Abstract
BACKGROUND:
The SPYRAL HTN-ON MED (Global Clinical Study of Renal Denervation With the Symplicity Spyral Multi-electrode Renal Denervation System in Patients With Uncontrolled Hypertension in the Absence of Antihypertensive Medications)trial showed significant office and nighttime systolic blood pressure (BP) reductions in patients with hypertension following renal denervation (RDN) compared with sham-control patients, despite similar 24-hour BP reductions. We compared antihypertensive medication and BP changes among prespecified subpopulations.
METHODS:
The multicenter, randomized, sham-controlled, blinded SPYRAL HTN-ON MED trial (n=337) evaluated BP changes after RDN compared with a sham procedure in patients with hypertension prescribed 1 to 3 antihypertensive drugs. Most patients (n=187; 54%) were enrolled outside the United States, while 156 (46%) US patients were enrolled, including 60 (18%) Black Americans.
RESULTS:
Changes in detected antihypertensive drugs were similar between RDN and sham group patients in the outside US cohort, while drug increases were significantly more common in the US sham group compared with the RDN group. Patients from outside the United States showed significant reductions in office and 24-hour mean systolic BP at 6 months compared with the sham group, whereas BP changes were similar between RDN and sham in the US cohort. Within the US patient cohort, Black Americans in the sham control group had significant increases in medication burden from baseline through 6 months (P=0.003) but not in the RDN group (P=0.44).
CONCLUSIONS:
Patients enrolled outside the United States had minimal antihypertensive medication changes between treatment groups and had significant office and 24-hour BP reductions compared with the sham group. Increased antihypertensive drug burden in the US sham cohort, especially among Black Americans, may have diluted the treatment effect in the combined trial population.
REGISTRATION:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT02439775.
Keywords: antihypertensive agents, Black or African American, blood pressure, hypertension, renal denervation
NOVELTY AND RELEVANCE.
What Is New?
Here, we assess outcomes from the SPYRAL HTN-ON MED trial investigating the safety and efficacy of renal denervation in patients taking antihypertensive medications in different patient subgroups based on geography (United States and outside the United States) and by race within the United States.
What Is Relevant?
Device antihypertensive trials in the presence of medications face an inherent limitation in isolating the treatment effect due to patient and clinician awareness of the primary end point via home blood pressure monitoring and ensuing adjustments to lifestyle or medications.
Clinical/Pathophysiological Implications?
The blood pressure–lowering effect of renal denervation was heterogenous in the Black American subgroup; however, this may be balanced by disproportionate and higher medication use in sham control patients among Black Americans. Further follow-up will be necessary to investigate long-term outcomes.
Blood pressure (BP) control rates in the treated hypertensive population are <50% and contribute substantially to global morbidity and mortality, despite the availability of safe and effective drug therapies.1,2 Within the United States, hypertension disproportionately affects Black Americans,3,4 highlighting the potential benefit of adjunctive therapies to augment drugs and lifestyle modification, especially in socioeconomic groups with unequal access to health care.5 Several recent clinical trials have shown highly consistent reductions in both office and ambulatory BP following catheter-based renal denervation (RDN) in untreated patients with hypertension compared with the sham control group.6–8 Likewise, BP reductions following RDN have been consistent in patients simultaneously treated with antihypertensive drugs.9–11 However, several on-medication trials of RDN have shown variable BP changes following randomization in the sham control group, obfuscating the comparative efficacy of RDN therapy in the presence of antihypertensive medications.12,13 In particular, primary analysis of the randomized sham-controlled SYMPLICITY HTN-3 (Renal Denervation in Patients With Uncontrolled Hypertension) trial showed similar BP reductions in the RDN-treated and sham control groups.12 A further subanalysis identified several potential confounding factors that may have contributed to the neutral result, including changes in adherence to antihypertensive drugs during the follow-up period in the sham group.13–15 Recent results from the SPYRAL HTN-ON MED (Global Clinical Study of Renal Denervation With the Symplicity Spyral Multi-electrode Renal Denervation System in Patients With Uncontrolled Hypertension in the Absence of Antihypertensive Medications) trial also showed substantial BP reductions in the sham control group.15 The present analysis of the SPYRAL HTN-ON MED trial compared the efficacy of RDN among various prespecified subgroups with particular focus on geographic location within and outside the United States. Differences in antihypertensive medication changes during the primary follow-up period were compared between geographies. Additional comparisons between Black and non-Black patients within the US population were also performed.
METHODS
The design of the global, randomized, blinded, sham-controlled SPYRAL HTN-ON MED study (https://www.clinicaltrials.gov; Unique identifier: NCT02439775) has been previously reported.16 Briefly, patients aged 20 to 80 years with uncontrolled hypertension, defined as office systolic BP ≥150 and <180 mm Hg, office diastolic BP ≥90 mm Hg, and a mean 24-hour ambulatory systolic BP ≥140 and <170 mm Hg, and prescribed 1, 2, or 3 standard antihypertensive medications, were enrolled. All patients provided written informed consent, and the protocol was approved by all local ethics committees. Reporting of race by individual subjects was not allowed by local law in the study centers outside the United States. The Black American group consisted of US patients who self-identified as Black on the study enrollment form. Subjects randomized to RDN received denervation via a multielectrode radiofrequency catheter system (Symplicity Spyral catheter and Symplicity G3 generator, Medtronic, Galway, Ireland) to provide circumferential ablation treatments in the 4 quadrants of the renal arteries and branch and accessory vessels between 3 and 8 mm in diameter. The sham procedure consisted of a renal angiogram with additional measures to ensure blinding.16 Office BP was assessed at the medication trough at 1, 3, and 6 months post-procedure. Likewise, 24-hour BP was recorded at 3 and 6 months immediately following the witnessed pill intake of all prescribed antihypertensive medications. Escape criteria were an office systolic BP ≥180 mm Hg, an office systolic BP <115 mm Hg with symptoms of hypotension, or other safety reasons as determined by the investigator. Patients who escaped were analyzed using the last observation carried forward for BP measures through 6 months when available. Medication adherence was assessed from plasma and urine samples via liquid chromatography–mass spectrometry at baseline and 6 months.
