ABSTRACT
Listeria monocytogenes is a Gram-positive pathogenic bacterium which can be found in soil or water. Infection with the organism can develop after ingestion of contaminated food products. Small and large outbreaks of listeriosis have been described. Listeria monocytogenes can cause a number of clinical syndromes, most frequently sepsis, meningitis, and rhombencephalitis, particularly in immunocompromised hosts. The latter syndrome mimics the veterinary infection in ruminants called “circling disease”. Neonatal infection can occur as a result of maternal chorioamnionitis (“early onset” sepsis) or through passage through a birth canal colonized with Listeria from the gastrointestinal tract. (“late onset” meningitis). Treatment of listeriosis is usually with a combination of ampicillin and an aminoglycoside but other regimens have been used. The mortality rate is high, reflecting the combination of an immunocompromised host and an often delayed diagnosis.
EPIDEMIOLOGY
Listeria monocytogenes is a Gram-positive motile facultative anaerobe that inhabits a broad ecologic niche (1–3). With selective media it can be readily isolated from soil, water, and vegetation, including raw produce designated for human consumption without further processing (4, 5). Newer chromogenic media may offer some advantages in the detection of contaminated foodstuffs (6, 7). Surface contamination of meat and vegetables is relatively common, with up to 15% of these foods harboring the organism. In addition, the organism is a transient inhabitant of both animal and human gastrointestinal tracts (8–10), and intermittent carriage suggests frequent exposure. The gut is the source for the organism in invasive listeriosis when it occurs, and the virulence factor ActA may promote carriage (11). The organism is psychrophilic and enjoys a competitive advantage against other Gram-positive and Gram-negative microorganisms in cold environments, such as refrigerators. It may also be amplified in spoiled food products, particularly when spoilage leads to increased alkalinity. Feeding of spoiled silage with a high pH has resulted in epidemics of listeriosis in sheep and cattle (12).
Several large foodborne outbreaks of listeriosis in humans have parallels to epidemic listeriosis in animals. The first proven foodborne outbreak occurred in Canada in 1980 to 1981 and was caused by ingestion of contaminated coleslaw (13). Subsequently, many other foodstuffs have been implicated in both small and large outbreaks, including unpasteurized and pasteurized cheeses (14–22), pasteurized milk (23, 24), butter (25), various fruits and vegetables (26–30), and several meat products (31–35) (Table 1). A large outbreak of listeriosis with over 900 cases and 200 deaths was reported from South Africa in 2017-2018. The source was a contaminated processed meat called “polony” (36). Recent evidence suggests that hospitalized patients are also at risk of acquiring invasive listeriosis (37). Tracking listeriosis cases and creating linkages to food products is now dependent on typing isolates using pulsed field gel electrophoresis and whole-genome sequencing, which have largely replaced older methods such as serotyping (38, 39). Uncertainty exists as to why outbreaks of listeriosis occur in human populations, although the 50% infective dose in sporadic disease is probably high. Enhancement of organism-specific virulence factors may play a role in epidemic disease, although all isolates of L. monocytogenes have the ability to produce all the virulence factors characteristic of the species.
TABLE 1.
Some foods implicated in published reports of foodborne listeriosis
| Dairy products | Fruits and vegetables | Meat products | Fish products |
|---|---|---|---|
| Pasteurized whole milkChocolate milkMexican-style cheeseSoft cheese (different types)Hard cheeseGoat cheeseIce creamFresh cream | Coleslaw (cabbage)LettuceCornRice saladStrawberriesCantaloupesNectarinesSalted mushroomsAlfalfa tabletsApplesBlueberriesStone fruitSprouts | Delicatessen foods (deli meats)PâtéUncooked hot dogsTurkey franks“Rillettes”Pork tongue in aspicPork pieBeefJellied porkCooked hamFoie grasOx tongueUndercooked chicken | Tuna saladSmoked fishShrimp salad |
Recent evidence has suggested that sporadic cases of listeriosis are also foodborne. Case-control studies of sporadic listeriosis cases in the absence of epidemic disease have implicated food products, including cold meats, turkey franks, and delicatessen-type foods, as vehicles for the development of sporadic invasive listeriosis in humans (40).
Our current understanding of the epidemiology of human listeriosis suggests that the organism is a common contaminant of food products and that ingestion of small numbers of L. monocytogenes occurs frequently in human populations. In one prospective study, a rate of 5 to 9 exposures per person-year was estimated (41). Amplification of the organism in biofilms or on food products undergoing processing but not pasteurization and kept at cold temperatures allows overgrowth of L. monocytogenes. Subsequent ingestion of large numbers of the organism may overwhelm innate host-defense systems in the gastrointestinal tract, liver, and spleen with subsequent development of invasive disease. The annual rate of sporadic listeriosis in Europe (42–44) and North America (45) is usually <1/100,000 population per year, and the disease is costly in both human (46) and economic terms (47). Sporadic listeriosis appears to be more common in the spring and summer months. This could be explained by seasonal variations in the types of food products eaten by human populations, with higher-risk products eaten in the warmer months. In addition, data suggest that preexisting damage to the gastrointestinal mucosa by other microorganisms, such as those that are associated with viral gastroenteritis, may allow translocation of L. monocytogenes from the gastrointestinal tract with subsequent development of invasive disease (48). These viral pathogens often have seasonal patterns that overlap with those of invasive listeriosis.
Demographic data from surveillance studies indirectly revealed several host-specific risk factors for invasive listeriosis (49, 50). Infection is most commonly seen in the first 30 days of life or in patients older than 60. In the first instance, the fetus is infected during maternal sepsis with L. monocytogenes or from perivaginal and perianal colonization of the mother by transition through the birth canal. Host defense against listeriosis is impaired in those infants with underdeveloped macrophage and cell-mediated immune function, and invasive listeriosis is more likely to occur if colonization of the liver, respiratory tract, or gastrointestinal tract has occurred.
The increased risk of invasive listeriosis in older patients reflects the increasing incidence of immunosuppressive conditions, such as solid tumors and hematologic malignancy, in this age group. Control of early infection in humans and in animal models is highly dependent on an intact gastrointestinal mucosa and effective macrophage function in the liver, spleen, and peritoneum following bacterial translocation from the gastrointestinal tract. Both these protective events can be impaired by the primary disease or by chemotherapy or radiation-induced damage. In addition, treatment of malignancy and the use of immunosuppressive agents with a specific effect on cell-mediated immune function, such as corticosteroids or cyclosporin A (51), predispose to invasive infection by diminishing L. monocytogenes-specific host responses that occur after the initial phase of infection. The recent proliferation of biologic treatments with immune modulators such as tumor necrosis factor-alpha inhibitors has also contributed to increases in invasive listeriosis (52–54).
The cell-mediated immune response to L. monocytogenes is impaired in pregnant women (55) and, with the decreased gastrointestinal motility (56) seen in pregnancy, may predispose to invasive listeriosis and subsequent transplacental infection of the infant. This results in “early-onset” listeriosis characterized by the delivery of an often premature and severely ill infant. Spontaneous recovery of the mother from Listeria sepsis normally occurs following delivery of the infant, although if recognized prepartum, appropriate antibiotic therapy can save the infant.
In “late-onset” listeriosis, the infant is infected through maternal gastrointestinal carriage of L. monocytogenes without sepsis, and the infant is infected during transition through a colonized birth canal. In these cases, clinical disease in the infant develops 7 to 14 days later. Direct cutaneous invasion is unlikely, and it is believed that aspiration of the organism into the respiratory tract or swallowing of the organism by the infant may occur during the incubation period. A unique outbreak of neonatal listeriosis in Costa Rica has been described: the vehicle was L. monocytogenes-contaminated mineral oil used to clean infants after delivery from healthy mothers, with cross contaminations of shared mineral oil (57). The index case was infected through the placental route of maternal-fetal transmission. Pregnancy-associated listeriosis has been recently reviewed (58, 59).
Several large outbreaks of a febrile gastroenteritis syndrome have further highlighted the importance of L. monocytogenes as a foodborne pathogen. In these outbreaks, with an average incubation period of approximately 24 h, attack rates (up to 72%) were much higher than those reported for outbreaks of invasive listeriosis. The reported vehicles for these more typical foodborne infections have included shrimp (60), rice salad (61), chocolate milk (24), corn salad (62), ready-to-eat meats (63, 64), jellied pork (65), and fresh cheese (15). The foods implicated were usually heavily contaminated (>109 CFU/ml of L. monocytogenes), and the amount of food ingested appeared to correlate with infection, suggesting that the high attack rates are not related to enhanced intrinsic virulence of the particular infecting strain of L. monocytogenes. A hospital-acquired outbreak of gastroenteritis has also been described from contaminated meat jelly (66).
While a predisposition to invasive listeriosis is seen in patients with malignancy or organ transplant, human immunodeficiency virus (HIV) infection is also an important risk factor in sporadic listeriosis (67). Earlier studies have reported that attack rates for invasive listeriosis in HIV-positive patients that were 500- to 1,000-fold greater than those in the general population. However, reductions in invasive listeriosis cases in HIV infection has been brought about by widely promulgated dietary recommendations to prevent foodborne illness and by the use of prophylaxis for Pneumocystis jiroveci pneumonia, primarily trimethoprim-sulfamethoxazole, to which L. monocytogenes is susceptible. Further reductions in HIV-associated cases may be due to better and more widespread antiretroviral therapy (68). Reductions in the overall incidence of listeriosis in non-HIV-positive patients may also be attributed to distribution of dietary recommendations to populations at risk, including pregnant women, patients with malignancies, and organ transplant recipients (69). Perhaps more importantly, the decreased incidence of listeriosis may be due to the promotion of guidelines to promote universal awareness of the problem in the food-processing industry, which has undertaken hazard analysis at critical control point (HACCP) (70–72) and microbial risk assessment (73, 74, 141) programs to reduce contamination of foods with L. monocytogenes as well as with other foodborne pathogens such as Salmonella spp., Campylobacter spp., and Escherichia coli. These activities have provided increased protection in the face of increased public demand for fresh, unprocessed food products that may not have been cooked or pasteurized and that by definition present a greater degree of risk for foodborne illness.
