Abstract
Background
Immune checkpoint inhibitors have changed previous treatment paradigm of advanced urothelial carcinoma (UC). The ARON-2 study (NCT05290038) aimed to assess the real-world effectiveness of pembrolizumab in patients recurred or progressed after platinum-based chemotherapy.
Patients and Methods
Medical records of patients with documented metastatic UC treated by pembrolizumab as second-line therapy were retrospectively collected from 88 institutions in 23 countries. Patients were assessed for overall survival (OS), progression-free survival (PFS) and overall response rate (ORR). Cox proportional hazards models were adopted to explore the presence of prognostic factors.
Results
In total, 836 patients were included: 544 patients (65%) received pembrolizumab after progression to first-line platinum-based chemotherapy in the metastatic setting (cohort A) and 292 (35%) after recurring within < 12 months since the completion of adjuvant or neoadjuvant chemotherapy (cohort B). The median follow-up time was 15.3 months. The median OS and the ORR were 10.5 months and 31% in the overall study population, 9.1 months and 29% in cohort A and 14.6 months and 37% in cohort B. At multivariate analysis, ECOG-PS ≥ 2, bone metastases, liver metastases and pembrolizumab setting (cohort A vs B) proved to be significantly associated with worst OS and PFS. Stratified by the presence of 0, 1–2 or 3–4 prognostic factors, the median OS was 29.4, 12.5 and 4.1 months (p < 0.001), while the median PFS was 12.2, 6.4 and 2.8 months, respectively (p < 0.001).
Conclusions
Our study confirms that pembrolizumab is effective in the advanced UC real-world context, showing outcome differences between patients recurred or progressed after platinum-based chemotherapy.
Supplementary information
The online version contains supplementary material available at 10.1007/s00262-024-03682-w.
Keywords: ARON-2 study, Pembrolizumab, Real-world data, Survival, Tumor response, Urothelial cancer
Introduction
The World Health Organization (WHO) has globally estimated 573,278 new cases of bladder cancer in 2020 [1]. In the same year, the number of bladder cancer-related deaths has been estimated at 212,536, the 75% of which are in men [1]. Urothelial cancer (UC) is the most frequent histologic variant of tumors of the upper and lower urinary tracts, representing approximately 90% of all new diagnoses [2]. The aggressive behavior of this tumor, which accounts for a dramatically low 5-year survival rate of around 7% [3], has pushed cancer researchers to develop novel therapeutic approaches with the aim of improving the management of UC patients and to exceed the results obtained by chemotherapy in this context [4]. The advent of immune checkpoint inhibitors has, at least in part, changed the previous treatment paradigm of advanced UC patients progressing after platinum-based chemotherapy [5]. The Food and Drugs Administration (FDA) approved pembrolizumab based on the results of the KEYNOTE-045 randomized phase III trial [6]. In this trial, patients were randomized to receive pembrolizumab at a dose of 200 mg every 3 weeks or the investigator's choice of chemotherapy with paclitaxel, docetaxel or vinflunine. Patients progressed after platinum-based chemotherapy or recurred within 12 months since completion of adjuvant or neoadjuvant therapy was eligible. The coprimary endpoints were overall survival (OS) and progression-free survival (PFS). Pembrolizumab, compared to chemotherapy, yielded a longer median OS (10.3 vs 7.4 months) and a lower rate of treatment-related adverse events (60.9 vs 90.2%). No statistically significant differences were found in terms of PFS. Of note, long-term results with > 2 years of follow-up were consistent with those reported by Bellmunt et al. in [7].
The ARON project was designed to globally share and analyze real-world experiences on the use of immunotherapy in patients with genitourinary tumors. Specifically, the ARON-2 study (NCT05290038) was conducted to assess the real-world efficacy of pembrolizumab in patients recurred or progressed after platinum-based chemotherapy.
Patients and methods
Study population
Patients aged 18 years or older, diagnosed with advanced UC confirmed through cytological and/or histologic tests, and experiencing progression (cohort A) or recurrence (cohort B) after receiving platinum-based therapy were part of the ARON-2 study and treated with pembrolizumab between January 1, 2016, and October 1, 2022. The study involved 88 institutions from 23 countries, as detailed in Supplementary Table 1.
The entire ARON-2 dataset was analyzed in this study. To ensure data security, information was anonymized, stored in a password-protected dataset and only accessible by authorized personnel. The dataset included various patient details like age, gender, Eastern Cooperative Oncology Group-Performance Status (ECOG-PS), tumor characteristics, treatment history and response to immunotherapy. Patients lacking sufficient information on treatment response, progression or follow-up were excluded from the study.
Follow-up procedures involved regular physical examinations, laboratory tests and imaging scans (CT or MRI) at specific intervals, typically every 2–4 months, as per individual physicians' practices or when there were clinical suspicions of disease progression.
The data supporting the study's findings can be obtained from the corresponding author upon reasonable request, following ethical guidelines.
Study endpoints
Study endpoints were determined based on the Response Evaluation Criteria in Solid Tumours version 1.1 (RECIST 1.1), categorizing disease response as complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD). The overall response rate (ORR) was calculated as the sum of CR and PR.
OS was measured from the initial administration of pembrolizumab until death, while PFS was calculated from the first pembrolizumab dose to documented disease progression or death, whichever came first. Patients without disease progression, death or lost to follow-up were censored at their last recorded visit.
