions, Tai et al. revealed that the injury-induced DCX expression in NG2 glia requires endogenous SOX2 [37]. Additionally, they found that ectopic SOX2 could reprogram NG2 glia into ASCL1+ neural progenitors, which further differentiated into DCX+ immature and neuronal nuclei+ mature neurons. The survival and maturation of these neurons were enhanced through the co-expression of neurotrophic factors such as BDNF-noggin (NOG) or a mutant form of NT3 (p75-2). These NG2 glia-derived neurons persisted for more than six months and differentiated into either excitatory or inhibitory propriospinal interneurons. To examine the monosynaptic connections of SOX2-induced neurons, pseudotyped recombinant rabies virus was used. The analysis revealed that these neurons received inputs from endogenous propriospinal neurons, as well as those located in the dorsal root ganglion and the brain stem. Importantly, reprogrammed NG2 glia also produced oligodendrocytes, which may be crucial for myelination of new neurons and remyelination of exposed axons of endogenous neurons. Notably, in vivo reprogramming of NG2 glia significantly reduced the glial scar and improved functional recovery after SCI. These findings demonstrate the potential of a regeneration-based therapeutic strategy for SCI [37].
Pitfalls, challenges, and opportunities
The adult spinal cord comprises various cell types, including glial cells and numerous neurons that are formed during neural development. In vivo reprogramming experiments face a critical challenge of ensuring that the alleged new neurons are indeed derived from resident glial cells. To address this issue, Wang et al. utilized Neurod1 as an example and performed multiple lineage mapping experiments by using well-characterized mouse lines and a retrograde tracing method [39]. They convincingly demonstrated that viral reporter-labeled neurons in the Neurod1 group were mislabeled endogenous neurons rather than converted from glia. This was mainly because the cell-type-specific promoter activity in the virus can be influenced by the insert gene sequences, such as Neurod1 [39–41]. In agreement with previous comments [42–45], Xie et al. recently demonstrated that reducing the virus dosage, using an alternative AAV serotype, or changing the virus-injection route did not correct the transgene-dependent leakage of the viral reporter in endogenous neurons [40]. Consequently, a mere comparison of the viral reporter expression between the control and the experimental group is insufficient to draw a definitive conclusion. Cautions should also be paid to retrovirus-based studies. Although retrovirus itself does not transduce postmitotic neurons, the virus-transduced microglia can fuse to and drive the viral reporter expression in nearby neurons [46], thereby resulting in an illusion of neuronal conversion of resident glial cells.
As such, genetic lineage tracing is essential for studying cell fate conversion in vivo [42,44,47,48]. For this purpose, the targeting cell type is uniquely and permanently tagged with a Cre-dependent reporter such that the cell fate can be followed by immunohistochemistry. The employed Cre- and reporter-containing mouse lines should be carefully characterized, because some mouse lines may exhibit region-dependent leakage of the reporter in neurons [39]. The tamoxifen-activatable CreERT2 (or CreERT or CreER™) lines are ideal such that the reporter could be only induced in the adult stage and before the reprogramming process. Owing to leaky expression in neurons, the virus-expressed Cre or Cre-dependent reporter is inappropriate for lineage tracing [39]. Of note, many reprogramming studies in the adult spinal cord cited in this concise review did not utilize appropriate lineage tracing methods, underscoring the need for future replication studies.
Another challenge of in vivo reprogramming is generating appropriate neuronal subtypes to improve spinal cord function. While some studies claim to obtain region-specific subtypes from resident glia, their true cell origin is debatable. As described above, endogenous neurons could be easily misidentified as glia-converted ones if not using stringent tracing methods. Because SCI frequently causes the loss of various neuronal subtypes critical for somatosensory and locomotive functions, the question arises whether it is possible to generate all these subtypes through cell fate reprogramming in vivo or whether one major subtype would suffice. It may be more practical to focus on the subtype that is most relevant to improving an SCI patient’s prime need. Alternatively, could the prime function be fulfilled with new neurons that are not subtype-specific but exhibit generic characteristics of excitatory or inhibitory neurons?
