Abstract
Fibrinogen-like protein 2 (FGL2) is an important immune regulator of both innate and adaptive response. It is present on the surface of macrophages and endothelial cells, and can be constitutively secreted by CD4+CD8+ T cells. Previous studies showed that FGL2 is a potential target for the treatment of experimental autoimmune myocarditis. However, the molecular mechanism of the roles of FGL2 in experimental autoimmune myocarditis is poorly understood. Here, we silenced FGL2 gene by using FGL2-RNAi lentivirus to reveal the heart function in experimental autoimmune myocarditis rats. We found that the cardiac myosin of pigs’ hearts induced Lewis rats to come into being as autoimmune myocarditis. TLR9 was upregulated in the heart of experimental autoimmune myocarditis rats. After primary immunization (21 day), the cardiac function of the myocarditis model group improved (P < 0.05). Significantly, the levels of INF-α and NF-κB in the FGL2-RNAi-treated group were lower compared to those in the myocarditis model (EAM) group (P < 0.05). Notably, the inflammation score correspondence with the protein and mRNA levels of TLR9 in myocardial tissues was markedly reduced compared to that in the EAM group (P < 0.05). These results support a role of FGL2 to alleviate inflammatory situation in the myocardium through regulation of the TLR9 signaling pathway in the experimental autoimmune myocarditis rats.
Keywords: Experimental autoimmune myocarditis, Toll receptor 9, Fibrinogen-like protein 2
Introduction
Dilated cardiomyopathy (DCM) is a common cause of heart failure and sudden death. Multiple autoimmune mechanisms are involved in DCM [1, 2]. Experimental autoimmune myocarditis is a model of inflammatory heart disease [3].
Fibrinogen-like protein 2 (FGL2), a member of the fibrinogen-related superfamily of proteins known to be secreted by T cells, has recently been reported by a number of groups to be highly expressed by Tregs and has been proposed to have a role in Treg effector function [4, 5]. Our recent study identified that FGL2 gene silencing induces myocardial microvascular endothelial cell (MMVEC) proliferation and cell migration by upregulation of Ang-1 and Ang-2 [6, 7]. Furthermore, overexpression of FGL2 deteriorates the heart function of experimental autoimmune myocarditis rats, suggesting that FGL2 is a potent target for the treatment of experimental autoimmune myocarditis [8, 9].
Toll receptor 9 (TLR9), a member of the toll-like receptor (TLR) family, plays critical roles in a variety of autoimmune diseases and inflammatory diseases. It is mainly involved in activating immune cell signal transduction by mediated unmethylated bacterial CpG DNA, causing a signaling cascade leading to the production of proinflammatory cytokine [10, 11]. TLR9 signaling could be nodal in experimental viral myocarditis [12]. Mathur et al. reported that activation of cardiac TLR9 prior to cardiac ischemia and reperfusion has been shown to limit subsequent myocardial damage and also improve cardiac function [13]. However, binding of microbial DNA to TLR9 can trigger inflammation in chronic periodontitis [14]. Oka et al. revealed that mitochondrial DNA that escaped from autophagy causes inflammation and heart failure by TLR9-mediated inflammatory responses in cardiomyocytes [15]. So far, there is no direct evidence to prove the role of TLR9 in experimental autoimmune myocarditis, and whether the TLR9 signaling pathway mediated by FGL2 remains largely unknown.
Here we hypothesized that FGL2, the CD4+CD25+ regulatory T cell effector molecule, is a potential target for the treatment of experimental autoimmune myocarditis rat. To answer this question, we tried to reveal whether FGL2 can downregulate TLR9 and improve heart function in experimental autoimmune myocarditis rat by silencing of FGL2 using FGL2-RNAi lentivirus. Experimental autoimmune myocarditis rats were randomly divided into three groups: myocarditis model group (EAM), GFP empty carrier lentivirus transfection group (GFP), and FGL2-RNAi-LV interference group (RNAi). Echocardiography was performed on 0 day prior to the initial immunization, and 21 days after the initial immunization. The inflammatory situation of heart tissues was stained with H&E staining. The serum IL-6, INF-a, and NF-κB levels were detected by using ELISA kit. The percentage of CD4+CD25+ T cells in total T cells was detected by flow cytometry. The protein and transcription levels of TLR9 and CLTA-4 mRNA were detected by RT-PCR or Western blot, respectively.
Materials and reagents
Ethics statement
The study protocol was approved by the Institutional Review Board of the First Affiliated Hospital of Nanchang University. All participants signed the informed consent prior to study participation. Laboratory animals were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing ICP 05076685). All efforts were made to minimize suffering.
Antibodies and reagents
Recombinant virus’ plasmid and the carriers of auxiliary packaging original plasmids were purchased from Shanghai Jikai Gene Chemical Co.. PCR re-agents were as follows: TRIzol kits were purchased from Invitrogen, retrovirus RNA kits from Promega, and RNase free from Axygen; PCR primer sequences were designed and synthesized by Shanghai Promega Biological company; P1250 Protein Extraction Kit I was purchased from Applygen Technologies Inc.; rat anti-Fgl2 antibody was purchased from Sigma; rat anti-TLR9 antibody was purchased from Novus Biologicals; cardiac myosin of pigs’ heart was purchased from Sigma, complete Freund’s adjuvant CFA was purchased from Sigma; and rat anti-β-actin antibody and goat anti-rat IgG antibody were purchased from ZSGB-BIO.
FGL2-RNAi-LV vector construction and packaging
For gene sequencing purposes, a multiple target sequence of RNA interference was designed according to the manufacturer’s instructions. Briefly, the best kinetic parameters were selected and used for targeting in subsequent experiment processes. Synthesis of the sequence of single-stranded oligonucleotides of DNA interference was used to produce the double-strand chain DNA through annealing. Both ends of DNAs that contain enzyme loci were directly ligated on the RNAi lentivirus’ carrier digested by enzymes. Products will be transformed into the preparation of bacteria cells. PCR products were confirmed by sequencing. The correct alignments of plasmids were extracted building a successful cloning gene RNAi lentivirus vector.
To get a high titer virus preparation, 293 T cells were used for the transfection of the different target gene RNA interference lentivirus’ vector according to the Invitrogen Lipofectamine 2000 instructions. Transfection effects were observed by a fluorescence microscope after a 24-h transfection. After a 36-h transfection, cells were harvested, and the target protein expression was detected by using Western blot. Optimization of the interference effects for the different targets was considered, and the high effective RNA interference target 5 -TGGAGAGTCAGGTGAACAA-3 was eventually selected. In final packaging, the virus containing FGL2-RNA1-LV was diluted to a series of concentrations to determine the virus drops to 1 × 108 TU/mL.