Statistical analysis
We utilized the Kaplan–Meier method with Rothman’s 95% confidence intervals (CI) to estimate OS and PFS. Comparison of survival curves was done using the log-rank test, and Cox proportional hazards models were employed to assess multivariable effects on patients’ survival and calculate hazard ratios (HRs) and 95% CIs. The Chi-square test was used for determining the difference between groups. Significance level was set at 0.05, with two-sided p values. The selection of explanatory variables of multivariate analysis was performed based on the data available in our database.
The statistical analyses were conducted using MedCalc version 19.6.4 (MedCalc Software, Broekstraat 52, 9030 Mariakerke, Belgium).
Results
Baseline characteristics
Eight hundred and thirty-six patients were included in our analysis. The median follow-up time was 15.3 months (95%CI 14.0 − 76.0); 613 patients (73%) were males and 223 females (27%). Median age was 71y (range 26 − 95). ECOG-PS was ≥ 2 in 143 patients (17%). Upper urinary tract carcinomas accounted for the 26% of all cases. Tumor histology was pure UC in 702 patients (84%). Variant histologies included: squamous in 64 (8%), poorly differentiated in 15 (2%), plasmacytoid in 12 (1%), neuroendocrine in 9 (1%), sarcomatoid in 7 (1%), clear cell in 6 (1%), glandular in 6 (1%), micropapillary in 5 (1%), nested in 4 (< 1%), microcystic in 2 (< 1%), lymphoepithelioma-like in 2 (< 1%) and giant cell in 2 (< 1%). Two hundred and fifty-four patients (30%) presented metastatic disease at the time of initial UC diagnosis (when in first-line). Visceral metastases were identified in 555 patients (66%).
In our analysis, 544 patients (65%) received pembrolizumab following progression to first-line platinum-based chemotherapy (cohort A) and 292 (35%) after recurring within 12 months since the completion of adjuvant or neoadjuvant chemotherapy (cohort B).
Four hundred and fifty-two (54%) were dead at time of the analysis. Treatment with pembrolizumab was ongoing in 317 patients (38%, 190 patients in cohort A, 127 in cohort B). One hundred and seventy (108 in cohort A and 62 in cohort B) of the 519 patients progressing during or after pembrolizumab treatment received further therapies. Patients’ baseline characteristics at the time of being assigned to receive pembrolizumab are summarized in Tables 1 and 2.
Table 1.
Patients’ characteristics
| Patients (n = 836) | |
|---|---|
| Sex | |
| Male | 613 (73) |
| Female | 223 (27) |
| Age, years (y) | |
| Median | 71 |
| Range | 26 − 95 |
| Interquartile ranges: | |
| 26–63 | 189 |
| 64–70 | 229 |
| 71–76 | 214 |
| 77–95 | 204 |
| ECOG Performance Status | |
| 0 | 268 (32) |
| 1 | 425 (51) |
| 2 | 132 (16) |
| 3 | 11 (1) |
| Current or former smokers | 549(66) |
| Primary tumor location | |
| Upper urinary tract | 220 (26) |
| Lower urinary tract | 616 (74) |
| Tumor histology | |
| Pure urothelial carcinoma | 702 (84) |
| Variants | 134 (16) |
| Metastatic disease | |
| Synchronous | 254 (30) |
| Metachronous | 582 (70) |
| Common sites of metastasis | |
| Lymph nodes | 586 (70) |
| Lung | 280 (33) |
| Bone | 243 (29) |
| Liver | 152 (18) |
| Brain | 15 (2) |
| Visceral metastases | |
| Yes | 555 (66) |
| No | 281 (34) |
| Patients progressed during first-line platinum-based chemotherapy (cohort A) | 544 (65) |
| Patients relapsed within < 1y since the completion of neoadjuvant/adjuvant chemotherapy (cohort B) | 292 (35) |
Statistically significant values were reported in bold
ECOG-PS, Eastern Cooperative Oncology Group-Performance Status; 1y, one year
Table 2.
Successive therapies in cohort A and B
| Successive therapies (cohort A) | |
| Paclitaxel | 46 (6) |
| Vinflunine | 30 (4) |
| Carboplatin and Gemcitabine | 6 (1) |
| Enfortumab vedotin | 6 (1) |
| Clinical trials | 6 (1) |
| Carboplitn and paclitaxel | 5 (1) |
| Docetaxel | 4 (< 1) |
| Gemcitabine | 3 (< 1) |
| Carboplatin | 1 (< 1) |
| MVAC | 1 (< 1) |
| Successive therapies (cohort B) | |
| Vinflunine | 14 (2) |
| Paclitaxel | 12 (1) |
| Carboplatin and Gemcitabine | 10 (1) |
| Clinical trials | 7 (1) |
| Enfortumab vedotin | 4 (< 1) |
| Gemcitabine and Palitaxel | 4 (< 1) |
| MVAC | 2 (< 1) |
| Cyclphosphamide | 2 (< 1) |
| Carboplitn and paclitaxel | 1 (< 1) |
| Gemcitabine | 1 (< 1) |
| Carboplatin | 1 (< 1) |
Survival analysis
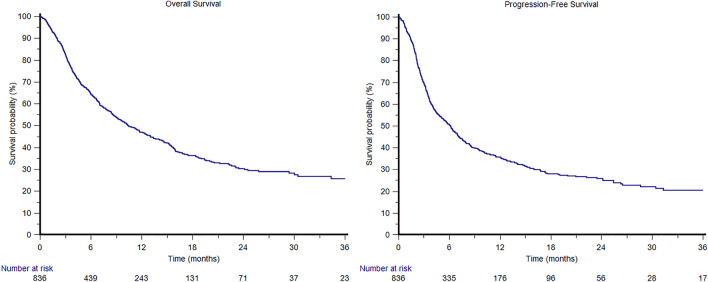
In the overall study population, the median OS was 10.5 months (95%CI 9.0 − 12.5, Fig. 1); 1-year and 2-year OS rates were 29 and 8%, respectively. The median OS was 9.1 months (95%CI 7.5 − 11.4) in cohort A and 14.6 months (95%CI 10.4 − 19.4) in cohort B (Fig. 2).
