Abstract
The sharing and documentation of cardiovascular research data are essential for efficient use and reuse of data, thereby aiding scientific transparency, accelerating the progress of cardiovascular research and healthcare, and contributing to the reproducibility of research results. However, challenges remain. This position paper, written on behalf of and approved by the German Cardiac Society and German Centre for Cardiovascular Research, summarizes our current understanding of the challenges in cardiovascular research data management (RDM). These challenges include lack of time, awareness, incentives, and funding for implementing effective RDM; lack of standardization in RDM processes; a need to better identify meaningful and actionable data among the increasing volume and complexity of data being acquired; and a lack of understanding of the legal aspects of data sharing. While several tools exist to increase the degree to which data are findable, accessible, interoperable, and reusable (FAIR), more work is needed to lower the threshold for effective RDM not just in cardiovascular research but in all biomedical research, with data sharing and reuse being factored in at every stage of the scientific process. A culture of open science with FAIR research data should be fostered through education and training of early-career and established research professionals. Ultimately, FAIR RDM requires permanent, long-term effort at all levels. If outcomes can be shown to be superior and to promote better (and better value) science, modern RDM will make a positive difference to cardiovascular science and practice. The full position paper is available in the supplementary materials.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00392-023-02303-3.
Keywords: Research data management, FAIR data, Metadata, Cardiac research, Cardiology
Introduction
Modern cardiovascular research has been associated with a rapid increase in the volume of data obtained by cardiovascular researchers as data are collected at ever finer levels of structural and functional complexity. At the same time, governments, funders, and journals have begun to encourage or require the sharing of research data in a findable, accessible, interoperable, and reusable (‘FAIR’) way [1–5].
However, there are significant barriers to effective and responsible research data management (RDM) in cardiovascular science that must be overcome. First, there is a lack of standardization in how data and metadata are collected, processed, and shared. Second, researchers often lack sufficient time, funding, or incentives to share their data. Third, the volume and complexity of data being collected makes the identification of meaningful and actionable data increasingly difficult. Finally, there are complex ethical and legal aspects of sharing data that researchers should understand, for example when dealing with sensitive data or sharing data across the borders of the European Union. Addressing these challenges will make it easier for scientists to use and understand their own data and the data of others.
The 3rd Joint German Cardiac Society (DGK) and German Centre for Cardiovascular Research (DZHK) Translational Workshop was held in Bonn, Germany in September 2022 to discuss the challenges and potential solutions associated with RDM in cardiovascular research; the topics, opinions, and findings discussed during the workshop are presented here. This position paper executive summary, written on behalf of and endorsed by the DGK and DZHK, identifies and describes challenges that scientists and clinicians currently face when collecting, using, and reusing data in the field of cardiovascular research and beyond. It then provides recommendations for improvements in RDM practices, informed by standardization efforts and guidelines from related domains. The full position paper can be found in the supplementary materials.
Data sharing and metadata
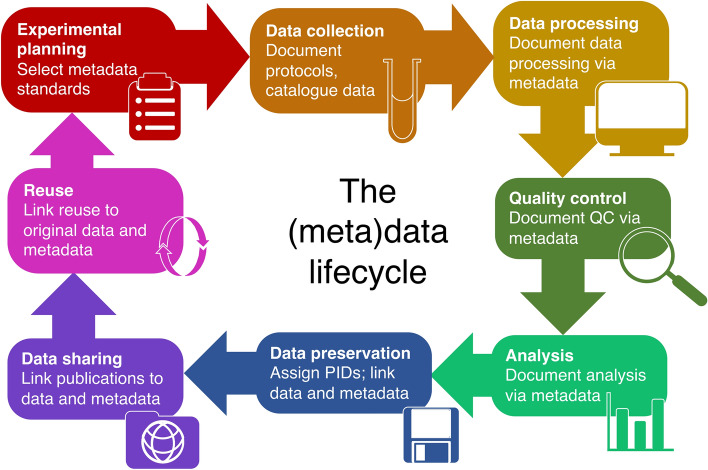
Effective RDM involves every step of the data lifecycle (Fig. 1) [6]. When planning a study, we recommend that researchers formulate a data management plan that considers how data and metadata will be collected, stored, annotated, analyzed, and shared [7–10]. Sharing data increases its impact [11] and allows researchers, peer-reviewers, and journals to understand exactly how work was carried out and accurately assess its validity even long after the conclusion of a study [10].
