Abstract
Trypanosoma cruzi (T. cruzi) is the causative agent of Chagas’ disease, a parasitic infection responsible for significant morbidity and mortality in Latin America. The current treatments have many serious drawbacks and new drugs are urgently required. In the UK, T. cruzi is classified by the Advisory Committee on Dangerous Pathogens (ACDP) as a Hazard Group 3 organism and strict safety practices must be adhered to when handling this pathogen in the laboratory. Validated inactivation techniques are required for safe T. cruzi waste disposal and removal from Containment Level 3 (CL3) facilities for storage, transportation and experimental analysis. Here we assess three T. cruzi. inactivation methods. These include three freeze-thaw cycles, chemical inactivation with Virkon disinfectant, and air drying on Whatman FTA cards (A, B, C, Elute) and on a Mitra microsampling device. After each treatment parasite growth was monitored for 4–6 weeks by microscopic examination. Three freeze-thaw cycles were sufficient to inactivate all T. cruzi CLBrener Luc life cycle stages and Silvio x10/7 A1 large epimastigote cell pellets up to two grams wet weight. Virkon treatment for one hour inactivated T. cruzi Silvio x10/7 subclone A1 and CLBrener Luc both in whole blood and cell culture medium when incubated at a final concentration of 2.5% Virkon, or at ≥1% Virkon when in tenfold excess of sample volume. Air drying also inactivated T. cruzi CLBrener Luc spiked blood when dried on FTA A, B or Elute cards for ≥30 minutes and on a Mitra Microsampler for two hours. However, T. cruzi CLBrener Luc were not inactivated on FTA C cards when dried for up to two hours. These experimentally confirmed conditions provide three validated T. cruzi inactivation methods which can be applied to other related ACDP Hazard Group 2–3 kinetoplastid parasites.
Introduction
Trypanosoma cruzi (T. cruzi) are flagellated protozoan parasites responsible for Chagas’ disease, also known as American trypanosomiasis. T. cruzi are transmitted to humans predominantly by the faeces of infected bloodsucking triatomine bugs at the bite site, through mucosal surfaces or broken skin. T. cruzi transmission can also occur congenitally, via organ transplantation, blood transfusion, or by ingestion of parasite contaminated food and drink [1, 2]. Cases of accidental laboratory-acquired infection have also been reported [3]. Chagas’ disease is endemic in Latin America with an estimated 8 million people infected resulting in 12,500 deaths per year [1, 4]. Chagas’ disease has an initial acute phase followed by a chronic phase which is life-long. Thirty percent of cases display severe clinical pathologies including cardiac problems and digestive disorders [5, 6]. The current treatments (benznidazole and nifurtimox) were developed over four decades ago and have many serious drawbacks [7, 8]. New treatments are urgently required; this has led to concerted research efforts to gain a better understanding of T. cruzi biology and Chagas’ disease pathogenesis as well as drug discovery and development. However, working with T. cruzi in research settings is challenging as it is classified as an ACDP Hazard Group 3 organism in the UK [9] and a Risk Group 3 in the EU [10], requiring cultivation in a Containment Level 3 (CL3) facility and adherence to a stringent code of practice that takes account of the nature of particular procedures and of the quantity of the agent involved (the USA, Canada, Australia and New Zealand categorize T. cruzi as Risk Group 2) [11]. The United Kingdom Health and Safety Executive (HSE) require validated T. cruzi inactivation methods for safe waste disposal within the CL3 facility and for safe removal of T. cruzi infected samples from the CL3 facility for storage, transportation and experimental analysis. Although many organisations will have established their own inactivation methods, limited evidence of their effectiveness against T. cruzi parasites is available in the literature. Here we assess three inactivation techniques for T. cruzi spiked mouse blood and cell culture samples including three freeze-thaw cycles, chemical inactivation with Virkon disinfectant and air drying on Whatman FTA cards (FTA A, B, C and Elute) and a Mitra microsampling device.
Methods
Cell culture
T. cruzi strain CL Brener expressing pTRIX2-PpyRE9h red-shifted firefly luciferase (CL Brener Luc) [12] known to be a discrete typing unit (DTU) TcVI strain, and Silvio X10/7 subclone A1 strain [13] (DTU TcI) were maintained as epimastigotes at 28ºC in RTH media [RPMI1640 (Sigma) supplemented with 0.4% trypticase peptone, 0.017 M HEPES, 25 μM haemin and 10% heat inactivated foetal calf serum (FCS)] [14–16].
CL Brener Luc trypomastigotes and intracellular amastigote cultures were obtained as previously described [17]. Briefly, 106 ml-1 epimastigotes were incubated in RTH at 28ºC for 10 days and subsequently used to infect a monolayer of Vero cells (African green monkey kidney cells, ECCAC 84113001) at a multiplicity of infection (MOI) of 10 overnight at 37ºC 5% CO2 in Dulbecco’s modified Eagles’s medium (Lonza) supplemented with 10% FCS. Extracellular parasites were removed by washing with serum-free DMEM three times, followed by addition of fresh complete DMEM. T. cruzi infected Vero flasks were maintained at 37ºC 5% CO2 and DMEM replaced every 2–3 days until trypomastigotes emerged after ~6–7 days.