Fig. 1.
Overall and progression-free survival curves in the overall ARON-2 study population
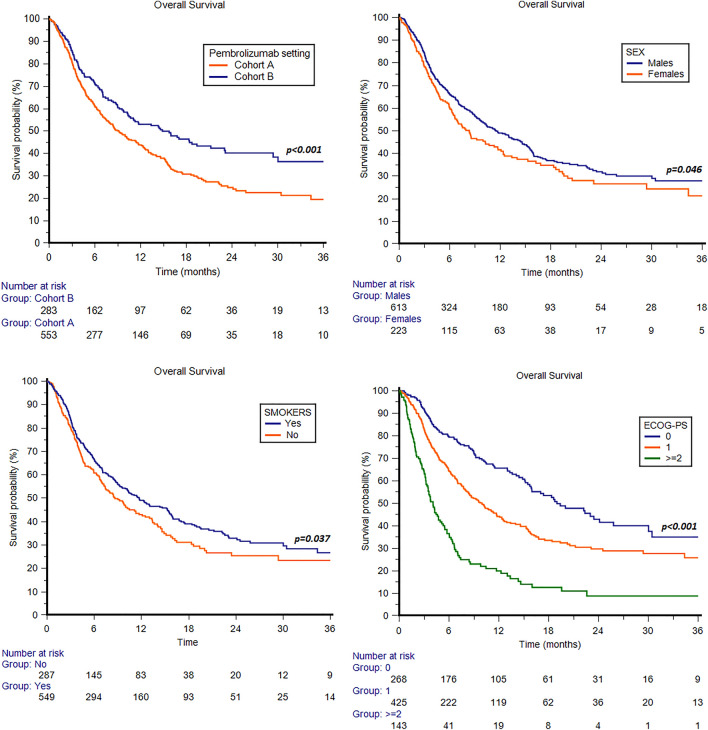
Fig. 2.
Overall survival in patients treated with pembrolizumab stratified by pembrolizumab setting (cohort A: patients progressed during first-line platinum base chemotherapy; cohort B: patients recurred within < 1y since the completion of adjuvant/neoadjuvant therapy), sex, smoking attitude and ECOG-PS
The median OS was significantly longer in males vs females (11.5 months, 95%CI 9.7 − 14.4, vs 8.3 months, 95%CI 6.4 − 11.3, p = 0.046, Fig. 2). Otherwise, no statistically significant differences were observed between patients aged < 65y and ≥ 65y (10.5 months, 95%CI 8.2 − 14.9 vs 10.4 months, 95%CI 8.7 − 12.7, p = 0.581).
Current or former smokers showed a longer median OS compared to non-smokers (11.7 months, 95%CI 9.5 − 15.3, vs 8.6 months, 95%CI 7.0 − 11.3, p = 0.037, Fig. 2).
Patients stratified by ECOG-PS (0,1 or 2) showed a median OS of 19.0 months (95%CI 15.9 − 25.8), 10.0 months (95%CI 8.2 − 11.7) and 4.1 months (95%CI 3.2 − 5.1) (p < 0.001, Fig. 2).
Patients with pure UC histology showed a median OS of 10.8 months (95%CI 19.2 − 13.0), while in patients with mixed variant histology was 8.6 months (95%CI 6.6 − 14.6, p = 0.511). Interestingly, no statistically significant differences were found between patients with tumors of the upper tract (8.6 months, 95%CI 6.6 − 12.4) and lower tract (11.3 months, 95%CI 9.5 − 13.4, p = 0.287).
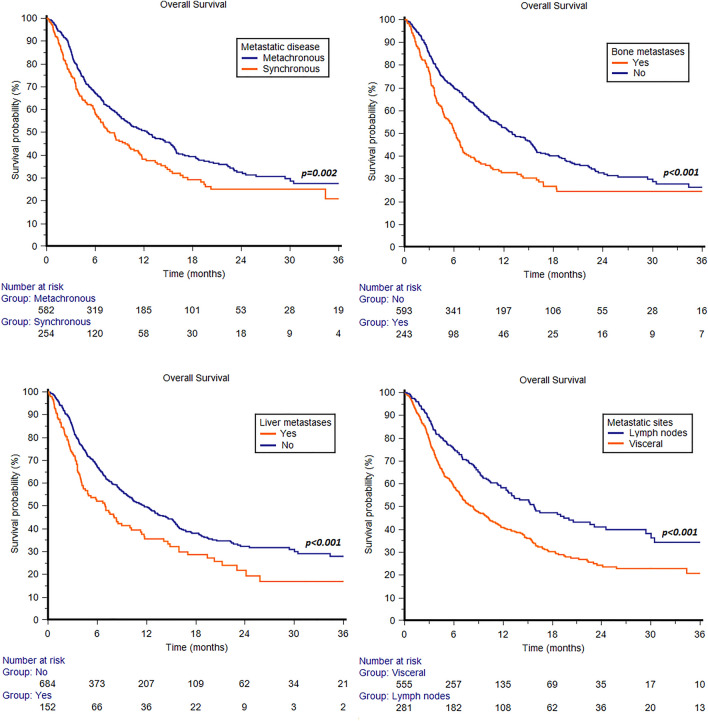
Synchronous metastatic disease was associated with shorter median OS (7.8 months, 95%CI 6.4 − 10.2, vs 12.5 months, 95%CI 10.0 − 15.3, p = 0.002, Fig. 3). By stratifying patients according to sites of metastasis, statistically significant differences were observed between patients with or without bone metastases (6.2 months, 95%CI 5.0 − 7.0, vs 13.0 months, 95%CI 11.3 − 15.4, p < 0.001, Fig. 3) and patients with or without liver metastases (7.0 months, 95%CI 4.3 − 9.0, vs 11.7 months, 95%CI 9.8 − 14.0, p < 0.001, Fig. 3). Patients with metastases confined to the lymph nodes showed longer median OS compared to those with visceral metastases (15.8 months, 95%CI 12.6 − 22.6, vs 8.4 months, 95%CI 7.0 − 10.2, p < 0.001, Fig. 3).