Fig. 1.
The data and metadata lifecycle. PID permanent identifier, QC quality control
Metadata (‘data about data’) provide essential information on the context, quality, structure, and condition of data [10, 12, 13]. Metadata and data should be linked together via unique and persistent identifiers and using standards such as FAIR digital objects (https://fairdo.org/) and the Research Object Crate (RO-Crate; https://www.researchobject.org) format [14], which aim to provide a mechanism to not only link data and metadata but also the associated analysis workflows, software, protocols, publications, presentations, and licensing information. Although metadata documentation is ultimately the responsibility of the investigators who collect the data, researchers may lack the time or expertise to generate good-quality metadata [13]. Training and education are needed, and the adoption or adaptation of community standards, such as the Recommended Metadata for Biological Images (REMBI) [15] and Investigation Study Assay (ISA) framework (https://isa-tools.org/) [16], is recommended to ensure consistent use of metadata. Where community standards do not yet exist, research teams are encouraged to self-organize and create common reporting formats for data and metadata, and to document them publicly [17]. Research teams may also enlist the services of specialist ‘data stewards’ to assist with data management, including metadata documentation; these roles require funds that are usually not available, however.
The FAIR principles
The FAIR principles for scientific data management and stewardship provide comprehensive and practical guidelines for ensuring data and metadata are FAIR (http://www.go-fair.org/fair-principles) [18]. Funders, journals, and policymakers are increasingly requiring the implementation of the FAIR principles for all research data and other related digital objects. While the FAIR principles provide a framework, it remains the responsibility of researchers to decide how they will ensure their data and metadata are FAIR. Fortunately, there are materials that provide guidance on implementing the FAIR principles at the project, group, and institutional levels (Table 1). However, to ensure widespread adoption of the FAIR principles, the threshold for implementation needs to be reduced substantially, such as with simple-to-use tools for easy data deposition and access via dedicated repositories.
Table 1.
Examples of standards and tools that researchers may use to make their data FAIR
| Example | Description |
|---|---|
|
Research Data Alliance |
A global, community-driven initiative to build social and technical infrastructure for open sharing and reuse of data, with several working groups in a number of disciplines |
|
FAIRsharing |
A searchable, interconnected registry of data standards, databases, and data policies across many research areas, allowing researchers to discover relevant repositories that meet their requirements [16] |
|
The FAIR Cookbook |
A collection of practical ‘recipes’ that provide guidance on the operational steps of FAIR data management, from creating unique, persistent identifiers to declaring data’s permitted uses [32] |
|
FAIRassist.org |
A repository aiming to offer personalized guidance to discover FAIR standards and other resources such as the Data Stewardship Wizard |
|
FAIR Data Self Assessment Tool https://ardc.edu.au/resource/fair-data-self-assessment-tool/ |
Self-assessment tool from the Australian Research Data Commons that allows users to assess how FAIR their research dataset is by answering simple questions |
|
ELIXIR Research Data Management Kit (RDMKit) |
Provides a set of best practices and guidelines for FAIR RDM across several life science domains, and journal research data policies [33] |
|
OpenAIRE |
Provides resources for researchers for the management and interoperability of data |
| Minimum Information about a Cardiac Electrophysiology Experiment (MICEE) [31] | An example of minimum reporting standards for recording, annotating, and reporting data from cardiac electrophysiology experiments |
|
FAIRsFAIR Data Policy Checklist https://www.fairsfair.eu/sites/default/files/FsF_Structured_Policy_Descriptions_17022022.pptx.pdf |
The FAIRsFAIR FAIR Data Policy Checklist and related structured policy description template provide support for the creation of structured policy documents at the project, institutional, and community level, helping policymakers to assess whether elements of their data policies are FAIR-enabling |
Data sharing and FAIR research to reduce the costs of scientific research
The financial costs of non-FAIR data can be quantified: a study by the European Commission estimated that non-FAIR research data costs €10.2 billion per year in Europe, with an additional estimated €16 billion impact on innovation; it is worth mentioning that these figures do not include the non-quantifiable benefits of making data FAIR [19]. Making data FAIR will increase the value of the data obtained, potentially accelerate progress in the improvement of therapeutics and diagnostics, and maximize the return on investment for funders.
