Abstract
We have studied the immune responses to the two glycoproteins of the Morbillivirus canine distemper virus (CDV) after DNA vaccination of BALB/c mice. The plasmids coding for both CDV hemagglutinin (H) and fusion protein (F) induce high levels of antibodies which persist for more than 6 months. Intramuscular inoculation of the CDV DNA induces a predominantly immunoglobulin G2a (IgG2a) response (Th1 response), whereas gene gun immunization with CDV H evokes exclusively an IgG1 response (Th2 response). In contrast, the CDV F gene elicited a mixed, IgG1 and IgG2a response. Mice vaccinated (by gene gun) with either the CDV H or F DNA showed a class I-restricted cytotoxic lymphocyte response. Immunized mice challenged intracerebrally with a lethal dose of a neurovirulent strain of CDV were protected. However, approximately 30% of the mice vaccinated with the CDV F DNA became obese in the first 2 months following the challenge. This was not correlated with the serum antibody levels.
Inoculation of plasmid DNA into muscle and the subsequent expression of the encoded protein have opened up new approaches in vaccination and gene therapy (for an extensive review see reference 25). Although initial studies used intramuscular (i.m.) inoculation to deliver the DNA, other routes have been shown to be equally or more efficient in inducing immune responses, which may be related to the types of antigen-presenting cells (APCs) which are involved (9). Recent observations suggest that after i.m. inoculation, muscle cells probably act as a reservoir for the foreign antigen, while the bone marrow cells seem to act as the APCs (8, 12, 26, 27). For DNA delivery to the skin, the APCs have not yet been identified but could well include cells of dendritic origin (21). Although both intradermal and i.m. DNA inoculations induce a strong Th1 response, inoculation of DNA precipitated onto gold beads and delivered by means of a gene gun favors a Th2 response (7). Whether this is due to the targeting of different APCs has not been determined.
We have been studying the possibility of using DNA vaccination to protect against canine distemper virus (CDV). CDV is a member of the genus Morbillivirus, in the Paramyxoviridae family, and although this virus primarily infects dogs, the disease has also been described in several animal species both in nature and in captivity (10, 18, 22). The currently available live attenuated vaccine efficiently protects dogs once maternal antibodies have disappeared, but it is not sufficiently attenuated for certain other animal species in which a fatal infection may ensue (6). This has led to a problem in protecting members of rare animal species living in captivity.
In the present study, we have expressed the two CDV glycoproteins, the attachment protein (hemagglutinin [H]) and the fusion protein (F), from plasmids driven by a cytomegalovirus (CMV) promoter. We show that i.m. and intradermal inoculation of the CDV H-encoding plasmid induces a Th1 response, whereas gene gun inoculation of the same plasmid induces a Th2-type response. In contrast, the CDV F gene administered with the gene gun elicited a mixed Th response. Mice immunized with either of the plasmids were protected against a lethal intracerebral (i.c.) infection.
MATERIALS AND METHODS
Plasmid construction.
cDNAs encoding CDV H and CDV F were subcloned into the BglII site of the pV1J plasmid (17), which is driven by the CMV promoter. To facilitate making these constructions, site-directed mutagenesis (15) was used to introduce BamHI sites at the 5′ and 3′ ends of the CDV F cDNA. Furthermore, site-directed mutagenesis was used to modify the context of the ATG in the CDV F cDNA in order to optimize the expression of the encoded proteins (14). In vitro expression of pV1J-CDV-H and pV1J-CDV-F was tested by transient transfection of murine Ltk− cells. Purified plasmid DNAs were transfected into Ltk− cells by Lipofectamine (Gibco BRL, Cergy Pontoise, France). At 48 h posttransfection, cells were examined for the production of proteins by immunofluorescence.
Gold bead-DNA preparations.
Approximately 30 mg of 1.6-μm-diameter gold powder (Bio-Rad, Ivry sur Seine, France) was mixed with 100 μl of 0.1 M spermidine (Sigma). After sonication in a water bath sonicator, plasmid DNAs were added at 0.1 to 2 μg/mg of gold powder. Two hundred microliters of 2.5 M CaCl2 was then added to the mixture with gentle spinning. Pellets were washed three times and suspended in cold 100% ethanol. Tubes with gold bead-DNA preparations were stored at 4°C under dry conditions.