Fig. 3.
Overall survival in patients treated with pembrolizumab stratified by synchronous or metachronous metastatic disease, bone or liver metastases or visceral metastases
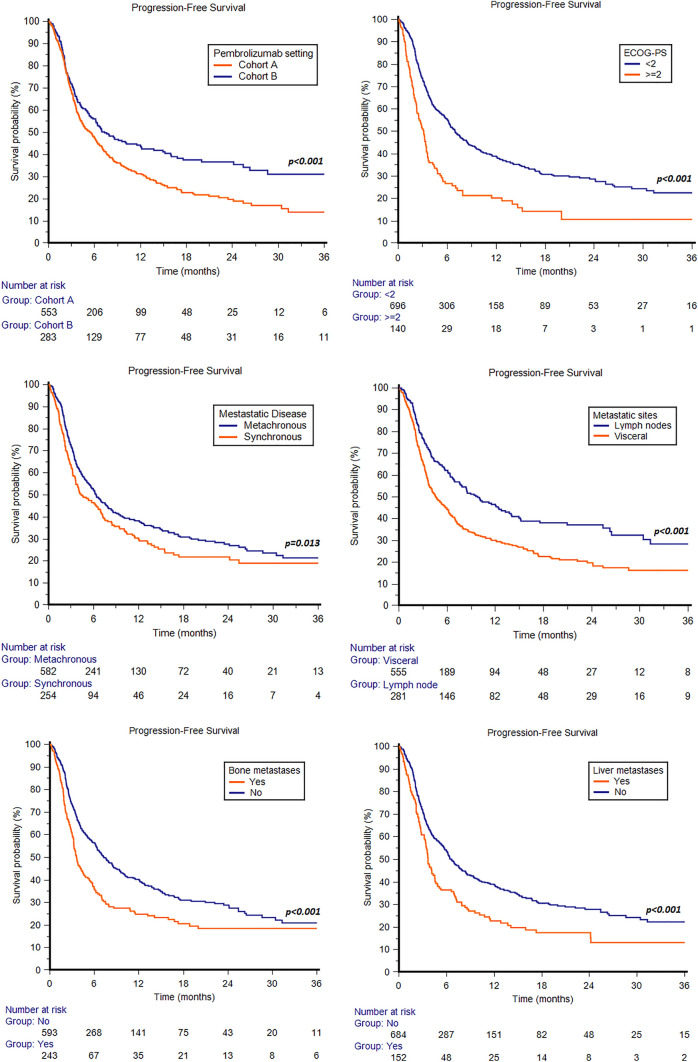
In the overall study population, the median PFS was 6.2 months (95%CI 5.1 − 6.9, Fig. 1); 1-year and 2-year PFS rates were 21 and 7%, respectively. The median PFS was 5.5 months (95%CI 4.4 − 6.4) in cohort A and 7.3 months (95%CI 5.8 − 12.0) in cohort B (p < 0.001, Fig. 4).
Fig. 4.
Progression-free survival in patients treated with pembrolizumab stratified by pembrolizumab setting (cohort A: patients progressed during first-line platinum base chemotherapy; cohort B: patients recurred within < 1y since the completion of adjuvant/neoadjuvant therapy), ECOG-PS, synchronous or metachronous metastatic disease, visceral metastases, bone or liver metastases
In terms of PFS, ECOG-PS ≥ 2 was associated with shorter median PFS when comparing with ECOG-PS 0–1 (3.1 months, 95%CI 2.3 − 3.5, vs 6.9 months, 95%CI 6.2 − 8.3, p < 0.001, Fig. 4). No statistically significant differences were observed between males and females (6.1 months, 95%CI 5.1 − 6.9 vs 6.3 months, 95%CI 4.0 − 7.9, p = 0.923), patients aged < 65y and ≥ 65y (6.2 months, 95%CI 4.3 − 7.5 vs 6.1 months, 95%CI 5.0 − 7.0, p = 0.439), smokers and non-smokers (5.5 months, 95%CI 4.2 − 6.9, vs 6.2 months, 95%CI 5.3 − 7.2, p = 0.441), pure and mixed UC histology (6.2 months, 95%CI 5.3 − 7.0, vs 5.0 months, 95%CI 3.9 − 44.0, p = 0.737), and upper and lower urinary tract (5.0 months, 95%CI 3.9 − 7.2, vs 6.3 months, 95%CI 5.4 − 7.1, p = 0.575).