Propriospinal and brain–spinal connections form the cellular basis for neuronal function. Recent studies using virus-based transsynaptic tracings have demonstrated that glia-derived new neurons can establish synaptic connections with neurons located in the spinal cord and brain [37], indicating the potential for these new neurons to contribute to the repair of spinal cord injuries by forming neuronal relays [49]. However, the question remains as to how to promote such relays. One possible approach is to utilize the concept of ‘use it or lose it,’ whereby rehabilitation or targeted chemogenetic or optogenetic stimulation can further enhance the synaptic connections formed by new neurons.
Current reprogramming strategies mainly focus on targeting resident NSCs, astrocytes, and NG2 glia, which are closely related to neurons and may exhibit a lower barrier for conversion into neurons. However, scars formed after SCI contain various other cell types, including fibroblasts, microglia, and macrophages, which are in the lesion core and contribute to axonal regeneration failure and impaired functional recovery after SCI [7]. The question arises whether these cells are also susceptible to neuronal conversion in vivo. Given that these cells are lineagewise distinct from neurons, they may be more resistant to reprogramming into neurons. Although there is a report of neuronal conversion of resident microglia in the adult striatum [50], a follow-up study has questioned these findings [51].
In summary, the in vivo reprogramming approach for spinal cord repair is still in its early stages of preclinical investigation, and there are some pitfalls and challenges that require attention. However, these challenges also present ample opportunities for future research. In addition to the challenges listed above, such as neuronal subtypes, synaptic integrations, and targeting cell types, there is still a lack of clear knowledge on the molecular mechanisms underlying the in vivo reprogramming process. As potential therapeutics, the virus-mediated delivery system may face safety issues such as toxicity, tumorigenesis, and mutagenesis. To address these concerns, delivery of the reprogramming factors through nanoparticles or other media may be potential strategies to consider.
Acknowledgements
The Zhang lab was supported by the Decherd Foundation, USA and the National Institutes of Health, USA (NS127375, NS117065, NS099073, NS111776, and NS092616).
Footnotes
Declaration of Competing Interest
The authors declare no competing interests.
Data Availability
No data were used for the research described in the article.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Kiyotake EA, Martin MD, Detamore MS: Regenerative rehabilitation with conductive biomaterials for spinal cord injury. Acta Biomater 2022, 139:43–64. [DOI] [PubMed] [Google Scholar]
- 2.Tai W, Xu XM, Zhang CL: Regeneration through in vivo cell fate reprogramming for neural repair. Front Cell Neurosci 2020, 14:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang LL, Zhang CL: Engineering new neurons: in vivo reprogramming in mammalian brain and spinal cord. Cell Tissue Res 2018, 371:201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen G, Wernig M, Berninger B, Nakafuku M, Parmar M, Zhang CL: In vivo reprogramming for brain and spinal cord repair. eNeuro 2015, 2:e0106–15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith DK, Zhang CL: Regeneration through reprogramming adult cell identity in vivo. Am J Pathol 2015, 185:2619–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anjum A, Yazid MD, Fauzi Daud M, Idris J, Ng AMH, Selvi Naicker A, Ismail OHR, Athi Kumar RK, Lokanathan Y: Spinal cord injury: pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int J Mol Sci 2020, 7533, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Z, Yu S, Hu X, Li Y, You