DNA immunizations.
Female BALB/c (H-2d) mice were purchased from IFFA-CREDO (Domaine des Oncins, France) and immunized at 6 to 8 weeks of age. For i.m. inoculations, DNA (50 μg) in 50 μl of phosphate-buffered saline (PBS) was inoculated into the quadriceps muscles of both hind legs. For intradermal inoculations, DNA (100 μg in 50 μl of PBS) was administered either with a multipuncture ring (similar to those used for tuberculin tests) in the abdominal skin or by tail scarification or subcutaneous injection in the abdomen. For gene gun inoculations, plasmid DNAs were injected into the shaved abdominal epidermis of the mice with a Helios gene gun system (Bio-Rad) at a helium pressure setting of 300 lb/in2. Two gene gun inoculations at different sites were given for each dose. Each inoculation delivered 0.5 mg of gold bead-DNA preparation.
Viruses and cells.
The Onderstepoort CDV strain was used for the neutralization assays, and the mouse neuroadapted CDV strain (2) was used for challenge studies. Stocks of the latter were prepared by passage in suckling-mouse brains. Mouse brain homogenates from infected suckling mice were used as a source of virus and were stored at −80°C. Cytotoxic T-lymphocyte (CTL) assays were performed in P815 (H-2d) cells (which express major histocompatibility complex class I but not class II molecules) and in CDV-infected P815 cells. These cells were also used for enzyme-linked immunosorbent assays (ELISAs).
Neutralization assay.
Serial twofold dilutions of mouse sera were prepared in a sterile 96-well flat-bottomed tissue culture plate. Dilutions were made in Dulbecco’s minimal essential medium (DMEM) supplemented with 10% fetal calf serum (FCS) and gentamicin (50 μg/ml). Fifty microliters of CDV (25 PFU per well) was added to each well (excluding cell controls), and plates were incubated for 90 min at 37°C. One hundred microliters of a suspension of Vero cells (2 × 105/ml) was added to each well. Plates were incubated for 4 to 6 days at 37°C.
Antibody assays.
Titers of antibodies against the CDV H and F proteins were determined by ELISAs. CDV-infected P815 cells were distributed in 96-well microtiter plates (1.5 × 105 cells per well). Diluted antisera were incubated with the intact cells for 90 min at 37°C. The specific antibody was developed with biotinylated anti-mouse immunoglobulin G1 (IgG1) or IgG2a and the streptavidin-phosphatase alkaline system (Sigma). Results are expressed with reference to control anti-H or anti-F monoclonal antibodies, which were used to standardize the assays for IgG1 and IgG2a. Titers were calculated with the SOFTmax program (Molecular Device Corporation, Menlo Park, Calif.). Data are given in nanograms of specific antibody per milliliter of serum sample.
CTL assays.
Spleens were removed from immunized animals and, after elimination of erythrocytes by perfusion with DMEM, cocultivated with CDV-infected P815 (H-2d) cells which had been incubated with mitomicin C (25 μg/ml) for 30 min at 37°C. The ratio of spleen cells to stimulator cells was 30:1. The cocultures were distributed in 24-well plates and incubated in DMEM–10% FCS containing 5 × 10−5 M 2-mercaptoethanol. On day 5 half the medium was changed, and the cytotoxic activity was tested on day 7.
For the CTL assays P815 cells and CDV-infected P815 cells were radiolabelled with 50 μCi of 51Cr for 90 min at 37°C, washed twice in DMEM–1% FCS, incubated for 1 h in DMEM–10% FCS, and washed once before use in a 4-h 51Cr release assay. The percent cell lysis was calculated as follows: {[experimental counts per minute (cpm) − spontaneous cpm]/[total cpm − spontaneous release]} × 100. Results are expressed as the percent lysis. Spontaneous release and total release were determined for target cells incubated with medium alone and after the addition of 100 μl of 1 M HCl, respectively.
Virus challenge.
Immunized mice were challenged by i.c. inoculation of a neuroadapted CDV strain. Briefly, 3 weeks after the first immunization, half of the mice in each group received boosters. Two weeks later, mice were inoculated i.c. with 50 μl of the neuroadapted strain (5 × 104 PFU/ml). Control animals consisted of either uninjected mice or mice immunized with blank vector. Survival was monitored daily for 30 days.
