Abstract
We previously showed that SerpinE2 and the serine protease HtrA1 modulate fibroblast growth factor (FGF) signaling in germ layer specification and head-to-tail development of Xenopus embryos. Here, we present an extracellular proteolytic mechanism involving this serpin-protease system in the developing neural crest (NC). Knockdown of SerpinE2 by injected antisense morpholino oligonucleotides did not affect the specification of NC progenitors but instead inhibited the migration of NC cells, causing defects in dorsal fin, melanocyte, and craniofacial cartilage formation. Similarly, overexpression of the HtrA1 protease impaired NC cell migration and the formation of NC-derived structures. The phenotype of SerpinE2 knockdown was overcome by concomitant downregulation of HtrA1, indicating that SerpinE2 stimulates NC migration by inhibiting endogenous HtrA1 activity. SerpinE2 binds to HtrA1, and the HtrA1 protease triggers degradation of the cell surface proteoglycan Syndecan-4 (Sdc4). Microinjection of Sdc4 mRNA partially rescued NC migration defects induced by both HtrA1 upregulation and SerpinE2 downregulation. These epistatic experiments suggest a proteolytic pathway by a double inhibition mechanism:
SerpinE2 ┤HtrA1 protease ┤Syndecan-4 → NC cell migration.
Research organism: Xenopus
Introduction
Collective cell migration is a fundamental process in the development and maintenance of multicellular organisms (Friedl and Gilmour, 2009; Shellard and Mayor, 2020). Embryonic development relies on the coordinated movement of cells to specific locations, and aberrant cell migration is linked to cancer metastasis. The acquisition of cell motility is associated with an epithelial-mesenchymal transition (EMT), in which cells disrupt epithelial adhesions and remodel junctional complexes in favor of cell-matrix adhesions to adopt a migratory behavior (Nieto et al., 2016; Piacentino et al., 2020). The EMT does not have to be complete, and cells can be motile while maintaining contact with one another. A prime example for the study of collective cell migration is the neural crest (NC) in vertebrate embryos (Szabó and Mayor, 2018). The NC is a multipotent cell population that is specified at the border of the neural plate (Le Douarin and Kalcheim, 1999; Schock et al., 2023). EMT initiates migration streams of NC cells toward their targets where they differentiate into diverse cell types and tissues, including peripheral nervous system, melanocytes, and craniofacial skeleton. The behavior of NC cells recapitulates certain stages of cancer progression and metastasis (Nieto et al., 2016). Defects in NC development can lead to many congenital syndromes and tumors of the NC lineage (Medina-Cuadra and Monsoro-Burq, 2021). These neurocristopathies highlight the need to better understand the molecular basis of key processes in NC development, including EMT and collective migration.
HtrA1 belongs to a conserved family of serine proteases that are homologous to the heat shock-induced HtrA (high temperature requirement A) peptidase from bacteria and primarily involved in protein quality control and degradation (Zurawa-Janicka et al., 2017). Vertebrate HtrA proteases, comprising the four members HtrA1–4, share a trypsin serine protease domain and a carboxyterminal PDZ domain with their bacterial counterpart. The HtrA family is implicated in various pathological conditions including cancer, arthritis, neurodegenerative diseases, and pregnancy disorders. HtrA1 modulates the extracellular matrix and cell signaling as a secreted protein but was found to be active in the cytoplasm and nucleus, too (Clawson et al., 2008; Chien et al., 2009a; Campioni et al., 2010). The function of HtrA1 in cell migration has been studied in vitro to reveal mainly a negative role, although also a positive role has been reported (Pei et al., 2015). How HtrA1 activities are regulated is poorly understood, and the mechanism by which this protease affects cell migration in vivo remains elusive.
Members of the serpin superfamily contain a carboxyterminal reactive center loop (RCL) that covalently binds to and inhibits target serine proteases inside and outside of cells (Olson and Gettins, 2011). Serpin peptidase inhibitor clade E member 2 (SerpinE2), also known as protease nexin-1 (PN1) or glia-derived nexin, has important roles in the nervous, blood, and reproductive systems (Arocas and Bouton, 2015; Monard, 2017). SerpinE2 also functions as a key factor in tumor dissemination, but the molecular mechanism by which this protease inhibitor governs cell migration and metastasis is largely unknown.
We previously showed that HtrA1 and SerpinE2 are transcriptionally induced by fibroblast growth factor (FGF) signals and act as feedback regulators of FGF/Erk signaling in germ layer and anteroposterior axis formation in the early Xenopus embryo (Hou et al., 2007; Acosta et al., 2015). HtrA1 activates Erk (extracellular signal-regulated kinase) and expression of the transcription factor Brachyury in the posterior mesoderm, whereas SerpinE2 suppresses Erk activation and Brachyury expression in the anterior ectoderm. The HtrA1 protease releases FGF ligands by triggering the cleavage of cell surface proteoglycans such as Syndecan-4 (Sdc4), thereby stimulating FGF/Erk signaling in mesoderm and trunk/tail formation (Hou et al., 2007). SerpinE2 binds to and inhibits HtrA1, thus restricting FGF/Erk signaling and allowing ectoderm and head formation to occur (Acosta et al., 2015). Since SerpinE2 and HtrA1 exhibit overlapping gene expression in the NC, we now asked whether these proteins might have a role in NC development.
Here, we introduce SerpinE2 as a key player in collective NC cell migration. We show that SerpinE2 promotes NC cell migration via inhibition of the secreted serine protease HtrA1. SerpinE2 de-represses the HtrA1-mediated block of NC migration in mRNA-injected embryos, and the ability of this protease inhibitor to rescue cell migration depends on its extracellular location and intact RCL. In epistatic experiments, Sdc4 mRNA can partly revert the NC migration defects that are induced by SerpinE2 knockdown or HtrA1 overexpression. We conclude that the SerpinE2/HtrA1/Sdc4 pathway regulates NC cell migration in the developing embryo.
Results
SerpinE2 and HtrA1 are expressed in NC cells
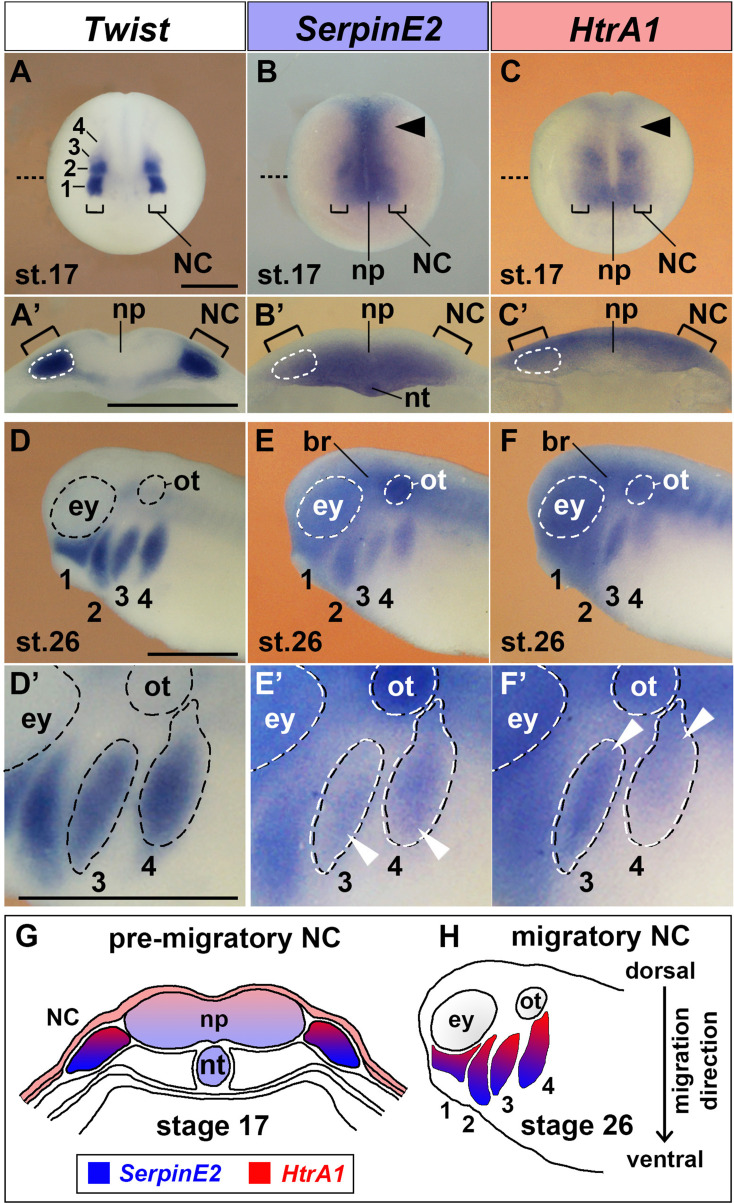
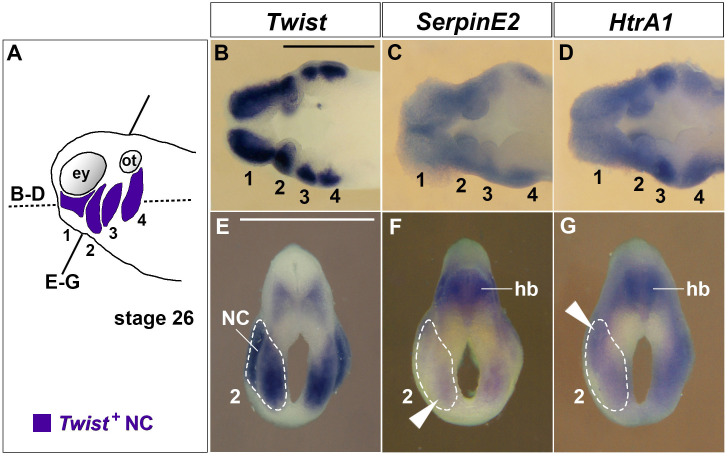
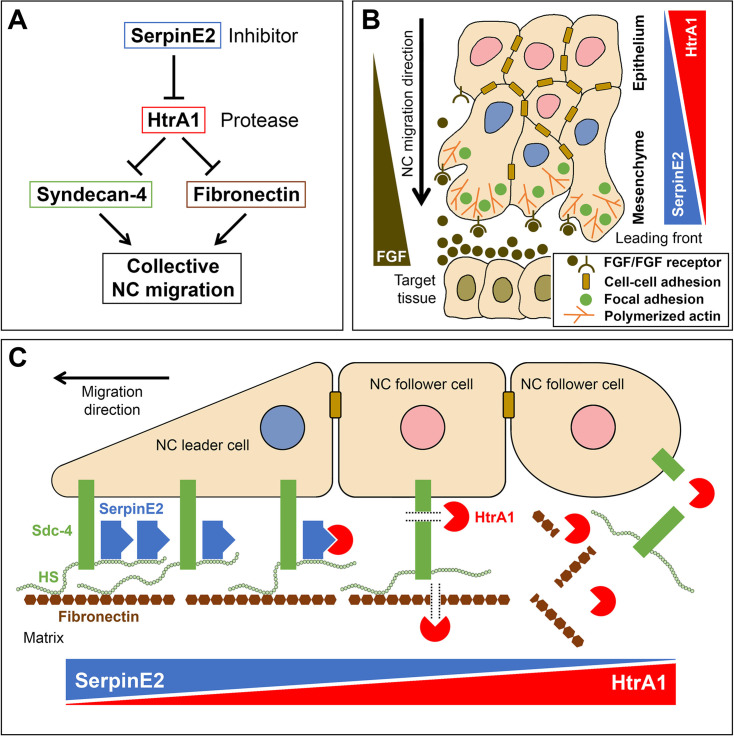
The expression of SerpinE2 and HtrA1 in early Xenopus embryos was previously reported by us and others (Pera et al., 2005; Onuma et al., 2006; Hou et al., 2007; Acosta et al., 2015). To investigate whether SerpinE2 and HtrA1 are expressed in the developing NC, we performed whole-mount in situ hybridization analysis of these genes side by side with the NC marker Twist (Figure 1). At neurula stage 17, Twist expression labeled pre-migratory NC cells in the deep layer of the ectoderm (Figure 1A and A’; Hopwood et al., 1989). SerpinE2 transcripts were found in the deep layer of the neural plate and adjacent NC cells (Figure 1B and B’). HtrA1 was transcribed in the superficial layer of the neural plate and in the NC (Figure 1C and C’). At stage 26, SerpinE2 and HtrA1 were co-expressed with Twist in ventrally migrating NC cells in the mandibular, hyoid, anterior branchial, and posterior branchial streams (numbered as 1–4 in Figure 1D–F; see also Figure 1—figure supplement 1A–D). Importantly, SerpinE2 transcripts accumulated at the ventral leading front, while HtrA1 expression was abundant in more dorsal follower cells within the migrating NC cohorts (Figure 1D’–F’; see also Figure 1—figure supplement 1E–G). In addition, SerpinE2 and HtrA1 shared overlapping expression in the brain, eye vesicles, and otic placodes (Figure 1E and F; see also Figure 1—figure supplement 1F and G). These results showed that SerpinE2 and HtrA1 are expressed in pre-migratory NC cells and adjacent tissues (Figure 1G), and that the SerpinE2 inhibitor transcripts prevail in the leading edge and the HtrA1 protease expression is predominant in the following migratory NC cells (Figure 1H).
Figure 1. SerpinE2 and HtrA1 are expressed in the neural crest.
Xenopus embryos were analyzed by whole-mount in situ hybridization. (A–C) Anterior view of embryos at stage 17. The brackets point to pre-migratory NC cells on each side of the neural plate. The numbers label the Twist-expressing cranial NC segments: 1, mandibular; 2, hyoid; 3, anterior branchial; 4, posterior branchial. Arrowheads show SerpinE2 and HtrA1 transcripts in the trunk NC. The stippled lines indicate the level of sections in A’–C’. (A’–C’) Transversally hemisectioned embryos. SerpinE2 and HtrA1 signals appear in the NC (strippled circle lines). Note that SerpinE2 is also expressed in the inner sensorial layer of the neural plate and underlying notochord, whereas HtrA1 expression is more abundant in the outer ependymal layer of the neural plate. (D–F) Lateral view of embryos at stage 26. SerpinE2 and HtrA1 are expressed in Twist+ NC cell streams (1–4). Transcripts of both genes can also be seen in the brain, eye, and otic placode. (D’–F’) Magnification of embryos. Arrowheads demarcate SerpinE2 transcripts near the front (E’) and HtrA1 transcripts at the rear end (F’) of the migrating NC cell collectives in the branchial arches. (G, H) Summary of gene expression domains. At stage 17, SerpinE2 is transcribed in ventral and HtrA1 in dorsal cells of the pre-migratory NC (G). At stage 26, SerpinE2 is expressed in leader cells and HtrA1 in follower cells of migrating NC streams (H).br, brain; ey, eye; NC, neural crest; np, neural plate; nt, notochord; ot, otic placode. Scale bars, 0.5 mm.
Figure 1—figure supplement 1. Twist, SerpinE2, and HtrA1 gene expression in sections of tailbud stage embryos after whole-mount in situ hybridization.