Statistical Analysis
Continuous variables, reported as mean (SD), were compared between treatment groups using t tests. Categorical variables, reported as counts and percentages, were compared between treatment groups using exact binomial tests and among subgroups using χ2 tests. Follow-up change measures were compared between treatment arms among prespecified subgroups including age, sex, body mass index, geography (United States and outside the United States), and race (Black Americans and non-Black Americans in the United States) using ANCOVA, adjusting for baseline measurements. Interactions between treatment arms for subgroups were evaluated using a linear regression model adjusted for baseline systolic BP, treatment indicator, subgroup indicator, and treatment by subgroup interaction. Antihypertensive medication burden, assessing both the number of medications and prescribed dosages, was calculated as previously described.15,17 Further details are provided in the Supplemental Material. Office and 24-h ambulatory BP measurements collected before protocol escape due to nonspecified antihypertensive medication increases were included in end point calculations (the last observation carried forward). Analyses were performed using SAS (Statistical Analysis Software Institute, Cary, North Carolina, USA) for Windows, version 9.4. The trial is registered with ClinicalTrials.gov.
RESULTS
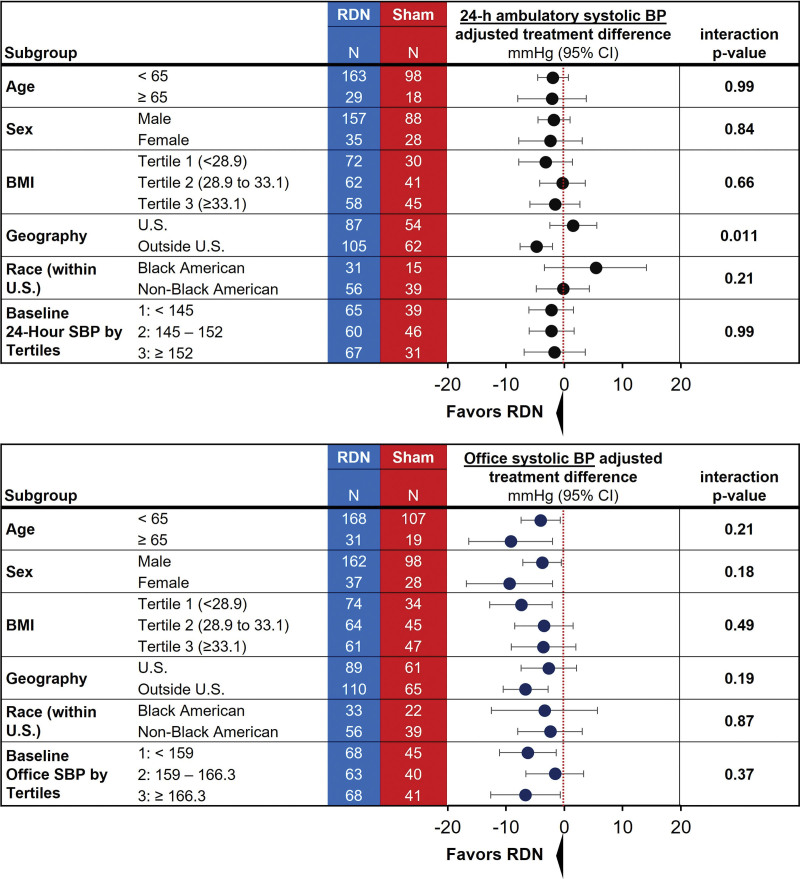
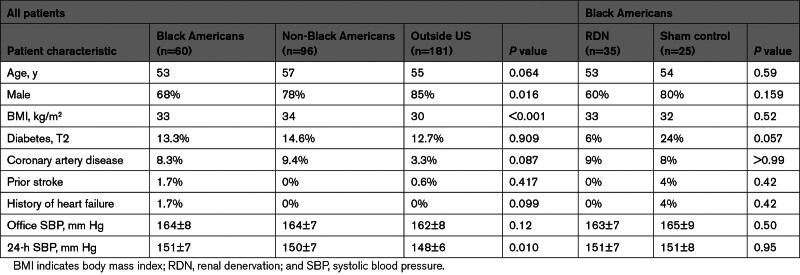
Demographic data are described in the Table. Most patients (n=181; 54%) were enrolled from outside the United States. The remaining patients (n=156; 46%) were enrolled within the United States, of whom Black Americans (n=60) comprised 18% of the study cohort (n=35/206 [17%] in the RDN group; n=25/131 [19%] in the sham group). Black American patients were more likely to be women, had a higher mean systolic 24-hour BP, and tended to be younger compared with non-Black Americans (n=96) and the rest of the cohort outside the United States (including Europe, Japan, and Australia). Procedural characteristics among subgroups are shown in Table S1. Variances in 6-month, 24-hour ambulatory systolic BP–adjusted treatment differences in predefined subgroups based on age (<65 and ≥65 years), sex, body index mass (by tertiles), race (Black Americans and non-Black Americans), and baseline systolic BP (by tertiles) were not significant (Figure 1). However, patients outside the United States had a significantly greater 24-hour ambulatory systolic BP treatment difference in favor of RDN compared with US patients (P=0.011). Notably, variances in office systolic BP–adjusted treatment differences were not significant for each of the subgroups (Figure 1). Nonetheless, the significantly different outcomes in 24-hour ambulatory systolic BP based on geography prompted further evaluation between subgroups.
Figure 1.
Twenty-four-hour systolic blood pressure (SBP) and office SBP forest plots of prespecified subgroups. BMI indicates body mass index; BP, blood pressure; and RDN, renal denervation.
Table.