RESULTS
Humoral and cellular response to pV1J-CDV-H.
The plasmid PV1J-CDV-H when transfected into either L or HeLa cells elicited a high level of expression of CDV H at the surface of the cells, as measured by immunofluorescence (data not shown).
In order to examine the efficiency of this plasmid as an immunogen in vivo, BALB/c mice were immunized by different routes of inoculation. A single i.m. inoculation (100 μg/mouse) induced high levels of antibody, mainly of the IgG2a isotype, which increased to maximum values by 3 months (Table 1). Application of the DNA by tail scarification (100 μg/mouse) or multipuncture ring induced lower antibody levels (Table 1). Whereas scarification induced mainly IgG2a antibodies, the multipuncture ring system induced predominantly IgG1.
TABLE 1.
Antibody response in mice immunized with pV1J-CDV-H by different routes
| DNA and route | Dose (μg) | Wk of sampling (no. of inoculations) | Level (mean ± SD; ng/ml) of:
|
|
|---|---|---|---|---|
| IgG1 | IgG2a | |||
| pV1J-CDV-H in PBSa | ||||
| Multipuncture ring | 100 | 7 | 305 ± 169 | 14 ± 7.5 |
| Tail scarification | 100 | 7 | 71 ± 10 | 216 ± 22 |
| Subcutaneous injection | 100 | 7 | —d | — |
| Intramuscular injection | 100 | 6 | 3,300 ± 2,067 | 7,150 ± 4,550 |
| 12 | 7,000 ± 7,970 | 9,000 ± 6,450 | ||
| pV1J-CDV-Hb | ||||
| Gene gun | 2 | 6 (1) | 9,700 ± 1,300 | — |
| 6 (2) | 18,200 ± 7,860 | — | ||
| 27 (1) | 14,000 ± 5,600 | — | ||
| 27 (2) | 26,000 ± 11,900 | — | ||
| 0.5 | 6 (1) | 7,400 ± 800 | — | |
| 6 (2) | 13,500 ± 2,410 | — | ||
| 27 (1) | 7,950 ± 2,000 | — | ||
| 27 (2) | 16,000 ± 1,000 | — | ||
| 0.1 | 6 (1) | 3,400 ± 1,400 | — | |
| 6 (2) | 9,000 ± 3,125 | — | ||
| 27 (1) | 2,200 ± 2,400 | — | ||
| 27 (2) | 10,500 ± 5,300 | — | ||
| pV1Jc | ||||
| Gene gun | 2 | 3 | — | — |
| 7 | — | — | ||
Groups of five mice were inoculated with the indicated quantity of DNA in PBS by different methods and routes of immunization.
Groups of eight mice were inoculated by the gene gun method with the indicated amounts of DNA. In each group, half of the mice received boosters 3 weeks after the first immunization.
Control mice were inoculated by the gene gun method with 2 μg of blank vector.
—, level of <1 ng/ml.
To study the efficiency of the gene gun, mice were inoculated with 2, 0.5, or 0.1 μg of pV1J-CDV-H, and groups of mice received boosters with the same quantity of plasmid DNA either at 3 weeks or at 24 weeks. The antibody response after a single inoculation increased during the first 6 weeks and then remained stable over the following 5 months (Table 1). The level of the response was related to the amount of DNA inoculated. Booster immunizations given at 3 weeks or 6 months increased the antibody titers at least twofold. After this method of vaccination only IgG1 antibodies were detected, IgG2a antibodies being present at concentrations of less than 0.1 μg/ml.
When the sera were examined for virus neutralization activity, only approximately half of the mice immunized with gene gun and 40% of the mice immunized i.m. had significant neutralizing antibody titers (Table 2).
TABLE 2.
