Abstract
The study proposes a dynamic spatio‐temporal profile of the distribution of tuberculosis incidence and air pollution in Romania, where this infectious disease induces more than 8,000 new cases annually. The descriptive analysis for the years 2012–2021 assumes an identification of the structuring patterns of mycobacterium tuberculosis risk in the Romanian population, according to gender and age, exploiting spatial modeling techniques of time series data. Through spatial autocorrelation, the degree of similarity between the analyzed territorial systems was highlighted and the relationships that are built between the analysis units in spatial proximity were investigated. By modeling the geographical distribution of tuberculosis, the spatial correlation with particulate matter (PM2.5) pollution was revealed. The identification of clusters of infected persons is an indispensable step in the construction of efficient tuberculosis management systems. The results highlight the link between the distribution of tuberculosis, air pollution and socio‐economic development, which requires a detailed analysis of the epidemiological data obtained in the national tuberculosis surveillance and control program from the perspective of geographical distribution.
Key Points
The study creates a dynamic spatio‐temporal profile of the tuberculosis frequency distribution (incidence) in Romania
The study analyses the impact of the COVID‐19 pandemic period on the evolution of the disease in Romania
The exploratory spatial autocorrelation method is used to identify spatial hotspots of tuberculosis concentration in Romania
1. Introduction
According to the Global Tuberculosis Report (GTR) (2022), tuberculosis (TB) is a globally prevalent infectious disease that is now among the leading causes of death worldwide and is the leading cause of death from a single infectious agent (GTR, 2022). Research shows that a quarter of the global population has been infected with Mycobacterium tuberculosis, but the majority will not develop TB or will clear the infection (Behr & Edelstein, 2019; Emery et al., 2021; Tiemersma et al., 2011). The frequency of pulmonary TB in Romania differs from region to region, making interdisciplinary research necessary to understand the determinants of these disparities. The literature highlights these geographical differentiations in numerous tuberculosis studies (Alene et al., 2017, 2021; Chen et al., 2021; Gao & Du, 2018; X. Li et al., 2021; Y. W. Li et al., 2016; Sun et al., 2022). Spatial heterogeneity points to the need for interdisciplinary analyses of disease transmission hubs in the community. Their knowledge is important because they become nodes of disease transmission to the outside of the area as well as within the community with new cases (Bishai et al., 1998; Munch et al., 2003; Small et al., 1994).
Numerous studies link the geographic factors (population density, lack of running water, quality of housing, geographical isolation, poverty, unemployment) to high levels of tuberculosis (Dangisso et al., 2014, 2015; Datiko et al., 2008; Koh et al., 2013; Liu et al., 2012; Munch et al., 2003; Shaweno et al., 2017; Tadesse et al., 2013; Touray et al., 2010; Trauer et al., 2019). Analysis of the spatial distribution of tuberculosis has been addressed in several territories globally with the aim of identifying the main risk factors. Thus, Guo et al. (2017) analyzing the distribution of tuberculosis in mainland China, revealed variations related to demographic, socio‐economic and meteorological factors. In Latin America and the Caribbean, the frequency of tuberculosis appears to be correlated with inequalities in per capita health expenditure and in access to sanitation, which seem to define the gradients of tuberculosis persistence in this area (Munayco et al., 2015).
Spatial modeling of tuberculosis has identified an important determinant in the development of this disease, pollution and particulate matter affecting the primary defense mechanisms against Mycobacterium tuberculosis (Huff et al., 2019; Ibironke et al., 2019; Lai et al., 2016; Popovic et al., 2019; Rivas‐Santiago et al., 2015; Smith et al., 2014; Zhu et al., 2018).
Knowledge of the spatial distribution of TB has an important role to play in developing strategies to eliminate TB from affected communities, and integrating the spatial dimension into public policy‐making in this area can help to increase the effectiveness of public policies. Thus, spatial analyses should distinguish between dispersion and diffusion patterns, an important differentiation in identifying spatial hotspots and the best TB management systems (Alene et al., 2021; Oxlade et al., 2015; Sattenspiel, 2009; Shaweno et al., 2018; Sotgiu et al., 2021).
Research in high TB prevalence countries has revealed a spatial distribution pattern characterized by low access to health services, high population density, high population mobility within and outside the community, poor socio‐economic conditions. These findings lead to a rethinking of public policies whose effectiveness depends on their adaptation to the specificities of each administrative unit (Alene & Clements, 2019; Alene et al., 2020; Carrasco‐Escobar et al., 2020; Dominkovics et al., 2011; Shaweno et al., 2018; Tiwari et al., 2010).
The COVID‐19 pandemic has had a significant impact on tuberculosis (TB) in various ways. Research studies have been conducted to understand the consequences of TB on COVID‐19 outcomes and vice versa during the pre‐vaccination period of the pandemic.
Here are some key findings from these studies:
Disruption of TB services: the COVID‐19 pandemic has severely disrupted TB services, leading to health facility closures, lockdowns, travel bans, overwhelmed healthcare systems, and restricted export of antituberculous drugs. These disruptions have affected the diagnosis, treatment, and management of TB patients (Jhaveri et al., 2022).
Impact on TB treatment initiation: Studies conducted in Canada showed a reduction in the initiation rates of latent TB infection treatment and active TB treatment during the COVID‐19 pandemic. This suggests that the focus on COVID‐19 may have led to a decrease in attention and resources allocated to TB prevention and treatment (Geric et al., 2022).
Co‐infection of TB and COVID‐19: co‐infection of TB and COVID‐19 has been reported in some cases. Studies from Turkey described the clinical features of 16 patients with co‐infection, highlighting the need for careful management and monitoring of these individuals (Gül et al., 2022). However, the overall prevalence of TB and COVID‐19 co‐infection appears to be relatively low (Daneshvar et al., 2023).
Impact on TB mortality and adverse outcomes: the association between TB and COVID‐19 mortality/adverse outcomes is still not fully understood. Some studies reported heterogenous results regarding the association between TB and COVID‐19 mortality. However, it is important to note that the effects of COVID‐19 on TB outcomes could not be assessed due to limited data on TB outcomes during the pandemic (Jhaveri et al., 2022).
Immune responses and co‐infection: patients with co‐infection of TB and COVID‐19 may exhibit dysregulated immune responses compared to those with only COVID‐19 or TB. Longitudinal studies are needed to confirm these findings and expand our knowledge of the immune response in co‐infected individuals (Flores‐Lovon et al., 2022).
Overall, the COVID‐19 pandemic has posed challenges to TB prevention, diagnosis, and treatment efforts. The disruptions in healthcare systems and the focus on COVID‐19 may have impacted the management and outcomes of TB patients. Further research is needed to fully understand the interaction between COVID‐19 and TB and its implications for public health.
This study aims to determine the spatial distribution of TB new cases and relapses in Romania, with the identification of a stratification algorithm of infection risks related to the geographical component of the country, with the identification of potential spatial hotspots for the spread of the disease in the community, through analysis at the nanostructural level (at the level of all territorial administrative units) and which will subsequently allow the creation of TB control guidelines for well‐defined territorial units, in correlation with the administrative organization of the country.
This study aims to determine the spatial distribution of TB cases in Romania, with the identification of a stratification algorithm of infection risks related to particulate matter (PM2.5) pollution, with the identification of potential spatial hotspots for the spread of the disease in the community, through analysis at the nanostructural level (at the level of all territorial administrative units) and which will subsequently allow the creation of TB control guidelines for well‐defined territorial units.
2. Materials and Methods
2.1. Study Area
The research was conducted at the level of all territorial administrative units (TAU) in Romania (Figure 1), with data interpretations being made at the TAU, county and the development region level.
Figure 1.
The territorial administrative structure of Romania.
2.2. Identification of Spatial Clusters
In order to spatialize the phenomenon, it was decided to aggregate the data at the level of territorial administrative unit (TAU). In Romania, the territorial administrative unit (TAU) is the smallest form of political‐administrative organization of communities in the form of a territorial system with a specialized decision‐making function regulated by specific legislation (mayor and local council). The use of the territorial system as a unit of analysis in the research approach plays a key role in the research design, providing interdisciplinarity with territorial systems studies in explaining the phenomenon, and at the same time, pragmatism in the valorization of the research results to decision makers.
Based on the aggregated data series at the level of the patient's territorial administrative unit of residence, the variable of the frequency of tuberculosis cases (incidence‐new cases and relapses) per 1,000 inhabitants was analyzed in cross‐sectional approach, by year, using thematic maps (choropleth) with fixed legend. The result is recorded in the maps in Figures 10a–10g. In order to identify spatial organization patterns in the tuberculosis phenomenon, the Global Spatial Autocorrelation and Local Spatial Autocorrelation for the tuberculosis frequency variable per 1,000 inhabitants were used for the values 2015–2021 (the sum of yearly records). We opted for the aggregation of values because the analysis aims to identify areas of persistence over time, and less of spread of TB infection.
Figure 10.