SerpinE2 knockdown reproduces the phenotype of NC extirpation in Xenopus embryos
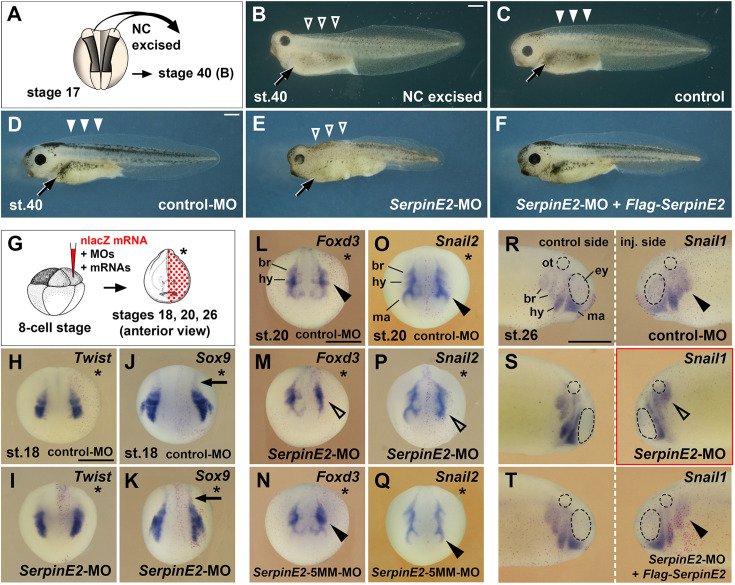
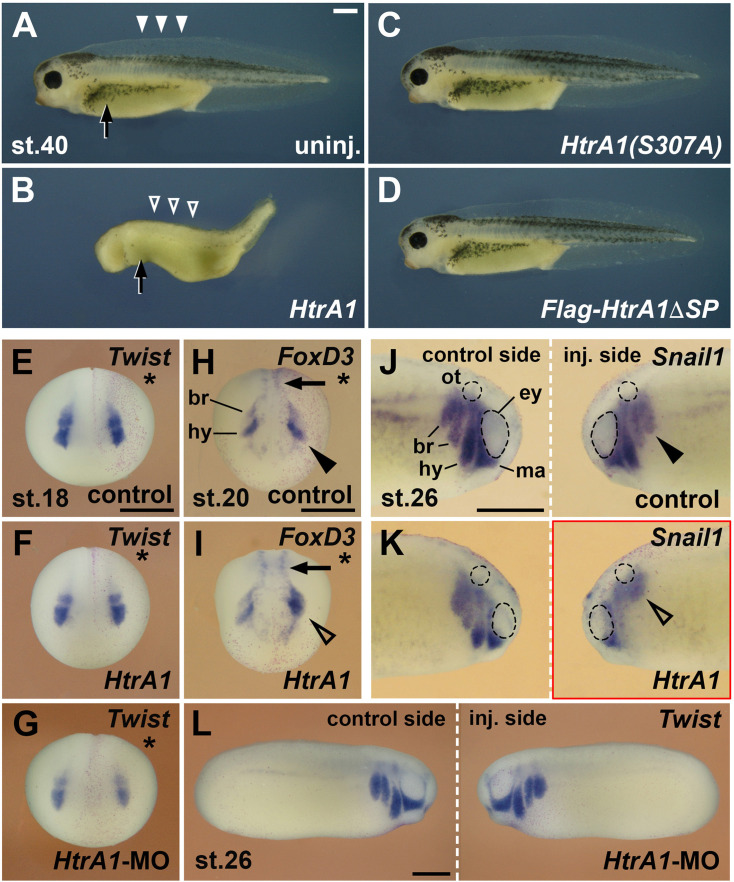
Classical extirpation experiments carried out in the urodele Amblystoma demonstrated that the cranial NC is important for the formation of the head tissue (Stone, 1922; Stone, 1926) and the trunk NC for dorsal fin and melanocyte development (DuShane, 1935). We reproduced these findings in Xenopus and showed that bilateral removal of the neural folds (anlagen of the NC) in the cranial and anterior trunk region of a mid-neurula embryo at stage 17 resulted in larvae displaying a reduced head size, missing dorsal fin (open arrowheads), and reduced melanocyte pigmentation (arrow) (Figure 2A–C).
Figure 2. Knockdown of SerpinE2 mimics the phenotype of neural excision and inhibits migration of neural crest cells.
(A) Scheme of extirpation. Dorsal view of Xenopus embryo at stage 17, from which NC tissue was removed on both sides. (B, C) Tadpole embryo at stage 40 following NC excision (B) and sibling control (C). Note the small head, absence of dorsal fin tissue (open arrowheads), and the reduced number of melanocytes (arrow) resulting from NC extirpation in A. (D) Unaffected tadpole after microinjection with control-MO into all animal blastomeres at the eight-cell stage. Highlighted are the pigmented melanocytes (arrow) and the intact dorsal fin (filled arrowheads). (E) SerpinE2-MO causes a reduction of head tissue, dorsal fin structures (open arrowheads), and melanocytes (arrow). (F) Co-injection of SerpinE2-MO and 2 ng non-targeted Flag-SerpinE2 mRNA restores a normal phenotype. (G) Scheme for microinjections in H–T. MOs and mRNAs were injected together with 100 pg nlacZ mRNA as lineage tracer (red nuclei) into one dorsal animal blastomere of embryos at the eight-cell stage. The injected side is marked with a star. (H–K) Anterior view of neurula embryos at stage 18. Neither control-MO nor SerpinE2-MO affect Twist and Sox9 expression in NC cells in the head and trunk (arrows) . (L–Q) SerpinE2-MO inhibits the epithelial-mesenchymal transition (EMT) of Foxd3+ and Snail2+ NC cells (open arrowheads) at stage 20, whereas the control-MO and SerpinE2-5MM-MO have no effect (filled arrowheads). (R–T) Lateral view of stage 26 embryos. A single injection of control-MO does not affect the migration of Snail1+ NC cells (filled arrowhead). SerpinE2-MO leads to a migration defect on the injected side (open arrowhead). 333 pg Flag-SerpinE2 mRNA rescues NC migration in the SerpinE2-morphant embryo. br, branchial crest segment; ey, eye primordium; hy, hyoid crest segment; ma, mandibular crest segment; MO, morpholino oligonucleotide; NC, neural crest; ot, otic vesicle. Doses of injected MOs per embryo were 40 ng (D–F) and 10 ng (H–T). Indicated phenotypes were shown in B, 10/11; D, 89/90; E, 64/83; F, 122/132; H, 29/29; I, 24/26; J, 7/7; K, 10/11; L, 9/9; M, 7/7; N, 7/9; O, 7/8; P, 8/9; Q, 9/11; R, 10/10; S, 13/15; T, 9/9. Scale bars, 0.5 mm.
Figure 2—figure supplement 1. SerpinE2 depletion does not affect the specification of neural crest cells.
The expression pattern prompted us to investigate the function of SerpinE2 in NC development. Xenopus laevis is allotetraploid (Session et al., 2016) and contains two SerpinE2 genes, namely SerpinE2.L (PN1.a) and SerpinE2.S (PN1.b), which encode two proteins that share 96% amino acid identity. A combination of two antisense morpholino oligonucleotides (MOs) that target the translation initiation site of SerpinE2.L and one MO directed against SerpinE2.S (collectively termed SerpinE2-MO) efficiently blocks SerpinE2 protein biosynthesis in Xenopus embryos (Acosta et al., 2015). We previously noted reduction of head structures in tadpoles upon microinjection of SerpinE2-MO into the animal pole blastomeres at the eight-cell stage. A closer analysis now revealed that these SerpinE2-morphant embryos also exhibited a loss of dorsal fin structures (open arrowheads) and a decreased number of melanocytes (arrow) at stage 40 (Figure 2E). These phenotypes were specific, since a standard control-MO had no phenotypic effect (Figure 2D), and co-injection of SerpinE2-MO together with a Flag-SerpinE2 mRNA that is not targeted by the MO rescued normal development (Figure 2F). The striking similarity of the phenotype obtained after knockdown of SerpinE2 with that of NC extirpation (Figure 2B and E) and supporting evidence from fate mapping studies that NC cells contribute to head mesenchyme, dorsal fin structures, and melanocytes (Tucker, 1986; Sadaghiani and Thiébaud, 1987; Tucker and Slack, 2004) suggest that SerpinE2 might be important for NC development.
SerpinE2 is dispensable for the specification but essential for the migration of NC cells
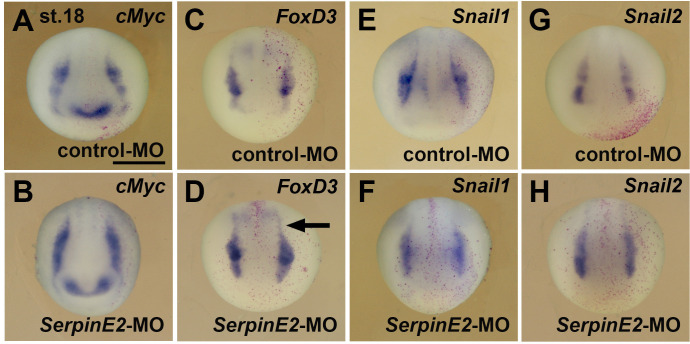
We injected MOs into the animal pole of embryos at the eight-cell stage together with nlacZ mRNA as lineage tracer (red nuclei) to identify the injected side and ensure that the MO is properly targeted (Figure 2G). SerpinE2-MO does not appear to affect NC specification in the head and trunk, as the expression of Twist, Sox9, cMyc, Foxd3, Snail1, and Snail2 in pre-migratory NC cells remained unchanged in mid-neurula embryos at stage 18 (Figure 2H–K; see also Figure 2—figure supplement 1).
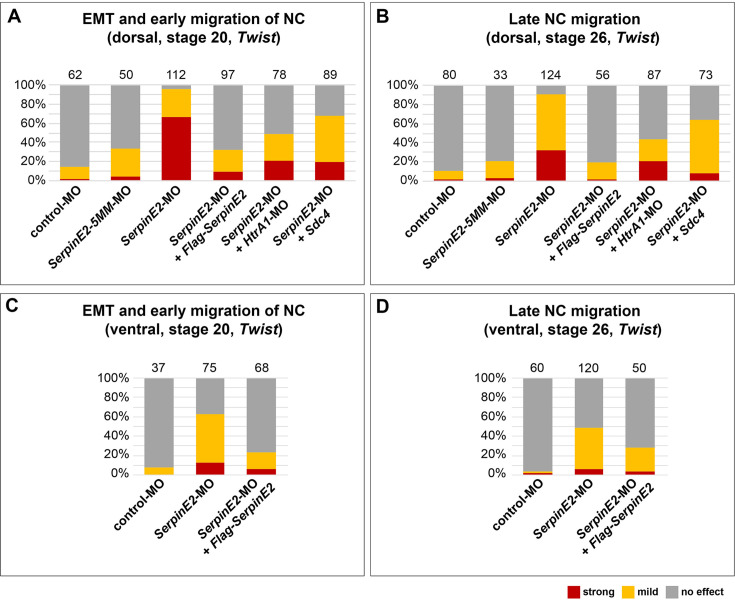
Since migration of the NC is initiated progressively from anterior to posterior in the neural folds of the closing neural tube (Sadaghiani and Thiébaud, 1987), we chose two distinct stages to monitor the EMT and migration of this cell population. At stage 20, NC cells of the mandibular crest segment are migrating from the mesencephalon around the eye primordium, while cells of the hyoid and branchial crest segments are undergoing EMT in the rhombencephalon (Figure 2L and O). At stage 26, NC cell migration occurs in the hyoid segment anterior to the otic vesicle, and in two split branchial segments posterior to the ear primordium (Figure 2R). A single dorsal injection of SerpinE2-MO caused a delay or failure of these NC cells to undergo EMT and migration (open arrowheads) in advanced neurula (Figure 2M and P) and tailbud embryos (Figure 2S). These knockdown effects were specific, because a SerpinE2-5MM-MO, which contains five mismatches with the SerpinE2.L and SerpinE2.S target mRNA sequences, as well as a combination of SerpinE2-MO and non-targeted Flag-SerpinE2 mRNA failed to disrupt NC migration (Figure 2N, Q, and T; see also Figure 8B–E’ and Figure 8—figure supplement 1A and B). Ventrally injected SerpinE2-MO was less efficient in reducing NC migration (Figure 8—figure supplement 1C and D), because expression of the SerpinE2 is highest in dorsal regions of post-gastrula embryos (Figure 1B). We conclude that SerpinE2 is dispensable for the initial specification but necessary for the migration of NC cells in Xenopus embryos.
HtrA1 inhibits the development and migration of NC cells
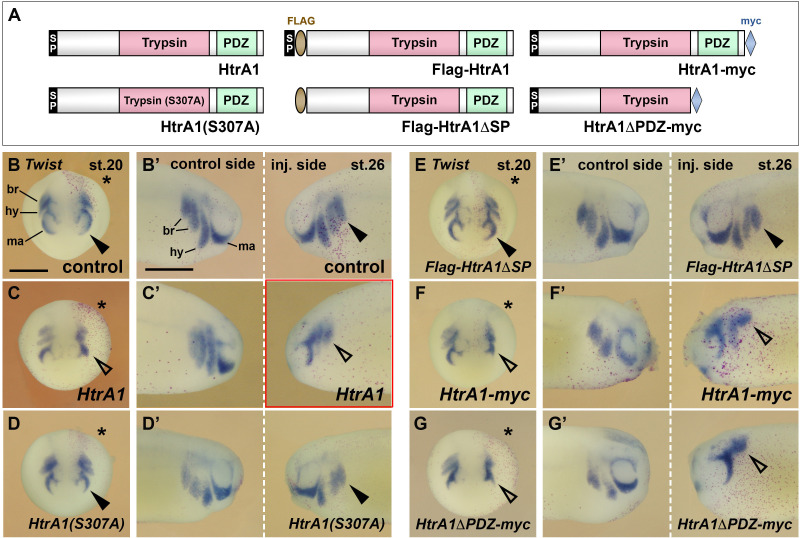
Next, we investigated whether HtrA1 affects NC development (Figure 3). Injection of HtrA1 mRNA into the animal pole blastomeres led to a decrease of head and eye structures in early tadpole embryos (Figure 3A and B) in accordance with the previously reported axis posteriorizing activity of this protease (Hou et al., 2007). HtrA1 overexpression also caused a reduction of dorsal fin tissue (open arrowheads) and fewer melanocytes (arrow), suggesting that HtrA1 inhibits the formation of NC-derived structures.
Figure 3. HtrA1 protease inhibits the formation of neural crest (NC)-derived structures and reduces NC migration.
Embryos were injected into all animal blastomeres (A–D) or a single dorsal animal blastomere (E–L) at the eight-cell stage. (A–D) Tadpoles at stage 40. HtrA1 mRNA causes reduction of head tissue, dorsal fin structures (arrowheads), and melanocytes (arrow), whereas HtrA1(S307A) and Flag-HtrA1ΔSP mRNA have no effect. (E–G) Anterior view of embryos at stage 18. Stars demarcate the injected sides. Neither 65 pg HtrA1 mRNA nor 10 ng HtrA1-MO do affect the specification of Twist+ cranial NC cells. (H, I) Anterior view of embryos at stage 20. 65 pg HtrA1 mRNA reduces the epithelial-mesenchymal transition (EMT) of Foxd3+ cranial NC cells (arrowheads) but does not affect the specification of trunk NC cells (arrows). (J–L) Lateral view of embryos at stage 26. The migration of NC cells (arrowheads) is reduced by 65 pg HtrA1 mRNA but not affected by 10 ng HtrA1-MO. br, branchial crest segments; ey, eye; hy, hyoid crest segment; ma, mandibular crest segment; ot, otic vesicle. Unless otherwise noted, the mRNA doses of HtrA1 and derived constructs per embryo were 100 pg. Indicated phenotypes were shown in B, 98/100; C, 74/83; D, 84/87; E, 31/31; F, 51/53; G, 59/60; H, 28/31; I, 23/26; J, 9/10; K, 21/21; L, 58/79 embryos; at least two independent experiments. Scale bars, 0.5 mm.