Experimental autoimmune myocarditis models
Thirty-two 6–8-week-old Lewis male rats were purchased from Beijing Vital River Laboratories (VRL) Animal Technology company. Lewis rats were randomly divided into four groups: the control group, the myocarditis model group (EAM), the GFP empty carrier lentivirus transfection group (GFP), and the FGL2-RNAi-LV interference group (Interference). Each group has eight rats (n = 8).
The EAM rat model was generated according to those previously described [8, 9]. Briefly, the purification of the pig heart muscle myosin is soluble in 0.15 mol/L PBS solution, controlling the concentration in 2 g/L, and then the solution, with complete Freund’s adjuvant (CFA), was mixed and emulsified completely according to a volume of 1:1, being made into homogeneous emulsion; then each one gives 1 mL emulsion (including myosin 1 mg) for injection of immune Lewis rats by subcutaneous of bilateral inguinal and axillary.
By a single subcutaneous administration in both foot pads, each rat was immunized on day 0 with 0.2 mL emulsion, containing 1 mg cardiac myosin with an equal volume of complete Freund’s adjuvant. Complete Freund’s adjuvant was supplemented with mycobacterium tuberculosis H37RA at a concentration of 10 mg/mL. The rats in the control group were only immunized with complete Freund’s adjuvant. FGL2-RNAi-LV was dissolved in 0.9% saline solution, with the final volume then measured to 4 mL and thoroughly incorporated. According to 80 μL FGL2-RNAi-LV, RNAi group rats were administrated from the rat tail intravenously on day 1 after initial immunization. GFP group rats were also administrated with the same volume of GFP carrying an empty carrier lentivirus mixture with 0.9% saline solution from the tail vein at the same time.
Echocardiography and cardiac function evaluation
Echocardiography and Doppler sonography using an American GE Vivid 7-type color Doppler flow imaging device (probe frequency 10.0 MHz) were performed together with electrocardiography on 0 and 21 days after the initial immunization. Rats were peritoneally administrated with 20% urethane solution (3 mL/kg). After anesthesia, rats were fixed to lie on the back slightly on the left side position. Ejection fraction (EF%), left ventricular end-diastolic diameter (LVEDd), left ventricular end systolic diameter (LVEDs), left ventricular short axis shortening fraction (FS %), and other indicators were detected.
On day 21 after initial immunization, four rats in each group were randomly selected and administrated with 20% urethane solution (3 mL/kg). After anesthesia, blood from eyeballs was collected and used in indirect ELISA to detect the inflammatory factor and also the percentage of CD4+CD25+ T cells in the rat serum. Rats were then dissected and their hearts removed, with incisions to the heart along the coronal slitting. Half of the heart was fixed in 4% paraformaldehyde for pathological examination; the other half of the heart was divided into two parts, one to be stored in − 80 °C for later Western blotting tests, and the other saved in tissue preservation solution for RT-PCR detection.
Histopathological analysis
Myocardial tissue was fixed in 4% paraformaldehyde and gradient ethanol dehydration, paraffin embedded, and continuously sliced in 4-μm thickness for conventional HE staining. The observation group and the normal group of rat myocardial inflammation were observed under light microscopy. Myocardial inflammation was given a histologic score from 0 to 4. Five high-magnification microscope images were randomly from the field of vision, where each field of vision of the inflammatory cell infiltration and necrosis area, proportional to the view area, were scored as follows: no involvement—0 point; less than or equal to 25%—1 point; more than 25%, less than or equal to 50%—2 points; more than 50%, less than or equal to 75%—3 points; more than 75%—4 points.
Enzyme-linked immunosorbent assay
Rat serum was separated after centrifugation of blood samples at 3000 rpm for 20 min. The serum IL-6, INF-α, and NF-κB levels were measured by ELISA kit according to the manufacturer’s instructions. Briefly, standard serum samples or blank was added to each well, and incubated at 37 °C for 1 h. After washing, the biotinylated detector antibody was added to each well, and incubated for 30 min followed by other washing. Avidin-HRP conjugation was added to each well and incubated for 30 min. After washing, the TMB substrate was added to each well and incubated in the dark for 10 min. Finally, stop solution was added into each well, followed by detection of O.D. absorbance at 450 nm with a microplate reader within 5 min.
Flow cytometry
Percentage of CD4+CD25+ T cells was detected by flow cytometry analysis. For in vivo experiments, the blood samples were obtained from different groups of the rats, which were prepared as a single-cell suspension in flow cytometry buffer (PBS with 2% FBS and 0.1% NaN3). The purified CD4+CD25− T cells were stained with CD4-FITC and CD25-PE antibodies. The samples were acquired with a FACSCalibur (BD Biosciences). The data were analyzed with FlowJo 7.6.1 software and FCS Express Version 4 software.
Quantitative reverse-transcription polymerase chain reaction
Total RNA was extracted from the heart tissues with TRIzol (Invitrogen, Carlsbad, CA, USA) according to the standard protocol and then reverse-transcribed. cDNA was then amplified using the ABI 7700 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) with a set of primers and probes corresponding to TLR9 and CTLA-4 and GAPDH. GAPDH was used as the control. The total reaction volume was 20 μL for PCR amplification. PCR primer sequences were designed and synthesized by Shanghai Promega Biological company. Primer sequences are the following: GAPDH, forward: 5′-GAAACCTGCCAAGTATG-3′, reverse: 5′-ACCAGGAAATGAGCTTGAGC-3′; CTLA-4: forward: 5′-AAGGACTGAGGGCTGCTGAAC-3′, reverse: 5′-GAATCTGGCATGGTTCTGGA-3′; TLR-9: forward: 5′-TGCAGG AGC TGAACATGAAC-3′, reverse: 5′-ATTGGACAGGTCCACAAAGC-3′. The PCR reaction program was as follows: 95 °C denaturation in advance 2 min, 95 °C degeneration 15 s, 62 °C annealing 30 s, and 72 °C extension 32 s, in total of 40 circulations, finally 72 °C extension 5 min, according to the 2−ΔΔCt method for quantitative analysis, each sample determination repeated five times.