X, Tian D, Cheng L, Zheng M, Jing J: Fibrotic scar after spinal cord injury: crosstalk with other cells, cellular origin, function, and mechanism. Front Cell Neurosci 2021, 15:720938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horky LL, Galimi F, Gage FH, Horner PJ: Fate of endogenous stem/progenitor cells following spinal cord injury. J Comp Neurol 2006, 498:525–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meletis K, Barnabe-Heider F, Carlen M, Evergren E, Tomilin N, Shupliakov O, Frisen J: Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol 2008, 6:e182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnabe-Heider F, Goritz C, Sabelstrom H, Takebayashi H, Pfrieger FW, Meletis K, Frisen J: Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell 2010, 7:470–482. [DOI] [PubMed] [Google Scholar]
- 11.Muthusamy N, Brumm A, Zhang X, Carmichael ST, Ghashghaei HT: Foxj1 expressing ependymal cells do not contribute new cells to sites of injury or stroke in the mouse forebrain. Sci Rep 2018, 8:1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren Y, Ao Y, O’Shea TM, Burda JE, Bernstein AM, Brumm AJ, Muthusamy N, Ghashghaei HT, Carmichael ST, Cheng L, et al. Ependymal cell contribution to scar formation after spinal cord injury is minimal, local and dependent on direct ependymal injury. Sci Rep 2017, 7:41122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah PT, Stratton JA, Stykel MG, Abbasi S, Sharma S, Mayr KA, Koblinger K, Whelan PJ, Biernaskie J: Single-cell transcriptomics and fate mapping of ependymal cells reveals an absence of neural stem cell function. Cell 2018, 173:1045–1057 e1049. [DOI] [PubMed] [Google Scholar]
- 14.Yang Z, Zhang A, Duan H, Zhang S, Hao P, Ye K, Sun YE, Li X: NT3-chitosan elicits robust endogenous neurogenesis to enable functional recovery after spinal cord injury. Proc Natl Acad Sci USA 2015, 112:13354–13359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hao F, Jia F, Hao P, Duan H, Wang Z, Fan Y, Zhao W, Gao Y, Fan OR, Xu F, et al. Proper wiring of newborn neurons to control bladder function after complete spinal cord injury. Biomaterials 2023, 292:121919. [DOI] [PubMed] [Google Scholar]
- 16.••.Wang Z, Duan H, Hao F, Hao P, Zhao W, Gao Y, Gu Y, Song J, Li X, Yang Z: Circuit reconstruction of newborn neurons after spinal cord injury in adult rats via an NT3-chitosan scaffold. Prog Neurobiol 2023, 220:102375. [DOI] [PubMed] [Google Scholar]; This study reported that NT3-chitosan scaffold facilitated maturation of spinal neurons from resident NSCs and promoted the formation of relay neural circuits for functional recovery after injury.
- 17.•.Zhao C, Rao JS, Duan H, Hao P, Shang J, Fan Y, Zhao W, Gao Y, Yang Z, Sun YE, et al. Chronic spinal cord injury repair by NT3-chitosan only occurs after clearance of the lesion scar. Signal Transduct Target Ther 2022, 7:184. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reported that the clearance of lesion core is essential for NT3-chitosan-mediated neural regeneration and functional repair after spinal cord injury.
- 18.Patel M, Anderson J, Lei S, Finkel Z, Rodriguez B, Esteban F, Risman R, Li Y, Lee KB, Lyu YL, et al. Nkx6.1 enhances neural stem cell activation and attenuates glial scar formation and neuroinflammation in the adult injured spinal cord. Exp Neurol 2021, 345:113826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.•.Patel M, Li Y, Anderson J, Castro-Pedrido S, Skinner R, Lei S, Finkel Z, Rodriguez B, Esteban F, Lee KB, et al. Gsx1 promotes locomotor functional recovery after spinal cord injury. Mol Ther 2021, 29:2469–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reported that Gsx1 overexpression increases the number of endogenous neural stem/progenitor cells, reduces reactive astrogliosis and glial scar formation, and improves the locomotor function of mice after SCI.