Induction of CDV-neutralizing antibodies after DNA vaccination
| Plasmid and route | Dose (μg) | No. of mice with neutralizing antibody titer of >80/total no. of mice vaccinated |
|---|---|---|
| pV1J-CDV-H | ||
| Gene gun | 2 | 4/8 |
| 0.5 | 4/8 | |
| 0.1 | 3/8 | |
| i.m. | 100 | 2/5 |
| pV1J-CDV-F | ||
| Gene gun | 2 | 0/8 |
| 0.5 | 0/8 | |
| 0.1 | 0/8 | |
| i.m. | 100 | 0/5 |
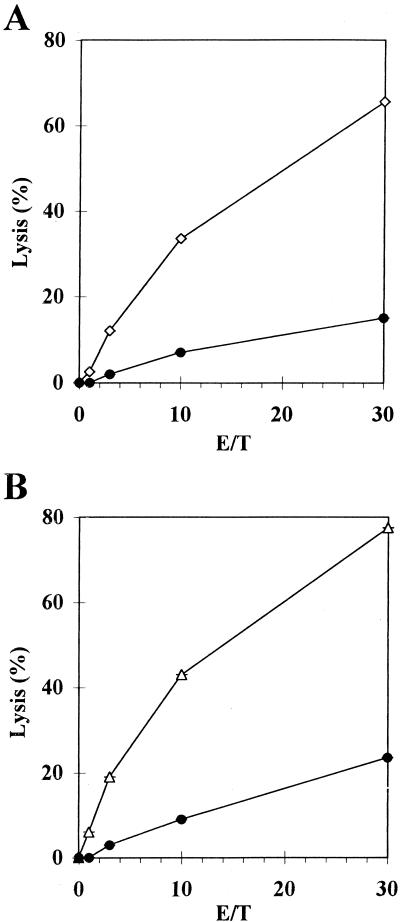
To study the potential of the pV1J-CDV-H to induce CTLs, BALB/c mice were immunized by the gene gun method and 3 weeks later their spleens were removed and the CTL response was examined. A good class I-restricted CTL response was observed (Fig. 1A). Furthermore, no NK activity could be detected in these effectors since they did not lyse NK-sensitive YAC-1 targets (data not shown).
FIG. 1.
Anti-CDV H (A) and F (B) CTL responses 30 days after a single immunization with pV1J-CDV-H or pV1J-CDV-F (2 μg) by the gene gun method, respectively. After in vitro stimulation with CDV-infected p815 cells, lysis was measured for CDV-infected P815 cells (open symbols) and P815 cells (circles), which were used as a negative control. The results are the percents lysis for pairs of animals at graded effector-to-target (E/T) ratios.
Humoral and cellular response to pV1J-CDV-F.
Transfection of L or HeLa cells with pV1J-CDV-F yielded a high level of expression of the CDV F protein at the surface of the cells as measured by immunofluorescence (results not shown). To investigate the immunizing potential of this plasmid, BALB/c mice were immunized i.m. with pV1J-CDV-F at 100 μg/mouse and the antibody responses were measured after 3 and 7 weeks. Immunization with 20 μg also elicited an antibody response (data not shown). Antibody concentrations of 1 to 2 μg/ml were induced with both concentrations of DNA by 3 weeks, decreasing slightly by 7 weeks (Table 3). The IgG2a antibody was the predominant isotype.
TABLE 3.
Antibody response in mice immunized with pV1J-CDV-F
| DNA and route | Dose (μg) | Wk of sampling (no. of inoculations) | Level (mean ± SD; ng/ml) of:
|
|
|---|---|---|---|---|
| IgG1 | IgG2a | |||
| pV1J-CDV-F in PBS, i.m. | 100 | 3 | 690 ± 230 | 1,670 ± 657 |
| 7 | 213 ± 100 | 950 ± 480 | ||
| 20 | 3 | 687 ± 530 | 1,009 ± 574 | |
| 7 | 448 ± 743 | 312 ± 187 | ||
| pV1J-CDV-F, gene gun | 2 | 6 (1) | 5,345 ± 2,400 | 8,232 ± 1,930 |
| 6 (2) | 15,400 ± 3,467 | 10,300 ± 3,420 | ||
| 15 (1) | 9,000 ± 990 | 4,000 ± 770 | ||
| 15 (2) | 21,000 ± 6,600 | 14,000 ± 4,000 | ||
| 0.5 | 6 (1) | 2,000 ± 1,000 | 2,500 ± 485 | |
| 6 (2) | 8,500 ± 5,480 | 13,500 ± 7,200 | ||
| 15 (1) | 5,200 ± 1,344 | 3,000 ± 1,484 | ||
| 15 (2) | 13,600 ± 6,500 | 11,000 ± 4,000 | ||
| 0.1 | 6 (1) | 2,300 ± 264 | 1,461 ± 840 | |
| 6 (2) | 3,000 ± 660 | 4,600 ± 1,250 | ||
| 15 (1) | —a | 600 ± 210 | ||
| 15 (2) | 1,600 ± 655 | 1,900 ± 1,153 | ||
—, level of <1 ng/ml.