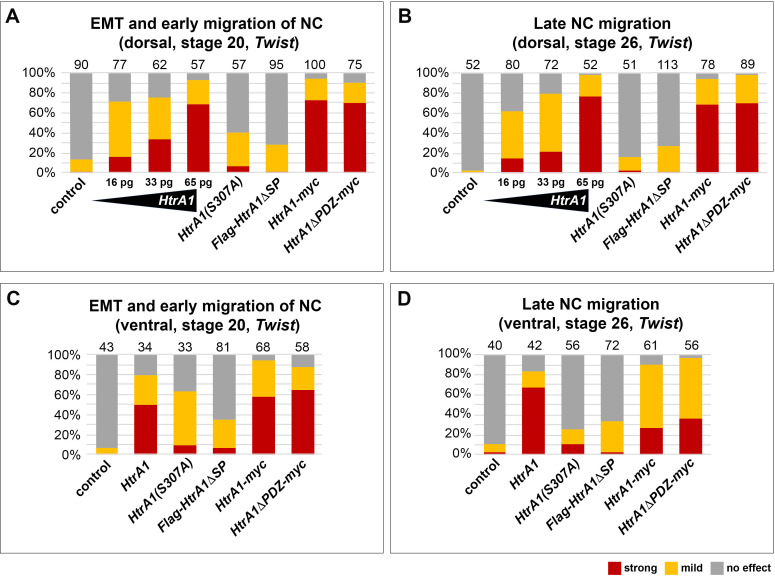
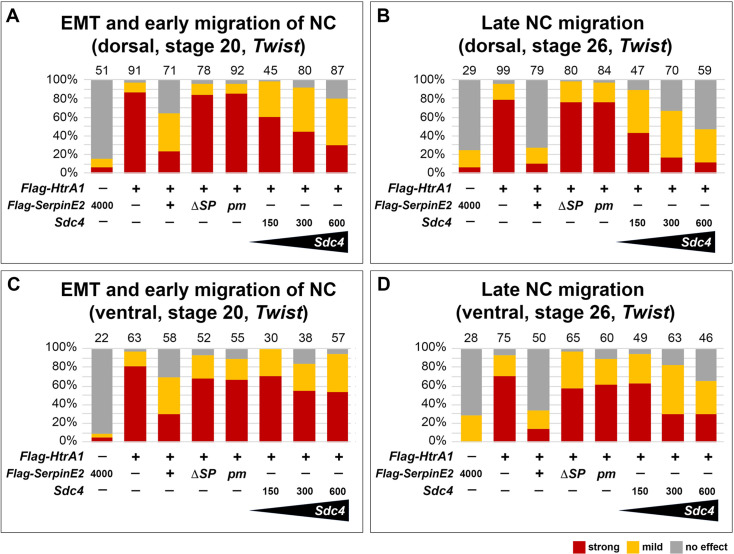
A single dorsal injection of HtrA1 mRNA did not affect the expression of cranial and trunk NC markers at stage 18 (Figure 3E and F) but blocked EMT and migration of NC cells at stages 20 and 26 (Figure 3H–K and Figure 6B–C’). Quantitative analysis showed that HtrA1 overexpression caused migratory defects in a concentration-dependent manner (Figure 6—figure supplement 1A and B). Ventral mRNA injection of HtrA1-derived constructs does not properly target the NC and therefore was less effective in inhibiting the migration of this cell population (Figure 6—figure supplement 1C and D).
Using western blot analysis with a polyclonal anti-HtrA1 antibody, we previously showed that a morpholino oligonucleotide directed against HtrA1.L and HtrA1.S, designated HtrA1-MO, efficiently blocks endogenous HtrA1 protein expression in Xenopus embryos (Hou et al., 2007). Dorsal injection of HtrA1-MO failed both to affect the induction of the NC marker Twist (Figure 3G) and to alter the migration of Twist+ cells (Figure 3L), suggesting that knockdown of HtrA1 appears not to affect NC development. We conclude that upregulation of HtrA1 protease activity inhibits the migration but not the specification of NC cells.
HtrA1 and SerpinE2 control the development of the head cartilage skeleton
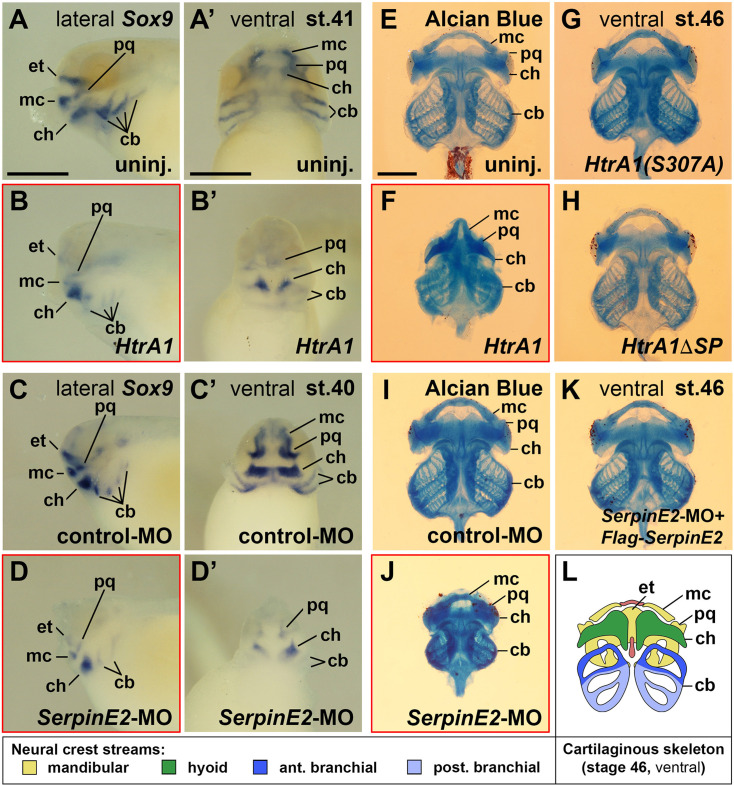
Cranial NC cells contribute to the craniofacial skeleton (Sadaghiani and Thiébaud, 1987). The mandibular crest stream gives rise to the ethmoid-traberculum (upper jaw), palatoquadrate and Meckel’s cartilage (lower jaw); the hyoid crest stream supplies the ceratohyal cartilage; and the branchial crest streams form the paired ceratobranchial cartilages (gills) (Figure 4L). The transcription factor Sox9 is a master regulator of chondrogenesis that mediates the condensation of chondrogenic mesenchyme and chondrocyte differentiation (Suzuki et al., 2012). In early tadpoles at stages 40/41, Sox9 expression demarcates chondrogenic precursors in the head mesenchyme (Figure 4A and A’). Upon microinjection of HtrA1 mRNA into the animal pole of all blastomeres at the four-cell stage, Sox9 expression was reduced in developing cartilage structures (Figure 4B and B’). A similar reduction of Sox9+ cartilaginous elements was induced by SerpinE2-MO, whereas the control-MO had no effect (Figure 4C–D’). The results suggest that HtrA1 overexpression and SerpinE2 knockdown impair with craniofacial cartilage formation.
Figure 4. HtrA1 overexpression and SerpinE2 knockdown decrease cartilaginous elements and craniofacial structures.
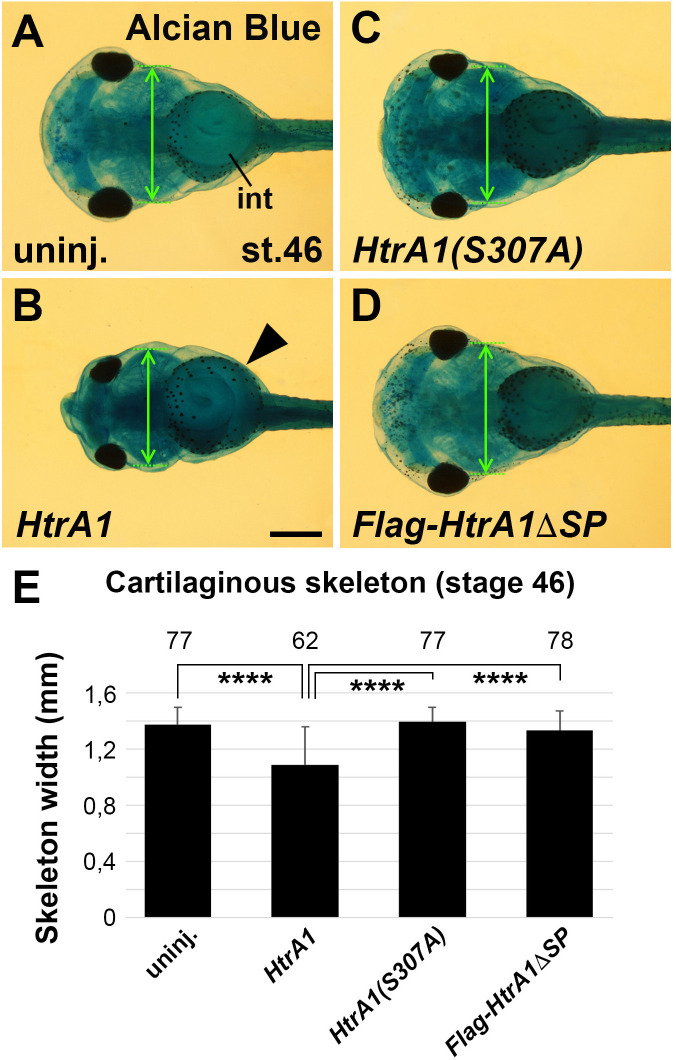
Xenopus embryos were injected into four animal blastomeres at the eight-cell stage with a total of 100 pg mRNA and 40 ng morpholino oligonucleotides (MOs). (A–D’) Tadpoles at stages 40/41 after whole-mount in situ hybridization in lateral (A–D) and ventral view (A’–D’). Note that HtrA1 mRNA and SerpinE2-MO reduce Sox9 expression, whereas control-MO has no effect on the labeled cartilaginous elements. (E–K) Ventral view of cartilaginous skeleton extracted from embryos at stage 46 after Alcian Blue staining. The dorsal ethmoid-trabecular cartilage was removed for better visibility. Note that HtrA1 mRNA, but not HtrA1(S307A) and Flag-HtrA1ΔSP mRNAs, diminishes craniofacial structures (E–H). SerpinE2-MO, but not control-MO nor a combination of SerpinE2-MO and Flag-SerpinE2 mRNA, reduce head skeleton structures (I–K). (L) Scheme of the cartilaginous skeleton at stage 46 in ventral view. Indicated is the contribution of neural crest streams to the craniofacial skeleton elements. cb, ceratobranchial; ch, ceratohyal; et, ethmoid-trabecular; mc, Meckel’s cartilage; pq, palatoquadrate. Indicated phenotypes were shown in A, 11/11; B, 17/19; C, 12/13; D, 15/17; E, 57/58; F, 17/21; G, 72/77; H, 67/78; I, 83/88; J, 73/77; K, 77/87 embryos; at least two independent experiments. Scale bars, 0.5mm.
Figure 4—figure supplement 1. Overexpression of HtrA1 decreases cartilaginous elements and craniofacial structures.
Figure 4—figure supplement 2. Knockdown of SerpinE2 reduces craniofacial skeleton formation.
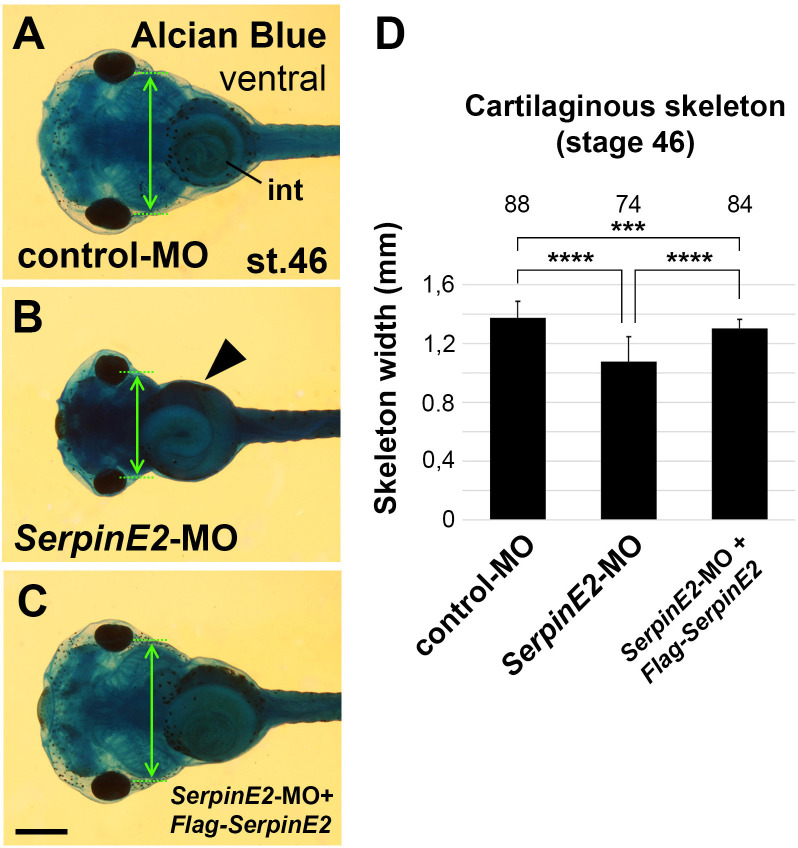
Alcian Blue staining was used to visualize the cartilaginous skeleton in Xenopus tadpoles at stage 46 (Figure 4E). For quantification of the skeleton size, we measured the width at the level of the anterior ceratobranchial cartilages (Figure 4—figure supplement 1A). HtrA1 mRNA injection reduced all cartilage structures and lowered the size of the head skeleton by 21% (Figure 4F, Figure 4—figure supplement 1B and E). Similarly, SerpinE2-MO decreased the skeleton size by 22% compared to control-morphant siblings (Figure 4I and J, Figure 4—figure supplement 2A, B, and D). The coiling pattern of the gut was not affected upon HtrA1 mRNA injection (Figure 4—figure supplement 1B, arrowhead) and in SerpinE2-morphant tadpoles (Figure 4—figure supplement 2B, arrowhead), ruling out an overall delay of embryonic development. Co-injection of SerpinE2-MO together with non-targeted Flag-SerpinE2 mRNA rescued craniofacial skeleton development (Figure 4K, Figure 4—figure supplement 2C and D). We conclude that HtrA1 and SerpinE2 regulate the formation of NC-derived cartilage structures.