Synchronous metastatic disease was associated with shorter median PFS compared to metachronous disease (4.4 months, 95%CI 3.6 − 6.6, vs 6.4 months, 95%CI 5.6 − 7.6, p = 0.013, Fig. 4). Patients with metastases confined to lymph nodes showed longer median PFS compared to those with visceral metastases (9.8 months, 95%CI 7.6 − 13.3, vs 4.6 months, 95%CI 3.9 − 5.8, p < 0.001, Fig. 4). By stratifying patients according to sites of metastasis, patients with bone (3.6 months, 95%CI 3.2 − 4.4, vs 7.3 months, 95%CI 6.4 − 8.6, p < 0.001, Fig. 4) or liver metastases (3.7 months, 95%CI 3.3 − 4.5, vs 6.6 months, 95%CI 6.0 − 7.7, p < 0.001, Fig. 4) showed a significantly shorter median PFS compared to patients without bone or liver metastases, respectively.
Response to therapy
According to RECIST 1.1, 84 patients (10%) experienced CR, 179 (21%) PR, 201 (24%) SD and 372 (44%) PD, with an ORR of 31%. The median OS was significantly different according to the type of response, being NR (95%CI NR − NR), 34.4 months (95%CI 22.4 − 47.2), 15.4 months (95%CI 12.4 − 19.4) and 4.3 months (95%CI 3.8 − 30.4) in patients with CR, PR, SD and PD, respectively (p < 0.001).
In cohort A, CR, PR, SD and PD were registered in 6, 23, 24 and 47%. In cohort B, we observed 18% of CR, % 19% of PR, 24% of SD and 39% of PD. The difference between the ORR in the two cohorts (29% vs 37%) was not statistically significant (p = 0.230), although cohort B reported a higher percentage of CR (p = 0.009).
Role of prognostic factors
At univariate analysis, gender, smoking attitude, ECOG-PS, synchronous metastatic disease, bone or liver metastases and pembrolizumab setting (cohort A vs cohort B) were significant predictors of OS (Table 3). As for PFS, the univariate analysis showed a prognostic role of ECOG-PS, synchronous metastatic disease, bone or liver metastases and pembrolizumab setting. At multivariate analysis, ECOG-PS ≥ 2, bone or liver metastases and pembrolizumab (cohort A vs B) proved to be significantly associated with both OS and PFS (Table 3).
Table 3.
Univariate and multivariate analyses of predictors of overall survival and Progression-free survival in UC patients treated by pembrolizumab
| Overall survival | Univariate Cox regression | Multivariate Cox regression | ||
|---|---|---|---|---|
| HR (95%CI) | p-value | HR (95%CI) | p-value | |
| Gender (females vs males) | 1.23(1.01 − 1.50) | 0.046 | 1.21 (0.98 − 1.50) | 0.080 |
| Age (≥ 65y vs < 65y) | 1.06 (0.86 − 1.31) | 0.581 | ||
| Smokers vs non-smokers | 0.82 (0.67 − 0.99) | 0.037 | 0.87 (0.71 − 1.06) | 0.166 |
| ECOG-PS (≥ 2 vs 0–1) | 2.71 (2.18 − 3.38) | < 0.001 | 2.56(2.04 − 3.20) | < 0.001 |
| Histology (mixed vs pure UC) | 1.08 (0.86 − 1.36) | 0.511 | ||
| Upper vs Lower urinary tract | 1.12 (0.91 − 1.37) | 0.287 | ||
| Synchronous metastatic disease (yes vs no) | 1.36 (1.12 − 1.66) | 0.002 | 1.15 (0.94 − 1.41) | 0.173 |
| Lymph node (Y vs N) | 0.87 (0.71 − 1.07) | 0.183 | ||
| Lung metastases (Y vs N) | 1.20 (0.99–1.45) | 0.069 | ||
| Liver metastases (Y vs N) | 1.49 (1.19 − 1.86) | < 0.001 | 1.45 (1.14 − 1.85) | 0.003 |
| Bone metastases (Y vs N) | 1.59 (1.30 − 1.93) | < 0.001 | 1.32 (1.06 − 1.67) | 0.014 |
| Patients progressed during first-line therapy vs recurred within < 1y from adjuvant/neoadjuvant therapy | 1.47 (1.20 − 1.80) | < 0.001 | 1.42 (1.15 − 1.75) | < 0.001 |
| Progression-free survival | Univariate Cox regression | Multivariate Cox regression | ||
|---|---|---|---|---|
| HR (95%CI) | p-value | HR (95%CI) | p-value | |
| Gender (females vs males) | 0.99 (0.81 − 1.20) | 0.923 | ||
| Age (≥ 65y vs < 65y) | 0.93 (0.77 − 1.12) | 0.440 | ||
| Smokers vs non-smokers | 0.93 (0.78 − 1.12) | 0.442 | ||
| ECOG-PS (≥ 2 vs 0–1) | 2.02 (1.63 − 2.51) | < 0.001 | 1.93 (1.55 − 2.40) | < 0.001 |
| Histology (mixed vs pure UC) | 1.04 (0.84 − 1.29) | 0.737 | ||
| Upper vs Lower urinary tract | 1.06 (0.87 − 1.28) | 0.576 | ||
| Synchronous metastatic disease (yes vs no) | 1.26 (1.05 − 1.51) | 0.014 | 1.13 (0.94 − 1.36) | 0.210 |
| Lymph node metastases (Y vs N) | 0.89 (0.74 − 1.07) | 0.222 | ||