In addition to hazard analysis at critical control point programs, regulatory agencies have aggressively pursued the control of L. monocytogenes contamination of food. The U.S. Food and Drug Administration has a zero-tolerance policy for L. monocytogenes in its industry sampling programs (75). Other countries have adopted less stringent guidelines, allowing a small amount of contamination (<102 CFU/g) to strike a balance between protection of public health and needless condemnation of otherwise edible food products. While invasive listeriosis may be more common in some countries in Europe than in the United States, it is not clear whether these differences can be attributed to less stringent standards in Europe that allow more L. monocytogenes in the food supply. The debate continues between zero tolerance advocates and those supporting a risk-assessment approach to Listeria contamination of food (76). However, these measures have not led to decreases in the incidence of L. monocytogenes infections in the developed world (77).
CLINICAL DISEASE DUE TO L. MONOCYTOGENES
A wide variety of clinical syndromes have been associated with L. monocytogenes infection in both animals and humans (Table 2). The earliest descriptions of L. monocytogenes sepsis were described in an epizootic affecting South African rodents (78) and in laboratory colonies of rabbits (79). One distinguishing characteristic of infection in rabbits was the production of monocytosis in blood, which suggested the species name monocytogenes. A monocytosis-producing antigen has been described as a virulence factor of L. monocytogenes (80), but monocytosis in the peripheral blood is not a characteristic of infection in humans.
TABLE 2.
Some clinical syndromes associated with L. monocytogenes infection
| Neonatal meningitis |
| Meningoencephalitis in adults |
| Rhombencephalitis |
| Sepsis (bacteremia) in infants or adults |
| Native or prosthetic valve endocarditis |
| Spontaneous bacterial peritonitis |
| Septic arthritis |
| Biliary tract disease |
| Hepatitis |
| Liver abscess |
| Cutaneous infections (in animal workers) |
| Endophthalmitis |
| Febrile gastroenteritis |
| Continuous ambulatory peritoneal dialysis peritonitis |
| Osteomyelitis |
Many wild and domesticated animals are subject to invasive listeriosis. Animals acquire the organism from the environment through grazing, amplified by fecal contamination of soil and vegetation. Specific syndromes with parallels in human disease have been recognized in animals. In New Zealand in the 1930s, Gill (81) described “circling disease,” a rhombencephalitis of sheep that may effect flocks fed spoiled silage. L. monocytogenes has also been implicated as a cause of abortion and prematurity in ruminants. Intravenous and oral models of L. monocytogenes infection in rodents can duplicate the illness seen in the natural state in animals, including maternal sepsis and abortion (82).
The clinical syndromes associated with listeriosis in humans were discovered later. Neonatal listeriosis was initially described in postwar Europe in premature septic newborns in East Germany (83). This description of early-onset listeriosis was followed by reports of neonatal meningitis (late-onset listeriosis) occurring later in the postpartum period. L. monocytogenes as a cause of meningitis in neonates is third to group B streptococci and E. coli in the developed world (84, 85). The use of antibiotic prophylaxis to prevent group B streptococcal infection may also have reduced cases of neonatal listeriosis (86). In less-developed countries, Gram-negative meningitis with E. coli or Salmonella spp. is more common, but Listeria meningitis still occurs.
PREGNANCY-ASSOCIATED LISTERIOSIS
Pregnant women are at high risk of infection, and occult or overt bacteremia can result in chorioamnionitis producing early-onset neonatal listeriosis (58). These infants have characteristic clinical features, including prematurity, sepsis at birth, fever, a diffuse maculopapular cutaneous eruption, and evidence of significant hepatic involvement with jaundice (87). The mortality rate of early-onset listeriosis, even with treatment, is very high, and stillbirth is also common in this setting. Autopsy findings in cases of early-onset listeriosis show significant chorioamnionitis in placental remnants and granulomas in multiple organs, particularly the liver and spleen, in infected infants. The original descriptions from East Germany characterized the entire syndrome as “granulomatosis infantiseptica.” (83).
The mothers of these septic infants may be asymptomatic but commonly have flu-like or pyelonephritis symptoms before the early onset of labor, and their blood cultures are frequently positive for L. monocytogenes. Symptoms in the mother include fever, chills, and malaise, which resolve spontaneously following delivery of the infected infant and placenta (87). Anecdotal case reports suggest that early treatment of the mother who has Listeria sepsis can prevent transplacental infection or treat the fetus in utero, with subsequent delivery of a normal uninfected infant (88). Unfortunately, this usually only happens when a community-based outbreak of L. monocytogenes has been identified and physicians are aware of the problem in a particular geographic region through public health alerts.
Late-onset neonatal meningitis due to L. monocytogenes has the typical features of the same syndrome caused by other organisms in this setting, including fever, irritability, bulging fontanelle, and meningismus (89). These symptoms usually develop 1 to 2 weeks following delivery. The mother has usually had an uncomplicated pregnancy, delivery, and postpartum course with no signs of sepsis. The clinical syndrome usually dictates a lumbar puncture, and the cerebrospinal fluid (CSF) in 50% of the cases reveals the organism by Gram’s stain. CSF cultures are usually positive, although the organism may be isolated simultaneously or only from the blood in some cases. The CSF shows other characteristics of bacterial meningitis, including a high polymorphonuclear leukocyte count, elevated protein, and low glucose with a decrease in the CSF-serum glucose ratio.
ADULT MENINGOENCEPHALITIS
L. monocytogenes is an uncommon cause of bacterial meningitis in adults. There are two major clinical presentations. The first is a typical subacute bacterial meningitis characterized by fever, headache, and neck stiffness (90). Because the organism is not commonly seen on Gram’s stain of CSF, and because the cell counts are lower than in other forms of bacterial meningitis, an initial diagnosis of viral meningitis is commonly made before culture of the organism from CSF or blood. The onset of the syndrome can occur over several days, unlike meningococcal or pneumococcal meningitis, which have more abrupt onsets. During epidemics of foodborne listeriosis, Listeria meningitis can occur in apparently healthy individuals of all ages. In sporadic disease, patients more commonly have obvious defects in cell-mediated immune function that predispose them to listeriosis.
The second form of central nervous system listeriosis in adults is a rhombencephalitis that has features characteristic of the same illness in animals, described as circling disease (91). Fever, headache, nausea, and vomiting occur early, with signs of meningeal irritation less commonly present. Subsequently, patients develop multiple cranial nerve abnormalities accompanied by cerebellar dysfunction, including ataxia. Fever may not be present in up to 15% of patients, which makes the diagnosis more difficult and more suggestive of noninfectious disorders. CSF pleocytosis may be minimal, and the organism is rarely seen on Gram’s stain. The diagnosis is established by culture of CSF or blood. Magnetic resonance imaging is the best diagnostic study and frequently demonstrates typical multiple microabscesses of the cerebellum and diencephalon (Fig. 1). The mortality rate in this condition approaches 50%, and despite treatment, residual morbidity, including permanent cranial nerve palsies and ataxia, may persist.
FIGURE 1.
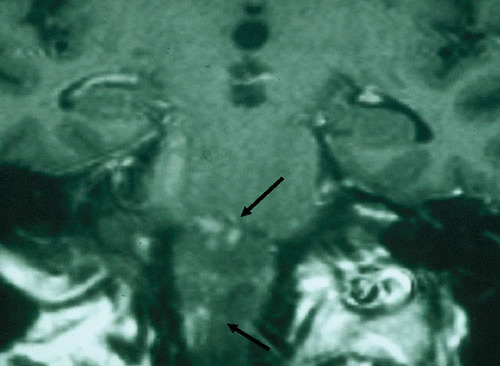
Computed tomography scan from a 72-year-old male with Listeria rhombencephalitis. The arrows point to multiple microabscesses.
L. monocytogenes can also be responsible for cerebritis or typical brain abscess in the supratentorial region (92). In these cases the typical rhombencephalitic symptoms due to microabscesses are absent. Host risk factors reflecting immune deficiency are commonly seen in these cases as they are in Listeria sepsis.
LISTERIA SEPSIS
Listeria sepsis, or bacteremia without central nervous system involvement, represents one-third of adult cases of invasive listeriosis. The symptoms are nonspecific but usually include fever and chills. As noted above, in pregnant women Listeria sepsis often masquerades as pyelonephritis or “flu” (87). The diagnosis is often established in retrospect following delivery of an infected infant. In nonpregnant adults, Listeria sepsis almost always occurs in patients with malignancy, organ transplant, or other immunocompromised states (93–95). In these settings, the presentation is also nonspecific and mimics sepsis with other Gram-positive and Gram-negative pathogens. The mortality rate for Listeria sepsis in these series is 25 to 30%.