HtrA1 and SerpinE2 act in the NC to regulate cell migration and adherence to fibronectin
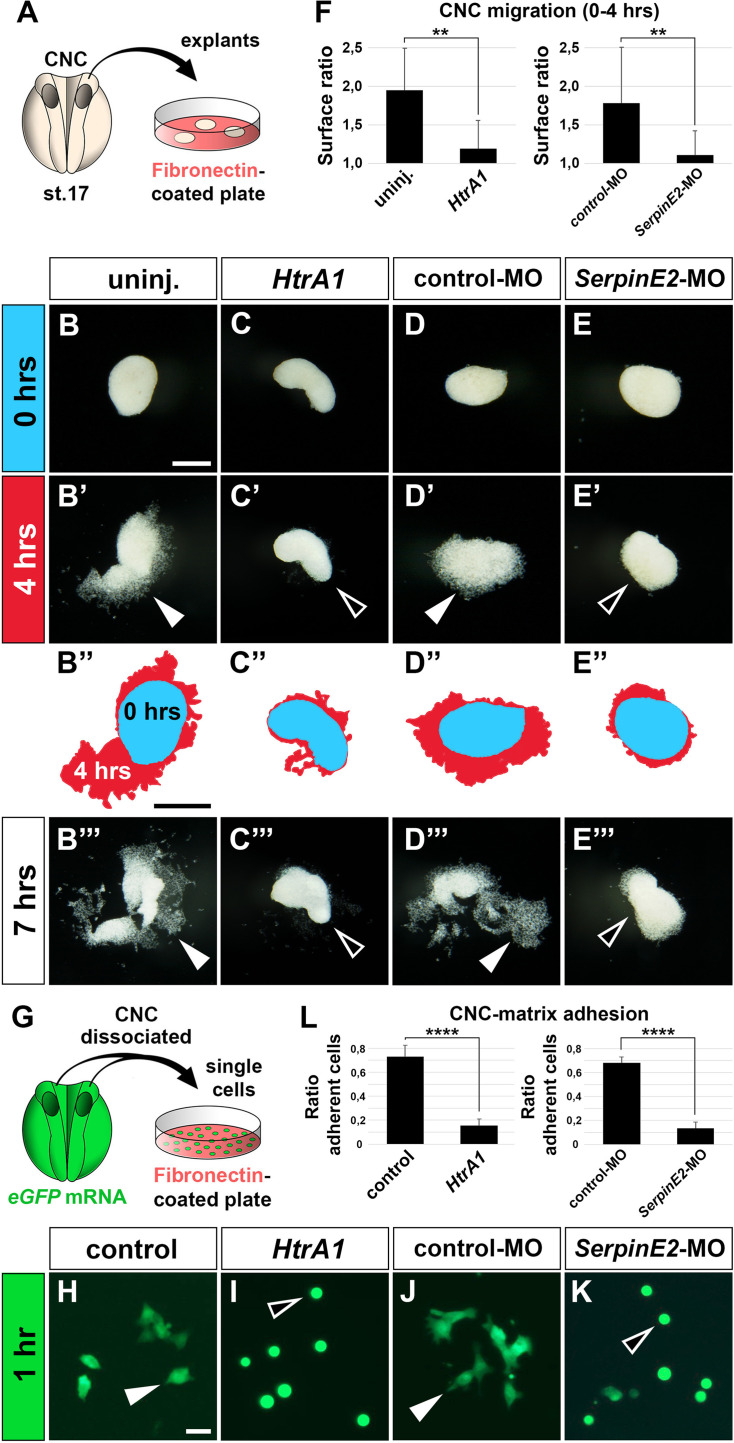
Since HtrA1 and SerpinE2 are expressed in both NC cells and surrounding tissue, we asked whether the proteins affect cell migration in an NC-autonomous or non-autonomous manner. The cranial NC can be dissected from Xenopus embryos at stage 17 and cultured on fibronectin in vitro to investigate collective cell migration in relative isolation, allowing for the identification of extrinsic versus intrinsic mechanisms (Figure 5A; Alfandari et al., 2003). After 4 hr, cells from uninjected explants spread as a coherent sheet toward one side and thereby doubled the surface area compared to the time of plating (Figure 5B–B’’ and F, filled arrowhead). In contrast, little cell migration was observed in explants upon injection with HtrA1 mRNA, contributing to only a 20% increase in surface area within this time frame (Figure 5C–C’’ and F, open arrowhead). Similarly, SerpinE2-MO inhibited cell migration, leading to only one-tenth of the increase in total surface area that was observed in control-morphant explants (Figure 5D–E’’ and F). At 7 hr after plating, distinct segments were seen in uninjected and control-MO-injected explants (Figure 5B’’’ and D’’’), resembling the mandibular, hyoid, and branchial streams seen in sibling control embryos at the tailbud stage (Figure 1D). In contrast, explants injected with either HtrA1 mRNA or SerpinE2-MO failed to display segmentation into distinct streams (Figure 5C’’’ and E’’’). In time-lapse video microscopy, control-MO-injected explants exhibited lamellipodia and filopodia at the leading front of the migratory NC cell clusters (Figure 5—video 1). SerpinE2-morphant NC cells lost polarity and took on a spherical appearance (Figure 5—video 2). These results show that both HtrA1 overexpression and SerpinE2 knockdown inhibit collective cell migration in vitro, providing evidence that HtrA1 and SerpinE2 regulate cell migration in the isolated NC.
Figure 5. HtrA1 overexpression and SerpinE2 knockdown inhibit cranial neural crest cell migration and adhesion to fibronectin in vitro.
(A) Scheme of migration experiment. The cranial neural crest was explanted from uninjected or injected embryos at stage 17 and cultured on a fibronectin-covered plastic plate. (B–E’’’) Time-lapse of cell migration in CNC explants after culturing for 0, 4, or 7 hr. Note collective cell migration (filled arrowheads) in uninjected controls and explants injected with control-MO, whereas HtrA1 mRNA and SerpinE2-MO block migration (open arrowheads). In B’’–E’’, the surface areas of explants at 0 hr (blue) and 4 hr (red) were determined by ImageJ and superimposed. Scale bar, 0.2 mm. (F) Quantification of initial CNC migration. Indicated is the surface ratio of explants 4 hr versus 0 hr after plating. 12 explants were analyzed per sample. (G) Scheme of adhesion experiment. Upon injection of eGFP mRNA, CNC explants were dissociated in Ca2+- and Mg2+-free medium, and single cells were cultured on a fibronectin plate. (H–K) Single eGFP-labeled CNC cells after 1 hr culture. Note adhering cells with extended cytoplasmic processes (filled arrowheads) in control sample and after co-injection with control-MO, whereas HtrA1 mRNA and SerpinE2-MO prevent adhesion causing injected cells to acquire a round phenotype (open arrowheads). Scale bar, 0.02 mm. (L) Quantification of CNC adhesion. Indicated is the ratio of adherent cells relative to the control. Analysis of n>1600 cells from at least six explants per sample. CNC, cranial neural crest; eGFP, enhanced green fluorescent protein. Embryos were injected with 100 pg mRNAs and 40 ng MOs. Data in all graphs are displayed as mean ± SD, n = 2; **p<0.01, ****p<0.0001, unpaired t-test.
Figure 5—video 1. Collective migration of neural crest (NC) cells in vitro.
Figure 5—video 2. Knockdown of SerpinE2 prevents adhesion and migration of neural crest (NC) cells.
To examine cell-matrix adhesion, we dissociated cranial NC explants in Ca2+/Mg2+-free medium and cultured them as single cells on fibronectin (Figure 5G). For better visibility of the NC cells, the donor embryos were injected with mRNA encoding enhanced green-fluorescent protein (eGFP). At 1 hr after plating, eGFP+ cells adhered to fibronectin and extended cytoplasmic processes on this extracellular matrix protein (Figure 5H, filled arrowhead). Upon injection of HtrA1 mRNA, nearly 80% of cells failed to attach and acquired a round morphology (Figure 5I and Figure 5L, open arrowhead). Similarly, SerpinE2-MO-injected cells lost adherence and cytoplasmic extensions, whereas the control-MO had no significant effect (Figure 5J–L). We conclude from these in vitro explant and single cell data that HtrA1 and SerpinE2 regulate in a NC-autonomous manner cell migration and adhesion on fibronectin.
HtrA1 controls NC migration as a secreted protease
HtrA1 contains a cleavable N-terminal signal peptide (SP), a trypsin-like serine protease domain and a carboxyterminal PDZ (post-synaptic density of 95kD, discs large, zona occludens-1) protein-protein interaction domain (Figure 6A, top left; Zurawa-Janicka et al., 2017). The SP is a short hydrophobic peptide sequence that destines proteins normally to the secretory pathway (Owji et al., 2018). We previously identified Xenopus HtrA1 as a protein in the supernatant of cDNA-transfected and metabolically labeled HEK 293T cells (Hou et al., 2007), suggesting that the protease might act in the extracellular space. On the other hand, human HtrA1 has been shown to associate with and stabilize microtubules in a PDZ domain-dependent manner (Chien et al., 2009b), raising the possibility for a non-proteolytic function of cytosolic HtrA1 in the regulation of cell motility. In a structure-function analysis, we investigated (1) whether HtrA1 acts as a protease in NC development, (2) whether HtrA1 operates in the extracellular space or inside the cell, and (3) whether its PDZ domain is involved in regulating NC migration. To this end, we compared with their cognate wild-type HtrA1 constructs three mutant derivatives, including HtrA1(S307A), in which the catalytic serine residue in amino acid position 307 is replaced by alanine (Hou et al., 2007), a newly generated Flag-tagged construct that lacks the secretory signal peptide (Flag-HtrA1ΔSP), and a myc-tagged construct that is devoid of the PDZ domain (HtrA1ΔPDZ-myc; Acosta et al., 2015; Figure 6A).
Figure 6. HtrA1 inhibits neural crest (NC) migration as an extracellular protease.
Embryos were injected into a single dorsal animal blastomere at the eight-cell stage. A star labels the injected side.Twist expression demarcates the NC in embryos at stage 20 (B–G; anterior view) and stage 26 (B’–G’; lateral view). (A) Overview of wild-type (top) and mutant (bottom) HtrA1 protein constructs. (B–E’) HtrA1 mRNA, but neither HtrA1(S307A) nor Flag-HtrA1ΔSP mRNAs, reduces epithelial-mesenchymal transition (EMT) and migration of NC cells on the injected side (arrowheads). Note that the diffusible HtrA1 protein reduces NC cell migration to a lower extent also on the non-injected side. (F–G’) Both HtrA1-myc and HtrA1ΔPDC-myc mRNAs reduce NC EMT and migration. br, branchial segments; hy, hyoid segment; ma, mandibular segment. Injected mRNA doses per embryos are 65 pg. Scale bars, 0.5mm. For quantification of NC migration defects, see Figure 6—figure supplement 1A and B.
Figure 6—figure supplement 1. HtrA1 mRNA inhibits neural crest (NC) migration in a concentration-dependent manner.
Figure 6—figure supplement 2. Cytoplasmic HtrA1 causes reduction of αTubulin protein levels in mammalian cells and Xenopus embryos.
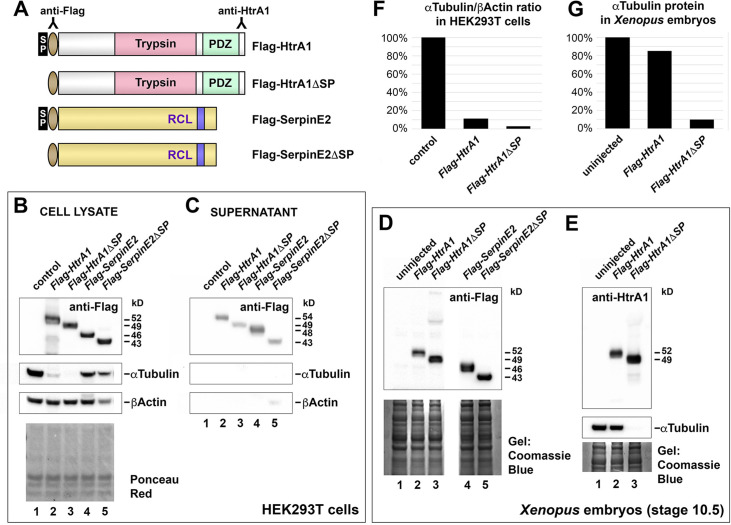
Using western blot analysis, we previously reported that HtrA1(S307A) (Hou et al., 2007) and HtrA1ΔPDZ-myc (Acosta et al., 2015) generate proteins of the expected sizes in mRNA-injected Xenopus embryos. These mutant HtrA1 constructs co-immunoprecipitate with Flag-SerpinE2 at protein levels similar to their corresponding wild-type HtrA1 and HtrA1-myc constructs (Acosta et al., 2015). Here, we used western blot analysis of lysates to show that the Flag-HtrA1 and Flag-HtrA1ΔSP protein constructs were expressed with the expected molecular weights in both cDNA-transfected HEK293T cells and mRNA-injected Xenopus embryos (Figure 6—figure supplement 2A, B, and D). However, while the Flag-HtrA1 protein accumulated at high levels in the supernatant of transfected cells, the Flag-HtrA1ΔSP protein failed to be efficiently secreted into the culture medium (Figure 6—figure supplement 2C). We also showed that wild-type Flag-HtrA1 and cytosolic Flag-HtrA1ΔSP degraded αTubulin, but not βActin, which validates the proteolytic activity and target specificity of these constructs (Figure 6—figure supplement 2B and E–G).
Neither HtrA1(S307A) nor Flag-HtrA1ΔSP affected NC migration in mRNA-injected embryos (Figure 6D–E’; see also Figure 6—figure supplement 1A and B). These two mutant constructs also failed to induce any defects in dorsal fin, melanocyte (Figure 3C and D) and craniofacial skeleton structures (Figure 4G and H; Figure 4—figure supplement 1C–E), suggesting that HtrA1 relies on an intact protease domain and transport to the secretory pathway to regulate the development of these NC derivatives. HtrA1ΔPDZ-myc efficiently inhibited EMT and NC migration to a degree comparable to that induced by HtrA1-myc control mRNA (Figure 6F–G’; Figure 6—figure supplement 1A and B). These results support the conclusions that HtrA1 acts as an extracellular protease and that an association of HtrA1 via its PDZ domain to microtubuli appears not to regulate the migratory behavior of NC cells in the Xenopus embryo.
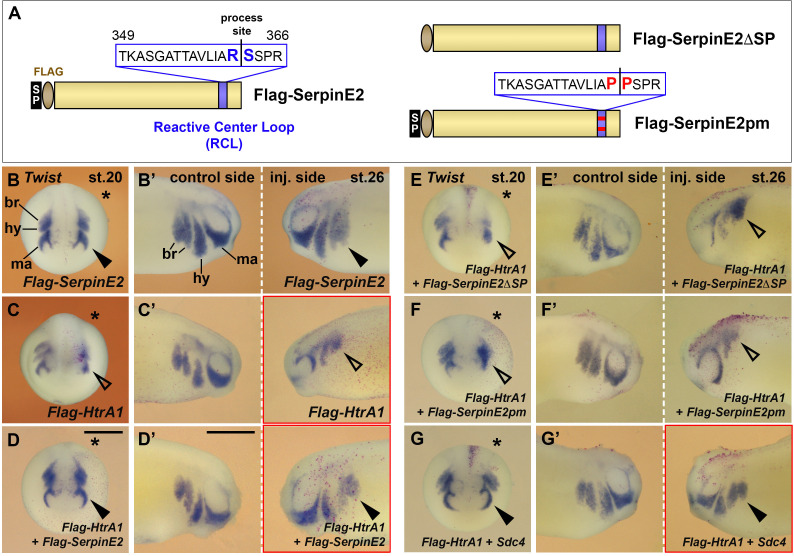
SerpinE2 interacts with HtrA1 in NC cell migration
The serpin superfamily comprises extracellular and intracellular members with an exposed RCL that is cleaved by a target protease and irreversibly inhibits the attacking enzyme by forming a covalent serpin-protease complex (Olson and Gettins, 2011). SerpinE2 contains an N-terminal signal peptide and a C-terminal RCL (Figure 7A). We previously showed that SerpinE2 via its RCL binds to the trypsin domain of HtrA1 (Acosta et al., 2015). Do SerpinE2 and HtrA1 interact in NC cell migration? To this end, we used Flag-tagged SerpinE2 (Flag-SerpinE2), a point mutant Flag-SerpinE2pm derivative, in which two proline residues replace the critical arginine and serine residues (R362P and S363P) at the process site of the RCL, and a newly generated truncated construct that lacks the N-terminal signal peptide (Flag-SerpinE2ΔSP) (Figure 7A). In previous western blot studies, we showed that Flag-SerpinE2 and Flag-SerpinE2pm are synthesized in similar protein amounts and at the expected sizes, but that overexpressed HtrA1 immunoprecipitates Flag-SerpinE2pm less efficiently than Flag-SerpinE2 (Acosta et al., 2015). Here, we use western blotting to validate that Flag-SerpinE2ΔSP protein is generated in expected amount and size upon mRNA injection in Xenopus embryos (Figure 6—figure supplement 2A, D, and E). However, unlike wild-type Flag-SerpinE2, the signal peptide-deficient Flag-SerpinE2ΔSP construct is not efficiently secreted in cDNA-transfected HEK293 cells (Figure 6—figure supplement 2B and C).
Figure 7. SerpinE2 and HtrA1 interact with Syndecan-4 (Sdc4) in neural crest (NC) cell migration.
mRNAs were injected into one dorsal animal blastomere at the eight-cell stage. Embryos are shown in anterior view (stage 20, injected side labeled with a star, B–G) and lateral view (stage 26, B’–G’). (A) Overview of wild-type (left) and mutant (right) SerpinE2 protein constructs. (B, B’) 4 ng Flag-SerpinE2 mRNA has no effect on the migration of Twist+ NC cells (filled arrowheads). (C, C’) Flag-HtrA1 inhibits NC cell migration robustly on the injected sides (open arrowheads). (D–F’) SerpinE2 mRNA, but neither Flag-SerpinE2ΔSP nor SerpinE2pm mRNA, rescues normal epithelial-mesenchymal transition (EMT) and migration of NC cells upon co-injection with Flag-HtrA1. (G, G’) Sdc4 mRNA restores normal NC migration in Flag-HtrA1-injected embryos. If not otherwise indicated, injected mRNA doses per embryos are 65 pg (Flag-HtrA1), 333 pg (Flag-SerpinE2 derived constructs), and 450 pg (Sdc4). Scale bars, 0.5mm. For quantification of NC migration defects, see Figure 7—figure supplement 1A and B.