Western blot analysis
For the determination of protein levels, equal amounts of protein extracts (30 mg) were separated by 10, 12.5, or 15% sodium dodecyl sulfate (SDS)-polyacryl-amide gel electrophoresis (Bio-Rad, CA, USA), respectively. Samples were electrophoretically transferred to nitrocellulose membranes (semi-dry transfer). Membranes were blocked with 5% non-fat dry milk in Tris-buffered saline (20 mM Tris, pH 7.6, 137 mM NaCl) with 0.1% Tween 20, washed, and then incubated with primary antibodies. According to standard protocols, the blots were probed with various primary antibodies, and the correspondence HRP-conjugated secondary antibodies were used to detect the specific protein bands with ECL detection reagents. The intensities of the various protein bands were quantified by densitometry. For each sample, the test was repeated three times.
Statistical analysis
Data were analyzed using SPSS software (version 18; SPSS, Chicago, USA). All values were expressed as means ± SE. Statistical analysis was determined using Student’s t test (when two groups were considered) or by one-way analysis of variance (ANOVA) followed by multiple comparisons with the LSD post hoc test. P < 0.05 was considered significant.
Results
FGL2 knockdown significantly reduces TLR9 expression at protein level in the myocardial tissue of rats
As shown in Fig. 1, FGL2 in myocardial tissues of the EAM group was significantly higher than both in the NC group and FGL2-RNAi group, on day 21 after initial immunization (P < 0.05). Notably, TLR9 protein expression in myocardial tissue of the EAM group was significantly higher than that in the control group on day 21 after initial immunization (P < 0.05). However, TLR9 was remarkably reduced in the FGL2-RNAi group compared to that in the EAM group (P < 0.05; Fig. 1). TLR9 expression was not different between EAM and GFP groups. Taken together, these data demonstrated that FGL2 knockdown can significantly reduce TLR9 protein expression in the myocardial tissue of rats, which indicated the pathological relevance of heart function mediated by FGL2 through the regulation of TLR9 signaling in the experimental autoimmune myocarditis rats. β-Actin was used as control. For each sample, the test was repeated three times. All values were expressed as means ± SE. Statistical analysis was determined using Student’s t test. P < 0.05 was considered significant.
Fig. 1.
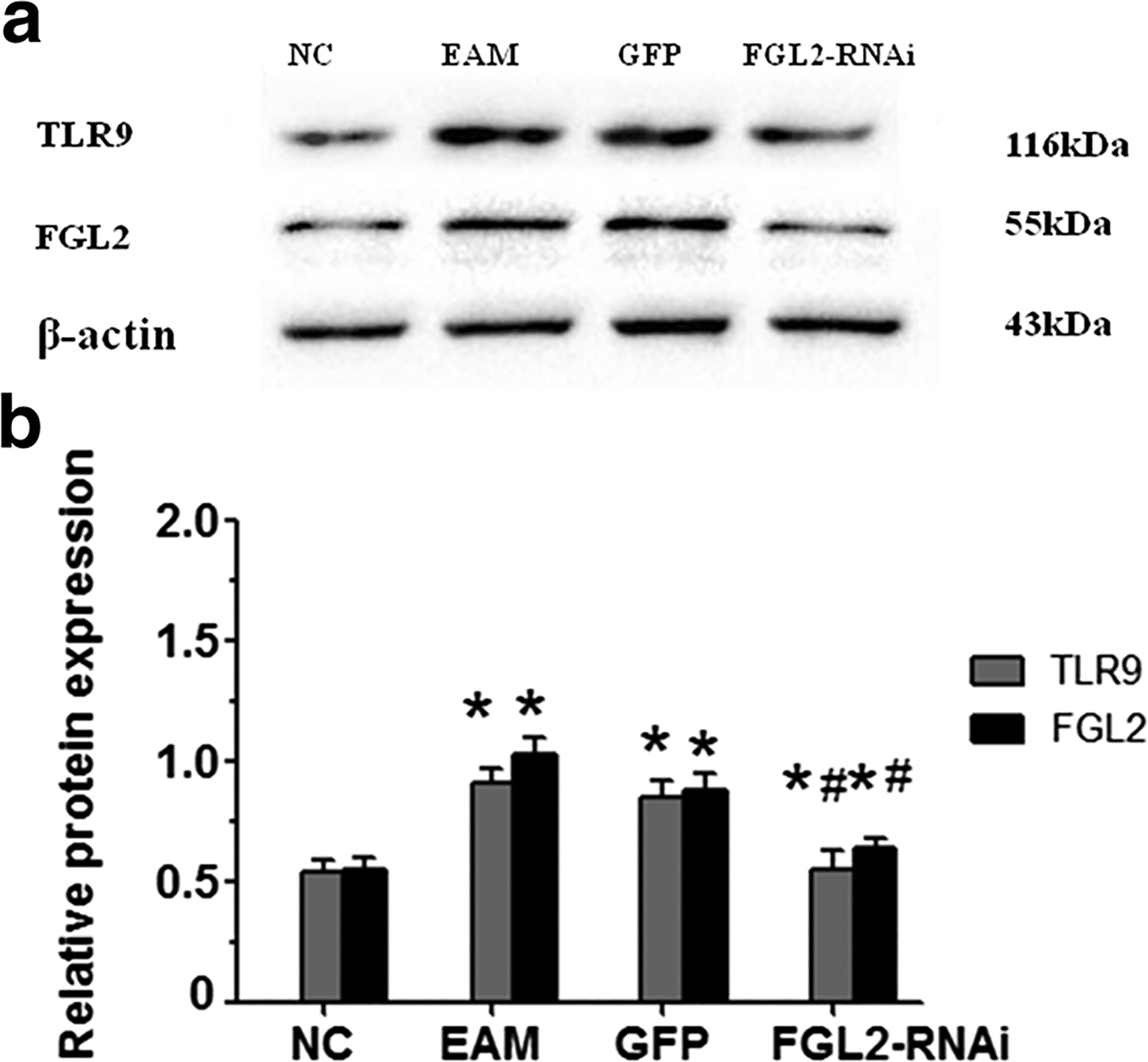
Western blotting analysis for TLR9 and Fgl2 protein expression in rat myocardial tissue. a TLR9 and Fgl2 protein expression stripe in each group. TLR9 protein expression in myocardial tissue of the EAM group was significantly higher than that of the control group on day 21 after initial immunization. TLR9 protein expression was significantly lower in the RNAi group compared with the EAM group, and also significantly higher in the RNAi group compared with the NC group. Fgl2 protein expression in myocardial tissue of the EAM group was significantly higher than that in the NC group on day 21 after initial immunization. Fgl2 protein expression was significantly lower compared with those in EAM and GFP groups. b β-Actin as control; the TLR9/β-actin and Fgl2/β-actin ratio of NC was considered as 100%. *P < 0.05 vs NC, group, #P < 0.05 vs EAM group
FGL2 silencing increases LVEDs and LVEDd, while decreases LVEF and FS after myosin immunization
As shown in Table 1, LVEDs, LVEDd, LVEF, and FS had no significant difference 0 day after the initial immune cardiac echocardiography among all groups. Compared with the control group, LVEDs and LVEDd were increased in the 21-day EAM group after myosin immunization, where the difference was statistically significantly (P < 0.05). However, LVEF and FS were decreased, and the difference was also statistically significantly (P < 0.05). There was no significance between the EAM group and the GFP group (P > 0.05). LVEF and FS of the FGL2-RNAi group were significantly higher compared to those of the EAM group, where the difference was statistically significantly (P < 0.05).