OTHER CLINICAL SYNDROMES
Cutaneous Listeriosis
Cutaneous listeriosis is an occupational hazard of veterinary workers exposed to infected amniotic fluid or placental remnants that are removed from the birth canal of animals (96–98). Occasionally, cutaneous infection, including conjunctivitis, has been seen in laboratory workers (99). Cutaneous listeriosis is characterized by low-grade fever and multiple papulopustular lesions of the skin from which the organism can be isolated. Its appearance is similar to the rash seen in infants with early-onset disseminated listeriosis. In adults, the condition may resolve spontaneously without treatment, but the infection itself should be entirely preventable with appropriate gloving and other protective wear.
Bacterial Endocarditis Caused by L. monocytogenes
Bacterial endocarditis presumably follows transient bacteremia from a gastrointestinal source, with subsequent establishment of endovascular infection on an abnormal heart valve. L. monocytogenes is an uncommon cause of native valve endocarditis, and over 50% of cases that have been described involve prosthetic valves (100–102). Infection with L. monocytogenes is usually found as part of the late prosthetic valve endocarditis syndrome. Diagnostic criteria for Listeria endocarditis include the presence of a prosthetic valve with or without vegetation and continuous bacteremia with L. monocytogenes. Septic emboli and abscess formations in other organs are relatively frequent. In native valve endocarditis, L. monocytogenes can sometimes follow previous episodes of streptococcal bacterial endocarditis or other valvular heart disease. Patients with malignancy, diabetes, steroid therapy, and renal and liver transplantation have been described with Listeria endocarditis. Their presentation is nonspecific for L. monocytogenes and includes prolonged fever, chills, and ultimately, signs of congestive heart failure. Septic embolization occurs in two-thirds of patients, and aortic and mitral valve involvement are most common. L. monocytogenes can also cause arterial infections that involve prosthetic abdominal and aortic grafts or native abdominal aortic aneurysms (103). The mortality of this condition approached 40% before 1985 but has been reduced to 12% with better recognition and surgical management.
Hepatitis and Liver Abscess Due to L. monocytogenes
L. monocytogenes has been described as a cause of acute hepatitis in several case reports (104). It occurs as acute onset of fever and jaundice accompanied by positive blood cultures for L. monocytogenes. The diagnosis is usually unsuspected. Severe disease with death has been described, and autopsy or liver biopsy generally reveals microabscesses and occasionally granulomas similar to those seen in severe early onset neonatal disease. Predisposing factors include cirrhosis and liver transplantation (105), although Listeria hepatitis can occur in a normal host.
Solitary and multiple liver abscesses with fever are also described (106, 107). Bacteremia occurs in half of these patients. Predisposing factors include diabetes mellitus, transplantation, cirrhosis, and alcoholism. Aspiration of the abscess demonstrates the organism. The mortality rate is 50%, and postmortem examination often reveals abscesses in other organs as well. Patients with multiple abscesses appear to do worse than those with a solitary abscess.
Listeria Peritonitis
L. monocytogenes can also cause isolated episodes of peritonitis (108, 109). It is most commonly seen in patients undergoing continuous ambulatory peritoneal dialysis (110), and the organisms are isolated from the dialysate or from blood culture. The organisms presumably cause infection through translocation from the gastrointestinal tract in patients who have ingested the organism with food. This complication is extremely rare and represents <1% of all cases of continuous ambulatory peritoneal dialysis peritonitis. It can also cause spontaneous bacterial peritonitis in advanced liver disease with ascites (111–113). The mortality rate is low, and laboratory and clinical features are typical of spontaneous bacterial peritonitis due to other organisms. The organism can rarely cause this disease in patients who have undergone liver transplantation.
Biliary Tract Infections
Recent reports have seen L. monocytogenes emerge as a biliary tract pathogen (114, 115, 142). Retrograde infection from contaminated food is the likely source. The organism is resistant to bile, and this may play some role in the pathogenesis of biliary disease. Most cases also have medical comorbidities, including immunosuppression from corticosteroid use or newer biologic agents to treat the underlying conditions. A number of deaths have been reported, often due to inappropriate antibiotic therapy.
Musculoskeletal Infection
L. monocytogenes is a very uncommon cause of osteomyelitis (116–118). Reports of Listeria osteomyelitis emphasize the role of diabetes mellitus or leukemia as predisposing factors, particularly when long-term corticosteroids are administered. Relapses have been described despite effective antibiotic therapy. Septic arthritis due to L. monocytogenes appears to be more common than bone infection, and rheumatoid arthritis appears to be a frequently associated condition. Low-dose methotrexate therapy may predispose to this infection. Infection may follow joint injection with corticosteroids. Infection has been described in prosthetic hips and knees as well as in native joints (119, 120). The organism can also cause vertebral osteomyelitis with epidural abscess (121). With prolonged antibiotic therapy, medical treatment alone, as opposed to removal of the prosthetic joint, may be successful. Deaths are rare and are normally due to the underlying disease.
Gastroenteritis
A febrile gastroenteritis syndrome has been described for listeriosis (15, 24, 25, 60–64, 143). Gastrointestinal prodromal symptoms, such as diarrhea or abdominal pain, have been common in large outbreaks of foodborne adult listeriosis, but sepsis and meningoencephalitis have been the usual presenting syndromes. Population-based attack rates for invasive listeriosis have been low in this setting. In Listeria gastroenteritis, a more typical foodborne illness occurs with high attack rates (up to 72%) among the individuals exposed to the vehicle of infection. Large numbers of organisms in the contaminated food may be responsible.
Most patients are well before development of the infection. While bacteremia has occurred in some patients, primary symptoms are diarrhea, fever, fatigue, chills, and myalgias occurring 24 h following exposure. This incubation period is considerably shorter than the 3- to 4-week period for more usual forms of invasive listeriosis.
Pregnant women appear to be more likely to have sepsis in these outbreaks. Isolation of L. monocytogenes with selective media from stool has been rare, but serologic tests have been used to help define the extent of the outbreaks. In the outbreaks reported to date, rice salad, shrimp salad, chocolate milk, corn, deli meats, and fresh cheese have been the reported vehicles, and high colony counts of the organism (up to 109 CFU/g) appear to be present in the contaminated food. Invasive listeriosis can also be a result of loss of gastrointestinal integrity due to other gastrointestinal tract pathogens such as Shigella spp. (122) or to presumed viral gastroenteritis (48). This may account for some sporadic and epidemic cases of Listeria sepsis and meningoencephalitis.
DIAGNOSIS OF LISTERIOSIS
Diagnosis of all forms of L. monocytogenes infection depends on isolation of the organism from a normally sterile site, usually blood or cerebrospinal fluid. Gram’s stain of specimens of sterile spinal fluid, peritoneal fluid, or joint aspirates occasionally, but unpredictably, reveals Gram-positive coccobacilli, characteristic of L. monocytogenes. In some forms of central nervous system infection, particularly rhombencephalitis, several samples may have to be obtained to isolate the organism. Focal neurologic findings that are characteristic of rhombencephalitis should prompt computed tomography or magnetic resonance imaging scanning. The finding of multiple microabscesses in the hindbrain is highly suspicious for Listeria rhombencephalitis, and empiric treatment for listeriosis should be started.
In situations where antibiotic therapy has already been administered, isolation of the organism from a nonsterile site may support a diagnosis of listeriosis. In pregnant women, stool or vaginal cultures may be positive when selective media for L. monocytogenes are used for culture. In febrile gastroenteritis syndromes, where traditional pathogens have not been isolated using standard media, culture of the stool with selective media for L. monocytogenes may also demonstrate the organism.
Direct detection of PCR products such as the HlyA from L. monocytogenes in CSF and other fluids has been studied. Sensitivity appears to be low but might be useful in patients already on treatment for listeriosis where cultures are negative (123, 124).
TREATMENT OF LISTERIOSIS
L. monocytogenes remains susceptible to most β-lactam antibiotics, with the exception of cephalosporins, to which the organism is usually resistant (125–127). Because newer cephalosporins are commonly used for the treatment of nonspecific sepsis syndromes or for the empiric treatment of bacterial meningitis, specific therapy for listeriosis may be delayed for some patients. Empiric therapy for bacterial meningitis with ampicillin is recommended for older adults but may not be necessary for children beyond the neonatal period (128). When listeriosis is a likely diagnosis, the use of ampicillin or, in penicillin-allergic patients, vancomycin provides empiric coverage for L. monocytogenes until the diagnosis is made by culture.
A combination of ampicillin and gentamicin is the current therapy of choice for all forms of listeriosis (129, 130). However, some data suggest that this combination is not useful and could be harmful (131). There has been a trend toward increasing resistance to penicillins (127). Ampicillin is not bactericidal for L. monocytogenes, and in vitro and in vivo data suggest that an additive or synergistic effect with gentamicin may improve the outcome (132). No randomized, controlled clinical trials of therapy in humans have been carried out, however.
Trimethoprim-sulfamethoxazole, with or without the addition of rifampin, is an alternative treatment regimen that has been recommended. Early “step-down” to oral trimethoprim-sulfamethoxazole has been successful in several cases (133). In one retrospective study, the combination of amoxicillin and cotrimoxazole was found to be more effective than ampicillin and gentamicin (134). High-level resistance to trimethoprim has been recognized, however (127, 135). New quinolones may also be effective, but data are limited to in vitro studies (136, 137). Rifampin is active against Listeria, but resistance has been found in cases of prosthetic joint infection (138, 139). For central nervous system infection, dexamethasone and phenytoin have been suggested as possible treatment adjuncts (140).