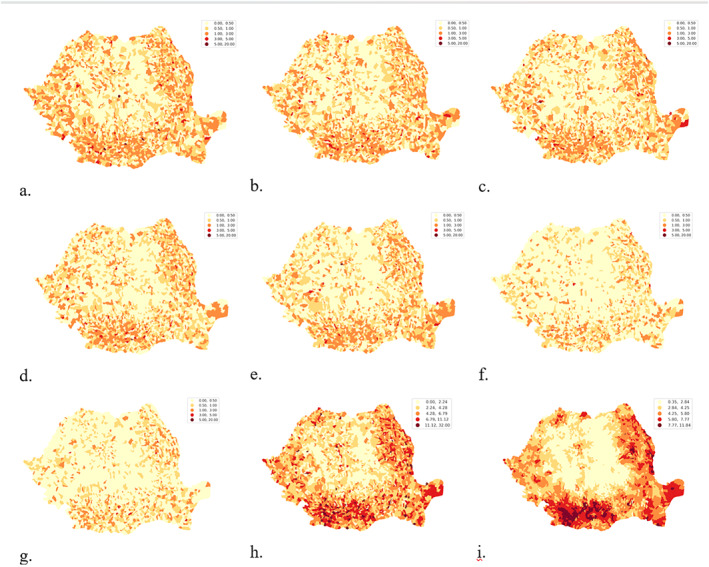
Frequency of TB new cases and relapses at territorial administrative unit (TAU) level: (a) 2015, (b) 2016, (c) 2017, (d) 2018, (e) 2019, (f) 2020, (g) 2021, (h) 2015–2021, (i) Spatial Lag for 2015–2021.
Spatial autocorrelation highlights the degree of similarity (Rey et al., 2023) between the territorial systems analyzed. As opposed to temporal autocorrelation, which involves time series of data on the same unit of analysis, spatial autocorrelation investigates the relationships that are built between units of analysis in spatial proximity on the observing variable. Overall spatial autocorrelation shows tendencies for similar values to cluster spatially or disperse (Rey et al., 2023) across the data set and provides analytical techniques to answer questions such as: are observed values randomly distributed or do observe similar values tend to cluster (positive correlation), or disperse (negative correlation)? Local spatial autocorrelation customizes the analysis at the level of each unit of analysis and their neighborhoods through scores that allow us to learn more about the spatial structure in our data. To measure the degree of spatial autocorrelation we used the libpysal and esda libraries from the PySAL (Rey & Anselin, 2007) family performing the operations: (a) determining the standardized spatial weighting matrix (W), (b) calculating the spatial lag of the variable, (c) calculating the Moran's I‐value and making the Moran's Plot to evaluate the null hypothesis.
The spatial weighting matrix (W) was constructed on the basis of spatial contiguity relations of the “Rook” type between analysis units, determining for each analysis unit the set of analysis units with which it adjoins, thus managing to incorporate the spatiality relation into the explanatory model. A contiguity relationship was chosen, where neighbors are identified by common boundaries, thus simulating the mode of spread of Mycobacterium tuberculosis, which involves direct contact between source and target and transmission from one subject to another in close proximity. The values in the contiguity‐based spatial weighting matrix are identified as follows (Sarrias, 2020): let n be the number of spatial analysis units. The spatial weighting matrix (W), an n × n positively symmetric and non‐stochastic matrix with w ij elements at position i,j. The w ij values, called weights for each location, are assigned according to spatial relational rules, in our case, according to spatial contiguity. By convention, w ij = 0 for the elements on the diagonal of the matrix (being the relations of the unit of analysis to itself). The matrix is populated according to the rule:
| (1) |
The resulting matrix determines the neighbors of each territorial unit, and assigns a weighting coefficient of one unit (Equation 1) to each identified neighbor, but in order for the spatial parameters obtained to be comparable between the analysis units, it is necessary to standardize on a per‐unit basis. This operation is performed by weighting the w ij values to one unit according to the formula (Equation 2):
| (2) |
For example, if the Buftea analysis unit has the following set of non‐null neighbors {“Buftea”: [1.0, 1.0, 1.0, 1.0, 1.0]} identified in the spatial weighting matrix, applying the row standardization operation to a unit yields the series {“Buftea”: [0.2, 0.2, 0.2, 0.2, 0.2]}.
With a structure of neighbors defined by nonzero values in the spatial weighting matrix (W), the spatial lag variable is a weighted sum or weighted average of the observed neighbor values on the same variable. The spatial lag variable is denoted by y and is the product of Wy (Anselin, 2018) (Equations 3 and 4):
| (3) |
or
| (4) |
where w ij are the elements of the rows i in the matrix W, correlated with the elements of the y vector. In other words, the spatial lag variable is a sum of the observed values in the neighbors. If the matrix W is standardized (Equation 5) by the row standardization operation (our case), then, the spatial lag operator is a weighted sum of the observed values at neighbors (Equations 6 and 7).
At this point we can assess global spatial autocorrelation on the frequency variable per 1,000 population cumulated between 2015 and 2020. Using the Moran plot (Figure 2a) we can observe each unit of analysis referenced in two axes: the variable analyzed (X‐axis) and the spatial lag variable associated to the variable analyzed (Y‐axis). In the case of the Moran chart, referencing is done by standardizing the values using the standard deviations determined for each variable at the level of the units of analysis. For the resulting point cloud, the linear regression line is determined, and the slope coefficient of the line, called Moran's I indicator, represents the degree of overall spatial autocorrelation at the level of the variable under analysis, and the sign of the slope coefficient indicates the direction of correlation (in our case, positive correlation), which means that large values at the level of the units of analysis tend to influence observations in the neighborhood.
Figure 2.
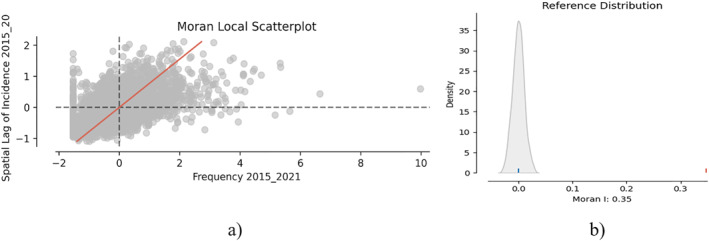
(a) Moran spatial autocorrelation chart; (b) Comparison of the p‐value with the reference distribution.
To perform the test of significance of spatial autocorrelation the null hypothesis is established (H0): There is no spatial correlation in the distribution of tuberculosis at the level of territorial TAU systems in Romania. The esda package (in PySAL) gives us the possibility to compute the p‐value (moran.p_sim) which expresses the probability of obtaining the same Moran's coefficient I under spatial permutation of existing values. Using the splot package (from PySAL) we were able to make the plots in Figure 2, which help us visualize the Moran plot and the Reference Distribution (Moran's I coefficient values for each simulation). Based on the p‐value and Figure 2a we reject the null hypothesis, that is, that the distribution of the variable is not random in the analyzed map.
Local spatial autocorrelation involves investigating the relationships at the level of each unit of analysis with its neighbors by determining Local Indicators of Spatial Autocorrelation. Local Indicators of Spatial Autocorrelation provides a calculated statistical value for each unit of analysis that is associated with a significance test. For the present analysis, Moran's local I indicator was used to determine the similarity or dissimilarity between the observed value and the spatial gap variable at the level of a unit of analysis. Using the esda and splot package (from PySAL), Moran's local I value were calculated, accompanied by an assessment of the significance of the p‐values. 952 are considered significant considering the p = 0.05 threshold, that is, approximately 30% of the territorial administrative units fall into spatial clusters. Units of analysis with p‐values ≤0.05 are classified into the following clusters: HH: high observed value, high spatial lag value, called hotspot; LL: low observed value, low spatial lag value, called coldspot; HL: high observed value, low spatial lag value; LH: low observed value, high spatial lag value (Figures 3a and 3b).
Figure 3.
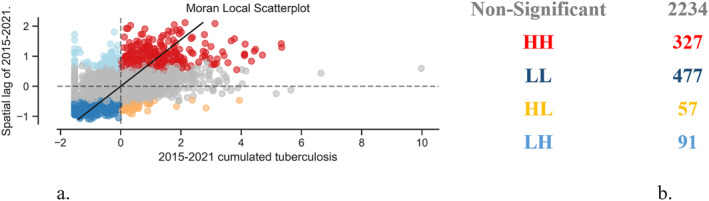
Tuberculosis recorded between 2015 and 2021: (a) graphical distribution of clusters and (b) space cluster statistics.
Local spatial autocorrelation analysis has been applied also to the data series of PM2.5 (Figures 4a and 4b). An average of the values was determined at the territorial administrative level for 2015–2021 interval and local spatial autocorrelation has been applied, resulting in the following outcomes: there is a very high level of spatial distribution of data (Moran I = 0.96) with the test of significance for spatial autocorrelation that has rejected the null hypothesis (H0: there is no a spatial distribution of PM2.5.)
Figure 4.