Table 1.
Echocardiographic analysis
| NC | EAM | GFP | FGL2-RANi | |
|---|---|---|---|---|
| 0 day | ||||
| LVEDs | 0.18 ±0.08 | 0.19 ± 0.02 | 0.17 ±0.02 | 0.18 ±0.05 |
| LVEDd | 0.42 ± 0.06 | 0.46 ± 0.03 | 0.42 ± 0.03 | 0.44 ± 0.03 |
| LVEF (%) | 90.02 ± 1.47 | 91.3 ±2.07 | 90.15 ±0.29 | 91.05 ±0.85 |
| FS (%) | 60.78 ± 1.04 | 61.38 ± 1.00 | 60.38 ± 1.12 | 60.38.02 ± 1.03 |
| 21 days | ||||
| LVEDs | 0.23 ± 0.03 | 0.36 ±0.05* | 0.37 ± 0.03* | 0.29 ± 0.04 |
| LVEDd | 0.46 ± 0.03 | 0.55 ±0.04* | 0.59 ± 0.05* | 0.53 ±0.05* |
| LVEF (%) | 89.83 ±0.68 | 48.65 ±3.08* | 50.03 ± 1.93* | 68.93 ± 1.51*,** |
| FS (%) | 58.85 ± 1.45 | 37.15 ±1.94* | 38.23 ± 1.85* | 48.3 ±3.54*,** |
Echocardiography was performed 0 and 21 days after the initial immunization .LVEDs, LVEDd, LVEF, and FS were detected. Compared with the EAM group 21 days after myosin immunization, LVEDs and LVEDd were decreased and LVEF and FS were increased in the RNAi group
P < 0.05 vs NC group;
P < 0.05 vs EAM
Silencing FGL2 significantly alleviates myocardial inflammatory in myocardial tissues
There was no inflammatory cell infiltration in the NC group. The EAM group 21 days after the appearance of initial immunization myocardial tissue had different degrees of inflammation and swelling in the myocardial cells, and a large number of focal inflammatory cell infiltrate, mainly lymphocytes accompanied by capillary expansion and myocardial cell necrosis. There was no significance between EAM and GFP groups. Myocardial inflammation was remarkably alleviated in the RNAi group compared with that in the EAM group 21 days after the initial immunization (Fig. 2a). Notably, inflammatory scores were significantly lower in the RNAi group compared with those in the EAM group (P < 0.05) (Fig. 2b).
Fig. 2.
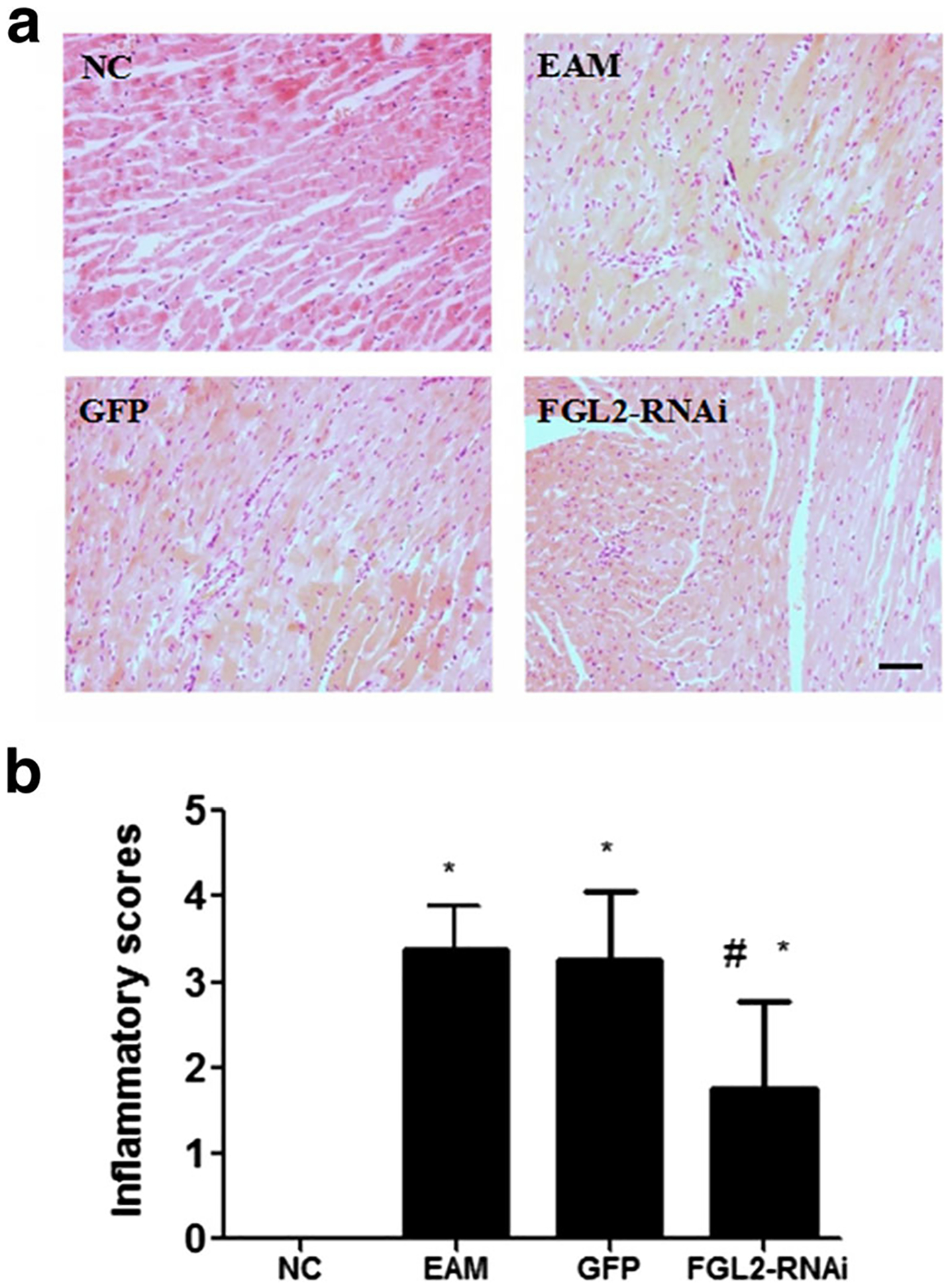