Research data management challenges in cardiovascular science
Sharing data via data repositories and encouraging data sharing
Data repositories are centralized storage spaces where datasets can be deposited for access and reuse by other users (although authorisation and authentication may be required). There are several thousand data repositories, ranging from generalist repositories to specialist repositories for specific kinds of data. At minimum, repositories should automatically provide a globally unique and persistent identifier to every element of each dataset [20]. Repositories should also require deposition of sufficient metadata to allow other users to understand, process, and compare the data in a meaningful way. Services such as re3data (https://www.re3data.org) or FAIRsharing (http://www.fairsharing.org, [16]) provide a means to discover relevant repositories that meet FAIR requirements.
If data repositories are to realize their potential, it will be necessary to further encourage contribution as outlined below. Publications that are linked to the underlying research data are already cited more often [11]. Researchers and journals should ensure that data are credited or cited wherever they are used or reused, for example by crediting the investigators who collected the original data and citing the original dataset [21–23]. Researchers who share well-annotated datasets via repositories should be recognized and rewarded by funding bodies and universities in a suitable manner [22, 23]. Citing data sources would allow some academic recognition and reward for data sharing, and help researchers satisfy funding obligations to share their data [24]. Researchers should work with their community to define expectations for the management, sharing, and reuse of data and associated metadata, for example by joining an already existing initiative such as one of the many working and interest groups within the Research Data Alliance (https://rd-alliance.org/).
Managing data heterogeneity: standardization and harmonization
Sharing and combining datasets can be challenging due to the heterogeneity of the data involved, particularly if data are obtained using team-specific protocols and with limited standardization across laboratories. Greater standardization of terminology and better adherence to existing standards is needed across cardiovascular science. Standardized collection, processing, quality assessment, and analysis pipelines are also needed to ensure interoperability and comparability of data. In evolving fields, there may be a need to develop and adopt community-wide standards for the collection and preservation of data and metadata ‘on-the-job’. Community reporting guidelines (or minimum information standards) that describe how to report everything from sample quality to the data processing protocols used can facilitate data sharing, streamline workflows, and allow for the long-term preservation of and access to information [17, 25, 26]. Where reporting standards do not exist, research communities can self-organize and create community-centric reporting formats for data and metadata [17]. Large-scale collaborative initiatives like the National Sleep Research Resource (http://www.sleepdata.org) and the UK Biobank (https://www.ukbiobank.ac.uk) have shown it is possible for researchers to organize and collaborate on the collection and sharing of large volumes of health data for their mutual benefit, despite the challenges. Although it is important to recognize that there is unlikely to be a ‘one-size-fits-all’ solution, these initiatives may provide a model for similar efforts in cardiovascular science.
Identifying meaningful, actionable data
Given the volume of data that can be collected with modern high-throughput techniques and novel technologies like mobile health devices, it is important that researchers focus on data that are meaningful (e.g., relevant to the disease being studied) and actionable (e.g., useful for answering a specific research question or to inform a specific treatment decision). Better guidance from manufacturers is needed to ensure researchers and clinicians can effectively use the most appropriate available technologies, better identify useful meaningful and actionable data, and improve treatment decision-making and risk assessment. Collaborations between scientific researchers, healthcare providers, manufacturers, software developers, and insurance companies may provide an opportunity to influence and guide the development of new technology to improve the quality and utility of data collected.
Managing sensitive data
Sharing sensitive data is rightfully strictly regulated, but levels of regulation differ internationally [27, 28]. Education is needed to ensure that researchers understand when and how data may be shared, what researchers need to do to ensure that they are in compliance with applicable laws (e.g., GDPR), and what technology is available for secure data sharing.
In research involving patients, consent must be managed and documented appropriately. Patients often support sharing of their data if it will improve diagnostic and therapeutic options [29, 30], but lack of information on the exact parameters of consent may prevent reuse of data where consent exists but is not easily traceable. Digitization and automation of (remote) patient consent is increasing and may help to improve access to samples and data as details of patient consent can be more efficiently traced.