Three freeze-thaw cycles
Epimastigotes in blood
Tissue culture derived CL Brener Luc epimastigotes were collected by centrifugation at 2000 × g for 10 min. Cell culture supernatant was removed and parasites resuspended at 108 ml-1 in BALB/c mouse blood collected in an EDTA vacutainer. We also assessed the effect of three freeze-thaw cycles on 10 μl of mouse blood containing CL Brener Luc epimastigotes (106) diluted with 20 μl sterile H2O (typical of a sample for mass spectrometry analysis). These were set-up in triplicate and subjected to three rapid snap freeze-thaw cycles by submerging in liquid nitrogen for 10 s followed by thawing at room temperature for 5 min until three cycles were completed. Subsequently samples were dispensed into a 24 well plate with 1 ml RTH media. Untreated epimastigote samples were included as controls. T. cruzi inactivation by this method was assessed by microscopic examination over 28 days for presence of motile parasites. T. cruzi were counted using a Neubauer haemocytometer after fixation 1:1 in 1% formaldehyde/PBS [limit of quantitation ≥2x104 ml-1].
Three rapid freeze-thaw cycles were also performed as described above on 100 μl of undiluted mouse blood spiked with 107 CL Brener Luc epimastigotes to determine the effect of this method on a larger volume of blood not under hypo-osmotic stress. This sample was placed in a T25 flask with 10 ml RTH media, incubated at 28ºC, and parasites were counted at day 28.
Epimastigote pellets
Three freeze-thaw cycles were assessed as a method to inactivate large cell pellets (2x1010 epimastigotes) using a dry ice bath for 10 min per step. Silvio X10/7 subclone A1 epimastigotes were grown in RTH in 1 l roller bottles for 3–4 d to a density of 2-4x107 ml-1 at 28ºC. Parasites were harvested by centrifugation at 900 × g for 35 min, supernatant removed, parasites resuspended in 50 ml PBS, counted and centrifuged at 900 × g for 15 min. The supernatant was aspirated to yield large cell pellets of 2x1010 parasites in 50 ml falcon tubes. These were submerged in a dry ice bath (dry ice pellets plus isopropanol) for 10 min and subsequently thawed for 10 min in a water bath at room temperature; this was repeated three times. Upon each thawing the pellets were visually inspected to ensure they were completely thawed. T. cruzi epimastigote inactivation by this method was assessed by placing ~10% (measured by weight) of each pellet in a T175 flask with 50 ml RTH for 6 weeks at 28ºC and monitoring weekly for presence of motile epimastigotes by microscopic examination. A control T175 flask was set-up with 10 epimastigotes to determine how long it would take to clearly observe motile parasites at a very low seeding density. In addition, a flask was set-up using the same batch of RTH media at a 1000-fold parasite dilution of the starting flask to demonstrate media competence. This process was performed on 43 sample pellets.
Trypomastigotes
The rapid freeze-thaw protocol in liquid nitrogen described above for epimastigotes in blood was also performed using CL Brener Luc trypomastigotes. Briefly, cell culture-derived trypomastigotes were harvested from the supernatant of infected Vero cell cultures after egress, centrifuged and resuspended in mouse blood at 108 ml-1. Sterile water (20 μl) was added to 10 μl of mouse blood (BALB/c) containing 106 CL Brener Luc trypomastigote parasites, set-up in triplicate and three rapid freeze-thaw cycles completed. As T. cruzi trypomastigotes are non-replicative forms, these samples were placed on a Vero cell monolayer (105 cells) plus 1 ml DMEM/10% FCS in 24 well plates overnight. They were subsequently washed with DMEM three times to remove any extracellular parasites and replenished with 1 ml DMEM/1% FCS twice weekly. Viable trypomastigotes can establish Vero cell infection, transform into intracellular replicating amastigotes and emerge from Vero cells as motile trypomastigotes, thus, presence of motile trypomastigotes was used to assess this inactivation method by microscopic examination over 28 days. Vero cells have a doubling time of ~24 h and are typically sub-cultured 2–3 times per week. In order to maintain cells during the 4 week monitoring period without sub-culture all Vero cell cultures were set-up in 1% FCS to reduce the Vero cell replication rate [18]. Untreated mouse blood spiked with trypomastigotes (10 μl plus 20 μl H2O) were used as controls to determine Vero cell cycling time of CL Brener Luc under these conditions.
Three rapid freeze-thaw cycles were also carried out on 100 μl of mouse blood spiked with 107 T. cruzi trypomastigotes to assess this method on a larger volume of blood not under osmotic stress. Treated and untreated samples were incubated overnight with a Vero cell monolayer (106 cells) in T25 flasks, DMEM/10% FCS at 37ºC 5% CO2. Extracellular parasites were removed the following day as described above. Flasks were maintained at 37ºC 5% CO2 in DMEM/1% FCS and media was replaced twice weekly. This assay was performed in triplicate and cultures were monitored for the emergence of parasites for 28 days.
Intracellular amastigotes
T. cruzi CL Brener Luc infected Vero cells were harvested after 5 days infection (multiplicity of infection of 10). Cell culture supernatant was aspirated, Vero cells washed with DMEM without FCS three times followed by 5 min incubation with Trypsin-EDTA (Gibco). After addition of DMEM/10% FCS, cells were resuspended at 106 ml-1 in triplicate and samples (1 ml) subjected to three rapid freeze-thaw cycles as described above. Treated and untreated samples were incubated with 106 Vero cells in T25 flasks, DMEM/10% at 37ºC 5% CO2 overnight. Flasks were subsequently washed and maintained as described above in DMEM/1% FCS. Vero cell cultures were monitored for the emergence of trypomastigotes for 28 days.