Trimethoprim-sulfamethoxazole has also been used as a prophylactic agent against a number of microorganisms, including P. jiroveci, in patients with HIV infection and in patients undergoing chemotherapy for leukemia or lymphoma. This drug would be effective in protecting against L. monocytogenes infections. The use of prophylaxis has been temporally associated with a decrease in the incidence of listeriosis in these compromised hosts in combination with dietary guidelines that have been issued for these patients in recent years (68).
The duration of treatment for invasive listeriosis has not been studied. Relapses appear to be uncommon, and 2 to 3 weeks of therapy with ampicillin and gentamicin is sufficient for most forms of listeriosis. Rhombencephalitis with abscess formation in the central nervous system may require more prolonged therapy, but data are not available that support treatment beyond 4 weeks (129, 130).
REFERENCES
- 1.Welshimer HJ, Donker-Voet J. 1971. Listeria monocytogenes in nature. Appl Microbiol 21:516–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Linke K, Rückerl I, Brugger K, Karpiskova R, Walland J, Muri-Klinger S, Tichy A, Wagner M, Stessl B. 2014. Reservoirs of Listeria species in three environmental ecosystems. Appl Environ Micro 80:5583–5592. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weis J, Seeliger HPR. 1975. Incidence of Listeria monocytogenes in nature. Appl Microbiol 30:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graves LM, Swaminathan B, Ajello GW, Malcolm GD, Weaver RE, Ransom R, Dever K, Plikaytis BD, Schuchat A, Wenger JD, Pinner RW, Broome CV, Listeria Study Group. 1992. Comparison of three selective enrichment methods for the isolation of Listeria monocytogenes from naturally contaminated foods. J Food Prot 55:952–959 10.4315/0362-028X-55.12.952. [DOI] [PubMed] [Google Scholar]
- 5.Law JW, Ab Mutalib NS, Chan KG, Lee LH. 2015. An insight into the isolation, enumeration, and molecular detection of Listeria monocytogenes in food. Front Microbiol 6:1227–1242 10.3389/fmicb.2015.01227. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reissbrodt R. 2004. New chromogenic plating media for detection and enumeration of pathogenic Listeria spp.: an overview. Int J Food Microbiol 95:1–9 10.1016/j.ijfoodmicro.2004.01.025. [PubMed] [DOI] [PubMed] [Google Scholar]
- 7.Bres V, Yang H, Hsu E, Ren Y, Cheng Y, Wisniewski M, Hanhan M, Zaslavsky P, Noll N, Weaver B, Campbell P, Reshatoff M, Becker M. 2014. Listeria monocytogenes LmG2 detection assay using transcription mediated amplification to detect Listeria monocytogenes in selected foods and stainless steel surfaces. J AOAC Int 97:1343–1358 10.5740/jaoacint.13-386. [PubMed] [DOI] [PubMed] [Google Scholar]
- 8.Kampelmacher EH, van Noorle Jansen LM. 1969. The isolation of Listeria monocytogenes from feces from clinically healthy humans and animals. Ned Tijdschr Geneeskd 113:1533–1536. (In Dutch.) [PubMed] [Google Scholar]
- 9.Grif K, Hein I, Wagner M, Brandl E, Mpamugo O, McLauchlin J, Dierich MP, Allerberger F. 2001. Prevalence and characterization of Listeria monocytogenes in the feces of healthy Austrians. Wien Klin Wochenschr 113:737–742. [PubMed] [Google Scholar]
- 10.Gahan CG, Hill C. 2014. Listeria monocytogenes: survival and adaptation in the gastrointestinal tract. Front Cell Infect Microbiol 4:9–16 10.3389/fcimb.2014.00009. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Travier L, Guadagnini S, Gouin E, Dufour A, Chenal-Francisque V, Cossart P, Olivo-Marin JC, Ghigo JM, Disson O, Lecuit M. 2013. ActA promotes Listeria monocytogenes aggregation, intestinal colonization and carriage. PLoS Pathog 9:e1003131 10.1371/journal.ppat.1003131. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Low JC, Renton CP. 1985. Septicaemia, encephalitis and abortions in a housed flock of sheep caused by Listeria monocytogenes type 1/2. Vet Rec 116:147–150 10.1136/vr.116.6.147. [PubMed] [DOI] [PubMed] [Google Scholar]
- 13.Schlech WF III, Lavigne PM, Bortolussi RA, Allen AC, Haldane EV, Wort AJ, Hightower AW, Johnson SE, King SH, Nicholls ES, Broome CV. 1983. Epidemic listeriosis: evidence for transmission by food. N Engl J Med 308:203–206 10.1056/NEJM198301273080407. [PubMed] [DOI] [PubMed] [Google Scholar]
- 14.Büla CJ, Bille J, Glauser MP. 1995. An epidemic of food-borne listeriosis in western Switzerland: description of 57 cases involving adults. Clin Infect Dis 20:66–72 10.1093/clinids/20.1.66. [PubMed] [DOI] [PubMed] [Google Scholar]
- 15.Carrique-Mas JJ, Hökeberg I, Andersson Y, Arneborn M, Tham W, Danielsson-Tham ML, Osterman B, Leffler M, Steen M, Eriksson E, Hedin G, Giesecke J. 2003. Febrile gastroenteritis after eating on-farm manufactured fresh cheese: an outbreak of listeriosis? Epidemiol Infect 130:79–86 10.1017/S0950268802008014. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention (CDC). 2001. Outbreak of listeriosis associated with homemade Mexican-style cheese: North Carolina, October 2000-January 2001. MMWR Morb Mortal Wkly Rep 50:560–562. [PubMed] [Google Scholar]
- 17.Linnan MJ, Mascola L, Lou XD, Goulet V, May S, Salminen C, Hird DW, Yonekura ML, Hayes P, Weaver R, Audurier A, Plikaytis BD, Fannin SL, Kleks A, Broome CV. 1988. Epidemic listeriosis associated with Mexican-style cheese. N Engl J Med 319:823–828 10.1056/NEJM198809293191303. [PubMed] [DOI] [PubMed] [Google Scholar]
- 18.Fretz R, Pichler J, Sagel U, Much P, Ruppitsch W, Pietzka AT, Stoger A, Huhulescu S, Heuberger S, Appl G, Werber D, Starl K, Prager R, Flieger A, Karpiskova R, Pfaff G, Allerberger F. 2010. Update: multinational listeriosis outbreak due to ‘quargel’, a sour milk curd cheese, caused by two different L. monocytogenes serotype 1/2a strains, 2009-2010. Euro Surveill 22:19543. [PubMed] [Google Scholar]
- 19.Jackson KA, Biggerstaff M, Tobin-D’Angelo M, Sweat D, Klos R, Nosari J, Garrison O, Boothe E, Saathoff-Huber L, Hainstock L, Fagan RP. 2011. Multistate outbreak of Listeria monocytogenes associated with Mexican-style cheese made from pasteurized milk among pregnant, Hispanic women. J Food Prot 74:949–953 10.4315/0362-028X.JFP-10-536. [PubMed] [DOI] [PubMed] [Google Scholar]
- 20.Choi MJ, Jackson KA, Medus C, Beal J, Rigdon CE, Cloyd TC, Forstner MJ, Ball J, Bosch S, Bottichio L, Cantu V, Melka DC, Ishow W, Slette S, Irvin K, Wise M, Tarr C, Mahon B, Smith KE, Silk BJ; Centers for Disease Control and Prevention (CDC). 2014. Multistate outbreak of listeriosis linked to soft-ripened cheese: United States, 2013. MMWR Morb Mortal Wkly Rep 63:294–295. [PMC free article] [PubMed] [Google Scholar]
- 21.Koch J, Dworak R, Prager R, Becker B, Brockmann S, Wicke A, Wichmann-Schauer H, Hof H, Werber D, Stark K. 2010. Large listeriosis outbreak linked to cheese made from pasteurized milk, Germany, 2006-2007. Foodborne Pathog Dis 7:1581–1584 10.1089/fpd.2010.0631. [PubMed] [DOI] [PubMed] [Google Scholar]
- 22.Heiman KE, Garalde VB, Gronostaj M, Jackson KA, Beam S, Joseph L, Saupe A, Ricotta E, Waechter H, Wellman A, Adams-Cameron M, Ray G, Fields A, Chen Y, Datta A, Burall L, Sabol A, Kucerova Z, Trees E, Metz M, Leblanc P, Lance S, Griffin PM, Tauxe RV, Silk BJ. 2016. Multistate outbreak of listeriosis caused by imported cheese and evidence of cross-contamination of other cheeses, USA, 2012. Epidemiol Infect 144:2698–2708 10.1017/S095026881500117X. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fleming DW, Cochi SL, MacDonald KL, Brondum J, Hayes PS, Plikaytis BD, Holmes MB, Audurier A, Broome CV, Reingold AL. 1985. Pasteurized milk as a vehicle of infection in an outbreak of listeriosis. N Engl J Med 312:404–407 10.1056/NEJM198502143120704. [PubMed] [DOI] [PubMed] [Google Scholar]