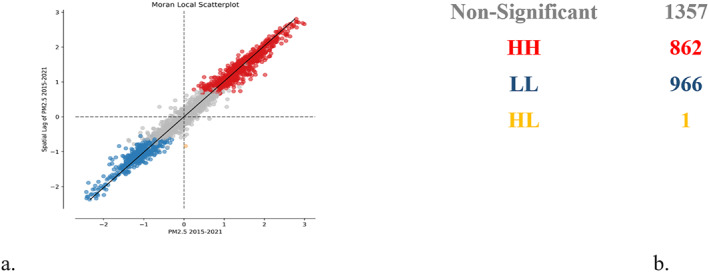
Average of PM 2.5 values for 2015–2021 in AQLI database (a) graphical distribution of clusters and (b) space cluster statistics.
The local spatial autocorelations applied analysis technique offered us two nominal variables (clusters of tuberculosis and clusters of PM2.5) that describe two independent phenomena on the same territory, both of variables referenced at the same analysis unit (territorial administrative unit) (Figure 5). In order to understand the correlation between these two independent nominal variables we applied Chi‐Square independence test, with H0: The two variables are independent.
Figure 5.
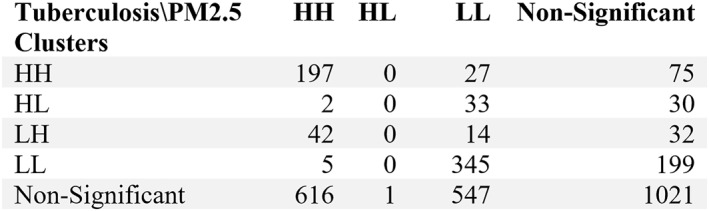
Contingency table matrix.
The null hypothesis is rejected with a Chi_square value 642.35, a p‐value <0.005 and 12 degree of freedom determined by scipy.stats python package. The Cramer’V indicator value is 0.259, giving us a level o association on a scale from 0 to 1 (where 0 indicates no association, and 1 indicates a perfect association.)
3. Descriptive Data Analysis
Information on tuberculosis cases was obtained from the Marius Nasta Institute of Pneumophthisiology, the unit that coordinates the National Tuberculosis Prevention, Surveillance and Control Program in Romania, representing the consolidated national database of all tuberculosis cases registered annually in Romania. The database contains annual information from 2012 to 2021, at the anonymized patient level, and has been used in compliance with GDPR policies. In determining the variable number of registered cases, new cases and relapses registered annually were entered, and records were aggregated at the level of the analysis unit (territorial administrative unit or county) on the analysis dimensions: age, gender, affected organ. For data spatialization, aggregation of records by county (for the whole 2012–2021 data set) and by territorial administrative unit (for 2015–2021) was used. To report the number of new cases and relapses to the population, annual data at the level of territorial administrative unit, by age and gender were taken from the national demographic statistics published on the official website of the National Institute of Statistics (Tempo online). The maps were produced using geospatial data sets published by the National Agency for Cadastre and Real Estate Publicity on the official open data website (https://data.gov.ro/), coordinated by the General Secretariat of the Romanian Government, and the official nomenclature of administrative territorial units of Romania, called SIRUTA. The data were integrated and processed in a PostgreSQL database where various views were created for data analysis and visualization. Office Excel 2019 software was used for the application of descriptive analysis techniques, and QuantumGIS and the geospatial statistical analysis packages PySAL and geosnap were used for geospatial analysis (Rey & Anselin, 2007).
The methodology of the first part of the paper replaces descriptive research and aims to identify patterns in the structuring of Mycobacterium tuberculosis disease in the Romanian population by gender and age, using time series visualization techniques. The exploratory approach of the research aims to obtain answers to the following questions: How is Mycobacterium tuberculosis disease distributed in the Romanian population? Are there patterns of structuring and how do these patterns behave over time? What was the impact of the COVID 19 pandemic period?
In the second part of the paper, the exploratory spatial autocorrelation method was used to analyze the data and to answer the question: are there spatial organization patterns in the tuberculosis phenomenon in Romania?
Obtaining answers to these questions can explain relationships and causalities in the morphology and dynamics of tuberculosis infection. It can also identify and explain structural characteristics of tuberculosis distribution, thus assisting national health policies, focusing decisions on prevention actions or guiding future research directions to understand the mechanisms of the onset and spread of tuberculosis infection.
In the analysis, the annual values of TB new cases and relapses were used, as well as the ratio of these values per 1,000 inhabitants according to the formula (Equation 5):
| (5) |
The two variables, the number of tuberculosis cases and the frequency of tuberculosis cases per 1,000 inhabitants, were disaggregated by age, gender and place of residence at the level of territorial administrative unit (2015–2021) or county (2012–2014) of the patient as well as by affected organ (pulmonary vs. extrapulmonary). Using data series visualization techniques, it was possible to identify the structuring of the tuberculosis disease phenomenon in the Romanian population.
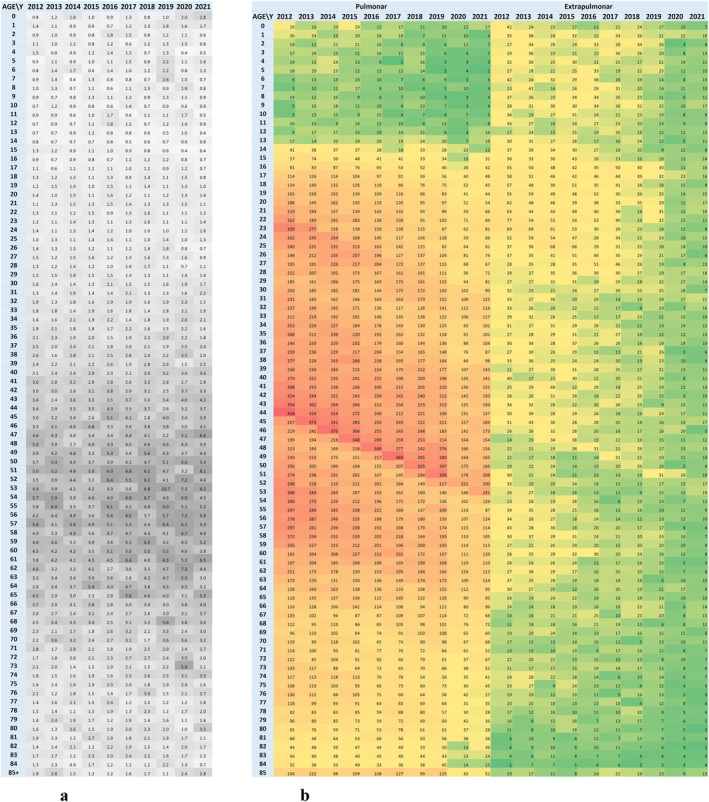
Line‐chart, bar chart and heatmap time series visualization techniques were used to present the structural evolution of tuberculosis cases. These visualization techniques show patterns of distribution and correlation between age, gender and affected organ. By using the heatmap technique, highly affected population cohorts and how they evolve over time are highlighted. Understanding the dynamics of how the Mycobacterium Tuberculosis disease phenomenon is structured along different dimensions of analysis was achieved by timeline visualizations of the main indicators in absolute values (number of tuberculosis cases) or relative values (shares of total registered cases) using the secondary axis (Figure 6a) and adding the trendline representation for the data series (Figure 6b).
Figure 6.
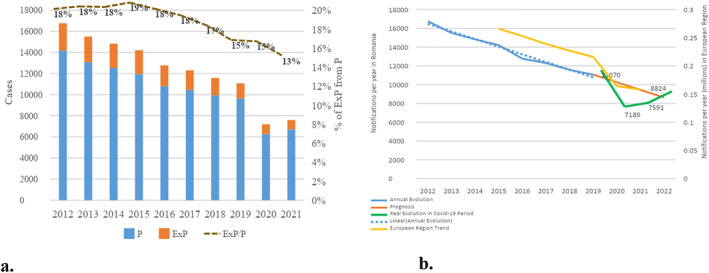
(a) Evolution of TB cases by location; (b) Specificity of the evolution of the number of TB cases during the COVID‐19 crisis. Abbreviations: P, Pulmonary; ExP, Extrapulmonary.
One of the research directions of this work is to understand the evolution of tuberculosis in the context of the COVID19 pandemic. Explaining the impact of the COVID pandemic was done by measuring the percentage difference between the number of cases actually registered and the number of cases estimated for the years 2020 and 2021 based on the trend determined by simple linear regression from 2012 to 2019 (Equation 6):
| (6) |
Applying the simple linear regression model for the values recorded between 2012 and 2019 the regression equation was determined (Equation 7).
| (7) |
The equation obtained (Equation 3) explains more than 98% (R2) of the variation in the number of tuberculosis cases between 2012 and 2019. Based on the slope coefficient (−815), the expected values for 2020 and 2021 were determined, and using formula (Equation 2), the percentage reduction in 2022 was calculated, where the observed value is the value recorded by official statistics and the expected value is that determined by the slope coefficient.
Understanding the spatial structuring of percentage reductions at the county level in Romania was achieved using spatial visualization techniques with choropleth maps (Figure 7, Figure 10).
Figure 7.