Who is responsible for RDM in cardiovascular science?
Researchers are ultimately responsible for ensuring that research data are suitably managed and shared according to the FAIR principles, including ensuring that data are adequately documented with metadata and made available for reuse as appropriate.
Journals and publishers should require authors to include links to all relevant raw or processed data, metadata, and other relevant materials in their submissions when publicly available. Where data are not publicly available, data sharing statements should indicate how the data can be accessed or requested, and authors or organizations should be expected to make data available upon request. Journals should also take greater responsibility for confirming that submitted materials include working links to the raw data, metadata, and other relevant materials, ideally via a persistent identifier (although the ultimate responsibility will continue to lie with authors to provide working links at submission).
Universities, funders, and government bodies should recognize and reward the collection and sharing of data in the same way that they recognize and reward publication activity. They should also support effective RDM via education and training programs, by defining and implementing data sharing policies, by employing data stewards, and by providing sufficient long-term funds for data storage and sharing. They should also establish data management training modules at the graduate level, with more advanced training at post-doctoral levels. In Germany, there is a chance to accelerate progress in the education of physicians in terms of the collection, use, quality assessment, and analysis of data via updates to the Approbationsordnung. Institutions, publishers, and state and national government bodies should commit to improving and future-proofing digital infrastructures for data storage and sharing, including funding for relevant personnel. Researchers, institutions, and journals should work together to develop low-threshold tools for data and metadata sharing during data acquisition (electronic records), processing (automated metadata annotation), and publication (low-level access to key data, such as contained in figures, via a ‘data container’). Coordination of RDM practices remains a challenge; currently, the German Research Foundation encourages RDM policy development by each ‘network grant’. Whether this is the most effective way forward remains to be seen, as parallel work, at times even within one university or faculty, would seem counterproductive. While the legal hurdles that need to be overcome when sharing data between, for example, EU- and non-EU-based research teams may be alleviated with technical solutions, a definitive solution will require the involvement of national funding bodies and governmental entities.
Finally, the cardiovascular research community should work to make sharing raw data and metadata the norm at all levels via the creation or adoption of cardiovascular reporting guidelines (an example of a well-intentioned and broadly endorsed—yet under-utilized—reporting guideline is MICEE [31]). As much cardiovascular research data and accompanying metadata should be made available via public repositories as possible to ensure the long-term and sustainable storage and reuse of data.
Conclusion
Data reuse should be factored in at every stage of scientific research, and researchers should foster a culture of open, FAIR science, through sharing good-quality, well-annotated data and metadata in repositories, defining and following agreed-upon standards, crediting and linking to the data of others, and publishing negative results. Community-driven standardization and harmonization at all stages of the data lifecycle is needed to reduce the heterogeneity of data and ensure good data quality. However, it is important to recognize that there is unlikely to be a one-size-fits-all solution for effective RDM in cardiovascular science, and the development, adoption, and application of RDM practices will require careful consideration at all levels and in all areas of cardiovascular research and should be part of the new Ärztliche Approbationsordnung. Standards should be considered living documents that need to be regularly adapted to new technologies or methods. Education, training, and funding are essential for widespread and enduring adoption of effective RDM.
It is not enough to simply recognize the importance of responsible and effective RDM: it must be put into practice. The authors encourage their professional societies and research organizations (including the DGK and DZHK), as well as funding and regulatory bodies, to spearhead a number of initiatives, including: (1) supporting initiatives and/or lobbying national funding bodies to aid a more concerted effort to develop relevant RDM processes and tools and FAIR data sharing approaches across the life sciences, including the development of and adherence to minimum reporting guidelines; (2) developing a generic (PDF- or HTML-compatible), pragmatic (focusing on data used to create figures in peer-reviewed publications), low-threshold (data container) tool to make a first but definitive step to data sharing that is independent of the research subject, methods used, and level of investigation involved; and (3) pushing for generalized ‘point-of-entry’ consenting of patients for the use of their data and any biological materials acquired in the process of diagnostic or therapeutic interventions that would otherwise be discarded, and probing the ethical acceptability of discarding healthy human donor tissue (the biological reference) that may not be used clinically (which must remain the primary aim of all donor organ utilization).