Patient Characteristics and Blood Pressure Measures at Baseline
Antihypertensive Medication Usage
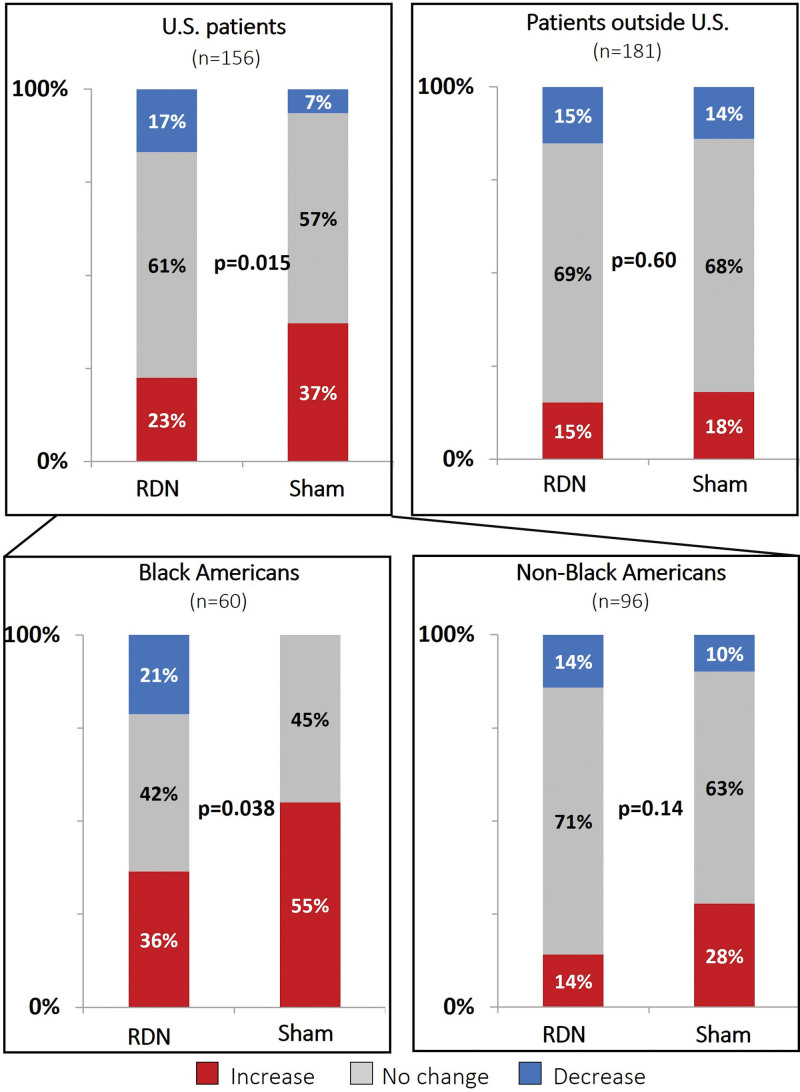
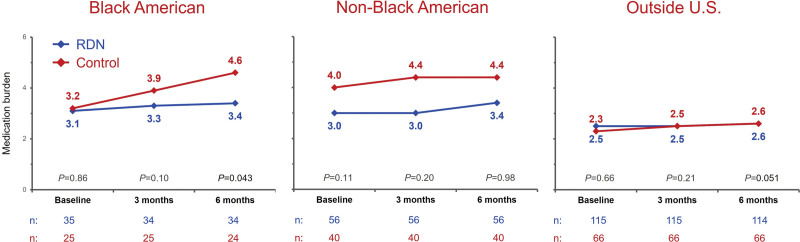
Changes in antihypertensive medication burden based on the number, dosage, and class of antihypertensive medication, as identified by blood and urine testing, occurred in both the RDN and sham groups (Supplemental Methods; Figure 2). The proportion of patients with either increases or decreases was similar between the RDN and sham control groups outside the US cohort (P=0.60) but not in the US cohort (P=0.015). More US sham group patients had an increased detected antihypertensive medication burden (37%) than RDN group patients (23%), while more US RDN patients had a decreased detected antihypertensive medication burden (17%) compared with sham group patients (7%). Within the US subgroup, the proportion of non-Black American patients with either increases, no change, or decreases in antihypertensive medications was similar between the RDN and sham groups (P=0.14; Figure 2). By comparison, there were significantly different trends between RDN and sham control groups among Black American patients (P=0.038). Fewer Black American patients in the RDN group had detected increases in antihypertensive medication burden (36%) than those in the sham control group (55%), whereas more Black American patients in the RDN group had detected decreases in antihypertensive medication burden (21%) compared with those in the sham control group (0%; Figure 2). The proportion of Black Americans with no detected changes in medication was similar between treatment groups. Evaluation of the prescribed antihypertensive medication burden across subgroups by treatment type revealed a similar trend among Black American patients. Prescribed medication changes from baseline to 6 months were not significant among Black Americans in the RDN group (P=0.44), whereas prescribed changes from baseline to 6 months significantly increased among those in the sham control group (P=0.003; Figure 3). This disparity in prescribed medication changes resulted in Black American patients in the sham control group having a significantly higher prescribed medication burden by the 6-month follow-up compared with Black Americans in the RDN group (P=0.043).
Figure 2.
Medication burden change from baseline to 6 months based on medication testing comparing renal denervation (RDN) and sham control groups in US patients and patients outside the United States as well as Black Americans and non-Black Americans. P values use the Mantel-Haenszel test for linear trend. Reported changes detected by blood and urine testing are based on the medication number, dosage, and class of antihypertensive medications.
Figure 3.
Changes in prescribed medication burden from baseline to 6 months in renal denervation (RDN) and sham control groups among Black Americans, non-Black Americans, and patients outside the United States.
Assessing antihypertensive medication use by medication testing also revealed that Black Americans were more likely to be taking angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and diuretics and were less likely to be taking β-blockers at baseline compared with non-Black Americans and patients outside the United States. When comparing medication class changes between treatment groups among Black Americans, both RDN and control patients had modest increases from baseline to 6 months in angiotensin-converting enzyme inhibitor/angiotensin receptor blocker (10% versus 11%) and diuretics (18% versus 12%; Figure S1). However, Black American control patients had a 28% increase in calcium channel blockers from baseline to 6 months, compared with a 1% increase in Black American RDN patients. In contrast, between-group medication change differences from baseline to 6 months among non-Black Americans and patients outside the United States were minimal (Figure S1).
Nine Black American patients had nonevaluable 6-month 24-hour ambulatory BP measures, and 5 Black American patients escaped before 6-month follow-up without available previous measures for the last observation carried forward analysis (Figure S2). A total of 24 of 55 (44%) patients in the Black American patient cohort had antihypertensive medication detected at 6 months that was also detected at baseline compared with 187 of 273 patients from the rest of the cohort (68%; P=0.0006). Medication testing identified 24 Black Americans (44%) with additional antihypertensive medications at 6 months that were not present at baseline. Among the rest of the cohort, additional medications were detected in 48 patients (18%; P<0.0001). The proportions of patients who were adherent to antihypertensive medications at 6 months as compared with baseline medications in each subgroup based on treatment type are shown in Figure S3.