| Lung metastases (Y vs N) | 1.14 (0.96 − 1.37) | 0.138 | ||
| Liver metastases (Y vs N) | 1.52 (1.24 − 1.88) | < 0.001 | 1.43 (1.15 − 1.79) | 0.002 |
| Bone metastases (Y vs N) | 1.52 (1.27 − 1.83) | < 0.001 | 1.27 (1.03 − 1.62) | 0.028 |
| Patients progressed during first-line therapy vs recurred within < 1y from adjuvant/neoadjuvant therapy | 1.40 (1.15 − 1.69) | < 0.001 | 1.33 (1.10 − 1.62) | 0.003 |
ECOG-PS Eastern Cooperative Oncology Group-Performance Status; UC urothelial carcinoma
Statistically significant values were reported in bold
The ARON prognostic factors
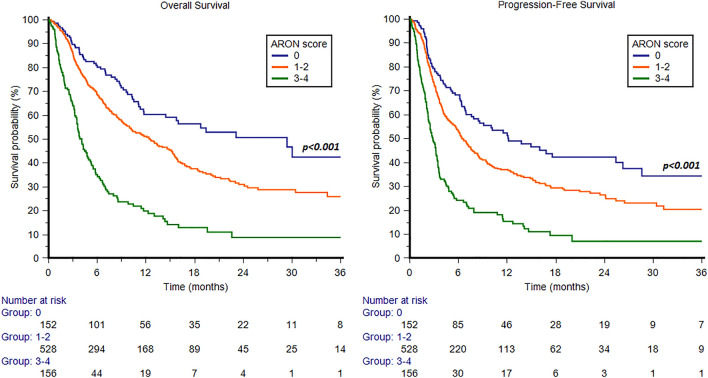
We further retrospectively analyzed patients according to the presence of the prognostic factors identified at multivariate analysis (ECOG-PS ≥ 2, bone or liver metastases and pembrolizumab pure second-line setting). We divided the study population into three groups, defined by the presence of 0, 1–2 or 3–4 prognostic factors. The median OS was 29.4 months (95%CI 14.4 − 45.9), 12.5 months (95%CI 10.0 − 15.2) and 4.1 months (95%CI 3.5 − 4.8), respectively (p < 0.001, c-index 0.629, 95%CI 0.596 − 0.662, Fig. 5). Accordingly, the median PFS was 12.2 months (95%CI 7.9 − 25.4), 6.4 months (95%CI 5.7 − 7.6) and 2.8 months (95%CI 2.3 − 3.4), in patients with 0, 1–2 or 3–4 prognostic factors (p < 0.001, c-index 0.612, 95%CI 0.578 − 0.646, Fig. 5). Furthermore, patients stratified into these 3 groups showed significantly different ORR, which was 45% in patients with 0 factors, 33% in patients with 1–2 factors and 12% in patients with 3–4 prognostic factors (p < 0.001). In particular, patients with 0 factors showed 23% CR, 22% PR, 26% SD and 29% PD. Patients with 1–2 factors registered 8% CR, 25% PR, 26% SD and 41% PD. On the other hand, patients with 3–4 factors reported 4% CR, 8% PR, 17% SD and 71% PD.
Fig. 5.
Overall survival and progression-free survival in patients stratified by ARON score
Discussion
Immune checkpoint inhibitors have contributed to change the therapeutic landscape of UC [6, 7, 9, 10] and is actively developed in all therapeutic settings, [11–14]. Nevertheless, a not negligible rate of patients presents primary resistance to immune checkpoint inhibitors [15] and the majority of UC patients will present disease progression to immunotherapy. The development of validated biomarkers of response to immune checkpoint inhibitors will be crucial in order to design personalized therapeutic approaches for patients with advanced UC. To date, PD-L1 expression seems more prognostic than predictive, while it has been recently showed that tumor mutational burden (TMB) and T-cell-inflamed gene expression profile (TcellinfGEP) are associated with the outcome of patients treated with pembrolizumab in both second-line therapy and first-line therapy for cisplatn-ineligible UC patients [16].
The ARON-2 study was designed to assess the real-world efficacy of pembrolizumab in patients with advanced UC and, to the best of our knowledge, represents the largest worldwide data collection in this setting and involving 88 institutions from 23 countries it could represent an original real-world study. In this study, we focused on the second-line setting, showing that in the overall patient population, median OS and PFS were 10.5 and 6.2 months, respectively. ECOG-PS ≥ 2 and the presence of bone or liver metastases were significantly associated with worst OS and PFS, while smoking attitude was associated with longer OS, in accordance with previous studies focused on the use of immunotherapy in cancer patients [17, 18]. These findings are consistent with a recent multicenter retrospective study that included 917 patients with mUC and treated with immune checkpoint inhibitors, which reported bone and liver metastases as strong predictors of worse oncologic outcome [19]. The ORR was 31% with 10% of CR. The type of tumor response according to RECIST 1.1 criteria was a significant predictor of OS, confirming previous exploratory analysis performed in KEYNOTE-045 trial [7].