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This position paper was written on behalf of the German Cardiac Society (Deutsche Gesellschaft für Kardiologie, DGK) and the German Centre for Cardiovascular Research (Deutsches Zentrum für Herz-Kreislauf-Forschung, DZHK) following discussion at the 3rd Joint DGK and DZHK Translational Workshop, held in Bonn on 28 September 2022. Writing and editing support was provided by James O'Reilly.
Sabine Steffens, Katrin Schröder, Matina Krüger, Christoph Maack, Katrin Streckfuss-Bömeke, and Peter Kohl are members of the Commission for Experimental Cardiology of the DGK and the Workshop Organising Committee.
Author contributions
All authors contributed to the conception of this position paper. The first draft of the manuscript was prepared by PK and ER-Z. All authors commented on subsequent versions of the manuscript. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The Workshop was funded by the DGK, DZHK, and the Department of Cardiology and Angiology at the University Heart Center Freiburg-Bad Krozingen. Writing of the consensus paper was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) Centres of Research Excellence SFB1123 (DFG #238187445), SFB1425 (DFG #422681845) and SFB1525 (DFG #453989101), as well as GRK 2824 (DFG #465640801).
Data availability
Not applicable.
Declarations
Conflict of interest
Christoph Maack is an advisor to Amgen, Boehringer Ingelheim, Bristol Myers Squibb, NovoNordisk and Servier; received speaker honoraria from AstraZeneca, Bayer, Bristol Myers Squibb, Boehringer Ingelheim, Berlin Chemie, Novartis, and NovoNordisk. Johannes Backs is a founder of Revier Therapeutics, co-founder of Artemes Bio, collaborates with the Lead Discovery Center Dortmund and NovoNordisk on a drug development program, and received a Golden Ticket from NovoNordisk for lab space in Biolabs Heidelberg. Bettina Baeßler has no competing interest directly related to this work, and is co-founder and CEO of Lernrad GmbH, and received modest lecture honorary from Bayer Vital GmbH. Rafael Kramann has no competing interest directly related to this work, and is co-founder and shareholder of Sequantrix GmBH, has grants from Travere Therapeutics, Galapagos, Chugai and Novo Nordisk and is a consultant for Bayer, Pfizer, Novo Nordisk and Gruenenthal. Maastricht University and The University of Copenhagen received modest lecture and consulting honorary and research support from Bayer, Biosense Webster, Astra Zeneca, Biotronik, Medtronic, Microport, NovoNordisk on behalf of Dominik Linz. Lars Maegdefessel is a scientific consultant for NovoNordisk, DrugFarm, and Angiolutions, and has received research funding from Roche Diagnostics and NovoNordisk. Benjamin Meder was a member of the Scientific Advisory Board for Fleischhacker (Malaysia) until 2020. Sabine Steffens, Katrin Schröder, Martina Krüger, Katrin Streckfuss-Bömeke, Ralf Backofen, Yvan Devaux, Ralf Gilsbach, Jordi Heijman, Jochen Knaus, Allyson L. Lister, Henrike Maatz, Manuel Mayr, Sara Y. Nussbeck, Marcel H. Schulz, Albert Sickmann, Eva A. Rog-Zielinska, Gökhan Yigit, and Peter Kohl have no conflicts to disclose.
Footnotes
The members of the Commission for Experimental Cardiology of the DGK and the Workshop Organizing Committee mentioned in “Acknowledgements”.
Sabine Steffens and Katrin Schröder contributed equally to this work.