Blood Pressure
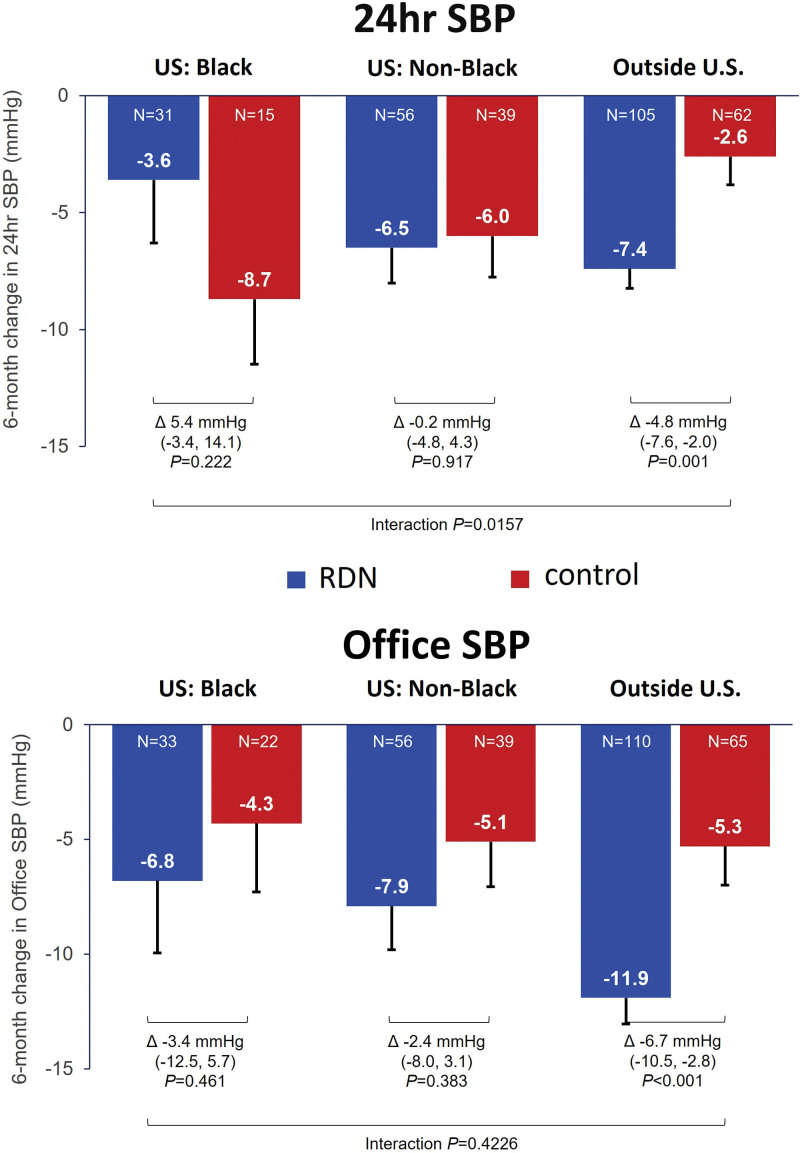
Among patients outside the US subgroup, 24-hour systolic BP decreased significantly in the RDN group (−7.4 [SD, 8.6] mm Hg) compared with the control group (−2.6 [9.5] mm Hg; adjusted treatment difference, −4.8 [95% CI, −7.6 to −2.0] mm Hg; P=0.0010; Figure 4). In contrast, baseline adjusted reductions in 24-hour ambulatory systolic BP from baseline to 6 months among Black Americans were nominally greater in the sham control group (−8.7 [10.8] mm Hg) compared with the RDN group (−3.6 [15.0] mm Hg; Figure 4), although the treatment difference between groups was not statistically significant (5.4 [95% CI, −3.4 to 14.1] mm Hg; P=0.22). Non-Black Americans similarly did not have significant differences in 24-hour systolic BP reductions from baseline to 6 months between the RDN (−6.5 [11.3] mm Hg) and sham control groups (−6.0 [11.0] mm Hg; adjusted treatment difference, −0.2 [95% CI, −4.8 to 4.3] mm Hg; P=0.92). While reductions in 24-hour ambulatory diastolic BP among Black Americans and non-Black Americans in the RDN and control groups were not significantly different through 6 months (Figure S4), a 3-way comparison showed significant differences between the RDN and control groups across Black Americans, non-Black Americans, and patients outside the United States (P for interaction, 0.016). Changes in daytime and nighttime ambulatory systolic BP are shown in Figure S5.
Figure 4.
Reduction in mean 24-hour ambulatory and office systolic blood pressure (SBP) at 6 months for renal denervation (RDN) and sham control groups among Black Americans, non-Black Americans, and patients outside the United States.
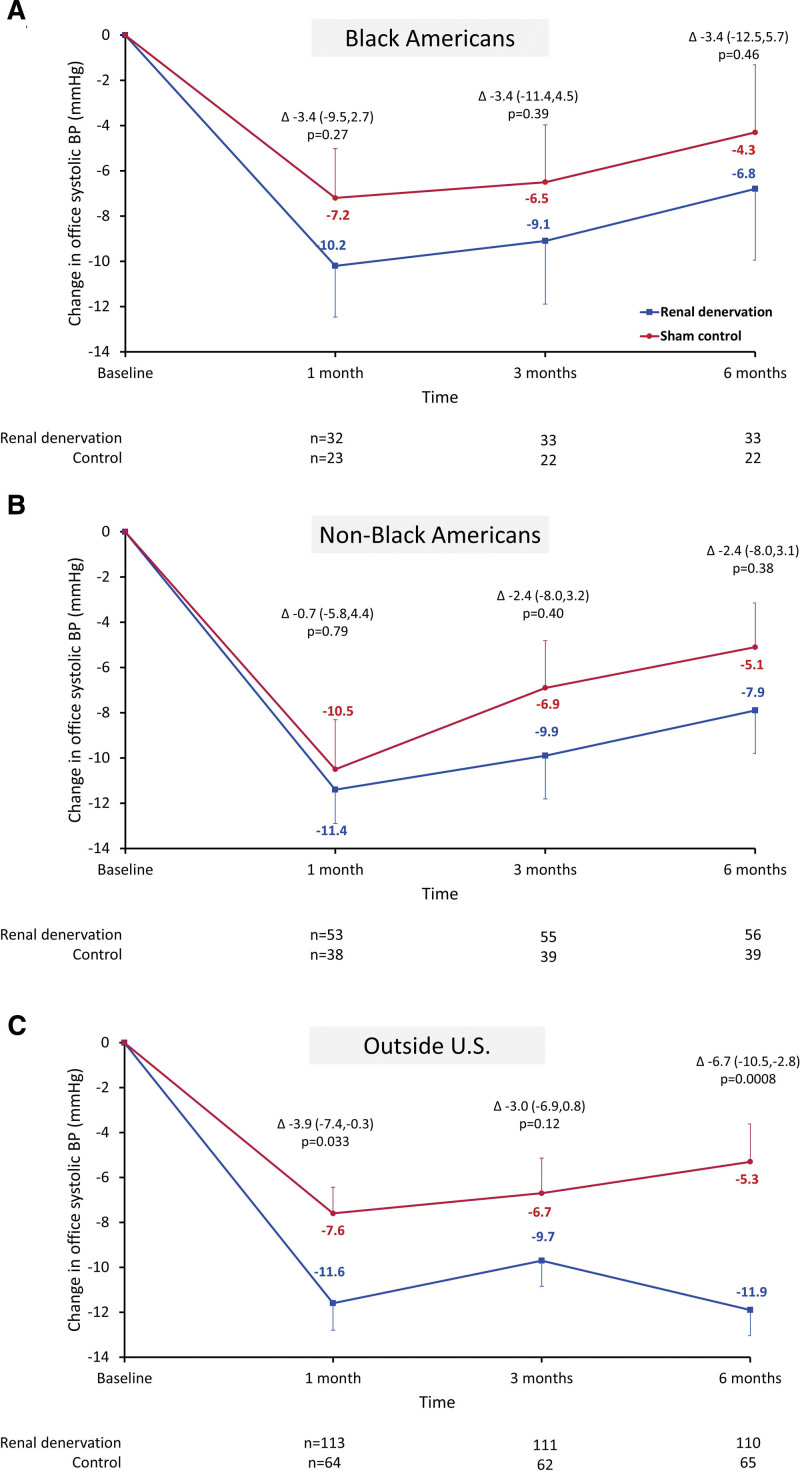
The changes in office systolic BP from baseline to 1, 3, and 6 months between RDN and control groups among Black Americans, non-Black Americans, and patients outside the United States are plotted in Figure 5. Among Black and non-Black Americans, reductions in office systolic BP were nominally lower at 1, 3, and 6 months in the RDN group compared with the control group, although treatment differences were not significant. However, among patients outside the United States, RDN patients had a significantly greater reduction in office systolic BP at 1 and 6 months compared with control patients (Figures 4 and 5). Similar trends from baseline through 6 months were observed in changes in office diastolic BP, with significant reductions between RDN and control groups among patients outside the United States but not among Black and non-Black Americans (Figure S4). Notably, among patients outside the United States on 3 more antihypertensive medications, significant treatment differences were observed in 24-hour ambulatory and office systolic BP between RDN and control groups at 6 months (Table S2).
Figure 5.
Change in office systolic blood pressure (BP) from baseline up to 6 months in select subgroups of renal denervation and sham control patients Mean office systolic blood pressure changes are depicted through 6 months in Black American patients (A), non-Black American patients (B), and patients outside the United States (C) in renal denervation (blue) and sham control groups (red). Error bars represent SE.
Hourly differences in ambulatory systolic BP between baseline and 6-month RDN and sham control groups are shown in Figures S6 and S7. Twenty-four-hour BP differences showed distinctive patterns in the Black Americans versus non-Black Americans and patients outside the United States (Figure S6).
DISCUSSION
This prespecified analysis of the SPYRAL HTN-ON MED trial identified significant differences in the 24-hour ambulatory systolic BP response to RDN in the subgroup of patients enrolled within the United States compared with those outside the United States. Interestingly, these geographic differences in outcomes were associated with corresponding differences in antihypertensive medication burden, which potentially biased BP reductions toward the sham treatment group. In the subgroup of patients enrolled outside the United States, including Europe, Australia, and Japan, fewer patients changed antihypertensive medication regimens (assessed by prescriptions and medication testing) within the sham control group through 6 months, and correspondingly, there was a significant treatment difference between the RDN and sham control groups in the mean 24-hour ambulatory and office systolic BP. Further analysis of antihypertensive use in the US patient cohort showed that Black Americans were more likely to have changed antihypertensive medication burden and classes detected by medication testing from baseline to 6 months. This likely contributed to the BP reductions in the Black American sham control subgroup.