- 24.Dalton CB, Austin CC, Sobel J, Hayes PS, Bibb WF, Graves LM, Swaminathan B, Proctor ME, Griffin PM. 1997. An outbreak of gastroenteritis and fever due to Listeria monocytogenes in milk. N Engl J Med 336:100–105 10.1056/NEJM199701093360204. [PubMed] [DOI] [PubMed] [Google Scholar]
- 25.Lyytikäinen O, Autio T, Maijala R, Ruutu P, Honkanen-Buzalski T, Miettinen M, Hatakka M, Mikkola J, Anttila VJ, Johansson T, Rantala L, Aalto T, Korkeala H, Siitonen A. 2000. An outbreak of Listeria monocytogenes serotype 3a infections from butter in Finland. J Infect Dis 181:1838–1841 10.1086/315453. [PubMed] [DOI] [PubMed] [Google Scholar]
- 26.Angelo KM, Conrad AR, Saupe A, Dragoo H, West N, Sorenson A, Barnes A, Doyle M, Beal J, Jackson KA, Stroika S, Tarr C, Kucerova Z, Lance S, Gould LH, Wise M, Jackson BR. 2017. Multistate outbreak of Listeria monocytogenes infections linked to whole apples used in commercially produced, prepackaged caramel apples: United States, 2014-2015. Epidemiol Infect 145:848–856 10.1017/S0950268816003083. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaul LK, Farag NH, Shim T, Kingsley MA, Silk BJ, Hyytia-Trees E. 2013. Hospital-acquired listeriosis outbreak caused by contaminated diced celery: Texas, 2010. Clin Infect Dis 56:20–26 10.1093/cid/cis817. [PubMed] [DOI] [PubMed] [Google Scholar]
- 28.McCollum JT, Cronquist AB, Silk BJ, Jackson KA, O’Connor KA, Cosgrove S, Gossack JP, Parachini SS, Jain NS, Ettestad P, Ibraheem M, Cantu V, Joshi M, DuVernoy T, Fogg NW Jr, Gorny JR, Mogen KM, Spires C, Teitell P, Joseph LA, Tarr CL, Imanishi M, Neil KP, Tauxe RV, Mahon BE. 2013. Multistate outbreak of listeriosis associated with cantaloupe. N Engl J Med 369:944–953 10.1056/NEJMoa1215837. [PubMed] [DOI] [PubMed] [Google Scholar]
- 29.Jackson BR, Salter M, Tarr C, Conrad A, Harvey E, Steinbock L, Saupe A, Sorenson A, Katz L, Stroika S, Jackson KA, Carleton H, Kucerova Z, Melka D, Strain E, Parish M, Mody RK; Centers for Disease Control and Prevention (CDC). 2014. Listeriosis associated with stone fruit: United States, 2014. MMWR Morb Mortal Wkly Rep 64:282–283. [PMC free article] [PubMed] [Google Scholar]
- 30.Self JL, Conrad A, Stroika S, Jackson A, Burnworth L, Beal J, Wellman A, Jackson KA, Bidol S, Gerhardt T, Hamel M, Franklin K, Kopko C, Kirsch P, Wise ME, Basler C. 2016. Outbreak of listeriosis associated with consumption of packaged salad: United States and Canada. MMWR Morb Mortal Wkly Rep 65:879–881 10.15585/mmwr.mm6533a6. [PubMed] [DOI] [PubMed] [Google Scholar]
- 31.MMWR. 2002. Public health dispatch: outbreak of listeriosis: northeastern United States, 2002. MMWR Morb Mortal Wkly Rep 51:950–951. [PubMed] [Google Scholar]
- 32.de Valk H, Vaillant V, Jacquet C, Rocourt J, Le Querrec F, Stainer F, Quelquejeu N, Pierre O, Pierre V, Desenclos JC, Goulet V. 2001. Two consecutive nationwide outbreaks of listeriosis in France, October 1999-February 2000. Am J Epidemiol 154:944–950 10.1093/aje/154.10.944. [PubMed] [DOI] [PubMed] [Google Scholar]
- 33.Currie A, Farber JM, Nadon C, Sharma D, Whitfield Y, Gaulin C, Galanis E, Bekal S, Flint J, Tschetter L, Pagotto F, Lee B, Jamieson F, Badiani T, MacDonald D, Ellis A, May-Hadford J, McCormick R, Savelli C, Middleton D, Allen V, Tremblay FW, MacDougall L, Hoang L, Shyng S, Everett D, Chui L, Louie M, Bangura H, Levett PN, Wilkinson K, Wylie J, Reid J, Major B, Engel D, Douey D, Huszczynski G, Di Lecci J, Strazds J, Rousseau J, Ma K, Isaac L, Sierpinska U, National Outbreak Investigation Team. 2015. Multi-province listeriosis outbreak linked to contaminated deli meat consumed primarily in institutional settings, Canada, 2008. Foodborne Pathog Dis 12:645–652 10.1089/fpd.2015.1939. [PubMed] [DOI] [PubMed] [Google Scholar]
- 34.Smith B, Larsson JT, Lisby M, Müller L, Madsen SB, Engberg J, Bangsborg J, Ethelberg S, Kemp M. 2011. Outbreak of listeriosis caused by infected beef meat from a meals-on-wheels delivery in Denmark 2009. Clin Microbiol Infect 17:50–52 10.1111/j.1469-0691.2010.03200.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 35.Hachler H, Marti G, Giannini P, Lehner A, Jost M, Beck J, Weiss F, Bally B, Jermini M, Stephan R, Baumgartner A. 2013. Outbreak of listeriosis due to imported cooked ham. Euro Surveill 18:20469. [PubMed] [Google Scholar]
- 36.National Institute for Communicable Diseases. 2017. Situation report on listeriosis outbreak, South Africa, 2017. 4 Dec 2017:1–3 https://www.who.int/csr/don/02-may-2018-listeriosis-south-africa/en/.
- 37.Silk BJ, McCoy MH, Iwamoto M, Griffin PM. 2014. Foodborne listeriosis acquired in hospitals. Clin Infect Dis 59:532–540 10.1093/cid/ciu365. [PubMed] [DOI] [PubMed] [Google Scholar]
- 38.Datta AR, Burall LS. 2017. Serotype to genotype: the changing landscape of listeriosis outbreak investigations. Food Microbiol 75:18–27. [PubMed] [DOI] [PubMed] [Google Scholar]
- 39.Kwong JC, Mercoulia K, Tomita T, Easton M, Li HY, Bulach DM, Stinear TP, Seemann T, Howden BP. 2016. Prospective whole genome sequencing enhances national surveillance of Listeria monocytogenes. J Clin Microbiol 54:333–342 10.1128/JCM.02344-15. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schuchat A, Deaver KA, Wenger JD, Plikaytis BD, Mascola L, Pinner RW, Reingold AL, Broome CV, The Listeria Study Group. 1992. Role of foods in sporadic listeriosis. I. Case-control study of dietary risk factors. JAMA 267:2041–2045 10.1001/jama.1992.03480150047035. [PubMed] [DOI] [PubMed] [Google Scholar]
- 41.Grif K, Patscheider G, Dierich MP, Allerberger F. 2003. Incidence of fecal carriage of Listeria monocytogenes in three healthy volunteers: a one-year prospective stool survey. Eur J Clin Microbiol Infect Dis 22:16–20. [DOI] [PubMed] [Google Scholar]
- 42.Goulet V, de Valk H, Pierre O, Stainer F, Rocourt J, Vaillant V, Jacquet C, Desenclos JC. 2001. Effect of prevention measures on incidence of human listeriosis, France, 1987-1997. Emerg Infect Dis 7:983–989 10.3201/eid0706.010610. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allerberger F, Wagner M. 2010. Listeriosis: a resurgent foodborne infection. Clin Microbiol Infect 16:16–23 10.1111/j.1469-0691.2009.03109.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 44.Koopmans MM, Bijlsma MW, Brouwer MC, van de Beek D, van der Ende A. 2017. Listeria monocytogenes meningitis in the Netherlands, 1985-2014: a nationwide surveillance study. J Infect 75:12–19 10.1016/j.jinf.2017.04.004. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Centers for Disease Control and Prevention (CDC). 2013. Vital signs: Listeria illnesses, deaths, and outbreaks: United States, 2009-2011. MMWR Morb Mortal Wkly Rep 62:448–452. [PubMed] [PMC free article] [PubMed] [Google Scholar]
- 46.de Noordhout CM, Devleesschauwer B, Angulo FJ, Verbeke G, Haagsma J, Kirk M, Havelaar A, Speybroeck N. 2014. The global burden of listeriosis: a systematic review and meta-analysis. Lancet Infect Dis 14:1073–1082 10.1016/S1473-3099(14)70870-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas MK, Vriezen R, Farber JM, Currie A, Schlech W, Fazil A. 2015. Economic cost of a Listeria monocytogenes outbreak in Canada, 2008. Foodborne Pathog Dis 12:966–971 10.1089/fpd.2015.1965. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwartz B, Hexter D, Broome CV, Hightower AW, Hirschhorn RB, Porter JD, Hayes PS, Bibb WF, Lorber B, Faris DG. 1989. Investigation of an outbreak of listeriosis: new hypotheses for the etiology of epidemic Listeria monocytogenes infections. J Infect Dis 159:680–685 10.1093/infdis/159.4.680. [PubMed] [DOI] [PubMed] [Google Scholar]
- 49.Dalton CB, Merritt TD, Unicomb LE, Kirk MD, Stafford RJ, Lalor K, OzFoodNet Working Group. 2011. A national case-control study of risk factors for listeriosis in Australia. Epidemiol Infect 139:437–445 10.1017/S0950268810000944. [PubMed] [DOI] [PubMed] [Google Scholar]