Histopathology analysis and inflammatory scores. a Inflammatory cell infiltration was not seen 21 days after the initial immunization in the NC group. There were a large number of focal inflammatory cell infiltrates in the GFP group 21 days after the initial immunization. There were also a large number of focal inflammatory cell infiltrates, mainly lymphocytes, accompanied by capillary expansion and myocardial cell necrosis in the EAM group 21 days after the initial immunization; the bar is representative of 50 μm. b Myocardial inflammation alleviated compared with the EAM group in the FGL2-RNAi group 21 days after the initial immunization. *P < 0.05 vs NC group, #P < 0.05 vs EAM group
FGL2 knockdown dramatically decreases mRNA expression levels of TLR9 and CTLA-4 in the myocardial tissue of rats
TLR9 and CTLA-4 mRNA expressions in myocardial tissue of the EAM group were significantly higher than those in the control group on day 21 after initial immunization (Fig. 3). The difference was statistically significantly (P < 0.05), while there was no significant difference compared with that in the GFP group. Notably, the mRNA expression levels were significantly higher in the FGL2-RNAi group compared with those in the control group (P < 0.05). However, the mRNA levels in the FGL2-RNAi group were dramatically decreased compared with those in the EAM group (P < 0.05).
Fig. 3.
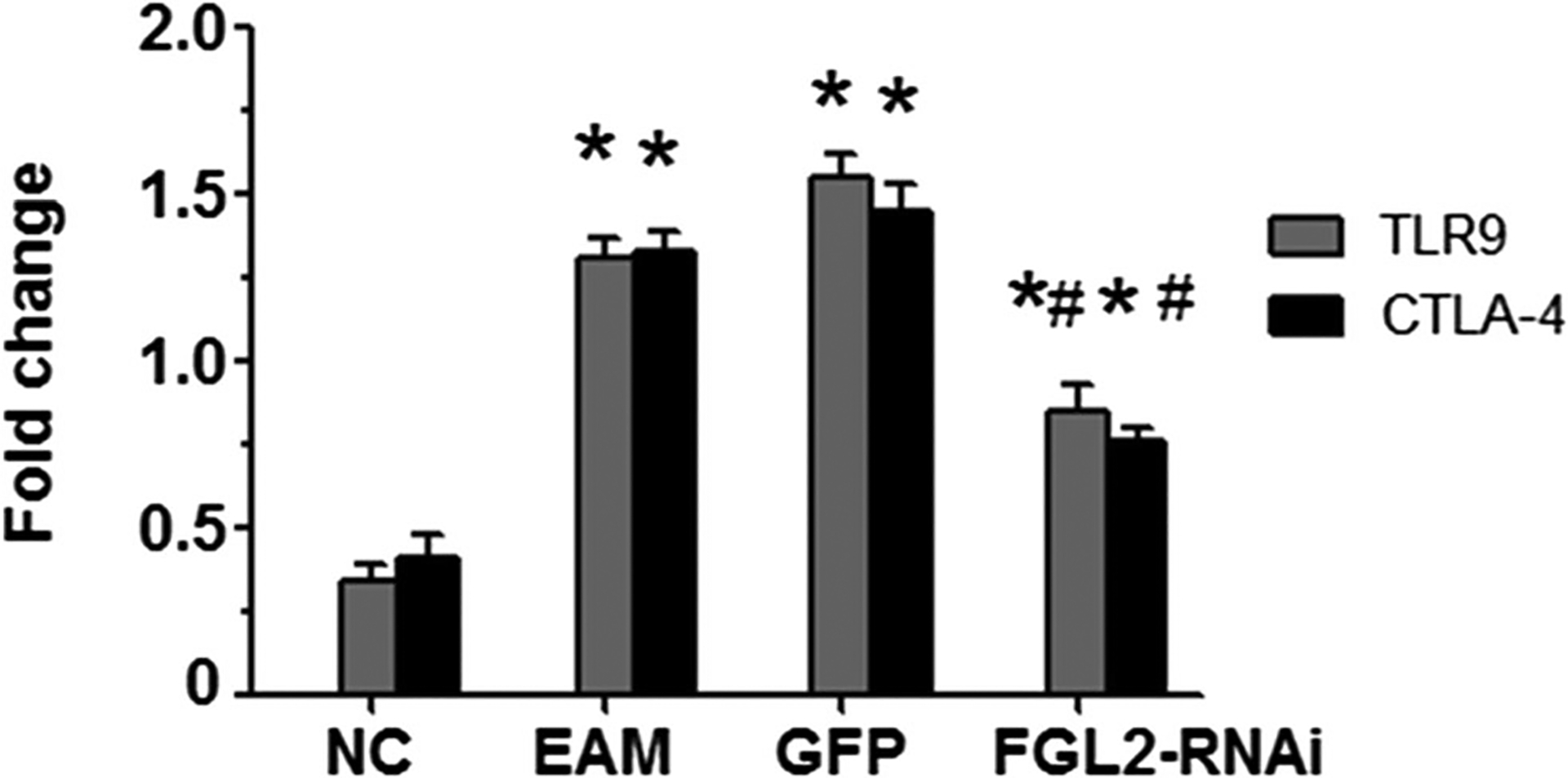
Quantitative RT-PCR was used to detect the level of TLR9 and CTLA-4 mRNA. TLR9 and CTLA-4 mRNA expressions in myocardial tissue of the EAM group were significantly higher than in the control group on day 21 after initial immunization. The expression level was significantly higher in the RNAi group compared with the control group but was lower compared with the EAM group. *P < 0.05 vs NC group, #P < 0.05 vs EAM group
FGL2 knockdown does not change the percentage of the CD4+CD25+ T cells
The percentage of CD4+CD25+ T cells in rat serums was detected by flow cytometry analysis. There was no statistical significant difference on day 21 after initial immunization in rat serum among different groups (P > 0.05, Fig. 4). For each sample, the test was repeated three times. All values were expressed as means ± SE. Statistical analysis was determined using Student’s t test. P < 0.05 was considered significant.