The wide variability of BP response in the sham group of this trial and previous10,12,13 trials has several potential explanations, including trial design, rigor of execution, communication between investigational staff and trial participants, as well as patient behavior. In a clinical trial setting, increased access to health care and amplified clinical attention can exacerbate the Hawthorne effect18 with a potentially variable impact on different subgroups. Unfortunately, trials of device-based hypertension therapies in the presence of prescribed antihypertensive medications face challenges from at least 2 factors. First, patients have awareness of the trial end point, in this case, systolic BP. Second, patients also have the means to influence the end point, intentionally or unintentionally, by monitoring BP at home and adjusting their intake of prescribed (or unprescribed) antihypertensive medications.19 Indeed, substantial placebo-group BP reductions have been observed recently in other recent antihypertensive trials.20–22 Awareness of this potential limitation led to the division of the clinical trial program into 2 distinct branches, including the SPYRAL HTN-OFF MED (Global Clinical Study of Renal Denervation With the Symplicity Spyral™ Multi-electrode Renal Denervation System in Patients With Uncontrolled Hypertension in the Absence of Antihypertensive Medications) and SPYRAL HTN-ON MED trials.16 The off med design specifically minimized the potential impact of changing patient behavior in the sham group on trial outcome. This strategy proved effective, as the SPYRAL HTN-OFF MED trial showed clear differences in BP reduction between the RDN and sham groups.7 However, despite a successful pilot phase,10 the SPYRAL HTN-ON MED Expansion trial reported sham group reductions in 24-hour systolic BP that were much greater than those observed in the SPYRAL HTN-OFF MED trial despite similar baseline BP in both cohorts.15 Drug testing results (Figure 2) suggest changes in antihypertensive medication, whether initiated by clinicians or patients, may have influenced BP-based end points. However, trialists only became aware of these medication changes after the primary end point, and thus they were not actionable.
Baseline demographics were dissimilar between the outside the United States, the US non-Black American, and the self-identified US Black American cohorts. The Black American cohort included more women and had a higher baseline 24-hour systolic BP. Consistent with guideline recommendations,23 the Black American cohort was prescribed different antihypertensive medication regimens at baseline, with a higher proportion of Black Americans receiving calcium channel blockers and diuretics compared with non-Black Americans.24 However, changes in both prescribed and detected medication during the follow-up period were reported in a significant proportion of patients beyond those who exited the study per protocol, especially within the Black American group. Prescribed medication burden increased significantly in Black Americans between baseline and 6 months but not in non-Black Americans and patients outside the United States. This antihypertensive medication burden increase may be reflected in the observed reduction in 24-hour ambulatory systolic BP in the sham control group among Black Americans at 6 months. Notably, both RDN and sham control patients outside the United States had minimal changes in antihypertensive medication from baseline to 6 months, while also demonstrating significant treatment differences between groups.