- 50.Pouillot R, Hoelzer K, Jackson KA, Henao OL, Silk BJ. 2012. Relative risk of listeriosis in Foodborne Diseases Active Surveillance Network (FoodNet) sites according to age, pregnancy, and ethnicity. Clin Infect Dis 54(Suppl 5):S405–S410 10.1093/cid/cis269. [PubMed] [DOI] [PubMed] [Google Scholar]
- 51.Schlech WF III. 1993. An animal model of foodborne Listeria monocytogenes virulence: effect of alterations in local and systemic immunity on invasive infection. Clin Invest Med 16:219–225. [PubMed] [Google Scholar]
- 52.Bodro M, Paterson DL. 2013. Listeriosis in patients receiving biologic therapies. Eur J Clin Microbiol Infect Dis 32:1225–1230 10.1007/s10096-013-1873-1. [PubMed] [DOI] [PubMed] [Google Scholar]
- 53.Abreu C, Magro F, Vilas-Boas F, Lopes S, Macedo G, Sarmento A. 2013. Listeria infection in patients on anti-TNF treatment: report of two cases and review of the literature. J Crohn’s Colitis 7:175–182 10.1016/j.crohns.2012.04.018. [PubMed] [DOI] [PubMed] [Google Scholar]
- 54.Holmøy T, von der Lippe H, Leegaard TM. 2017. Listeria monocytogenes infection associated with alemtuzumab: a case for better preventive strategies. BMC Neurol 17:65–69 10.1186/s12883-017-0848-8. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sridama V, Pacini F, Yang SL, Moawad A, Reilly M, DeGroot LJ. 1982. Decreased levels of helper T cells: a possible cause of immunodeficiency in pregnancy. N Engl J Med 307:352–356 10.1056/NEJM198208053070606. [PubMed] [DOI] [PubMed] [Google Scholar]
- 56.Wald A, Van Thiel DH, Hoechstetter L, Gavaler JS, Egler KM, Verm R, Scott L, Lester R. 1982. Effect of pregnancy on gastrointestinal transit. Dig Dis Sci 27:1015–1018 10.1007/BF01391748. [PubMed] [DOI] [PubMed] [Google Scholar]
- 57.Schuchat A, Lizano C, Broome CV, Swaminathan B, Kim C, Winn K. 1991. Outbreak of neonatal listeriosis associated with mineral oil. Pediatr Infect Dis J 10:183–189 10.1097/00006454-199103000-00003. [PubMed] [DOI] [PubMed] [Google Scholar]
- 58.Girard D, Leclercq A, Laurent E, Lecuit M, de Valk H, Goulet V. 2014. Pregnancy-related listeriosis in France, 1984 to 2011, with a focus on 606 cases from 1999 to 2011. Euro Surveill 19:20909 10.2807/1560-7917.ES2014.19.38.20909. [PubMed] [DOI] [PubMed] [Google Scholar]
- 59.Madjunkov M, Chaudhry S, Ito S. 2017. Listeriosis during pregnancy. Arch Gynecol Obstet 296:143–152 10.1007/s00404-017-4401-1. [PubMed] [DOI] [PubMed] [Google Scholar]
- 60.Riedo FX, Pinner RW, Tosca ML, Cartter ML, Graves LM, Reeves MW, Weaver RE, Plikaytis BD, Broome CV. 1994. A point-source foodborne listeriosis outbreak: documented incubation period and possible mild illness. J Infect Dis 170:693–696 10.1093/infdis/170.3.693. [PubMed] [DOI] [PubMed] [Google Scholar]
- 61.Salamina G, Dalle Donne E, Niccolini A, Poda G, Cesaroni D, Bucci M, Fini R, Maldini M, Schuchat A, Swaminathan B, Bibb W, Rocourt J, Binkin N, Salmaso S. 1996. A foodborne outbreak of gastroenteritis involving Listeria monocytogenes. Epidemiol Infect 117:429–436 10.1017/S0950268800059082. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aureli P, Fiorucci GC, Caroli D, Marchiaro G, Novara O, Leone L, Salmaso S. 2000. An outbreak of febrile gastroenteritis associated with corn contaminated by Listeria monocytogenes. N Engl J Med 342:1236–1241 10.1056/NEJM200004273421702. [PubMed] [DOI] [PubMed] [Google Scholar]
- 63.Frye DM, Zweig R, Sturgeon J, Tormey M, LeCavalier M, Lee I, Lawani L, Mascola L. 2002. An outbreak of febrile gastroenteritis associated with delicatessen meat contaminated with Listeria monocytogenes. Clin Infect Dis 35:943–949 10.1086/342582. [PubMed] [DOI] [PubMed] [Google Scholar]
- 64.Sim J, Hood D, Finnie L, Wilson M, Graham C, Brett M, Hudson JA. 2002. Series of incidents of Listeria monocytogenes non-invasive febrile gastroenteritis involving ready-to-eat meats. Lett Appl Microbiol 35:409–413 10.1046/j.1472-765X.2002.01207.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 65.Pichler J, Much P, Kasper S, Fretz R, Auer B, Kathan J, Mann M, Huhulescu S, Ruppitsch W, Pietzka A, Silberbauer K, Neumann C, Gschiel E, de Martin A, Schuetz A, Gindl J, Neugschwandtner E, Allerberger F. 2009. An outbreak of febrile gastroenteritis associated with jellied pork contaminated with Listeria monocytogenes. Wien Klin Wochenschr 121:149–156 10.1007/s00508-009-1137-3. [PubMed] [DOI] [PubMed] [Google Scholar]
- 66.Jacks A, Pihlajasaari A, Vahe M, Myntti A, Kaukoranta SS, Elomaa N, Salmenlinna S, Rantala L, Lahti K, Huusko S, Kuusi M, Siitonen A, Rimhanen-Finne R. 2016. Outbreak of hospital-acquired gastroenteritis and invasive infection caused by Listeria monocytogenes, Finland, 2012. Epidemiol Infect 144:2732–2742 10.1017/S0950268815002563. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jurado RL, Farley MM, Pereira E, Harvey RC, Schuchat A, Wenger JD, Stephens DS. 1993. Increased risk of meningitis and bacteremia due to Listeria monocytogenes in patients with human immunodeficiency virus infection. Clin Infect Dis 17:224–227 10.1093/clinids/17.2.224. [PubMed] [DOI] [PubMed] [Google Scholar]
- 68.Ewert DP, Lieb L, Hayes PS, Reeves MW, Mascola L. 1995. Listeria monocytogenes infection and serotype distribution among HIV-infected persons in Los Angeles County, 1985-1992. J Acquir Immune Defic Syndr Hum Retrovirol 8:461–465 10.1097/00042560-199504120-00005. [DOI] [PubMed] [Google Scholar]
- 69.Bennion JR, Sorvillo F, Wise ME, Krishna S, Mascola L. 2008. Decreasing listeriosis mortality in the United States, 1990-2005. Clin Infect Dis 47:867–874 10.1086/591131. [PubMed] [DOI] [PubMed] [Google Scholar]
- 70.Tappero JW, Schuchat A, Deaver KA, Mascola L, Wenger JD. 1995. Reduction in the incidence of human listeriosis in the United States. Effectiveness of prevention efforts? The Listeriosis Study Group. JAMA 273:1118–1122 10.1001/jama.1995.03520380054035. [PubMed] [DOI] [PubMed] [Google Scholar]
- 71.Buchanan RL, Whiting RC. 1998. Risk assessment: a means for linking HACCP plans and public health. J Food Prot 61:1531–1534 10.4315/0362-028X-61.11.1531. [PubMed] [DOI] [PubMed] [Google Scholar]
- 72.Majewski MC. 1992. Food safety: the HACCP approach to hazard control. Commun Dis Rep CDR Rev 2:R105–R108. [PubMed] [Google Scholar]
- 73.Panisello PJ, Rooney R, Quantick PC, Stanwell-Smith R. 2000. Application of foodborne disease outbreak data in the development and maintenance of HACCP systems. Int J Food Microbiol 59:221–234 10.1016/S0168-1605(00)00376-7. [DOI] [PubMed] [Google Scholar]
- 74.McLauchlin J, Mitchell RT, Smerdon WJ, Jewell K. 2004. Listeria monocytogenes and listeriosis: a review of hazard characterisation for use in microbiological risk assessment of foods. Int J Food Microbiol 92:15–33 10.1016/S0168-1605(03)00326-X. [DOI] [PubMed] [Google Scholar]
- 75.Thompson P, Salsbury PA, Adams C, Archer DL. 1990. US food legislation. Lancet 336:1557–1559 10.1016/0140-6736(90)93320-O. [DOI] [PubMed] [Google Scholar]
- 76.Donnelly CW. 2001. Listeria monocytogenes: a continuing challenge. Nutr Rev 59:183–194 10.1111/j.1753-4887.2001.tb07011.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 77.Lomonaco S, Nucera D, Filipello V. 2015. The evolution and epidemiology of Listeria monocytogenes in Europe and the United States. Infect Genet Evol 35:172–183 10.1016/j.meegid.2015.08.008. [PubMed] [DOI] [PubMed] [Google Scholar]
- 78.Pirie JHH. 1927. A new disease of veld rodents, Tiger River disease. Publ S Afr Inst Med Res 3:163–186. [Google Scholar]
- 79.Murray EGD, Webb RA, Swann MBR. 1926. A disease of rabbits characterized by large mononuclear leucocytosis, caused by a hitherto undescribed bacillus: Bacterium monocytogenes. J Pathol Bacteriol 29:407–439 10.1002/path.1700290409. [DOI] [Google Scholar]
- 80.Shum DT, Galsworthy SB. 1982. Stimulation of monocyte production by an endogenous mediator induced by a component from Listeria monocytogenes. Immunology 46:343–351. [PMC free article] [PubMed] [Google Scholar]