Figure 7—figure supplement 1. SerpinE2 and Sdc4 mRNAs partially rescue neural crest (NC) migration defects that are induced by HtrA1 overexpression.
Microinjection of Flag-SerpinE2 mRNA at doses of up to 4 ng did not affect the migration of Twist+ NC cells (marked with nuclear lacZ lineage tracer) at stages 20 and 26 (Figure 7B and B’; see also Figure 7—figure supplement 1). However, co-injection of Flag-HtrA1 and Flag-SerpinE2 mRNAs reverted the EMT and NC migration defects that were induced by Flag-HtrA1 mRNA alone (Figure 7C–D’; Figure 7—figure supplement 1), suggesting that SerpinE2 can relieve HtrA1-mediated suppression of NC migration. Flag-SerpinE2ΔSP failed to rescue the Flag-HtrA1-induced NC migration defects (Figure 7E and E’; Figure 7—figure supplement 1), underscoring that entry into the secretory pathway is important for the function of SerpinE2. Moreover, Flag-SerpinE2pm did not inhibit Flag-HtrA1 mRNA from blocking NC migration (Figure 7F and F’; Figure 7—figure supplement 1). We conclude that SerpinE2 functions in the extracellular space and requires an intact RCL for efficient interaction with HtrA1 to modulate NC cell migration.
Double knockdown of SerpinE2 and HtrA1 rescues NC migration
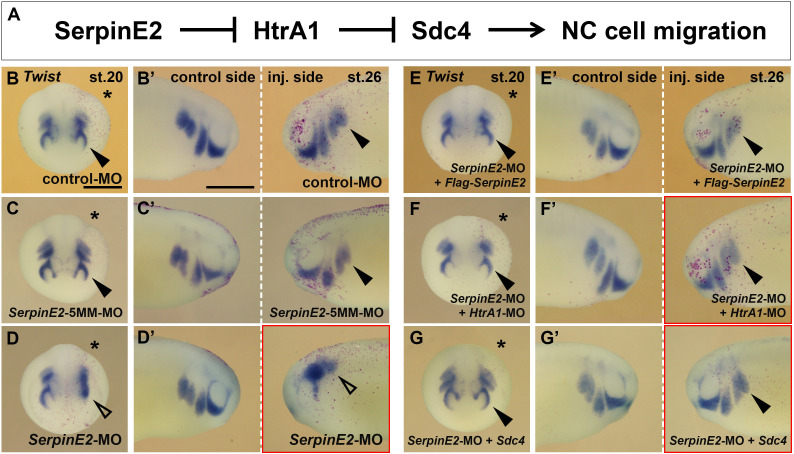
We next asked whether the NC migration defects induced by SerpinE2 depletion are dependent on endogenous HtrA1 protein. In loss-of-function experiments, we co-injected SerpinE2-MO and HtrA1-MO into a single dorsal blastomere and assessed the effects on NC cells. Importantly, knockdown of HtrA1 significantly reduced the EMT and migration defects in SerpinE2-depleted embryos (Figure 8D, D’, F, and F’; see also Figure 8—figure supplement 1A and B). These epistatic experiments support the existence of an extracellular proteolytic regulatory system, in which SerpinE2 stimulates NC cell migration through the inhibition of HtrA1.
Figure 8. SerpinE2 functions in neural crest (NC) cell migration in an HtrA1- and Syndecan-4 (Sdc4)-dependent manner.
mRNAs and morpholino oligonucleotides (MOs, 10 ng) were injected into one dorsal animal blastomere at the eight-cell stage. Embryos are shown in anterior view (stage 20, injected side labeled with a star, B–G) and lateral view (stage 26, B’–G’). (A) Proposed mechanism for the regulation of NC migration by SerpinE2, HtrA1, and Sdc4. (B–D’) SerpinE2-MO blocks epithelial-mesenchymal transition (EMT) and migration of Twist+ NC cells (arrow) on the injected side, while control-MO and SerpinE2-5MM-MO have no effect. (E–G’) Flag-SerpinE2 mRNA, HtrA1-MO, and Sdc4 mRNA restore normal NC migration in SerpinE2-morphant embryos. Injected mRNA doses per embryos are 333 pg (Flag-SerpinE2) and 450 pg (Sdc4). Scale bars, 0.5mm. For quantification of NC migration defects, see Figure 8—figure supplement 1A and B.
Figure 8—figure supplement 1. SerpinE2-MO causes neural crest (NC) migration defects which are rescued by HtrA1-MO and Sdc4 mRNA.
HtrA1 and SerpinE2 regulate cranial NC migration via Sdc4
Syndecans comprise a family of single-pass transmembrane proteoglycans with four members in vertebrates (Keller-Pinter et al., 2021). Sdc4 is a major component of focal adhesions and interacts with fibronectin during cell-matrix adhesion and cell movement (Woods and Couchman, 2001). We previously showed that HtrA1 triggers the proteolytic cleavage of Sdc4 and that SerpinE2 protects this proteoglycan from degradation by HtrA1 (Hou et al., 2007; Acosta et al., 2015). In Xenopus embryos, Sdc4 is abundant in the NC; knockdown of Sdc4 does not affect the induction of the NC but reduces its cell migration (Matthews et al., 2008). The effects of HtrA1 overexpression and SerpinE2 downregulation described in this study mimic the effects of Sdc4 depletion (Matthews et al., 2008). To investigate the relationship between the SerpinE2-HtrA1 axis and Sdc4 in the NC, we dorsally injected Sdc4 mRNA and found it reduced in a concentration-dependent manner the EMT and NC migration defects induced by Flag-HtrA1 mRNA (Figure 7C, C’, G, and G’; see also Figure 7—figure supplement 1A and B). Ventral co-injection of Sdc4 and Flag-HtrA1 mRNAs was less efficient in rescuing NC cell migration (Figure 7—figure supplement 1C and D), because the transmembrane Sdc4 protein - unlike the secreted HtrA1 protease - remains anchored to ventral regions of the embryo and does not reach the dorsally located NC cells. Importantly, dorsally injected Sdc4 mRNA partially restored normal EMT and cell migration in SerpinE2-morphant embryos (Figure 8D, D’, G and G’; see also Figure 8—figure supplement 1A and B). Therefore, our epistatic studies in Xenopus embryos suggest that SerpinE2 promotes NC cell migration by inhibiting HtrA1-mediated degradation of Sdc4.
Discussion
This study reveals, for the first time, a role for the SerpinE2 and HtrA1 proteolytic pathway in embryonic cell migration. Several lines of evidence support the conclusion that this inhibitor/protease pair functionally interact in the control of NC cell motility. (1) SerpinE2 and HtrA1 were co-expressed in pre-migratory and migrating NC cells in the Xenopus embryo. (2) In gain-of-function studies, wild-type HtrA1, but not a protease-defective construct, inhibited NC migration and development of NC-derived structures, such as branchial arch cartilage, dorsal fin tissue, and melanocytes. (3) SerpinE2 reverted the HtrA1-induced migration defects in mRNA-injected embryos. (4) In loss-of-function studies, SerpinE2 knockdown inhibited NC migration and development of NC-derived structures. (5) Concomitant knockdown of SerpinE2 and HtrA1 restored normal NC migration in MO-injected embryos. Additional epistatic experiments showed that Sdc4 mRNA partially rescues the migration defects induced by HtrA1 overexpression or SerpinE2 knockdown. Thus, SerpinE2, HtrA1, and Sdc4 form an important regulatory axis to control NC development. SerpinE2 promotes NC cell migration by inhibiting endogenous HtrA1 and preventing this protease from degrading the transmembrane proteoglycan Sdc4 in the Xenopus embryo. Hence, our study reveals a critical role for the SerpinE2-HtrA1-Sdc4 axis of extracellular proteins to regulate collective cell migration in vivo (Figure 8A).
Proteolytic control of morphogens
Secreted proteases, particularly of the astacin class of zinc metalloproteases, are important for the formation of morphogen gradients. In Hydra, the HAS-7 protease normally processes Wnt3 and restricts head organizer formation; its knockdown results in ectopic organizers (Ziegler et al., 2021; Holstein, 2022). In Xenopus, Tolloid regulates Spemann’s organizer function by cleaving Chordin and de-repressing BMP signaling (Piccolo et al., 1997). The secreted Frizzled-related protein Sizzled binds to and inhibits Tolloid (Lee et al., 2006) and controls patterning of the dorsoventral axis through the following pathway:
Sizzled ┤Tolloid protease ┤Chordin ┤ BMP
Our group previously showed that the serine protease HtrA1 induces mesoderm and ectopic tail formation by triggering the cleavage of Sdc4 and releasing active FGF messages (Pera et al., 2005). SerpinE2 (previously named PN1) binds to and inhibits HtrA1 (Acosta et al., 2015) and controls germ layer formation and patterning of the anteroposterior axis as follows:
SerpinE2 ┤HtrA1 protease ┤Syndecan-4 ┤ FGF
Extracellular proteases in cell migration
Migrating NC cells face major challenges to overcome physical barriers, such as the basal membrane or the extracellular matrix, suggesting proteolysis as an important mechanism for these cells to invade other tissues and reach their destined targets in the embryo. Matrix metalloproteases have been well studied in extracellular matrix remodeling during NC development (Gouignard et al., 2018). Less understood are other classes of proteases in the control of NC cell migration and invasion. The laboratory of Nicole Le Douarin was first to show that migrating NC cells produce serine proteases (Valinsky and Le Douarin, 1985). Using interspecific quail-chick grafting and enzyme-specific zymography, the group demonstrated high plasminogen activator activity in lysates of cranial NC compared to adjacent embryonic tissues.
Subsequent studies by other laboratories showed that mouse NC cells secrete urokinase and tissue plasminogen activators (uPA and tPA) into the culture medium (Menoud et al., 1989) and that uPA promotes chick NC migration in vitro via activation of plasmin and TGFβ signaling (Brauer and Yee, 1993; Agrawal and Brauer, 1996). Mutations in the lectin complement pathway gene MASP1/3, encoding for Mannose-associated serine protease-1 and -3, cause 3MC (Mingarelli, Malpuech, Michels and Carnevale) syndrome, a rare autosomal recessive disorder that is characterized by a spectrum of developmental features including craniofacial abnormalities (Rooryck et al., 2011). Zebrafish morphants exhibit craniofacial cartilage and pigment defects as well as abnormal NC migration, suggesting that MASP1 is a guidance cue that directs the migration of NC cells in the early embryo. Of note, all secreted serine proteases listed above have a positive role in NC migration. As we now demonstrate, HtrA1 is the only serine protease identified so far that acts as a negative regulator of NC migration.
SerpinE2 has a broad spectrum of target serine proteases that it binds to and inhibits, including uPA, tPA, and plasmin (Arocas and Bouton, 2015). Given the pro-migratory properties of these serine proteases, one should expect that SerpinE2 would inhibit NC migration by antagonizing their activity. However, overexpression of SerpinE2 at mRNA doses of up to 4 ng did not affect the migration of NC cells in the Xenopus embryo. Instead, knockdown by MOs that block endogenous SerpinE2 protein biosynthesis (Acosta et al., 2015) efficiently inhibited NC migration and the development of NC-derived structures, as shown in this study. The finding that microinjection of SerpinE2 mRNA reverted migration defects in these morphant embryos supports the view that SerpinE2 specifically promotes NC migration. It therefore appears that uPA, tPA, and plasmin are not target proteases of SerpinE2 in the modulation of NC migration in the Xenopus embryo.
SerpinE2 and HtrA1 form a proteolytic pathway in NC migration
In Xenopus embryos at the neurula stage, HtrA1 transcripts were most abundant in the superficial (ependymal) layer of the ectoderm containing non-motile epithelial cells, whereas SerpinE2 expression was confined to the deep (sensorial) layer of the ectoderm that gives rise to motile mesenchymal NC cells (Figure 1G). In post-neurula embryos, transcripts of these genes appeared in the collective of migrating NC cells, with SerpinE2 accumulating near the front of the cell streams and HtrA1 being enriched at their rear ends (Figure 1H). We propose that only in regions where the SerpinE2 concentration is sufficiently high, HtrA1-mediated repression of cell motility is relieved so that NC cell migration can occur. In support of this conclusion, microinjection of HtrA1 mRNA reduced in a concentration-dependent manner EMT and migration of NC cells, leading to defects in craniofacial skeleton structures. A key experiment was that EMT and migration of NC cells were restored by HtrA1 knockdown in SerpinE2-depleted embryos, suggesting that SerpinE2 promotes NC migration via inhibiting endogenous HtrA1 protease activity in the embryo. Our finding that HtrA1 mRNA and SerpinE2-MO diminished cell migration in isolated NC explants provided evidence that the two proteins act in an NC-autonomous manner. HtrA1 overexpression and SerpinE2 knockdown also reduced Sox9-expressing chondrogenic precursors in the NC-derived head mesenchyme and caused defects in the cartilaginous skull and hyobranchial skeleton in Xenopus tadpoles. Interestingly, increased HtrA1 expression has been detected in cranial sutures of mice with thyroid hormone-induced craniofacial disruptions (Howie et al., 2016). Since proper migration of NC cells is essential for the formation of bones, cartilage, and soft tissue in the head (Minoux and Rijli, 2010), elevated HtrA1 levels might disturb NC migration not only in Xenopus, but also in mammalian embryos.
SerpinE2 and HtrA1 regulate NC migration as extracellular proteins
The expression patterns were consistent with loss- and gain-of-function data that SerpinE2 promoted EMT and NC cell migration, whereas HtrA1 had the opposite effect. The actin cytoskeleton and microtubules play important roles in cell migration (Seetharaman and Etienne-Manneville, 2020). Two cytoskeletal proteins with functions in cell movement, i.e., fascin (actin bundling) and talin1 (regulation of actin assembly in focal adhesions), were previously identified as proteolytic substrates of HtrA1 (An et al., 2010). It has also been shown that the protease cleaves tubulins (Chien et al., 2009a) and that HtrA1 binds to and stabilizes microtubules via its PDZ domain (Chien et al., 2009b). However, the significance of the degradation of cytoskeletal proteins by HtrA1 remains unclear and it is unknown whether the association between HtrA1 and microtubules is important for cell motility. Here, we showed that wild-type HtrA1, but not a derived construct that was lacking a secretory signal peptide (HtrA1ΔSP), inhibited NC migration as well as craniofacial, dorsal fin, and melanocyte development. An HtrA1 construct with a deletion of the PDZ domain (HtrA1ΔPDZ) efficiently reduced EMT and migration of NC cells. The results strongly suggest that HtrA1 acts primarily as an extracellular protease during NC collective cell migration.
SerpinE2 shares with other members of the Serpin family an RCL that is cleaved by target proteases at the process site and forms a covalent acyl-enzyme complex leading to irreversible inhibition of the protease (Olson and Gettins, 2011; Arocas and Bouton, 2015). The introduction of prolines to residues P1 and P1’ at the RCL cleavage site reduces the ability of SerpinE2 to physically interact with the catalytic trypsin domain of HtrA1 and inhibits its protease activity (Acosta et al., 2015). Here, we demonstrated that wild-type SerpinE2 restored normal EMT and migration of NC cells in HtrA1 mRNA-injected embryos, whereas a point mutant SerpinE2 construct with the mutations Arg362Pro and Ser363Pro at P1 and P1’ of the scissile site (SerpinE2pm) failed to show any effect. In addition, a SerpinE2 construct without a secretory signal peptide (SerpinE2ΔSP) did not revert the HtrA1-induced NC migration defects. The results underscore that SerpinE2 promotes NC migration as an extracellular protease inhibitor.
The SerpinE2-HtrA1-Sdc4 axis functions in NC migration
Sdc4 is a central component of focal adhesion complexes that regulate cell-matrix adhesion and cell migration in cooperation with members of the integrin family of transmembrane proteins (Keller-Pinter et al., 2021). In zebrafish and Xenopus embryos, Sdc4 is expressed in migrating NC cells and promotes directional NC cell migration via regulation of the small GTPase Rac1 and activation of non-canonical Wnt signaling in the planar cell polarity pathway (Matthews et al., 2008). Similarly, human SDC4 favors cell migration and invasion through activation of Wnt5A signals in melanoma (O’Connell et al., 2009), indicating conserved signaling downstream of this proteoglycan in NC cell migration and malignant progression of an NC-derived cancer.