Chemical inactivation- Virkon
Epimastigotes in blood and cell culture media
For qualitative experiments, T. cruzi Silvio X10/7 subclone A1 epimastigotes (5x107) suspended in 1 ml rat blood were mixed with 1 volume of 1% Virkon (w/v) solution, sterile tap water (0.2 micron filtered) or PBS. After incubation for 1 h at room temperature, samples were centrifuged to pellet parasites, washed twice with PBS and re-suspended in 1 ml RTH/FCS. Parasites were then serially diluted tenfold into 24 well plates at 107 to 101/ml in RTH/FCS, incubated at 28°C and microscopically examined for motile parasites on day 1, 8, 15, 20, 25 and 32.
For quantitative experiments, CL Brener Luc epimastigotes were centrifuged at 2000 × g 10 min, supernatant removed, washed in PBS and re-suspended at 2x107 ml-1 in mouse blood (C57/BL6) and in RTH (10% FCS) media. Samples (100 μl, in triplicate) were mixed with 100 μl and 900 μl of 1%, 2% and 5% Virkon solution (Rely+On™VIRKON™, LanXESS Corporation) and PBS. Samples were incubated for 1 h at room temperature, subsequently centrifuged, washed twice with PBS and re-suspended in 1 ml RTH. These were dispensed into 24-well plates, incubated at 28 ºC 5% CO2 and monitored for the presence of motile parasites over 28 days by microscopic examination. Parasite density was determined using a Neubauer haemocytometer as described above.
Drying techniques
Epimastigotes in blood- Mitra microsampling device
A Mitra Microsampler (Phenomenex) [19] was used to collect mouse blood (10 μl) spiked with 108 ml-1 CL Brener Luc epimastigote parasites in triplicate. Samples were left to dry at room temperature for 2 h. The tip containing the sample was subsequently added to a 24 well plate with 1 ml RTH and incubated at 28 ºC 5% CO2. Epimastigote inactivation by this drying method was determined by monitoring cultures for presence of motile parasites by microscopic examination over 28 days.
Epimastigotes in blood- Whatman® FTA® cards
Whatman FTA DMPK A, B, C and Elute cards (GE Healthcare Life Sci) were sterilised prior to use by lightly spraying with 70% ethanol and air drying. The sample application area was cut out and placed into a 24 well plate. Mouse blood (20 μl) containing 108 ml-1 CL Brener Luc epimastigotes was spotted onto each card and allowed to air dry for 15, 30, 60 and 120 min before addition of 1 ml RTH. Wells were monitored for presence of motile parasites for 28 days by microscopic examination. This assay was performed in duplicate for each time point.
Results
Three freeze-thaw cycles
Epimastigotes in blood
Pharmacokinetic studies play a pivotal role in drug development; these include mass spectrometry analysis of blood samples taken from animal models and typically require dilution in water prior to evaluation. We therefore assessed the effect of three freeze-thaw cycles on 10 μl of mouse blood containing CL Brener Luc epimastigotes (106) diluted with sterile H2O (20 μl). Three rapid freeze-thaw cycles successfully inactivated CL Brener Luc epimastigotes blood / water samples with no motile parasites observed after 28 days in culture. Epimastigote growth was recorded in the spiked blood / water controls (Fig 1, S1 Table). Three rapid freeze-thaw cycles were also sufficient to inactivate undiluted epimastigote (107) spiked blood samples with no motile parasites detected in cultures over 28 days (S1 Table).
Fig 1. T. cruzi epimastigote growth after three rapid freeze-thaw cycles.
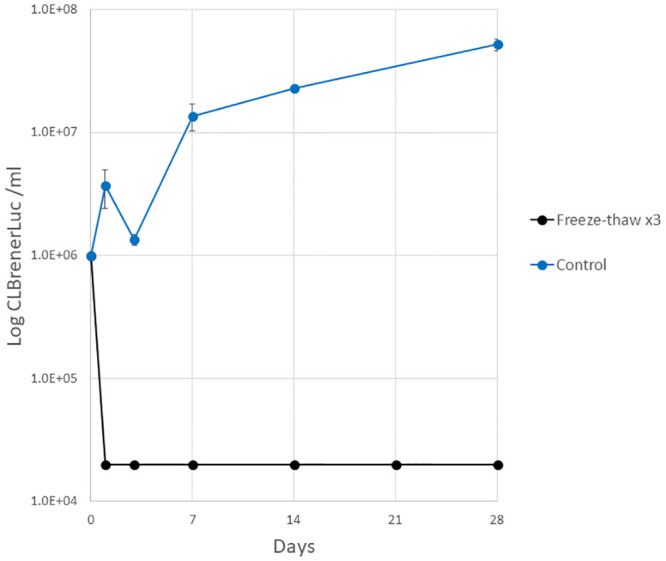
Samples of mouse blood spiked with T. cruzi CLBrener Luc were subjected to three freeze-thaw cycles and monitored for parasite growth over 28 days (three technical replicates, mean ± standard deviation, limit of quantitation 2×104 ml-1).