- 81.Gill DA. 1937. Ovine bacterial encephalitis (circling disease) and the bacterial genus Listerella. Aust Vet J 13:46–56 10.1111/j.1751-0813.1937.tb01148.x. [DOI] [Google Scholar]
- 82.Lammerding AM, Glass KA, Gendron-Fitzpatrick A, Doyle MP. 1992. Determination of virulence of different strains of Listeria monocytogenes and Listeria innocua by oral inoculation of pregnant mice. Appl Environ Microbiol 58:3991–4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Potel J. 1952. Granulomatosis infantiseptica. Zentralbl Bakteriol Orig 158:329–332. [PubMed] [Google Scholar]
- 84.Dawson KG, Emerson JC, Burns JL. 1999. Fifteen years of experience with bacterial meningitis. Pediatr Infect Dis J 18:816–822 10.1097/00006454-199909000-00014. [PubMed] [DOI] [PubMed] [Google Scholar]
- 85.Okike IO, Johnson AP, Henderson KL, Blackburn RM, Muller-Pebody B, Ladhani SN, Anthony M, Ninis N, Heath PT, Galiza EP, Cameron JC, Smith-Palmer A, McDonald E, Sinka K, Jones L, Cunney R, Borgulya G, Borrow R. 2014. Incidence, etiology, and outcome of bacterial meningitis in infants aged <90 days in the United Kingdom and Republic of Ireland: prospective, enhanced, national population-based surveillance. Clin Infect Dis 59:150–157 10.1093/cid/ciu514. [PubMed] [DOI] [PubMed] [Google Scholar]
- 86.Baltimore RS, Huie SM, Meek JI, Schuchat A, O’Brien KL. 2001. Early-onset neonatal sepsis in the era of group B streptococcal prevention. Pediatrics 108:1094–1098 10.1542/peds.108.5.1094. [PubMed] [DOI] [PubMed] [Google Scholar]
- 87.Mylonakis E, Paliou M, Hohmann EL, Calderwood SB, Wing EJ. 2002. Listeriosis during pregnancy: a case series and review of 222 cases. Medicine (Baltimore) 81:260–269 10.1097/00005792-200207000-00002. [PubMed] [DOI] [PubMed] [Google Scholar]
- 88.Charlier-Woerther C, Lecuit M. 2014. Listeriosis and pregnancy. Presse Med 43:676–682 10.1016/j.lpm.2014.03.006. (In French.) [PubMed] [DOI] [PubMed] [Google Scholar]
- 89.Kessler SL, Dajani AS. 1990. Listeria meningitis in infants and children. Pediatr Infect Dis J 9:61–63 10.1097/00006454-199001000-00016. [PubMed] [DOI] [PubMed] [Google Scholar]
- 90.Mylonakis E, Hohmann EL, Calderwood SB. 1998. Central nervous system infection with Listeria monocytogenes. 33 years’ experience at a general hospital and review of 776 episodes from the literature. Medicine (Baltimore) 77:313–336 10.1097/00005792-199809000-00002. [PubMed] [DOI] [PubMed] [Google Scholar]
- 91.Armstrong RW, Fung PC. 1993. Brainstem encephalitis due to Listeria monocytogenes: case report and review. Clin Infect Dis 16:689–702 10.1093/clind/16.5.689. [PubMed] [DOI] [PubMed] [Google Scholar]
- 92.Eckburg PB, Montoya JG, Vosti KL. 2001. Brain abscess due to Listeria monocytogenes: five cases and a review of the literature. Medicine (Baltimore) 80:223–235 10.1097/00005792-200107000-00001. [PubMed] [DOI] [PubMed] [Google Scholar]
- 93.Goulet V, Marchetti P. 1996. Listeriosis in 225 non-pregnant patients in 1992: clinical aspects and outcome in relation to predisposing conditions. Scand J Infect Dis 28:367–374 10.3109/00365549609037921. [PubMed] [DOI] [PubMed] [Google Scholar]
- 94.Rivero GA, Torres HA, Rolston KVI, Kontoyiannis DP. 2003. Listeria monocytogenes infection in patients with cancer. Diagn Microbiol Infect Dis 47:393–398 10.1016/S0732-8893(03)00116-0. [DOI] [PubMed] [Google Scholar]
- 95.Huang SL, Chou YT, Hsieh YC, Huang YC, Lin TY, Chiu CH. 2010. Epidemiology and clinical characteristics of Listeria monocytogenes bacteremia in a Taiwanese medical center. J Microbiol Immunol Infect 43:485–490 10.1016/S1684-1182(10)60075-8. [DOI] [PubMed] [Google Scholar]
- 96.McLauchlin J, Low JC. 1994. Primary cutaneous listeriosis in adults: an occupational disease of veterinarians and farmers. Vet Rec 135:615–617. [PubMed] [Google Scholar]
- 97.Zelenik K, Avberšek J, Pate M, Lušicky M, Krt B, Ocepek M, Zdovc I. 2014. Cutaneous listeriosis in a veterinarian with the evidence of zoonotic transmission: a case report. Zoonoses Public Health 61:238–241 10.1111/zph.12075. [PubMed] [DOI] [PubMed] [Google Scholar]
- 98.Godshall CE, Suh G, Lorber B. 2013. Cutaneous listeriosis. J Clin Microbiol 51:3591–3596 10.1128/JCM.01974-13. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hof H. 2017. Listeria infections of the eye. Eur J Ophthalmol 27:115–121 10.5301/ejo.5000884. [PubMed] [DOI] [PubMed] [Google Scholar]
- 100.Fernández Guerrero ML, Rivas P, Rábago R, Núñez A, de Górgolas M, Martinell J. 2004. Prosthetic valve endocarditis due to Listeria monocytogenes. Report of two cases and reviews. Int J Infect Dis 8:97–102 10.1016/j.ijid.2003.06.002. [PubMed] [DOI] [PubMed] [Google Scholar]
- 101.Spyrou N, Anderson M, Foale R. 1997. Listeria endocarditis: current management and patient outcome: world literature review. Heart 77:380–383 10.1136/hrt.77.4.380. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.García-Granja PE, López J, Vilacosta I, Olmos C, Sarriá C, San Román JA. 2016. Infective endocarditis due to Listeria monocytogenes: a report of 4 patients. Rev Esp Cardiol (Engl Ed) 69:700–702 10.1016/j.rec.2016.04.006. [PubMed] [DOI] [PubMed] [Google Scholar]
- 103.Gauto AR, Cone LA, Woodard DR, Mahler RJ, Lynch RD, Stoltzman DH. 1992. Arterial infections due to Listeria monocytogenes: report of four cases and review of world literature. Clin Infect Dis 14:23–28 10.1093/clinids/14.1.23. [PubMed] [DOI] [PubMed] [Google Scholar]
- 104.Bourgeois N, Jacobs F, Tavares ML, Rickaert F, Deprez C, Liesnard C, Moonens F, Van de Stadt J, Gelin M, Adler M. 1993. Listeria monocytogenes hepatitis in a liver transplant recipient: a case report and review of the literature. J Hepatol 18:284–289 10.1016/S0168-8278(05)80271-5. [DOI] [PubMed] [Google Scholar]
- 105.Vargas V, Alemán C, de Torres I, Castells L, Gavaldá J, Margarit C, Esteban R, Guardia J. 1998. Listeria monocytogenes-associated acute hepatitis in a liver transplant recipient. Liver 18:213–215 10.1111/j.1600-0676.1998.tb00153.x. [DOI] [PubMed] [Google Scholar]
- 106.Braun TI, Travis D, Dee RR, Nieman RE. 1993. Liver abscess due to Listeria monocytogenes: case report and review. Clin Infect Dis 17:267–269 10.1093/clinids/17.2.267. [PubMed] [DOI] [PubMed] [Google Scholar]
- 107.Brönnimann S, Baer HU, Malinverni R, Büchler MW. 1998. Listeria monocytogenes causing solitary liver abscess. Case report and review of the literature. Dig Surg 15:364–368 10.1159/000018633. [PubMed] [DOI] [PubMed] [Google Scholar]
- 108.Sivalingam JJ, Martin P, Fraimow HS, Yarze JC, Friedman LS. 1992. Listeria monocytogenes peritonitis: case report and literature review. Am J Gastroenterol 87:1839–1845. [PubMed] [Google Scholar]
- 109.Bierhoff M, Krutwagen E, van Bommel EFH, Verburgh CA. 2011. Listeria peritonitis in patients on peritoneal dialysis: two cases and a review of the literature. Neth J Med 69:461–464. [PubMed] [Google Scholar]
- 110.Moscovici A, Kogan M, Kliers I, Kukuy O, Segal G. 2016. Listeria peritonitis in a patient treated with peritoneal dialysis. Isr Med Assoc J 18:129–130. [PubMed] [Google Scholar]
- 111.Jayaraj K, Di Bisceglie AM, Gibson S. 1998. Spontaneous bacterial peritonitis caused by infection with Listeria monocytogenes: a case report and review of the literature. Am J Gastroenterol 93:1556–1558 10.1111/j.1572-0241.1998.00482.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 112.El Sayed Zaki M, El Shabrawy WO, El-Eshmawy MM, Aly Eletreby S. 2011. The high prevalence of Listeria monocytogenes peritonitis in cirrhotic patients of an Egyptian medical center. J Infect Public Health 4:211–216 10.1016/j.jiph.2011.06.002. [PubMed] [DOI] [PubMed] [Google Scholar]
- 113.How J, Azar MM, Meyer JP. 2015. Are nectarines to blame?: a case report and literature review of spontaneous bacterial peritonitis due to Listeria monocytogenes. Conn Med 79:31–36. [PMC free article] [PubMed] [Google Scholar]