The Black American population was not adequately represented, or reported separately, in many landmark placebo-controlled trials of medication for hypertension.25 However, similar to the present analysis, previous studies have also demonstrated changes in health-seeking behaviors in Black Americans participating in randomized controlled hypertension trials. Notably, a trial of community and technology-based methods to improve patient self-care in the Black American community also reported improvements in systolic BP in the control group with health education that were attributed to increased awareness of BP due to trial participation.26 Substantial reductions in control group systolic BP (−9.3 [16.0] mm Hg) were also reported in a study conducted in Los Angeles barbershops in community-based health promotion in non-Hispanic Black men.27
Similar behavioral bias between the RDN and sham groups was not observed in the SPYRAL HTN-ON MED pilot trial,10 possibly due to a relatively smaller Black American subpopulation (n=5/80; 6%). The observations from the SPYRAL HTN-ON MED full cohort are consistent with similar observed changes reported in the SYMPLICITY HTN-3 trial.24 The hourly ambulatory systolic BP among Black American sham control patients was noticeably variable from time point to time point, whether the measure began at the time the ambulatory cuff was placed (Figure S6) or was aligned to the time of day (Figure S7). Some degree of variability may be attributed to the relatively fewer Black American sham control patients with evaluable 24-hour ambulatory BP measures at 6 months (n=15). Among Black American sham control patients, 40% (n=10) had nonevaluable 24-hour ambulatory BP measures at 6 months. However, some variability may also stem from the medication burden increases documented in the Black American sham control group. Patients ingested their medications twice at times that could impact the ABPM readings: first at the time of witnessed pill intake immediately before ambulatory monitor cuff placement, and again the next morning at home before patients returned their ambulatory monitor. Therefore, the impact of medication changes on mean 24-hour ambulatory BP is expected to be greater than it is for office and nighttime BP.15 This hypothesis, however, remains speculative.
There are several limitations to this analysis. The collection of demographic data on patient race was not reported outside the United States, thus further analysis based on race was not possible within this subgroup. Within the United States, self-identified race is a social contract and is not considered a biologic or genetically defined category.28 Importantly, there were sizeable differences in detected medication classes at baseline and 6 months between Black Americans, non-Black Americans, and patients outside the United States, which in part reflect differences in treatment per guideline recommendations.23 Therefore, observed differences between groups likely reflect behavioral and cultural factors, as well as possible differences in health care access and different guidelines between subgroups, rather than genetic differences mediating different physiological responses to the procedure. In addition, baseline 24-hour ambulatory BP patterns were different between patients who enrolled before and during the COVID-19 pandemic, in line with patterns observed in other trials across the United States during the pandemic.29–31 Likewise, over 60% of 6-month follow-up data were collected during the COVID-19 pandemic, and 80% of patients in the Expansion cohort enrolled during the pandemic. Moreover, this analysis is limited since the primary end point (between-group difference in mean 24-hour systolic BP at 6 months) was not achieved.
Conclusions
In summary, the cohort of patients enrolled outside the United States, including Europe, Japan, and Australia, showed significant treatment benefit in the RDN group compared with the sham control group for 24-hour ambulatory and office systolic BP, reflective of minimal and balanced medication changes between treatment groups. Increases in hypertensive medication burden and adherence observed in the US sham group, especially among Black Americans, were associated with unexpected BP reductions from baseline that may have diluted the beneficial treatment effect in the overall cohort. Therefore, medication adherence is important to advocate for with better patient education and monitoring in all stages of antihypertensive management. RDN should be considered as an adjunctive therapy to antihypertensive medications but may also be considered in patients who are nonadherent to their medications in the uncontrolled hypertension population.
Perspectives
RDN is an emerging adjunctive therapy for the treatment of uncontrolled hypertension. However, device trials in an on med setting face an inherent limitation of patients and clinicians influencing the primary end point through BP monitoring and adjusting antihypertensive medications. In the SPYRAL HTN-ON MED study, Black Americans in particular had differential medication adjustments before the primary end point ascertainment by treatment group, possibly diluting the treatment effect of RDN. It will be important to continue to monitor this through the long-term follow-up of Black American patients receiving RDN.
ARTICLE INFORMATION
Author Contributions
Benjamin Woods, PhD, and Maria Min-young Kim, MB ChB, MRCP, PhD, of Medtronic provided editorial support under the direction of the first author, including creation of tables, figures, and copy editing of text. R.R. Townsend, D.E. Kandzari, K. Kario, F. Mahfoud, M.A. Weber, R.E. Schmieder, S. Pocock, K. Tsioufis, and M. Böhm participated in the design of the study. R.R. Townsend, D.E. Kandzari, K. Kario, F. Mahfoud, R.E. Schmieder, S. David, S. Steigerwalt, A. Walton, I. Hopper, B. Bertolet, F. Sharif, K. Fengler, and M. Böhm participated in patient data collection. All authors were involved in interpretation of the data. M. Fahy was the study biostatistician responsible for the statistical analyses. R.R. Townsend wrote the first draft of the report with significant input from K.C. Ferdinand, F. Mahfoud, D.E. Kandzari, K. Kario, S. Pocock, D.A. Hettrick, S. Brar, and M. Böhm. All authors agreed on the content of the manuscript, reviewed drafts, and approved the final version.
Sources of Funding
This study was funded by Medtronic.