Epimastigote pellets
Large Silvio X10/7 subclone A1 epimastigote pellets (2x1010) were inactivated in 41 of 43 samples by three 10 min freeze-thaw cycles with no motile parasites observed after 6 weeks in culture. Epimastigotes were generally visible by day 7 in the very low seeding density control flasks with media competence confirmed in all runs. It was noted that the average weight of pellets was 1.3 g. However, the two pellets that failed the inactivation procedure weighed 2.3 and 3.1 g despite the same parasite numbers. The increased weight is likely due to insufficient removal of supernatant after centrifugation. It is, therefore, important to remove as much supernatant as possible before performing freeze-thaw cycles. This inactivation method is validated for T. cruzi Silvio X10/7 subclone A1 pellets weighing ≤2 g, longer duration freeze thaw cycles may be required for larger pellets. In addition, in previous experiments when large pellets (≤2 g) were frozen on dry ice without isopropanol, 1 out of 12 failed inactivation, possibly due to less efficient heat transfer in solid CO2.
Trypomastigotes
Three rapid freeze-thaw cycles inactivated CL Brener Luc trypomastigote (106) spiked blood / water samples with no motile parasites observed emerging from Vero cells after 28 days (Table 1). In control samples trypomastigotes were detected after 10 days incubation with Vero cells confirming media and cell viability.
Table 1. Effect of freeze thawing on survival of T. cruzi trypomastigote and amastigote stages.
CL Brener Luc trypomastigote spiked mouse blood and T. cruzi-infected Vero cells were subjected to three freeze-thaw cycles (F-T), before being incubated with fresh Vero cells and monitored for trypomastigote emergence over 28 days. Three technical replicates, mean (standard deviation).
| Sample | CL Brener Luciferase Trypomastigote Counts ml-1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Day | |||||||||
| 0 | 1 | 3 | 7 | 10 | 14 | 21 | 24 | 28 | |
| F-T Spiked blood + H2O | 106 | -/-/- | -/-/- | -/-/- | -/-/- | -/-/- | -/-/- | -/-/- | -/-/- |
| F-T Spiked blood | 107 | -/-/- | -/-/- | -/-/- | -/-/- | -/-/- | -/-/- | -/-/- | -/-/- |
| F-T Infected Vero cells | * | -/-/- | -/-/- | -/-/- | -/-/- | -/-/- | -/-/- | -/-/- | -/-/- |
| Control Spiked blood + H2O | 106 | -/-/- | -/-/- | -/-/- | +/+/+ | 3x104 (2x104) | 3x104 (1x104) | nd | nd |
| Control Spiked blood | 107 | -/-/- | -/-/- | +/+/+ | 2x104 (0) | 2x105 (6x104) | nd | nd | nd |
| Control Infected Vero Cells | * | -/-/- | -/-/- | +/+/+ | 3x105 (2x104) | nd | nd | nd | nd |
* Infected Vero cells were harvested after 5 days T. cruzi infection (MOI10), resuspended at 106 ml-1, freeze-thawed and control samples were subsequently incubated with 105 ml-1 Vero cells
+/+/+ Motile trypomastigotes observed in all three replicates, but below limit of quantitation 2x104 ml-1
-/-/- No motile trypomastigotes observed in all three replicates
nd Not done
Three rapid freeze-thaw cycles were also sufficient to inactivate 100 μl trypomastigote (107) undiluted spiked blood samples. No motile trypomastigotes emerged from Vero cells after 28 days (Table 1). In control samples trypomastigotes started to emerge from Vero cells at day 7. Growth of Vero cells plus 100 μl blood (not spiked) in 1% FCS was recorded at day 0 (105 ml-1) to day 28 (2x106 ml-1, doubling time 6 days). This is in line with reduced Vero cell growth under low FCS conditions suggesting whole mouse blood did not adversely affect Vero cells.
Intracellular amastigotes
Three rapid freeze-thaw cycles inactivated intracellular T. cruzi CLBrener Luc. No parasites emerged from Vero cells infected with freeze-thawed T. cruzi infected Vero cells after 4 weeks, while trypomastigotes started to emerge from Vero cells at day 7 in the untreated control cultures (Table 1).
Chemical inactivation- Virkon
Virkon is a disinfectant containing an oxidising agent (potassium peroxymonosulfate), a detergent (sodium dodecylbenzenesulfonate) and organic acids (sulfamic acid and malic acid) plus sodium hexametaphosphate to buffer at low pH [20]. Preliminary experiments with Virkon showed that exposure to a 0.5% solution for 10 min was unable to kill all T. cruzi Silvio X10/7 subclone A1 epimastigotes in whole blood or serum as assessed by microscopic examination for motile parasites. Consequently, all subsequent experiments involved a 1 h exposure. Parasites (107 ml-1) in whole blood or whole serum also survived exposure for 1 h in a 1:1 mixture with 1% Virkon likely due to the neutralising effect of the high organic matter on Virkon’s active components. A subsequent experiment showed that heavily infected whole blood or serum samples must be diluted ten-fold with 1% Virkon for 1 h for complete inactivation (Table 2). Using a ten-fold dilution of the treated sample into fresh medium it is possible to assess the log reduction in viable parasites following long-term culture. Viable parasites could be detected in PBS control cultures down to a dilution of 102 ml-1 from 15 days onwards, whereas no motile parasites were detected in Virkon-treated blood after 32 days with an initial inoculum of 107 ml-1. This equates to a 5 log (105-fold) effective kill. African trypanosomes are considered to be particularly susceptible to osmotic shock, but a 1 h exposure to tap water (10:1) has only a 2 log (100-fold) effective kill in the case of T. cruzi (Table 2).
Table 2. Effect of Virkon treatment on T. cruzi epimastigote viability in rat blood.