- 114.Charlier C, Fevre C, Travier L, Cazenave B, Bracq-Dieye H, Podevin J, Assomany D, Guilbert L, Bossard C, Carpentier F, Cales V, Leclercq A, Lecuit M. 2014. Listeria monocytogenes-associated biliary tract infections: a study of 12 consecutive cases and review. Medicine (Baltimore) 93:e105 10.1097/MD.0000000000000105. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Descy J, De Mol P, Hayette MP, Huynen P, Meex C, Melin P. 2012. Acute cholecystitis with Listeria monocytogenes. Acta Clin Belg 67:295–297. [DOI] [PubMed] [Google Scholar]
- 116.Louthrenoo W, Schumacher HR Jr. 1990. Listeria monocytogenes osteomyelitis complicating leukemia: report and literature review of Listeria osteoarticular infections. J Rheumatol 17:107–110. [PubMed] [Google Scholar]
- 117.Charlier C, Leclercq A, Cazenave B, Desplaces N, Travier L, Cantinelli T, Lortholary O, Goulet V, Le Monnier A, Lecuit M. 2012. Listeria monocytogenes: associated joint and bone infections: a study of 43 consecutive cases. Clin Infect Dis 54:240–248 10.1093/cid/cir803. [PubMed] [DOI] [PubMed] [Google Scholar]
- 118.Del Pozo JL, de la Garza RG, de Rada PD, Ornilla E, Yuste JR. 2013. Listeria monocytogenes septic arthritis in a patient treated with mycophenolate mofetil for polyarteritis nodosa: a case report and review of the literature. Int J Infect Dis 17:e132–e133 10.1016/j.ijid.2012.11.005. [PubMed] [DOI] [PubMed] [Google Scholar]
- 119.Bader G, Al-Tarawneh M, Myers J. 2016. Review of prosthetic joint infection from Listeria monocytogenes. Surg Infect (Larchmt) 17:739–744 10.1089/sur.2016.067. [PubMed] [DOI] [PubMed] [Google Scholar]
- 120.Seo Y, Noh YS, Wie SH, Chang UI. 2016. Prosthetic knee joint infection due to Listeria monocytogenes bacteremia in a diabetic female. Korean J Intern Med 31:616–619 10.3904/kjim.2014.361. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Khan KM, Pao W, Kendler J. 2001. Epidural abscess and vertebral osteomyelitis caused by Listeria monocytogenes: case report and literature review. Scand J Infect Dis 33:714–716 10.1080/00365540110027033. [DOI] [PubMed] [Google Scholar]
- 122.Lorber B. 1991. Listeriosis following shigellosis. Rev Infect Dis 13:865–866 10.1093/clinids/13.5.865. [DOI] [PubMed] [Google Scholar]
- 123.Bäckman A, Lantz P, Rådström P, Olcén P. 1999. Evaluation of an extended diagnostic PCR assay for detection and verification of the common causes of bacterial meningitis in CSF and other biological samples. Mol Cell Probes 13:49–60 10.1006/mcpr.1998.0218. [PubMed] [DOI] [PubMed] [Google Scholar]
- 124.Le Monnier A, Abachin E, Beretti JL, Berche P, Kayal S. 2011. Diagnosis of Listeria monocytogenes meningoencephalitis by real-time PCR for the hly gene. J Clin Microbiol 49:3917–3923 10.1128/JCM.01072-11. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hof H, Nichterlein T, Kretschmar M. 1997. Management of listeriosis. Clin Microbiol Rev 10:345–357 10.1128/CMR.10.2.345. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Safdar A, Armstrong D. 2003. Antimicrobial activities against 84 Listeria monocytogenes isolates from patients with systemic listeriosis at a comprehensive cancer center (1955-1997). J Clin Microbiol 41:483–485 10.1128/JCM.41.1.483-485.2003. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Morvan A, Moubareck C, Leclercq A, Hervé-Bazin M, Bremont S, Lecuit M, Courvalin P, Le Monnier A. 2010. Antimicrobial resistance of Listeria monocytogenes strains isolated from humans in France. Antimicrob Agents Chemother 54:2728–2731 10.1128/AAC.01557-09. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Okike IO, Awofisayo A, Adak B, Heath PT. 2015. Empirical antibiotic cover for Listeria monocytogenes infection beyond the neonatal period: a time for change? Arch Dis Child 100:423–425 10.1136/archdischild-2014-307059. [PubMed] [DOI] [PubMed] [Google Scholar]
- 129.Lorber B. 1997. Listeriosis. Clin Infect Dis 24:1–9, quiz 10–11 10.1093/clinids/24.1.1. [PubMed] [DOI] [PubMed] [Google Scholar]
- 130.Temple ME, Nahata MC. 2000. Treatment of listeriosis. Ann Pharmacother 34:656–661 10.1345/aph.19315. [PubMed] [DOI] [PubMed] [Google Scholar]
- 131.Mitjà O, Pigrau C, Ruiz I, Vidal X, Almirante B, Planes AM, Molina I, Rodríguez D, Pahissa A. 2009. Predictors of mortality and impact of aminoglycosides on outcome in listeriosis in a retrospective cohort study. J Antimicrob Chemother 64:416–423 10.1093/jac/dkp180. [PubMed] [DOI] [PubMed] [Google Scholar]
- 132.MacGowan A, Wootton M, Bowker K, Holt HA, Reeves D. 1998. Ampicillin-aminoglycoside interaction studies using Listeria monocytogenes. J Antimicrob Chemother 41:417–418 10.1093/jac/41.3.417. [PubMed] [DOI] [PubMed] [Google Scholar]
- 133.Grant MH, Ravreby H, Lorber B. 2010. Cure of Listeria monocytogenes meningitis after early transition to oral therapy. Antimicrob Agents Chemother 54:2276–2277 10.1128/AAC.01815-09. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Merle-Melet M, Dossou-Gbete L, Maurer P, Meyer P, Lozniewski A, Kuntzburger O, Wéber M, Gérard A. 1996. Is amoxicillin-cotrimoxazole the most appropriate antibiotic regimen for Listeria meningoencephalitis? Review of 22 cases and the literature. J Infect 33:79–85 10.1016/S0163-4453(96)92929-1. [DOI] [PubMed] [Google Scholar]
- 135.Bertsch D, Muelli M, Weller M, Uruty A, Lacroix C, Meile L. 2014. Antimicrobial susceptibility and antibiotic resistance gene transfer analysis of foodborne, clinical, and environmental Listeria spp. isolates including Listeria monocytogenes. Microbiologyopen 3:118–127 10.1002/mbo3.155. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Marco F, Almela M, Nolla-Salas J, Coll P, Gasser I, Ferrer MD, de Simon M, The Collaborative Study Group of Listeriosis of Barcelona. 2000. In vitro activities of 22 antimicrobial agents against Listeria monocytogenes strains isolated in Barcelona, Spain. Diagn Microbiol Infect Dis 38:259–261 10.1016/S0732-8893(00)00208-X. [DOI] [PubMed] [Google Scholar]
- 137.Pupo I, Lepe JA, Smani Y, Aznar J. 2017. Comparison of the in vitro activity of ampicillin and moxifloxacin against Listeria monocytogenes at achievable concentrations in the central nervous system. J Med Microbiol 66:713–720 10.1099/jmm.0.000486. [PubMed] [DOI] [PubMed] [Google Scholar]
- 138.Chenal-Francisque V, Charlier C, Mehvish S, Dieye H, Leclercq A, Courvalin P, Lecuit M. 2014. Highly rifampin-resistant Listeria monocytogenes isolated from a patient with prosthetic bone infection. Antimicrob Agents Chemother 58:1829–1830 10.1128/AAC.02449-13. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Isnard C, Fines-Guyon M, Toublet FX, Guerin F, Rogowski C, Vergnaud M, Cattoir V. 2016. In vivo emergence of rifampicin resistance by rpoB mutation in Listeria monocytogenes during therapy of prosthetic joint infection. Int J Antimicrob Agents 48:572–574 10.1016/j.ijantimicag.2016.07.016. [PubMed] [DOI] [PubMed] [Google Scholar]
- 140.Pelegrín I, Moragas M, Suárez C, Ribera A, Verdaguer R, Martínez-Yelamos S, Rubio-Borrego F, Ariza J, Viladrich PF, Cabellos C. 2014. Listeria monocytogenes meningoencephalitis in adults: analysis of factors related to unfavourable outcome. Infection 42:817–827 10.1007/s15010-014-0636-y. [PubMed] [DOI] [PubMed] [Google Scholar]
- 141.Rocourt J, Hogue A, Toyofuku H, Jacquet C, Schlundt J. 2001. Listeria and listeriosis: risk assessment as a new tool to unravel a multifaceted problem. Am J Infect Control 29:225–227 10.1067/mic.2001.115681. [PubMed] [DOI] [PubMed] [Google Scholar]
- 142.Bruminhent J, Lynch TK, Gefen J, Santoro J. 2013. Listeria monocytogenes cholecystitis: a possible new syndrome. Am J Med Sci 345:414–417 10.1097/MAJ.0b013e3182761cda. [PubMed] [DOI] [PubMed] [Google Scholar]
- 143.Ooi ST, Lorber B. 2005. Gastroenteritis due to Listeria monocytogenes. Clin Infect Dis 40:1327–1332 10.1086/429324. [PubMed] [DOI] [PubMed] [Google Scholar]