References
- 1.Holdren JP (2013) Increasing access to the results of federally funded scientific research. Office of Science and Technology Policy. Executive Office of the President. USA. https://obamawhitehouse.archives.gov/blog/2016/02/22/increasing-access-resultsfederally-funded-science. Accessed 22 Nov 2022
- 2.Organization for Economic Co-Operation and Development (2004). Declaration on access to research data from public funding. https://legalinstruments.oecd.org/en/instruments/157. Accessed 22 Nov 2022
- 3.Taichman DB, Sahni P, Pinborg A, Peiperl L, Laine C, James A, Hong ST, Haileamlak A, Gollogly L, Godlee F, Frizelle FA, Florenzano F, Drazen JM, Bauchner H, Baethge C, Backus J. Data sharing statements for clinical trials—a requirement of the international committee of medical journal editors. N Engl J Med. 2017;376(23):2277–2279. doi: 10.1056/NEJMe1705439. [DOI] [PubMed] [Google Scholar]
- 4.Federer LM, Belter CW, Joubert DJ, Livinski A, Lu YL, Snyders LN, Thompson H. Data sharing in PLOS ONE: an analysis of data availability statements. PLoS ONE. 2018;13(5):e0194768. doi: 10.1371/journal.pone.0194768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stall S, Yarmey L, Cutcher-Gershenfeld J, Hanson B, Lehnert K, Nosek B, Parsons M, Robinson E, Wyborn L. Make scientific data FAIR. Nature. 2019;570(7759):27–29. doi: 10.1038/d41586-019-01720-7. [DOI] [PubMed] [Google Scholar]
- 6.Tenopir C, Rice NM, Allard S, Baird L, Borycz J, Christian L, Grant B, Olendorf R, Sandusky RJ. Data sharing, management, use, and reuse: Practices and perceptions of scientists worldwide. PLoS ONE. 2020;15(3):e0229003. doi: 10.1371/journal.pone.0229003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jansen P, van den Berg L, van Overveld P, Boiten JW. Research data stewardship for healthcare professionals. In: Kubben P, Dumontier M, Dekker A, editors. Fundamentals of clinical data science. Cham: Springer; 2018. pp. 37–53. [PubMed] [Google Scholar]
- 8.Nature Everyone needs a data-management plan. Editor Nat. 2018;555(7696):286. doi: 10.1038/d41586-018-03065-z. [DOI] [PubMed] [Google Scholar]
- 9.Schiermeier Q. Data management made simple. Nature. 2018;555:403–405. doi: 10.1038/d41586-018-03071-1. [DOI] [PubMed] [Google Scholar]
- 10.Dijkers MP. A beginner's guide to data stewardship and data sharing. Spinal Cord. 2019;57(3):169–182. doi: 10.1038/s41393-018-0232-6. [DOI] [PubMed] [Google Scholar]
- 11.Colavizza G, Hrynaszkiewicz I, Staden I, Whitaker K, McGillivray B. The citation advantage of linking publications to research data. PLoS ONE. 2020;15(4):e0230416. doi: 10.1371/journal.pone.0230416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ulrich H, Kock-Schoppenhauer AK, Deppenwiese N, Gött R, Kern J, Lablans M, Majeed RW, Stöhr MR, Stausberg J, Varghese J, Dugas M, Ingenerf J. Understanding the nature of metadata: systematic review. J Med Internet Res. 2022;24(1):e25440. doi: 10.2196/25440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perrier L, Blondal E, MacDonald H. The views, perspectives, and experiences of academic researchers with data sharing and reuse: a meta-synthesis. PLoS ONE. 2020;15(2):e0229182. doi: 10.1371/journal.pone.0229182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soiland-Reyes S, Sefton P, Crosas M, Castro LJ, Coppens F, Fernández JM, Garijo D, Grüning B, La Rosa M, Leo S, Carragáin EÓ, Portier M, Trisovic A, RO-Crate Community. Groth P, Goble C. Packaging research artefacts with RO-Crate. Data Sci. 2022 doi: 10.3233/DS-210053. [DOI] [Google Scholar]