T. cruzi strain Silvio X10/7 subclone A1 infected rat blood, was treated for 1 h with a 10-fold (v/v) excess of 1% Virkon. Samples were pelleted and resuspended in the same volume of culture medium before 10-fold dilution into culture medium. Viable parasites were detected by microscopic examination for motile epimastigotes and scored qualitatively. Parasite positive samples are highlighted as a heat map.
| T. cruzi infected sample | Treatment | Day | Silvio X10/7 A1 Starting Density, cells ml-1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 107 | 106 | 105 | 104 | 103 | 102 | 101 | |||
| Rat blood | PBS control | 8 | NV | ++ | +++ | ++ | + | - | - |
| 15 | ++ | ++++ | +++ | ++ | ++ | + | - | ||
| 20 | ++ | ++++ | ++++ | +++ | + | + | - | ||
| 25 | +++ | ++++ | ++++ | +++ | ++ | + | - | ||
| 32 | - | +++ | ++++ | ++++ | ++++ | - | - | ||
| Rat blood | Water | 8 | - | - | - | - | - | - | - |
| 15 | - | - | - | - | - | - | - | ||
| 20 | +/- | + | + | + | - | - | - | ||
| 25 | ++ | ++ | ++ | + | - | - | - | ||
| 32 | - | - | + | + | - | - | - | ||
| Rat blood | 1% Virkon | 8 | NV | - | - | - | - | - | - |
| 15 | NV | - | - | - | - | - | - | ||
| 20 | NV | - | - | - | - | - | - | ||
| 25 | NV | - | - | - | - | - | - | ||
| 32 | NV | - | - | - | - | - | - | ||
Only motile parasites scored as ‘+’
NV = Suspension too thick to detect motile parasites
- no motile parasites seen in ≥20 fields
+<1 per field (20 fields searched)
++ 1–5 per field
+++ >5 per field
++++ Numerous parasites with rosette formation
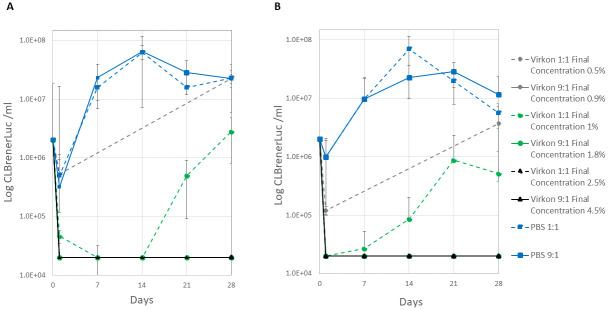
Further quantitative experiments were carried out with T. cruzi CLBrener Luc epimastigotes (2x106) in whole mouse blood and cell culture media (RTH 10% FCS). These confirmed that parasites were not inactivated by 1 h treatment 1:1 with 1% Virkon (final concentration 0.5% w/v) in whole blood. Although a 2- and 8-fold decrease in parasite counts was observed at day 1 in spiked blood and in RTH samples, respectively, parasite growth was recorded at day 28 (Fig 2A and 2B). A larger decrease in parasite counts was observed in spiked blood samples following 1 h treatment 1:1 with 2% Virkon at day 1 (21-fold) that declined to below the limit of quantitation at day 7 and 14. However, motile parasites were observed from day 21 (Fig 2A and 2B). In T. cruzi cell culture samples no motile parasites were observed on day 1 after treatment at a final concentration of 1% Virkon, however, growth was observed from day 7 (Fig 2B). Treatment for 1 h with 5% Virkon at a ratio 1:1 (final concentration 2.5%) successfully inactivated both T. cruzi spiked blood and RTH samples (Fig 2A and 2B). When spiked blood and RTH samples were treated for 1 h with a 9-fold excess volume of Virkon (final concentration 0.9, 1.8 and 4.5% Virkon) no viable parasites were detected over 28 days at all 3 concentrations tested (Fig 2A and 2B). Epimastigote growth was observed in all PBS treated controls.
Fig 2. Virkon inactivation of T. cruzi epimastigotes in blood (A) and RTH media (B).
T. cruzi CLBrener Luc epimastigotes were incubated for 1 h with 1, 2 and 5% Virkon 1:1 (dashed lines) and 9:1 (solid lines) volumetric excess giving a final Virkon concentration range of 0.5–4.5%. Cultures were monitored for parasite growth over 28 days (three technical replicates, mean ± standard deviation, limit of quantitation 2e4 ml-1). PBS controls were included.
Drying techniques
Air drying T. cruzi CLBrener Luc spiked blood (106) for 2 h after collection using the Mitra Microsampler was sufficient to inactivate these samples, with no motile epimastigotes detected in cultures up to 28 days (S2 Table).
No motile T. cruzi were observed in cultures of epimastigote spiked blood (2x106) collected on FTA A and B cards and dried for 15 to 120 min (S2 Table). One parasite was detected by microscopic examination of the well of one replicate after 15 min drying on FTA Elute cards at day 1, 3 and 7 but was not detected at day 14 to 28 of cell culture. No parasites were detected in FTA Elute card samples up to 28 days in culture when dried for 30, 60 or 120 min (S2 Table). However, for all drying times (15 to 120 min), motile parasites were detected in FTA C card cultures at all timepoints of the monitoring period (Fig 3, S2 Table).
Fig 3. T. cruzi epimastigote growth after air drying on FTA C cards.