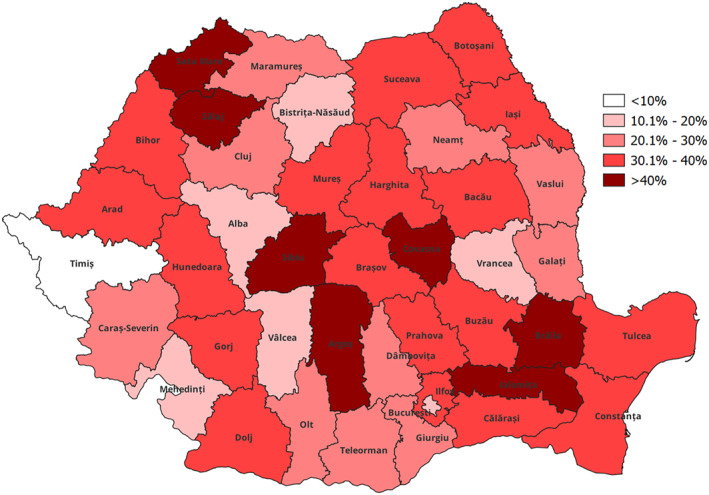
Loss level (%) of recorded new cases and relapses of TB in 2020 compared to 2019.
By using heatmap visualization techniques, tabular representation on two axes each representing a variable (x‐axis for the annual time series and y‐axis for the analyzed variable), and coloring the cells according to the value entered in the cell on an intensity scale, the behavior of the phenomenon recorded over time by different age and gender categories was highlighted (Figure 6). In Figure 7, the heatmap visualization was used to understand the relationship between the frequency of tuberculosis in men and that recorded in women (Figure 7a), and to visualize the distribution in the population time‐series structure of recorded values by affected organ (pulmonary vs. extrapulmonary, Figure 7b).
For the spatial modeling of (PM2.5), the Donkelaar et al. (2021) database, version V5.GL.03 was used, public data that can be accessed directly at: https://sites.wustl.edu/acag/datasets/surface-pm2-5/. The representation of the data set in spatial models was done in order to highlight the geographical areas where values above 20 μg/m3 were recorded for the period analyzed, the limit set by law no. 104 of 15 June 2011. QGis, a free and open sources geographic information system, was used to produce the maps.
4. Results
4.1. Tuberculosis Evolution in Romania
In Romania, by 2020, the trend of TB new cases and relapses recorded in official statistics was to decrease by −5.7% (−815 cases) annually. During the pandemic period, the number of cases decreased by an average of 30%, practically 10,557 cases were expected to be identified, but 7,189 cases were registered, compared to the possible trend without the impact of the pandemic (Figure 6b). This downward trend is more pronounced in Romania than in the rest of the European Region, as recorded in the Global Tuberculosis Report 2022. According to the recorded statistics, extrapulmonary TB represents 18% of cases, with a slightly decrease in recent years, a trend that continues during the pandemic period, when no significant changes are observed in the ratio of pulmonary to extrapulmonary TB (Figure 6a).
Comparing the loss of recorded new cases and relapses in 2020 against 2019 at the county level, there is a significant variation in the TB statistics behavior across the country. Seven counties have lost more than 40% of records, while only one county keep a level in an expected trend (Figure 7). The data obtained require a two‐way assessment of the situation, both in terms of the number of COVID‐19 cases recorded, the restrictions applied in the respective territories, and an analysis of the health system and how resilient it has been during the pandemic period.
Analyzing the distribution of tuberculosis new cases and relapses by age and gender, although in Romania, tuberculosis affects all ages, regardless of gender, it can be seen that certain age groups are more often affected (Figure 8a, Table 1).
Figure 8.
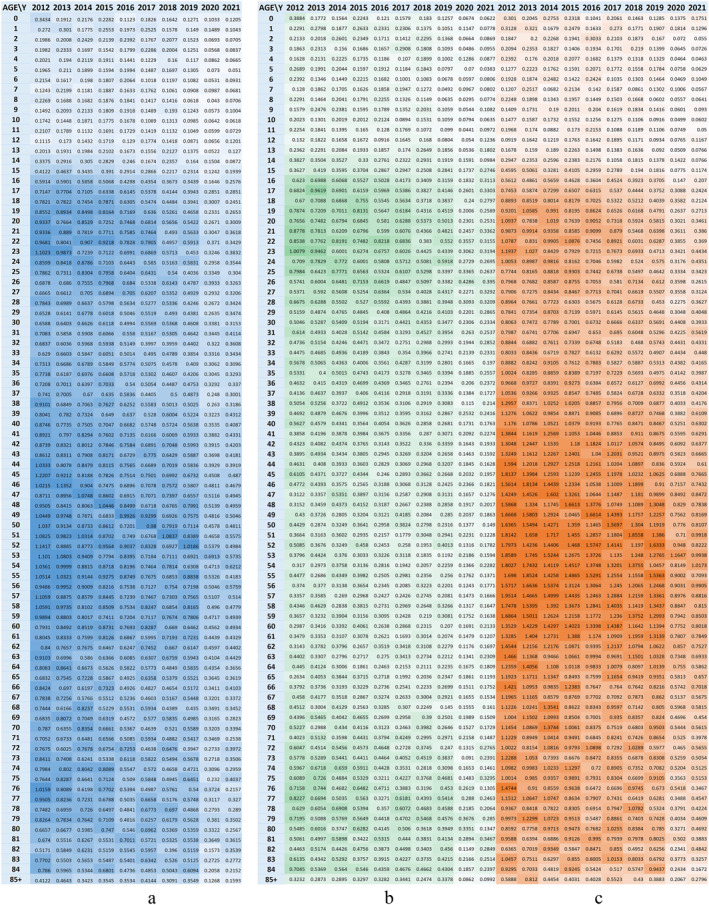
Evolution of TB frequency per 1,000 inhabitants by age; (a) total; (b) female; (c) male.
Table 1.
Structural Characteristics of Affected Cohorts
| Age | Cohort characteristics |
|---|---|
| 16–23 | It is characterized by a strong onset around the age of 15–16, regardless of generation. The behavior over time of this segment of the population affected by tuberculosis shows that the triggers of tuberculosis are linked to the dynamics of social life, the socio‐economic context of the population in this age group and there are no other aspects linked to the characteristics of the generation in question |
| 45–55 | The population included in the age segment of 45 years, as well as those aged 48–55 years in the year 2021, respectively people born in 1976 and between 1973 and 1966 have a different behavior, new cases and relapses occur predominantly within the generation and do not trigger at a certain age, which leads us to focus our research in investigating this population segment in its historical evolution, in specific aspects related to that generation (specific medical vaccination policies applied, specific socio‐economic conditions of the generation, etc.), highlighting possible intrinsic causes and less on current living conditions |
From the gender distribution of tuberculosis new cases and relapses we see a uniform distribution in the first age segment analyzed (16–23 years), but in the older age groups males predominate as the most affected by the disease (Figures 6b and 6c).
The gender ratio between the affected populations is measured in Figure 9a, where we see that in the +30 generations, men are the most affected by this infection, with values as high as 7:1 around the age of 50, while the younger and adolescent generations are similarly affected. Furthermore, the adolescent generation is the segment with extrapulmonary forms of tuberculosis, with a 1:3 ratio of extrapulmonary to pulmonary cases, shown in Figure 9b. The same Figure 9b further nuances the characteristics of the 16–23 age segment, as follows: pulmonary TB follows a generational trend, similar to trends at older ages, but extrapulmonary TB is the one that starts in an age segment, regardless of generation.
Figure 9.
Structural elements of TB frequency: (a) The ratio of the frequency of cases in the male and female population (M/F) per 1,000 inhabitants and (b) Evolution of TB cases by body location.
4.2. Spatial Distribution of TB in Romania
From a spatial perspective, tuberculosis is present throughout Romania, with higher presence and continuity in the Oltenia, South‐West Muntenia, South Moldavia and Danube Delta areas. The annual distributions in time series with fixed legend show a decreasing trend (Figures 10a–10g), without showing regional trends. The map in Figure 10h shows the frequency per 1,000 inhabitants for the years 2015–2021 revealing the areas where tuberculosis records predominate, these are better identified by the spatial autocorrelation operation (Figure 10i) which more clearly identifies the areas where tuberculosis is significant.
A manually defined legend was used to locate the extreme TB areas to highlight areas with less than 1 case per 1,000 population and areas with more than 10 cases per 1,000 population over the 2015–2021 timeframe (Figure 11a). Thus, qualitative research to identify the enabling/disabling causes or conditions for the occurrence/non‐occurrence of TB will be able to focus on specific communities, identified exactly on the map in Figure 11a. Figure 11b shows the distribution of spatial clusters identified on the basis of local spatial autocorrelation. The 327 (Figure 3b) administrative territorial units that are part of the HH cluster are now spatially located and considered as tuberculosis prevalence areas in Romania, and specific prevention policies can be formulated for them. Prevention policies can be decentralized and focused on the areas identified as a problem.
Figure 11.