Several metalloproteases (MMP-3, -9, and -14) and serine proteases (plasmin, thrombin) cleave Sdc4 preferentially at the juxtamembrane site, so that its ectodomain can be released from the cell surface (Manon-Jensen et al., 2013). We previously showed that HtrA1 triggers the proteolytic cleavage of Xenopus Sdc4 (Hou et al., 2007). Whether HtrA1 directly cleaves this transmembrane protein or induces its cleavage through activation of other proteases remains to be shown. We further reported that SerpinE2 through physical interaction prevents HtrA1-mediated Sdc4 degradation, and that endogenous SerpinE2 is needed to protect the integrity of Sdc4 in embryos (Acosta et al., 2015). Here, we showed that Sdc4 mRNA rescued NC migration defects that were induced by co-injection of HtrA1 mRNA, indicating that Sdc4 is a relevant target of HtrA1 in vivo. The finding that Sdc4 mRNA also rescued migration defects in SerpinE2-morphant embryos suggests a proteolytic pathway by the following double inhibition mechanism:
SerpinE2 ┤HtrA1 protease ┤Syndecan-4 → NC cell migration.
SerpinE2 and HtrA1 might modulate FGF signals in directed NC migration
FGF signals including FGF8 stimulate NC migration by increasing cell motility and guiding cell movement (Kubota and Ito, 2000; Sato et al., 2011). In Xenopus embryos, the target tissues of NC streams secrete FGF8 and other FGF ligands, while the migrating NC cells express FGF receptors, including FGFR1 and FGFR4 (Brändli and Kirschner, 1995; Lea et al., 2009). Once released from the cells, FGFs are bound by heparan sulfate (HS) chains of cell surface proteoglycans such as the transmembrane Sdc4, which limit their diffusion (Matsuo and Kimura-Yoshida, 2013). We previously reported that the HtrA1-mediated proteolytic cleavage of Sdc4 mobilizes FGFs complexed to its soluble ectodomain, allowing these FGF-proteoglycan messages to activate FGFRs at distance (Hou et al., 2007). HS protects FGFs against cleavage by serine proteases (Saksela et al., 1988; Sommer and Rifkin, 1989), and HtrA1 can degrade free FGF8 protein (Kim et al., 2012). We showed that the HtrA1 inhibitor SerpinE2 stabilizes Sdc4 and regulates long-range FGF signaling in the Xenopus embryo (Acosta et al., 2015). The expression data presented here suggest that opposing gradients of SerpinE2 and HtrA1 form in the collective of migrating NC cells (Figure 1H). By controlling the mobilization of FGF signals, the SerpinE2/HtrA1 pair might help to establish a chemotactic FGF gradient and thereby facilitate collective migration of NC cells (Figure 9B).
Figure 9. Model for a proteolytic pathway of SerpinE2 and HtrA1 that regulates collective neural crest migration.
(A) SerpinE2 stimulates collective NC migration by a double-inhibitory mechanism involving the secreted serine protease HtrA1 and its proteolytic substrates Syndecan-4 and fibronectin. (B) Opposing gradients of SerpinE2 and HtrA1 activities regulate the directed migration in a collective of NC cells. The SerpinE2/HtrA1 pair contributes to the formation of a chemoattractant gradient that guides the NC stream toward a source of FGF signals in the target tissue. High SerpinE2 and low HtrA1 levels coincide with abundant focal adhesion sites and polymerized actin that drive mesenchymal migration at the leading edge. (C) SerpinE2 anchored to the heparan sulfate chains of the transmembrane proteoglycan Syndecan-4 protects the integrity of focal adhesions at the leading front and allows collective cell migration to occur (left side). Unbound HtrA1 triggers the proteolytic cleavage of Syndecan-4 and degrades the matrix protein fibronectin (middle), causing loss of cell-matrix adhesion at the rear end of the NC cell collective (right side). FGF, fibroblast growth factor; HS, heparan sulfate; NC, neural crest; Sdc4, Syndecan-4.
Fibronectin is a possible target of the SerpinE2-HtrA1 axis in NC cell adhesion and migration
Binding of fibronectin to the HS chains of Sdc4 and to the extracellular domain of the integrin adhesion receptors is critical for the activation of intracellular signaling that affects actin polymerization and contraction (Keller-Pinter et al., 2021). Fibronectin is ubiquitously expressed along NC migration pathways in the Xenopus embryo (Davidson et al., 2004). This glycoprotein is the only known extracellular matrix component that promotes Xenopus cranial NC cell migration as an adhesive substrate (Alfandari et al., 2003). Our study showed that HtrA1 overexpression and SerpinE2 knockdown reduced adhesion of cranial NC cells to fibronectin in vitro. Since fibronectin is a proteolytic substrate of HtrA1 (Grau et al., 2006; Hadfield et al., 2008), degradation of this matrix component might contribute to the HtrA1-mediated inhibition of NC migration.
A gradient of serine protease activity may act in collective NC migration
We are proposing a mechanism, in which SerpinE2 and HtrA1 constitute a proteolytic pathway that regulates collective NC migration by targeting the focal adhesion protein Sdc4 and the matrix protein fibronectin (Figure 9A). Our structure-function analyses and epistatic experiments led us suggest a model in which SerpinE2 and HtrA1 regulate directed migration of the NC collective via remodeling of cell-matrix adhesions (Figure 9C). Since SerpinE2 has a high affinity for HS through its heparin binding domain (Li and Huntington, 2012), the transmembrane Sdc4 proteoglycan likely recruits this protease inhibitor to the pericellular space. Elevated SerpinE2 concentration keeps HtrA1 activity low near the leading edge of the NC collective; this protects the integrity of both Sdc4 and fibronectin in focal adhesions, which is critical for the leader cells to attach to the matrix and drive cell migration (left side in Figure 9C). Gradually decreasing SerpinE2 protein levels and a concomitant increase in HtrA1 protease activity behind the leader cells triggers degradation of Sdc4 and cleavage of fibronectin, causing disruption of focal adhesion complexes in follower cells and loss of cell-matrix binding at the rear end of the NC cluster (right side in Figure 9C).
SERPINE2, HTRA1, and SDC4 might interact in human placenta development and pre-eclampsia
An interaction of SerpinE2, HtrA1, and Sdc4 in cell migration might not be confined to the NC as shown in this study but could also occur in other aspects of development and disease. In the developing placenta, extravillous trophoblast (EVT) cells from the human embryo migrate into the inner uterus wall (endometrium) and invade the uterine spiral arteries, converting them into large blood vessels so that the blood flow to the embryo is enhanced. Inadequate EVT migration results in insufficient maternal artery remodeling, causing hypoxia of the placenta and hypertension in a pathological condition called pre-eclampsia that is potentially life-threatening for both fetus and mother. The placenta expresses the highest levels of SERPINE2 among all probed human tissues (The Human Protein Atlas; https://www.proteinatlas.org/), with abundant protein expression in migratory EVTs and spiral arteries (Chern et al., 2011). HTRA1 expression is low in EVTs, high in less motile villous trophoblasts and upregulated in the placenta of patients with pre-eclampsia (Ajayi et al., 2008). Interestingly, the migration and invasion of cultured EVTs is inhibited by silencing of SERPINE2 (Chern et al., 2011), overexpression of HTRA1 (Ajayi et al., 2008), and knockdown of SDC4 (Jeyarajah et al., 2019). The results suggest that the SERPINE2-HTRA1-SDC4 axis might regulate trophoblast motility during placental development and a serious pregnancy condition in humans.
The SERPINE2-HTRA1-SDC4 regulatory axis might act in cancer
Cell migration is a hallmark of tumor malignancy and essential for metastasis formation (Hanahan and Weinberg, 2011). SERPINE2 is upregulated in aggressive variants of several cancer types, and high expression levels correlate with poor prognosis in patients (Arocas and Bouton, 2015). Downregulation of SERPINE2 impairs the metastatic spread of melanoma and breast cancer cells in xenografted mice (Wagenblast et al., 2015; Wu, 2016; Tang et al., 2019), suggesting a positive role of SERPINE2 in cancer cell migration and metastasis. On the other hand, HTRA1 is downregulated in many primary tumors and metastatic foci, while low levels correlate with poor clinical outcome (Zurawa-Janicka et al., 2017). Overexpression of HTRA1 inhibits the invasion and migration of melanoma and breast cancer cells, respectively (Baldi et al., 2002; Wang et al., 2012). SDC4 is overexpressed in several tumor types such as melanoma, and high expression levels are associated with poor prognosis in breast cancer (Keller-Pinter et al., 2021). Gene silencing confirms the requirement of SDC4 in the metastasis of human breast carcinoma cells in mice (Leblanc et al., 2018). Together, the double-inhibitory mechanism involving the molecular interaction of SERPINE2, HTRA1, and SDC4 as suggested here for NC cell migration in the Xenopus embryo might also regulate cancer cell migration in human metastasis.
Conclusions
This is the first study to demonstrate an interaction of SerpinE2 and HtrA1 in cell migration in vivo. We showed that SerpinE2 and HtrA1 acted in the extracellular space at least partly through the cell surface proteoglycan Sdc4 to govern NC migration in Xenopus embryos. The secreted SerpinE2 and HtrA1 proteins, as well as the ectodomain of Sdc4, are amenable to neutralizing antibodies. Our structure-function analysis unraveled important roles for the RCL of SerpinE2 and the catalytic serine residue of its target protease, whereas the PDZ domain of HtrA1 was not involved in the control of NC cell migration. The identification of critical domains in SerpinE2 and HtrA1 provides an important basis for the development of effective therapeutics in craniofacial anomalies and other neurocristopathies. Given the roles of these proteins in trophoblast migration in the developing placenta, together with their implication in metastasis of numerous tumor types, the SERPINE2-HTRA1-SDC4 axis constitutes a potential therapeutic target for the treatment of pre-eclampsia and various aggressive cancers.
Materials and methods
Key resources table.
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Cell line (Homo sapiens) | HEK293T | TakaraBio | Cat# 632180 | Used for transient transfection and protein production |
| Biological sample (X. laevis, male) | Sperm | Nasco | Cat# LM00715M | Used for in vitro fertilization |
| Biological sample (X. laevis, male) | Sperm | Xenopus 1 | Cat# 5215 | Albino; used for in vitro fertilization |
| Biological sample (X. laevis, female) | Egg | Nasco | Cat# LM00535M | Used for in vitro fertilization |
| Biological sample (X. laevis, female) | Egg | Nasco | Cat# LM00510M | Albino; used for in vitro fertilization |
| Biological sample (X. laevis, female) | Egg | Xenopus 1 | Cat# 4280 | Used for in vitro fertilization |
| Antibody | Anti-flag M2-Peroxidase (HRP) (Mouse monoclonal) | Sigma-Aldrich | Cat# A8592 | WB (1:1000) |
| Antibody | Anti-αTubulin (Mouse monoclonal) | Sigma-Aldrich | Cat# T5168 clone B-5-1-2 | WB (1:1000) |
| Antibody | Anti-βActin (Mouse monoclonal) | Sigma-Aldrich | Cat# A5441 clone AC-15 | WB (1:10,000) |
| Antibody | Anti-HtrA1 (Rabbit polyclonal) | Hou et al., 2007 PMID:17681134 | Immuno-purified GST5057, G22-7 | WB (1:2500) |
| Antibody | Mouse IgG HRP (Goat polyclonal) | R&D Systems | Cat# AF007 | WB (1:2000) |
| Antibody | Rabbit IgG HRP (Goat polyclonal) | R&D Systems | Cat# HAF008 | WB (1:2000) |
| Commercial assay or kit | NucleoBond PC 100, Midi Kit | Macherey-Nagel | Cat# 740573.100 | DNA purification |
| Commercial assay or kit | NucleoSpin Gel and PCR Clean-up, Mini kit | Macherey-Nagel | Cat# 740609.50 | DNA purification |
| Commercial assay or kit | mMessage mMachine SP6 Transcription kit | Invitrogen | Cat# AM1340 | mRNA synthesis |
| Commercial assay or kit | RNeasy Mini Kit | QIAGEN | Cat# 74104 | RNA purification |
| Commercial assay or kit | Pierce BCA Protein Assay Kit | Thermo Fisher Scientific | Cat# 23225 | Protein measurement |
| Chemical compound, drug | Pfu DNA Polymerase | Thermo Fisher Scientific | Cat# EP0572 | DNA synthesis |
| Chemical compound, drug | Lipofectamin 3000 Transfection Reagent | Thermo Fisher Scientific | Cat# L3000001 | Transfection |
| Chemical compound, drug | RIPA Lysis and Extraction Buffer | Thermo Fisher Scientific | Cat# 89901 | Protein purification |
| Chemical compound, drug | Halt Protease and Phosphatase Inhibitor Cocktail | Thermo Fisher Scientific | Cat# 78442 | Protein purification |
| Chemical compound, drug | cOmplete, EDTA-free Protease Inhibitor Cocktail | Roche | Cat# 11873580001 | Protein purification |
| Chemical compound, drug | Bolt Bis-Tris Plus Mini Protein Gels, 4–12% | Thermo Fisher Scientific | Cat# NW04125BOX | Protein electrophoresis |
| Chemical compound, drug | Bolt Sample Reducing Agent (10×) | Thermo Fisher Scientific | Cat# B0009 | Protein electrophoresis |
| Chemical compound, drug | Bolt LDS Sample Buffer (4×) | Thermo Fisher Scientific | Cat# B0007 | Protein electrophoresis |
| Chemical compound, drug | Bolt MES SDS Running Buffer (20×) | Thermo Fisher Scientific | Cat# B0002 | Protein electrophoresis |
| Chemical compound, drug | PageRuler Prestained Protein Ladder, 10–180 kDa | Thermo Fisher Scientific | Cat# 26616 | Protein electrophoresis |
| Chemical compound, drug | Ponceau S Solution | Sigma-Aldrich | Cat# P7170 | Western blotting |
| Chemical compound, drug | Pierce ECL Western Blotting Substrate | Thermo Fisher Scientific | Cat# 32106 | Western blotting |
| Chemical compound, drug | Restore PLUS Western Blot Stripping Buffer | Thermo Fisher Scientific | Cat# 46430 | Western blotting |
| Chemical compound, drug | Human Plasma Fibronectin Purified Protein | Sigma-Aldrich | Cat# FC010 | Neural crest explant culture |
| Chemical compound, drug | Gentamycin Solution | Sigma-Aldrich | Cat# G1272 | Embryo culture |
| Software, algorithm | ImageJ | NIH https://imagej.net/ij/ |
RRID:SCR_003070 | Neural crest explant measurement |
| Software, algorithm | AxioVision 4.8 | Zeiss https://www.zeiss.com/ |
RRID:SCR_002677 | Neural crest explant imaging |
| Other | 35 mm Dish, No. 0 Coverslip, 10 mm Glass Diameter, uncoated |
MatTek | Cat# P35G-0-10C | Neural crest explant culture, time-lapse imaging |
| Other | Vivaspin 2 MWCO 10,000 | Cytiva | Cat# 28932247 | Protein concentration |
| Other | iBlot Transfer Stack, PVDF, mini | Thermo Fisher Scientific | Cat# IB401002 | Western blotting |
Materials availability statement
Requests for resources and further information should be directed and will be fulfilled by the corresponding author, Edgar M Pera.