Fig. 4.
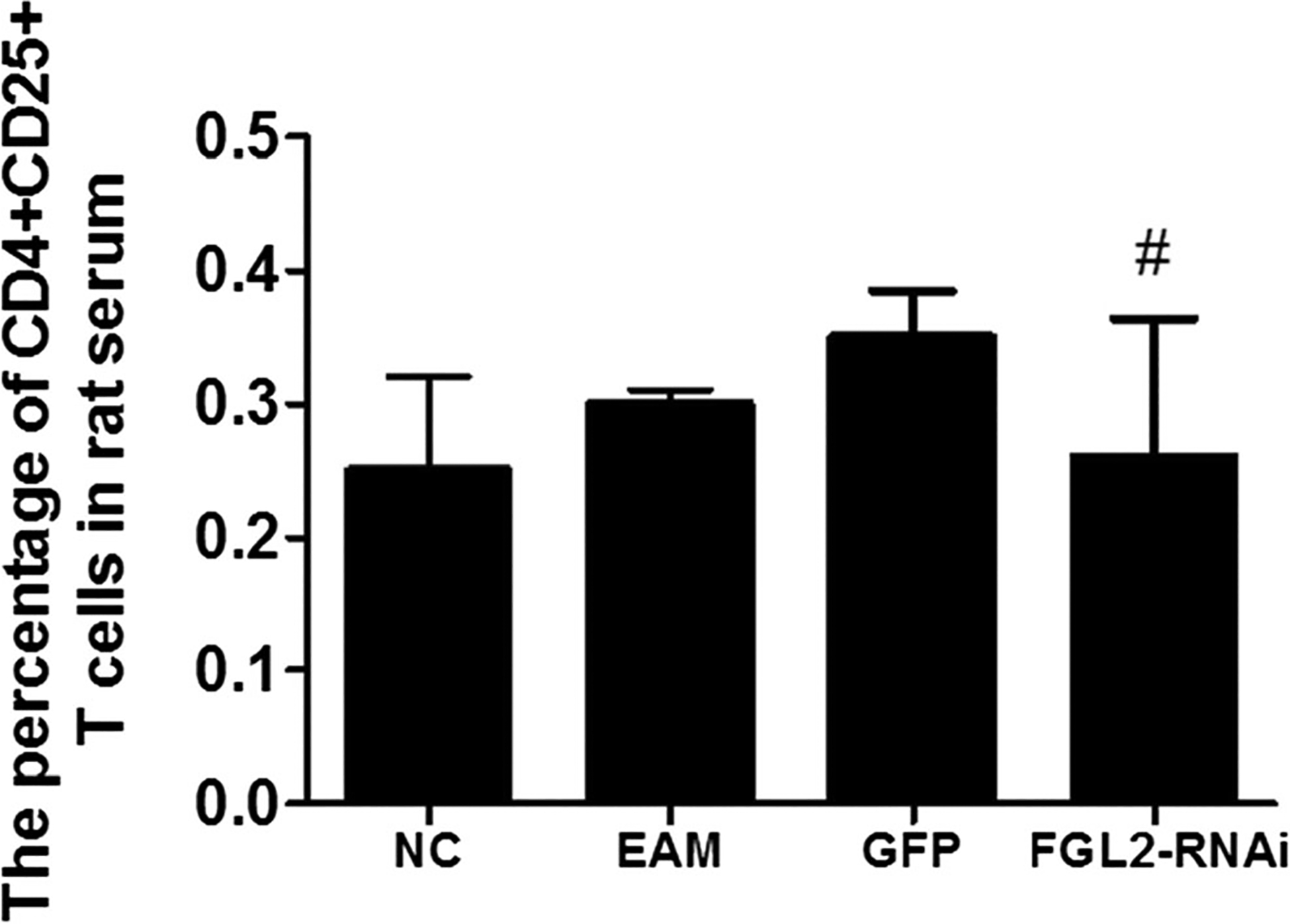
The percentage of CD4+CD25+ T cells in rat serum 21 days after T cells in rat serum 21 days after the initial immunization. The percentage of CD4+CD25+ T cells in rat serum was detected by FCM. There was no statistically significant difference among all groups (#P > 0.05)
FGL2 knockdown significantly decreases inflammatory factor levels of INF-α and NF-κB in rat serum
The inflammation factors of INF-α and NF-κB levels in serums were significantly increased 21 days after the initial immunization in the EAM group (P < 0.05; Fig. 5). Interestingly, the inflammatory factor of IL-6 level in serum on day 21 has no obvious changes, compared with that in other groups (P > 0.05). For each sample, the test was repeated three times. All values were expressed as means ± SE. Statistical analysis was determined using Student’s t test. P < 0.05 was considered significant.
Fig. 5.