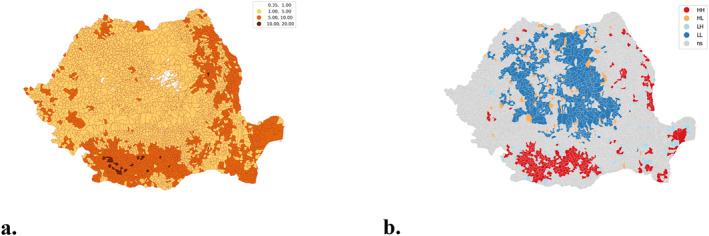
Spatial clustering. (a) spatial autocorrelation of cumulative tuberculosis frequency (2015–2021); (b) spatial clustering.
4.3. Geographical Distribution of Particulate Matter in Romania
Analysis of PM2.5 distribution revealed the association with an increased risk of tuberculosis infection, a link already highlighted in the literature (Elf et al., 2018), with the results validating the hypothesis of correlation between PM 2.5 and risk of tuberculosis infection. The geographical distribution of particulate matter (PM2.5) highlights the link between high TB values and the maintenance of air pollution with particulate matter over long periods of time. In the south‐west of Romania, Bucharest, Timișoara, Iași and their emerging area, values above or close to the maximum concentration allowed (20 μg/m3) are recorded (Figure 12a).
Figure 12.
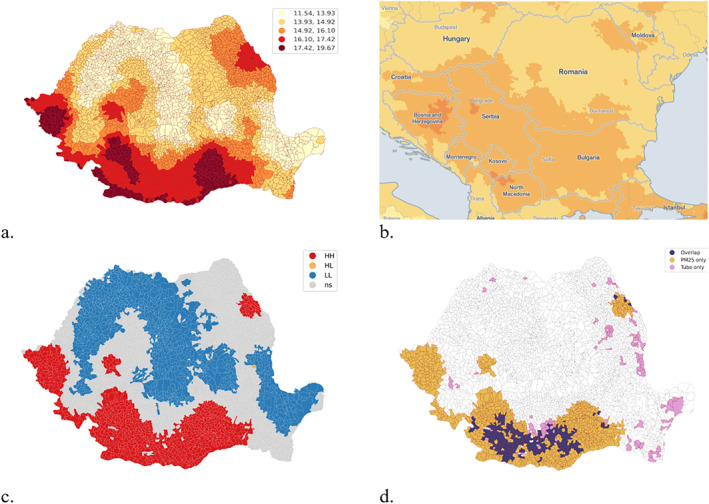
(a) Geographical distribution of annual average values of particulate matter (PM2.5) in Romania between 2015 and 2021. Local spatial autocorrelation of PM2.5. (b) Balkan context of PM2.5. Source: https://aqli.epic.uchicago.edu/the-index/. (c) PM2.5 Local Spatial Clusters. (d) Overlap between tuberculosis and PM2.5 local clusters.
The geographical distribution of particulate matter (PM2.5) emphasize the relationship with industrialized areas in Romania (Figure 12a) and the regional context of Balkans (Figure 12b). Local geospatial clusters of PM2.5 have been determined and overlapped with local geospatial clusters of tuberculosis calculated for the same period of time (2015–2021). Both of the phenomena are geospatially related and, based on chi‐square independence test, both of them are corelated with a level of association of 0.26 (Cramer's V indicator) on a scale from 0 to 1 (Figures 12c and 12d).
5. Conclusions and Discussion
The results show the same trends in the management of TB diagnosis in Romania as those observed globally in various countries in 2020, with the TB frequency rate falling in the first year of the pandemic globally by 9%, while the global rate of decline in frequency was 2%, according to the Global TB Report. Differences in Romania are significantly different from the global report, with an average decrease of 30% in the loss level in Romania, which requires a careful analysis of both the health system and the way in which local authorities have managed the need for medical care for patients with tuberculosis (Chakaya et al., 2020; Jain et al., 2020). In the North region of Brazil, for example, the tuberculosis detection rate in 2020 fell by 4.5%–16% (Costa et al., 2023).
Another research direction of our study aims to analyze age‐gender paternity in the frequency of tuberculosis cases. Most studies point out that the main factors that induce an increase in tuberculosis cases are malnutrition, poverty, diabetes, smoking and air pollution, with no correlation identified with gender or age (Lee et al., 2020; Noubiap et al., 2019; Reid et al., 2019; Stoichita et al., 2021). The statistically analyzed data show a distribution of pulmonary tuberculosis cases in favor of men, similar to other studies in the literature, probably as a result of the social characteristics that the male sex has: involvement in heavy work, choice of jobs involving longer commutes, excluding a genetic determinism component in the increased prevalence (Shaweno et al., 2021). In our study, three such clusters of increased frequency are noted for the period analyzed, in the population born in specific periods such as 1950–1952, 1967–1969, and 1989–1991. These periods can be associated with important social and economic changes in Romania. The population of the first cluster 1950–1952, is the one impacted by political changes, with the establishment of the communist regime and increasing urbanization. The second cluster, 1967–1969, coincides with the period immediately following the national decree banning abortions in 1966, leading to an increase in the birth rate while the socio‐economic difficulties specific to the communist period in 1980s Romania increased. In the case of the third cluster, 1989–1991, the generation born during the 1989 revolution was influenced by the years of transition to capitalism and the inherent challenges of the period, which impacted the socio‐medical system. The data obtained, however, require further analysis of socio‐economic and educational conditions. The third line of assessment of the frequency data identified geographical areas of concentration of tuberculosis. Spatial patterns identified TB concentration in poor regions of Romania, where access to health services is limited by lack of health care, low income and poor education, a situation scientifically confirmed by previous studies. The spatial correlation between high values of TB and particulate matter (PM2.5) opens an important direction for interdisciplinary research, as the determination relationship is evident. Future research needs to establish the weighting level of the pollution degree in the TB determinant matrix.
The comparative evaluation of the two spatial distribution maps of tuberculosis and PM2.5 particles reveals three distinct areas on the territory of Romania. On the one hand, we are talking about clear areas where the risk of tuberculosis is not correlated with the level of pollutants, or areas where, although PM 2.5 pollution is increased, the number of tuberculosis cases is below the national medicine. However, our study highlights two areas with increased risk of TB infection that overlap with two geographical territories where pollution is increased, as is the incidence of tuberculosis. The evaluation of the risk of tuberculosis infection from the perspective of air quality and not only of predisposing factors related to socio‐economic conditions creates a different perspective on the needs that health policies must adopt. Similar research demonstrates an increased risk of tuberculosis infection after exposure to fine PM2.5 particles (Lai et al., 2016). The study by Lu and co‐workers, claims that a 10 μg/m3 increase in PM 2.5 concentration increases the cumulative relative risks of tuberculosis by up to 1.10 over a period of 10 months, without bringing into question other risk factors (Lu et al., 2023).
Our study highlights that tuberculosis infection respects the known risk patterns, namely social conditions, low living standards, the degree of control through health policies, but through the analysis it brings into question in the most obvious way the relationship that air polluted with PM2.5 it has to do with TB infection, an aspect highlighted by Ma Z's study which establishes the role of PM 2.5 as essential, but alongside other social and environmental factors (Ma & Fan, 2023).
The main contribution of this study is to highlight the importance of research on the spatial dimension of TB case frequency for the development of control policies based on complex data sets that address both the disease and the geographical context of its occurrence and spread. Research on the environmental conditions in a geographical area is an important avenue for analyzing the factors that determine the development of TB, with high concentrations of pollution being an important factor in the onset of respiratory diseases (Ibironke et al., 2019; Lai et al., 2016; Mahler et al., 2023; Popovic et al., 2019).
Moreover, the methodology used in this research could contribute to the development of methodologies for analyzing processes that are strongly influenced by spatial factors, such as fractal analysis, the relevance of which is highlighted in numerous studies (Andronache et al., 2017, 2019; Diaconu et al., 2019; Petrișor et al., 2016; Simion et al., 2021), and the development of interdisciplinary approaches to understanding highly complex processes (Branisteanu et al., 2022; Busnatu et al., 2022; Halip et al., 2022; Ion et al., 2022; Ionescu et al., 2021; Păduraru et al., 2018).
The analysis of the distribution of tuberculosis at the level of administrative territorial units is the main advantage of this research because it allows the stratification of tuberculosis risk not only at the county level, but at the level of the administrative territorial units, which would allow a much more efficient management of the medical and social assistance that these cases require. Overlapping the risk maps produced in scientific research provides two distinct patterns visible in Romania. Our study provides, through the spatial patterns used and the period analyzed, the delineation of areas of increased risk of tuberculosis frequency, a situation that can be the basis for the future recommendation of active tuberculosis screening programmes, reforming the health system so that tuberculosis management focuses on these areas with specific particularities, in accordance with the allocation of funds for communication and education campaigns to the local community according to the specificity of the region.
The downward trend in the frequency of tuberculosis cases over the last 20 years creates the need to adapt the analysis model in order to make the best use of socio‐medical resources in a country where both social inequalities and access to the health network are uneven. The distribution of TB shows a high degree of heterogeneity determined by a complex of factors: individual factors associated with risk of disease, environmental conditions, social marginalization, limited access to health services, which increase the need for psychological support in these patients.