Immunization with the gene gun (2, 0.5, or 0.1 μg/mouse) induced high levels of anti-F antibodies, with increases during a 6-week period (Table 3). The response was greater with higher DNA concentrations. A booster inoculation at 3 or 12 weeks increased the antibody titers two- to fourfold. The antibody isotype profiles were mixed, with both IgG1 and IgG2a being induced. The different protocols (DNA concentration and booster inoculation) did not alter radically the antibody isotype profile.
Although CTL responses for CDV F have not yet been demonstrated, we examined the possibility that DNA vaccination with pV1J-CDV-F may induce a CTL response. BALB/c mice were inoculated by the gene gun method, and the CTL response was examined 3 weeks later. A class I-restricted CTL response of similar magnitude to that induced by the DNA for the H protein was observed (Fig. 1B). Furthermore, no NK activity could be detected in these effectors, since they did not lyse NK-sensitive YAC-1 targets (data not shown).
Survival study following i.c. viral challenge.
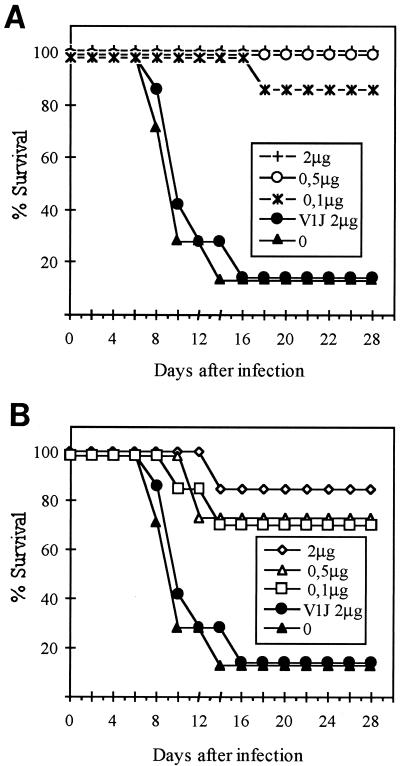
In order to evaluate the level of protection induced by the CDV H and F DNAs, mice were immunized by the gene gun method with 2, 0.5, or 0.1 μg of pV1J-CDV-H or pV1J-CDV-F. Control mice were either not injected or inoculated with blank vector. Two weeks after the last immunization, the mice were challenged i.c. with a lethal dose of a neuroadapted strain of CDV (Fig. 2). In the pV1J-CDV-H-immunized mice, the protection was almost complete, with only one of eight mice receiving the lowest DNA concentration dying (Fig. 2A). The pV1J-CDV-F gave 70 to 90% protection (Fig. 2B).
FIG. 2.
Survival of mice immunized by the gene gun method with pV1J-CDV-H (A) or pV1J-CDV-F (B) after lethal i.c. challenge. Mice were immunized once or twice with pV1J-CDV-H or pV1J-CDV-F at the indicated doses. Controls either were not injected (closed triangles) or were inoculated with blank vector (V1J) (closed circles). All animals were challenged i.c. 2 weeks after the final immunization, and survival was assessed daily for 30 days.
We observed previously that with mice inoculated with sublethal doses of the neuroadapted strain of CDV, 20 to 40% of the animals became obese (2). We therefore kept the vaccinated-challenged mice under observation for a further 3 months to see if they developed such a syndrome. Of the 21 pV1J-CDV-H-immunized animals, only one mouse became obese (5%). In contrast, 5 of 16 surviving mice immunized with pV1J-CDV-F did so (31%) (Table 4). This phenomenon was not related either to the concentration of DNA used for vaccination or to the serum antibody titers attained (Table 4).
TABLE 4.