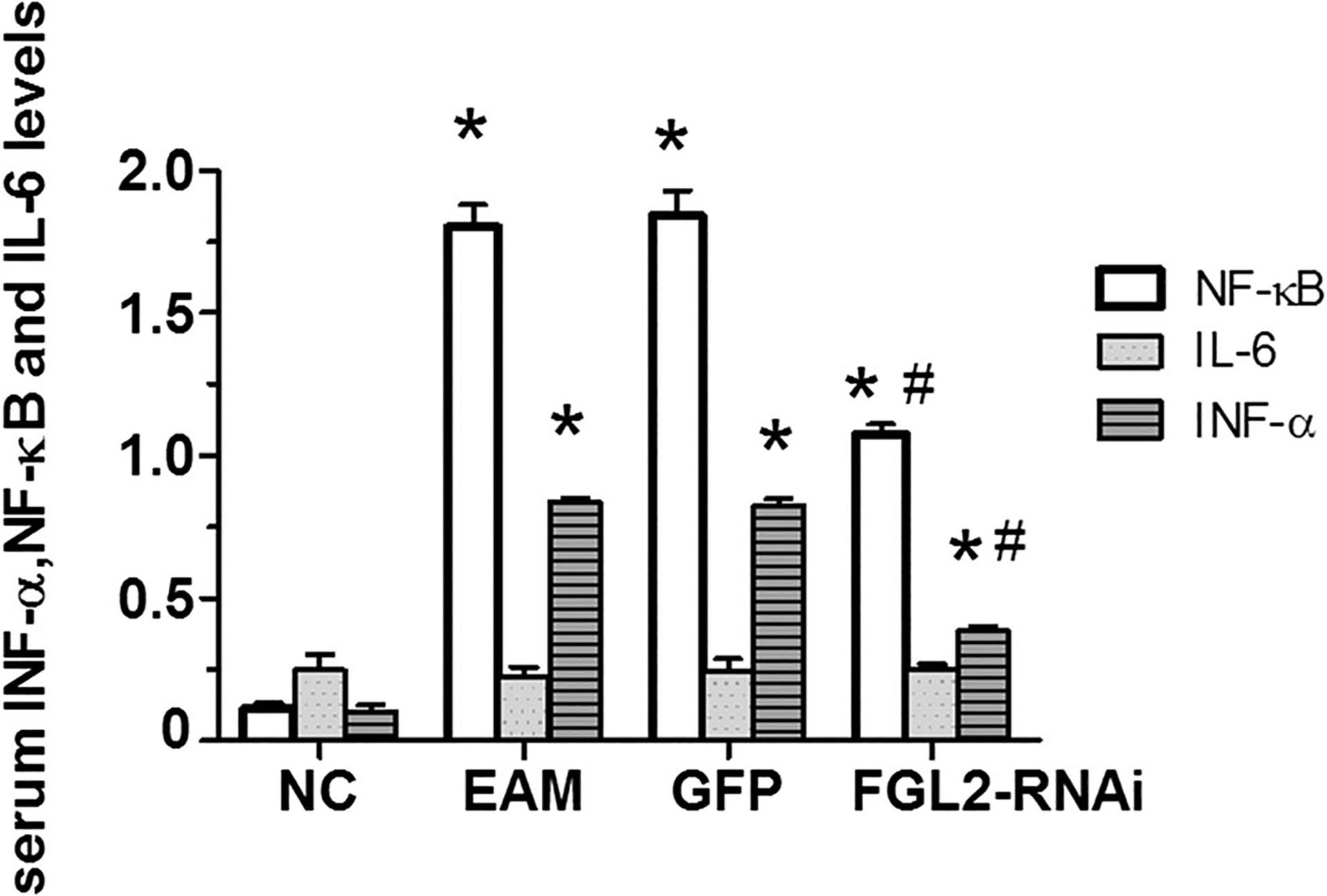
INF-α, NF-κB, and IL-6 levels in rat serum. Serum INF-α level in different groups. There was no change in IL-6 level 21 days after the initial immunization in the EAM group. *P < 0.05 vs NC group, #P < 0.05 vs EAM group
Discussion
In recent years, it was discovered that Toll-like receptor 9 (TLR9) was involved not only in a variety of autoimmune diseases but also in the abnormal immune response of inflammatory diseases, such as systemic lupus erythematosus, cancer, and ulcerative colitis [4–6]. DCM is a common cause of heart failure and sudden death, its development being closely related to autoimmune mechanism, and it is one of the most common heart diseases. So far, there is no effective therapeutic strategy to prevent myocarditis from becoming into DCM clinically. Fibrinogen-like protein 2 (FGL2) is a potent target for the treatment of experimental autoimmune myocarditis [8, 9].
In the present study, we established autoimmune myocarditis rat models to simulate the pathogenesis of DCM, by using the immunized Lewis rats with purified cardiac myosin of pigs’ heart. Our findings showed that FGL2 knockdown in autoimmune myocarditis significantly decreased TLR9 at both protein and mRNA levels, suggesting that the TLR9 signal pathway plays an important role in the process of myocarditis. Furthermore, echocardiography revealed that the rat heart ejection fraction was lower in experimental autoimmune myocarditis rats. This data significantly showed that heart function was obviously impaired, which is in consistent with previous studies [16]. FGL2 silencing increases LVEDs and LVEDd, while it decreases LVEF and FS after myosin immunization indicating that FGL2 may be used for the prevention and treatment of myocarditis.
During an inflammation in the body, NF-κB was activated by TLR9 signaling which is induced by the pathogen CpG DNA or CpG-ODN. NF-κB signaling can further trigger the downstream inflammatory cytokines, such as TNF-β, IL-2, IL-12, and INF-α, which can induce TH1 cells’ immune response [17–21]. The present study shows that inflammation in the myocardium with a large number of infiltrated lymphocytes and swelling myocardial cells, and even necrosis, was observed in experimental autoimmune myocarditis rats by pathological examination. In addition, except for NF-κB, we also observed that another transcription factor CTLA-4 was decreased after FGL2 knockdown. Our findings emphasize the inhibition of TLR9/CpG DNA signaling pathways by FGL2 knockdown in rat autoimmune myocarditis. Further, levels of inflammatory factors INF-α and NF-κB dropped, and inflammation and immune response were alleviated, but there was no difference in the level of IL-6. More details on the mechanism of inflammation factors involved in experimental autoimmune myocarditis during FGL2 knockdown need to be further investigated.
In experimental autoimmune myocarditis, the higher the expression of TLR9, the higher the myocardial inflammation scores, accompanied with inflammatory factor levels, which suggested that TLR9 plays an important role in myocarditis mediated by FGL2. Previous studies showed that, as one of the effectors, FGL2 can increase the levels of CD4+CD25+ T cells [9, 10]. However, we observed that CD4+CD25+ T cells had no obvious increase by FCM during FGL2 knockdown in experimental autoimmune myocarditis rats. The discrepancy on why CD4+CD25+ T cells have no change under FGL2 knockdown in experimental autoimmune myocarditis rats warrants further investigation.
Cumulatively, the current study shows that TLR9 in myocarditis plays an important role in the process of acute myocardial injury. FGL2 knockdown can be used for the preventive treatment of myocarditis, and for the study on the pathogenesis of autoimmune myocarditis and dilated cardiomyopathy. Our study provides a new direction of therapeutic strategists to combat the immune injury of dilated cardiomyopathy.