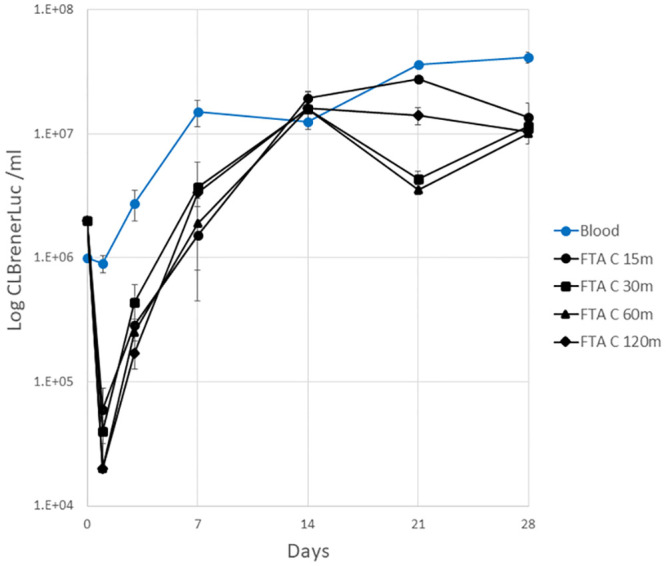
CL Brener Luc epimastigote spiked mouse blood (0.02 ml) spotted on FTA C cards and air dried for 15–120 min. T. cruzi infected blood control (not dried) was also included and parasite growth recorded over 28 days (mean ±SD, limit of quantitation 2×104 ml-1).
Discussion
Many laboratories use disinfectants and autoclaving as methods to inactivate waste contaminated with human and animal pathogens. It is required that these methods are validated for efficacy against pathogens before work is permitted. Published articles in this area are limited with most laboratories performing validation experiments for the pathogens they work on without publishing the data. We have now successfully validated three methodologies to inactivate T. cruzi in cell culture and blood samples. These include three freeze-thaw cycles, Virkon treatment (final concentration 2.5% for 1:1 dilutions, or ≥1% when Virkon solution is applied in 10-fold volumetric excess) and air drying on FTA A, B and Elute cards (≥30 min) or on a Mitra Microsampler (2 h).
We also established that three freeze-thaw cycles are capable of inactivating Silvio X10/7 A1 epimastigote pellets up to 2 g wet weight (~2x1010 parasites). Furthermore, three freeze-thaw cycles are effective against the more clinically relevant mammalian stages of the T. cruzi CLBrener Luc life cycle. Although both three rapid freeze-thaw cycles in liquid nitrogen and 10-min cycles in a dry ice/isopropanol bath were effective against T. cruzi epimastigotes, when inactivating large parasite pellets the combined weight of the pellet and remaining supernatant prior to freeze-thaw is critical and must be ≤2 g as two heavier pellets failed inactivation. It is therefore crucial that large parasite pellets are frozen and submerged in a dry ice/ isopropanol bath as performed in this study to ensure consistent heat transfer and complete freezing of the pellet and contact with the entire pellet containing tube. Furthermore, it is vital that a minimum of three freeze-thaw cycles are carried out. Previous work showed that when a thick suspension of 1010 T. cruzi Silvio X10/7 A1 epimastigotes was dispensed dropwise into liquid nitrogen to form ‘noodles’ for cryogrinding purposes, motile parasites could be recovered after 20 days in culture (RTH). Thus, one rapid freeze-thaw cycle is not sufficient to inactivate T. cruzi (S. Wyllie, personal communication).
A study assessing the ability of T. cruzi to survive the processing and storage conditions of human cells, tissues and tissue-based products found that T. cruzi trypomastigotes survived 24 h at room temperature, at least 10 days at 4ºC and for long periods as stabilates at -80 ºC [21]. They also showed that trypomastigotes in media or cryoprotectant were viable after 4 freeze-thaw cycles. However, in this case trypomastigotes were frozen in a Mr Frosty (Nalgene) giving a slow freezing rate of 1 ºC per min in a -80 ºC freezer and thawed at weekly intervals. This suggests that it is not only the number of freeze-thaw cycles, but also rapid freezing that is important causing rigid ice crystal formation damaging cell membranes during the freeze-thaw procedure leading to cell death.
Virkon was first developed in the 1980’s as a broad-spectrum disinfectant. It is a mix of six biocides acting in different ways against viruses and many bacteria and fungi [20, 22]. The principal active components are oxidising agents that combine to form hypochlorous acid and a detergent. The biocidal activity of both of these components is attenuated by the presence of organic material and this is borne out in the present study in that it is the ratio of blood or serum to Virkon that is critical for effective disinfection.
Whatman FTA DMPK-A, B and FTA Elute cards are cellulose paper impregnated with chemicals to lyse cells and denature proteins on contact. They are designed to inactivate organisms, including blood-borne pathogens, allowing safe transport and storage of samples at room temperature. FTA DMPK- A and B cards are typically used for analyte quantitation by HPLC-MS/MS, while FTA Elute cards capture nucleic acids on the matrix for downstream experiments. FTA DMPK-C cards can be used when the coating on FTA A and B cards interferes with analyte quantitation. However, FTA DMPK-C cards contain pure cellulose and are not impregnated with chemicals, meaning that air drying is the only method used for pathogen inactivation. Notably drying techniques were only effective at inactivating T. cruzi epimastigote infected blood when dried on FTA DMPK-A, B or Elute cards for ≥30 min. These cards are not visibly dry until they have been incubated at room temperature for 1 h, thus, it is recommended that T. cruzi samples collected on these cards are dried for a minimum of 1 h before removal from a biosafety facility. Motile epimastigotes were observed at all drying times on FTA DMPK- C cards, thus, 2 h air drying alone is not sufficient to kill T. cruzi. This may be due to insufficient drying time, but the cards were visibly dry and a prolonged drying period was not tested to confirm this. Although we have not assessed parasite viability beyond 2 h it is interesting to note that in a separate study T. cruzi trypomastigotes were still infective 14 days after Triatoma infestans vector death [23] highlighting the need for validation for specific inactivation methods. T. cruzi epimastigote infected blood was inactivated after 2 h air drying on a Mitra Microsampler. It is unclear what material this device is made of, but the manufacturers state it is a ‘hydrophilic porous material that rapidly wicks fluid and dries in 2 h or less’.