- 15.Sarkans U, Chiu W, Collinson L, Darrow MC, Ellenberg J, Grunwald D, Hériché JK, Iudin A, Martins GG, Meehan T, Narayan K, Patwardhan A, Russell MRG, Saibil HR, Strambio-De-Castillia C, Swedlow JR, Tischer C, Uhlmann V, Verkade P, Barlow M, Bayraktar O, Birney E, Catavitello C, Cawthorne C, Wagner-Conrad S, Duke E, Paul-Gilloteaux P, Gustin E, Harkiolaki M, Kankaanpää P, Lemberger T, McEntyre J, Moore J, Nicholls AW, Onami S, Parkinson H, Parsons M, Romanchikova M, Sofroniew N, Swoger J, Utz N, Voortman LM, Wong F, Zhang P, Kleywegt GJ, Brazma A. REMBI: Recommended Metadata for Biological Images—enabling reuse of microscopy data in biology. Nat Methods. 2021;18(12):1418–1422. doi: 10.1038/s41592-021-01166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sansone SA, McQuilton P, Rocca-Serra P, Gonzalez-Beltran A, Izzo M, Lister AL, Thurston M, FAIRsharing Community FAIRsharing as a community approach to standards, repositories and policies. Nat Biotechnol. 2019;37(4):358–367. doi: 10.1038/s41587-019-0080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crystal-Ornelas R, Varadharajan C, O'Ryan D, Beilsmith K, Bond-Lamberty B, Boye K, Burrus M, Cholia S, Christianson DS, Crow M, Damerow J, Ely KS, Goldman AE, Heinz SL, Hendrix VC, Kakalia Z, Mathes K, O'Brien F, Pennington SC, Robles E, Rogers A, Simmonds M, Velliquette T, Weisenhorn P, Welch JN, Whitenack K, Agarwal DA. Enabling FAIR data in Earth and environmental science with community-centric (meta)data reporting formats. Sci Data. 2022;9(1):700. doi: 10.1038/s41597-022-01606-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilkinson MD, Dumontier M, Aalbersberg IJ, Appleton G, Axton M, Baak A, Blomberg N, Boiten JW, Bourne PE, Bouwman J, Brookes AJ, Clark T, Crosas M, Dillo I, Dumon O, Edmunds S, Evelo CT, Finkers R, Gonzalez-Beltran A, Gray AJ, Groth P, Goble C, Grethe JS, Heringa J, Hoen PA, Hooft R, Kuhn T, Kok R, Kok J, Lusher SJ, Martone ME, Mons A, Packer AL, Persson B, Rocca-Serra P, Roos M, van Schaik R, Sansone SA, Schultes E, Sengstag T, Slater T, Strawn G, Swertz MA, Thompson M, van der Lei J, van Mulligen E, Velterop J, Waagmeester A, Wittenburg P, Wolstencroft K, Zhao J, Mons B. The FAIR Guiding Principles for scientific data management and stewardship. Sci Data. 2016;3:160018. doi: 10.1038/sdata.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.European Commission, Directorate-General for Research and Innovation (2019) Cost-benefit analysis for FAIR research data: cost of not having FAIR research data. Publications Office, Brussels, Belgium. 10.2777/02999. Accessed 4 Jan 2023
- 20.McMurry JA, Juty N, Blomberg N, Burdett T, Conlin T, Conte N, Courtot M, Deck J, Dumontier M, Fellows DK, Gonzalez-Beltran A, Gormanns P, Grethe J, Hastings J, Hériché JK, Hermjakob H, Ison JC, Jimenez RC, Jupp S, Kunze J, Laibe C, Le Novère N, Malone J, Martin MJ, McEntyre JR, Morris C, Muilu J, Müller W, Rocca-Serra P, Sansone SA, Sariyar M, Snoep JL, Soiland-Reyes S, Stanford NJ, Swainston N, Washington N, Williams AR, Wimalaratne SM, Winfree LM, Wolstencroft K, Goble C, Mungall CJ, Haendel MA, Parkinson H. Identifiers for the 21st century: How to design, provision, and reuse persistent identifiers to maximize utility and impact of life science data. PLoS Biol. 2017;15(6):e2001414. doi: 10.1371/journal.pbio.2001414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bierer BE, Crosas M, Pierce HH. Data Authorship as an Incentive to Data Sharing. N Engl J Med. 2017;377(4):402. doi: 10.1056/NEJMc1707245. [DOI] [PubMed] [Google Scholar]
- 22.Cousijn H, Kenall A, Ganley E, Harrison M, Kernohan D, Lemberger T, Murphy F, Polischuk P, Taylor S, Martone M, Clark T. A data citation roadmap for scientific publishers. Sci Data. 2018;5:180259. doi: 10.1038/sdata.2018.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pierce HH, Dev A, Statham E, Bierer BE. Credit data generators for data reuse. Nature. 2019;570:30–32. doi: 10.1038/d41586-019-01715-4. [DOI] [PubMed] [Google Scholar]