Funding information
This work was supported by a grant from the National Nature Science Foundation of China (Nos. 81660067 and 81260044), Young Scientists Training Project of Jiangxi Province (No. 2015 BCB23036) to Z. Zheng; and an American Heart Association (AHA) SDG grant 17SDG33410868 to H. Wu.
Abbreviations
- CTLA-4
Cytotoxic T lymphocyte-associated antigen-4
- DCM
Dilated cardiomyopathy
- EAM
Experimental autoimmune myocarditis
- EF%
Ejection fraction
- ELISA
Enzyme-linked immunosorbent assay
- FGL2
Fibrinogen-like protein 2
- FS %
Left ventricular short axis shortening fraction
- GFP
Green fluorescent protein
- LVEDd
Left ventricular end-diastolic diameter
- LVEDs
Left ventricular end systolic diameter
- TLR9
Toll receptor 9
Footnotes
Compliance with ethical standards The study protocol was approved by the Institutional Review Board of the First Affiliated Hospital of Nanchang University. All participants signed the informed consent prior to study participation. Laboratory animals were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing ICP 05076685). All efforts were made to minimize suffering.
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Yasukawa H, Yajima T, Duplain H, Iwatate M, Kido M, Hoshijima M, et al. The suppressor of cytokine signaling-1 (SOCS1) is a novel therapeutic target for enterovirus-induced cardiac injury. J Clin Invest. 2003;111(4):469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao P, Sharma AC, Ren J. Pathogenesis and therapy of autoimmunity-induced dilated cardiomyopathy. Front Biosci. 2009;14:1708–15. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe K, Sukumaran V, Veeraveedu PT, Thandavarayan RA, Gurusamy N, Ma M, et al. Regulation of inflammation and myocardial fibrosis in experimental autoimmune myocarditis. Inflamm Allergy Drug Targets. 2011;10(3):218–25. [DOI] [PubMed] [Google Scholar]
- 4.Shalev I, Wong KM, Foerster K, Zhu Y, Chan C, Maknojia A, et al. The novel CD4+CD25+ regulatory T cell effector molecule fibrinogen-like protein 2 contributes to the outcome of murine fulminant viral hepatitis. Hepatology. 2009;49(2):387–97. [DOI] [PubMed] [Google Scholar]
- 5.Han M, Yan W, Guo W, Xi D, Zhou Y, Li W, et al. Hepatitis B virus-induced hFGL-transcription is dependent on c-Ets-2 and MAPK signal pathway[J]. J Biol Chem. 2008;283(47):32715–29. [DOI] [PubMed] [Google Scholar]
- 6.Zheng Z, Wang L, Wu Y, Wang H, Gao D, Fa Y, et al. Fibrinogen-like protein 2 gene silencing activates angiopoietin/Tie system and induces myocardial microvascular endothelial cell proliferation and cell migration. Biomed Res. 2012;23:37–42. [Google Scholar]
- 7.Zheng Z, Huang H, Yu Y, Liang J. SerumFgl2 levels elevated in patients with acute coronary syndrome. Health MED J. 2012;6: 2062–5. [Google Scholar]
- 8.Zheng Z, Huang H, Yu Y, Liang J. Overexpression of Fgl2 deteriorates the heart function of experimental autoimmune myocarditis rats. Eur J Inflamm. 2015;13:66–71. [Google Scholar]
- 9.Zhenzhong Z, Yafa Y, Jin L. Fibrinogen-like protein 2 gene silencing inhibits cardiomyocytes apoptosis, improves heart function of streptozotocin-induced diabetes rats and the molecular mechanism involved. Biosci Rep. 2015;35(3):e00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Platz J, Beisswenger C, Dalpke A, Koczulla R, Pinkenburg O, Vogelmeier C, et al. Microbial DNA induces a host defense reaction of human respiratory epithelial cells. J Immunol. 2004;173:1219–23. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen G, Andresen L, Matthiessen MW, Rask-Madsen J, Brynskov J. Expression of Toll-like receptor 9 and response to bacterial CpG oligodeoxynucleotides in human intestinal epithelium. Clin Exp Immunol. 2005;141(2):298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pagni PP, Traub S, Demaria O, Chasson L, Alexopoulou L. Contribution of TLR7 and TLR9 signaling to the susceptibility of MyD88-deficient mice to myocarditis. Autoimmunity. 2010;430: 275–87. [DOI] [PubMed] [Google Scholar]
- 13.Mathur S, Walley KR, Boyd JH. The Toll-like receptor 9 ligand CPG-C attenuates acute inflammatory cardiac dysfunction. Shock. 2010;36:478–83. [DOI] [PubMed] [Google Scholar]
- 14.Kim PD, Xia-Juan X, Crump KE, Abe T, Hajishengallis G, Sahingur SE. Toll-like receptor 9-mediated inflammation triggers alveolar bone loss in experimental murine periodontitis. Infect Immun. 2015;83(7):2992–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485(7397):251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wakisaka Y, Niwano S, Niwano H, Saito J, Yoshida T, Hirasawa S, et al. Structural and electrical ventricular remodeling in rat acute myocarditis and subsequent heart failure. Cardiovasc Res. 2004;63(4):689–99. [DOI] [PubMed] [Google Scholar]
- 17.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4(7):499–511. [DOI] [PubMed] [Google Scholar]
- 18.Huang LY, Ishii KJ, Akira S, Aliberti J, Golding B. Th1-like cytokine induction by heat-killed Brucella abortus is dependent on triggering of TLR9. J Immunol. 2005;175:3964–70. [DOI] [PubMed] [Google Scholar]
- 19.Macedo GC, Magnani DM, Carvalho NB, Bruna-Romero O, Gazzinelli RT, Oliveira SC. Central role of MyD88-dependent dendritic cell maturation and proinflammatory cytokine production to control Brucella abortus infection. J Immunol. 2008;180:1080–7. [DOI] [PubMed] [Google Scholar]
- 20.Bhan U, Trujillo G, Lyn-Kew K, Newstead MW, Zeng X, Hogaboam CM, et al. Toll-like receptor 9 regulates the lung macrophage phenotype and host immunity in murine pneumonia caused by Legionella pneumophila. Infect Immunol. 2008;7: 2895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu CC, Sabet M, Hayashi T, Tawatao R, Fierer J, Carson DA, et al. In vivo efficacy of a phosphodiester TLR-9 aptamer and its beneficial effect in a pulmonary anthrax-infection model. Cell Immunol. 2008;251:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]