Disclosures
R.R. Townsend is a consultant for Medtronic, Cytel, Novartis, Regeneron, and Janssen. He also receives royalties from UpToDate. K.C. Ferdinand is a consultant for Medtronic, Novartis, and Janssen. D.E. Kandzari reports institutional research/grant support from Biotronik, Boston Scientific, Cardiovascular Systems, Inc, Orbus Neich, Teleflex, Medtronic, and Ablative Solutions and personal consulting honoraria from Cardiovascular Systems, Inc, Medtronic, and Abbott Vascular. K. Kario receives personal fees from Medtronic during the conduct of the study; grants from A&D, Omron Healthcare, Fukuda Denshi, CureApp, Sanwa Kagaku Kenkyusho, Teijin Pharma, Boehringer Ingelheim Japan, and Fukuda Lifetec; consulting fees from A&D, JIMRO, Omron Healthcare, CureApp, Terumo, and Fukuda Denshi; honoraria from Otsuka Pharmaceuticals and Omron Healthcare; and participation in Advisory Board of Fukuda Denshi, outside the submitted work. F. Mahfoud is supported by Deutsche Gesellschaft für Kardiologie, Deutsche Forschungsgemeinschaft (SFB TRR219), and Deutsche Herzstiftung. He has received scientific support from Ablative Solutions, Medtronic, and ReCor Medical and speaker honoraria/consulting fees from Ablative Solutions, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Inari, Medtronic, Merck, ReCor Medical, Servier, and Terumo. M.A. Weber has received consulting fees from Medtronic, ReCor Medical, Ablative Solutions, Johnson & Johnson, and Urovant. R.E. Schmieder reports grants and personal fees from Medtronic, ReCor Medical, and Ablative Solutions. S. Pocock reports personal fees from Medtronic outside the submitted work. K. Tsioufis reports institutional research/grant support from Medtronic and ReCor Medical and personal consulting honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Medtronic, ReCor Medical, Servier, WinMedica, and ELPEN. A. Walton is a proctor, on the medical advisory board, and has received grant support from Medtronic, Edwards, and Abbott. I. Hopper has received honoraria from Boehringer Ingelheim, Eli Lilly, AstraZeneca, and Vifor. B. Bertolet serves on the Medtronic Renal Denervation Advisory Committee as a consultant. F. Sharif is supported by Science Foundation Ireland Research Infrastructure (17/RI/5353) and is a consultant and advisory board member for Medtronic. K. Fengler received institutional grants from Medtronic, ReCor Medical, and Biotronic. M. Fahy, D.A. Hettrick, and S. Brar are employees of Medtronic. M. Böhm is supported by the Deutsche Forschungsgemeinschaft (German Research Foundation; TTR 219, project number 322900939) and reports personal fees from Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Medtronic, Novartis, ReCor Medical, Servier, and Vifor during the conduct of the study. The other authors report no conflicts.
Supplemental Material
Figures S1–S7
Tables S1 and S2
Supplementary Material
Nonstandard Abbreviations and Acronyms
- BP
- blood pressure
- RDN
- renal denervation
For Sources of Funding and Disclosures, see page 1104.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.123.22251.
REFERENCES
- 1.Zhou B, Carrillo-Larco RM, Danaei G, Riley LM, Paciorek CJ, Stevens GA, Gregg EW, Bennett JE, Solomon B, Singleton RK, et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398:957–980. doi: 10.1016/S0140-6736(21)01330-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16:223–237. doi: 10.1038/s41581-019-0244-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 4.Muntner P, Hardy ST, Fine LJ, Jaeger BC, Wozniak G, Levitan EB, Colantonio LD. Trends in blood pressure control among US adults with hypertension, 1999-2000 to 2017-2018. JAMA. 2020;324:1190–1200. doi: 10.1001/jama.2020.14545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aggarwal R, Chiu N, Wadhera RK, Moran AE, Raber I, Shen C, Yeh RW, Kazi DS. Racial/ethnic disparities in hypertension prevalence, awareness, treatment, and control in the United States, 2013 to 2018. Hypertension. 2021;78:1719–1726. doi: 10.1161/HYPERTENSIONAHA.121.17570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Townsend RR, Mahfoud F, Kandzari DE, Kario K, Pocock S, Weber MA, Ewen S, Tsioufis K, Tousoulis D, Sharp ASP, et al. ; SPYRAL HTN-OFF MED Trial Investigators*. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet. 2017;390:2160–2170. doi: 10.1016/S0140-6736(17)32281-X [DOI] [PubMed] [Google Scholar]
- 7.Böhm M, Kario K, Kandzari DE, Mahfoud F, Weber MA, Schmieder RE, Tsioufis K, Pocock S, Konstantinidis D, Choi JW, et al. ; SPYRAL HTN-OFF MED Pivotal Investigators. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet. 2020;395:1444–1451. doi: 10.1016/S0140-6736(20)30554-7 [DOI] [PubMed] [Google Scholar]
- 8.Azizi M, Schmieder RE, Mahfoud F, Weber MA, Daemen J, Davies J, Basile J, Kirtane AJ, Wang Y, Lobo MD, et al. ; RADIANCE-HTN Investigators. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet. 2018;391:2335–2345. doi: 10.1016/S0140-6736(18)31082-1 [DOI] [PubMed] [Google Scholar]
- 9.Azizi M, Sapoval M, Gosse P, Monge M, Bobrie G, Delsart P, Midulla M, Mounier-Véhier C, Courand P-Y, Lantelme P, et al. ; Renal Denervation for Hypertension (DENERHTN) Investigators. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet. 2015;385:1957–1965. doi: 10.1016/S0140-6736(14)61942-5 [DOI] [PubMed] [Google Scholar]
- 10.Kandzari DE, Böhm M, Mahfoud F, Townsend RR, Weber MA, Pocock S, Tsioufis K, Tousoulis D, Choi JW, East C, et al. ; SPYRAL HTN-ON MED Trial Investigators. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391:2346–2355. doi: 10.1016/S0140-6736(18)30951-6 [DOI] [PubMed] [Google Scholar]
- 11.Azizi M, Sanghvi K, Saxena M, Gosse P, Reilly JP, Levy T, Rump LC, Persu A, Basile J, Bloch MJ, et al. ; RADIANCE-HTN Investigators. Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN TRIO): a randomised, multicentre, single-blind, sham-controlled trial. Lancet. 2021;397:2476–2486. doi: 10.1016/S0140-6736(21)00788-1 [DOI] [PubMed] [Google Scholar]
- 12.Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, et al. ; SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–1401. doi: 10.1056/NEJMoa1402670 [DOI] [PubMed] [Google Scholar]