In this study we have used a common in vitro T. cruzi strain Silvio X10/7 A1 and animal model strain CLBrener Luc. Although it is unlikely resistance to inactivation techniques would vary between T. cruzi strains, it may be prudent for those using different strains or different experimental conditions to consider validation in a similar way as described here.
A previous study assessed the effect common laboratory disinfectants and 50 ºC heat on a range of trypanosomatid parasites including bloodstream form T. brucei, T. rangeli epimastigotes and L. major promastigotes [24]. After 5 min of each treatment, live cells were counted on a haemocytometer. Using this method, it was determined that all treatments were able to kill all three parasites stating a 100% lethal concentration of 0.05% for bleach, 0.2% for TriGene, 15–17.5% for ethanol, 0.1% for liquid hand soap and 80–90% for water alone suggesting these parasites do not endure hypo-osmotic stress. Furthermore, a report verified killing of T. brucei by 20-sec treatment with ≥0.1% TriGene and complete cell lysis at ≥1% TriGene [25]. Wang et al [24] propose that other trypanosomatids are likely to be susceptible to these inactivation methods including T. cruzi which, like T. rangeli, is a stercorarian trypanosome. However, our data has shown that even when very small numbers of T. cruzi survive (below the limit of quantitation), they are able to establish cultures with high parasite densities at later time points. In the case of T. cruzi, therefore, growth must be monitored for at least 4 weeks after treatment to ensure 100% killing. In addition, although T. rangeli were slightly more resistant to water inactivation than African trypanosomes, they still succumbed to treatment. Thus, T. cruzi appears to be considerably more robust than other kinetoplastid parasites, suggesting the successful inactivation methods validated here can be extrapolated to other kinetoplastid parasites.
Conclusions
For successful T. cruzi inactivation the following methods have been validated:
Three consecutive freeze-thaw cycles on T. cruzi Silvio X10/7 A1 and CLBrener Luc
Incubation of T. cruzi Silvio X10/7 A1 and CLBrener Luc for 1 h with Virkon disinfectant at a final concentration of 2.5% or at a final concentration of ≥1% when in tenfold volumetric excess
Air drying T. cruzi CLBrener Luc infected blood samples on FTA cards A, B and Elute for ≥1 h
Air drying T. cruzi CLBrener Luc infected blood samples on a Mitra microsampling device for 2 h
Notes
For T. cruzi Silvio X10/7 A1 epimastigote pellets >2 g three 10 min freeze cycles were not sufficient to achieve full inactivation
Air drying T. cruzi CLBrener Luc infected blood samples for 2 h on FTA DMPK-C cards was not sufficient to achieve inactivation
Supporting information
(XLSX)
Mouse blood samples spiked with T. cruzi were subjected to three freeze-thaw cycles and monitored for parasite growth over 28 days. Three technical replicates, mean (standard deviation).
(DOCX)
Parasite growth was monitored over 28 days, mean (standard deviation).
(DOCX)
Acknowledgments
The authors wish to thank members of the DDU DMPK group Liam Ferguson, Laste Stojanovski, Frederick Simeons for supply of rodent blood, and John Kelly (London School Hygiene and Tropical Medicine) for CL Brener expressing pTRIX2-PpyRE9h red-shifted firefly luciferase. We would also like to thank Susan Wyllie for critical appraisal of this manuscript.
Data Availability
All relevant data are within the manuscript and its Supporting information files.
Funding Statement
Wellcome Awards 204672/Z/16/Z, 203134/Z/16/Z and 224024/Z/21/Z.
References
- 1.WHO. Research Priorities for Chagas Disease, Human African Trypanosomiasis and Leishmaniasis. WHO Technical Report Series. Geneva2012. p. 11. [PubMed]
- 2.Gomez LA, Gutierrez FRS, Penuela OA. Trypanosoma cruzi infection in transfusion medicine. Hematol Transfus Cell Ther. 2019;41(3):262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herwaldt BL. Laboratory-acquired parasitic infections from accidental exposures. Clin Microbiol Rev. 2001;14(4):659–88. doi: 10.1128/CMR.14.3.659-688.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moncayo A, Silveira AC. Current epidemiological trends for Chagas disease in Latin America and future challenges in epidemiology, surveillance and health policy. Memórias do Instituto Oswaldo Cruz. 2009;104(Supl. 1):17–30. doi: 10.1590/s0074-02762009000900005 [DOI] [PubMed] [Google Scholar]
- 5.Nunes MC, Dones W, Morillo CA, Encina JJ, Ribeiro AL, Council on Chagas Disease of the Interamerican Society of C. Chagas disease: an overview of clinical and epidemiological aspects. Journal of the American College of Cardiology. 2013;62(9):767–76. [DOI] [PubMed] [Google Scholar]
- 6.Rassi A, Rassi A, Marin-Neto JA. Chagas’ disease. Lancet. 2010;375(9723):1388–402. doi: 10.1016/S0140-6736(10)60061-X [DOI] [PubMed] [Google Scholar]
- 7.Cancado JR. Long term evaluation of etiological treatment of Chagas disease with benznidazole. Revista do Instituto de Medicina Tropical de Sao Paulo. 2002;44(1):29–37. [PubMed] [Google Scholar]
- 8.Bahia-Oliveira LM, Gomes JA, Cancado JR, Ferrari TC, Lemos EM, Luz ZM, et al. Immunological and clinical evaluation of chagasic patients subjected to chemotherapy during the acute phase of Trypanosoma cruzi infection 14–30 years ago. The Journal of Infectious Diseases. 2000;182(2):634–8. [DOI] [PubMed] [Google Scholar]
- 9.The Approved List of Biololgical Agents: Health and Safety Executive; 2013 [http://www.hse.gov.uk/pubns/misc208.pdf.