- 20.Fukuoka T, Kato A, Hirano M, Ohka F, Aoki K, Awaya T, Adilijiang A, Sachi M, Tanahashi K, Yamaguchi J, et al. Neurod4 converts endogenous neural stem cells to neurons with synaptic formation after spinal cord injury. iScience 2021, 24:102074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tran AP, Warren PM, Silver J: New insights into glial scar formation after spinal cord injury. Cell Tissue Res 2022, 387:319–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okada S, Hara M, Kobayakawa K, Matsumoto Y, Nakashima Y: Astrocyte reactivity and astrogliosis after spinal cord injury. Neurosci Res 2018, 126:39–43. [DOI] [PubMed] [Google Scholar]
- 23.Niu W, Zang T, Smith DK, Vue TY, Zou Y, Bachoo R, Johnson JE, Zhang CL: SOX2 reprograms resident astrocytes into neural progenitors in the adult brain. Stem Cell Rep 2015, 4:780–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niu W, Zang T, Zou Y, Fang S, Smith DK, Bachoo R, Zhang CL: In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat Cell Biol 2013, 15:1164–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su Z, Niu W, Liu ML, Zou Y, Zhang CL: In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat Commun 2014, 5:3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang LL, Su Z, Tai W, Zou Y, Xu XM, Zhang CL: The p53 pathway controls SOX2-mediated reprogramming in the adult mouse spinal cord. Cell Rep 2016, 17:891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puls B, Ding Y, Zhang F, Pan M, Lei Z, Pei Z, Jiang M, Bai Y, Forsyth C, Metzger M, et al. Regeneration of functional neurons after spinal cord injury via in situ NeuroD1-mediated astrocyte-to-neuron conversion. Front Cell Dev Biol 2020, 8:591883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu F, Zhang YJ, Chen FL, Yuan JC, Li SL, Han S, Lu DY, Geng JL, Rao ZP, Sun L, et al. Neurog2 directly converts astrocytes into functional neurons in midbrain and spinal cord. Cell Death Dis 2021, 12:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou M, Tao X, Sui M, Cui M, Liu D, Wang B, Wang T, Zheng Y, Luo J, Mu Y, et al. Reprogramming astrocytes to motor neurons by activation of endogenous Ngn2 and Isl1. Stem Cell Rep 2021, 16:1777–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magnusson JP, Goritz C, Tatarishvili J, Dias DO, Smith EM, Lindvall O, Kokaia Z, Frisen J: A latent neurogenic program in astrocytes regulated by Notch signaling in the mouse. Science 2014, 346:237–241. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Li B, Cananzi S, Han C, Wang LL, Zou Y, Fu YX, Hon GC, Zhang CL: A single factor elicits multilineage reprogramming of astrocytes in the adult mouse striatum. Proc Natl Acad Sci USA 119:e2107339119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.••.Tan Z, Qin S, Yuan Y, Hu X, Huang X, Liu H, Pu Y, He C, Su Z: NOTCH1 signaling regulates the latent neurogenic program in adult reactive astrocytes after spinal cord injury. Theranostics 2022, 12:4548–4563. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identified NOTCH1 as a key signaling pathway in controlling neuronal conversion of reactive astrocytes in the adult mouse spinal cord. Overcoming the NOTCH1 inhibition with small molecules may provide a promising therapeutic application.
- 33.Kirdajova D, Anderova M: NG2 cells and their neurogenic potential. Curr Opin Pharmacol 2020, 50:53–60. [DOI] [PubMed] [Google Scholar]
- 34.Tripathi RB, Rivers LE, Young KM, Jamen F, Richardson WD: NG2 glia generate new oligodendrocytes but few astrocytes in a murine experimental autoimmune encephalomyelitis model of demyelinating disease. J Neurosci 2010, 30:16383–16390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto S, Yamamoto N, Kitamura T, Nakamura K, Nakafuku M: Proliferation of parenchymal neural progenitors in response to injury in the adult rat spinal cord. Exp Neurol 2001, 172:115–127. [DOI] [PubMed] [Google Scholar]
- 36.Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE: NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron 2010, 68:668–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.••.Tai W, Wu W, Wang LL, Ni H, Chen C, Yang J, Zang T, Zou Y, Xu XM, Zhang CL: In vivo reprogramming of NG2 glia enables adult neurogenesis and functional recovery following spinal cord injury. Cell Stem Cell 2021, 28:923–937 e924. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that injury can activate the neurogenic potential of resident NG2 glia in the adult mouse spinal cord. This potential is dependent on endogenous SOX2. Significantly, ectopic SOX2 can effectively reprogram NG2 glia into neural progenitors that mature and establish synaptic connections with neurons in the spinal cord and brain. Additionally, SOX2-mediated reprogramming reduces scarring and enhances functional recovery following SCI.