Constructs
pCS2-Flag-HtrA1 (identical with pCS2-xHtrA1*; Hou et al., 2007) and pCS2-Flag-SerpinE2 (identical with pCS2-Flag-PN1; Acosta et al., 2015) were previously described. Briefly, N-terminally truncated open reading frames of X. laevis HtrA1.S (amino acids 17–459) and SerpinE2.L (amino acids 20–395) were each introduced into a modified pCS2 expression vector in frame and downstream of a secretion cassette with the sequence MQCPPILLVWTLWIMAVDCSRPKVFLPIQPEQEPLQSKT(DYKDDDDK)LE that contains a cleavable signal peptide (underlined) and N-terminus until Thr39 of X. laevis Chordin, followed by a Flag-tag (in brackets) and two amino acids (LE) representing an XhoI cloning site (pCS2-ChdSP-Flag, constructed by Stefano Piccolo in the laboratory of Eddy De Robertis, UCLA). Using pCS2-Flag-HtrA1 and pCS2-Flag-SerpinE2 as templates, the N-terminally truncated open reading frames of HtrA1 and SerpinE2 were PCR-amplified, using a forward primer that inserts a start codon before the N-terminal Flag-tag, and subcloned into the expression vector pCS2 to generate pCS2-Flag-HtrA1ΔSP and pCS2-Flag-SerpinE2ΔSP. The PCRs were performed with high fidelity Pfu DNA polymerase (Thermo Fisher, EP0572), and correct sequences were validated by sequencing in sense and antisense directions (Eurofins, Germany). Protein bands were quantified using Image Lab (Bio-Rad).
Antisense morpholino oligonucleotides (purchased from Gene Tools LLC)
| Name | Forward | Reference |
|---|---|---|
| Standard control-MO | 5’- CCT CTT ACC TCA GTT ACA ATT TAT A | Gene Tools LLC |
| SerpinE2.L (PN1.a)-MO1 | 5’- GAA GTC AAG TAA GAA TAC TCC CGG C | Acosta et al., 2015 |
| SerpinE2.L (PN1.a)-MO2 | 5’- ACT AGT CGC CTC ATG ATC GTA CAA C | Acosta et al., 2015 |
| SerpinE2.S (PN1.b)-MO | 5’- CAT GAT CGT AGA ACT GGA TAG AAG T | Acosta et al., 2015 |
| SerpinE2-5MM-MO | 5’-ACT ACT CAC CTA ATG ATA GTA AAA C | Acosta et al., 2015 |
| HtrA1-MO | 5’- ACA CCG CCA GCC ACA ACA TGG TCA T | Hou et al., 2007 |
Xenopus embryo microinjection
To prepare mRNA, pCS2 constructs containing HtrA1, HtrA1-S307A, and Flag-HtrA1 (Hou et al., 2007), HtrA1-myc, HtrA1ΔPDZ-myc, Flag-SerpinE2 and Flag-SerpinE2pm (Acosta et al., 2015), Flag-HtrA1ΔSP and Flag-SerpinE2ΔSP (this study), nlacZ (a kind gift from Dr. Tomas Pieler, University Göttingen, Germany), eGFP (a kind gift from Dr. Eric Bellefroid, Université Libre de Bruxelles, Belgium) and Flag-Sdc4 (Muñoz et al., 2006) were linearized with NotI and transcribed with Sp6 RNA polymerase (mMessage mMachine, Invitrogen). Embryos were injected in 1× MBS (Modified Barth’s saline) and cultured in 0.1× MBS. Pigmented embryos were injected into all four animal blastomeres at the eight-cell Muñoz et al., 2006 stage. Albino embryos were injected into a single animal blastomere with nlacZ mRNA as a lineage tracer and stained with Magenta-Red (Sigma B8931, red nuclei) after fixation. Dorsally and ventrally injected embryos were identified based on the lineage tracer pattern. Embryos were prepared, fixed, and processed by whole-mount in situ hybridization as described (Pera et al., 2015).
Neural crest explantation, in vitro culture, and time-lapse imaging
For NC extirpation, the vitelline membrane was removed with fine forceps from pigmented embryos at stage 17 and the neural folds were extracted in 1× MMR (Marc’s Minimal Ringers) buffer, using eye lashes mounted with nail polisher to pipette tips. Operated embryos were cultured in 0.3× MMR and 50 µg/ml Gentamycin (Sigma G1272) until stage 41.
CNC explants and single fluorescently labeled cells from eGFP mRNA-injected embryos were cultured in Danilchik’s for Amy media (53 mM NaCl, 5 mM Na2CO3, 4.5 K gluconate, 32 mM Na gluconate, 1 mM MgSO4, 1 mM CaCl2, 0.1% bovine serum albumin, adjusted with 1 M bicine to pH 8.3) in fibronectin-coated plastic dishes as described (Gouignard et al., 2016).
For video time-lapse imaging, CNC explants were cultured in 23% Leibovitz’s L15 medium in fibronectin-coated glass-bottom culture dishes (MatTek Corp. P35G-0-10C). The video was taken after 7 hr in culture. Filming was done with a Zeiss inverted microscope, and AxioVision 4.8.2 SP3 software was used for imaging.
Cell culture and transfection
HEK293T cells were cultured in DMEM (Dulbecco’s modified Eagle medium) supplemented with 10% heat-inactivated fetal bovine serum and penicillin/streptavidin. The cells were seeded at a density of 20–30% in Opti-MEM (Thermo Fisher Scientific) with penicillin/streptavidin in six-well plates, and transient transfection with plasmid DNAs was performed using Lipofectamin 3000 (Thermo Fisher Scientific), when cells reached between 60% and 70% confluency. Following a medium exchange 6 hr after transfection, the supernatant was harvested, and the cells were lysed 70 hr after transfection.
Sample preparations and western blotting
Microinjected embryos and transfected HEK293T cells were lysed using RIPA-buffer (Thermo Fisher Scientific, Cat# 89901) supplemented with protease/phosphatase inhibitor cocktail (Thermo Fisher Scientific, Cat# 78442). The cell lysate was sonicated, using the Sonicator Bioruptor Plus (Diagenode) with 40 cycles for 15 s on and 15 s off. The supernatant was cleared from cells by centrifugations first at 2500 rpm and then at 8000 rpm for each 5 min at 4°C. After addition of a protease inhibitor cocktail (Roche, Cat# 11873580001), proteins in the supernatant were concentrated, using Vivaspin2 10 kDa molecular weight cut-off columns (Cytiva, Cat# 28932247). Proteins were measured in the lysates and supernatants, using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Cat# 23225). Gel electrophoresis was performed with 20 μg protein per lane, using Bolt Bis-Tris Plus Mini Protein gels (4–12%, Thermo Fisher Scientific, Cat# NW04125BOX). Western blots were performed, using the following primary antibodies: anti-flag HRP-conjugated (1:1000; Sigma-Aldrich, A8592), anti-αTubulin (1:1000; Sigma-Aldrich, T5168), anti-βActin (1:10,000; Sigma-Aldrich, A5441), immunopurified anti-HtrA1 (5 μg/ml; Hou et al., 2007).
Statistical analysis
Results are shown as mean ± standard deviation (SD). Unpaired t-test was employed for comparing means of two treatment groups. For multiple comparisons, one-way ANOVA followed by Tukey’s post hoc test was used. Statistical significance was defined as **p<0.01, ***p<0.001, and ****p<0.0001.
Acknowledgements
We are indebted to Drs. E Bellefroid, EM De Robertis, J Larraine, J-P Saint Jeannet, and T Pieler for plasmids, to Drs. Erika Velásquez, Isak Martinsson, Oxana Klementieva, Gunnar Gouras for sharing advice and equipment, and to J Monka for language improvement of the manuscript. EMP wishes to thank Dr. Eddy De Robertis for valuable comments on the manuscript, suggestion of the neural crest extirpation experiment, generous support, and being a superb host during a memorable 3-month sabbatical stay (May to July 2023) in his laboratory at UCLA in Los Angeles (USA). Funding: This paper was supported by the following grants: Swedish Research Council 2009-4951 to Edgar M Pera. Swedish Childhood Cancer Fund. PROJ11/101 to Edgar M Pera. OE and Edla Johansson foundation to Edgar M Pera. Albert Påhlsson foundation to Edgar M Pera. Pia Ståhl foundation to Edgar M Pera. MultiPark to Laurent Roybon. Swedish Research Council 2021-02284 to Laurent Roybon.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Edgar M Pera, Email: edgar.pera@med.lu.se.
Yuji Mishina, University of Michigan, United States.
Jonathan A Cooper, Fred Hutchinson Cancer Research Center, United States.
Funding Information
This paper was supported by the following grants:
Swedish Research Council 2009-4951 to Edgar M Pera.
Swedish Childhood Cancer Fund ProJ11/101 to Edgar M Pera.
O. E. och Edla Johanssons Vetenskapliga Stiftelse to Edgar M Pera.
Albert Påhlsson Foundation to Edgar M Pera.
Pia Stahl Foundation to Edgar M Pera.
Swedish Research Council 2021-02284 to Laurent Roybon.
Lund University MultiPark to Laurent Roybon.
Additional information
Competing interests
No competing interests declared.
Author contributions
Conceptualization, Resources, Formal analysis, Supervision, Funding acquisition, Validation, Investigation, Visualization, Methodology, Writing - original draft, Project administration, Writing - review and editing.
Conceptualization, Formal analysis, Validation, Investigation, Visualization.
Resources, Formal analysis, Validation, Investigation.
Resources, Funding acquisition.
Conceptualization, Formal analysis, Validation, Investigation, Methodology.
Ethics
This study was conducted in strict accordance with all local, national, and European regulations and ARRIVE guidelines. All Xenopus laevis experiments reported in this study were approved by the Lund/Malmö; regional ethical committee (Dnr. 5.8.18-15884/2019).
Additional files
Data availability
All data generated or analysed during this study are included in the manuscript and supporting files. Three source data files have been provided for Figure 6—figure supplement 2.
References
- Acosta H, Iliev D, Grahn THM, Gouignard N, Maccarana M, Griesbach J, Herzmann S, Sagha M, Climent M, Pera EM. The serpin PN1 is a feedback regulator of FGF signaling in germ layer and primary axis formation. Development. 2015;142:1146–1158. doi: 10.1242/dev.113886. [DOI] [PubMed] [Google Scholar]
- Agrawal M, Brauer PR. Urokinase-type plasminogen activator regulates cranial neural crest cell migration in vitro. Developmental Dynamics. 1996;207:281–290. doi: 10.1002/(SICI)1097-0177(199611)207:3<281::AID-AJA5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Ajayi F, Kongoasa N, Gaffey T, Asmann YW, Watson WJ, Baldi A, Lala P, Shridhar V, Brost B, Chien J. Elevated expression of serine protease HtrA1 in preeclampsia and its role in trophoblast cell migration and invasion. American Journal of Obstetrics and Gynecology. 2008;199:557. doi: 10.1016/j.ajog.2008.04.046. [DOI] [PubMed] [Google Scholar]
- Alfandari D, Cousin H, Gaultier A, Hoffstrom BG, DeSimone DW. Integrin alpha5beta1 supports the migration of Xenopus cranial neural crest on fibronectin. Developmental Biology. 2003;260:449–464. doi: 10.1016/s0012-1606(03)00277-x. [DOI] [PubMed] [Google Scholar]
- An E, Sen S, Park SK, Gordish-Dressman H, Hathout Y. Identification of novel substrates for the serine protease HTRA1 in the human RPE secretome. Investigative Ophthalmology & Visual Science. 2010;51:3379–3386. doi: 10.1167/iovs.09-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arocas V, Bouton MC. In: The Serpin Family. Geiger M, Wahlmüller F, Furtmüller M, editors. Cham: Springer International Publishing; 2015. Protease Nexin-1: a Serpin involved in pathophysiology; pp. 179–196. [DOI] [Google Scholar]
- Baldi A, De Luca A, Morini M, Battista T, Felsani A, Baldi F, Catricalà C, Amantea A, Noonan DM, Albini A, Natali PG, Lombardi D, Paggi MG. The HtrA1 serine protease is down-regulated during human melanoma progression and represses growth of metastatic melanoma cells. Oncogene. 2002;21:6684–6688. doi: 10.1038/sj.onc.1205911. [DOI] [PubMed] [Google Scholar]
- Brändli AW, Kirschner MW. Molecular cloning of tyrosine kinases in the early Xenopus embryo: identification of Eck-related genes expressed in cranial neural crest cells of the second (hyoid) arch. Developmental Dynamics. 1995;203:119–140. doi: 10.1002/aja.1002030202. [DOI] [PubMed] [Google Scholar]
- Brauer PR, Yee JA. Cranial neural crest cells synthesize and secrete a latent form of transforming growth factor beta that can be activated by neural crest cell proteolysis. Developmental Biology. 1993;155:281–285. doi: 10.1006/dbio.1993.1026. [DOI] [PubMed] [Google Scholar]
- Campioni M, Severino A, Manente L, Tuduce IL, Toldo S, Caraglia M, Crispi S, Ehrmann M, He X, Maguire J, De Falco M, De Luca A, Shridhar V, Baldi A. The serine protease HtrA1 specifically interacts and degrades the tuberous sclerosis complex 2 protein. Molecular Cancer Research. 2010;8:1248–1260. doi: 10.1158/1541-7786.MCR-09-0473. [DOI] [PubMed] [Google Scholar]
- Chern SR, Li SH, Chiu CL, Chang HH, Chen CP, Tsuen Chen EI. Spatiotemporal expression of SERPINE2 in the human placenta and its role in extravillous trophoblast migration and invasion. Reproductive Biology and Endocrinology. 2011;9:106. doi: 10.1186/1477-7827-9-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien J, He X, Shridhar V. Identification of tubulins as substrates of serine protease HtrA1 by mixture-based oriented peptide library screening. Journal of Cellular Biochemistry. 2009a;107:253–263. doi: 10.1002/jcb.22121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien J, Ota T, Aletti G, Shridhar R, Boccellino M, Quagliuolo L, Baldi A, Shridhar V. Serine protease HtrA1 associates with microtubules and inhibits cell migration. Molecular and Cellular Biology. 2009b;29:4177–4187. doi: 10.1128/MCB.00035-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clawson GA, Bui V, Xin P, Wang N, Pan W. Intracellular localization of the tumor suppressor HtrA1/Prss11 and its association with HPV16 E6 and E7 proteins. Journal of Cellular Biochemistry. 2008;105:81–88. doi: 10.1002/jcb.21804. [DOI] [PubMed] [Google Scholar]
- Davidson LA, Keller R, DeSimone DW. Assembly and remodeling of the fibrillar fibronectin extracellular matrix during gastrulation and neurulation in Xenopus laevis. Developmental Dynamics. 2004;231:888–895. doi: 10.1002/dvdy.20217. [DOI] [PubMed] [Google Scholar]
- DuShane GP. An experimental study of the origin of pigment cells in Amphibia. Journal of Experimental Zoology. 1935;72:1–31. doi: 10.1002/jez.1400720102. [DOI] [Google Scholar]
- Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nature Reviews. Molecular Cell Biology. 2009;10:445–457. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- Gouignard N, Maccarana M, Strate I, von Stedingk K, Malmström A, Pera EM. Musculocontractural Ehlers-Danlos syndrome and neurocristopathies: dermatan sulfate is required for Xenopus neural crest cells to migrate and adhere to fibronectin. Disease Models & Mechanisms. 2016;9:607–620. doi: 10.1242/dmm.024661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouignard N, Andrieu C, Theveneau E. Neural crest delamination and migration: Looking forward to the next 150 years. Genesis. 2018;56:e23107. doi: 10.1002/dvg.23107. [DOI] [PubMed] [Google Scholar]
- Grau S, Richards PJ, Kerr B, Hughes C, Caterson B, Williams AS, Junker U, Jones SA, Clausen T, Ehrmann M. The role of human HtrA1 in arthritic disease. The Journal of Biological Chemistry. 2006;281:6124–6129. doi: 10.1074/jbc.M500361200. [DOI] [PubMed] [Google Scholar]