The median OS observed in the present study is very similar to the median OS reported in the pivotal KEYNOTE-045 trial (10.1 months), despite the ARON-2 population may be more representative of pembrolizumab use in real-world context. In our ARON-2 analysis, 65% of patients received pembrolizumab as second-line therapy vs 88.2% receiving pembrolizumab as second/third-line therapy in the KEYNOTE-045. Additionally, 66% had visceral metastases (18% liver) vs 89.2% (33.7% liver), and 17% had ECOG-PS ≥ 2 vs 0.7%, in ARON-2 study compared to KEYNOTE-045 trial [6, 7].
Our study presents several limitations, mainly due to its retrospective nature. A centralized review of radiological imaging was not performed and patient not assessable for response were excluded. Furthermore, we had no available data on hemoglobin levels, concomitant medications or other comorbidities that could affect the efficacy of pembrolizumab. Consequently, our results should be interpreted with caution and are in need of a larger prospective validation.
Furthermore, an important limitation related to overall survival is the low rate of successive therapies with enfortumab vedotin, which is currently the standard third-line treatment, but at the time of data collection, this treatment was not available in most of the countries enrolled in the study.
Another important point must be considered: in a Presidential Symposium at the ESMO Congress 2023 (Madrid, 20–24 October), practice-changing results were presented from the phase III trial EV-302/KEYNOTE-A39. In this trial, the combination of enfortumab vedotin with pembrolizumab almost doubled median progression-free survival (PFS) (12.5 versus 6.3 months, respectively; hazard ratio [HR] 0.45; 95% confidence interval [CI] 0.38–0.54; p < 0.00001) and median overall survival (OS) (31.5 versus 16.1 months, respectively; HR 0.47; 95% CI 0.38–0.58; p < 0.00001) compared with chemotherapy (cisplatin or carboplatin plus gemcitabine) in patients with previously untreated, locally advanced or metastatic urothelial carcinoma [20].
Nevertheless, this real-world data analysis shows that pembrolizumab was effective as second-line therapy for advanced UC patients. Further studies investigating the biological and immunological characteristics of UC patients are warranted in order to optimize the outcome of patients receiving immunotherapy in this setting.
Supplementary information
Authors contributions
Conceptualization, material preparation, data collection, methodology, analysis, validation and writing of the original draft were performed by Francesco Massari, Matteo Santoni, Camillo Porta and Joaquim Bellmunt. All authors commented on previous versions of the manuscript and contributed to review. All authors read and approved the final manuscript.
Funding
Open access funding provided by Alma Mater Studiorum - Università di Bologna within the CRUI-CARE Agreement. The authors declare that no funds, grants or other support were received during the preparation of this manuscript.
Data availability
The data that support the findings of this study are available from the corresponding author, F.M., upon reasonable request.
Declarations
Conflict of interests
Matteo Santoni has received research support and honoraria from Janssen, Bristol Myers Squibb, Ipsen, MSD, Astellas and Bayer, all unrelated to the present paper. Francesco Massari has received research support and/or honoraria from Astellas, BMS, Janssen, Ipsen, MSD and Pfizer outside the submitted work. R. Kanesvaran has received fees for speaker bureau and advisory board activities from the following companies; Pfizer, MSD, BMS, Eisai, Ipsen, Johnson and Johnson, Merck, Amgen, Astellas and Bayer. Enrique Grande has received honoraria for speaker engagements, advisory roles or funding of continuous medical education from Adacap, AMGEN, Angelini, Astellas, Astra Zeneca, Bayer, Blueprint, Bristol Myers Squibb, Caris Life Sciences, Celgene, Clovis-Oncology, Eisai, Eusa Pharma, Genetracer, Guardant Health, HRA-Pharma, IPSEN, ITM-Radiopharma, Janssen, Lexicon, Lilly, Merck KGaA, MSD, Nanostring Technologies, Natera, Novartis, ONCODNA (Biosequence), Palex, Pharmamar, Pierre Fabre, Pfizer, Roche, Sanofi-Genzyme, Servier, Taiho and Thermo Fisher Scientific. EG has received research grants from Pfizer, Astra Zeneca, Astellas and Lexicon Pharmaceuticals. Fernando Sabino Marques Monteiro has received research support from Janssen, Merck Sharp Dome and honoraria from Janssen, Ipsen, Bristol Myers Squibb and Merck Sharp Dome. Camillo Porta has received honoraria from Angelini Pharma, AstraZeneca, BMS, Eisai, General Electric, Ipsen and MSD and acted as a Protocol Steering Committee Member for BMS, Eisai and MSD. Sebastiano Buti received honoraria as speaker at scientific events and advisory role by BMS, Pfizer, MSD, Ipsen, AstraZeneca, Merck. Joaquim Bellmunt reports institutional and personal, paid and unpaid support for research, advisory boards, consultancy and honoraria (all outside this work) from: AstraZeneca, Bristol Myers Squibb, EMD Serono, Merck, Pfizer, Roche, Sanofi/Aventis, Up-To-Date, CME events (Peerview, OncLive,), outside the submitted work. No speaker’s bureau. Javier Molina-Cerrillo declares consultant, advisory or speaker roles for IPSEN, Roche, Pfizer, Sanofi, Janssen and BMS. JMC has received research grants from Pfizer, IPSEN and Roche. Jose Carlos Tapia has received travel, accommodations, expenses from Roche, Pfizer, Merck and Lily. Zin Myint has received research support from Merck but not related to the present paper. Patrizia Giannatempo has received research support from Ipsen, Astra Zeneca e MSD and honoraria for speaker engagements, advisory roles from Astellas, MSD, Janssen, Pfizer. The other authors declare to have no conflicts of interest.