- 10.Directive 2000/54/EC of the European Parliament and of the Council of 18 September 2000 on the protection of workers from risks related to exposure to biological agents at work (seventh individual directive within the meaning of Article 16(1) of Directive 89/391/EEC): Publications Office of the European Union EUR-Lex & Legal Information Unit; 2000 [http://data.europa.eu/eli/dir/2000/54/2020-06-24
- 11.Risk Group Database: American Biological Safety Association; 2021 [https://my.absa.org/Riskgroups.
- 12.Lewis MD, Fortes Francisco A, Taylor MC, Burrell-Saward H, McLatchie AP, Miles MA, et al. Bioluminescence imaging of chronic Trypanosoma cruzi infections reveals tissue-specific parasite dynamics and heart disease in the absence of locally persistent infection. Cell Microbiol. 2014;16(9):1285–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silveira FT, Viana Dias MG, Pereira Pardal P, Oliveira Lobâo A, Britto Melo G. Nono caso-autóctone de doença de Chagas registrado no estado do Pará, Brasil (Nota prévia). Hiléia Médica. 1979;1:61–2. [Google Scholar]
- 14.Gibson WC, Miles MA. The karyotype and ploidy of Trypanosoma cruzi. The EMBO journal. 1986;5(6):1299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunter KJ, Le Quesne SA, Fairlamb AH. Identification and biosynthesis of N1,N9-bis(glutathionyl)aminopropylcadaverine (homotrypanothione) in Trypanosoma cruzi. European journal of biochemistry / FEBS. 1994;226(3):1019–27. [DOI] [PubMed] [Google Scholar]
- 16.Kendall G, Wilderspin AF, Ashall F, Miles MA, Kelly JM. Trypanosoma cruzi glycosomal glyceraldehyde-3-phosphate dehydrogenase does not conform to the ’hotspot’ topogenic signal model. The EMBO journal. 1990;9(9):2751–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacLean LM, Thomas J, Lewis MD, Cotillo I, Gray DW, De Rycker M. Development of Trypanosoma cruzi in vitro assays to identify compounds suitable for progression in Chagas’ disease drug discovery. PLoS Negl Trop Dis. 2018;12(7):e0006612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Rycker M, Thomas J, Riley J, Brough SJ, Miles TJ, Gray DW. Identification of Trypanocidal Activity for Known Clinical Compounds Using a New Trypanosoma cruzi Hit-Discovery Screening Cascade. PLoS Negl Trop Dis. 2016;10(4):e0004584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.A Novel Dried Matrix Microsampling Device that Eliminates the Volume Based Hematocrit Bias Associated with DBS Sub-punch Workflows [Internet]. Phenomenex, 411 Madrid Ave., Torrance, CA 90501, USA. 2014. http://www.phenomenex.com/Products/Part/9R-K002-CA.
- 20.VIRKON Background Information: Antec International Limited; 1994 [https://www.fishersci.co.uk/webfiles/uk/web-docs/SLSGD05.PDF.
- 21.Martin DL, Goodhew B, Czaicki N, Foster K, Rajbhandary S, Hunter S, et al. Trypanosoma cruzi survival following cold storage: possible implications for tissue banking. PLoS One. 2014;9(4):e95398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ares-Mazas E, Lorenzo MJ, Casal JA, Fernandez da Ponte B, Castro JA, Freire F. Effect of a commercial disinfectant (’Virkon’) on mouse experimental infection by Cryptosporidium parvum. J Hosp Infect. 1997;36(2):141–5. doi: 10.1016/s0195-6701(97)90120-1 [DOI] [PubMed] [Google Scholar]
- 23.Asin SN, Catala SS. Are dead Triatoma infestans a competent vector of Trypanosoma cruzi? Mem Inst Oswaldo Cruz. 1991;86(3):301–5. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Jobe M, Tyler KM, Steverding D. Efficacy of common laboratory disinfectants and heat on killing trypanosomatid parasites. Parasit Vectors. 2008;1(1):35. doi: 10.1186/1756-3305-1-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolfreys K, Field M. Verification of the efficiency of killing Trypanosoma brucei by TriGENE reagent. http://fieldlab.org/Reprints_files/TriGENE%20verification.pdf; 2005.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Mouse blood samples spiked with T. cruzi were subjected to three freeze-thaw cycles and monitored for parasite growth over 28 days. Three technical replicates, mean (standard deviation).
(DOCX)
Parasite growth was monitored over 28 days, mean (standard deviation).
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting information files.