The distribution of TB is very heterogeneous due to several factors: individual factors associated with the risk of the disease, environmental conditions, social marginalization, limited access to health services. The analysis of the risk of illness from the perspective of the effect of PM2.5 and the impact of air quality on the degree of illness brings important information not only to restore tuberculosis control measures but also to draw up the main necessary health policies.
The spatial design of tuberculosis case frequency, correlated with risk levels at the level of the Autonomous Community, complements the classical methods of analysis of this disease by including the spatial dimension in understanding the geographical and socioeconomic context of the people affected. The results obtained here pave the way for future research in geographical areas with different TB concentrations. In addition, it is expected that this approach will provide valuable information in identifying the extent to which socio‐economic and environmental factors contribute to the maintenance of high TB values in certain geographical areas.
The study also has some important limitations related to information related to the degree of coverage with medical services unevenly distributed in all territories, although the health policies provide for this condition, on the other hand the geographical structure of some areas such as the Danube Delta does not allow for medical assistance permanent, which increases the risk of disease, even if the pollution level is reduced. The western area of Romania is practically on the edge of an extended European area with a high level of pollution, which would probably require a more careful monitoring of the level of pollutants at the border of Romania, but also of the movement of air in the mentioned territories.
In conclusion, knowledge of the spatial and geographical distribution of tuberculosis, and the level of PM2,5 in different geographic area from Romania is an essential tool to develop more effective public health policies and to implement active screening projects with maximum impact, but also to create effective diagnostic networks in areas of potential risk induced by the proximity of high‐frequency TAUs. This data‐driven approach can help reduce illness, increase early diagnosis rates and improve the health status of the population, and is an important step in the fight against tuberculosis in Romania. Spatial analysis allows the identification of spatial clusters or hotspots of cases. This information can help identify specific areas or populations that may be at higher risk of transmission. By focusing efforts on these clusters, interventions such as active case detection, contact tracing and targeted education campaigns can be implemented to reduce transmission and prevent further spread of the disease. Spatial analysis also allows exploring the relationship between tuberculosis frequency and environmental conditions and socio‐economic context. Understanding these associations can provide insights into the underlying determinants of TB and help inform policies and interventions to address the social and environmental factors that contribute to the burden of disease. In addition, spatial dispersion analysis can help monitor changes in frequency over time. By analyzing temporal trends, patterns and changes in the spatial distribution of TB cases, public health authorities can assess the effectiveness of control measures and identify areas where interventions may need to be intensified or modified.
Conflict of Interest
The authors declare no conflicts of interest relevant to this study.
Acknowledgments
This research received logistic support from the University of Bucharest, Romania, through projects 10680UB and 10681UB and from Graphit Innovation Factory.
Peptenatu, D. , Băloi, A. M. , Andronic, O. , Bolocan, A. , Cioran, N. , Gruia, A. K. , et al. (2024). Spatio‐temporal pattern of tuberculosis distribution in Romania and particulate matter pollution associated with risk of infection. GeoHealth, 8, e2023GH000972. 10.1029/2023GH000972
Contributor Information
A. M. Băloi, Email: aurel-mihail.baloi@s.unibuc.ro.
A. Pistol, Email: adriana.pistol@umfcd.ro.
Data Availability Statement
Data are available on request from the Marius Nasta Institute of Pneumophthisiology. The address where data can be requested is: secretariat@marius-nasta.ro.
References
- Alene, K. A. , & Clements, A. C. (2019). Spatial clustering of notified tuberculosis in Ethiopia: A nationwide study. PLoS One, 14(18), e0221027. 10.1371/journal.pone.0221027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alene, K. A. , Viney, K. , McBryde, M. S. , & Clements, A. C. A. (2017). Spatial patterns of multidrug resistant tuberculosis and relationships to socio‐economic, demographic and household factors in northwest Ethiopia. PLoS One, 12(2), 0171800. 10.1371/journal.pone.0171800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alene, K. A. , Wagaw, Z. A. , & Clements, A. C. (2020). Mapping tuberculosis prevalence in Ethiopia: Protocol for a geospatial meta‐analysis. BMJ Open, 10(5), e034704. 10.1136/bmjopen-2019-034704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alene, K. A. , Xu, Z. , Bai, L. , Yi, H. , Tan, Y. , Gray, D. J. , et al. (2021). Spatiotemporal patterns of tuberculosis in Hunan Province, China. International Journal of Environment Research and Public Health, 18(13), 6778. 10.3390/ijerph18136778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andronache, I. , Fensholt, R. , Ahammer, H. , Ciobotaru, A. M. , Pintilii, R. D. , Peptenatu, D. , et al. (2017). Assessment of textural differentiations in forest resources in Romania using fractal analysis. Forests, 8(54), 54. 10.3390/f8030054 [DOI] [Google Scholar]
- Andronache, I. , Marin, M. , Fischer, R. , Ahammer, H. , Radulovic, M. , Ciobotaru, A. M. , et al. (2019). Dynamics of forest fragmentation and connectivity using particle and fractal analysis. Scientific Reports, 9(1), 12228. 10.1038/s41598-019-48277-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselin, L. (2018). GeoDa. An introduction to spatial data science (online‐version). Retrieved from https://geodacenter.github.io/workbook/4d_weights_applications/lab4d.html#fn1
- Behr, M. A. , & Edelstein, P. H. (2019). Ramakrishnan L. Is Mycobacterium tuberculosis infection life long? BMJ, 367, l5770. 10.1136/bmj.l5770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishai, W. R. , Graham, N. M. , Harrington, S. , Pope, D. S. , Hooper, N. , Astemborski, J. , et al. (1998). Molecular and geographic patterns of tuberculosis transmission after 15 years of directly observed therapy. JAMA, 280(19), 1679–1684. 10.1001/jama.280.19.1679 [DOI] [PubMed] [Google Scholar]
- Branisteanu, D. E. , Dirzu, D. S. , Toader, M. P. , Branisteanu, D. C. , Nicolescu, A. C. , Brihan, I. , et al. (2022). Phototherapy in dermatological maladies (Review). Experimental and Therapeutic Medicine, 23(4), 259. 10.3892/etm.2022.11184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busnatu, S. , Niculescu, A. G. , Bolocan, A. , Petrescu, G. E. D. , Păduraru, D. N. , Năstasă, I. , et al. (2022). Clinical applications of artificial intelligence—An updated overview. Journal of Clinical Medicine, 11(8), 2265. 10.3390/jcm11082265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco‐Escobar, G. , Schwalb, A. , Tello‐Lizarraga, K. , Vega‐Guerovich, P. , & Ugarte‐Gil, C. (2020). Spatio‐temporal co‐occurrence of hotspots of tuberculosis, poverty and air pollution in Lima, Peru. Infectious Diseases of Poverty, 9(32), 1–6. 10.1186/s40249-020-00647-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakaya, J. , Khan, M. , Ntoumi, F. , Aklillu, E. , Fatima, R. , Mwaba, P. , et al. (2020). Global Tuberculosis Report 2020 ‐ Reflections on the Global TB burden, treatment and prevention efforts. International Journal of Infectious Diseases, 113, S7–S12. 10.1016/j.ijid.2021.02.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, D. , Lu, H. , Zhang, S. , Yin, J. , Liu, X. , Zhang, Y. , et al. (2021). The association between extreme temperature and pulmonary tuberculosis in Shandong Province, China, 2005–2016: A mixed method evaluation. BMC Infectious Diseases, 21(402), 402. 10.1186/s12879-021-06116-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa, G. F. , Garcez, J. C. D. , Marcos, W. , Ferreira, A. L. d.S. , Andrade, J. A. A. , Rodrigues, Y. C. , et al. (2023). Factors associated with tuberculosis outcome in a hyperendemic city in the north of Brazil. Healthcare, 2023(508), 11. 