Ethical approval
The study was approved by the ethical committee of the Marche Region.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Footnotes
Camillo Porta, Joaquim Bellmunt co-senior authors.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Francesco Massari and Matteo Santoni have contributed equally to his work.
References
- 1.https://gco.iarc.fr/ Accessed on October 8th, 2022
- 2.Mohanty SK, Lobo A, Cheng L. The 2022 revision of World Health Organization classification of tumors of the urinary system and male genital organs: advances and challenges. Hum Pathol. 2022;S0046–8177(22):00224–226. doi: 10.1016/j.humpath.2022.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Cronin KA, Lake AJ, Scott S, Sherman RL, Noone AM, Howlader N, et al. Annual report to the Nation on the status of cancer, part I: national cancer statistics. Cancer. 2018;124(13):2785–2800. doi: 10.1002/cncr.31551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mollica V, Rizzo A, Montironi R, Cheng L, Giunchi F, Schiavina R, et al. Current strategies and novel therapeutic approaches for metastatic urothelial carcinoma. Cancers (Basel) 2020;12(6):1449. doi: 10.3390/cancers12061449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powles T, Smith K, Stenzl A, Bedke J. Immune checkpoint inhibition in metastatic urothelial cancer. EurUrol. 2017;72:477–481. doi: 10.1016/j.eururo.2017.03.047. [DOI] [PubMed] [Google Scholar]
- 6.Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fradet Y, Bellmunt J, Vaughn DJ, Lee JL, Fong L, Vogelzang NJ, et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: results of >2 years of follow-up. Ann Oncol. 2019;30(6):970–976. doi: 10.1093/annonc/mdz127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz LH, Litière S, de Vries E, Ford R, Gwyther S, Mandrekar S, et al. RECIST 1.1-Update and clarification: from the RECIST committee. Eur J Cancer. 2016;62:132–137. doi: 10.1016/j.ejca.2016.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Heijden MS, Loriot Y, Durán I, Ravaud A, Retz M, Vogelzang NJ, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma: a long-term overall survival and safety update from the phase 3 IMvigor211 clinical trial. Eur Urol. 2021;80(1):7–11. doi: 10.1016/j.eururo.2021.03.024. [DOI] [PubMed] [Google Scholar]
- 10.Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383(13):1218–1230. doi: 10.1056/NEJMoa2002788. [DOI] [PubMed] [Google Scholar]
- 11.Bajorin DF, Witjes JA, Gschwend JE, Schenker M, Valderrama BP, Tomita Y, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med. 2021;384(22):2102–2114. doi: 10.1056/NEJMoa2034442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Necchi A, Anichini A, Raggi D, Briganti A, Massa S, Lucianò R, et al. Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma (PURE-01): an open-label, single-arm, Phase II study. J Clin Oncol. 2018;36(34):3353–3360. doi: 10.1200/JCO.18.01148. [DOI] [PubMed] [Google Scholar]
- 13.Szabados B, Kockx M, Assaf ZJ, van Dam PJ, Rodriguez-Vida A, Duran I, et al. Final results of neoadjuvant atezolizumab in cisplatin-ineligible patients with muscle-invasive urothelial cancer of the bladder. Eur Urol. 2022;82(2):212–222. doi: 10.1016/j.eururo.2022.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Meghani K, Cooley LF, Choy B, Kocherginsky M, Swaminathan S, Munir SS, et al. First-in-human intravesical delivery of pembrolizumab identifies immune activation in bladder cancer unresponsive to bacillus Calmette-Guérin. Eur Urol. 2022;82(6):602–610. doi: 10.1016/j.eururo.2022.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer. 2018;118(1):9–16. doi: 10.1038/bjc.2017.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bellmunt J, de Wit R, Fradet Y, Climent MA, Petrylak DP, Lee JL, et al. Putative biomarkers of clinical benefit with pembrolizumab in advanced urothelial cancer: results from the KEYNOTE-045 and KEYNOTE-052 landmark trials. Clin Cancer Res. 2022;28(10):2050–2060. doi: 10.1158/1078-0432.CCR-21-3089. [DOI] [PubMed] [Google Scholar]
- 17.Lee KWC, Lord SJ, Kasherman L, Marschner I, Stockler M, Gralla R, et al. The impact of smoking on the effectiveness of immune checkpoint inhibitors - a systematic review and meta-analysis. Acta Oncol. 2020;59(1):96–100. doi: 10.1080/0284186X.2019.1670354. [DOI] [PubMed] [Google Scholar]
- 18.Hu D, Pang X, Luo J, Zhou J, Wang N, Tang H, et al. The correlation between the influencing factors and efficacy of immune checkpoint inhibitor therapy: an umbrella meta-analysis of randomized controlled trials. Ann Med. 2023;55(1):2215543. doi: 10.1080/07853890.2023.2215543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makrakis D, Talukder R, Lin GI, Diamantopoulos LN, Dawsey S, Gupta S, et al. Association between sites of metastasis and outcomes with immune checkpoint inhibitors in advanced urothelial carcinoma. Clin Genitourin Cancer. 2022;20(5):e440–e452. doi: 10.1016/j.clgc.2022.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Powles TB, Perez Valderrama B, Gupta S, Bedke J, Kikuchi E, Hoffman-censits J, et al. Ann Oncol. 2023;34(suppl_2):S1254–S1335. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, F.M., upon reasonable request.