- 24.Devriendt T, Shabani M, Borry P. Data sharing in biomedical sciences: a systematic review of incentives. Biopreserv Biobank. 2021;19(3):219–227. doi: 10.1089/bio.2020.0037. [DOI] [PubMed] [Google Scholar]
- 25.Goodman A, Pepe A, Blocker AW, Borgman CL, Cranmer K, Crosas M, Di Stefano R, Gil Y, Groth P, Hedstrom M, Hogg DW, Kashyap V, Mahabal A, Siemiginowska A, Slavkovic A. Ten simple rules for the care and feeding of scientific data. PLoS Comput Biol. 2014;10(4):e1003542. doi: 10.1371/journal.pcbi.1003542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lowndes JSS, Best BD, Scarborough C, Afflerbach JC, Frazier MR, O'Hara CC, Jiang N, Halpern BS. Our path to better science in less time using open data science tools. Nat Ecol Evol. 2017;1(6):160. doi: 10.1038/s41559-017-0160. [DOI] [PubMed] [Google Scholar]
- 27.The European Parliament and the Council of the European Union. General Data Protection Regulation (EU) 2016/679 (GDPR) https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:02016R0679-20160504. Accessed 4 Jan 2023
- 28.Bentzen HB, Høstmælingen N. Balancing protection and free movement of personal data: the New European Union General Data Protection Regulation. Ann Intern Med. 2019;170(5):335–337. doi: 10.7326/M18-2782. [DOI] [PubMed] [Google Scholar]
- 29.Seltzer E, Goldshear J, Guntuku SC, Grande D, Asch DA, Klinger EV, Merchant RM. Patients' willingness to share digital health and non-health data for research: a cross-sectional study. BMC Med Inform Decis Mak. 2019;19(1):157. doi: 10.1186/s12911-019-0886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mello MM, Lieou V, Goodman SN. Clinical trial participants' views of the risks and benefits of data sharing. N Engl J Med. 2018;378(23):2202–2211. doi: 10.1056/NEJMsa1713258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quinn TA, Granite S, Allessie MA, Antzelevitch C, Bollensdorff C, Bub G, Burton RA, Cerbai E, Chen PS, Delmar M, Difrancesco D, Earm YE, Efimov IR, Egger M, Entcheva E, Fink M, Fischmeister R, Franz MR, Garny A, Giles WR, Hannes T, Harding SE, Hunter PJ, Iribe G, Jalife J, Johnson CR, Kass RS, Kodama I, Koren G, Lord P, Markhasin VS, Matsuoka S, McCulloch AD, Mirams GR, Morley GE, Nattel S, Noble D, Olesen SP, Panfilov AV, Trayanova NA, Ravens U, Richard S, Rosenbaum DS, Rudy Y, Sachs F, Sachse FB, Saint DA, Schotten U, Solovyova O, Taggart P, Tung L, Varró A, Volders PG, Wang K, Weiss JN, Wettwer E, White E, Wilders R, Winslow RL, Kohl P. Minimum Information about a Cardiac Electrophysiology Experiment (MICEE): standardised reporting for model reproducibility, interoperability, and data sharing. Prog Biophys Mol Biol. 2011;107(1):4–10. doi: 10.1016/j.pbiomolbio.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rocca-Serra P, Gu W, Ioannidis V, Daloii TA, Capella-Gutierrez S, Chandramouliswaran I, Splendiani A, Burdett T, Giessmann RT, Henderson D, Batista D, Lister A, Emam I, Gadiya Y, Giovanni L, Willighagen E, Evelo C, Gray AJG, Gribbon P, Juty N, Welter D, Quast K, Peeters P, van Vlijmen H, Plasterer T, Reilly D, van der Horst E, Sansone SA, the FAIR Cookbook Recipes' Authors (2022) The FAIR Cookbook—the essential resource for and by FAIR doers. Zenodo. 10.5281/zenodo.7156792. https://zenodo.org/record/7156792
- 33.Hrynaszkiewicz I, Simons N, Hussain A, Grant R, Goudie S. Developing a research data policy framework for all journals and publishers. Data Sci J. 2020;19(1):5. doi: 10.5334/dsj-2020-005. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.