- 13.Kario K, Yokoi Y, Okamura K, Fujihara M, Ogoyama Y, Yamamoto E, Urata H, Cho JM, Kim CJ, Choi SH, et al. Catheter-based ultrasound renal denervation in patients with resistant hypertension: the randomized, controlled REQUIRE trial. Hypertens Res. 2022;45:221–231. doi: 10.1038/s41440-021-00754-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kandzari DE, Bhatt DL, Brar S, Devireddy CM, Esler M, Fahy M, Flack JM, Katzen BT, Lea J, Lee DP, et al. Predictors of blood pressure response in the SYMPLICITY HTN-3 trial. Eur Heart J. 2015;36:219–227. doi: 10.1093/eurheartj/ehu441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kandzari DE, Townsend RR, Kario K, Mahfoud F, Weber MA, Schmieder RE, Pocock S, Tsioufis K, Konstantinidis D, Choi J, et al. ; SPYRAL HTN-ON MED Investigators. Safety and efficacy of renal denervation in patients taking antihypertensive medications. J Am Coll Cardiol. 2023;82:1809–1823. doi: 10.1016/j.jacc.2023.08.045 [DOI] [PubMed] [Google Scholar]
- 16.Böhm M, Townsend RR, Kario K, Kandzari D, Mahfoud F, Weber MA, Schmieder RE, Tsioufis K, Hickey GL, Fahy M, et al. Rationale and design of two randomized sham-controlled trials of catheter-based renal denervation in subjects with uncontrolled hypertension in the absence (SPYRAL HTN-OFF MED Pivotal) and presence (SPYRAL HTN-ON MED Expansion) of antihypertensive medications: a novel approach using Bayesian design. Clin Res Cardiol. 2020;109:289–302. doi: 10.1007/s00392-020-01595-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahfoud F, Kandzari DE, Kario K, Townsend RR, Weber MA, Schmieder RE, Tsioufis K, Pocock S, Dimitriadis K, Choi JW, et al. Long-term efficacy and safety of renal denervation in the presence of antihypertensive drugs (SPYRAL HTN-ON MED): a randomised, sham-controlled trial. Lancet. 2022;399:1401–1410. doi: 10.1016/S0140-6736(22)00455-X [DOI] [PubMed] [Google Scholar]
- 18.McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol. 2014;67:267–277. doi: 10.1016/j.jclinepi.2013.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kandzari DE, Mahfoud F, Bhatt DL, Böhm M, Weber MA, Townsend RR, Hettrick DA, Schmieder RE, Tsioufis K, Kario K. Confounding factors in renal denervation trials: revisiting old and identifying new challenges in trial design of device therapies for hypertension. Hypertension. 2020;76:1410–1417. doi: 10.1161/HYPERTENSIONAHA.120.15745 [DOI] [PubMed] [Google Scholar]
- 20.Freeman MW, Halvorsen YD, Marshall W, Pater M, Isaacsohn J, Pearce C, Murphy B, Alp N, Srivastava A, Bhatt DL, et al. ; BrigHTN Investigators. Phase 2 trial of baxdrostat for treatment-resistant hypertension. N Engl J Med. 2023;388:395–405. doi: 10.1056/NEJMoa2213169 [DOI] [PubMed] [Google Scholar]
- 21.Schlaich MP, Bellet M, Weber MA, Danaietash P, Bakris GL, Flack JM, Dreier RF, Sassi-Sayadi M, Haskell LP, Narkiewicz K, et al. ; PRECISION Investigators. Dual endothelin antagonist aprocitentan for resistant hypertension (PRECISION): a multicentre, blinded, randomised, parallel-group, phase 3 trial. Lancet. 2022;400:1927–1937. doi: 10.1016/S0140-6736(22)02034-7 [DOI] [PubMed] [Google Scholar]
- 22.Bakris G. Top-line results of the first-in-class aminopeptidase-A inhibitor firibastat in treatment-resistant hypertension (FRESH) study. Late Breaking Clinical Trial presented at: American Heart Association’s Scientific Sessions 2022. 2022. https://www.ahajournals.org/doi/10.1161/CIR.0000000000001116#d1e2983 [Google Scholar]
- 23.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. 2018;71:1269–1324. doi: 10.1161/HYP.0000000000000066 [DOI] [PubMed] [Google Scholar]
- 24.Flack JM, Bhatt DL, Kandzari DE, Brown D, Brar S, Choi JW, D’Agostino R, East C, Katzen BT, Lee L, et al. ; SYMPLICITY HTN-3 Investigators. An analysis of the blood pressure and safety outcomes to renal denervation in African Americans and non-African Americans in the SYMPLICITY HTN-3 trial. J Am Soc Hypertens. 2015;9:769–779. doi: 10.1016/j.jash.2015.08.001 [DOI] [PubMed] [Google Scholar]
- 25.Flack JM, Sica DA, Bakris G, Brown AL, Ferdinand KC, Grimm RH, Hall WD, Jones WE, Kountz DS, Lea JP, et al. ; International Society on Hypertension in Blacks. Management of high blood pressure in Blacks: an update of the International Society on Hypertension in Blacks consensus statement. Hypertension. 2010;56:780–800. doi: 10.1161/HYPERTENSIONAHA.110.152892 [DOI] [PubMed] [Google Scholar]
- 26.Still CH, Margevicius S, Harwell C, Huang MC, Martin L, Dang PB, Wright Jnr JT. A community and technology-based approach for hypertension self-management (COACHMAN) to improve blood pressure control in African Americans: results from a pilot study. Patient Prefer Adherence. 2020;14:2301–2313. doi: 10.2147/PPA.S283086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Victor RG, Lynch K, Li N, Blyler C, Muhammad E, Handler J, Brettler J, Rashid M, Hsu B, Foxx-Drew D, et al. A cluster-randomized trial of blood-pressure reduction in Black barbershops. N Engl J Med. 2018;378:1291–1301. doi: 10.1056/NEJMoa1717250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flanagin A, Frey T, Christiansen SL, Bauchner H. The reporting of race and ethnicity in medical and science journals: comments invited. JAMA. 2021;325:1049–1052. doi: 10.1001/jama.2021.2104 [DOI] [PubMed] [Google Scholar]
- 29.Azzouzi S, Stratton C, Muñoz-Velasco LP, Wang K, Fourtassi M, Hong BY, Cooper R, Balikuddembe JK, Palomba A, Peterson M, et al. The impact of the COVID-19 pandemic on healthy lifestyle behaviors and perceived mental and physical health of people living with non-communicable diseases: an international cross-sectional survey. Int J Environ Res Public Health. 2022;19:8023. doi: 10.3390/ijerph19138023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bress AP, Cohen JB, Anstey DE, Conroy MB, Ferdinand KC, Fontil V, Margolis KL, Muntner P, Millar MM, Okuyemi KS, et al. Inequities in hypertension control in the United States exposed and exacerbated by COVID-19 and the role of home blood pressure and virtual health care during and after the COVID-19 pandemic. J Am Heart Assoc. 2021;10:e020997. doi: 10.1161/JAHA.121.020997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vasquez Reyes M. The disproportional impact of COVID-19 on African Americans. Health Hum Rights. 2020;22:299–307. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.