- 38.Ohori Y, Yamamoto S, Nagao M, Sugimori M, Yamamoto N, Nakamura K, Nakafuku M: Growth factor treatment and genetic manipulation stimulate neurogenesis and oligodendrogenesis by endogenous neural progenitors in the injured adult spinal cord. J Neurosci 2006, 26:11948–11960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.••.Wang LL, Serrano C, Zhong X, Ma S, Zou Y, Zhang CL: Revisiting astrocyte to neuron conversion with lineage tracing in vivo. Cell 2021, 184:5465–5481 e5416. [DOI] [PMC free article] [PubMed] [Google Scholar]; Contrary to prior publications, this study employed rigorous lineage tracing methods to demonstrate that endogenous neurons were incorrectly identified as the astrocyte-converted following ectopic NEUROD1 expression in the adult mouse brain. Additionally, genetically traced resident astrocytes did not convert into neurons despite efficient knockdown of PTBP1 in vivo. This work underscores the necessity of implementing lineage tracing strategies in studies of cell fate conversions in vivo.
- 40.Xie Y, Zhou J, Wang L-L, Zhang C-L, Chen B: New AAV tools fail to detect Neurod1-mediated neuronal conversion of Müller glia and astrocytes in vivo. eBioMedicine 2023, 90:104531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le N, Appel H, Pannullo N, Hoang T, Blackshaw S: Ectopic insert-dependent neuronal expression of GFAP promoter-driven AAV constructs in adult mouse retina. Front Cell Dev Biol 2022, 10:914386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Svendsen CN, Sofroniew MV: Lineage tracing: the gold standard to claim direct reprogramming in vivo. Mol Ther 2022, 30:988–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang LL, Zhang CL: Reply to in vivo confusion over in vivo conversion. Mol Ther 2022, 30:986–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang LL, Zhang CL: In vivo glia-to-neuron conversion: pitfalls and solutions. Dev Neurobiol 2022, 82:367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blackshaw S, Sanes JR: Turning lead into gold: reprogramming retinal cells to cure blindness. J Clin Investig 2021, 131:e146134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ackman JB, Siddiqi F, Walikonis RS, LoTurco JJ: Fusion of microglia with pyramidal neurons after retroviral infection. J Neurosci 2006, 26:11413–11422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang LL, Zhang CL: Therapeutic potential of PTBP1 inhibition, if any, is not attributed to glia-to-neuron conversion. Annu Rev Neurosci 2023, 46:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Calzolari F, Berninger B: cAAVe phaenomena: beware of appearances!. Cell 2021, 184:5303–5305. [DOI] [PubMed] [Google Scholar]
- 49.Bonner JF, Steward O: Repair of spinal cord injury with neuronal relays: from fetal grafts to neural stem cells. Brain Res 2015, 1619:115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsuda T, Irie T, Katsurabayashi S, Hayashi Y, Nagai T, Hamazaki N, Adefuin AMD, Miura F, Ito T, Kimura H, et al. Pioneer factor NeuroD1 rearranges transcriptional and epigenetic profiles to execute microglia-neuron conversion. Neuron 2019, 101:472–485.e477. [DOI] [PubMed] [Google Scholar]
- 51.Rao Y, Du S, Yang B, Wang Y, Li Y, Li R, Zhou T, Du X, He Y, Wang Y, et al. NeuroD1 induces microglial apoptosis and cannot induce microglia-to-neuron cross-lineage reprogramming. Neuron 2021, 109:4094–4108.e4095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used for the research described in the article.