10.3390/healthcare11040508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneshvar, P. , Hajikhani, B. , Sameni, F. , Noorisepehr, N. , Zare, F. , Bostanshirin, N. , et al. (2023). COVID‐19 and tuberculosis coinfection: An overview of case reports/case series and meta‐analysis of prevalence studies. Heliyon, 9(2), 13637. 10.1016/j.heliyon.2023.e13637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangisso, M. H. , Datiko, D. G. , & Lindtjorn, B. (2014). Trends of tuberculosis case notification and treatment outcomes in the Sidama Zone, southern Ethiopia: Ten‐year retrospective trend analysis in urban‐rural settings. PLoS One, 9(12), e114225. 10.1371/journal.pone.0114225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangisso, M. H. , Datiko, D. G. , & Lindtjørn, B. (2015). Accessibility to tuberculosis control services and tuberculosis programme performance in southern Ethiopia. Global Health Action, 8(1), 29443. 10.3402/gha.v8.29443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datiko, D. G. , Yassin, M. A. , Chekol, L. T. , Kabeto, L. E. , & Lindtjørn, B. (2008). The rate of TB‐HIV co‐infection depends on the prevalence of HIV infection in a community. BMC Public Health, 8(1), 266. 10.1186/1471-2458-8-266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaconu, D. C. , Andronache, I. , Pintilii, R. D. , Brețcan, P. , Simion, A. G. , Drăghici, C. C. , et al. (2019). Using fractal fragmentation and compaction index in analysis of the deforestation process in Bucegi Mountains Group, Romania. Carpathian Journal of Earth and Environmental Sciences, 14(2), 431–438. 10.26471/cjees/2019/014/092 [DOI] [Google Scholar]
- Dominkovics, P. , Granell, C. , Pérez‐Navarro, A. , Casals, M. , Orcau, A. , & Caylà, J. A. (2011). Development of spatial density maps based on geoprocessing web services: Application to tuberculosis incidence in Barcelona, Spain. International Journal of Health Geographics, 10(62), 62. 10.1186/1476-072X-10-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donkelaar, A. , Hammer, M. S. , Bindle, L. , Brauer, M. , Brook, J. R. , Garay, M. J. , et al. (2021). Monthly global estimates of fine particulate matter and their uncertainty. Environmental Science & Technology, 55(22), 15287–15300. 10.1021/acs.est.1c05309 [DOI] [PubMed] [Google Scholar]
- Elf, J. L. , Kinikar, A. , Khadse, S. , Mave, V. , Suryavanshi, N. , Gupte, N. , et al. (2018). The association of household fine particulate matter and kerosene with tuberculosis in women and children in Pune, India. Occupational and Environmental Medicine, 76(1), 40–47. 10.1136/oemed-2018-105122 [DOI] [PubMed] [Google Scholar]
- Emery, J. C. , Richards, A. S. , Dale, K. D. , McQuaid, C. F. , White, R. G. , Denholm, J. T. , & Houben, R. M. G. J. (2021). Self‐clearance of Mycobacterium tuberculosis infection: Implications for lifetime risk and population at‐risk of tuberculosis disease. Proceedings of the Royal Society B, 288(1943), 20201635. 10.1098/rspb.2020.1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores‐Lovon, K. , Ortiz‐Saavedra, B. , Cueva‐Chicaña, L. A. , Aperrigue‐Lira, S. , Montes‐Madariaga, E. S. , Soriano‐Moreno, D. R. , et al. (2022). Immune responses in COVID‐19 and tuberculosis coinfection: A scoping review. Frontiers in Immunology, 13, 992743. 10.3389/fimmu.2022.992743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, J. , & Du, J. (2018). Temporal and spatial characteristics of pulmonary tuberculosis based on spatial epidemiology. Modern Preventive Medicine, 45(15), 2694–2696. [Google Scholar]
- Geric, C. , Saroufim, M. , Landsman, d. , Richard, J. , Benedetti, A. , Batt, J. , et al. (2022). Impact of COVID‐19 on tuberculosis prevention and treatment in Canada: A multicenter analysis of 10 833 patients. The Journal of Infectious Diseases, 225(8), 1317–1320. 10.1093/infdis/jiab608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gül, Ş. , Karaca, E. S. A. , Niksarlıoğlu, E. Y. Ö. , Çınarka, H. , & Uysal, M. A. (2022). Coexistence of tuberculosis and COVID‐19 pneumonia: A presentation of 16 patients from Turkey with their clinical features. Tuberkuloz ve Toraks, 70(1), 8–14. 10.5578/tt.20229902 [DOI] [PubMed] [Google Scholar]
- Guo, C. , Du, Y. , Shen, S. Q. , Lao, X. Q. , Qian, J. , & Ou, C. Q. (2017). Spatiotemporal analysis of tuberculosis incidence and its associated factors in mainland China. Epidemiology and Infection, 145(12), 2510–2519. 10.1017/S0950268817001133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halip, I. A. , Vata, D. , Statescu, L. , Salahoru, P. , Patrascu, A. I. , Olinici, D. T. , et al. (2022). Assessment of basal cell carcinoma using dermoscopy and high frequency ultrasound examination. Diagnostics, 12(3), 735. 10.3390/diagnostics12030735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff, R. D. , Carlsten, C. , & Hirota, J. A. (2019). An update on immunologic mechanisms in the respiratory mucosa in response to air pollutants. Journal of Allergy and Clinical Immunology, 143(6), 1989–2001. 10.1016/j.jaci.2019.04.012 [DOI] [PubMed] [Google Scholar]
- Ibironke, O. , Carranza, C. , Sarkar, S. , Torres, M. , Choi, H. T. , Nwoko, J. , et al. (2019). Urban air pollution particulates suppress human T‐cell responses to mycobacterium tuberculosis. International Journal of Environmental Research and Public Health, 16(21), 4112. 10.3390/ijerph16214112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ion, D. , Niculescu, A. G. , Păduraru, D. N. , Andronic, O. , Mușat, F. , Grumezescu, A. M. , & Bolocan, A. (2022). An up‐to‐date review of natural nanoparticles for cancer management. Pharmaceutics, 14(1), 18. 10.3390/pharmaceutics14010018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu, S. , Nicolescu, A. C. , Madge, O. L. , Marincaş, M. , Radu, M. , Simion, L. , et al. (2021). A small bowel metastasis from an achromic melanoma causing intussusception and bowel obstruction ‐ A case presentation. Oncolog‐Hematolog, 57, 36. 10.26416/OnHe.57.4.2021.5799 [DOI] [Google Scholar]
- Jain, V. K. , Iyengar, K. P. , Samy, D. A. , & Vaishya, R. (2020). Tuberculosis in the era of COVID‐19 in India. Diabetes & Metabolic Syndrome: Clinical Research Reviews, 14(5), 1439–1443. 10.1016/j.dsx.2020.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri, T. A. , Fung, C. , LaHood, A. N. , Lindeborg, A. , Zeng, C. , Rahman, R. , et al. (2022). Clinical outcomes of individuals with COVID‐19 and tuberculosis during the pre‐vaccination period of the pandemic: A systematic review. Journal of Clinical Medicine, 11(19), 5656. 10.3390/jcm11195656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh, G. C. , Hawthorne, G. , Turner, A. M. , Kunst, H. , & Dedicoat, M. (2013). Tuberculosis incidence correlates with sunshine: An ecological 28‐year time series study. PLoS One, 8(3), e57752. 10.1371/journal.pone.0057752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, T. C. , Chiang, C. Y. , Wu, C. F. , Yang, S. L. , Liu, D. P. , Chan, C. C. , & Lin, H. H. (2016). Ambient air pollution and risk of tuberculosis: A cohort study. Occupational and Environmental Medicine, 73(1), 56–61. 10.1136/oemed-2015-102995 [DOI] [PubMed] [Google Scholar]
- Lee, K. K. , Bing, R. , Kiang, J. , Bashir, S. , Spath, N. , Stelzle, D. , et al. (2020). Adverse health effects associated with household air pollution: A systematic review, meta‐analysis, and burden estimation study. Lancet Global Health, 8(11), e1427–e1434. 10.1016/S2214-109X(20)30343-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Chen, D. , Zhang, Y. , Xue, X. , Zhang, S. , Chen, M. , et al. (2021). Analysis of spatial‐temporal distribution of notifiable respiratory infectious diseases in Shandong Province, China during 2005–2014. BMC Public Health, 21(1), 1597. 10.1186/s12889-021-11627-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. W. , Cheng, J. , Wang, H. , Zhao, F. , Li, X. X. , Tao, W. W. , et al. (2016). Spatial‐temporal analysis of pulmonary tuberculosis in Shandong province. Zhonghua Liu Xing Bing Xue Za Zhi, 37, 1257–1261. 10.3760/cma.j.issn.0254-6450.2016.09.015 [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Li, X. , Wang, W. , Li, Z. , Hou, M. , He, Y. , et al. (2012). Investigation of space‐time clusters and geospatial hot spots for the occurrence of tuberculosis in Beijing. International Journal of Tuberculosis & Lung Disease, 16(4), 486–491. 10.5588/ijtld.11.0255 [DOI] [PubMed] [Google Scholar]