Induction of obesity syndrome in mice after i.c. challenge with CDVa
| DNA dose (μg) | No. of inoculations | pV1J-CDV-H
|
pV1J-CDV-F
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mortalityb | Frequency of obesityb | Mean titer of specific IgG (ng/ml) in:
|
Mortalityb | Frequency of obesityb | Mean titer of specific IgG (ng/ml) in:
|
||||
| Normal mice | Obese mice | Normal mice | Obese mice | ||||||
| 2 | 1 | 0/4 | 0/3 | 10,160 | —c | 1/4 | 1/3 | 13,570 | 13,600 |
| 2 | 0/3 | 0/4 | 18,210 | — | 0/3 | 1/3 | 25,800 | 32,580 | |
| 0.5 | 1 | 0/4 | 0/4 | 7,780 | — | 1/3 | 0/2 | 4,980 | — |
| 2 | 0/4 | 0/4 | 13,570 | — | 1/4 | 2/3 | 22,150 | 21,000 | |
| 0.1 | 1 | 1/4 | 0/3 | 2,320 | — | 1/3 | 0/2 | 3,890 | — |
| 2 | 0/3 | 1/3 | 9,020 | 11,260 | 1/4 | 1/3 | 7,690 | 6,490 | |
Mean body weights were 20.8 ± 0.9 g in the normal mice and 32.5 ± 2.08 g in the obese mice.
No. of mice showing mortality or obesity/total no. of mice.
—, level of <1 ng/ml.
DISCUSSION
CDV has a wide host range in animals that has recently been shown to extend to certain members of the Felidae family (lions and tigers) both in the wild and in captivity (18, 22). The currently available CDV vaccines are of the live attenuated type and are based on passage in either canine or avian cells. The latter vaccine is more attenuated in dogs, but its efficiency is lower than the canine-cell-passaged vaccine. Although both these vaccines have an attenuated phenotype in dogs, they may induce a more virulent infection in other species (3). Thus, a vaccine which does not consist of infectious virus may have certain advantages in terms of such a risk. However, the mechanisms of protection that such vaccines induce remain to be investigated.
In the present study, we have examined the possibility of protecting against CDV infection by DNA vaccination. Our studies on the closely related measles virus have shown that both humoral and cell-mediated immunity are important in protection. Antibodies to either of the two viral glycoproteins neutralize infection in vitro and passively protect mice in vivo (28). Furthermore, immunization of mice with a single class I-restricted CTL epitope protects them from a lethal i.c. dose of CDV (1). In contrast, in a monkey model the CTL response could not be shown to reduce the virus load.
In the present study, we have examined the effects of the route of DNA inoculation on the quantity and quality of the immune response against CDV. Both i.m. and gene gun immunizations induced high levels of antibodies. In agreement with previous observations obtained with measles virus glycoproteins (4, 5) and those made by others with different viral systems, the gene gun induced an IgG1 (Th2) response, whereas inoculation by the other routes induced an IgG2a (Th1) response (20, 26). These results do not appear to be related to the site of inoculation, as CDV H DNA given by the gene gun directly into the muscle still induced IgG1 antibodies (24a). Similar results have been reported with influenza virus H DNA (7). Thus, it was surprising to find that the CDV F DNA inoculated with the gene gun induced a mixed IgG1 and IgG2a response. This is in contrast to our studies with measles virus F, which despite having 70% sequence homology with CDV F induces only IgG1 antibodies. Recent studies have reported that certain DNA sequences can induce different cytokines and so influence the subsequent immune response (13, 23, 24). It is possible that such sequences favoring Th1 cytokines may be present in the CDV F sequence, and it should therefore be possible to identify them by using measles virus–CDV-F DNA chimeras.
Although mice immunized with either CDV H or F were protected from a lethal i.c. challenge, of the CDV F-immunized animals, more than 30% became obese within the 3 months following the challenge. Our group and others have previously described this phenomenon in mice infected with sublethal doses of CDV (2, 16), and it seems to result in loss of critical populations of hypothalamic neurons (19). In our study, an obesity syndrome was observed in animals challenged with CDV which had been vaccinated with different quantities of DNA, and this did not correlate with serum antibody levels. The major difference between the two groups of animals was the presence of virus-neutralizing antibodies, detectable in vitro only, for the CDV H-immunized mice. These results are compatible with the previous report by Hirayama et al. (11), but it is not clear if neutralizing antibodies are the main marker for protection against subsequent virus-induced pathologies or whether an alternative type of immune response is implicated in protection.