- Hadfield KD, Rock CF, Inkson CA, Dallas SL, Sudre L, Wallis GA, Boot-Handford RP, Canfield AE. HtrA1 inhibits mineral deposition by osteoblasts: requirement for the protease and PDZ domains. The Journal of Biological Chemistry. 2008;283:5928–5938. doi: 10.1074/jbc.M709299200. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Holstein TW. The role of cnidarian developmental biology in unraveling axis formation and Wnt signaling. Developmental Biology. 2022;487:74–98. doi: 10.1016/j.ydbio.2022.04.005. [DOI] [PubMed] [Google Scholar]
- Hopwood ND, Pluck A, Gurdon JB. A Xenopus mRNA related to Drosophila twist is expressed in response to induction in the mesoderm and the neural crest. Cell. 1989;59:893–903. doi: 10.1016/0092-8674(89)90612-0. [DOI] [PubMed] [Google Scholar]
- Hou S, Maccarana M, Min TH, Strate I, Pera EM. The Secreted Serine Protease xHtrA1 Stimulates Long-Range FGF Signaling in the Early Xenopus Embryo. Developmental Cell. 2007;13:226–241. doi: 10.1016/j.devcel.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Howie RN, Durham EL, Black L, Bennfors G, Parsons TE, Elsalanty ME, Yu JC, Weinberg SM, Cray JJ. Effects of in utero thyroxine exposure on murine cranial suture growth. PLOS ONE. 2016;11:e0167805. doi: 10.1371/journal.pone.0167805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyarajah MJ, Jaju Bhattad G, Kops BF, Renaud SJ. Syndecan-4 regulates extravillous trophoblast migration by coordinating protein kinase C activation. Scientific Reports. 2019;9:10175. doi: 10.1038/s41598-019-46599-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller-Pinter A, Gyulai-Nagy S, Becsky D, Dux L, Rovo L. Syndecan-4 in Tumor Cell Motility. Cancers. 2021;13:3322. doi: 10.3390/cancers13133322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GY, Kim HY, Kim HT, Moon JM, Kim CH, Kang S, Rhim H. HtrA1 is a novel antagonist controlling fibroblast growth factor (FGF) signaling via cleavage of FGF8. Molecular and Cellular Biology. 2012;32:4482–4492. doi: 10.1128/MCB.00872-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Ito K. Chemotactic migration of mesencephalic neural crest cells in the mouse. Developmental Dynamics. 2000;217:170–179. doi: 10.1002/(SICI)1097-0177(200002)217:2<170::AID-DVDY4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Lea R, Papalopulu N, Amaya E, Dorey K. Temporal and spatial expression of FGF ligands and receptors during Xenopus development. Developmental Dynamics. 2009;238:1467–1479. doi: 10.1002/dvdy.21913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc R, Sahay D, Houssin A, Machuca-Gayet I, Peyruchaud O. Autotaxin-β interaction with the cell surface via syndecan-4 impacts on cancer cell proliferation and metastasis. Oncotarget. 2018;9:33170–33185. doi: 10.18632/oncotarget.26039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin N, Kalcheim C. The Neural Crest. Cambridge: Cambridge University Press; 1999. [DOI] [Google Scholar]
- Lee HX, Ambrosio AL, Reversade B, De Robertis EM. Embryonic dorsal-ventral signaling: secreted frizzled-related proteins as inhibitors of tolloid proteinases. Cell. 2006;124:147–159. doi: 10.1016/j.cell.2005.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Huntington JA. Crystal structures of protease nexin-1 in complex with heparin and thrombin suggest a 2-step recognition mechanism. Blood. 2012;120:459–467. doi: 10.1182/blood-2012-03-415869. [DOI] [PubMed] [Google Scholar]
- Manon-Jensen T, Multhaupt HAB, Couchman JR. Mapping of matrix metalloproteinase cleavage sites on syndecan-1 and syndecan-4 ectodomains. The FEBS Journal. 2013;280:2320–2331. doi: 10.1111/febs.12174. [DOI] [PubMed] [Google Scholar]
- Matsuo I, Kimura-Yoshida C. Extracellular modulation of Fibroblast Growth Factor signaling through heparan sulfate proteoglycans in mammalian development. Current Opinion in Genetics & Development. 2013;23:399–407. doi: 10.1016/j.gde.2013.02.004. [DOI] [PubMed] [Google Scholar]
- Matthews HK, Marchant L, Carmona-Fontaine C, Kuriyama S, Larraín J, Holt MR, Parsons M, Mayor R. Directional migration of neural crest cells in vivo is regulated by Syndecan-4/Rac1 and non-canonical Wnt signaling/RhoA. Development. 2008;135:1771–1780. doi: 10.1242/dev.017350. [DOI] [PubMed] [Google Scholar]
- Medina-Cuadra L, Monsoro-Burq AH. Xenopus, an emerging model for studying pathologies of the neural crest. Current Topics in Developmental Biology. 2021;145:313–348. doi: 10.1016/bs.ctdb.2021.03.002. [DOI] [PubMed] [Google Scholar]
- Menoud PA, Debrot S, Schowing J. Mouse neural crest cells secrete both urokinase-type and tissue-type plasminogen activators in vitro. Development. 1989;106:685–690. doi: 10.1242/dev.106.4.685. [DOI] [PubMed] [Google Scholar]
- Minoux M, Rijli FM. Molecular mechanisms of cranial neural crest cell migration and patterning in craniofacial development. Development. 2010;137:2605–2621. doi: 10.1242/dev.040048. [DOI] [PubMed] [Google Scholar]
- Monard D. SERPINE2/Protease Nexin-1 in vivo multiple functions: Does the puzzle make sense? Seminars in Cell & Developmental Biology. 2017;62:160–169. doi: 10.1016/j.semcdb.2016.08.012. [DOI] [PubMed] [Google Scholar]
- Muñoz R, Moreno M, Oliva C, Orbenes C, Larraín J. Syndecan-4 regulates non-canonical Wnt signalling and is essential for convergent and extension movements in Xenopus embryos. Nature Cell Biology. 2006;8:492–500. doi: 10.1038/ncb1399. [DOI] [PubMed] [Google Scholar]
- Nieto MA, Huang RY-J, Jackson RA, Thiery JP. EMT: 2016. Cell. 2016;166:21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- O’Connell MP, Fiori JL, Kershner EK, Frank BP, Indig FE, Taub DD, Hoek KS, Weeraratna AT. Heparan sulfate proteoglycan modulation of Wnt5A signal transduction in metastatic melanoma cells. The Journal of Biological Chemistry. 2009;284:28704–28712. doi: 10.1074/jbc.M109.028498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson ST, Gettins PGW. Regulation of proteases by protein inhibitors of the serpin superfamily. Progress in Molecular Biology and Translational Science. 2011;99:185–240. doi: 10.1016/B978-0-12-385504-6.00005-1. [DOI] [PubMed] [Google Scholar]
- Onuma Y, Asashima M, Whitman M. A Serpin family gene, protease nexin-1 has an activity distinct from protease inhibition in early Xenopus embryos. Mechanisms of Development. 2006;123:463–471. doi: 10.1016/j.mod.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Owji H, Nezafat N, Negahdaripour M, Hajiebrahimi A, Ghasemi Y. A comprehensive review of signal peptides: Structure, roles, and applications. European Journal of Cell Biology. 2018;97:422–441. doi: 10.1016/j.ejcb.2018.06.003. [DOI] [PubMed] [Google Scholar]
- Pei X, Ma K, Xu J, Wang N, Liu N. Inhibition of cell proliferation and migration after HTRA1 knockdown in retinal pigment epithelial cells. Graefe’s Archive for Clinical and Experimental Ophthalmology = Albrecht von Graefes Archiv Fur Klinische Und Experimentelle Ophthalmologie. 2015;253:565–572. doi: 10.1007/s00417-014-2901-2. [DOI] [PubMed] [Google Scholar]
- Pera EM, Hou S, Strate I, Wessely O, De Robertis EM. Exploration of the extracellular space by a large-scale secretion screen in the early Xenopus embryo. The International Journal of Developmental Biology. 2005;49:781–796. doi: 10.1387/ijdb.052003ep. [DOI] [PubMed] [Google Scholar]
- Pera EM, Acosta H, Gouignard N, Climent M. In: In Situ Hybridization Methods. Neuromethods. Hauptmann G, editor. New York, NY: Humana Press; 2015. Whole-mount in situ hybridization and immunohistochemistry in Xenopus embryos; pp. 151–167. [DOI] [Google Scholar]
- Piacentino ML, Li Y, Bronner ME. Epithelial-to-mesenchymal transition and different migration strategies as viewed from the neural crest. Current Opinion in Cell Biology. 2020;66:43–50. doi: 10.1016/j.ceb.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Agius E, Lu B, Goodman S, Dale L, De Robertis EM. Cleavage of Chordin by Xolloid metalloprotease suggests a role for proteolytic processing in the regulation of Spemann organizer activity. Cell. 1997;91:407–416. doi: 10.1016/s0092-8674(00)80424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooryck C, Diaz-Font A, Osborn DPS, Chabchoub E, Hernandez-Hernandez V, Shamseldin H, Kenny J, Waters A, Jenkins D, Kaissi AA, Leal GF, Dallapiccola B, Carnevale F, Bitner-Glindzicz M, Lees M, Hennekam R, Stanier P, Burns AJ, Peeters H, Alkuraya FS, Beales PL. Mutations in lectin complement pathway genes COLEC11 and MASP1 cause 3MC syndrome. Nature Genetics. 2011;43:197–203. doi: 10.1038/ng.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani B, Thiébaud CH. Neural crest development in the Xenopus laevis embryo, studied by interspecific transplantation and scanning electron microscopy. Developmental Biology. 1987;124:91–110. doi: 10.1016/0012-1606(87)90463-5. [DOI] [PubMed] [Google Scholar]
- Saksela O, Moscatelli D, Sommer A, Rifkin DB. Endothelial cell-derived heparan sulfate binds basic fibroblast growth factor and protects it from proteolytic degradation. The Journal of Cell Biology. 1988;107:743–751. doi: 10.1083/jcb.107.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Scholl AM, Kuhn EB, Stadt HA, Decker JR, Pegram K, Hutson MR, Kirby ML. FGF8 signaling is chemotactic for cardiac neural crest cells. Developmental Biology. 2011;354:18–30. doi: 10.1016/j.ydbio.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schock EN, York JR, LaBonne C. The developmental and evolutionary origins of cellular pluripotency in the vertebrate neural crest. Seminars in Cell & Developmental Biology. 2023;138:36–44. doi: 10.1016/j.semcdb.2022.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seetharaman S, Etienne-Manneville S. Cytoskeletal crosstalk in cell migration. Trends in Cell Biology. 2020;30:720–735. doi: 10.1016/j.tcb.2020.06.004. [DOI] [PubMed] [Google Scholar]
- Session AM, Uno Y, Kwon T, Chapman JA, Toyoda A, Takahashi S, Fukui A, Hikosaka A, Suzuki A, Kondo M, van Heeringen SJ, Quigley I, Heinz S, Ogino H, Ochi H, Hellsten U, Lyons JB, Simakov O, Putnam N, Stites J, Kuroki Y, Tanaka T, Michiue T, Watanabe M, Bogdanovic O, Lister R, Georgiou G, Paranjpe SS, van Kruijsbergen I, Shu S, Carlson J, Kinoshita T, Ohta Y, Mawaribuchi S, Jenkins J, Grimwood J, Schmutz J, Mitros T, Mozaffari SV, Suzuki Y, Haramoto Y, Yamamoto TS, Takagi C, Heald R, Miller K, Haudenschild C, Kitzman J, Nakayama T, Izutsu Y, Robert J, Fortriede J, Burns K, Lotay V, Karimi K, Yasuoka Y, Dichmann DS, Flajnik MF, Houston DW, Shendure J, DuPasquier L, Vize PD, Zorn AM, Ito M, Marcotte EM, Wallingford JB, Ito Y, Asashima M, Ueno N, Matsuda Y, Veenstra GJC, Fujiyama A, Harland RM, Taira M, Rokhsar DS. Genome evolution in the allotetraploid frog Xenopus laevis. Nature. 2016;538:336–343. doi: 10.1038/nature19840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shellard A, Mayor R. Rules of collective migration: from the wildebeest to the neural crest. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2020;375:20190387. doi: 10.1098/rstb.2019.0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer A, Rifkin DB. Interaction of heparin with human basic fibroblast growth factor: Protection of the angiogenic protein from proteolytic degradation by a glycosaminoglycan. Journal of Cellular Physiology. 1989;138:215–220. doi: 10.1002/jcp.1041380129. [DOI] [PubMed] [Google Scholar]
- Stone LS. Experiments on the development of the cranial ganglia and the lateral line sense organs in Amblystoma punctatum. Journal of Experimental Zoology. 1922;35:420–496. doi: 10.1002/jez.1400350403. [DOI] [Google Scholar]
- Stone LS. Further experiments on the extirpation and transplantation of mesectoderm in Amblystoma punctatum. Journal of Experimental Zoology. 1926;44:95–131. doi: 10.1002/jez.1400440104. [DOI] [Google Scholar]
- Suzuki T, Kusakabe M, Nakayama K, Nishida E. The protein kinase MLTK regulates chondrogenesis by inducing the transcription factor Sox6. Development. 2012;139:2988–2998. doi: 10.1242/dev.078675. [DOI] [PubMed] [Google Scholar]
- Szabó A, Mayor R. Mechanisms of neural crest migration. Annual Review of Genetics. 2018;52:43–63. doi: 10.1146/annurev-genet-120417-031559. [DOI] [PubMed] [Google Scholar]
- Tang T, Zhu Q, Li X, Zhu G, Deng S, Wang Y, Ni L, Chen X, Zhang Y, Xia T, Zen K, Pan Y, Jin L. Protease Nexin I is a feedback regulator of EGF/PKC/MAPK/EGR1 signaling in breast cancer cells metastasis and stemness. Cell Death & Disease. 2019;10:649. doi: 10.1038/s41419-019-1882-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker RP. The role of glycosaminoglycans in anuran pigment cell migration. Development. 1986;92:145–164. doi: 10.1242/dev.92.1.145. [DOI] [PubMed] [Google Scholar]
- Tucker AS, Slack JMW. Independent induction and formation of the dorsal and ventral fins in Xenopus laevis. Developmental Dynamics. 2004;230:461–467. doi: 10.1002/dvdy.20071. [DOI] [PubMed] [Google Scholar]
- Valinsky JE, Le Douarin NM. Production of plasminogen activator by migrating cephalic neural crest cells. The EMBO Journal. 1985;4:1403–1406. doi: 10.1002/j.1460-2075.1985.tb03793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenblast E, Soto M, Gutiérrez-Ángel S, Hartl CA, Gable AL, Maceli AR, Erard N, Williams AM, Kim SY, Dickopf S, Harrell JC, Smith AD, Perou CM, Wilkinson JE, Hannon GJ, Knott SRV. A model of breast cancer heterogeneity reveals vascular mimicry as A driver of metastasis. Nature. 2015;520:358–362. doi: 10.1038/nature14403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Eckert KA, Zomorrodi AR, Xin P, Pan W, Shearer DA, Weisz J, Maranus CD, Clawson GA. Down-regulation of HtrA1 activates the epithelial-mesenchymal transition and ATM DNA damage response pathways. PLOS ONE. 2012;7:e39446. doi: 10.1371/journal.pone.0039446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Couchman JR. Syndecan-4 and focal adhesion function. Current Opinion in Cell Biology. 2001;13:578–583. doi: 10.1016/s0955-0674(00)00254-4. [DOI] [PubMed] [Google Scholar]
- Wu QW. Serpine2, a potential novel target for combating melanoma metastasis. American Journal of Translational Research. 2016;8:1985–1997. [PMC free article] [PubMed] [Google Scholar]
- Ziegler B, Yiallouros I, Trageser B, Kumar S, Mercker M, Kling S, Fath M, Warnken U, Schnölzer M, Holstein TW, Hartl M, Marciniak-Czochra A, Stetefeld J, Stöcker W, Özbek S. The Wnt-specific astacin proteinase HAS-7 restricts head organizer formation in Hydra. BMC Biology. 2021;19:120. doi: 10.1186/s12915-021-01046-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawa-Janicka D, Wenta T, Jarzab M, Skorko-Glonek J, Glaza P, Gieldon A, Ciarkowski J, Lipinska B. Structural insights into the activation mechanisms of human HtrA serine proteases. Archives of Biochemistry and Biophysics. 2017;621:6–23. doi: 10.1016/j.abb.2017.04.004. [DOI] [PubMed] [Google Scholar]