- Lu, J. W. , Mao, J. J. , Zhang, R. R. , Li, C. H. , Sun, Y. , Xu, W. Q. , et al. (2023). Association between long‐term exposure to ambient air pollutants and the risk of tuberculosis: A time‐series study in Nantong, China. Heliyon, 9(6), e17347. 10.1016/j.heliyon.2023.e17347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Z. , & Fan, H. (2023). Influential factors of tuberculosis in mainland China based on MGWR model. PLoS One, 18(8), e0290978. 10.1371/journal.pone.0290978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler, B. , Băiceanu, D. , Panciu, T. C. , Florea, R. M. , Iorga, A. L. , Gnat, M. , et al. (2023). Air pollutants and their impact on chronic diseases—A retrospective study in Bucharest, Romania. Atmosphere, 14(5), 867. 10.3390/atmos14050867 [DOI] [Google Scholar]
- Munayco, C. V. , Mújica, O. J. , León, F. X. , del Granado, M. , & Espinal, M. A. (2015). Social determinants and inequalities in tuberculosis incidence in Latin America and the Caribbean. Revista Panamericana de Salud Pública, 38(3), 177–185. [PubMed] [Google Scholar]
- Munch, Z. , Van Lill, S. W. P. , Booysen, C. N. , Zietsman, H. L. , Enarson, D. A. , & Beyers, N. (2003). Tuberculosis transmission patterns in a high incidence area: A spatial analysis. International Journal of Tuberculosis & Lung Disease, 7(3), 271–277. [PubMed] [Google Scholar]
- Noubiap, J. J. , Nansseu, J. R. , Nyaga, U. F. , Nkeck, J. R. , Endomba, F. T. , Kaze, A. D. , et al. (2019). Global prevalence of diabetes in active tuberculosis: A systematic review and meta‐analysis of data from 2·3 million patients with tuberculosis. Lancet Global Health, 7(4), e448–e460. 10.1016/S2214-109X(18)30487-X [DOI] [PubMed] [Google Scholar]
- Oxlade, O. , Piatek, A. , Vincent, C. , & Menzies, D. (2015). Modeling the impact of tuberculosis interventions on epidemiologic outcomes and health system costs. BMC Public Health, 15(141), 141. 10.1186/s12889-015-1480-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Păduraru, D. N. , Alexandra, B. , Ion, D. , Dumitrascu, M. C. , Nitipir, C. , Stoian, A. P. , et al. (2018). Latest news and trends in what concerns the risk factors of endometrial cancer. Romanian Biotechnological Letters, 23(5), 14056–14066. 10.26327/RBL2018.177 [DOI] [Google Scholar]
- Petrișor, A. I. , Andronache, I. , Petrisor, L. E. , Ciobotaru, A. M. , & Peptenatu, D. (2016). Assessing the fragmentation of the green infrastructure in Romanian cities using fractal models and numerical taxonomy. Procedia Environmental Sciences, 32, 110–123. 10.1016/j.proenv.2016.03.016 [DOI] [Google Scholar]
- Popovic, I. , Soares Magalhaes, R. J. , Ge, E. , Marks, G. B. , Dong, G.‐H. , Wei, X. , & Knibbs, L. D. (2019). A systematic literature review and critical appraisal of epidemiological studies on outdoor air pollution and tuberculosis outcomes. Environmental Research, 170, 33–45. 10.1016/j.envres.2018.12.011 [DOI] [PubMed] [Google Scholar]
- Reid, M. J. A. , Arinaminpathy, N. , Bloom, A. , Bloom, B. R. , Boehme, C. , Chaisson, R. , et al. (2019). Building a tuberculosis‐free world: The Lancet Commission on tuberculosis. Lancet, 393(10178), 1331–1384. 10.1016/S0140-6736(19)30024-8 [DOI] [PubMed] [Google Scholar]
- Rey, S. , Arribas‐Bel, D. , & Wolf, L. J. (2023). Geographic data science with Python (1st ed.). Chapman and Hall/CRC. 10.1201/9780429292507 [DOI] [Google Scholar]
- Rey, S. J. , & Anselin, L. (2007). PySAL: A Python Library of Spatial Analytical Methods. Review of Regional Studies, 37(1), 5–27. 10.52324/001c.8285 [DOI] [Google Scholar]
- Rivas‐Santiago, C. E. , Sarkar, S. , Cantarella, IV, P. , Osornio‐Vargas, Á. , Quintana‐Belmares, R. , Meng, Q. , et al. (2015). Air pollution particulate matter alters antimycobacterial respiratory epithelium innate immunity. Infection and Immunity, 83(6), 2507–2517. 10.1128/IAI.03018-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrias, M. (2020). Lecure 1: Introduction to Spatial Econometric, Course Support, 2020. University of Talca. Retrieved from https://www.msarrias.com/uploads/3/7/7/8/37783629/lecture1.pdf [Google Scholar]
- Sattenspiel, L. (Ed.). (2009). The geographic spread of infectious diseases: Models and applications. Princeton University Press. [Google Scholar]
- Shaweno, D. , Horton, K. C. , Hayes, R. J. , & Dodd, P. J. (2021). Assortative social mixing and sex disparities in tuberculosis burden. Scientific Reports, 11(1), 7530. 10.1038/s41598-021-86869-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaweno, D. , Shaweno, T. , Trauer, J. , Denholm, J. , & McBryde, E. (2017). Heterogeneity of distribution of tuberculosis in Sheka Zone, Ethiopia: Drivers and temporal trends. International Journal of Tuberculosis & Lung Disease, 21(1), 79–85. 10.5588/ijtld.16.0325 [DOI] [PubMed] [Google Scholar]
- Shaweno, D. , Trauer, J. M. , Denholm, J. T. , & McBryde, E. S. (2018). The role of geospatial hotspots in the spatial spread of tuberculosis in rural Ethiopia: A mathematical model. Royal Society Open Science, 5(9), 180887. 10.1098/rsos.180887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simion, A. G. , Andronache, I. , Ahammer, H. , Marin, M. , Loghin, V. , Nedelcu, I. D. , et al. (2021). Particularities of forest dynamics using Higuchi dimension. Parâng mountains as a case study. Fractal and Fractional, 5(3), 96. 10.3390/fractalfract5030096 [DOI] [Google Scholar]
- Small, P. M. , Hopewell, P. C. , Singh, S. P. , Paz, A. , Parsonnet, J. , Ruston, D. C. , et al. (1994). The epidemiology of tuberculosis in San Francisco—a population‐based study using conventional and molecular methods. New England Journal of Medicine, 330(24), 1703–1709. 10.1056/NEJM199406163302402 [DOI] [PubMed] [Google Scholar]
- Smith, G. , Schoenbach, V. J. , Richardson, D. B. , & Gammon, M. D. (2014). Particulate air pollution and susceptibility to the development of pulmonary tuberculosis disease in North Carolina: An ecological study. International Journal of Environmental Health Research, 24(2), 103–112. 10.1080/09603123.2013.800959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotgiu, G. , Rosales‐Klintz, S. , Centis, R. , D'Ambrosio, L. , Verduin, R. , Correia, A. M. , et al. (2021). TB management in the European Union/European Economic Area: A multi‐centre survey. International Journal of Tuberculosis & Lung Disease, 25(2), 126–133. 10.5588/ijtld.20.0849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoichita, A. , Dumitrescu, A. , Ciobanu, A. , Oancea, C. , Petronela, F. , Dabja, R. , et al. (2021). Depression and anxiety symptoms among people with rifampicin‐resistant tuberculosis receiving in‐patient care in the National Pulmonology Reference Institute in Romania. Monaldi Archives for Chest Disease, 91(1). 10.4081/monaldi.2021.1704 [DOI] [PubMed] [Google Scholar]
- Sun, Y. H. , Tian, M. Z. , & Nie, Y. W. (2022). Application of spatial panel data model in the analysis of national tuberculosis surveillance data from 2015 to 2019. Chinese Preventive Medicine, 23(06), 436–441. [Google Scholar]
- Tadesse, T. , Demissie, M. , Berhane, Y. , Kebede, Y. , & Abebe, M. (2013). The clustering of smear‐positive tuberculosis in Dabat, Ethiopia: A population‐based cross sectional study. PLoS One, 8(5), e65022. 10.1371/journal.pone.0065022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Global Tuberculosis Report 2022 . (2022). Retrieved from https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022
- Tiemersma, E. W. , van der Werf, M. J. , Borgdorff, M. W. , Williams, B. G. , & Nagelkerke, N. J. (2011). Natural history of tuberculosis: Duration and fatality of untreated pulmonary tuberculosis in HIV negative patients: A systematic review. PLoS One, 6(4), e17601. 10.1371/journal.pone.0017601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari, N. , Kandpal, V. , Tewari, A. , Rao, K. R. M. , & Tolia, V. (2010). Investigation of tuberculosis clusters in Dehradun city of India. Asian Pacific Journal of Tropical Medicine, 3(6), 486–490. 10.1016/S1995-7645(10)60117-4 [DOI] [Google Scholar]
- Touray, K. , Adetifa, I. M. , Jallow, A. , Rigby, J. , Jeffries, D. , Cheung, Y. B. , et al. (2010). Spatial analysis of tuberculosis in an urban west African setting: Is there evidence of clustering? Tropical Medicine and International Health, 15(6), 664–672. 10.1111/j.1365-3156.2010.02533.x [DOI] [PubMed] [Google Scholar]
- Trauer, J. M. , Dodd, P. J. , Gomes, M. G. M. , Gomez, G. B. , Houben, R. M. G. J. , McBryde, E. S. , et al. (2019). The importance of heterogeneity to the epidemiology of tuberculosis. Clinical Infectious Diseases, 69(1), 159–166. 10.1093/cid/ciy938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, S. , Xia, L. , Wu, J. , Chen, S. , Chen, F. , Zeng, F. , et al. (2018). Ambient air pollutants are associated with newly diagnosed tuberculosis: A time‐series study in Chengdu, China. Science of the Total Environment, 631–632, 47–55. 10.1016/j.scitotenv.2018.03.017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request from the Marius Nasta Institute of Pneumophthisiology. The address where data can be requested is: secretariat@marius-nasta.ro.