There are numerous examples in which antibodies do not neutralize virus in vitro but protect against it in vivo and in which they neutralize it in vitro but do not protect against it in vivo. In the CDV model, it is necessary to study the contributions of the different types of immune responses.
ACKNOWLEDGMENTS
These studies were supported by a grant from the French Rhône-Alpes Région (Emergence Programme).
We thank C. Orvell for supplying CDV monoclonal antibodies for calibrating our ELISAs, F. Henry for animal care, and B. Maret for editorial assistance.
REFERENCES
- 1.Beauverger P, Cardoso A I, Daviet L, Buckland R, Wild T F. Analysis of the contribution of CTL epitopes in the immunobiology of morbillivirus infection. Virology. 1996;219:133–139. doi: 10.1006/viro.1996.0230. [DOI] [PubMed] [Google Scholar]
- 2.Bernard A, Wild T F, Tripier M F. Canine distemper infection in mice: characterization of a neuro-adapted virus strain and its long-term evolution in mice. J Gen Virol. 1983;64:1571–1579. doi: 10.1099/0022-1317-64-7-1571. [DOI] [PubMed] [Google Scholar]
- 3.Bush M, Montali R J, Brownstein D, James A E, Appel M J G. Vaccine-induced canine distemper in a lesser panda. J Am Vet Med Assoc. 1976;169:959–960. [PubMed] [Google Scholar]
- 4.Cardoso A I, Blixenkrone-Moller M, Fayolle J, Liu M, Buckland R, Wild T F. Immunization with plasmid DNA encoding for the measles virus hemagglutinin and nucleoprotein leads to humoral and cell-mediated immunity. Virology. 1996;225:293–299. doi: 10.1006/viro.1996.0603. [DOI] [PubMed] [Google Scholar]
- 5.Cardoso A I, Sixt N, Vallier A, Fayolle J, Buckland R, Wild T F. Measles virus DNA vaccination: antibody isotype is determined by the method of immunization and by the nature of both the antigen and the coimmunized antigen. J Virol. 1998;72:2516–2518. doi: 10.1128/jvi.72.3.2516-2518.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carpenter J W, Appel M J G, Erickson R C, Novilla M N. Fatal vaccine induced canine distemper virus infection in black-footed ferrets. J Am Vet Med Assoc. 1976;169:961–964. [PubMed] [Google Scholar]
- 7.Feltquate D M, Heaney S, Webster R G, Robinson H L. Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J Immunol. 1997;158:2278–2284. [PubMed] [Google Scholar]
- 8.Fu T, Ulmer J B, Caulfield M J, Deck R R, Friedman A, Wang S, et al. Priming of cytotoxic T lymphocytes by DNA vaccines; requirement for professional antigen presenting cells and evidence for antigen transfer from myocytes. Mol Med. 1997;3:362–371. [PMC free article] [PubMed] [Google Scholar]
- 9.Fynan E F, Webster R G, Fuller D H, Haynes J R, Santoro J C, Robinson H L. DNA vaccines: protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc Natl Acad Sci USA. 1993;90:11478–11482. doi: 10.1073/pnas.90.24.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harder T C, Kenter M, Vos H, Siebelink K, Huisman W, Van Amerongen G, et al. Canine distemper virus from diseased large felids: biological properties and phylogenetic relationships. J Gen Virol. 1996;77:397–405. doi: 10.1099/0022-1317-77-3-397. [DOI] [PubMed] [Google Scholar]
- 11.Hirayama N, Senda M, Nakashima N, Takagi M, Sugiyama M, Yoshikawa Y, et al. Protective effects of monoclonal antibodies against lethal canine distemper virus infection in mice. J Gen Virol. 1991;72:2827–2830. doi: 10.1099/0022-1317-72-11-2827. [DOI] [PubMed] [Google Scholar]
- 12.Iwasaki A, Torres C A T, Ohashi P S, Robinson H L, Barber B H. The dominant role of bone marrow-derived cells in CTL induction following plasmid DNA immunization at different sites. J Immunol. 1997;159:11–14. [PubMed] [Google Scholar]
- 13.Klinman D M, Yamshchikov G, Ishigatsubo Y. Contribution of CpG motifs to the immunogenicity of DNA vaccines. J Immunol. 1997;158:3635–3639. [PubMed] [Google Scholar]
- 14.Kozak M. Bifunctional messenger RNAs in eukaryotes. Cell. 1986;47:481–483. doi: 10.1016/0092-8674(86)90609-4. [DOI] [PubMed] [Google Scholar]
- 15.Kunkel T A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci USA. 1985;82:488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyons M, Faust I M, Hemmes R B, Buskirk D R, Hirsh J, Zabriskie J B. A virally induced obesity syndrome in mice. Science. 1982;216:82–85. doi: 10.1126/science.7038878. [DOI] [PubMed] [Google Scholar]
- 17.Montgomery D L, Shiver J W, Leander K R, Perry H C, Friedman A, Martinez D, et al. Heterologous and homologous protection against influenza A by DNA vaccination: optimization of DNA vectors. DNA Cell Biol. 1993;12:777–783. doi: 10.1089/dna.1993.12.777. [DOI] [PubMed] [Google Scholar]
- 18.Morell V. Serengeti’s big cats going to the dogs. Science. 1994;264:1664. doi: 10.1126/science.8209243. [DOI] [PubMed] [Google Scholar]
- 19.Nagashima K, Zabriskie J B, Lyons M J. Virus-induced obesity in mice: association with a hypothalamic lesion. J Neuropathol Exp Neurol. 1992;51:101–109. doi: 10.1097/00005072-199201000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Pertmer T M, Roberts T R, Haynes J R. Influenza virus nucleoprotein-specific immunoglobulin G subclass and cytokine responses elicited by DNA vaccination are dependent on the route of vector DNA delivery. J Virol. 1996;70:6119–6125. doi: 10.1128/jvi.70.9.6119-6125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raz E, Carson D A, Parker S E, Parr T B, Abai A M, Aichinger G, et al. Intradermal gene immunization: the possible role of DNA uptake in the induction of cellular immunity to viruses. Proc Natl Acad Sci USA. 1994;91:9519–9523. doi: 10.1073/pnas.91.20.9519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roelke-Parker M E, Munson L, Packer C, Kock R, Cleaveland S, Carpenter M, et al. A canine distemper virus epidemic in Serengeti lions (Panthera leo) Nature. 1996;379:441–445. doi: 10.1038/379441a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roman M, Martin-Orozco E, Goodman J S, Nguyen M, Sato Y, Ronaghy A, et al. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat Med. 1997;3:849–854. doi: 10.1038/nm0897-849. [DOI] [PubMed] [Google Scholar]
- 24.Sato Y, Roman M, Tighe H, Lee D, Corr M, Nguyen M D, et al. Immunostimulatory DNA sequences necessary for effective intradermal gene immunization. Science. 1996;273:352–354. doi: 10.1126/science.273.5273.352. [DOI] [PubMed] [Google Scholar]
- 24a.Sixt, N., et al. Unpublished observations.
- 25.Tighe H, Corr M, Roman M, Raz E. Gene vaccination: plasmid DNA is more than just a blueprint. Immunol Today. 1998;19:89–97. doi: 10.1016/s0167-5699(97)01201-2. [DOI] [PubMed] [Google Scholar]
- 26.Torres C A T, Iwasaki A, Barber B H, Robinson H L. Differential dependence on target site tissue for gene gun and intramuscular DNA immunizations. J Immunol. 1997;158:4529–4532. [PubMed] [Google Scholar]
- 27.Ulmer J B, Deck R R, Dewitt C M, Donnelly J J, Liu M A. Generation of MHC class-I restricted cytotoxic T lymphocytes by expression of a viral protein in muscle cells: antigen presentation by non-muscle cells. Immunology. 1996;89:59–67. doi: 10.1046/j.1365-2567.1996.d01-718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wild T, Bernard A, Spehner D, Drillien R. Construction of vaccinia virus recombinants expressing several measles virus proteins and analysis of their efficacy in vaccination of mice. J Gen Virol. 1992;73:359–367. doi: 10.1099/0022-1317-73-2-359. [DOI] [PubMed] [Google Scholar]